Abstract
We developed an efficient and modular route to 2- and 6-(1,2,3-triazol-1-yl)azulenes to expand the synthetic accessibility and functional scope of azulene-based π-systems with stimulus-responsive photophysics. Readily accessible 2- and 6-azidoazulenes, prepared in excellent yields via SNAr reactions of haloazulenes, were subjected to Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition with a broad range of terminal alkynes to afford the corresponding triazolylazulenes in good to high yields, followed by acid-mediated decarboxylation and Staudinger reduction to enable further diversification to 2-azulenyltriazoles and a 6-aminoazulene derivative. Single-crystal X-ray diffraction analysis revealed substitution-position-dependent torsional arrangements and variations in π-conjugation between the azulene and triazole units. Photophysical characterization by UV/Vis absorption and fluorescence spectroscopy showed pronounced halochromism under acidic conditions, and selected derivatives displayed substantially enhanced fluorescence quantum yields. Overall, these results establish the azulene–1,2,3-triazole motif as a versatile building block for designing optoelectronic π-systems with acid-responsive emission properties.
1. Introduction
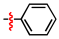
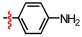
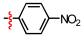
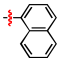
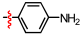
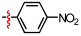
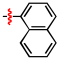
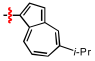
1,2,3-Triazoles are frequently encountered as structural motifs in pharmaceuticals, such as Rufinamide [1], Tazobactam [2], Solithromycin [3], and others [4,5], owing to their ability to function as bioisosteres of imidazoles and various carboxylic acid derivatives (Figure 1). In addition, 1,2,3-triazoles have attracted considerable attention as ligands for metal coordination [6,7,8] and as functional components for electrolyte applications such as ionic liquids [9,10]. Among the established synthetic methods, the most widely employed approach to the 1,2,3-triazoles is the Huisgen [3 + 2] dipolar cycloaddition between azides and alkynes. In the early development of this reaction, azides and alkynes were thermally activated to promote the cycloaddition reaction [11,12]. Subsequently, Sharpless introduced the concept of “click chemistry” for the Huisgen cycloaddition, now extensively utilized in the construction of pharmaceuticals, biological probes, and functional organic materials because of its high efficiency, broad functional-group tolerance, and operational simplicity [13]. The Huisgen reaction—classified as a click transformation—enables the rapid formation of the 1,2,3-triazole derivatives via the Cu(I)-catalyzed [3 + 2] cycloaddition of azides with terminal alkynes [14,15,16].
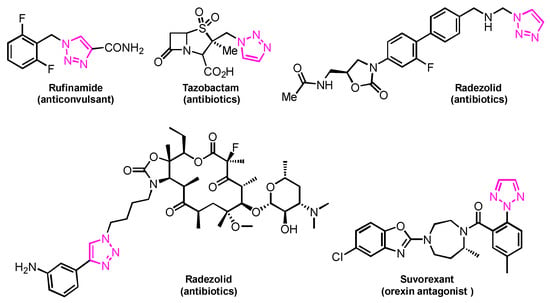
Figure 1.
Pharmaceuticals containing a 1,2,3-triazole substructure.
Previously, Nozoe, Horino and Toda reported that, in the course of investigating the reactivity of 5-N-(1,2,3-triazol-1-yl)tropolone, the reaction of this ether derivative with malononitrile or ethyl cyanoacetate afforded the corresponding 6-(1,2,3-triazol-1-yl)azulenes [17,18]. In 2014, Emrick and co-workers also reported the synthesis of a 2-(1,2,3-triazol-1-yl)azulene derivative as a monomer precursor for functional polymer development [19]. This compound was obtained via a copper(I) iodide (CuI)-catalyzed Huisgen cycloaddition between 2-trimethylsilylethynylazulene and the corresponding azide. However, despite the fact that azidoazulenes themselves are relatively accessible, their reactivity in the Huisgen cycloadditions with alkynes has not been systematically explored, and no examples of such transformations have been disclosed to date.
In the present study, as part of our ongoing investigation into the reactivity of diethyl 2- and 6-azidoazulene-1,3-dicarboxyltes, we explored the CuI-catalyzed Huisgen cycloaddition with a range of alkynes, establishing an efficient synthetic route to 2- and 6-(1,2,3-triazol-1-yl)azulene drerivatives. In addition, we developed a convenient protocol for the preparation of 6-aminoazulene derivative via the Staudinger reaction of the corresponding 6-azidoazulene derivative. The synthesis of 2-(1,2,3-triazol-1-yl)azulenes without the ester substituents was also investigated, and optical measurements revealed that several of these derivatives exhibit relatively strong fluorescence with relatively high quantum yields. Furthermore, the detailed molecular structures of the 2- and 6-(1,2,3-triazol-1-yl)azulenes were established by single-crystal X-ray diffraction analysis.
2. Results and Discussion
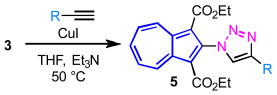
Synthesis: Prior to the synthesis of azulene-substituted triazoles, azidoazulenes were prepared from 2-chloro and 6-bromoazulene derivatives 1 and 2 bearing two-ethoxycarbonyl functions at the 1,3-positions of the azulene ring. Since the synthesis of 6-azidoazulene derivative 4 was previously reported by us, a similar approach was adopted for the preparation of 2-azidoazulene derivative 3 [20]. Thus, the preparation of 2- and 6-azidoazulene derivatives 3 and 4 [21] were accomplished in 96% and 97% yields, respectively, by the aromatic nucleophilic substitution (SNAr) reaction of 1 and 2 with sodium azide (NaN3) in dimethyl sulfoxide (DMSO) at 70 °C (Scheme 1).
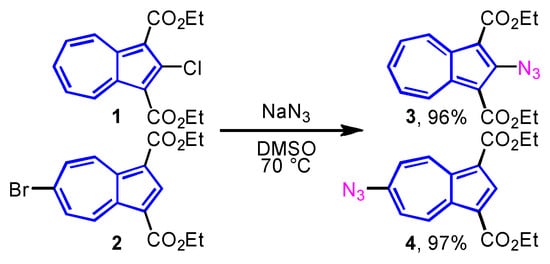
Scheme 1.
Preparation of 2- and 6-azidoazulenes 3 and 4.
The Huisgen cycloaddition of 3 with various alkynes in the presence of a CuI catalyst furnished 2-(1,2,3-triazol-1-yl)azulenes 5a–5g, bearing an aromatic or an alkyl substituent at the 4-position, in good to excellent yields (Table 1). The electronic nature of the substituents at the para-position of the arylalkynes exerted little influence on the reaction outcome, and 5a–5c were obtained in almost comparable yields (entries 1–3). The reaction of 1-ethynylnaphthalene afforded 5d in 74% yield, similar to those of phenylacetylenes (entry 4). In contrast, electron-rich alkynes such as 1-ethynylazulene and ethynylferrocene [22] gave the corresponding cycloadducts 5e and 5f in 88% and 85% yields, respectively, indicating enhanced reactivity under these conditions (entries 5 and 6). Furthermore, the cycloaddition of 3 with an alkyl-substituted alkyne, 1-hexyne, also proceeded smoothly to afford 5g in 72% yield (entry 7).

Table 1.
Huisgen cycloaddition of 2-azidoazulene 3.
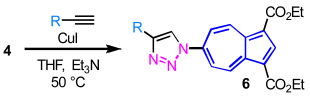
Similarly, 6-azidoazulene derivative 4 also underwent smooth Huisgen cycloaddition with a range of alkynes to afford the corresponding 6-azulenyltriazoles 6a–6g in good yields (Table 2). Reactions of 4 with benzene-substituted alkynes in the presence of catalytic amount of CuI provided 6a (84%), 6b (86%), and 6c (69%) (entries 1–3). The reaction of 4 with 1-ethynylnaphthalene, followed by chromatographic purification on silica gel, furnished 6d in 82% yield (entry 4). Furthermore, the reactions of 4 with 1-ethynylazulene and ethynylferrocene afforded di(azulenyl)triazole 6e (76%) and ferrocenyl derivative 6f (74%), respectively (entries 5 and 6). The cycloaddition of 4 with 1-hexyne also proceeded efficiently to give n-butyl-substituted triazole 6g in 65% yield (entry 7).

Table 2.
Huisgen cycloaddition of 6-azidoazulene derivative 4.
As summarized in Table 1 and Table 2, the Huisgen cycloaddition of both azides 3 and 4 with diverse alkynes proceeded efficiently to furnish the corresponding 1,2,3-triazoles in consistently high yields, demonstrating that 3 and 4 possess high intrinsic reactivity toward the [3 + 2] dipolar cycloaddition.
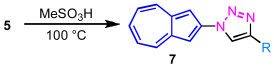
The decarboxylation reactions of 5a–5g were investigated under acidic conditions. In general, 100% phosphoric acid (H3PO4) is widely employed for the decarboxylation of ester groups in azulene derivatives. However, owing to the limited solubility of 5a–5g in 100% H3PO4, methanesulfonic acid (MeSO3H) was selected as an alternative reaction medium [23]. This modification was expected to improve the solubility of the substrates and thereby facilitate a more efficient decarboxylation process. In practice, the decarboxylation reaction of 5a–5g proceeded smoothly to afford the corresponding products 7a–7g in excellent yields (88–95%) in most cases (Table 3). Notably, in the case of 5e, decarboxylation also occurred at the ester group on the substituted 1-azulenyl moiety, affording the fully decarboxylated product 7e’ in 89% yield (entry 5). In contrast, the ferrocene-substituted derivative 5f underwent significant decomposition under the reaction conditions, and no isolable product could be obtained (entry 6).

Table 3.
Decarboxylation of 5 to 2-(1,2,3-triazol-1-yl)azulenes 7.
Similarly, the decarboxylation of 6a was also examined under the conditions comparable to those used for the decarboxylation of 5a–5g. However, unlike 5a–5g, the reaction of 6a was accompanied by extensive decomposition, and the desired product 8 was isolated in only 7% yield (Scheme 2). This result indicates that the 6-substituted derivative is less stable than the corresponding 2-substituted analogues under the decarboxylation conditions.
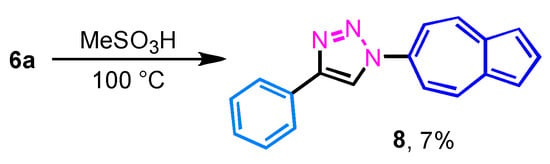
Scheme 2.
Decarboxylation of 6a to 6-(4-phenyl-1,2,3-triazol-1-yl)azulene 8.
The Staudinger reaction is a well-established method for the conversion of azides into primary amines under relatively mild conditions [24,25,26,27]. In this transformation, azides react with phosphines to afford iminophosphorane intermediates, which can subsequently be hydrolyzed to yield the corresponding primary amines. Although the synthesis of 6-aminoazulene derivative 10 via the reduction of 4 was previously reported by us [17], that method required the use of Raney-Ni—a highly reactive reducing agent. To develop a more convenient and safer alternative, we explored the Staudinger reaction as a method for converting 4 into 10.
The reaction of 4 with triphenylphosphine (PPh3) proceeded smoothly to afford the corresponding iminophosphorane 9 in 85% yield. The detailed structure of 9 was confirmed by single-crystal X-ray diffraction analysis, as described later. Subsequent acidic hydrolysis of 9 with aqueous HCl in THF furnished the desired 6-aminoazulene derivative 10 in 82% yield (Scheme 3). This two-step sequence provides a safer and more convenient alternative for the synthesis of the 6-aminoazulene derivative compared with the previously reported Raney-Ni reduction protocol.
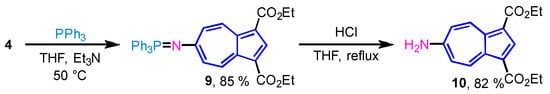
Scheme 3.
Transformation to 6-aminoazulene derivative 10 via Staudinger reaction.
Spectroscopic properties: The 2- and 6-(1,2,3-triazol-1-yl)azulenes obtained in this study were fully characterized based on their spectroscopic data, as summarized in Section 3. The 1H NMR spectral assignments of the reported compounds were confirmed by COSY experiments (see Supplementary Materials). The high-resolution mass spectra (HRMS) of the newly prepared compounds, recorded using ESI-TOF or MALDI-TOF ionization, displayed the expected molecular ion peaks, consistent with the proposed molecular formulas. These spectroscopic results collectively support the assigned structures of the synthesized products.
Since suitable single crystals for X-ray crystallographic analysis were obtained for 5a, 5d, 8, and 9, their molecular structures were elucidated as summarized in Figure 2. In compounds 5a and 5d, the dihedral angle between the azulene and triazole rings was determined to be 85.69° (5a) and 85.98° (5d), indicating that the two rings are nearly orthogonal. These large torsions are attributed to steric repulsion between the triazole moiety and the two ester substituents substituted at the 1,3-positions of the azulene ring, which reduces the overall planarity of the π-system. In contrast, the dihedral angles between the triazole and the pendant phenyl and 1-naphtyl groups were found to be 8.02° (5a) and 36.55° (5d), signifying a coplanarity as a result of reduced steric hindrance.
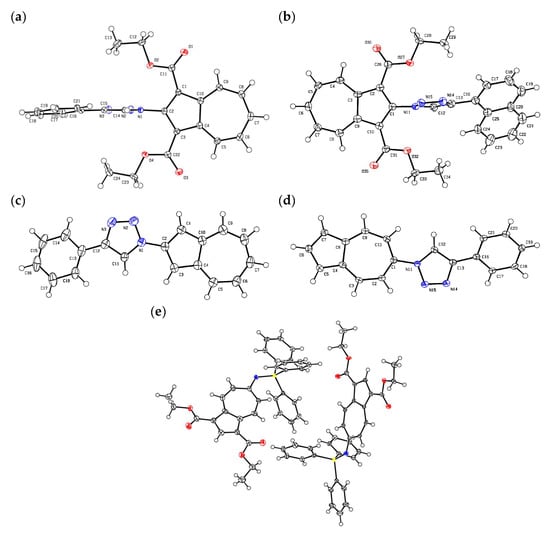
Figure 2.
ORTEP drawing of (a) 5a (CCDC 2087438), (b) 5d (CCDC 2447293), (c) 7a (CCDC 2502616), (d) 8 (CCDC 2447292), and (e) 9 (CCDC 2088856); ellipsoids are drawn at the 50% probability level.
Upon decarboxylation, the steric environment changed markedly. In the decarboxylated derivative 7a, the dihedral angle between the azulene and triazole rings was significantly reduced to 8.13°. In contrast, 8 exhibited larger dihedral angles of 33.23° (azulene–triazole) and 18.87° (triazole–phenyl). The increased torsion between the azulene and triazole rings in 8 is plausibly attributed to steric repulsion between the hydrogen atoms on the seven-membered ring of the azulene moiety and the adjacent triazole ring.
A partially planar conformation was also observed in the crystal structure of 9, in which the dihedral angle between the azulene framework and the iminophosphorane moiety was only 0.41°, suggesting efficient conjugation between these two π-systems.
To investigate the effects of the substitution position on the triazole ring and the substituents on the introduced aryl groups on the optical properties, UV/Vis absorption spectra of compounds 5–8 were measured. The maximum absorption wavelengths (λmax) and the molar absorption coefficients (log ε) for compounds 5 and 6 in dichloromethane (CH2Cl2) as well as 7 in 10% CF3CO2H (TFA)/CH2Cl2 are summarized in Table 4 and Table 5, respectively.

Table 4.
The maximum absorption wavelength (λmax) and molar absorption coefficient (log ε) of 5 and 6 in CH2Cl2.

Table 5.
The maximum absorption wavelength (λmax), molar absorption coefficient (log ε), maximum fluorescence wavelength (λFlu), and fluorescence quantum yield (ΦFlu) of 7 and 8 in 10% TFA/CH2Cl2.
Overall, the absorption maxima of 6a–6g were red-shifted in the visible region compared with those of 5a–5g (Table 4). Compound 5b (λmax = 524 nm), bearing an electron-donating p-anilino group, exhibited a slightly red-shifted absorption maximum relative to 5a (λmax = 513 nm). In contrast, 5c (λmax = 512 nm), containing an electron-withdrawing p-nitrophenyl group, displayed an absorption maximum nearly identical to that of 5a. For 1-naphthyl derivative 5d (λmax = 508 nm), which possesses a π-extended aromatic ring, the absorption maximum also showed little deviation from that of phenyl analogue 5a. This behavior is likely attributable to the near-orthogonal orientation of the azulene and triazole rings, as revealed by single-crystal X-ray structural analysis, where steric hindrance from the two ester substituents minimizes π-conjugation overlap between the two systems. Compound 5e (λmax = 522 nm) exhibited a prominent absorption band in the visible region with approximately twice the molar absorption coefficient of 5a, a feature reasonably ascribed to the overlap of π–π* transitions of the two azulene units. Meanwhile, the absorption maxima of 5f (λmax = 517 nm) and 5g (λmax = 514 nm) were similar to that of 5a, indicating that the absorption characteristics are governed predominantly by the 1,3-diethoxycarbonylazulene core, with only minor electronic contributions from the substituents.
Conversely, despite the relatively high planarity observed in the X-ray crystallographic structures of 6a–6g, neither the electronic effects nor the conjugation extension of the aryl substituents on the triazole ring were reflected in their absorption maxima. This behavior may be attributed to the weak electronic influence of substituents at the 6-position of the azulene ring, where electronic communication with the π-system is comparatively limited.
The UV/Vis spectra of 7a–7g in CH2Cl2 are also measured (see Section 3). All compounds exhibited two distinct absorption bands in the range of λmax = 540–585 nm, accompanied by a weak shoulder around 630–655 nm. These absorptions can be assigned to the π–π* transitions derived from the azulene ring. Notably, even for 7b (λmax = 593 nm) and 7c (λmax = 598 nm), bearing electron-donating and electron-withdrawing substituents, respectively, the absorption maxima appeared at nearly identical wavelengths. These observations suggest that both conjugation and substituent effects are electronically decoupled by the triazole spacer, preventing efficient transmission of the substituent influence to the azulene core. Since compound 8 (λmax = 593 nm) exhibited an absorption maximum similar to those of 7a–7g, the substitution position of the triazole ring on the azulene framework may not significantly influence its electronic properties.
Azulene derivatives are well known for exhibiting a color change dependent on pH, so called halochromism, under acidic conditions [27,28,29]. We have also found that several azulene derivatives not only induce halochromism but also display remarkable fluorescence under acidic conditions [30,31,32]. Since a series of 7 might also exhibit halochromism, UV/Vis absorption and fluorescence spectra were also measured under acidic conditions. The maximum absorption (λmax) and fluorescence (λFlu) wavelengths, and fluorescence quantum yields (ΦFlu) in 10% TFA/CH2Cl2 are presented in Table 5.
All compounds exhibited distinct spectral changes in both band shape and absorption intensity under the acidic conditions. In CH2Cl2, 7a–7g showed absorption bands at around 540–585 nm, accompanied by weak shoulders at near 630–655 nm. In contrast, when measured in 10% TFA/CH2Cl2, a new strong absorption band emerged at approximately 410–420 nm, while the absorption intensity in the longer-wavelength visible region (550–600 nm) increased slightly. These pronounced spectral changes could be attributed to the protonation of the azulene core or triazole ring by TFA, which perturbs the π-electronic structure and changes electronic character. Regarding the substituent effects, both 7a and 7b exhibited the emergence of a pronounced new absorption band at approximately 410–421 nm, whereas the position of the long-wavelength visible band remained nearly identical to that observed under neutral conditions. In sharp contrast, 7c displayed distinct new absorption bands at λmax = 412 nm and 472 nm, together with a pronounced enhancement of the absorption intensity in the visible region. In 10% TFA/CH2Cl2, the absorption maximum of 8 (λmax = 619 nm) showed a slight red shift compared with those of 7a–7g.
As is typical for azulene derivatives, 7a–7g and 8 exhibited exceedingly weak fluorescence in CH2Cl2, presumably arising from the S2→S0 transition. However, the fluorescence spectra displayed only faint, poorly resolved emission features, and the corresponding fluorescence quantum yields were below the instrumental detection limit. By contrast, in 10% TFA/CH2Cl2, 7a–7g and 8 showed visually discernible fluorescence. The maximum fluorescence wavelength (λFlu) and fluorescence quantum yields (ΦFlu) of 7 in 10% TFA/CH2Cl2 are also summarized in Table 5. All compounds exhibited multiple emission peaks within the range of λFlu = 470–540 nm. However, the ΦFlu values varied substantially depending on the aryl substituents. Compounds 7a (λFlu = 475, 489, and 540 nm), 7b (λFlu = 491 and 534 nm), and 7d (λFlu = 463, 489, and 529 nm) showed extremely weak fluorescence (ΦFlu < 1%), whereas 7e, despite its low overall efficiency (ΦFlu = 2.3%), displayed clear fluorescence at λFlu = 472 and 523 nm. In contrast, 7c (λFlu = 492 and 533 nm) and 7g (λFlu = 472 and 530 nm) exhibited markedly enhanced ΦFlu values of 27.0% and 10.8%, respectively (Figure 3).
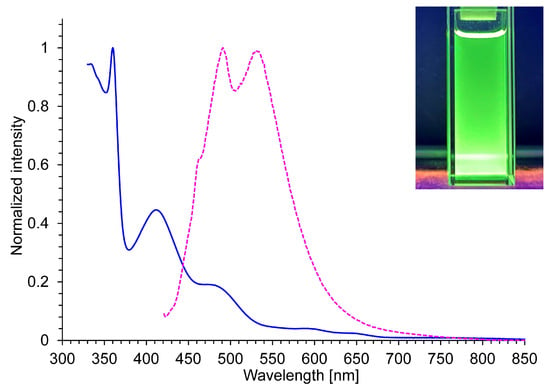
Figure 3.
UV/Vis (blue line) and fluorescent spectra (λex = 405 nm, pink dotted-line) of 7c in 10% TFA/CH2Cl2; Photograph: 7c in 10% TFA/CH2Cl2 under the UV-light irradiation at λex = 365 nm.
To investigate these emission behaviors, the fluorescence spectra of 7c in 10% TFA/CH2Cl2 were measured while varying the excitation wavelength from 350 nm to 405 nm (Figure 4). If the intensity ratio between the shorter and longer-wavelength emissions changes upon varying the excitation wavelength, it can be considered that the fluorescence originates from multiple emissive species. In practice, the fluorescence spectra of 7c showed a decrease in the near-infrared emission band and a simultaneous increase in the fluorescence intensity at λFlu = 533 nm as the excitation wavelength was shifted to longer wavelengths. This excitation-wavelength dependence of the fluorescence spectra indicates that the fluorescence of 7c arises from multiple emissive species.
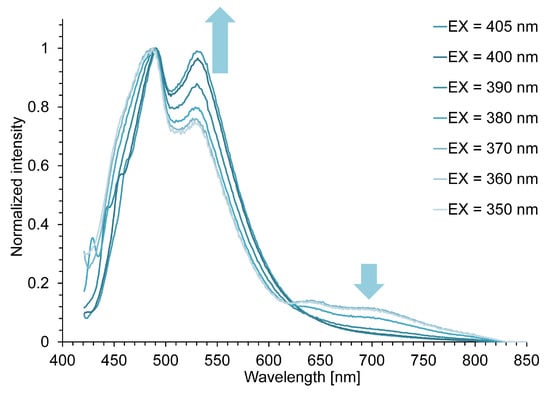
Figure 4.
Fluorescence spectra of 7c in 10% TFA/CH2Cl2 under different excitation wavelengths.
To elucidate the structure of the species formed in acidic medium, the 1H NMR spectra of 7a and 7c were recorded in 10% CF3CO2D/CDCl3 (Figure 5 and Figure 6). Formation of azulenium ion in acidic media induces a pronounced downfield shift of proton signals on the seven-membered ring [33,34,35], and the protonation at the five-membered ring disrupts the inherent symmetry of the azulene skeleton. In the present case, the azulene proton signals for 7a and 7c were observed while retaining symmetry even in 10% CF3CO2D/CDCl3. This indicates that protonation preferentially occurs at the triazole nitrogen rather than at the azulene ring. The proton signals of 7a and 7c in 10% CF3CO2D/CDCl3 were shifted downfield overall relative to those in CDCl3, which can be attributed to the increased electron-withdrawing nature by the protonation of the triazole ring such as the structure in Figure 5 and Figure 6. Notably, the H1 and H3 protons of both 7a and 7c disappeared due to proton-deuterium exchange, suggesting transient formation of azulenium ion intermediates whose contribution is not dominant on the 1H NMR timescale. Accordingly, the differences in ΦFlu observed across the series are plausibly governed by the electronic states of the protonated species and/or the generation of the transient azulenium ion intermediates. Accordingly, emission maxima observed in the fluorescence spectra may originate from the coexistence of two chemically distinct emissive species, namely, triazolium and azulenium ions in equilibrium, or from an excited-state intramolecular proton transfer (ESIPT) process from the triazole moiety to the azulene unit [36,37,38]. Moreover, the higher ΦFlu of 7c may be rationalized by a greater contribution of the azulenium ion, stemming from either an equilibrium shift or an ESIPT, facilitated by destabilization of the triazolium cation induced by the strongly electron-withdrawing p-nitrophenyl substituent.
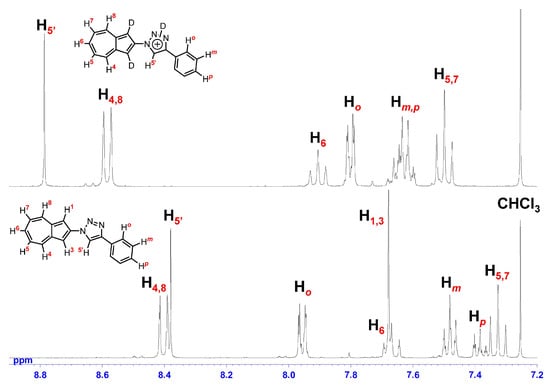
Figure 5.
1H NMR spectra (400 MHz) of 7a in CDCl3 (bottom) and 10% CF3CO2D/CDCl3 (top).
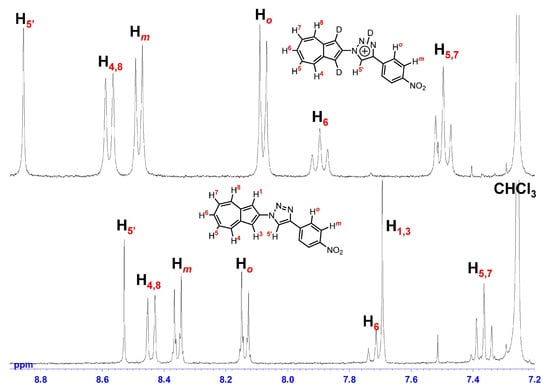
Figure 6.
1H NMR spectra (400 MHz) of 7c in CDCl3 (bottom) and 10% CF3CO2D/CDCl3 (top).
3. Materials and Methods
General: Melting points were determined with a Yanagimoto MPS3 micromelting apparatus. UV/Vis spectra were measured with JASCO V-670 spectrophotometer. The fluorescence spectra and fluorescence quantum yield were measured with JASCO FP-8350 and Hamamatsu C11347 absolute photoluminescence quantum yield spectrometer, respectively. The UV/Vis spectra, fluorescence spectra, and fluorescence quantum yields were measured at room temperature. 1H and 13C NMR spectra were recorded with a JEOL ECA500 at 500 MHz (1H NMR) and 125 MHz (13C NMR) or a JEOL ECZ400 at 400 MHz (1H NMR) and 100 MHz (13C NMR), respectively. The HRMS data were obtained with a Waters Xevo G2-XS QTof (ESI-TOF) or a Bruker Daltonics autoflex maX (MALDI-TOF).
Diethyl 2-azidoazulene-1,3-dicarboxylate (3): A mixture of 3-chloro-1,3-diethoxycarbonylazulene (1) (1.54 g, 5.02 mmol) and sodium azide (503 mg, 7.72 mmol) in DMSO (30 mL) was heated at 70 °C in an oil bath for 4 h under an Ar atmosphere. The reaction mixture was poured into water and extracted with AcOEt. The organic layer was washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure to afford the product 3 (1.53 g, 97%) as a red oil. IR (ATR): νmax = 2998 (w), 2979 (w), 2110 (m), 1694 (s), 1676 (m), 1592 (w), 1533 (w), 1498 (w), 1434 (s), 1411 (m), 1382 (w), 1296 (m), 1211 (s), 1180 (s), 1113 (w), 1093 (w), 1069 (m), 1056 (m), 1031 (m), 966 (w), 883 (w), 848 (w), 824 (w), 789 (m), 768 (w), 737 (w), 674 (w) cm−1; 1H NMR (500 MHz, CDCl3): δH = 9.45 (dd, J = 9.7, 1.1 Hz, 2H), 7.84–7.80 (m, 1H), 7.68 (t, J = 9.9 Hz, 2H), 4.49 (q, J = 7.1 Hz, 4H), 1.47 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 164.32, 149.20, 142.52, 138.97, 137.12, 131.41, 109.42, 60.70, 14.57 ppm; HRMS (ESI-TOF, Positive): calcd for [C16H15N3O4 + H]+ 314.1135, found: 314.1147.
Diethyl 6-azidoazulene-1,3-dicarboxylate (4): A solution of 2 (358 mg, 1.02 mmol) and sodium azide (170 mg, 2.61 mmol) in DMSO (30 mL) was heated at 70 °C in an oil bath for 2 h under an Ar atmosphere. The reaction mixture was poured into water and extracted with ethyl acetate. The combined organic layers were washed with water, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using ethyl acetate as eluent to afford 4 (304 mg, 95%) as a yellow solid. M.p. 150–152 °C (lit. 150.5–152 °C); 1H NMR (500 MHz, CDCl3): δH = 9.65 (dd, J = 9.9, 1.3 Hz, 2H), 8.68 (s, 1H), 7.32 (dt, J = 11.1, 1.1 Hz, 2H), 4.42 (q, J = 7.1 Hz, 4H), 1.44 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 165.02, 152.43, 141.75, 140.99, 138.41, 120.99, 117.69, 60.25, 14.63 ppm; HRMS (ESI-TOF, Positive): calcd for [C16H15N3O4 + H]+ 314.1135; found: 314.1147.
Diethyl 2-(4-phenyl-1H-1,2,3-triazol-1-yl)azulene-1,3-dicarboxylate (5a): To a degassed solution of 3 (158 mg, 0.50 mmol) and ethynylbenzene (115 mg, 1.13 mmol) in THF (2.5 mL) and triethylamine (2.5 mL) was added CuI (11 mg, 0.06 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using toluene as eluent to afford 5a (160 mg, 77%) as a purple solid. M.p. 140–143 °C; IR (ATR): νmax = 3143 (w), 2978 (w), 1678 (s), 1510 (w), 1479 (w), 1459 (w), 1431 (s), 1414 (m), 1385 (m), 1354 (w), 1322 (w), 1299 (w), 1256 (m), 1214 (m), 1180 (s), 1154 (m), 1112 (w), 1084 (w), 1060 (m), 1042 (m), 1025 (m), 881 (w), 826 (w), 792 (m), 761 (m), 741 (w), 718 (w), 690 (m), 656 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 236 (4.62), 272 (4.47), 291 sh (4.61), 302 (4.73), 361 (4.01), 495 sh (2.99), 513 (3.00), 570 sh (2.60) nm; 1H NMR (500 MHz, CDCl3): δH = 9.96–9.94 (m, 2H), 8.17–8.12 (m, 2H), 7.95–7.89 (m, 4H), 7.49–7.46 (m, 2H), 7.39–7.36 (m, 1H), 4.18–4.14 (m, 4H), 1.01 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 163.63, 146.61, 143.65, 142.87, 141.84, 141.65, 131.94, 130.82, 129.04, 128.16, 125.88, 123.38, 113.15, 60.67, 13.93 ppm; HRMS (ESI-TOF, Positive): calcd for [C24H21N3O4 + H]+ 416.1605; found: 416.1608.
Diethyl 2-[4-(4-aminophenyl)-1H-1,2,3-triazol-1-yl]azulene-1,3-dicarboxylate (5b): To a degassed solution of 3 (158 mg, 0.50 mmol) and 4-ethynylaniline (103 mg, 0.88 mmol) in THF (2.5 mL) and triethylamine (2.5 mL) was added CuI (12 mg, 0.06 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using toluene/AcOEt as eluent to afford 5b (162 mg, 75%) as a red solid. M.p. 199–202 °C; IR (ATR): νmax = 3415 (w), 3347 (w), 2977 (w), 1692 (m), 1674 (m), 1643 (w), 1613 (m), 1563 (w), 1513 (m), 1489 (m), 1474 (m), 1460 (m), 1432 (s), 1386 (m), 1352 (w), 1332 (w), 1293 (w), 1252 (s), 1223 (m), 1212 (m), 1189 (s), 1141 (m), 1117 (w), 1080 (m), 1057 (m), 1038 (s), 888 (w), 865 (w), 847 (w), 829 (m), 816 (m), 792 (m), 764 (w), 730 (m), 712 (w), 700 (w), 683 (m), 655 (w), cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 236 (4.48), 272 (4.57), 294 (4.69), 333 sh (4.00), 362 (3.97), 499 (3.00), 524 sh (2.98), 574 (2.57) nm; 1H NMR (500 MHz, CDCl3): δH = 9.92 (d, J = 9.7 Hz, 2H), 8.13 (t, J = 9.9 Hz, 1H), 7.97 (s, 1H), 7.90 (t, J = 10.2 Hz, 2H), 7.73 (d, J = 8.6 Hz, 2H), 6.78 (d, J = 8.6 Hz, 2H), 4.15 (q, J = 7.1 Hz, 4H), 3.79 (s, 2H), 1.02 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 163.72, 146.94, 146.52, 143.90, 142.72, 141.70, 141.66, 131.86, 127.12, 122.16, 121.32, 115.45, 113.17, 60.67, 13.94 ppm; HRMS (ESI-TOF, Positive): calcd for [C24H22N4O4 + H]+ 431.1714; found: 431.1733.
Diethyl 2-[4-(4-nitrophenyl)-1H-1,2,3-triazol-1-yl]azulene-1,3-dicarboxylate (5c): To a degassed solution of 3 (158 mg, 0.50 mmol) and 4-ethynylnitrobenzene (147 mg, 1.00 mmol) in THF (2.5 mL) and triethylamine (2.5 mL) was added CuI (11 mg, 0.06 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using toluene/AcOEt as eluent to afford 5c (178 mg, 77%) as a red solid. M.p. 222–225 °C; IR (ATR): νmax = 3139 (w), 3111 (w), 2988 (w), 1703 (m), 1684 (m), 1604 (w), 1591 (w), 1512 (s), 1473 (w), 1436 (s), 1385 (w), 1341 (s), 1313 (w), 1286 (w), 1254 (m), 1215 (m), 1183 (m), 1144 (w), 1110 (w), 1077 (w), 1054 (w), 1040 (m), 967 (w), 887 (w), 853 (m), 795 (m), 768 (w), 757 (w), 745 (w), 728 (w), 711 (w), 688 (w), 680 (w), 661 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 232 (4.46), 273 (4.31), 303 (4.57), 335 sh (4.40), 365 sh (4.13), 493 sh (2.98), 512 (3.00) nm; 1H NMR (500 MHz, CDCl3): δH = 9.96 (d, J = 10.0 Hz, 2H), 8.35 (d, J = 8.6 Hz, 2H), 8.28 (s, 1H), 8.24–8.16 (m, 2H), 8.12 (d, J = 8.9 Hz, 2H), 7.95 (t, J = 10.0 Hz, 2H), 7.69 (d, J = 8.9 Hz, 1H), 4.17 (q, J = 7.1 Hz, 4H), 1.01 (t, J = 7.0 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 163.42, 147.45, 144.44, 143.23, 142.16, 141.57, 133.50, 132.16, 126.18, 124.90, 124.61, 123.86, 113.06, 60.73, 13.96 ppm; HRMS (ESI-TOF, Positive): calcd for [C24H20N4O6 + H]+ 461.1456; found: 461.1480.
Diethyl 2-[4-(naphthalen-1-yl)-1H-1,2,3-triazol-1-yl]azulene-1,3-dicarboxylate (5d): To a degassed solution of 3 (158 mg, 0.50 mmol) and 1-ethynylnaphthalene (158 mg, 1.04 mmol) in THF (2.5 mL) and triethylamine (2.5 mL) was added CuI (11 mg, 0.06 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using toluene/AcOEt as eluent to afford 5d (171 mg, 74%) as an orange solid. M.p. 174–176 °C; IR (ATR): νmax = 3135 (w), 3063 (w), 2998 (w), 2978 (w), 1693 (m), 1679 (s), 1591 (w), 1506 (m), 1434 (s), 1414 (m), 1397 (m), 1384 (m), 1354 (w), 1317 (w), 1298 (w), 1265 (m), 1254 (m), 1215 (s), 1180 (s), 1152 (m), 1116 (w), 1062 (m), 1049 (m), 1033 (w), 998 (w), 943 (w), 885 (w), 839 (w), 800 (s), 793 (s), 776 (s), 744 (m), 715 (w), 687 (w), 669 (w), 660 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 273 (4.40), 294 sh (4.61), 304 (4.74), 362 (3.94), 493 sh (2.99), 508 (3.00) nm; 1H NMR (500 MHz, CDCl3): δH = 9.99 (d, J = 9.7 Hz, 2H), 8.59 (dd, J = 6.6, 2.9 Hz, 1H), 8.21 (s, 1H), 8.17 (t, J = 9.9 Hz, 1H), 7.96–7.90 (m, 5H), 7.61–7.53 (m, 3H), 4.21 (q, J = 7.2 Hz, 4H), 1.07 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 163.71, 145.49, 142.93, 141.92, 141.76, 134.12, 132.01, 131.21, 128.94, 128.70, 128.15, 127.22, 126.71, 126.18, 126.12, 125.65, 125.42, 113.23, 60.75, 14.10 ppm; HRMS (ESI-TOF, Positive): calcd for [C28H23N3O4 + H]+ 466.1761; found: 466.1747.
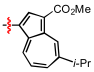
Diethyl 2-[4-(5-isopropyl-3-methoxycarbonylazulen-1-yl)-1H-1,2,3-triazol-1-yl]azulene-1,3-dicarboxylate (5e): To a degassed solution of 3 (122 mg, 0.39 mmol) and 1-ethynyl-5-isopropyl-3-methoxycarbonylazulene (192 mg, 0.76 mmol) in THF (2 mL) and triethylamine (2 mL) was added CuI (11 mg, 0.06 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using toluene as eluent to afford 5e (193 mg, 88%) as a brown solid. M.p. 208–211 °C; IR (ATR): νmax = 2974 (w), 1685 (s), 1592 (w), 1518 (m), 1434 (s), 1386 (m), 1362 (w), 1321 (w), 1275 (w), 1191 (s), 1170 (m), 1136 (m), 1099 (w), 1053 (m), 1027 (m), 937 (w), 902 (m), 879 (w), 792 (m), 776 (m), 740 (w), 719 (w), 710 (w), 687 (w), 659 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 237 (4.71), 273 (4.65), 305 (4.97), 318 sh (4.71), 365 (4.20), 388 (4.10), 403 sh (4.03), 522 (3.18), 570 (3.03) nm; 1H NMR (500 MHz, CDCl3): δH = 9.98–9.95 (m, 2H), 9.82 (d, J = 2.0 Hz, 1H), 9.62 (d, J = 9.5 Hz, 1H), 8.63 (s, 1H), 8.20 (s, 1H), 8.15 (t, J = 9.7 Hz, 1H), 7.92 (t, J = 10.2 Hz, 2H), 7.83 (d, J = 10.6 Hz, 1H), 7.56 (t, J = 10.0 Hz, 1H), 4.17 (q, J = 7.2 Hz, 4H), 3.98 (s, 3H), 3.26 (t, J = 6.9 Hz, 1H), 1.45 (d, J = 6.9 Hz, 6H), 1.01–0.99 (m, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 165.90, 163.70, 149.64, 143.86, 143.59, 142.83, 142.26, 141.81, 141.71, 139.95, 139.28, 138.79, 138.23, 137.32, 131.93, 127.85, 124.17, 117.09, 114.97, 113.23, 60.67, 51.20, 39.19, 24.74, 13.98 ppm; HRMS (ESI-TOF, Positive): calcd for [C33H31N3O6 + H]+ 566.2286, found: 566.2260.
Diethyl 2-[4-ferrocenyl-1H-1,2,3-triazol-1-yl]azulene-1,3-dicarboxylate (5f): To a degassed solution of 3 (161 mg, 0.51 mmol) and ethynylferrocene (221 mg, 1.05 mmol) in THF (2.5 mL) and triethylamine (2.5 mL) was added CuI (12 mg, 0.06 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using toluene/AcOEt as eluent to afford 5f (226 mg, 85%) as a red solid. M.p. 191–194 °C; IR (ATR): νmax = 2987 (w), 1686 (s), 1592 (w), 1525 (w), 1507 (w), 1457 (w), 1434 (s), 1385 (m), 1335 (w), 1319 (w), 1299 (w), 1252 (m), 1239 (w), 1214 (s), 1186 (s), 1154 (w), 1099 (m), 1061 (m), 1036 (s), 974 (w), 877 (m), 825 (m), 781 (w), 762 (w), 742 (w), 725 (w), 714 (w), 703 (w), 683 (w), 669 (w), 654 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 273 (4.47), 291 sh (4.59), 302 (4.71), 362 (4.00), 490 (3.30), 517 sh (3.26) nm; 1H NMR (500 MHz, CDCl3): δH = 9.89 (d, J = 9.7 Hz, 2H), 8.13 (t, J = 9.9 Hz, 1H), 7.89 (t, J = 10.2 Hz, 2H), 7.83 (s, 1H), 4.78 (t, J = 1.7 Hz, 2H), 4.34 (t, J = 1.9 Hz, 2H), 4.21 (q, J = 7.2 Hz, 4H), 4.17 (s, 5H), 1.14 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 163.68, 145.31, 143.67, 142.66, 141.62, 141.53, 131.79, 123.01, 113.15, 75.86, 69.66, 68.71, 67.04, 60.69, 14.20 ppm; HRMS (ESI-TOF, Positive): calcd for [C28H25FeN3O4 + H]+ 524.1267, found: 524.1282; calcd: [C28H25FeN3O4]+ 523.1189, found: 523.1216.
Diethyl 2-(4-butyl-1H-1,2,3-triazol-1-yl)azulene-1,3-dicarboxylate (5g): To a degassed solution of 3 (157 mg, 0.50 mmol) and 1-hexyne (84 mg, 1.02 mmol) in THF (2.5 mL) and triethylamine (2.5 mL) was added CuI (10 mg, 0.05 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using toluene/AcOEt as eluent to afford 5g (142 mg, 72%) as a red solid. M.p. 99–101 °C; IR (ATR): νmax = 3144 (w), 2983 (w), 2954 (w), 2931 (w), 2855 (w), 1678 (s), 1591 (w), 1508 (m), 1459 (w), 1433 (s), 1415 (m), 1383 (m), 1355 (w), 1317 (w), 1298 (w), 1254 (m), 1221 (s), 1182 (s), 1132 (w), 1111 (w), 1059 (m), 1044 (s), 1027 (w), 880 (w), 820 (w), 793 (m), 743 (w), 716 (w), 687 (w), 669 (w), 660 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 273 (4.47), 292 sh (4.61), 303 (4.73), 363 (3.94), 495 sh (2.99), 514 (3.00) nm; 1H NMR (500 MHz, CDCl3): δH = 9.89 (dd, J = 10.7, 1.0 Hz, 2H), 8.11 (t, J = 9.7 Hz, 1H), 7.88 (dd, J = 10.7, 9.9 Hz, 2H), 7.62 (s, 1H), 4.16 (q, J = 7.2 Hz, 4H), 2.86 (t, J = 7.7 Hz, 2H), 1.80–1.74 (m, 2H), 1.49 (q, J = 7.4 Hz, 2H), 1.07 (t, J = 7.2 Hz, 6H), 0.98 (t, J = 7.3 Hz, 3H) ppm; 13C NMR (125 MHz, CDCl3): δC = 163.73, 146.86, 144.16, 142.58, 141.56, 131.76, 124.29, 113.18, 60.56, 31.91, 25.38, 22.52, 13.96, 13.92 ppm; HRMS (ESI-TOF, Positive): calcd for [C22H25N3O4 + H]+ 396.1918; found: 396.1927.
Diethyl 6-(4-phenyl-1H-1,2,3-triazol-1-yl)azulene-1,3-dicarboxylate (6a): To a degassed solution of 4 (269 mg, 0.86 mmol) and ethynylbenzene (182 mg, 1.78 mmol) in THF (4 mL) and triethylamine (4 mL) was added CuI (17 mg, 0.09 mmol). The mixture was heated at 50 °C in an oil bath for 18 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using CH2Cl2/ethyl acetate as eluent to afford 6a (299 mg, 84%) as a purple solid. M.p. 177–180 °C; IR (ATR): νmax = 3116 (w), 3092 (w), 2976 (w), 2930 (w), 1692 (s), 1592 (w), 1550 (w), 1522 (w), 1480 (w), 1467 (w), 1437 (s), 1406 (w), 1393 (w), 1382 (w), 1362 (w), 1302 (w), 1237 (w), 1199 (s), 1173 (m), 1092 (w), 1080 (w), 1047 (w), 1036 (s), 988 (m), 909 (w), 867 (w), 842 (m), 820 (w), 766 (s), 708 (w), 691 (m), 671 (w), 656 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 239 (4.80), 265 sh (4.51), 309 (4.77), 336 (4.67), 374 sh (4.42), 522 (3.00), 565 sh (2.87), 623 (2.32) nm; 1H NMR (500 MHz, CDCl3): δH = 9.76 (d, J = 11.2 Hz, 2H), 8.90 (s, 1H), 7.93 (s, 1H), 7.77 (dd, J = 10.0, 1.1 Hz, 2H), 7.41–7.33 (m, 3H), 7.22–7.21 (m, 2H), 4.44 (q, J = 7.1 Hz, 4H), 1.45 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 164.71, 145.14, 144.92, 142.96, 138.62, 137.91, 134.02, 129.92, 129.40, 128.78, 127.40, 126.15, 118.26, 60.52, 14.61 ppm; HRMS (ESI-TOF, Positive): calcd for [C24H21N3O4 + H]+ 416.1605, found: 416.1608.
Diethyl 6-[4-(4-aminophenyl)-1H-1,2,3-triazol-1-yl]azulene-1,3-dicarboxylate (6b): To a degassed solution of 4 (162 mg, 0.52 mmol) and 4-ethynylaniline (120 mg, 1.02 mmol) in THF (3 mL) and triethylamine (3 mL) was added CuI (11 mg, 0.06 mmol). The mixture was stirred at 50 °C for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using CH2Cl2/AcOEt as eluent to afford 6b (192 mg, 86%) as a purple solid. M.p. 223–227 °C; IR (ATR): νmax = 3432 (w), 3348 (w), 3095 (w), 2979 (w), 1701 (m), 1673 (m), 1633 (w), 1612 (w), 1589 (w), 1549 (w), 1498 (w), 1436 (s), 1406 (w), 1387 (m), 1298 (w), 1239 (w), 1200 (s), 1179 (m), 1091 (m), 1035 (s), 990 (m), 907 (w), 871 (w), 850 (w), 828 (m), 765 (m), 720 (w), 670 (w), 655 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 239 (4.32), 270 (4.30), 315 (4.50), 347 (3.89), 378 (3.90), 523 sh (2.78), 565 sh (2.62), 624 sh (2.13) nm; 1H NMR (500 MHz, DMSO-d6): δH = 9.74 (d, J = 11.2 Hz, 2H), 9.04 (s, 1H), 8.65 (s, 1H), 8.50 (d, J = 11.2 Hz, 2H), 7.63 (d, J = 8.3 Hz, 2H), 6.71 (d, J = 8.0 Hz, 2H), 4.98 (s, 2H), 4.41 (q, J = 7.2 Hz, 4H), 1.40 (t, J = 7.0 Hz, 6H) ppm; Low solubility of compound humped the measurement of 13C NMR; HRMS (ESI-TOF, Positive): calcd for [C24H22N4O4 + H]+ 431.1714, found: 431.1733.
Diethyl 6-[4-(4-nitrophenyl)-1H-1,2,3-triazol-1-yl]azulene-1,3-dicarboxylate (6c): To a degassed solution of 4 (159 mg, 0.51 mmol) and 4-ethynylnitrobenzene (81 mg, 0.55 mmol) in THF (3 mL) and triethylamine (3 mL) was added CuI (10 mg, 0.05 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using CH2Cl2/AcOEt as eluent to afford 6c (162 mg, 69%) as a purple solid. M.p. 258–262 °C; IR (ATR): νmax = 3162 (w), 2979 (w), 1688 (m), 1606 (w), 1588 (w), 1550 (w), 1518 (m), 1429 (s), 1406 (w), 1389 (w), 1344 (m), 1246 (w), 1201 (s), 1110 (w), 1095 (w), 1080 (w), 1043 (m), 1019 (m), 996 (w), 963 (w), 912 (w), 868 (w), 855 (w), 810 (w), 768 (w), 755 (w), 708 (w), 690 (w), 657 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 237 (4.52), 268 (4.26), 333 (4.69), 374 sh (4.26), 523 (2.83), 570 sh (2.68), 630 sh (2.17) nm; 1H NMR (500 MHz, DMSO-d6): δH = 9.77 (dd, J = 10.0, 1.1 Hz, 2H), 9.57 (d, J = 2.0 Hz, 1H), 8.69 (s, 1H), 8.50 (dd, J = 11.3, 0.7 Hz, 2H), 8.33 (d, J = 8.9 Hz, 2H), 8.21 (dd, J = 6.9, 2.0 Hz, 2H), 4.41 (q, J = 7.1 Hz, 4H), 1.40 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, DMSO-d6): δC = 164.27, 148.14, 146.76, 145.51, 143.52, 142.05, 138.79, 136.79, 127.11, 124.78, 123.68, 123.61, 118.35, 60.58, 14.75 ppm; HRMS (ESI-TOF, Positive): calcd for [C24H20N4O6 + H]+ 461.1456, found: 461.1436.
Diethyl 6-[4-(naphthalen-1-yl)-1H-1,2,3-triazol-1-yl]azulene-1,3-dicarboxylate (6d): To a degassed solution of 4 (143 mg, 0.46 mmol) and 1-ethynylnaphthalene (140 mg, 0.92 mmol) in THF (2.5 mL) and triethylamine (2.5 mL) was added CuI (10 mg, 0.05 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using CH2Cl2 as eluent to afford 6d (175 mg, 82%) as a purple solid. M.p. 179–182 °C; IR (ATR): νmax = 3132 (w), 3060 (w), 2979 (w), 1685 (s), 1587 (w), 1551 (w), 1518 (w), 1435 (s), 1378 (w), 1350 (w), 1201 (s), 1128 (w), 1109 (w), 1086 (w), 1046 (m), 1021 (w), 993 (m), 912 (w), 874 (w), 859 (w), 797 (m), 769 (m), 742 (w), 714 (w), 674 (w), 662 (w), cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 270 (4.27), 316 (4.74), 358 sh (4.30), 375 sh (4.23), 522 (3.00), 565 (2.87), 625 sh (2.29) nm; 1H NMR (500 MHz, CDCl3): δH = 9.93 (dd, J = 10.0, 1.4 Hz, 2H), 8.89 (s, 1H), 8.46–8.43 (m, 2H), 8.30 (dd, J = 9.9, 1.3 Hz, 2H), 7.96–7.93 (m, 2H), 7.83 (dd, J = 6.9, 1.1 Hz, 1H), 7.60–7.54 (m, 3H), 4.47 (q, J = 7.2 Hz, 4H), 1.48 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 164.76, 148.77, 145.05, 144.43, 142.43, 138.61, 134.04, 131.16, 129.74, 128.72, 127.69, 127.11, 127.04, 126.34, 125.46, 125.28, 122.62, 121.68, 118.43, 60.54, 14.63 ppm; HRMS (ESI-TOF, Positive): calcd for [C28H23N3O4 + H]+ 466.1761, found: 466.1747.
Diethyl 6-{4-[5-isopropyl-3-(methoxycarbonyl)azulen-1-yl]-1H-1,2,3-triazol-1-yl}azulene-1,3-dicarboxylate (6e): To a degassed solution of 4 (53 mg, 0.17 mmol) and 1-ethynyl-5-isopropyl-3-methoxycarbonylazulene (86 mg, 0.34 mmol) in THF (1 mL) and triethylamine (1 mL) was added CuI (3 mg, 0.016 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using toluene as eluent to afford 6e (73 mg, 76%) as a purple solid. M.p. 170–174 °C; IR (ATR): νmax = 2978 (w), 1686 (s), 1587 (w), 1571 (w), 1547 (w), 1522 (w), 1434 (s), 1421 (m), 1404 (m), 1239 (m), 1207 (s), 1171 (m), 1119 (m), 1082 (w), 1063 (w), 1041 (m), 1028 (m), 983 (m), 940 (w), 908 (w), 876 (m), 857 (m), 817 (w), 777 (m), 768 (w), 739 (w), 705 (w), 676 (w), 662 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 237 (4.79), 270 (4.51), 324 (4.98), 364 sh (4.41), 381 (4.12), 560 sh (2.85), 613 sh (2.52) nm; 1H NMR (500 MHz, CDCl3): δH = 9.92 (d, J = 11.2 Hz, 2H), 9.83 (d, J = 1.7 Hz, 1H), 9.55 (d, J = 9.7 Hz, 1H), 8.88 (s, 1H), 8.64 (s, 1H), 8.46 (s, 1H), 8.29 (dd, J = 10.0, 1.4 Hz, 2H), 7.85 (d, J = 10.3 Hz, 1H), 7.60 (t, J = 10.0 Hz, 1H), 4.47 (q, J = 7.2 Hz, 4H), 3.99 (s, 3H), 3.27 (t, J = 6.9 Hz, 1H), 1.48 (t, J = 7.2 Hz, 6H), 1.45 (d, J = 6.9 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 165.74, 164.79, 150.27, 146.83, 145.09, 144.28, 142.50, 142.38, 140.16, 139.61, 139.12, 138.60, 138.54, 137.32, 128.34, 122.46, 119.03, 118.35, 115.58, 115.23, 60.52, 51.28, 39.21, 24.73, 14.65 ppm; HRMS (ESI-TOF, Positive): calcd for [C33H31N3O6 + H]+ 566.2286, found: 566.2260.
Diethyl 6-(4-ferrocenyl-1H-1,2,3-triazol-1-yl)azulene-1,3-dicarboxylate (6f): To a degassed solution of 4 (158 mg, 0.50 mmol) and ethynylferrocene (210 mg, 1.00 mmol) in THF (2.5 mL) and triethylamine (2.5 mL) was added CuI (10 mg, 0.05 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using CH2Cl2 as eluent to afford 6f (194 mg, 74%) as a red solid. M.p. 247–250 °C; IR (ATR): νmax = 3119 (w), 2975 (w), 1691 (s), 1598 (w), 1550 (w), 1436 (s), 1409 (w), 1390 (m), 1352 (w), 1306 (w), 1237 (m), 1194 (s), 1118 (w), 1078 (w), 1043 (m), 1036 (s), 1020 (m), 988 (m), 870 (m), 840 (m), 820 (m), 764 (m), 716 (w), 670 (w), 658 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 240 (4.41), 266 sh (4.11), 309 (4.38), 336 (4.27), 374 sh (4.03), 501 (2.99), 565 sh (2.73) nm; 1H NMR (500 MHz, CDCl3): δH = 9.90 (d, J = 11.5 Hz, 2H), 8.87 (s, 1H), 8.23 (d, J = 11.5 Hz, 2H), 8.08 (s, 1H), 4.83 (t, J = 1.7 Hz, 2H), 4.46 (q, J = 7.2 Hz, 4H), 4.39 (t, J = 1.9 Hz, 2H), 4.15 (s, 4H), 1.47 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 164.79, 149.20, 145.03, 144.19, 142.30, 138.60, 122.27, 118.30, 117.32, 74.15, 69.80, 69.30, 67.04, 60.49, 14.63 ppm; HRMS (ESI-TOF, Positive): calcd for [C28H25FeN3O4 + H]+ 524.1267, found: 524.1282; [C28H25FeN3O4]+ 523.1189, found: 523.1216.
Diethyl 6-(4-butyl-1H-1,2,3-triazol-1-yl)azulene-1,3-dicarboxylate (6g): To a degassed solution of 4 (158 mg, 0.50 mmol) and 1-hexyne (84 mg, 1.02 mmol) in THF (5 mL) and triethylamine (5 mL) was added CuI (15 mg, 0.08 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The reaction mixture was poured into a 10% NH4Cl solution and extracted with toluene. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using toluene as eluent to afford 6g (129 mg, 65%) as a purple solid. M.p. 172–173 °C; IR (ATR): νmax = 3125 (w), 2960 (w), 2931 (w), 2872 (w), 1693 (s), 1590 (w), 1549 (w), 1519 (w), 1479 (w), 1436 (s), 1409 (w), 1394 (w), 1381 (w), 1352 (w), 1325 (w), 1308 (w), 1236 (w), 1198 (s), 1164 (m), 1077 (m), 1039 (s), 1020 (w), 994 (m), 932 (w), 910 (w), 869 (w), 845 (w), 804 (w), 763 (m), 701 (w), 659 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 237 (4.24), 270 (3.96), 324 (4.43), 362 sh (3.88), 381 sh (3.57), 519 (2.73), 561 sh (2.53), 616 sh (1.93) nm; 1H NMR (500 MHz, CDCl3): δH = 9.87 (dd, J = 10.0, 1.4 Hz, 2H), 8.85 (s, 1H), 8.17 (dd, J = 10.0, 1.4 Hz, 2H), 7.92 (s, 1H), 4.45 (q, J = 7.2 Hz, 4H), 2.85 (t, J = 7.7 Hz, 2H), 1.79–1.73 (m, 2H), 1.48–1.44 (m, 8H), 0.98 (t, J = 7.4 Hz, 3H) ppm; 13C NMR (125 MHz, CDCl3): δC = 164.78, 150.46, 145.32, 144.14, 142.31, 138.58, 122.50, 119.97, 118.20, 60.47, 31.45, 25.46, 22.43, 14.62, 13.91 ppm; HRMS (ESI-TOF, Positive): calcd for [C22H25N3O4 + H]+ 396.1918, found: 396.1927.
1-(Azulen-2-yl)-4-phenyl-1H-1,2,3-triazole (7a): A solution of 5a (104 mg, 0.25 mmol) in methanesulfonic acid (3 mL) was heated at 90 °C in an oil bath for 2 h. The reaction mixture was poured into water. The precipitate was collected by filtration to afford 7a (63 mg, 93%) as a purple solid. M.p. 228–231 °C; IR (ATR): νmax = 3142 (m), 3115 (w), 3084 (w), 3055 (w), 3023 (w), 1575 (m), 1536 (m), 1522 (s), 1477 (m), 1452 (m), 1421 (m), 1399 (m), 1299 (w), 1230 (m), 1223 (m), 1213 (m), 1172 (w), 1155 (w), 1071 (w), 1040 (m), 1016 (m), 959 (w), 915 (w), 899 (m), 810 (m), 760 (m), 736 (m), 706 (w), 692 (m), 660 (w), 632 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 239 (4.33), 285 (4.75), 301 (4.64), 344 (3.85), 373 (4.17), 549 (2.65), 569 (2.63), 582 (2.64), 640 sh (2.24) nm; 1H NMR (400 MHz, CDCl3): δH = 8.40 (d, J = 9.2 Hz, 2H), 8.38 (s, 1H), 7.96 (dd, J = 8.3, 1.3 Hz, 2H), 7.70–7.64 (m, 3H), 7.50–7.46 (m, 2H), 7.41–7.36 (m, 1H), 7.33 (t, J = 9.9 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3): δC = 148.44, 142.64, 140.04, 137.76, 137.62, 130.24, 129.03, 128.57, 126.01, 125.33, 117.96, 106.28 ppm; HRMS (ESI-TOF, Positive): calcd for [C18H13N3 + H]+ 272.1182; found: 272.1210.
4-[1-(Azulen-2-yl)-1H-1,2,3-triazol-4-yl]aniline (7b): A solution of 5b (108 mg, 0.25 mmol) in methanesulfonic acid (3 mL) was heated at 90 °C in an oil bath for 2 h. The reaction mixture was poured into water. The precipitate was collected by filtration to afford 7b (63 mg, 88%) as a purple solid. M.p. 237–239 °C; IR (ATR): νmax = 3464 (w), 3333 (s), 3210 (w), 1625 (s), 1612 (m), 1579 (w), 1535 (w), 1519 (m), 1489 (s), 1441 (w), 1417 (m), 1397 (w), 1296 (m), 1230 (m), 1218 (w), 1202 (w), 1184 (w), 1169 (w), 1128 (w), 1041 (m), 1020 (w), 958 (w), 946 (w), 901 (w), 833 (m), 801 (s), 789 (s), 769 (w), 729 (m), 700 (w), 657 (w), 630 (w) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 237 (4.30), 292 (4.93), 380 (4.16), 510 sh (2.55), 547 (2.69), 582 (2.66), 638 sh (2.30) nm; 1H NMR (400 MHz, CDCl3): δH = 8.40–8.38 (m, 2H), 8.24 (s, 1H), 7.76–7.74 (m, 2H), 7.68–7.63 (m, 3H), 7.32 (t, J = 9.8 Hz, 2H), 6.79–6.77 (m, 2H), 3.81 (s, 2H) ppm; 13C NMR (100 MHz, CDCl3): δC = 148.77, 146.85, 142.85, 140.05, 137.54, 137.42, 127.26, 125.25, 120.67, 116.58, 115.37, 106.24 ppm; HRMS (ESI-TOF, Positive): [C18H14N4 + H]+ 287.1291; found: 287.1290.
1-(Azulen-2-yl)-4-(4-nitrophenyl)-1H-1,2,3-triazole (7c): A solution of 5c (115 mg, 0.25 mmol) in methanesulfonic acid (3 mL) was heated at 90 °C in an oil bath for 2 h. The reaction mixture was poured into water. The precipitate was collected by filtration to afford 7c (75 mg, 95%) as a purple solid. M.p. 321–323 °C; IR (ATR): νmax = 3134 (w), 3102 (w), 3053 (w), 1604 (s), 1579 (w), 1536 (m), 1508 (s), 1477 (s), 1453 (m), 1415 (m), 1398 (m), 1376 (w), 1337 (s), 1315 (s), 1296 (s), 1272 (m), 1236 (s), 1222 (s), 1208 (s), 1199 (s), 1164 (w), 1109 (s), 1032 (m), 1016 (s), 1006 (s), 962 (m), 899 (w), 860 (s), 852 (m), 836 (m), 793 (s), 769 (m), 752 (s), 742 (s), 703 (m), 688 (m), 652 (w), 627 (m) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 299 (4.65), 357 (4.35), 373 (4.45), 549 (2.70), 582 (2.68), 637 sh (2.31) nm; 1H NMR (400 MHz, CDCl3): δH = 8.53 (s, 1H), 8.44 (d, J = 9.2 Hz, 2H), 8.36 (dd, J = 7.0, 2.0 Hz, 2H), 8.14 (dd, J = 6.9, 2.0 Hz, 2H), 7.74–7.69 (m, 3H), 7.36 (t, J = 9.9 Hz, 2H) ppm; Low solubility of compound hampered the measurement of 13C NMR; HRMS (ESI-TOF, Positive): [C18H12N4O2 + H]+ 317.1033; found: 317.1062.
1-(Azulen-2-yl)-4-(naphthalen-1-yl)-1H-1,2,3-triazole (7d): A solution of 5d (116 mg, 0.25 mmol) in methanesulfonic acid (3 mL) was heated at 90 °C in an oil bath for 2 h. The reaction mixture was poured into water, and the resulting solid was collected by filtration to afford 7d (73 mg, 91%) as a purple solid. M.p. 159–161 °C; IR (ATR): νmax = 3115 (m), 3045 (m), 3016 (w), 1581 (m), 1536 (m), 1503 (s), 1454 (m), 1392 (m), 1338 (w), 1298 (w), 1277 (w), 1258 (w), 1237 (m), 1218 (m), 1201 (m), 1163 (w), 1105 (w), 1034 (m), 1010 (m), 955 (w), 900 (w), 861 (w), 836 (m), 813 (m), 792 (s), 769 (s), 731 (s), 716 (m), 658 (w), 637 (w), 625 (m) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 289 (4.80), 340 (3.90), 358 (4.02), 372 (4.18), 549 (2.66), 582 (2.65), 639 sh (2.27) nm; 1H NMR (400 MHz, CDCl3): δH = 8.49–8.46 (m, 1H), 8.45–8.42 (m, 3H), 7.95–7.93 (m, 2H), 7.85 (dd, J = 7.1, 1.2 Hz, 1H), 7.74 (s, 2H), 7.69 (t, J = 9.9 Hz, 1H), 7.60–7.55 (m, 3H), 7.35 (t, J = 9.9 Hz, 2H) ppm; 13C NMR (100 MHz, CDCl3): δC = 147.62, 142.63, 140.10, 137.82, 137.70, 134.00, 131.26, 129.30, 128.62, 127.69, 127.52, 126.89, 126.20, 125.50, 125.36, 121.05, 106.34 ppm, one signal is overlapped with other signals; HRMS (ESI-TOF, Positive): [C22H15N3 + H]+ 322.1339; found: 322.1357.
1-(Azulen-2-yl)-4-(azulen-1-yl)-1H-1,2,3-triazole (7e): A solution of 5e (113 mg, 0.20 mmol) in methanesulfonic acid (5 mL) was heated at 90 °C in an oil bath for 2 h. The reaction mixture was poured into water, and the resulting solid was collected by filtration to afford 7e (65 mg, 89%) as a purple solid. M.p. 252–254 °C; IR (ATR): νmax = 3136 (m), 3112 (m), 3084 (m), 3045 (m), 3023 (m), 3004 (m), 2958 (s), 2922 (m), 2884 (m), 2865 (m), 1580 (m), 1562 (m), 1536 (m), 1522 (s), 1502 (m), 1484 (m), 1453 (m), 1428 (m), 1394 (s), 1363 (m), 1325 (w), 1295 (m), 1261 (m), 1247 (w), 1220 (m), 1176 (m), 1161 (w), 1106 (m), 1070 (w), 1054 (w), 1040 (m), 1004 (m), 978 (w), 955 (w), 943 (w), 919 (w), 908 (w), 900 (m), 886 (w), 870 (w), 859 (w), 820 (m), 802 (s), 770 (s), 730 (m), 700 (m), 632 (m) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 299 (4.89), 341 (4.12), 386 (4.22), 550 (3.15), 585 (3.15), 655 sh (2.93) nm; 1H NMR (400 MHz, CDCl3): δH = 9.28 (d, J = 9.9 Hz, 1H), 8.40 (d, J = 10.2 Hz, 3H), 8.33 (d, J = 1.7 Hz, 1H), 8.21 (d, J = 4.0 Hz, 1H), 7.72 (s, 2H), 7.67–7.59 (m, 2H), 7.37–7.28 (m, 4H), 3.12 (t, J = 6.9 Hz, 1H), 1.40 (d, J = 6.9 Hz, 6H) ppm; 13C NMR (100 MHz, CDCl3): δC = 146.43, 144.27, 143.21, 142.83, 140.33, 137.39, 137.32, 136.06, 135.62, 135.23, 125.24, 124.15, 117.97, 117.45, 117.28, 106.52, 38.55, 24.53 ppm; HRMS (ESI-TOF, Positive): [C25H21N3 + H]+ 364.1808; found: 364.1826.
1-(Azulen-2-yl)-4-butyl-1H-1,2,3-triazole (7g): A solution of 5g (79 mg, 0.20 mmol) in methanesulfonic acid (5 mL) was heated at 90 °C in an oil bath for 3 h. The reaction mixture was poured into water, and the resulting solid was collected by filtration to afford 7g (45 mg, 90%) as a purple solid. M.p. 97–100 °C; IR (ATR): νmax = 3142 (m), 3115 (m), 3084 (w), 3055 (w), 3023 (w), 1575 (m), 1536 (s), 1522 (s), 1477 (m), 1452 (m), 1421 (m), 1399 (s), 1299 (w), 1230 (m), 1223 (m), 1213 (m), 1172 (m), 1155 (w), 1071 (w), 1040 (m), 1016 (s), 959 (w), 915 (w), 899 (m), 810 (s), 760 (m), 736 (m), 706 (w), 692 (s), 660 (w), 632 (m) cm−1; UV/Vis (CH2Cl2): λmax (log ε) = 239 (4.21), 290 (4.78), 298 (4.77), 340 (3.82), 354 (3.94), 370 (4.10), 548 sh (2.65), 581 (2.63), 635 sh (2.28) nm; 1H NMR (400 MHz, CDCl3): δH = 8.36 (d, J = 9.1 Hz, 2H), 7.89 (s, 1H), 7.66–7.60 (m, 3H), 7.29 (t, J = 10.1 Hz, 2H), 2.83 (t, J = 7.5 Hz, 2H), 1.74 (quint, J = 7.5 Hz, 2H), 1.44 (sextet, J = 7.5 Hz, 2H), 0.96 (t, J = 7.5 Hz, 3H) ppm; 13C NMR (100 MHz, CDCl3): δC = 149.30, 143.06, 140.07, 137.43, 137.32, 125.19, 119.34, 106.21, 31.55, 25.45, 22.42, 13.95 ppm; HRMS (ESI-TOF, Positive): [C16H17N3 + H]+ 252.1495; found: 252.1497.
1-(Azulen-2-yl)-4-(azulen-1-yl)-1H-1,2,3-triazole (8): A solution of 5e (208 mg, 0.50 mmol) in methanesulfonic acid (5 mL) was heated at 90 °C in an oil bath for 1 h. The reaction mixture was poured into water, and the resulting solid was collected by filtration. The crude solid was dissolved in CH2Cl2 and purified by column chromatography on silica gel using CH2Cl2 as eluent to afford 8 (9 mg, 7%) as a purple solid. M.p. 228–231 °C; IR (ATR): νmax = 3125 (m), 3093 (w), 3080 (w), 3058 (w), 1574 (m), 1551 (m), 1477 (m), 1454 (m), 1422 (m), 1398 (m), 1379 (m), 1346 (m), 1308 (m), 1286 (m), 1239 (m), 1211 (m), 1197 (m), 1161 (m), 1085 (m), 1073 (m), 1050 (m), 1032 (m), 1019 (m), 977 (m), 965 (m), 916 (m), 905 (m), 837 (m), 821 (m), 792 (m), 764 (s), 740 (s), 706 (m), 689 (s), 662 (m), 644 (m) cm−1; 1H NMR (400 MHz, CDCl3): δH = 8.46 (d, J = 10.3 Hz, 2H), 8.30 (d, J = 0.6 Hz, 1H), 8.00 (t, J = 3.8 Hz, 1H), 7.95–7.93 (m, 2H), 7.65 (d, J = 10.6 Hz, 2H), 7.54 (d, J = 3.7 Hz, 2H), 7.48 (t, J = 7.7 Hz, 2H), 7.41–7.37 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3): δC = 148.81, 142.82, 139.18, 138.78, 135.46, 130.11, 129.09, 128.71, 125.99, 120.74, 119.10, 116.23 ppm; HRMS (MALDI-TOF, Positive): calcd for [C18H13N3 + H]+ 272.1182, found: 272.1175.
Diethyl 6-[(triphenyl-λ5-phosphaneylidene)amino]azulene-1,3-dicarboxylate (9): To a degassed solution of 4 (605 mg, 1.93 mmol) in THF (10 mL) was added triphenylphosphane (1.02 g, 3.89 mmol). The mixture was heated at 50 °C in an oil bath for 17 h under an Ar atmosphere. The generated solid was filtered and washed with EtOH and hexane to afford 9 (908 mg, 86%) as a yellow solid. M.p. 175–178 °C; IR (ATR): νmax = 2980 (w), 1686 (w), 1666 (m), 1579 (w), 1562 (w), 1535 (w), 1479 (w), 1459 (w), 1425 (s), 1368 (w), 1323 (s), 1241 (w), 1196 (s), 1142 (w), 1106 (m), 1094 (m), 1047 (m), 999 (w), 952 (s), 889 (s), 868 (w), 846 (w), 828 (w), 806 (w), 761 (w), 721 (m), 693 (m), 669 (w), 655 (w) cm−1; 1H NMR (500 MHz, CDCl3): δH = 9.11 (d, J = 10.9 Hz, 2H), 8.22 (s, 1H), 7.77–7.73 (m, 6H), 7.62–7.59 (m, 3H), 7.53–7.50 (m, 6H), 7.14 (d, J = 11.7 Hz, 2H), 4.33 (q, J = 7.1 Hz, 4H), 1.38 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 168.86, 168.84, 166.07, 137.43, 137.12, 134.80, 132.90, 132.88, 132.68, 132.60, 129.33, 129.23, 128.45, 127.84, 127.68, 127.65, 114.58, 59.34, 14.71 ppm; HRMS (ESI-TOF, Positive): calcd for [C34H30NO4P + H]+ 548.1985; found: 548.1978.
Diethyl 6-aminoazulene-1,3-dicarboxylate (10): To a solution of 9 (551 mg, 1.01 mmol) in THF (20 mL) was added hydrochloric acid (2 mL). The mixture was heated at reflux temperature in an oil bath for 17 h. The reaction mixture was poured into water and neutralized with K2CO3. The resulting solid was collected by filtration and washed with EtOH and hexane to afford 10 (235 mg, 81%) as a yellow solid. M.p. 252–254 °C (lit. 236–237 °C); 1H NMR (500 MHz, CDCl3): δH = 9.41 (d, J = 11.2 Hz, 2H), 8.34 (s, 1H), 6.87 (d, J = 11.5 Hz, 2H), 5.05 (s, 2H), 4.38 (q, J = 7.1 Hz, 4H), 1.42 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (125 MHz, CDCl3): δC = 165.78, 159.38, 139.96, 137.49, 136.51, 132.26, 128.62, 116.50, 77.10, 59.70, 14.70 ppm; HRMS (ESI-TOF, Positive): calcd for [C16H17NO4 + H]+ 288.1230; found: 288.1253.
4. Conclusions
In summary, we have demonstrated that both 2- and 6-azidoazulenes serve as highly efficient dipoles for Cu(I)-catalyzed Huisgen cycloaddition with terminal alkynes, enabling rapid access to structurally diverse 1,2,3-triazole-functionalized azulenes. Subsequent transformations, including acid-mediated decarboxylation and the Staudinger reaction, further expanded the synthetic utility of these intermediates to furnish 2-azulenyltriazoles and 6-aminoazulene derivative.
Single-crystal X-ray diffraction analysis revealed that steric congestion around the azulene 1,3-positions enforces near-orthogonality between the azulene and triazole rings, whereas partial planarization is achieved in decarboxylated derivatives, providing direct structural insight into the control of π-conjugation within these scaffolds. UV/Vis and fluorescence measurements of a series of 7 disclosed pronounced effects of protonation, leading to distinct spectral changes and, in some cases, fluorescence with markedly enhanced quantum yields.
Taken together, these results establish azidoazulenes as versatile building blocks for modular construction of triazole-based π-extended azulenes, and reveal that fine control of substitution pattern, conformation, and protonation state provides an effective design handle for tuning their optical responses. We anticipate that these findings will contribute to the future development of azulene-based functional chromophores and electro-/photoresponsive organic materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules31020221/s1, Figures S1–S114: 1H, 13C NMR, UV/Vis and fluorescent spectra of new compounds; Table S1: Selected crystal data of 5a, 5d, 7a, 8, and 9.
Author Contributions
Methodology, T.S.; analysis, T.S., M.Y., M.I., M.F., S.M., T.O., I.U., R.S. and S.I.; investigation, T.S., M.Y., M.I., M.F., R.S. and S.I.; writing, original draft preparation, T.S.; writing, review and editing, T.S., M.Y., M.I., M.F., S.M., T.O., I.U., R.S. and S.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number 21K05037 and 25K08627.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hakimian, S.; Cheng-Hakimian, A.; Anderson, G.D.; Miller, J.W. Rufinamide: A New Anti-Epileptic Medication. Expert Opin. Pharmacother. 2007, 8, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Gorbach, S.L. Piperacillin/Tazobactam in the Treatment of Polymicrobial Infections. Intensive Care Med. 1994, 20, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Martens, E.; Bertrand, D.; Pereira, D. The Solithromycin Journey—It Is All in the Chemistry. Bioorg. Med. Chem. 2016, 24, 6420–6428. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.B.; Finn, J.; Hilgers, M.; Morales, G.; Rahawi, S.; Kedar, G.C.; Picazo, J.J.; Im, W.; Shaw, K.J.; Stein, J.L. Structure-Activity Relationships of Diverse Oxazolidinones for Linezolid-Resistant Staphylococcus Aureus Strains Possessing the Cfr Methyltransferase Gene or Ribosomal Mutations. Antimicrob. Agents Chemother. 2010, 54, 5337–5343. [Google Scholar] [CrossRef]
- Cox, C.D.; Breslin, M.J.; Whitman, D.B.; Schreier, J.D.; McGaughey, G.B.; Bogusky, M.J.; Roecker, A.J.; Mercer, S.P.; Bednar, R.A.; Lemaire, W.; et al. Discovery of the Dual Orexin Receptor Antagonist [(7R)-4-(5-Chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the Treatment of Insomnia. J. Med. Chem. 2010, 53, 5320–5332. [Google Scholar] [CrossRef]
- Shi, W.; Chen, X.; Xu, N.; Song, H.; Zhao, B.; Cheng, P.; Liao, D.; Yan, S. Synthesis, Crystal Structures, and Magnetic Properties of 2D Manganese(II) and 1D Gadolinium(III) Coordination Polymers with 1H-1,2,3-Triazole-4,5-dicarboxylic Acid. Eur. J. Inorg. Chem. 2006, 2006, 4931–4937. [Google Scholar] [CrossRef]
- Schulze, B.; Friebe, C.; Jäger, M.; Görls, H.; Birckner, E.; Winter, A.; Schubert, U.S. PtII Phosphors with Click-Derived 1,2,3-Triazole-Containing Tridentate Chelates. Organometallics 2017, 37, 145–155. [Google Scholar] [CrossRef]
- Moreno-Latorre, M.; de la Torre, M.C.; Gornitzka, H.; Hemmert, C.; Sierra, M.A. Mono- and Dinuclear 1-(2-Pyridyl)-4-phenyl-1,2,3-triazole-Based Ir(III) and Rh(III) Complexes. Organometallics 2024, 43, 1128–1136. [Google Scholar] [CrossRef]
- Lau, G.P.S.; Tsao, H.N.; Zakeeruddin, S.M.; Grätzel, M.; Dyson, P.J. Highly Stable Dye-Sensitized Solar Cells Based on Novel 1,2,3-Triazolium Ionic Liquids. ACS Appl. Mater. Interfaces 2014, 6, 13571–13577. [Google Scholar] [CrossRef]
- Stroppa, P.H.F.; Martins, J.S.; Dias, R.C.; Salla, C.A.M.; Bechtold, I.H.; Legnani, C.; Fragneaud, B.; Quirino, W.G.; David da Silva, A. High Efficient Light-Emitting Electrochemical Cells Based on Ionic Liquids 1,2,3-Triazolium. Org. Electron. 2019, 73, 172–181. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Proc. Chem. Soc. 1961, 357–396. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kacprzak, K.; Skiera, I.; Piasecka, M.; Paryzek, Z. Alkaloids and Isoprenoids Modification by Copper(I)-Catalyzed Huisgen 1,3-Dipolar Cycloaddition (Click Chemistry): Toward New Functions and Molecular Architectures. Chem. Rev. 2016, 116, 5689–5743. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Mishra, B.B.; Mishra, K.B.; Mishra, N.; Singh, A.S.; Chen, X. Cu-Catalyzed Click Reaction in Carbohydrate Chemistry. Chem. Rev. 2016, 116, 3086–3240. [Google Scholar] [CrossRef]
- Xie, S.; Sundhoro, M.; Houk, K.N.; Yan, M. Electrophilic Azides for Materials Synthesis and Chemical Biology. Acc. Chem. Res. 2020, 53, 937–948. [Google Scholar] [CrossRef]
- Nozoe, T.; Horino, H.; Toda, T. Synthesis and Reactions of 5-Azidotropolones. Tetrahedron Lett. 1967, 8, 5349–5353. [Google Scholar] [CrossRef]
- Horino, H.; Toda, T. Reactions of 5-N-(1,2,3-Triazol-1-yl)tropolones. Bull. Chem. Soc. Jpn. 1972, 45, 584–587. [Google Scholar] [CrossRef]
- Puodziukynaite, E.; Wang, H.-W.; Lawrence, J.; Wise, A.J.; Russell, T.P.; Barnes, M.D.; Emrick, T. Azulene Methacrylate Polymers: Synthesis, Electronic Properties, and Solar Cell Fabrication. J. Am. Chem. Soc. 2014, 136, 11043–11049. [Google Scholar] [CrossRef]
- Ito, S.; Okujima, T.; Kabuto, C.; Morita, N. Preparation, characterization, and cycloaddition reaction of the heterocumulenes attached directly to azulenes. An efficient strategy for the preparation of azulene-substituted heterocycles. Tetrahedron 2003, 59, 4651–4659. [Google Scholar] [CrossRef]
- Nozoe, T.; Takase, K.; Tada, M. The Anionoid Substitution Reaction of Diethyl 6-Bromoazulene-1,3-dicarboxylate. Bull. Chem. Soc. Jpn. 1965, 38, 247–251. [Google Scholar] [CrossRef]
- Johann, P.; Herwig, S. Conversion of Methyl Ketones into Terminal Acetylenes: Ethynylferrocene. Org. Synth. 1996, 73, 262. [Google Scholar] [CrossRef]
- Shoji, T.; Sakata, N.; Ishikawa, R.; Mori, S.; Okujima, T.; Sekiguchi, R.; Ito, S. AzUlene-Fused Polycyclic Aromatic Hydrocarbons: Synthesis, Structural, Optical, and Electrochemical Properties of Azuleno [2,1-a]phenalenones. ChemistryEurope 2025, 3, e202500039. [Google Scholar] [CrossRef]
- Staudinger, H.; Meyer, J. Über Neue Organische Phosphorverbindungen III. Phosphinmethylenderivate Und Phosphinimine. Helv. Chim. Acta 1919, 2, 635–646. [Google Scholar] [CrossRef]
- Köhn, M.; Breinbauer, R. The Staudinger Ligation—A Gift to Chemical Biology. Angew. Chem. Int. Ed. 2004, 43, 3106–3116. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions. Acc. Chem. Res. 2011, 44, 666–676. [Google Scholar] [CrossRef]
- Xiang, J.; Vu, D.; He, M.; Duan, C.; Ge, C.; McNeill, C.R.; Gao, X. Poly(2,6-Azulene Vinylene)s: Azulene Orientation Control and Property Studies. Macromolecules 2023, 56, 9475–9488. [Google Scholar] [CrossRef]
- Ren, P.; Chen, L.; Sun, C.; Hua, X.; Luo, N.; Fan, B.; Chen, P.; Shao, X.; Zhang, H.-L.; Liu, Z. Linear Non-Benzenoid Isomer of Acene Fusing Chrysene with Azulene Units. J. Phys. Chem. Lett. 2024, 15, 8410–8419. [Google Scholar] [CrossRef]
- Zhang, H.; An, P. Azulene-Fused Seco-Hexabenzocoronene-Based Nanographenes. Org. Lett. 2025, 27, 4411–4416. [Google Scholar] [CrossRef]
- Shoji, T.; Yamazaki, A.; Katoh, R.; Shimamura, K.; Sakai, R.; Yasunami, M.; Okujima, T.; Ito, S. Synthesis, Reactivity, and Properties of Benz[a]azulenes via the [8 + 2] Cycloaddition of 2H-Cyclohepta[b]furan-2-ones with an Enamine. J. Org. Chem. 2022, 87, 5827–5845. [Google Scholar] [CrossRef]
- Shoji, T.; Sakata, N.; Ariga, Y.; Yamazaki, A.; Katoh, R.; Okujima, T.; Sekiguchi, R.; Ito, S. Construction of a 2,2′-Biazulene Framework via Brønsted Acid-Promoted Annulation of 2,3-Di(1-azulenyl)benzofurans. Chem. Commun. 2023, 59, 3447–3450. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Sasahara, N.; Hamasaki, A.; Mori, S.; Okujima, T.; Sekiguchi, R.; Ito, S. Synthesis, Structural, and Optical Properties of Azuleno[1,2-c]pyran-1-ones: Brønsted Acid-Mediated Cyclization of 2-Azulenylalkynes. J. Org. Chem. 2023, 88, 15739–15749. [Google Scholar] [CrossRef] [PubMed]
- Hou, I.C.; Berger, F.; Narita, A.; Müllen, K.; Hecht, S. Proton-Gated Ring-Closure of a Negative Photochromic Azulene-Based Diarylethene. Angew. Chem. Int. Ed. 2020, 59, 18532–18536. [Google Scholar] [CrossRef]
- Williams, G.E.; Kociok-Köhn, G.; James, T.D.; Lewis, S.E. C4-Aldehyde of Guaiazulene: Synthesis and Derivatisation. Org. Biomol. Chem. 2021, 19, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, M.; Yoshida, M.; Ando, D.; Mori, S.; Okujima, T.; Sekiguchi, R.; Ito, S.; Yasunami, M.; Shoji, T. Synthesis of 11H-Indeno[2,1-a]azulenes and Transformation into Azulene-Fused Dicyanobenzofulvenes. J. Org. Chem. 2025, 90, 15972–15996. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef]
- Suzuki, N.; Kubota, T.; Ando, N.; Yamaguchi, S. Photobase-Driven Excited-State Intramolecular Proton Transfer (ESIPT) in a Strapped π-Electron System. Chem. Eur. J. 2021, 28, e202103584. [Google Scholar] [CrossRef]
- Qiao, Q.; Wang, C.; Wang, H.; Ruan, Y.; Liu, W.; Chen, J.; Wu, Z.; Liu, X.; Xu, Z. Tail-Assisted Excited-State Intramolecular Proton Transfer (Ta-ESIPT) Fluorophores: A Universal Ratiometric Platform for Hydration-Sensitive Biomolecular Imaging and Sensing. J. Am. Chem. Soc. 2025, 147, 15602–15613. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.