Structural and Spectroscopic Study of Benzoperimidines Derived from 1-Aminoanthraquinone and Their Application to Bioimaging
Abstract
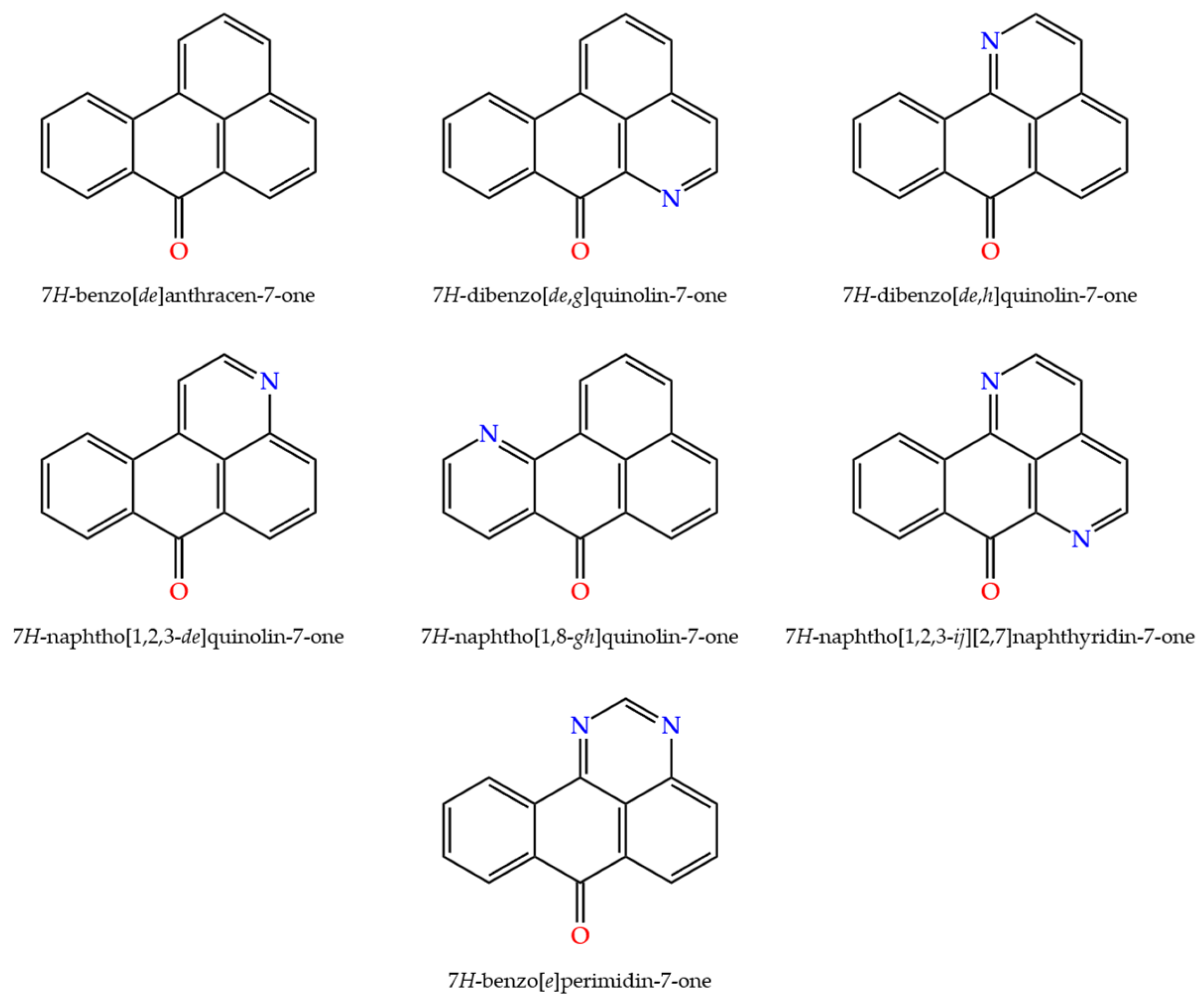
1. Introduction
2. Results and Discussion
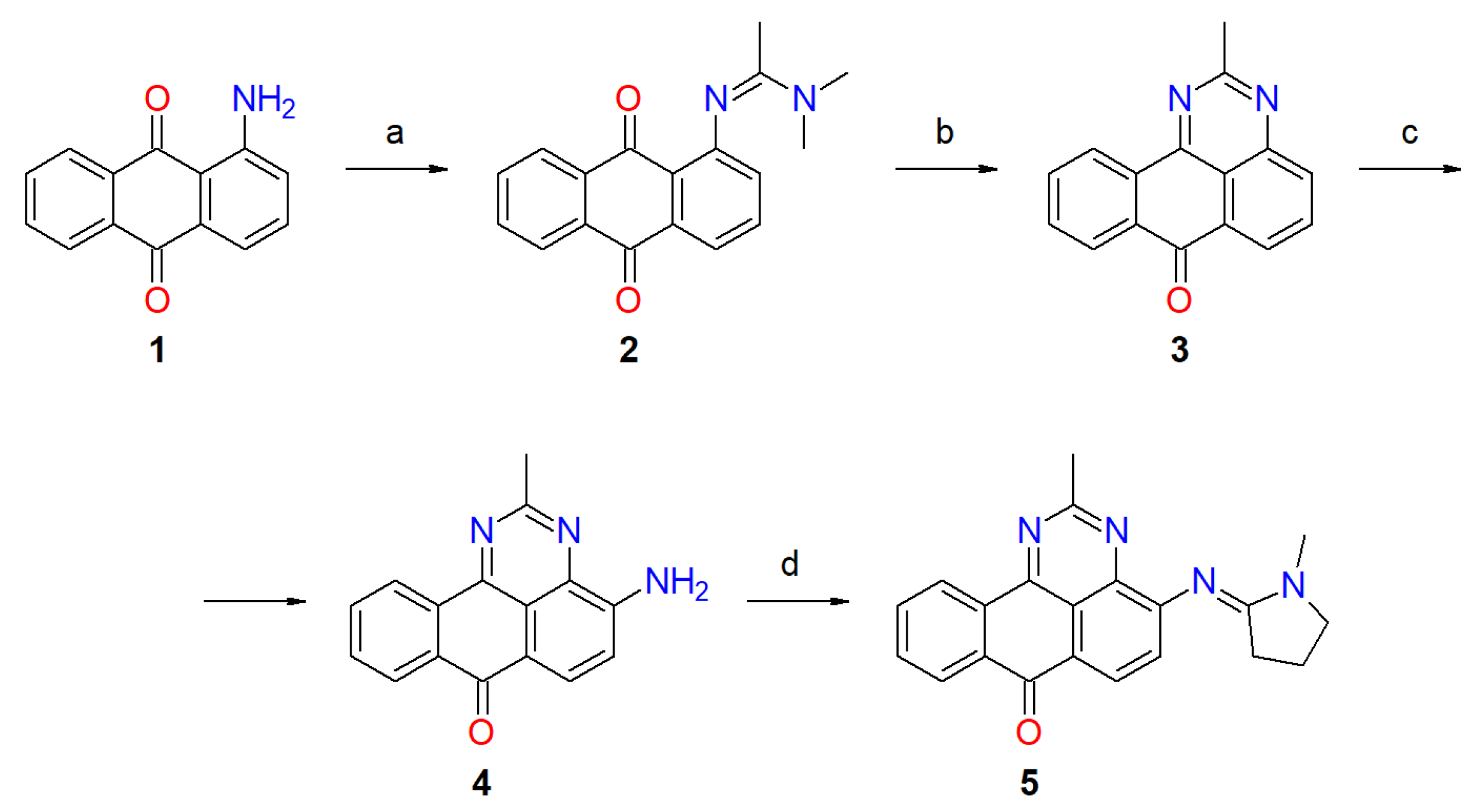
2.1. Synthesis
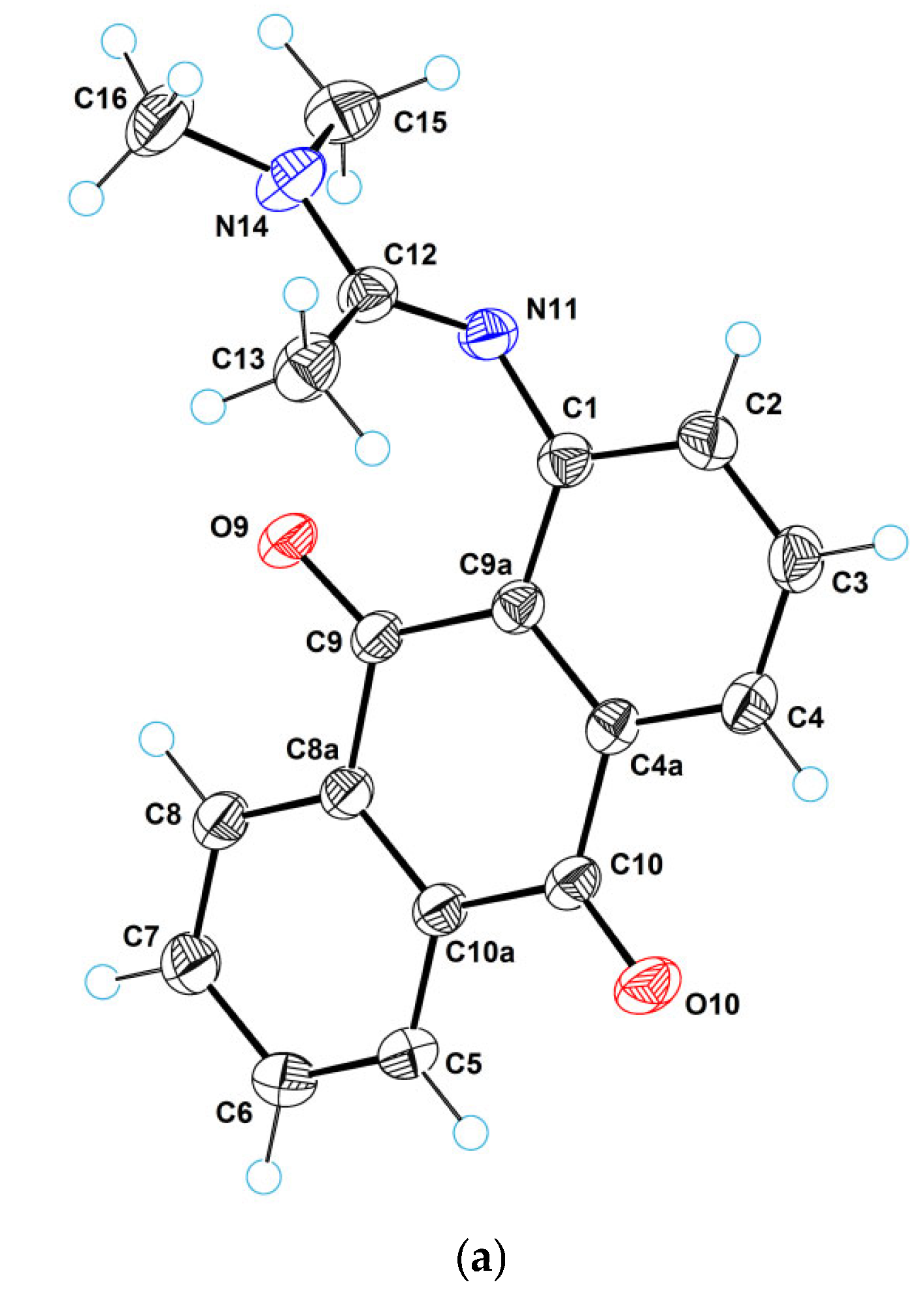
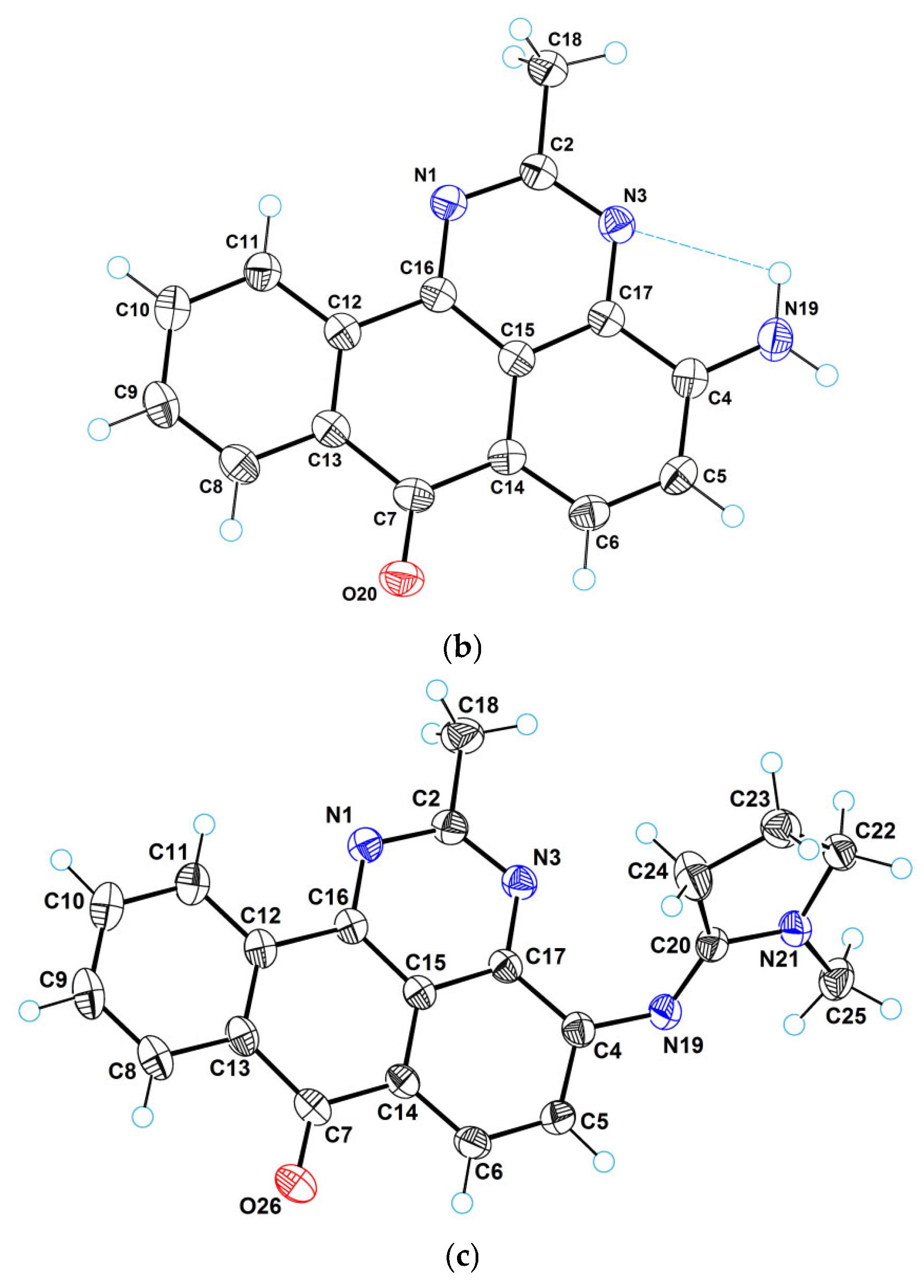
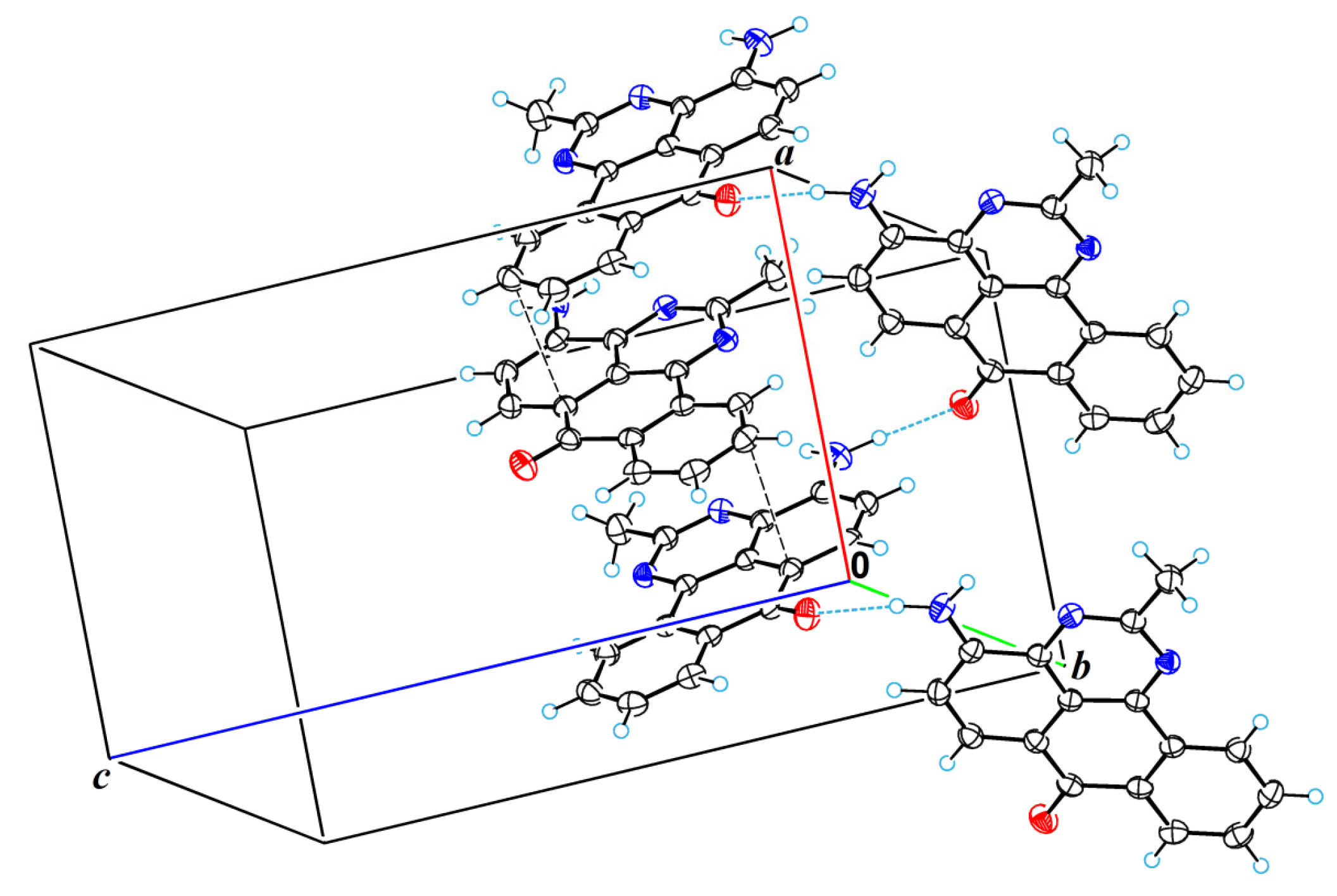
2.2. X-Ray Crystallographic Study
2.3. Photophysical Properties
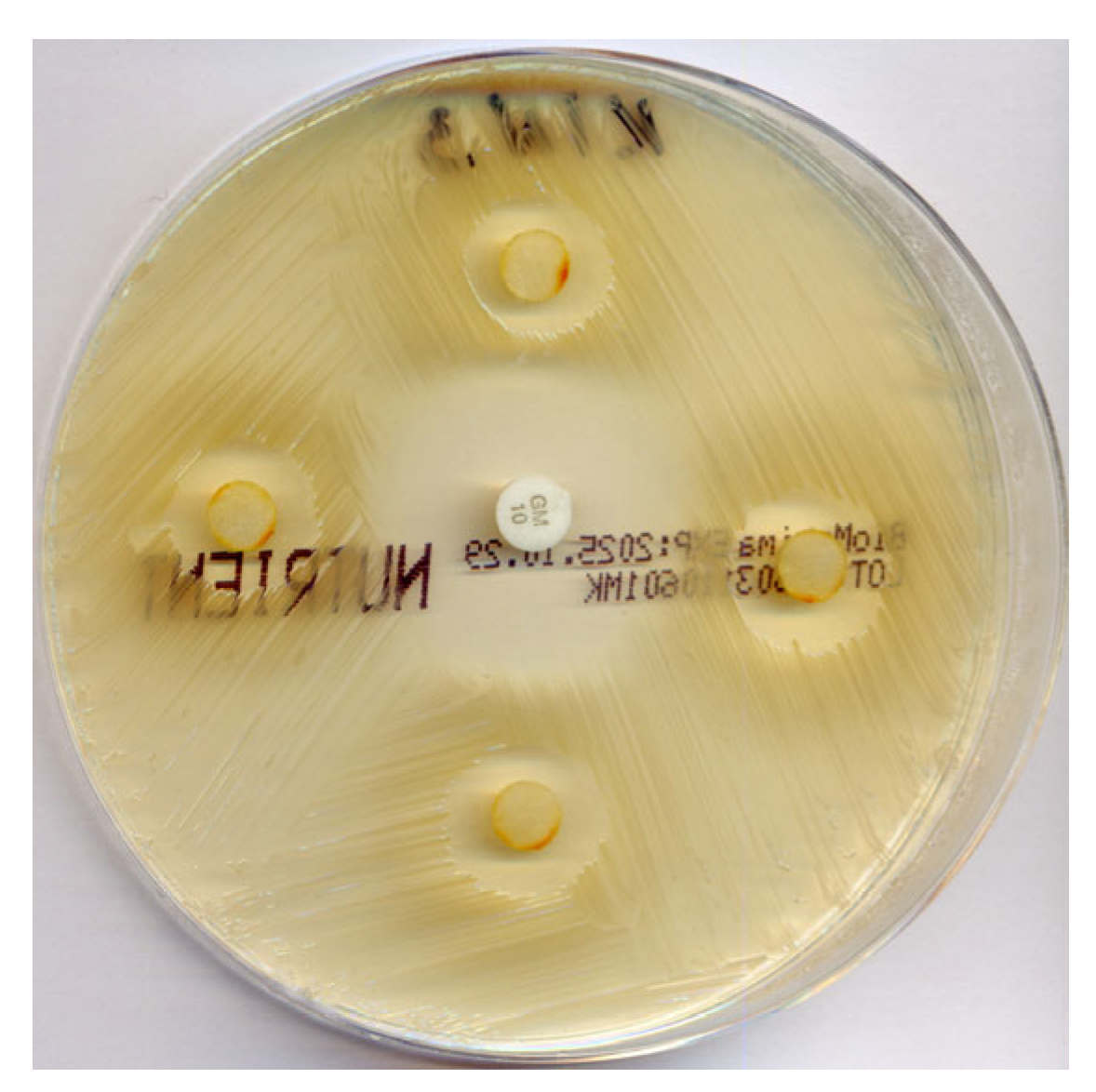
2.4. Antimicrobial Activity
2.5. Imaging of Opisthorchis felineus
3. Materials and Methods
3.1. Materials and Measurements
3.2. Synthesis and Characterization
3.3. Antimicrobial Activity Test
3.4. Confocal Laser Scanning Microscopy Imaging
3.5. Single Crystal X-Ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grabchev, I.; Bojinov, V.; Moneva, I. The synthesis and application of fluorescent dyes based on 3-amino benzanthrone. Dyes Pigm. 2001, 48, 143–150. [Google Scholar] [CrossRef]
- Cao, L.; Xu, L.; Zhang, D.; Zhou, Y.; Zheng, Y.; Fu, Q.; Jiang, X.-F.; Lu, F. D-A dyad and D-A-D triad incorporating triphenylamine, benzanthrone and perylene diimide: Synthesis, electrochemical, linear and nonlinear optical properties. Chem. Phys. Lett. 2017, 682, 133–139. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. pH sensor potential and antimicrobial activity of a new PPA dendrimer modified with benzanthrone fluorophores in solution and on viscose fabric. J. Photochem. Photobiol. A Chem. 2019, 375, 24–29. [Google Scholar] [CrossRef]
- Staneva, D.; Betcheva, R. Synthesis and functional properties of new optical pH sensor based on benzo[de]anthracen-7-one immobilized on the viscose. Dyes Pigm. 2006, 74, 148–153. [Google Scholar] [CrossRef]
- Kirilova, E.M.; Puckins, A.I.; Romanovska, E.; Fleisher, M.; Belyakov, S.V. Novel amidine derivatives of benzanthrone: Effect of bromine atom on the spectral parameters. Acta Part A Mol. Biomol. Spectrosc. 2018, 202, 41–49. [Google Scholar] [CrossRef]
- Maļeckis, A.; Griškjāns, E.; Cvetinska, M.; Savicka, M.; Belyakov, S.; Kirilova, E. Synthesis, characterization, spectroscopic studies and evaluation of toxicological effect on growth of wheat sprouts (Triticum aestivum) of new benzanthrone α-aryl-α-aminophosphonates. J. Mol. Struct. 2022, 1277, 134838. [Google Scholar] [CrossRef]
- Maļeckis, A.; Cvetinska, M.; Kirjušina, M.; Mežaraupe, L.; Kecko, S.; Gavarāne, I.; Kiyan, V.; Lider, L.; Pavlova, V.; Savicka, M.; et al. A Comparative Study of New Fluorescent Anthraquinone and Benzanthrone α-Aminophosphonates: Synthesis, Spectroscopy, Toxicology, X-ray Crystallography, and Microscopy of Opisthorchis felineus. Molecules 2024, 29, 1143. [Google Scholar] [CrossRef] [PubMed]
- Grabchev, I.; Moneva, I. Synthesis and properties of benzanthrone derivatives as luminophore dyes for liquid crystals. Dyes Pigm. 1998, 37, 155–164. [Google Scholar] [CrossRef]
- Grabtchev, I.K.; Bojinov, V.B.; Moneva, I.T. Functional properties of azomethine substituted benzanthrone dyes for use in nematic liquid crystals. J. Mol. Struct. 1998, 471, 19–25. [Google Scholar] [CrossRef]
- Maļeckis, A.; Cvetinska, M.; Griškjāns, E.; Kirilova, E. Exploring dual solvatochromic traits in novel fluorescent benzanthrone ethynyl derivatives. J. Solution Chem. 2024, 53, 1074–1088. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I.; Wolarz, E.; Bauman, D. Fluorescent 3-oxy benzanthrone dyes in liquid crystalline media. Dyes Pigm. 2003, 58, 1–6. [Google Scholar] [CrossRef]
- Maļeckis, A.; Cvetinska, M.; Griškjāns, E.; Dmitrijevs, K.; Traskovskis, K.; Belyakov, S.; Kirilova, E. Benzanthrone sulfides: Synthesis, solvatochromism characterization and analysis of experimental photophysical parameters and theoretical calculations. Dyes Pigm. 2023, 219, 111599. [Google Scholar] [CrossRef]
- Maļeckis, A.; Romanovska-Dzalbe, E. Recent progress of benzanthrone chemistry: A condensed review. Chem. Pap. 2025, 79, 3463–3473. [Google Scholar] [CrossRef]
- Rodríguez-Arce, E.; Cancino, P.; Arias-Calderón, M.; Silva-Matus, P.; Saldías, M. Oxoisoaporphines and Aporphines: Versatile Molecules with Anticancer Effects. Molecules 2019, 25, 108. [Google Scholar] [CrossRef]
- Sun, J.; Zhan, X.; Wang, W.; Yang, X.; Liu, Y.; Yang, H.; Deng, J.; Yang, H. Natural aporphine alkaloids: A comprehensive review of phytochemistry, pharmacokinetics, anticancer activities, and clinical application. J. Adv. Res. 2023, 63, 231–253. [Google Scholar] [CrossRef]
- Liao, L.-S.; Tan, L.-J.; Chen, Y.; Yang, Q.-Y.; Choudhary, M.I.; Pan, Y.-M.; Tang, H.-T.; Su, G.-F.; Liang, H.; Chen, Z.-F. One-pot synthesis of oxoaporphines as potent antitumor agents and investigation of their mechanisms of actions. Eur. J. Med. Chem. 2022, 231, 114141. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, X.-D.; Wei, Y.-B.; Huang, S.-L.; Huang, Z.-S.; Tan, J.-H.; An, L.-K.; Wu, J.-Y.; Chan, A.S.-C.; Gu, L.-Q. Oxoisoaporphine alkaloid derivatives: Synthesis, DNA binding affinity and cytotoxicity. Eur. J. Med. Chem. 2007, 43, 973–980. [Google Scholar] [CrossRef]
- Guo, R.; Wang, C.-L.; Cao, X.-J.; Yao, X.-J.; Qiao, X.; Meng, Y.-T.; Zhang, T.; Zhang, Q. Rare oxoisoaporphine alkaloids from Menispermum dauricum with potential anti-inflammatory activity. Phytochemistry 2024, 225, 114170. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, F.-Y.; Levine, M.S.; Shi, H.-R.; Wang, Y.; Xiong, X.; Yang, L.-M.; Shi, Y.-Q.; Zou, T.; Sessler, J.L.; et al. Oxoisoaporphine alkaloid Iridium(III) derivative: An immunogenic cell death inducer that engages the Autophagy-Dependent regulator Cathepsin D. J. Am. Chem. Soc. 2025, 147, 15216–15228. [Google Scholar] [CrossRef]
- Zhong, H.; Zhao, M.; Wu, C.; Zhang, J.; Chen, L.; Sun, J. Development of oxoisoaporphine derivatives with topoisomerase I inhibition and reversal of multidrug resistance in breast cancer MCF-7/ADR cells. Eur. J. Med. Chem. 2022, 235, 114300. [Google Scholar] [CrossRef]
- Melzer, B.; Bracher, F. A divergent approach to benzylisoquinoline-type and oxoaporphine alkaloids via regioselective direct ring metalation of alkoxy isoquinolines. Org. Biomol. Chem. 2015, 13, 7664–7672. [Google Scholar] [CrossRef]
- Qin, J.-L.; Meng, T.; Chen, Z.-F.; Xie, X.-L.; Qin, Q.-P.; He, X.-J.; Huang, K.-B.; Liang, H. Facile total synthesis of lysicamine and the anticancer activities of the RuII, RhIII, MnII and ZnII complexes of lysicamine. Oncotarget 2017, 8, 59359–59375. [Google Scholar] [CrossRef] [PubMed]
- Hufford, C.D.; Sharma, A.S.; Oguntimein, B.O. Antibacterial and antifungal activity of liriodenine and related oxoaporphine alkaloids. J. Pharm. Sci. 1980, 69, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hao, N.; Wang, Q.; Li, R.; Zhang, G.; Chen, G.; Liu, S.; Che, Z. Non-food bioactive forest product liriodenine: Sources, chemistry, and bioactivities. Ind. Crops Prod. 2022, 187, 115447. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Sun, J. Oxoisoaporphine alkaloids: Prospective Anti-Alzheimer’s disease, anticancer, and antidepressant agents. ChemMedChem 2018, 13, 1262–1274. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Qin, J.-L.; Meng, T.; Yang, G.-A.; Wei, Z.-Z.; Liu, Y.-C.; Liang, H.; Chen, Z.-F. Preparation of 6/8/11-Amino/Chloro-Oxoisoaporphine and group-10 metal complexes and evaluation of their in vitro and in vivo antitumor activity. Sci. Rep. 2016, 6, 37644. [Google Scholar] [CrossRef]
- Qin, J.-L.; Shen, W.-Y.; Chen, Z.-F.; Zhao, L.-F.; Qin, Q.-P.; Yu, Y.-C.; Liang, H. Oxoaporphine Metal Complexes (CoII, NiII, ZnII) with High Antitumor Activity by Inducing Mitochondria-Mediated Apoptosis and S-phase Arrest in HepG2. Sci. Rep. 2017, 7, 46056. [Google Scholar] [CrossRef]
- Melzer, B.C.; Bracher, F. A novel approach to oxoisoaporphine alkaloids via regioselective metalation of alkoxy isoquinolines. Beilstein J. Org. Chem. 2017, 13, 1564–1571. [Google Scholar] [CrossRef]
- Wei, Y.-B.; Li, Y.-X.; Song, H.; Feng, X.-J. Design, synthesis and anticancer activity of oxoaporphine alkaloid derivatives. J. Enzyme Inhib. Med. Chem. 2014, 29, 722–727. [Google Scholar] [CrossRef][Green Version]
- Tatke, D.R.; Seshadrif, S. Nucleophilic substitution reactions of azabenzanthrone derivatives. Dyes Pigm. 1986, 7, 153–158. [Google Scholar] [CrossRef]
- Lokhande, P.K.M.; Patil, D.S.; Kadam, M.M.; Sekar, N. Theoretical Investigation of Optical and Nonlinear optical (NLO) properties of 3-Azabenzanthrone analogues: DFT and TD-DFT Approach. ChemistrySelect 2019, 4, 10033–10045. [Google Scholar] [CrossRef]
- Zang, Q.; Yu, J.; Yu, W.; Qian, J.; Hu, R.; Tang, B.Z. Red-emissive azabenzanthrone derivatives for photodynamic therapy irradiated with ultralow light power density and two-photon imaging. Chem. Sci. 2018, 9, 5165–5171. [Google Scholar] [CrossRef]
- Rao, J.U.M.; Giri, G.S.; Hanumaiah, T.; Rao, K.V.J. Sampangine, a New Alkaloid from Cananga odorata. J. Nat. Prod. 1986, 49, 346–347. [Google Scholar] [CrossRef]
- Plodek, A.; König, M.; Bracher, F. Synthesis of the Azaoxoaporphine Alkaloid Sampangine and Ascididemin-Type Pyridoacridines through TMPMgCl·LiCl-Mediated Ring Closure. Eur. J. Org. Chem. 2015, 2015, 1302–1308. [Google Scholar] [CrossRef]
- Mahdi, F.; Morgan, J.B.; Liu, W.; Agarwal, A.K.; Jekabsons, M.B.; Liu, Y.; Zhou, Y.-D.; Nagle, D.G. Sampangine (a Copyrine Alkaloid) Exerts Biological Activities through Cellular Redox Cycling of Its Quinone and Semiquinone Intermediates. J. Nat. Prod. 2015, 78, 3018–3023. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Deady, L.W.; Finlay, G.J.; Baguley, B.C.; Denny, W.A. Synthesis and Cytotoxic Activity of 7-Oxo-7H-dibenz[f,ij]isoquinoline and 7-Oxo-7H-benzo[e]perimidine Derivatives. J. Med. Chem. 2001, 44, 2004–2014. [Google Scholar] [CrossRef]
- Rubenina, I.; Gavarane, I.; Kirilova, E.; Mezaraupe, L.; Kirjusina, M. Comparison of the Benzanthrone Luminophores: They Are Not Equal for Rapid Examination of Parafasciolopsis fasciolaemorpha (Trematoda: Digenea). Biomolecules 2021, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments. Angew. Chem. Int. Ed. 2004, 43, 5291–5292. [Google Scholar] [CrossRef]
- Sahiba, N.; Agarwal, S. Recent Advances in the Synthesis of Perimidines and their Applications. Top. Curr. Chem. 2020, 378, 44. [Google Scholar] [CrossRef]
- Pozharskii, A.F.; Gulevskaya, A.V.; Claramunt, R.M.; Alkorta, I.; Elguero, J. Perimidines: A unique π-amphoteric heteroaromatic system. Russ. Chem. Rev. 2020, 89, 1204–1260. [Google Scholar] [CrossRef]
- Li, Y.P.; Weng, X.; Ning, F.X.; Bin Ou, J.; Hou, J.Q.; Bin Luo, H.; Li, D.; Huang, Z.S.; Huang, S.L.; Gu, L.Q. 3D-QSAR studies of azaoxoisoaporphine, oxoaporphine, and oxoisoaporphine derivatives as anti-AChE and anti-AD agents by the CoMFA method. J. Mol. Graph. Model. 2013, 41, 61–67. [Google Scholar] [CrossRef]
- Baranov, D.S.; Fadeev, D.S. Synthesis of 2-octyloxy-7H-benzo[e]perimidin-7-one and 3-substituted 3H-benzo[e]perimidine-2,7-diones. Mendeleev Commun. 2016, 26, 174–176. [Google Scholar] [CrossRef]
- Ning, F.X.; Weng, X.; Huang, S.L.; Gu, L.J.; Huang, Z.S.; Gu, L.Q. A facile and efficient method for hydroxylation of azabenzanthrone compounds. Chin. Chem. Lett. 2010, 22, 41–44. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans 1987, 2, S1–S19. [Google Scholar]
- Yordanova, S.; Vasileva-Tonkova, E.; Staneva, D.; Stoyanov, S.; Grabchev, I. Synthesis and characterization of new water soluble 9,10-anthraquinone and evaluation of its antimicrobial activity. J. Mol. Struct. 2018, 1168, 22–27. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Kukeva, R.; Stoyanova, R.; Grabchev, I. Synthesis, spectral characteristics and microbiological activity of benzanthrone derivatives and their Cu(II) complexes. J. Mol. Struct. 2019, 1197, 576–582. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. A New Bioactive Complex between Zn(II) and a Fluorescent Symmetrical Benzanthrone Tripod for an Antibacterial Textile. Materials 2019, 12, 3473. [Google Scholar] [CrossRef] [PubMed]
- Tsanova, A.; Stoyanova, V.; Jordanova, A.; Grabchev, I. Study of the Mechanism of the Antimicrobial Activity of Novel Water Soluble Ammonium Quaternary Benzanthrone on Model Membranes. J. Membr. Biol. 2020, 253, 247–256. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Liu, L.; Lu, L.D.; Yang, G.J.; Qian, M.B.; Yang, K.; Tan, F.; Zhou, X.N. Global, regional and national disease burden of food-borne trematodiases: Projections to 2030 based on the Global Burden of Disease Study 2021. Infect. Dis. Poverty 2024, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, M.Y.; Mordvinov, V.A. The liver fluke Opisthorchis felineus: Biology, epidemiology and carcinogenic potential. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 28–36. [Google Scholar] [CrossRef]
- Hu, Y.; Zhan, R.J.; Lu, S.L.; Zhang, Y.Y.; Zhou, M.Y.; Huang, H.; Wang, D.D.; Zhang, T.; Huang, Z.X.; Zhou, Y.F.; et al. Global distribution of zoonotic digenetic trematodes: A scoping review. Infect. Dis. Poverty 2024, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Sripa, J.; Chaiwong, T. Multi-epitope protein production and its application in the diagnosis of opisthorchiasis. Parasites Vectors 2024, 17, 206. [Google Scholar] [CrossRef] [PubMed]
- Bulashev, A.K.; Borovikov, S.N.; Serikova, S.S.; Suranshiev, Z.A.; Kiyan, V.S.; Eskendirova, S.Z. Development of an ELISA using anti-idiotypic antibody for diagnosis of opisthorchiasis. Folia Parasitol. 2016, 63, 025. [Google Scholar] [CrossRef]
- Taron, W.; Jamnongkan, W.; Kamjanlard, C.; Phetcharaburanin, J.; Wangwiwatsin, A.; Klanrit, P.; Namwat, N.; Techasen, A.; Titapun, A.; Boonmars, T.; et al. Development of ready-to-use microtiter plates for enhanced ELISA detection of Opisthorchis viverrini antigens in urine samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 343, 126589. [Google Scholar] [CrossRef]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef]
- Machicado, C.; Marcos, L.A. Carcinogenesis associated with parasites other than Schistosoma, Opisthorchis and Clonorchis: A systematic review. Int. J. Cancer 2016, 138, 2915–2921. [Google Scholar] [CrossRef]
- Gouveia, M.J.; Pakharukova, M.Y.; Laha, T.; Sripa, B.; Maksimova, G.A.; Rinaldi, G.; Brindley, P.J.; Mordvinov, V.A.; Amaro, T.; Santos, L.L.; et al. Infection with Opisthorchis felineus induces intraepithelial neoplasia of the biliary tract in a rodent model. Carcinogenesis 2017, 38, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Fürst, T.; Keiser, J.; Utzinger, J. Global burden of human food-borne trematodiasis: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 210–221. [Google Scholar] [CrossRef]
- Kokina, I.; Mickeviča, I.; Jahundoviča, I.; Ogurcovs, A.; Krasovska, M.; Jermaļonoka, M.; Mihailova, I.; Tamanis, E.; Gerbreders, V. Plant Explants Grown on Medium Supplemented with Fe3O4 Nanoparticles Have a Significant Increase in Embryogenesis. J. Nanomater. 2017, 3, 4587147. [Google Scholar] [CrossRef]
- Kirilova, E.M.; Kecko, S.; Mežaraupe, L.; Gavarāne, I.; Pučkins, A.; Mickeviča, I.; Rubeniņa, I.; Osipovs, S.; Bulanovs, A.; Pupiņš, M.; et al. Novel luminescent dyes for confocal laser scanning microscopy used in parasite Trematoda diagnostics. Acta Biochim. Pol. 2018, 65, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Gavarane, I.; Kirilova, E.; Rubeniņa, I.; Mežaraupe, L.; Osipovs, S.; Deksne, G.; Pučkins, A.; Kokina, I.; Bulanovs, A.; Kirjušina, M. A Simple and Rapid Staining Technique for Sex Determination of Trichinella Larvae Parasites by Confocal Laser Scanning Microscopy. Microsc. Microanal. 2019, 25, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Kirilova, E.; Mickeviča, I.; Mežaraupe, L.; Pučkins, A.; Rubeniņa, I.; Osipovs, S.; Kokina, I.; Bulanovs, A.; Kirjušina, M.; Gavarāne, I. Novel dye for detection of callus embryo by confocal laser scanning fluorescence microscopy. Luminescence 2019, 34, 353–359. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. 2015, 71, 59–75. [Google Scholar]

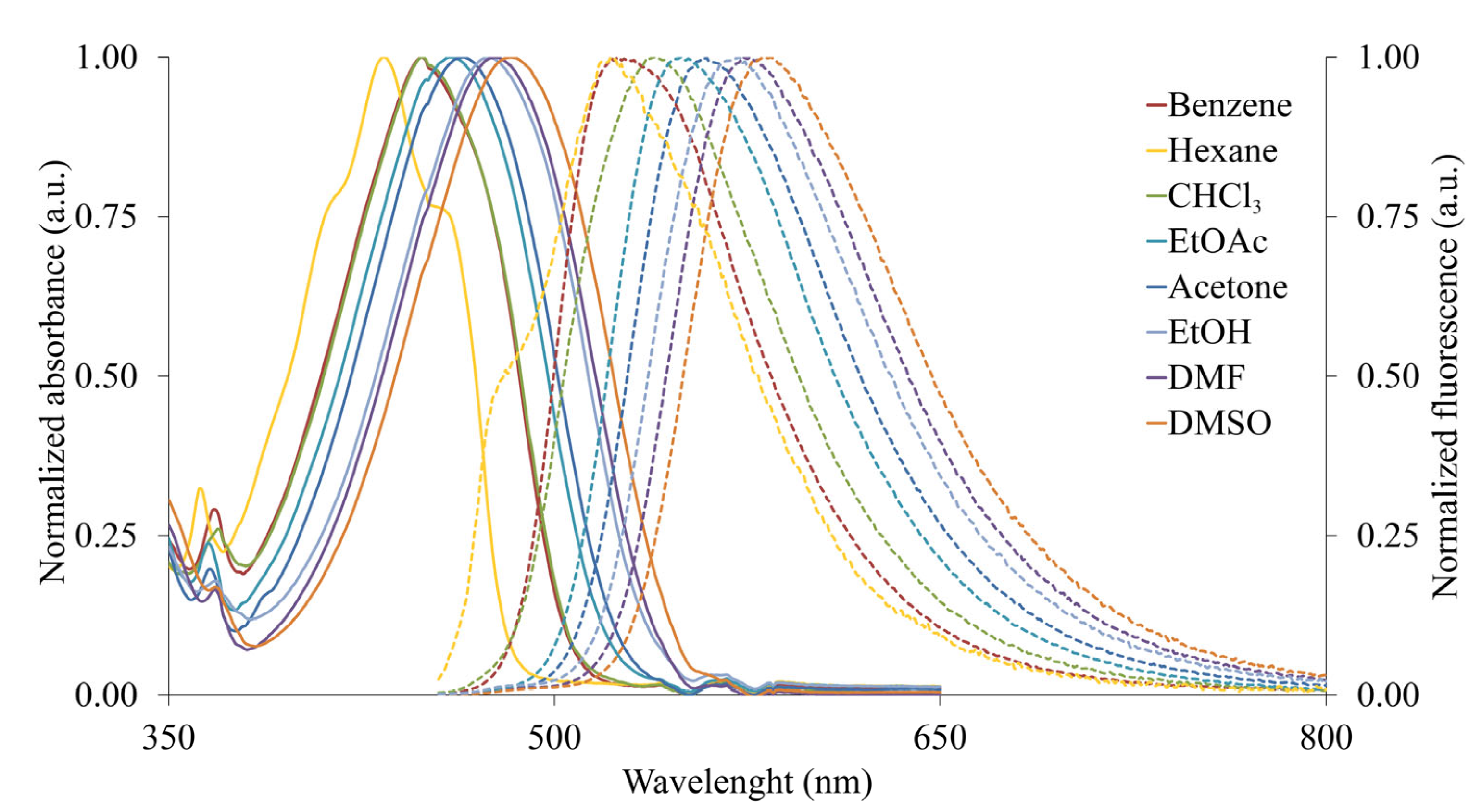


| Solvent | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| λabs, nm (lgε) | λabs, nm (lgε) | λabs, nm (lgε) | λem (Q), nm | λabs, nm (lgε) | λem (Q), nm | |
| Hexane | 410 (3.60) | 447 (3.96) | 434 (4.33) | 522 (0.001) | 448 (4.26) | 582 (0.47) |
| Benzene | 412 (3.61) | 461 (3.95) | 448 (4.32) | 527 (0.05) | 455 (4.34) | 599 (0.43) |
| Chloroform | 411 (3.49) | 489 (3.76) | 449 (4.32) | 540 (0.01) | 458 (4.35) | 600 (0.54) |
| EtOAc | 412 (3.55) | 469 (3.91) | 460 (4.33) | 550 (0.08) | 457 (4.32) | 607 (0.48) |
| Acetone | 412 (3.52) | 469 (3.91) | 464 (4.37) | 559 (0.06) | 462 (4.30) | 615 (0.20) |
| DMF | 417 (3.38) | 480 (3.94) | 477 (4.35) | 575 (0.04) | 469 (4.28) | 620 (0.16) |
| DMSO | 417 (3.31) | 484 (3.88) | 483 (4.37) | 583 (0.04) | 471 (4.42) | 622 (0.12) |
| EtOH | 400 (3.61) | 477 (3.98) | 473 (4.35) | 570 (0.015) | 455 (4.23) | 614 (0.07) |
| Compound | Zones, mm/diameter |
|---|---|
| Gentamicin | 22 ± 4 |
| Compound 1 | 0 |
| Compound 2 | 0 |
| Compound 3 | 0 |
| Compound 4 | 0 |
| Compound 5 | 13 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirilova, E.; Maļeckis, A.; Kirjušina, M.; Mežaraupe, L.; Rubeniņa, I.; Brakovska, A.; Pavlova, V.; Kecko, S.; Umbraško, I.; Kiyan, V.; et al. Structural and Spectroscopic Study of Benzoperimidines Derived from 1-Aminoanthraquinone and Their Application to Bioimaging. Molecules 2025, 30, 4472. https://doi.org/10.3390/molecules30224472
Kirilova E, Maļeckis A, Kirjušina M, Mežaraupe L, Rubeniņa I, Brakovska A, Pavlova V, Kecko S, Umbraško I, Kiyan V, et al. Structural and Spectroscopic Study of Benzoperimidines Derived from 1-Aminoanthraquinone and Their Application to Bioimaging. Molecules. 2025; 30(22):4472. https://doi.org/10.3390/molecules30224472
Chicago/Turabian StyleKirilova, Elena, Armands Maļeckis, Muza Kirjušina, Ligita Mežaraupe, Ilze Rubeniņa, Aija Brakovska, Veronika Pavlova, Sanita Kecko, Inta Umbraško, Vladimir Kiyan, and et al. 2025. "Structural and Spectroscopic Study of Benzoperimidines Derived from 1-Aminoanthraquinone and Their Application to Bioimaging" Molecules 30, no. 22: 4472. https://doi.org/10.3390/molecules30224472
APA StyleKirilova, E., Maļeckis, A., Kirjušina, M., Mežaraupe, L., Rubeniņa, I., Brakovska, A., Pavlova, V., Kecko, S., Umbraško, I., Kiyan, V., Lider, L., Pučkins, A., & Belyakov, S. (2025). Structural and Spectroscopic Study of Benzoperimidines Derived from 1-Aminoanthraquinone and Their Application to Bioimaging. Molecules, 30(22), 4472. https://doi.org/10.3390/molecules30224472






