Abstract
Background: Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chow. (ZS) is a valuable plant with diverse economic applications, as all its organs contain bioactive secondary metabolites. The seeds, known as Suanzaoren in traditional Chinese medicine, are utilized as both a medicinal and edible resource, while the fruit pulp and leaves serve as significant raw materials in the food industry. Increasing market demand for Suanzaoren has led to expanded cultivation, though current production practices emphasize seed utilization, resulting in the underutilization of pulp and leaf tissues. In agricultural systems, developing elite varieties is an effective strategy for enhancing crop yield and quality. Breeding initiatives should establish specific objectives aligned with particular end uses, such as seed, pulp, or leaf production. Germplasm serves as the foundational material for breeding programs, so its selection must correspond to intended applications. Evaluating existing germplasm resources based on chemical composition profiles will provide a basis for developing improved ZS varieties. Objective: This study aimed to systematically compare the characteristic chemical composition in the seeds, pulp, and leaves of ZS. By quantifying key chemical components—such as flavonoid glycosides and saponins in seeds, organic acids and phenolic compounds in pulp, and flavonol glycosides and phenolic acids in leaves—we evaluated the quality of ZS germplasm resources. The resulting compositional profiles provide a concrete basis for selecting and breeding elite cultivars tailored to specific end uses, including seed, pulp, or leaf production. Methods: Chemical characterization was performed using ultra-high-performance liquid chromatography coupled with hybrid quadrupole-orbitrap mass spectrometry (UPLC-Q-Exactive Orbitrap MS/MS). Quantitative analysis of chemical composition was conducted using high-performance liquid chromatography with evaporative light scattering detection (HPLC-ELSD). Multivariate statistical analyses—including principal component analysis (PCA), orthogonal partial least squares discriminant analysis (OPLS-DA), and entropy-weighted technique for order preference by similarity to an ideal solution (entropy-weighted TOPSIS)(EWT)—were employed for comprehensive data evaluation. Results: A comprehensive phytochemical analysis of Ziziphi spinosae (ZS) was conducted, identifying 144 distinct compounds across the seeds, pulp, and leaves. Of these, 114 were found in the seeds, 84 in the leaves, and 79 in the pulp. The seeds were particularly rich in flavonoid glycosides, such as spinosin and 6‴-feruloylspinosin, as well as saponins like jujuboside A and B. The pulp was dominated by organic acids, including citric acid, and phenolic compounds, while the leaves were abundant in flavonol glycosides, including rutin, and phenolic acids such as isochlorogenic acid B. Based on the chemical composition profiles, the ZS germplasms were evaluated for specific applications. ZS24, ZS22, and ZS3 were identified as the most suitable for seed production, ZS3, ZS6, and ZS9 for pulp utilization, and ZS20, ZS3, and ZS18 for leaf-based applications. With respect to the integrated utilization of multiple plant parts (roots, stems, and leaves), ZS6, ZS3, and ZS24 demonstrated the highest potential. Conclusions: The identification of superior germplasm resources provides strategic direction for the breeding of elite ZS cultivars. These findings will enable the comprehensive utilization of ZS plant resources and support the high-quality development of related industries.
1. Introduction
Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chow., belonging to the Rhamnaceae family, holds significant economic and medicinal value and is found across Asia, Europe, and the Americas [1]. The entire plant possesses medicinal and edible utility: its seed, known as Suanzaoren in traditional Chinese medicine, is a medicinal food homolog. It is sweet in taste and neutral in nature, and is used to nourish the liver, calm the heart, arrest sweating, and promote fluid production, primarily for treating conditions such as insomnia, palpitations, night sweats, and thirst due to fluid deficiency. It also shows potential in alleviating anxiety, depression, and Alzheimer’s disease [2,3,4]. The pulp, rich in vitamins, trace elements, and various bioactive compounds, can tonify Qi and strengthen the spleen, improve complexion, and is consumed fresh or used as a food additive [5,6,7]. The leaves, valued for their nutritional and bioactive content, are used to make tea and are renowned as the “Oriental Sleep Leaf” for their preventive effects against coronary heart disease [8,9].
Modern research has systematically elucidated its chemical constituents and pharmacological activities. Chemically, the seeds are characterized by flavonoid-C-glycosides (e.g., spinosin), dammarane-type triterpenoid saponins (e.g., jujubosides A and B), and cyclic peptide alkaloids [10,11,12]. The pulp is marked by organic acids, polyphenols, and cyclic nucleotides, while the leaves are a rich source of flavonol glycosides (e.g., rutin) and phenolic acids [13,14,15]. Pharmacologically, the sedative-hypnotic effects of the seeds are largely attributed to their saponins and flavonoids, while the cyclic peptide alkaloids are associated with anti-anxiety activity [16,17]. In quality control, alongside the pharmacopoeia-stipulated HPLC methods, UPLC-Q-TOF-MS/MS-based metabolomics has emerged as a vital tool for precise compound identification, germplasm differentiation, and comprehensive quality assessment [2,18,19].
The recent increase in the market value of ZS seeds has driven a focus on seed production, leading to the disposal of significant amounts of pulp and leaf biomass as waste, raising concerns about resource inefficiency and environmental impact [20,21]. This underutilization reveals a key deficiency in the ZS value chain, underscoring the need for cultivars optimized for the comprehensive use of all plant components. This study integrated UPLC-Q-Exactive Orbitrap MS/MS and HPLC-ELSD technologies to systematically profile metabolites, conduct comparative analysis, and evaluate the comprehensive chemical quality of three main parts (seeds, leaves, and pulp) from 26 different Ziziphus jujuba var. spinosa germplasms for the first time. Principal component analysis (PCA), orthogonal partial least squares-discriminant analysis (OPLS-DA), and the entropy-weighted TOPSIS model were employed for multi-indicator decision-making. The research aims to provide a more comprehensive and reliable chemical basis for selecting superior germplasms tailored to specific application targets, thereby holding significant practical implications for promoting high-quality development in the Ziziphus jujuba var. spinosa industry.
2. Results
2.1. Identification and Characterization of Chemical Composition in ZS Tissues
A comprehensive phytochemical analysis of ZS seeds, pulp, and leaves was performed using UPLC-Q-Exactive Orbitrap MS/MS, focusing on retention time, exact mass, and MS/MS fragmentation patterns. A total of 144 compounds were identified, revealing distinct metabolic profiles across tissues: seeds showed the greatest compositional diversity with 114 compounds, compared to 84 in leaves and 79 in pulp (Table 1). All metabolite identifications were assigned a Level 2 confidence rating based on the Metabolomics Standards Initiative (MSI) framework. The chemical composition were categorized into five primary classes: flavonoids (40), terpenoids (21), organic acids (34), alkaloids (15), and amino acids (12), along with trace compounds such as lignans, coumarins, nucleosides, and amides. This detailed compositional profile provides a crucial basis for further quantitative analysis of key chemical composition.

Table 1.
Chemical constituents identified in the seeds, pulp, and leaves of ZS.
2.2. Quantification of Major Chemical Composition Across ZS Germplasms
2.2.1. Validation of the Quantitative Analytical Method
The results of the methodological validation for quantitative analysis of major chemical composition in ZS seeds, pulp, and leaves are presented in Table A1, Appendix A. Excellent linearity (R2 > 0.999) was observed for all calibration curves across the tested concentration ranges. The relative standard deviation (RSD) values for precision, repeatability, and stability were all below 3%. Average recoveries ranged from 97.50% to 102.98%, with RSD values below 3.00%, confirming the validity and reliability of the established method.
2.2.2. Content of Major Chemical Composition in Seeds
The contents of six major chemical composition—cryptochlorogenic acid, caffeic acid, spinosin, 6‴-feruloylspinosin, jujuboside A, and jujuboside B—were quantified in seeds across 26 ZS germplasms (Table A2). Considerable variation was observed among germplasms, with concentration ranges and coefficients of variation (CV) as follows: cryptochlorogenic acid (0.0394–0.1818 mg/g; CV = 43.38%), caffeic acid (0.1842–2.1300 mg/g; CV = 46.62%), spinosin (0.3413–1.8418 mg/g; CV = 35.32%), 6‴-feruloylspinosin (1.4580–5.5312 mg/g; CV = 36.00%), jujuboside A (0.2396–0.9615 mg/g; CV = 36.45%), and jujuboside B (0.1250–0.1942 mg/g; CV = 11.98%). The highest content of each compound was detected in ZS15 (cryptochlorogenic acid), ZS24 (caffeic acid), ZS4 (spinosin), ZS22 (6‴-feruloylspinosin), ZS24 (jujuboside A), and ZS4 (jujuboside B), while the lowest values were observed in ZS20, ZS26, ZS21, ZS20, ZS21, and ZS8, respectively. Germplasm ZS24 exhibited the highest total content of the six chemical composition, whereas ZS21 showed the lowest. Notably, ZS24 also contained the highest levels of spinosin and jujuboside A, two compounds of significant pharmacological relevance, highlighting its potential as a superior germplasm for seed applications.
2.2.3. Content of Major Chemical Composition in Pulp
Quantitative analysis of seven major chemical composition in the pulp—citric acid, gallic acid, catechin, caffeic acid, ferulic acid, rutin, and quercetin-3-O-glucoside—was performed across the 26 germplasms (Table A3). The content ranges and corresponding coefficients of variation were as follows: citric acid (1.4692–20.4395 mg/g; CV = 53.70%), gallic acid (0.0326–0.2219 mg/g; CV = 41.11%), catechin (0.0406–0.1485 mg/g; CV = 30.44%), caffeic acid (0.0017–0.0181 mg/g; CV = 52.41%), ferulic acid (0.0006–0.0081 mg/g; CV = 74.87%), rutin (0.0280–0.3270 mg/g; CV = 58.92%), and quercetin-3-O-glucoside (0.0025–0.0148 mg/g; CV = 28.00%). The germplasms with the highest individual compound content were ZS1 (citric acid), ZS26 (gallic acid), ZS3 (catechin), ZS3 (caffeic acid), ZS11 (ferulic acid), ZS6 (rutin), and ZS3 (quercetin-3-O-glucoside). The lowest values were identified in ZS23, ZS16, ZS7, ZS13, ZS3, ZS26, and ZS16, respectively. ZS1 displayed the highest total content of the seven compounds, while ZS23 exhibited the lowest.
2.2.4. Content of Major Chemical Composition in Leaves
Thirteen major chemical compositions were quantified in the leaves: citric acid, neochlorogenic acid, catechin, caffeic acid, rutin, quercetin-3-O-glucoside, kaempferol-3-O-rutinoside, isochlorogenic acid B, astragalin, quercetin, jujuboside A, jujuboside B, and kaempferol (Table A4). The concentrations and variability among germplasms were as follows: citric acid (0.3820–1.0655 mg/g; CV = 30.81%), neochlorogenic acid (0.0046–0.0478 mg/g; CV = 55.73%), catechin (0.0088–0.1344 mg/g; CV = 93.65%), caffeic acid (0.0126–0.1302 mg/g; CV = 51.23%), rutin (2.8404–18.8823 mg/g; CV = 46.99%), quercetin-3-O-glucoside (0.2564–0.8187 mg/g; CV = 30.71%), kaempferol-3-O-rutinoside (0.0598–0.7043 mg/g; CV = 57.13%), isochlorogenic acid B (0.0024–3.1403 mg/g; CV = 92.25%), astragalin (0.0104–0.1644 mg/g; CV = 62.53%), quercetin (0.0910–0.3637 mg/g; CV = 39.21%), jujuboside A (0.1028–0.8472 mg/g; CV = 52.26%), jujuboside B (0.1099–0.4504 mg/g; CV = 35.05%), and kaempferol (0.2105–1.5576 mg/g; CV = 61.45%). The highest contents were found in ZS20 (citric acid), ZS4 (neochlorogenic acid), ZS3 (catechin), ZS6 (caffeic acid), ZS6 (rutin), ZS20 (quercetin-3-O-glucoside), ZS6 (kaempferol-3-O-rutinoside), ZS18 (isochlorogenic acid B), ZS20 (astragalin), ZS20 (quercetin), ZS11 (jujuboside A), ZS13 (jujuboside B), and ZS20 (kaempferol). The lowest values corresponded to ZS22, ZS3, ZS12, ZS11, ZS7, ZS6, ZS7, ZS12, ZS22, ZS7, ZS15, ZS5, and ZS7, respectively. Germplasm ZS6 exhibited the highest total content of the thirteen compounds, while ZS7 showed the lowest.
2.3. Quality Evaluation of ZS Germplasm
ZS possesses significant economic value due to its well-documented medicinal applications across multiple plant organs. The seeds, commercially known as Suanzaoren, are established therapeutic agents and nutritional supplements. Similarly, the pulp is directly consumable or serves as a key raw material in food processing, while the leaves are traditionally used in herbal tea production. Consequently, quality evaluation of ZS germplasm must be rigorously aligned with these specific end-use applications to ensure functional efficacy and economic viability.
2.3.1. Quality Evaluation of Seed-Use ZS Germplasm Based on Major Seed Chemical Composition
The clinical effectiveness of Suanzaoren is fundamentally determined by the composition and concentration of its chemical composition. Therefore, quantifying these constituents is essential for robust quality assessment of seed-oriented ZS germplasm.
Hierarchical Cluster Analysis (HCA)
HCA was conducted using the concentrations of six major chemical composition in seeds as classification variables, resulting in a cluster heatmap that delineates the 26 ZS germplasms (Figure A1A). In this heatmap, color intensity directly corresponds to relative abundance, with red and green indicating high and low levels, respectively. Based on distinct phytochemical profiles, the germplasms were decisively segregated into three groups: A, B, and C. Group A, containing the highest chemical composition, included ZS24, ZS3, and ZS22. Group B, with intermediate levels, comprised ZS4, ZS13, ZS14, ZS1, ZS15, ZS16, ZS6, ZS10, ZS17, ZS19, ZS18, and ZS9. Group C, showing the lowest accumulation, contained the remaining accessions. Each cluster demonstrated strong internal consistency, validating the classification reliability.
Principal Component Analysis (PCA)
To further verify the cluster structure, PCA was performed using the same six chemical composition. Data processed through SIMCA 14.1 produced two principal components explaining R2X [1] = 0.680 and R2X [2] = 0.128 of total variance. The resulting score plot (Figure 1A) conclusively affirmed the HCA-derived classification, similarly separating germplasms into three discrete groups, thereby reinforcing the robustness of the identified clusters.
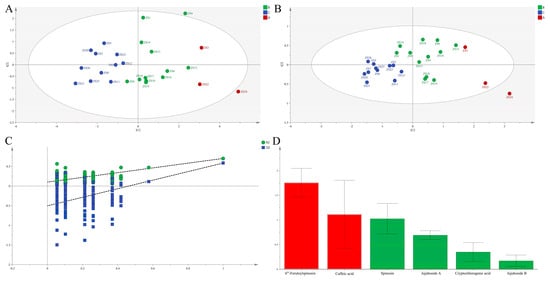
Figure 1.
PCA score plots (A) and OPLS-DA score plots (B), permutation validation (C), and variable importance in projection (VIP) (D) analysis of different ZS germplasms based on chemical composition in seeds.
Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
To minimize within-group variation and identify discriminatory chemical composition, OPLS-DA was applied following PCA. The model exhibited strong explanatory power, with R2X = 0.985, R2Y = 0.540, and Q2 = 0.324 (Figure 1B). Clear group separation in the score plot confirmed significant inter-germplasm heterogeneity in chemical composition. Model validity was unequivocally established through a rigorous 200-iteration permutation test, which effectively ruled out overfitting. VIP analysis definitively identified two key discriminatory compounds—6‴-feruloylspinosin and caffeic acid—both with VIP values exceeding 1.
Entropy-Weighted TOPSIS Analysis (EWT)
Significant chemotypic diversity was observed among the 26 germplasms based on the six major chemical composition. To comprehensively evaluate overall quality, an EWT approach was employed. Weighting coefficients were systematically derived from compound abundance data using the entropy method. Subsequent normalization of the raw data matrix was performed according to Equation (1), as all indicators were beneficial. Indicator proportions and respective weights were calculated using Equations (2)–(5), followed by the construction of a weighted normalization matrix. Positive and negative ideal solutions were definitively established using Equations (6) and (7), respectively. Euclidean distances to these ideals and relative closeness coefficients (Cᵢ) were calculated using Equations (8)–(10), with higher Cᵢ values unequivocally indicating superior germplasm quality. The eight elite accessions—ZS24, ZS22, ZS3, ZS15, ZS6, ZS16, ZS4, and ZS10—achieved a mean Cᵢ of 0.627, confirming their superior phytochemical quality. These results demonstrate strong concordance with the HCA-based classification (Figure 2A).
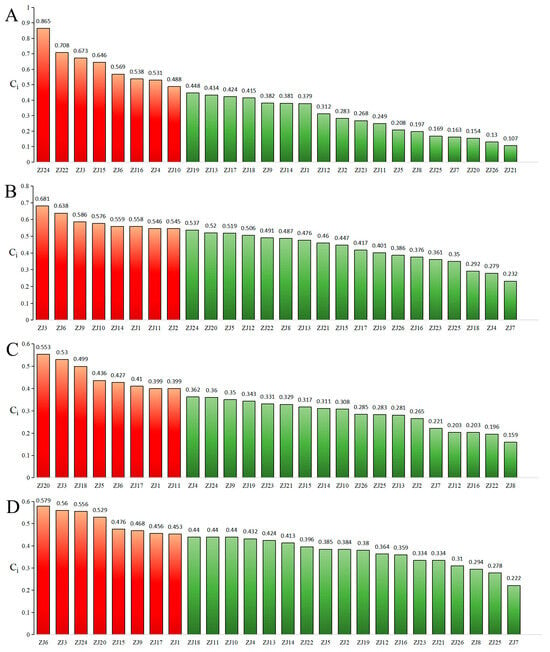
Figure 2.
Comprehensive quality ranking of ZS germplasms based on chemical composition ((A): seed-use; (B): pulp-use; (C): leaf-use; (D): comprehensive utilization).
Yij: Data normalization; : the original data; : the characteristic weight; : the entropy value; : the utility value; wj: the weight; : the positive ideal solution; : the negative ideal solution; : the positive ideal distance; : the negative ideal distance; : the closeness coefficient.
2.3.2. Quality Evaluation of Pulp-Use ZS Germplasm Based on Major Chemical Composition in Pulp
The health benefits of ZS pulp consumption are directly attributable to its chemical composition. Thus, precise quantification of these compounds is crucial for evaluating pulp-use germplasm quality.
HCA
HCA conducted using seven major chemical compositions in pulp generated a cluster heatmap of germplasms (Figure A1B), revealing substantial inter-germplasm variation. Germplasms were classified into four groups (D–G). Group D, with the highest content, included ZS3, ZS6, ZS10, ZS9, and ZS24; Group E, showing moderately high levels, contained ZS11, ZS12, and ZS13; Group F demonstrated intermediate accumulation (ZS5, ZS21, ZS26, ZS23, ZS25); Group G, with minimal content, comprised the remaining accessions.
PCA
PCA based on the seven compounds yielded R2X [1] = 0.350 and R2X [2] = 0.237 (Figure 3A), robustly corroborating the four-group structure identified by HCA.
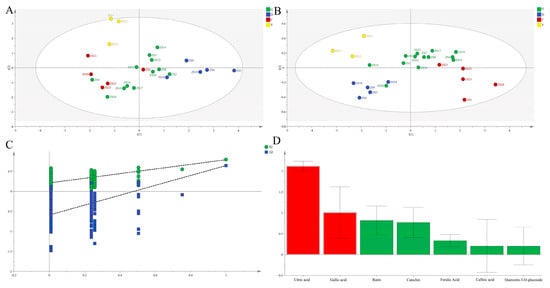
Figure 3.
PCA score plots (A) and OPLS-DA score plots (B), permutation validation (C), and variable importance in projection (VIP) (D) analysis of different ZS germplasms based on chemical composition in pulp.
OPLS-DA
Subsequent OPLS-DA modeling demonstrated excellent goodness-of-fit (R2X = 1.000, R2Y = 0.735, Q2 = 0.459; Figure 3B), revealing significant inter-group phytochemical differences. Model robustness was confirmed through permutation testing. Citric acid and gallic acid (VIP >1) were definitively identified as key discriminators.
EWT
EWT analysis clearly identified ZS3, ZS6, ZS9, ZS10, ZS14, ZS1, ZS11, and ZS2 as superior germplasms (mean Cᵢ = 0.586), consistent with HCA classification (Figure 2B).
2.3.3. Quality Evaluation of Leaf-Use ZS Germplasm Based on Major Chemical Composition in Leaves
ZS leaves are utilized in functional tea production, where chemical composition serve as primary quality determinants. Therefore, their accumulation levels are a critical criterion for evaluating leaf-use germplasm.
HCA
HCA using 13 leaf chemical composition effectively classified germplasms into three distinct groups (H–J) (Figure A1C). Group H (highest content) included ZS20 and ZS6; Group I (intermediate) comprised eight accessions, included ZS18, ZS17, ZS4, ZS24, ZS9, ZS15, ZS11, and ZS1.; Group J (lowest) contained the remainder.
PCA
PCA (R2X [1] = 0.406, R2X [2] = 0.138; Figure 4A) conclusively affirmed the three-group classification.
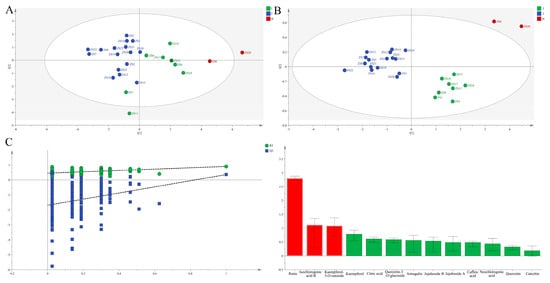
Figure 4.
PCA score plots (A) and OPLS-DA score plots (B), permutation validation (C), and variable importance in projection (VIP) (D) analysis of different ZS germplasms based on chemical composition in leaves.
OPLS-DA
OPLS-DA (R2X = 1.000, R2Y = 0.870, Q2 = 0.226; Figure 4B) confirmed significant inter-group differentiation. Rutin, isochlorogenic acid B, and kaempferol-3-O-rutinoside (VIP > 1) were identified as discriminatory markers.
EWT
TOPSIS analysis definitively identified ZS20, ZS3, ZS18, ZS5, ZS6, ZS17, ZS1, and ZS11 as elite germplasms (mean Cᵢ = 0.457), aligning with HCA groupings (Figure 2C).
2.3.4. Comprehensive Quality Evaluation of ZS Germplasm Integrating Major Chemical Composition from Seeds, Pulp, and Leaves
Conventional germplasm evaluation typically focuses on single-organ utilization, primarily seeds. However, exclusive reliance on seeds may lead to diminished economic returns with expanded cultivation, highlighting the necessity of whole-plant valorization. A comprehensive quality assessment using EWT incorporated chemical composition from all three organs as positive indicators (Figure 2D). The top-eight germplasms—ZS6, ZS3, ZS24, ZS20, ZS15, ZS9, ZS17, and ZS1 (mean Cᵢ = 0.510)—were conclusively identified as possessing superior holistic quality, underscoring their significant potential in breeding programs aimed at multi-purpose crop development.
3. Discussion
Ziziphus jujuba var. spinosa is an economically significant crop with well-established medicinal value. While its seeds (Suanzaoren) are a cornerstone of traditional Chinese medicine, the pulp and leaves also contain potentially bioactive metabolites, offering substantial potential for edible and medicinal applications. This study employed an integrated approach using UPLC-Q-Exactive Orbitrap MS/MS and HPLC-ELSD to systematically characterize the chemical constituents in these three plant parts. A total of 144 compounds were identified, revealing the highest phytochemical diversity in the seeds (114 compounds), followed by the leaves (84 compounds) and pulp (79 compounds). These findings provide a chemical basis confirming the seeds as the primary medicinal component, while also highlighting the substantial potential for broader utilization of the pulp and leaves.
The three plant parts exhibited distinct chemical profiles supporting their differential applications: Seeds were rich in flavonoid glycosides and saponins, with 6‴-feruloylspinosin being the most abundant (1.46–5.53 mg/g). The feruloyl moiety in this compound enhances blood–brain barrier permeability [1,53] and, together with structurally related compounds like spinosin, contributes to the sedative, hypnotic, anxiolytic, and cognitive-enhancing effects of Suanzaoren, including documented potential in Alzheimer’s disease management [54,55]. Pulp was characterized by high levels of organic acids and polyphenols. Its elevated citric acid content imparts a characteristic sourness while providing preservative, antioxidant, and flavor-enhancing properties [56]. Phenolic compounds further strengthen its antioxidant capacity, supporting its traditional use as a food additive, acidulant, and nutritional supplement rich in polysaccharides, amino acids, and vitamins [57]. Leaves contained substantial amounts of flavonol glycosides, known to confer anti-inflammatory, anti-allergic, and antioxidant effects, in addition to enhancing capillary resilience [58,59,60].
4. Materials and Methods
4.1. Materials
4.1.1. Plant Material
Samples of ZS were collected from a standardized cultivation base operated by Shandong Zhongchang Yuan Investment Group Co., Ltd., located in the Yellow River Delta Agricultural High-tech Industrial Demonstration Zone, Dongying City, Shandong Province, China. This site was selected to ensure environmental consistency and genetic representativeness of the samples.
A total of twenty-six germplasms (labeled ZS1 to ZS26) were selected based on observable phenotypic variation among the plants. On 12 August 2024, fruits and leaves were randomly harvested from each germplasm to reduce sampling bias. Samples were rinsed with purified water and air-dried. The pulp was manually separated from the endocarp, and seeds were extracted by mechanically cracking the stones. Seeds, pulp, and leaves were dried to constant weight at 50 °C, ground into a fine powder with an electric grinder, sieved through a 60-mesh sieve, and stored in a desiccator for further analysis.
Taxonomic authentication was authoritatively performed by Professor Yongqing Zhang of Shandong University of Traditional Chinese Medicine, which confirmed all germplasms as Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chow. (family Rhamnaceae). These accessions represent distinct germplasms primarily distinguished by macroscopic morphological differences in plant and fruit characteristics.
4.1.2. Chemicals and Reagents
All reference compounds—including cryptochlorogenic acid (1), caffeic acid (2), spinosin (3), 6‴-feruloylspinosin (4), jujuboside A (5), jujuboside B (6), citric acid (7), gallic acid (8), catechin (9), ferulic acid (10), rutin (11), quercetin-3-O-glucoside (12), neochlorogenic acid (13), kaempferol-3-O-rutoside (14), isochlorogenic acid B (15), astragalin (16), quercetin (17), and kaempferol (18)—were sourced from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Each compound was rigorously certified to possess a purity of ≥98% through HPLC analysis, thereby ensuring reliability and reproducibility of analytical results.
High-purity formic acid (guaranteed reagent grade) and HPLC-grade acetonitrile were procured from Thermo Fisher Scientific (Waltham, MA, USA), while purified water was supplied by Watsons Food & Beverage Co., Ltd. (Guangzhou, China).
4.2. Methods
4.2.1. Preparation of Sample Solutions
Accurately weighed powdered samples (1.0 g) were transferred into sealed conical flasks and combined with 10 mL of methanol. The flasks were reweighed to account for any solvent loss, then subjected to optimized ultrasonication (KQ-500DE CNC Ultrasonic Cleaner, Jiangsu, China) at 30 °C for 60 min (500 W, 40 kHz) to maximize compound extraction. After cooling to room temperature, the total weight was recorded, and methanol was added to compensate for any solvent loss, ensuring volumetric accuracy. The mixtures were thoroughly vortexed to achieve homogeneity, centrifuged (SorvallST8R, Thermo Scientific, Waltham, MA, USA) at 12,000× g r/min for 15 min to remove particulates, and the supernatants were filtered through 0.22 μm membranes to eliminate residual impurities prior to chromatographic analysis.
4.2.2. Preparation of Standard Solutions
Individual stock solutions were prepared by dissolving accurately weighed reference standards in methanol, followed by dilution to volume in calibrated volumetric flasks to ensure precise concentration levels. The concentrations of each stock solution were established as follows (in mg/mL): (1) 0.960, (2) 1.200, (3) 0.990, (4) 1.090, (5) 0.960, (6) 0.950, (7) 1.292, (8) 0.980, (9) 0.985, (10) 1.075, (11) 2.250, (12) 1.020, (13) 1.115, (14) 1.130, (15) 1.080, (16) 1.090, (17) 1.120, and (18) 1.030.
Working standard solutions were prepared by diluting aliquots of stock solutions with methanol to achieve concentrations within the linear range of detection. All solutions were stored at 4 °C and filtered through a 0.22 μm membrane before injection to prevent column contamination and ensure analytical consistency.
4.2.3. UPLC-Q-Exactive-Orbitrap-MS/MS Conditions for Qualitative Analysis
Chromatographic separation was performed using a Thermo Vanquish-Orbitrap Exploris 120 system equipped with a HALO C18 column (2.1 mm × 100 mm, 2.7 μm; Agilent, Santa Clara, CA, USA). The mobile phase consisted of 0.1% formic acid in water (phase A) and 0.1% formic acid in acetonitrile (phase B), delivered at a constant flow rate of 0.3 mL/min with the column temperature maintained at 30 °C. The gradient elution profile was programmed as follows: 0–3 min, 5–11% B; 3–3.5 min, 11–14% B; 3.5–11.5 min, 14–33% B; 11.5–12 min, 33–36% B; 12–15 min, 36–44% B; 15–18 min, 44–74% B; 18–26 min, 74–95% B; 26–28 min, 95% B.
Ionization was performed using a heated electrospray ionization (HESI) source in both positive and negative ion modes. The parameters were optimized for sensitivity and spectral accuracy: capillary voltage at 350 V, capillary and vaporizer temperatures at 350 °C, mass range m/z 80–121, resolution at 70,000, S-Lens RF level at 55, sheath gas flow at 50 arb, and auxiliary gas flow at 10 arb.
Figure 5 comprehensively illustrates representative chromatograms of seed, pulp, and leaf extracts in both ionization modes, providing a clear basis for compound identification.
Figure 5.
Base peak chromatograms (BPCs) of seed, pulp, and leaf extracts from S acquired in positive (left) and negative (right) ion modes.
4.2.4. HPLC-ELSD Conditions for Quantitative Analysis
Quantitative analysis was conducted using an Agilent 1260 Infinity II HPLC system coupled with an Agilent 1290 Infinity II Evaporative Light Scattering Detector (ELSD). Separation was achieved on a ZORBAX SB-C18 column (4.6 mm × 250 mm, 5 μm; Agilent, Santa Clara, CA, USA) maintained at 30 °C, with a mobile phase flow rate of 1.0 mL/min. The mobile phase comprised 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient elution conditions were specifically optimized for each tissue type: Seeds: 0–5 min, 15–25% B; 5–8 min, 25–36% B; 8–9 min, 36–39% B; 9–17 min, 39–42% B; 17–18 min, 42–43% B. Pulp: 0–15 min, 5–13% B; 15–30 min, 13–15% B; 30–45 min, 15–20% B. Leaves: 0–11 min, 5–25% B; 11–12 min, 25–29% B; 12–20 min, 29–50% B; 20–24 min, 50–73% B.
Chromatograms for reference standards and sample extracts are explicitly provided in Figure 6 and Figure 7, respectively, facilitating direct comparative analysis.
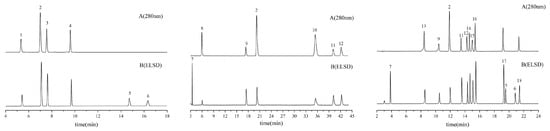
Figure 6.
Representative HPLC-ELSD chromatogram of the mixed reference standard solution.
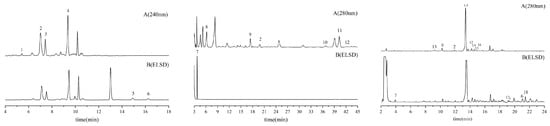
Figure 7.
Representative HPLC-ELSD chromatograms of seed (left), pulp (middle), and leaf (right) extracts from ZS under detection conditions: A. 240 nm and B. ELSD.1 Cryptochlorogenic acid, 2 Caffeic acid, 3 Spinosin, 4 6‴-Feruloylspinosin, 5 Jujuboside A, 6 Jujuboside B,7 Citric acid, 8Gallic acid, 9 Catechin, 10 Ferulic Acid, 11 Rutin, 12 Quercetin-3-O-glucoside, 13 Neochlorogenic acid, 14 Kaempferol-3-O-rutoside, 15 Isochlorogenic acid B, 16 Astragalin, 17 Quercetin, 18 Kaempferol.
4.2.5. Validation of the Quantitative Method
The analytical method was comprehensively validated for linearity, precision, stability, repeatability, and recovery in accordance with ICH guidelines. A six-point calibration curve was constructed through serial dilution of a mixed standard solution, and linear regression analysis was performed by plotting peak area (Y) against concentration (X), consistently yielding correlation coefficients (R2) exceeding 0.999. Precision was unequivocally demonstrated through six consecutive injections of the same standard, resulting in RSD values below 2%. Stability was assessed by analyzing identical samples over 0, 2, 4, 8, 12, and 24 h, confirming analyte integrity under storage conditions. Repeatability was validated using six independently prepared sample replicates, with RSD values consistently under 3%. Recovery tests were performed using spiked samples in six replicates, yielding recovery rates between 97.5% and 102.5%, calculated as:
Recovery (%) = (measured amount in spiked sample − original amount)/amount added × 100%
4.2.6. Statistical Analysis
Qualitative data were analyzed using Xcalibur™ 4.3 software (Thermo Fisher Scientific, Waltham, MA, USA) with a mass accuracy tolerance of ≤10 ppm. Multivariate analyses, including PCA and OPLS-DA, were performed in SIMCA 14.1 (Umetrics AB, Umea, Sweden). HCA and heatmap visualization were carried out using the Metware Cloud platform (https://cloud.metware.cn, accessed on 18 July 2025). EWT was executed in Microsoft Excel 2021.
5. Conclusions
Building on the distinct chemical profiles established in this study, elite germplasms were successfully identified for specific applications through multi-component quantification. The optimal germplasms for different utilization purposes were determined as follows: ZS24, ZS3, and ZS22 were optimal for seed production; ZS3, ZS6, and ZS9 for pulp utilization; and ZS20, ZS3, and ZS18 for leaf applications. For integrated use of all three organs, ZS6, ZS3, and ZS24 demonstrated superior performance.
The recent market-driven expansion in ZS cultivation necessitates sustainable development strategies as supply approaches demand. This study provides the first systematic chemical profiling of three plant parts across multiple germplasms, establishing a scientific foundation for breeding specialized cultivars. Our findings not only advance the understanding of ZS’s chemical composition but also directly support the high-value, comprehensive utilization of Ziziphus jujuba var. spinosa resources for sustainable industrial development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30224470/s1.
Author Contributions
Conceptualization, L.Z. and Y.Z.; methodology, L.Z. and Y.Z.; software, X.S.; validation, L.Z., Y.Z. and X.S.; formal analysis, X.S.; investigation, X.S.; resources, Y.Z.; data curation, X.S.; writing—original draft preparation, X.S.; writing—review and editing, L.Z. and Y.Z.; visualization, X.S.; supervision, Y.Z.; project administration, L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by Research on the Quality Improvement of Ziziphi spinosae Seed as a Medicinal Material, SYZX2025043.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Results of methodological validation for the quantitative analysis of major chemical composition in the seeds, pulp, and leaves of ZS.
Table A1.
Results of methodological validation for the quantitative analysis of major chemical composition in the seeds, pulp, and leaves of ZS.
| Part | NO. | Compounds | Regression Equation | R2 | Linear Range (mg/mL) | Precision (RSD%; n = 6) | Repeatability (RSD%; n = 6) | Stability (RSD%; n = 6) | Recovery | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | RSD% | |||||||||
| Seed | 1 | Cryptochlorogenic acid | y = 181.7x − 8.2576 | 0.999 | 4.80 × 10−5–9.60 × 10−4 | 0.63 | 0.29 | 2.93 | 98.48 | 0.63 |
| 2 | Caffeic acid | y = 331.74x + 16.201 | 0.999 | 1.20 × 10−3–2.40 × 10−2 | 0.57 | 0.52 | 2.82 | 98.01 | 0.01 | |
| 3 | Spinosin | y = 169.99x + 9.557 | 1.000 | 9.90 × 10−4–1.98 × 10−2 | 0.22 | 0.07 | 0.97 | 99.28 | 1.08 | |
| 4 | 6‴-Feruloylspinosin | y = 142.31x + 7.0431 | 0.999 | 1.09 × 10−3–2.18 × 10−2 | 0.31 | 0.03 | 0.98 | 99.14 | 0.85 | |
| 5 | Jujuboside A | y = 20.72x − 44.533 | 0.999 | 9.60 × 10−4–1.92 × 10−2 | 0.37 | 0.21 | 2.99 | 98.51 | 0.32 | |
| 6 | Jujuboside B | y = 16.945x − 37.072 | 0.999 | 9.50 × 10−4–1.90 × 10−2 | 0.44 | 0.32 | 2.64 | 99.20 | 1.05 | |
| Pulp | 7 | Citric acid | y = 259.6x + 95.312 | 0.999 | 1.48 × 10−3–4.44 × 10−2 | 1.82 | 2.91 | 1.45 | 99.55 | 2.05 |
| 8 | Gallic acid | y = 194.22x − 4.8952 | 0.999 | 1.41 × 10−5–1.41 × 10−3 | 0.35 | 2.64 | 2.08 | 100.79 | 0.93 | |
| 9 | Catechin | y = 85.116x + 10.774 | 0.999 | 1.34 × 10−5–1.34 × 10−3 | 0.43 | 2.34 | 2.03 | 100.11 | 0.50 | |
| 2 | Caffeic acid | y = 484.79x + 11.078 | 0.999 | 1.49 × 10−6–2.97 × 10−4 | 0.38 | 2.78 | 2.36 | 102.12 | 0.38 | |
| 10 | Ferulic Acid | y = 439.01x + 12.786 | 0.999 | 1.54 × 10−6–3.07 × 10−4 | 0.57 | 1.42 | 1.44 | 100.75 | 0.97 | |
| 11 | Rutin | y = 91.964x + 13.828 | 0.999 | 1.34 × 10−5–1.34 × 10−3 | 0.47 | 1.96 | 2.44 | 99.76 | 1.35 | |
| 12 | Quercetin-3-O-glucoside | y = 129x + 6.4427 | 0.999 | 7.29 × 10−6–7.29 × 10−4 | 0.42 | 2.95 | 2.78 | 102.32 | 2.06 | |
| Leaf | 7 | Citric acid | y = 22.597x − 28.666 | 0.999 | 2.59 × 10−4–2.59 × 10−3 | 1.98 | 2.98 | 2.92 | 101.40 | 2.97 |
| 13 | Neochlorogenic acid | y = 16.42x + 3.6476 | 0.999 | 5.58 × 10−6–1.12 × 10−4 | 0.89 | 2.97 | 2.03 | 101.10 | 2.55 | |
| 9 | Catechin | y = 68.325x + 2.3997 | 0.999 | 1.97 × 10−5–7.88 × 10−4 | 0.34 | 2.89 | 2.90 | 102.12 | 0.66 | |
| 2 | Caffeic acid | y = 62.608x + 8.4197 | 0.999 | 9.85 × 10−6–3.94 × 10−4 | 0.54 | 2.99 | 2.59 | 100.50 | 2.85 | |
| 11 | Rutin | y = 1371.3x + 1479 | 0.999 | 5.50 × 10−3–5.50 × 10−2 | 0.43 | 2.92 | 2.73 | 99.86 | 2.64 | |
| 12 | Quercetin-3-O-glucoside | y = 92.852x + 9.7727 | 0.999 | 2.04 × 10−4–2.04 × 10−3 | 0.46 | 2.34 | 2.31 | 100.14 | 2.71 | |
| 14 | Kaempferol-3-O-rutoside | y = 108.22x + 13.042 | 0.999 | 5.65 × 10−5–2.26 × 10−3 | 0.15 | 2.99 | 2.74 | 102.98 | 0.80 | |
| 15 | Isochlorogenic acid B | y = 1898.4x + 29.204 | 1.000 | 1.08 × 10−6–1.08 × 10−2 | 2.95 | 2.32 | 2.72 | 97.50 | 1.02 | |
| 16 | Astragalin | y = 35.406x + 2.4954 | 0.999 | 1.09 × 10−5–4.36 × 10−4 | 2.94 | 2.82 | 2.86 | 101.46 | 2.66 | |
| 17 | Quercetin | y = 100.09x − 122.13 | 0.999 | 1.12 × 10−4–1.68 × 10−3 | 2.96 | 2.71 | 2.72 | 101.74 | 0.47 | |
| 5 | Jujuboside A | y = 46.259x − 55.494 | 0.999 | 1.18 × 10−4–2.35 × 10−3 | 2.21 | 2.94 | 2.09 | 100.73 | 1.90 | |
| 6 | Jujuboside B | y = 29.055x − 28.964 | 0.999 | 1.07 × 10−4–2.13 × 10−3 | 1.41 | 2.74 | 2.51 | 100.76 | 2.01 | |
| 18 | Kaempferol | y = 102.2x − 81.167 | 0.999 | 1.03 × 10−4–4.12 × 10−3 | 2.21 | 2.69 | 2.45 | 102.65 | 0.83 | |

Table A2.
Contents of major chemical composition in seeds of different ZS germplasms (n = 3; mg/g).
Table A2.
Contents of major chemical composition in seeds of different ZS germplasms (n = 3; mg/g).
| No. | Cryptochlorogenic Acid | Caffeic Acid | Spinosin | 6‴-Feruloyl-Spinosyn | Jujuboside A | Jujuboside B | Total Content |
|---|---|---|---|---|---|---|---|
| ZS1 | 0.1062 | 0.7112 | 1.2886 | 2.3730 | 0.3141 | 0.1938 | 4.9869 |
| ZS2 | 0.0727 | 0.6286 | 0.7910 | 2.2690 | 0.4826 | 0.1521 | 4.3960 |
| ZS3 | 0.1646 | 0.9589 | 1.4800 | 5.0200 | 0.6344 | 0.1938 | 8.4517 |
| ZS4 | 0.0725 | 1.0770 | 1.8418 | 3.9023 | 0.6084 | 0.1942 | 7.6962 |
| ZS5 | 0.0587 | 0.5209 | 0.8595 | 1.8880 | 0.3145 | 0.1618 | 3.8034 |
| ZS6 | 0.1395 | 0.6972 | 1.3440 | 3.6170 | 0.7288 | 0.1564 | 6.6829 |
| ZS7 | 0.0565 | 0.6188 | 0.8110 | 1.6747 | 0.2615 | 0.1494 | 3.5719 |
| ZS8 | 0.0623 | 0.6414 | 1.0281 | 2.5806 | 0.2980 | 0.1250 | 4.7354 |
| ZS9 | 0.1281 | 0.5376 | 0.8448 | 2.8698 | 0.4869 | 0.1448 | 5.0120 |
| ZS10 | 0.1466 | 0.5632 | 1.1062 | 2.8243 | 0.6565 | 0.1462 | 5.4430 |
| ZS11 | 0.0741 | 0.8066 | 0.7994 | 2.9534 | 0.3466 | 0.1328 | 5.1129 |
| ZS12 | 0.0537 | 0.6482 | 0.9176 | 3.7911 | 0.4211 | 0.1499 | 5.9816 |
| ZS13 | 0.0602 | 0.7359 | 1.3451 | 4.6438 | 0.5297 | 0.1584 | 7.4731 |
| ZS14 | 0.0909 | 0.6125 | 1.4105 | 2.7143 | 0.5073 | 0.1621 | 5.4976 |
| ZS15 | 0.1818 | 0.9821 | 1.4245 | 3.8416 | 0.6744 | 0.1721 | 7.2765 |
| ZS16 | 0.1577 | 0.7805 | 1.1325 | 4.0440 | 0.5537 | 0.1585 | 6.8269 |
| ZS17 | 0.1129 | 1.0943 | 0.9379 | 3.2048 | 0.4883 | 0.1541 | 5.9923 |
| ZS18 | 0.1069 | 0.8839 | 0.7133 | 3.7788 | 0.4744 | 0.1578 | 6.1151 |
| ZS19 | 0.1139 | 0.9753 | 0.7203 | 3.9951 | 0.4863 | 0.1590 | 6.4499 |
| ZS20 | 0.0394 | 0.5856 | 0.7227 | 1.4580 | 0.2964 | 0.1523 | 3.2544 |
| ZS21 | 0.0639 | 0.5653 | 0.3413 | 1.5143 | 0.2396 | 0.1308 | 2.8552 |
| ZS22 | 0.1498 | 1.4030 | 0.9837 | 5.5312 | 0.7107 | 0.1792 | 8.9576 |
| ZS23 | 0.0592 | 0.6142 | 0.9316 | 2.6552 | 0.4238 | 0.1551 | 4.8391 |
| ZS24 | 0.1591 | 2.1300 | 1.6016 | 5.1558 | 0.9615 | 0.1695 | 10.1775 |
| ZS25 | 0.0801 | 0.4998 | 0.6658 | 2.0807 | 0.2968 | 0.1283 | 3.7515 |
| ZS26 | 0.0497 | 0.1842 | 0.4621 | 2.2039 | 0.3340 | 0.1410 | 3.3749 |
| CV(%) | 43.38 | 46.62 | 35.32 | 36.00 | 36.45 | 11.98 |

Table A3.
Contents of major chemical composition in pulp of different ZS germplasms (n = 3; mg/g).
Table A3.
Contents of major chemical composition in pulp of different ZS germplasms (n = 3; mg/g).
| No. | Citric Acid | Gallic Acid | Catechin | Caffeic Acid | Ferulic Acid | Rutin | Quercetin-3-O-Glucoside | Total Content |
|---|---|---|---|---|---|---|---|---|
| ZS1 | 20.4395 | 0.1283 | 0.0878 | 0.0069 | 0.0042 | 0.1586 | 0.0125 | 20.8378 |
| ZS2 | 12.6134 | 0.0907 | 0.1239 | 0.0146 | 0.0023 | 0.1455 | 0.0124 | 13.0028 |
| ZS3 | 18.0934 | 0.1619 | 0.1485 | 0.0181 | 0.0006 | 0.3071 | 0.0148 | 18.7444 |
| ZS4 | 7.2853 | 0.0814 | 0.0738 | 0.0018 | 0.0025 | 0.0533 | 0.0091 | 7.5072 |
| ZS5 | 10.0898 | 0.2129 | 0.1340 | 0.0030 | 0.0032 | 0.1538 | 0.0093 | 10.606 |
| ZS6 | 14.6059 | 0.1527 | 0.1240 | 0.0136 | 0.0011 | 0.3270 | 0.0137 | 15.238 |
| ZS7 | 5.2575 | 0.0778 | 0.0406 | 0.0089 | 0.0007 | 0.0855 | 0.0063 | 5.4773 |
| ZS8 | 13.9532 | 0.1101 | 0.1133 | 0.0150 | 0.0009 | 0.1079 | 0.0090 | 14.3094 |
| ZS9 | 16.7025 | 0.1355 | 0.1187 | 0.0111 | 0.0014 | 0.2884 | 0.0101 | 17.2677 |
| ZS10 | 13.7535 | 0.0947 | 0.1371 | 0.0131 | 0.0007 | 0.3081 | 0.0114 | 14.3186 |
| ZS11 | 18.1177 | 0.1323 | 0.0413 | 0.0175 | 0.0081 | 0.1208 | 0.0062 | 18.4439 |
| ZS12 | 17.0808 | 0.0799 | 0.0936 | 0.0078 | 0.0076 | 0.2349 | 0.0025 | 17.5071 |
| ZS13 | 14.4683 | 0.0714 | 0.0739 | 0.0017 | 0.0075 | 0.2126 | 0.0091 | 14.8445 |
| ZS14 | 17.0504 | 0.1662 | 0.1129 | 0.0148 | 0.0028 | 0.1450 | 0.0063 | 17.4984 |
| ZS15 | 13.0702 | 0.0674 | 0.1149 | 0.0095 | 0.0018 | 0.2273 | 0.0059 | 13.497 |
| ZS16 | 8.8191 | 0.0326 | 0.1123 | 0.0017 | 0.0024 | 0.1333 | 0.0114 | 9.1128 |
| ZS17 | 3.4939 | 0.0597 | 0.1281 | 0.0109 | 0.0027 | 0.0834 | 0.0107 | 3.7894 |
| ZS18 | 2.4508 | 0.0464 | 0.0983 | 0.0056 | 0.0011 | 0.0428 | 0.0103 | 2.6553 |
| ZS19 | 4.1632 | 0.1063 | 0.0947 | 0.0106 | 0.0021 | 0.0596 | 0.0120 | 4.4485 |
| ZS20 | 4.9013 | 0.1578 | 0.0803 | 0.0163 | 0.0022 | 0.2230 | 0.0112 | 5.3921 |
| ZS21 | 6.9057 | 0.1775 | 0.0510 | 0.0077 | 0.0070 | 0.0678 | 0.0103 | 7.227 |
| ZS22 | 12.9828 | 0.1075 | 0.0840 | 0.0112 | 0.0036 | 0.1039 | 0.0122 | 13.3052 |
| ZS23 | 1.4692 | 0.1441 | 0.0815 | 0.0041 | 0.0028 | 0.0831 | 0.0112 | 1.796 |
| ZS24 | 10.1719 | 0.1142 | 0.1165 | 0.0087 | 0.0023 | 0.2351 | 0.0131 | 10.6618 |
| ZS25 | 6.5215 | 0.1217 | 0.0835 | 0.0038 | 0.0023 | 0.0653 | 0.0108 | 6.8089 |
| ZS26 | 2.2088 | 0.2219 | 0.0586 | 0.0090 | 0.0025 | 0.0280 | 0.0081 | 2.5369 |
| CV(%) | 53.70 | 41.11 | 30.44 | 52.41 | 74.87 | 58.92 | 28.00 |

Table A4.
Contents of major chemical composition in leaves of different ZS germplasms (n = 3; mg/g).
Table A4.
Contents of major chemical composition in leaves of different ZS germplasms (n = 3; mg/g).
| No. | Citric Acid | Neochlorogenic Acid | Catechin | Caffeic Acid | Rutin | Quercetin-3-O-Glucoside | Kaempfer-ol-3-O-Rutoside | Isochlorogenic Acid B | Astragalin | Querce-Tin | Jujuboside A | Jujuboside B | Kaempferol | Total Content |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZS1 | 0.5709 | 0.0275 | 0.0897 | 0.0308 | 11.1865 | 0.3571 | 0.0921 | 0.0261 | 0.0693 | 0.2753 | 0.6068 | 0.3064 | 0.3229 | 13.9614 |
| ZS2 | 0.5089 | 0.0157 | 0.0424 | 0.0457 | 7.5060 | 0.6052 | 0.3063 | 0.0386 | 0.0610 | 0.1344 | 0.5541 | 0.2446 | 0.3106 | 10.3735 |
| ZS3 | 0.9195 | 0.0046 | 0.1344 | 0.0489 | 5.7306 | 0.5200 | 0.2046 | 2.2140 | 0.0849 | 0.1150 | 0.4081 | 0.2854 | 0.4080 | 11.078 |
| ZS4 | 0.7810 | 0.0478 | 0.0300 | 0.0709 | 10.0390 | 0.5569 | 0.2977 | 1.7245 | 0.0544 | 0.1227 | 0.3168 | 0.2025 | 0.2656 | 14.5098 |
| ZS5 | 0.5405 | 0.0155 | 0.1039 | 0.0157 | 3.0968 | 0.6526 | 0.3868 | 1.5529 | 0.1065 | 0.0967 | 0.2268 | 0.1099 | 0.4777 | 7.3823 |
| ZS6 | 1.0364 | 0.0133 | 0.0379 | 0.1302 | 18.8823 | 0.6733 | 0.7043 | 0.0416 | 0.1379 | 0.1540 | 0.4177 | 0.4441 | 0.4645 | 23.1375 |
| ZS7 | 0.4347 | 0.0107 | 0.0150 | 0.0429 | 2.8404 | 0.3405 | 0.0598 | 1.2509 | 0.0439 | 0.0910 | 0.5921 | 0.1577 | 0.2105 | 6.0901 |
| ZS8 | 0.5221 | 0.0106 | 0.0248 | 0.0526 | 5.4711 | 0.2564 | 0.1500 | 0.7973 | 0.0143 | 0.1135 | 0.1440 | 0.1711 | 0.3087 | 8.0365 |
| ZS9 | 0.8177 | 0.0415 | 0.0281 | 0.0620 | 13.3891 | 0.5901 | 0.3803 | 0.1045 | 0.0529 | 0.1316 | 0.1063 | 0.3690 | 0.9751 | 17.0482 |
| ZS10 | 0.5645 | 0.0099 | 0.0659 | 0.0830 | 9.6662 | 0.4719 | 0.3710 | 0.0332 | 0.0844 | 0.1036 | 0.2724 | 0.2821 | 0.3268 | 12.3349 |
| ZS11 | 0.8775 | 0.0410 | 0.0605 | 0.0126 | 17.7091 | 0.3422 | 0.1191 | 0.0306 | 0.0461 | 0.2499 | 0.8472 | 0.1333 | 0.4671 | 20.9362 |
| ZS12 | 0.4080 | 0.0106 | 0.0088 | 0.0391 | 11.0067 | 0.3394 | 0.2339 | 0.0024 | 0.0312 | 0.1687 | 0.4293 | 0.3416 | 0.4284 | 13.4481 |
| ZS13 | 0.5045 | 0.0136 | 0.0176 | 0.0520 | 12.2706 | 0.3904 | 0.2340 | 0.0119 | 0.0373 | 0.2217 | 0.5657 | 0.4504 | 0.5333 | 15.303 |
| ZS14 | 0.5120 | 0.0122 | 0.0102 | 0.0955 | 5.3304 | 0.4209 | 0.1608 | 2.0755 | 0.0403 | 0.1677 | 0.2860 | 0.2943 | 0.3945 | 9.8003 |
| ZS15 | 0.7865 | 0.0354 | 0.0129 | 0.0732 | 12.2471 | 0.5399 | 0.3339 | 0.0844 | 0.0909 | 0.1405 | 0.1028 | 0.2935 | 0.9070 | 15.648 |
| ZS16 | 0.5200 | 0.0141 | 0.0252 | 0.0266 | 11.1758 | 0.3514 | 0.1402 | 0.0164 | 0.0214 | 0.1681 | 0.4261 | 0.2741 | 0.3892 | 13.5486 |
| ZS17 | 0.9237 | 0.0296 | 0.0089 | 0.0769 | 10.3866 | 0.6467 | 0.2408 | 2.2678 | 0.0505 | 0.2046 | 0.4654 | 0.4048 | 0.4513 | 16.1576 |
| ZS18 | 0.9343 | 0.0261 | 0.0382 | 0.0915 | 8.7731 | 0.7476 | 0.2424 | 3.1403 | 0.0495 | 0.2369 | 0.3470 | 0.3817 | 0.5601 | 15.5687 |
| ZS19 | 0.5011 | 0.0211 | 0.0270 | 0.0514 | 5.8081 | 0.5051 | 0.1189 | 2.1670 | 0.0380 | 0.1515 | 0.5541 | 0.2828 | 0.3797 | 10.6058 |
| ZS20 | 1.0655 | 0.0296 | 0.0109 | 0.1216 | 14.7244 | 0.8187 | 0.4353 | 2.1744 | 0.1644 | 0.3637 | 0.1360 | 0.4444 | 1.5576 | 22.0465 |
| ZS21 | 0.6110 | 0.0220 | 0.0176 | 0.0498 | 5.3191 | 0.3270 | 0.1322 | 2.1690 | 0.0293 | 0.1277 | 0.2187 | 0.3844 | 0.6718 | 10.0796 |
| ZS22 | 0.3820 | 0.0133 | 0.0187 | 0.0363 | 3.1398 | 0.3311 | 0.0652 | 1.3516 | 0.0104 | 0.1034 | 0.3316 | 0.1694 | 0.2585 | 6.2113 |
| ZS23 | 0.6581 | 0.0120 | 0.0358 | 0.0180 | 7.8348 | 0.4324 | 0.3036 | 1.4796 | 0.0425 | 0.2302 | 0.6041 | 0.2018 | 0.2860 | 12.1389 |
| ZS24 | 0.9091 | 0.0458 | 0.0094 | 0.0655 | 16.1573 | 0.5281 | 0.3047 | 0.0699 | 0.0793 | 0.1554 | 0.1221 | 0.3515 | 1.0696 | 19.8677 |
| ZS25 | 0.4940 | 0.0239 | 0.0107 | 0.0611 | 6.1704 | 0.3545 | 0.2018 | 1.9754 | 0.0509 | 0.1355 | 0.3014 | 0.1707 | 0.2315 | 10.1818 |
| ZS26 | 0.8259 | 0.0216 | 0.0088 | 0.0500 | 9.0060 | 0.3979 | 0.1736 | 1.6415 | 0.0248 | 0.1255 | 0.1866 | 0.2935 | 0.4068 | 13.1625 |
| CV(%) | 30.81 | 55.73 | 93.65 | 51.23 | 46.99 | 30.71 | 57.13 | 92.25 | 62.53 | 39.21 | 52.26 | 35.05 | 61.45 | 13.9614 |
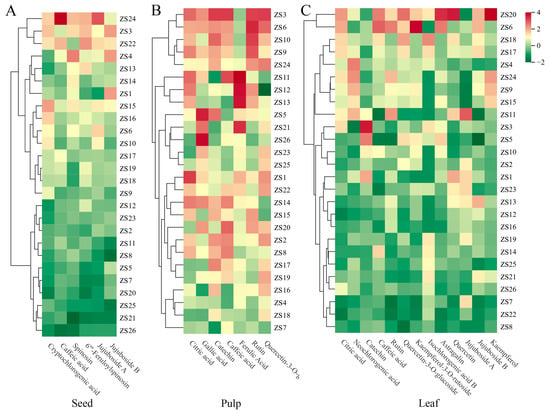
Figure A1.
Hierarchical cluster analysis (HCA) heatmap of different ZS germplasms based on major chemical composition in seeds (A), pulp (B), and leaves (C).
References
- Hua, Y.; Xu, X.; Guo, S.; Xie, H.; Yan, H.; Ma, X.; Niu, Y.; Duan, J. Wild Jujube (Ziziphus jujuba var. spinosa): A Review of Its Phytonutrients, Health Benefits, Metabolism, and Applications. J. Agric. Food Chem. 2022, 70, 7871–7886. [Google Scholar] [CrossRef]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2025; p. 394. [Google Scholar]
- Zhang, Y.; Zhang, H.; Meng, X.; Qu, T.; Li, N.; Wang, D.; Zhang, Y.; Chen, J. Mechanism of Polygalae Radix-Ziziphi spinosae Semen drug Pair in the Treatment of Anxiety and Insomnia Based on Network Pharmacology and Cell Validation Experiment. Nat. Prod. Res. Dev. 2025, 37, 1942–1952. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, H.; Wen, C.; Zhao, C.; Tian, Y.; Liu, Y.; Yang, J. Mechanism of jujuboside A on improving cognitive function of mice with Alzheimer’s disease based on transcriptomic. Chin. Tradit. Herb. Drugs 2023, 54, 8094–8104. [Google Scholar]
- Wei, H.; Wang, X.; Wang, J.; Ren, S.; Mur, L.A.J.; Lu, D. Flavonoids from sour jujube leaves: Ultrasound-assisted extraction, UPLC-QQQ-MS/MS quantification, and ameliorative effect on DSS-induced ulcerative colitis in mice. Ultrason. Sonochem 2025, 114, 107279. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, W.; Li, X.; Gao, X.; Fu, K.; Zhang, J. Phenotypic Diversity and Nutrient Composition Analysis of Ziziphus jujuba var. spinosa Germplasm Resources. Seed 2024, 43, 120–126. [Google Scholar]
- Shang, Z.; Ye, Z.; Li, M.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Dynamics of microbial communities, flavor, and physicochemical properties of pickled chayote during an industrial-scale natural fermentation: Correlation between microorganisms and metabolites. Food Chem. 2022, 377, 132004. [Google Scholar] [CrossRef]
- Dong, J.; Liu, X.; Li, C.; Qi, X.; Zhu, Y.; Wang, Y.; Shan, H. Effect of jujube leaves alcohol extract on M1 and M2 polarization of mouse macrophages. Nat. Prod. Res. Dev. 2020, 32, 961–967. [Google Scholar]
- Tang, J.; Li, T.; Niu, Z.; Du, H.; Pei, X.; Du, C. Chemical constituents from the leaves of Ziziphus jujuba var. spinosa and their anti-inflammatory activities. Chin. Tradit. Pat. Med. 2024, 46, 4029–4035. [Google Scholar]
- Cheng, G.; Bai, Y.; Zhao, Y.; Tao, J.; Liu, Y.; Tu, G.; Ma, L.; Liao, N.; Xu, X. Flavonoids from Ziziphus jujuba Mill. var. spinosa. Tetrahedron 2000, 56, 8915–8920. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Ikebata, A.; Wakao, S.; Murakami, N.; Matsuda, H.; Yamahara, J. Bioactive saponins and glycosides. X. On the constituents of zizyphi spinosi semen, the seeds of Zizyphus jujuba Mill. var. spinosa Hu (1): Structures and histamine release-inhibitory effect of jujubosides A1 and C and acetyljujuboside B. Chem. Pharm. Bull. 1997, 45, 1186–1192. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.; Tang, Y.; Qiang, Y.; Zhao, J.; Qian, D.; Su, S.; Shang, E. Simultaneous qualitative and quantitative analysis of triterpenic acids, saponins and flavonoids in the leaves of two Ziziphus species by HPLC–PDA–MS/ELSD. J. Pharm. Biomed. Anal. 2011, 56, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yuan, M.; Liu, H.; Wang, L.; Zhao, X. Analysis of Metabolites and Metabolic Pathways of Three Chinese Jujube Cultivar. Metabolites 2023, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Li, D.; Wang, Y.; Sui, C.; Cao, Y.; Liang, Q. Study on the contents of cAMP and cGMP in different cultivars, growing periods and organs in Chinese jujube. Acta Hortic. Sin. 2009, 36, 1134–1139. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Nie, X.; Li, X.; Ye, Y. Research Progress on Active Ingredients and Applications of Choerospondias axillaris. Food Sci. Technol. 2024, 49, 82–87. [Google Scholar]
- Wang, F.; Wang, Z.; Luo, M.; Tang, H.; Liu, R.; Tang, X. Identification Study of Ziziphi spinosae Semen and Its Adulterants Based on Characteristic Chromatogram and Content Determination. J. Chin. Med. Mater. 2025, 9, 2273–2278. [Google Scholar]
- Chen, S.; Ye, X.; Zhang, J.; Wang, Y.; Huang, S.; Wen, N.; Yang, H.; Yang, P.; Zuo, Z.; Liu, Y. Research Progress of Zaoren Anshen Priscription and Its Predictive Analysis of Quality Markers. Chin. Pharm. J. 2025, 60, 1898–1907. [Google Scholar]
- Chen, W.; Zheng, S.; Cao, Y.; Tian, Y.; Zhang, C.; Wang, B.; Feng, H.; Tang, H.; Zhang, Q. Phytochemical components of Qin medicine Ziziphi spinosae Semen by GC-MS and UHPLC-TOF-MS. Cent. South. Pharm. 2025, 23, 901–907. [Google Scholar]
- Yang, R.; Li, Z.; Dong, L.; Heng, Y.; Song, L.; Guo, L.; Pei, X.; Yan, Y.; Du, C. Analysis of Components in Ziziphi spinosae Semen Before and After Processing Based on Targeted and Untargeted Metabolomics. Foods 2025, 14, 3771. [Google Scholar] [CrossRef]
- Gao, Q.; Dong, J.; Cui, R.; Muraki, I.; Yamagishi, K.; Sawada, N.; Iso, H.; Tsugane, S. Consumption of flavonoid-rich fruits, flavonoids from fruits and stroke risk: A prospective cohort study. Br. J. Nutr. 2021, 126, 1717–1724. [Google Scholar] [CrossRef]
- Cai, H.; You, S.; Xu, Z.; Li, Z.; Guo, J.; Ren, Z.; Fu, C. Novel extraction methods and potential applications of polyphenols in fruit waste: A review. J. Food Meas. Charact. 2021, 15, 3250–3261. [Google Scholar] [CrossRef]
- Song, H.; Xia, J.; Ji, G.; Ren, Z.; Zhang, X.; Liu, F.; Chen, L. Determination of 9 Amino Acid Endogenous Substances in Morning Urine of Depression Patients by LC-MS/MS. China Pharm. 2020, 31, 91–98. [Google Scholar]
- Sheng, J.; Zhong, H.; Zhou, Q.; Sun, L.; Jiang, H.; Zhang, S.; Guo, F.; Hou, L.; Zhang, C.; Dong, S. Analysis on chemical constituents of fruit of Chaenomeles speciosa by UPLC-Q-Exactive Orbitrap-Ms. Chin. Tradit. Herb. Drugs 2018, 49, 4773–4779. [Google Scholar]
- Chen, J.; Wu, H.; Liu, R.; Zhang, C.; Sun, Y. Study on the Chemical Constituents in Wuling Capsules by UPLC-QE-Orbitrap-MS. J. Chin. Med. Mater. 2022, 45, 639–646. [Google Scholar]
- Zhou, D.; Duan, H.; Wa, Q.; Wu, Y.; Peng, X.; Sun, X. Analysis of Chemical Constituents in Aerial Parts of Anemone vitifolia Based on UHPLC-Q-Exactive Orbitrap MS. Mod. Chin. Med. 2025, 27, 1018–1030. [Google Scholar]
- Liang, H.; Li, H.; Ren, Y.; Tian, D.; Zhang, M. Mechanism of Rehmanniae Radix-Salviae Miltiorrhizae Radix in the treatment of diabetic retinopathy based on network pharmacology and experimental verification. Nat. Prod. Res. Dev. 2025, 37, 1341–1355+1340. [Google Scholar]
- Zhang, X.; Yu, X.; Sun, X.; Meng, X.; Fan, J.; Zhang, F.; Zhang, Y. Comparative study on chemical constituents of different medicinal parts of Lonicera japonica Thunb. Based on LC-MS com-bined with multivariate statistical analysis. Heliyon 2024, 10, e31722. [Google Scholar] [CrossRef]
- Liu, P.; Wang, N.; Xie, S.; Wang, L.; Liao, Z.; Lai, R.; Qin, M. Rapid Determination of Fatty Acids in Ziziphi spinosae Semen from Different Origins by Internal Extractive Electrospray Ionization Mass Spectrometry. J. Chin. Mass. Spectrom. Soc. 2025, 46, 503–510. [Google Scholar]
- Niu, Y.; Wang, S. Analysis on chemical constituents in Danggui-Shaoyao-San by LC-Q- TOF-MS and LC-IT-MSn. Chin. Tradit. Herb. Drugs 2014, 45, 1056–1062. [Google Scholar]
- Chen, Y.; Yu, M.; Dai, X.; Jia, H.; Zhang, H.; Ma, Z.; Zou, J. Characterization of major constituents and determination of protocatechuic acid in Chinese herbal medicine Cibotii Rhizoma. Chin. Tradit. Herb. Drugs 2023, 54, 2254–2261. [Google Scholar]
- Yang, L.; Yang, L.; Jia, P.; Lan, W.; Zhang, Y.; Wang, S.; Zhang, P.; Zheng, X. HPLC-Q-TOF-MS/MS-based analysis of chemical constituents in Choerospondiatis fructus. Acad. J. Nav. Med. Univ. 2016, 37, 159–166. [Google Scholar]
- Cai, W.; Wang, Y.; Jin, W.; Yang, L.; Wang, J.; Zhang, Z. Chemical constituents of Qiang medicine Primula chungensis by UPLC-Q-Exactive-Orbitrap-MS. Nat. Prod. Res. Dev. 2023, 35, 986–996. [Google Scholar]
- Wang, X.; Liu, H.; Ding, S.; Luo, J.; Zhao, C.; Yao, Z.; Li, Q.; Liu, J.; Yu, J.; Wei, X.; et al. Comparison of Chemical Components between Ziziphi spinosae Semen or Schisandrae Chinensis Fructus Monodecoction and Their Codecoction by HPLC-LTQ-Orbitrap-MSn. Mod. Chin. Med. 2021, 23, 1949–1966. [Google Scholar]
- Li, B.; Qie, L.; Wang, X.; Niu, L. Simultaneous determination of fifteen constituents in Suanzao Anshen Granules by UPLC-MS/MS. Chin. Tradit. Pat. Med. 2022, 4406, 1751–1755. [Google Scholar]
- Li, R.; Li, S.; Liu, W.; Qi, S.; Peng, Y. Analysis of chemical constituents of Agrimonia pilosa based on UHPLC-Q-Exactive Orbitrap HRMS technology. J. Shenyang Pharm. Univ. 2025, 4204, 342–357. [Google Scholar]
- Qu, T.; Li, N.; Lu, J.; Ren, H.; Cui, X.; Hu, J.; Chen, Z.; Zhang, H. Analysis on chemical constituents in Ziziphi spinosae Semen by UPLC-Q-Exactive Focus-MS/MS and network pharmacology study on anti-Alzheimer’s disease mechanism. Drug Eval. Res. 2023, 46, 2563–2579. [Google Scholar]
- Liu, J.; Wei, J.; Wu, J.; Du, C.; Yan, Y. Identification of Chemical Constituents in Suanzaoren Tang Granules by UPLC-Q-TOF-MS/MS. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 1–12. [Google Scholar]
- Ren, T.; Li, Z.; Lian, J.; Su, M.; Cheng, S.; Nie, Z.; Liu, P.; Shi, J. Determination of components of 12 species of Ziziphi spinosi Semen before and after processing by UPLC-MS. West. China J. Pharm. Sci. 2023, 38, 300–305. [Google Scholar]
- Shi, Y.; Nan, Y.; Zheng, W.; Yao, L.; Lian, H.; Chen, X.; Song, J.; Zhang, J.; Jia, D.; Wang, Q.; et al. Qualitative and semiquantitative analyses of the chemical components of the seed coat and kernel of Ziziphi spinosae Semen. Chin. J. Chromatogr. 2024, 42, 234–244. [Google Scholar] [CrossRef]
- Li, Y.; Cao, L.; Chen, Z. Comparison of chemical compositions before and after charcoal-frying of Scutellariae Radix based on HPLC fingerprint and UPLC-Q Exactive Focus MS/MS. Chin. Tradit. Herb. Drugs 2024, 55, 8425–8434. [Google Scholar]
- Sun, J.; He, S. Identification of metabolites of (+)-catechin in rat liver microsomes based on UPLC-LTQ-Orbitrap and multiple mass defect filter method. Chin. J. Hosp. Pharm. 2016, 36, 1264–1268. [Google Scholar]
- Li, Z.; Du, H.; Xie, Y.; Heng, Y.; Duan, H.; Pei, X.; Yan, Y.; Du, C. Prediction and analysis of Q-Markers of fried Ziziphi spinosae Semen pieces based on multivariate statistical analysis and network pharmacology. Chin. Tradit. Herb. Drugs 2021, 52, 4811–4824. [Google Scholar]
- Li, X.; Chen, Q.; Chen, C.; Zhang, T.; Xu, L.; Zheng, P.; Yang, P. Pharmacokinetic Studies of Total Flavonoids of Desmodium Styracifolium After Oral Administration of Different Doses in SD Rats by HPLC-QTRAP-MS/MS. Chin. Pharm. J. 2021, 56, 1810–1817. [Google Scholar]
- Huang, X.; Mao, Y.; Li, H.; Wang, H.; Liu, Y. Difference in Chemical Constituents of Ziziphi spinosae Semen from Different Producing Areas by UHPLC-LTQ-Qrbitrap-Ms-based Metabolomics. Mod. Chin. Med. 2021, 23, 2077–2087. [Google Scholar]
- Yan, Y.; Du, C.; Feng, H.; Qi, J.; Qin, X. Integrated strategy of UHPLC-Q Exactive Orbitrap-HRMS and HPLC-CL to study with constituents in Ziziphi spinosae Folium. Chin. Tradit. Herb. Drugs 2016, 47, 3109–3114. [Google Scholar]
- Guo, X.; Li, H.; Feng, H.; Qi, H.; Zhang, L.; Xu, W.; Wu, Y.; Wang, C.; Liang, X. Quality analysis of Ziziphi spinosae Semen extracts based on high-performance liquid chromatography quantitative fingerprint and ultra-high performance liquid chromatography-tandem mass spectrometry quantification. Chin. J. Chromatogr. 2021, 39, 989–997. [Google Scholar] [CrossRef]
- Ye, J.; Yang, M.; Yang, X.; Zhang, H.; Zan, L. Analysis of chemical constituents in Ziziphus jujuba var. spinosa folium by UPLC-QTOF-MS. Nat. Prod. Res. Dev. 2019, 31, 1183–1191. [Google Scholar]
- Liu, J.; Long, K.; Ma, Z.; Du, X.; Zhang, H. Analysis of chemical constituents of Yifei jiedu Granules by UHPLC-Q Exactive Focus MS/MS. Chin. Pharm. J. 2023, 58, 1940–1954. [Google Scholar]
- Cai, Z.; Hu, Y.; Liu, W.; Wang, S.; Kong, X.; Yang, Y.; Qian, M.; Cao, L.; Wang, Z. Analysis of chemical components of Yin-Qiao-Qing-Re Tablets by UPLC-Q-TOF-MS/MS and GC-MS. J. Nanjing Univ. Tradit. Chin. Med. 2025, 1198–1212. [Google Scholar] [CrossRef]
- Ma, Z.; Sang, S.; Duo, J. Content Determination of 6 Kinds of Triterpene Acid in Tibetan Medicine Rubus biflorus by Pre-column Derivatization HPLC/FLD APCI/MS. China Pharm. 2019, 30, 2243–2247. [Google Scholar]
- Yan, Y.; Chu, Y.; Duan, H.; Wang, H.; Qin, X.; Du, C. Study on the tissue distribution of eight effective components of Ziziphi spinosae Semen aqueous extract by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Acta Pharm. Sin. 2023, 58, 740–749. [Google Scholar] [CrossRef]
- Kang, Q.; Li, Z.; Fan, S.; Rong, R.; Jiang, H.; Jiang, X.; Zhang, J.; Gong, L. Qualitative Analysis on Perilla frutescens Leaves and Stalks by UPLC-Q-Exactive-Orbitrap-MS. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 156–162. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Xie, J.; Wang, Q.; Qi, W. Protective Effect of 6’’’-Feruloylspinosin on Aβ1-42-Induced SH-SY5Y Cells Injury. Sci. Technol. Food Ind. 2022, 43, 373–380. [Google Scholar]
- Guo, S.; Duan, J.; Li, Y.; Wang, Y.; Yan, H.; Qian, D.; Tang, Y.; Su, S. Comparison of the Bioactive Components in Two Seeds of Ziziphus Species by Different Analytical Approaches Combined with Chemometrics. Front. Pharmacol. 2017, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Bian, T.; Chen, J.; Liang, H.; Xu, M.; Wei, X.; Fu, K.; Li, X. Research progress on Ziziphi spinosae Semen and their developmentand application in prevention and treatment of insomnia. Chin. Tradit. Herb. Drugs 2025, 56, 6823–6832. [Google Scholar]
- Turan, E.; Gules, O.; Kilimci, F.S.; Kara, M.E.; Dilek, O.G.; Sabanci, S.S.; Tatar, M. The mixture of liquid foam soap, ethanol and citric acid as a new fixative–preservative solution in veterinary anatomy. Ann. Anat.-Anat. Anz. 2017, 209, 11–17. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, F.; Ramírez-Rodrigues, M.M. Influence of time-temperature in the antioxidant activity, anthocyanin and polyphenols profile, and color of Ardisia compressa K. extracts, with the addition of sucrose or citric acid. Food Chem. 2024, 440, 138181. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.; Sui, Y.; Wang, Y.; Qiu, X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed. Pharmacother. 2017, 86, 32–40. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Zhang, X.; Fang, Q.; Xu, Y.; Wang, H. Rutin alleviates Pb-induced oxidative stress, inflammation and cell death via activating Nrf2/ARE system in SH-SY5Y cells. NeuroToxicology 2024, 104, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Niu, Z.; Mei, D.; Zhang, B. Mechanisms and Protective Effect of lsochlorogenic Acid B on Liver Injury in Mice. Her. Med. 2020, 39, 895–899. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).