Ti-Supported Oxide Coatings Based on MWO4 (M = Fe, Co, Ni): Plasma Electrolytic Synthesis, Characterization and Catalytic Properties in S, N-Heterocycles Peroxide Oxidation
Abstract
1. Introduction
2. Results and Discussion
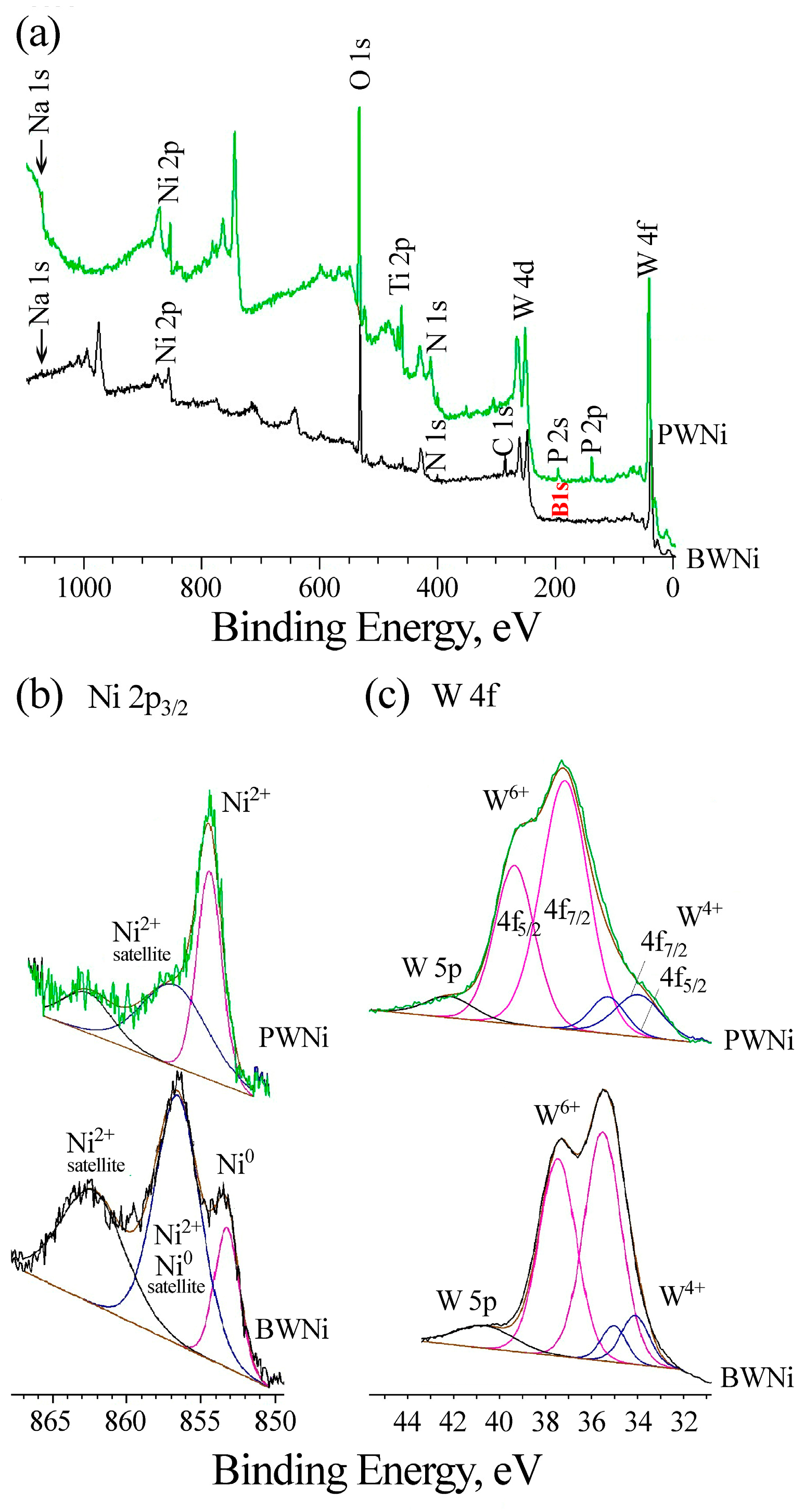
2.1. Preparation and Characterization of Catalysts
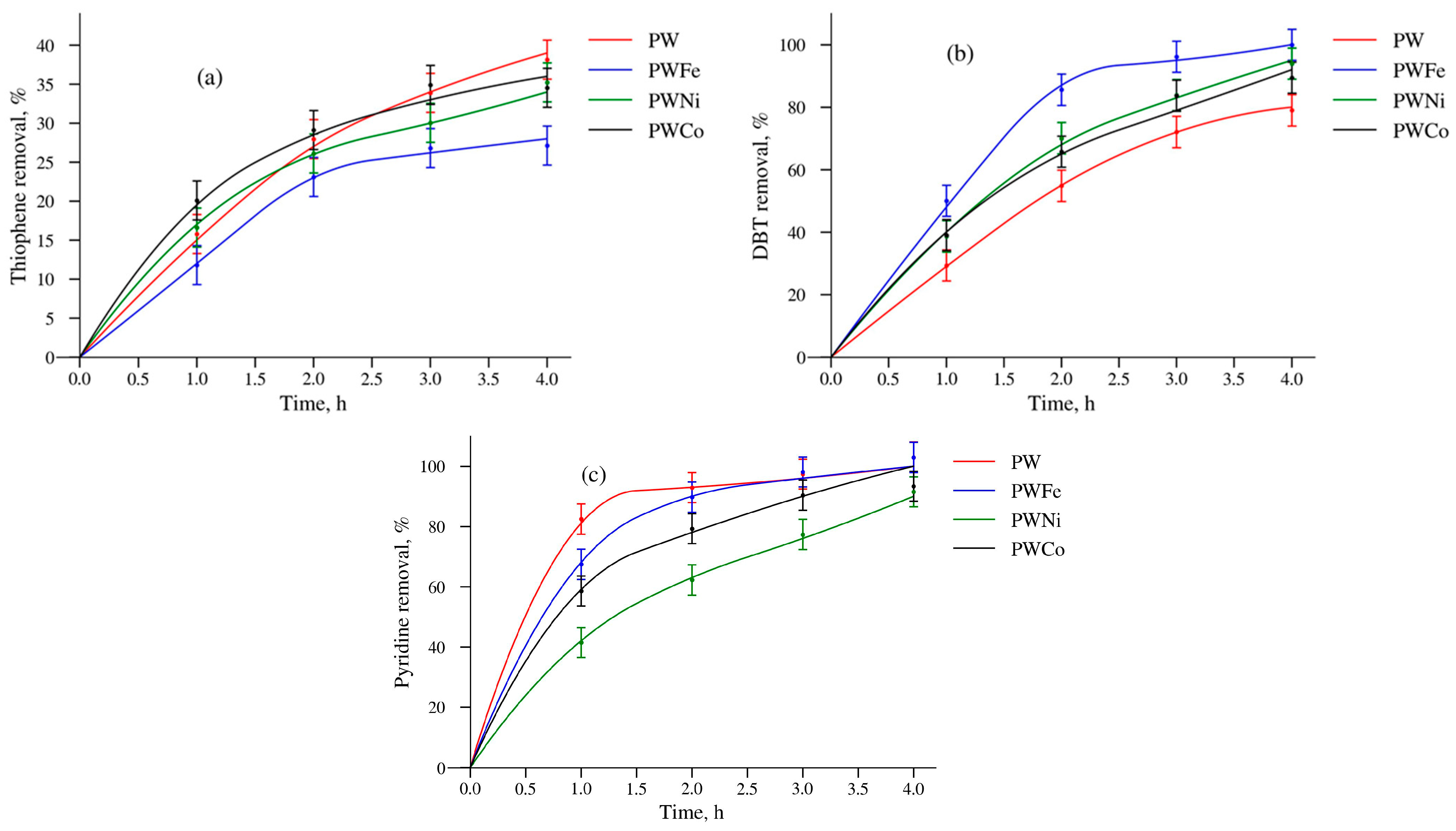
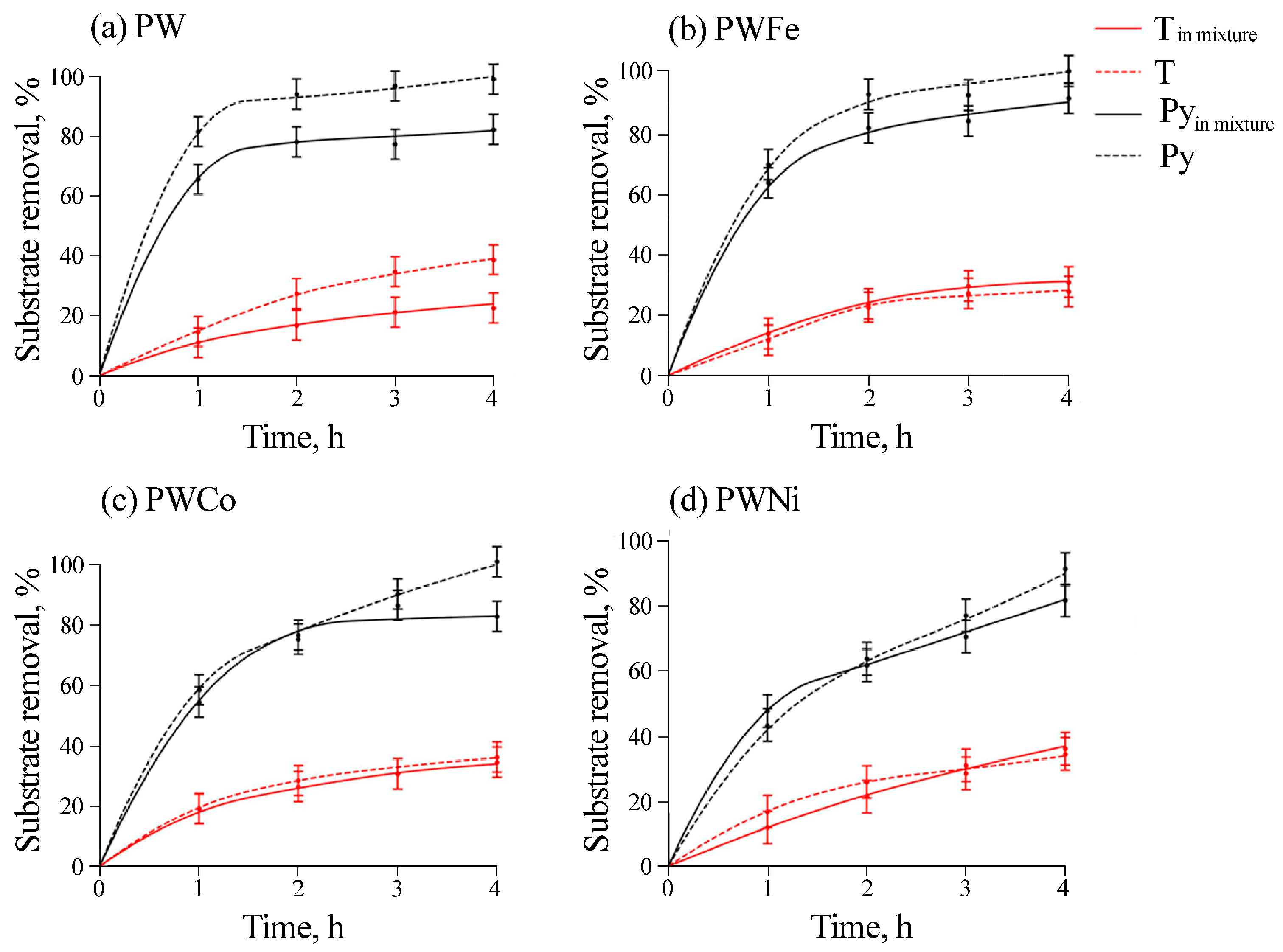
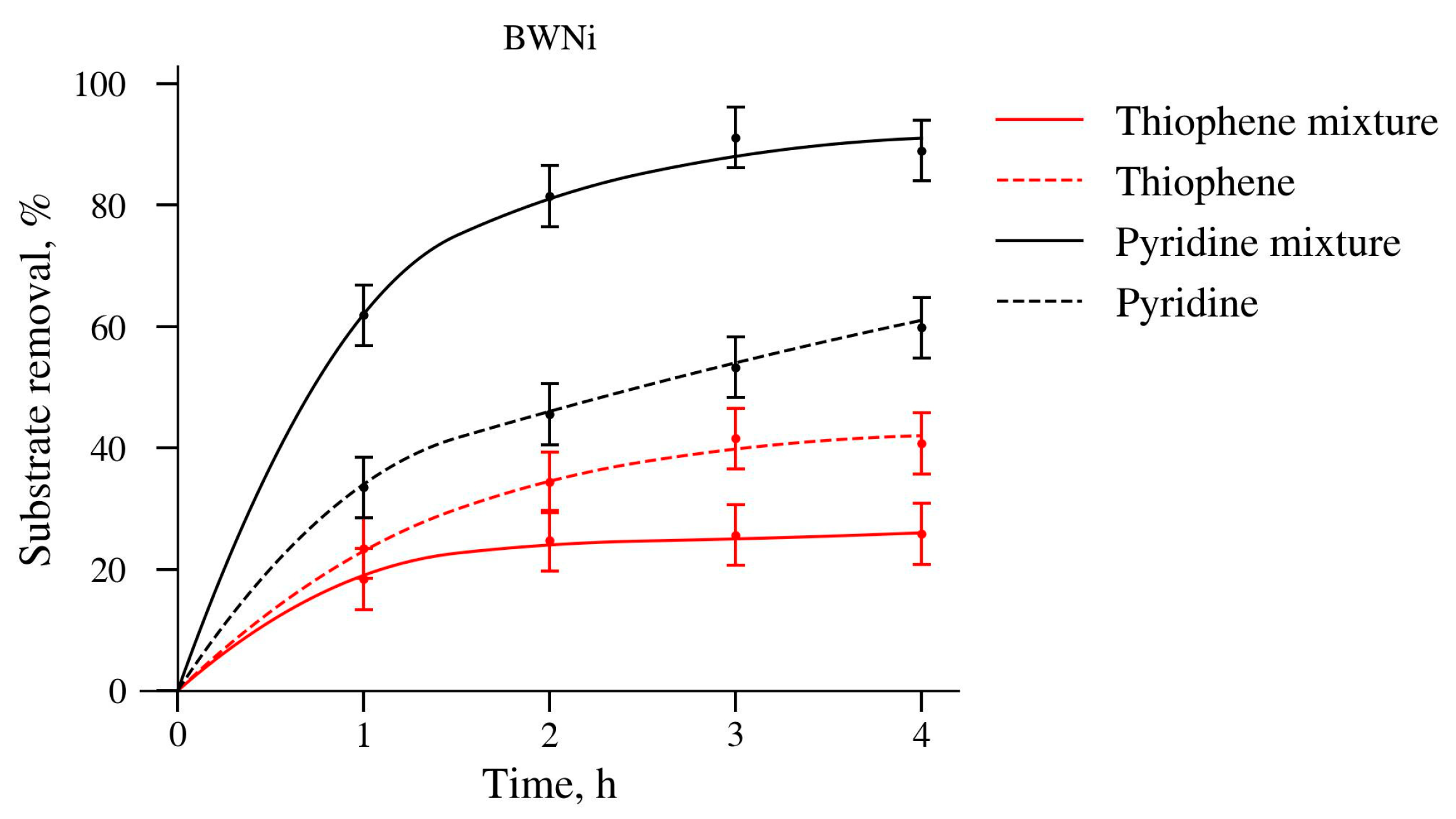
2.2. Catalytic Properties
3. Materials and Methods
3.1. Pre-Treatment of Titanium Samples and Preparation of Catalysts
3.2. Characterization of Catalyst Surfaces
3.3. Catalytic Testing and Reaction Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEO | plasma electrolytic oxidation |
| ODS | oxidative desulfurization |
| ODN | oxidative denitrogenation |
| EDX | energy-dispersive X-ray spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction analysis |
References
- Rudnev, V.S. Multiphase anodic layers and prospects of their application. Prot. Met. 2008, 44, 263–272. [Google Scholar] [CrossRef]
- Famiyeh, L.; Huang, X. Plasma electrolytic oxidation coatings on aluminum alloys: Microstructures, properties, and applications. Mod. Concept. Mater. Sci. 2019, 2, 000526. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications, and limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Dikici, B.; Kaseem, M. A comprehensive review of plasma electrolytic oxidation (PEO) of tantalum (Ta): Mechanisms, properties, and applications. Int. J. Refract. Met. Hard Mater. 2025, 128, 107059. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Hou, F.; Gorthy, R.; Mardon, I.; Tang, D.; Goode, C. Protecting light metal alloys using a sustainable plasma electrolytic oxidation process. ACS Omega 2022, 7, 8570–8580. [Google Scholar] [CrossRef] [PubMed]
- Pourshadloo, M.; Rezaei, H.A.; Saeidnia, M.; Alkokab, H.; Bathaei, M.S. Effect of g-family incorporation on corrosion behavior of PEO-treated titanium alloys: A review. Surf. Innov. 2023, 11, 5–14. [Google Scholar] [CrossRef]
- Ming, X.; Wu, Y.; Zhang, Z.; Li, Y. Micro-arc oxidation in titanium and its alloys: Development and potential of implants. Coatings 2023, 13, 2064. [Google Scholar] [CrossRef]
- Karbasi, M.; Nikoomanzari, E.; Hosseini, R.; Bahramian, H.; Chaharmahali, R.; Giannakis, S.; Kaseem, M.; Fattah-alhosseini, A. A review on plasma electrolytic oxidation coatings for organic pollutant degradation: How to prepare them and what to expect of them? J. Environ. Chem. Eng. 2023, 11, 110027. [Google Scholar] [CrossRef]
- Ogur, E.; Alves, A.C.; Toptan, F. Advancing titanium-based surfaces via micro-arc oxidation with solid substance incorporation: A systematic review. Mater. Today Commun. 2024, 41, 110343. [Google Scholar] [CrossRef]
- Samadi, P.; Witonska, I.A. Plasma electrolytic oxidation layers as alternative supports for metallic catalysts used in oxidation reaction for environmental application. Catal. Commun. 2023, 181, 106722. [Google Scholar] [CrossRef]
- Borisov, V.A.; Sigaeva, S.S.; Anoshkina, E.A.; Ivanov, A.L.; Litvinov, P.V.; Vedruchenko, V.R.; Temerev, V.L.; Arbuzov, A.B.; Kuznetsov, A.A.; Mukhin, V.A.; et al. Plasma electrolytic oxide coatings on silumin for oxidation CO. AIP Conf. Proc. 2017, 1876, 020001. [Google Scholar] [CrossRef]
- Karakurkchi, A.; Sakhnenko, M.; Ved, M.; Gorokhyvskiy, A. Nanostructured PEO coatings on silumin as environmental catalysts. Mater. Today Proc. 2022, 50, 502–506. [Google Scholar] [CrossRef]
- Quintero, D.; Galvis, O.; Calderón, J.A.; Castaño, J.G.; Echeverría, F. Effect of electrochemical parameters on the formation of anodic films on commercially pure titanium by plasma electrolytic oxidation. Surf. Coat. Technol. 2014, 258, 1223–1231. [Google Scholar] [CrossRef]
- Adelinia, A.; Yerokhin, A.; Matthews, D.T.A.; de Rooij, M.B.; Zanjani, J.S.M. Pore formation and pore inter-connectivity in plasma electrolytic oxidation coatings on aluminium alloy. Surf. Coat. Technol. 2025, 496, 131597. [Google Scholar] [CrossRef]
- Sharifi, H.; Aliofkhazraei, M.; Darband, G.B.; Shrestha, S. A review on adhesion strength of PEO coatings by scratch test method. Surf. Rev. Lett. 2018, 25, 1830004. [Google Scholar] [CrossRef]
- Patcas, F.; Krysmann, W. Efficient catalysts with controlled porous structure obtained by anodic oxidation under spark-discharge. Appl. Catal. A Gen. 2007, 316, 240–249. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, G.; Lv, Y.; Wu, Y.; Dong, Z. Growth mechanism of titania on titanium substrate during the early stage of plasma electrolytic oxidation. Surf. Coat. Technol. 2020, 400, 126202. [Google Scholar] [CrossRef]
- Xu, J.; Holthaus, P.; Yang, N.; Jiang, S.; Heupel, A.; Schönherr, H.; Yang, B.; Krumm, W.; Jiang, X. Catalytic tar removal using TiO2/NiWO4-Ni5TiO7 films. Appl. Catal. B Environ. 2019, 249, 155–162. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Chen, C.; Xia, Q.; Yao, Z. Enhanced Fenton-like degradation of phenol by sulfur modified α-Fe2O3/Fe3O4/R-TiO2 composite coating on Ti alloy prepared by plasma electrolytic oxidation. Mater. Res. Express 2019, 6, 095532. [Google Scholar] [CrossRef]
- Vasilyeva, M.S.; Lukiyanchuk, I.V.; Sergeev, A.A.; Sergeeva, K.A.; Ustinov, A.Y.; Tkachev, V.V.; Arefieva, O.D. Plasma electrolytic synthesis and characterization of oxide coatings with MWO4 (M =Co, Ni, Cu) as photo-Fenton heterogeneous catalysts. Surf. Coat. Technol. 2021, 424, 127640. [Google Scholar] [CrossRef]
- Budnikova, Y.B.; Vasilyeva, M.S.; Lukiyanchuk, I.V.; Egorkin, V.S.; Tkachev, V.V.; Korochentsev, V.V.; Shlyk, D.H.; Arefieva, O.D.; Marchenko, A.V.; Myagchilov, A.V. Plasma electrolytic preparation of film CoWO4/WO3 p-n heterostructures and their photocatalytic and electrochemical properties. J. Photochem. Photobiol. A Chem. 2025, 467, 116414. [Google Scholar] [CrossRef]
- Lukiyanchuk, I.V.; Tarkhanova, I.G.; Vasilyeva, M.S.; Yarovaya, T.P.; Ustinov, A.Y.; Vyaliy, I.E.; Kuryavyi, V.G. Plasma electrolytic formation of TiO2-VOx-MoOy-P2O5 coatings on titanium and their application as catalysts for the oxidation of S- and N-containing substances. Mater. Chem. Phys. 2024, 311, 128520. [Google Scholar] [CrossRef]
- Said, S.; Mikhail, S.; Riad, M. Recent trends for clean fuel using environmental protecting oxidative desulfurization process. Clean. Chem. Eng. 2025, 11, 100140. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Ishaq, M.; Yang, W.; Hussain, M. Recent breakthroughs in deep aerobic oxidative desulfurization of petroleum refinery products. J. Clean. Prod. 2021, 294, 125731. [Google Scholar] [CrossRef]
- Haruna, A.; Merican, Z.M.A.; Musa, S.G. Recent advances in catalytic oxidative desulfurization of fuel oil—A review. J. Ind. Eng. Chem. 2022, 112, 20–36. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Mehrvarz, E.; Taghipour, A. Polyoxometalate as an effective catalyst for the oxidative desulfurization of liquid fuels: A critical review. Rev. Chem. Eng. 2020, 36, 831–858. [Google Scholar] [CrossRef]
- Saladino, R.; Botta, G.; Crucianelli, M. Advances in Nanostrostructured Transition Metal Catalysis Applied to Oxidative Desulfurization. In Applying Nanotechnology to the Desulfurization Process in Petroleum Engineering; Tawfik, A.S., Ed.; IGI Global: Hershey, PA, USA, 2016; pp. 180–215. [Google Scholar]
- Mohammed, A.E.; Mohammed, W.T.; Gheni, S.A. Scale-up of oxidative desulfurization for sour diesel fuel: Modeling, simulation, and reactor design using Fe/AC catalyst. Case Stud. Chem. Environ. Eng. 2025, 11, 101024. [Google Scholar] [CrossRef]
- Saeed, M.; Munir, M.; Intisar, A.; Waseem, A. Facile synthesis of a novel Ni-WO3@g-C3N4 nanocomposite for efficient oxidative desulfurization of both model and real fuel. ACS Omega 2022, 7, 15809–15820. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Sprague, M.; Song, C.S. Deep desulfurization of gasoline by selective adsorption over nickel-based adsorbent for fuel cell applications. Ind. Eng. Chem. Res. 2005, 44, 5768–5775. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Hemmatzadeh, A.; Oroumi, G.; Miri, O.F. Enhanced catalytic activity of PMo11V/NiO/PAN nanosphere composite for real fuel oxidation desulfurization: Synthesis and characterization. Mater. Chem. Phys. 2024, 311, 128504. [Google Scholar] [CrossRef]
- Rezvani, M.; Bakhtiyari, A.; Rahmani, P. Synthesis and characterization of new nanocomposite PW11Mn@CuO@PAN as an efficient nanocatalyst for deep oxidative desulfurization of real/model fuel. Mater. Sci. Eng. B 2024, 303, 117314. [Google Scholar] [CrossRef]
- Rudnev, V.S.; Lukiyanchuk, I.V.; Vasilyeva, M.S.; Morozova, V.P.; Zelikman, V.M.; Tarkhanova, I.G. W-containing oxide layers obtained on aluminum and titanium by PEO as catalysts in thiophene oxidation. Appl. Surf. Sci. 2017, 422, 1007–1014. [Google Scholar] [CrossRef]
- Faria, R.G.; Silva, D.; Mirante, F.; Gago, S.; Cunha-Silva, L.; Balula, S.S. Advanced technologies conciliating desulfurization and denitrogenation to prepare clean fuels. Catalysts 2024, 14, 137. [Google Scholar] [CrossRef]
- Tochtermann, J.; Tietze, F.; Huber, M.; Korth, W.; Albert, J.; Jess, A. Extraction-coupled oxidative desulfurization (ECODS) and denitrogenation (ECODN) of real fuels under mild conditions using a polyoxometalate catalyst and molecular oxygen. Energy Fuels 2025, 39, 781–797. [Google Scholar] [CrossRef]
- Silva, D.F.; Viana, A.M.; Mirante, F.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Removing simultaneously sulfur and nitrogen from fuel under a sustainable oxidative catalytic system. Sustain. Chem. 2021, 2, 382–391. [Google Scholar] [CrossRef]
- Palomeque-Santiago, J.F.; López-Medina, R.; Oviedo-Roa, R.; Navarrete-Bolaños, J.; Mora-Vallejo, R.; Montoya-de la Fuente, J.A.; Martínez-Magadán, J.M. Deep oxidative desulfurization with simultaneous oxidative denitrogenation of diesel fuel and straight run gas oil. Appl. Catal. B Environ. 2018, 236, 326–337. [Google Scholar] [CrossRef]
- Prado, G.H.C.; Rao, Y.; de Klerk, A. Nitrogen removal from oil: A review. Energy Fuels 2017, 31, 14–36. [Google Scholar] [CrossRef]
- Kumari, S.; Sengupta, S. Non-hydrogen processes for simultaneous desulfurization and denitrogenation of light petroleum fuels—An elaborative review. Environ. Sci. Pollut. Res. 2021, 28, 61873–61907. [Google Scholar] [CrossRef]
- Lokeshkumar, E.; Premchand, C.; Manojkumar, P.; Shishir, R.; Krishna, L.R.; Prashanth, K.G.; Rameshbabu, N. Effect of electrolyte composition on the surface characteristics of plasma electrolytic oxidation coatings over Ti–40Nb alloy. Surf. Coat. Technol. 2023, 465, 129591. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Liu, H.; Lei, J.; Zhang, J.; Zhou, H.; Qi, M. Formation and in vitro/in vivo performance of “cortex-like” micro/nano-structured TiO2 coatings on titanium by micro-arc oxidation. Mater. Sci. Eng. C 2018, 87, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, X.; Yu, F.; Li, Y.; Xue, X.; Qi, M.; Li, Y. Formation and cytocompatibility of a hierarchical porous coating on Ti-20Zr-10Nb-4Ta alloy by micro-arc oxidation. Surf. Coat. Technol. 2020, 404, 126471. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 105203. [Google Scholar] [CrossRef]
- Mortazavi, G.; Jiang, J.; Meletis, E.I. Investigation of the plasma electrolytic oxidation mechanism of titanium. Appl. Surf. Sci. 2019, 488, 370–382. [Google Scholar] [CrossRef]
- Pershina, S.V. Conductivity studies of glasses in the system WO3–P2O5. Russ. J. Appl. Chem. 2019, 92, 482–489. [Google Scholar] [CrossRef]
- Moore, L.; Dutta, I.; Wheaton, B.; Stapleton, E.; Parysek, R.; Aitken, B. A new identification of the conducting phase in tungsten-titanium-phosphate glass-ceramics. J. Am. Ceram. Soc. 2020, 103, 3552–3561. [Google Scholar] [CrossRef]
- Vovna, V.I.; Gnedenkov, S.V.; Gordienko, P.S.; Kuznetsov, M.V.; Sinebryukhov, S.L.; Cherednichenko, A.I.; Khrisanfova, O.A. Surface layers produced on titanium by the microarc oxidation: An X-ray diffractometry study. Russ. J. Electrochem. 1998, 34, 1090–1093. [Google Scholar]
- Tu, W.; Cheng, Y.; Wang, X.; Zhan, T.; Han, J.; Cheng, Y. Plasma electrolytic oxidation of AZ31 magnesium alloy in aluminate-tungstate electrolytes and the coating formation mechanism. J. Alloys Compd. 2017, 725, 199–216. [Google Scholar] [CrossRef]
- Zhu, Z.; Tu, W.; Cheng, Y.; Cheng, Y. The formation of metallic W and amorphous phase in the plasma electrolytic oxidation coatings on an Al alloy from tungstate-containing electrolyte. Surf. Coat. Technol. 2019, 361, 176–187. [Google Scholar] [CrossRef]
- Snizhko, L.O. The nature of anodic gas at plasma electrolytic oxidation. Prot. Met. Phys. Chem. Surf. 2014, 50, 705–708. [Google Scholar] [CrossRef]
- Motekaitis, R.J.; Cox III, X.B.; Taylor, P.; Martell, A.E.; Miles, B.; Tvedt, T.J., Jr. Thermal degradation of EDTA chelates in aqueous solution. Can. J. Chem. 1982, 60, 1207–1213. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Akopyan, A.; Eseva, E.; Polikarpova, P.; Kedalo, A.; Vutolkina, A.; Glotov, A. Deep oxidative desulfurization of fuels in the presence of brönsted acidic polyoxometalate-based ionic liquids. Molecules 2020, 25, 536. [Google Scholar] [CrossRef]
- Polikarpova, P.; Akopyan, A.; Shigapova, A.; Glotov, A.; Anisimov, A.; Karakhanov, E. Oxidative desulfurization of fuels using heterogeneous catalysts based on MCM-41. Energy Fuels 2018, 32, 10898–10903. [Google Scholar] [CrossRef]
- Gao, H.; Wu, X.; Sun, D.; Niu, G.; Guan, J.; Meng, X.; Liu, C.; Xia, W.; Song, X. Preparation of core–shell PW12@TiO2 microspheres and oxidative desulfurization performance. Dalton Trans. 2019, 48, 5749–5755. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Jiaheng, L.; Lina, Z.; Ran, G.Z.; Xiaodi, D.; Junsheng, L. Highly efficient deep desulfurization of fuels by meso/macroporous H3PW12O40/TiO2 at room temperature. Mater. Res. Bull. 2018, 105, 210–219. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Jiang, Z.X.; Li, J.; Zhang, Y.N.; Lin, F.; Liu, Y.; Li, C. Catalytic oxidation of thiophene and its derivatives via dual activation for ultra-deep desulfurization of fuels. J. Catal. 2012, 287, 5–12. [Google Scholar] [CrossRef]
- Lukiyanchuk, I.V.; Vasilyeva, M.S.; Ustinov, A.Y.; Bryzhin, A.A.; Tarkhanova, I.G. Ti/TiO2/NiWO4 +WO3 composites for oxidative desulfurization and denitrogenation. Surf. Coat. Technol. 2022, 434, 128200. [Google Scholar] [CrossRef]
- Saha, B.; Sengupta, S. Influence of different hydrocarbon components in fuel on the oxidative desulfurisation of thiophene: Deactivation of catalyst. Fuel 2015, 150, 679–686. [Google Scholar] [CrossRef]

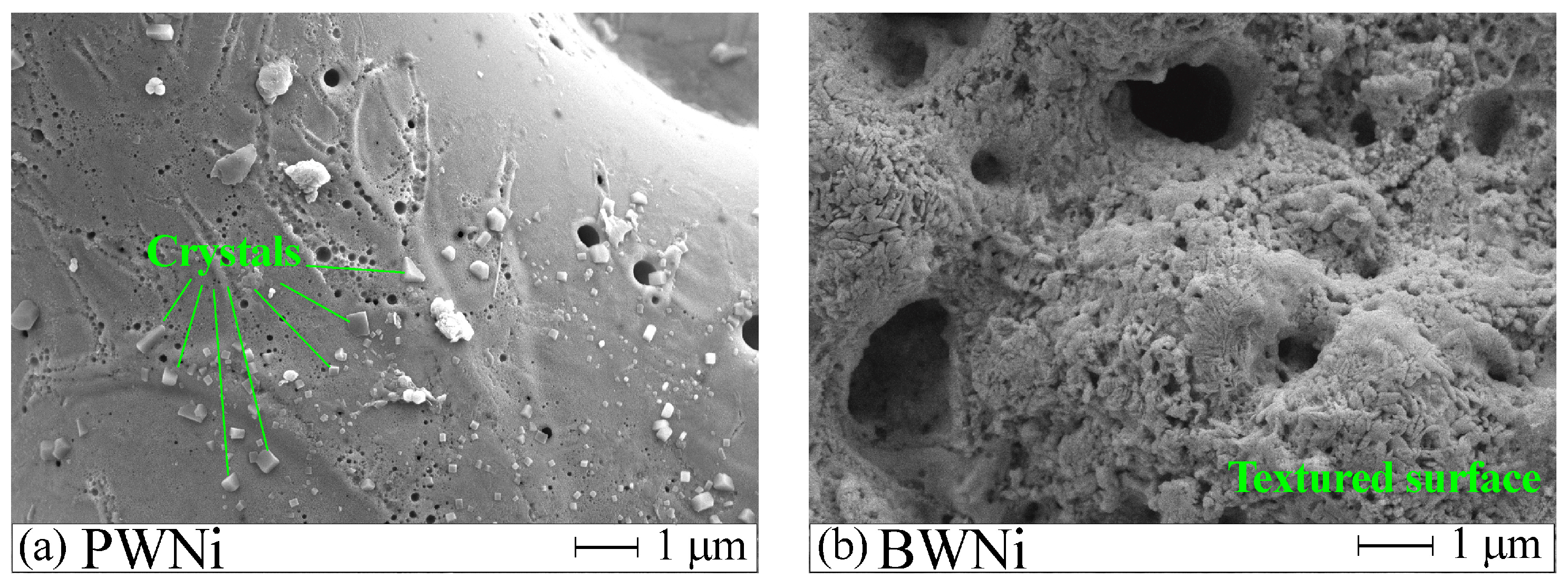
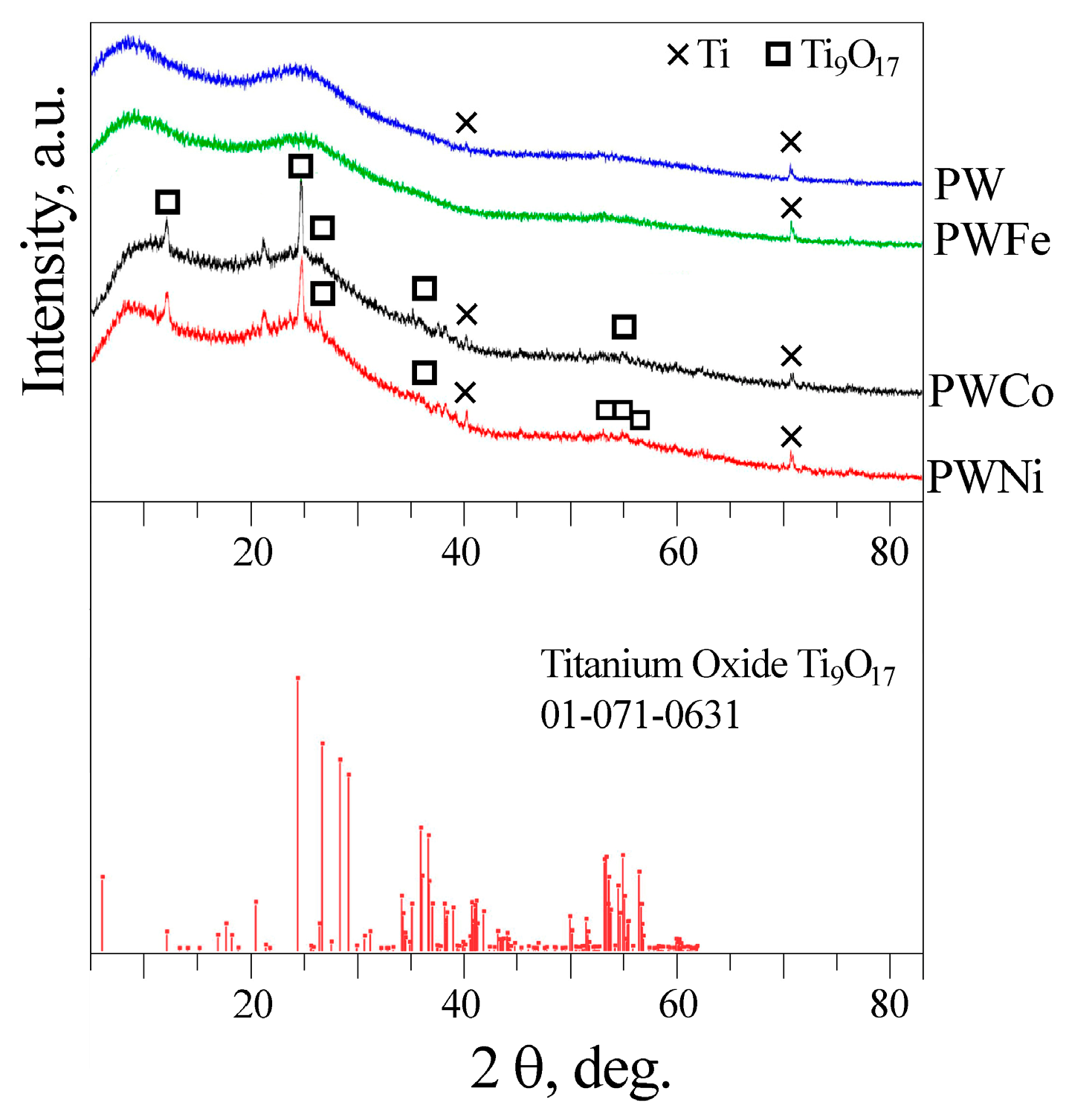
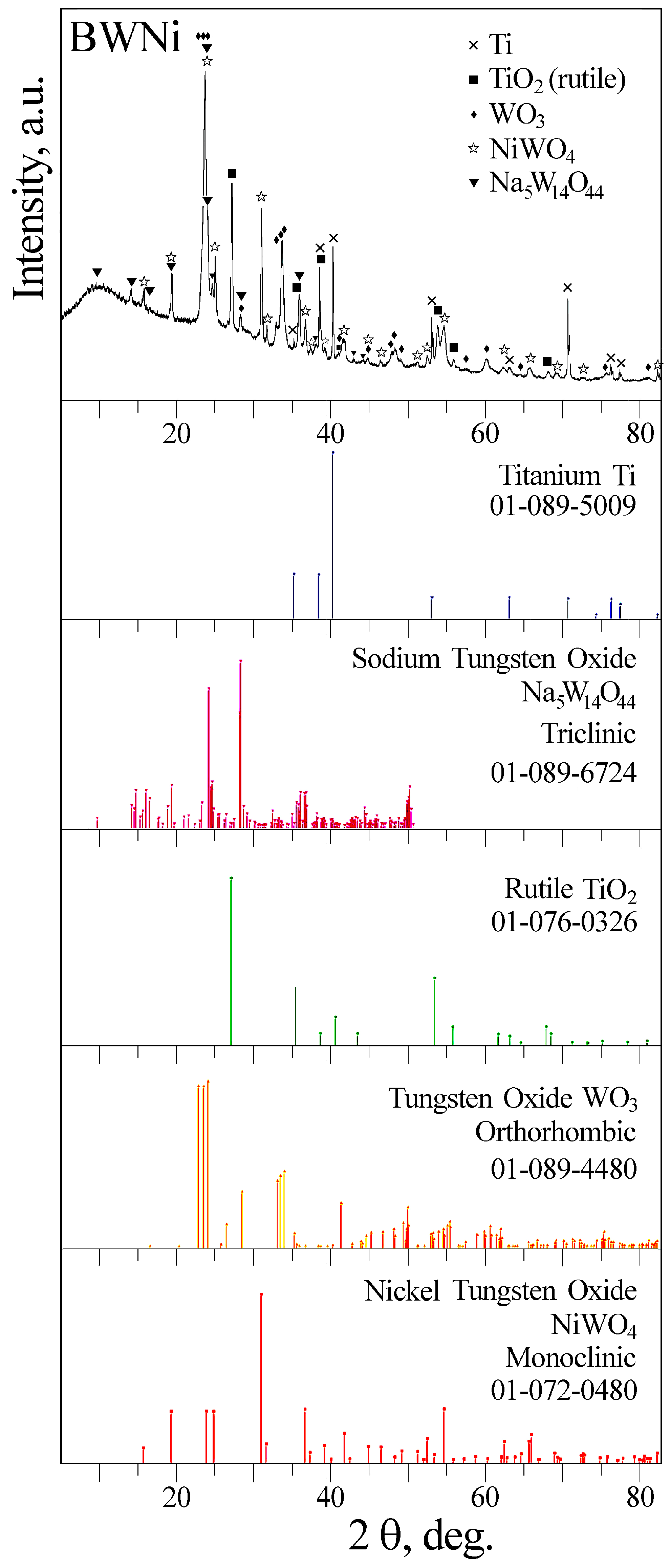
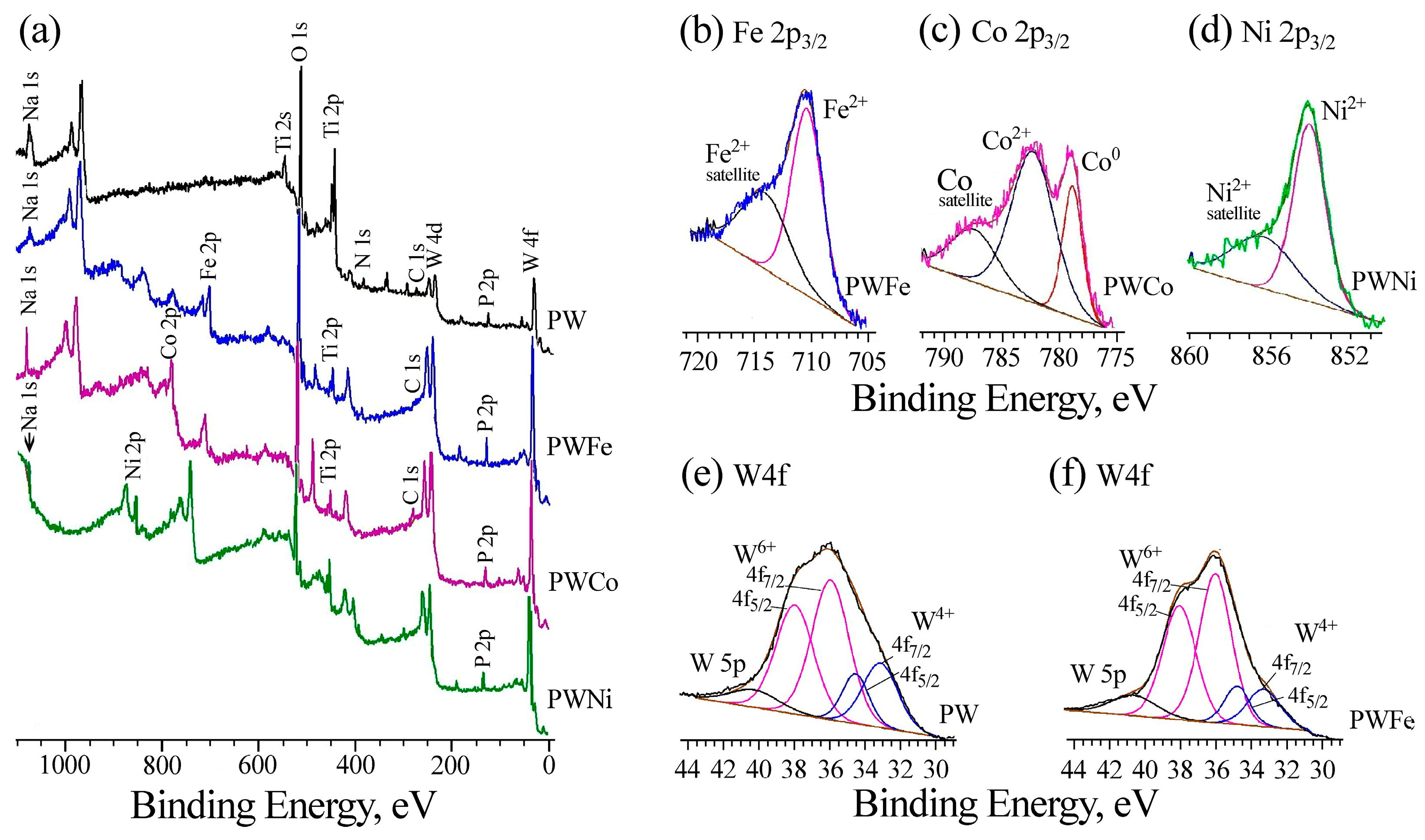
| Label | Base Solution | Additives | i [A/cm2] | t [min] | Umax [V] | Uf [V] | h [μm] |
|---|---|---|---|---|---|---|---|
| PW | 0.05 M Na3PO4 + 0.1 M Na2WO4 | No | 0.1 | 10 | 106 ± 2 | 94 ± 2 | 29 ± 3 |
| PWFe | 0.05 M Na3PO4 + 0.1 M Na2WO4 | 0.05 M Na2H2EDTA + 0.05 M Fe(NH4)2(SO4)2 | 0.05 | 10 | 98 ± 1 | 92 ± 2 | 36 ± 3 |
| PWCo | 0.05 M Na3PO4 + 0.1 M Na2WO4 | 0.05 M Na2H2EDTA + 0.05 M Co(CH3COO)2 | 0.1 | 10 | 145 ± 3 | 141 ± 5 | 35 ± 4 |
| PWNi | 0.05 M Na3PO4 + 0.1 M Na2WO4 | 0.05 M Na2H2EDTA + 0.05 M Ni(CH3COO)2 | 0.1 | 10 | 150 ± 2 | 144 ± 3 | 32 ±3 |
| BWNi | 0.05 M Na2B4O7 + 0.1 M Na2WO4 | 0.05 M Na2H2EDTA + 0.05 M Ni(CH3COO)2 | 0.1 | 10 | 90 ± 2 | 89 ± 2 | 17 ± 3 |
| Catalyst | Layer | Elemental Composition [at. %] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Na | P | B | Ti | W | M | ||
| PW | Outer | 8.2 | 69.2 | 2.2 | 5.3 | - | 9.7 | 5.4 | - |
| Inner | 3.4 | 68.2 | 0.1 | 1.5 | - | 25.1 | 1.7 | - | |
| PWFe | Outer | 7.9 | 68.8 | 1.9 | 6.0 | - | 6.1 | 5.5 | 3.8 Fe |
| Inner | 4.3 | 67.8 | 0.2 | 2.3 | - | 22.1 | 2.5 | 0.8 Fe | |
| PWCo | Outer | 7.6 | 66.2 | 3.2 | 4.8 | - | 5.6 | 8.1 | 4.5 Co |
| Inner | 3.1 | 68.9 | 0.2 | 2.1 | - | 20.2 | 4.7 | 0.8 Co | |
| PWNi | Outer | 7.4 | 66.4 | 3.3 | 4.9 | - | 5.4 | 8.5 | 4.1 Ni |
| Inner | 3.1 | 68.7 | 0.7 | 2.4 | - | 19.2 | 5.0 | 0.9 Ni | |
| BWNi | Outer | 12.3 | 59.4 | 0.5 | - | 8.1 | 1.9 | 10.6 | 7.3 Ni |
| Inner | 3.8 | 66.3 | 0.3 | - | 0.0 | 26.2 | 3.1 | 0.3 Ni | |
| Line | PW | PWFe | PWCo | PWNi | BWNi | Group | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Eb | C | Eb | C | Eb | C | Eb | C | Eb | ||
| Na1s | 3.6 | 1071.8 | 0.7 | 1072.0 | 4.6 | 1072.3 | 1.2 | 1072.0 | 0.6 | 1072.0 | Na+ |
| Fe 2p3/2 | 6.6 | 710.4 | Fe2+ | ||||||||
| Co 2p3/2 | 7.6 | 782.0 | Co2+ | ||||||||
| 1.9 | 778.5 | Co0 | |||||||||
| Ni 2p3/2 | 3.2 | 854.2 | 6.6 | 856.5 | Ni2+ | ||||||
| 2.0 | 853.2 | Ni0 | |||||||||
| O 1s | 14.6 | 532.1 | 9.6 | 532.7 | 15.2 | 532.6 | 9.7 | 532.0 | 12.7 | 531.9 | POx, COx, BOx |
| 46.3 | 530.6 | 48.0 | 531.4 | 37.2 | 531.5 | 50.7 | 530.8 | 36.3 | 530.9 | MOx | |
| Ti 2p3/2 | 13.2 | 459.0 | 3.6 | 459.5 | 3.4 | 459.2 | 4.2 | 458.9 | 1.7 | 459.2 | Ti4+ |
| 2.1 | 457.8 | 1.2 | 458.1 | 0.5 | 457.9 | 1.1 | 457.7 | 0.3 | 458.1 | Ti−N | |
| N 1s | 0.9 | 399.8 | 1.2 | 400.2 | 1.0 | 399.7 | 0.7 | 399.5 | 2.9 | 400.0 | N−Ti |
| 2.3 | 397.2 | 3.0 | 397.7 | 1.2 | 397.4 | 3.0 | 397.4 | 0.9 | 397.0 | N−N, N−H | |
| C 1s | 1.3 | 288.5 | 0.8 | 287.1 | 1.7 | 287.0 | 3.6 | 289.0 | O=C(H)−O, COC | ||
| 4.7 | 285.0 | 1.1 | 285.0 | 5.1 | 284.9 | 2.6 | 285.0 | 15.0 | 285.0 | CC, CH | |
| 1.2 | 281.5 | 0.6 | 283.6 | 1.0 | 283.6 | 0.8 | 283.4 | CW, CTi | |||
| P 2p | 5.9 | 133.6 | 10.53 | 134.0 | 6.7 | 133.5 | 6.7 | 134.8 | P5+ (P2O5) | ||
| B 1s | 3.5 | 191.0 | B3+ (B2O3) | ||||||||
| W 4f7/2 | 2.7 | 36.1 | 10.6 | 36.1 | 9.0 | 35.7 | 13.3 | 36.1 | 11.0 | 35.4 | W6+ |
| 1.2 | 33.1 | 2.7 | 33.4 | 3.9 | 33.3 | 3.5 | 33.4 | 2.0 | 34.0 | W4+ | |
| Total | 100 | 100.2 | 100.0 | 99.9 | 99.9 | ||||||
| Catalysts | Thiophene | DBT | Pyridine |
|---|---|---|---|
| PW | 6 | 5 | 4 |
| PWFe | 1.5 | 2 | 4 |
| PWCo | 11 | 13 | 5 |
| PWNi | 16 | 14 | 14 |
| BWNi | 2 | 4 | 2 |
| Catalysts | Thiophene | DBT | Pyridine |
|---|---|---|---|
| PW | 0.045 | 0.073 | 0.34 |
| PWFe | 0.036 | 0.18 | 0.36 |
| BWNi | 0.048 | 0.2 | 0.13 |
| Line | After Interaction with Thiophene | After Interaction with Thiophene + Pyridine Mixture | Group | ||
|---|---|---|---|---|---|
| C | Eb | C | Eb | ||
| O 1s | 4.4 | 532.8 | 4.0 | 533.0 | C-O |
| 15.8 | 531.7 | 20.9 | 531.6 | POx, COx, BOx | |
| 29.4 | 530.5 | 23.2 | 530.4 | O2− (M-O) | |
| Ti 2p3/2 | 4.4 | 458.8 | 6.7 | 458.8 | Ti4+ |
| N 1s | 0.3 | 402.8 | 1.8 | 402.8 | O-NR3 |
| 3.0 | 401.6 | 1.5 | 401.5 | NR4+ | |
| 0.9 | 399.7 | 1.2 | 399.8 | N-Ti | |
| 2.3 | 288.3 | 3.9 | 288.5 | O-C=O | |
| C 1s | 7.9 | 286.0 | 9.9 | 286.2 | C-O, C-N |
| 23.9 | 284.4 | 21.6 | 284.7 | C-C | |
| S 2p3/2 | 0.8 | 167.7 | 0.2 | 167.7 | SO42− |
| P 2p | 2.5 | 133.3 | 2.7 | 133.3 | P5+ |
| W 4f7/2 | 4.4 | 35.5 | 2.4 | 35.5 | W6+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarkhanova, I.G.; Zelikman, V.M.; Lukiyanchuk, I.V.; Vasilyeva, M.S.; Tkachev, V.V.; Korochentsev, V.V.; Shlyk, D.H. Ti-Supported Oxide Coatings Based on MWO4 (M = Fe, Co, Ni): Plasma Electrolytic Synthesis, Characterization and Catalytic Properties in S, N-Heterocycles Peroxide Oxidation. Molecules 2025, 30, 1998. https://doi.org/10.3390/molecules30091998
Tarkhanova IG, Zelikman VM, Lukiyanchuk IV, Vasilyeva MS, Tkachev VV, Korochentsev VV, Shlyk DH. Ti-Supported Oxide Coatings Based on MWO4 (M = Fe, Co, Ni): Plasma Electrolytic Synthesis, Characterization and Catalytic Properties in S, N-Heterocycles Peroxide Oxidation. Molecules. 2025; 30(9):1998. https://doi.org/10.3390/molecules30091998
Chicago/Turabian StyleTarkhanova, Irina G., Vladimir M. Zelikman, Irina V. Lukiyanchuk, Marina S. Vasilyeva, Vladimir V. Tkachev, Vladimir V. Korochentsev, and Daria H. Shlyk. 2025. "Ti-Supported Oxide Coatings Based on MWO4 (M = Fe, Co, Ni): Plasma Electrolytic Synthesis, Characterization and Catalytic Properties in S, N-Heterocycles Peroxide Oxidation" Molecules 30, no. 9: 1998. https://doi.org/10.3390/molecules30091998
APA StyleTarkhanova, I. G., Zelikman, V. M., Lukiyanchuk, I. V., Vasilyeva, M. S., Tkachev, V. V., Korochentsev, V. V., & Shlyk, D. H. (2025). Ti-Supported Oxide Coatings Based on MWO4 (M = Fe, Co, Ni): Plasma Electrolytic Synthesis, Characterization and Catalytic Properties in S, N-Heterocycles Peroxide Oxidation. Molecules, 30(9), 1998. https://doi.org/10.3390/molecules30091998







