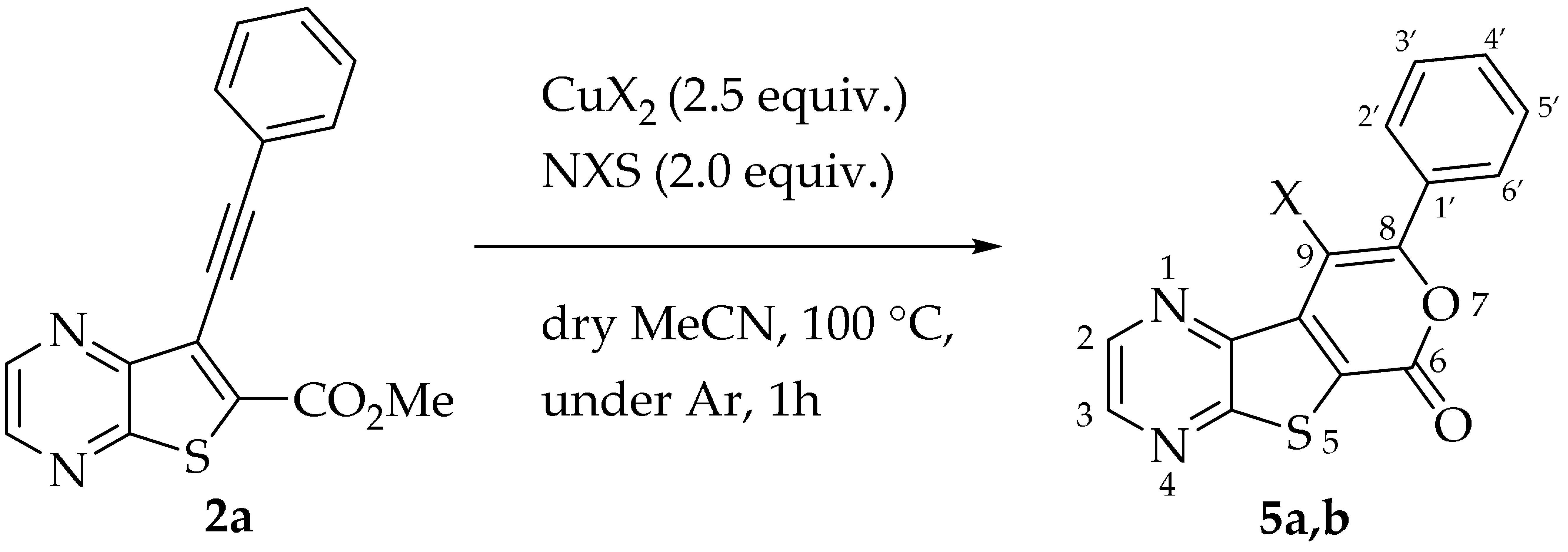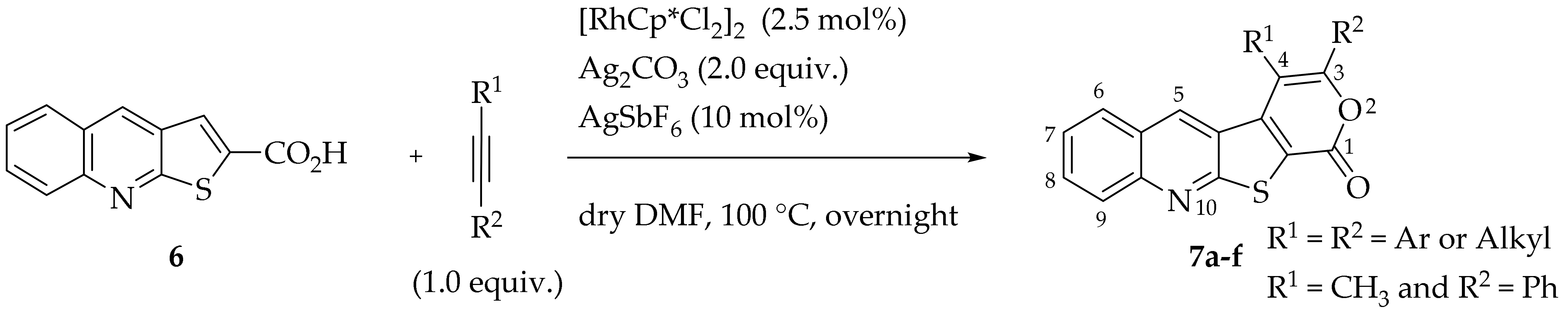Abstract
Tricyclic and tetracyclic lactone derivatives of thieno[2,3-b]pyrazine or thieno[2,3-b]quinoline, and 2H-pyrones were prepared using different methodologies. Pd/Cu-catalyzed Sonogashira coupling using Et3N as a base, of methyl 7-bromothieno[2,3-b]pyrazine-6-carboxylate and (het)arylalkynes to yield the Sonogashira ester products, gave also the corresponding tricyclic lactones as minor products. However, the major products did not cyclize with TFA. Tricyclic lactones were then obtained by a tandem one-pot Sonogashira coupling and 6-endo-dig lactonization of 7-bromothieno[2,3-b]pyrazine-6-carboxylic acid with (het)arylalkynes, in good yields. Halogenated tricyclic lactones were synthesized by halocyclization using CuX and NXS. Tetracyclic lactones were synthesized through a Rh(III)-catalyzed formal [4+2] cycloaddition, between thieno[2,3-b]quinoline-2-carboxylic acid and internal alkynes, triggered by C-H activation, with the carboxylic group acting as a directing group. Using the SRB assay, the antitumor activity of both Sonogashira products and lactones was evaluated across five human cancer cell lines (CaCo-2, MCF-7, AGS, HeLa, NCI-H460). The best-performing compound was a Sonogashira product showing a GI50 < 10 µM in all tumor cell lines and low toxicity in PLP2 cells. Additionally, antiparasitic testing against Trypanosoma brucei and Leishmania infantum revealed some compounds with IC50 < 11 µM, though some level of cytotoxicity was observed in THP-1—derived macrophages.
1. Introduction
Thieno[2,3-b]pyrazine derivatives constitute a significant class of aromatic heterocyclic compounds, largely due to the important biological activities exhibited by some of their derivatives. The synthesis and biological activities of these compounds have been predominantly reported in patents as inhibitors of the serine/threonine kinase B-Raf, which plays a role in the signal transduction pathways regulating cell growth and proliferation [1], as inhibitors of cancer cell growth and proliferation of different human tumor cell lines at low concentrations, as well as inducing apoptosis [2], as selective inhibitors of interleukin-1 receptor-associated kinase 4 (IRAK4) [3], or, as inhibitors of ubiquitin-proteasome system components, ubiquitin specific peptidase 28 (USP28) and/or ubiquitin specific peptidase 25 (USP25), that are involved in the treatment of cancer and inflammatory, autoimmune, and infectious diseases [4].
Thieno[2,3-b]quinolines have also attracted significant attention in medicinal chemistry due to their diverse biological activities [5]. Some derivatives have been described as selective inhibitors of the interaction between protein kinase C isoenzyme PKCε and the receptor for activated C-kinase 2 (RACK2) [6], as antioxidant and anti-inflammatory agents [7], or, as epidermal growth factor receptor (EGFR) inhibitors of tyrosine kinase domain (TKIs) [8].
2H-pyran-2-ones and their benzo derivatives, isocoumarins, are significant components of various lactone-based natural products that exhibit a broad variety of biological activities, including antifungal and antimicrobial properties [9,10].
In 2009, some of us reported the synthesis of 3-(aryl)benzothieno[2,3-c]pyran-1-ones. These compounds were synthesized via Pd/Cu-catalyzed Sonogashira coupling reactions of methyl 3-bromobenzo[b]thiophene-2-carboxylates with aryl alkynes followed by an electrophilic 6-endo-dig cyclization to give the aryl substituted pyranone ring iodinated or not using I2 or TFA. In the same study, the corresponding tricyclic lactones were also synthesized through a tandem one-pot reaction, which involved Pd/Cu-catalyzed Sonogashira coupling between 3-bromobenzo[b]thiophene-2-carboxylic acid and arylalkynes, followed by 6-endo-dig cyclization (Scheme 1). All the compounds prepared including the Sonogashira ester products were submitted to antitumor evaluation in tumor cell lines and some of them showed to be promising [11].
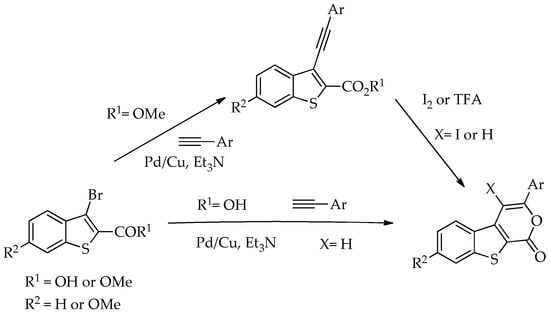
Scheme 1.
Synthesis of tricyclic lactones derivatives of benzo[b]thiophene [11].
More recently, we have focused on synthesizing tricyclic lactones that are disubstituted in the pyranone ring. This process involves a Rh(III)-catalyzed formal [4+2] cycloaddition between thienopyridine or thieno[2,3-b]pyrazine carboxylic acids and internal alkynes, initiated by C-H activation (Scheme 2) [12].
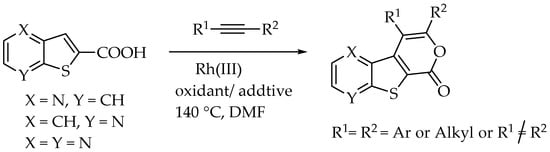
Scheme 2.
Synthesis of tricyclic lactones derivatives of thienopyridines and thieno[2,3-b]pyrazine [12].
Herein, we report the synthesis of fused 2H-pyran-2-ones with thieno[2,3-b]pyrazine (tricyclic lactones) or thieno[2,3-b]quinoline (tetracyclic lactones) through the thiophene ring, using different methodologies. The resulting lactones could possess potential biological properties. The work initially describes the synthesis of Sonogashira coupling products from methyl 7-bromothieno[2,3-b]pyrazine-6-carboxylate and terminal (hetero)alkynes, as well as the formation of tricyclic lactones as minor products through 6-endo-dig lactonization of the coupling products. The Sonogashira ester products were prepared for cyclization into (halo)tricyclic lactones. The tricyclic lactones were also obtained in better yields in a tandem one-pot reaction of Sonogashira coupling followed by 6-endo-dig lactonization, by reaction of 7-bromothieno[2,3-b]pyrazine-6-carboxylic acid with terminal alkynes.
Furthermore, disubstituted tetracyclic lactones were prepared by Rh(III)-catalyzed formal [4+2] cycloaddition between thieno[2,3-b]quinoline-2-carboxylic acid and internal alkynes, triggered by C-H activation.
The synthesized compounds are new and were fully characterized. Furthermore, they were evaluated for their potential antitumor and antiparasitic activities.
2. Results and Discussion
2.1. Synthesis of Thieno[2,3-b]pyrazine Derivatives by Pd/Cu-Catalyzed Sonogashira Cross-Coupling Followed by Intramolecular Cyclization to Tricyclic Lactones
Sonogashira coupling products are usually precursors of molecules used as pharmaceuticals and/or organic materials [13,14]. Additionally, they are commonly employed in the synthesis of heterocycles through intramolecular cyclization [15].
2.1.1. Pd/Cu-Catalyzed Sonogashira Cross-Coupling
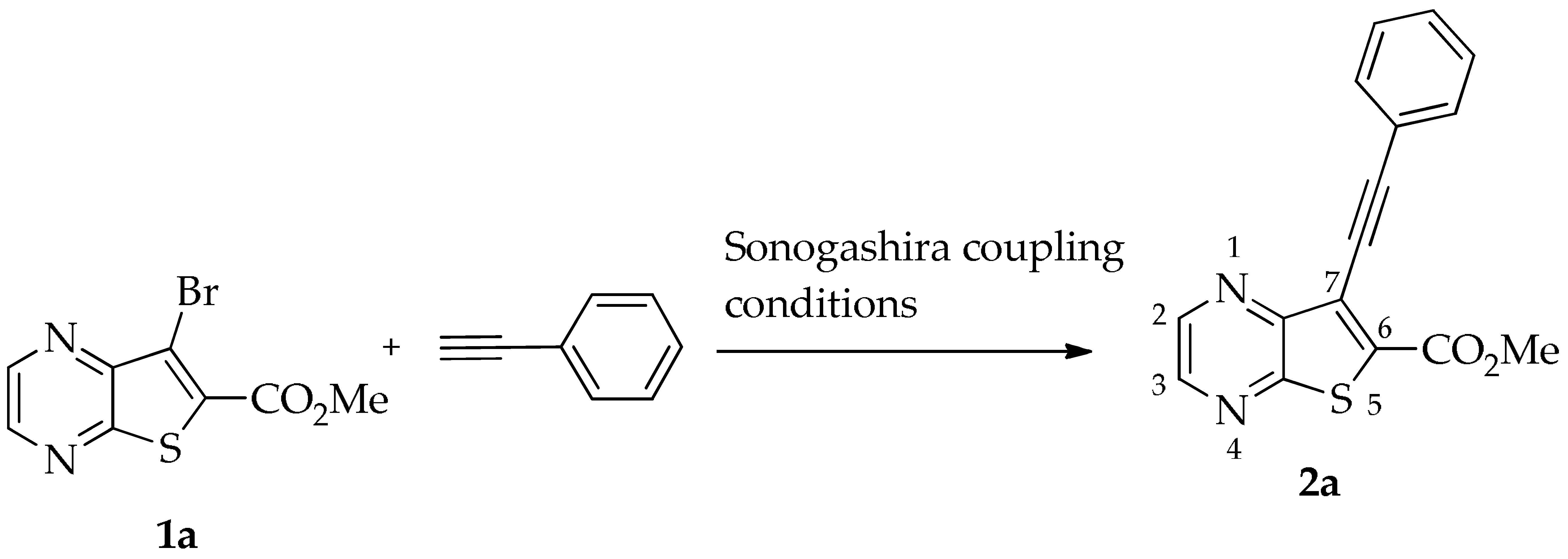
The Sonogashira reaction for the coupling of methyl 7-bromothieno[2,3-b]pyrazine-6-carboxylate (1a) [16] with phenylacetylene to yield 2a was performed using Pd/Cu and Et3N as a base in DMF, conditions previously used by us to reduce the formation of the alkyne dimer [11]. We compared these conditions with heterogeneous catalysis using Pd/C without copper, 2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl (XPhos) as a ligand [17], K2CO3 as a base, and eucalyptol as a solvent [18], which is a more sustainable approach. Under these conditions, compound 2a was obtained in only a moderate yield (30%), and a major byproduct that was not identified was also formed (Table 1).

Table 1.
Comparison of the Sonogashira conditions previously used by us with heterogeneous catalysis, excluding copper, using a more environmentally friendly solvent, in the synthesis of 2a.
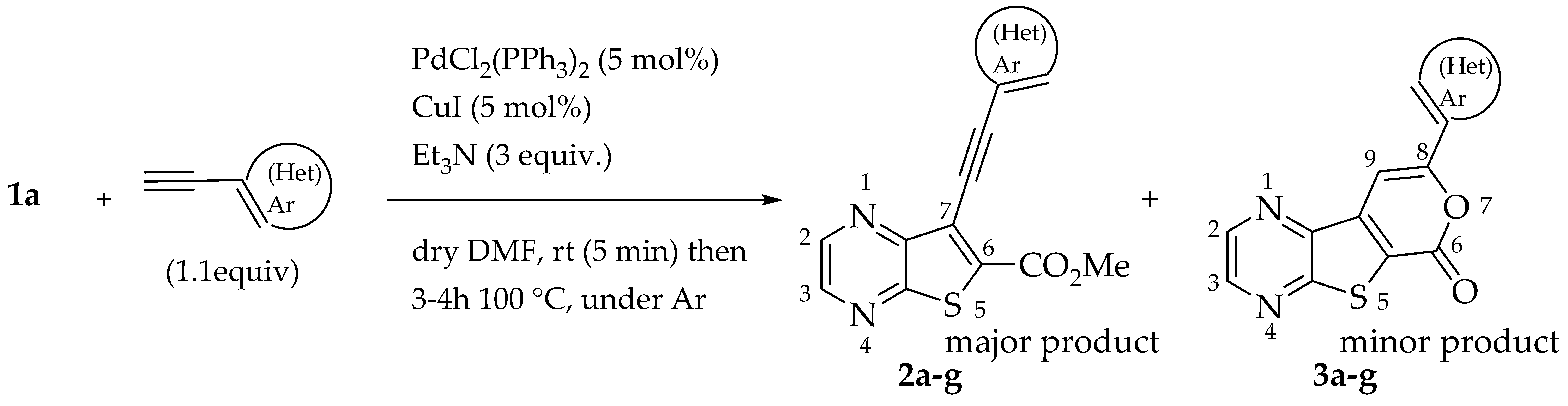
The best conditions (Table 1, entry 1) for the synthesis of the Sonogashira product 2a were used for the coupling of compound 1a with several terminal alkynes, to afford the corresponding Sonogashira coupling products 2a–h, in good to high yields (50–95%), and the tricyclic lactones 3a–c,e–g as minor products (5–12%), by regioselective 6-endo-dig cyclization [19], as depicted in Table 2. The tricyclic lactones may be formed via an electrophile-promoted nucleophilic cyclization (EPNC) with Et3N+H as source of H+ [20].

Table 2.
Synthesis of Sonogashira products 2 and tricyclic lactones 3 as minor products.
The highest yield was obtained for compound 2h (95%, entry 8, Table 2) followed by compounds 2d and 2g (75%, entries 4 and 7, Table 2). Compound 2h bears an electron-withdrawing group (EWG), while compound 2d bears an electron-donating group (EDG) on the phenyl ring and compound 2g contains a thiophene ring (electron rich). Compound 2a was obtained in 65% (entry 1, Table 2), and compound 2b bearing an amino group (EDG) was obtained in 60%, (entry 2, Table 2). The lowest yield (50%, entry 6, Table 2) was observed for compound 2f, containing a pyridine ring (electron deficient), followed by compounds 2c and 2e (55%), bearing a F (weakly EWG) or a methyl group (EDG by inductive effect) (entries 3 and 5, Table 2). Overall, it is not possible to depict a definitive conclusion about the electronic nature of the substituents that enhance the reaction yield. However, among the heterocyclic rings, the electron-rich thiophene ring appears to favor the reaction more than the electron-poor pyridine ring.
For alkynes, Baldwin’s rules help in determining whether a five-membered (5-exo-dig) or six-membered (6-endo-dig) ring closure will be favored when an oxygen nucleophile attacks the alkyne, typically under EPNC conditions. These principles are essential in the design of efficient cyclization strategies in organic synthesis, as both 5-exo-dig and 6-endo-dig products can be obtained with regioselectivity, depending on the nature of the substituents and the nucleophile involved. Moreover, fused systems consisting of a six-membered ring following a five-membered ring, or vice versa, tend to be more stable [21]. Thus, the corresponding 6-endo-dig tricyclic lactones 3a–c, e–g were formed regioselectively, as minor products.
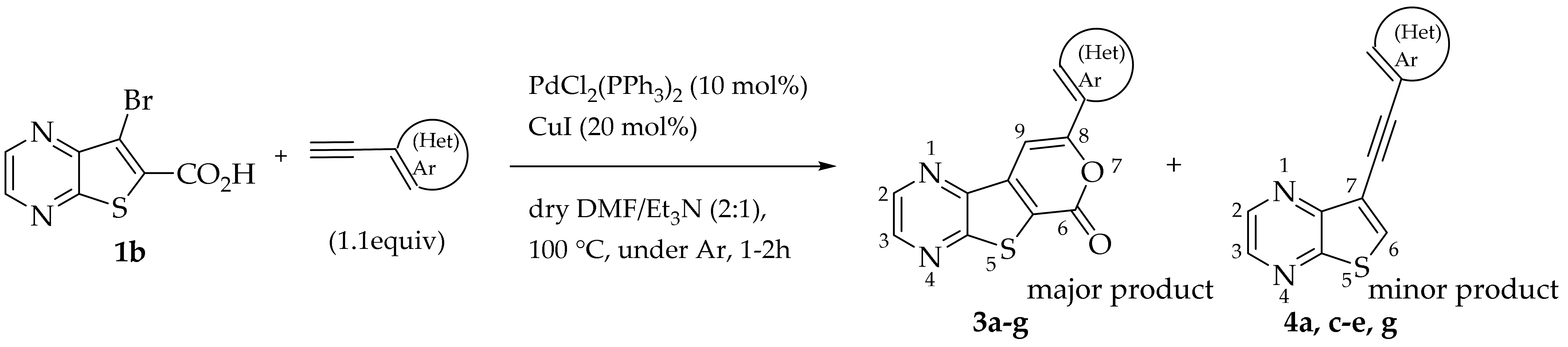
To synthesize tricyclic lactones 3 via 6-endo-dig cyclization of the Sonogashira ester coupling products, compound 2a was treated with TFA [11]. However, the cyclization did not proceed, possibly due to protonation of the nitrogens of the thienopyrazine. The strategy of using 7-bromothieno[2,3-b]pyrazine-6-carboxylic acid (1b) instead of 1a as the starting material was used to obtain tricyclic lactones 3 in higher yields.
2.1.2. Tandem One-Pot Reaction: Pd/Cu-Catalyzed Sonogashira Cross-Coupling Followed by 6-Endo-dig Cyclization to Tricyclic Lactones
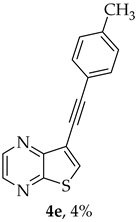
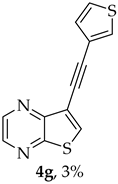
Compound 1b was reacted with various (hetero)arylalkynes via a tandem one-pot reaction, that involves a Sonogashira coupling followed by an electrophile-promoted nucleophilic cyclization [14,20]. The 6-endo-dig tricyclic lactones 3a–g were isolated as the exclusive cyclization product in good yields (50–63%). Additionally, 7-[(hetero)arylethynyl]thieno[2,3-b]pyrazines (4a, c–e, g) were obtained as minor products in very low yields (3–12%) due to decarboxylation of the non-isolable intermediate Sonogashira products (Table 3).

Table 3.
Synthesis of 6-endo-dig tricyclic lactones 3 and decarboxylation products 4 of the non-isolable Sonogashira intermediate, as minor products.
The highest yield was achieved for compound 3b (63%, entry 2) derivative of a terminal alkyne bearing an amine on the phenyl ring. This was followed by compound 3a (59%, entry 1, Table 3) derived from phenylacetylene, and compounds 3g (54%, entry 7, Table 3) and 3d (53%, entry 4, Table 3), using 3-ethynylthiophene and 4-ethynylanisole as terminal alkynes, respectively. The lowest yields were observed for compounds 3c, 3e, 3f (51%-50%, entries 3, 5, 6 Table 3), using 1-ethynyl-4-fluorobenzene, 4-ethynyltoluene and 2-ethynylpyridine. The reaction of compound 1b with 4-ethynylbenzonitrile gave a complex mixture of spots on TLC after 1 h of reaction. The 1H NMR spectrum of the crude mixture showed numerous signals that were difficult to assign to the expected 6-endo-dig lactone, so we did not proceed with purification. The presence of an EWG in the phenyl ring may reduce the availability of the triple bond for polarization, in contrast to the presence of EDGs, especially the amino group, which may enhance that polarization, as suggested by the following proposed mechanism. The EPNC may occur after deprotonation of the carboxylic group of the intermediate Sonogashira product by Et3N. The Et3N+H generated can coordinate with the π-bond, allowing for subsequent nucleophilic attack by an internal nucleophile, as shown in Scheme 3 and suggested by Uchiyama et al. in intramolecular cyclization studies of alkynes with adjacent carboxylic acids promoted by weak bases [20].
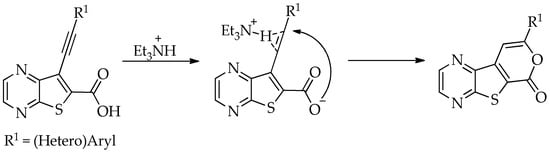
Scheme 3.
Plausible mechanism for electrophile-promoted nucleophilic lactonization using weak bases, adapted from Uchiyama et al. [20].
Compounds 3a–g exhibited a 1H NMR signal for the 9-H proton between 7.57 and 8.13 ppm when using DMSO-d6 as the solvent, as shown in Table 4. The shifts (δ) are in the aromatic zone, indicating that 6-endo-dig lactones were formed.

Table 4.
Chemical shifts (δ) of 9-H in 1H NMR spectra (DMSO-d6) of compounds 3a–3g.
In some experiments, the decarboxylated Sonogashira product intermediates 4a, 4c–e, and 4g were also isolated. The 13C NMR spectra of these compounds revealed carbon signals corresponding to their structures, including those for the triple bond carbons (~82 ppm and ~93 ppm, in DMSO-d6). The HRMS (ESI) data for all compounds 4 also confirmed their structures (see Section 3.1.1).
2.1.3. Halocyclization to 9-Halo Tricyclic Lactones
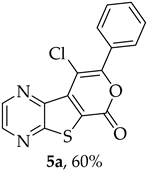
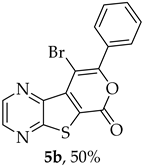
Efforts to synthesize 9-iodotricyclic lactones from the Sonogashira ester coupling product 2a using I2 and ICl have so far been unsuccessful. Therefore, the 9-halo-8-phenyl-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-ones 5a and 5b were successfully synthesized in good yields (50–60%) (Table 5) through halocyclization, using CuX2 and NXS (X = Cl, entry 1; Br, entry 2) [10].

Table 5.
Synthesis of halogenated tricyclic lactones 5.
In 2010, Miyata et al. proposed a reaction pathway for this type of chlorocyclization using ο-alkynyl amides, although the precise mechanism has not been fully elucidated. They proposed that CuCl2 activates the alkyne, facilitating nucleophilic addition of the carbonyl oxygen, which leads to the formation of a Cu(II) intermediate through a 6-endo-dig cyclization. Subsequent chlorination at the copper center by NCS generates a Cu(III) intermediate, which undergoes reductive elimination of CuCl to yield the chlorinated product [10]. An adapted mechanism for the halocyclization of compound 2a, leading to the formation of compounds 5a or 5b, is presented in Scheme 4.
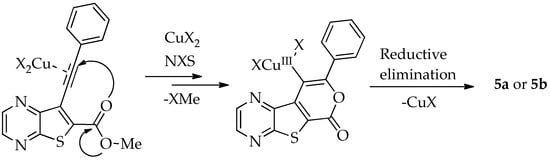
Scheme 4.
Adapted mechanism for halocyclization (X = Cl or Br) of compound 2a from Miyata et al. [10].
2.2. Synthesis of Tetracyclic Lactones Derived from Thieno[2,3-b]quinoline by Rh(III)-Catalyzed Formal [4+2] Cycloaddition, Triggered by C-H Activation
Synthetic methodologies based on C–H activation processes are among the most versatile and impactful approaches in contemporary organic chemistry. These techniques enable a rapid and atom-economical enhancement of molecular complexity starting from simple, unfunctionalized substrates rather than pre-functionalized precursors such as halogenated compounds [22,23]. However, given the abundance of various C–H bonds in organic molecules, achieving regioselective C–H activation and subsequent functionalization remains a formidable challenge. A highly promising approach to achieving high selectivity is utilizing existing functional groups within organic molecules as directing groups (DGs) [24,25]. For instance, functional groups like carboxylic acids can participate in subsequent cycloaddition reactions, acting as dynamic DGs [26,27]. Cycloaddition reactions are highly attractive due to their ability to simultaneously form two bonds and a ring in a single step. Extensive research has demonstrated that metal catalysts can effectively facilitate the cycloannulation of substrates that would otherwise remain unreactive [28].
Rh(III)-Catalyzed formal [4+2] cycloadditions between thieno[2,3-b]quinoline-2-carboxylic acid (6) and various internal alkynes, either symmetrical (aryl-aryl or alkyl-alkyl) or unsymmetrical (aryl-alkyl), were performed to synthesize 3,4-disubstituted-1H-pyrano[4′,3′:4,5]thieno[2,3-b]quinolin-1-ones (7a–f), in good to high yields (50–85%). The formation of these tetracyclic lactones was achieved using [RhCp*Cl2]2 as catalyst, Ag2CO3 as a base/oxidant and AgSbF6 as an additive, in dry DMF at 100 °C (Table 6). These conditions were selected based on our previous application of the same method to the thieno[2,3-b]pyridine system, where nitrogen and sulfur occupy the same relative positions [12]. Unlike the earlier experiments conducted at 140 °C, these reactions were carried out at 100 °C in dry DMF due to the high level of decarboxylation observed in the thieno[2,3-b]quinoline system (6) at 120 and 140 °C.

Table 6.
Synthesis of tetracyclic lactones 7.
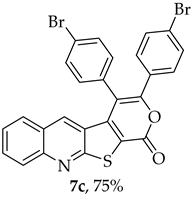
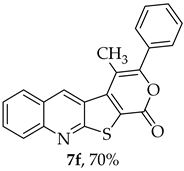
The highest yield was obtained for compound 7b (85%, entry 2, Table 6), using 5-decyne as the internal alkyne. This was followed by the dibrominated compound 7c (75%, entry 3, Table 6) and compound 7f (70%, entry 6, Table 6), the latter obtained regioselectively from the unsymmetrical 1-phenyl-1-propyne. The regioisomer 7f was assigned using nOe showing a 10% enhancement of the 5-H signal at 9.49 ppm upon saturation of the methyl group signal at 2.71 ppm (see Supplementary Materials). The lowest yield was observed for compound 7e (50%, entry 5), which was synthesized using 1,2-bis(4-(trifluoromethyl)phenyl)ethyne. This reduced yield is likely attributed to the EWGs on the phenyl rings. Meanwhile, using 1,2-bis(4-methoxyphenyl)ethyne as the internal alkyne resulted in the formation of compound 7d in 61% yield (entry 4), despite the presence of EDGs on the phenyl rings.
Table 7 presents the chemical shifts (δ) of the 5-H signal and 5-CH in 1H NMR and 13C NMR spectra, respectively, for all compounds 7, recorded in DMSO-d6 at 80 °C or 100 °C. The assignments were made based on HSQC 1H-13C 2D correlations, which enabled unambiguous identification of the 5-H and 5-CH signals (see Supplementary Materials). The lower δ values for the 5-H signal in compounds bearing aryl substituents can be attributed to the shielding cone generated by the aromatic ring. In contrast, compounds 7b and 7f, which feature alkyl substituents, exhibit the 5-H signal at higher δ values, indicating a more deshielded environment due to the absence of aromatic ring anisotropy—particularly in 7f, where the spatial proximity of a methyl group likely contributes to this effect. A similar trend is observed for the 5-CH carbon signals. The structures of all compounds 7 were further corroborated by HRMS (ESI) data (see Section 3.1.2).

Table 7.
Chemical shifts (δ) of 5-H in 1H NMR and the corresponding CH in the 13C NMR spectra (DMSO-d6 at 80 °C or 100 °C) of compounds 7a–7f.
The use of eucalyptol instead of DMF, using diphenylacetylene or 5-decyne in the same conditions gave only traces of the corresponding tetracyclic lactones 7a and 7b.
The reaction mechanism begins with the C-H activation, facilitated by the proximity of the bond to the metal center, typically guided by a directing group. Concurrently, a base coordinated to the metal center promotes deprotonation of the C-H bond in a concerted process, resulting in the formation of the C-metal bond. This step is followed by the insertion of the internal alkyne. Subsequently, reductive elimination of the metal takes place, yielding the cycloannulation product. An external oxidant regenerates the active Rh(III) catalyst from Rh(I), enabling the catalytic cycle to continue. Scheme 5 illustrates the general mechanism proposed by You et al. for Rh(III)-catalyzed C-H activation and cycloaddition, using a carboxylic acid as a transient directing group and internal alkynes [29].
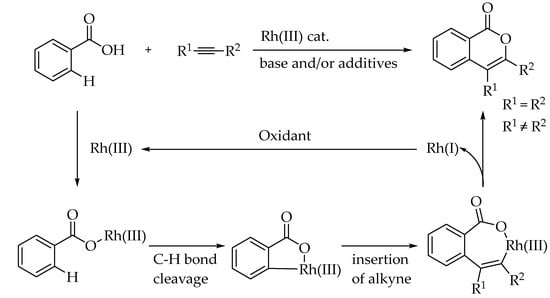
Scheme 5.
General mechanism for rhodium (III)-catalyzed formal [4+2] cycloaddition for benzoic acid adapted from You et al. [29].
This reaction enabled the synthesis of tetracyclic lactone derivatives of thieno[2,3-b]quinolines through an atom-economical C-H activation methodology, facilitated by a carboxylic group that participates in the subsequent reaction. Using Rh(III), a formal [4+2] cycloaddition took place.
An attempt to carry out this reaction under the same conditions, but using the terminal alkyne phenylacetylene, resulted only in the formation of the alkyne dimer, rather than the corresponding monosubstituted tetracyclic lactone.
2.3. Cell Growth Inhibitory Effect of Compounds 2, 3, 5 and 7 on CaCo-2, MCF-7, AGS, HeLa, NCI-H460 Cell Lines and on a Non-Tumor Cell Line (PLP2)
The antitumor potential of the Sonogashira coupling products 2, tricyclic lactones 3 and 5, and tetracyclic lactones 7 was evaluated using the sulforhodamine B (SRB) assay [16,30] against five human tumor cell lines: colorectal adenocarcinoma (CaCo-2), breast adenocarcinoma (MCF-7), gastric adenocarcinoma (AGS), cervical carcinoma (HeLa), and non-small cell lung cancer (NCI-H460). A primary pig liver cell culture (PLP2) was used to assess the toxicity of compounds. Doxorubicin served as the positive control and the results are expressed as GI50 values (µM), as shown in Table 8.

Table 8.
GI50 concentrations of compounds 2, 3, 5, and 7 were evaluated against five human tumor cell lines and a non-tumor cell line (PLP2).
All the compounds tested against these human tumor cell lines are not very promising, as their GI50 values are very much higher than 10 μM. Compound 2f, as highlighted in Table 8, is the only promising antitumor agent within all series, showing activity against all tested human cell lines (GI50 < 10 μM) without significant toxicity in PLP2 at their GI50 concentrations. Notably, it exhibited higher activity against the Caco-2 and MCF-7 cell lines (GI50 < 5 μM) and warrants further investigation.
2.4. Cell Growth Inhibitory Effect of Compounds 2c–g, 3a, 5a and 7 on T. brucei and L. infantum Promastigotes and THP-1-Derived Macrophage Cell Line as a Toxicity Model
The antiparasitic activity of compounds 2c–g, 3a, 5a and 7 was evaluated at a single concentration of 20 μM, against Trypanosoma brucei (T. brucei) and Leishmania infantum (L. infantum) promastigotes. The results are shown in Table 9 together with the cytotoxicity data (CC50) in THP-1–derived macrophages cells [31]. Compounds exhibiting more than 50% inhibition undergo further testing, where dose-response curves were conducted to determine their IC50 values.

Table 9.
Antiparasitic activity against T. brucei and L. infantum promastigotes and cytotoxity in THP-1—derived macrophages cells for compounds 2c–g, 3a, 5a and 7.
For T. brucei, two compounds, 2f and 5a, exhibited IC50 at low μM range, however, both displayed some level of toxicity as highlighted in Table 9. None of the compounds tested demonstrated activity against L. infantum promastigotes below 10 μM, which is conventionally used to define anti-Leishmanial hits.
3. Materials and Methods
3.1. Chemistry
The reactions were monitored by thin layer chromatography (TLC). The melting points were determined on a Stuart MP3 melting point apparatus and are uncorrected. Ether refers to diethyl ether and petroleum ether refers to petroleum ether 40–60 °C. 1H and 13C NMR were measured on a Bruker Avance III 400 (Bruker, Bremen, Germany), 400 MHz for 1H and 100.6 MHz for 13C, and using TopSpin 2.1 software. DEPT θ 135° was used to differentiate the type of carbons and 1H-13C bidimensional correlations, HSQC and HMBC, to identify some signals. The 19F NMR spectra were performed in a Bruker Avance III 300 (Bruker, Bremen, Germany) at 282.85 MHz. HRMS (ESI) results were obtained at the CACTI University of Vigo service, using a mass spectrometer Bruker FTMS SolariX XR. HPLC to determine the purity of compounds 3b–e was performed using reverse-phase chromatography on a Jasco (Tokyo, Japan) PU-980/UV-975 system with a LiChrospher 100 RP-18 (5 µm) column. The mobile phase for these compounds consisted of acetonitrile (MeCN)/H2O in a 60:40 (v/v) ratio, with 0.1% trifluoroacetic acid (TFA) as an additive. For compound 7e, the mobile phase was MeCN/H2O in a 90:10 (v/v) ratio. Detection was carried out at a λ = 350 nm.
3.1.1. Synthesis of Thieno[2,3-b]pyrazine Derivatives by Pd/Cu-Catalyzed Sonogashira Cross-Coupling and Intramolecular Cyclization to Tricyclic Lactones
- 7-bromothieno[2,3-b]pyrazine-6-carboxylic acid (1b): Compound 1a (0.617 g, 2.26 mmol) was dissolved in THF/MeOH/H2O (6:1:1, 60 mL) and LiOH (0.203 g, 8.47 mmol) was added and the mixture was stirred for 3 h at rt, monitored by TLC. After partial removal of the solvents, the mixture was acidified using HClconc till pH = 5 and a precipitate came out. This was filtered under vacuum, washed with H2O and dried in the oven at 50 °C for several hours. Compound 1b was obtained as a white solid (0.530 g, 90%). 1H NMR (400 MHz, DMSO-d6): δ = 8.85 (1 H, d, J = 2.0 Hz, HetAr-H), 8.95 (1 H, d, J = 2.0 Hz, HetAr-H) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C7H479BrN2O2S: 258.9172, found 258.9169. Calculated for C7H481BrN2O2S: 260.9151, found 260.9146.
General Procedure for the Synthesis of Sonogashira Cross-Coupling Products (2a–g)
In a vacuum-dried Schlenk tube with dry DMF (2 mL), compound 1a (1.0 equiv.) and PdCl2(PPh3)2 (5 mol%) were added and stirred, under Ar. In a small Schlenk flask with dry DMF (2 mL), the alkyne (1.1 equiv.), Et3N (3.0 equiv.) and CuI (5 mol%) were added and stirred, under Ar. This mixture was added to the one in the Schlenk tube, and it was left stirring for 5 min at rt. After this, the final mixture was heated at 100 °C with stirring under Ar for 3–4 h, monitoring by TLC. After cooling, water (5 mL) was added and a precipitate came out. This was filtered under vacuum and submitted to column chromatography using solvent gradients of ether/petroleum ether increasing 10% each time, to give compounds 2 and 3 as minor products, unless stated otherwise.
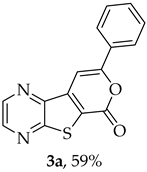
- Methyl 7-(phenylethynyl)thieno[2,3-b]pyrazine-6-carboxylate (2a) and 8-phenyl-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3a): Compound 1a (0.100 g, 0.366 mmol), phenylacetylene (0.0460 g, 0.0490 mL, 0.403 mmol) heating for 3.5 h. Column chromatography using a solvent gradient from 30 to 50% ether/petroleum ether gave compound 2a as a light yellow solid (0.0700 g, 65%), m.p. 145–147 °C. 1H NMR (400 MHz, DMSO-d6): δ = 3.98 (3 H, s, OCH3), 7.48–7.52 (3 H, m, Ar-H), 7.63–7.65 (2 H, m, Ar-H), 8.86 (1 H, d, J = 2.4 Hz, HetAr-H), 8.97 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 53.3 (OCH3), 81.8 (C), 99.6 (C), 121.5 (C), 121.7 (C), 129.0 (2 × ArCH), 129.8 (4′-CH), 131.7 (2 × Ar-CH), 136.3 (C), 144.3 (HetAr-CH), 144.7 (HetAr-CH), 147.8 (C), 154.3 (C), 161.1 (C=O) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C16H11N2O2S: 295.0536; found: 295.0540.
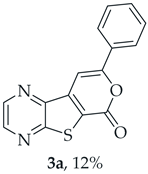
A more polar compound, 3a, was also isolated as light orange solid (0.0120 g, 12%). 1H NMR (400 MHz, DMSO-d6): δ = 7.53–7.58 (3 H, m, Ar-H), 7.93 (1 H, s, 9-H), 8.04–8.06 (2 H, m, Ar-H), 8.94 (1 H, d, J = 2.4 Hz, HetAr-H), 9.03 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. This compound was fully characterized when obtained as a major product from the carboxylic acid 1b.
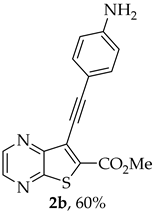
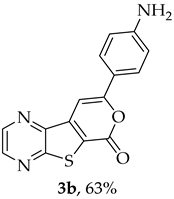
- Methyl 7-[(4-aminophenyl)ethynyl]thieno[2,3-b]pyrazine-6-carboxylate (2b) and 8-(4-aminophenyl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3b): Compound 1a (0.100 g, 0.366 mmol), 4-ethynylaniline (0.0490 g, 0.403 mmol) heating for 3 h. Column chromatography using a solvent gradient from 20 to 60% ethyl acetate/petroleum ether gave compound 2b as an orange solid (0.0683 g, 60%), m.p. 203–204 °C. 1H NMR (400 MHz, DMSO-d6): δ = 3.97 (3 H, s, OCH3), 5.80 (2 H, s, NH2), 6.61 (2 H, d, J = 8.8 Hz, 3′ and 5′-H), 7.31 (2 H, d, J = 8.8 Hz, 2′ and 6′-H), 8.84 (1 H, d, J = 2.4 Hz, HetAr-H), 8.95 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 53.0 (OCH3), 80.4 (C), 103.3 (C), 107.1 (C), 113.7 (3′ and 5′-CH), 122.9 (C), 133.2 (C), 133.4 (2′ and 6′-CH), 144.0 (HetAr-CH), 144.6 (HetAr-CH), 147.8 (C), 150.6 (C), 154.4 (C), 161.4 (C=O) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C16H12N3O2S: 310.0645; found: 310.0645.
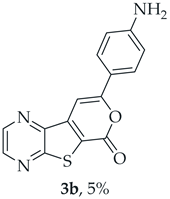
A more polar compound, 3b, was also isolated as a dark orange solid (0.00500 g, 5%). 1H NMR (400 MHz, DMSO-d6): δ = 5.89 (2 H, s, NH2), 6.66 (2 H, d, J = 8.8 Hz, 3′ and 5′-H), 7.57 (1 H, s, 9-H), 7.72 (2 H, d, J = 8.8 Hz, 2′ and 6′-H), 8.91 (1 H, d, J = 2.4 Hz, HetAr-H), 8.99 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. This compound was fully characterized when obtained as a major product from the carboxylic acid 1b.
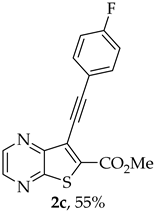
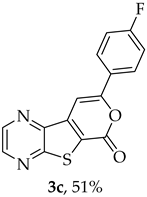
- Methyl 7-[(4-fluorophenyl)ethynyl]-thieno[2,3-b]pyrazine-6-carboxylate (2c) and 8-(4-fluorophenyl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3c): Compound 1a (0.0760 g, 0.278 mmol), 1-ethynyl-4-fluorobenzene (0.0370 g, 0.306 mmol) heating for 4 h. Column chromatography using a solvent gradient from 30 to 50% ether/petroleum ether gave compound 2c as a white solid (0.0473 g, 55%), m.p. 175–176 °C. 1H NMR (400 MHz, DMSO-d6): δ = 3.99 (3 H, s, OCH3), 7.32–7.38 (2 H, m, 3′ and 5′-H), 7.68–7.73 (2 H, m, 2′ and 6′-H), 8.88 (1 H, d, J = 2.0 Hz, HetAr-H), 8.98 (1 H, d, J = 2.0 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 53.3 (OCH3), 81.6 (C), 98.5 (C), 116.4 (d, J = 22.1 Hz, 3′ and 5′-CH), 118.1 (d, J = 3.0 Hz, 1′-C), 121.3 (C), 134.2 (d, J = 9.1 Hz, 2′ and 6′-CH), 136.4 (C), 144.3 (HetAr-CH), 144.7 (HetAr-CH), 147.7 (C), 154.3 (C), 161.0 (C=O), 162.6 (d, J = 248.5 Hz, CF) ppm. 19F NMR (282.85 MHz, DMSO-d6) δ = −104.9 ppm. HRMS (ESI) [M + H]+, m/z Calculated for C16H10FN2O2S: 313.0441; found: 313.0441.
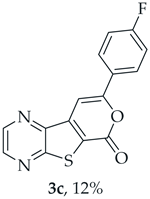
A more polar compound, 3c, was also isolated as beige solid (0.00900 g, 12%). 1H NMR (400 MHz, DMSO-d6): δ = 7.37–7.42 (2 H, m, 3′ and 5′-H), 7.96 (1 H, s, 9-H), 8.12-8.15 (2 H, m, 2′ and 6′-H), 8.95 (1 H, d, J = 2.4 Hz, HetAr-H), 9.04 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. This compound was fully characterized when obtained as a major product from the carboxylic acid 1b.
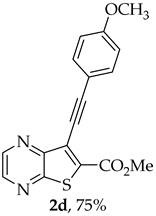
- Methyl 7-[(4-methoxyphenyl)ethynyl]thieno[2,3-b]pyrazine-6-carboxylate (2d): Compound 1a (0.0800 g, 0.290 mmol), 4-ethynylanisole (0.0420 g, 0.0410 mL, 0.320 mmol) heating for 4 h. Column chromatography using a solvent gradient from 10 to 40% ether/petroleum ether gave compound 2d as a yellow solid (0.0710 g, 75%), m.p. 150–152 °C. 1H NMR (400 MHz, DMSO-d6): δ = 3.82 (3 H, s, 4′-OCH3), 3.98 (3 H, s, OCH3), 7.04–7.07 (2 H, d, J = 8.8 Hz, 3′ and 5′-H), 7.58–7.60 (2 H, d, J = 8.8 Hz, 2′ and 6′-H), 8.86–8.87 (1 H, d, J = 2.4 Hz, HetAr-H), 8.97 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 53.2 (OCH3), 55.4 (4′-OCH3), 80.9 (C), 100.3 (C), 113.5 (C), 114.7 (3′ and 5′-CH), 122.0 (C), 133.5 (2′ and 6′-CH), 135.2 (C), 144.2 (HetAr-CH), 144.7 (HetAr-CH), 147.8 (C), 154.3 (C), 160.4 (C), 161.1 (C=O) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C17H13N2O3S: 325.0641; found: 325.0642.
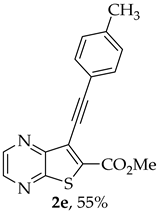
- Methyl 7-(p-tolylethynyl)thieno[2,3-b]pyrazine-6-carboxylate (2e) and 8-(p-Tolyl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3e): Compound 1a (0.100 g, 0.366 mmol), 4-ethynyltoluene (0.0470 g, 0.403 mmol) heating for 4 h. Column chromatography using a solvent gradient from 10 to 20% ether/petroleum ether gave compound 2e as a yellow solid (0.0616 g, 55%) m.p. 146–147 °C. 1H NMR (400 MHz, DMSO-d6): δ = 2.37 (3 H, s, CH3), 3.99 (3 H, s, OCH3), 7.31 (2 H, d, J = 8.0 Hz, 3 ’and 5′-H), 7.53 (2 H, d, J = 8.0 Hz, 2′ and 6′-H), 8.87 (1 H, d, J = 2.0 Hz, HetAr-H), 8.98 (1 H, d, J = 2.0 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 21.2 (CH3), 53.2 (OCH3), 81.4 (C), 100.0 (C), 118.6 (C), 121.7 (C), 129.6 (3′ and 5′-CH), 131.7 (2′ and 6′-CH), 135.9 (C), 139.8 (C), 144.2 (HetAr-CH), 144.7 (HetAr-CH), 147.8 (C), 154.3 (C), 161.1 (C=O) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C17H13N2O2S: 309.0692; found: 309.0696.
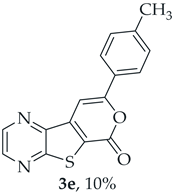
A more polar compound, 3e, was also isolated as yellow solid (0.0110 g, 10%). 1H NMR (400 MHz, DMSO-d6): δ = 2.39 (3 H, s, CH3), 7.36 (2 H, d, J = 8.0 Hz, 3′ and 5′-H), 7.78 (1 H, s, 9-H), 7.90 (2 H, d, J = 8.0 Hz, 2′ and 6′-H), 8.90 (1 H, d, J = 2.4 Hz, HetAr-H), 8.99 (1H, d, J = 2.4 Hz, HetAr-H) ppm. This compound was fully characterized when obtained as a major product from the carboxylic acid 1b.
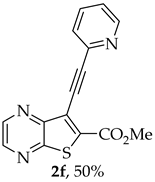
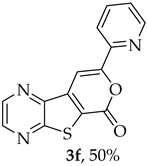
- Methyl 7-(pyridin-2-ylethynyl)thieno[2,3-b]pyrazine-6-carboxylate (2f) and 8-(Pyridin-2-yl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3f): Compound 1a (0.0800 g, 0.290 mmol), 2-ethynylpyridine (0.0340 g, 0.0330 mL, 0.320 mmol) heating for 5 h. Column chromatography using a solvent gradient from 10 to 60% ethyl acetate/petroleum ether gave compound 2f as a white solid (0.0420g, 50%), m.p. 183–185°C. 1H NMR (400 MHz, DMSO-d6): δ = 3.99 (3 H, s, OCH3), 7.48–7.51 (1 H, ddd, J = 7.6, 4.8 and 1.2 Hz, 5′-H), 7.72–7.74 (1 H, dt, J = 7.6, 1.2 and 0.8 Hz, 3′-H), 7.90–7.94 (1 H, app. td, J= 7.6 and 1.6 Hz, 4′-H), 8.67–8.69 (1 H, dq, J = 4.8, 1.6 and 0.8 Hz, 6′-H), 8.89 (1 H, d, J = 2.4 Hz, HetAr-H), 8.99 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 53.3 (OCH3), 80.4 (C), 98.4 (C), 120.6 (C), 124.3 (5′-CH), 128.0 (3′-CH), 137.0 (4′-CH), 137.7 (C), 141.7 (C), 144.4 (HetAr-CH), 144.8 (HetAr-CH), 147.9 (C), 150.5 (6′-CH), 154.2 (C), 160.9 (C=O) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C15H10N3O2S: 296.0488; found: 296.0488.
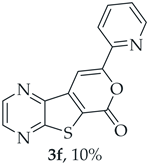
A more apolar compound, 3f, was also isolated as white solid (0.00800 g, 10%). 1H NMR (400 MHz, DMSO-d6): δ = 7.53–7.56 (1 H, m, 5′-H), 8.02–8.03 (2 H, m, 3′ and 4′-H), 8.13 (1 H, s, 9-H), 8.74–8.76 (1 H, m, 6′-H), 8.95 (1 H, d, J = 2.4 Hz, HetAr-H), 9.05 (1H, d, J = 2.4 Hz, HetAr-H) ppm. This compound was fully characterized when obtained as a major product from the carboxylic acid 1b.
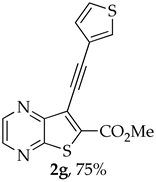
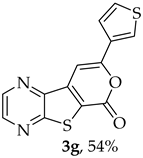
- Methyl 7-(thiophen-3-ylethynyl)thieno[2,3-b]pyrazine-6-carboxylate (2g) and 8-(thiophen-3-yl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3g): Compound 1a (0.100 g, 0.366 mmol), 3-ethynylthiophene (0.0450 g, 0.0410 mL, 0.403 mmol) heating for 3.5 h. Column chromatography using a solvent gradient from 20 to 60% ether/petroleum ether gave compound 2g as a dark orange solid (0.0840 g, 75%), m.p. 158–160 °C. 1H NMR (400 MHz, DMSO-d6): δ = 3.98 (3 H, s, OCH3), 7.33 (1 H, dd, J = 5.2 and 1.2 Hz, 4′-H), 7.72 (1 H, dd, J = 5.2 and 2.8 Hz, 5′-H), 8.05 (1 H, dd, J = 2.8 and 1.2 Hz, 2′-H), 8.87 (1 H, d, J = 2.4 Hz, HetAr-H), 8.96 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 53.2 (OCH3), 81.2 (C), 95.2 (C), 120.5 (C), 121.6 (C), 127.5 (5′-CH), 129.6 (4′-CH), 131.7 (2′-CH), 135.9 (C), 144.2 (HetAr-CH), 144.7 (HetAr-CH), 147.8 (C), 154.2 (C), 161.0 (C=O) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C14H9N2O2S2: 301.0100; found: 301.0096.
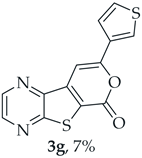
A more polar compound, 3g, was also isolated as a light yellow solid (0.00700 g, 7%). 1H NMR (400 MHz, DMSO-d6): δ = 7.73–7.75 (1 H, dd, J = 5.2 and 2.8 Hz, 5′-H), 7.81 (1 H, dd, J = 5.2 and 1.2 Hz, 4′-H), 7.84 (1 H, s, 9-H), 8.27 (1 H, dd, J = 2.8 and 1.2 Hz, 2′-H), 8.93 (1 H, d, J = 2.4 Hz, HetAr-H), 9.02 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. This compound was fully characterized when obtained as a major product from the carboxylic acid 1b.
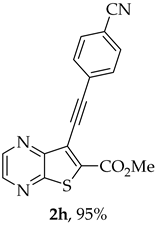
- Methyl 7-[(4-cyanophenyl)ethynyl]thieno[2,3-b]pyrazine-6-carboxylate (2h): Compound 1a (0.050 g, 0.183 mmol), 4-ethynylbenzonitrile (0.0256 g, 0.201 mmol) heating for 4 h. After cooling, H2O was added and a precipitate came out. This was filtered under vacuum, dried in the oven overnight at 50 °C giving a light brown solid, that after some washes with dry ether gave compound 2h as a beige solid (0.0555 g, 95%), m.p. 281–282 °C. 1H NMR (400 MHz, DMSO-d6, 60 °C): δ = 4.00 (3 H, s, OCH3), 7.80 (2 H, d, J = 8.0 Hz, 2′ and 6’-H), 7.92 (2 H, d, J = 8.0 Hz, 3′ and 5’-H), 8.86 (1 H, d, J = 2.0 Hz, HetAr-H), 8.96 (1 H, d, J = 2.0 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6, 60 °C): δ = 52.9 (OCH3), 84.9 (C), 97.2 (C), 111.6 (C), 117.8 (C), 120.3 (C), 126.2 (C), 132.1 (2′ and 6’-CH), 132.4 (3′ and 5’-CH), 137.4 (C), 144.0 (HetAr-CH), 144.4 (HetAr-CH), 147.4 (C), 154.0 (C), 161.5 (C=O) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C17H10N3O2S: 320.0489; found: 320.0492.
General Procedure for Tricyclic Lactones (3a–g) by One Pot Tandem Sonogashira Coupling and Intramolecular Cyclization
In a vacuum-dried Schlenk tube with dry DMF/Et3N (2:1, 3 mL), compound 2b (1.0 equiv.), PdCl2(PPh3)2 (10 mol%), CuI (20 mol%) and the alkyne (1.1 equiv.) were added and stirred, under Ar. After this, the mixture was heated at 100 °C with stirring under Ar for 1–2 h, monitoring by TLC. After cooling, water was added (10 mL) and the mixture was extracted with ethyl acetate (3 × 10 mL). The organic phase was washed successively with water (3 × 10 mL) and brine (2 × 10 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The resulting residue was submitted to column chromatography to give compounds 3 and 4 as minor products.
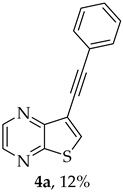
- 8-Phenyl-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3a) and 7-(phenylethynyl)thieno[2,3-b]pyrazine (4a): Compound 1b (0.100 g, 0.386 mmol), phenylacetylene (0.0430 g, 0.0466 mL, 0.425 mmol) and heating for 1 h. Column chromatography using a solvent gradient from 10 to 20% ether/petroleum ether gave compound 3a as a yellow solid (0.0630 g, 59%), m.p. 202–204 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.53–7.58 (3 H, m, Ar-H), 7.93 (1 H, s, 9-H), 8.04–8.06 (2 H, m, Ar-H), 8.94 (1 H, d, J = 2.4 Hz, HetAr-H), 9.03 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 96.6 (9-CH), 122.9 (C), 125.5 (2 × Ar-CH), 129.2 (2 × Ar-CH), 130.8 (4′-CH), 130.9 (C), 141.5 (C), 143.8 (C), 143.9 (HetAr-CH), 145.6 (HetAr-CH), 157.5 (C), 157.6 (C), 158.1 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C15H9N2O2S: 281.0379; found: 281.0379.
A more apolar compound, 4a, was also isolated as white solid (0.0110 g, 12%). 1H NMR (400 MHz, DMSO-d6): δ = 7.45–7.46 (3 H, m, Ar-H), 7.59–7.62 (2 H, m, Ar-H), 8.66 (1 H, s, 6-CH), 8.72 (1 H, d, J = 2.4 Hz, HetAr-H), 8.86 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 82.0 (C), 92.6 (C), 116.3 (C), 121.8 (C), 128.9 (2 × Ar-CH), 129.2 (4′-CH), 131.5 (Ar-CH), 136.4 (6-CH), 141.8 (HetAr-CH), 142.8 (HetAr-CH), 147.7 (C), 153.9 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C14H9N2S: 237.0481, found 237.0478.
- 8-(4-Aminophenyl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3b): Compound 1b (0.100 g, 0.386 mmol), 4-ethynylaniline (0.0410 g, 0.340 mmol) and heating for 2 h. Column chromatography using a solvent gradient from 20 to 50% ethyl acetate/petroleum ether gave compound 3b as a dark orange solid (0.0570 g, 63%), m.p. 286–287 °C. 1H NMR (400 MHz, DMSO-d6): δ = 5.89 (2 H, s, NH2), 6.66 (2 H, d, J = 8.8 Hz, 3′ and 5′-H), 7.57 (1 H, s, 9-H), 7.72 (2 H, d, J = 8.8 Hz, 2′ and 6′-H), 8.91 (1 H, d, J = 2.4 Hz, HetAr-H), 8.99 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 92.6 (9-CH), 113.7 (3′ and 5′-CH), 117.5 (C), 119.3 (C), 127.1 (2′ and 6′-CH), 142.5 (C), 143.7 (HetAr-CH), 143.9 (C), 145.5 (HetAr-CH), 151.7 (C), 157.9 (C), 158.3 (C), 159.7 (C) ppm. Calculated for C15H10N3O2S: 296.0488; found: 296.0488. HPLC retention time = 4.02 min, 3b purity is 100%.
- 8-(4-Fluorophenyl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3c) and 7-[(4-Fluorophenyl)ethynyl]thieno[2,3-b]pyrazine (4c): Compound 1b (0.100 g, 0.386 mmol), 1-ethynyl-4-fluorobenzene (0.0520 g, 0.425 mmol) and heating for 2 h. Column chromatography using a solvent gradient from 10 to 50% ether/petroleum ether gave compound 3c as a yellow solid (0.0580 g, 35%), m.p. 269–271 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.37–7.42 (2 H, m, 3′ and 5′-H), 7.96 (1 H, s, 9-H), 8.12-8.15 (2 H, m, 2′ and 6′-H), 8.95 (1 H, d, J = 2.4 Hz, HetAr-H), 9.04 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 96.7 (9-CH), 116.4 (d, J = 22.1 Hz, 3′ and 5′-CH), 122.9 (C), 127.7 (d, J =3.0 Hz, 1′-C), 128.2 (d, J = 9.1 Hz, 2′ and 6′-CH), 141.6 (C), 143.9 (C), 144.1 (HetAr-CH), 145.8 (HetAr-CH), 156.9 (C), 157.6 (C), 158.3 (C), 163.6 (d, J = 250.5 Hz, CF) ppm. 19F NMR (282.85 MHz, DMSO-d6) δ = −105.5 ppm. HRMS (ESI) [M + H]+, m/z Calculated for C15H8FN2O2S: 299.0285; found: 299.0280. HPLC retention time = 12.31 min, 3c purity is 96%.
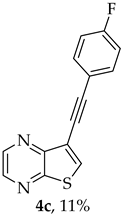
A more apolar compound, 4c, was also isolated as yellow solid (0.0110g, 11%). 1H NMR (400 MHz, DMSO-d6): δ= 7.26–7.31 (2 H, m, 3′ and 5′-CH), 7.63–7.68 (2 H, m, 2′ and 6′-CH), 8.63 (1 H, s, 6-CH), 8.70 (1 H, d, J = 2.4 Hz, HetAr-H), 8.84 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 81.9 (C), 91.7 (C), 116.3 (C), 116.34 (d, J = 20.1Hz, 3′ and 5′-CH), 118.4 (d, J = 3.0 Hz, 1′-C), 134.1 (d, J = 8.0 Hz, 2′ and 6′-CH), 136.6 (6-CH), 141.9 (HetAr-CH), 142.9 (HetAr-CH), 147.8 (C), 154.0 (C), 162.4 (d, J = 248.5 Hz, CF) ppm. 19F NMR (282.85 MHz, DMSO-d6) δ = −105.9 ppm. HRMS (ESI) [M + H]+, m/z Calculated for C14H8FN2S: 255.0387, found 255.0389.
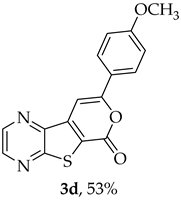
- 8-(4-Methoxyphenyl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3d) and 7-[(4-methoxyphenyl)ethynyl]thieno[2,3-b]pyrazine (4d): Compound 1b (0.0900 g, 0.347 mmol), 4-ethynylanisole (0.0520 g, 0.382 mmol) and heating for 1 h. Column chromatography using a solvent gradient from 10 to 40% ether/petroleum ether gave compound 3d as a yellow solid (0.0570 g, 53%), m.p. 222–224 °C. 1H NMR (400 MHz, DMSO-d6): δ = 3.83 (3 H, s, OCH3), 7.09 (2 H, d, J = 8.8 Hz, 3′ and 5′-H), 7.80 (1 H, s, 9-H), 7.99 (2 H, d, J = 8.8 Hz, 2′ and 6-H), 8.96 (1 H, d, J = 2.4 Hz, HetAr-H), 9.05 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 55.6 (OCH3), 95.2 (9-CH), 114.8 (3′ and 5′-CH), 121.7 (C), 123.5 (C), 127.4 (2′ and 6′-CH), 142.1 (C), 143.96 (C), 143.98 (HetAr-CH), 145.8 (HetAr-CH), 157.8 (C), 158.1 (C), 158.4 (C), 161.5 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C16H11N2O3S: 311.0485; found: 311.0486. HPLC retention time = 12.68 min, 3d purity is 97%.
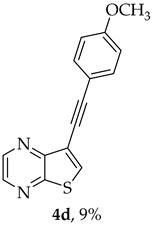
A more apolar compound, 4d, was also isolated as yellow solid (0.00900 g, 9%). 1H NMR (400 MHz, DMSO-d6): δ = 3.80 (3 H, s, OCH3), 7.00 (2 H, d, J = 8.8, Ar-H), 7.53 (2 H, d, J = 8.8, Ar-H), 8.59 (1 H, s, 6-CH), 8.71 (1 H, d, J = 2.4 Hz, HetAr-H), 8.85 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 55.4 (OCH3), 80.7 (C), 92.8 (C), 113.8 (C), 114.6 (2 × Ar-CH), 116.7 (C), 133.2 (2 × Ar-CH), 135.6 (6-CH), 141.7 (HetAr-CH), 142.8 (HetAr-CH), 147.8 (C), 153.9 (C), 159.9 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C15H11N2OS: 266.0514; found: 266.0510.
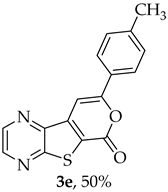
- 8-(p-Tolyl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3e) and 7-(p-tolylethynyl)thieno[2,3-b]pyrazine (4e): Compound 1b (0.100 g, 0.386 mmol), 4-ethynyltoluene (0.0490 g, 0.425 mmol) and heating for 2 h. Column chromatography using a solvent gradient from 5 to 40% ether/hexane gave compound 3e as a yellow solid (0.0560 g, 50%), m.p. 241–243 °C. 1H NMR (400 MHz, DMSO-d6): δ = 2.39 (3 H, s, CH3), 7.36 (2 H, d, J = 8.0 Hz, 3′ and 5′-H), 7.78 (1 H, s, 9-H), 7.90 (2 H, d, J = 8.0 Hz, 2′ and 6′-H), 8.90 (1 H, d, J = 2.4 Hz, HetAr-H), 8.99 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 20.5 (CH3), 95.6 (9-CH), 122.1 (C), 125.1 (2′ and 6′-CH), 128.1 (C), 129.4 (3′ and 5′-CH), 140.5 (C), 141.3 (C), 143.45 (HetAr-CH), 143.5 (C), 145.1 (HetAr-CH), 157.1 (C), 157.9 (C), 158.0 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C16H11N2O2S: 295.0536; found: 295.0534. HPLC retention time = 18.5 min, 3e purity is 99%.
A more apolar compound, 4e, was also isolated as yellow solid (0.00400 g, 4%). 1H NMR (400 MHz, DMSO-d6): δ = 2.34 (3 H, s, CH3), 7.27 (2 H, d, J = 8.0 Hz, Ar-H), 7.49 (2 H, dd, J = 8.0 Hz, Ar-H), 8.64 (1 H, s, 6-H), 8.72 (1 H, d, J = 2.4 Hz, HetAr-H), 8.86 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C15H11N2S: 251.0637, found 251.0634.
- 8-(Pyridin-2-yl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3f): Compound 1b (0.100 g, 0.386 mmol), 2-ethynylpyridine (0.0450 g, 0.0440 mL, 0.425 mmol) and heating for 1 h. Column chromatography using a solvent gradient from 5 to 30% ethyl acetate/petroleum ether gave compound 3f as a yellow solid (0.040 g, 50%), m.p. 238–239 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.53–7.56 (1 H, m, 5′-H), 8.02–8.03 (2 H, m, 3′ and 4′-H), 8.13 (1 H, s, 9-H), 8.74–8.76 (1 H, m, 6′-H), 8.95 (1 H, d, J = 2.4 Hz, HetAr-H), 9.05 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 97.6 (9-CH), 120.1 (3′ or 4′-CH), 124.6 (C), 125.4 (5′-CH), 138.0 (3′ or 4′-CH), 141.0 (C), 143.9 (C), 144.3 (HetAr-CH), 145.8 (HetAr-CH), 148.1 (C), 150.3 (6′-CH), 156.5 (C), 157.2 (C), 158.2 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C14H8N3O2S: 282.0332; found: 282.0333.
- 8-(Thiophen-3-yl)-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (3g) and 7-(thiophen-3-ylethynyl)thieno[2,3-b]pyrazine (4g): Compound 1b (0.0900 g, 0.347 mmol), 3-ethynylthiophene (0.0430 g, 0.0390 mL, 0.382 mmol) and heating for 1 h. Column chromatography using a solvent gradient from 20 to 40% ethyl ether/petroleum ether gave compound 3g as a yellow solid (0.0600 g, 54%), m.p. 247–249 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.73–7.75 (1 H, dd, J = 5.2 and 2.8 Hz, 5′-H), 7.81 (1 H, dd, J = 5.2 and 1.2 Hz, 4′-H), 7.84 (1 H, s, 9-H), 8.27 (1 H, dd, J = 2.8 and 1.2 Hz, 2′-H), 8.93 (1 H, d, J = 2.4 Hz, HetAr-H), 9.02 (1H, d, J = 2.4 Hz, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 96.3 (9-CH), 122.2 (C), 125.2 (4′-CH), 126.1 (2′-CH), 128.4 (5′-CH), 133.4 (C), 141.8 (C), 143.88 (C), 143.91 (HetAr-CH), 145.7 (HetAr-CH), 154.6 (C), 157.5 (C), 158.2 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C14H9N2O2S2: 301.0100; found: 301.0096.
A more apolar compound, 4g, was also isolated as yellow solid (0.00200 g, 3%), 1H NMR (400 MHz, DMSO-d6): δ = 7.31 (1H, dd, J = 5.2 and 1.2 Hz, 4′-H), 7.67–7.69 (1 H, dd, J = 5.2 and 2.8 Hz, 5′-H), 7.97–7.98 (1 H, dd, J = 2.8 and 1.2 Hz, 2′-H), 8.64 (1 H, s, 6-H), 8.72 (1 H, d, J = 2.4 Hz, HetAr-H), 8.85 (1 H, d, J = 2.4 Hz, HetAr-H) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C13H7N2O2S2: 286.9943; found: 286.9944.
General Procedure for the 9-HaloTricyclic Lactones (5a and 5b)
In a vacuum-dried Schlenk tube with dry acetonitrile (3 mL), compound 2a (1.0 equiv.), NXS (2.0 equiv.) and CuX2 (2.5 equiv.) were added and the mixture was heated and stirred at 100 °C, under Ar. After 1 h a precipitate came out, the mixture was cooled and filtered under vacuum to give compounds 5a or 5b.
- 9-Chloro-8-phenyl-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (5a): Compound 2a (0.0500 g, 0.170 mmol), NCS (0.0450 g, 0.340 mmol) and CuCl2 (0.0580 g, 0.425 mmol), gave compound 5a as a yellow solid (0.0324 g, 60%), m.p. 313–314 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.57–7.59 (3 H, m, Ar-H), 7.82–7.84 (2 H, m, Ar-H), 8.98 (1 H, broad s, HetAr-H), 9.08 (1 H, broad s, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 108.2 (C), 125.2 (C), 128.3 (2 × Ar-CH), 129.0 (2 × Ar-CH), 130.2 (C), 130.4 (4′-CH), 136.6 (C), 143.6 (HetAr-CH), 143.8 (C), 144.8 (HetAr-CH), 153.5 (C), 156.5 (C), 157.5 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C15H835ClN2O2S: 314.9989; found: 314.9989.
- 9-Bromo-8-phenyl-6H-pyrano[4′,3′:4,5]thieno[2,3-b]pyrazin-6-one (5b): Compound 2a (0.0560 g, 0.190 mmol), NBS (0.0680 g, 0.381 mmol) and CuBr2 (0.107 g, 0.478 mmol), gave compound 5b as a yellow solid (0.0340 g, 50%), m.p. 313–315 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.56 (3 H, m, Ar-H), 7.77 (2 H, m, Ar-H), 8.95 (1 H, broad s, HetAr-H), 9.06 (1 H, broad s, HetAr-H) ppm. 13C NMR (100.6 MHz, DMSO-d6): δ = 94.4 (C), 125.3 (C), 127.9 (2 × Ar-CH), 129.2 (2 × Ar-CH), 130.1 (4′-CH), 131.5 (C), 136.9 (C), 142.9 (HetAr-CH), 143.8 (C), 144.6 (HetAr-CH), 154.5 (C), 156.6 (C), 157.5 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C15H879BrN2O2S: 358.9484; found: 358.9484. m/z Calculated for C15H881BrN2O2S: 360.9464; found: 360.9464.
3.1.2. Synthesis of Tetracyclic Lactones Derived from Thieno[2,3-b]quinoline by Rh(III)-Catalyzed Formal [4+2] Cycloaddition, Triggered by C-H Activation
General Procedure for the C-H Activation/Cycloaddition (7a–f)
In a Schlenk tube with dry DMF (0.8 mL), compound 6 (1.0 equiv.), [RhCp*Cl2]2 (2.5 mol%), Ag2CO3 (2.0 equiv.), AgSbF6 (10 mol%) and the internal alkyne (1.0 equiv.) were added. The reaction was left stirring at 100 °C, overnight, monitoring by TLC. After cooling, ethyl acetate (10 mL) was added, and the solvents were removed under vacuum. The resulting residue was then submitted to column chromatography to give compounds 7.
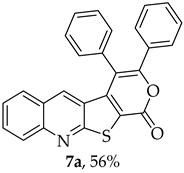
- 3,4-Diphenyl-1H-pyrano[4′,3′:4,5]thieno[2,3-b]quinolin-1-one (7a): Compound 6 (0.0500 g, 0.218 mmol) and diphenylacetylene (0.0400 g, 0.218 mmol). Column chromatography using a solvent gradient from 5 to 40% ether/petroleum ether gave compound 7a as a yellow solid (0.0500 g, 56%), m.p. 304–305 °C. 1H NMR (400 MHz, DMSO-d6, 80 °C): δ = 7.25 (1 H, s, 5-H), 7.30–7.35 (3 H, m, Ar-H), 7.44–7.47 (2 H, m, Ar-H), 7.50–7.66 (7 H, m, Ar-H), 7.87–7.91 (1 H, m, Ar-H), 8.07–8.10 (1 H, broad d, J = 8.4 Hz, Ar-H) ppm. 13C NMR (100.6 MHz, DMSO-d6, 80 °C): δ = 115.8 (C) 122.0 (C), 124.0 (C), 126.2 (CH), 127.0 (C), 127.3 (CH), 127.7 (2 × CH), 128.6 (2 × CH), 128.7 (2 × CH), 129.0 (2 × CH), 129.1 (CH), 130.6 (2 × CH), 131.7 (CH), 131.8 (C), 133.0 (C), 134.2 (5-CH), 141.2 (C), 147.3 (C), 154.3 (C), 157.1 (C), 162.2 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C26H16NO2S: 406.0896; found: 406.0897.
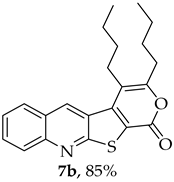
- 3,4-Dibutyl-1H-pyrano[4′,3′:4,5]thieno[2,3-b]quinolin-1-one (7b): Compound 6 (0.0500 g, 0.218 mmol) and 5-decyne (0.0300 g, 0.0390 mL, 0.218 mmol). Column chromatography using a solvent gradient from 5 to 20% ether/petroleum ether afforded compound 7b as a yellow solid (0.0680 g, 85%), m.p.172–173 °C. 1H NMR (400 MHz, DMSO-d6, 80 °C): δ = 0.92–0.99 (6 H, m, 2 × CH3), 1.41–1.43 (2 H, m, CH2), 1.59–1.67 (6 H, m, 3 × CH2), 2.72–2.76 (2 H, t, J = 7.6 Hz, CH2), 3.05–3.09 (2 H, m, CH2), 7.71–7.75 (1 H, m, Ar-H), 7.94–7.99 (1 H, m, Ar-H), 8.11–8.13 (1H, broad d, J = 8.4 Hz, Ar-H), 8.31–8.33 (1 H, broad d, J = 8.4 Hz, Ar-H), 9.26 (1 H, s, 5-H) ppm. 13C NMR (100.6 MHz, DMSO-d6, 80 °C): δ = 13.7 (CH3), 13.9 (CH3), 21.7 (CH2), 21.8 (CH2), 25.8 (CH2), 29.5 (CH2) 29.8 (CH2), 31.5 (CH2), 113.6 (C), 121.8 (C), 125.1 (C), 126.5 (CH), 127.0 (C), 127.5 (CH), 130.0 (CH), 132.2 (CH), 135.7 (5-CH), 141.6 (C), 147.4 (C), 158.0 (C), 158.4 (C), 162.3 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C22H24NO2S: 366.1522; found: 366.1526.
- 3,4-Bis(4-bromophenyl)-1H-pyrano[4′,3′:4,5]thieno[2,3-b]quinolin-1-one (7c): Compound 6 (0.0500 g, 0.218 mmol) and bis(4-bromophenyl)acetylene (0.0730 g, 0.218 mmol). Column chromatography using a solvent gradient from 5 to 20% ethyl acetate/petroleum ether gave compound 7c as a yellow solid (0.0920 g, 75%), m.p. 307–308 °C. 1H NMR (400 MHz, DMSO-d6, 80 °C): δ = 7.36 (2 H, d, J = 8.4 Hz, Ar-H), 7.40 (1 H, s, 5-H), 7.51 (2 H, d, J = 8.4 Hz, Ar-H), 7.56 (2 H, d, J = 8.4 Hz, Ar-H), 7.61–7.62 (2 H, m, Ar-H), 7.79 (2 H, d, J = 8.4 Hz, Ar-H), 7.88–7.92 (1 H, m, Ar-H), 8.08 (1 H, broad d, J = 8.8 Hz, Ar-H) ppm. 13C NMR (100.6 MHz, DMSO-d6, 80 °C): δ = 115.0 (C), 122.4 (C), 122.9 (C), 123.9 (C), 126.3 (CH), 126.7 (C), 127.2 (CH), 128.9 (CH), 130.7 (2 × CH), 130.8 (C), 130.9 (2 × CH), 131.8 (CH), 132.0 (C), 132.1 (2 × CH), 132.7 (2 × CH), 134.0 (5-CH), 140.5 (C), 147.3 (C), 153.4 (C), 156.8 (C), 162.0 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C26H1479Br2NO2S: 561.9106; found: 561.9105; m/z Calculated for C26H1479Br81BrNO2S: 563.9086; found: 563.9085; m/z Calculated for C26H1481Br2NO2S: 565.9066; found: 565.9064.
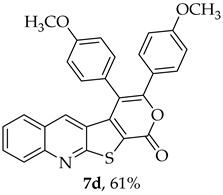
- 3,4-Bis(4-methoxyphenyl)-1H-pyrano[4′,3′:4,5]thieno[2,3-b]quinolin-1-one (7d): Compound 6 (0.0500 g, 0.218 mmol) and 1,2-bis(4-methoxyphenyl)ethyne (0.0520 g, 0.218 mmol). Column chromatography using a solvent gradient from 5 to 15% ethyl acetate/petroleum ether gave compound 7d as a yellow solid (0.0620 g, 61%), m.p. 304–306 °C.1H NMR (400 MHz, DMSO-d6, 100 °C): δ = 3.77 (3 H, s, OCH3), 3.93 (3 H, s, OCH3), 6.88 (2 H, d, J = 8.8 Hz, Ar-H), 7.16 (2 H, d, J = 8.8 Hz, Ar-H), 7.38–7.45 (5 H, m, 5-H and 4 × Ar-H), 7.57–7.62 (2 H, m, Ar-H), 7.86–7.90 (1 H, m, Ar-H), 8.08 (1 H, broad d, J = 8.6 Hz, Ar-H) ppm. 13C NMR (100.6 MHz, DMSO-d6, 100 °C): δ = 54.8 (OCH3), 55.1 (OCH3), 113.3 (2 × CH), 114.6 (C), 114.7 (2 × CH), 121.0 (C), 123.9 (C), 124.2 (C), 125.2 (C), 126.0 (CH), 127.1 (C), 127.14 (CH), 128.7 (CH), 130.0 (2 × CH), 131.4 (CH), 131.7 (2 × CH), 134.2 (5-CH), 141.8 (C), 147.2 (C), 154.4 (C), 157.0 (C), 159.7 (C), 159.8 (C), 162.2 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C28H20NO4S: 466.1107; found: 466.1107.
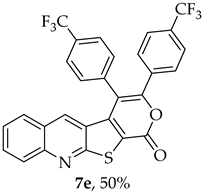
- 3,4-Bis(4-(trifluoromethyl)phenyl)-1H-pyrano[4’,3’:4,5]thieno[2,3-b]quinolin-1-one (7e): Compound 6 (0.0400 g, 0.175 mmol) and 1,2-bis(4-(trifluoromethyl)phenyl)ethyne (0.0550 g, 0.175 mmol). Column chromatography using a solvent gradient from 5 to 30% ether/petroleum ether gave compound 7e as a yellow solid (0.0470 g, 50%), m.p. 276–278 °C. 1H NMR (400 MHz, DMSO-d6, 80 °C): δ = 7.23 (1 H, s, 5-H), 7.50 (1 H, broad d, J = 8.4 Hz, Ar-H), 7.59–7.71 (5 H, m, Ar-H), 7.81 (2 H, d, J = 8.0 Hz, Ar-H), 7.83–7.93 (1 H, m, Ar-H), 9.94 (2 H, d, J = 8.0 Hz, Ar-H) 8.09 (1 H, broad d, J = 8.4 Hz, Ar-H) ppm. 13C NMR (100.6 MHz, DMSO-d6, 80 °C): δ = 115.6 (C), 122.3 (C), 123.1 (C) 123.4 (q, J = 271.2 Hz, CF3), 123.7 (q, J = 271.2 Hz, CF3), 124.6 (q, J = 3.0 Hz, 2 × CH), 125.8 (q, J = 3.0Hz, 2 × CH), 126.3 (CH), 126.6 (C), 127.3 (CH), 128.6 (CH), 129.6 (q, J = 32.2 Hz, CCF3), 129.7 (2 × CH), 129.9 (q, J = 32.2 Hz, CCF3), 131.8 (2 × CH), 131.9 (CH), 133.9 (5-CH), 135.4 (C), 137.0 (C), 140.1 (C), 147.4 (C) 152.9 (C), 156.7 (C), 162.0 (C) ppm. 19F NMR (282.85 MHz, DMSO-d6) δ = -57.4 (s), −57.0 (s) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C28H14F6NO2S: 542.0644; found: 542.0643. HPLC retention time = 7.9 min, 7e purity is 96%.
- 4-Methyl-3-phenyl-1H-pyrano[4’,3’:4,5]thieno[2,3-b]quinolin-1-one (7f): Compound 6 (0.0500 g, 0.218 mmol) and 1-phenyl-1-propyne (0.0250 g, 0.0270 mL, 0.218 mmol). Column chromatography using a solvent gradient from 5 to 10% ethyl acetate/petroleum ether gave compound 7f as a yellow solid (0.0520 g, 70%), m.p. 301–302 °C. 1H NMR (400 MHz, DMSO-d6, 80 °C): δ = 2.72 (3 H, s, CH3), 7.55–7.61 (3 H, m, Ar-H), 7.66–7.73 (3 H, m, Ar-H), 7.93–7.97 (1 H, m, Ar-H), 8.12 (1 H, broad d, J = 8.8 Hz, Ar-H), 8.32–8.35 (1 H, broad, J = 8.8 Hz, Ar-H), 9.52 (1 H, s, 5-H) ppm. The regioisomer 7f was assigned using nOe. NMR (100.6 MHz, DMSO-d6, 80 °C): δ = 15.0 (CH3), 110.1 (C), 122.2 (C), 124.9 (C), 126.0 (CH), 127.2 (CH), 127.6 (C), 128.2 (2 × CH), 129.1 (2 × CH); 129.5 (CH), 129.6 (CH), 131.7 (CH), 131.9 (C), 135.7 (5-CH), 142.2 (C), 147.3 (C), 154.3 (C), 157.3 (C), 162.2 (C) ppm. HRMS (ESI) [M + H]+, m/z Calculated for C21H14NO2S: 344.0740; found: 344.0740.
3.2. Biological Activities
3.2.1. In Vitro Antitumor Evaluation (SRB Assay)
The human tumor cell lines used were CaCo-2 (colorectal adenocarcinoma), MCF-7 (breast adenocarcinoma), AGS (gastric adenocarcinoma), HeLa (cervical carcinoma), NCI-H460 (lung carcinoma). Non-tumor cell lines were also tested: PLP2 (primary pig liver culture) [32]. All the cell lines were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 mg/mL), with the exception of PLP2, which was maintained in DMEM medium supplemented with fetal bovine serum (10%), glutamine and antibiotics. The culture flasks were incubated at 37 °C and with 5% CO2, under a humid atmosphere. The cells were used only when they had 70 to 80% confluence. A known mass of each compound was dissolved in DMSO/H2O (1:1, 1 mL), in order to obtain the stock solutions with a concentration of 1 mM. From the latter, successive dilutions were made to obtain the concentrations to be tested (0.16–10 μM). Each compound concentration (10 μL) was incubated with the cell suspension (190 μL) of the cell lines tested in 96-well microplates for 72 h. The microplates were incubated at 37 °C and with 5% CO2 in a humid atmosphere after checking the adherence of the cells. All cell lines were tested at a concentration of 10,000 cells/well. After the incubation period, the cells were treated: TCA (10% w/v; 100 μL) was previously cooled, and plates were incubated for 1 h at 4 °C, washed with water, and, after drying, a SRB solution (0.057%, m/v; 100 μL) was added and left to stand at room temperature for 30 min. To remove non-adhered SRB, plates were washed three times with a solution of acetic acid (1% v/v) and placed to dry. Finally, an adhered SRB was solubilized with Tris (10 mM, 200 μL) and the absorbance at a wavelength of 540 nm was read in the Biotek ELX800 microplate reader. The results obtained by the SRB assay [16] were expressed regarding the concentration compounds that can inhibit cell growth by 50%—GI50. Doxorubicin was used as a positive control.
3.2.2. In Vitro Anti-Parasitic Evaluation
Parasite Cultures
T. brucei Lister 427 bloodstream forms were grown in a humidified incubator at 37 °C, 5% CO2 in complete HMI-9 medium 1 supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS) and 100 UI/mL penicillin/streptomycin. Parasites maintenance was performed in T25 ventilated flasks by subpassage at a concentration of 1 × 104/mL every 2 days on T25 ventilated flasks.
L. infantum MHOM/MA/67/ITMAP-263 promastigotes forms were grown at 27 °C, in complete RPMI-1640 medium supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS), 2 mM L-glutamine, 100 UI/mL penicillin/streptomycin, 20 mM HEPES. Parasites maintenance was performed in T25 non-ventilated flasks by subpassage at a concentration of 1 × 106/mL every 5 days on T25 non-ventilated flasks [33].
Cell Cultures
Human leukemia cell line, THP-1 (ATCC® TIB-202™, Manassas, VA, USA) was cultured in RPMI-1640 medium supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS), 2 mM L-glutamine, 100 UI/mL penicillin/streptomycin, 20 mM HEPES. The cell line was maintained in a humidified incubator at 37 °C and 5% CO2 by subculture every three days in 20 mL of media at a concentration of 1 × 105/mL in a T75 flask. All cell culture reagents were purchased from Lonza-Bioscience (Morrisville, NC, USA).
In Vitro Evaluation of Anti-T. brucei Activity
The efficacy of compounds against T. brucei bloodstream forms was evaluated using a modified resazurin-based assay previously described in the literature [34]. Parasites were added to 100 µL of serial dilutions of compounds in supplemented complete medium at a cell density of 5 × 103/mL for. As a quality control, a dose–response curve for the antitrypanosomal pentamidine was included in all the assays. The final volume of the assay is 200 µL/well. Each condition was carried out in duplicate. Following 72 h incubation at the specific conditions for T. brucei, 20 µL of a 0.5 mM resazurin solution was added and plates were incubated for a further 4 h under the same conditions. Fluorescence was measured at 544 nm and 590 nm excitation and emission wavelength, respectively, using a Synergy 2 Multi-Mode Reader (Biotek, Winooski, VT, USA). Results were shown as % of parasite growth inhibition compared to control (untreated parasites) and represent the average of at least three independent experiments. The effect was evaluated by the determination of the IC50 value (concentration required to inhibit growth in 50%) and calculated by non-linear regression curves using GraphPad Prism version 8.1.1 for Windows (GraphPad Software, San Diego CA, USA).
Evaluation of Activity Against L. infantum MHOM/MA/67/ITMAP-263 Promastigotes
The compounds’ efficacy against L. infantum promastigotes was evaluated using a resazurin-based assay [35]. Parasites were added to 100 µL of serial dilutions of compounds in supplemented complete medium at a cell density of 5 × 105/mL. As a quality control, a dose–response curve to the antileishmanial drug miltefosine was included in all the assays. The final volume of the assay was 200 µL/well. Each condition was carried out in duplicate. Following 72 h of incubation at the specific conditions for L. infantum, 20 µL of a 0.5 mM resazurin solution was added, and the plates were incubated for a further 4 h under the same conditions. The methodology to obtain the results was the same described in Section 3.2.2. for T. brucei.
Cytotoxicity in THP-1 Cells
The cytotoxicity effect of compounds on THP-1-derived macrophages was assessed by the colorimetric MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) [35]. Briefly, THP-1 cells were suspended in RPMI complete medium at a density of 1 × 106 cells/mL and 100 μL/well were seeded in a 96-well plate and were differentiated into macrophages by addition of 40 ng/mL of phorbol-myristate 13-acetate (PMA, Sigma, Saint Louis, MI, USA) for 24 h followed by replacement with fresh medium for more 24 h. Subsequently, cells were incubated with 100 μL of compounds ranging from 100 to 12.5 μM after dilution in the RPMI complete medium. Each condition was carried out in quadruplicate. After 72 h of incubation at 37 °C 5% CO2, the medium was removed and 200 μL of 0.5 mg/mL MTT solution diluted in RPMI was added. Plates were incubated for an additional 4 h. Then 160 μL of media was removed and the same volume of 2-propanol was added. Absorbance was read at 570 nm using a Synergy 2 Multi-Mode Reader (Biotek, Winooski, VT, USA). Cytotoxicity was evaluated by the determination of the CC50 value (drug concentration that reduced the percentage of viable cells in 50%) and calculated by non-linear regression analysis using GraphPad Prism version 8.1.1 for Windows (GraphPad Software, San Diego, CA, USA). The results represent the average of at least three independent experiments. For each compound, the Selectivity Index (SI) was calculated as the ratio between cytotoxicity in THP-1 (CC50, 72 h) and activity against parasites (IC50, 72 h).
4. Conclusions
We successfully synthesized tricyclic and tetracyclic lactone derivatives of thieno[2,3-b]pyrazine or thieno[2,3-b]quinoline, and 2H-pyrones. First Pd/Cu-catalyzed Sonogashira coupling, using Et3N as a base, of methyl 7-bromothieno[2,3-b]pyrazine-6-carboxylate with (het)arylalkynes, which were either unsubstituted or substituted with EDGs or EWGs on the phenyl ring, and with either electron-rich or electron-deficient heteroaryl rings, were performed to obtain the Sonogashira ester products 2, being the corresponding tricyclic lactones 3 isolated as minor products in some cases. An attempt was made to conduct the Sonogashira coupling using heterogeneous catalysis (Pd/C), excluding Cu, with a ligand and eucalyptol as the solvent for the synthesis of 2a. However, 2a was only obtained in moderate yield, along with a major unidentified product. Further studies are required to optimize and apply these more environmentally friendly conditions.
It was not possible to cyclize the Sonogashira product 2a with TFA to lactone 3a. The tricyclic lactones 3a–g were obtained in good yields by a tandem one-pot Sonogashira coupling and 6-endo-dig lactonization of 7-bromothieno[2,3-b]pyrazine-6-carboxylic acid with (het)arylalkynes. Additionally, halogenated tricyclic lactones were synthesized from Sonogashira ester product 2a by halocyclization using CuX2 and NXS, enabling further functionalization. With I2 or ICl the halocyclization did not occur.
Tetracyclic lactones 7 were synthesized through a Rh(III)-catalyzed formal [4+2] cycloaddition of thieno[2,3-b]quinoline-2-carboxylic acid with symmetrical or unsymmetrical internal alkyl or arylalkynes. The reaction was driven by C-H activation, with the carboxylic acid serving as a dynamic directing group to facilitate the cycloaddition. This methodology is atom-efficient and enables late-stage functionalization.
The biological activity of the Sonogashira ester products and lactones was assessed for both antitumor and antiparasitic potential. Antitumor activity was evaluated against five human tumor cell lines using the SRB assay. The most active compound was a Sonogashira ester product bearing a pyridine ring 3f, exhibiting a GI50 value below 10 µM across all tested cell lines, with low toxicity, at their GI50 values, in non-cancerous PLP2 cells. The antiparasitic activity was assessed against T. brucei and L. infantum. Two compounds, 3f and the 9-chloro tricyclic lactone 5a, displayed notable activity against T. brucei, with IC50 values below 11 µM, though some degree of cytotoxicity was observed in THP-1-derived macrophages. These findings highlight the potential of the most promising compounds as lead candidates for the further development of antitumor and antiparasitic drugs, including investigations into their mechanisms of action. Additionally, all the compounds may be subjected to further biological activity assays to explore their full therapeutic potential.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30091999/s1, and contains 1H and 13C NMR spectra of the compounds 1b, 2a–2g, 3a–3g, 4a, 4c–4e, 4g, 5a–5b and 7a–7f, a nOe experiment on 7f, 1H-13C 2D correlations HSQC spectra of compounds 7, 19F NMR spectra of compounds 2c, 3c, 4c and 7e and HPLC chromatograms of compounds 3b, 3c, 3d, 3e and 7e are available online.
Author Contributions
Conceptualization, M.-J.R.P.Q. and M.F.M.; methodology, M.-J.R.P.Q., R.C.C. and A.C.-d.-S.; validation, M.F.M., F.R., A.B. and N.S.; formal analysis, R.C.C., N.S. and A.C.-d.-S.; investigation, M.F.M., F.R., A.B. and N.S.; resources, M.-J.R.P.Q., R.C.C. and A.C.-d.-S.; writing—original draft preparation, M.-J.R.P.Q., M.F.M., F.R., R.C.C., N.S. and A.C.-d.-S.; writing—review and editing, M.-J.R.P.Q., R.C.C. and A.C.-d.-S.; visualization, M.-J.R.P.Q. and M.F.M.; supervision, M.-J.R.P.Q., R.C.C. and A.C.-d.-S.; project administration, M.-J.R.P.Q.; funding acquisition, M.-J.R.P.Q., R.C.C. and A.C.-d.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Portuguese Foundation for Science and Technology (FCT, Portugal) through CQ-UM (strategic funding UID/00686/2020), CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2021); the institutional scientific employment program-contract for RCC; NS is funded by national funds through FCT and co-funded through the European Social Fund within the Human Potential Operating Programme 2021.04285.CEECIND/CP1663/CT0004. The work on anti-parasitic activity is performed under the scope of COST Action CA21111: One Health Drugs against Parasitic Vector-Borne Diseases in Europe and Beyond (OneHealthDrugs).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Folmer, B.J.B.; Man, A.P.A.; Gernette, E.S.; Azevedo, R.C.R.G.; Ibrahim, H. Thieno[2,3-b]pyrazine Compounds as B-RAF Inhibitors. WO 2011147764, A1, 1 December 2011. [Google Scholar]
- Gong, Y.D.; Kwak, S.; Lee, E.S. Substituted Thieno[3,2-b]Pyrazines for Inhibiting Cancer Cell Proliferation and Inducing Cancer Cell Apoptosis. WO 2016093554, A1, 16 June 2016. [Google Scholar]
- Lim, J.; Altman, M.D.; Gibeau, C.R. Thienopyrazine Inhibitors of IRAK4 Activity. WO 2016144849, A1, 15 September 2016. [Google Scholar]
- Guerin, D.J.; Bair, K.W.; Caravella, J.A.; Ioannidis, S.; Lancia, D.R., Jr.; Li, H.; Mischke, S.; Ng, P.Y.; Richard, D.; Sciller, S.E.R.; et al. Thienopyrazine Carboxamides as Ubiquitin-Specific Proteases Inhibitors. WO 2017139779, A1, 17 August 2017. [Google Scholar]
- Teja, C.; Nawaz Khan, F.R. Recent Advances in the Synthesis of Thienoquinolines (Quinoline-fused heterocycle). Asian. J. Org. Chem. 2020, 9, 1889–1900. [Google Scholar] [CrossRef]
- Rechfeld, F.; Gruber, P.; Kirchmair, J.; Boehler, M.; Hauser, N.; Hechenberger, G.; Garczarczyk, D.; Lapa, G.B.; Preobrazhenskaya, M.N.; Goekjian, P.; et al. Thienoquinolines as novel disruptors of the PKCε/RACK2 protein-protein interaction. J. Med. Chem. 2014, 57, 3235–3246. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Nikam, M.; Asrondkar, A.; Bobade, A.; Gill, C. Synthesis, Antioxidant, and Anti-Inflammatory Evaluation of Novel Thiophene-Fused Quinoline Based β-Diketones and Derivatives. J. Heterocycl. Chem. 2017, 54, 1415–1422. [Google Scholar] [CrossRef]
- Abdelbaset, M.S.; Abdel-Aziz, M.; Ramadan, M.; Abdelrahman, M.H.; Abbas Bukhari, S.N.; Ali, T.F.S.; Abuo-Rahma, G.E.D.A. Discovery of novel thienoquinoline-2-carboxamide chalcone derivatives as antiproliferative EGFR tyrosine kinase inhibitors. Bioorg. Med. Chem. 2019, 27, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Larock, R.C. Synthesis of isocoumarins and α-pyrones via electrophilic cyclization. J. Org. Chem. 2003, 68, 5936–5942. [Google Scholar] [CrossRef]
- Jithunsa, M.; Ueda, M.; Miyata, O. Copper(II)chloride-mediated cyclization reaction of N-alkoxy- ortho-alkynylbenzamides. Org. Lett. 2011, 13, 518–521. [Google Scholar] [CrossRef]
- Calhelha, R.C.; Vale-Silva, L.A.; Pinto, E.; São-José Nascimento, M.; Queiroz, M.J.R.P. Synthesis of Novel 3-(Aryl)Benzothieno[2,3-c]Pyran-1-ones from Sonogashira Products and Intramolecular Cyclization: Antitumoral Activity Evaluation. Eur. J. Med. Chem. 2009, 44, 1893–1899. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Cendón, B.; Gulías, M.; Mascareñas, J.L.; Queiroz, M.J.R.P. Rhodium(III)-Catalyzed Formal Cycloaddition between Thienopyridine/Thienopyrazine Carboxylic Acids and Alkynes, Triggered by C−H Activation. Eur. J. Org. Chem. 2021, 2021, 3234–3240. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U.; Nasir, N.M. Palladium and Copper Catalyzed Sonogashira cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A review. Catalysts 2020, 10, 443. [Google Scholar] [CrossRef]
- Yogeshwaran, V.; Dhayanithi, S.; Vinoth Kumar, P.; Mohana Roopan, S. 6-Endo-Dig Cyclization: Flexible Enforce to Develop Synthetic Route in Organic Syntheses. Results Chem. 2023, 6, 101131. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Calhelha, R.C.; Nogueira, A.; Ferreira, I.C.F.R.; Barros, L.; Queiroz, M.J.R.P. Synthesis of novel methyl 7-[(Hetero)arylamino]thieno[2,3-b]pyrazine-6-carboxylates and antitumor activity evaluation: Effects in human tumor cells growth, cell cycle analysis, apoptosis and toxicity in non-tumor cells. Molecules 2021, 26, 4823. [Google Scholar] [CrossRef] [PubMed]
- Duplais, C.; Forman, A.J.; Baker, B.A.; Lipshutz, B.H. UC Pd: A New form of Pd/C for Sonogashira Couplings. Chem. Eur. J. 2010, 16, 3366–3371. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol, an All-Purpose Product. Catalysts 2022, 12, 48. [Google Scholar] [CrossRef]
- Gilmore, K.; Mohamed, R.K.; Alabugin, I.V. The Baldwin Rules: Revised and Extended. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 487–514. [Google Scholar] [CrossRef]
- Uchiyama, M.; Ozawa, H.; Takuma, K.; Matsumoto, Y.; Yonehara, M.; Hiroya, K.; Sakamoto, T. Regiocontrolled Intramolecular Cyclizations of Carboxylic Acids to Carbon−Carbon Triple Bonds Promoted by Acid or Base Catalyst. Org. Lett. 2006, 8, 5517–5520. [Google Scholar] [CrossRef]
- Gilmore, K.; Alabugin, I.V. Cyclizations of Alkynes: Revisiting Baldwins Rules for Ring Closure. Chem. Rev. 2011, 111, 6513–6556. [Google Scholar] [CrossRef]
- Song, G.; Wang, F.; Li, X. C-C, C-O and C-N Bond Formation via Rhodium(III)-Catalyzed Oxidative C-H Activation. Chem. Soc. Rev. 2012, 41, 3651–3678. [Google Scholar] [CrossRef]
- Roudesly, F.; Oble, J.; Poli, G. Metal-Catalyzed C-H Activation/Functionalization: The Fundamentals. J. Mol. Catal. A Chem. 2017, 426, 275–296. [Google Scholar] [CrossRef]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A Comprehensive Overview of Directing Groups Applied in Metal-Catalysed C-H Functionalisation Chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef]
- Rej, S.; Chatani, N. Rhodium-Catalyzed C(sp2)- or C(sp3)-H Bond Functionalization Assisted by Removable Directing Groups. Acc. Chem. Res. 2019, 131, 8390–8416. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, M.P.; Gooßen, L.J. Carboxylic Acids as Directing Groups for C−H Bond Functionalization. Chem. Eur. J. 2016, 22, 18654–18677. [Google Scholar] [CrossRef] [PubMed]
- Font, M.; Quibell, J.M.; Perry, G.J.P.; Larrosa, I. The Use of Carboxylic Acids as Traceless Directing Groups for Regioselective C-H Bond Functionalisation. Chem. Commun. 2017, 53, 5584–5597. [Google Scholar] [CrossRef]
- Gulías, M.; Mascareñas, J.L. Metal-Catalyzed Annulations through Activation and Cleavage of C-H Bonds. Angew. Chem. Int. Ed. Engl. 2016, 128, 11164–11184. [Google Scholar] [CrossRef]
- Yang, Y.; Li, K.; Cheng, Y.; Wan, D.; Li, M.; You, J. Rhodium-catalyzed annulation of arenes with alkynes through weak chelation-assisted C-H activation. Chem. Commun. 2016, 52, 2872–2884. [Google Scholar] [CrossRef]
- Long, S.; Resende, D.I.S.P.; Kijjoa, A.; Silva, A.M.S.; Pina, A.; Fernández-Marcelo, T.; Helena Vasconcelos, M.; Sousa, E.; Pinto, M.M.M. Antitumor Activity of Quinazolinone Alkaloids Inspired by Marine Natural Products. Mar. Drugs 2018, 16, 261. [Google Scholar] [CrossRef]
- Long, S.; Duarte, D.; Carvalho, C.; Oliveira, R.; Santarém, N.; Palmeira, A.; Resende, D.I.S.P.; Silva, A.M.S.; Moreira, R.; Kijjoa, A.; et al. Indole-Containing Pyrazino[2,1-b]quinazoline-3,6-diones Active against Plasmodium and Trypanosomatids. ACS Med. Chem. Lett. 2022, 13, 225–235. [Google Scholar] [CrossRef]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R.P. Anti-Hepatocellular Carcinoma Activity Using Human HepG2 Cells and Hepatotoxicity of 6-Substituted Methyl 3-Aminothieno[3,2-b]Pyridine-2- Carboxylate Derivatives: In Vitro Evaluation, Cell Cycle Analysis and QSAR Studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef]
- Santarém, N.; Cunha, J.; Silvestre, R.; Silva, C.; Moreira, D.; Ouellette, M.; Cordeiro-Da-Silva, A. The Impact of Distinct Culture Media in Leishmania infantum Biology and Infectivity. Parasitology 2014, 141, 192–205. [Google Scholar] [CrossRef]
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human African trypanosomiasis. Int. J. Parasitol. Drugs Resist. 2012, 2, 262–270. [Google Scholar] [CrossRef]
- Lopes, A.; Teixeira, S.; Santarém, N.; Greco, A.; Pagliaro, A.; Keminer, O.; Gul, S.; Cordeiro-Da-Silva, A.; Carvalho, M.A. SAR Study of 4,8-Disubstituted Pyrimido[5,4-d]Pyrimidines Exhibiting Antitrypanosomal and Antileishmanial Activity. ACS Med. Chem. Lett. 2024, 15, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).