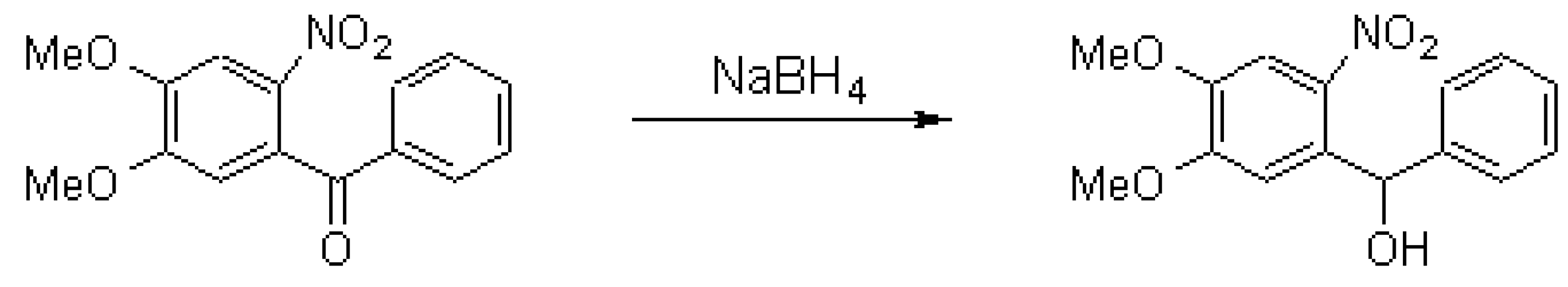
The general part of the experimental section [1] has been presented elsewhere. To a boiling solution of 4,5-dimethoxy-4-nitrobenzophenone (10.0 g, 35 mmol) in ethanol (85 ml), NaBH4 (0.61 g) was added gradually. After an hour the reaction mixture was diluted with water (300 ml) and then acidified with hydrochloric acid (20 %) to pH 6. The precipitate obtained was filtered off and recrystallized from ethanol to yield 7.75 g (77 %) of 4,5-dimethoxy-2-nitrobenzhydrol as yellow needles.
M.p. 130¡ãC (ethanol).
IR (cm-1): 3500 (OH).
1H NMR (CDCl3, 80 MHz): 7.47 (s, 1H,3-HAr); 7.17 (s, 5H, HPh); 7.13 (s, 1H, 6-HAr); 6.37 (s, 1H, CH); 3.80 (s, 6H, OCH3); 3.12 (broad s, 1H, OH).
Anal. calc. for C15H15NO5 (289.30): C 62.28, H 5.23; Found: C 62.03, H 5.41.
Supplementary materials
Supplementary File 1Supplementary File 2Reference
- Gutnov, A.V.; Butin, A.V.; Abaev, V.T.; Krapivin, G.D.; Zavodnik, V.E. Furyl(aryl)alkanes and Their Derivatives.19. Synthesis of Benzofuran Derivatives via 2-Hydroxyaryl-R-(5-methylfur-2-yl)methanes. Reaction of Furan Ring Opening - Benzofuran Ring. Molecules 1999, 4, 204–218. [Google Scholar] [CrossRef]
- Sample availability: available from the authors and from MDPI.
© 1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/