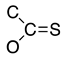
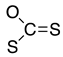
Abstract
Heterocyclic sulfur and nitrogen containing compounds capable of forming an equilibrium: thiol/imine = thione/amine (N=C-S-H ⇌ H-N-C=S) were reacted with levoglucosenone (LG) in the presence of triethylamine. Unexpectedly, the only isolated products were the result of the aza-Michael addition. No S-adducts were detected. All products were crystalline with good to excellent yields. The structure of products was determined using NMR, MS, and single-crystal X-ray analysis.
1. Introduction
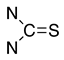
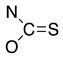
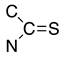
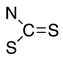
Thiols bonded to the five membered rings of thiazole, thiazoline, or thiadiazole are nucleophiles capable of reacting with a conjugated unsaturated system of levoglucosenone 1 [1]. Michael addition could proceed through either the thiol or amine groups to produce the corresponding S or N adducts (or both).
We have investigated the conjugate Michael addition reaction of thiols for many years [2,3,4], but recently [5] we noticed that heterocyclic iminethiols (N=C-S-H ⇌ H-N-C=S) form only N-adducts when reacted in chloroform with levoglucosenone, as depicted in Scheme 1. This is unexpected, since thiols are excellent nucleophiles in Michael additions [6]. Moreover, under certain conditions when thiophenol and aniline addition to acrylonitrile was promoted by triethylammonium acetate, the only product was an S-adduct [7].
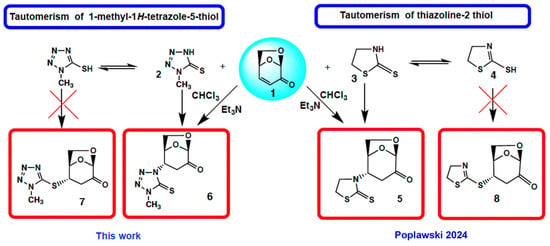
Scheme 1.
Michael N-aza-addition of enolizable heterocyclic thiols to levoglucosenone (LG). The synthesis of compound 5 has been previously described [5].
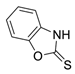
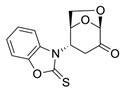
An observation of only N-addition of a similar compound (2-mercapto-benzoxazole) to levoglucosenone was previously reported by Spanevello et al. [8]. Recently, 2-mercapto-benzoxazole and 2-mercapto-benzothiazole were used in a Michael addition reaction to α,β-unsaturated esters [9]. Various aspects of thiono-thiol tautomerism of thiadiazole and oxadiazole have also been reported [10,11]. These data and our earlier observation of tautomeric nucleophile 4-thioxo-pyridine acylation [12] prompted us to further investigate the reactivity of enolizable/tautomeric imino-thiols. Interestingly, some substituted thioureas belonging to enolizable/tautomeric imino-thiols are well known FDA-approved drugs, such methimazole (Thiamazole) or propylthiouracil (Propycil), both used to treat hyperthyroidism (Figure 1). Drug disposition, delivery, and toxicity are known to be influenced by the presence of tautomers in some drugs and also by their respective metabolites [13,14].
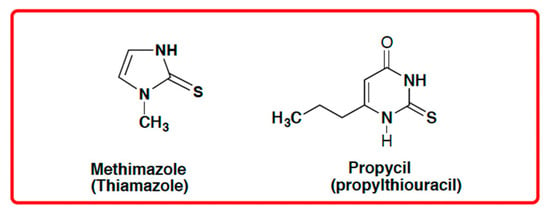
Figure 1.
FDA-approved drugs with enolizable thiol functional motifs.
The aza-Michael addition of imino-thiols to levoglucosenone (LG) can be considered as a type of glycorandomization [15] in which the LG molecule serves as a representative 1,6-anhydro protected sugar. Glycorandomization [15,16,17] has been reported as a universal platform methodology to modify the physico-chemical character of many natural products, and drugs. Scheme 2 provides an example of glycorandomization of levoglucosenone and representative thiones with six various functional motifs.
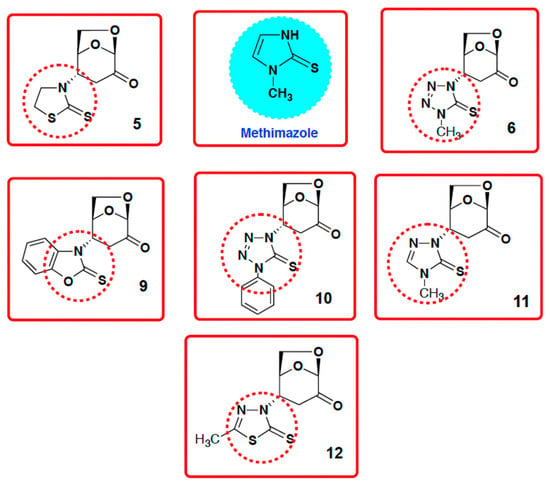
Scheme 2.
Products of glycorandomization of levoglucosenone after reaction with representative thiols with six different functional motifs (thiazoline, benzoxazole, tetrazole, triazole, and thiadiazole). Note the similarity of methimazole structure to discussed adducts.
Other authors [18] synthesized relevant N-adducts via Michael reaction addition to levoglucosenone under aqueous conditions. Interestingly, several of the synthesized levoglucosenone N-adducts exhibited promising properties against disorders such as cancer, autoimmune, and heart diseases. Greatrex et al. [19] published some new data on the synthesis of aza LG adducts from the reaction with reactive amines. Mroczek et al. [20] reported some chromatographic characteristics of tautomers of 1,2,4-triazole-3-thione and 3-thiol tautomers. Mloston et al. [21] recently published important studies of the reactivity of enolizable 1-substituted 5-mercapto-1H-tetrazoles in the cycloaddition reaction with dimethyl 2-arylcyclopropane dicarboxylates. Ring-opening reactions of donor–acceptor cyclopropanes with enolizable aza-heterocyclic thiones were also reported [22]. Published studies on 1H-benzo[d]imidazole-2-thiols [23] and 2-thioxo-4-thiazolidinone [24] present interesting applications and discuss their structural features and reactivities.
2. Results and Discussion
In order to study the addition of enolizable/tautomeric imine-thiols to levoglucosenone (LG), we initiated a study with a series of five-member heterocyclic thiols bearing a nitrogen atom in the α-position to the C=S group, as shown in Table 1. In each case, only N-adducts, and no trace of S-adducts, were observed as products. Additionally, the stereoselectivity of the addition reaction was influenced by the structure of the levoglucosenone moiety, directing the addition to take place from the bottom of the molecule via exo-attack.

Table 1.
Michael addition of enolizable heterocyclic thiols to levoglucosenone.
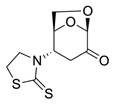
Products were characterized by 1H, 13C NMR along with single-crystal X-ray diffraction of compound 6. Crystals of 6 were grown from methanol, and the X-ray analysis revealed the structure presented in Figure 2. The ORTEP diagram for 6 confirms the N-linkage of tetrazole moiety connected to C4 of the bicyclic 1,6-anhydrosugar unit, the presence of the thiono group C=S, and the stereospecificity of the addition reaction.
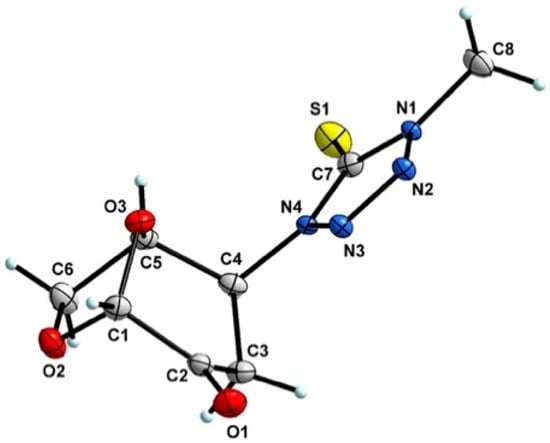
Figure 2.
ORTEP diagram of N-methyl-2-thioxo-tetrazole (6) determined by means of a single-crystal X-ray diffraction. Atoms are represented by thermal ellipsoids (50%) for clarity.
The summary of the aza-Michael addition is presented in Scheme 3. One possible explanation for the aza-Michael addition being favored over the thia-Michael addition would be that the starting material exists essentially as only the thione-containing tautomer.
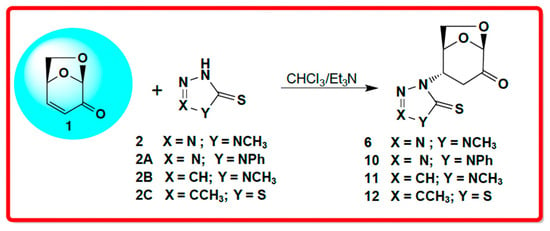
Scheme 3.
Summary of the aza-Michael addition products with five-membered heterocycles.
To explore this possibility, we examined the NMR, Raman, and IR spectra of the reacting substrates and found no evidence for the presence of the thiol-enamine tautomer. While it is not definitive proof, this explanation is likely to be correct.
The 13C chemical shifts for the thiocarbonyl groups for compounds (6, 10–12) were compared with those observed for the enolizable thiols used in this study and chemical shift correlation tables as summarized in Table 2. These specific observations, together with all analytical data, are consistent with the aza-Michael addition reaction of the enolizable heterocyclic thiols to levoglucosenone.

Table 2.
The 13C NMR chemical shifts in thiocarbonyl functional groups as a diagnostic tool.
It is worth adding that we also investigated the Michael addition reaction of a masked thiol in the form of thiouronium salt 13 to levoglucosenone [25]. The only product formed under mild basic conditions was polycyclic compound 14. It must have derived from the initial Michael addition at C4 of LG followed by cyclization to form the six-membered ring. Interestingly, the reaction with corresponding thiol 15 gave the expected Michael S-adduct 16, as depicted in Scheme 4 [25].
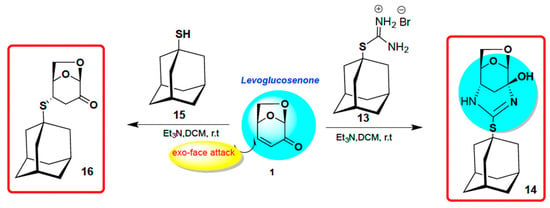
Scheme 4.
S-Adamantane derivative 16, and N-aza adducts of LG 14, from thiol 15 and masked thiol 13. Ref. [25]. Published in 2021.
3. Material and Methods
CCDC Deposition: Complete crystallographic data for compound 6 have been deposited at the Cambridge Crystallographic Data Centre as supplementary publication numbers CCDC-2212586. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/ (accessed on 10 December 2025) see Supplementary Materials.
General information: All reagents and solvents were used as purchased without further purification.
1H NMR and 13C NMR spectra were recorded on a Bruker Avance III 400 Ultrashield Plus spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). Two-dimensional (COSY and HSQC) experiments were performed to enhance assignments. Chemical shifts (δ–scale) are reported in ppm with TMS (0 ppm) and the residual solvent signals (CDCl3: 7.26 ppm) for 1H NMR and (CDCl3: 77.16 ppm) for 13C NMR as internal standards.
Thin-layer chromatography was performed on silica gel-coated TLC plates and visualized under UV light (at 254 nm); detection was executed by exposing to iodine (I2) vapor. The melting points (mps) were obtained on an ElectroThermal FARGO MP-2D (Electrothermal Engineering Ltd, Rochford, England, UK) capillary melting point apparatus and were uncorrected. Chemical names were generated by ChemDraw Professional V.15.1.0.144 software.
Starting materials: Levoglucosenone (1) was prepared from cellulose by sulfuric acid-assisted pyrolysis, following a known procedure [26] and provided as a generous gift from Circa Ltd. (Parkville, Victoria, Australia). All imine-thiols used in the aza-Michael reaction addition were purchased from Sigma-Aldrich (St. Louis, MO, USA).
4. Synthetic Procedures and Characterization Data
General Procedure for the Synthesis of 4-N-Functionalized Dihydrolevoglucosenones
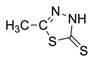
To a solution of levoglucosenone (1) 1.26 g (0.01 mole) in 35 mL of chloroform, 0.01 mole of thiol 2 (2A–2C) was added and magnetically stirred for 5 min. After that time, 1 mL of triethylamine was added dropwise, and the solution was stirred for 24–48 h at room temperature. Upon overnight cooling at 5 °C the crystalline residue was filtered off and recrystallized from methanol. The crystalline products were air-dried.
Characterization data
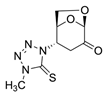
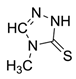
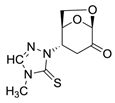
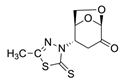
(1S,2S,5R)-2-(4-methyl-5-thioxo-4,5-dihydro-1H-tetrazol-1-yl)-6,8-dioxabicyclo[3.2.1]octan-4-one (6)
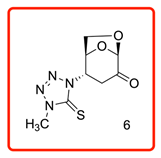
The reaction was carried at room temperature for 24 h. White crystals m. p. 192–194 °C; (1.58 g, 65% yield); Rf = 0.26 (Hex/EA 1/1), [α]D30-54.6 (c 1.0, CHCl3); 1H NMR (400 MHz CDCl3) δ 2.90 (ddd, J = 16.0 Hz, J = 5.4 Hz, J = 1.4 Hz 1H), 3.19 (dd, J = 16.0 Hz, 8.4 Hz, 1H), 3.93 (s, 3H), 4.12 (dd, J = 8.4 Hz, J = 5.4 Hz, 1H), 4.23 (dd, J = 8.4 Hz, J = 5.4 Hz, 1 H), 5.05 (d, J = 4.0 Hz, 1H), 5.25 (d, J = 1.4 Hz, 1H), 5.26 (d, J = 2.8 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3) δ 34.67, 34.70, 58.08, 65.88, 73.11, 101.57, 163.59 (C=S), 194.94 (C=O); APCI-MS calculated monoisotopic mass for [M+H]+ 243.06 u with detected mass 243.1 u.
(1S,2S,5R)-2-(4-phenyl-5-thioxo-4,5-dihydro-1H-tetrazol-1-yl)-6,8-dioxabicyclo[3.2.1]octan-4-one (10)
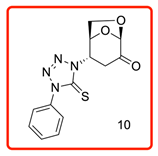
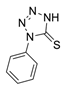
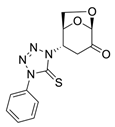
The reaction was carried out under ambient temperature for 48 h. White crystals m. p. 158–160 °C; (1.95 g, 64% yield); Rf = 0.22 (Hex/EA 1/1), [α]D30-51.6 (c 1.0, CHCl3); 1H NMR 400 MHz CDCl3) δ 2.96 (d, J = 17.2 Hz, 1H), 3.24(dd, J = 17.2 Hz, 7.6 Hz, 1H), 4.14 (dd J = 8.0 Jz, J = 6.0 Hz, 1H), 4.25 (d, J = 8.0 Hz, 1H), 5.14 (d, J = 4.8 Hz, 1H), 5.32 (s, 1H), 5.39 (d, J = 7.6 Hz, 1H), 7.46–7.59 (m, aromatic, 3 H), 7.95 (d, J = 8.0 Hz, aromatic 1H); 13C{1H} NMR (100 MHz, CDCl3) δ 34.82, 58.00, 65.94, 73.12, 101.63, 123.73, 129.36, 129.88 134.41 162.52 (C=S), 194.95 (C=O); APCI-MS calculated monoisotopic mass for [M+H]+ 305.07 u with detected mass 305.1 u.
(1S,5R)-2-(4-methyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-1-yl)-6,8-dioxabicyclo[3.2.1]oct-2-en-4-one (11)
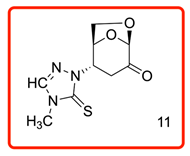
The reaction was carried out under ambient temperature for 24 h. White crystals m. p. 178–180 °C; (1.63 g, 68% yield); Rf = 0.29 (Hex/Acetone 1/1), [α]D30-52.6 (c 1.0, CHCl3); 1H NMR (400 MHz CDCl3) δ 2.79 (d, J = 16.0 Hz, 1H), 3.09 (dd, J = 16.0, 7.6 Hz, 1H), 3.62 (s, 3H), 4.07 (dd, J = 8.0, J = 5.6 Hz, 1H), 4.1 9 (d, J = 8.4, Hz, 1H), 5.07 (d, J = 4.8 Hz, 1H), 5.23 (s, 1H), 5.34 (d, J = 8.0 Hz, 1H), 7.82 (s, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 32.61, 35.01, 58.06, 66.00, 74.07, 101.51, 139.67, 166.15 (C=S), 196.75 (C=O); APCI-MS calculated monoisotopic mass for [M+H]+ 242.06 u with detected mass 242.0 u.
(1S,2S,5R)-2-(5-methyl-2-thioxo-1,3,4-thiadiazol-3(2H)-yl)-6,8-dioxabicyclo[3.2.1]octan-4-one (12)
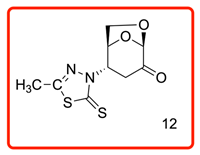
The reaction was carried out at room temperature for 24 h. White crystals m. p. 214–216 °C; (1.85 g, 72% yield); Rf = 0.28 (Hex/EA 1/1)), [α]D30-50.6 (c 1.0, CHCl3); 1H NMR (400 MHz CDCl3) δ 2.50 (s,3H), 2.81 (dd, J = 16.0, 6.4 Hz, 1H), 3.99 (dd, J = 15.0, 14.0 Hz), 3.88 (dt, J = 12.4, 6.0 Hz, 1H), 4.56 (d, J = 8.0 Hz, 1H), 4.94 (br s,1H), 5.20 (s, 1H), 5.77 (dd, J =5.2, 4.0 Hz,1H); 13C{1H} NMR (100 MHz, CDCl3) δ 34.81, 57.99, 65.93, 73.11, 73.4, 101.62, 123.73, 129.36, 129.87 134.41 162.52, 194.9, 5 (C=O); Minor peaks present are due to an unidentified impurity. APCI-MS calculated monoisotopic mass for [M+H]+ 259.01 with detected positive ion [M+H]+ 259.0 u.
5. Conclusions
The present study shows that the enolizable/tautomeric imine-thiols 2 (2A–2C) undergo Michael addition to levoglucosenone (LG) with the formation of N-adducts in good yield (64–72%). Only N-adducts were isolated and no S-adducts were found. In studied solvents the substrates exist only in the amine/thione tautomeric form and, arguably, this is why only the aza-Michael addition products are formed. However, it should be noted that only five-membered heterocyclic thiols were investigated and reported here. Future studies are underway which will attempt to determine under what conditions thia-additions might become favored and why aza additions are dominant in the discussed processes. The results will be published elsewhere.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules31010164/s1, X-Ray single crystal analysis and ORTEP of compound 6 and 1H, 13C NMR spectra.
Author Contributions
A.M.: Synthesis, Analysis, Characterization; R.M.: Synthesis, Analysis, Characterization; Z.J.W.: Preparation procedure, Synthesis, Analysis, Characterization, Writing—original draft, Writing—review and editing; D.E.M.: NMR, and MS Analysis, Writing—original draft, Writing—review and editing; R.B.: Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We gratefully acknowledge that the project was generously supported by an internal grant from Wilkes University. We would like to thank Circa Group Pty. Ltd. Melbourne, Victoria, 3133, Australia, for a generous gift of levoglucosenone and Cyrene. We thank Peter Andreana and Kristen Kirschbaum, University of Toledo, for their help in the X-ray diffraction analysis. Any M. Barrios Gomez and Karina Soto completed the NMR experiments of the thiol substrates used in this study.
Conflicts of Interest
The authors declare no competing financial interests. We affirm that there are no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Essig, M.G. Michael additions of thiols to levoglucosenone. Carbohydr. Res. 1986, 156, 225–231. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Sun, J.; Mielguj, R. Synthesis of L-fucopyranosyl-4-thiodisaccharides from levoglucosenone and their inhibitory activity on α-L-fucosidase. Bioorg. Med. Chem. Lett. 1995, 5, 2169–2174. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Chhabra, R.; Chen, H.; Xie, X.-Q. Thio-sugars II. The Novel Approach to Thiodisaccharides. The Synthesis of 3-deoxy-4-thiocellobiose from Levoglucosenone. Carbohydr. Res. 1997, 301, 167–175. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Kaplon, P.; Dey, P.M. Thio-sugars VII. Effect of 3-deoxy-4-S-(β-ᴅ-gluco- and β-ᴅ-galactopyranosyl)-4-thiodisaccharides and Their Sulfoxides and Sulfones on the Viability and Growth of Selected Murine and Human Tumor Cell Lines. Carbohydr. Res. 2003, 338, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, T.; Galita, G.; Sarnik, J.; Macieja, A.; Bielski, R.; Mencer, D.E.; Witczak, Z.J. New N-Adducts of Thiadiazole and Thiazoline with Levoglucosenone and Evaluation of Their Significant Cytotoxic (Anti-Cancer) Activity. Cancers 2024, 16, 216. [Google Scholar] [CrossRef]
- Khiste, S.A.; Pakhare, D.S.; More, V.S.; Chakibanda, G.; Lokhande, M.N. Thia-Michael Addition in Diverse Organic Synthesis. Croat. Chem. Acta 2023, 96, 141–152. [Google Scholar] [CrossRef]
- Verma, A.K.; Attri, P.; Chopra, V.; Tiwari, R.K.; Chandra, R. Triethylammonium acetate (TEAA): A recyclable inexpensive ionic liquid promotes the chemoselective aza- and thia-Michael reactions. Monatsh Chem. 2008, 139, 1041–1047. [Google Scholar] [CrossRef]
- Giri, G.F.; Danielli, M.; Marinelli, R.A.; Spanevello, R.A. Cytotoxic effect of levoglucosenone and related derivatives against human hepatocarcinoma cell lines. Bioorganic Med. Chem. Lett. 2016, 26, 3955–3957. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, C.; Lin, W.; Wang, D.; Ma, J.; Dong, Z. Michael Addition Reaction of Benzothiazol-2-thiol/Benoxazol-2-thiol with α, β-Unsaturated Esters: Chemoselective Construction of C-S and C-N Bonds. ChemistrySelect 2023, 8, e202300552. [Google Scholar] [CrossRef]
- Pop, R.O.; Ilici, M.; Andoni, M.; Bercean, V.N.; Muntean, C.; Venter, M.M.; Julean, I. Theoretical Considerations Regarding the Thione-thiol Tautomerism in 2-(5-mercapto-1,3,4-thiadiazol-2-ylthio)acetic Acid. Acta Chim. Slov. 2015, 62, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Arslan, N.B.; Özdemir, N.; Dayan, O.; Dege, N.; Koparır, M.; Koparır, P.; Muğlu, H. Direct and Solvent-assisted thione-thiol tautomerism in 5-(thiophen-2-yl)-1,3,4-oxadiazole-2(3H)-thione: Experimental and molecular modeling study. Chem. Phys. 2014, 439, 1–11. [Google Scholar] [CrossRef]
- Jordan, F.; Kudzin, Z.; Witczak, Z.; Hoops, P. Nuclear Magnetic Resonance Determination of the Site of Acylation of the Tautomeric Nucleophile 4-Thioxopyridine. J. Org. Chem. 1986, 51, 571–573. [Google Scholar] [CrossRef]
- Bharatam, P.V.; Valanju, O.R.; Wani, A.A.; Dhaked, D.K. Importance of tautomerism in drugs. Drug Discov. Today 2023, 28, 103494. [Google Scholar] [CrossRef]
- Allegretti, P.E.; Castro, E.A.; Furlong, J.J.P. Tautomeric equilibrium of amides and related compounds: Theoretical and spectral evidences. J. Mol. Struct. 2000, 499, 121–126. [Google Scholar] [CrossRef]
- Griffith, B.R.; Langenhan, J.M.; Thorson, J.S. Sweetening natural products via glycorandomization. Curr. Opin. Biotechnol. 2005, 16, 622–630. [Google Scholar] [CrossRef]
- Langenhan, J.M.; Griffith, B.R.; Thorson, J.S. Neoglycorandomization and Chemoenzymatic Glycorandomization: Two Complementary Tools for Natural Product Diversification. J. Nat. Prod. 2005, 68, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, D.; Kushwaha, A.K.; Kumar, R.; Chauhan, D. Recent advances in the synthesis of Glyconjugated heterocycles: A promising strategy for accessing bioactive compounds. Bioorganic Chem. 2025, 162, 108559. [Google Scholar] [CrossRef]
- Westman, J.; Wiman, K.; Mohell, N. Levoglucosenone Derivatives for the Treatment of Disorders Such as Cancer, Autoimmune Diseases and Heart Diseases. PCT Application WO/2007/139497, 6 December 2007. [Google Scholar]
- Kim, S.; Ledingham, E.T.; Kudo, S.; Greatrex, B.W.; Sperry, J. Bio-Based Chiral Amines via Aza-Michael Additions to (–)-Levoglucosenone Under Aqueous Conditions. Eur. J. Org. Chem. 2018, 17, 2028–2038. [Google Scholar] [CrossRef]
- Mroczek, T.; Plech, T.; Wujec, M. Novel Concept of Discrimination of 1,2,4-Triazole-3-thione and 3-Thiol Tautomers. J. Chromatogr. Sci. 2017, 55, 117–129. [Google Scholar] [CrossRef]
- Mlostoń, G.; Celeda, M.; Palusiak, M.; Heimgartner, H.; Denel-Bobrowska, M.; Olejniczak, A.B. Ambident Reactivity of Enolizable 5-Mercapto-1H-tetrazoles in Trapping Reactions with in situ-Generated Thiocarbonyl S-Methanides Derived from Sterically Crowded Cycloaliphatic Thioketones. Beilstein J. Org. Chem. 2025, 21, 1508–1519. [Google Scholar] [CrossRef]
- Mlostoń, G.; Kowalczyk, M.; Celeda, M.; Oliver, G.A.; Werz, D.B. Ring-Opening Reactions of Donor-Acceptor Cyclopropanes with Some Enolizable Azaheterocyclic Thiones: S-versus N-Attack. Synlett 2025, 36, 1548–1552. [Google Scholar] [CrossRef]
- Cen, K.; Wei, J.; Feng, Y.; Liu, Y.; Wang, X.; Liu, Y.; Yin, Y.; Yu, J.; Wang, D.; Cai, J. Synthesis of fused 3-trifluoromethyl-1,2,4- triazoles via base-promoted [3 + 2] cycloaddition of nitrile imines and 1H-benzo[d]imidazole-2-thiols. Org. Biomol. Chem. 2023, 21, 7095–7099. [Google Scholar] [CrossRef]
- Metwally, N.H.; Abdalla, M.A.; Mosselhi, M.A.N.; El-Desoky, E.A. Synthesis and antimicrobial activity of some new N-glycosides of 2-thioxo-4-thiazolidinone derivatives. Carbohydr. Res. 2010, 345, 1135–1141. [Google Scholar] [CrossRef]
- Witczak, Z.J.; Mauger, A.; Bielski, R.; Mencer, D.E. Thioglycomimetics with enhanced lipophilicity and their biological activity. ARKIVOC 2021, iv, 268–279. [Google Scholar] [CrossRef]
- Klepp, J.; Dillon, W.; Lin, Y.; Feng, P.; Greatrex, B.W. Preparation of (-)-levoglucosenone from cellulose using sulfuric acid in polyethylene glycol. Org. Synth. 2020, 97, 38–53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.