Activated Carbons Derived from Brewing Cereal Residues and Pineapple Peelings for Removal of Acid Orange 7 (AO7) Dye
Abstract
1. Introduction
2. Results and Discussion
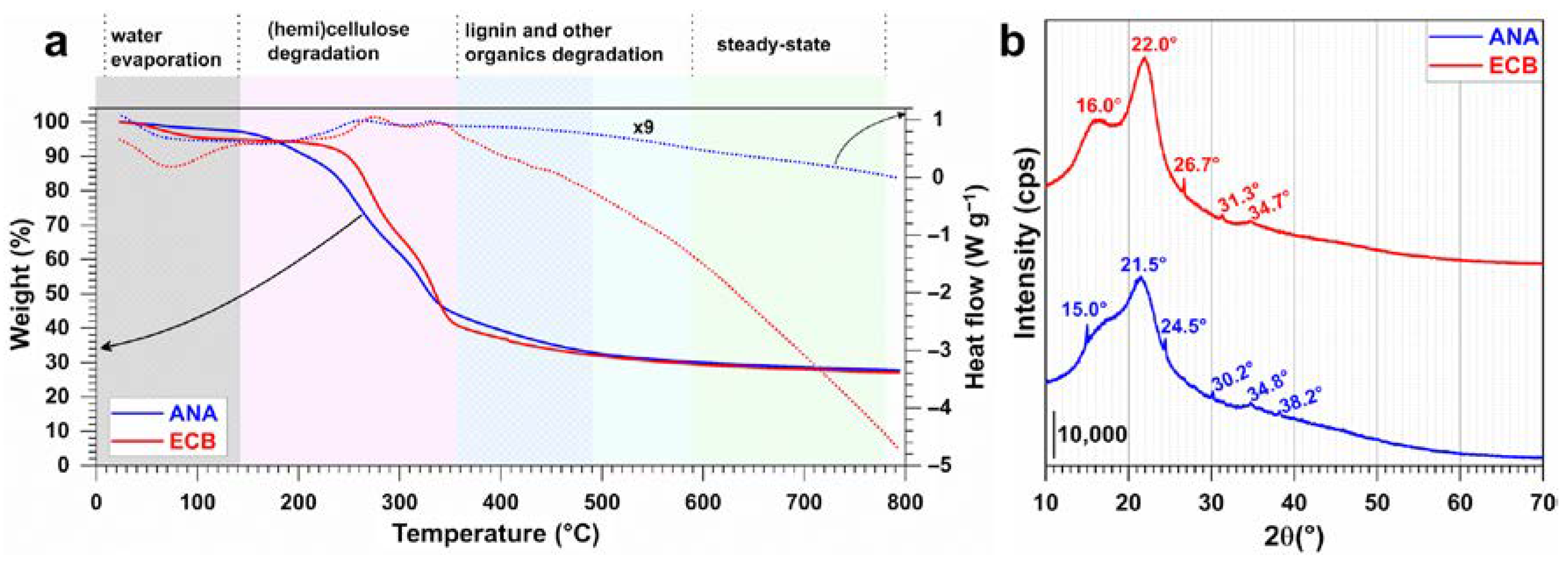
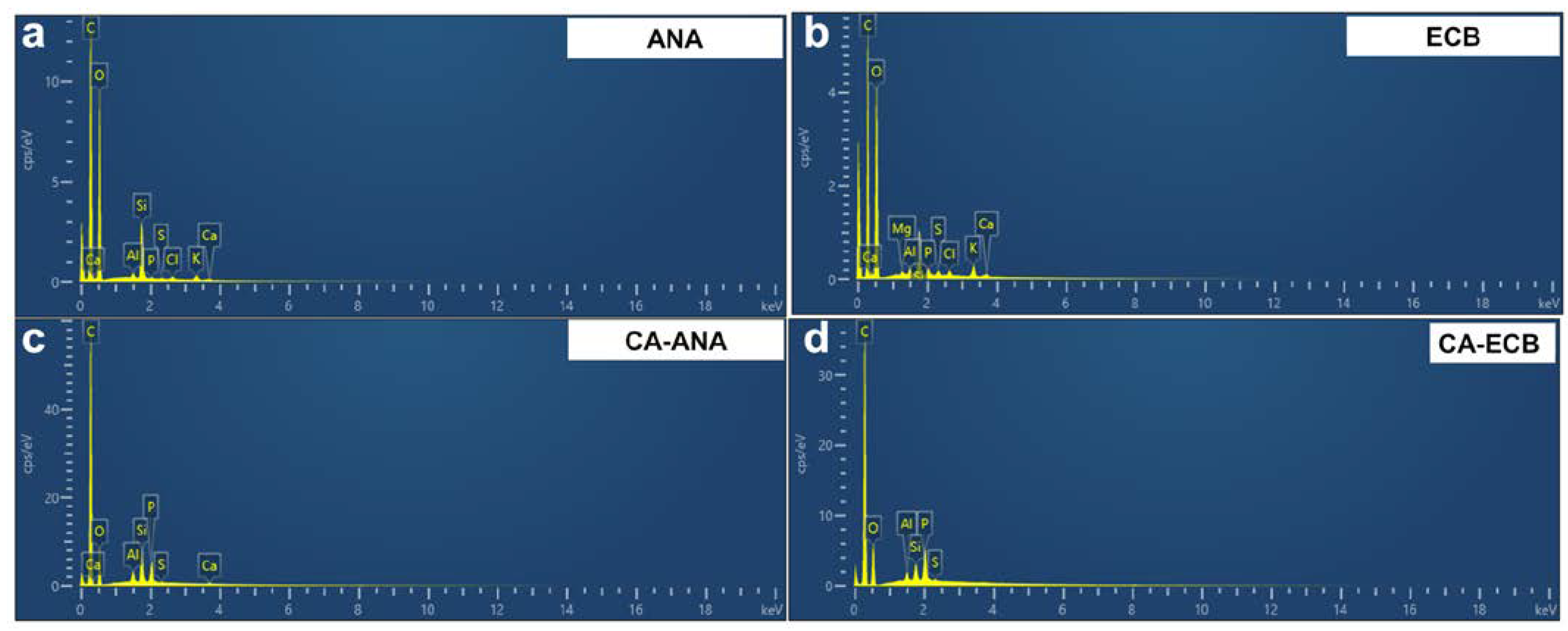
2.1. Characterization of Raw Materials
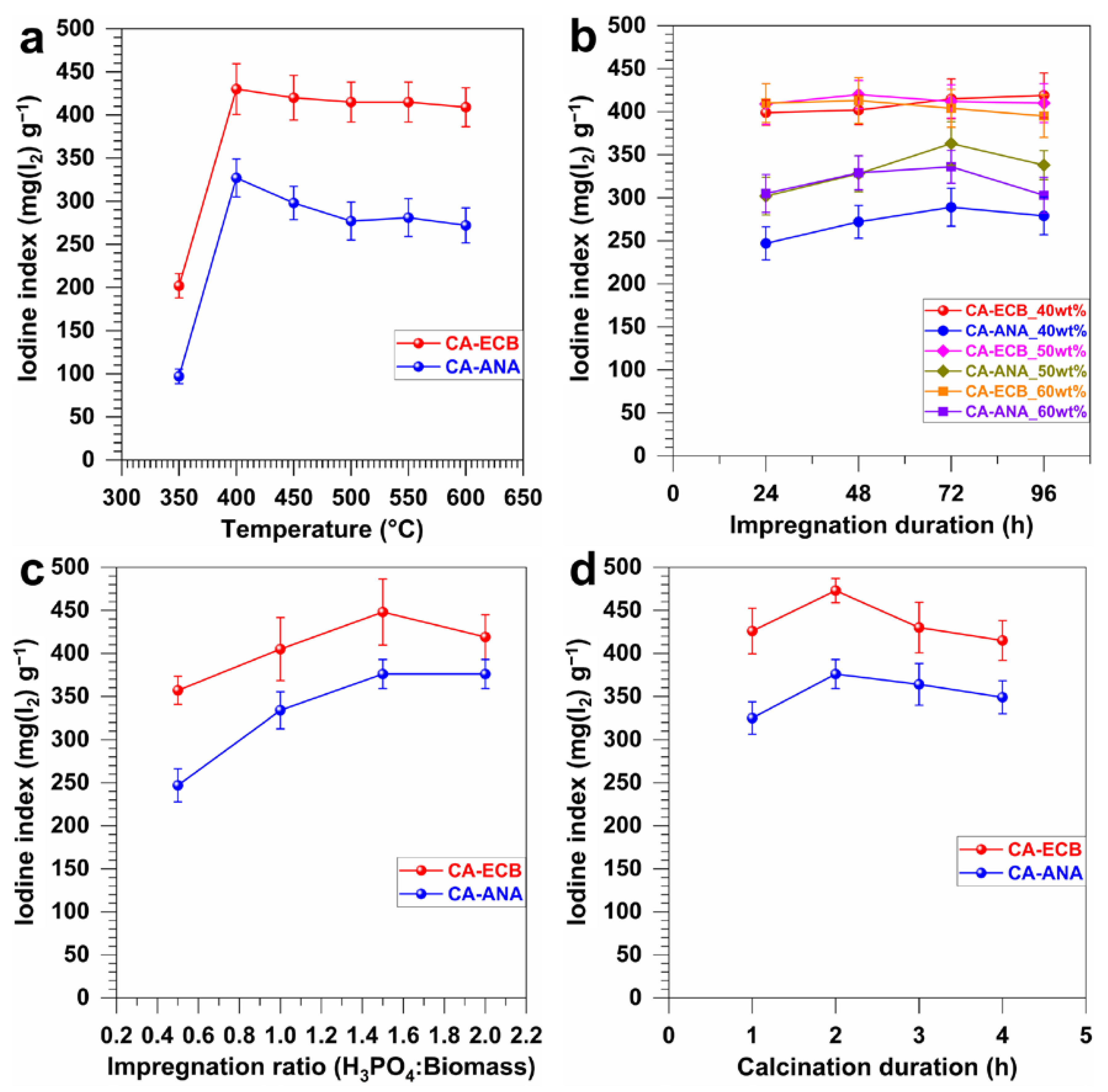
2.2. Optimized Parameters for Activated Carbon Synthesis
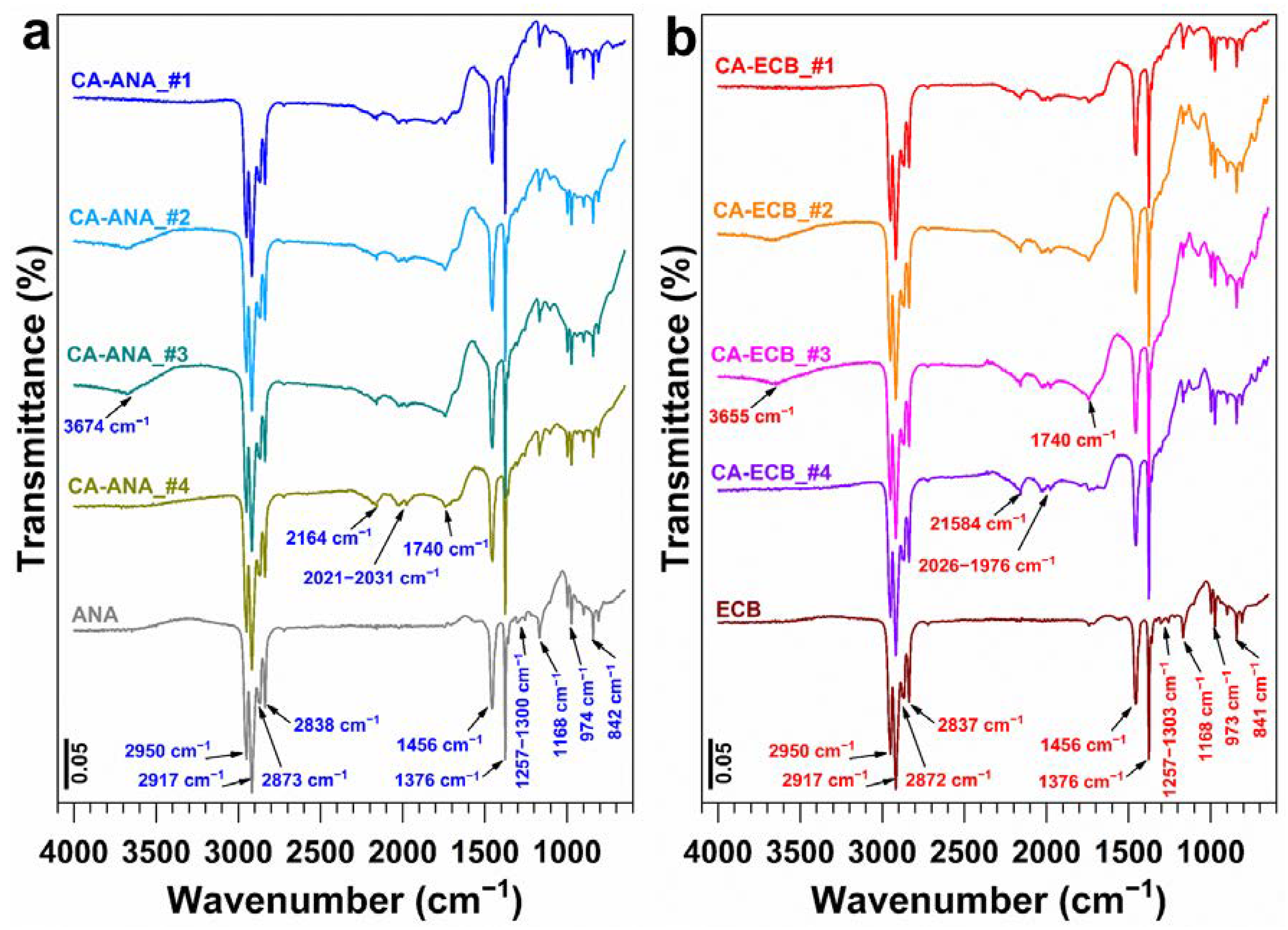
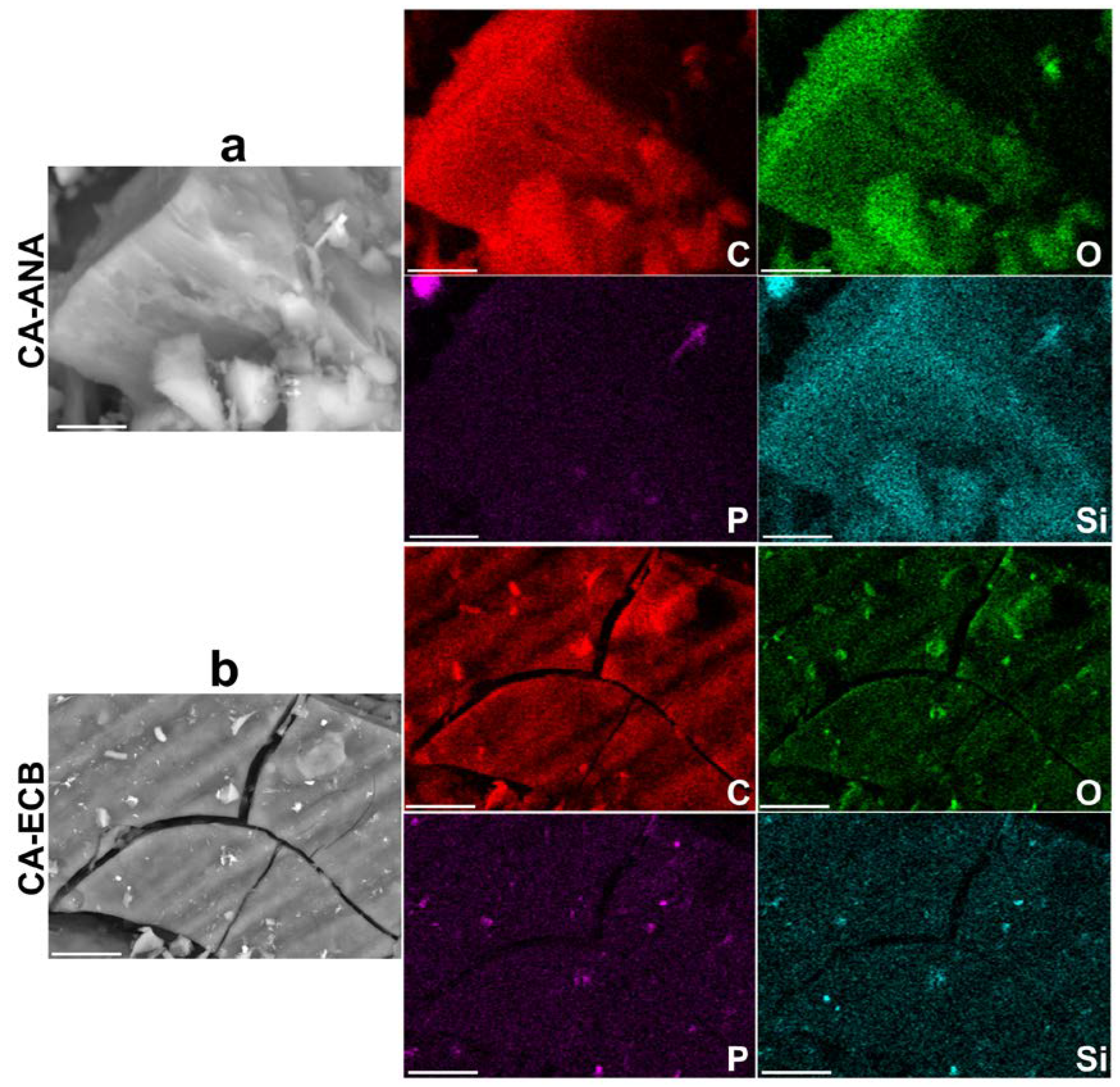
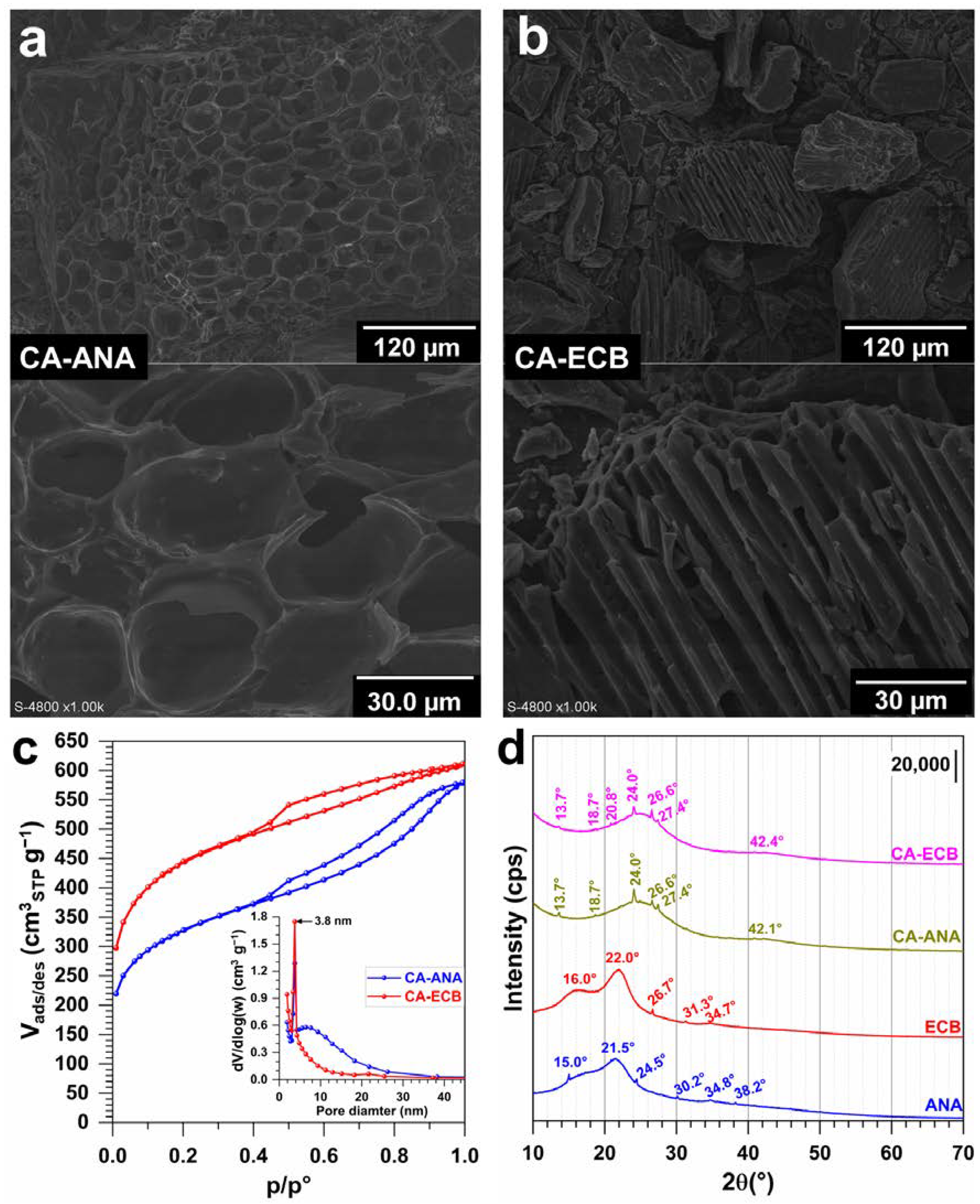
2.3. Results of the Physicochemical Characterization of Activated Carbon
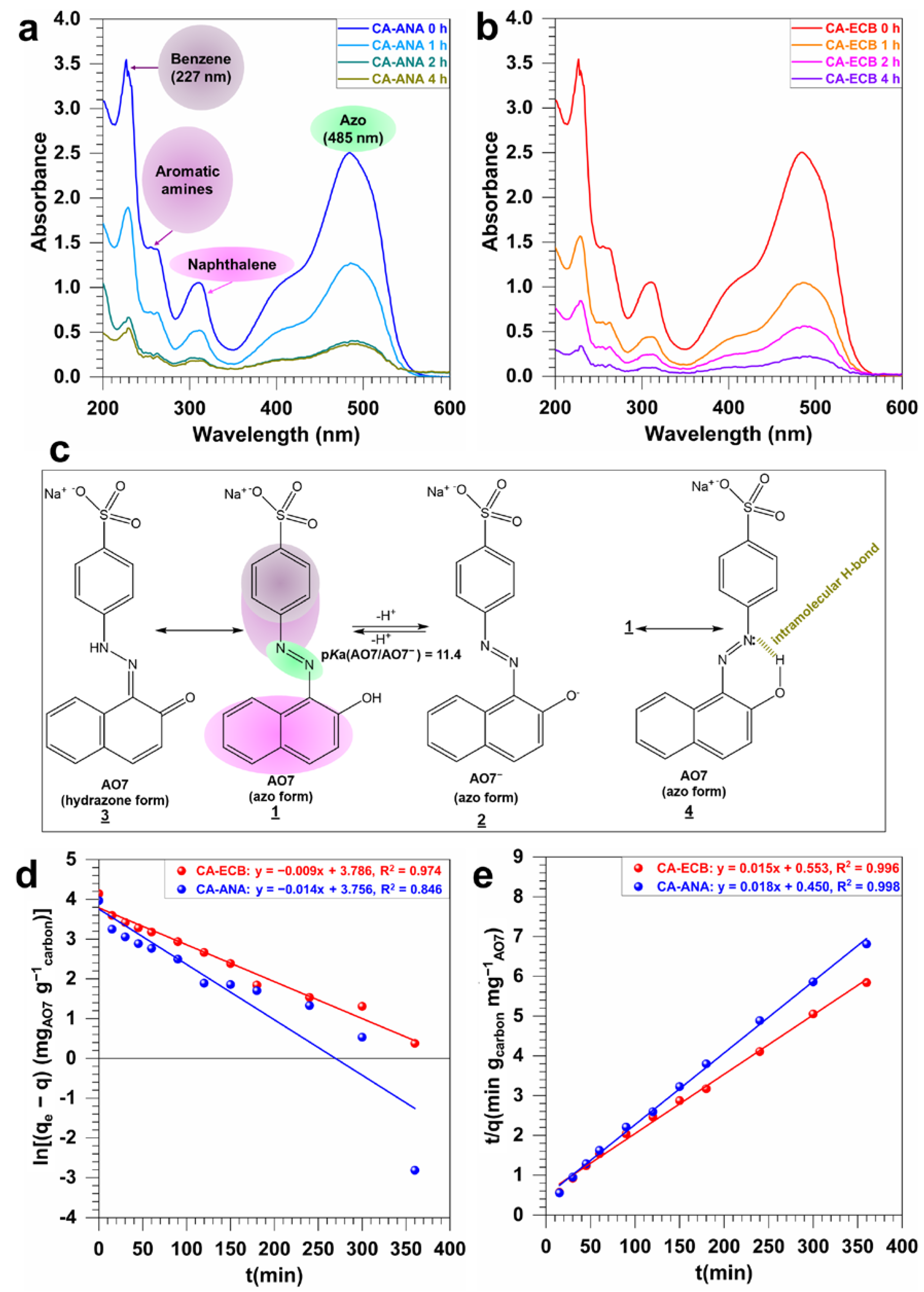
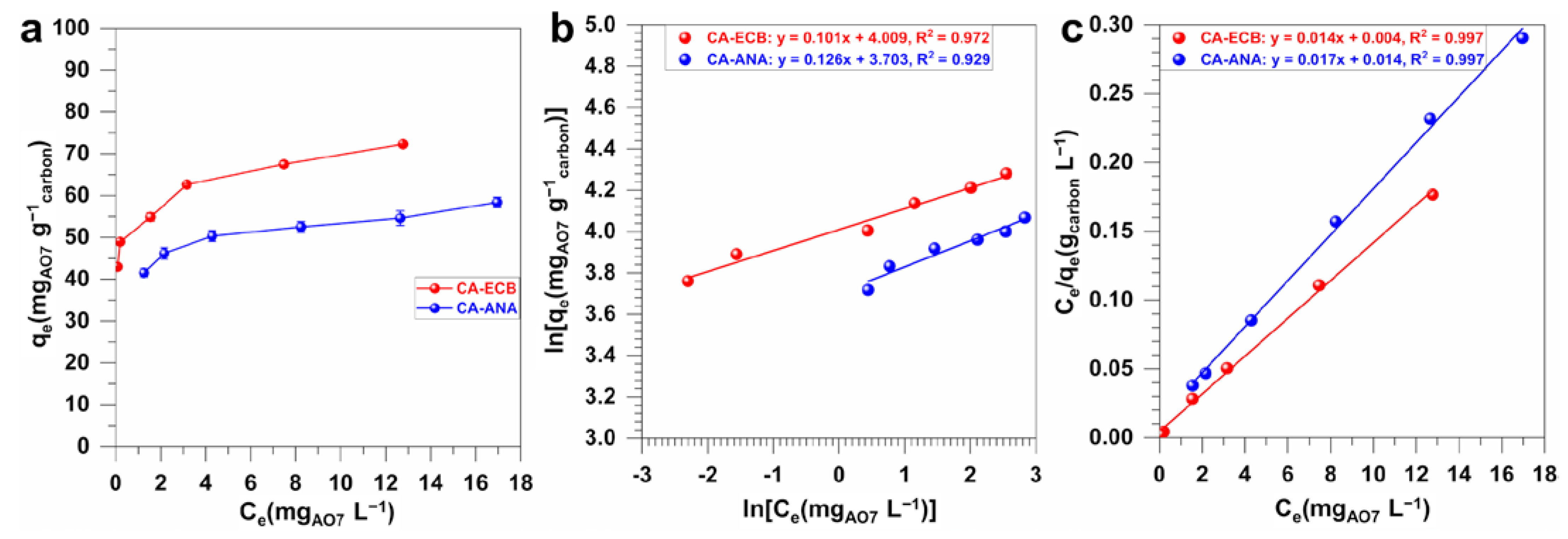
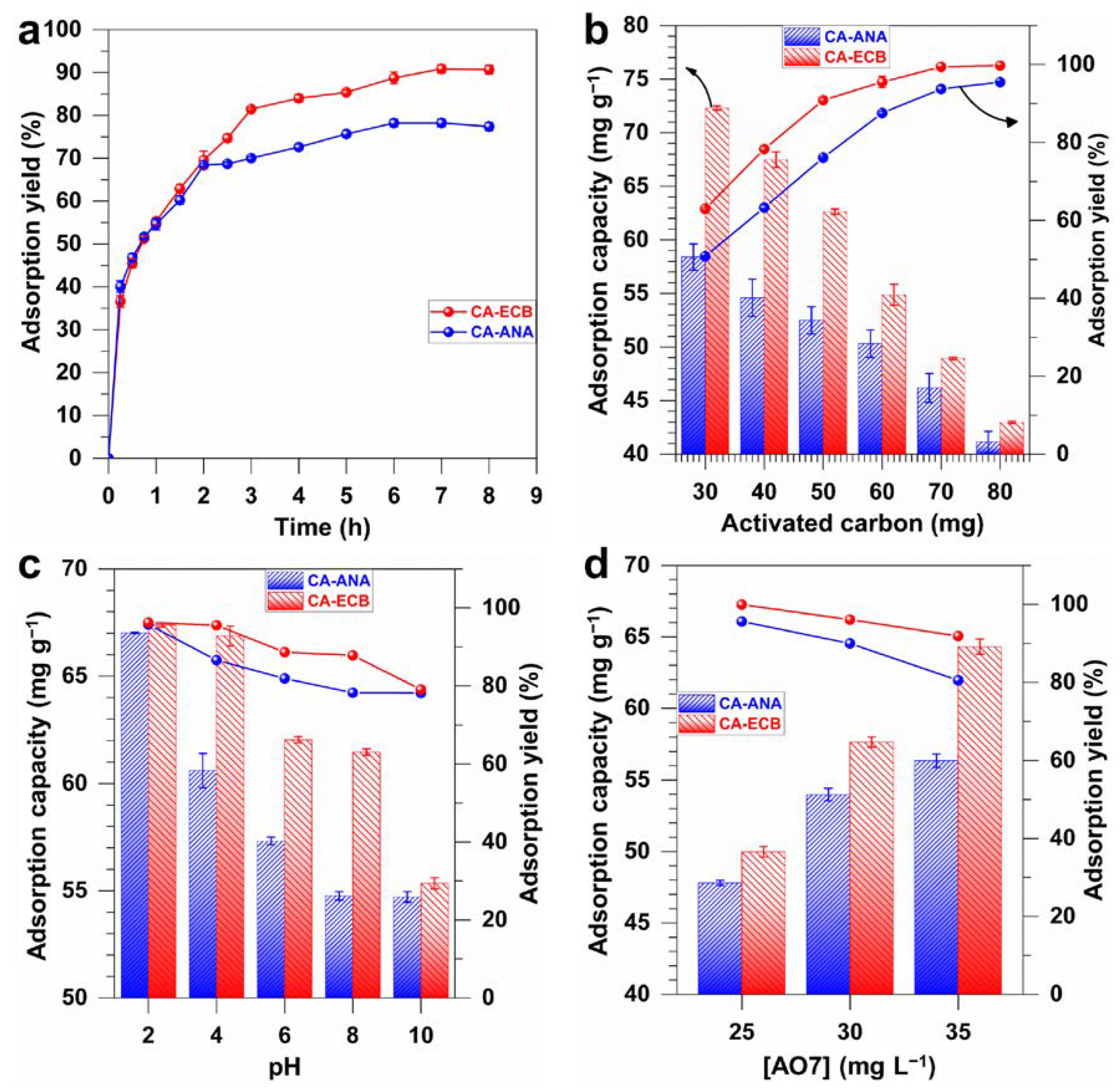
2.4. Acid Orange 7 Dye Adsorption Study
2.4.1. Adsorption Kinetics of AO7 on CA-ANA and CA-ECB Activated Carbons
2.4.2. Adsorption Isotherm of AO7 on CA-ANA and CA-ECB Activated Carbons
2.4.3. Study of the Influence of Contact Time, Adsorbent Mass, pH, and Initial Concentration on the Adsorption of AO7 onto Activated Carbons (CA-ANA, CA-ECB)
3. Materials and Methods
3.1. Chemicals
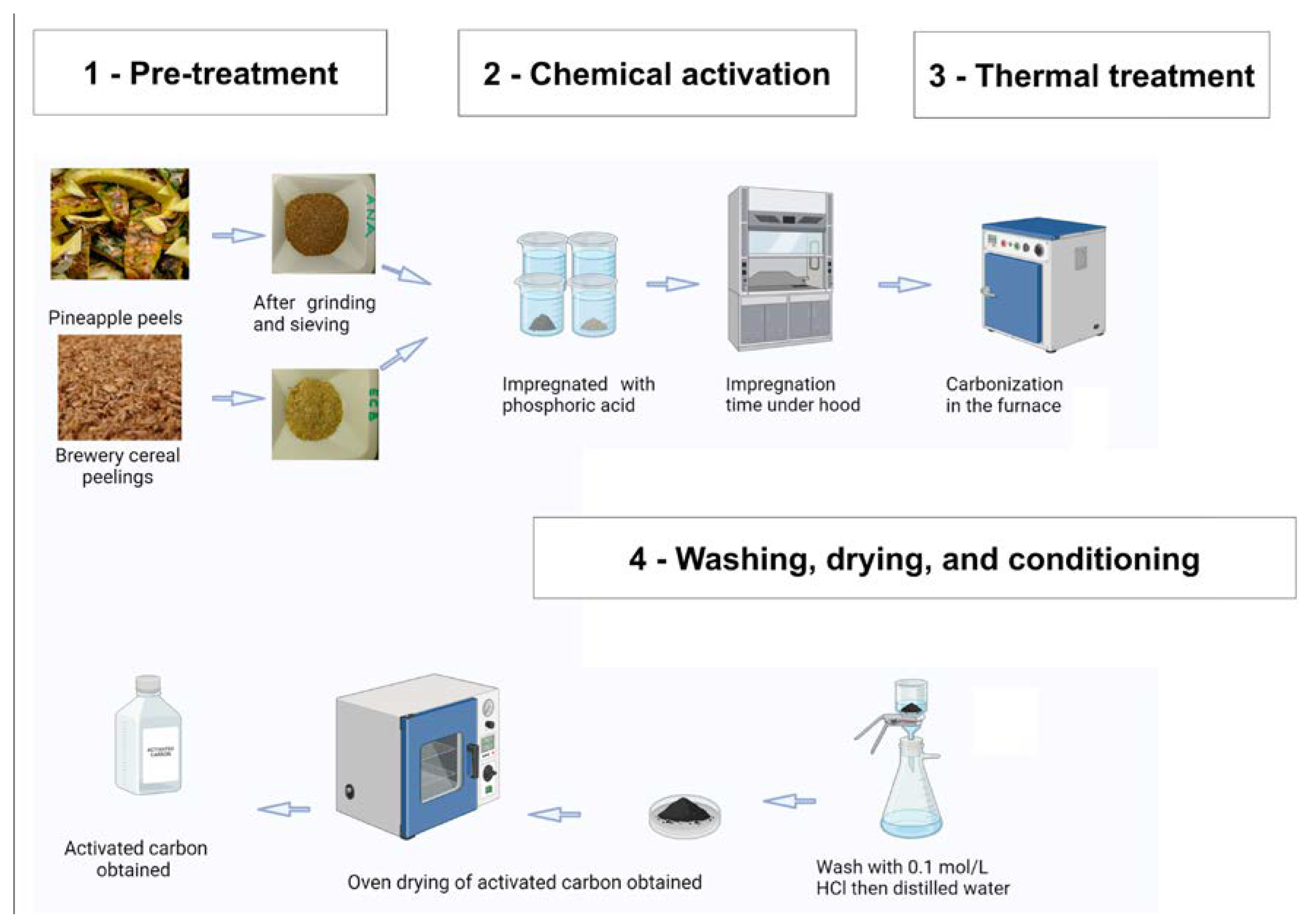
3.2. Selection, Collection, and Pre-Treatment of Raw Materials
3.3. Optimization and Thermal Processing of Raw Materials
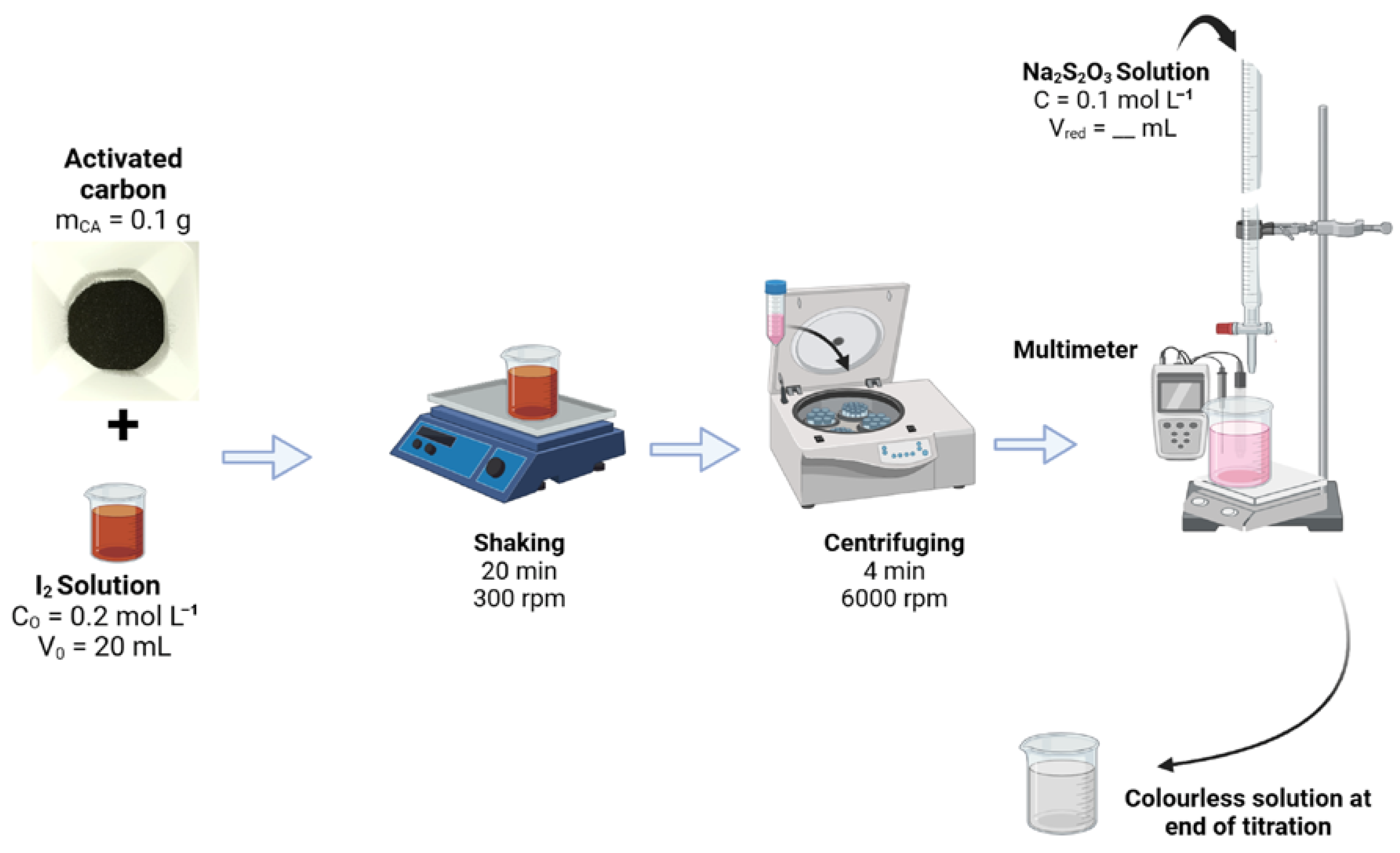
3.4. Physicochemical Characterization
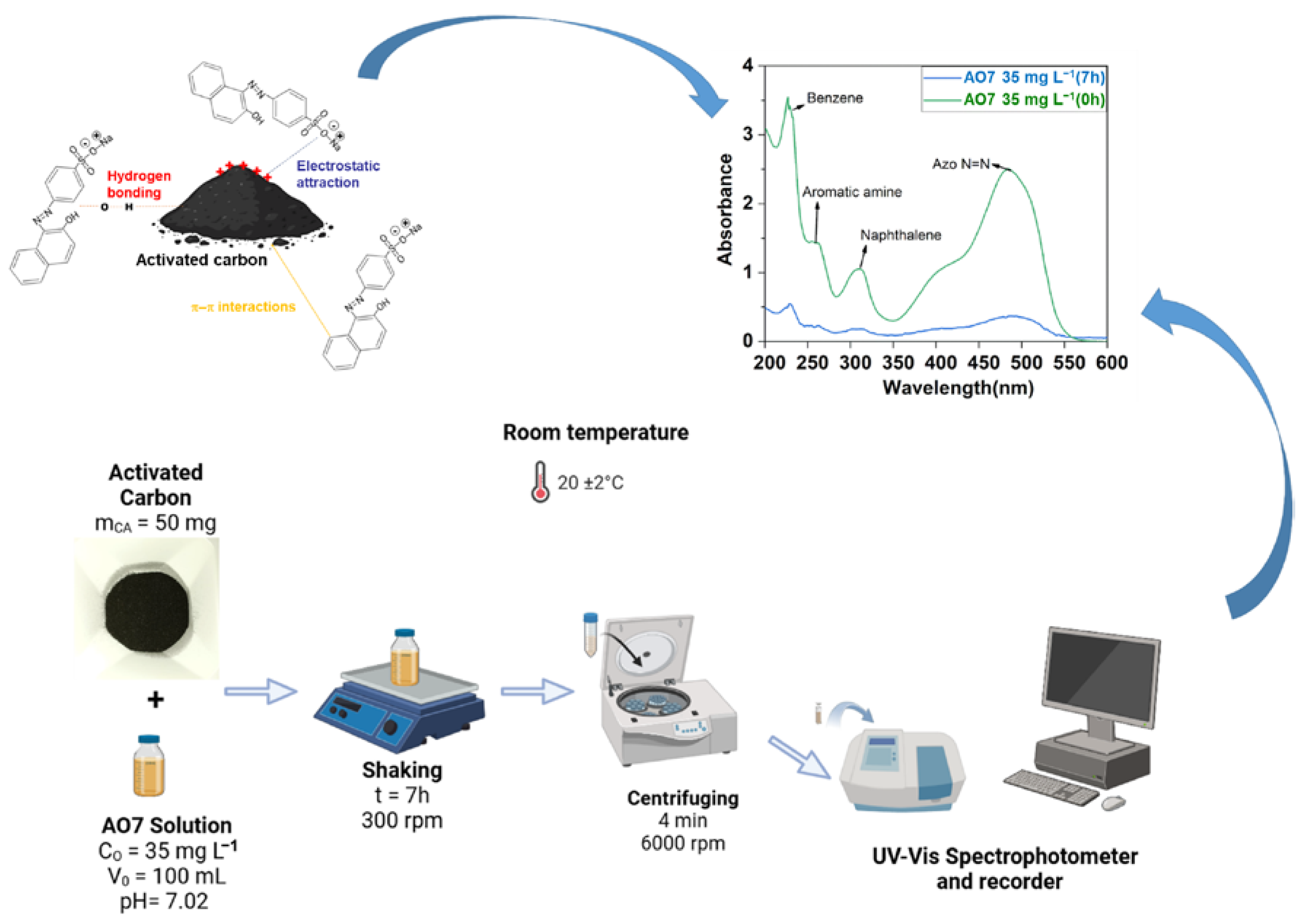
3.5. Removal of Acid Orange 7 (AO7) as a Model Pollutant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, R.; Liang, Z.; Yang, C.; Zhao, Z.; Cui, F. Selective adsorption of organic pigments on inorganically modified mesoporous biochar and its mechanism based on molecular structure. J. Colloid Int. Sci. 2020, 573, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Aouay, F.; Attia, A.; Dammak, L.; Ben Amar, R.; Deratani, A. Activated Carbon Prepared from Waste Coffee Grounds: Characterization and Adsorption Properties of Dyes. Materials 2024, 17, 3078. [Google Scholar] [CrossRef] [PubMed]
- Ouro-Agoro, F.; Dougna Akpénè, A.; Tchakala, I.; Sanni, S.; Kodom, T.; Alfa-Sika Mande, S.-L.; Bawa Limam, M. Synthesis of Activated Carbon from Cassava Peeling and Its Applications for Removal of Methylene Blue. Am. J. Phys. Chem. 2024, 13, 9–16. [Google Scholar]
- Aguilar, M.I.; Lloréns, M.; Ortuño, J.F.; Meseguer, V.F.; Pérez-Marín, A.B.; Cases, A. Use of the Box–Behnken Experimental Design for the Optimization of Orange II (Acid Orange 7) Adsorption on Aloe vera. Sustainability 2023, 15, 15727. [Google Scholar] [CrossRef]
- Aber, S.; Daneshvar, N.; Soroureddin, S.M.; Chabok, A.; Asadpour-Zeynali, K. Study of acid orange 7 removal from aqueous solutions by powdered activated carbon and modeling of experimental results by artificial neural network. Desalination 2007, 211, 87–95. [Google Scholar] [CrossRef]
- Yadav, A.K.; Chaubey, A.K.; Kapoor, S.; Pratap, T.; Preetiva, B.; Vimal, V.; Mohan, D. Sustainable Napier Grass (Pennisetum purpureum) Biochar for the Sorptive Removal of Acid Orange 7 (AO7) from Water. Processes 2024, 12, 1115. [Google Scholar] [CrossRef]
- Batista, A.C.L.; Silva, M.C.F.; Batista, J.B.; Nascimento, A.E.; Campos-Takaki, G.M. Eco-Friendly Chitosan Production by Syncephalastrum racemosum and Application to the Removal of Acid Orange 7 (AO7) from Wastewaters. Molecules 2013, 18, 7646–7660. [Google Scholar] [CrossRef]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Pathways of solar light-induced photocatalytic degradation of azo dyes in aqueous TiO2 suspensions. Appl. Catal. B Environ. 2003, 40, 271–286. [Google Scholar] [CrossRef]
- Eleryan, A.; Hassaan, M.A.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; El-Nemr, M.A.; Ragab, S.; Hossain, I.; El Nemr, A. Kinetic and isotherm studies of Acid Orange 7 dye absorption using sulphonated mandarin biochar treated with TETA. Biomass Convers. Biorefinery 2024, 14, 10599–10610. [Google Scholar] [CrossRef]
- Momeni, S.; Nematollahi, D. New insights into the electrochemical behavior of acid orange 7: Convergent paired electrochemical synthesis of new aminonaphthol derivatives. Sci. Rep. 2017, 7, 41963. [Google Scholar] [CrossRef]
- Sabnis, R.W. Handbook of Biological Dyes and Stains: Synthesis and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Oakes, J.; Gratton, P. Kinetic investigations of azo dye oxidation in aqueous media. J. Chem. Soc. Perkin Trans. 2 1998, 1857–1864. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Mirzaee, S.A.; Martinez, S.S.; Alavi, S.; Ahmadi, M.; Jaafarzadeh, N. Adsorption of textile dye in activated carbons prepared from DVD and CD wastes modified with multi-wall carbon nanotubes: Equilibrium isotherms, kinetics and thermodynamic study. Chem. Eng. Res. Des. 2019, 141, 290–301. [Google Scholar] [CrossRef]
- Boulika, H.; El Hajam, M.; Hajji Nabih, M.; Idrissi Kandri, N.; Zerouale, A. Activated carbon from almond shells using an eco-compatible method: Screening, optimization, characterization, and adsorption performance testing. RSC Adv. 2022, 12, 34393–34403. [Google Scholar] [CrossRef] [PubMed]
- El-Nemr, M.A.; El Nemr, A.; Hassaan, M.A.; Ragab, S.; Tedone, L.; De Mastro, G.; Pantaleo, A. Microporous Activated Carbon from Pisum sativum Pods Using Various Activation Methods and Tested for Adsorption of Acid Orange 7 Dye from Water. Molecules 2022, 27, 4840. [Google Scholar] [CrossRef]
- Huo, Y.; Zheng, H.; Jiang, Y.; Chen, H.; Cao, W.; Mameda, N.; Nghiem, L.D.; Zhang, X.; Liu, Q. Comparison and Characterization of Nitrogen/Sulfur-Doped Activated Carbon for Activating Peroxydisulfate to Degrade Acid Orange 7: An Experimental and Theoretical Study. Ind. Eng. Chem. Res. 2023, 62, 11894–11904. [Google Scholar] [CrossRef]
- Liang, P.; Rivallin, M.; Cerneaux, S.; Lacour, S.; Petit, E.; Cretin, M. Coupling cathodic Electro-Fenton reaction to membrane filtration for AO7 dye degradation: A successful feasibility study. J. Membr. Sci. 2016, 510, 182–190. [Google Scholar] [CrossRef]
- Guo, Y.; Rockstraw, D.A. Physicochemical properties of carbons prepared from pecan shell by phosphoric acid activation. Bioresour. Technol. 2007, 98, 1513–1521. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Yu, S.; Wang, L.; Li, Q.; Zhang, Y.; Zhou, H. Sustainable carbon materials from the pyrolysis of lignocellulosic biomass. Mater. Today Sustain. 2022, 19, 100209. [Google Scholar] [CrossRef]
- Piazza, V.; Batista da Silva Junior, R.; Frassoldati, A.; Lietti, L.; Gambaro, C.; Rajendran, K.; Chen, D.; Faravelli, T.; Beretta, A. Unravelling the complexity of hemicellulose pyrolysis: Quantitative and detailed product speciation for xylan and glucomannan in TGA and fixed bed reactor. Chem. Eng. J. 2024, 497, 154579. [Google Scholar] [CrossRef]
- Drané, M.; Zbair, M.; Hajjar-Garreau, S.; Josien, L.; Michelin, L.; Bennici, S.; Limousy, L. Unveiling the Potential of Corn Cob Biochar: Analysis of Microstructure and Composition with Emphasis on Interaction with NO2. Materials 2024, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Bouzikri, S.; Ouasfi, N.; Khamliche, L. Bifurcaria bifurcata activated carbon for the adsorption enhancement of Acid Orange 7 and Basic Red 5 dyes: Kinetics, equilibrium and thermodynamics investigations. Energy Nexus 2022, 7, 100138. [Google Scholar] [CrossRef]
- Sathasivam, K.V.; Haris, M.R.H.M.; Fuloria, S.; Fuloria, N.K.; Malviya, R.; Subramaniyan, V. Chemical Modification of Banana Trunk Fibers for the Production of Green Composites. Polymers 2021, 13, 1943. [Google Scholar] [CrossRef] [PubMed]
- Lewoyehu, M. Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J. Anal. Appl. Pyrolysis 2021, 159, 105279. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, C.; Zeng, G.; Huang, D.; Tan, X.; Duan, A. Lignocellulosic biomass carbonization for biochar production and characterization of biochar reactivity. Renew. Sustain. Energy Rev. 2022, 157, 112056. [Google Scholar] [CrossRef]
- Wasilewska, M.; Grządka, E. Activated Carbons as Effective Adsorbents of Non-Steroidal Anti-Inflammatory Drugs. Appl. Sci. 2024, 14, 743. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Shi, S.; Li, Y.; Luo, Y.; Huang, Z.; Xiao, W.; Wang, S.; Zhang, P.; Shu, Y.; et al. Embedding Hierarchical Pores by Mechanochemistry in Carbonates with Superior Chemoselective Catalysis and Stability. Inorg. Chem. 2023, 62, 12920–12930. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, L.; Cheng, W.; Shi, S.; Yuan, M.; Luo, Y.; Mei, L.; Xu, T.; Wang, S.; Zhao, D.; et al. Self-Stirring Microcatalysts: Large-Scale, High-Throughput, and Controllable Preparation and Application. Inorg. Chem. 2022, 61, 11757–11765. [Google Scholar] [CrossRef]
- Hegazy, S.; Ibrahim, H.H.; Weckman, T.; Hu, T.; Tuomikoski, S.; Lassi, U.; Honkala, K.; Srivastava, V. Synergistic pyrolysis of Cellulose/Fe-MOF Composite: A Combined experimental and DFT study on dye removal. Chem. Eng. J. 2025, 504, 158654. [Google Scholar] [CrossRef]
- Tchakala, I.; Bawa, L.; Djaneye-Boundjou, G.; Doni, K.; Nambo, P. Optimisation du procédé de préparation des Charbons Actifs par voie chimique (H3PO4) à partir des tourteaux de Karité et des tourteaux de Coton. Int. J. Biol. Chem. Sci. 2012, 6, 461–478. [Google Scholar]
- Jawad, A.H.; Rashid, R.A.; Ishak, M.A.M.; Wilson, L.D. Adsorption of methylene blue onto activated carbon developed from biomass waste by H2SO4 activation: Kinetic, equilibrium and thermodynamic studies. Desalin. Water Treat. 2016, 57, 25194–25206. [Google Scholar] [CrossRef]
- Balogoun, C.; Bawa, M.; Osseni, S.; Aina, M.P. Préparation des charbons actifs par voie chimique à l’acide phosphorique à base de coque de noix de coco. Int. J. Biol. Chem. Sci. 2015, 9, 563–580. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, R.; Wu, D.; Su, H.; Liu, Z.; Chen, Z. How hydrogen bonding and π–π interactions synergistically facilitate mephedrone adsorption by bio-sorbent: An in-depth microscopic scale interpretation. Environ. Pollut. 2024, 342, 123044. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Li, H. Kinetics and equilibrium study of phenol adsorption by activated carbon derived from pig blood. Carbon Trends 2023, 12, 100281. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Effect of pyrolysis temperature on polycyclic aromatic hydrocarbons toxicity and sorption behaviour of biochars prepared by pyrolysis of paper mill effluent treatment plant sludge. Bioresour. Technol. 2015, 192, 312–320. [Google Scholar] [CrossRef]
- Sait, H.H.; Hussain, A.; Salema, A.A.; Ani, F.N. Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour. Technol. 2012, 118, 382–389. [Google Scholar] [CrossRef]
- Dziejarski, B.; Hernández-Barreto, D.F.; Moreno-Piraján, J.C.; Giraldo, L.; Serafin, J.; Knutsson, P.; Andersson, K.; Krzyżyńska, R. Upgrading recovered carbon black (rCB) from industrial-scale end-of-life tires (ELTs) pyrolysis to activated carbons: Material characterization and CO2 capture abilities. Environ. Res. 2024, 247, 118169. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Okano, T.; Sugiyama, J. Allomorphs of native crystalline cellulose I evaluated by two equatoriald-spacings. J. Wood Sci. 2001, 47, 124–128. [Google Scholar] [CrossRef]
- Wada, M.; Hori, R.; Kim, U.-J.; Sasaki, S. X-ray diffraction study on the thermal expansion behavior of cellulose Iβ and its high-temperature phase. Polym. Degrad. Stab. 2010, 95, 1330–1334. [Google Scholar] [CrossRef]
- Darmawan, S.; Wistara, N.J.; Pari, G.; Maddu, A.; Syafii, W. Characterization of Lignocellulosic Biomass as Raw Material for the Production of Porous Carbon-based Materials. BioResources 2016, 11, 3561–3574. [Google Scholar] [CrossRef]
- Galvano, F.; Pietri, A.; Bertuzzi, T.; Piva, A.; Chies, L.; Galvano, M. Activated Carbons: In Vitro Affinity for Ochratoxin A and Deoxynivalenol and Relation of Adsorption Ability to Physicochemical Parameters. J. Food Prot. 1998, 61, 469–475. [Google Scholar] [CrossRef]
- González, J.F.; Encinar, J.M.; Ramiro, A.; Sabio, E. Regeneration by Wet Oxidation of an Activated Carbon Saturated with p-Nitrophenol. Ind. Eng. Chem. Res. 2002, 41, 1344–1351. [Google Scholar] [CrossRef]
- Galvano, F.; Pietri, A.; Fallico, B.; Bertuzzi, T.; Scirè, S.; Galvano, M.; Maggiore, R. Activated Carbons: In Vitro Affinity for Aflatoxin B1 and Relation of Adsorption Ability to Physicochemical Parameters. J. Food Prot. 1996, 59, 545–550. [Google Scholar] [CrossRef]
- Keirsse, H.; Van Hoof, F.; Janssens, J.; Buekens, A. Water Treatment by Means of Activated Carbon, Prepared from Locally Available Waste Materials (II). Water Sci. Technol. 1986, 18, 55–66. [Google Scholar] [CrossRef]
- Ruiz Bevia, F.; Prats Rico, D.; Marcilla Gomis, A.F. Activated carbon from almond shells. Chemical activation. 1. Activating reagent selection and variables influence. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 266–269. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Y.; Wang, D.; Sun, Z.; Chen, M.; Zhou, Z.; Chen, W. Performance and mechanism of low-frequency ultrasound to regenerate the biological activated carbon. Ultrason. Sonochem. 2017, 34, 142–153. [Google Scholar] [CrossRef]
- Babas, H.; Khachani, M.; Warad, I.; Ajebli, S.; Guessous, A.; Guenbour, A.; Safi, Z.; Berisha, A.; Bellaouchou, A.; Abdelkader, Z.; et al. Sofosbuvir adsorption onto activated carbon derived from argan shell residue: Optimization, kinetic, thermodynamic and theoretical approaches. J. Mol. Liq. 2022, 356, 119019. [Google Scholar] [CrossRef]
- Guanfeng, L.; WU, K.K.; Huang, B. Effects of Small Amounts of Phosphoric Acid as Additive in the Preparation of Microporous Activated Carbons. Mater. Sci. 2018, 24, 362–366. [Google Scholar]
- Shahcheragh, S.K.; Bagheri Mohagheghi, M.M.; Shirpay, A. Effect of physical and chemical activation methods on the structure, optical absorbance, band gap and urbach energy of porous activated carbon. SN Appl. Sci. 2023, 5, 313. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Husien, S.; El-taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Curr. Res. Green Sustain. Chem. 2022, 5, 100325. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, A.H.; Pham, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption isotherms and kinetic modeling of methylene blue dye onto a carbonaceous hydrochar adsorbent derived from coffee husk waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef]
- Ghasemi, A.; Shams, M.; Qasemi, M.; Afsharnia, M. Data on efficient removal of acid orange 7 by zeolitic imidazolate framework-8. Data Brief 2019, 23, 103783. [Google Scholar] [CrossRef]
| Biomass | Humidity (105 °C) wt% | Dry Matter (110 °C) wt% | Volatile Matter (550 °C) wt% | Ash Content (700 °C) wt% |
|---|---|---|---|---|
| ECB | 4.6 | 95.4 | 69.6 | 27.9 |
| ANA | 2.0 | 98.0 | 69.0 | 28.7 |
| Optimization Parameters | Iodine Index mg(I2) g−1 | |||||
|---|---|---|---|---|---|---|
| Carbonization Temperature (°C) | Phosphoric Acid (wt%) | Impregnation Duration | Impregnation Ratio H3PO4/Biomass (w/w) | Carbonization Time (h) | CA-ANA | CA-ECB |
| Blank | - | - | - | - | 9 ± 1 | 17 ±1 |
| 350 | 50 | 48 | 1:1 | 2 | 97 ± 8 | 202 ± 14 |
| 400 | 50 | 48 | 1:1 | 2 | 328 ± 21 | 430 ± 29 |
| 450 | 50 | 48 | 1:1 | 2 | 298 ± 19 | 420 ± 26 |
| 500 | 50 | 48 | 1:1 | 2 | 277 ± 21 | 415 ± 23 |
| 550 | 50 | 48 | 1:1 | 2 | 281 ± 21 | 415 ± 23 |
| 600 | 50 | 48 | 1:1 | 2 | 272 ± 20 | 409 ± 23 |
| 400 | 40 | 24 | 1:1 | 2 | 247 ± 19 | 399 ± 15 |
| 400 | 40 | 48 | 1:1 | 2 | 272 ± 19 | 402 ± 17 |
| 400 | 40 | 72 | 1:1 | 2 | 289 ± 22 | 415 ± 23 |
| 400 | 40 | 96 | 1:1 | 2 | 279 ± 22 | 419 ± 26 |
| 400 | 50 | 24 | 1:1 | 2 | 302 ± 22 | 409 ± 24 |
| 400 | 50 | 48 | 1:1 | 2 | 328 ± 21 | 421 ± 16 |
| 400 | 50 | 72 | 1:1 | 2 | 363 ± 25 | 412 ± 19 |
| 400 | 50 | 96 | 1:1 | 2 | 338 ± 17 | 410 ± 23 |
| 400 | 60 | 24 | 1:1 | 2 | 305 ± 22 | 410 ± 23 |
| 400 | 60 | 48 | 1:1 | 2 | 329 ± 20 | 413 ± 27 |
| 400 | 60 | 72 | 1:1 | 2 | 336 ± 19 | 404 ± 22 |
| 400 | 60 | 96 | 1:1 | 2 | 303 ± 21 | 395 ± 25 |
| 400 | 50 | 48 (ECB) 72 (ANA) | 0.5:1 | 2 | 247 ± 19 | 357 ± 16 |
| 400 | 50 | 48 (ECB) 72 (ANA) | 1:1 | 2 | 334 ± 21 | 405 ± 37 |
| 400 | 50 | 48 (ECB) 72 (ANA) | 1.5:1 | 2 | 376 ± 17 | 473 ± 14 |
| 400 | 50 | 48 (ECB) 72 (ANA) | 2:1 | 2 | 376 ± 17 | 419 ± 26 |
| 400 | 50 | 48 (ECB) 72 (ANA) | 1.5:1 | 1 | 325 ± 18 | 426 ± 27 |
| 400 | 50 | 48 (ECB) 72 (ANA) | 1.5:1 | 2 | 376 ± 17 | 473 ± 14 |
| 400 | 50 | 48 (ECB) 72 (ANA) | 1.5:1 | 3 | 364 ± 24 | 430 ± 29 |
| 400 | 50 | 48 (ECB) 72 (ANA) | 1.5:1 | 4 | 349 ± 19 | 415 ± 23 |
| Before Calcination | After Calcination | |||||||
|---|---|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | |||||
| Element | ECB | ANA | ECB | ANA | CA-ECB | CA-ANA | CA-ECB | CA-ANA |
| C | 55.7 ± 0.1 | 54.8 ± 0.1 | 63.2 ± 0.1 * | 62.5 ± 1.7 | 79.3 ± 0.4 | 79.3 ± 1.5 | 84.1 ± 0.3 | 84.1 ± 1.3 |
| O | 41.8 ± 0.1 | 42.4 ± 1.7 | 35.6 ± 0.1 * | 36.3 ± 1.7 | 19.1 ± 0.4 | 19.1 ± 1.7 | 15.2 ± 0.3 | 15.2 ± 1.3 |
| Mg | 0.1± 0.1 * | 0.1 ± 0.1 | <0.1 | <0.1 | - | - | - | - |
| Al | 0.3 ± 0.1 | 0.2 ± 0.1 | - | - | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 * | 0.1 ± 1.3 |
| Si | 1.0 ± 0.3 | 1.4 ± 0.7 | 0.5 ± 0.1 | 0.7 ± 0.3 | 0.4 ± 0.1 * | 0.9 ± 0.1 * | 0.2 ± 0.1 * | 0.4 ± 1.3 |
| P | 0.2 ± 0.1 * | 0.1 ± 0.1 * | <0.1 | <0.1 | 0.9 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 * | 0.2 ± 0.1 * |
| S | 0.1 ± 0.1 * | 0.1 ± 0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Cl | 0.2 ± 0.1 * | 0.2 ± 0.1 | 0.1 ± 0.1 * | 0.1 ± 0.1 * | <0.1 | <0.1 | <0.1 | <0.1 |
| K | 0.5 ± 0.1 * | 0.5 ± 0.3 | 0.2 ± 0.1 * | 0.2 ± 0.1 * | <0.1 | <0.1 | <0.1 | <0.1 |
| Ca | 0.1 ± 0.1 * | 0.2 ± 0.2 | 0.1 ± 0.1 * | 0.1 ± 0.1 * | <0.1 | <0.1 | <0.1 | <0.1 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Entry | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Diameter (nm) |
|---|---|---|---|
| CA-ANA | 1147 | 0.6 | 3.8 |
| CA-ECB | 1626 | 0.4 | 3.8 |
| Model 1: Pseudo-First-Order Kinetics (PFO) | |||||
|---|---|---|---|---|---|
| qe1-exp (mgAO7 g−1carbon) | qe1-th (mgAO7 g−1carbon) | R2 | k1 (10−3 min−1) | Error Rate * (%) | |
| CA-ANA | 52.9 | 42.8 | 0.846 | 14.0 | 19.1 |
| CA-ECB | 63.1 | 44.1 | 0.974 | 9.0 | 30.1 |
| Model 2: Pseudo-Second-Order Kinetics (PSO) | |||||
| qe2-exp (mgAO7 g−1carbon) | qe2-th (mgAO7 g−1carbon) | R2 | k2 (10−4 gcarbon mg−1AO7 min−1) | Error Rate (%) | |
| CA-ANA | 52.9 | 55.6 | 0.998 | 72.0 | 5.1 |
| CA-ECB | 63.1 | 66.7 | 0.996 | 4.1 | 5.7 |
| Freundlich | Langmuir | ||||||
|---|---|---|---|---|---|---|---|
| n | KF (mgAO7(1−1/n) L−1/n g−(1+1/n)carbon) | R2 | Q0 (mgAO7 g−1carbon) | KL (L mg−1AO7) | RL | R2 | |
| CA-ECB | 9.9 | 55.1 | 0.97 | 71.4 | 3.5 | 1.0 × 10−2 | 0.99 |
| CA-ANA | 7.9 | 40.6 | 0.93 | 58.8 | 1.2 | 2.3 × 10−2 | 0.99 |
| Adsorbent | Surface Area (m2 g−1) | Experimental Conditions 1 | Maximum Adsorption Capacity (mgAO7 g−1carbon) | Reference |
|---|---|---|---|---|
| Activated carbon derived from brewing cereals residues | 1626 | Batch sorption C0 = 35 mg L−1, T = ambient (20 ± 2 °C), pH = 7, teq = 420 min, AC dosage = 0.5 g L−1 | 63.1 | This study |
| Activated carbon derived from pineapple peels | 1147 | Batch sorption C0 = 35 mg L−1, T = ambient (20 ± 2 °C), pH = 7, teq = 420 min, AC dosage = 0.5 g L−1 | 52.9 | This study |
| Sustainable Napier Grass (Pennisetum purpureum) biochar | 108 | Batch sorption C0 = 10–30 mg L−1, T = 25 °C, pH = 6.5, teq = 720 min, AC dosage = 2 g L−1 | 12.7 | [6] |
| Activated carbon from Pisum sativum pods | 1500 | Batch sorption C0 = 400 mg L−1, T = ambient, pH = 1.5, teq = 60 min, AC dosage = 2.5 g L−1 | 467.2 | [15] |
| Activated carbon from waste coffee grounds | 29 | Batch sorption C0 = 20 mg L−1, T = ambient, pH = 7.5, teq = 40 min, AC dose = 0.285 g L−1 | 119.5 | [2] |
| Activated carbon from the Bifurcaria bifurcata algae | 157 | Batch sorption C0 = 10 mg L−1, T = 25 °C, pH = 7.5, teq = 120 min, AC dose = 0.2 g L−1 | 44.3 | [23] |
| Zeolitic imidazolate framework-8 (ZIF-8) | 978 | Batch sorption C0 = 100 mg L−1, T = 25 °C, pH = 6, teq = 720 min, AC dose = 0.6 g L−1 | 80.5 | [60] |
| Fe3O4 modified biochar from sorghum straw | 216.6 | Batch sorption C0 = 50 mg L−1, T = 25° C, pH = 6, teq = 720 min, AC dose = 0.6 g L−1 | 59.3 | [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanni, S.; Tchakala, I.; Kodom, T.; Karamoko, B.A.; Bawa, L.M.; Holade, Y. Activated Carbons Derived from Brewing Cereal Residues and Pineapple Peelings for Removal of Acid Orange 7 (AO7) Dye. Molecules 2025, 30, 881. https://doi.org/10.3390/molecules30040881
Sanni S, Tchakala I, Kodom T, Karamoko BA, Bawa LM, Holade Y. Activated Carbons Derived from Brewing Cereal Residues and Pineapple Peelings for Removal of Acid Orange 7 (AO7) Dye. Molecules. 2025; 30(4):881. https://doi.org/10.3390/molecules30040881
Chicago/Turabian StyleSanni, Samadou, Ibrahim Tchakala, Tomkouani Kodom, Bonito Aristide Karamoko, Limam Moctar Bawa, and Yaovi Holade. 2025. "Activated Carbons Derived from Brewing Cereal Residues and Pineapple Peelings for Removal of Acid Orange 7 (AO7) Dye" Molecules 30, no. 4: 881. https://doi.org/10.3390/molecules30040881
APA StyleSanni, S., Tchakala, I., Kodom, T., Karamoko, B. A., Bawa, L. M., & Holade, Y. (2025). Activated Carbons Derived from Brewing Cereal Residues and Pineapple Peelings for Removal of Acid Orange 7 (AO7) Dye. Molecules, 30(4), 881. https://doi.org/10.3390/molecules30040881









