Abstract
Gas sensors are vital tools in areas such as environmental monitoring, industrial safety, and personal healthcare. Among various sensing materials, semiconductor metal oxides (SMOs) are widely studied owing to their high sensitivity, good stability, and notable catalytic activity. To overcome inherent drawbacks of pure SMOs—such as high operating temperatures, limited selectivity, sluggish response/recovery behavior, and inadequate long-term stability—functionalization with noble metals has emerged as a powerful modification strategy. This review systematically outlines the primary mechanisms through which noble metals enhance gas sensing performance and analyzes the key factors influencing sensor behavior. Finally, we discuss the current challenges and future directions in the development of noble metal-modified SMO gas sensors.
1. Introduction
Gas sensors play an important role in numerous fields. They aid in environmental protection by regulating gases such as carbon monoxide and ozone [1,2,3]. They detect hydrogen and methane leaks [4,5] and provide early warnings on hazardous gases such as hydrogen sulfide and ammonia in factories [6,7]. They assist in the breath examination in relation to illness in healthcare [8]. They are sensitive to formaldehyde and smoke in smart homes. Sensitizing these tools, enhancing them to be more accurate, fast, and reliable, is one of the ways to improve these issues, and new concepts of gas sensors are formed.
Gas sensors are available in various types such as electrochemical [9], optical [10], surface acoustic wave [11] and solid electrolyte-type [12] gas sensors. Resistive sensors with semiconductor metal oxides (SMOs) are one such type, and have been studied intensively because of their relatively easy preparation, low cost, large response signals, compatibility with integration and capability of miniaturization.
But unmodified single-metal oxide sensors do have significant limitations in real-world practice that limits their potential performance: lack of selectivity means that it is very hard to discriminate between specific target gases in mixed-gas streams, or in a humid environment; being operated at high temperatures not only increases power consumption, but also reduces their ability to maintain long-term stability; and the slow response/recovery times and susceptibility to environmental interference (especially to humidity) prevent their broader applicability.
As such, to overcome these drawbacks, researchers have examined a wide range of strategies, including optimization of the morphology and microstructure of metal oxides (e.g., the creation of nanomaterials and porous materials) [13,14,15], building heterojunctions between metal oxides [16,17,18], elemental doping [19], and functionalizing surfaces [20]. Notably, the deployment of noble metal nanoparticles—including Au, Pt, Pd, Ag, Rh, and Ru (Table 1)—has proven to be a highly promising route for the enhancement of SMO gas sensors, yielding multifarious and significant benefits to their sensing capabilities. The affinity of the noble metals enables adsorption, dissociation and surface reaction of target gas molecules which in turn makes the sensor more sensitive and have, for example, faster reaction/recovery dynamics. Moreover, electronic sensitization effects (e.g., formation of Schottky barriers at the noble metal–metal oxide interface) effectively control charge transfer and enhance resistance variations by gas interactions [5,21,22,23]. The chemical sensitization effect is especially significant, allowing specific noble metals (e.g., Pd with H2, Au with H2S) [24] to create selective bonds or a strong adsorbent with the particular target gases [25,26]; therefore, high sensor selectivity is significantly enhanced. Moreover, in the photo-assisted gas sensors, the surface plasmon resonance (SPR) effect of noble metals such as Au and Ag can significantly improve light absorption [27,28], which gives a significant argument to the promotion of the work of light-driven gas sensing.

Table 1.
Comparative analysis of the characteristic properties of six noble metal modifiers.
As such, the functionalization of noble metals is crucial. It directly addresses the historic weaknesses of SMO sensors, their typically poor selectivity, requirement of high operating temperatures and slow response kinetics. In addition to resolving these fundamental problems, this strategy also paves the way to the creation of the new generation of high-performance sensors that are energy-efficient and highly specific.
A considerable number of existing review articles have provided insightful summaries of the research progress in noble metal-modified gas sensing materials from various perspectives [29,30], such as those focusing on specific noble metals or particular metal oxides. Building upon this foundation, this review aims to systematically integrate and synthesize the common mechanisms (e.g., electronic and chemical effects) of noble metal modification and their universal applicability across different material systems (e.g., oxides, sulfides). It will elaborately discuss the core mechanisms of noble metal modification and provide an in-depth analysis of key influencing factors, including the optimization of noble metal loading, the construction of catalytic interfaces, and the effects of conditions such as light illumination and humidity (Figure 1). The review also incorporates relevant work from our group on the Pt-MoS2 composite system [31], further validating the broad applicability of this modification strategy across different types of semiconductor materials. Furthermore, it explores performance optimization strategies in the context of various application scenarios and outlines future challenges and development directions in the field, hoping to offer a more systematic reference for researchers.
Figure 1.
Schematic outline of this review.
2. Intrinsic Properties of Noble Metals and Sensing Mechanisms
The oxygen adsorption model [32] is the most effective model that explains the operating mechanism of resistive-type solid-state metal oxide (SMO) gas sensors that mainly focus on reactions of the surface that occur between chemisorbed oxygen species with the target gas molecules [33]. In the case of n-type semiconducting oxides, including SnO2, ZnO and TiO2, the ambient air baseline electrical resistance is increased as a result of the capture of conduction electrons by adsorbed dioxin (O2ads) [34] forming surface ions (O2−, O−, etc.) [35,36] and creating an electron depletion layer at the sensor face. A surface reaction when the sensor is exposed to reducing gases (e.g., CO, H2) releases the previously trapped electrons; the reduction in the width of the depletion layer results in a measurable drop in resistance. On the other hand, encounters with oxidizing gases (e.g., NO2, O3) cause further withdrawal of the electrons, further depleting the depletion layer, thus adding to the sensor resistance [37,38,39].
In substances with p-type semiconductors like NiO and CuO, holes serve as the dominant charge carriers. The adsorption of oxygen in these materials tends to increase the concentration of holes creating a low-resistance baseline. Exposure to reducing gases causes the resistance to take the increase in form of holes consumed whilst that of oxidizing gases causes it to take the decrease in form of increased population of holes.
2.1. Electronic Sensitization Effect
Noble metals, e.g., platinum (Pt), act as an important enhancement of the sensing properties of metal oxide semiconductor (MOS) sensors via the electronic sensitization effect [40,41]. This effect originates from the disparity in work function between the noble metal and the MOS; noble metals typically exhibit a higher work function (>5.0 eV) than n-type MOS materials (e.g., ZnO at approximately 4.2 eV), which leads to spontaneous electron transfer at the interface. Movement of electrons occurs as Fermi levels of the MOS-noble metal are matched until a Schottky barrier is created [42,43], and the electron depletion layer on the MOS surface is significantly increased [44] (Figure 2).
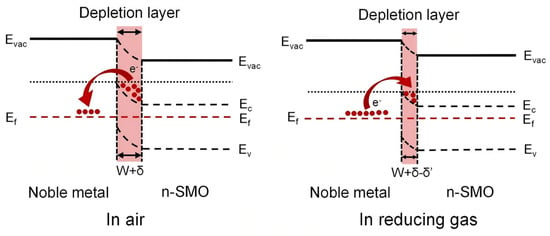
Figure 2.
Schematic energy band diagram of noble metal-decorated n-SMO reproduced with permission from Ref. [30]. Copyright 2023, open access.
A fundamental aspect of electronic sensitization is that it does not scale the resistance under air (Rair) and gas (Rgas) environments equally. Instead, its primary action is to profoundly alter the initial electronic state by depleting the MOS of charge carriers, thereby establishing a very high Rair. When a reducing gas is introduced, the ensuing surface reactions reduce the height of the pre-existing Schottky barrier. Crucially, this barrier modulation effect means that the relative resistance change (ΔR/Rair) for a given quantity of released electrons is amplified. Although Rgas for the decorated sensor may also be higher than that of its pristine counterpart, the increase in Rair is disproportionately larger. This disproportionate increase is quantitatively reflected in the sensor response (for an n-type MOS to reducing gases, S = Rair/Rgas − 1), leading to a greater Rair/Rgas ratio and thus enhanced sensitivity.
For oxidizing gases, where the response is typically defined as S = Rgas/Rair − 1, the impact of noble metal decoration is more complex and often dominated by catalytic sensitization. The noble metal catalyzes the dissociation of oxygen and the target gas, increasing the density of surface-adsorbed oxygen ions and leading to a dramatic rise in Rgas. While the electronic sensitization effect also raises Rair, the catalytic-driven increase in Rgas is usually dominant, resulting in a net enhancement of the response. However, in highly depleted nanostructures, the pre-existing low electron concentration due to electronic sensitization can sometimes limit the dynamic range for further electron trapping.
The extent of depletion layer broadening can be quantified using a physical model. The depletion layer width, LD, is related to the charge carrier concentration n and the built-in potential Vbi by the following expression:
where εr denotes the relative dielectric constant of the material. Noble metal decoration increases Vbi, thereby leading to a substantial expansion of LD. When LD approaches or exceeds the characteristic dimensions of the MOS structure (e.g., the thickness of a nanosheet), the charge carrier depletion can become nearly complete, resulting in a significant increase—by one to two orders of magnitude—in the baseline resistance of the sensor.
The electronic sensitization effect makes sensor responses much faster via band engineering of the noble metal–semiconductor interface, whose physical reality is the work function differentiated interfacial electron transfer and band modulation.
The most common example of the electronic sensitization effect can be observed in the article by Zhou Li [45] et al. Pt-decorated ZnO nanorods (Figure 3a–d) were discovered as having a mixed assemblage of Pt0 and PtO (Pt2+/Pt4+ oxides) in this inquiry. The significantly lower working energy of an n ZnO compared to that of Pt(0) (5.65 eV) leads to thermodynamic equilibrium thus driving spontaneous electron transfer between ZnO and Pt0 thus creating a substantially thickened electron depletion layer on the ZnO surface, creating an abrupt increase in intrinsic resistance (Figure 3f). This interfacial electronic reconstruction has a great influence on the dynamic range of the gas response: the number of electrons released by the reaction of H2S with adsorbed oxygen (O−) effectively modulates the depletion barrier when the gas is exposed to a reducing agent, H2S, and the sensor is able to respond to 23.1 to 100 ppb H2S at 260 °C, a more than 580 percentage point improvement from pristine ZnO (response = 3.4) with a limit of 1.1 ppb.
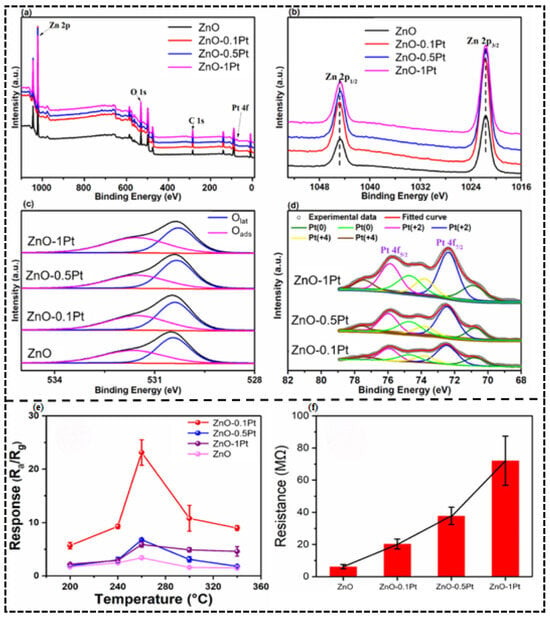
Figure 3.
XPS spectra of pristine and Pt-loaded nanorods. (a) Survey spectrum and high resolution spectra of (b) Zn 2p, (c) O 1s, (d) Pt 4f. (e) Response of pristine and Pt-decorated sensors to 100 ppb of H2S at various temperatures. (f) Electrical resistances of pristine and Pt-loaded ZnO sensors in air at 260 °C. Reproduced with permission from Ref. [45]. © 2021 Elsevier B.V. All rights reserved.
In addition to the metallic modifications made during the basis of platinum, single-atom Pt-based modification is also evidenced of strong effects of electronic sensitization. In another study by Lingyue Liu et al. [46], single-atom Pt2+-modified ZnO performed remarkably. Pt, which consists of single atoms, facilitates electron transfer between ZnO and Pt2+ because of the work–function difference and makes the electron depletion line much more thick (Figure 4c). This increases the barrier height change caused by electrons released when triethylamine signal (TEA) reaction occurs leading to a 92-fold increase in response value (up to 4170) at 200 °C and a limit of 7.5 ppb (Figure 4b and Table 2).
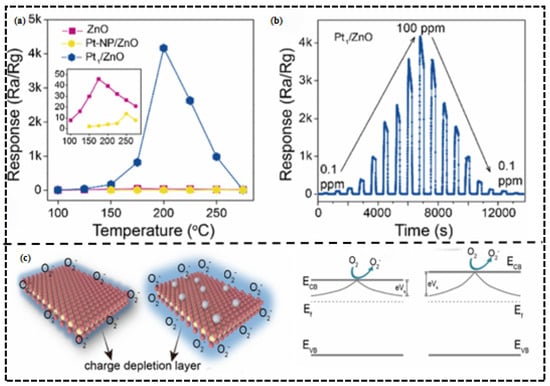
Figure 4.
(a) Response of the three sensors to 100 ppm TEA at 100–275 °C (relative humidity: 40%). (b) Dynamic response–recovery curves of Pt1/ZnO sensor to TEA in concentrations of 0.1–100 ppm at 200 °C. (c) Band structure of ZnO and Pt1/ZnO. Reproduced with permission from Ref. [46]. Copyright © 2023, American Chemical Society.
2.2. Chemical Sensitization Effect
Chemical sensitization, also known as the “spillover effect”, centers on the catalytic role of noble metals (e.g., Pt [47], Pd [48], Au [49] (Table 3)) in optimizing the kinetics of chemical reactions on the metal oxide surface, rather than altering its bulk electrical properties. Unlike electronic sensitization, which directly modulates the depletion layer, chemical sensitization enhances performance via these pathways: Noble metal nanoparticles act as highly active sites that efficiently dissociate oxygen molecules to generate highly reactive atomic oxygen species (e.g., O−). These active species then “spill over” and diffuse onto the metal oxide surface, increasing the concentration of sites available for gas reactions. Concurrently, the noble metals significantly lower the activation energy barrier for the oxidation of target gases (e.g., ethanol, acetone). The synergy between these two effects drastically accelerates the entire kinetic cycle of “gas adsorption–surface reaction–electron transfer.” The ultimate manifestations are significantly faster response/recovery speeds, markedly improved sensitivity at low temperatures, and enhanced selectivity toward specific gases, as the reaction pathway is altered to promote deep oxidation.
As illustrated in Figure 5, the process begins with the preferential adsorption and dissociation of O2 on the noble metal NPs. The resulting oxygen ions subsequently diffuse onto the SMO surface. When a reducing gas is introduced, this enlarged reservoir of O− ions can react with a greater number of target gas molecules. This reaction releases electrons back into the conduction band of the SMO, leading to a rapid and substantial change in electrical resistance, which translates to enhanced sensor response.
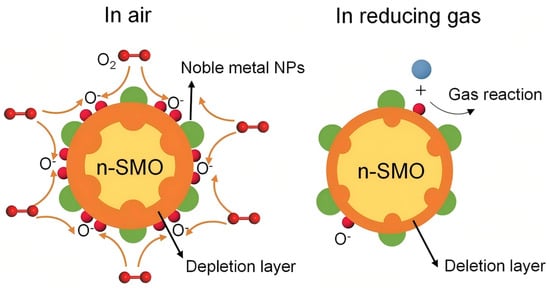
Figure 5.
Schematic illustration of the chemical sensitization of noble metal-decorated n-SMO. Ref. [30]. Copyright 2023, Open access.
In a study by Taro Ueda et al. [50], in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) analysis (Figure 6a–c) of porous In2O3 loaded with Au nanoparticles (0.5 wt%) revealed that Au significantly enhanced the chemical adsorption activity and the quantity of NO2 on the material surface. Au catalyzed the formation of more highly reactive adsorbed species at the interface, such as monodentate nitrite (characteristic IR peak at ~1108 cm−1) and chelating bidentate nitrite (peaks at ~1284 cm−1 and ~1188 cm−1). The energy barrier of the forming of these negatively charged adsorbed species was also lesser with the presence of Au. This alteration in surface chemistry directly resulted in a notably higher response of the Au/In2O3 sensor to low concentrations of NO (0.6–5 ppm) at 100 °C compared to pure In2O3 (Figure 6d), along with faster response/recovery kinetics. The fundamental nature of chemical sensitization, as is evident in this case, is that noble metal catalysts can increase reaction rates and selectivity, by decreasing the activation energy of important surface reaction steps (e.g., gas dissociation, oxygen activation, and intermediate formation), controlling reaction pathways, and, in the end, yielding high sensitivity and rapid-response gas sensing behavior.
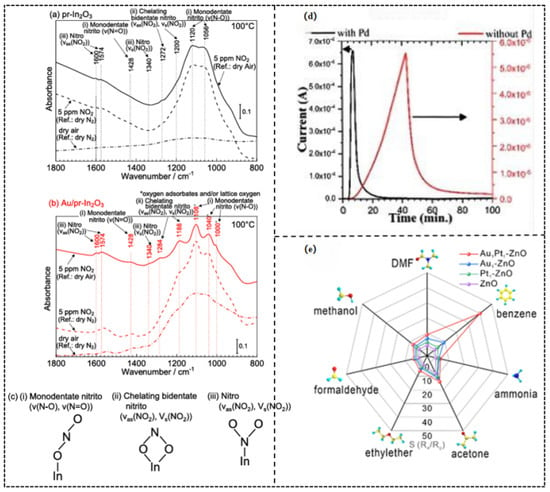
Figure 6.
(a–c) DRIFT spectra of the (a) pr-In2O3 sensor and (b) Au/pr In2O3 sensor in 5 ppm NO2 in dry air at 100 °C (reference: in dry air and N2 at 100 °C) together with their spectra in dry air (reference: in dry N2 at 100 °C). (c) Illustration of (i) monodentate nitrito, (ii) chelating bidentate nitrito, and (iii) nitro. Reproduced with permission from Ref. [50] Copyright © 2021, American Chemical Society. (d) Dynamic sensor responses of Z0.5 with and without Pd catalysis at different operating temperatures. Reproduced with permission from Ref. [51] Copyright © 2022 The Authors. Published by American Chemical Society. (e) Responses of pure ZnO (350 °C), Au1-ZnO (350 °C), Pt1-ZnO (350 °C), and Au1Pt1-ZnO (300 °C) porous nanobelts to seven typical VOCs (50 ppm). Reproduced with permission from Ref. [52] © 2024 Elsevier B.V. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
2.3. Selectivity: Targeted Recognition and Anti-Interference Mechanisms
The enhancement of gas selectivity through noble metal modification stems from their unique chemical bonding interactions with specific gas molecules [53,54,55]. For instance, the interaction between palladium (Pd) and hydrogen (H2) involves dissociative adsorption, where Pd surface atoms cleave the H–H bond of H2 molecules [24,56]. This process arises from the distinct electronic configuration of Pd atoms, which selectively activate H2 without similar effects on other gases such as CO. Gold (Au), on the other hand, exhibits high selectivity toward aromatic compounds via π-complexation, where Au atoms form planar coordination structures with benzene rings. This interaction stabilizes benzene adsorption on the Au surface, while linear molecules like ethanol are repelled due to incompatible electron cloud distributions. Silver (Ag) recognizes formaldehyde (HCHO) through coordination bonds, where Ag+ ions form strong interactions with the carbonyl oxygen atom of HCHO. Such bonding is ineffective against molecules like acetone, which lack lone-pair donors. These specific chemical interactions significantly enhance the selectivity of metal oxide sensors toward particular gases when modified with the corresponding noble metal.
The unique selectivity enabled by Pd’s dissociative adsorption of H2 is clearly demonstrated in the study by Soheil Mobtakeri et al. [51]. Here, Pd modification increased the response of MoO3 nanowall sensors to 1000 ppm H2 by 754 times at 300 °C (compared to the unmodified sample), achieving a response value as high as 3.3 × 105 (Figure 6d). This activation process is highly specific to H2, as other gases like CO cannot be effectively dissociated and adsorbed by Pd (Table 4).
On the other hand, the remarkable selectivity for aromatic compounds conferred by π-complexation is evident in the research by Tianyu Yang et al. [52]. In their work, Au-Pt bimetallic-decorated ZnO sensors exhibited a high response of 39 toward 50 ppm benzene, far exceeding their responses to other VOCs (e.g., only 8.2 for 50 ppm methanol) (Figure 6e). This selectivity originates from the specific interaction between the delocalized π-electron cloud of benzene and the d-orbitals of Au atoms, while linear or non-conjugated molecules (e.g., ethanol) cannot form equally stable complexes.
The gas sensing characteristics of SMOs functionalized with different noble metals, such as Au, Pt, and Pd, are detailed in Table 2, Table 3 and Table 4, which compiles critical parameters including target gas, operating temperature, response magnitude, and detection limit.

Table 2.
GasNAsensitive response of PtNAdecorated SMONAbased gas sensors.
Table 2.
GasNAsensitive response of PtNAdecorated SMONAbased gas sensors.
| Materials/The Loading Amount/Types of Precious Metals | Target Gas | Critical Parameters (O.T. Conc. Response Tres/Tre LOD) | Refs. |
|---|---|---|---|
| ZnO/2 wt% 1:1/AuNAPt | benzene | 300 °C 50 ppm 39 8/30 NA | [52] |
| rGONAFe2(MoO4)3/5 wt%/Pt | xylene | 175 °C 100 ppm 41.3 NA 500 ppb | [57] |
| ZnO/NA/Ag | NO2 | 250 °C 1 ppm 434.3% NA 1 ppb | [58] |
| SnO2/NA/Pt | H2 | 79 °C 1000 ppm 197 < 1/20 s NA | [59] |
| ZnO/NA/Pt | ethanol | 150 °C 50 ppm 843 4/22 min NA | [60] |
| WO3/1 at%/AuNAPt | MeSa | 450 °C 50 ppm 5.4 NA 41 ppb | [61] |
| GONAZnO/NA/Pt | acetone | 220 °C 50 ppm 150.2 4/7 s NA | [62] |
| SnO2/NA/Ga | NH3 | 275 °C 1000 ppm 5980, 115/30 s 0.1 ppm | [63] |
| WO3−x/NA/Pt | acetone | 350 °C 5 ppm 43.4 NA 0.1 ppm | [64] |
| ZnO/NA/Pt | TEA | 200 °C 100 ppm 4170 34/76 s 100 ppb | [46] |
| ZnO/1 at%/Pt | H2 | 150 °C 100 ppm 63.8% NA NA | [47] |

Table 3.
GasNAsensitive response of AuNAdecorated SMONAbased gas sensors.
Table 3.
GasNAsensitive response of AuNAdecorated SMONAbased gas sensors.
| Materials/The Loading Amount/Types of Precious Metals | Target Gas | Critical Parameters (O.T. Conc. Response Tres/Tre LOD) | Refs. |
|---|---|---|---|
| In2O3/NA/Au | n-butanol | 325 °C 100 ppm 1054 8/15 s 50 ppb | [49] |
| SnO2/0.1 at%/Au | NO | 130 °C 100 ppb 183 163/146 s 1 ppb | [65] |
| WO3/1 wt%/Au | NO2 | 30 °C 1000 ppb 64.19 55/24 s NA | [66] |
| In2O3/0.2 wt%/Au | CO | 240 °C 50 ppm 18.2 37/86 s NA | [67] |
| WO3/5 at%/Au | H2S | 400 °C 1 ppb 2.01 NA 1 ppb | [68] |
| SnO2/NA/Au | H2S | 24 ± 1 °C 500 ppb~270% NA/126 s 2 ppb | [69] |
| W18O49/NA/Pd@Au | H2S | 100 °C 50 ppm 55.5 NA NA | [70] |
| SnO2/NA/Au | DMMP | 200 °C 680 ppb 1.88 25/72 s 34 ppb | [71] |
| Co3O4/NA/Au | acetone | 250 °C 10 ppm 27.05 NA NA | [72] |
| ZnO/0.5 wt%/Au | isoprene | 350 °C 50 ppb 42 NA NA | [73] |
| ZnO/NA/Au | ethanol | 200 °C 50 ppm 159 NA NA | [74] |
| ZnO/4 wt%/Au | formaldehyde | 70 °C 100 ppm 68.8 216/106 s 0.25 ppm | [75] |
| ZnO/exfoliated WSe2/0.5 wt%/Au | Benzene | 25 °C 30 ppm 255.64% 40/58 s 0.1 ppm | [76] |
| ZnO/2 wt% 1:1/AuNAPt | Benzene | 300 °C 50 ppm 39 8/30 NA | [52] |

Table 4.
GasNAsensitive response of PdNAdecorated SMONAbased gas sensors.
Table 4.
GasNAsensitive response of PdNAdecorated SMONAbased gas sensors.
| Materials/The Loading Amount/Types of Precious Metals | Target Gas | Critical Parameters (O.T. Conc. Response Tres/Tre LOD) | Refs. |
|---|---|---|---|
| SnO2/Pd/In2O3 | CH3COCH3 | 400 °C 200 ppb 2.9 4/47 NA | [19] |
| ZnO/Pd@ZIFNA8 | CH4 | 210 °C 500 ppm 57.9% NA NA | [77] |
| SnO2NArGO/5 at%/Pd | H2 | 360 °C 200 ppm 32.38 NA NA | [78] |
| nNAZnO/PdNA30 wt%Ag | CH4 | 50–100 °C 100–10,000 ppm 45–80% <1–67 s 80–270 ppm | [48] |
| CuCrO2/NA/Pd | H2S | 150 °C 50 ppm 72.3% 35.9/182.7 s 500 ppb | [79] |
| ZnO/NA/Pd | H2 | RT 50 ppm 70% 137/165 s 10 ppb | [80] |
| MoO3/NA/Pd | H2 | 250 °C 1000 ppm 3.3 × 105 379/304 s NA | [51] |
| NiONAZnO/0.31 wt%/Pd | H2 | 150 °C 50 ppm 65% 48/216 s 0.1 ppm | [81] |
| InNAZnNAO/NA/Pd | H2 | 250 °C 1% 15,900 20/51 s 100 | [82] |
| Ga2O3/NA/Pd | NO2 | RT 100 ppm 146.56% 12/23 s 51 ppb | [83] |
| ZnO/0.3 wt%/Pd@Pt | NO2 | 80 °C 50 ppb 60.3 160/230 300 ppt | [84] |
| WO3/NA/Pd | H2 | 110 °C 10 ppm 40.63 49/73 s NA | [85] |
| MILNA125NATiO2/NA/Pd | CH2O | RT 100 ppm 15 37/12 NA | [86] |
| αNAFe2O3/0.59 wt%/Pd | H2 | 300 °C 200 ppm 41,000 49/533 s 50 ppb | [87] |
| WO3 NSs/2 wt%/Pd | MustardGas | 260 °C 700 ppb 8.5 9/92 s 15 ppb | [88] |
| ZnO/NA/Pd | CH3NH2 | 250 °C 400 ppm 99.5%–25 ppm | [89] |
3. Factors Influencing Noble Metal Modification
3.1. Optimization of Noble Metal Loading Amount
The loading amount of noble metal is a critical factor affecting the gas sensing performance of materials. In optimizing the loading, a key threshold exists: loading that is too low leads to an insufficient number of active sites, failing to effectively catalyze reactions, whereas excessive loading readily induces agglomeration of noble metal particles, significantly reducing the effective specific surface area and potentially forming a continuous conductive metallic network [58,67]. This not only diminishes catalytic efficiency but also weakens the resistance modulation capability of the semiconductor substrate in response to gases, and may even reduce selectivity [85,90]. Therefore, the ideal loading amount must achieve highly dispersed noble metals to maximize exposed active sites while avoiding masking the intrinsic gas sensing properties of the base semiconductor material.
Our group employed high-throughput experimental technology to rapidly screen the NO2 sensing performance of various noble metal/In2O3 sensors (Table 5) [90]. It identified 0.5 mol% as the optimal Ag loading. This work confirms that the ideal loading is a result of the synergy between the noble metal properties, support interface, and target gas molecules, which must be determined through systematic performance testing rather than predicted by any single theoretical parameter.

Table 5.
Performance comparison of noble metal-modified In2O3 sensors to 5 ppm NO2.
A research paper by Zong-Ying Shen et al. [58] regarding Ag-modified ZnO nanorods to detect NO2 demonstrates how crucial the loading of the metal material is in the optimization of the sensor parameter. When sputtered using a power of 25 W which corresponds to a 0.11 at% loading of Ag, the nanoparticles had a mean diameter of 3.2 pm and a homogeneous spread (Figure 7a), thus giving high density of catalytic active sites with a sensor response of 434.3 percent at 1 ppm of NO2. Conversely, as the loading was increased to 0.39 at% (sputtering power 100 W) the agglomeration was substantially induced, the particle size increased to 22.4 nm (Figure 7d), and a continuous conductive network developed that hindered the diffusion of gases, which also lowered the response to 315%. This loading-dependent threshold effect shows the dynamic compensation between catalytic efficiency and dispersion; optimum loading reduces agglomeration of metals and maintains the inherent gas sensing properties of the semiconductor substrate, and makes the most efficient use of the active sites.
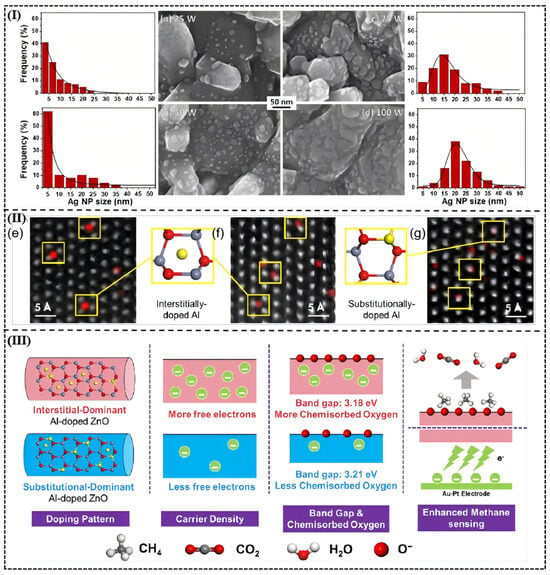
Figure 7.
(I) SEM images of ZnO nanostructures with AgNPs deposited at (a) 25 W, (b) 50 W, (c) 75 W and (d) 100 W, along with their Ag NP size distribution statistics measured based on SEM images. Reproduced with permission from Ref. [58] © 2025 Elsevier B.V. All rights are reserved, including those for text and data mining, AI training, and similar technologies. (II) ACTEM images and Al EDS mappings of (e) AZO-0.1%, (f) AZO-0.2%, and (g) AZO-1%. The gray, red, and yellow balls represent Zn, O, and Al atoms, respectively. (III) Schematic of the mechanism underlying the enhanced CH4 sensing performance of interstitially Al-doped ZnO. Reproduced with permission from Ref. [91].Copyright © 2025, American Chemical Society.
Noble metal loading optimization is a standard technique applied to improve the work of gas sensors. As illustrated by Chen et al. [91] in the research of our group, the effects of the careful formation of the doping locations, and, therefore, on the electronic structure can be achieved through the purposeful control of the dopant loading levels. Through a careful manipulation of NaAlO2 additive, the Al concentration was adjusted to the point where interstitial-dominant to substitutional-dominant doping regime was changed. Direct observation of the atomic-level doping locations was used to make the use of X-ray diffraction (XRD) and aberration-corrected transmission electron microscopy (ACTEM) with energy dispersive spectroscopy (EDS) mapping. The interstitial-doped AZO-0.2(100)-0.2 sample has also shown a lattice expansion (i.e., the (100) peak was moved to 34.34°), and substitutional-doped AZO-1(100)-0.2 sample decreased the lattice, proving that the doping mode could be controlled. ACTEM images showed that the electrons of the interstitial arrangement fit the Zn atoms with the Al atoms at the center of three faces whereas in the substitutional arrangement, an Al atom took the place of Zn atoms so that the electrons are more optimized in distribution (Figure 7II).
It was also found that using the interstitial-dominant AZO-0.2% sensor, which responded at 42.4% to 500 ppm of CH4, at 280 °C, was much higher than the responses of pure ZnO (20.2% response) and the substitutional-dominant AZO-1% sensor (39.2% response). In addition, the interstitial-doped sensor achieved a response time and recovery time of 3.8 and 5.0 s, respectively, which is the best, compared to the majority of doped metal oxide semiconductors.
Mechanism analysis found that interstitial doling added more free electrons (each Al atom adding three net electrons), thus making its carrier density be 2.46 × 1020 cm−3, and substitutional doling added only one net electron which amounts to carrier density being 8.46 × 1019 cm−3 (Figure 7III). Calculations using density functional theory (DFT) showed that interstitial doping reduced the band gap to 2.998 eV (as compared to 3.299 eV in the undoped ZnO) and allowed charge transfer between the conduction and the valence band. Further experiments involving oxygen temperature-programmed desorption (O2-TPD) experiments showed that the interstitial-doped sample was sixfold that of chemically adsorbed pure ZnO and therefore O2 oxidation reaction of CH4 was accelerated (CH4 + 4O−ads → CO2 + 2H2O + 4e−) and the response time was reduced. Electronic-state modulation effect was visually represented by band structure and density-of-states diagrams.
3.2. Construction of Catalytic Interfaces
Noble metal and metal oxide interface engineering is needed to improve the room temperature gas sensing performance [77,84,92]. Through accurate regulation of size, morphology, and distribution of noble metal nanoparticles on the oxide surface, atomic-level manipulation of gas–molecule adsorption–reaction pathways can be attained; this will increase the electron transfer and catalytic reaction kinetics and therefore, simultaneously increase the sensor sensitivity and selectivity. This plan gives a different orientation of a traditional material design and forms basis of further interface engineering.
Based on this, interface engineering avoids classical performance bottlenecks by the synergism design of core and shell structures as well as heterojunctions [19,57,69,70,74]. The core–shell structure utilizes atomic-scale coating technology to inhibit high-temperature Ostwald ripening—a product-loss process whereby atoms on the surface of small particles are dissolved by high curvature energy barriers, diffuse through the substrate, and adsorb to large particles, thus being lost [93,94]. A thick shell is appropriate to prevent the lineages of atomic migration, protecting the dispersion and activity of the metal particles. It can also screen physically in terms of size and chemically in terms of adsorption competition based on its shell structure.
A good example of this is the Pt-shell-modified Pd-core structures of Li, Y. R. et al. [92]. Pt-shell-modified Pd-core structures are based on the high oxygen adsorption affinity of Pt (the energy of the Pt-O bond is much greater than the energy of the Pd-O bond). When exposed to air, oxygen molecules are captured and immobilized mostly by Pt at the surface, forming a localized oxygen isolation layer. This physically impedes diffusion of oxygen to the Pd core and what is more important is that this process is a screening process on a molecular level due to chemical adsorption energy differences. Small dementia (reducing, 0.289 nm in diameter) hydrogen molecules, therefore, can enter the shell to react with Pd core to give Pd-H bonds, but larger oxidizing poisoning molecules (e.g., H2O 0.26 nm not shown, but permeable) or organic contaminants will be excluded (Figure 8c,d). It is a dual-level filtration system, comprising physical size screening and chemical adsorption competition that allows Pd active sites to specialize in target gas reactions, eventually providing ultra-high sensitivity detection and anti-interference of hydrogen in the air compared to the situation with single-size screening.
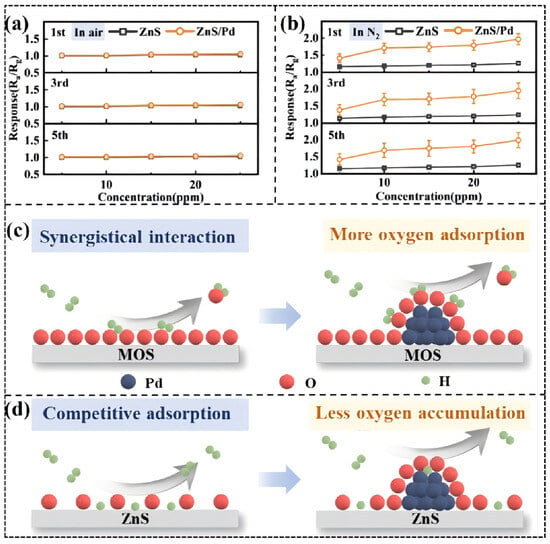
Figure 8.
(a) Response of ZnS and ZnS/Pd NPs exposed to H2 in air; (b) response of ZnS and ZnS/Pd NPs in N2; and schematic diagram of (c) MOS and (d) ZnS-sensing process. Reproduced with permission from Ref. [92]. Copyright © 2024, American Chemical Society.
Furthermore, the interface band modulation mechanism of heterojunctions enhances gas response through directional carrier migration [95]. A typical example is demonstrated in the Pd@Pt core–shell modified ZnO system studied by Chengming Sui et al. [84]: the Pt shell (work function 5.65 eV) forms a Schottky barrier upon contact with ZnO (4.5 eV), driving electron transfer from ZnO to Pt and significantly increasing the surface-adsorbed oxygen concentration. When exposed to NO2, its strong electron-acceptor character increases the barrier height from 0.7 eV to 1.2 eV (Figure 9a–f), causing a drastic change in resistance and achieving a high response of 60.3 (Rg/Ra) to 50 ppb NO2 with a detection limit at the 300 ppt level (Figure 9g,h).
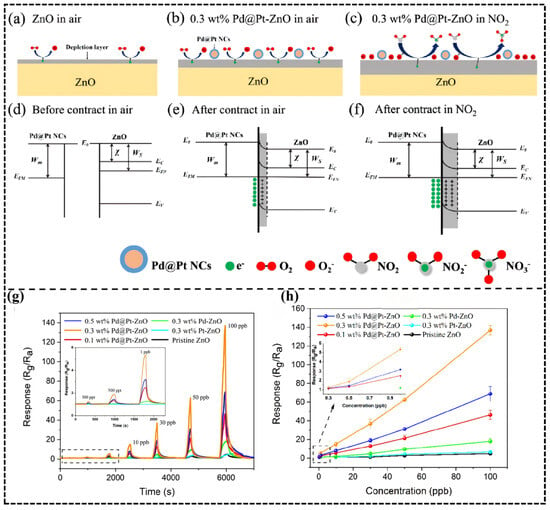
Figure 9.
(a–f) Schematic diagram of sensing mechanism (WM, the work function of metal; WS, the work function of semiconductor; χ, electron affinity; E0, vacuum level; EC, conduction band; EFM, EFN, Fermi level; and EV, valence band); (g,h) dynamic response and response relationship to NO2 from 0.3 to 100 ppb of the sensors at 80 °C in 25% RH. Reproduced with permission from Ref. [84] Copyright © 2024, American Chemical Society.
Single-atom doping (e.g., with Au, Pt) has emerged as a revolutionary strategy in recent years, constructing highly efficient catalytic interfaces through atomically dispersed noble metal sites [96,97,98,99]. This approach addresses the issue of active site blocking common in traditional core–shell or heterojunction structures. By leveraging strong coordination between metal atoms and the semiconductor substrate, it forms uniform and high-density active centers, greatly improving atomic utilization and interfacial charge transfer efficiency.
A study by Wei et al. [100] on single-atom Au-modified mesoporous SnO2 serves as a representative case: Au/Ce-SnO2 material synthesized via a self-templating strategy was clearly shown by HAADF imaging to have atomically uniform dispersion of Au (no agglomeration) (Figure 10g, the red circles is single-atom Au). This precise interface design enabled each Au atom to form an Au-O2 coordination configuration with two oxygen atoms, optimizing the adsorption and dissociation pathways of oxygen molecules.
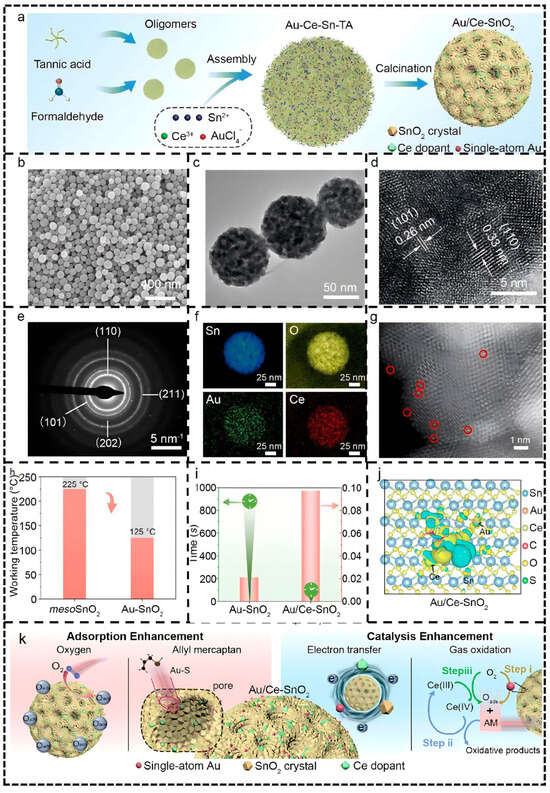
Figure 10.
(a) The synthesis strategy of Au/Ce–SnO2. (b) SEM, (c) TEM, (d) HRTEM, (e) SAED pattern, (f) element mapping, and (g) HADDF image of the typical sample (Au/Ce–SnO2). (h) The sensitization of single-atom Au on mesoporous SnO2 nanospheres. (i) The response time and sensitivity for Au/Ce–SnO2 and Au–SnO2 materials. (j) The charge transfer between Au/Ce–SnO2 (101) and allyl mercaptan. The blue region: the consumption of charge; the yellow region: the accumulation of charge. (k) The proposed synergistic sensitization effects of the sensor towards allyl mercaptan (AM). Oads: the surface-adsorbed oxygen. Reproduced from Ref. [100] with permission from the Royal Society of Chemistry.
Synchrotron EXAFS analysis further confirmed the atomic state characteristics of Au (Au–O bond length 2.02 Å), and Bader charge calculations revealed enhanced charge transfer (0.53 eV), providing direct evidence for interfacial electronic reconstruction. This atomic-level interface engineering significantly promoted the adsorption and activation of target gases (e.g., allyl mercaptan). Combined with the redox synergy of Ce doping (Ce(III)/Ce(IV) cycle), the sensor achieved ultra-high sensitivity (0.097 ppb−1) and an ultra-low detection limit (0.74 ppb) at 125 °C (Figure 10h). The performance enhancement mechanism can be summarized as a synergistic “adsorption enhancement–catalytic enhancement” pathway—single-atom Au preferentially adsorbs and dissociates oxygen molecules, while Ce doping accelerates target gas oxidation and electron release (Figure 10k).
Compared to traditional noble metal nanoparticle modification, single-atom interfaces avoid high-temperature Ostwald ripening, providing a theoretical and technical foundation for the next generation of low-power, high-selectivity gas sensors.
3.3. Illumination
In the context of gas sensing studies, the light of the sensing element is an external source of energy that alters the functioning of noble metal-modified materials in various physicochemical processes. When irradiated, the semiconductor substrate absorbs photon energy, which is initially used to excite electrons located in the valence band to the conduction band, and increases the intrinsic carrier concentration as a result. Meanwhile, light is known to promote the photocatalytic generation of surface-adsorbed oxygen species, which enhances the rate of the redox reaction between gas molecules and active sites [101,102]. It has to be said that not every type of photosensitive material can demonstrate such a great improvement in performance; its effectiveness is influenced by the ratio between the material band gap and the wavelength of incident light and ability of interfacial defect states to eliminate the recombination of electrons and holes.
Focusing on this fact, the addition of noble metal nanoparticles thereof (e.g., Au, Ag, Pd) provides a new light responsivity mechanism—surface plasmon resonance (SPR) [103,104,105,106,107]. SPR is a natural reaction of metal nanostructures of noble elements (e.g., Au, Ag, Pt) to certain optical excitation and produces an impressive localized electromagnetic field growth at the metal–dielectric interface due to the synchronization of all electrons in the specimen. This effect significantly enhances the light-harvesting efficiency of semiconductor metal oxides by synergistic interactions: metal nanoparticles are considered subwavelength optical antennas, increasing the pathlength of incident photons within the semiconductor through Mie scattering; the field produced by SPR directly stimulated the growth of the number of carriers in the semiconductor by overcoming the metal–semiconductor interface, directly enhancing the number of photoreduced carriers by non-equilibrium hot electrons produced by SPR decay. This boosts the factors leading to enhanced efficiency of carriers through the utilization of optical energy within the visible to near-infrared wavelength range, which allows photoactivated gas sensors to achieve higher concentrations of carriers under low-energy illumination to facilitate what reduces the operation temperature penalty and enhances sensitivity to trace gases. The root cause of this performance gain is the optimization of the processes of light–matter interaction as opposed to changes in the inherent band structure of the material.
A typical example can be found in the Pd-modified ZnO/rGO ternary composite material developed by our research group [108]. This study constructed 2–6 nm Pd nanoparticles on a ZnO/rGO substrate via photodeposition, forming a visible light-responsive ternary heterostructure. UV-vis spectra revealed a significant absorption peak at 470 nm, confirming the SPR effect of Pd nanoparticles, and the rGO/ZnO/Pd hybrids exhibited markedly higher absorption intensity in the visible region compared to other control samples.
Photoelectrochemical tests further showed that the photocurrent density of the ternary heterostructure reached 66.5 μA (at 2.0 V) under 470 nm illumination, six times higher than that of pure ZnO, indicating significantly improved charge separation efficiency. Under 470 nm visible light illumination, the sensor exhibited a high room temperature response of 63.4% to 1% CH4, with response/recovery times shortened to 74 s/78 s, significantly outperforming its performance in dark conditions (response only 4.1%). This enhancement is attributed to the multiple effects induced by SPR: Pd nanoparticles act as photosensitizers promoting hot electron injection into the ZnO conduction band, increasing the generation of active oxygen species (·O2− and O−); rGO nanosheets serve as efficient charge transport networks accelerating electron migration; the multiple Schottky barriers formed at the ternary heterointerfaces optimize carrier separation efficiency (Figure 11a,b). Moreover, in situ FT-IR analysis demonstrated the reaction pathway of CH4 oxidation to CO2 and H2O, with the apparent rate constant (k) showing a positive correlation with the sensing response value (R2 = 0.98), establishing a quantitative relationship between photocatalytic activity and sensing performance. This work provides direct experimental evidence for the mechanism of noble metal SPR effects in gas sensing, promoting the application of room temperature-photoactivated sensors in coal mine safety monitoring.
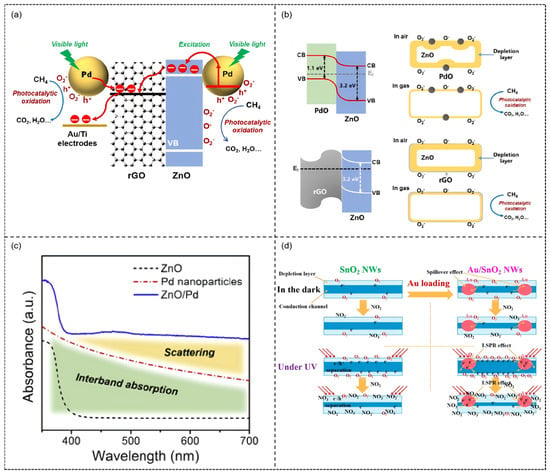
Figure 11.
Schematic illustration of the mechanism for (a) photocatalytic oxidation of CH4, the improved electron transfer and (b) the multiple heterojunctions formed in the ternary rGO/ZnO/Pd hybrid under visible light illumination. Reproduced from Ref. [108] © 2019 Elsevier B.V. All rights reserved. (c) UV–vis absorption spectra of the Pd nanoparticles in a colloidal sus pension (red dashed line), ZnO/Pd hybrid (blue solid line) and pure ZnO reference (black dashed line). Reproduced from Ref. [109] © 2019 Elsevier Ltd. and Techna Group S.r.l. All rights reserved. (d) Schematic illustration of the sensing mechanism in this study. Reproduced with permission from Ref. [110]. Copyright 2022, open access.
This exemplary case demonstrates powerful synergy between electronic sensitization, chemical sensitization and plasmonic effects. The SPR of Pd nanoparticles acts as an “energy switch” that simultaneously enhances chemical catalysis and modulates charge transfer. The rational ternary design provides optimal pathways for both surface reactions and electron transport. Most importantly, the quantitative correlation between photocatalytic activity and sensing response establishes a direct link between microscopic mechanisms and macroscopic performance. This work provides a paradigm for multi-mechanism integration in high-performance gas sensors, demonstrating that deliberate synergy between different enhancement strategies yields superior results compared to any single mechanism alone.
In gas sensing research, noble metal modification and photoactivation strategies also significantly enhance performance through other mechanisms. First, the study by J. Wang et al. exemplifies the non-plasmonic photoactivation mechanism [109]. The Pd/ZnO system exhibits a characteristic absorption peak at 475 nm, corresponding to the interband transition of Pd; under 475 nm visible light irradiation (Figure 11c), Pd nanoparticles generate non-plasmonic hot electrons via this transition and inject them into the ZnO conduction band, thereby greatly increasing the room temperature response to 100 ppb NO2 to 160%. In contrast, the study by B. Zhang et al. highlights the role of the LSPR effect [110]. Specifically, under 365 nm UV excitation, the LSPR of Au nanoparticles induces collective oscillations of surface-free electrons, generating abundant hot electrons that are injected into the SnO2 conduction band, which in turn significantly raises the carrier concentration (Figure 11d). This leads to a response of 65 to 5 ppm NO2 at room temperature, along with markedly accelerated response/recovery speeds. These two cases reveal the different underlying mechanisms by which noble metals enhance photoactivated gas sensing.
3.4. Humidity
The influence of humid environments on sensing performance is particularly complex: water molecules not only compete with target gases for adsorption sites but also alter the surface hydroxyl concentration and ionic conductivity of the material, often leading to baseline drift and sensitivity degradation [111,112,113]. For instance, in unmodified SnO2 sensors, the response to formaldehyde typically decays by more than 60% under 70% RH.
Noble metal modification offers a promising pathway to address this challenge. A study by Zhou et al. [114]. demonstrated that a Pt/TiO2 sensor exhibits p-type response (resistance increase) at low humidity (<30% RH) due to preferential adsorption of H2 on Pt forming PtHx, while at high humidity (>65% RH), H2O occupies the Pt active sites, forcing H2 to react directly with TiO2 and release electrons, resulting in an n-type response (resistance decrease). This humidity-triggered p-n transition mechanism (Figure 12a) causes a signal inversion at a H2 concentration threshold of 40 ppm. Although the response time increased from 25 s under dry conditions to 72 s, the sensor’s adaptability to humid environments was significantly enhanced.
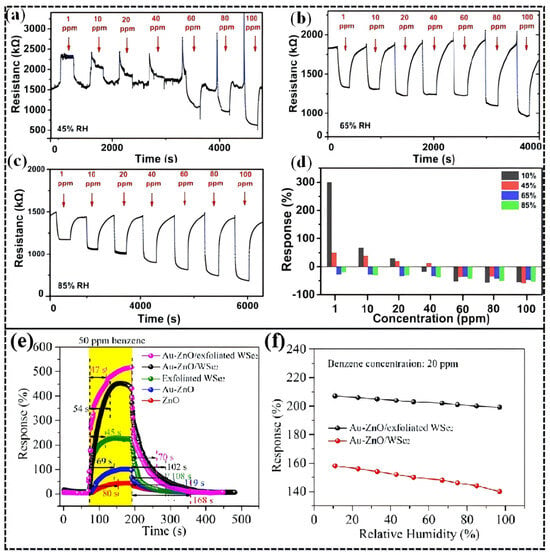
Figure 12.
H2-sensing performance of the 15 s sputtered Pt/TiO2 in 45% RH (a), 65% RH (b), and 85% RH (c), and comparison of the response in different humidity conditions (d). Reproduced from Ref. [114] Copyright © 2021, American Chemical Society. (e,f) Sensing properties of ZnO, Au-ZnO, exfoliated WSe2, Au-ZnO/WSe2,and Au-ZnO/exfoliated WSe2 sensors toward 30 and 50 ppm benzene gas. Reproduced from Ref. [76] Copyright © 2021, American Chemical Society.
The suppression of humidity interference by noble metal composite structures is also notable in benzene sensing. Research by Zhang et al. [76]. showed that a Au nanoparticle-modified ZnO/WSe2 heterojunction, leveraging the catalytic activity of Au and interfacial charge modulation (Figure 13a–c), achieved a high response of 520% to 50 ppm benzene at 25 °C without light illumination (Figure 12e). More importantly, as shown in (Figure 12f), when the environmental humidity increased from 11% RH to 97% RH, the sensor’s response decay was only about 10%, far lower than the >50% decay observed in traditional materials. DFT calculations further revealed that the introduction of Au significantly enhanced the adsorption energy of benzene molecules from −0.92 eV (pure ZnO) to −2.58 eV, greatly reducing the impact of competitive water molecule adsorption and highlighting the anti-interference advantage of noble metal modification in extreme humidity conditions.
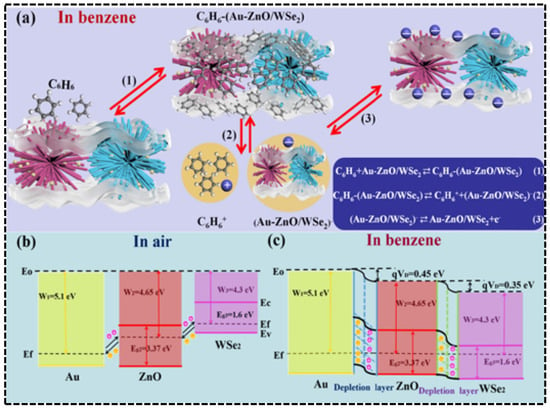
Figure 13.
(a) Schematic of the sensing mechanism of Au-ZnO/exfoliated WSe2 composites in a benzene atmosphere. Energy band schematic (b) in air and (c) under benzene conditions. Reproduced from Ref. [76] Copyright © 2021, American Chemical Society.
3.5. Other Factors
In the framework of gas sensor development, solid solution materials offer new opportunities in the optimization of the sensing characteristics, as they possess rich physicochemical characteristics and internal structures, which can be tuned in a natural fashion.
Our team of researchers reported In/Fe oxide nanowires solid solutions that were fabricated through a co-spinning system utilized in a part of the composition α-(Fe1−xInx)2O3: In/Fe oxide nano-structured materials are crystal-phase oxide nanowires with this type [115]. Analysis of X-ray diffraction showed that a single material of the solid solution phase was formed when the level of In3+ was 10–20%; the diffractions peaks became situated at lower values of 2θ with In content and the lattice constant increasing with the substitution of the larger In3+ (0.092 nm) with the smaller Fe3+ (0.067 nm).
Microstructural analysis showed that the incorporation of In3+ had significant morphological changes on the material: Fe1.8In0.2O3 nanowires showed interconnected pore channels which allowed better gas permeability, compared to the amorphous regions of FeInO3 nanowires which hindered the movement of gases. The performance testing showed that Fe1.6In0.4O3 created a response at a concentration of 100 ppm triethylamine at 260 °C with the response time of 4s only (Figure 14c), an improvement from the response of pure α-Fe2O3. This improvement was achieved by means of the In doping band structure modulation, which was observed through the Fermi level value of Fe1.6In0.4O3 increasing by 0.3 eV above that of α-Fe2O3, thus facilitating the creation of the surface-adsorbed oxygen species.
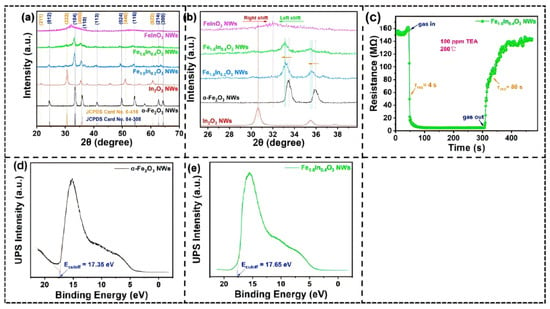
Figure 14.
(a) XRD patterns of α-Fe2O3, In2O3, and three In/Fe composite NWs. (b) Locally amplified XRD patterns of all as-synthesized samples. (c) A comparison of single-cycle response–recovery transient curves of sensors based on Fe1.6In0.4O3. (d,e) UPS spectra (He I) of α-Fe2O3 and Fe1.6In0.4O3 NWs. Reproduced from Ref. [115] Copyright © 2022, American Chemical Society.
It was also noted that formation of solid solution had reached a threshold at 20% In3+ content; after that, the phase of segregation was observed and regions of amorphous have formed thus deteriorating recovery properties (FeInO3 took 395 s to recover 77%). The study clarifies the inherent relationship between composition, structure and performance in solid solutions and can be useful in design of high-performance gas sensing materials.
On the other hand, the study on ZnO/Pd@ZIF-8 core–shell structures reflects innovative applications of noble metal modification in traditional metal oxide systems. The ZnO/Pd@ZIF-8 core–shell structure developed by our research team exemplifies this strategy [77]. Successfully synthesized via a self-templating method, the ZIF-8 shell acts not only as a physical barrier but also achieves intelligent molecular sieving of gas molecules through its unique pore structure and chemical properties (Figure 15i). The sensor’s exceptional performance originates from the dual filtering mechanism of the ZIF-8 shell. Firstly, there is the size sieving effect: the effective aperture of ZIF-8 (~4.0–4.2 Å) physically hinders the diffusion of larger molecules like NO2 (kinetic diameter 4.5 Å) toward the internal ZnO/Pd sensing layer. More importantly, there is also the polarity sieving effect: for polar molecules CO (3.76 Å) and NH3 (2.6 Å) with kinetic diameters smaller than the aperture, their molecular polarity causes strong interaction with the ZIF-8 framework, thereby increasing diffusion resistance. In contrast, non-polar CH4 molecules (3.8 Å) are virtually unaffected and diffuse the fastest. Performance tests revealed that at the optimal operating temperature of 210 °C, the sensor exhibited a high response of 57.9% to 500 ppm CH4, with selectivity coefficients of 20.6, 11.8, and 113.1 against the interfering gases CO (50 ppm), NH3 (50 ppm), and NO2 (5 ppm), respectively (Figure 15a–h). This synergistic sieving mechanism was confirmed through in situ FT-IR and TPD experiments, which clarified the order of interaction strength between gas molecules and ZIF-8 as CH4 < CO < NH3.
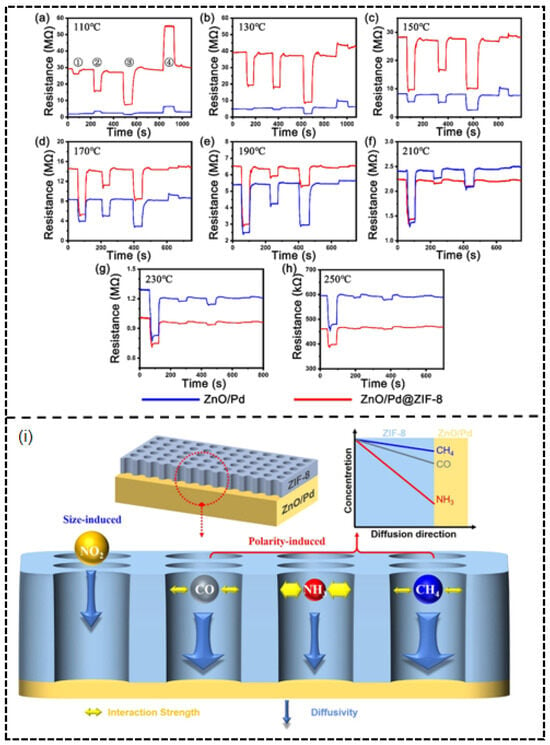
Figure 15.
Dynamic resistance curve of the sensors based on ZnO/Pd@ZIF-8 and ZnO/Pd to ➀ CH4, ➁ CO, ➂ NH3, ➃ NO2 at (a) 110 °C, (b) 130 °C, (c) 150 °C, (d) 170 °C, (e) 190 °C, (f) 210 °C, (g) 230 °C, (h) 250 °C. (i) Schematic illustration of size and polarity-induced dual filtering effect of ZIF-8. Reproduced from Ref. [77] © 2023 Elsevier B.V. All rights reserved.
4. Challenges and Future Perspectives
- Lagged Research of Bimetallic/Multimetallic Synergistic Effects.
Current research heavily favors single-metal systems, leaving the synergistic effects in bimetallic/multimetallic alloys significantly underexplored, which hinders the rational design of high-performance materials [62,116]. Future efforts should focus on employing advanced characterization techniques such as in situ X-ray Photoelectron Spectroscopy (XPS) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), combined with theoretical calculations, to uncover synergistic mechanisms—including electronic effects, interfacial effects, and catalytic synergy—at the atomic/molecular level. The precise synthesis of novel nanostructures like core–shell, intermetallic compounds, and even high-entropy alloys will be crucial for systematically establishing composition–structure–property relationships, providing a new paradigm and a theoretical blueprint for designing sensors with high selectivity and stability.
- 2.
- An outstanding Long-term Stability and Humidity Resistance Bottlenecks.
Fluctuating environmental humidity causes baseline drift and sensitivity loss, posing a major obstacle for practical applications. Overcoming this challenge requires a combination of material innovation, device design, and intelligent algorithms—developing intrinsically hydrophobic materials (e.g., via surface fluorination) or incorporating Metal–Organic Frameworks (MOFs) as molecular sieve protective layers to enhance intrinsic humidity resistance, while advancing dynamic humidity compensation algorithms. Exploring hardware solutions that integrate sensors with micro-scale temperature control modules is promising, aiming to collectively improve long-term reliability and enable lifespan prediction under complex varying conditions through both physical isolation and advanced signal processing.
- 3.
- Lack of adequacy of recognition of complex gas interference.
In environments with multiple gas components, the selectivity of single sensors is limited, and signals are easily interfered with. Future work should emphasize the deep integration of heterogeneous sensor arrays and intelligent identification algorithms. By incorporating diverse sensing elements (e.g., MOS, conducting polymers, 2D materials) to obtain rich dynamic response fingerprints, and utilizing advanced algorithms like Graph Neural Networks (GNNs) and Temporal Convolutional Networks to decode high-dimensional, non-linear data, significant progress can be made. Furthermore, integrating physicochemical models—such as surface reaction kinetics and adsorption–desorption energy barriers—as constraints into machine learning frameworks will foster the development of highly interpretable, data-efficient physics-informed algorithms, ultimately achieving accurate identification and concentration inversion of specific target gases within complex backgrounds.
- 4.
- Material System Restrictions and Cost-Effectiveness Problems.
The high cost and susceptibility to poisoning of noble metals, coupled with a research focus limited mainly to traditional oxides, restrict material diversity. Solutions lie in promoting material innovation and a shift in R&D paradigms—vigorously developing Single-Atom Catalysts (SACs) to maximize noble metal utilization and potentially unlock unique activities; actively exploring the performance boundaries of non-precious alternatives (e.g., sulfuret [117], transition-metal carbides, nitrides [118], and of MXenes [119,120]); and leveraging machine learning-assisted high-throughput computation and screening to map the relationships between composition, structure, and properties. This approach facilitates the inverse design of new materials meeting specific application needs (e.g., high selectivity, poison resistance), accelerating the practical application and industrialization of high-performance, cost-effective gas sensing technologies.
5. Conclusions
In summary, this review systematically elucidates the performance enhancement mechanisms, key influencing factors, and future challenges of noble metal-modified metal oxide (MOS) gas sensors. The main conclusions are as follows:
- 1.
- Performance Enhancement Mechanisms:
Noble metals play a crucial role in supporting sensor sensitivity, selectivity and response speed through simultaneous synergistic effects of electronic sensitization–interface electron transfer, and depletion layer modulation due to work function differences, chemical sensitization—catalytic weakening of activation energy barriers of reactions with gases and surface plasmon resonance—amplification of local electromagnetic field and injection of hot electrons. Multimetallic modification systems which include Pd@Pt core–shell structures are even better refinements in reaction pathways through synergistic catalysis, and, as such, they are breakthrough performances.
- 2.
- Key Influencing Factors:
- Loading amount: The loading (threshold ≈0.1–0.5 wt%) determines selectivity and active site density.
- Catalytic interface: Core–shell structures (e.g., Pt-shell/Pd-core) achieve specific recognition through size sieving and chemical adsorption competition; heterojunction band modulation (e.g., Schottky barrier in Pd@Pt/ZnO) amplifies response signals.
- External stimuli: Humidity-triggered p-n response transition (e.g., Pt/TiO2) and light-enhanced carrier generation (e.g., UV-Pd/Ga2O3) can dynamically optimize sensing behaviors.
- 3.
- Challenges and Perspectives:
New advances must be made in inadequately understood principles of multimetallic synergy, inadequacy of stability at high humidity (>90% RH and >60% decay), and cross-interference in multigas mixtures. Future engineering aims at performing in situ characterization methods in order to address interface processes, anti-humidity interface engineering, and intelligent decoupling of sensor arrays based on AI.
Finally, functionalized metal oxide nanostructures with noble metals have turned out to be one of the prospective methods in the sphere of gas sensing with great improvement in sensitivity, selectivity and response/recovery times. As the research and development continues, such sensors will transform different fields, such as environmental monitoring, the control of industries and personal safety. The prognosis of the noble metal-modified metal oxide gas sensor is bright and its common usage will help create the world of safety, health and sustainability.
Author Contributions
R.Y.: Writing—original draft. Y.X.: Writing—review and editing, Supervision. L.Y.: Project administration, Methodology. J.X.: Software, Resources. Q.Z.: Writing—review and editing, Project administration. S.G.: Writing—review and editing, Visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Natural Science Foundation of China (grant No. 62561031, 62101225, and 52274410) and Project of the “Xingdian Talent Support Program” of Yunnan Province (XDYC-QNRC-2022-0055), Yunnan Fundamental Research Projects (202501CF070183, 202401BE070001-005), Yunnan Major Scientific and Technological Projects (grant No. 202302AG050002), Key Technology Research and Development Program of Shandong Province (grant No. 2023CXGC010903). The authors (Shenghui Guo, Yunling Scholar; Li Yang, Industrial Innovation Scholar) would like to acknowledge Yunnan Province Xingdian Talent Support Plan Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fletcher, S.E.M.; Schaefer, H. Rising methane: A new climate challenge. Science 2019, 364, 932–933. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.; Umar, A.; Chen, W.; Kumar, R. Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications. Sens. Actuators B Chem. 2018, 256, 656–664. [Google Scholar] [CrossRef]
- Sui, N.; Zhang, P.; Zhou, T.; Zhang, T. Selective ppb-level ozone gas sensor based on hierarchical branch-like In2O3 nanostructure. Sens. Actuators B Chem. 2021, 336, 129612. [Google Scholar] [CrossRef]
- Fu, L.; You, S.; Li, G.; Li, X.; Fan, Z. Application of Semiconductor Metal Oxide in Chemiresistive Methane Gas Sensor: Recent Developments and Future Perspectives. Molecules 2023, 28, 6710. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, H.J.; Jung, H.J.; Park, J.Y.; Seok, T.J.; Choa, Y.H.; Park, T.J.; Lee, S.W. High-Performance, Transparent Thin Film Hydrogen Gas Sensor Using 2D Electron Gas at Interface of Oxide Thin Film Heterostructure Grown by Atomic Layer Deposition. Adv. Funct. Mater. 2018, 29, 1807760. [Google Scholar] [CrossRef]
- Park, K.-R.; Cho, H.-B.; Lee, J.; Song, Y.; Kim, W.-B.; Choa, Y.-H. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuators B Chem. 2020, 302, 127179. [Google Scholar] [CrossRef]
- Yu, X.F.; Li, Y.C.; Cheng, J.B.; Liu, Z.B.; Li, Q.Z.; Li, W.Z.; Yang, X.; Xiao, B. Monolayer Ti2CO2: A Promising Candidate for NH3 Sensor or Capturer with High Sensitivity and Selectivity. ACS Appl. Mater. Interfaces 2015, 7, 13707–13713. [Google Scholar] [CrossRef]
- Jang, W.B.; Yi, D.; Nguyen, T.M.; Lee, Y.; Lee, E.J.; Choi, J.; Kim, Y.H.; Choi, E.J.; Oh, J.W.; Kwon, S.M. Artificial Neural Processing-Driven Bioelectronic Nose for the Diagnosis of Diabetes and Its Complications. Adv. Healthc. Mater. 2023, 12, e2300845. [Google Scholar] [CrossRef]
- Pang, X.; Shaw, M.D.; Lewis, A.C.; Carpenter, L.J.; Batchellier, T. Electrochemical ozone sensors: A miniaturised alternative for ozone measurements in laboratory experiments and air-quality monitoring. Sens. Actuators B Chem. 2017, 240, 829–837. [Google Scholar] [CrossRef]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Carbon monoxide (CO) optical gas sensor based on ZnO thin films. Sens. Actuators B Chem. 2017, 250, 679–685. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Mei, S.; Jia, Y.; Liu, M.; Xue, X.; Yang, D. Development of a Pd/Cu nanowires coated SAW hydrogen gas sensor with fast response and recovery. Sens. Actuators B Chem. 2019, 287, 157–164. [Google Scholar] [CrossRef]
- Ishihara, T.; Iwata, J. Effects of additives on RuO2 (10 wt%)/La0.6Sr0.4CoO3 anode for increasing sensitivity of solid oxide amperometric CO Sensor. Sens. Actuators B Chem. 2016, 223, 535–539. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Xia, Y.; Zhang, B. Mesoporous ZnO nanosheets with rich surface oxygen vacancies for UV-activated methane gas sensing at room temperature. Sens. Actuators B Chem. 2021, 333, 129547. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R Rep. 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas–sensor applications: A review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Nurdiwijayanto, L.; Choi, P.G.; Jiang, D.; Tsuruta, A.; Masuda, Y. 2D/2D hierarchical heterojunction of SnO2 nanosheets and reduced graphene oxide for highly sensitive gas sensors. Ceram. Int. 2025, 51, 54381–54391. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Yang, S.; Lei, G.; Xu, H.; Lan, Z.; Wang, Z.; Gu, H. Metal oxide based heterojunctions for gas sensors: A review. Nanomaterials 2021, 11, 1026. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, R.; Li, Y.; Li, Y.; Wang, K.; Liu, Y.; Sun, J.; Huang, K.; Zhong, L.; Wang, J.; et al. A portable breath acetone analyzer using a low-power and high-selectivity MEMS gas sensor based on Pd/In2O3-decorated SnO2 nanocomposites. Sens. Actuators B Chem. 2025, 439, 137854. [Google Scholar] [CrossRef]
- Yang, X.; Deng, Y.; Yang, H.; Liao, Y.; Cheng, X.; Zou, Y.; Wu, L.; Deng, Y. Functionalization of mesoporous semiconductor metal oxides for gas sensing: Recent advances and emerging challenges. Adv. Sci. 2023, 10, 2204810. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Suman, P.H.; Kim, J.J.; Tuller, H.L.; Orlandi, M.O. Investigation of electronic and chemical sensitization effects promoted by Pt and Pd nanoparticles on single-crystalline SnO nanobelt-based gas sensors. Sens. Actuators B Chem. 2019, 301, 127055. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, C.; Zhang, J.; Peng, Y.; Xie, E. Enhanced gas sensing performance of electrospun Pt-functionalized NiO nanotubes with chemical and electronic sensitization. ACS Appl. Mater. Interfaces 2013, 5, 7410–7416. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Naik, G.K.; Lee, H.-J.; Song, H.-G.; Lee, C.-R.; Lee, I.-H.; Yu, Y.-T. Au@NiO core-shell nanoparticles as a p-type gas sensor: Novel synthesis, characterization, and their gas sensing properties with sensing mechanism. Sens. Actuators B Chem. 2018, 268, 223–231. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yousefi, H.R.; Falsafi, F.; Bonyani, M.; Lee, J.-H.; Kim, J.-H.; Kim, H.W.; Kim, S.S. An overview on how Pd on resistive-based nanomaterial gas sensors can enhance response toward hydrogen gas. Int. J. Hydrogen Energy 2019, 44, 20552–20571. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, D.-H.; Kim, I.-D. Controlled synthesis of electrospun hollow Pt-loaded SnO2 microbelts for acetone sensing. Sens. Actuators B Chem. 2021, 344, 130208. [Google Scholar] [CrossRef]
- Alev, O.; Büyükköse, S. Effect of Pt catalyst on the sensor performance of WO3 nanoflakes towards hazardous gases. J. Mater. Sci. Mater. Electron. 2021, 32, 25376–25384. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Wang, Y.; Qin, C.; Cao, J.; Wang, Y. Light-driven room temperature methane gas sensor based on Ag modified flower-like ZnO microsphere. Sens. Diagn. 2023, 2, 878–886. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. UV-LED Photo-activated Chemical Gas Sensors: A Review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 416–432. [Google Scholar] [CrossRef]
- Gao, D.-H.; Yu, Q.-C.; Kebeded, M.A.; Zhuang, Y.-Y.; Huang, S.; Jiao, M.-Z.; He, X.-J. Advances in modification of metal and noble metal nanomaterials for metal oxide gas sensors: A review. Rare Met. 2025, 44, 1443–1496. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, S.; Yang, L.; He, S.; Zhou, L.; Wang, M.; Gao, J.; Hou, M.; Wang, J.; Komarneni, S. Enhanced Free-Radical Generation on MoS2/Pt by Light and Water Vapor Co-Activation for Selective CO Detection with High Sensitivity. Adv. Mater. 2023, 35, e2303523. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, S.; Guo, Y.; Yang, L.; Zhang, S.; Xia, Y.; Xiang, M.; Hou, M. Waste honeycomb in-situ derived N-doped TiO2 with hierarchical porous nanostructure for rapid and selective H2 detection. J. Alloys Compd. 2025, 1016, 179016. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Diversiform metal oxide-based hybrid nanostructures for gas sensing with versatile prospects. Coord. Chem. Rev. 2020, 413, 213272. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Lu, W.; Zhao, S.; Li, T.; Zhong, X.; Cui, B.; Wei, D.; Zhang, Y. Highly selective NO2 chemiresistive gas sensor based on hierarchical In2O3 microflowers grown on clinoptilolite substrates. J. Alloys Compd. 2020, 828, 154395. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chen, H.; Li, G.D.; Shi, Z.; Zou, H.; Zou, X. Ultrathin In2O3 Nanosheets with Uniform Mesopores for Highly Sensitive Nitric Oxide Detection. ACS Appl. Mater. Interfaces 2017, 9, 16335–16342. [Google Scholar] [CrossRef]
- Horprathum, M.; Srichaiyaperk, T.; Samransuksamer, B.; Wisitsoraat, A.; Eiamchai, P.; Limwichean, S.; Chananonnawathorn, C.; Aiempanakit, K.; Nuntawong, N.; Patthanasettakul, V.; et al. Ultrasensitive hydrogen sensor based on Pt-decorated WO3 nanorods prepared by glancing-angle dc magnetron sputtering. ACS Appl. Mater. Interfaces 2014, 6, 22051–22060. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.; Kan, Z.; Yang, L.; Chang, Z.; Dong, B.; Bai, X.; Lu, G.; Song, H. A multi-platform sensor for selective and sensitive H2S monitoring: Three-dimensional macroporous ZnO encapsulated by MOFs with small Pt nanoparticles. J. Hazard. Mater. 2022, 426, 128075. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B Chem. 2021, 331, 129425. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Sung, S.-H.; Lee, G.; Kim, J.; Seong, J.; Shim, Y.-S.; Jun, S.C.; Jeon, S. High-performance gas sensor array for indoor air quality monitoring: The role of Au nanoparticles on WO3, SnO2, and NiO-based gas sensors. J. Mater. Chem. A 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- Koo, W.T.; Choi, S.J.; Kim, S.J.; Jang, J.S.; Tuller, H.L.; Kim, I.D. Heterogeneous Sensitization of Metal-Organic Framework Driven Metal@Metal Oxide Complex Catalysts on an Oxide Nanofiber Scaffold Toward Superior Gas Sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Liang, Y. Pd-loaded In2O3 nanowire-like network synthesized using carbon nanotube templates for enhancing NO2 sensing performance. RSC Adv. 2015, 5, 30038–30045. [Google Scholar] [CrossRef]
- Yu, A.; Li, Z.; Yi, J. Selective detection of parts-per-billion H2S with Pt-decorated ZnO nanorods. Sens. Actuators B Chem. 2021, 333, 129545. [Google Scholar] [CrossRef]
- Liu, L.; Mao, C.; Fu, H.; Qu, X.; Zheng, S. ZnO Nanorod-Immobilized Pt Single-Atoms as an Ultrasensitive Sensor for Triethylamine Detection. ACS Appl. Mater. Interfaces 2023, 15, 16654–16663. [Google Scholar] [CrossRef]
- Kumar, S.; Lawaniya, S.D.; Agarwal, S.; Yu, Y.-T.; Nelamarri, S.R.; Kumar, M.; Mishra, Y.K.; Awasthi, K. Optimization of Pt nanoparticles loading in ZnO for highly selective and stable hydrogen gas sensor at reduced working temperature. Sens. Actuators B Chem. 2023, 375, 132943. [Google Scholar] [CrossRef]
- Gupta, S.; Knoepfel, A.; Zou, H.; Ding, Y. Investigations of methane gas sensor based on biasing operation of n-ZnO nanorods/p-Si assembled diode and Pd functionalized Schottky junctions. Sens. Actuators B Chem. 2023, 392, 134030. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Guo, L.; Chen, T.; Zhao, W.; Liu, X.; Wang, J.; Wang, X.; Yang, Y. Chemiresistive n-butanol gas sensors based on Au@In2O3 hollow-sphere-array thin films. Sens. Actuators B Chem. 2025, 422, 136579. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Effects of Gas Adsorption Properties of an Au-Loaded Porous In2O3 Sensor on NO2-Sensing Properties. ACS Sens. 2021, 6, 4019–4028. [Google Scholar] [CrossRef]
- Mobtakeri, S.; Habashyani, S.; Gur, E. Highly Responsive Pd-Decorated MoO3 Nanowall H2 Gas Sensors Obtained from In-Situ-Controlled Thermal Oxidation of Sputtered MoS2 Films. ACS Appl. Mater. Interfaces 2022, 14, 25741–25752. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-Y.; Yu, G.-X.; Liu, J.; Li, X.; Chen, L.; Guo, Z. Bimetallic Au-Pt nanoparticle-supported ZnO porous nanobelts for selective gas sensing enhancement to benzene. Sens. Actuators B Chem. 2025, 426, 137125. [Google Scholar] [CrossRef]
- Ishak, S.; Johari, S.; Ramli, M.M. Formaldehyde detection using Sn doped ZnO thin film. J. Sol-Gel Sci. Technol. 2020, 95, 265–275. [Google Scholar] [CrossRef]
- Bhati, V.S.; Ranwa, S.; Fanetti, M.; Valant, M.; Kumar, M. Efficient hydrogen sensor based on Ni-doped ZnO nanostructures by RF sputtering. Sens. Actuators B Chem. 2018, 255, 588–597. [Google Scholar] [CrossRef]
- Woo, H.-S.; Kwak, C.-H.; Kim, I.-D.; Lee, J.-H. Selective, sensitive, and reversible detection of H2S using Mo-doped ZnO nanowire network sensors. J. Mater. Chem. A 2014, 2, 6412–6418. [Google Scholar] [CrossRef]
- Darmadi, I.; Nugroho, F.A.A.; Langhammer, C. High-Performance Nanostructured Palladium-Based Hydrogen Sensors—Current Limitations and Strategies for Their Mitigation. ACS Sens. 2020, 5, 3306–3327. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Z.; Li, J.; Zhang, R.; Meng, F. Fast-response xylene gas sensor based on Pt-modified Fe2(MoO4)3 nano-hollow spheres compounded on rGO. Sens. Actuators B Chem. 2025, 439, 137849. [Google Scholar] [CrossRef]
- Shen, Z.-Y.; Wang, J.-T.; Chiang, W.-H.; Hsiao, C.-N.; Wuu, D.-S.; Chen, G.-Y.; Wang, T.-W.; Liu, P.-L.; Chu, J.P.; Horng, R.-H. Study on performance improvement of zinc oxide gas sensor with silver nanoparticles surface modification. Sens. Actuators B Chem. 2025, 438, 137803. [Google Scholar] [CrossRef]
- Jing, J.; Li, J.; Xue, Q.; Wang, P.; Li, W.; Meng, Y.; Zhan, Z.; Zhang, Y.; Li, F. Stable Pt/PtO2-enhanced 3D inverse opal SnO2 gas sensor for high sensitivity and fast H2 sensing at low temperatures. Sens. Actuators B Chem. 2025, 431, 137462. [Google Scholar] [CrossRef]
- Inomata, Y.; Koga, K.; Shinkai, T.; Kida, T. Pt-Decorated ZnO Nanorods for Light-Assisted Ethanol Sensing and In Situ Analysis of the Sensing Mechanism under Light Irradiation. ACS Appl. Mater. Interfaces 2025, 17, 1399–1407. [Google Scholar] [CrossRef]
- Yang, B.; Thi Hanh To, D.; Sobolak, D.; Mendoza, E.R.; Myung, N.V. High performance methyl salicylate gas sensor based on noble metal (Au, Pt) decorated WO3 nanofibers. Sens. Actuators B Chem. 2024, 413, 135741. [Google Scholar] [CrossRef]
- Qiao, L.; Jia, X.; Zhang, J.; Yang, J.; Shao, D.; Feng, L.; Song, H. A highly responsive, moisture resistant diabetes diagnostic gas sensor with Pt-loaded porous GO/ZnO. Sens. Actuators B Chem. 2024, 418, 136275. [Google Scholar] [CrossRef]
- Kuo, C.-K.; Luo, K.-H.; Chu, P.-Y.; Wang, J.-C.; Tan, S.-W.; Liu, W.-C. Study of a highly sensitive ammonia gas sensor based on a sputtered Ga-doped SnO2 (GTO) thin film decorated with evaporated platinum nanoparticles. Sens. Actuators B Chem. 2024, 417, 136043. [Google Scholar] [CrossRef]
- Yuan, T.; Xue, Z.; Chen, Y.; Xu, J. Single Pt atom-based gas sensor: Break the detection limit and selectivity of acetone. Sens. Actuators B Chem. 2023, 397, 134139. [Google Scholar] [CrossRef]
- Haotian, J.; Jie, Z.; Li, W. Ultrasensitive nitric oxide gas sensor based on gold/tin oxide composite nanofibers prepared by electrospinning. Sens. Actuators B Chem. 2025, 427, 137225. [Google Scholar] [CrossRef]
- Ganesan, M.; Harish, S.; Shanmugasundaram, K.; Mohan, M.K.; Archana, J.; Navaneethan, M. Highly sensitive and selective Au-loaded WO3 nanoplates for NO2 gas detection. Sens. Actuators B Chem. 2025, 440, 137900. [Google Scholar] [CrossRef]
- Zhao, F.; Yu, L.; Wang, J.; Cao, W.; Zhang, H.; Wang, H.; Wang, P.H.; Qiao, Z. Metal-Organic Framework-Derived Au-Doped In2O3 Nanotubes for Monitoring CO at the ppb Level. ACS Sens. 2024, 9, 4007–4016. [Google Scholar] [CrossRef]
- Yang, B.; To, D.T.H.; Resendiz Mendoza, E.; Myung, N.V. Achieving One Part Per Billion Hydrogen Sulfide (H2S) Level Detection through Optimizing Composition and Crystallinity of Gold-Decorated Tungsten Trioxide (Au-WO3) Nanofibers. ACS Sens. 2024, 9, 292–304. [Google Scholar] [CrossRef]
- Deb, M.; Lu, C.J.; Zan, H.W. Achieving Room-Temperature ppb-Level H2S Detection in a Au-SnO2 Sensor with Low Voltage Enhancement Effect. ACS Sens. 2024, 9, 4568–4577. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, T.; Wang, X.; Cheng, Z.; Xu, J. Coal mine gases sensors with dual selectivity at variable temperatures based on a W18O49 ultra-fine nanowires/Pd@Au bimetallic nanoparticles composite. Sens. Actuators B Chem. 2022, 354, 131004. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, L.; Zhang, Y.; Xing, Y.; Fei, T.; Liu, S.; Zhang, T. DMMP sensors based on Au-SnO2 hybrids prepared through colloidal assembly approach: Gas sensing performances and mechanism study. Sens. Actuators B Chem. 2022, 369, 132278. [Google Scholar] [CrossRef]
- Lee, H.Y.; Bang, J.H.; Majhi, S.M.; Mirzaei, A.; Shin, K.Y.; Yu, D.J.; Oum, W.; Kang, S.; Lee, M.L.; Kim, S.S.; et al. Conductometric ppb-level acetone gas sensor based on one-pot synthesized Au @Co3O4 core-shell nanoparticles. Sens. Actuators B Chem. 2022, 359, 131550. [Google Scholar] [CrossRef]
- Saito, N.; Haneda, H.; Watanabe, K.; Shimanoe, K.; Sakaguchi, I. Highly sensitive isoprene gas sensor using Au-loaded pyramid-shaped ZnO particles. Sens. Actuators B Chem. 2021, 326, 128999. [Google Scholar] [CrossRef]
- Lei, M.; Gao, M.; Yang, X.; Zou, Y.; Alghamdi, A.; Ren, Y.; Deng, Y. Size-Controlled Au Nanoparticles Incorporating Mesoporous ZnO for Sensitive Ethanol Sensing. ACS Appl. Mater. Interfaces 2021, 13, 51933–51944. [Google Scholar] [CrossRef]
- Huang, J.; Liang, H.; Ye, J.; Jiang, D.; Sun, Y.; Li, X.; Geng, Y.; Wang, J.; Qian, Z.; Du, Y. Ultrasensitive formaldehyde gas sensor based on Au-loaded ZnO nanorod arrays at low temperature. Sens. Actuators B Chem. 2021, 346, 130568. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, W.; Zhou, L.; Yu, S. Room-Temperature Benzene Sensing with Au-Doped ZnO Nanorods/Exfoliated WSe2 Nanosheets and Density Functional Theory Simulations. ACS Appl. Mater. Interfaces 2021, 13, 33392–33403. [Google Scholar] [CrossRef]
- Luo, S.; Chen, R.; Wang, J.; Xiang, L. Conductometric methane gas sensors based on ZnO/Pd@ZIF-8: Effect of dual filtering of ZIF-8 to increase the selectivity. Sens. Actuators B Chem. 2023, 383, 133600. [Google Scholar] [CrossRef]
- Duan, P.; Duan, Q.; Peng, Q.; Jin, K.; Sun, J. Design of ultrasensitive gas sensor based on self-assembled Pd-SnO2/rGO porous ternary nanocomposites for ppb-level hydrogen. Sens. Actuators B Chem. 2022, 369, 132280. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Kwoka, M.; Gang, M.; Kumar, M. IoT-enabled surface-active Pd-anchored metal oxide chemiresistor for H2S gas detection. Sens. Actuators B Chem. 2024, 402, 135065. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Park, S.J.; Ha, T.J. Functionalization of Zinc Oxide Nanoflowers with Palladium Nanoparticles via Microwave Absorption for Room Temperature-Operating Hydrogen Gas Sensors in the ppb Level. ACS Appl. Mater. Interfaces 2021, 13, 25082–25091. [Google Scholar] [CrossRef]
- Barala, S.; Kumar, A.; Kwoka, M.; Gupta, A.; Kumar, M. Spillover effect in Pd anchored NiO-ZnO nanostructures improves hydrogen gas sensor’s performance. Sens. Actuators B Chem. 2025, 433, 137534. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Kuo, C.-K.; Chu, P.-Y.; Shih, D.-H.; Liu, W.-C. Study of a palladium nanoparticle/In-Zn-O thin film-based hydrogen gas sensor. Sens. Actuators B Chem. 2024, 415, 136015. [Google Scholar] [CrossRef]
- Feng, C.; Qiao, G.; Peng, Y.; Hao, L.; Guo, H.; Bai, H.; Ye, J.; Zheng, Y.; Zhang, R.; Chen, D. Study on unusual response behavior of NO2 sensor based on UV enhanced Pd-functionalized Ga2O3 microrods. Sens. Actuators B Chem. 2025, 429, 137266. [Google Scholar] [CrossRef]
- Sui, C.; Zhang, M.; Li, Y.; Wang, Y.; Liu, Y.; Liu, Z.; Bai, J.; Liu, F.; Lu, G. Pd@Pt Core-Shell Nanocrystal-Decorated ZnO Nanosheets for ppt-Level NO2 Detection. ACS Sens. 2024, 9, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Zhao, Z.G.; Yue, L.J.; Xie, K.F.; Jin, G.X.; Fang, S.M.; Zhang, Y.H. Pd Decoration with Synergistic High Oxygen Mobility Boosts Hydrogen Sensing Performance at Low Working Temperature on WO3 Nanosheet. ACS Sens. 2023, 8, 4293–4306. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; He, Z.K.; Zhang, W.; Du, Y.; Li, X.; Wang, S.; Zhao, J.; Song, Y.Y.; Gao, Z. Pd Nanoclusters-Sensitized MIL-125/TiO2 Nanochannel Arrays for Sensitive and Humidity-Resistant Formaldehyde Detection at Room Temperature. ACS Sens. 2024, 9, 4166–4175. [Google Scholar] [CrossRef]
- Mo, T.; Xu, X.; Fang, T.; Tao, H.; Wang, H.; Jin, M.L.; Yu, B.; Qian, L.; Zhao, Z.J. High Response and ppb-Level Detection toward Hydrogen Sensing by Palladium-Doped alpha-Fe2O3 Nanotubes. ACS Sens. 2024, 9, 5976–5984. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Wu, J.; Shen, J.; Chen, L.; Zhang, J.; Mao, Y.; Cheng, H.; Zhang, M.; Ma, Q.; et al. Ultrathin WO3 Nanosheets/Pd with Strong Metal-Support Interactions for Highly Sensitive and Selective Detection of Mustard-Gas Simulants. ACS Sens. 2024, 9, 3773–3782. [Google Scholar] [CrossRef]
- Bruce, J.; Bosnick, K.; Kamali Heidari, E. Pd-decorated ZnO nanoflowers as a promising gas sensor for the detection of meat spoilage. Sens. Actuators B Chem. 2022, 355, 131316. [Google Scholar] [CrossRef]
- Zhang, D.; Du, Q.; Yang, L.; Gao, J.; Yi, J.; Hou, M.; Guo, S.; Zeng, H. High-Throughput Experimental Technology: Rapid Identification of the Precious Metal Modified In2O3 for NO2 Low-Temperature Sensing. IEEE Sens. J. 2023, 23, 8101–8108. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Xia, Y.; Xiang, L. Controlled Doping Sites to Enhance Charge Transfer of ZnO for Ultrarapid Methane Sensing. ACS Appl. Mater. Interfaces 2025, 17, 21410–21418. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Jia, X.; Jiang, Y.; Xue, Z.; Xu, J. Inhibiting Emulative Oxygen Adsorption via Introducing Pt-Segregated Sites into the Pd Surface for Enhanced H2 Sensing in Air. ACS Sens. 2024, 9, 5405–5413. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Challa, S.R.; Delariva, A.T.; Hansen, T.W.; Helveg, S.; Sehested, J.; Hansen, P.L.; Garzon, F.; Datye, A.K. Relating rates of catalyst sintering to the disappearance of individual nanoparticles during Ostwald ripening. J. Am. Chem. Soc. 2011, 133, 20672–20675. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406. [Google Scholar] [CrossRef]
- Fan, M.; Cui, J.; Wu, J.; Vajtai, R.; Sun, D.; Ajayan, P.M. Improving the catalytic activity of carbon-supported single atom catalysts by polynary metal or heteroatom doping. Small 2020, 16, e1906782. [Google Scholar] [CrossRef]
- Lei, Y.; Butler, D.; Lucking, M.C.; Zhang, F.; Xia, T.; Fujisawa, K.; Granzier-Nakajima, T.; Cruz-Silva, R.; Endo, M.; Terrones, H. Single-atom doping of MoS2 with manganese enables ultrasensitive detection of dopamine: Experimental and computational approach. Sci. Adv. 2020, 6, eabc4250. [Google Scholar] [CrossRef]
- Luo, X.; Wei, X.; Wang, H.; Gu, W.; Kaneko, T.; Yoshida, Y.; Zhao, X.; Zhu, C. Secondary-atom-doping enables robust Fe–N–C single-atom catalysts with enhanced oxygen reduction reaction. Nano-Micro Lett. 2020, 12, 163. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Cheng, Y.; Li, K.; Yao, Y.; Zhang, Q.; Dong, C.; Wang, P.; Schwingenschlögl, U.; Yang, W. Doping monolayer graphene with single atom substitutions. Nano Lett. 2012, 12, 141–144. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Feng, Y.; Feng, B.; Cheng, D.; Wei, J. Synergistic sensitization effects of single-atom gold and cerium dopants on mesoporous SnO2 nanospheres for enhanced volatile sulfur compound sensing. Mater. Horiz. 2024, 11, 3038–3047. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Widmann, D.; Behm, R.J. Activation of molecular oxygen and the nature of the active oxygen species for CO oxidation on oxide supported Au catalysts. Acc. Chem. Res. 2014, 47, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Chen, Y.; Ming, H. Review of surface plasmon resonance and localized surface plasmon resonance sensor. Photonic Sens. 2012, 2, 37–49. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Xu, L.; Li, X.; Huang, S. A room-temperature methane sensor based on Pd-decorated ZnO/rGO hybrids enhanced by visible light photocatalysis. Sens. Actuators B Chem. 2020, 304, 127334. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Xia, Y.; Komarneni, S. Highly sensitive, fast and reversible NO2 sensors at room-temperature utilizing nonplasmonic electrons of ZnO/Pd hybrids. Ceram. Int. 2020, 46, 8462–8468. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Xia, Y.; Yu, P.; Xu, Y.; Dong, Y.; Wei, Q.; Wang, J. High-Performance Room-Temperature NO2 Gas Sensor Based on Au-Loaded SnO2 Nanowires under UV Light Activation. Nanomaterials 2022, 12, 4062. [Google Scholar] [CrossRef]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. based sensors for gas, humidity, and strain detections: A review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxidebased gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter 2003, 15, R813. [Google Scholar] [CrossRef]
- Zhou, X.; Tao, T.; Bao, Y.; Xia, X.; Homewood, K.; Wang, Z.; Lourenco, M.; Huang, Z.; Shao, G.; Gao, Y. Dynamic Reaction Mechanism of P-N-Switched H2-Sensing Performance on a Pt-Decorated TiO2 Surface. ACS Appl. Mater. Interfaces 2021, 13, 25472–25482. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Yu, P.; Guo, L.; Xu, Y.; Dong, Y.; Ni, Y.; Ao, J.; Wei, Q.; Xia, Y. In/Fe Cospinning Nanowires for Triethylamine Gas Sensing. ACS Appl. Nano Mater. 2022, 5, 9554–9566. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Xu, P.; Chen, Y.; Yu, H.; Li, X. In Situ TEM Technique Revealing the Deactivation Mechanism of Bimetallic Pd–Ag Nanoparticles in Hydrogen Sensors. Nano Lett. 2022, 22, 3157–3164. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, Z.; Li, X.; Yang, L.; Guo, S.; Xia, Y. In2O3/MoS2 composite prepared by electrostatic self-assembly for NO2 sensing at room-temperature. J. Porous Mater. 2025, 32, 1365–1374. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Chaudhary, V.; Dahiya, M.S.; Nehra, S.; Duhan, S.; Kailasam, K. A low temperature, highly sensitive and fast response toluene gas sensor based on In (III)-SnO2 loaded cubic mesoporous graphitic carbon nitride. Sens. Actuators B Chem. 2018, 255, 3564–3575. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, X.; Hui, Z.; Zhou, J.; Huang, X.; Sun, G.; Huang, W. Ti3C2TX MXene for sensing applications: Recent progress, design principles, and future perspectives. ACS Nano 2021, 15, 3996–4017. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Hazra, A. MXene-based gas sensors. J. Mater. Chem. C 2021, 9, 15735–15754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).