Design of a Novel Class of N-Heterocyclic Carbene Cycloplatinated Complexes Containing Pyrene Chromophores
Abstract
1. Introduction
2. Results and Discussion
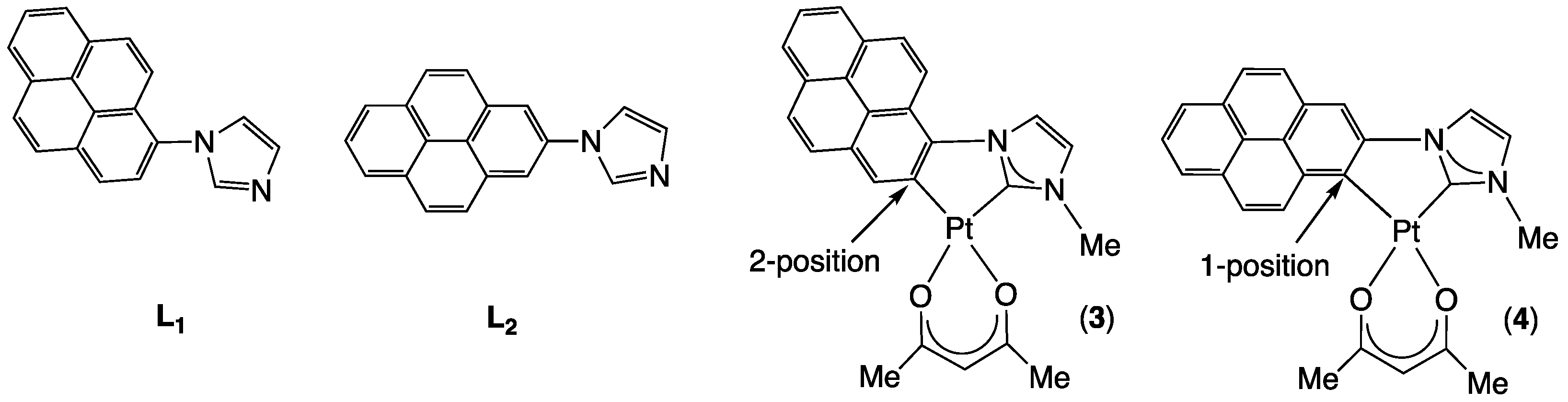
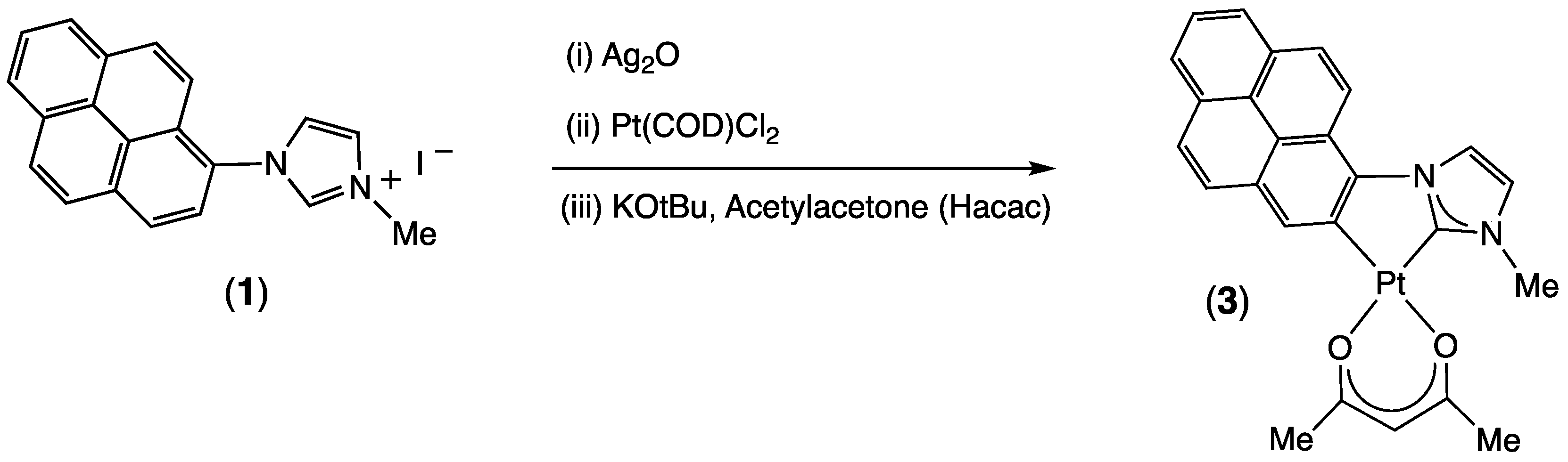
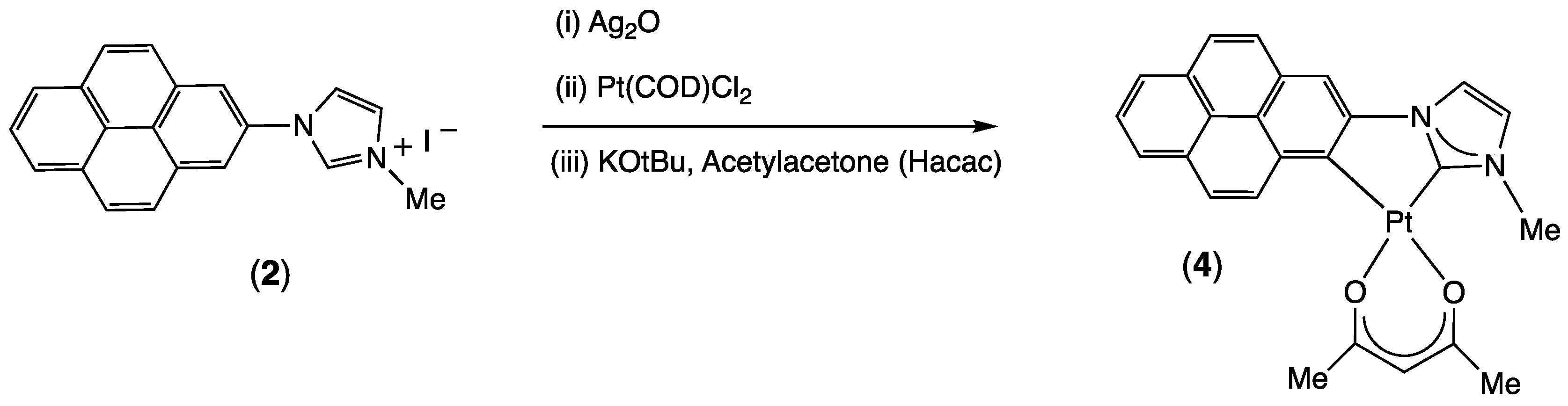
2.1. Synthesis and Characterization
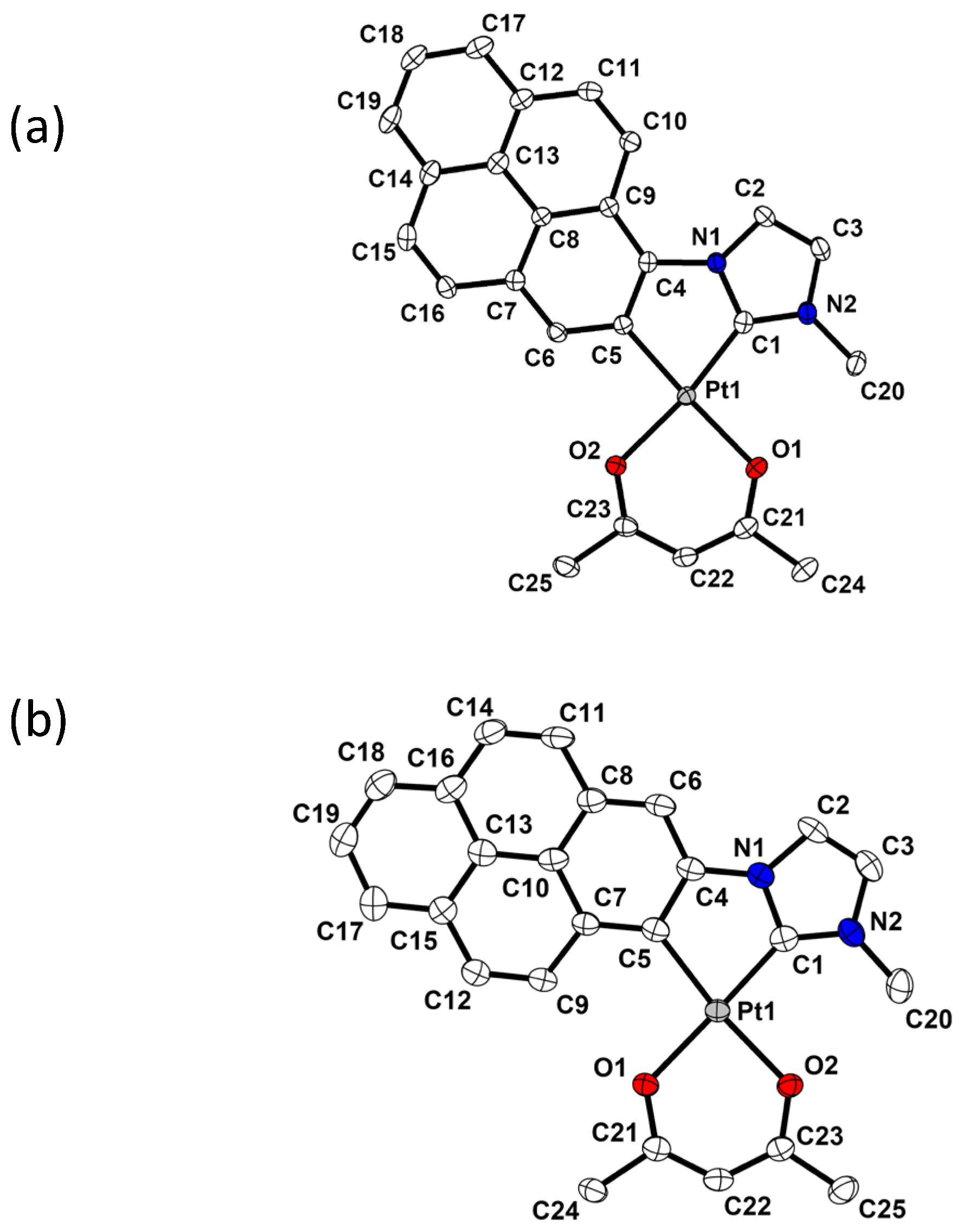
2.2. X-Ray Molecular Structures of (Pyrenyl-NHC-Me)-Pt(acac) Isomers 3 and 4
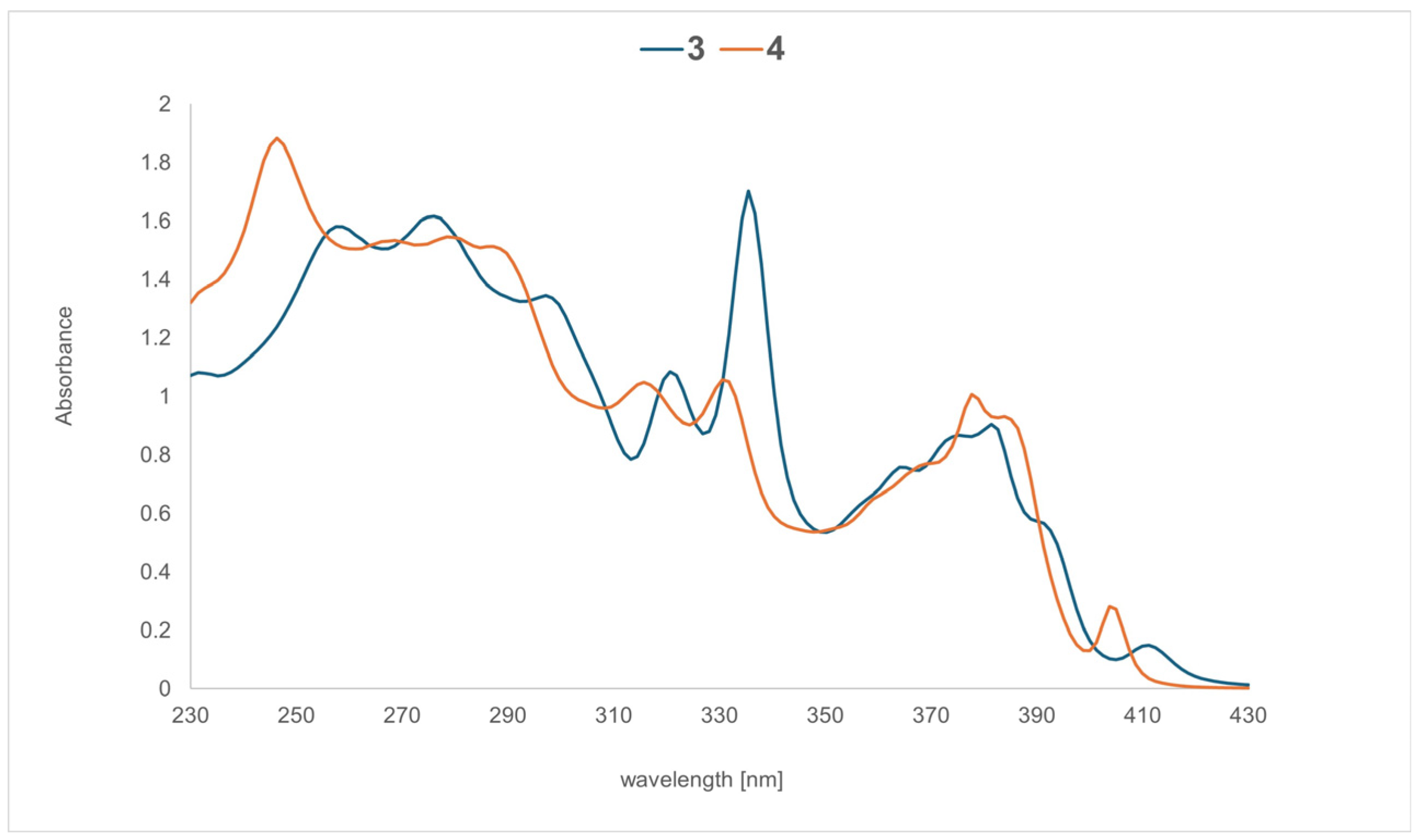
2.3. Absorption Properties
2.4. Luminescence Properties
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strassner, T. Phosphorescent Platinum(II) Complexes with C^C* Cyclometalated NHC Ligands. Acc. Chem. Res. 2016, 49, 2680–2689. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; El Moll, H.; Alenezi, K.M.; Khan, M.S.; Wong, W.Y. Functional Materials Based on Cyclometalated Platinum(II) β-Diketonate Complexes: A Review of Structure-Property Relationships and Applications. Materials 2021, 14, 4236. [Google Scholar] [CrossRef] [PubMed]
- You, Y. Pt(II) complexes with tetradentate ligands: Toward commercially applicable blue organic electroluminescence devices. Coord. Chem. Rev. 2025, 526, 216374. [Google Scholar] [CrossRef]
- Canadas, P.; Forzatti, M.; Fuertes, S.; Martin, A.; Sessolo, M.; Tordera, D.; Sicilia, V. Platinum(II) complexes bearing functionalized NHC-based pincer ligands: Synthesis and application in phosphorescent OLEDs. Mat. Chem. Front. 2025, 9, 2559–2570. [Google Scholar] [CrossRef]
- Kang, J.; Zaen, R.; Park, K.M.; Lee, K.H.; Lee, J.Y.; Kang, Y. Cyclometalated Platinum(II) β-Diketonate Complexes as Single Dopants for High-Efficiency White OLEDs: The Relationship between Intermolecular Interactions in the Solid State and Electroluminescent Efficiency. Cryst. Growth. Des. 2020, 20, 6129–6138. [Google Scholar] [CrossRef]
- Amouri, H. Luminescent Complexes of Platinum, Iridium, and Coinage Metals Containing N-Heterocyclic Carbene Ligands: Design, Structural Diversity, and Photophysical Properties. Chem. Rev. 2023, 123, 230–270. [Google Scholar] [CrossRef]
- Pinter, P.; Soellner, J.; Strassner, T. Sky-Blue Triplet Emitters with Cyclometalated Imidazopyrazine-Based NHC-Ligands and Aromatic Bulky Acetylacetonates. Chem. Eur. J. 2019, 25, 14495–14499. [Google Scholar] [CrossRef]
- Pinter, P.; Strassner, T. Prediction of the Efficiency of Phosphorescent Emitters: A Theoretical Analysis of Triplet States in Platinum Blue Emitters. Chem. Eur. J. 2019, 25, 4202–4205. [Google Scholar] [CrossRef]
- Stipurin, S.; Strassner, T. C^C* Platinum(II) Complexes with Electron-Withdrawing Groups and Beneficial Auxiliary Ligands: Efficient Blue Phosphorescent Emission. Inorg. Chem. 2021, 60, 11200–11205. [Google Scholar] [CrossRef]
- Riesebeck, T.; Strassner, T. Blue Light-Emitting Cyclometalated Spirofluorenexanthene Imidazolylidene Platinum(II) Complexes. Organometallics 2023, 42, 3275–3282. [Google Scholar] [CrossRef]
- Wurl, F.; Stipurin, S.; Kollar, J.I.; Strassner, T. Synthesis and Photophysical Properties of Monometallic C^C* Platinum(II) Formamidinate Complexes using Sterically Demanding Ligands. Angew. Chem. Int. Ed. 2023, 62, e202301225. [Google Scholar] [CrossRef]
- Riesebeck, T.; Strassner, T. Synthesis and Characterization of Platinum(II) Complexes with Bis(3-(trifluoromethyl)-pyrazolyl)-borate Auxiliary Ligands. Inorg. Chem. 2025, 64, 15881–15891. [Google Scholar] [CrossRef]
- Wu, W.T.; Wu, W.H.; Ji, S.M.; Guo, H.M.; Zhao, J.Z. Observation of Room-Temperature Deep-Red/Near-IR Phosphorescence of Pyrene with Cycloplatinated Complexes: An Experimental and Theoretical Study. Eur. J. Inorg. Chem. 2010, 2010, 4470–4482. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, T.Y.; Wu, T.W.; Ding, Z.M.; Zhang, Q.; Zhu, W.G.; Liu, Y. An effective strategy to obtain near-infrared emission from shoulder to shoulder-type binuclear platinum(ii) complexes based on fused pyrene core bridged isoquinoline ligands. J. Mat. Chem. C. 2021, 9, 2282–2290. [Google Scholar] [CrossRef]
- Huynh, H.V. The Organometallic Chemistry of N-Heterocyclic Carbenes; Wiley: Chichester, UK, 2017. [Google Scholar]
- Bourissou, D.; Guerret, O.; Gabbaie, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–91. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.A. N-heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Neuditschko, B.; Zappe, K.; Schaier, M.; Gerner, M.C.; Schmetterer, K.G.; Del Favero, G.; Bonsignore, R.; Cichna-Markl, M.; Koellensperger, G.; et al. An Organometallic Gold(I) Bis-N-Heterocyclic Carbene Complex with Multimodal Activity in Ovarian Cancer Cells. Chem. Eur. J. 2020, 26, 15528–15537. [Google Scholar] [CrossRef]
- Nelson, D.J.; Nolan, S.P. Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef]
- Hossain, J.; Akhtar, R.; Khan, S. Luminescent coinage metal complexes of carbenes. Polyhedron 2021, 201, 115151–115187. [Google Scholar] [CrossRef]
- Lanoe, P.H.; Najjari, B.; Hallez, F.; Gontard, G.; Amouri, H. N-Heterocyclic Carbene Coinage Metal Complexes Containing Naphthalimide Chromophore: Design, Structure, and Photophysical Properties. Inorganics 2017, 5, 58. [Google Scholar] [CrossRef]
- Cheng, Y.; Gontard, G.; Khatyr, A.; Knorr, M.; Amouri, H. N-Heterocyclic Carbene Copper (I) Complexes Incorporating Pyrene Chromophore: Synthesis, Crystal Structure, and Luminescent Properties. Molecules 2023, 28, 4025. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gontard, G.; Rager, M.N.; Khatyr, A.; Knorr, M.; Amouri, H. A Novel Family of Luminescent Pyrenyl-(N-Heterocyclic Carbene)- Halogenated Coinage Metal Complexes: Synthesis, Crystal Structures, and Optical Properties. Eur. J. Inorg. Chem. 2024, 27, e202400247. [Google Scholar] [CrossRef]
- Chow, A.L.-F.; So, M.-H.; Lu, W.; Zhu, N.-Y.; Che, C.-M. Synthesis, Photophysical Properties, and Molecular Aggregation of Gold(I) Complexes Containing Carbon-Donor Ligands. Chem. Asian J. 2011, 6, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Peter, L.; Vaubel, G. Phosphorescence of Pure Crystalline Pyrene. Chem. Phys. Lett. 1973, 21, 158–160. [Google Scholar] [CrossRef]
- Omary, M.A.; Kassab, R.M.; Haneline, M.R.; Elbjeirami, O.; Gabbai, F.P. Enhancement of the phosphorescence of organic luminophores upon interaction with a mercury trifunctional Lewis acid. Inorg. Chem. 2003, 42, 2176–2178. [Google Scholar] [CrossRef]
- Marwitz, A.C.; Dutta, A.K.; Conner, R.L.; Sanz, L.A.; Jacobsohn, L.G.; Knope, K.E. Unlocking Arene Phosphorescence in Bismuth-Organic Materials. Inorg. Chem. 2024, 63, 11053–11062. [Google Scholar] [CrossRef]
- Harriman, A.; Hissler, M.; Khatyr, A.; Ziessel, R. A ruthenium(II) tris(2,2-bipyridine) derivative possessing a triplet lifetime of 42μs. Chem. Commun. 1999, 8, 735–736. [Google Scholar] [CrossRef]
- Howarth, A.J.; Majewski, M.B.; Wolf, M.O. Photophysical properties and applications of coordination complexes incorporating pyrene. Coord. Chem. Rev. 2015, 282, 139–149. [Google Scholar] [CrossRef]
- Lanoe, P.-H.; Chan, J.; Gontard, G.; Monti, F.; Armaroli, N.; Barbieri, A.; Amouri, H. Deep-Red Phosphorescent Iridium(III) Complexes with Chromophoric N-Heterocyclic Carbene Ligands: Design, Photophysical Properties, and DFT Calculations. Eur. J. Inorg. Chem. 2016, 2016, 1631–1634. [Google Scholar] [CrossRef]
- Lanoe, P.-H.; Chan, J.; Groue, A.; Gontard, G.; Jutand, A.; Rager, M.-N.; Armaroli, N.; Monti, F.; Barbieri, A.; Amouri, H. Cyclometalated N-heterocyclic carbene iridium(III) complexes with naphthalimide chromophores: A novel class of phosphorescent heteroleptic compounds. Dalton Trans. 2018, 47, 3440–3451. [Google Scholar] [CrossRef]
- Groue, A.; Montier-Sorkine, E.; Cheng, Y.P.; Rager, M.N.; Jean, M.; Vanthuyne, N.; Crassous, J.; Lopez, A.C.; Moncada, A.S.; Barbieri, A.; et al. Enantiopure, luminescent, cyclometalated Ir(III) complexes with N-heterocyclic carbene-naphthalimide chromophore: Design, vibrational circular dichroism and TD-DFT calculations. Dalton Trans. 2022, 51, 2750–2759. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Li, X.Y.; Liu, S.H.; Zhang, L.T.; Xie, W.F. Near-infrared organic light-emitting materials, devices and applications. Mat. Chem. Front. 2023, 7, 4744–4767. [Google Scholar] [CrossRef]
- Walesa-Chorab, M. Towards red-NIR emission of platinum(II) complexes. J. Photochem. Photobiol. 2024, 59, 100664. [Google Scholar] [CrossRef]
- Unger, Y.; Meyer, D.; Molt, O.; Schildknecht, C.; Muenster, I.; Wagenblast, G.; Strassner, T. Green-Blue Emitters: NHC-Based Cyclometalated [Pt(C^C*)(acac)] Complexes. Angew. Chem. Int. Ed. 2010, 49, 10214–10216. [Google Scholar] [CrossRef]
- Tronnier, A.; Risler, A.; Langer, N.; Wagenblast, G.; Muenster, I.; Strassner, T. A Phosphorescent C^C* Cyclometalated Platinum(II) Dibenzothiophene NHC Complex. Organometallics 2012, 31, 7447–7452. [Google Scholar] [CrossRef]
- Tronnier, A.; Wagenblast, G.; Muenster, I.; Strassner, T. Phosphorescent Platinum(II) Complexes with C^C* Cyclometalated NHC Dibenzofuranyl Ligands: Impact of Different Binding Modes on the Decay Time of the Excited State. Chem. Eur. J. 2015, 21, 12881–12884. [Google Scholar] [CrossRef]
- Stipurin, S.; Strassner, T. Phosphorescent Bimetallic C^C Platinum(II) Complexes with Bridging Substituted Diphenylformamidinates. Chem. Eur. J. 2022, 28, e202202227. [Google Scholar] [CrossRef]
- Auerhammer, N.; Schulz, A.; Schmiedel, A.; Holzapfel, M.; Hoche, J.; Röhr, M.I.S.; Mitric, R.; Lambert, C. Dynamic exciton localisation in a pyrene-BODIPY-pyrene dye conjugate. Phys. Chem. Chem. Phys. 2019, 21, 9013–9025. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d(8) and d(10) Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Moussa, J.; Wong, K.M.-C.; Chamoreau, L.-M.; Amouri, H.; Yam, V.W.-W. Luminescent 1D chain of platinum(II) terpyridyl units with p-dithiobenzoquinone organometallic linker: Self-aggregation imparted from Pt···Pt/π-π interactions. Dalton Trans. 2007, 32, 3526–3530. [Google Scholar] [CrossRef]
- Sesolis, H.; Dubarle-Offner, J.; Chan, C.K.M.; Puig, E.; Gontard, G.; Winter, P.; Cooksy, A.L.; Yam, V.W.W.; Amouri, H. Highly Phosphorescent Crystals of Square-Planar Platinum Complexes with Chiral Organometallic Linkers: Homochiral versus Heterochiral Arrangements, Induced Circular Dichroism, and TD-DFT Calculations. Chem. Eur. J. 2016, 22, 8032–8037. [Google Scholar] [CrossRef]
- Sesolis, H.; Chan, C.K.-M.; Gontard, G.; Fu, H.L.-K.; Yam, V.W.-W.; Amouri, H. Dinuclear (N C N) Pincer Pt(II) Complexes with Bridged Organometallic Linkers: Synthesis, Structures, Self-Aggregation, and Photophysical Properties. Organometallics 2017, 36, 4794–4801. [Google Scholar] [CrossRef]
- Pinter, P.; Soellner, J.; Strassner, T. Photophysical properties of phosphorescent mono- and bimetallic platinum(II) complexes with C^C* cyclometalating NHC ligands. Organometallics 2021, 40, 557–563. [Google Scholar] [CrossRef]
- Pokhrel, M.R.; Bossmann, S.H. Synthesis and Characterization of Poly(NIPAM-co-AA) Polymers Possessing Perfluorinated Side Chains and Chemically Linked Pyrene Labels. J. Nepal. Chem. Soc. 2011, 27, 67–73. [Google Scholar] [CrossRef]
- Tronnier, A.; Poethig, A.; Metz, S.; Wagenblast, G.; Muenster, I.; Strassner, T. Enlarging the π System of Phosphorescent (C^C*) Cyclometalated Platinum(II) NHC Complexes. Inorg. Chem. 2014, 53, 6346–6356. [Google Scholar] [CrossRef]
- Drew, D.; Doyle, J.R.; Shaver, A.G. Cyclic Diolefin Complexes of Platinum and Palladium. Inorg. Synth. 2007, 258, 346–349. [Google Scholar]

| PL in a PMMA Matrix | PL in 2-MeTHF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Complex | λem a [nm] | Φ b [%] | τ0 c [μs] | kr [×103 s−1] d | knr [×103 s−1] e | CIExy f | λem a [nm] | Φ g [%] | CIExy f |
| 3 | 622 | 7 | * | * | * | 0.489; 0.311 | 618 | 12 | 0.400; 0.261 |
| 4 | 619 | 17 | 10 | 105 | 502 | 0.528; 0.330 | 614 | 26 | 0.480; 0.259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Cheng, Y.; Gontard, G.; Riesebeck, T.; Fornal, S.; Strassner, T.; Amouri, H. Design of a Novel Class of N-Heterocyclic Carbene Cycloplatinated Complexes Containing Pyrene Chromophores. Molecules 2025, 30, 4473. https://doi.org/10.3390/molecules30224473
Zhang Z, Cheng Y, Gontard G, Riesebeck T, Fornal S, Strassner T, Amouri H. Design of a Novel Class of N-Heterocyclic Carbene Cycloplatinated Complexes Containing Pyrene Chromophores. Molecules. 2025; 30(22):4473. https://doi.org/10.3390/molecules30224473
Chicago/Turabian StyleZhang, Zeping, Yaping Cheng, Geoffrey Gontard, Tim Riesebeck, Sandy Fornal, Thomas Strassner, and Hani Amouri. 2025. "Design of a Novel Class of N-Heterocyclic Carbene Cycloplatinated Complexes Containing Pyrene Chromophores" Molecules 30, no. 22: 4473. https://doi.org/10.3390/molecules30224473
APA StyleZhang, Z., Cheng, Y., Gontard, G., Riesebeck, T., Fornal, S., Strassner, T., & Amouri, H. (2025). Design of a Novel Class of N-Heterocyclic Carbene Cycloplatinated Complexes Containing Pyrene Chromophores. Molecules, 30(22), 4473. https://doi.org/10.3390/molecules30224473









