Abstract
Dietary bioactive compounds are increasingly explored as complementary cardioprotective strategies, and the nitration of unsaturated fatty acids has emerged as a process capable of enhancing antiplatelet properties. This study investigated whether Phaseolus vulgaris L. extracts can generate nitrated fatty acids under gastric-like conditions and evaluated their effects on human platelet function. Bean extracts and major fatty acids were nitrated in vitro and tested using washed platelets to assess cytotoxicity, TRAP-6 and collagen-induced aggregation, activation markers (P-selectin, CD63), and mitochondrial responses including membrane potential, ROS production, and Ca2+ dynamics. Nitrated extracts markedly inhibited TRAP-6 induced aggregation (IC50 ≈ 1.8 mg/mL), whereas non-nitrated extracts showed minimal activity; this effect was reversed by β-mercaptoethanol, indicating dependence on electrophilic nitroalkenes. Fractionation revealed that the lipidic fraction accounted for most of the antiplatelet effect, and isolated nitrated fatty acids (NO2-LN, NO2-LA, NO2-OA) displayed stronger inhibition than their native counterparts without increasing cytotoxicity. Nitrated species additionally reduced mitochondrial membrane potential and granule secretion without elevating ROS. These findings identify Phaseolus vulgaris L. as a natural source of bioactive nitrated fatty acids and support their potential as nutraceutical agents capable of modulating platelet activation and contributing to cardiovascular risk reduction.
1. Introduction
Cardiovascular diseases (CVDs) continue to pose a significant global public health burden because of their high morbidity and mortality rates. These conditions, which include hypertension, coronary artery disease, cerebrovascular disease, heart failure, and other cardiac disorders, are among the leading causes of death worldwide [1]. The global prevalence of coronary artery disease ranges from approximately 5–8%, while peripheral artery disease affects 10–20% of the population, depending on variables such as study design, population age, sex, and geographical location [2]. The socioeconomic impact of these diseases is profound, highlighting the need for changes in clinical management through increased adherence to treatment guidelines, lifestyle modification, and pharmacological or non-pharmacological interventions [3].
Platelets play a central role in the pathogenesis of cardiovascular events. These anucleate blood components, derived from bone marrow megakaryocytes, are essential for hemostasis because of their capacity to adhere to damaged endothelium and aggregate at injury sites [4,5]. While platelet activation and aggregation are crucial for vascular repair following atherosclerotic plaque rupture, excessive or uncontrolled platelet activation can lead to thrombus formation, vascular occlusion, and subsequent ischemic events such as myocardial infarction and stroke [6]. Given the key role of platelets in thrombotic complications, targeting platelet function remains a cornerstone of pharmacological intervention [7]. In parallel, there is increasing interest in dietary strategies that can complement traditional therapies. The development of functional foods and nutraceuticals with cardioprotective properties is gaining momentum, particularly in response to the growing global prevalence of CVDs [8].
Phaseolus vulgaris L., commonly known as the common bean, is a staple legume recognized for its nutritional richness and health benefits. It is a major source of plant based protein (16–33%) and is especially valuable in vegetarian diets and low-income populations [9]. Besides its protein content, beans are abundant in essential vitamins (e.g., B-group vitamins, vitamin E, and vitamin C), minerals, complex carbohydrates, and bioactive compounds such as polyphenols, which contribute to antioxidant activity [10,11]. Previous studies have suggested that the cardioprotective effects of bean extracts may be linked to their antioxidant content, particularly polyphenols that can neutralize reactive oxygen species (ROS) and mitigate platelet hyperactivity [12,13]. Recent interest has emerged in enhancing the bioactivity of such extracts through processing techniques such as nitration, which may increase endogenous nitric oxide (NO) production. NO is a key regulator of vascular tone and platelet function because of its vasodilatory and antiplatelet effects [14]. Although beans are naturally low in fat, they contain essential fatty acids, notably the polyunsaturated fatty acids linoleic acid (LA) and alpha-linolenic acid (ALA), which are beneficial for cardiovascular and neurological health [15].
During digestion, unsaturated fatty acids can undergo endogenous nitration through interactions with reactive nitrogen species derived from NO oxidation. Nitrogen dioxide (NO2), formed from dietary nitrites in the acidic environment of the stomach, acts as a key nitrating agent in this process [16]. This reaction results in the formation of nitro-fatty acids (NO2-FAs), including nitro-oleic acid (NO2-OA), nitro-linoleic acid (NO2-LA), and nitro-linolenic acid (NO2-LN), which have been shown to modulate vascular tone, inflammation, and platelet activation [17]. Extensive experimental evidence has shown that NO2-FAs offer protection against various cardiovascular and metabolic insults, including atherosclerosis, cardiac ischemia–reperfusion injury, diabetes, hypertension, and vascular inflammation [18,19,20]. These compounds can attenuate endothelial dysfunction by inhibiting membrane adhesion molecule expression and reducing platelet activation [21]. Moreover, NO2-FAs exert cytoprotective effects under conditions of mitochondrial dysfunction by forming electrophilic adducts with critical residues in key regulatory proteins [22,23]. Their impact on mitochondrial function ranges from respiratory inhibition to protective effects in models of ischemia–reperfusion injury. Moreover, NO2-FAs derived from dietary sources such as extra virgin olive oil have been linked to improved mitochondrial function in non-alcoholic fatty liver disease [23].
The global rise in chronic diseases associated with sedentary lifestyles and the shift from traditional diets to those rich in refined carbohydrates and unhealthy fats underscore the need for sustainable dietary strategies to promote cardiovascular health. In this context, Phaseolus vulgaris L., a widely consumed, nutritionally dense legume, may serve as a valuable dietary source of NO2-FAs. The present study aimed to investigate the generation of NO2-FAs from Phaseolus vulgaris L. under simulated gastric conditions and to evaluate their antiplatelet properties. By exploring the potential of bean derived NO2-FAs, this research seeks to enhance the nutritional and therapeutic value of a widely accessible food crop in Chile and beyond.
2. Results
2.1. Effect of the Nitration Process on the Extract’s Antiplatelet Activity
Lipids within platelets do not directly trigger aggregation; instead, they play a crucial role in modulating the response to other aggregating agents. They act as fine tuning regulators, functioning either as inhibitors, such as omega-3 fatty acids, or as pro-thrombotic mediators, such as thromboxane A2 precursors. Since dietary lipids preferentially react with nitrating agents during digestion, changes in the biological activity of nutraceuticals are commonly observed.
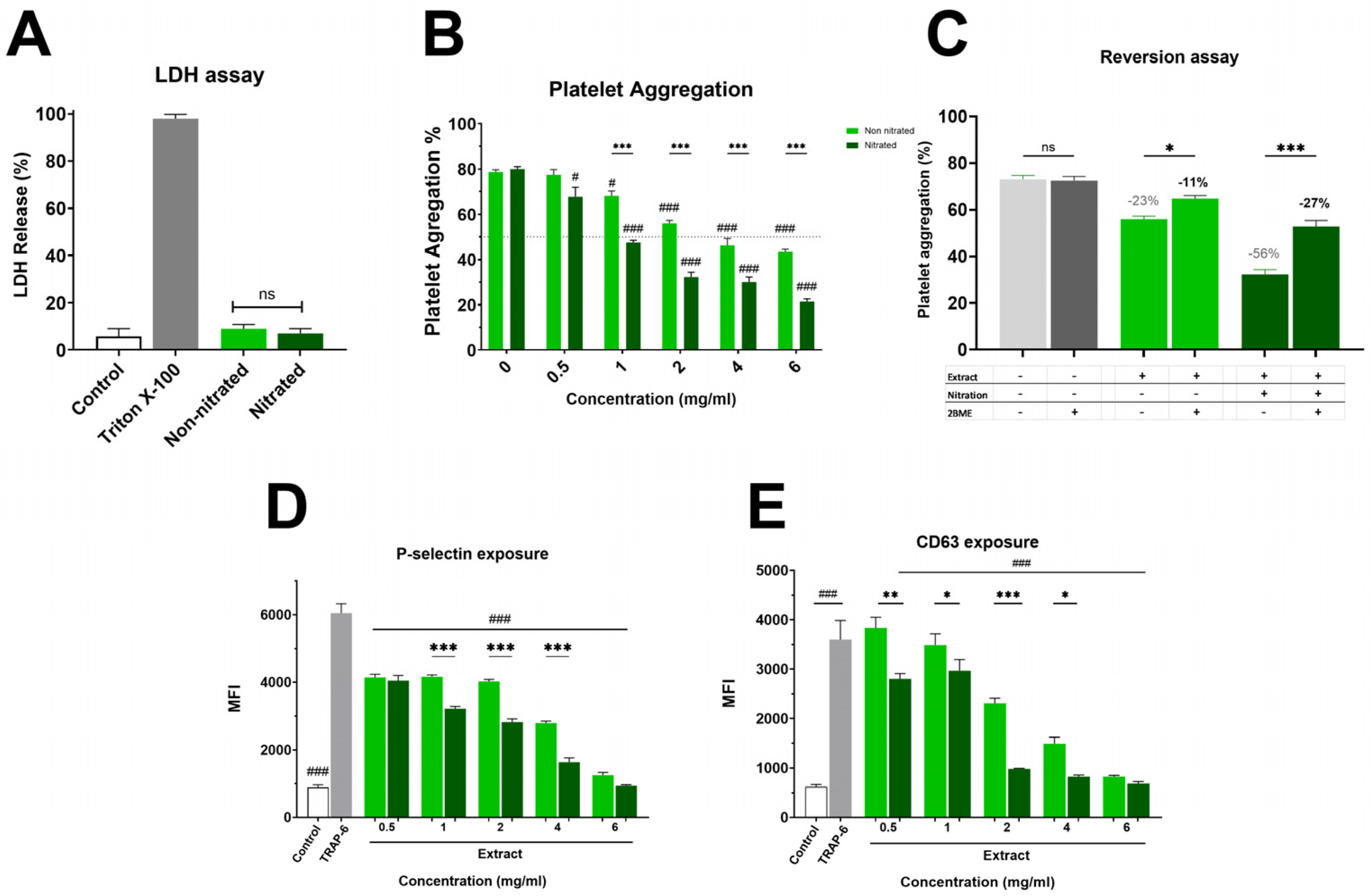
Figure 1A and Supplementary Material Figure S1 show the impact on platelet viability. The non-nitrated extract induced slightly higher LDH release than the nitrated extract; however, this difference was not statistically significant, showing that neither extract caused platelet damage. Both extracts were evaluated in the platelet aggregation assay, where a marked difference in antiplatelet activity was observed (Figure 1B). The nitrated extract exhibited strong inhibition of platelet aggregation, with an IC50 of 1.8 ± 0.1 mg/mL. In contrast, the non-nitrated extract showed a much weaker effect, with an IC50 exceeding the highest concentration tested (6 mg/mL). This enhanced antiplatelet effect of the nitrated extract was also clear in granule secretion markers. As shown in Figure 1C, P-selectin externalization was lower in platelets treated with the nitrated extract compared to the non-nitrated extract. Both extracts inhibited P-selectin expression relative to the control, with the nitrated extract showing a greater inhibitory effect. Finally, Figure 1D illustrates platelet activation assessed by CD63 surface expression. The analysis suggests that the nitrated extract significantly reduces CD63 exposure, further supporting its inhibitory effect on both platelet activation and aggregation. Overall, nitration enhances the antiplatelet properties of the extract without increasing cytotoxicity in TRAP-6 activated platelets.
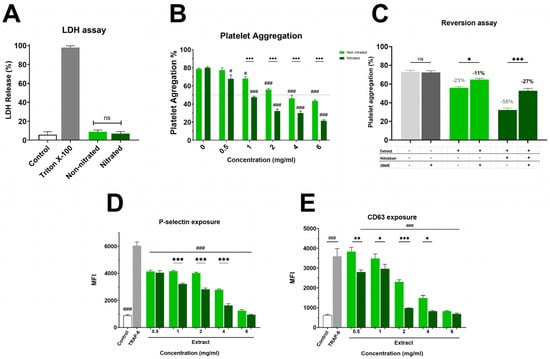
Figure 1.
Cytotoxicity and Platelet Aggregation by Nitrated vs. Non-nitrated Extracts. (A) LDH release following incubation with either nitrated or non-nitrated extracts was evaluated. Green represents the non-nitrated extract, and deep green shows the nitrated extract. Triton X-100 was used as a positive control. To evaluate platelet aggregation and activation, washed platelets were pre-incubated with increasing concentrations of each extract (0.5-6.0 mg/mL) and activated with TRAP-6 (5 µM). (B) Platelet aggregation in response to nitrated and non-nitrated extracts at various concentrations, using the same color scheme. TRAP-6 (5 µM) served as a positive control. (C) Platelet aggregation was assessed after treatment with control, nitrated, or non-nitrated extracts, in the presence or absence of β-mercaptoethanol (BME). (D) P-selectin expression in TRAP-6 stimulated platelets following pre-incubation with nitrated and non-nitrated extracts at various concentrations. (E) CD63 exposure on the platelet membrane under the same conditions as in (D). Data are presented as mean ± SEM (n = 6 per group). The statistical significance was assessed as follows: comparison against TRAP-6 control is showed by # (# p < 0.05 and ### p < 0.001); comparison against the corresponding non-nitrated concentration is indicated by * (* p < 0.05, ** p < 0.01 and *** p < 0.001). “ns” shows no statistically significant difference.
While the extracts significantly inhibited TRAP-6 induced aggregation, no significant effects were observed when collagen (2 µg/mL) was used as the agonist (Table S1). This finding is consistent with a preferential modulation of PAR-1 associated pathways rather than GPVI mediated platelet activation.
2.2. Role of Nitration Bonds on Antiplatelet Activity
The platelet aggregation data revealed a clear difference in biological activity between nitrated and non-nitrated Hallado Alemán extracts. To determine whether the presence of electrophilic nitroalkenes was responsible for the increase in antiplatelet activity, the extracts were pre-incubated with an excess of BME, followed by addition to platelets, and aggregation was evaluated (Figure 1C). Control samples with TRAP-6 plus BME showed aggregation values around 69–73%, indicating that BME alone does not influence platelet activation induced by TRAP-6, thus ruling out confounding effects on platelet function. When the nitrated extract was pre-incubated with 1 mM BME, platelet aggregation increased notably, showing a substantial loss of antiplatelet activity. This suggests that electrophilic nitroalkenes (nitrated fatty acids with the NO2 group at the double bond) handle the inhibition of platelet aggregation. In the presence of BME, the nitrated extract behaved similarly to the non-nitrated one. In contrast, for the non-nitrated extract, incubation with BME did not significantly alter platelet aggregation, confirming that the observed BME effect is specifically related to blocking the effects of nitrated fatty acids rather than any non-specific effects on the extract.
2.3. Comparative Antiplatelet Effects of Lipidic, Phenolic, and Combined Extract Fractions
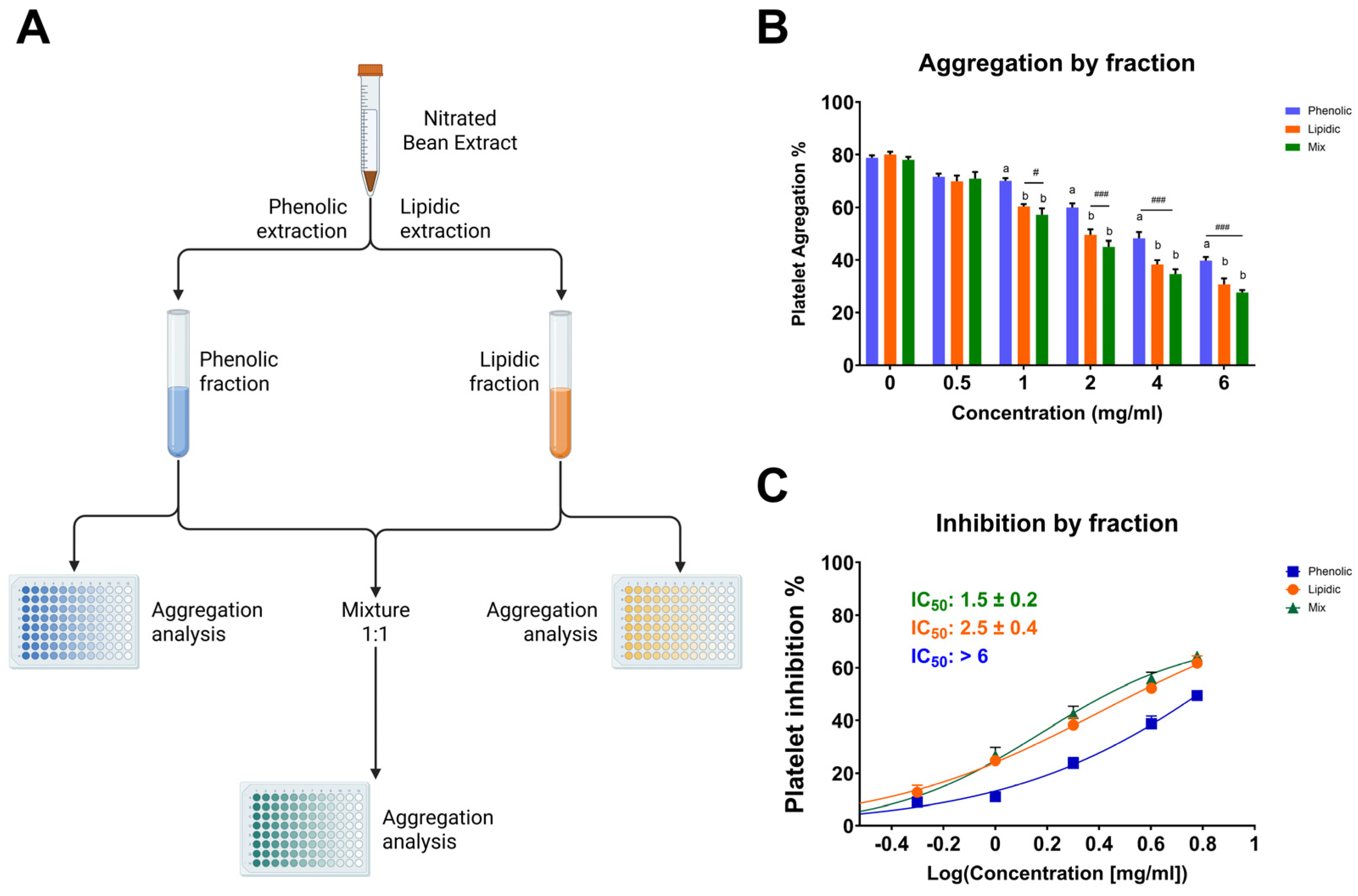
Among the tested fractions, the lipidic extract exhibited the most potent antiplatelet activity, suggesting that the bioactive components responsible for inhibiting platelet aggregation are mainly concentrated in the lipidic portion (Figure 2). At a concentration of 6 mg/mL, the lipidic fraction reduced platelet aggregation to 34.2 ± 2.3%, in contrast to 43.7 ± 1.4% for the phenolic fraction and 30.7 ± 1.7% for the combination of both (lipidic and phenolic). This trend was consistent across all concentrations tested. Notably, the mixture of fractions did not show a markedly synergistic effect compared to the lipidic extract alone, reinforcing the hypothesis that the lipidic fraction contains the primary compounds responsible for the antiplatelet activity. Since this fraction was subjected to nitration, it is plausible that nitrated lipid species, such as NO2-FAs, play a central role in the observed bioactivity. These results underscore the significance of the nitration process in augmenting antiplatelet efficacy and reinforce the notion that targeted chemical modifications of natural lipid matrices can yield novel antithrombotic agents.
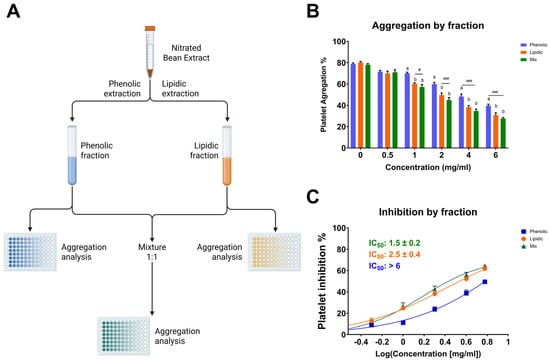
Figure 2.
Antiplatelet activity of lipidic, phenolic, and combined (1:1) fractions of the nitrated extract. (A) Schematic representation of the extraction process used to obtain the phenolic, lipidic, and mixed (1:1) fractions. (B) Platelet aggregation induced by TRAP-6 (5 µM) in the presence of fractions at various concentrations. (C) IC50 values were calculated for phenolic, lipidic, and mixed fractions. Washed platelets were pre-incubated with increasing concentrations of each fraction (0.5-6.0 mg/mL), followed by stimulation with TRAP-6 (5 µM). Data are presented as mean ± SEM (n = 6 per group). Statistical significance was assessed as follows: comparison against TRAP-6 control is indicated by # (# p < 0.05, and ### p < 0.001). The different letters (a and b) in the column show significant differences between them according to Tukey’s test (p < 0.05).
2.4. Determination of Fatty Acid Content of Bean Extract
As shown in Table 1, polyunsaturated fatty acids (PUFAs) account for 60.91% of the total fatty acids present in the bean extract, followed by monounsaturated fatty acids (MUFAs) and saturated fatty acids (SFAs). Figure S2 shows the chemical structure of the saturated and unsaturated fatty acids identified in the extract. The major unsaturated fatty acids in bean extract are LN, LA, and OA in that order. These results support that the bean extract is a relevant food substrate for NO2-FAs formation under acidic gastric conditions, impacting its nutraceutical properties.

Table 1.
Fatty acid composition of the Phaseolus vulgaris L. (Hallado Alemán variety) bean extract.
2.5. Antiplatelet Activity of Major Nitrated Fatty Acids in the Bean Extract
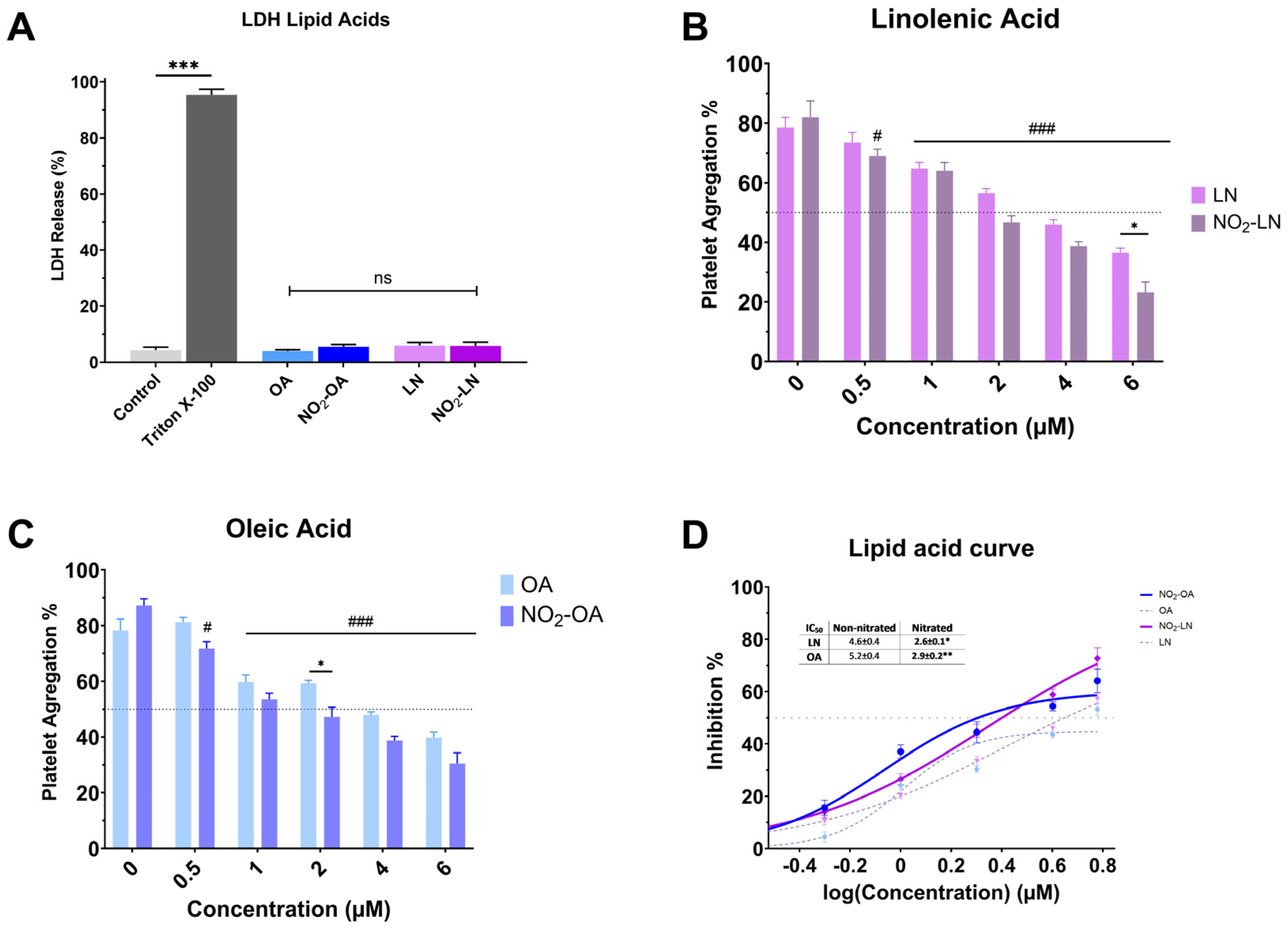
Nitration alters the chemical characteristics of fatty acids, potentially increasing their bioactivity. Nitrated fatty acids have been shown to have a variety of biological effects, including antibacterial, anti-inflammatory, and vasodilator properties. We analyzed the cytotoxicity of individual NO2-FAs (Figure 3A). Nitration does not increase the toxicity levels of the NO2-FAs since the levels of free LDH (%) obtained are significantly lower (p < 0.001) when compared to the positive control (Triton X-100). The antiplatelet activity of each nitrated PUFA was higher compared to the non-nitrated fatty acid (Figure 3B–D).
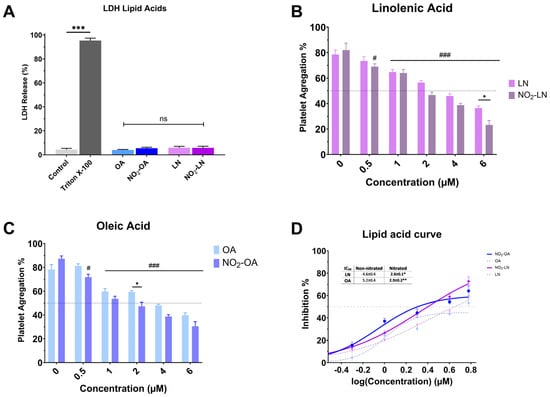
Figure 3.
Effect of isolated nitrated fatty acids on platelet aggregation. (A) LDH release from platelets exposed to nitrated and non-nitrated fatty acids. (B,C) correspond to the dose-dependent evaluation of platelet aggregation of NO2-LN and NO2-OA. (D) IC50 values were calculated for major nitrated and non-nitrated fatty acids. Washed platelets were pre-incubated with increasing concentrations of each compound (0.5–6.0 mg/mL), followed by stimulation with TRAP-6 (5 µM). Data are presented as mean ± SEM (n = 6 per group). Statistical significance was assessed as follows: comparison against TRAP-6 control is indicated by # (# p < 0.05, and ### p < 0.001); comparison of the nitrated vs. non-nitrated concentration is indicated with * (* p < 0.05, ** p < 0.01 and *** p < 0.001). “ns” indicates no statistically significant difference.
2.6. Mitochondrial Effect of Nitrated Bean Extract
The association between cardiovascular disease (CVD) development and mitochondrial dysfunction is well established. Several NO2-FAs have been reported to modulate mitochondrial metabolism, either by enhancing mitochondrial efficiency or by inhibiting specific respiratory complexes. In this context, we evaluated whether the nitrated fatty acids tested in this study influence platelet activation and mitochondrial related parameters under pro-aggregatory conditions. All results shown in Figure 4A–D were obtained using TRAP-6 activated platelets following pre-incubation with the shown nitrated fatty acids, ensuring consistency across experimental readouts.
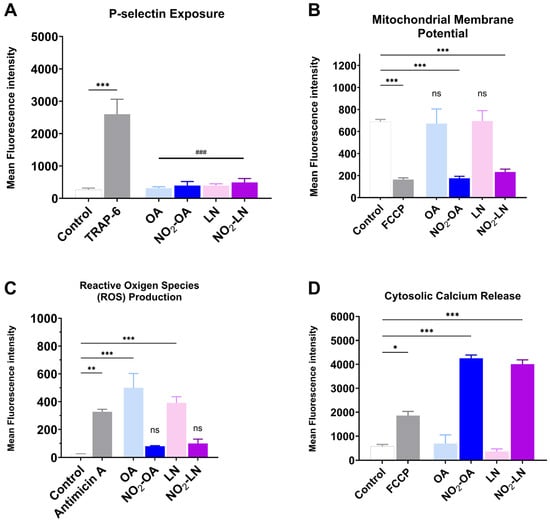
Figure 4.
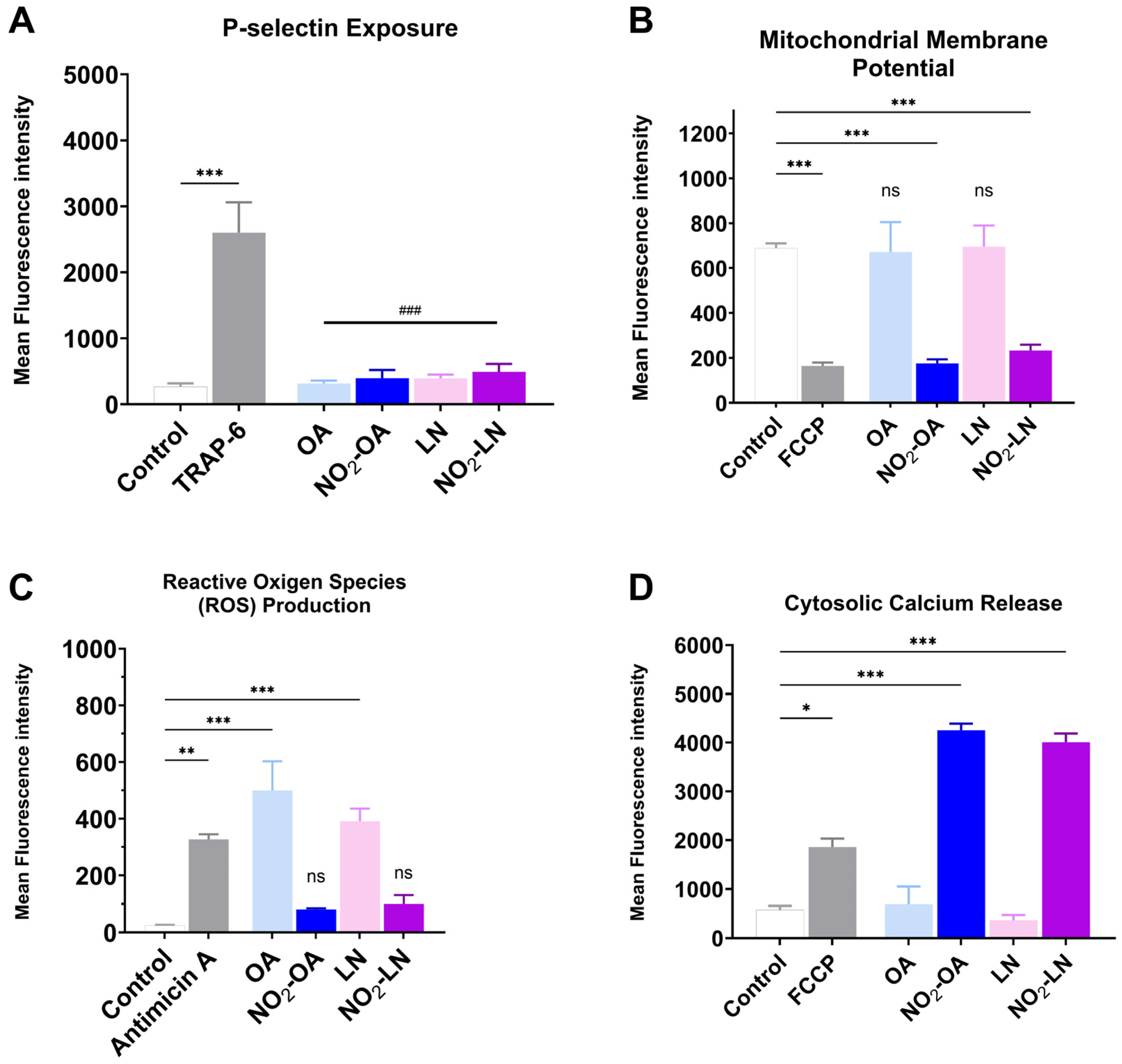
Effect on mitochondrial dysfunction of the nitrated fatty acids present in the Hallado Alemán extract. (A) P-selectin exposure induced by incubation with LN, OA, NO2-LN, and NO2-OA was evaluated by flow cytometry. Positive control corresponds to the condition of TRAP-6-activated platelets. (B) Mitochondrial membrane potential (MMP) modulation was determined as explained in the Methods section, with FCCP as a positive control. (C) Reactive oxygen species (ROS) production with Antimicin A as a positive control. (D) Calcium levels induced by fatty acids with FCCP as a positive control were also determined. Washed platelets were pre-incubated with the IC50 concentration of compounds (OA, NO2-OA, LN, and NO2-LN), followed by stimulation with TRAP-6 (5 µM). Data are presented as mean ± SEM (n = 6 per group). The statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test. * p < 0.05; ** p < 0.01 and *** p < 0.001. ### p < 0.001 versus TRAP-6 stimulated platelets (comparison of nitrated and non-nitrated fatty acids with the agonist). “ns” indicates no statistically significant difference. FCCP: carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone. Control corresponds to DMSO 0.2% (vehicle).
Figure 4A shows how each type of NO2-FAs tested reduces granule secretion, as observed by P-selectin membrane exposure. The effect on mitochondrial membrane potential, shown in Figure 4B, shows that both NO2-OA and NO2-LN decrease the mitochondrial potential, without increasing intracellular ROS (Figure 4C). Interestingly, Ca2+ homeostasis was affected by a significant increase (Figure 4D), which did not induce aggregation.
3. Discussion
Excessive platelet aggregation and thrombus formation are well-established risk factors for CVD, including coronary artery disease and stroke [24]. Recent attention has focused on NO2-FAs as promising antiplatelet agents with potential therapeutic implications [25,26]. In this study, we investigated the antiplatelet properties of NO2-FAs derived from Phaseolus vulgaris L. (common bean), with a focus on the subspecies Hallado Alemán, which is characterized by a favorable PUFAs profile [27].
The fatty acid composition of Phaseolus vulgaris L. is influenced by cultivation conditions and extraction methods. Our gas chromatography/mass spectrometry (GC/MS) analysis confirmed that the bean lipid profile is dominated by LN (33.2 g/100 g), LA (27.7 g/100 g), and OA (3.8 g/100 g), accounting for approximately 66% of total fatty acids. These findings support the potential of Phaseolus vulgaris L. as a dietary source of beneficial PUFAs, which confer cardioprotective effects.
Gastric nitration under acidic conditions facilitated the generation of NO2-FAs from these PUFAs. In vitro synthesis enabled controlled production of these bioactive compounds for further characterization [28]. NO2-FAs are known for their anti-inflammatory and antioxidant properties; here we provide evidence for their antiplatelet activity. Platelet aggregation induced by thrombin receptor-activating peptide (TRAP-6) was significantly inhibited by nitrated extracts in a concentration-dependent manner, with a stronger effect than non-nitrated controls. Interestingly, the extracts had minimal impact on collagen-induced aggregation, suggesting selective inhibition of G-protein-coupled receptor (GPCR) pathways, such as those mediated by protease-activated receptor-1 (PAR-1), rather than GPVI mediated collagen signaling.
These findings are consistent with those reported by Coles et al. [29], who showed that NO2-LA inhibits thrombin mediated platelet activation by increasing intracellular cAMP levels and reducing calcium mobilization. The receptor selectivity observed in our study, whereby nitrated extracts selectively inhibited TRAP-6 induced aggregation but not collagen induced responses, supports the hypothesis that nitrated compounds derived from Phaseolus vulgaris L. preferentially modulate thrombin receptor-associated signaling pathways, as previously described for purified nitrated fatty acids. Such selectivity may be advantageous, as it allows modulation of platelet activation without broadly suppressing collagen-dependent hemostatic mechanisms.
Mechanistically, the inhibitory effect was reversed by BME, a reducing agent that disrupts nitroalkene moieties, confirming the specificity of nitration in mediating the biological activity. The non-nitrated extract showed no such reversal, reinforcing this conclusion. Fractionation of the extract further revealed that the lipid fraction was primarily responsible for the antiplatelet activity, achieving inhibition levels of ~58% at 6 mg/mL. In contrast, the phenolic fraction showed only modest effects. Notably, co-administration of both fractions did not produce additive or synergistic inhibition, showing that phenolic compounds may not significantly enhance the bioactivity of NO2-FAs in this context. NO2-FAs also suppressed key markers of platelet activation, including P-selectin and CD63, in a concentration dependent manner. While previous studies have reported that NO2-FAs may act through nitric oxide-related mechanisms [30,31], the present study does not directly address NO production or signaling. Therefore, alternative or complementary mechanisms such as modulation of phospholipase A2 activity, integrin GPIIb/IIIa signaling, or other redox-sensitive pathways may contribute to the observed effects and warrant further investigation [32].
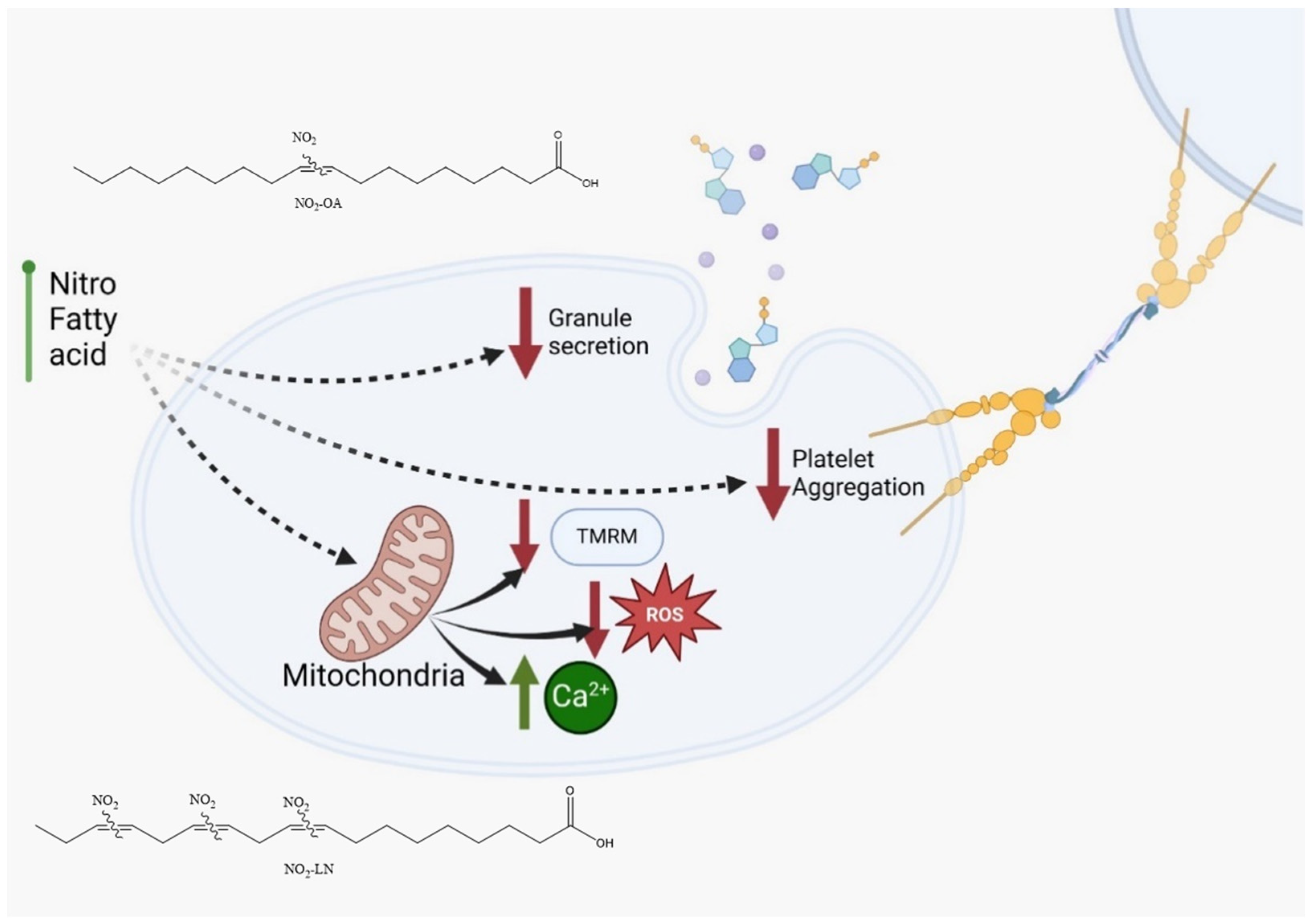
Among the fatty acids evaluated, LN emerged as a key contributor to the antiplatelet effects observed, consistent with its abundance in Hallado Alemán beans. Our data support the hypothesis that LN, particularly when nitrated, significantly contributes to the bioactivity of the extract. This aligns with other studies linking dietary PUFAs to reduced inflammatory and thrombotic activity [33]. The interaction of NO2-FAs with other bioactive compounds, such as resveratrol or polyphenols found in red wine and apples, has been reported to potentiate their antiplatelet effects [34]. Similar synergy might be explored in future studies involving dietary matrices rich in antioxidants and PUFAs. Notably, we observed that NO2-FAs from Phaseolus vulgaris L. inhibited platelet activation and mitochondrial membrane potential elevation without increasing ROS production (Figure 5). This paradoxical finding suggests that NO2-FAs may function as mild mitochondrial uncouplers or selectively modulate components of the electron transport chain. Similar effects have been reported for NO2-OA, which shifts mitochondrial metabolism toward glycolysis. This mitochondrial reprogramming may underlie some of the protective effects of NO2-FAs against oxidative stress and thrombotic risk.
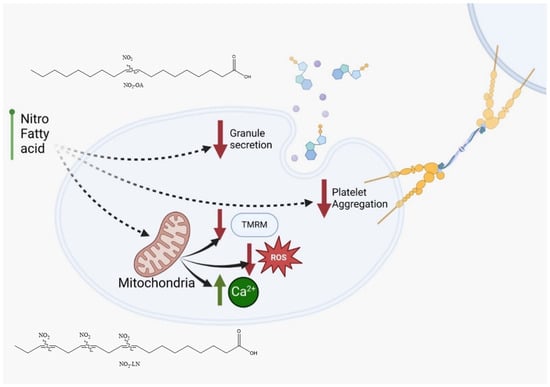
Figure 5.
NO2-FAs identified in Phaseolus vulgaris L. are proposed to possess antithrombotic properties based on their ability to inhibit platelet activation and attenuate mitochondrial membrane potential (TMRM) elevation without inducing reactive oxygen species (ROS) release. NO2-OA: nitro-oleic acid; NO2-LN: nitro-linolenic acid.
Importantly, Hallado Alemán beans offer a cost effective and widely available dietary source of PUFAs and NO2-FAs, potentially providing a practical alternative to more expensive sources such as olive oil or specialized supplements. However, despite promising in vitro data, several limitations must be addressed. Our models employed platelet-rich plasma and washed platelets, which do not fully replicate the complexity of whole blood physiology. Some assays used supra-physiological concentrations of extracts, and sample sizes were limited. Therefore, future studies should include in vivo validation, pharmacokinetic analyses, and dose–response evaluations under physiologically relevant conditions. Explaining the precise molecular pathways by which NO2-FAs exert their effects will also be critical for advancing their clinical development. These insights will be essential to establishing whether Phaseolus vulgaris L. derived NO2-FAs can serve as effective, food-based interventions for CVD prevention and therapy.
Experimental Limitations and Challenges for Physiological Extrapolation
A rigorous interpretation of the present findings requires acknowledgment of several methodological and translational limitations that constrain the extent to which the results can be generalized. Although direct mass spectrometry based characterization of nitrated species in the whole extract was not performed, several converging lines of evidence support the occurrence of fatty acid nitration and its contribution to the enhanced antiplatelet activity observed. Specifically, the nitrated extract exhibited a markedly greater inhibitory effect on platelet aggregation than the non-nitrated control, showing that the nitration process increased biological activity. Pre-incubation of the nitrated extract with β-mercaptoethanol completely abolished its antiplatelet effect, a response consistent with the presence of electrophilic nitroalkene moieties known to undergo thiol dependent Michael addition reactions. This characteristic property of nitrated unsaturated fatty acids, together with the enrichment of activity in the lipid fraction and the ability of previously synthesized and fully characterized nitrated fatty acids (e.g., NO2-LN and NO2-OA) to reproduce the observed effects, provides strong indirect chemical and biological evidence for nitration. While direct LC–MS/MS or GC–MS analyses would strengthen structural confirmation, the combined use of a validated nitration method, thiol reactivity assays, lipid fractionation, and comparative biological analyses supports the conclusion that nitration of unsaturated fatty acids contributes to the potentiated antiplatelet activity of the extract.
There is an inherent discrepancy between the effective concentrations identified in vitro and those realistically achievable in vivo. Functional assays, including platelet aggregation, activation markers, and mitochondrial bioenergetics, required concentrations of extracts or nitroalkene species that may exceed physiologically attainable levels derived from dietary intake. This discrepancy introduces uncertainty when extrapolating parameters such as IC50 to clinically relevant scenarios. The IC50 values reported here should be interpreted as comparative, system dependent parameters reflecting inhibitory behavior under the specific experimental conditions, rather than as absolute potency constants of individual chemical species. Therefore, complementary studies examining bioavailability, metabolism, and pharmacokinetics are essential to determine maximal plasma concentrations and to assess the potential endogenous formation of NO2-FAs under gastrointestinal conditions [35].
A second limitation arises from the use of simplified in vitro models, such as washed platelets and platelet-rich plasma, which do not fully capture the complexity of the human circulatory environment. Physiological modulators, including interactions with erythrocytes and leukocytes, plasma protein binding, vascular shear stress, and hepatic biotransformation, can markedly influence platelet and mitochondrial responses. As a result, the magnitude and even the direction of the effects observed in vitro may differ under physiological conditions. This underscores the need for future studies employing whole blood under shear flow, ex vivo perfusion systems, and in vivo animal models to improve the ecological validity of the findings [36].
Another limitation relates to the intrinsically low lipid content of Phaseolus vulgaris L., which restricts the extractive yield of PUFAs and consequently limits the formation of bioactive NO2-FAs. Although the lipid profile dominated by ALA, LA, and OA supports the generation of nitroalkene species, the absolute amount of lipids is low and subject to substantial variability across cultivars, environmental conditions, and processing methods. Previous studies have shown that NO2-FA formation depends critically on both lipid abundance and the redox environment [37]. Thus, the food matrix itself may inherently restrict endogenous NO2-FA generation in vivo. Strategies such as lipid enrichment, germination, enzymatic hydrolysis, or lipid fractionation should be explored to enhance PUFA bioavailability and nitration potential [38].
Finally, the absence of deeper mechanistic analyses including redox proteomics, identification of molecular targets, and validation in animal models limits our ability to fully explain the signaling pathways modulated by NO2-FAs and their potential effects on inflammation, redox homeostasis, platelet activation, and endothelial function. Previous studies show that nitrated fatty acids can modulate key regulatory pathways such as NF-κB, Nrf2, and eNOS, highlighting their biological relevance; however, systematic validation under physiological conditions remains necessary [39,40]. Together, these limitations highlight the need for future multidisciplinary research to better understand the physiological relevance, therapeutic potential, and practical applications of NO2-FAs derived from Phaseolus vulgaris L.
4. Materials and Methods
4.1. Chemicals
Thrombin receptor activating peptide 6 (TRAP-6), Antimycin (AA), dihydroethidium (DHE), intracellular calcium fluorescence indicator (Fluo-4-AM), and trifluoromethoxyphenylhydrazone (FCCP) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-CD62P-PE, anti-CD61-FITC, and anti-CD63-PE antibodies were purchased from BD Pharmingen (BD Biosciences, San Diego, CA, USA). The HPLC-grade solvents used were purchased from Burdick and Jackson (Muskegon, MI, USA).
4.2. Extraction Process of the Bean Sample
To obtain the extracts, the extraction was performed using a bean to water ratio of 1:40 for 1 h. Ultrasound-assisted extraction (UAE) was conducted at 50% amplitude (10 kHz) for a sonication duration of 60 min. The resulting samples were then centrifuged at 3500 rpm for 15 min at 20 °C. The supernatant was subsequently filtered using Corning Falcon cell filters with pore sizes of 100, 70, and 40 µm, respectively. The filtered supernatant was then frozen at −86 °C for 48 h and subsequently lyophilized (Operon, FDU 7024, Gimpo, South Korea) for 20 h before analysis [12].
4.3. Fatty Acid Composition of Bean Extract
The fatty acid composition of Phaseolus vulgaris L. was determined using gas chromatography. A minimum sample quantity of 2 g was required for analysis. Lipid extraction followed the AOAC Official Method 996.06 for Fat (Total, Saturated, and Unsaturated) in Foods, with specific modifications [41]. Briefly, 2 g of the sample was weighed, and a solution containing 2 mL of acidified methanol and 1 mL of toluene was added. The samples were subjected to rotary tube shaking at 1700 rpm for 10 min and then placed in a water bath at 90 °C for 2 h, with temperature control during the initial 30 min. After this, the samples were cooled to room temperature, mixed with 5 mL of saturated NaCl, and centrifuged for 8 min at 4000 rpm. The toluene phase obtained was then injected into the gas chromatography system for further analysis.
Fatty acid composition analysis was performed by gas chromatography using a flame ionization detector (FID) maintained at 285 °C. Separation was achieved using a 100 m × 0.25 mm capillary column with a 0.20 µm bis-cyanopropyl stationary phase (HP-88 or SP-2560). The oven temperature program started at 100 °C with a 4 min hold, followed by a ramp of 3 °C/min to 240 °C, which was maintained for 15 min.
4.4. Nitration of the Bean Extract and Its Major Fatty Acids
Lyophilized bean extract (Phaseolus vulgaris) (100 mg) was transferred to a glass reaction tube and resuspended in 1.0 mL of sodium phosphate buffer adjusted to pH 3.0 to simulate acidic gastric conditions. The suspension was vortex-mixed for 1 min to ensure homogenization. A magnetic stirring bar was added, and the tube was placed in a water bath on a magnetic stirrer maintained at 37 °C. The temperature of the water bath was gradually increased and monitored until reaching 41 °C [42].
Before initiating the reaction, dissolved oxygen was removed by purging the reaction mixture, and the glass tube was immediately sealed to maintain an oxygen-depleted environment. The nitration reaction was initiated by the addition of sodium nitrite (NaNO2) to reach a final concentration of 5 mM, followed by continuous stirring at 37 °C. The reaction mixture was maintained under these conditions for a total reaction time of 60 min. Control samples (non-nitrated extracts) were processed in parallel under identical experimental conditions, except that NaNO2 was omitted. In addition, the major fatty acids oleic acid (OA), linoleic acid (LA), and linolenic acid (LN) were nitrated and extracted as previously reported [16,28].
Extraction of Nitrated Lipids
Following completion of the nitration reaction, nitrated lipids were extracted using an organic solvent system. Briefly, 2.5 mL of a hexane/isopropanol/acetic acid mixture was added to each reaction tube using glass pipettes, and the mixture was vortexed for 1 min. Subsequently, 2.5 mL of hexane was added, followed by vortex mixing for an additional 1 min. Samples were then centrifuged at 1800 rpm for 5–6 min to achieve phase separation.
The upper organic phase was carefully collected and transferred to clean glass tubes. The remaining aqueous phase was re-extracted with an additional 2.5 mL of hexane, vortexed for 1 min, and centrifuged under the same conditions. The organic phases were combined and evaporated to dryness under controlled conditions, and the resulting extracts were weighed to determine the extraction yield. Dried samples were stored at −20 °C until further analysis [21,43].
4.5. Preparation of Human Platelets
A total of six healthy volunteers aged 20–65 years were recruited for this study. Inclusion criteria consisted of individuals with no clinical history of cardiovascular, metabolic, or hematological diseases. Exclusion criteria were: (i) use of any medication, specifically antiplatelet or anti-inflammatory drugs (e.g., NSAIDs), within 7 days before blood collection; (ii) smoking; and (iii) consumption of dietary supplements that could interfere with platelet function. All participants provided written informed consent before blood withdrawal, and the study was conducted in accordance with the Declaration of Helsinki and approved by the Local Ethics Committee (Protocol 16-2023) [44]. After reading and signing the informed consent, 20 mL venous blood samples were collected by phlebotomy using a 21 G needle [5]. Each sample (5 mL) was centrifuged using a DCS-16 Centrifugal Presvac RV centrifuge (Presvac, Buenos Aires, Argentina) at 240 g for 10 min to obtain platelet-rich plasma (PRP). Two-thirds of the PRP was then removed, and the remaining portion was centrifuged at 650× g for 10 min to obtain platelet-poor plasma (PPP). The remaining 5 mL was processed similarly, first centrifuged to obtain PRP and then further centrifuged at 650× g for 10 min. The pellet was washed with HEPES-Tyrode’s buffer containing Prostaglandin E1 (PGE1, 120 nmol/L). PRP and washed platelets were adjusted to a concentration of 200–300 × 106 platelets/mL using a Bayer Advia 60 Hematology System (Bayer Diagnostics, Tarrytown, NY, USA). Data are expressed as the mean ± standard error of the mean (SEM) from at least six independent experiments (n = 6), each performed using platelet samples obtained from different healthy volunteers.
4.6. Cytotoxicity of Extracts
Washed platelets (3 × 108 platelets/mL) were incubated for 10 min at 37 °C with the highest concentration of extracts tested (6 mg/mL). Then, platelets were centrifuged at 800× g for 8 min, and the resulting supernatant was analyzed with the lactate dehydrogenase (LDH) cytotoxicity assay kit (Cayman Chemical, Ann Arbor, MI, USA). The absorbance of the reaction was measured at 490 nm in a microplate reader (Microplate Reader (Thermo Scientific MultiskanTM Go, Thermo Fisher Scientific, Vantaa, Finland), with 10% TritonX-100 used as a positive control [45].
4.7. Inhibition of Platelet Aggregation of Bean Extract and Fatty Acids
Platelet aggregation was assessed using a changed microplate assay. Washed platelets were incubated with nitrated or non-nitrated Phaseolus vulgaris L. (bean) extracts at varying concentrations (0.5, 1, 2, 4, and 6 mg/mL) or vehicle control (0.2% DMSO) at 37 °C for 6 min. Aggregation was then stimulated with either TRAP-6 (5 μM) or collagen (2 μg/mL). Samples were transferred to 96-well plates and shaken at 1200 rpm for 5 min at 37 °C. Platelets treated with 0.2% DMSO were used as the control for maximal aggregation. All treatments were prepared using DMSO as a vehicle to ensure equivalent solvent conditions across experimental groups; the final DMSO concentration did not exceed 0.2% (v/v), a level confirmed not to affect platelet viability or function. Absorbance was measured at 450 nm using a microplate spectrophotometer (Thermo Scientific Multiskan™ GO, Thermo Fisher Scientific, Vantaa, Finland). To determine the concentration required to inhibit 50% of platelet aggregation (IC50), dose–response curves were constructed for both nitrated and non-nitrated bean extracts. To determine the half-maximal inhibitory concentration (IC50), dose–response curves were generated by fitting the experimental data to a nonlinear regression model (log[inhibitor] vs. normalized response with variable slope) using GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA). Each IC50 value corresponds to the concentration required to inhibit 50% of the maximal platelet aggregation response and is expressed as the mean ± SEM from six independent experiments (n = 6). A concentration range of 0.5 to 6 mg/mL was selected for the extracts based on previous studies on lipid bioactivity, allowing evaluation from minimal inhibition to saturation of the inhibitory effect [42].
Additionally, the antiplatelet activity of individual nitrated fatty acids identified in the extracts OA, LA, and LN was evaluated [42,46]. To investigate the role of nitration in the biological activity of the extracts, washed platelets were pre-incubated with nitrated or non-nitrated extracts in the presence of 1 mM β-mercaptoethanol (BME) at 37 °C for 6 min to potentially reduce nitration bonds. Platelet aggregation was subsequently induced with TRAP-6 (5 μM) and measured as described above [42].
For assessment of platelet activation, the expression of P-selectin (CD62P) and secretion of CD63 were evaluated by flow cytometry. Washed platelets (200 × 109/L) were pre-incubated for 10 min at 37 °C with nitrated fatty acids from bean extracts (0.5, 1, 2, 4, and 6 mg/mL) or vehicle (0.2% DMSO), followed by stimulation with TRAP-6 (5 μM). A 50 μL aliquot of each sample was then incubated for 30 min at room temperature in the dark with saturating concentrations of anti-CD61-FITC (platelet marker), anti-CD62P-PE (P-selectin), and anti-CD63-PE antibodies. Samples were analyzed using a BD FACSLyric™ flow cytometer (BD Biosciences, San Jose, CA, USA) [47].
4.8. Mechanism of Platelet Activation and Secretion Mediated by Mitochondrial Nitrated Compounds
Intracellular calcium levels were measured in washed platelets (5 × 107 platelets/mL) using the calcium sensitive fluorescent dye Fluo-4-AM (0.4 μM). Platelets were incubated with the dye at room temperature for 30 min, followed by a 5 min incubation with either vehicle (0.2% DMSO) or nitrated fatty acids (NO2-OA and NO2-LN). Fluorescence data were recorded for 15 s to establish a baseline, after which carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP, 1 μM) was added to induce calcium mobilization. Data were then collected for an additional 60 s. The effects of the treatments on cytosolic calcium levels were calculated relative to the vehicle control [45,48].
Reactive oxygen species production was assessed in washed platelets (50 × 106 platelets/mL) using 10 μM dihydroethidium (DHE). Platelets were pre-incubated with individual compounds (1 and 2 μM) for 30 min at 37 °C. Antimycin A (20 μM) served as a positive control to increase ROS levels. Fluorescence was analyzed using a BD FACSLyric™ flow cytometer (BD Biosciences, San Jose, CA, USA) [5,49].
Mitochondrial membrane potential (ΔΨm) was evaluated using the cell-permeant dye tetramethylrhodamine methyl ester perchlorate (TMRM, 100 nM). Washed platelets were incubated with 0.2% DMSO (control), NO2-fatty acids, or FCCP (1 μM) at 37 °C for 20 min. Changes in ΔΨm were detected by flow cytometry using the BD FACSLyric™ flow cytometer (BD Biosciences, San Jose, CA, USA) [50].
4.9. Statistical Analysis
All results are expressed as mean ± standard error of the mean (SEM), based on six independent experiments (n = 6). Statistical analysis was performed using GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA). Differences between multiple groups were assessed using one-way ANOVA followed by Bonferroni or Tukey’s post hoc tests, as indicated in the figure legends. Statistical significance was considered at p < 0.05, p < 0.01, and p < 0.001. Comparisons versus the TRAP-6 stimulated control are denoted with # (# p < 0.05, ### p < 0.001), while comparisons between nitrated and corresponding non-nitrated treatments are indicated with asterisks (p < 0.05, * p < 0.01, ** p < 0.001). Different lowercase letters (a, b, c) denote significant differences among groups according to Tukey’s test (p < 0.05), and “ns” indicates no statistically significant difference.
5. Conclusions
This work establishes that Phaseolus vulgaris L. extracts can be selectively enriched in NO2-FAs with potent and mechanistically distinct antiplatelet effects. Nitrated extracts, and particularly NO2-LN and NO2-OA, consistently suppressed TRAP-6 mediated aggregation, granule secretion, and mitochondrial polarization, effects because of electrophilic nitroalkenes concentrated in the lipid fraction. These findings reveal that even low-lipid food matrices can yield bioactive nitrated species capable of modulating platelet activation pathways without exacerbating cytotoxicity or oxidative stress. While the in vitro nature of the work limits direct physiological extrapolation, the data provide a compelling biochemical rationale for considering legumes as precursors of endogenous NO2-FAs with cardioprotective properties. Future studies will be required to determine the extent to which such species are formed in vivo, their bioavailability, and their contribution to diet-derived modulation of thrombotic risk.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules31030488/s1, Figure S1: Cytotoxicity by Nitrated vs. Non-nitrated Extracts; Figure S2: Chemical structures of saturated and unsaturated fatty acids identified in the Phaseolus vulgaris L. extract; Table S1: Effect of Hallado Alemán Extracts and Their Fractions on Collagen-Induced Platelet Aggregation.
Author Contributions
Conceptualization, L.R. and E.F.; methodology, L.R.; formal analysis, L.R. and H.L.M.-G.; investigation, L.R. and F.L.; data curation, H.L.M.-G.; visualization, H.L.M.-G.; writing—original draft preparation, L.R.; writing—review and editing, L.R., H.L.M.-G., P.O., A.T. and E.F.; supervision, B.C., I.P. and E.F.; project administration, B.C. and I.P.; funding acquisition, B.C. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funding by ANID-R20F0001.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Universidad de Talca (No. 29-2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The article contains all data necessary to support the conclusion. All data is contained within the article.
Acknowledgments
We appreciate the support of ANID-FONDECYT REGULAR N° 1220339, ANID-FOVI240019, ANID-FOVI240035, ANID/FONDEQUIP N° EQM200049, ANID-FONDEQUIP N° EQM240025, and Fpta-INIA (Uruguay). Also, we thank Universidad de Talca, Dirección de Investigación, PIA: “Mito-Platelet: Interdisciplinary Group on Mitochondrial Function for Prevention and Precision Medicine in Platelet-Associated Thrombosis”. Also, we thank the Interuniversity Center for Healthy Aging, Code RED211993, and Red Interuniversitaria de Envejecimiento Saludable, Latinoamérica y Caribe (RIES-LAC). During the preparation of this work, the authors used ChatGPT 4.0 to check the grammar of the writing. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | Antimycin A |
| ALA | Alpha-linolenic acid |
| BME | β-mercaptoethanol |
| CD61 | Glycoprotein IIIa (platelet marker) |
| CD62P | P-selectin |
| CD63 | Lysosomal granule marker |
| CVD | Cardiovascular disease |
| DHE | Dihydroethidium |
| DMSO | Dimethyl sulfoxide |
| FCCP | Carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone |
| GC | Gas chromatography |
| GPCR | G protein-coupled receptor |
| GPVI | Glycoprotein VI (collagen receptor) |
| HPLC | High-performance liquid chromatography |
| IC50 | Half maximal inhibitory concentration |
| LA | Linoleic acid |
| LDH | Lactate dehydrogenase |
| LN | Linolenic acid |
| MMP (ΔΨm) | Mitochondrial membrane potential |
| MUFA | Monounsaturated fatty acid |
| NO | Nitric oxide |
| NO2-FAs | Nitrated fatty acids |
| NO2-LA | Nitro-linoleic acid |
| NO2-LN | Nitro-linolenic acid |
| NO2-OA | Nitro-oleic acid |
| OA | Oleic acid |
| PAR-1 | Protease-activated receptor-1 |
| PBS | Phosphate-buffered saline (if needed; appears indirectly) |
| PPP | Platelet-poor plasma |
| PRP | Platelet-rich plasma |
| PUFA | Polyunsaturated fatty acid |
| ROS | Reactive oxygen species |
| SEM | Standard error of the mean |
| SFA | Saturated fatty acid |
| TMRM | Tetramethylrhodamine methyl ester |
| TRAP-6 | Thrombin receptor-activating peptide 6 |
| UAE | Ultrasound-assisted extraction |
References
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef]
- Bauersachs, R.; Zeymer, U.; Brière, J.-B.; Marre, C.; Bowrin, K.; Huelsebeck, M. Burden of coronary artery disease and peripheral artery disease: A literature review. Cardiovasc. Ther. 2019, 2019, 8295054. [Google Scholar] [CrossRef] [PubMed]
- Huseynov, A.; Reinhardt, J.; Chandra, L.; Dürschmied, D.; Langer, H.F. Novel Aspects Targeting Platelets in Atherosclerotic Cardiovascular Disease—A Translational Perspective. Int. J. Mol. Sci. 2023, 24, 6280. [Google Scholar] [CrossRef] [PubMed]
- Machlus, K.R.; Italiano, J.E., Jr. Megakaryocyte development and platelet formation. In Platelets; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–46. [Google Scholar]
- Rodriguez, L.; Badimon, L.; Mendez, D.; Padro, T.; Vilahur, G.; Pena, E.; Carrasco, B.; Vogel, H.; Palomo, I.; Fuentes, E. Antiplatelet activity of isorhamnetin via mitochondrial regulation. Antioxidants 2021, 10, 666. [Google Scholar] [CrossRef]
- Gehin, M.; Storey, R.F.; Bernaud, C.; Dingemanse, J. Clinical pharmacology of selatogrel for self-administration by patients with suspected acute myocardial infarction. Expert Opin. Drug Metab. Toxicol. 2023, 19, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Wang, X.; Peter, K. Platelets in cardiac ischaemia/reperfusion injury: A promising therapeutic target. Cardiovasc. Res. 2019, 115, 1178–1188. [Google Scholar] [CrossRef]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef]
- Mariotti, F.; Gardner, C.D. Dietary protein and amino acids in vegetarian diets—A review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef]
- Andac-Ozturk, S.; Garipoğlu, G.; Çatak, J.; Yaman, M. Investigation of the vitamins B1, B2, and B6 vitamers bioaccessibilities of canned, dried legumes after in vitro gastrointestinal digestion system. Food Res. Int. 2022, 160, 111671. [Google Scholar] [CrossRef]
- Rodríguez, L.; Mendez, D.; Montecino, H.; Carrasco, B.; Arevalo, B.; Palomo, I.; Fuentes, E. Role of Phaseolus vulgaris L. in the Prevention of Cardiovascular Diseases—Cardioprotective Potential of Bioactive Compounds. Plants 2022, 11, 186. [Google Scholar] [CrossRef]
- Rodríguez, L.; Plaza, A.; Méndez, D.; Carrasco, B.; Tellería, F.; Palomo, I.; Fuentes, E. Antioxidant Capacity and Antiplatelet Activity of Aqueous Extracts of Common Bean (Phaseolus vulgaris L.) Obtained with Microwave and Ultrasound Assisted Extraction. Plants 2022, 11, 1179. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Zam, W.; Kumar, M.; Cardoso, S.M.; Pereira, O.R.; Ademiluyi, A.O.; Adeleke, O.; Moreira, A.C.; Živković, J.; et al. Phenolic bioactives as antiplatelet aggregation factors: The pivotal ingredients in maintaining cardiovascular health. Oxid. Med. Cell. Longev. 2021, 2021, 2195902. [Google Scholar] [CrossRef]
- Ally, A.; Powell, I.; Ally, M.M.; Chaitoff, K.; Nauli, S.M. Role of neuronal nitric oxide synthase on cardiovascular functions in physiological and pathophysiological states. Nitric Oxide 2020, 102, 52–73. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef]
- Trostchansky, A.; Mastrogiovanni, M.; Miquel, E.; Rodriguez-Bottero, S.; Martinez-Palma, L.; Cassina, P.; Rubbo, H. Profile of Arachidonic Acid-Derived Inflammatory Markers and Its Modulation by Nitro-Oleic Acid in an Inherited Model of Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2018, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Batthyany, C.; Schopfer, F.J.; Baker, P.R.S.; Durán, R.; Baker, L.M.S.; Huang, Y.; Cerveñansky, C.; Branchaud, B.P.; Freeman, B.A. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J. Biol. Chem. 2006, 281, 20450–20463. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, F.J.; Cole, M.P.; Groeger, A.L.; Chen, C.S.; Khoo, N.K.; Woodcock, S.R.; Golin-Bisello, F.; Motanya, U.N.; Li, Y.; Zhang, J.; et al. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: Selective ligand activity and anti-diabetic signaling actions. J. Biol. Chem. 2010, 285, 12321–12333. [Google Scholar] [CrossRef]
- Klinke, A.; Moller, A.; Pekarova, M.; Ravekes, T.; Friedrichs, K.; Berlin, M.; Scheu, K.M.; Kubala, L.; Kolarova, H.; Ambrozova, G.; et al. Protective effects of 10-nitro-oleic acid in a hypoxia-induced murine model of pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2014, 51, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, L.; Chang, L.; Salvatore, S.R.; Ichikawa, T.; Zhang, J.; Petrovic-Djergovic, D.; Jia, L.; Carlsen, H.; Schopfer, F.J.; Freeman, B.A.; et al. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc. Res. 2013, 98, 116–124. [Google Scholar] [CrossRef]
- Trostchansky, A.; Bonilla, L.; Thomas, C.P.; O’Donnell, V.B.; Marnett, L.J.; Radi, R.; Rubbo, H. Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. J. Biol. Chem. 2011, 286, 12891–12900. [Google Scholar] [CrossRef]
- Koenitzer, J.R.; Bonacci, G.; Woodcock, S.R.; Chen, C.-S.; Cantu-Medellin, N.; Kelley, E.E.; Schopfer, F.J. Fatty acid nitroalkenes induce resistance to ischemic cardiac injury by modulating mitochondrial respiration at complex II. Redox Biol. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Sánchez-Calvo, B.; Cassina, A.; Mastrogiovanni, M.; Santos, M.; Trias, E.; Kelley, E.E.; Rubbo, H.; Trostchansky, A. Olive oil-derived nitro-fatty acids: Protection of mitochondrial function in non-alcoholic fatty liver disease. J. Nutr. Biochem. 2021, 94, 108646. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Ahmad, F.; Saxena, R. Platelet activation markers in evaluation of thrombotic risk factors in various clinical settings. Blood Rev. 2019, 37, 100583. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, M.S. Nitric Oxide Signal Transduction and Its Role in Skin Sensitization. Biomol. Ther. 2023, 31, 388. [Google Scholar] [CrossRef]
- Koutoulogenis, G.S.; Kokotos, G. Nitro fatty acids (NO2-FAs): An emerging class of bioactive fatty acids. Molecules 2021, 26, 7536. [Google Scholar] [CrossRef]
- Skwarek, P.; Karwowska, M. Fatty Acids Profile and Antioxidant Properties of Raw Fermented Sausages with the Addition of Tomato Pomace. Biomolecules 2022, 12, 1695. [Google Scholar] [CrossRef]
- Sánchez-Calvo, B.; Mastrogiovanni, M.; Santos, M.; Petingi, S.; Conde-Innamorato, P.; Arias-Sibillotte, M.; Ibáñez, F.; Trostchansky, A.; Rubbo, H. Detection of nitro-conjugated linoleic acid and nitro-oleic acid in virgin olive oil under gastric conditions: Relationship to cultivar, fruit ripening, and polyphenol content. ACS Food Sci. Technol. 2022, 2, 673–681. [Google Scholar] [CrossRef]
- Coles, B.; Bloodsworth, A.; Eiserich, J.P.; Coffey, M.J.; McLoughlin, R.M.; Giddings, J.C.; Lewis, M.J.; Haslam, R.J.; Freeman, B.A.; O’Donnell, V.B. Nitrolinoleate Inhibits Platelet Activation by Attenuating Calcium Mobilization and Inducing Phosphorylation of Vasodilator-stimulated Phosphoprotein through Elevation of cAMP*. J. Biol. Chem. 2002, 277, 5832–5840. [Google Scholar] [CrossRef]
- Trostchansky, A.; Moore-Carrasco, R.; Fuentes, E. Oxidative pathways of arachidonic acid as targets for regulation of platelet activation. Prostaglandins Other Lipid Mediat. 2019, 145, 106382. [Google Scholar] [CrossRef]
- Srihirun, S.; Sriwantana, T.; Unchern, S.; Kittikool, D.; Noulsri, E.; Pattanapanyasat, K.; Fucharoen, S.; Piknova, B.; Schechter, A.N.; Sibmooh, N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLoS ONE 2012, 7, e30380. [Google Scholar] [CrossRef]
- Trostchansky, A.; Rubbo, H. Formation of Nitrated 5 Lipids and Their Biological Relevance. In Lipid Oxidation in Health and Disease; Spickett, C.M., Forman, H.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 101–118. ISBN 978-1-4398-5153-3. [Google Scholar]
- Cruz, M.R.; Tovar, A.R.; del Prado, M.; Torres, N. Molecular mechanisms of action and health benefits of polyunsaturated fatty acids. Rev. Investig. Clínica 2005, 57, 457–472. [Google Scholar]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective actions of grape polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef]
- Michelson, A.D. Antiplatelet therapies for the treatment of cardiovascular disease. Nat. Rev. Drug Discov. 2010, 9, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Trostchansky, A.; Rubbo, H. Nitrated fatty acids: Mechanisms of formation, chemical characterization, and biological properties. Free Radic. Biol. Med. 2008, 44, 1887–1896. [Google Scholar] [CrossRef]
- Augusto, P.E.D. Editorial overview: Food physics & materials science Vol. 37–39—2021. Curr. Opin. Food Sci. 2021, 38, iii–iv. [Google Scholar] [CrossRef]
- Schopfer, F.J.; Khoo, N.K.; Wittmann, B.M.; Baker, P.R.S.; Freeman, B.A. Nitro-fatty acids: Formation, redox signaling, and therapeutic potential. Chem. Rev. 2011, 111, 5983–6006. [Google Scholar]
- Schopfer, F.J.; Vitturi, D.A.; Jorkasky, D.K.; Freeman, B.A. Nitro-fatty acids: New drug candidates for chronic inflammatory and fibrotic diseases. Nitric Oxide 2018, 79, 31–37. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, H.; Park, J.J.; Cho, I.H. Validation of the AOAC method for analyzing fatty acids in meat by-products for the Korean Food Composition Database. Food Sci. Biotechnol. 2023, 32, 847–854. [Google Scholar] [CrossRef]
- Rodríguez, L.; Lagos, F.; Mastrogiovanni, M.; Flores, A.; Plaza, A.; Telleria, F.; Palomo, I.; Fuentes, E.; Trostchansky, A. Tomato pomace-derived nitrated fatty acids: Synthesis and antiplatelet activity. Biomed. Pharmacother. 2024, 177, 117154. [Google Scholar] [CrossRef]
- Trostchansky, A.; Souza, J.M.; Ferreira, A.; Ferrari, M.; Blanco, F.; Trujillo, M.; Castro, D.; Cerecetto, H.; Baker, P.R.; O’Donnell, V.B.; et al. Synthesis, isomer characterization, and anti-inflammatory properties of nitroarachidonate. Biochemistry 2007, 46, 4645–4653. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. Human experimentation. Code of Ethics of the World Medical Association (Declaration of Helsinki). Br. Med. J. 1964, 2, 177. [Google Scholar] [CrossRef]
- Montecino-Garrido, H.; Sepúlveda, M.; Méndez, D.; Monroy-Cárdenas, M.; Alfaro, S.; González-Avendaño, M.; Caballero, J.; Urra, F.A.; Araya-Maturana, R.; Fuentes, E. Assessing mitochondria-targeted acyl hydroquinones on the mitochondrial platelet function and cytotoxic activity: Role of the linker length. Free Radic. Biol. Med. 2023, 208, 26–36. [Google Scholar] [CrossRef] [PubMed]
- De Simone, I.; Baaten, C.C.F.M.J.; Gibbins, J.M.; Ten Cate, H.; Heemskerk, J.W.M.; Jones, C.I.; van der Meijden, P.E.J. Repeated platelet activation and the potential of previously activated platelets to contribute to thrombus formation. J. Thromb. Haemost. 2023, 21, 1289–1306. [Google Scholar] [CrossRef]
- Rodriguez, L.; Muñoz-Bernal, Ó.A.; Fuentes, E.; Alvarez-Parrilla, E.; Palomo, I.; Wall-Medrano, A. Phenolic profile, cheminformatics, and antiplatelet aggregation activity of orange and purple sweet potato (Ipomoea batatas L.) storage roots. Food Chem. 2024, 454, 139794. [Google Scholar] [CrossRef]
- Méndez, D.; Urra, F.A.; Millas-Vargas, J.P.; Alarcón, M.; Rodriguez-Lavado, J.; Palomo, I.; Trostchansky, A.; Araya-Maturana, R.; Fuentes, E. Synthesis of antiplatelet ortho-carbonyl hydroquinones with differential action on platelet aggregation stimulated by collagen or TRAP-6. Eur. J. Med. Chem. 2020, 192, 112187. [Google Scholar] [CrossRef]
- Méndez, D.; Arauna, D.; Fuentes, F.; Araya-Maturana, R.; Palomo, I.; Alarcón, M.; Sebastián, D.; Zorzano, A.; Fuentes, E. Mitoquinone (MitoQ) Inhibits Platelet Activation Steps by Reducing ROS Levels. Int. J. Mol. Sci. 2020, 21, 6192. [Google Scholar] [CrossRef] [PubMed]
- Méndez, D.; Donoso-Bustamante, V.; Millas-Vargas, J.P.; Pessoa-Mahana, H.; Araya-Maturana, R.; Fuentes, E. Synthesis and pharmacological evaluation of acylhydroquinone derivatives as potent antiplatelet agents. Biochem. Pharmacol. 2021, 183, 114341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.