Abstract
In previous quests to synthesize fragments of tumor-associated carbohydrate antigens (TACAs), we determined that bromoalkyl β glycosides of N-acetylglucosamine were labile and incompatible with some of the synthetic conditions required for the preparation of oligosaccharides. While N-acetylglucosamine chloroalkyl β glycosides are common intermediates for oligosaccharide synthesis, they exhibit poor yields upon subsequent reactions used to introduce the oxyamine required for further conjugation. Thus, we looked to synthesize these TACAs using chloroalkyl β glycosides and substitute the chlorine for bromine at a later synthetic stage. Upon substitution of the bromine for chlorine using sodium bromide in a dibromomethane (DBM) dimethylformamide (DMF) mixture, we observed the unexpected anomerization of the N-acetylglucosamine β glycosides, yielding up to 90% of the α glycosides. We describe our studies of this unexpected anomerization and report on how the anomeric ratios can be controlled experimentally. Interestingly, we also report the anomerization of alkyl β glycosides of N-acetylglucosamine in a mixture of DBM and DMF without sodium bromide. Further studies are being conducted to determine the mechanism of this anomerization and the scope of this reaction.
1. Introduction
Glucosamines are a class of monosaccharides that are a major building block of many biological molecules [1]. Specifically, N-acetyl-d-glucosamine (GlcNAc) is commonly found at the reducing end of oligosaccharides, especially in cell-surface antigens such as tumor-associated carbohydrate antigens (TACAs) [2]. It has been well established by the Crich group and others that the N-acetyl group in GlcNAc has a major impact on the reactivity and function of the saccharide in comparison to its glucose analog [3,4,5,6,7,8,9]. It is important to consider these effects during oligosaccharide synthesis. We are interested in conjugating different fragments of TACAs to carrier molecules through these terminal β GlcNAc residues to allow for immunochemical testing. In particular, we intended to conjugate TACAs (Lewis B and Lewis A) to the immunostimulant PS A1 zwitterionic polysaccharide using an oxime linkage as described by Andreana and co-workers [10,11,12,13]. Our strategy involved the synthesis of the TACAs bearing a 9-bromononyl glycoside at the reducing-end GlcNAc residue with the intent to displace the bromide with N-hydroxyphthalimide in mild conditions [14], which would, after deprotection, yield the aminooxy functional group needed for conjugation to PS A1. However, we observed that 9-bromononyl glycosides were easily reduced to their nonyl derivatives in the reaction conditions required in our multistep synthetic scheme to obtain the desired TACAs [15]. Thus, our synthetic strategy was revised, and we converted the 9-bromononyl GlcNAc glycoside to the 9-chlorononyl glycoside analog, which we knew would be stable throughout our synthetic scheme [15]. In turn, we investigated the displacement of the chlorine atom by bromide to further allow for its displacement by N-hydroxyphthalimide in mild conditions. Thus, we report below our attempts to displace the chloride of chloroalkyl β GcNAc glycosides using sodium bromide in a mixture of dimethylformamide (DMF) and dibromomethane (DBM) and our observation that these conditions promoted anomerization of the β glycosides to the α anomers.
2. Results
2.1. Substitution and Anomerization of Chloroalkyl β Glycosides
Our investigation into the phenomena began with studying the substitution of bromide for chloride in chloroalkyl glycosides with six- and eight-membered chains (Scheme 1). We first prepared (Supplementary Material) the known [16] 6-chlorohexyl β glycoside 1 and the 8-chlorohexyl β glycoside 2 from known [17] Horton’s chloride. The nucleophilic displacement of the chlorine atoms in 1 and 2 was then attempted using the conditions established by Babler and Spina [18]. In these conditions, the chloride displacement with NaBr is carried out in a 1:2 (v/v) ratio of DBM-DMF, in which the DBM facilitates the displacement by acting as a trap for chloride ions [18]. Thus, β glycosides 1 and 2 at a 0.4 M concentration were reacted in sealed flasks with NaBr (10 eq) in a 1:2 mixture of DBM and DMF at 100 °C for 24 h (Scheme 1).
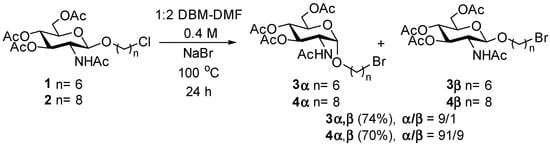
Scheme 1.
Displacement of chloride for bromide in chloroalkyl β glycosides 1 and 2.
Under these conditions, the displacement of the chloride by bromide was complete for both compounds 1 and 2, giving, after work-up, the bromoalkyl glycosides 3 and 4 in 74 and 70% yields, respectively. This was confirmed by the shift of the methylene triplets from ~3.5 ppm in the 1H NMR spectra correlating to ~45 ppm in the 13C NMR spectra for the CH2Cl in compounds 1 and 2 to ~3.4 ppm in the 1H NMR spectra correlating at ~34 ppm in the 13C NMR spectra for the CH2Br in compounds 3 and 4. However, careful examination of the GlcNAc ring signals revealed the presence of two glycosides in both compounds 3 and 4, which were faintly separated on TLC. In both compounds, we found that the α anomers 3α and 4α were the major products (α/β ≈ 9:1), characterized by anomeric signals appearing as doublets at ~4.8 ppm with the characteristic coupling constants 3JH-1,H-2 = 3.7 Hz in the 1H NMR spectra and that correlated to anomeric signals at 97.1 ppm in the 13C NMR spectra. In contrast, the minor β anomers 3β and 4β were characterized by anomeric signals appearing as doublets at ~4.6 ppm in the 1H NMR spectra with the characteristic coupling constants 3JH-1,H-2 = 8.3 Hz and that correlated to anomeric signals at 100.7 ppm in the 13C NMR spectra. After careful chromatography, analytical samples were obtained for all compounds, and their NMR spectra are given in the Supplementary Material.
This unexpected anomerization became the focus of our study. We examined the effect of the concentration and presence of sodium bromide in the reaction mixture at 100 °C and the effect of temperature on the substitution and anomerization of compound 1. All reactions were allowed to proceed for 24 h in sealed flasks, and the results are listed in Table 1.

Table 1.
Effect of the temperature, concentration, and presence of NaBr (10 equiv) on the substitution and anomerization of the 6-chlorohexyl β glycoside 1 (ratios assessed by 1H NMR).
The degree of substitution of chloride by bromide and the anomeric ratios were estimated from the 1H NMR spectra of the crude products through the relative integrations of the methylene signals and the anomeric protons, respectively. As can be seen in Table 1, diluting the reaction from 0.4 M to 0.04 M had no impact on the substitution or anomerization reactions; we observed full displacement of the chloride and a 9/1 ratio of α/β anomers in both reactions (Table 1, entries 1 and 2). Interestingly, omitting sodium bromide from the reaction mixture had no impact on the degree of substitution and only a negligible effect on the degree of anomerization. Indeed, at 100 °C, the chlorine atom was again fully displaced, and the α/β ratio was 85/15 (Table 1, entry 3). Lowering the temperature only had a moderate impact on the degree of substitution of chloride by bromide; it was complete at 100 and 90 °C (entries 1 and 2) and only dropped down to a ratio 82/18 in favor of the bromoalkyl glycoside at 70 °C (entry 6). In contrast, lowering the temperature had a major impact on the degree of anomerization. At 90 °C, the anomerization occurred only for half of the β glycoside, leading to an α/β ratio of 1/1 (entry 4). At 80 °C, the glycoside remained mainly β, with a 15/85 α/β ratio, while at 70 °C, very little β glycoside anomerized to the α configuration, leading to an α/β ratio of 6/94. It is important to point out that given the remote position of the halogen from the ring signals, NMR does not allow for assigning chlorine or bromine substitution to a specific anomer; thus, we were unable at this point to conclude whether any of the chloroalkyl or bromoalkyl glycosides were anomerizing preferentially.
To observe the kinetics of product formation during the reaction, a time trial was conducted by reacting the 6-chlorohexyl β glycoside 1 (0.04 M) in a sealed flask at 100 °C in a 1:2 DBM-DMF mixture with NaBr (10 equiv). Samples were taken every 30 min for the first 2 h and then hourly until reaching 6 h of reaction and then at 12 h, and finally, the reaction was stopped after 24 h. These samples were worked-up, the ratios were determined using 1H NMR as described above, and the results plotted against time are shown in Figure 1. From the graph, it is clear that the chloride displacement happened quickly, i.e., within the first 2 h of reaction, to give the 6-bromoalkyl glycoside. In contrast, the anomerization happened slowly in the first hour of reaction and then faster in the next 6 h to reach a plateau after 12 h of reaction. As can be seen in this plot, it appeared that anomerization happened preferentially from the 6-bromohexyl β glycoside. Furthermore, this tracked reaction showed an optimal time to isolate the 6-bromohexyl β glycoside (3β) in high yields prior to anomerization.
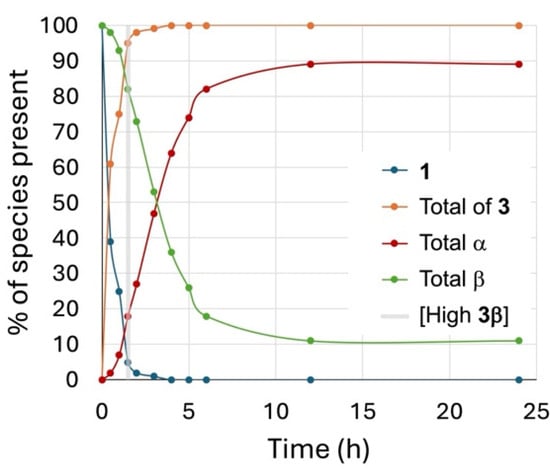
Figure 1.
Tracked reaction of the conversion from 1 to 3 at 100 °C, 0.04 M in a 1:2 DBM-DMF mixture containing NaBr (10 equiv) in a sealed flask.
2.2. Anomerization of Alkyl β Glycosides
To assess whether the bromine atom in compounds 2 and 4 was involved in the anomerization reaction, we synthesized known [19] hexyl GlcNAc β glycoside 5β and known [20] methyl GlcNAc β glycoside 6β from known [17] Horton’s chloride (Supplementary Material). The β glycosides 5β and 6β were then submitted to the reaction conditions described above (1:2, DBM-DMF, 100 °C, sealed flasks, 24 h) omitting the sodium bromide (Scheme 2), which did not significantly impact anomerization.
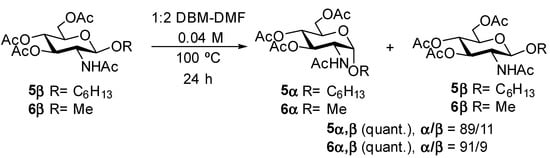
Scheme 2.
Anomerization of alkyl glycosides without halide displacement.
After work-up, glycosides 5 and 6 were recovered in quantitative yields, and they were analyzed by NMR. To our surprise, we found for both compounds that the known α anomers 5α [21] and 6α [22] were again the major products of the reactions, with α/β ratios of ~9/1. Full assignment of the 1H and 13C NMR signals for the glycosides 5α and 6α in the mixtures were obtained by irradiating the anomeric signals in 1D TOCSY experiments and correlating these signals to carbon signals using an HSQC experiment (Supplementary Material). Thus, these α glycosides were characterized by anomeric signals appearing as doublets at 4.80 and 4.70 ppm with characteristic coupling constants 3JH-1,H-2 = 3.7 and 3.6 Hz in the 1H NMR spectra and that correlated to anomeric signals at 97.1 and 98.3 ppm in the 13C NMR spectra for compounds 5α and 6α, respectively. In contrast, the minor β glycosides were characterized by anomeric signals appearing as doublets at 4.66 and 4.56 ppm in the 1H NMR spectra with the characteristic coupling constants 3JH-1,H-2 = 8.3 Hz and that correlated to anomeric signals at 100.7 and 101.6 ppm in the 13C NMR spectra for compounds 5β and 6β, respectively. Attempts to anomerize the β glycosides 5β and 6β in either DBM or DMF alone at 100 °C for 24 h were unsuccessful. Adding sodium bromide to solutions of glycosides 5β and 6β in DBM or DMF at 100 °C did not lead either to anomerization. Thus, it appeared that the anomerization reaction only proceeded in a mixture of DBM and DMF.
Time trials were completed with the β glycosides 5β and 6β to track the formation of the α anomer over time. The % amount of α anomers 5α and 6α formed was assessed by relative integration of the anomeric signals in the 1H NMR. The results of these time trials are plotted against time in Figure 2 and compared to the anomerization of the 6-chlorohexyl β glycoside 1, giving the 6-bromo/6-chlorohexyl glycosides 1/3α shown in Figure 1.
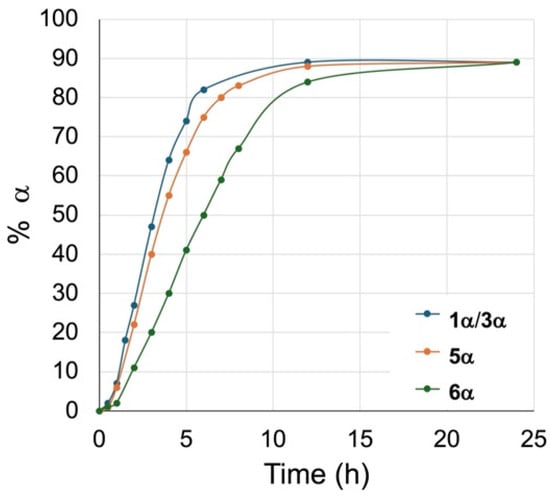
Figure 2.
Comparison of reaction rates for the anomerization for compounds 1/3, 5, and 6, 100 °C, 0.04 M, DBM-DMF (1:2). Assessed by 1H NMR.
All β glycosides anomerized to 90% over 24 h. Notably, the hexyl glycosides 1/3β and 5β anomerized faster than the methyl glycoside 6β, with 74% and 66% of 1/3α and 5α, respectively, formed after 5 h, while the α methyl glycoside only reached 41% 6α after the same length of time. Similarly to the anomerization of the 6-chlorohexyl β glycoside 1, we also observed that anomerization of the β alkyl glycosides was slower in the first hour of reaction. In our study of the anomerization of the 6-chlorohexyl β glycoside (see above), we attributed this delay in the kinetics of anomerization to the initial displacement of the chloride by bromide, postulating that it was preferentially the 6-bromohexyl β glycoside 3β that underwent anomerization. However, given that this delay was also observed with the alkyl β glycosides, the bromine in the 6-bromohexyl β glycoside 3β had no significant impact on the anomerization.
We attempted two time trials in parallel (Figure 3). In one reaction, the 1:2 DBM-DMF mixture was preheated at 100 °C for 24 h in a sealed flask before the addition of the methyl β glycoside 6β dissolved in a minimum amount of fresh solvent. The other reaction was carried out as usual; the methyl β glycoside 6β was dissolved in DBM-DMF (1:2), and the solution was placed in a sealed flask at 100 °C. The amount of anomerization in each reaction was followed at regular intervals by taking aliquots, working them up, running 1H NMR, and integrating the anomeric signals. The percentage formation of the 6α anomer in these reactions was plotted against time, and the results are shown in Figure 3. From this graph, it is clear that when the DBM-DMF mixture was preheated, the anomerization occurred readily, while it was slower when the solvents had not been preheated.
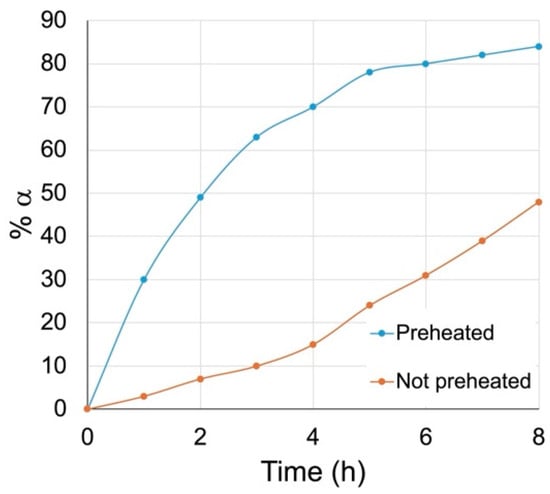
Figure 3.
Anomerization of methyl β glycoside 6β in DBM-DMF with (blue curve) or without (orange curve) preheating of the DBM-DMF.
To assess the reactivity of DBM with DMF, the two solvents were stirred at a ratio 1:2 in a sealed flask at 100 °C in an oil bath for 24 h. After cooling the reaction mixture to room temperature, we observed the formation of a crystalline solid suggesting that the two solvents had reacted together. This hygroscopic solid was isolated by centrifugation, washed with diethyl ether, dried and added to a solution of methyl β glycoside 6β in pure DMF. Heating the solution to 100 °C led to 4% anomerization after 24 h and 7% after 48 h. Although anomerization did not reach the levels observed in the DBM-DMF mixture, this was a significant indication that the solid was able to promote anomerization, since heating the β glycoside 6β in pure DMF did not lead to any anomerization.
Six additional time trials were conducted in parallel: three with the β hexyl glycoside 5β (Figure 4A) and three with the β methyl glycoside 6β (Figure 4B). For two of these, the mixtures of DBM-DMF (1:2) were heated at 100 °C for 24 h, and the glycosides were then added to the mixture without delay (Figure 4, blue curves) while the temperature was maintained at 100 °C. For two others, the mixtures of DBM-DMF (1:2) were heated at 100 °C for 24 h, and then cooled to room temperature as we observed the solid forming. The glycosides were then added to the cold mixtures, which were then placed again at 100 °C (Figure 4, orange curves). For the last two experiments, a mixture of DBM-DMF (1:2) was heated at 100 °C for 24 h, then cooled to room temperature, the solid was decanted, and the supernatant was retrieved and added to the glycosides. The mixtures were then placed at 100 °C (Figure 4, green curves). Aliquots of all reactions were taken at 1 h intervals, they were worked-up, the % anomerization giving 5α and 6α was assessed by NMR, and the results are given in Figure 4A and 4B, respectively. As can be seen from Figure 4, anomerization began without delay in all cases. Anomerization of the methyl glycoside 6β was confirmed to be slower than that of the hexyl glycoside 5β in all cases. For instance, after 2 h of reaction, the relative ratios of 5α to 6α were 60 to 44 (blue curve) and 80 to 66 (orange and green curves). Interestingly, heating the DBM-DMF mixtures (100 °C, 24 h) and then cooling them to room temperature before adding the glycosides and then placing the reactions at 100 °C led to faster anomerization of 5β and 6β (Figure 4, orange curves). Similarly, decanting the cooled supernatant of a preheated mixture of DBM-DMF (100 °C, 24 h) and adding it to the glycosides and then placing the mixtures at 100 °C also increased the kinetics of anomerization for both 5β and 6β (green curves), albeit a bit more slowly than when the β glycosides were added to the heated-then-cooled mixtures of DBM-DMF.
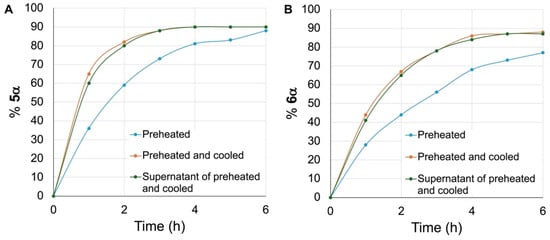
Figure 4.
Anomerization of hexyl (A) and methyl (B) glycosides 5β and 6β. Blue curves: 5β and 6β were added at 100 °C to preheated (24 h) mixtures of DBM-DMF. Orange curves: 5β and 6β were added at room temperature to preheated (100 °C, 24 h)-then-cooled mixtures of DBM-DMF, and the reactions were then placed at 100 °C. Green curves: 5β and 6β were added at room temperature to the supernatant of a preheated (100 °C, 24 h)-then-cooled mixture of DBM-DMF, and the reactions were then placed at 100 °C.
3. Discussion
We have discovered simple and cost-effective conditions to promote the anomerization of N-acetylglucosamine β glycosides to their α anomers with up to 90% conversion. These conditions involve dissolving the β glycosides in DBM-DMF (1:2) and placing the solutions at 100 °C for 24 h in a sealed flask. Our study suggests that heating the DBM-DMF mixture at 100 °C leads to the formation of a compound that promotes this anomerization. Upon cooling of a DBM-DMF (1:2) mixture heated at 100 °C for 24 h, we observed the formation of a crystalline solid. We have shown that this hygroscopic solid promoted the anomerization of methyl β glycoside 6β in pure DMF to 4% after 24 h and 7% after 48 h. Since heating the β glycoside in pure DMF did not lead to any anomerization, this result suggests that this solid could have promoted the anomerization. We have shown that the anomerization was faster when the hexyl and methyl β glycosides (5β and 6β) were stirred at 100 °C in the supernatant of a DBM-DMF mixture preheated at 100 °C for 24 h and then cooled to room temperature and decanted before being added to the β glycosides. Interestingly, cooling preheated (24 h, 100 °C) mixtures of DBM-DMF and then adding the β glycosides at room temperature before placing the mixtures at 100 °C further increased the kinetics of anomerization for both hexyl and methyl glycosides 5β and 6β. We propose that the formation of the crystalline solid during the heating/cooling cycle of the mixture prior to the addition of the β glycosides increased the concentration of the active species in solution, thereby enhancing the kinetics of anomerization.
It is known [23] that DMF and methyl bromide heated to 80 °C in a sealed vessel lead to the formation tetramethylammonium bromide. Indeed, the reaction of N,N-disubstituted amides with alkyl halides is a known way to access various quaternary ammonium halide salts [24]. In these reactions (Scheme 3A), the amides first degrade to give carbon monoxide and the corresponding dialkylamines (I), which then undergo two successive nucleophilic displacements by alkyl halides, yielding the quaternary ammonium salts [24]. It is also known that the reaction of dihalomethane (CH2I2 or CH2Br2) with trimethylamine (Scheme 3B, [25]) or N,N,N′,N′-tetramethylmethylenediamine (TMMD) (Scheme 3C, [26]) produces dimethymethyleniminium salts (II and III).
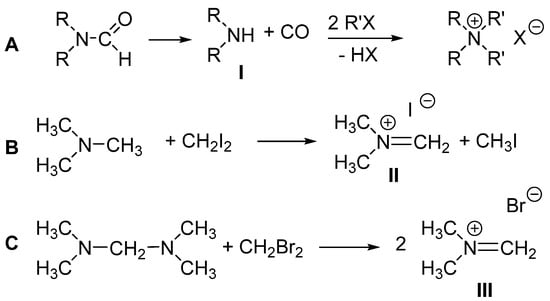
Scheme 3.
(A) Known [23,24] reaction of N,N-disubstituted amides with alkyl halides; (B) known [25] reaction of trimethylamine with diiodomethane; (C) known [26] reaction of TMMD with dibromomethane.
We observed constant gas evolution while heating the sealed DBM-DMF reaction mixtures, which strongly suggested that DMF dissociated into dimethylamine and carbon monoxide. The 1H NMR (d6-DMSO) spectrum of the dried solid showed four products (Supplementary Material). The major compound was characterized by two signals of a 3-to-1 relative integration at 3.65 ppm and 8.18 ppm, correlating in the HSQC to methyl and methylene carbons at 48 and 168 ppm, respectively. These signals are in agreement with those reported for the iminium iodide salt (II, Scheme 3) described by Schreiber et al. [25] at 3.67 and 8.18 ppm, respectively. Further examination of the 1H NMR spectrum of the solid also revealed two signals of a 3-to-1 relative integration that, depending on the sample, appeared as doublets or broad singlets at 2.66 ppm and 4.44 ppm, both correlating in the COSY spectrum to a broad NH signal at 9.5 ppm. In the HSQC, these signals correlated to methyl and methylene carbons at 38 and 79 ppm, respectively. We propose that these signals correspond respectively to the two methyl and the hydroxymethyl substituents in hydroxymethyl(dimethyl)ammonium bromide (Scheme 4, VI), which was likely formed by the reaction of the iminium salt (III) with water contained in the NMR solvent. Finally, NMR of the solid in d6-DMSO also showed two unrelated signals: a doublet at 2.77 ppm and a triplet at 2.54 ppm, which correlated in the COSY spectrum to broad NH signals at 9.6 and 8.4 ppm, respectively. In the HSQC, these signals correlated to methyl carbons at 44 and 34 ppm, respectively. We propose that these signals correspond to dimethylamine (Scheme 4, IV) and dimethylammonium bromide, respectively.
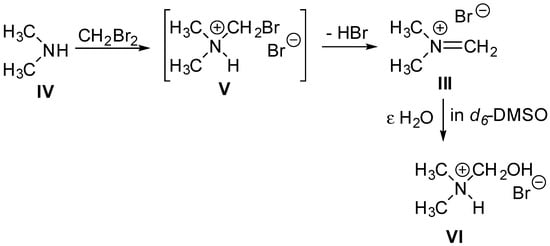
Scheme 4.
Proposed mechanism of formation of iminium salt III and hydroxymethyl(dimethyl)ammonium bromide VI observed in “wet” d6-DMSO.
X-ray crystallography of the crystalline solid confirmed that it was indeed the dimethylmethyleniminium bromide salt III (Figure 5). Crystallographic data and experimental details are given in the Supplementary Material. The structure obtained was deposited at the Cambridge Crystallographic Data Centre (CCDC) under the number 2425977 and is in complete agreement with that previously reported by Clark et al. [26] for the same compound.
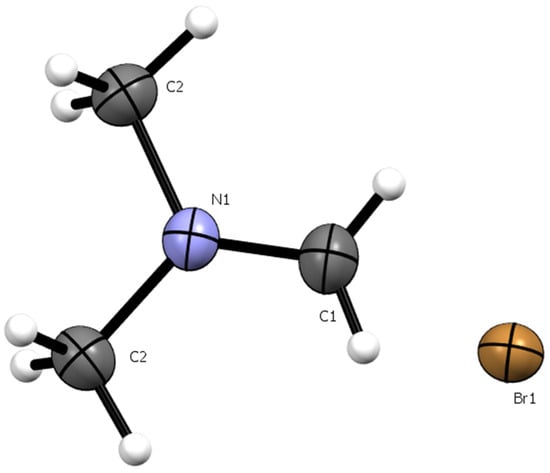
Figure 5.
Crystal structure of dimethylmethyleniminium bromide salt III with thermal ellipsoids shown at 50% probability with non-H atoms labelled.
Concentration of the supernatant and 1H NMR (d6-DMSO) analysis (Supplementary Material) of the residue showed the presence of what we assume to be dimethylamine (doublet at 2.77 ppm) and dimethylammonium bromide (triplet at 2.54 ppm) as major compounds. We also observed the presence of what we proposed to be hydroxymethyl(dimethyl)ammonium bromide (VI), resulting from the hydration of the dimethylmethyleniminium bromide salt by water contained in DMSO and characterized by the two doublets mentioned above (2.66 ppm and 4.44 ppm).
We propose (Scheme 4) that the main reaction taking place between DMF and DBM results from the nucleophilic displacement of bromide in dibromomethane by the dimethylamine IV formed after decomposition of the DMF. This reaction leads to the bromomethyl(dimethyl)ammonium bromide intermediate V, which promptly loses HBr to give dimethylmethyleniminium bromide III. Thus, we conclude that anomerization could be promoted either by the HBr released during the formation of the dimethylmethyleniminium bromide III or by the highly reactive dimethylmethyleniminium bromide salt III itself. Without attempting to optimize the reaction, we observed up to 7% anomerization when adding dry dimethylmethyleniminium bromide III salt to a solution of the β glycoside 6β in pure DMF and heating the solution at 100 °C for 48 h. Since stirring the β glycoside 6β in pure DMF at 100 °C did not lead to any anomerization, this initial result strongly suggests that the highly reactive Mannich salt III is indeed capable of promoting anomerization. Further experiments are ongoing to confirm this hypothesis and clarify if the HBr formed is involved in the anomerization.
It has been known since the late 1920s that Lewis acids can promote the anomerization of β glucosides, as Pacsu [27,28,29] described the anomerization of methyl, hexyl, and cyclohexyl tetraacetyl β-d-glucopyranosides using SnCl4 or TiCl4. Subsequently, Ikemoto et al. [30] described the anomerization of various acylated β glycosides using FeCl3 at room temperature and reported that acetylated and benzoylated β glycosides yielded the best results. Interestingly when attempting the anomerization of peracetylated β methyl N-acetylglucosamine glycoside 6β, they observed the formation of the oxazoline in a 32% yield, which suggested an exocyclic cleavage of the glycosidic bond. Expanding on these early results, the Murphy group studied the TiCl4- and SnCl4-promoted anomerization of various β glycosides [31,32,33,34]. These studies confirmed the early work by Pacsu that showed that TiCl4 was more efficient at promoting anomerization than SnCl4 [32]. The Murphy group discovered that β glucuronic acids were more easily anomerized than glycosides, 6-deoxy glycosides, or xylopyranosides and that fuco-, xylo-, arabino-, gluco-, and galactopyranosides, including β glycosides of N-acetylglucosamine, could be anomerized in these conditions [31,32,34]. Furthermore, they also studied the impact of the O-2 protecting group, showing that 2-O-acetylated β glycosides were less reactive toward anomerization than their 2-O-benzoylated analogues [33]. Interestingly, Crich and Vinod made the serendipitous discovery that a β methyl glycoside of 2-N-acetyl-2-N,3-O-oxazolidinone of glucosamine underwent anomerization to the α anomer when treated with NaCNBH3 and HCl in diethyl ether [35]. The Oscarson and Ito groups also independently reported the anomerization of N-acetyl or N-benzyl 2,3-trans-oxazolidinones of glucosamine promoted by AgOTf or BF3•Et2O, respectively [36,37]. Crich [35] suggested that the anomerization of these 2,3-oxazolidinones was assisted by the strain created by the 2,3-trans-fused ring, which facilitated a O5-C1 endo cleavage, eventually leading to the more stable α anomer. Indeed, subsequent calculations by the Ito group showed that the N-benzyl-2,3-trans-oxazolidinone group gave lower energies for the transition state and endo O5-C1 cleavage than pyranosides not carrying this 2,3-trans-fused ring [38]. More recently, Frem et al. [39] also reported anomerization of the n-butyl β glycoside of peracetylated GlcNAc promoted by Cu(OTf)2 or triflic acid at 130 °C in 1,2-dichloroethane. In the latter case, they observed considerable degradation of the glycoside. They also reported transglycosylation of the n-butyl β glycoside to the methyl β glycoside when adding excess methanol to the reaction mixture. Interestingly, they observed limited anomerization of the β methyl glycoside, only reaching an α/β ratio of 1/6 in this reaction.
Glycosidic bonds can be cleaved either via an exocyclic C1-O1 or endocyclic O5-C1 cleavage (Scheme 5 (a and b)).
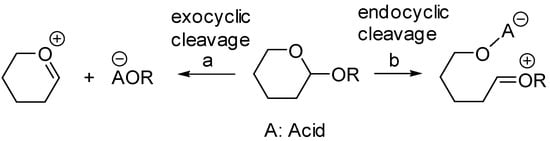
Scheme 5.
Possible path of glycosidic bond cleavage: a. exocyclic cleavage, b. endocyclic cleavage.
It is generally accepted that when submitted to solvolysis in protic conditions, α glycosides undergo exocyclic C1-O1 cleavage assisted by the stereoelectronic effect of the ring oxygen O-5 [40,41,42]. In contrast, the path of cleavage for β glycosides in protic acid has been shown to occur through both exo (C1-O1) and endo (O5-C1) cleavage [40,41,42]. Furthermore, the ring opening of β glycosides under Lewis acid catalysis such as Me2BBr [43], AlMe3 [44], and AlMe3/MeTiCl3 [45] was shown to proceed mostly through endo cleavage. In his studies, Murphy [33] proposed that anomerization of β glycosides promoted by TiCl4 or SnCl4 occurred via an endocyclic O5-C1 cleavage induced by the chelation of the O5 and O6 oxygens by the metal catalyst, as shown in Scheme 6.
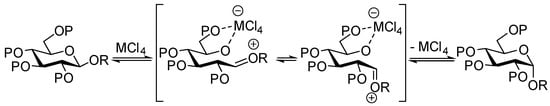
Scheme 6.
Proposed mechanism by Murphy [33] for the anomerization of β glucosides catalyzed by Lewis acids.
We do not have unquestionable evidence that the anomerizations described here follow endocyclic opening at the O5-C1 bond. However, the fact that we do not observe either hemiacetal formation (even when adding traces of water to our reactions) or the formation of oxazoline as reported by Ikemoto et al. [30] strongly suggests that the glycosidic C1-O1 bond is not cleaved during the anomerization. Thus, our results suggest that the anomerizations follow an endocyclic mechanism.
As we have described above, while all β glycosides 1, 2, 5, and 6 reached a 9/1 α/β ratio after 24 h of reaction, the β methyl glycoside 6β was slower to anomerize that the β hexyl glycoside 5β (Figure 2 and Figure 4), suggesting that the size of the glycoside impacts the reaction. These results are in agreement with the results reported by Pacsu [28], who observed faster anomerization of the peracetylated β hexyl glucopyranoside than that of its methyl glucoside analog under TiCl4 activation.
Further experiments are ongoing in our laboratory to confirm whether dimethylmethyleniminium bromide III, HBr, or the combination of both promotes anomerization. Furthermore, we are currently investigating the scope of this reaction and its applicability to other glycosides, especially those that do not carry an acetamido group at C-2. Preliminary results have shown that the presence of a more electron-withdrawing N-Troc, N-trichloroacetyl (N-TCA), or O-Ac had a significant impact on the degree of anomerization. These results will be reported in due course.
4. Materials and Methods
4.1. General Experimental
All chemicals were purchased from Fisher Scientific (Ottawa, ON, Canada) or Sigma Aldrich (Oakville, ON, Canada) and were used without purification. Anhydrous dichloromethane was obtained by distillation from phosphorus pentoxide. 1H NMR and 13C NMR spectra were recorded at 298 K on Bruker Avance-400 or 600 spectrometers (Bruker Biospin, Boston, MA, USA) for solutions in CDCl3 (calibrated at δC 77.0 ppm, using residual CHCl3 at δH 7.24 ppm) or d6-DMSO (calibrated at 13C δC 39.5 ppm, using residual CHD2SOCD3 at δH 2.50 ppm). All chemical shifts are reported in parts per million (ppm). All coupling constants (J) are reported in hertz (Hz) and were obtained from analysis of 1H NMR spectra. Structural assignments were made with additional information from gCOSY, gHSQC. 1D TOCSY experiments were acquired with mixing times of 150 ms and pre-scan delays of 2 s. Multiplicities are abbreviated as singlet (s), broad singlet (bs), doublet (d), doublet of a doublet (dd), triplet (t), and multiplet (m). Organic solutions were dried over Na2SO4 and concentrated under reduced pressure. Thin layer chromatography (TLC) was performed on aluminum plates coated with silica gel and charred with 20% H2SO4 in EtOH. Compounds were purified by flash column chromatography using Silica Gel 60 (230–400 mesh). Mass spectra were obtained under electrospray ionization (ESI) on a Thermo Scientific Orbitrap Velos Pro spectrometer equipped with a TOF analyzer at the Queen’s Mass Spectrometry Facility (Kingston, ON, Canada). The reported masses are for 35Cl isotope of chlorine and the 79Br isotope for bromine. Optical rotations for new compounds were measured on a Autopol® IV automatic polarimeter by Rudolph Research Analytical (Hackettstown, NJ, USA).
4.2. General Method for the Anomerization Reactions
Glycosides 1, 2, 5β, and 6β (10–100 mg) were dissolved in a 1:2 volumetric ratio of DBM-DMF at concentrations of 0.04 M or 0.4 M. For glycosides 1 and 2, 10 equiv of NaBr was added in all reactions except for that described in Table 1, entry 3. The reaction mixtures were sealed and placed in an oil bath preheated to the desired temperature (70–100 °C), and the reactions were left to proceed for 24 h. The solutions were diluted in 5 mL of DCM, washed with water (2 × 5 mL), and the water phases were re-extracted with DCM (3 × 5 mL). The combined organic layers were dried on Na2SO4, concentrated under reduced pressure at 40 °C to yield tan syrups or amorphous solids. The products were analyzed by TLC (hexanes:EtOAc) and NMR.
4.3. 8-Chlorooctyl 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-d-glucopyranoside (2)
Known [17] 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-d-glucopyranosyl chloride (Horton’s chloride, 4 g, 11 mmol) was dissolved in anhyd DCM (40 mL) under N2. Molecular sieves (4 Å, 5 g) were added along with 8-chloro-1-octanol (2.8 mL, 16 mmol, 1.5 equiv), and the reaction was stirred for 1 h. HgCl2 (3.86 g, 14 mmol, 1.3 equiv) was added to the reaction, which was left to stir for 16 h at rt. The mixture was diluted with DCM (50 mL), filtered over Celite®, and the filtrate was washed with satd aq NaHCO3 (2 × 50 mL) and water (3 × 50 mL), and the water phases were re-extracted with DCM (3 × 20 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The pure 8-chlorooctyl glycoside 2 was precipitated in cold 1:1 Et2O–toluene and filtered off as a white solid (3.6 g, 67%). Chromatography of the mother liquor (EtOAc-hexanes, 8:2) gave additional pure 8-chlorooctyl glycoside 2 (0.32 g, 6%). [α]D −8.80 (c 0.5, MeOH). 1H NMR (CDCl3, 400 MHz) δH 5.42 (d, 1H, J = 8.7 Hz, NH), 5.28 (dd, 1H, J = 9.3, 10.6 Hz, H-3), 5.04 (t, 1H, J = 9.7 Hz, H-4), 4.66 (d, 1H, J = 8.3 Hz, H-1), 4.24 (dd, 1H, J = 4.8, 11.9 Hz, H-6a), 4.11 (dd, 1H, J = 2.5, 12.2 Hz, H-6b), 3.86–3.75 (m, 2H, H-2, OCHHCH2), 3.69–3.65 (m, 1H, H-5), 3.51 (t, 2H, J = 6.7 Hz, CH2Cl), 3.47–3.41 (m, 1H, OCHHCH2), 2.06, 2.01, 2.00, 1.92 (4s, 12H, 4 × COCH3), 1.73 (m, 2H, J = 7.8 Hz, CH2CH2Cl), 1.56–1.50 (m, 2H, OCH2CH2), 1.41–1.35 (m, 2H, CH2CH2CH2Cl), 1.29–1.25 (m, 6H, CH2(CH2)3CH2). 13C NMR (CDCl3, 100 MHz) δC 170.9, 170.7, 170.0, 169.4 (C=O), 100.7 (C-1), 72.3 (C-4), 71.8 (C-5), 69.8 (OCH2CH2), 68.7 (C-3), 62.2 (C-6), 54.9 (C-2), 45.1 (CH2Cl), 32.6 (CH2CH2Cl), 29.4, 29.1, 28.8, 26.8, 25.7 (CH2(CH2)5CH2), 23.4, 20.8, 20.7, 20.6 (COCH3). HRESIMS m/z: [M + H]+ calcd for C22H37ClNO9 494.2157; found 494.2151.
4.4. 6-Bromohexyl 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-d-glucopyranoside (3α) and 6-Bromohexyl 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-d-glucopyranoside (3β)
Known [16] 6-chlorohexyl 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-d-glucopyranoside 1 (Supplementary Material, 100 mg, 0.215 mmol) was reacted in DBM-DMF (1:2) at a 0.4 M concentration with 10 equiv of NaBr in a sealed flask as described above in the general method (100 °C, 24 h). Work-up as described above gave a brown syrup (82 mg, 74%). The α/β (9:1) ratio was determined by NMR. Purification of the residue with chromatography (EtOAc-hexanes, 7:3) gave analytical samples of pure 3α and 3β.
Analytical data for compound 3α: [α]D +73.1 (c 1.0, MeOH). 1H NMR (CDCl3, 400 MHz) δH 5.64 (d, 1H, J = 9.4 Hz, NH), 5.18 (dd, 1H, J = 9.3, 10.5 Hz, H-3), 5.09 (t, 1H, J = 9.6 Hz, H-4), 4.81 (d, 1H, J = 3.7 Hz, H-1), 4.31 (m, 1H, H-2), 4.21 (dd, J = 4.8, 11.9 Hz, H-6a), 4.07 (dd, 1H, J = 2.5, 12.2 Hz, H-6b), 3.93–3.89 (m, 1H, H-5), 3.69–3.63 (m, 1H, OCHHCH2), 3.44–3.38 (m, 3H, CH2Br, OCHHCH2), 2.08, 2.01, 2.00, 1.92 (4s, 12H, 4 × COCH3), 1.86 (m, 2H, CH2CH2Br), 1.65–1.58 (m, 2H, OCH2CH2), 1.51–1.43 (m, 2H, CH2CH2CH2Br), 1.40–1.33 (m, 2H, OCH2CH2CH2). 13C NMR (CDCl3, 100 MHz) δC 171.4, 170.7, 169.8, 169.3 (C=O), 97.1 (C-1), 71.4 (C-4), 68.2 (OCH2), 68.1 (C-5), 67.7 (C-3), 62.0 (C-6), 51.9 (C-2), 33.7 (CH2Br), 32.5, 29.1, 27.8, 25.3 (CH2(CH2)4CH2Br), 23.2, 20.7, 20.7, 20.6 (COCH3). HRESIMS m/z: [M + H]+ calcd for C20H33BrNO9 510.1339; found 510.1323.
Analytical data for compound 3β: [α]D −11.1 (c 0.54, MeOH). 1H NMR (CDCl3, 400 MHz) δH 5.44 (d, 1H, J = 8.9 Hz, NH), 5.27 (dd, 1H, J = 9.3, 10.6 Hz, H-3), 5.04 (t, 1H, J = 9.6 Hz, H-4), 4.65 (d, 1H, J = 8.3 Hz, H-1), 4.23 (dd, 1H, J = 4.8, 11.9 Hz, H-6a), 4.10 (dd, 1H, J = 2.5, 12.2 Hz, H-6b), 3.87–3.75 (m, 2H, H-2, OCHHCH2), 3.69–3.65 (m, 1H, H-5), 3.48–3.42 (m, 1H, OCHHCH2), 3.38 (t, 2H, J = 6.7 Hz, CH2Br), 2.09, 2.03, 2.02, 1.93 (4s, 12H, 4 × COCH3), 1.82 (m, 2H, CH2CH2Br), 1.63–1.52 (m, 2H, OCH2CH2), 1.47–1.28 (m, 4H, CH2(CH2)2CH2). 13C NMR (CDCl3, 100 MHz) δC 170.9, 170.7, 170.1, 169.4 (C=O), 100.7 (C-1), 72.3 (C-4), 71.8 (C-5), 69.6 (OCH2), 68.6 (C-3), 62.1 (C-6), 54.9 (C-2), 33.8 (CH2Br), 32.6, 29.2, 27.7 25.0 (CH2(CH2)4CH2Br), 23.4, 20.7, 20.7, 20.6 (COCH3). HRESIMS m/z: [M + H]+ calcd for C20H33BrNO9 510.1339; found 510.1339.
4.5. 8-Bromooctyl 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-d-glucopyranoside (4α) and 8-Bromooctyl 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-d-glucopyranoside (4β)
The 8-chlorooctyl glycoside 2 (105 mg, 0.213 mmol) was reacted in DBM-DMF (1:2) at a 0.4 M concentration with 10 equiv of NaBr in a sealed flask as described above in the general method (100 °C, 24 h). Work-up as described above gave a brown syrup (80 mg, 70%. The α/β (91:9) ratio was determined by NMR. Analytical samples of 4α and 4β were obtained by chromatography (EtOAc/hexanes, 7:3).
Analytical data for compound 4α: [α]D +49.0 (c 0.69, MeOH). 1H NMR (CDCl3, 400 MHz) δH 5.64 (d, 1H, J = 8.7 Hz, NH), 5.18 (dd, 1H, J = 9.3, 10.5 Hz, H-3), 5.09 (t, 1H, J = 9.6, 10.5 Hz, H-4), 4.80 (d, 1H, J = 3.7 Hz, H-1), 4.34–4.28 (m, 1H, H-2), 4.21 (dd, J = 4.6, 12.3 Hz, H-6a), 4.07 (dd, 1H, J = 2.5, 12.3 Hz, H-6b), 3.93–3.89 (m, 1H, H-5), 3.68–3.62 (m, 2H, H-2, OCHHCH2), 3.43–3.37 (m, 3H, OCHHCH2, CH2Br), 2.07, 2.01, 2.00, 1.93 (4s, 12H, 4 × COCH3), 1.84 (m, 2H, CH2CH2Br), 1.61–1.55 (m, 2H, OCH2CH2), 1.46–1.39 (m, 2H, CH2CH2CH2Br), 1.37–1.28 (m, 6H, CH2(CH2)3CH2). 13C NMR (CDCl3, 100 MHz) δC 171.4, 170.7, 169.8, 169.3 (C=O), 97.1 (C-1), 71.4 (C-4), 68.5 (OCH2), 68.1 (C-5), 67.7 (C-3), 62.0 (C-6), 51.9 (C-2), 33.9 (CH2Br), 32.7 (CH2CH2Br), 29.2, 29.1, 28.6, 28.0, 26.0 (CH2(CH2)5CH2CH2Br), 23.2, 20.7, 20.7, 20.6 (COCH3). HRESIMS m/z: [M + H]+ calcd for C22H37BrNO9 538.1652; found 538.1646.
Analytical data for compound 4β: [α]D -12.1 (c 0.69, MeOH). 1H NMR (CDCl3, 400 MHz) δH 5.42 (d, 1H, J = 8.7Hz, NH), 5.28 (dd, 1H, J = 9.3, 10.6 Hz, H-3), 5.04 (t, 1H, J = 9.7 Hz, H-4), 4.66 (d, 1H, J = 8.3 Hz, H-1), 4.24 (dd, 1H, J = 4.8, 12.2 Hz, H-6a), 4.11 (dd, 1H, J = 2.5, 12.2 Hz, H-6b), 3.86–3.75 (m, 2H, H-2, OCHHCH2), 3.69–3.65 (m, 1H, H-5), 3.47–3.41 (m, 1H, OCHHCH2), 3.38 (t, 2H, J = 6.8 Hz, CH2Br), 2.06, 2.01, 2.00, 1.92 (4s, 12H, 4 × COCH3), 1.82 (m, 2H, CH2CH2Br), 1.57–1.50 (m, 2H, OCH2CH2), 1.43–1.35 (m, 2H, CH2CH2CH2Br), 1.31–1.23 (m, 6H, CH2(CH2)3CH2). 13C NMR (CDCl3, 100 MHz) δC 170.9, 170.7, 170.0, 169.4 (C=O), 100.7 (C-1), 72.3 (C-4), 71.8 (C-5), 69.8 (OCH2), 68.7(C-3), 62.2 (C-6), 54.9 (C-2), 34.0 (CH2Br), 32.7 (CH2CH2Br), 29.4, 29.1, 28.7, 28.0, 25.7 (CH2(CH2)5CH2CH2Br), 23.4, 20.8, 20.7, 20.6 (COCH3). HRESIMS m/z: [M + H]+ calcd for C22H37BrNO9 538.1652; found 538.1652.
4.6. Hexyl 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-d-glucopyranoside (5α) and Hexyl 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-d-glucopyranoside (5β)
The known [19] hexyl glycoside 5β (Supplementary Material, 12 mg, 0.027 mmol) was reacted in DBM-DMF (1:2) at a 0.04 M concentration in a sealed flask as described above in the general method (100 °C, 24 h). Work-up as described above gave a brown syrup (12 mg, quant). The α/β (89:11) ratio was determined by NMR.
NMR data for known major compound 5α are in agreement with those previously reported [21] and were assigned in the mixture using 1D TOCSY (irradiation of H-1) and HSQC experiments (Supplementary Material): 1H NMR (CDCl3, 400 MHz) δH 5.63 (d, 1H, J = 9.5 Hz, NH), 5.19 (dd, 1H, J = 9.6, 10.6 Hz, H-3), 5.09 (t, 1H, J = 9.6 Hz, H-4), 4.80 (d, 1H, J = 3.7 Hz, H-1), 4.31 (m, 1H, H-2), 4.21 (dd, 1H, J = 4.8, 12.3 Hz, H-6a), 4.07 (dd, 1H, J = 2.5, 12.1 Hz, H-6b), 3.91 (m, 1H, H-5), 3.68–3.63 (m, 1H, OCHHCH2), 3.43–3.38 (m, 1H, OCHHCH2), 2.07, 2.01, 2.00, 1.93 (4 s, 12H, 4 × COCH3), 1.59 (m, 2H, OCH2CH2), 1.36–1.21 (m, 6H, CH2(CH2)3CH3), 0.88 (m, 3H, (CH2)5CH3). 13C NMR (CDCl3, 100 MHz) δC 171.4, 170.7, 169.8, 169.3 (C=O), 97.1 (C-1), 71.5 (C-4), 68.7 (OCH2CH2), 68.6 (C-5), 67.7 (C-3) 62.1 (C-6), 51.9 (C-2), 31.5 (OCH2CH2), 29.2, 25.8 (CH2(CH2)2CH2), 23.3 (COCH3), 22.6 (CH2CH3), 20.7, 20.7, 20.6 (COCH3), 14.0 (CH2CH3).
NMR data for known minor compound 5β are in agreement with those previously reported [19]: 1H NMR (CDCl3, 400 MHz) δH 5.46 (d, 1H, J = 8.7 Hz, NH), 5.29 (dd, 1H, J = 9.4, 10.5 Hz, H-3), 5.04 (t, 1H, J = 9.6 Hz, H-4), 4.66 (d, 1H, J = 8.3 Hz, H-1), 4.23 (dd, 1H, J = 4.7, 12.2 Hz, H-6a), 4.10 (dd, 1H, J = 2.4, 12.2 Hz, H-6b), 3.96–3.75 (m, 2H, H-2, OCHHCH2), 3.69–3.65 (m, 1H, H-5), 3.48–3.41 (m, 1H, OCHHCH2), 2.10, 2.02, 2.01, 1.92 (4 s, 12H, 4 × COCH3), 1.55 (m, 2H, OCH2CH2), 1.31–1.20 (m, 6H, CH2(CH2)3CH3), 0.85 (m, 3H, (CH2)5CH3). 13C NMR (CDCl3, 100 MHz) δC 170.9, 170.7, 170.1, 169.4 (C=O), 100.7 (C-1), 72.3 (C-4), 71.7 (C-5), 70.0 (OCH2CH2), 68.7 (C-3) 62.2 (C-6), 54.9 (C-2), 31.5 (OCH2CH2), 29.4, 25.5, (CH2(CH2)2CH2), 23.3 (COCH3), 22.6 (CH2CH3), 20.7, 20.7, 20.6 (COCH3), 14.0 (CH2CH3).
4.7. Methyl 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-d-glucopyranoside (6α) and Methyl 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-d-glucopyranoside (6β)
The known [20] methyl glycoside 6β (Supplementary Material, 10 mg, 0.028 mmol) was reacted in DBM-DMF (1:2) at a 0.04 M concentration in a sealed flask as described above in the general method (100 °C, 24 h). Work-up as described above gave a brown syrup (10 mg, quant.). The α/β (91:9) ratio was determined by NMR.
NMR data for known major compound 6α are in agreement with those previously reported [22] and were assigned using 1D TOCSY (irradiation of H-1) and HSQC experiments (Supplementary Material): 1H NMR (CDCl3, 400 MHz) δH 5.69 (d, 1H, J = 9.7 Hz, NH), 5.19 (dd, 1H, J = 9.6, 10.6 Hz, H-3), 5.09 (t, 1H, J = 9.8 Hz, H-4), 4.70 (d, 1H, J = 3.6 Hz, H-1), 4.32 (m, 1H, H-2), 4.21 (dd, 1H, J = 3.7, 9.7 Hz, H-6a), 4.08 (dd, 1H, J = 3.6, 9.6 Hz, H-6b), 3.89 (m, 1H, H-5), 3.38 (s, 3H, OCH3), 2.07, 2.00, 1.99, 1.93 (4 s, 12H, 4 × COCH3). 13C NMR (CDCl3, 100 MHz) δC 171.4, 170.7, 169.9, 169.3 (C=O), 98.3 (C-1), 71.3 (C-4), 68.1 (C-5), 67.6 (C-3), 62.0 (C-6), 55.4 (CH3), 54.6 (C-2), 23.2, 22.7, 20.7, 20.6 (COCH3).
NMR data for known minor compound 6β are in agreement with those previously reported [20]: 1H NMR (CDCl3, 400 MHz) δH 5.45 (d, 1H, J = 8.9 Hz, NH), 5.25 (dd, 1H, J = 9.3, 10.4 Hz, H-3), 5.06 (t, 1H, J = 9.8 Hz, H-4), 4.56 (d, 1H, J = 8.3 Hz, H-1), 4.26 (dd, 1H, J = 4.7, 12.3 Hz, H-6a), 4.13 (dd, 1H, J = 2.5, 12.3 Hz, H-6b), 3.84 (m, 1H, H-2), 3.68 (m, 1H, H-5), 3.48 (s, 3H, OCH3), 2.08, 2.01, 2.00, 1.94 (4 s, 12H, 4 × COCH3). 13C NMR (CDCl3, 100 MHz) δC 171.0, 170.7, 170.2, 169.4 (C=O), 101.6 (C-1), 72.4 (C-4), 71.8 (C-5), 68.5 (C-3), 62.1 (C-6), 56.8 (CH3), 54.6 (C-2), 23.4, 20.7, 20.7, 20.6 (COCH3).
5. Conclusions
We have described the synthesis of a new 8-chlorooctyl β GlcNAc glycoside. We have established that the chlorine atom in this compound as well as in the 6-chlorohexyl analog can be swiftly displaced by bromide in a mixture of DBM and DMF at 90–100 °C with or without the addition of sodium bromide. However, these reactions must be carefully monitored to maximize the nucleophilic displacement while limiting anomerization of the glycosidic bond. At 100 °C, the reaction is best stopped after ~2 h. The bromide in these glycosides should then be easily displaced with N-hydroxyphthalimide in mild conditions [14], leading, after deprotection, to the corresponding aminooxy functional group needed for conjugation to PS A1 [10,11,12,13] or other carrier molecules.
In the course of this work, we discovered that these mild conditions led to the anomerization of the β glycosidic bond in GlcNAc to the corresponding α anomer. At 100 °C, the α/β was 9/1 after 24 h of reaction in a mixture (1:2) of DBM and DMF. While we initially assumed that anomerization happened preferentially from the bromoalkyl glycosides, we established that the alkyl glycosides also anomerized in these conditions. We discovered that applying a heating–cooling cycle to the solvent mixtures prior to adding the β glycosides and placing the reaction at 100 °C increased the kinetics of anomerization. Upon cooling the preheated DBM-DMF mixtures, we observed the formation of a crystalline solid, which was characterized by NMR spectroscopy and X-ray crystallography to be the known [25,26] Mannich salt dimethylmethyleniminium bromide. Although we only observed limited anomerization (4–7%) when the solid was reacted with the β glycoside 6β in pure DMF at 100 °C, we propose that this highly reactive carbocation could play the role of a Lewis acid in promoting anomerization in these reactions. However, it is also possible that the HBr formed together with the dimethylmethyleniminium bromide plays a role in the anomerization. Further experiments are ongoing in our laboratory to clarify the mechanism of this reaction. Our studies strongly suggest that anomerization of these GlcNAc β glycosides occurs via an endo O5-C1 cleavage, followed by bond rotation and cyclization to the more stable α anomer. We have also shown that the nature of the aglycon impacted the rate of anomerization, with hexyl β glycosides of GlcNAc anomerizing at a faster rate than the analogous methyl β glycoside. The conditions discovered and described here are cost-effective and simple when compared to using heavy-metal-containing Lewis acids such as TiCl4 or SnCl4 [27,28,29,31,32,33,34] to promote the anomerization of β glycosides. Furthermore, these conditions are mild and do not lead to the degradation of the glycoside like the use of a strong protic acid such as TfOH does [39]. In the present work, we focused our investigation on the anomerization of β glycosides of peracetylated N-acetylated glucosamine. Following this serendipitous discovery, additional experiments are ongoing to optimize the reaction conditions and further maximize the anomerization. Furthermore, we are currently investigating the scope of this reaction and its applicability to other glycosides, including disaccharides. Preliminary results suggest that the presence of a more electron-withdrawing N-Troc, N-TCA, or O-Ac at C-2 of a β-d-glucopyranoside has a significant impact on the degree of anomerization. Finally, further mechanistic studies are also ongoing to clarify the involvement of the Mannich salt dimethylmethyleniminium bromide and HBr in the anomerization; our results will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30071483/s1, Experimental section for the synthesis of known compounds: 1, 5β, and 6β. X-ray crystallography of dimethylmethyleniminium bromide (III). Tables of crystallographic parameters for dimethylmethyleniminium bromide (III). 1H, JMOD, COSY, HSQC, and NMR spectra for new compounds: 2, 3α, 3β, 4α, and 4β. 1H, JMOD, COSY, HSQC, and NMR spectra for known compounds: 1, 5β, and 6β. 1H, JMOD, COSY, HSQC, and TOCSY for end mixtures for compounds 5α/β (9/1) and 6α/β (9/1). 1H, JMOD, COSY, and HSQC of dried solids formed upon heating/cooling of DBM-DMF (1:2) mixture. 1H, JMOD, COSY, and HSQC of supernatant from heated/cooled DBM-DMF (1:2) mixture. Refs. [46,47,48,49,50,51] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, J.D., E.M., D.R., N.B.C. and F.-I.A.; methodology, N.B.C., D.R. and E.M.; formal analysis, N.B.C., D.R. and E.M.; investigation, N.B.C., D.R. and E.M.; resources, F.-I.A.; data curation, N.B.C., D.R. and E.M.; X-ray crystallography, L.K.W.; writing—original draft preparation, N.B.C.; writing—review and editing, F.-I.A.; supervision, F.-I.A.; project administration, F.-I.A.; funding acquisition, F.-I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Sciences and Engineering Research Council of Canada (NSERC), grant number 03820.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors acknowledge McMaster University for allowing them to use their X-ray facility (MAX).
Conflicts of Interest
Authors Doriane Rousseau, Esperance Mutoni, and Jeffrey Davidson are currently affiliated with Fareva Valdepharm, Sun Pharma Canada Inc., and Toronto Research Chemicals, respectively. However, their contributions to this research were conducted entirely during their time as students at the University of Guelph, prior to their current employment. The remaining authors declare no commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TACA | Tumor-associated carbohydrate antigen |
| DMF | Dimethylformamide |
| DBM | Dibromomethane |
| GlcNAc | N-acetyl glucosamine |
| TMMD | N,N,N′,N′-tetramethylmethylenediamine |
References
- Lindhorst, T.K. Essentials of Carbohydrate Chemistry and Biochemistry; Wiley-VCH: Weinheim, Germany, 2007; pp. 79–82. [Google Scholar]
- Hakomori, S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989, 52, 257–331. [Google Scholar]
- Crich, D.; Dudkin, V. Why are the hydroxy groups of partially protected N-acetylglucosamine derivatives such poor glycosyl acceptors, and what can be done about it? A comparative study of the reactivity of N-acetyl-, N-phthalimido-, and 2-azido-2-deoxy-glucosamine derivatives in glycosylation. 2-Picolinyl ethers as reactivity-enhancing replacements for benzyl ethers. J. Am. Chem. Soc. 2001, 123, 6819–6825. [Google Scholar]
- Bohn, M.L.; Colombo, M.I.; Stortz, C.A.; Ruveda, E.A. A comparative study of the influence of some protecting groups on the reactivity of D-glucosamine acceptors with a galactofuranosyl donor. Carbohydr. Res. 2006, 341, 1096–1104. [Google Scholar] [PubMed]
- Enugala, R.; Carvalho, L.C.R.; Dias Pires, M.J.; Marques, M.M.B. Stereoselective Glycosylation of Glucosamine: The Role of the N-Protecting Group. Chem.—Asian J. 2012, 7, 2482–2501. [Google Scholar]
- Green, A.R.; Li, K.; Lockard, B.; Young, R.P.; Mueller, L.J.; Larive, C.K. Investigation of the Amide Proton Solvent Exchange Properties of Glycosaminoglycan Oligosaccharides. J. Phys. Chem. B 2019, 123, 4653–4662. [Google Scholar] [PubMed]
- Liao, L.; Auzanneau, F.-I. The amide group in N-acetylglucosamine glycosyl acceptors affects glycosylation outcome. J. Org. Chem. 2005, 70, 6265–6273. [Google Scholar] [PubMed]
- Liao, L.; Robertson, V.; Auzanneau, F.-I. Unusual conformational behavior of trisaccharides containing N-acetylglucosamine. Carbohydr. Res. 2005, 340, 2826–2832. [Google Scholar] [CrossRef]
- Hendel, J.L.; Wang, J.W.; Jackson, T.A.; Hardmeier, K.; De Los Santos, R.; Auzanneau, F.I. How the Substituent at O-3 of N-Acetylglucosamine Impacts Glycosylation at O-4: A Comparative Study. J. Org. Chem. 2009, 74, 8321–8331. [Google Scholar]
- De Silva, R.A.; Wang, Q.L.; Chidley, T.; Appulage, D.K.; Andreana, P.R. Immunological Response from an Entirely Carbohydrate Antigen: Design of Synthetic Vaccines Based on Tn-PS A1 Conjugates. J. Am. Chem. Soc. 2009, 131, 9622–9623. [Google Scholar] [CrossRef]
- De Silva, R.A.; Appulage, D.K.; Pietraszkiewicz, H.; Bobbitt, K.R.; Media, J.; Shaw, J.; Valeriote, F.A.; Andreana, P.R. The entirely carbohydrate immunogen Tn-PS A1 induces a cancer cell selective immune response and cytokine IL-17. Cancer Immunol. Immunother. 2012, 61, 581–585. [Google Scholar]
- Bourgault, J.P.; Trabbic, K.R.; Shi, M.C.; Andreana, P.R. Synthesis of the tumor associative alpha-aminooxy disaccharide of the TF antigen and its conjugation to a polysaccharide immune stimulant. Org. Biomol. Chem. 2014, 12, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.C.; Kleski, K.A.; Trabbic, K.R.; Bourgault, J.P.; Andreana, P.R. Sialyl-Tn Polysaccharide A1 as an Entirely Carbohydrate Immunogen: Synthesis and Immunological Evaluation. J. Am. Chem. Soc. 2016, 138, 14264–14272. [Google Scholar] [CrossRef]
- Pulsipher, A.; Dutta, D.; Luo, W.; Yousaf, M.N. Cell-Surface Engineering by a Conjugation-and-Release Approach Based on the Formation and Cleavage of Oxime Linkages upon Mild Electrochemical Oxidation and Reduction. Angew. Chem. Int. Ed. Engl. 2014, 53, 9487–9492. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Gauthier-Signore, C.; Auzanneau, F.I. Synthesis of Dimeric Lewis A and Lewis B-Lewis A Tumor- Associated Carbohydrate Antigen Oligosaccharide Fragments. J. Org. Chem. 2023, 88, 5554–5562. [Google Scholar] [CrossRef]
- Zemlyakov, A.E. Immobilization of synthetic glycopeptides on polymeric supports. Chem. Nat. Compd. 1998, 34, 80–85. [Google Scholar] [CrossRef]
- Horton, D. 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-D-glucopyranosyl choride. Org. Synth. 1966, 46, 1–5. [Google Scholar]
- Babler, J.H.; Spina, K.P. A Facile Method for the Conversion of Primary Alkyl Chlorides to the Corresponding Bromides. Synth. Commun. 1984, 14, 1313–1319. [Google Scholar] [CrossRef]
- Heskamp, B.M.; Veeneman, G.H.; Vandermarel, G.A.; Vanboeckel, C.A.A.; Vanboom, J.H. Synthesis of a Sle(X) Mimic—A Potential E-Selectin Binding Antagonist. Recl. Trav. Chim. Pays-Bas 1995, 114, 398–402. [Google Scholar] [CrossRef]
- Myers, W.H.; Robertson, G.J. The synthesis of amino sugars I. J. Am. Chem. Soc. 1943, 65, 8–11. [Google Scholar] [CrossRef]
- Kadokawa, J.; Nagaoka, T.; Ebana, J.; Tagaya, H.; Chiba, K. New α-selective thermal glycosylation of acetyl-protected 2-acetamido-2-deoxy-β-D-glucopyranosyl diphenylphosphinate. Carbohydr. Res. 2000, 327, 341–344. [Google Scholar] [CrossRef]
- Gorbach, V.I.; Krasikova, I.N.; Lukyanov, P.A.; Soloveva, T.F.; Ovodov, Y.S. Migration of an Acetyl Group in Glucosamine and Glucose Derivatives by the Action of Butyllithium—The Synthesis of an N-Acetylglucosamine 4-Phosphate Derivative. Bull. Acad. Sci. USSR Div. Chem. Sci. 1987, 36, 1957–1960. [Google Scholar] [CrossRef]
- Neumeyer, J.L.; Cannon, J.G. Reaction of Methyl Bromide with Dimethylformamide. J. Org. Chem. 1961, 26, 4681–4682. [Google Scholar]
- Ropponen, J.; Lahtinen, M.; Busi, S.; Nissinen, M.; Kolehmainen, E.; Rissanen, K. Novel one-pot synthesis of quaternary ammonium halides: New route to ionic liquids. New J. Chem. 2004, 28, 1426–1430. [Google Scholar] [CrossRef]
- Schreiber, J.; Maag, H.; Hashimoto, N.; Eschenmoser, A. Synthetic Methods 2. Dimethyl(Methylene)Ammonium Iodide. Angew. Chem. Int. Ed. Engl. 1971, 10, 330–331. [Google Scholar] [CrossRef]
- Clark, G.R.; Shaw, G.L.; Surman, P.W.J.; Taylor, M.J.; Steele, D. Preparation, Structure and Vibrational-Spectrum of the Dimethylmethyleniminium Ion, Including the Role of Cationic Polymers in Its Formation. J. Chem. Soc. Faraday Trans. 1994, 90, 3139–3144. [Google Scholar] [CrossRef]
- Pacsu, E. Rearrangement of β-glycoside and β-acetyl sugars in their α-form. Ber. Dtsch. Chem. Ges. 1928, 61, 137–144. [Google Scholar] [CrossRef]
- Pacsu, E. Action of titanium tetrachloride on derivatives of sugars. II. Preparation of tetra-acetyl-beta normal-hexylglucoside and its transformation to the alpha form. J. Am. Chem. Soc. 1930, 52, 2563–2567. [Google Scholar]
- Pacsu, E. Action of titanium tetrachloride on derivatives of sugars. III. Transformation of tetra-acetyl-beta-cyclohexylglucoside to the alpha form and the preparation of alpha-cyclohexylglucoside. J. Am. Chem. Soc. 1930, 52, 2568–2571. [Google Scholar]
- Ikemoto, N.; Kim, O.K.; Lo, L.C.; Satyanarayana, V.; Chang, M.; Nakanishi, K. Ferric-Chloride, an Anomerization Catalyst for the Preparation of Alkyl Alpha-Glycopyranosides. Tetrahedron Lett. 1992, 33, 4295–4298. [Google Scholar]
- O’Brien, C.; Poláková, M.; Pitt, N.; Tosin, M.; Murphy, P.V. Glycosidation-anomerisation reactions of 6,1-anhydroglucopyranuronic acid and anomerisation of β-D-glucopyranosiduronic acids promoted by SnCl4. Chem. Eur. J. 2007, 13, 902–909. [Google Scholar]
- Pilgrim, W.; Murphy, P.V. SnCl4- and TiCl4-Catalyzed Anomerization of Acylated O- and S-Glycosides: Analysis of Factors That Lead to Higher α:β Anomer Ratios and Reaction Rates. J. Org. Chem. 2010, 75, 6747–6755. [Google Scholar]
- Farrell, M.P.; Doyle, L.M.; Murphy, P.V. Influence of acyl groups on glucopyranoside reactivity in Lewis acid promoted anomerisation. Tetrahedron Lett. 2018, 59, 2726–2731. [Google Scholar]
- Doyle, L.M.; Meany, F.B.; Murphy, P.V. Lewis acid promoted anomerisation of alkyl O- and S-xylo-, arabino- and fucopyranosides. Carbohydr. Res. 2019, 471, 85–94. [Google Scholar] [PubMed]
- Crich, D.; Vinod, A.U. 6-benzyl- and 6-silyl-acetyl-2-amino-2,3-carbonyl-2-deoxyglucosides:: Effective glycosyl acceptors in the glucosamine 4-OH series.: Effect of anomeric stereochemistry on the removal of the oxazolidinone group. J. Org. Chem. 2005, 70, 1291–1296. [Google Scholar]
- Olsson, J.D.M.; Eriksson, L.; Lahmann, M.; Oscarson, S. Investigations of glycosylation reactions with 2-acetyl-2,3-oxazolidinone-protected glucosamine donors. J. Org. Chem. 2008, 73, 7181–7188. [Google Scholar] [PubMed]
- Manabe, S.; Ishii, K.; Hashizume, D.; Koshino, H.; Ito, Y. Evidence for Endocyclic Cleavage of Conformationally Restricted Glycopyranosides. Chem. Eur. J. 2009, 15, 6894–6901. [Google Scholar] [CrossRef]
- Satoh, H.; Hutter, J.; Lüthi, H.P.; Manabe, S.; Ishii, K.; Ito, Y. Low-Barrier Pathway for endo-Cleavage Induced Anomerization of Pyranosides with N-Benzyl-2,3-trans-oxazolidinone Groups. Eur. J. Org. Chem. 2009, 2009, 1127–1131. [Google Scholar]
- Frem, D.; Urban, D.; Norsikian, S.; Beau, J.M. From Chitin to α-Glycosides of N-Acetylglucosamine Using Catalytic Copper Triflate in a Heated Sealed-Vessel Reactor. Eur. J. Org. Chem. 2017, 2017, 5094–5101. [Google Scholar] [CrossRef]
- Liras, J.L.; Anslyn, E.V. Exocyclic and Endocyclic Cleavage of Pyranosides in Both Methanol and Water Detected by a Novel Probe. J. Am. Chem. Soc. 1994, 116, 2645–2646. [Google Scholar]
- Liras, J.L.; Lynch, V.M.; Anslyn, E.V. The ratio between endocyclic and exocyclic cleavage of pyranoside acetals is dependent upon the anomer, the temperature, the aglycon group, and the solvent. J. Am. Chem. Soc. 1997, 119, 8191–8200. [Google Scholar]
- Deslongchamps, P.; Li, S.G.; Dory, Y.L. Hydrolysis of α- and β-glycosides.: New experimental data and modeling of reaction pathways. Org. Lett. 2004, 6, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Guindon, Y.; Anderson, P.C. Stereoelectronic Effects in the Ring Cleavage of Methyl Glycopyranosides Using Dimethylboron Bromide. Tetrahedron Lett. 1987, 28, 2485–2488. [Google Scholar] [CrossRef]
- Olsson, R.; Berg, U.; Frejd, T. Endocyclic cleavage of glycosides. VI. Substituent effects of the alkylative endocyclic cleavage of glycosides. Tetrahedron 1998, 54, 3935–3954. [Google Scholar] [CrossRef]
- Olsson, R.; Rundström, P.; Persson, B.; Frejd, T. Organotitanium-induced stereoselective alkylative endo-cleavage of benzyl pentopyranosides. Carbohydr. Res. 1998, 307, 13–18. [Google Scholar] [CrossRef]
- Bruker. APEX 5; Bruker AXS Inc.: Madison, WI, USA, 2023. [Google Scholar]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2020. [Google Scholar]
- Bruker. SADABS; Bruker AXS: Madison, WI, USA, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.K.A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).