Abstract
Supercapacitors are widely studied for their high energy density, low cost, and exceptional cycling durability. However, the decisive factor in determining the performance of supercapacitors is the electrode material. Among emerging materials, metal glycerates stand out as tunable organic-inorganic hybrids with well-controlled structures. Yet, progress in tailoring metal glycerates for supercapacitors has not been organized or consolidated into a coherent framework. Herein, we systematically summarize recent advances in the synthesis, structural evolution, and electrochemical applications of metal glycerates and their derivatives (including hydroxides, oxides, sulfides, phosphides, selenides, and composites) as electrodes for supercapacitors, emphasizing the intrinsic structure-performance correlations. Finally, the key challenges and future prospects, covering controlled synthesis, interfacial stability, mechanistic insight, and device-level integration, are discussed to guide the rational design of next-generation MG-based materials for high-performance, sustainable supercapacitor technologies.
1. Introduction
The rapid growth of global population, along with accelerated industrialization and economic expansion, has placed enormous strain on both energy resources and the environment [1,2,3,4,5,6,7]. Currently, fossil fuels (such as coal, oil, and natural gas) remain the dominant energy sources, yet they are nonrenewable and are being rapidly consumed, releasing substantial amounts of greenhouse gases and hazardous pollutants that severely damage the environment [8,9,10,11,12,13,14]. In response to these critical issues, the global energy landscape is undergoing a profound transformation, marked by a surge in the development and deployment of renewable energy sources such as solar, wind, and hydrogen energy [15,16,17,18,19,20,21]. Despite their environmental benefits and theoretical abundance, these renewable resources are inherently intermittent and unevenly distributed, which poses serious challenges to ensuring a stable and continuous energy supply on a large scale [22,23,24,25,26]. Consequently, converting renewable energy into electricity and storing it for reliable use is therefore considered a promising strategy to overcome these limitations [27,28]. This underscores the urgent need for advanced, low-cost, and multifunctional electrochemical energy storage (EES) devices that can effectively bridge the gap between energy generation and utilization [29,30,31,32].
Among various EES technologies, supercapacitors (SCs) have emerged as promising devices that bridge the performance gap between conventional capacitors and batteries due to their rapid charge–discharge capability, high power density, long cycle life, and environmental friendliness [33,34,35]. Based on their energy storage mechanisms, SCs are primarily classified into three categories: electric double-layer capacitors (EDLCs), pseudocapacitors (PCs), and hybrid supercapacitors (HSCs) [36,37]. EDLCs store energy through electrostatic ion adsorption at the electrode-electrolyte interface, without involving Faradaic reactions [38,39,40]. Carbon-based materials, such as activated carbon, graphene, and carbon nanotubes, are commonly used as EDLC electrodes owing to their large specific surface area, high electrical conductivity, and adjustable pore structures [40,41,42,43]. In contrast, PCs store charge via fast and reversible Faradaic redox reactions occurring at or near the surface of electrode materials, typically transition metal oxides or conducting polymers, which offer higher energy density but often suffer from structural instability and limited cycle life [44,45,46]. HSCs, meanwhile, integrate the advantages of both EDLCs and PCs by employing an asymmetric configuration consisting of a capacitive carbon-based electrode and a battery-type Faradaic electrode [47]. This combination allows HSCs to achieve enhanced energy density without sacrificing the characteristic high power output and cycle stability of EDLCs [48].
Due to their advantageous characteristics, SCs have been implemented in a variety of emerging applications, including hybrid electric vehicles, wearable electronics, and smart grids [37]. Nevertheless, their practical deployment is still constrained by their intrinsically low energy density [49,50]. Structurally, SCs typically comprise electrode materials, current collectors, separators, and electrolytes, among which the electrode materials play a pivotal role in determining electrochemical performance of the devices [39,51]. The energy density of SCs is primarily governed by the specific capacitance and operational voltage of the electrode materials, highlighting the critical need for advanced materials with optimized electrochemical properties and broader voltage windows [52,53].
In this context, metal-glycerate (MG)-based materials have garnered considerable attention as promising electrode materials for SCs due to their unique combination of inorganic and organic characteristics [54,55,56]. MGs are composed of glycerate anions and metal ions and are typically regarded as inorganic polymers that integrate the structural and chemical features of both organic and inorganic components, similar to metal–organic frameworks (MOFs) [57,58,59,60,61,62,63]. The coordination between polyhydroxy ligands and metal centers imparts MGs with notable structural stability, tunable composition, and electrochemical activity [64,65]. In addition to their direct application in SCs, MGs serve as versatile precursors or templates for fabricating advanced electrode materials [66,67,68]. For example, MGs can be converted into various metal-based compounds, including oxides, sulfides, phosphide, and other derivatives, through thermal treatment processes such as oxidation, sulfuration, or phosphorization [69,70,71]. The diversity of MGs and their derivatives largely stems from the wide selection of transition metals (e.g., Co, Ni, Fe, Mn, Mo, and V) that can be used as metal centers [50,72,73,74,75,76,77]. These ions not only construct the coordination framework but also impart rich physicochemical properties essential for energy storage applications.
Through continuous efforts in tailoring MGs and their derivatives to meet the specific requirements of SC systems, researchers have successfully developed a range of high-performance SCs. However, despite significant progress in the design and synthesis of these materials for SC applications, comprehensive reviews that systematically summarize recent advances and critically analyze the underlying structure-property relationships remain scarce. Moreover, unlike previous reviews that either take a broad view of various energy-related applications or concentrate mainly on electrocatalytic water splitting, this review focuses exclusively on metal-glycerates and their derivatives for supercapacitor applications, offering a comprehensive and in-depth analysis to better inform and guide future research in this emerging field.
In this review, we provide a comprehensive summary of recent advances in MGs and their derivatives as electrode materials for SCs, with particular interest in synthetic methods, and especially SC applications. We begin by outlining the synthesis strategies for MGs and their derivatives, followed by an in-depth discussion of their application as high-performance electrodes in SCs (Figure 1). Finally, we outline the current challenges and provide future perspectives on the rational design of MG-based materials for next-generation supercapacitor technologies. This review aims to offer valuable insights and guidance for advancing the development of MG-based materials and accelerating their practical deployment in high-performance SC systems.
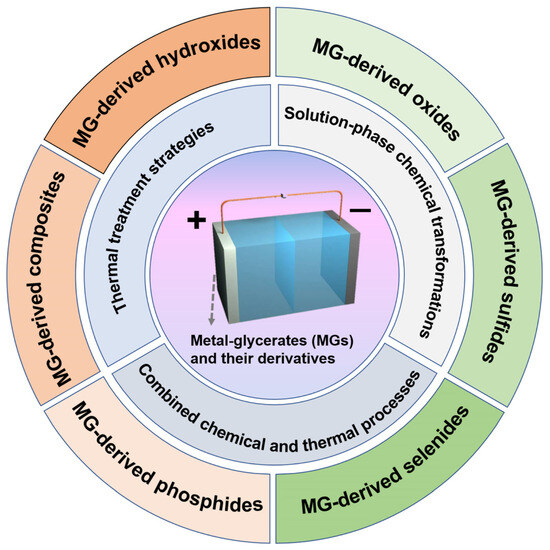
Figure 1.
Schematic diagram outlining the strategy for employing metal glycerates and their derivatives in supercapacitor applications.
2. Synthetic Strategies of MGs and Their Derivatives
2.1. Synthetic Strategies of MGs
Solvothermal synthesis is a widely employed and effective method for preparing MGs [55,65]. Under solvothermal conditions, multidentate glycerol ligands chelate metal cations to form MG frameworks that, aided by viscosity and hydrogen bonding of glycerol, self-assemble into low-surface-energy spherical aggregates, giving rise to monodisperse microspheres [54,57,64,78]. The type and concentration of metal precursors, surfactants, reaction parameters such as temperature and duration play pivotal roles in determining the morphological and structural characteristics of the resulting MGs [79,80,81]. By precisely adjusting the molar ratios of metal ions, surfactants, and reaction conditions, the nucleation and crystal growth processes can be carefully controlled. Numerous studies have reported the use of various metal salts as metal sources and glycerol as an organic linker in the solvothermal synthesis of MGs, enabling the tailored design of their composition and structure.
The selection of metal precursors allows for the solvothermal synthesis of MGs with a wide range of metal compositions, encompassing single-metal MGs (e.g., Co-glycerate [82], Mn- glycerate [83], Mo-glycerate [84], Fe-glycerate [76]), bimetallic MGs (e.g., NiCo-glycerate [56], CoMn-glycerate [85], CoMo-glycerate [86], NiMn-glycerate [87]), trimetallic MGs (e.g., NiCoMn-glycerate [88], NiCoMo-glycerate [68], ZnMnCo-glycerate [89], NiCoZn-glycerate [72]), and even high-entropy MGs incorporating multiple metal elements [90,91].
Beyond compositional diversity, the concentration of metal precursors has been shown to significantly influence the particle size of MGs. For example, our group successfully synthesized monodisperse NiCoMn-glycerate solid spheres with tunable diameters ranging from 650 to 1100 nm by varying the amount of Mn(NO3)2, without employing any surfactants [50]. As illustrated in Figure 2a,c, the approach involved a solvothermal process using Co(NO3)2, Ni(NO3)2, and glycerol in isopropanol to produce uniform NiCo-glycerate spheres. Remarkably, upon introducing Mn(NO3)2 into the reaction system, the spherical morphology was preserved, yet the particle size noticeably increased (Figure 2b,d–k). To gain further insight, we investigated Co-glycerate spheres synthesized at varying concentrations of Co(NO3)2. The results revealed that higher Co2+ concentrations led to larger particle sizes, suggesting that an increased metal ion content enhances collision frequency between metal ions and glycerate molecules, thereby accelerating crystal growth and resulting in larger MG spheres.
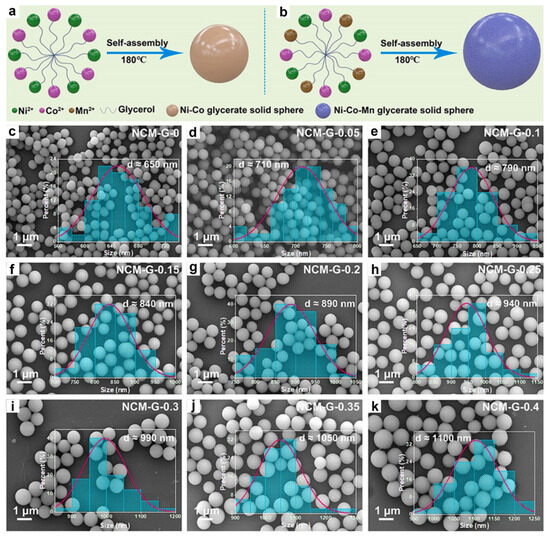
Figure 2.
Schematics of sphere formation for (a) Ni-Co glycerate and (b) Ni-Co-Mn glycerate. FESEM images and size histograms for products prepared with Mn(NO3)2 at (c) 0, (d) 0.05, (e) 0.10, (f) 0.15, (g) 0.20, (h) 0.25, (i) 0.30, (j) 0.35, and (k) 0.40 mmol. Reproduced with permission [50]. Copyright 2023, Elsevier.
In another study, Kong et al. successfully synthesized a series of CoFe-glycerates featuring yolk-shell microsphere architectures assembled from ultrathin nanosheets, by precisely adjusting the molar ratios of cobalt nitrate and ferric nitrate precursors while maintaining the total metal precursor amount [92]. As shown in Figure 3a–f, the morphology of these CoFe-glycerates varied significantly with the Co/Fe ratio. Co1Fe3-glycerate formed larger spheres (~1.1 μm) with a dense, three-dimensional flower-like structure composed of vertically aligned nanosheets. As the cobalt content increased, Co1Fe1-glycerate exhibited smaller spheres (~900 nm) with larger nanosheets and more noticeable gaps, whereas Co3Fe1-glycerate yielded even smaller spheres (~700 nm) characterized by loosely arranged nanosheets. In addition, Li and co-workers reported the synthesis of three distinct CoCu-glycerate structures by varying the duration of the solvothermal reaction [80]. Remarkably, the number of shells in the resulting materials could be precisely controlled by adjusting the reaction time. As illustrated in Figure 3g–i, single-, double-, and triple-shelled CoCu-glycerates were obtained after reaction times of 3, 6, and 8 h, respectively.
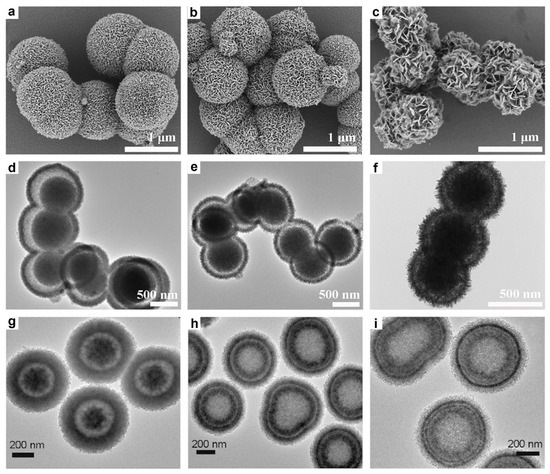
Figure 3.
SEM images of (a) Co1Fe3-glycerates, (b) Co1Fe1-glycerates, and (c) Co1Fe1-glycerates; TEM images of (d) Co1Fe3-glycerates, (e) Co1Fe1-glycerates and (f) Co1Fe1-glycerates. Reproduced with permission [92]. Copyright 2025, Elsevier. TEM images of time-evolved precursors collected at (g) 3 h, (h) 6 h, and (i) 8 h. Reproduced with permission [80]. Copyright 2019, Elsevier.
Furthermore, the incorporation of surfactants during the synthesis of MGs plays a crucial role in controlling particle size. For instance, Zhang et al. synthesized a series of NiCo-glycerates via a polyvinylpyrrolidone (PVP)-assisted solvothermal method, employing nickel nitrate, cobalt nitrate, and glycerol in an isopropanol solution to produce highly uniform and monodisperse solid nanospheres (Figure 4a) [81]. The particle size of the NiCo-glycerates was precisely tuned by varying the amount of PVP added during synthesis, with quantities ranging from 0 to 500 mg resulting in precursor diameters decreasing from 550 nm to 97 nm. As shown in Figure 4(b1)–(h3), all samples exhibited consistent spherical morphologies and smooth surfaces across different sizes, demonstrating good uniformity and dispersion.
Figure 4.
(a) Schematic of the PVP-assisted, size-controllable synthesis of NiCo-glycerates. (b1–h3) SEM images of NiCo-glycerates obtained with varying PVP mass; scale bars: (b1–h1) 1 μm, (b2–h2) 500 nm, and (b3–h3) 100 nm. Reproduced with permission [81]. Copyright 2020, Wiley-VCH.
2.2. Synthesis Strategies of Metal-Glycerate Derivatives
Beyond the precise design of MG precursors, the strategies employed for their conversion into MG-derived materials are crucial for precisely tailoring the nanostructure and composition of the resulting functional materials. Due to their relatively weak coordination environments, MGs possess high chemical reactivity, enabling controlled structural transformations during subsequent processing [54]. This inherent reactivity makes MGs versatile precursors for synthesizing a wide range of nanostructured materials, including various metal compounds and composites [64]. Nevertheless, the intrinsic chemical and thermal instability of MGs necessitate careful selection of conversion methods to ensure the preservation of desired morphological and structural features [93]. Broadly, the synthesis routes for MG-derived materials can be classified into three primary categories: (1) thermal treatment strategies; (2) solution-phase chemical transformations; and (3) combined chemical and thermal processes.
2.2.1. Thermal Treatment Strategies
Thermal treatment is widely employed for converting MG precursors into their derivatives due to its simplicity and precise control over material properties [94]. This process eliminates moisture and volatile components at elevated temperatures and often induces the formation of hollow nano- and microscale structures, driven by shrinkage and adhesion effects under nonequilibrium conditions [95]. The choice of atmosphere plays a crucial role in determining the final product composition and morphology: oxidative environments typically yield metal oxides that retain the precursor morphology and develop porous structures, whereas inert atmospheres promote partial carbonization, leading to metal/carbon composites [96,97,98]. Moreover, integrating gas–solid reactions such as sulfidation, phosphidization, and selenization during thermal treatment enables the formation of metal sulfides, phosphides, or selenides with tailored compositions and enhanced electrochemical performance [99,100].
In 2024, Xie and co-workers synthesized Mn-doped Co3O4 (Mn-Co3O4) with a porous hollow spherical architecture via the calcination of CoMn-glycerate precursors in air (Figure 5a) [101]. As shown in Figure 5b,c, the resulting Mn-Co3O4 exhibited a uniform single-shelled hollow structure with an average diameter of ~1.15 μm. Similarly, Zhang et al. prepared hollow Co3O4/CuO nanospheres by calcining hollow CuCo-glycerate precursors in air [94]. They systematically examined the influence of the Co/Cu atomic ratio on the microstructural evolution of both the glycerate precursors and the resulting oxide materials. Notably, the incorporation of copper not only facilitated the transformation from solid Co-glycerate to hollow CuCo-glycerate nanospheres but also significantly enhanced the electrochemical performance of the resulting Co3O4/CuO composites.
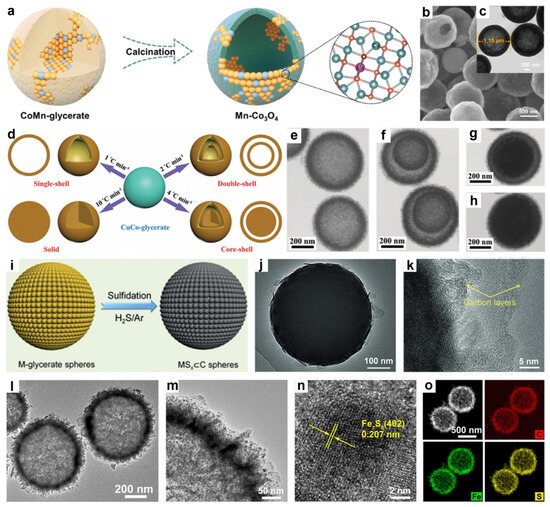
Figure 5.
(a) Schematic of the sequential route to Mn-Co3O4, (b) SEM and (c) TEM images of Mn-Co3O4. Reproduced with permission [101]. Copyright 2024, Elsevier. (d) Synthesis of CuCo2O4 microspheres with hierarchical internal structures. (e–h) TEM images of the single-shell, double-shell, core–shell and solid CuCo2O4 microspheres. Reproduced with permission [102]. Copyright 2017, Royal Society of Chemistry. (i) Schematic of the hierarchical MSx⊂C sphere formation. (j) TEM and (k) HRTEM images of V2S3⊂C spheres. Reproduced with permission [103]. Copyright 2019, Wiley-VCH. (l,m) TEM images, (n) HRTEM image, and (o) element mapping images of Fe7S8@HCSs. Reproduced with permission [97]. Copyright 2022, Elsevier.
In another study, Kaverlavani and colleagues synthesized CuCo2O4 microspheres with diverse hollow architectures using a simple and cost-effective self-template method (Figure 5d) [102]. In this process, CuCo-glycerate microspheres were first fabricated via a solvothermal approach, followed by calcination at different heating rates. This method produced CuCo2O4 microspheres with varied internal structures, including single-shell, double-shell hollow spheres, core–shell spheres, and solid spheres. TEM images demonstrated that the heating rate significantly influenced the resulting morphologies, yielding single-shell (Figure 5e), double-shell (Figure 5f), core–shell (Figure 5g), and solid sphere (Figure 5h) structures.
In addition, Yu’s group developed a general strategy for synthesizing carbon-coated metal sulfide (MS⊂C) spheres by first preparing uniform MG precursors via solvothermal synthesis, followed by thermal sulfidation in an H2S/Ar atmosphere (Figure 5i) [103]. For instance, annealing V-glycerate yielded uniform V2S3⊂C spheres, in which nanoscale V2S3 crystals were embedded within an in situ formed carbon matrix. Specifically, the V-glycerate spheres initially decompose to form V2O3 nanocrystals embedded within an in situ generated carbon matrix. Upon further heating, sulfidation occurred through the replacement of O2− ligands by S2−, leading to the formation of V2S3 nanocrystals confined within the carbon matrix. This confinement effectively prevented excessive particle growth, resulting in uniformly dispersed nanoscale V2S3 embedded in a conductive carbon shell (Figure 5j,k).
Similarly, Xu et al. synthesized ultrafine Fe7S8 nanocrystals embedded within hollow carbon nanospheres (Fe7S8@HCSs) through low-temperature sulfurization of hollow Fe-glycerate nanospheres [97]. In the synthesis, hollow Fe-glycerate spheres were first prepared via a solvothermal method, where the amount of deionized water was critical in achieving the hollow structure. Subsequent calcination in an Ar atmosphere converted the glycerate into carbon and transformed Fe3+ into Fe3O4 nanoparticles, forming Fe3O4@HCSs. Finally, sulfurization at 350 °C in an H2/Ar atmosphere with sublimed sulfur powder produced Fe7S8@HCSs, effectively preventing nanoparticle aggregation typically observed during high-temperature sulfidation. As shown in Figure 5l,m, the resulting Fe7S8@HCSs exhibited a hollow spherical structure with a diameter of ~670 nm, featuring a nanosheet-assembled shell and uniformly dispersed Fe7S8 nanoparticles ~10 nm in size within the carbon matrix. HRTEM (Figure 5n) revealed a lattice spacing of 0.207 nm, corresponding to the d402 planes of Fe7S8, while EDX elemental mapping (Figure 5o) confirmed the uniform distribution of C, S, and Fe, with weight ratios of 9.1%, 35.2%, and 55.7%, respectively.
2.2.2. Solution-Phase Chemical Transformations
In addition to thermal treatment methods, solution-phase chemical transformations such as ion exchange offer an effective route for converting MG precursors into nanostructured derivatives with tunable compositions and architectures [85,87]. These solid–liquid reactions often proceed under relatively mild conditions, particularly when the interaction between metal ions and organic ligands in the MG framework is weaker than that between the metal ions and incoming inorganic anions [104,105]. Such transformations can induce significant changes in the morphology and internal structure of the materials, enabling the formation of diverse functional nanostructures. Moreover, solution-phase processes preserve delicate nanostructures and allow precise control over composition and particle size, which is crucial for optimizing electrochemical properties.
In 2021, Lou’s group demonstrated a self-templating synthesis of cobalt-substituted Mn-rich Prussian blue analog hollow spheres (CoMn-PBA HSs), in which solvothermally prepared CoMn-glycerate solid spheres were converted into hierarchical hollow architectures through an anion-exchange process, forming a shell composed of interconnected nanocubes (Figure 6a) [106]. The TEM (Figure 6b) and SEM (Figure 6c) images revealed that CoMn-PBA HSs possessed a well-defined hierarchical hollow structure with ~70 nm nanocube subunits uniformly assembled on the surface, forming shells ~200 nm thick and an overall diameter of ~1.2 μm. Moreover, Guo et al. developed a self-templated method to synthesize Co-Mn mixed oxide double-shelled hollow spheres by transforming solid Co-glycerate spheres through redox reactions with KMnO4 followed by Ostwald ripening at mild temperatures [107]. The formation mechanism of the Co-Mn mixed oxide double-shelled hollow spheres was elucidated through a time-resolved morphological evolution study (Figure 6d–f). Initially, the Co-glycerate precursors retain a nearly intact spherical shape with slight surface roughening (Figure 6d). After 10 min of hydrothermal treatment, internal voids begin to emerge, leading to the formation of single-shelled hollow structures (Figure 6e). Following a 1 h reaction with KMnO4, a well-defined double-shelled morphology is observed, featuring two distinct shells and internal cavities (Figure 6f). The appearance of randomly oriented nanoflakes on the outer shell indicates the formation of MnCo2O4 via an Ostwald ripening process, wherein smaller nanoparticles dissolve and redeposit to construct the secondary shell. A schematic representation of this formation pathway is shown in Figure 6g.
In one representative study, Zhang and co-workers reported the synthesis of NiCo-LDH hollow spheres through a simple ion-exchange strategy using Co-glycerate spheres as sacrificial templates [105]. The Co-glycerate precursors were first obtained via a solvothermal method, followed by treatment with Ni(NO3)2 in an ethanol-based solution. During this process, ion exchange occurred, leading to the formation of NiCo-LDH hollow structures. Notably, protons, hydroxyl groups, and Ni2+ ions generated from the hydrolysis of Ni(NO3)2 played dual roles: etching the Co-glycerate core and reacting with released Co ions to facilitate the in situ growth of the NiCo-LDH shell. Similarly, Che’s group synthesized trimetallic CoNiFe carbonate hydroxide hierarchical hollow microflowers (CN-xFe HMs) employing CoNi-glycerate nanospheres as sacrificial templates [108]. Specifically, uniform CoNi-glycerate nanospheres were first prepared via a modified solvothermal method. Upon hydrothermal treatment, partial oxidation of Co2+ to Co3+ occurred, and the in situ generated OH− and CO32− species reacted with Co2+, Co3+, and Ni2+ to form nanosheet-like carbonate hydroxides on the surface, leading to yolk-shell structures. These intermediates were subsequently immersed in Fe(NO3)3 solution, enabling cation exchange and partial substitution of Co species by Fe3+, ultimately resulting in well-defined hollow architectures. The hierarchical hollow microflower morphology of CN-xFe HMs was confirmed by the SEM (Figure 6h) and TEM (Figure 6i) images. In a more recent example, Jia and co-workers synthesized Ce-doped nickel/cobalt hydroxide hierarchical hollow spheres (NiCoCe-OH) through a two-step strategy [109]. First, NiCo-glycerate solid spheres with smooth surfaces were obtained via a solvothermal method and subsequently converted into hierarchical hollow structures composed of interconnected nanosheets through a hydrolysis reaction, driven by the Kirkendall effect. In the second step, a cation-exchange process was conducted by introducing Ce ions into the NiCo-OH framework, with reaction durations ranging from 1 to 8 h to modulate Ce content and morphological characteristics. Among the resulting materials, NiCoCe-OH-4 (4 represents 4 h) exhibited a well-defined hollow structure, featuring ultrathin, curly nanosheets and a uniform distribution of Ce dopants.
In addition, yolk-shelled amorphous Ni-Co-Mn sulfide spheres were synthesized by our group via a sulfidation treatment of Ni-Co-Mn glycerate solid spheres using thioacetamide (TAA) in ethanol solution [50]. During this process, S2− released from the thermal decomposition of TAA reacted with the metal glycerate precursor. Initially, an anion exchange between S2− and the metal species at the surface led to the formation of a core–shelled Ni-Co-Mn glycerate@Ni-Co-Mn sulfide structure. As the reaction progressed, inward diffusion of S2− combined with a faster outward diffusion of metal ions resulted in the growth of the sulfide shell and the formation of an internal void. Eventually, a secondary sulfide layer formed around the core, giving rise to well-defined yolk-shelled Ni-Co-Mn sulfide spheres upon completion of the transformation. FESEM images (Figure 6j) distinctly demonstrate the yolk-shelled hollow structure of the Ni-Co-Mn sulfide with an average diameter of ~900 nm, while fractured spheres provide further evidence of its hollow structure. TEM image (Figure 6k) further confirms the yolk-shelled architecture, revealing an inner core (yolk) diameter of about 320 nm and an outer shell thickness of approximately 40 nm. Similarly, Yang and colleagues reported the synthesis of Cu7Se4-CuxCo1−xSe2 double-shelled hollow nanospheres via a facile self-templated hydrothermal anion exchange method [110]. In this approach, CuCo-glycerate nanospheres act as sacrificial templates and undergo a one-step hydrothermal reaction during which Se2− replace glycerate anions, leading to the in situ formation of well-defined double-shelled hollow architectures. By precisely controlling the reaction time, the morphology and composition of the composite can be optimized without requiring separate synthesis of the individual components.
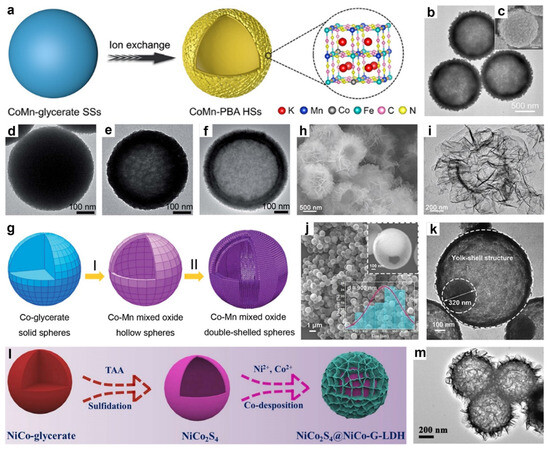

Figure 6.
(a) The synthetic process of CoMn-PBA HSs. (b) TEM image and (c) SEM image of CoMn-PBA HSs. Reproduced with permission [106]. Copyright 2019, Wiley-VCH. TEM images of products from the Co-glycerate/KMnO4 reaction at (d) 0, (e) 10, and (f) 60 min. (g) Schematic illustrating the evolution to Co-Mn mixed-oxide double-shell hollow spheres. Reproduced with permission [107]. Copyright 2019, Royal Society of Chemistry. (h) SEM image and (i) TEM image of CN-xFe HMs. Reproduced with permission [108]. Copyright 2022, Wiley-VCH. (j) FESEM of NCM-S-0.25 with insets showing a broken-structure SEM (top right) and the size distribution profile (bottom right). (k) TEM image of NCM-S-0.25. Reproduced with permission [50]. Copyright 2023, Elsevier. (l) Schematic of NiCo2S4@NiCo-G-LDH formation. (m) TEM image of NiCo2S4@NiCo-G-LDH. Reproduced with permission [111]. Copyright 2022, Elsevier.
In another work, Li and co-workers synthesized NiCo2S4@NiCo-G-LDH hierarchical core–shelled heterostructure via a two-step strategy (Figure 6l) [111]. Firstly, uniform NiCo2S4 hollow nanospheres were obtained by hydrothermal vulcanization of NiCo-glycerate precursors. Then, ultrathin NiCo-LDH nanosheets were grown on the surface of the hollow NiCo2S4 nanospheres via a co-deposition process, during which glucose molecules were introduced to serve as interlayer guests. As shown in Figure 6m, the NiCo2S4@NiCo-G-LDH exhibited a well-defined core–shelled hollow architecture, in which glucose-intercalated NiCo-LDH nanosheets are vertically anchored onto the surface of NiCo2S4 hollow nanospheres. Similarly, Wang et al. synthesized hierarchical multi-cavity hollow Ni-Co bimetallic sulfide composite (NixCo1−xS2/NiCo2S4) via a multistep approach involving solvothermal coordination, interfacial self-assembly, and in situ sulfurization [33]. Specifically, NiCo-glycerate microspheres were first prepared from Ni2+/Co2+ ions and glycerol. Upon treatment with 2-methylimidazole, surface metal ions partially dissociated and coordinated with the ligand, forming NiCo-ZIF nanoparticles on the NiCo-glycerate surface, yielding a dual-template structure. Subsequent sulfurization with TAA under solvothermal conditions produced S2− that reacted with metal species to form sulfides. The Kirkendall effect facilitated hollow structure formation, with NixCo1−xS2 nanoparticles inside and NiCo2S4 on the outer shell, resulting in a hierarchical multicavity architecture. This well-defined structure offered abundant active sites and enhanced mechanical integrity, contributing to improved electrochemical performance.
2.2.3. Combined Chemical and Thermal Processes
Combined chemical and thermal strategies synergize solution-phase reactivity with high-temperature treatment, enabling refined control over composition, crystallinity, and hierarchical architecture [76,112]. Typically, these methods involve sequential chemical modification and thermal conversion steps, performed in either order, to tailor complex hollow architectures. In one route, MG precursors undergo chemical transformation such as ion exchange, coordination, or hydrolysis, facilitating the incorporation of hetero-elements or the formation of hollow or yolk-shell intermediates [66,113]. Subsequent thermal treatment (e.g., calcination, phosphorization, sulfurization) induces phase conversion and crystallization, often via the Kirkendall effect, yielding hollow structures with preserved external morphology [100,114]. Alternatively, thermal pretreatment can first generate stable metal oxide frameworks, followed by post-synthetic chemical modifications (e.g., anion exchange or surface functionalization) to produce sulfides, phosphides, or hybrid composites without compromising structural integrity [96,115,116]. Such combined approaches effectively retain delicate architectures during processing and enable the design of multi-shelled, hierarchical, and multicavity systems. The synergistic tuning of composition and architecture enhances electronic conductivity, structural robustness, and active site accessibility, all of which are critical for high-performance SCs.
In 2023, Zhang et al. proposed a stepwise synthesis strategy for constructing CuCo2O4@MoNi-LDH core–shelled hollow nanostructures via sequential thermal calcination and hydrothermal treatment (Figure 7a) [117]. Specifically, the solvothermally prepared CuCo-glycerate solid spheres (Figure 7b) were first annealed at elevated temperatures, leading to the formation of hollow CuCo2O4 microspheres with roughened surfaces (Figure 7c). The shell exhibited evenly distributed crystalline grains, indicating a uniform structural transformation. Subsequently, MoNi-LDH nanosheets were grown on the surface through a one-step hydrothermal process. As shown in Figure 7d, the nanosheets were conformally coated, forming a well-defined core–shelled hollow architecture. In a related study, Xu and co-workers developed a multistep synthesis strategy to fabricate yolk-shelled hollow NiMnSe3 nanospheres by integrating solvothermal treatment, thermal oxidation, and hydrothermal selenification [118]. Initially, Ni-Mn glycerate precursors were prepared via solvothermal synthesis using glycerol as a key structure-directing agent. Glycerol not only facilitated the formation of uniform spheres but also enabled Ostwald ripening during the reaction, wherein the internal glycerate cores gradually shrank while the outer shell thickened, ultimately giving rise to a distinct yolk-shelled hollow configuration. Subsequent calcination in air converted the organic framework into Ni-Mn oxide microspheres with preserved hollow interiors and enhanced structural rigidity. In the final step, the oxide spheres were exposed to selenide ions under hydrothermal conditions, resulting in the formation of porous NiMnSe3. As shown in Figure 7e, the yolk-shelled hollow structures were maintained post-selenization, while nanosheets composed of MnSe and NiSe2 (Figure 7f) uniformly emerged on the shell surface, forming a hierarchical composite architecture.
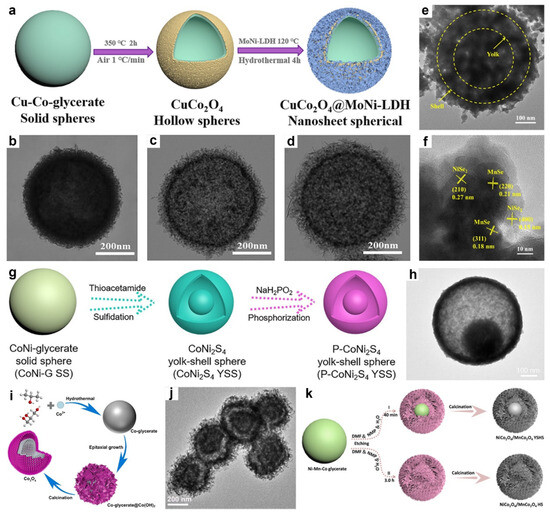
Figure 7.
(a) Schematic of the synthesis method followed to obtain CuCo2O4@MoNi-LDH formation and (b–d) corresponding images at each stage. Reproduced with permission [117]. Copyright 2023, Elsevier. (e) TEM image and (f) HRTEM image of NiMnSe3. Reproduced with permission [118]. Copyright 2022, American Chemical Society. (g) Illustration of the preparation of P-CoNi2S4 YSSs. (h) TEM image of P-CoNi2S4 YSSs. Reproduced with permission [119]. Copyright 2021, Wiley-VCH. (i) Schematic illustration of structural evolution of hollow double-shelled Co3O4 sphere. (j) TEM image of hollow double-shelled Co3O4 sphere. Reproduced with permission [120]. Copyright 2023, Elsevier. (k) Schematic representation of a controllable strategy to construct NiCo2O4/MnCo2O4 hollow structures. Reproduced with permission [88]. Copyright 2023, Elsevier.
Lou’s group synthesized phosphorized CoNi2S4 (P-CoNi2S4) yolk-shelled hollow spheres via a sequential two-step strategy involving hydrothermal sulfidation followed by gas-phase phosphorization (Figure 7g) [119]. Initially, CoNi-glycerate precursors were converted into CoNi2S4 yolk-shelled hollow structure through anion exchange and chemical etching in a TAA solution under hydrothermal conditions, resulting in microspheres with rough surfaces and well-defined interior voids. In the subsequent phosphorization step, these sulfides were phosphorized via a gas–solid reaction, during which phosphorus atoms were introduced to partially substitute the chalcogen components. As shown in Figure 7h, the resulting P-CoNi2S4 preserved its yolk-shelled hollow structure with a ~35 nm porous shell composed of ultrafine nanoparticles, effectively promoting ion diffusion and active site accessibility.
In addition, Fu’s group developed a multi-step synthetic route to construct hierarchical double-shelled hollow Co3O4 nanospheres, integrating solvothermal precursor formation, solution-phase shell growth, and controlled thermal treatment (Figure 7i) [120]. In the first step, Co-glycerate solid spheres were synthesized through a one-step solvothermal method and used as sacrificial templates. Subsequently, the introduction of Co(NO3)2 and 2-methylimidazole at room temperature induced the in situ growth of Co(OH)2 nanosheets on the surface of the Co-glycerate spheres, forming a well-defined Co-glycerate@Co(OH)2 core–shelled structure. The composite was then subjected to calcination, during which a sequence of thermally induced structural transformations occurred. Initially, the outer Co(OH)2 shell was rapidly oxidized to form a rigid Co3O4 layer firmly attached to the shrinking core. As heating progressed, the Co-glycerate core underwent oxygenolysis and significant inward contraction, leading to its gradual detachment from the outer shell. This mismatch in contraction behavior, governed by the different thermal responses of the inner and outer layers, ultimately resulted in the formation of hierarchical double-shelled hollow Co3O4 architecture (Figure 7j).
Moreover, Li et al. developed a two-step strategy to construct Co9S8-x core–shelled hollow spheres with tunable sulfur vacancy concentrations, using Co-glycerate as the starting precursor [121]. In the first step, the solvothermally prepared Co-glycerate underwent sulfidation with TAA, during which S2− reacted with cobalt centers to form Co9S8 featuring a core–shelled hollow structures. These Co9S8 were subsequently annealed under an Ar/H2 atmosphere at 300 °C, 400 °C, or 500 °C, yielding core–shelled hollow Co9S8-x spheres with controlled sulfur deficiency, designated as Co9S8-x-L, Co9S8-x-M, and Co9S8-x-H, respectively.
In a related work, hollow and yolk-shelled NiCo2O4/MnCo2O4 spheres were synthesized by Shi and co-workers via a template-assisted hydrothermal etching strategy followed by calcination (Figure 7k) [88]. Initially, solid NiMnCo-glycerate spheres were synthesized via a solvothermal route and served as sacrificial templates. These were then subjected to hydrothermal treatment in a mixed solvent system of the water, Nmethylpyrrolidone (NMP), and N, N-dimethylformamide (DMF), where partial dissolution of the core accompanied by nanosheet shell formation induced progressive structural transformation. By precisely adjusting the reaction duration, the morphology evolved from dense spheres to yolk-shelled structures, and eventually to completely hollow architectures. This transition was governed by sequential surface reconfiguration, inward etching-driven core–shell separation, and full template degradation. Final thermal annealing converted the intermediate structures into crystalline NiCo2O4 and MnCo2O4, while preserving the hollow or yolk-shelled structures.
3. Application of Metal-Glycerates and Their Derivatives in Supercapacitor Electrode Materials
3.1. Metal-Glycerate as Electrode Materials for Supercapacitor
MGs, a subclass of metal-alkoxides, have emerged as promising electrode materials for SCs owing to their layered and amorphous structures [54]. Their lamellar architecture provides abundant interlayer spacing, which facilitates efficient ion diffusion and electrolyte access. Concurrently, the amorphous nature introduces abundant grain boundaries and disordered coordination environments, providing a high density of electroactive sites and multiple ion transport channels [78]. Under alkaline conditions, MGs can be in situ transformed into metal oxyhydroxides, the actual redox-active species during charge–discharge processes [122]. This structural adaptability, combined with their flexible framework, contributes to enhanced cycling stability.
Several studies have demonstrated the feasibility of using pristine metal glycerates directly as SCs electrode materials. For example, Ding et al. synthesized uniform NiMn-glycerate (NiMn-Gly) microspheres via a solvothermal coordination reaction between glycerate ligands and Ni2+/Mn2+, which were subsequently used to assemble an all-solid-state NiMn-Gly//activated carbon (AC) SC device with a PVA/KOH gel electrolyte (Figure 8a) [78]. SEM image (Figure 8b) revealed that the microspheres possess a uniform spherical morphology with diameters of 3–6 μm and smooth surfaces. TEM image (Figure 8c) further confirmed their solid internal structure with well-defined, nonporous boundaries. As shown in Figure 8d, the all-solid-state device demonstrated stable galvanostatic charge–discharge (GCD) behavior within a voltage window of 0–1.6 V at varying current densities, delivering specific capacitances of 244.9, 201.0, 173.7, 130.5, and 72.0 C g−1 at 1, 2, 3, 5, and 10 A g−1, respectively. The nearly negligible IR drop observed in the GCD curves reflects low internal resistance and efficient charge transport. Notably, the device achieved a maximum energy density of 54.4 Wh kg−1 at a power density of 800 W kg−1 (Figure 9e). As shown in Figure 9f, the device retained nearly 100% capacitance and coulombic efficiency after 20,000 cycles at 10 A g−1, maintaining intact microsphere morphology and good structural stability.
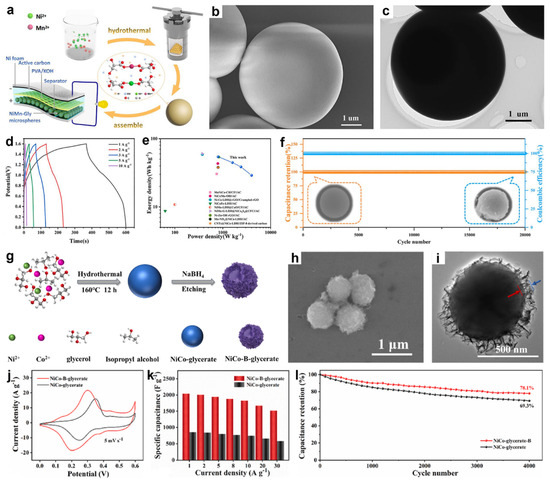
Figure 8.
(a) Schematic of NiMn-Gly formation and the NiMn-Gly-1//AC solid-state device. (b) SEM and (c) TEM images of NiMn-Gly-1. (d) GCD curves at different current densities, (e) Ragone plots, and (f) cycling stability of NiMn-Gly-1//AC device. Reproduced with permission [78]. Copyright 2022, Elsevier. (g) Schematic of the formation process of NiCo-B-glycerate. (h) SEM and (i) TEM images of NiCo-B-glycerate. (j) The CV curves at 5 mV s−1 of NiCo-B-glycerate. (k) Specific capacities and (l) cycling stability of NiCo-B-glycerate and NiCo-glycerate. Reproduced with permission [55]. Copyright 2024, Elsevier.
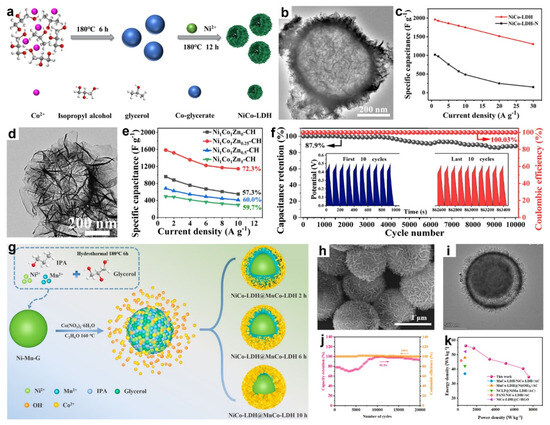
Figure 9.
(a) Schematic of NiCo-LDH hollow sphere fabrication routes. (b) TEM image of NiCo-LDH hollow sphere. (c) Specific capacities at different current densities. Reproduced with permission [105]. Copyright 2022, Elsevier. (d) TEM image of NiCoZn-CH. (e) Specific capacitance of the electrodes plotted against current density. (f) Cycling stability of Ni1Co1Zn0.25-CH electrode. Reproduced with permission [72]. Copyright 2023, Royal Society of Chemistry. (g) Schematic diagram of the synthesis of NiCo-LDH@MnCo-LDH. (h) SEM and (i) TEM images of NiCo-LDH@MnCo-LDH. (j) Cycling stability and coulombic efficiency, and (k) Ragone plot of NiCo-LDH@MnCo-LDH//AC ASC. Reproduced with permission [87]. Copyright 2025, Elsevier.
In another study, Zhang et al. developed boron-doped NiCo-glycerate spheres (NiCo-B-glycerate) with a distinctive sphere@nanosheet structure via a two-step strategy (Figure 8g) [55]. Initially, uniform NiCo-glycerate solid spheres were synthesized through a solvothermal method. A subsequent NaBH4 treatment served dual roles, acting both as a boron source and a chemical etchant, simultaneously incorporating B atoms into the framework and inducing surface reconstruction. This process led to the formation of wrinkled nanosheets on the particle surface, as confirmed by FESEM (Figure 8h) and TEM (Figure 8i) images. The transformation was attributed to the oxygen-scavenging effect of hydride ions (H−), which generated surface defects and promote local structural rearrangement. The resulting architecture shortened ion diffusion pathways and improved mechanical adaptability during cycling. As a result, the electrochemical performance was significantly improved. As shown in Figure 8j, both NiCo-glycerate and NiCo-B-glycerate exhibited distinct redox peaks in their CV curves at 5 mV s−1, indicative of typical pseudocapacitive behavior. Notably, NiCo-B-glycerate displayed smaller redox peak separation, indicating reduced polarization and faster electron transfer kinetics. Furthermore, NiCo-B-glycerate delivered a markedly higher specific capacitance of 2036 F g−1 at 1 A g−1, significantly surpassing the 856 F g−1 recorded for the undoped NiCo-glycerate (Figure 8k). At a high current density of 30 A g−1, it retained 74.6% of its initial capacitance, compared to 68.2% retention for the undoped sample. Durability tests at 10 A g−1 further demonstrated superior stability of NiCo-B-glycerate, retaining 78.1% of its initial capacity after 4000 cycles versus 69.3% for the undoped counterpart (Figure 8l). An asymmetric supercapacitor (ASC) constructed with NiCo-B-glycerate achieved an energy density of 58.3 Wh kg−1 at 0.8 kW kg−1. The superior electrochemical performance of NiCo-B-glycerate was mainly attributed to B doping, which generated a defective coordination environment in NiCo-glycerate and modulated its electronic structure, together with the 2D nanosheet morphology that provided additional accessible active sites and shortened charge-transport pathways.
Overall, pristine MGs as electrodes demonstrate that even without further conversion, their lamellar-amorphous frameworks can deliver competitive capacitance and durability compared with many conventional hydroxides and oxides. The comparison between NiMn-Gly and NiCo-/NiCo-B-glycerates indicates that introducing multimetal synergy, defect engineering (e.g., B doping), and controlled surface reconstruction is crucial for simultaneously enhancing redox kinetics and structural robustness. At the same time, the relatively modest rate capability of pristine MGs highlights their intrinsic constraints, motivating subsequent transformation into derivatives with higher electronic conductivity and richer redox chemistry.
3.2. Metal-Glycerate Derivatives as Electrode Materials for Supercapacitor
Recent advancements in metal-glycerate (MG)-derived materials have demonstrated their great promise as electrode candidates for supercapacitors, primarily owing to their tunable morphology, diverse composition, and favorable redox properties [94,123]. Upon thermal or chemical conversion, MG precursors can be transformed into a variety of high-performance electroactive materials such as hydroxides metal oxides, sulfides, phosphides, and selenides, all while retaining or enhancing their original hierarchical architectures [54,124,125]. These derivatives exhibit multiple electrochemical advantages, including abundant electroactive sites, shortened ion/electron transport paths, and improved structural stability during long-term cycling [111,126,127].
3.2.1. MG-Derived Hydroxides
Layered double hydroxides (LDHs), a class of anionic layered functional materials, have attracted attention for use in SCs because of their superior anion-exchange ability, rich redox-active sites, and favorable ion-intercalation characteristics [38,128,129,130]. Building upon these merits, MG-derived LDHs have emerged as particularly promising electrode materials for SCs [87,124]. The incorporation of MGs as precursors offers a controllable route to tailor the composition and morphology of LDHs, thereby enhancing their electrochemical activity. During the conversion process, the in situ generation of hollow or/and porous nanosheet architectures effectively increases the accessible surface area and facilitates rapid ion diffusion [105]. Furthermore, the synergistic effects between multiple metal cations (e.g., Ni, Co, Zn or Mn) in the LDH framework can significantly improve the intrinsic redox kinetics and electrical conductivity [72,122]. As a result, MG-derived LDHs often deliver high specific capacitance, enhanced rate capability, and robust cycling durability, making them attractive candidates for high-performance SCs.
In 2022, Zhang and co-workers synthesized the NiCo-LDH hollow spheres via a facile ion-exchange method, using Co-glycerate as both the cobalt source and sacrificial template (Figure 9a) [105]. The TEM image (Figure 9b) showed that the NiCo-LDH hollow sphere possess an internal cavity surrounded by outer nanosheet shells approximately 50 nm thick. This nanosheet-assembled hollow architecture suppressed nanosheet agglomeration, enlarged the electrolyte-accessible surface area, and shortened ion-diffusion paths by facilitating electrolyte penetration into the interior cavity. Benefiting from these compositional and structural features, the NiCo-LDH hollow spheres delivered a high specific capacitance of 1962 F g−1 at 1 A g−1 with 66.4% capacitance retention at 30 A g−1 (Figure 9c). The NiCo-LDH//AC ASC further achieved an energy density of 62.9 Wh k g−1 at 0.8 kW k g−1. Thus, with the merits of composition and hollow structure, in a subsequent study, Cheng et al. synthesized NiMn-glycerate solid spheres as sacrificial templates and selectively etched them in a 1-methyl-2-pyrrolidone/water mixture to yield hierarchical Ni-Mn hydroxide hollow spheres [122]. Employed as electrode material for SCs, the Ni-Mn hydroxide delivered a high specific capacitance of 1680 F·g−1 at 2.0 A·g−1 and 1068 F·g−1 at 15 A·g−1, while retaining 96.6% of their capacitance after 5500 cycles at 10 A·g−1. A Ni-Mn hydroxide//AC ASC reached 42.8 Wh kg−1 at 1703 W kg−1. The superior electrochemical performance stemmed from the combined advantages of a mesoporous, high surface-area architecture and ultrathin nanosheet subunits that expose abundant active sites and shorten ion/electron diffusion paths; the hollow morphology accommodated volume changes during cycling, enhancing structural stability; and the amorphous, layered character of the nanosheets further facilitated rapid ion/electron transport.
In addition, Liu et al. developed novel flower-like NiCoZn-carbonate hydroxide (NiCoZn-CH) hollow nanospheres through a two-step solvothermal process [72]. As shown in Figure 9d, the resulting Ni1Co1Zn0.25-CH exhibited a flower-like hollow nanospheres with an overall diameter of approximately 800 nm and an internal cavity of about 400 nm. This unique flower-like architecture provided a large electrode-electrolyte contact area, abundant active sites, and shortened ion transport pathways, while the hollow interior effectively mitigated structural stress, enhancing cycling stability. Moreover, the incorporation of Zn2+ ions facilitated ion transfer and boosted electrochemical activity. As a result, the Ni1Co1Zn0.25-CH electrode delivered high specific capacitances of 1585.2, 1520.6, 1356.8, 1223.4, 1173.6, and 1145.6 F g−1 from 1 to 10 A g−1, respectively, retaining ~72.3% of its capacitance between 1 and 10 A g−1 (Figure 9e). It also showed outstanding durability and charge efficiency, maintaining 87.9% of its initial capacitance (from 962.8 to 846.5 F g−1) with a coulombic efficiency of ~100% after 10,000 cycles at 10 A g−1 (Figure 9f). The Ni1Co1Zn0.25-CH//AC ASC delivered 33.7 Wh kg−1 at 400 W kg−1 and showed 99.9% capacity retention after 15,000 cycles at 10 A g−1.
Recently, Zhang et al. reported the synthesis of NiCo-LDH@MnCo-LDH yolk-shell heterostructures via a simple two-step hydrothermal method [87]. As shown in Figure 9g, Ni-Mn glycerate (Ni-Mn-G) spheres were first prepared via a solvothermal reaction of Ni2+ and Mn2+ in glycerol/isopropanol. Subsequent hydrothermal treatment with cobalt nitrate in ethanol partially etched the spheres through Co2+ hydrolysis, releasing Ni2+ and Mn2+ that coprecipitated with Co2+. The combination of slower Co2+ and OH− diffusion into the sphere interior and proton-induced etching promoted the growth of NiCo-LDH and MnCo-LDH nanosheets on the outer surfaces, yielding the NiCo-LDH@MnCo-LDH composites. As shown in Figure 9h,i, the resulting material exhibited a yolk-shell nanosheet-sphere architecture. An ASC using NiCo-LDH@MnCo-LDH as the positive electrode and AC as the negative electrode retained 93.2% of its capacitance after 20,000 cycles at 6 A g−1 (Figure 9j), and delivered a power density of 831.2 W kg−1, an energy density of 56.1 Wh kg−1 (Figure 9k).
3.2.2. MG-Derived Oxides
Beyond hydroxides, MG precursors can be transformed into a variety of metal oxides, which are widely explored as electrode materials for SCs due to their high theoretical capacitance, chemical stability, and tunable nanostructures [67,115,123]. MG-derived oxides often retain the hierarchical architectures of the original precursors, such as hollow spheres, nanosheets, or porous frameworks, which provide a large surface area and facilitate rapid ion/electron transport [88,94,107]. Moreover, the uniform distribution of multiple metal cations within the oxide lattice can enhance redox activity and improve electronic conductivity, leading to superior electrochemical performance [68,69,131,132].
Recently, Zhang et al. reported a simple template strategy to synthesize hybrid Ni-Mn-Ce oxide hierarchical hollow architectures, in which Ni-Mn-Ce glycerate solid spheres were first prepared via a one-pot solvothermal method, then transformed into hierarchical hollow intermediates through treatment in a N-methylpyrrolidone/H2O mixture, and finally converted to the hybrid Ni-Mn-Ce oxide hierarchical hollow architectures by calcination (Figure 10a) [69]. The SEM (Figure 10b) and TEM (Figure 10c) images revealed that the hybrid Ni-Mn-Ce oxide possessed a hollow structure, with clear inner voids and a hierarchical shell. Structurally, the mesoporous texture and high surface area enhanced the electrochemical active sites and facilitated electrolyte diffusion. The inner cavities effectively alleviated volume changes during charge–discharge cycles, while the nanosheet-based hierarchical shell shortened ion/electron transport distances, improving rate performance. Compositionally, the hybrid oxide configuration created abundant heterointerfaces, enhancing structural stability and accelerating ion/electron diffusion. Therefore, a solid-state ASC was constructed using Ni-Mn-Ce oxides, achieving a high energy density of 78.9 Wh kg−1 at 1199.5 W kg−1 (Figure 10d).
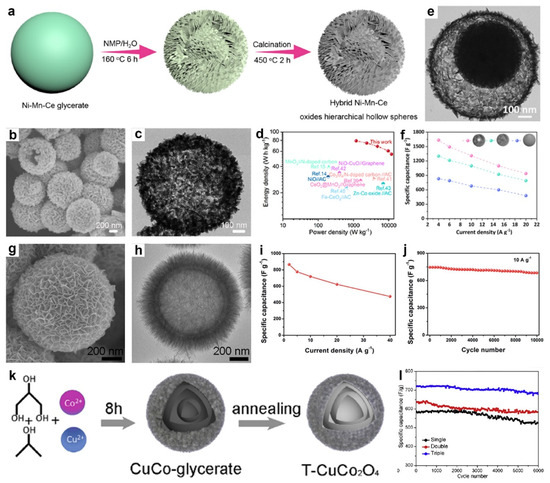
Figure 10.
(a) Preparation of hybrid Ni-Mn-Ce oxide hierarchical hollow structures. (b) SEM and (c) TEM images of hierarchical hybrid Ni-Mn-Ce oxide hollow spheres. (d) Ragone plot of the solid-state asymmetric device. Reproduced with permission [69]. Copyright 2025, American Chemical Society. (e) TEM image of NiCo2O4/MnCo2O4 YSHS. (f) Specific capacitance of different NiCo2O4/MnCo2O4 structures. Reproduced with permission [88]. Copyright 2023, Elsevier. (g) FESEM and (h) TEM images of Co-Mn mixed oxide double-shelled hollow spheres. (i) Specific capacitance as a function of current density and (j) cycling performance of Co-Mn mixed oxide double-shelled hollow spheres. Reproduced with permission [107]. Copyright 2023, Royal Society of Chemistry. (k) The formation process of T-CuCo2O4 spheres. (l) Cycling stability of different CuCo2O4. Reproduced with permission [80]. Copyright 2019, Elsevier.
In a related study, Hao’s group synthesized hierarchical yolk-shelled NiCo2O4 spheres via controlled hydrolysis followed by thermal annealing [133]. The resulting structure, featuring an inner spherical core enveloped by nanosheet shells, provided a high surface area of 169.6 m2 g−1. The NiCo2O4 exhibited a high capacitance of 835.7 F g−1 at 0.5 A g−1 and retained 93% of its capacitance after 10,000 cycles. An HSC integrating the NiCo2O4 with graphene delivered a high energy density of 34.7 Wh kg−1 at 395.0 W kg−1. Similarly, Shi and colleagues employed Ni-Mn-Co glycerate templates, followed by etching and calcination, to obtain NiCo2O4/MnCo2O4 yolk-shelled hollow spheres (YSHS) [88]. As shown in Figure 10e, the as-synthesized NiCo2O4/MnCo2O4 YSHS exhibited the well-defined yolk-shelled hollow structures. Correspondingly, the specific capacitance of the NiCo2O4/MnCo2O4 YSHS reached 1636, 1494, 1310, and 1100 F g−1 at current densities of 4.0–15 A g−1, and retained 940 F g−1 at 20 A g−1 (Figure 10f), indicating good rate capability. In comparison, the solid- and hollow-sphere counterparts exhibited comparable rate behavior but lower capacitance, underscoring the performance advantage imparted by the yolk-shelled hollow architecture. A solid-state ASC employing NiCo2O4/MnCo2O4 YSHS was assembled, achieving an energy density of 62.8 Wh kg−1 at 1650.4 W kg−1.
In addition, Guo et al. reported a facile self-templated route to Co-Mn mixed oxide double-shelled hollow structures [107]. Specifically, Co-glycerate solid spheres were used as templates, then were converted in KMnO4 solution via surface redox reactions followed by low-temperature Ostwald ripening. The SEM image (Figure 10g) showed the Co-Mn mixed oxide present a hierarchical nanosheet-assembled exterior. TEM image (Figure 10h) confirmed a double-shelled hollow structure with a distinct gap between the inner and outer shells. Leveraging these compositional and architectural merits, the Co-Mn mixed oxide electrode delivered 860, 778, 720, 624, and 475 F g−1 at 2, 5, 10, 20, and 40 A g−1 (Figure 10i), respectively, evidencing high-rate capability. Remarkably, as shown in Figure 10j, the electrode exhibits a high reversible capacity of 684 F g−1 after 10,000 cycles at 10 A g−1 (8.2% loss).
In another work, Li et al. prepared triple-shelled hollow CuCo2O4 microspheres (T-CuCo2O4) by a solvothermal-calcination route (Figure 10k) [80]. The shell number can be precisely tuned by varying the reaction time. The triple-shelled architecture provided abundant electroactive sites and enhanced mechanical robustness, thereby delivering both high specific capacitance and durability. Consequently, T-CuCo2O4 achieved 691 F g−1 at 1 A g−1 and 470 F g−1 at 20 A g−1, whereas the double- and single-shelled counterparts (D-CuCo2O4 and S-CuCo2O4) reached 580 and 524 F g−1 at 1 A g−1, respectively. Moreover, T-CuCo2O4 retained 68.1% of its specific capacitance upon a tenfold increase in current density, surpassing D-CuCo2O4 (65.5%) and S-CuCo2O4 (57.3%), which was attributed to more favorable ion-diffusion kinetics in the hollow framework. As shown in Figure 11l, its specific capacitance decreased only slightly from 702 to 653 F g−1 after 6000 cycles (∼7.0% loss), notably outperforming S-CuCo2O4 (12%) and D-CuCo2O4 (9.3%).
3.2.3. MG-Derived Sulfides
Transition metal sulfides derived from MG precursors have recently attracted significant attention as promising electrode materials for SCs owing to their high electrical conductivity, rich redox activity, and structural tunability [53,134,135,136]. Typically, MG precursors are sulfurized via thermal or hydrothermal/solvothermal treatment in sulfur-containing media [137,138]. This transformation enhances intrinsic redox kinetics, charge-transfer and ion diffusion efficiency, thereby delivering superior electrochemical performance.
In 2021, Li and co-workers rationally engineered flower-like NiCo2S4 hollow nanospheres (F-NCS HNS) by coupling surface self-reconstruction with subsequent sulfidation in hydrothermal media [139]. As shown in Figure 11a, uniform NiCo-glycerate nanospheres were first prepared as self-sacrificing templates. During hydrothermal treatment, surface metal ions dissolved and recrystallized into flower-like nanosheet architectures, which were then converted to F-NCS HNS via a facile sulfidation step. Owing to this unique architecture, the F-NCS HNS offered an enlarged surface area with abundant active sites and a porous framework that facilitated ion diffusion, thereby enhancing its electrochemical performance. Consequently, an HSC (F-NCS HNS//AC) delivered an energy density of 47.7 W h kg−1 at a power density of 399.9 W h kg−1 (Figure 11b).
In another work, Mohammadi et al. synthesized nanoporous CuCo2S4 hollow spheres by first preparing CuCo-glycerate microspheres through a solvothermal reaction of Cu(NO3)2 and Co(NO3)2 in isopropanol/glycerol, followed by sulfurization with thioacetamide in ethanol under solvothermal conditions [140]. TEM image (Figure 11c) distinctly showed a hollow interior surrounded by a porous shell, where the dark edges and lighter centers indicate the cavity structure. Nanoporous CuCo2S4 hollow spheres excel due to thiospinel conductivity and multi-redox chemistry, hollow microspheres that shorten ion paths and enhance stability, porous shells providing abundant active sites and higher utilization, and nanoscale shell thickness that accelerates electron transport and overall reaction kinetics (Figure 11d). As a result, the CuCo2S4 electrode delivered higher specific capacitance than CuCo2O4 (1566 vs. 1216 F g−1 at 2 A g−1) and superior rate performance compared with CuCo2O4 (Figure 11e). It also exhibited good durability, with only 4.3% capacitance loss after 5000 cycles at 10 A g−1 (Figure 11f). In addition, a CuCo2S4//AC ASC attained maximum power and energy densities of 16 and 43.65 W h kg−1, respectively.
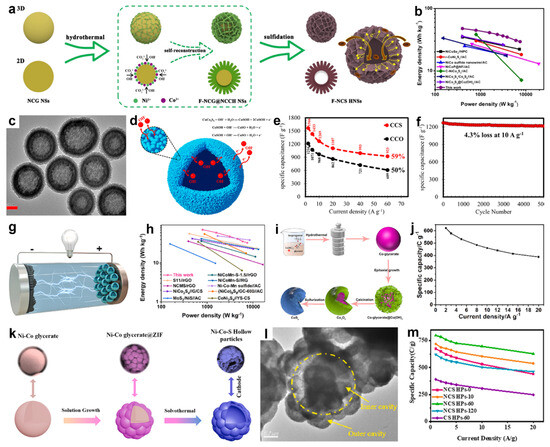

Figure 11.
(a) Formation mechanism of flower-like NiCo2S4 hollow nanospheres. (b) Ragone diagram of the device. Reproduced with permission [139]. Copyright 2021, American Chemical Society. (c) TEM image of CuCo2S4. (d) Schematic of ion transport within the CuCo2S4 electrode. (e) The specify capacitance of the CuCo2O4 and CuCo2S4 electrodes at various current densities. (f) Cycling stability of CuCo2S4 electrode. Reproduced with permission [140]. Copyright 2018, American Chemical Society. (g) Schematic illustration of the assembled NCM-S-0.25//AC HSC device. (h) Ragone plots of the device. Reproduced with permission [50]. Copyright 2023, Elsevier. (i) Schematic illustration of the synthesis of hollow double-shell CoSx heterostructures. (j) Variation in specific capacity with discharge current density. Reproduced with permission [113]. Copyright 2024, Elsevier. (k) Synthesis-route schematic for NixCo1−xS2/NiCo2S4. (l) TEM image of NixCo1−xS2/NiCo2S4. (m) Specific capacity of the synthesized materials. Reproduced with permission [33]. Copyright 2022, Elsevier.
In 2023, our group developed a surfactant-free two-step solvothermal strategy to synthesize size-controllable yolk-shell amorphous Ni-Co-Mn sulfide spheres by first forming Ni-Co-Mn glycerate spheres and subsequently converting them via solvothermal sulfidation [50]. Leveraging trimetallic synergy and the amorphous yolk-shell architecture, the assembled hybrid supercapacitor (NCM-S-0.25//AC, Figure 11g) delivered a high energy density of 71.86 Wh kg−1 at 793.56 W kg−1 (Figure 11h). In a related study, Chen et al. synthesized double-shelled hollow CoSx spheres employing Co-glycerate templates via a controlled sequence of epitaxial growth, thermal treatment, and sulfuration, with shell formation governed by the Kirkendall effect (Figure 11i) [113]. The hollow double shell architecture afforded robust structural stability, ample electroactive sites, and accelerated electron transport. Therefore, the CoSx electrode delivered specific capacities of 622 and 461 C g−1 at 2 and 10 A g−1, respectively, retaining 74.1% capacity upon a tenfold increase in current density (Figure 11j). Although the capacity gradually decreased during cycling, 74% of the initial value was still maintained after 10,000 cycles. The assembled CoSx//AC HSC achieved an energy density of 38.2 Wh kg−1 at a power density of 794.7 W kg−1.
In addition, Wang and co-workers synthesized hierarchical multicavity hollow Ni-Co bimetallic sulfide composites using a dual-template-assisted solvothermal approach [33]. As shown in Figure 11k, NiCo-glycerate microspheres were first prepared solvothermally, then dispersed in aqueous 2-methylimidazole to induce surface dissolution and in situ growth of NiCo-ZIF particles, followed by sulfidation with thioacetamide in ethanol under solvothermal conditions, where S2− reacted with Ni/Co species and an ion-diffusion imbalance derived outward deposition to yield hollow multicavity Ni-Co sulfide spheres. Figure 11l confirmed a multicavity hollow architecture in NCS HPs-60, with outer chambers connected to an inner hollow shell and hemispherical subunits (~150 nm radius). This carefully engineered structure maximizes active-site utilization, shortens charge-transport pathways, and mitigates structural collapse during cycling, yielding a high specific capacity of 797 C g−1 at 1 A g−1 and outstanding rate retention of 78.8% at 20 A g−1 (Figure 11m).
3.2.4. MG-Derived Selenides
Compared with oxides, hydroxides, and sulfides, metal selenides derived from MG precursors have recently emerged as highly promising electrode materials for supercapacitors owing to their superior electrical conductivity, higher intrinsic redox activity, and lower bandgap energy [141,142,143]. The substitution of oxygen with selenium effectively enhances the charge-transfer rate and improves reaction reversibility, leading to faster redox kinetics and enhanced capacitance performance [144,145]. Meanwhile, the MG-derived route provides precise control over composition, morphology, and hollow architecture, which helps maintain mechanical integrity and ensures stable long-term cycling.
In 2021, Zhang et al. synthesized hollow biphase cobalt-nickel perselenide spheres consisting of ~20.86 wt% metastable marcasite-type CoSe2 and 79.14 wt% stable pyrite-type NiCoSe4 using NiCo-glycerate precursors, with the Ni/Co ratio precisely controlled to tailor the phase composition [146]. As illustrated in Figure 12a, Ni(NO3)2 6H2O and Co(NO3)2 6H2O were solvothermally reacted in a glycerol/isopropanol mixed solvent to yield NiCo-glycerate with tunable Ni/Co ratios, which were subsequently converted to Co-Ni perselenides (NxCySe) via a second solvothermal step. The hollow biphase architecture, coupled with bimetallic synergy, enhanced electron transport and accelerated ion/electron kinetics, affording a specific capacitance of 1008 F g−1 at 0.5 A g−1 and a high-rate performance of 859 F g−1 at 20 A g−1 (Figure 12b). Accordingly, an HSC assembled with the NxCySe cathode, and a hierarchical porous carbon anode delivered a maximum power density of 7272 W kg−1 and a maximum energy density of 34.8 Wh kg−1.
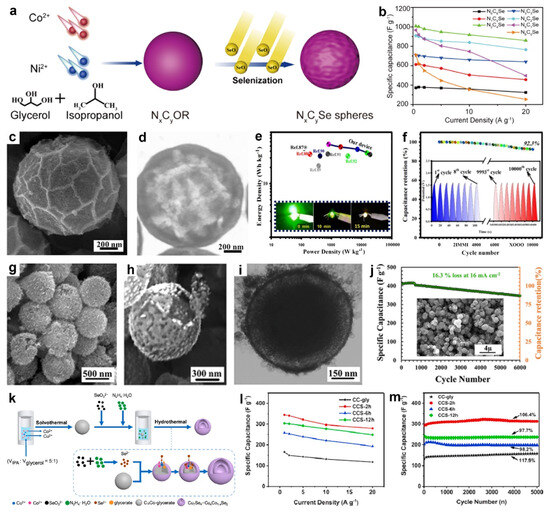
Figure 12.
(a) Synthesis schematic of NxCySe spheres. (b) Specific capacitance at different current densities of NxCySe spheres. Reproduced with permission [146]. Copyright 2021, American Chemical Society. (c) FESEM and (d) TEM images of CCSe-HS. (e) Ragone plot of the CCSe-HS//AC device. (f) Durability performance of CCSe-HS//AC; inset shows GCD traces from cycles 1–8 and 9993–10,000. Reproduced with permission [147]. Copyright 2020, Elsevier. (g,h) SEM and (i) TEM images of yolk-shelled CuCo2Se4 microspheres. (j) Cycling stability of the electrode (inset presents the SEM morphology after cycling). Reproduced with permission [142]. Copyright 2020, American Chemical Society. (k) Preparation schematic of copper-cobalt selenide double-shell hollow nanospheres. (l) Specific capacitance and (m) cycle properties of different samples. Reproduced with permission [110]. Copyright 2020, Elsevier.
Similarly, Zardkhoshoui et al. developed a straightforward self-templated route to copper-cobalt selenide hollow spheres [147]. Specifically, CuCo-glycerate spheres were solvothermally synthesized in isopropanol/glycerol from Cu(NO3)2 6H2O and Co(NO3)2 6H2O, then oxidized with KMnO4 to yield CuCo2O4 hollow spheres (CCO-HS). Subsequent dispersion of CCO-HS in ethanol containing Na2SeO3, followed by dropwise addition of N2H4 H2O under solvothermal conditions, generated Se2− in situ and converted the oxide into CuCo2Se4 hollow spheres (CCSe-HSs). As shown in Figure 12c,d, CCSe-HSs exhibited wrinkled, nanoparticle-decorated shells and a well-defined hollow, mesoporous interior that provided high surface area and accessible ion-transport channels. The asymmetric device (CCSe-HSs//AC) delivered 53.86 Wh kg−1 at 800 W kg−1 and reliably lights LEDs (Figure 12e). It also exhibited remarkable durability, retaining ~92.3% of its initial capacitance after 10,000 GCD cycles at 24 A g−1, with nearly overlapping first and last eight curves confirming robust stability (Figure 12f).
In a related study, Tavakoli et al. synthesized yolk-shelled CuCo2Se4 microspheres via a facile hydrothermal route using CuCo-glycerate as the template [142]. During selenization, Se2− first reacted with surface metal ions of CuCo-glycerate, initiating an anion-exchange process that generated a CuCo2Se4 shell while simultaneous inward Se2− diffusion and faster outward metal-cation diffusion create a core–shell gap (Kirkendall effect). Continued selenization formed a secondary CuCo2Se4 layer and, upon complete exchange of the core, yielded well-defined yolk-shelled CuCo2Se4 structures. As shown in Figure 12g,h, the yolk-shelled CuCo2Se4 microspheres exhibited uniform spherical morphology with an average diameter of ~750 nm and a porous surface composed of densely packed nanoparticles. TEM image (Figure 12i) further revealed distinct contrast between the dark outer shell and the bright inner cavity, confirming the well-defined yolk-shelled hollow architecture. The assembled ASC delivers an energy density of ~9.45 Wh kg−1, a power density up to 850 W kg−1, and robust cycling stability with only ~16.3% capacitance loss after 6000 charge–discharge cycles (Figure 12j). Similarly, Shirvani et al. synthesized tri-metallic MnNiCoSe yolk-shell spheres confined within nanosheets via a straightforward self-templating route, employing uniform NiCo-glycerate precursors as sacrificial templates followed by a controlled selenization step [148]. Benefiting from abundant redox-active sites, high-surface-area mesoporosity, strong electronic conductivity, and Mn, Ni, Co synergies, the MnNiCoSe electrode delivered 263.67 mAh g−1 at 1 A g−1, retained 76.63% capacity at 20 A g−1, and maintained 84.28% after 10,000 cycles. Moreover, the HSC using MnNiCoSe as the cathode and AC as the anode delivered an energy density of 53.32 Wh kg−1 at a power density of 1031.39 W kg−1.
In addition, Yang et al. synthesized Cu7Se4-CuxCo1−xSe2 double-shell hollow nanospheres via a hydrothermal anion-exchange process using CuCo-glycerate nanospheres as templates [110]. As shown in Figure 12k, CuCo-glycerate nanospheres were first synthesized in a mixed organic solvent and subsequently subjected to a hydrothermal process with Na2SeO3 as the selenium source and N2H4 H2O as the reducing agent, where SeO32− was in situ reduced to Se2−, initiating anion exchange with CuCo-glycerate template to yield Cu7Se4-CuxCo1−xSe2 double-shell hollow nanospheres. The resulting Cu7Se4-CuxCo1−xSe2 double-shell hollow nanospheres delivered a specific capacitance of 349.1 F g−1 at 1 A g−1, retained 80.1% of its capacitance at 20 A g−1 (Figure 12l), and exhibited outstanding cycling stability with 106.4% retention after 5000 cycles (Figure 12m).
3.2.5. MG-Derived Phosphides
Transition-metal phosphides converted from MG precursors also have emerged as a compelling class of SC electrodes due to their high electrical conductivity and multi-electron Faradaic redox [149,150]. For instance, Shirvani et al. synthesized hollow trimetallic MnNiCoP yolk-shelled spheres composed of interconnected nanosheets via a facile self-templated strategy, employing highly uniform Co-glycerate spheres as sacrificial precursors followed by controlled phosphorization [71]. FETEM (Figure 13a) and TEM (Figure 13b,c) images confirmed a well-defined yolk-shelled hollow architecture, with the spherical shells constructed from closely assembled nanosheets. The hierarchical yolk-shelled hollow architecture with mesoporous channels and high surface area together with the synergistic effect of Mn, Ni, and Co atoms and good electronic conductivity affords markedly enhanced electrochemical performance. Figure 13d confirmed Faradaic behavior in the MnNiCoP electrode. The comparable charge/discharge times across current densities reflect high coulombic efficiency, strong rate performance, and near-reversible Faradaic reactions. MnNiCoP electrode delivered 291.24 mAh g−1 at 1 A g−1, 80% capacity retention at 20 A g−1 (Figure 13e), and long-term stability with 91.3% retention after 14,000 cycles at 5 A g−1 (Figure 13f). Moreover, an HSC device (MnNiCoP//AC) showed high specific capacitance of 162.92 F g−1 at 1 A g−1 and preserved 75.40% of its capacitance at 20 A g−1 (Figure 13g). Moreover, the device maintained 88.41% capacity after 14,000 cycles at 5 A g−1 (Figure 13h) and achieved an energy density of 57.03 Wh kg−1 at ~800 W kg−1 (Figure 13i).
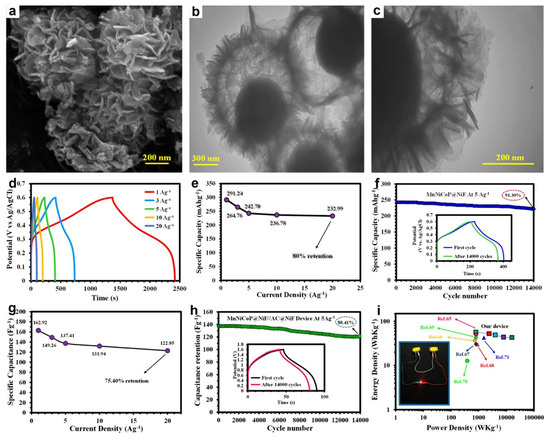
Figure 13.
(a) FESEM and (b,c) TEM images of MnNiCoP. (d) GCD profiles, (e) specific capacitances, and (f) cycling stability of the MnNiCoP electrode. (g) Specific capacitances, (h) durability and (i) Ragone plot of the MnNiCoP//AC device. Reproduced with permission [71]. Copyright 2023, Royal Society of Chemistry.
3.2.6. MG-Derived Composites
MG-derived composites integrate MG-derived compounds with conductive matrices or secondary active phases to unify fast charge transport with robust, multi-electron redox kinetics [115,134,149]. Exploiting the morphology programmability of MG precursors, these hybrids retain hierarchical porosity while constructing continuous electron- and ion-conduction networks, providing mechanical buffering against volumetric strain and activating rich heterointerfaces [70,73,117]. Broadly, MG-derived composites fall into two categories: first, hybrids that couple MG-derived compounds with conductive media such as carbon materials or conducting polymers, which suppress particle agglomeration, raise accessible surface area, and improve electrical conductivity; second, heterostructures that combine distinct transition-metal compounds, where well-defined interfacial coupling delivers synergistic redox activity and accelerated charge transport.
Integrating MG-derived transition-metal compounds with conductive components such as reduced graphene oxide (rGO) [124,151], carbon nanotubes (CNTs) [137,152], or conducting polymer [153,154,155] effectively enhanced electrochemical performance. These hybrid structures mitigated particle agglomeration, increased specific surface area, improved electronic conductivity, and buffered structural stress during repeated charge–discharge processes. For instance, Yin and co-workers devised a facile route to a “donor-bridge-acceptor” interface by conformally growing graphdiyne (GDY) on CoNi2S4 yolk-shell spheres, yielding CoNi2S4/GDY YSSs [156]. As shown in Figure 14a, CoNi-glycerate solid spheres were first vulcanized to form pristine CoNi2S4 yolk-shell spheres, followed by in situ GDY deposition. The GDY bridge promoted efficient electron transfer from Ni to Co, generating more interfacial Co2+ sites and enabling reversible Co-S/Co-S-OH/Co-S-O redox, thereby boosting capacitance. Simultaneously, the conformal all-carbon layer enhanced structural and interfacial stability, suppressing framework degradation and improving durability. Consequently, CoNi2S4/GDY YSSs delivered 856 C g−1 at 1 A g−1 and retained 61.9% (530 C g−1) at 50 A g−1, versus 27.1% for pristine CoNi2S4 (Figure 14b), and maintained 82.2% capacitance after 10,000 cycles at 20 A g−1, far exceeding the 51.3% of the uncoated counterpart (Figure 14c). Also, the CoNi2S4/GDY//AC ASC delivered a high energy density of 91.7 Wh kg−1 at 793 W kg−1, while maintaining 90.8% capacitance after 10,000 charge–discharge cycles.
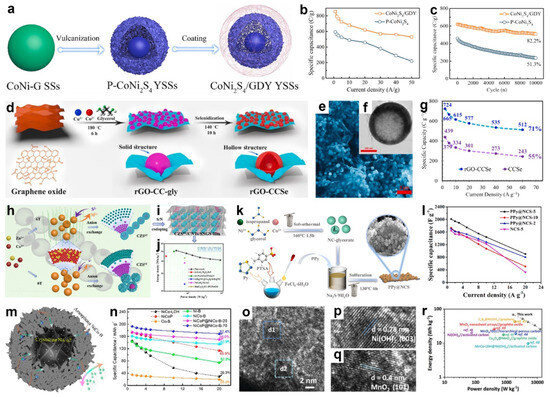
Figure 14.
(a) Schematic illustration of the preparation for CoNi2S4/GDY. (b) specific capacitance and (c) cycling stability of CoNi2S4/GDY and P-CoNi2S4. Reproduced with permission [156]. Copyright 2023, Elsevier. (d) Schematic representation of the synthesis route to rGO-CCSe. (e) SEM and (f) TEM images of rGO-CCSe. (g) Specific capacitance of rGO-CCSe and CCSe. Reproduced with permission [141]. Copyright 2021, Elsevier. (h,i) Schematic illustration of the formation of CZS6T/CNTs/SNGS. (j) Ragone plot of CZS6T/CNTs/SNGS//CNTs/SNGS ASC device. Reproduced with permission [157]. Copyright 2020, American Chemical Society. (k) Schematic illustration of the preparation of PPy@NCS. (l) Specific capacitance of different electrodes. Reproduced with permission [153]. Copyright 2024, Elsevier. (m) Ideal microstructures of NiCoP@NiCo-B. (n) Specific capacity of different electrodes. Reproduced with permission [116]. Copyright 2024, American Chemical Society. (o–q) HRTEM images of hierarchical Ni(OH)2-MnO2 hollow spheres. (r) Ragone plots of hierarchical Ni(OH)2-MnO2 hollow spheres//AC device. Reproduced with permission [158]. Copyright 2022, Royal Society of Chemistry.
Moreover, Moosavifard et al. designed a facile self-templated strategy to synthesize porous hollow copper-cobalt selenide microspheres uniformly anchored on conductive networks of reduced graphene oxide (rGO-CCSe) [141]. As outlined in Figure 14d, rGO-CuCo-glycerate precursors were mixed with a selenization solution of ethylene glycol, hydrazine hydrate, and SeO2. During the hydrothermal step, hydrazine reduced SeO2 to Se2−, which converted the Cu/Co precursor into the selenide phase to yield rGO-CCSe. SEM (Figure 14e) and TEM (Figure 14f) images confirmed rGO-CCSe showed a well-defined hollow sphere and firmly anchored on the rGO sheets. The synergistic integration of conductive rGO and hollow porous CCSe architecture provides high surface area and rapid charge pathways, enhancing ion transport, active-site accessibility. Therefore, the rGO-CCSe delivered 724 to 512 C g−1 as current density increases from 2 to 60 A g−1, outperforming CCSe (439 to 243 C g−1) and retaining 71% capacity at 60 A g−1 versus 55% for CCSe (Figure 14g). Furthermore, an HSC based on the rGO-CCSe electrode delivered a high energy density of 57.8 Wh kg−1 at a power density of 1800 W kg−1. In another work, Ye et al. prepared NiCo2S4@N-CNT via a two-step hydrothermal route, in which hollow NiCo2S4 nanoparticles (derived from Ni-Co glycerates) were bridged by conductive N-doped carbon nanotubes (N-CNT) [137]. The unique structure afforded a rough, high-area surface, abundant heterointerfaces, and electrolyte-accessible porous channels, enabling rapid ion/electron transport while buffering volume changes during cycling. Benefiting from these features, an all-solid-state asymmetric device (NiCo2S4@N-CNTs//AC) delivered an energy density of 59.37 Wh kg−1 at a power density of 750 W kg−1.
In addition, Yu and co-workers synthesized flexible S, N codoped carbon nanotube/graphene film embedded with the one-dimensional (1D) bunched Zn-Co-S yolk-shell spheres (CZS6T/CNTs/SNGS) via a high magnetic field-controlled anion-exchange strategy followed by vacuum filtration and one-step S, N codoping/reduction (Figure 14h,i) [157]. The high magnetic field-directed Zn-Co-S yolk-shell balls provided enlarged surface area and porosity, higher crystallinity and conductivity, and accelerated axial ion/electron transport. Integrating these subunits into an S, N-codoped CNT/graphene network suppressed agglomeration and ensures binder-free, intimate contact, thereby increasing redox-active site accessibility, shortening diffusion paths, and enhancing charge transfer. Consequently, the asymmetric device (CZS6T/CNTs/SNGS//CNTs/SNGS) achieved an energy density of 41.1 Wh kg−1 at a power density of 9022 W kg−1 (Figure 14j).
Yu et al. fabricated a conductive polymer polypyrrole (PPy)-anchored Ni-Co bimetallic sulfide composite (PPy@NCS) as electrode material for hybrid SCs [153]. As shown in Figure 14k, NiCo-glycerate was first synthesized through a one-step solvothermal process, while polypyrrole (PPy) was separately prepared under ice-bath conditions. The resulting NiCo-glycerate was then subjected to a one-pot solvothermal sulfidation in Na2S solution, during which in situ polymerization and anchoring of PPy occurred, yielding the PPy@NCS composite. The unique surface is more conducive to exposing more active sites. The PPy@NCS composite couples a conductive PPy network with porous Ni-Co sulfide nanosheets, yielding homogeneous dispersion, strong interfacial bonding, and efficient electron pathways. Its rough, high-surface-area architecture exposed abundant redox sites and accelerated ion transport, while the flexible PPy coating buffered volume changes. Therefore, PPy@NCS-5 delivered a high specific capacitance of 2011.6 F g−1 at 1 A g−1 (Figure 14l) and maintains 93.3% of its initial capacitance after 5000 cycles at 20 A g−1, demonstrating high long-term stability. An HSC assembled with PPy@NCS-5 as the positive electrode and AC as the negative electrode delivered an energy density of 44.6 Wh kg−1 at 15,005.6 W kg−1.
The coupling of different transition-metal compounds can form well-defined heterojunctions that integrate complementary functionalities. For instance, Shi et al. fabricated crystalline-amorphous core–shell nanospheres (NiCoP@NiCo-B, Figure 14m) by coating nanosheet-assembled NiCoP hollow cores with an amorphous Ni-Co-B shell [116]. Specifically, uniform NiCo-glycerate solid spheres were first prepared by one-pot solvothermal synthesis, then hydrolyzed in deionized water/ethanol to 2D NiCo-LDH. Subsequent NaH2PO2-mediated phosphidation yielded nanosheet-assembled NiCoP hollow spheres. After redispersion to adsorb Ni2+/Co2+, an ice-bath NaBH4 reduction under N2 deposited an amorphous NiCo-B shell, affording crystalline/amorphous NiCoP@NiCo-B core–shell nanospheres. The crystalline NiCoP core ensured mechanical stability, whereas the amorphous NiCo-B shell accelerated electrolyte-ion diffusion; their well-coupled interface furnished abundant redox sites and enabled rapid charge/ion transport. Benefiting from this synergistic design, the optimized electrode delivered a high specific capacity of 193.1 mAh g−1 at 1 A g−1 and retained 87.4% of its initial capacity at 20 A g−1 (Figure 14n), evidencing outstanding rate capability. Moreover, Wei and co-workers synthesized hierarchical Ni(OH)2-MnO2 hollow spheres via a straightforward template-conversion route [158]. Ni-Mn glycerate solid spheres were first transformed into Ni-Mn hydroxide hollow spheres, followed by O2 oxidation to yield Ni(OH)2-MnO2. The Ni(OH)2-MnO2 retained their hierarchical hollow architecture. HRTEM (Figure 14o–q) resolved lattice fringes of 0.78 nm and 0.40 nm, indexed to the (003) planes of Ni(OH)2 and the (101) planes of MnO2, respectively. The resulting architecture offered multiple internal voids, enlarged surface area, and plentiful redox-active sites, facilitating ion/electron transport. An asymmetric solid-state device using a PVA-KOH gel electrolyte, with these hollow spheres as the cathode and activated carbon as the anode, delivered a high energy density of 68.7 Wh kg−1 at a power density of 1650 W kg−1 (Figure 14r).
4. Conclusions and Outlook
In conclusion, this exhaustive review has focused on recent developments in the preparation, design, and supercapacitor application of electrode materials based on metal-glycerates and their derivatives. Their distinct metal ions/glycerolate coordination, compositional tunability, and morphological diversity provide an ideal platform for designing advanced functional materials. Tailored synthetic routes, including solvothermal self-assembly, ion-exchange transformations, and controlled thermal conversions, enable precise construction of solid, hollow, yolk-/multi-shelled, and other multiscale architectures. Subsequent conversion of MGs into functional derivatives (hydroxides, oxides, sulfides, phosphides, selenides, and composites) preserves or amplifies structural hierarchy while introducing complementary electronic structures and redox chemistries.
Despite significant advances (Table 1), several scientific and technological challenges remain before MG-based materials can achieve large-scale implementation in commercial supercapacitors:

Table 1.
Representative MG precursors and their derivatives as electrode materials for SCs.
(1) Controlled synthesis and structural precision.
Although the effects of metal precursor type/concentration, surfactant dosage, and reaction time on MG morphology have been systematically reported, the complex coordination environment in MGs still demands deeper mechanistic understanding of nucleation and growth to achieve truly uniform morphology, compositional homogeneity, and scalable synthesis. In particular, quantitative control over shell number, inter-shell spacing, defect density, and hierarchical porosity in hollow and yolk-/multi-shelled architectures of MG-based materials remains limited, which hampers rigorous correlation between structure and ion/electron transport behavior. Furthermore, the potential effects of autoclave pressure, temperature, and solvent composition on MG formation have been scarcely explored and thus represent an important direction for future studies on pressure/solvent/temperature modulation in the solvothermal synthesis of MGs.
(2) Interfacial and phase stability
Many MG-derived materials suffer from partial dissolution, phase transformation, volume expansion, or structural degradation under high potentials and high current densities. For sulfides, selenides, and phosphides in particular, redox-driven conversion reactions, interfacial corrosion, dissolution/precipitation, and surface reconstruction can gradually deteriorate active phases and increase internal resistance, yet their detailed cycling degradation pathways remain largely unresolved. Rational interface engineering (e.g., conformal carbon or polymer coatings) and robust heterostructure design are therefore crucial to suppress side reactions, alleviate mechanical stress, and maintain structural integrity during long-term operation.
(3) Mechanistic understanding
Current research on MG-based electrodes is still dominated by “materials fabrication-performance evaluation”, with relatively limited exploration of charge-storage mechanisms, interfacial processes, and dynamic structural evolution. For example, amorphous/crystalline coexisting architectures have been shown to enhance capacitance, yet the underlying synergistic mechanisms (such as defect-mediated redox and internal electric-field effects) remain poorly quantified. Likewise, multi-shelled hollow structures can effectively buffer volume change, but the quantitative relationships among shell number, inter-shell spacing, tortuosity, and ion-diffusion kinetics are not yet established. Future work should therefore place greater emphasis on combining in situ/operando characterization (e.g., XRD, TEM, Raman, XPS, XAS, and synchrotron-based techniques), advanced electrochemical diagnostics (e.g., GITT, EIS modeling, and Dunn analysis), and theoretical simulations (DFT and ab initio molecular dynamics) to elucidate potential-dependent structural evolution, ion/electron transport pathways, and true charge-storage mechanisms in MG-derived systems.
(4) Electrolyte selection and device-level optimization
To date, nearly all reported MG-based and MG-derived supercapacitors operate in aqueous alkaline electrolytes, predominantly KOH solutions or PVA-KOH gels, and systematic studies in non-aqueous or wide-voltage electrolyte systems are, to the best of our knowledge, still lacking. Advancing MG-based devices toward higher energy density without sacrificing power output or durability will therefore require not only sophisticated electrode architectures but also rigorous device engineering. Key directions include rationally matching mass loading and kinetics between positive and negative electrodes, integrating high-voltage aqueous, water-in-salt, or non-aqueous/gel electrolytes with wider electrochemical windows, mitigating the loss of areal capacitance at practical loadings, and ensuring mechanical robustness in flexible or solid-state configurations. It is also crucial to evaluate MG-based devices under realistic operating conditions, such as high areal loading, limited electrolyte, and broad temperature ranges, in order to accurately assess their practical application potential.
Author Contributions
Conceptualization, Y.Z., F.Q., Z.W. and X.W.; methodology, Q.L., Z.Z. and M.L.; validation, Y.Z., Q.L. and Z.Z.; investigation, J.S., J.F., K.Z., Z.Y. and H.X.; resources, J.C., S.P. and M.Z.; writing—original draft preparation, Y.Z., Q.L. and Z.Z.; writing—review and editing, Y.Z. and Q.L.; supervision, F.Q., Z.W. and X.W.; project administration, X.W.; funding acquisition, Y.Z., J.C., M.Z., Z.W. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52402117, 52502098, and 52502100; Natural Science Foundation of Jiangsu Province, China, grant number BK20240864, BK20240863, and BK20230538; Changzhou Sci&Tech Program, grant number CJ20250088; Qujiang District Science and Technology Program Projects, grant number QJ2024067.
Data Availability Statement
The datasets used and analyzed in this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, C.; Deng, C.; Zhai, P.; Shi, X.; Liu, W.; Jin, D.; Shang, B.; Gao, J.; Sun, L.; Hou, J. Tracking the correlation between spintronic structure and oxygen evolution reaction mechanism of cobalt-ruthenium-based electrocatalyst. Nat. Commun. 2025, 16, 215. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Shao, S.; Yu, Y.; Wang, W.; Sun, T.; Wu, S. A Ca-modified Ni/CeO2·Al2O3 bifunctional catalyst for two-stage steam reforming of biomass pyrolysis oil for hydrogen production. Ind. Crops Prod. 2025, 228, 120891. [Google Scholar]
- Ji, Q.; Zhou, C.; Li, Z.; Boateng, I.D.; Liu, X. Is nanocellulose a good substitute for non-renewable raw materials? A comprehensive review of the state of the art, preparations, and industrial applications. Ind. Crops Prod. 2023, 202, 117093. [Google Scholar]
- Yuan, J.; Zhu, Y.; Wang, J.; Gan, L.; He, M.; Zhang, T.; Li, P.; Qiu, F. Preparation and application of Mg–Al composite oxide/coconut shell carbon fiber for effective removal of phosphorus from domestic sewage. Food Bioprod. Process. 2021, 126, 293–304. [Google Scholar]
- Gong, C.X.; Meng, X.Z.; Jin, C.D.; Yang, M.; Liu, J.L.; Sheng, K.C.; Pu, Y.Q.; Ragauskas, A.J.; Ji, G.Y.; Zhang, X.M. Green synthesis of cellulose formate and its efficient conversion into 5-hydroxymethylfurfural. Ind. Crops Prod. 2023, 192, 115985. [Google Scholar]
- Chen, S.R.; Zhao, Y.F.; Tang, Z.; Ding, H.T.; Su, Z.; Ding, Z. Structural model of straw briquetting machine with vertical ring die and optimization of briquetting performance. Agriculture 2022, 12, 736. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, Y.H.; Yu, J.G.; Xue, L.H.; Li, H.B.; Yang, L.Z. Roles of bulk and rhizosphere denitrifying bacteria in denitrification from paddy soils under straw return condition. J. Soils Sediments 2021, 21, 2179–2191. [Google Scholar] [CrossRef]
- Xu, Y.; Du, Y.; Chen, H.; Chen, J.; Ding, T.; Sun, D.; Kim, D.H.; Lin, Z.; Zhou, X. Recent advances in rational design for high-performance potassium-ion batteries. Chem. Soc. Rev. 2024, 53, 7202–7298. [Google Scholar]
- Welsby, D.; Price, J.; Pye, S.; Ekins, P. Unextractable fossil fuels in a 1.5 °C world. Nature 2021, 597, 230–234. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Zhang, L.; Yu, X.; Liu, H.; Yagoub, A.E.A.; Zhou, C. Synthesis of 5-HMF from an ultrasound-ionic liquid pretreated sugarcane bagasse by using a microwave-solid acid/ionic liquid system. Ind. Crops Prod. 2020, 149, 112361. [Google Scholar]
- Hassan, G.; Shabbir, M.A.; Ahmad, F.; Pasha, I.; Aslam, N.; Ahmad, T.; Rehman, A.; Manzoor, M.F.; Inam-Ur-Raheem, M.; Aadil, R.M. Cereal processing waste, an environmental impact and value addition perspectives: A comprehensive treatise. Food Chem. 2021, 363, 130352. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.J.; Li, G.; Nazir, M.M.; Zulfiqar, F.; Siddique, K.H.M.; Iqbal, B.; Du, D. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Till. Res. 2024, 237, 105959. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.C.; Zheng, X.Q.; Ding, L.; Ming, F.; Pan, A.; Lv, W.G.; Tang, X.M. Rice straw decomposition affects diversity and dynamics of soil fungal community, but not bacteria. J. Soils Sediments 2018, 18, 248–258. [Google Scholar] [CrossRef]
- Shao, S.S.; Ma, L.; Li, X.; Zhang, H.; Xiao, R. Preparation of activated carbon with heavy fraction of bio-oil from rape straw pyrolysis as carbon source and its performance in the aldol condensation for aviation fuel as carrier. Ind. Crops Prod. 2023, 192, 115912. [Google Scholar] [CrossRef]
- Zhu, J.; Tie, Z.; Bi, S.; Niu, Z. Towards more sustainable aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2024, 63, e202403712. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Zhao, Y.; Zhu, X.; Ge, A.; Gordon, K.C.; Wang, F.; Xu, G.; Zhu, M. Enhancing robustness and charge transfer kinetics of sodium-ion batteries through introduction of anionic anchoring separators. J. Am. Chem. Soc. 2025, 147, 8488–8499. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.; Wu, Z.; Wang, X.; Guan, R.; Li, C.; Qiao, F.; Wang, J.; Fu, Y.; Baek, J.-B. Amorphous/crystalline heterostructured nanomaterials: An emerging platform for electrochemical energy storage. Small 2025, 21, 2411941. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Chen, L.; Ren, M.; Fakayode, O.A.; Han, J.; Zhou, C. Efficient hydrogen evolution reaction performance using lignin-assisted chestnut shell carbon-loaded molybdenum disulfide. Ind. Crops Prod. 2023, 193, 116214. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Chen, L.; Yarley, O.P.N.; Zhou, C. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crops Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Xu, G.; Wang, S.; Cai, X.; Mei, J.; Zou, H.; Zhang, L.; Qian, H. Study on characteristics, hydrogen-rich gas, bio-oil production, and process optimization of co-pyrolysis of sewage sludge and wheat straw. Case Stud. Therm. Eng. 2023, 45, 102888. [Google Scholar] [CrossRef]
- Pan, S.; Zabed, H.M.; Wei, Y.; Qi, X. Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: Current status, challenges and future outlook. Ind. Crops Prod. 2022, 188, 115684. [Google Scholar] [CrossRef]
- Jiang, H.; Yao, L.; Qin, J.; Bai, Y.; Brandt, M.; Lian, X.; Davis, S.J.; Lu, N.; Zhao, W.; Liu, T.; et al. Globally interconnected solar-wind system addresses future electricity demands. Nat. Commun. 2025, 16, 4523. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, R.; Yang, J.; Wang, Y.; Zhang, A.; Hu, Z.; Lv, M.; Wu, C.; Bai, Y. High-entropy materials for aqueous zinc metal batteries. Energy Environ. Sci. 2025, 18, 97–129. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Y.; Wang, Q.; Zou, X.; Pan, Z.; Leung, M.K.H.; An, L. Direct seawater electrolysis for green hydrogen production: Electrode designs, cell configurations, and system integrations. Energy Environ. Sci. 2025, 18, 4596–4624. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Chen, T.; Yun, J.; Zhao, M.; Zabed, H.M.; Zhang, C.; Qi, X. Metabolic remodulation of chassis and corn stover bioprocessing to unlock 3-hydroxypropionic acid biosynthesis from agrowaste-derived substrates. J. Agric. Food Chem. 2024, 72, 2536–2546. [Google Scholar] [CrossRef]
- Wang, G.X.; Chen, A.; Chen, Y.; Qiao, F.; Wang, J.F.; Yang, N.J.; Zhang, H.; Wen, Z.H. Advancements in electrochemical synthesis: Expanding from water electrolysis to dual-value-added products. eScience 2025, 5, 100333. [Google Scholar] [CrossRef]
- Fagiolari, L.; Sampò, M.; Lamberti, A.; Amici, J.; Francia, C.; Bodoardo, S.; Bella, F. Integrated energy conversion and storage devices: Interfacing solar cells, batteries and supercapacitors. Energy Storage Mater. 2022, 51, 400–434. [Google Scholar] [CrossRef]
- Shafiei, K.; Seifi, A.; Hagh, M.T. A novel multi-objective optimization approach for resilience enhancement considering integrated energy systems with renewable energy, energy storage, energy sharing, and demand-side management. J. Energy Storage 2025, 115, 115966. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Zhou, Y.; Wang, J.; Qiao, F.; Wu, Z.; Zhou, J. Boosting reaction kinetics in vanadium oxide cathode via carboxylated conjugate multi-ring molecular intercalation. J. Energy Storage 2025, 117, 116173. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, M.; He, W.; Guo, T.; Tong, W.; Kan, E.; Ouyang, X.; Qiao, F.; Wang, J.; Sun, X.; et al. Unveiling the autocatalytic growth of Li2S crystals at the solid-liquid interface in lithium-sulfur batteries. Nat. Commun. 2024, 15, 9535. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Xu, H.; Qiao, Y.; He, G.; Chen, H. Engineering improved strategies for spinel cathodes in high-performing zinc-ion batteries. Nanoscale 2024, 16, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, B.; Lu, Y.; Xu, H.; Qiao, Y.; Xu, H.; He, G.; Chen, H. One-step construction of FeNi LDH/FeNi2S4 heterojunctions for boosting electrocatalytic oxygen evolution reaction and hybrid capacitive storage. Appl. Surf. Sci. 2023, 610, 155480. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Wang, X.; Guo, Y.; Ni, C.; Wu, J.; Hao, C. High performance hybrid supercapacitors assembled with multi-cavity nickel cobalt sulfide hollow microspheres as cathode and porous typha-derived carbon as anode. Ind. Crops Prod. 2022, 189, 115863. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Li, C.E.; Yan, X.H.; Zhang, Y.Y.; Yin, R.B.; Wei, Y.F.; Gao, K.Z.; Gao, H.L. Advanced strategies for enhancing electrochemical performance of NiAl LDH electrodes in supercapacitors. Coord. Chem. Rev. 2025, 531, 216497. [Google Scholar] [CrossRef]
- Wu, Q.; Zhong, Y.; Chen, R.; Ling, G.; Wang, X.; Shen, Y.; Hao, C. Cu-Ag-C@Ni3S4 with core shell structure and rose derived carbon electrode materials: An environmentally friendly supercapacitor with high energy and power density. Ind. Crops Prod. 2024, 222, 119676. [Google Scholar] [CrossRef]
- Kumar, R.; Ranjan, B.; Kaur, D. Recent advancements in zero, one, two, and three-dimensional transition metal nitride-based supercapacitor electrodes. J. Energy Storage 2025, 121, 116580. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, X.; Yan, X.-h.; Gao, H.-l.; Gao, K.-z.; Cao, Y.; Hu, S.; Zhang, Y.-y. Rational design of NiMn-based electrode materials for high-performance supercapacitors. Coord. Chem. Rev. 2024, 499, 215494. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, R.B.; Wei, Y.F.; Fan, Z.Y.; Gao, H.L. Co-based LDH for high-performance supercapacitors: Preparation methods, structural design, and performance optimization. Coord. Chem. Rev. 2025, 541, 216804. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.Y.; Li, C.E.; Yan, X.H.; Hu, S.; Yin, R.B.; Wei, Y.F.; Gao, K.Z.; Gao, H.L. Research progress of NiFe2O4 electrode materials in supercapacitors: Preparation, modification, structural regulation, and future challenges. Coord. Chem. Rev. 2024, 519, 216103. [Google Scholar] [CrossRef]
- Ni, G.; Yang, Y.; Chen, K.; Liu, C.; Wang, S. Optimization of pore structure and surface chemical properties of activated carbon fiber via liquid-phase stabilization for enhanced supercapacitor performance. Diamond Relat. Mater. 2024, 149, 111607. [Google Scholar] [CrossRef]
- Mahmood, F.; Ali, M.; Khan, M.; Mbeugang, C.F.M.; Isa, Y.M.; Kozlov, A.; Penzik, M.; Xie, X.; Yang, H.; Zhang, S.; et al. A review of biochar production and its employment in synthesizing carbon-based materials for supercapacitors. Ind. Crops Prod. 2025, 227, 120830. [Google Scholar] [CrossRef]
- Chai, L.; Li, R.; Sun, Y.; Zhou, K.; Pan, J. MOF-derived carbon-based materials for energy-related applications. Adv. Mater. 2025, 37, 2413658. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Yang, Y.; Qu, S.; Zuo, P.; Niu, H.; Qin, F. Exploring oxygen migration and capacitive mechanisms: Crafting oxygen-enriched activated carbon fiber from low softening point pitch. Surf. Interfaces 2024, 52, 124970. [Google Scholar] [CrossRef]
- Patil, A.M.; Moon, S.; Jadhav, A.A.; Hong, J.; Kang, K.; Jun, S.C. Modifying electronic structure of cationex-changed bimetallic sulfide/metal oxide heterostructure through in situ inclusion of silver (Ag) nanoparticles for extrinsic pseudocapacitor. Adv. Funct. Mater. 2023, 33, 2305264. [Google Scholar] [CrossRef]
- Liu, C.; Gong, W.; Iftikhar, T.; Liu, W.; Su, L.; Zhang, X. Iron-based metal-organic frameworks and their derivatives for high-performance supercapacitors. Next Mater. 2025, 7, 100362. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, L.; Liu, Y.; Chen, H.; Li, H.; Yang, M.; Qiu, J. Triazine-containing covalent organic polymer-derived grid-like multilocular spheres for aqueous supercapacitors. Adv. Mater. 2025, 37, 2419124. [Google Scholar] [CrossRef]
- Arumugam, G.; Chettiannan, B.; Mathan, S.; Selvaraj, M.; Assiri, M.A.; Rajendran, R. Recent progress and prospects on freestanding metal-organic frameworks and its derivatives for supercapacitor applications. J. Alloys Compd. 2025, 1027, 180508. [Google Scholar] [CrossRef]
- Ji, B.; Li, W.; Zhang, F.; Geng, P.; Li, C.M. MOF-derived transition metal phosphides for supercapacitors. Small 2025, 21, 2409273. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Patra, A.; Manasa, G.; Belgami, M.A.; Mun Jeong, S.; Rout, C.S. Borocarbonitride-based emerging materials for supercapacitor applications: Recent advances, challenges, and future perspectives. Adv. Sci. 2024, 11, 2305325. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, Y.; Zhang, T.; Hu, C.; Qiao, F.; Wang, J.; Noh, H.-J.; Baek, J.-B. Synthesis of size-controllable, yolk-shell metal sulfide spheres for hybrid supercapacitors. Chem. Eng. J. 2023, 476, 146377. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Y.; Poisson, J.; He, Y.; Zhang, H.; Zhang, Y.; Bao, Y.; Chen, S.; Chen, Y.M.; Zhang, K. Recent advances in biopolymer-based hydrogel electrolytes for flexible supercapacitors. ACS Energy Lett. 2024, 9, 1803–1825. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Peng, X.; Chen, J.; Kang, L.; Yin, X.; Yusuke, Y.; Ding, B. Emerging issues and opportunities of 2D layered transition metal dichalcogenide architectures for supercapacitors. ACS Nano 2025, 19, 13591–13636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, D.; Ge, Z.; Pan, L.; Chen, Y.; Wang, W.; Mitsuzaki, N.; Jia, S.; Chen, Z. Recent advances in transition metal sulfide-based electrode materials for supercapacitors. Chem. Commun. 2025, 61, 8314–8326. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.M.; Hennemann, A.L.; Ruiz-Montoya, J.G.; Martins, P.R.; Araki, K.; Angnes, L.; Shahbazian-Yassar, R. Metal-glycerolates and their derivatives as electrode materials: A review on recent developments, challenges, and future perspectives. Coord. Chem. Rev. 2023, 477, 214954. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, W.; Li, C.; Mi, P.; Liu, Z.; Li, Y.; Tian, Y.; Ma, X.; Liu, F.; Jin, X. Rational design of boron-doped NiCo-glycerate spheres with covered nanosheets for high performance supercapacitors. Mater. Today Chem. 2024, 35, 101826. [Google Scholar] [CrossRef]
- Jiao, A.-J.; Duan, Y.-K.; Li, Z.-W.; Zhang, S.-C.; Zhang, Y.-M.; Su, T.; Fu, Z.-H. Nanostructure engineering of cobalt-nickel glycerate (CoNi-G) spheres as anodes for constructing high-performance lithium-ion capacitors. J. Power Sources 2025, 627, 235838. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Ruiz-Montoya, J.G. Emerging high-entropy coordination compounds and their derivatives for energy application. J. Mater. Chem. A 2023, 11, 20872–20885. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Jayan, H.; Gao, S.; Zhou, R.; Yosri, N.; Zou, X.; Guo, Z. Recent and emerging trends of metal-organic frameworks (MOFs)-based sensors for detecting food contaminants: A critical and comprehensive review. Food Chem. 2024, 448, 139051. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Chen, Q.; Ouyang, Q. Multifunctional metal-organic frameworks driven three-dimensional folded paper-based microfluidic analysis device for chlorpyrifos detection. J. Agric. Food Chem. 2024, 72, 14375–14385. [Google Scholar] [CrossRef]
- Liang, N.; Shi, B.; Hu, X.; Li, W.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. A ternary heterostructure aptasensor based on metal-organic framework and polydopamine nanoparticles for fluorescent detection of sulfamethazine. Food Chem. 2024, 460, 140570. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar] [CrossRef]
- Shi, B.Q.; Zhang, X.A.; Li, W.T.; Liang, N.N.; Hu, X.T.; Xiao, J.B.; Wang, D.Y.; Zou, X.B.; Shi, J.Y. An intrinsic dual-emitting fluorescence sensing toward tetracycline with self-calibration model based on luminescent lanthanide-functionalized metal-organic frameworks. Food Chem. 2023, 400, 133995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Zhou, R.Y.; Ke, L.J.; Li, J.B.; Jayan, H.; El-Seedi, H.R.; Zou, X.B.; Guo, Z.M. Development of multifunctional metal-organic frameworks (MOFs)-based nanofiller materials in food packaging: A comprehensive review. Trends Food Sci. Technol. 2024, 154, 104771. [Google Scholar] [CrossRef]
- Liu, X.; Gong, M.; Deng, S.; Zhao, T.; Zhang, J.; Wang, D. Recent advances on metal alkoxide-based electrocatalysts for water splitting. J. Mater. Chem. A 2020, 8, 10130–10149. [Google Scholar] [CrossRef]
- Qiu, S.; Cui, C.; Li, G.; Wang, Y.; Duan, L.; Chu, H.; Xu, F.; Li, H.; Ma, L.; Luo, Y.; et al. Core-shell structured nickel glycerolate spheres@ amorphous ZIF-67 nanosheets to enhance electrochemical performance of supercapacitors. J. Energy Storage 2025, 114, 115736. [Google Scholar] [CrossRef]
- Jia, S.; Zhao, Q.; He, M.; Zhang, T. Hierarchical construction of amorphous NiCo-OHS@ZnS hollow spheres with optimized reaction kinetics via ion exchange-etching strategy for energy storage. Chem. Eng. J. 2024, 494, 153215. [Google Scholar] [CrossRef]
- Wang, C.; Sui, G.; Guo, D.; Li, J.; Zhuang, Y.; Guo, W.; Zhou, Y.; Yang, X.; Chai, D.-F. Inverted design of oxygen vacancies modulated NiCo2O4 and Co3O4 microspheres with superior specific surface area as competitive bifunctional materials for supercapacitor and hydrogen evolution reaction. J. Energy Storage 2022, 49, 104083. [Google Scholar] [CrossRef]
- Heidari Gourji, F.; Rajaramanan, T.; Frette, Ø.; Velauthapillai, D. Fabrication of ternary NiCoMoOx with yolk-shell hollow structure as a positive electrode material for high-performance electrochemical capacitor applications. Nanoscale 2023, 15, 16178–16187. [Google Scholar] [CrossRef]
- Wei, C.; Cheng, C.; Wei, D.; Du, W.; Chen, J.; Cheng, T.; Duan, Y. Hybrid Ni-Mn-Ce oxide hierarchical hollow architectures for high-performance supercapacitors. ACS Appl. Nano Mater. 2025, 8, 19786–19795. [Google Scholar] [CrossRef]
- Chen, K.; Xie, H.; Yang, H.; Han, G.; Chen, J.; Liu, H. Construction of crystalline/amorphous heterostructures to enhance the supercapacitor performance of a high-entropy sulfide. J. Alloys Compd. 2025, 1015, 178921. [Google Scholar] [CrossRef]
- Shirvani, M.; Hosseiny Davarani, S.S. Self -templated construction of hollow trimetallic MnNiCoP yolk–shell spheres assembled with nanosheets as a satisfactory positive electrode material for hybrid supercapacitors. Nanoscale 2023, 15, 11115–11130. [Google Scholar] [CrossRef]
- Liu, R.; Gao, X.; Xie, Y.; Liu, Q.; Zhang, K.; Sun, Y.; Bai, H.; Yao, F.; Yue, H. Self-templated flower-like NiCoZn-carbonate hydroxide hollow nanospheres for asymmetric supercapacitors with high performance. Nanoscale 2023, 15, 16795–16802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zang, Q.; Shi, Q.; Xiao, Z.; Wang, K.-P.; Zong, L.; Wang, L. Formation of V6O11@Ni(OH)2/NiOOH hollow double-shell nanoflowers for the excellent cycle stability of supercapacitors. Dalton Trans. 2021, 50, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Ameri, B.; Zardkhoshoui, A.M.; Davarani, S.S.H. Engineering raspberry-like CuCo2S4@ZnS hollow particles encapsulated with reduced graphene oxide for hybrid supercapacitors. Mater. Chem. Front. 2023, 7, 3127–3145. [Google Scholar] [CrossRef]
- Li, Q.; Lu, W.; Li, Z.; Ning, J.; Zhong, Y.; Hu, Y. Hierarchical MoS2/NiCo2S4@C urchin-like hollow microspheres for asymmetric supercapacitors. Chem. Eng. J. 2020, 380, 122544. [Google Scholar] [CrossRef]
- Li, Y.; Lu, F.; Zong, K.; Wang, J.; Pan, L.; Nie, Y.; Wang, X.; Yang, Y.; Yang, L.; Jin, M.; et al. Fe3O4/Fe2N heterostructured hollow microspheres as functional electrocatalysts for high stability lithium-sulfur batteries. Nano Energy 2025, 139, 110949. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zhang, Y.; Li, N.; Zhang, D.; Zhao, S.; Zhao, Y. NiCo2V2O8@NC spheres with mesoporous yolk-bilayer hierarchical structure for enhanced lithium storage. Langmuir 2024, 40, 15161–15170. [Google Scholar] [CrossRef]
- Ding, S.S.; An, J.; Ding, D.; Zou, Y.N.; Zhao, L.J. Micron-sized NiMn-glycerate solid spheres as cathode materials for all-solid-state asymmetric supercapacitor with superior energy density and cycling life. Chem. Eng. J. 2022, 431, 134100. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, W.; Xiao, Y.; Wang, Y.; Luan, C.; Qin, C.; Dong, Y.; Li, M.; Dai, X.; Zhang, X. One-pot-synthesized CoFe-glycerate hollow spheres with rich oxyhydroxides for efficient oxygen evolution reaction. ACS Sustain. Chem. Eng. 2020, 8, 5464–5477. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Pan, Y.; Zhou, T.; Ding, J.; Sun, Y.; Wang, Y. Self-templated formation of CuCo2O4 triple-shelled hollow microspheres for all-solid-state asymmetric supercapacitors. J. Alloys Compd. 2019, 787, 694–699. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Chu, X.; Liu, F.; Guo, C.; Yang, W. Synthesis of size-controllable NiCo2S4 hollow nanospheres toward enhanced electrochemical performance. Energy Environ. Mater. 2020, 3, 421–428. [Google Scholar] [CrossRef]
- Chong, B.; Xia, M.; Lv, Y.; Li, H.; Yan, X.; Lin, B.; Yang, G. Hierarchical phosphorus-oxygen incorporated cobalt sulfide hollow micro/nano-reactor for highly-efficient electrocatalytic overall water splitting. Chem. Eng. J. 2023, 465, 142853. [Google Scholar] [CrossRef]
- Zhong, L.; Yue, M.; Chen, H.; Xie, W.; Xiao, Y.; Cheng, B.; Lin, L.; Lei, S. Hierarchical flower-like MnPS3 hollow spheres for high-rate and long-cycle sodium storage. J. Power Sources 2024, 614, 234990. [Google Scholar] [CrossRef]
- Cai, R.; Qin, H.; Yu, X.; Yan, F.; Wang, X.; Zhao, Y.; Wang, B.; Zhang, X. Kirkendall effect-assisted synthesis of hollow MoS2 nanospheres with interlayer expansion for improved magnesium diffusion kinetics and durability. J. Mater. Chem. A 2025, 13, 2574–2582. [Google Scholar] [CrossRef]
- Ju, H.; Lang, H.; Yi, T.; Tian, K.; Yue, J.; Zhang, T.; Zhang, M.; Ye, Y.; Chen, N.; Li, Q. Formation of yolk-shell Co-Mn Prussian blue analog spheres with enhanced electrochemical performance for supercapacitors. J. Alloys Compd. 2025, 1010, 177897. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, T.; He, Q.; Wang, H.; Liu, Z.; Wang, D.; Wang, H.; Meng, L. Enhancing lithium-ion storage performance of hollow CoS2/MoS2 nanospheres via N-doped carbon-coating. J. Energy Storage 2023, 72, 108639. [Google Scholar] [CrossRef]
- Zhang, C.; Qing, X.; Wu, Y.; Zhan, D.; Xu, F.; Sun, L.; Xiang, C.; Xiao, Z.; Zou, Y. Rational design of layered double hydroxides with yolk-shell heterostructures for novel supercapacitor electrodes. Electrochim. Acta 2025, 524, 146026. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, Y.; Zhang, Y.; Sun, J.; Zheng, J.; Wei, C.; Du, W.; Liu, L.; Cheng, C. Designed synthesis of yolk-shelled NiCo2O4/MnCo2O4 hollow sphere with boosted performance for supercapacitors. Appl. Surf. Sci. 2023, 611, 155758. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Wang, Y.; Gu, S.; Yang, C.; Gao, T.; Cao, P.; Zhou, G. Preparation of yolk-double shell Mn0.5Zn0.5Co2O4/C nanomaterials as anodes for high-performance lithium-ion batteries. Appl. Mater. Today 2022, 27, 101452. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Su, Y.H.; Lin, C.C.; Ruan, J.; Ting, J.M. A new high entropy glycerate for high performance oxygen evolution reaction. Adv. Sci. 2021, 8, 2002446. [Google Scholar] [CrossRef]
- Ting, N.-H.; Nguyen, T.X.; Lee, C.-H.; Chen, Y.-C.; Yeh, C.-H.; Chen, H.-Y.T.; Ting, J.-M. Composition-controlled high entropy metal glycerate as high-performance electrocatalyst for oxygen evolution reaction. Appl. Mater. Today 2022, 27, 101398. [Google Scholar] [CrossRef]
- Kong, L.; Hao, L.; Hu, M.; Su, M.; Meng, Q.; Zhang, Y. A one-pot hydrothermal synthesis of morphologically controllable yolk-shell structured CoFe glycerate spheres for oxygen evolution reaction. J. Colloid Interface Sci. 2025, 677, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, Y.; Zhao, Z.; Wang, X.; Guan, R.; Li, C.; Yang, L.; Qiao, F.; Wang, J.; Wu, Z.; et al. Hollow structures derived from metal-glycerates toward efficient electrochemical energy storage and conversion. Nano Energy 2025, 144, 111348. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, H.; Yao, T.; Ji, X.; Zhang, Q.; Meng, L.; Feng, J.; Wang, H. Copper-induced formation of heterostructured Co3O4/CuO hollow nanospheres towards greatly enhanced lithium storage performance. Chin. Chem. Lett. 2024, 35, 108450. [Google Scholar] [CrossRef]
- Shen, L.; Yu, L.; Yu, X.Y.; Zhang, X.; Lou, X.W. Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors. Angew. Chem. Int. Ed. 2014, 54, 1868–1872. [Google Scholar] [CrossRef]
- Sun, L.; Yan, M.; Xiao, L.; Liu, Y.; Bai, H.; Xie, L.; Shi, W. Synthesis of C/Co3O4 composite mesoporous hollow sphere sandwich graphene films for high-performance supercapacitors. Inorg. Chem. Front. 2018, 5, 2554–2562. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Sun, J.; Duan, L.; Xu, J.; Sun, D.; Zhou, X. Implantation of Fe7S8 nanocrystals into hollow carbon nanospheres for efficient potassium storage. J. Colloid Interface Sci. 2022, 615, 840–848. [Google Scholar] [CrossRef]
- Osman, S.; Peng, C.; Li, F.; Chen, H.; Shen, J.; Zhong, Z.; Huang, W.; Xue, D.; Liu, J. Defect-induced dense amorphous/crystalline heterophase enables high-rate and ultrastable sodium storage. Adv. Sci. 2022, 9, 2205575. [Google Scholar] [CrossRef]
- Pan, R.; Liu, S.; Guo, X.; Duan, Z.; Fan, Q.; Liu, Y.; Zheng, X.; Li, C.; Kong, Q.; Zhang, J. Non-carbonization annealing toward regulation of cobalt-based organic-inorganic hybrids as advanced electrocatalysts for water splitting. J. Colloid Interface Sci. 2025, 682, 671–679. [Google Scholar] [CrossRef]
- Xu, B.; Duan, M.; Shen, K.; Guo, X.; Yang, X.; Zhang, M.; Yue, B.; Zhang, M.; Zhang, J.; Jin, Z. Hydrothermal hydrolyzation-driven topological transformation of Ni-Co bimetallic compounds with hollow nanoflower structure for optimizing hydrogen evolution catalysis. ACS Appl. Mater. Interfaces 2024, 16, 16399–16407. [Google Scholar] [CrossRef]
- Xie, J.Y.; Wang, F.L.; Zhai, X.J.; Li, X.; Zhang, Y.S.; Fan, R.Y.; Lv, R.Q.; Chai, Y.M.; Dong, B. Manganese doped hollow cobalt oxide catalysts for highly efficient oxygen evolution in wide pH range. Chem. Eng. J. 2024, 482, 148926. [Google Scholar] [CrossRef]
- Kamari Kaverlavani, S.; Moosavifard, S.E.; Bakouei, A. Self-templated synthesis of uniform nanoporous CuCo2O4 double-shelled hollow microspheres for high-performance asymmetric supercapacitors. Chem. Commun. 2017, 53, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, Y.; Wu, F.; Moudrakovski, I.; van Aken, P.A.; Maier, J.; Yu, Y. Hierarchical metal sulfide/carbon spheres: A generalized synthesis and high sodium-storage performance. Angew. Chem. Int. Ed. 2019, 58, 7238–7243. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Lv, Y.; Wei, J.; Guan, J.; Zhai, Y.; Shao, Z. Amorphous nanosheets constructed nickel cobalt hydroxysulfide hollow spheres as cathode materials for hybrid supercapacitors. Chem. Eng. J. 2023, 456, 141120. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, W.; Tian, Y.; Yang, S.; Zhang, Q.; Lei, D.; Zhao, Y. Nanosheet-assembled NiCo-LDH hollow spheres as high-performance electrodes for supercapacitors. J. Colloid Interface Sci. 2022, 606, 1120–1127. [Google Scholar] [CrossRef]
- Zeng, Y.; Lu, X.F.; Zhang, S.L.; Luan, D.; Li, S.; Lou, X.W. Construction of Co-Mn Prussian blue analog hollow spheres for efficient aqueous Zn-ion batteries. Angew. Chem. Int. Ed. 2021, 60, 22189–22194. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, C.; Li, N.-W.; Yuan, S.; Yu, L. Formation of Co-Mn mixed oxide double-shelled hollow spheres as advanced electrodes for hybrid supercapacitors. J. Mater. Chem. A 2019, 7, 25247–25253. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; Ge, R.; Pei, K.; Song, C.; Li, W.; Chen, Y.; Zhang, Y.; Feng, L.; Che, R. Construction of CoNiFe trimetallic carbonate hydroxide hierarchical hollow microflowers with oxygen vacancies for electrocatalytic water oxidation. Adv. Funct. Mater. 2022, 32, 2200726. [Google Scholar] [CrossRef]
- Jia, S.; Zhao, D.; Ren, Y.; Wang, J.; Hui, W.; Yin, G.; Zhang, T.; Li, Y. Critical role of cation exchange in hierarchical hollow NiCoCe-hydroxides cathode with multi-effects for high-performance alkaline Zn battery. Adv. Funct. Mater. 2025, 35, e08372. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Cao, H.; Li, C.; Wang, L.; Wu, Y.; Wang, C.; Li, Y. Rational synthesis of Cu7Se4-CuxCo1−xSe2 double-shell hollow nanospheres for high performance supercapacitors. J. Power Sources 2020, 480, 228741. [Google Scholar] [CrossRef]
- Li, P.; Liu, X.; Arif, M.; Yan, H.; Hu, C.; Chen, S.-M.; Liu, X. In situ growth of glucose-intercalated LDHs on NiCo2S4 hollow nanospheres to enhance energy storage capacity for hybrid supercapacitors. Colloid Surf. A 2022, 644, 128823. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Yin, M.; Li, J.; Yang, H.; Liu, W.; Wang, X.; Xi, G. Multilayered hollow transition metal nitride spheres made from single-source precursors for SERS analytics. Nat. Commun. 2025, 16, 2678. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Q.; Pan, S.; Lu, Z.; Fu, Y. Stepwise construction of hollow double shell cobalt sulfide spheres for enhanced hybrid supercapacitors. J. Energy Storage 2024, 104, 114417. [Google Scholar] [CrossRef]
- Amiri, M.; Davarani, S.S.H.; Kaverlavani, S.K.; Moosavifard, S.E.; Shamsipur, M. Construction of hierarchical nanoporous CuCo2V2O8 hollow spheres as a novel electrode material for high-performance asymmetric supercapacitors. Appl. Surf. Sci. 2020, 527, 146855. [Google Scholar] [CrossRef]
- Wu, Y.; Qing, X.; Cao, Y.; Geng, B.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L. Core-shell manganese cobalt oxide-nickel molybdenum layered double hydroxide composites for supercapacitor electrodes. J. Energy Storage 2025, 131, 117529. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, M.; Zheng, Q.; Li, F.; Jiao, L.; Su, Z.; Li, M.; Song, X. Constructing crystalline NiCoP@amorphous Nickel-Cobalt boride core-shell nanospheres with enhanced rate capability for aqueous supercapacitors and rechargeable Zn-based batteries. Energy Fuel 2024, 38, 1525–1537. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, Q.; Lu, L.; Zou, Y.; Xu, F.; Sun, L.; Cai, D.; Xiang, C. Hollow core-shell CuCo2O4@MoNi-layered double hydroxides as an electrode material for supercapacitors. J. Energy Storage 2023, 61, 106691. [Google Scholar] [CrossRef]
- Xu, J.; Xiang, C.; Yu, S.; Zou, Y.; Fang, S.; Hu, Z.; Xu, F.; Sun, L. Synthesis of porous yolk-shelled NiSe2-MnSe heterojunctions for high-cycling-stability asymmetric supercapacitor electrode materials. ACS Appl. Energy Mater. 2022, 5, 6194–6205. [Google Scholar] [CrossRef]
- Lu, X.F.; Zhang, S.L.; Sim, W.L.; Gao, S.; Lou, X.W. Phosphorized CoNi2S4 yolk-shell spheres for highly efficient hydrogen production via water and urea electrolysis. Angew. Chem. Int. Ed. 2021, 133, 23067–23073. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Sun, J.; Zhu, J.; Wang, X.; Fu, Y. The construction of hierarchical hollow Double-Shelled Co3O4 for the enhanced thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2022, 571, 151342. [Google Scholar] [CrossRef]
- Li, L.; Sheng, Z.; Xiao, Q.; Hu, Q. Co9S8 core-shell hollow spheres for enhanced oxygen evolution and methanol oxidation reactions by sulfur vacancy engineering. Dalton Trans. 2023, 53, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wei, C.; He, Y.; Liu, L.; Hu, J.; Du, W. Etching strategy synthesis of hierarchical Ni-Mn hydroxide hollow spheres for supercapacitors. J. Energy Storage 2021, 33, 102105. [Google Scholar] [CrossRef]
- Nagaraju, M.; Ramulu, B.; Girija Shankar, E.; Yu, J.S. Rational design of hierarchical zeolitic imidazolate framework-67@Cu2CoO3 core-shell architectures for hybrid supercapacitor applications. Appl. Surf. Sci. 2023, 640, 158339. [Google Scholar] [CrossRef]
- Tong, H.; Li, L.; Wu, C.; Tao, Z.; Fang, J.; Guan, C.; Zhang, X. Sea urchin-like NiCo-LDH hollow spheres anchored on 3D graphene aerogel for high-performance supercapacitors. ChemSusChem 2024, 17, e202400142. [Google Scholar] [CrossRef]
- Fan, J.; Zheng, H.; Chen, A.; Gu, L.; Xie, X.; Liu, Y.; Ding, Z. Substantially boosted energy density of NiCoMn layered double hydroxides with nanocrystalline-amorphous domains. J. Energy Storage 2024, 97, 112663. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Li, C.; Cao, H.; Wang, C.; Cheng, S.; Zhang, Q. A novel strategy of multi-element nanocomposite synthesis for high performance ZnO-CoSe2 supercapacitor material development. Chin. J. Chem. 2021, 39, 2441–2450. [Google Scholar] [CrossRef]
- Sekar, K.; Raji, G.; Chen, S.; Liu, S.; Xing, R. Ultrathin VS2 nanosheets vertically aligned on NiCo2S4@C3N4 hybrid for asymmetric supercapacitor and alkaline hydrogen evolution reaction. Appl. Surf. Sci. 2020, 527, 146856. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Z.; Zhang, T.; Chen, Q.; Qiu, F.; Peng, Y.; Tang, S. Fabrication of functional biomass carbon aerogels derived from sisal fibers for application in selenium extraction. Food Bioprod. Process. 2018, 111, 93–103. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Zou, X.; Giampieri, F.; Battino, M.; Zhang, D. A phenol-interference decoupling method for hydroxyl-sanshools detection based on a modified electrode with magnesium-aluminum layered double hydroxide. J. Food Compos. Anal. 2025, 141, 107365. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, B.; Chen, Q.; Peng, X.; Yang, D.; Qiu, F. Layered double hydroxide functionalized biomass carbon fiber for highly efficient and recyclable fluoride adsorption. Appl. Biol. Chem. 2019, 62, 12. [Google Scholar] [CrossRef]
- En Han, T.G.; Wang, T.; Bao, M.; Cai, J. Synergy of yttrium single atom doped carbon nitride and nickel/cobalt spinel ferrites in electrochemical sensing: A novel sensor for simultaneous detection of antioxidants. J. Food Compos. Anal. 2025, 143, 107597. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.P.; Qiu, F.X.; Zhang, T.; Xu, J.C. Controllable preparation of FeOOH/CuO@ WBC composite based on water bamboo cellulose applied for enhanced arsenic removal. Food Bioprod. Process. 2020, 123, 177–187. [Google Scholar] [CrossRef]
- Wang, L.; Jiao, X.; Liu, P.; Ouyang, Y.; Xia, X.; Lei, W.; Hao, Q. Self-template synthesis of yolk-shelled NiCo2O4 spheres for enhanced hybrid supercapacitors. Appl. Surf. Sci. 2018, 427, 174–181. [Google Scholar] [CrossRef]
- Naveenkumar, P.; Yesuraj, J.; Maniyazagan, M.; Yang, H.W.; Kim, K.; Kim, S.J. Synthesis of MoS2 nanosheet-encapsulated CuCo2S4 nanoparticle clusters derived from metal glycerate for high-performance supercapacitor applications. J. Alloys Compd. 2025, 1014, 178737. [Google Scholar] [CrossRef]
- Hsiang, H.-I.; Chiou, Y.Y.; Chung, S.H. Synthesis and electrochemical mechanisms of yolk-shell ZnCo2S4 for high-performance supercapacitors. J. Energy Storage 2022, 55, 105402. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Qian, L.; Yin, Y.; Yuan, Z.; Dai, Y.; Zhang, T.; Yang, D.; Qiu, F. Lamellar Ti3C2 MXene composite decorated with platinum-doped MoS2 nanosheets as electrochemical sensing functional platform for highly sensitive analysis of organophosphorus pesticides. Food Chem. 2024, 459, 140379. [Google Scholar] [CrossRef]
- Ye, Y.; Luo, Y.; Lou, J.; Chen, X.; Cheng, Y.-J.; Xia, J.; Li, Y.; Guo, K. Hollow spherical NiCo2S4@N-CNT composites with high energy density for all-solid-state supercapacitors. ACS Appl. Energy Mater. 2023, 6, 6742–6751. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Ameri, B.; Davarani, S.S.H. α-MnS@Co3S4 hollow nanospheres assembled from nanosheets for hybrid supercapacitors. Chem. Eng. J. 2021, 422, 129953. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Mao, Y.; Yin, H.; Chen, S.M.; Liu, X. Surface self-reconstruction and sulfidation strategy to fabricate flower-like NiCo2S4 Hollow nanospheres: Formation, storage mechanism, and application in hybrid supercapacitors. ACS Appl. Energy Mater. 2021, 4, 9178–9189. [Google Scholar] [CrossRef]
- Mohammadi, A.; Moosavifard, S.E.; Goljanian Tabrizi, A.; Abdi, M.M.; Karimi, G. Nanoporous CuCo2S4 microspheres: A novel positive electrode for high-performance hybrid energy storage devices. ACS Appl. Energy Mater. 2018, 2, 627–635. [Google Scholar] [CrossRef]
- Moosavifard, S.E.; Mohammadi, A.; Ebrahimnejad Darzi, M.; Kariman, A.; Abdi, M.M.; Karimi, G. A facile strategy to synthesis graphene-wrapped nanoporous copper-cobalt-selenide hollow spheres as an efficient electrode for hybrid supercapacitors. Chem. Eng. J. 2021, 415, 128662. [Google Scholar] [CrossRef]
- Tavakoli, F.; Rezaei, B.; Taghipour Jahromi, A.R.; Ensafi, A.A. Facile synthesis of yolk-shelled CuCo2Se4 microspheres as a Novel electrode material for supercapacitor application. ACS Appl. Mater. Interfaces 2019, 12, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Roushani, M. Rational design of hollow core-double shells hybrid nanoboxes and nanopipes composed of hierarchical Cu-Ni-Co selenides anchored on nitrogen-doped carbon skeletons as efficient and stable bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2020, 402, 126174. [Google Scholar] [CrossRef]
- Tang, G.; Liang, J.; Wu, W. Transition metal selenides for supercapacitors. Adv. Funct. Mater. 2024, 34, 2310933. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, F.; Yan, K.; Pu, Y.; Li, G.; Tian, D. Massive growth of Ni3Se4 on electrospun carbon nanofibers induced by lignin and Co for high-performance supercapacitor. Mater. Today Chem. 2025, 49, 103043. [Google Scholar] [CrossRef]
- Xie, Z.; Qiu, D.; Xia, J.; Wei, J.; Li, M.; Wang, F.; Yang, R. Hollow biphase cobalt nickel perselenide spheres derived from metal glycerol alkoxides for high-performance hybrid supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 12006–12015. [Google Scholar] [CrossRef]
- Zardkhoshoui, A.M.; Davarani, S.S.H. Construction of complex copper-cobalt selenide hollow structures as an attractive battery-type electrode material for hybrid supercapacitors. Chem. Eng. J. 2020, 402, 126241. [Google Scholar] [CrossRef]
- Shirvani, M.; Nasr Esfahani, D. From NiCo-glycerate to tri-metallic selenide: Engineering yolk-shell MnNiCoSe spheres with nanosheet arrays for hybrid supercapacitors. Mater. Chem. Front. 2025, 9, 2200–2212. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xu, Y.; Xue, Y.; Si, D.; Zhu, R.; Liu, J.; Zhou, C.; Chen, Y.; Wang, G. NiCoP/C composite with hollow sphere as electrodes for high performance supercapacitors. Electrochim. Acta 2022, 434, 141313. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, X.; Wang, P.; Chen, H.-S.; Yang, P. Hierarchical nickel-cobalt phosphide hollow spheres embedded in P-doped reduced graphene oxide towards superior electrochemistry activity. Carbon 2019, 149, 222–233. [Google Scholar] [CrossRef]
- Kaverlavani, S.K.; Moosavifard, S.E.; Bakouei, A. Designing graphene-wrapped nanoporous CuCo2O4 hollow spheres electrodes for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 14301–14309. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, X.; Wang, Z.; Wang, X.; Li, Z.; Wang, L.; Zhou, C.; Chen, D.; Luo, Y. Yolk-shell structured nickel cobalt sulfide and carbon nanotube composite for high-performance hybrid supercapacitors. Energy Fuel 2021, 35, 5342–5351. [Google Scholar] [CrossRef]
- Yu, T.; Li, S.; Zhang, L.; Li, F.; Pan, H.; Zhang, D. Design and construction of conductive polymer PPy anchored NiCo bi-metal sulfide composite electrode materials for high-performance hybrid supercapacitor and electrochemical hydroquinone sensor. J. Energy Storage 2024, 87, 111427. [Google Scholar] [CrossRef]
- Hsiang, H.I.; Chang, M.H.; Chung, S.H. Optimizing yolk–shell ZnCo2S4 nanoparticles for enhanced supercapacitor performance: Failure mechanism analysis and material design. Batter. Supercaps 2024, 8, e202400403. [Google Scholar] [CrossRef]
- Abuali, M.; Arsalani, N.; Ahadzadeh, I. On the effect of polypyrrole on electrochemical performance of micro-sized hollow spheres of NiCo2S4 and CuCo2S4 nanoparticles. Electrochim. Acta 2022, 427, 140840. [Google Scholar] [CrossRef]
- Yin, B.; Hao, L.; Li, X.; Yang, Q. Efficient charge transfer at the donor-bridge-acceptor interface for excellent supercapacitors. Chem. Eng. J. 2023, 476, 146569. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Liu, L.; Fautrelle, Y.; Lu, X.; Li, X. High magnetic field-engineered bunched Zn-Co-S yolk-shell balls intercalated within S, N codoped CNT/graphene films for free-standing supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 33690–33701. [Google Scholar] [CrossRef]
- Wei, C.; Sun, J.; Zhang, Y.; Liu, Y.; Guo, Z.; Du, W.; Liu, L.; Zhang, Y. Hierarchical Ni(OH)2-MnO2 hollow spheres as an electrode material for high-performance supercapacitors. Inorg. Chem. Front. 2022, 9, 3542–3551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).