Abstract
The present study explores novel alternatives for the exploitation of sugarcane bagasse ash by obtaining and modifying SiO2 nanoparticles through a green synthesis method. The hydrophilic nature of the nanoparticles was modified using oleic acid. The nanoparticles were characterized using FTIR, FESEM, and DLS, and their performance in the stabilization of Pickering emulsions was also studied. FESEM micrographs of the nanoparticles revealed an irregular and agglomerated structure. EDS confirmed that their main components are oxygen and silicon, and ATR-FTIR spectra demonstrated that oleic acid effectively modified the nanoparticles. Subsequently, O/W Pickering emulsions were fabricated by combining rotor–stator homogenization and probe ultra-sonication, using dodecane and liquid paraffin as model oil phases and SiO2 NPs as stabilizers. Static light scattering measurements showed that the emulsions exhibited polydispersity, while photographic monitoring confirmed that their physical stability was affected by the concentrations of oleic acid and nanoparticles: concentrations of up to 20.0 wt% and 1.0 wt%, respectively, produced emulsions that remained stable for 7 to 15 days. This study identifies the behavior and challenges associated with novel pathways for the valorization of sugarcane bagasse ash. The stabilization of Pickering emulsions using the obtained SiO2 NPs highlights their potential in pharmaceutical, cosmetic, and food applications.
1. Introduction
Sugarcane is one of the most important crops worldwide, with annual production exceeding 1.9 billion tons in 2023 [1]. It is processed by the mechanical extraction of sugarcane juice, which is further transformed to obtain refined sugar or to produce bioethanol; during this process, different by-products are generated [2]. Among them, sugarcane bagasse, the fibrous material derived after the sugarcane juice extraction, is an interesting material, as it is rich in hemicelluloses, cellulose, lignin, and minerals [3]. It is estimated that nearly 250 kg of sugarcane bagasse is generated for each ton of sugarcane processed [4].
Besides its potential as a raw material of different biomolecules, sugarcane bagasse is commonly used as a biofuel in traditional furnaces, such as those used in the processing of panela or piloncillo, a sweetening alternative derived from sugarcane; it is typical of Latin America and produced mostly by small communities [5]. After combustion, 1 ton of bagasse is reduced to 25 kg of sugarcane bagasse ash (SBA), which is rich in minerals, mainly silica, with a content of up to 96.93 wt.%, depending on soil composition and plant growth [6]. This ash has been traditionally used as fertilizer; however, its mineral profile and low concentration of nutrients require its mixing with other compounds before its application, highlighting the need for different studies into its exploitation. These reports have focused on its potential to reinforce cementitious materials [6,7,8]; nevertheless, they overlook other alternatives for this by-product.
Its silica content has prompted its evaluation as a raw material to produce silica-based ordered mesoporous material [9] and silica nanoparticles (SiO2 NPs) [3]; these nanoparticles consist of a colloidal dispersion of SiO2 particles with ranges from 1 to 100 nm, and they are usually synthesized by the Störber method, which uses a silica precursor, such as Tetraethyl Orthosilicate (TEOS), and a basic catalyzer, such as NH4OH [3,10]. The development of different pathways using renewable sources is of interest as it helps to strengthen the productive chain of sugarcane and provides alternative high-value applications where small communities could access as raw material providers for the obtention of SiO2 NPs, a material with an estimated market value of 8.8 billion USD in 2020 [3,10]. Several studies have reported the inhalation toxicity [11] and nephrotoxicity [12] of TEOS. Consequently, the development of different pathways using renewable sources as silica precursor is of interest as it helps to strengthen the productive chain of sugarcane and provides alternative high-value applications where small communities could access as raw material providers for the obtention of SiO2 NPs.
The valorization of sugarcane bagasse ash through the synthesis of SiO2 NPs represents not only an environmentally friendly alternative but also an opportunity to strengthen and diversify the sugarcane production chain through technological development. In this context, hard technologies [13], such as nanomaterials synthesis, and soft technologies, which aim to implement biorefinery models that integrate traditional production with the development of new inputs for emerging sectors through knowledge management [13], play a key role in innovation and sustainability within agro-industrial systems. The sugarcane sector, traditionally focused on sugar and energy production, still faces technological gaps that limit its diversification and the integral valorization of its by-products. The incorporation of nanotechnology in this production chain contributes to closing these gaps, promoting technology transfer, the generation of new business models based on circular economy principles [14] and fostering research and institutional collaboration, as well as increasing the economic value of this by-product [15,16]. This approach contributes to achieving the Sustainable Development Goals (SDG 9, 12 and 13) by encouraging sustainable innovation, efficient resource management and reduction in environmental impacts associated with sugarcane production [17].
Nevertheless, the synthesis of SiO2 NPs comes with important challenges: the intended application impacts the synthesis and adsorption modification of these nanoparticles, as changes in the nanoparticle surface can affect their performance [10]. Among the application of these nanoparticles, their use as an emulsifier is of interest for agricultural and food industries, where there is mounting pressure to reduce the consumption of synthetic emulsifiers, such as surfactants, in different formulations, resulting in a cleaner label [18]. In this context, the use of solid-stabilized emulsions, also known as Pickering emulsions, is an important and evolving field of application for SiO2 NPs [19].
Pickering emulsions are obtained when a solid particle is adsorbed at the oil/water interphase at the edge of the droplets [19,20]. The energy required to reverse this adsorption depends on particle size and the contact angle of the particle at the O/W interphase, θ, while a theoretical maximum is observed at 90 °; however, this value shall be a little lower for O/W emulsions and a little higher for W/O, allowing for the particle to move towards the continuous phase, while both phases wet the particle surface [20]. SiO2 NPs are traditionally hydrophilic, allowing the unmodified particles to stabilize emulsions with polar oils while reducing their ability to stabilize non-polar phases [19,21]. Therefore, the properties of the synthesized SiO2 NPs shall be tailored to satisfy these requirements and stabilize the resultant emulsions. This implies that the nanoparticles shall be modified.
There are different strategies to achieve this modification, while traditional methods rely on chemical functionalization and the formation of covalent bonds of non-polar aliphatic chains to a silane atom; compounds as polyethylene glycol silanes [21] have been hydrolyzed and reacted with unmodified SiO2 NPs. Novel techniques, aiming to reduce the reliance on chemical synthesis, have been proposed, achieving the adsorption of different emulsifiers, such as cetyl trimethyl ammonium, sodium dodecyl sulfate [22], and oleic acid [23], among other compounds. Oleic acid has been reported as an interesting option as it is a biodegradable, biocompatible fatty acid and has been used as an emulsifier in water in oil emulsions [24], appearing as a sustainable alternative to synthetic compounds, showing interesting results in promoting the stabilization of Pickering emulsions with different oils. Nevertheless, most of these works evaluate its adsorption over synthetic monodisperse SiO2 NPs [22,25,26] and rice paddy husk [23]. To the authors knowledge, there is no exploration of the effect of this type of adsorption modification using nanoparticles derived from sugarcane bagasse ash using green methods, which might result in a wide arrange of nanoparticle distribution, thus hindering the exploration of these nanoparticles in food and agricultural applications.
This work aims to provide novel alternatives to exploit sugarcane bagasse ash by synthesizing and modifying SiO2 NPs using green methods and evaluating their physical and morphological characteristics by attenuated total reflection–Fourier transform infrared spectroscopy (ATR–FTIR), field emission scanning electron microscopy and energy-dispersive X-ray spectroscopy (FESEM-EDS) and static light scattering (SLS) analysis, as well as their performance in the formation of Pickering emulsions using two model oils, dodecane and paraffin, assessing their stability by physical observation, in order to identify the effect of nanoparticle and oleic acid concentration in emulsion formation and stability. We aim to identify the behavior and challenges of novel pathways of sugarcane bagasse ash valorization.
2. Results
2.1. Nanoparticle Characterization
2.1.1. Field Emission Scanning Electron Microscopy (FESEM)
Morphological and compositional characterization were carried out through FESEM and EDS analyses of the obtained SiO2 NPs. Figure 1a,b show the presence of particle agglomerations of irregular morphologies, which is explained by the presence of micropores between silica grains, highlighted by yellow dotted circles in Figure 1b [27]. This type of conformation is favored by the destabilization of the three-dimensional network formed by the NPs during the drying process, which results in particle agglomeration and the formation of a xerogel [4].
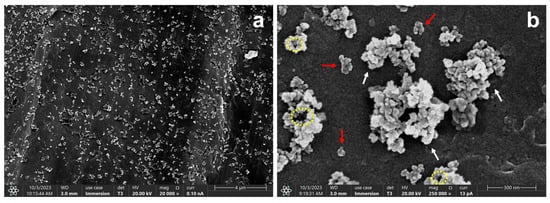
Figure 1.
FESEM micrographs of the unmodified nanoparticles at (a) 20,000× and (b) 250,000×. Red arrows indicate the presence of smaller or less aggregated particles, while white arrows represent larger clusters. Yellow dotted circles indicate the presence of micropores.
A mean Feret diameter of 526.36 ± 262.36 nm was obtained, indicating a broad size distribution. This large variability can be attributed to the coexistence of both isolated nanoparticles and agglomerated structures formed during sample preparation. The isolated nanoparticles exhibited Feret diameters in the range of 43.60–120.54 nm, whereas larger particles, corresponding to aggregated structures or clusters, showed sizes between 146.90 and 603.10 nm.
Additionally, EDS analysis, Figure 2, indicates that samples are mainly composed of silicon and oxygen, with traces of elements such as carbon, iron and manganese, mostly on the background of the pictures which could come from the sample holder, or traces of dissolved ions. Results obtained in both FESEM and EDS analyses are consistent whit previous reports that produced silicon nanoparticles from sugarcane bagasse and SBA, such as the work of Falk et al. [4] and Rovani et al. [28]. They obtained a similar microstructure and heterogeneous morphology with the presence of agglomeration of nanoparticles, as well as an elemental composition that included the presence of impurities such as carbon, iron, aluminum, manganese and sodium [4,27,28,29,30].
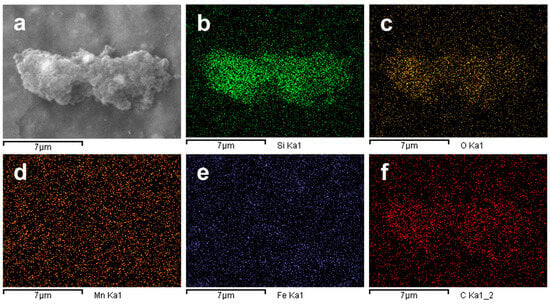
Figure 2.
EDS compositional mapping of nanoparticles: (a) FESEM-EDS micrograph; (b) silicon (Si) mapping; (c) oxygen (O) mapping; (d) manganese (Mn) mapping; (e) iron (Fe) mapping; (f) carbon (C) mapping.
In summary, these particles present an irregular, agglomerated structure, mainly composed of oxygen and silicon. According to these results, the green synthesis of silica nanoparticles provides a pathway for nanoparticle production, which reduces the need for the reagents used in conventional synthesis processes [3,31], and it increases the value of agricultural byproducts, contributing to a more efficient use of resources. Furthermore, the synthesis method described in this study utilizes only HCl and NaOH as reagents and demonstrates the possibility of obtaining nanoparticles composed primarily of oxygen and silicon.
2.1.2. Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy (ATR–FTIR)
While EDS made it possible to identify that the main elements present in the synthesized material are oxygen and silicon, ATR-FTIR was used to verify that these elements formed chemical bonds characteristic of silica nanoparticles [23,25,30].
FTIR spectra, shown in Figure 3a reveals distinctive characteristics of nanoparticles obtained through green synthesis methods. According to Ni’mah et al. [30], absorption peaks in the region of 3421 cm−1 and 1636 cm−1 correspond to the stretching vibration and bending vibration, respectively, of –OH and H2O silanol groups present in silica nanoparticles, indicating their formation from sugarcane bagasse ash. Additionally, the characteristic peaks at 1087 cm−1 and 804 cm−1 are attributed to the asymmetric and symmetric stretching vibration of the Si-O-Si bond, respectively, confirming the silica structure. This evidence suggests the synthesis of silica nanoparticles through the selected approach [30].
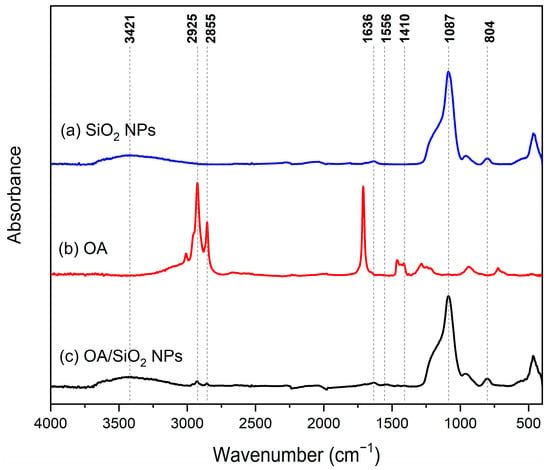
Figure 3.
FTIR spectra of (a) unmodified SiO2 NPs; (b) pure oleic acid, (c) SiO2 NPs modified with oleic acid following the nanoparticle/oleic acid ratio of sample P-HH.
However, characterization carried out through FESEM and EDS analyses revealed agglomeration of nanoparticles, indicating the need for their modification to enhance sol stability. The need for adsorption modification extends to improve the stability of the developed Pickering emulsions. Similarly, a literature review shows that silica nanoparticles are highly hydrophilic in character [22,23,32]. Therefore, an adsorption modification strategy to enhance the lipophilic character of the nanoparticles must be developed to improve their performance in the formulation of Pickering emulsions.
ATR-FTIR was used as well, to evaluate silica nanoparticles before and after their modification with 20 wt.% oleic acid (based on nanoparticle weight), comparing the results with the spectrum of pure oleic acid. ATR-FTIR spectroscopic analysis provides insights into the modification of silica nanoparticles with oleic acid and the significance of this modification in Pickering emulsion stabilization.
FTIR spectrum of oleic acid is presented in Figure 3b. Two distinct peaks are evident at 2853 cm−1 and 2921 cm−1, corresponding to symmetric and asymmetric stretching vibrations of –CH2 groups, respectively [23]. Additionally, a moderately sharp peak at 3005 cm−1, overlapping with the O–H stretching band, is attributed to C–H stretching within the vinyl moiety [23]. Furthermore, a sharp peak appearing at 1714 cm−1 predominantly signifies C=O stretching, while the peak around 1285 cm−1 is associated with C–O stretching within the carboxylic group [23].
FTIR analysis reveals significant changes between the spectra of silica nanoparticles modified with oleic acid and the unmodified nanoparticles (see Figure 3a,c). Premaratne et al. noted that the characteristic bands at 1093 cm−1, 800 cm−1, and 460 cm−1 correspond to the Si-O-Si vibration modes, which persist in both modified and unmodified nanoparticles [23]. The absorption peaks at 2855 and 2925 cm−1 depicted in Figure 3b,c are indicative of stretching vibrations specific to the –CH2 group [23,25], which are absent in the spectrum of pure silica nanoparticles shown in Figure 3a. This observation suggests the presence of long alkyl chains within the modified SiO2 NPs incorporating oleic acid. These results confirm the successful modification of silica nanoparticles, which is crucial for the effective stabilization of Pickering emulsions [23,30].
2.2. Emulsion Characterization
2.2.1. Physical Stability
Multiple factors can influence the stability of emulsions; therefore, analyzing their behavior over time is crucial in its understanding. In the present article, a photographic record was used to evaluate the qualitative stability of the obtained emulsions. Photographs of the physical stability test of dodecane and liquid paraffin emulsions are presented in Figure 4 and Figure 5. For both types of emulsions, it is observed that those prepared with low concentrations of nanoparticles and/or devoid of oleic acid, destabilized immediately after preparation. In contrast, emulsions using dodecane prepared with medium and high concentrations of SiO2 NPs modified with adsorbed oleic acid remained stable for up to 7 d, with minimal creaming, and those prepared with liquid paraffin PL-H (0.5% SiO2 NPs and 20 wt.% oleic acid) remained stable for up to 15 d with no evident creaming. To confirm the effect of the SiO2 NPs, the stabilization of dodecane using pure oleic acid was also tested, resulting in a mixture that did not form a stable emulsion, as can be seen in Figure S1. Oleic acid is insoluble in water and has an HLB of 1, being used in stabilizing water in oil emulsions [33], while the stabilization of oil-in-water emulsions with oleic acid is frequently achieved with the addition of cosurfactants, such as polyoxyethylene sorbitan monolaurate (Tween 20) [34]. This result is coherent with the findings of Sadeghpour et al. [22], who reported that oleic acid shows a very low stabilizing effect for O/W emulsions due to its very low hydrophilic-lipophilic balance value. To summarize, the influence of nanoparticle concentration, their adsorption modification, and type of oil phase used on emulsion stability is evident, while none of the materials alone could achieve a stable emulsion. Further characterizations were only performed in samples that remained stable for more than one day.
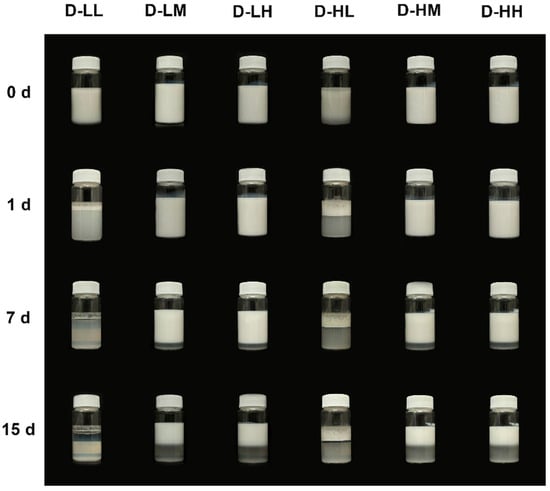
Figure 4.
Physical stability of different formulations of dodecane-water emulsions stabilized with SiO2 NPs modified with adsorbed OA, where: LL refers to low concentration of SiO2 NPs and low concentration of OA; LM refers to low concentration of SiO2 NPs and medium concentration of OA; LH refers to low concentration of SiO2 NPs and high concentration of OA; HL refers to high concentration of SiO2 NPs and low concentration of OA; HM refers to high concentration of SiO2 NPs and medium concentration of OA; and HH refers to high concentration of SiO2 NPs and high concentration of OA. Pictures were taken after immediately after emulsification (0 h), 1 day after (1 d), 7 days (7 d) and 15 days (15 d).
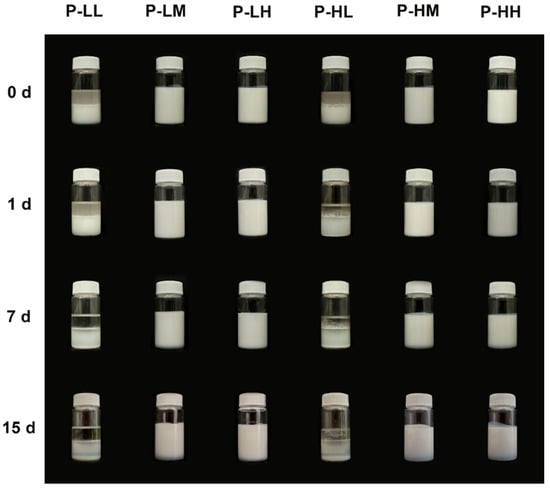
Figure 5.
Physical stability of different formulations of liquid paraffin-water emulsions stabilized with SiO2 NPs modified with adsorbed OA, where: LL refers to low concentration of SiO2 NPs and low concentration of OA; LM refers to low concentration of SiO2 NPs and medium concentration of OA; LH refers to low concentration of SiO2 NPs and high concentration of OA; HL refers to high concentration of SiO2 NPs and low concentration of OA; HM refers to high concentration of SiO2 NPs and medium concentration of OA; and HH refers to high concentration of SiO2 NPs and high concentration of OA. Pictures were taken after immediately after emulsification (0 h), 1 day after (1 d), 7 days (7 d) and 15 days (15 d).
As previously mentioned, silica nanoparticles alone do not effectively stabilize emulsions due to their high hydrophilicity, hindering their adsorption at the water/oil interface [35]. The adsorption modification of silica nanoparticles with oleic acid results in a significant increase in emulsion stability, demonstrating the synergism between the nanoparticles and oleic acid in stabilizing the emulsions, preventing phase separation even after 15 d in the case of emulsions prepared with liquid paraffin, which showed greater stability. Similar results were reported by Lin et al. [36], who synthesized Fe3O4 coated with oleic acid to first produce W/O Pickering emulsions and used the modified particles to stabilize W1/O/W2 emulsions. Sadeghpour et al. [22] also fabricated emulsions with a similar behavior, modifying hydrophilic silica nanoparticles using oleic acid to produce O/W Pickering emulsions; the modified silica nanoparticles enabled the stabilization of emulsions containing various oil phases, such as long-chain alkanes and liquid paraffin.
Therefore, the modification of silica nanoparticles with oleic acid is crucial to achieve Pickering emulsions that remain stable for longer periods. The literature suggests that the presence of long alkyl chains in the modified nanoparticles allows for a more hydrophobic nanoparticles, with organic chains surrounding the surface of silica NPs [37], promoting their interaction with the oil phase of the emulsion, thereby facilitating its stabilization. In other words, oleic acid reduces the hydrophilic behavior of silica nanoparticles, enhancing their compatibility with organic solvents and oil [22,23,25,36,38].
On the other hand, the enhanced stability to creaming of liquid paraffin emulsions compared to those utilizing dodecane can be elucidated through Stokes’ Law (Equation (3)) [39], a mathematical model that represents the gravitational separation rate of spherical droplets.
denotes the gravitational separation rate, with as the gravitational constant, representing the droplet radius, and and the densities of the continuous and dispersed phases, respectively. A higher value, while being lower than , results in a reduced value alongside . Essentially, owing to greater density of liquid paraffin (0.880 g/cm3) relative to dodecane (0.753 g/cm3), it exhibits a diminished gravitational separation rate, thereby improving its stability. Multiple studies have proved the correlation between increased oil density and enhanced emulsion stability [40,41,42].
2.2.2. Emulsification Index
To quantitatively rectify the aforementioned observations, the emulsification index was calculated. The emulsification index (EI) is a measure of the emulsion’s stability during storage and primarily indicates the emulsion’s capacity to resist flocculation and sedimentation caused by gravity [43]. In other words, the EI increments alongside the emulsion stability, with an EI of 100% indicating a completely emulsified system. The EI was calculated for all samples that remained stable longer than 1 day; samples D-LL, D-HL, P-LL, and P-HL destabilized right after fabrication, thus not producing relevant data. The results are shown in Table 1.

Table 1.
Effect of time on the emulsification index of emulsions with modified SiO2 NPs for oil phases dodecane and liquid paraffin. Values with different superscript letters within the table are significantly different ().
Firstly, for measurements taken in 1 d, samples do not present statistically different EI, with all values being close to 100%. Nonetheless, samples at 15 d exhibit significantly different behavior between oil phases; dodecane samples present lower EI values, ranging from 58.296% ± 1.794% to 66.858% ± 10.985%, whereas liquid paraffin samples present significantly higher indexes, ranging from 88.638% ± 0.639% to 93.998% ± 0.923%. As previously mentioned, this can be explained by the difference in density between both oil phases, where the higher density of liquid paraffin is associated with a diminished gravitational separation rate. Furthermore, sample P-HH is particularly interesting, as its EI does not change significantly between 1 d and 15 d, which can be associated with a higher content of both nanoparticles and oleic acid, thus resulting in a higher stability. Calculations of EI for 0 d and 7 d are available in the Supplementary Information (Table S1).
Figure 6 shows representative samples for the EI behavior of both oil phases; Figure S2 presents data for the rest of the samples. Paraffin samples’ EI decrease slower over time compared with dodecane’s, as depicted in the inset photographs: the P-HH 15 d (Figure 6a) sample remains mostly uniform, while the D-HH 15 d (Figure 6b) sample shows two distinct phases.
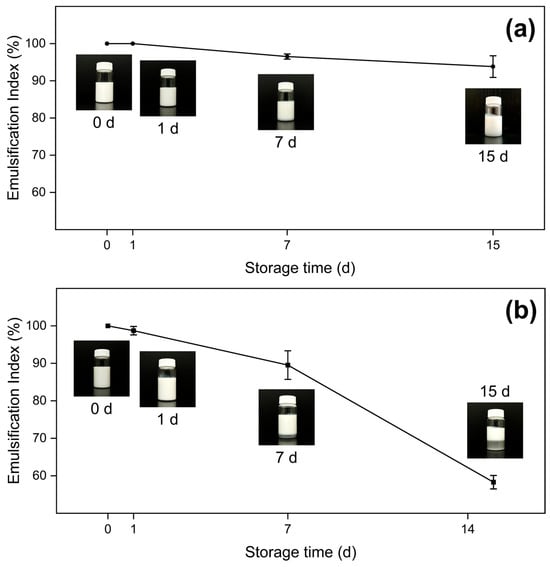
Figure 6.
Emulsification index over time for the samples (a) P-HH, and (b) D-HH.
2.2.3. Particle Size Distribution
Another parameter to consider when characterizing emulsions is droplet size [44]. Only the most stable emulsions for each oil phase were analyzed, considering the previous stability analysis, namely P-HH and D-HH. Figure 7a,b show the size distributions of paraffin and dodecane emulsions, respectively, measured at three times: immediately after preparation (0 d), after one day (1 d), and after one week (7 d).
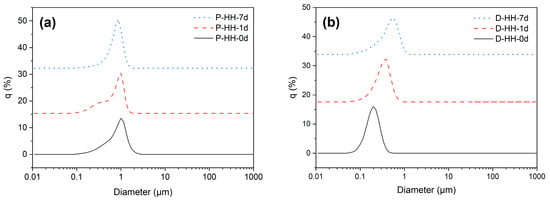
Figure 7.
Size distribution at different time stamps: after emulsification (0 d), 1 day after (1 d) and 7 days after (7 d), for emulsions with (a) paraffin for oil phase and (b) dodecane for oil phase.
Table 2 shows the d10, d50, d90, D4,3, and δ values obtained for these samples. The values of d10, d50, and d90 were used to calculate δ as indicated in Table 2. For all analyzed samples, both paraffin and dodecane, it is evident that δ is greater than 0.5, indicating polydispersity in droplet sizes [44]. Overall, emulsions with a dodecane oil phase tend to show lower δ and, therefore, lower polydispersity [44].

Table 2.
d10, d50, d90, D4,3, and δ values for emulsions with two different oil phases at different times after preparation. Values with different superscript letters in the same row are significantly different ().
The mean particle diameter (D4,3) was used to monitor the stability of the emulsions and their aggregation [40,45]. Dodecane emulsions showed a greater increase in diameter (from 0.181 ± 0.007 μm to 0.558 ± 0.039 μm) compared to paraffin emulsions (from 0.512 ± 0.257 μm to 0.675 ± 0.047 μm). While the droplet size for paraffin emulsions did not present statistically significant changes during storage, dodecane emulsions exhibited a significant increment in the mean particle diameter, which can be attributed to coalescence, as suggested by Zhang et al. [40]. The authors suggest that higher density oils, like liquid paraffin, reduce the buoyancy and velocity of droplets following the initial homogenization, as well as possessing greater mass per unit volume, which increases friction during droplet motion and limits the rate of coalescence and size enlargement, thus limiting emulsion destabilization [40]. These results are coherent with the EI calculations, where dodecane samples show more rapid destabilization, while paraffin samples remain stable for longer periods of time. Additionally, Ostwald ripening is unlikely to contribute to the droplet size growth considering the low solubility of dodecane in water of only 8.9 × 10−10 mol/mol [46].
To summarize, this study evaluates the relationship between silicon nanoparticle concentration, oil phase, and droplet size distribution in determining emulsion stability, which provides insights for various applications. The concentration of nanoparticles and oleic acid significantly affects stability, with low concentrations leading to rapid destabilization, while medium to high concentrations result in stability lasting up to 7 or 15 d. Nanoparticle modification, particularly with adsorbed oleic acid, plays a key role in enhancing stability, preventing phase separation even after an extended period, especially in emulsions with liquid paraffin. Modification of silica nanoparticles by physical adsorption of oleic acid is a simple method to enhance the nanoparticle hydrophobicity of the nanoparticles, as demonstrated in previous studies [22,47]. Consequently, the modified particles exhibit a strong affinity for the oil/water interface, facilitating the formation of stable emulsions [47]. According to Sadeghpour et al. [22], the stability of these droplets is attributed to the considerable energy required for particle detachment from the interface [22].
Additionally, droplet size distribution evidence polydispersity for both samples, with dodecane emulsions exhibiting a lower polydispersity profile. Despite some separation, stable emulsions were maintained over time, as evidenced by consistent droplet size measurements and physical stability analysis. These results offer a better understanding of the behavior of Pickering emulsions stabilized with silica nanoparticles modified with oleic acid, where the oil phases—dodecane and liquid paraffin—serve as models to evaluate the behavior of the modified nanoparticles as stabilizer. This approach opens the possibility for further studies, where the modified nanoparticles can be used to stabilize emulsions with more complex oil phases. Such systems may find applications in various fields, including pharmaceuticals, drug delivery, cosmetics, food industry, among others [20,32,48,49].
3. Materials and Methods
3.1. Materials
All aqueous solutions were prepared using distilled water. Sugarcane bagasse ash was provided by La Calandria, a panela-producing company located in San Roque, Antioquia, Colombia. Hydrochloric acid (35–37%) and sodium hydroxide (reagent grade) were obtained from Merck KGaA, Darmstadt, Germany, while dodecane, liquid paraffin, and oleic acid (>90%) were purchased from Sigma-Aldrich, St. Louis, MO, USA.
3.2. Nanoparticle Synthesis
Synthesis of silica nanoparticles was carried out using a method based on previous works with some modifications [4,27], Figure 8 presents a scheme of the synthesis process. Initially, the raw material was sieved with a #100 mesh equivalent to an average particle size of 150 μm to achieve a uniform size distribution. Subsequently, the product underwent an acid pretreatment with 1 M hydrochloric acid (HCl) at 80 °C, continuously stirred for 1 h, with the purpose of removing metal ions and oxides. The insoluble matter was washed with deionized water until a pH of 5 was reached, then dried at 105 °C for 2 h. An ash/acid solution ratio of 1:10 by weight was used for this reaction.
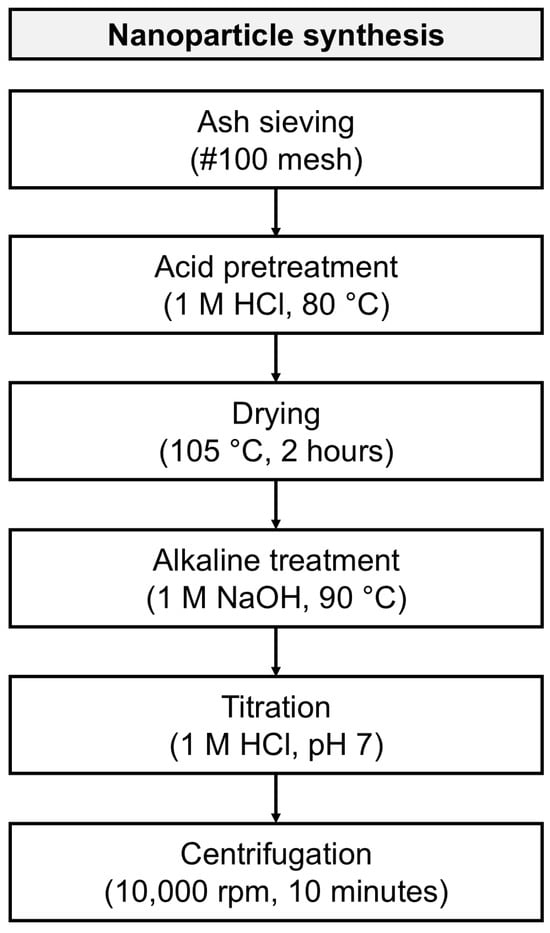
Figure 8.
Flow diagram for silica nanoparticles production.
In order to obtain a sodium silicate solution, the product underwent an alkaline reaction using a 1M sodium hydroxide (NaOH) solution at a 1:10 weight ratio, at 90 °C, and a continuous stirring for 1 h. The mixture was filtered, and the resulting sodium silicate solution was allowed to rest at room temperature for 24 h.
The solution was titrated with 1 M HCl, added dropwise under slow and constant stirring until reaching a pH of 7, to obtain silicon dioxide (SiO2). The resulting solution was aged for 24 h. After this time passed, the gel was centrifuged four times with distilled water and four times with deionized water, the supernatant was discarded to remove water-soluble impurities, using consistent centrifugation parameters (10,000 rpm for 10 min) (Hermle Z 326K, Hermle, Gosheim, Germany). Finally, the remaining solid products were stored in a refrigerator at 4 °C for subsequent characterization.
3.3. Adsorption Modification of Nanoparticles
Adsorption modification of the obtained nanoparticles was performed using oleic acid, based on the methodology proposed by Sadeghpour et al. [22]. The obtained nanoparticles were dispersed in distilled water using a sonic probe (Qsonica Q700, Qsonica, Newtown, CT, USA) at 20 kHz for 180 s at 30% power. Separately, oleic acid was also dispersed in distilled water by sonication for 180 s at 30% power. Subsequently, both solutions were mixed and sonicated for 180 s at 30% power to achieve the adsorption modification of silica nanoparticles.
The concentrations of nanoparticles and oleic acid were varied to study the effect of these variables on the emulsion properties. Nanoparticles concentration of 0.5 or 1 wt.% with respect to the continuous phase were chosen, while for oleic acid, 10 or 20 wt.% with respect to the quantity of nanoparticles; these conditions were selected according to the results observed in the literature [25,35,44].
3.4. Nanoparticles Characterization
3.4.1. Field Emission Scanning Electron Microscope (FESEM)
The morphological characteristics of silica nanoparticles were examined using a field emission scanning electron microscope (FESEM) (Apreo 2 S LoVac, Thermo Fisher Scientific, Waltham, MA, USA) with a typical acceleration voltage of 20 kV and a beam current of 0.10 nA. Prior to analysis, sample dilutions were prepared following a 1:1000 ratio. A 10 μL drop of the sample was deposited onto a sample holder and allowed to rest for 24 h, to allow water evaporation at room temperature. Subsequently, the samples were coated with gold, using vacuum sputtering. Additionally, energy-dispersive X-ray spectroscopy (EDS) analysis was performed using the same equipment to study the elemental composition of the sample.
To evaluate the particle size and morphology, measurements were performed on FE-SEM micrographs using Fiji 2.16.0 [50]. Image processing included contrast enhancement, background subtraction, and threshold-based segmentation, allowing the measurement of Feret mean diameter of individual nanoparticles and aggregates. Measurements were performed on 85 particles (n = 85) from images at 20,000× magnification.
3.4.2. Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy (ATR–FTIR)
ATR-FTIR spectroscopy analysis of both modified (OA/SiO2 NPs) and unmodified (SiO2 NPs) nanoparticles was performed with an FTIR spectrometer (Thermo Scientific Nicolet iS50, Waltham, MA, USA) with a single-reflection ATR and type-IIA diamond crystal mounted on tungsten carbide. The diamond ATR had a sampling area of 0.5 mm2, and a consistent reproducible pressure was applied to every sample. The FTIR spectra were collected between 4000 and 400 cm−1 for 64 scans at a 4 cm−1 resolution. Each spectrum represents the average of four spectra, and all spectra were corrected using the advanced ATR correction of OMNIC 9TM software (Thermo Scientific), to eliminate the diamond crystal effect and ease comparison with the transmission spectra.
3.5. Pickering Emulsions Fabrication
Pickering emulsions were processed, using two model oil phases, dodecane and liquid paraffin. These oil phases were selected as model phases since they are frequently used in the literature [22,35,44]. Pickering emulsions using an aqueous/oil phase ratio of 4:1 were prepared following the methodology proposed by Gao et al. [44]. The oil phase was added to the previously obtained continuous phase, and the mixture was processed using a rotor–stator homogenizer (Ultra Turrax T25, IKA, Cologne, Germany) at 16,000 rpm for 120 s. The homogenizer facilitates the dispersion of oil droplets in the continuous phase through high shear forces [49]. Subsequently, the sample was sonicated at 60% power for 240 s. This equipment induces cavitation, enabling the reduction in oil droplet size and achieving a more uniform size distribution [44].
Following the emulsification process, samples were labeled according to the type of oil phase used and the concentration of nanoparticles and oleic acid, as detailed in Table 3. Experiments were conducted in triplicate to ensure data reproducibility () and statistical analysis (Student’s t-test) for corresponding characterizations was performed.

Table 3.
Labels for the emulsions, according to their type of oil phase, SiO2 NPs concentration and oleic acid concentration.
3.6. Pickering Emulsions Characterization
3.6.1. Physical Stability
The stability of all the emulsions was evaluated by visual observation with photographs on days 0, 1, 7 and 15 after their preparation [51]. Image acquisition was performed using a mobile device (iPhone 15TM, Apple Inc., Cupertino, CA, USA).
3.6.2. Emulsification Index
The emulsification index (EI) is defined as the ratio between the height of the emulsion layer () and the total height of the sample (). All samples were stored at room temperature for 15 days, and their EI were calculated using Equation (2).
3.6.3. Particle Size Distribution
The size distribution of adsorption-modified SiO2 particles and the droplets of the obtained Pickering emulsions were determined using a static light scattering particle size analyzer (LA-960, Horiba, Kyoto, Japan) at room temperature [52]. Results were analyzed based on the average diameter (d50), volume mean diameter D4,3 as shown in Equation (3), and the relative span (δ) [53] given in Equation (4).
where n is the number of particles with diameter d.
where d90, d10, d50 are droplet diameters corresponding to 90%, 10%, and 50 vol.% on the accumulation curve, respectively. The relative span is employed to characterize emulsion dispersion, where values below 0.5 indicate a high degree of monodispersity [44].
4. Conclusions
This work proposed a green synthesis methodology for obtaining silica nanoparticles from sugarcane bagasse ash, followed by oleic acid adsorption modification to develop amphiphilic particles suitable for Pickering emulsion stabilization. The synthesized nanoparticles, primarily composed of silicon and oxygen, exhibited a degree of agglomeration, as observed through FESEM-EDS analysis. Adsorption modification with oleic acid was successfully confirmed by ATR–FTIR, revealing characteristic absorption bands of the modifier. The amphiphilic nanoparticles were then used as solid stabilizers in oil-in-water Pickering emulsions formulated with liquid paraffin and dodecane as model oil phases. The results showed that both the concentration of nanoparticles and the presence of oleic acid significantly influenced the physical stability of the emulsions, producing higher stability in emulsions formulated with liquid paraffin rather than those with dodecane. Low concentrations led to rapid destabilization, while medium to high concentrations yielded stable emulsions over 15 days in liquid paraffin emulsions. Oleic acid modification played a key role in maintaining emulsion stability, especially in formulations containing liquid paraffin.
This research contributes to the valorization of agro-industrial by-products by demonstrating that high-value materials such as silica nanoparticles can be obtained from by-products like sugarcane bagasse ash. The ash used as source material originates directly from the conventional processing of sugarcane and was used without any alteration to the existing process, enhancing the suitability of the proposed approach. The results showed that emulsions prepared with high concentrations of silica nanoparticles and oleic acid remained physically stable over time. This highlights the potential of adsorption-modified silica nanoparticles as effective stabilizers in the formulation of surfactant-free emulsions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30224464/s1, Figure S1: Dodecane emulsions stabilized with oleic acid at (a) 1 d and (b) 5 d; Table S1: Effect of time on the emulsification index of emulsions with modified SiO2 NPs for oil phases dodecane and liquid paraffin; Figure S2: Effect of time on the emulsification index for dodecane (D) and liquid paraffin (P) emulsions with different concentrations of SiO2 NPs and oleic acid as stabilizers. The graph shows that dodecane emulsions decrease their emulsification index faster than liquid paraffin emulsions, indicating that liquid paraffin emulsions are more stable over time.
Author Contributions
D.J.-V., A.F.-C., M.O.-C. and L.D.B. carried out the synthesis of the nanoparticles, experimental procedures, materials characterization, and data analysis. J.A.V.-C., E.U.-B., R.A.V.-C. and C.G.-H. contributed to the conceptualization of the study, experimental design, and the writing and review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 100,000 Strong in the Americas Innovation Fund under grant number 808C-04/23-49, Universidad Pontificia Bolivariana, Medellín, Colombia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors acknowledge Trapiche La Calandria for providing the sugarcane ash used in this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| SBA | Sugarcane Bagasse Ash |
| SiO2 NPs | Silica nanoparticles |
| O/W | Oil/Water |
| W/O | Water/Oil |
| ATR-FTIR | Attenuated total reflection—Fourier transform infrared spectroscopy |
| FESEM-EDS | Field emission scanning electron microscopy and energy-dispersive X-ray spectroscopy |
| HCl | Hydrochloric acid |
| NaOH | Sodium hydroxide |
References
- FAO. FAOSTAT: Food Supply Quantity—Crops and Livestock Products [Internet]. 2024. Available online: https://www.fao.org/faostat/en/#home (accessed on 31 July 2025).
- Chindaprasirt, P.; Rattanasak, U. Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci. Rep. 2020, 10, 9890. Available online: https://www.nature.com/articles/s41598-020-66885-y (accessed on 12 October 2025). [CrossRef] [PubMed]
- September, L.A.; Kheswa, N.; Seroka, N.S.; Khotseng, L. Green synthesis of silica and silicon from agricultural residue sugarcane bagasse ash—A mini review. RSC Adv. 2023, 13, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Falk, G.; Shinhe, G.P.; Teixeira, L.B.; Moraes, E.G.; de Oliveira, A.P.N. Synthesis of silica nanoparticles from sugarcane bagasse ash and nano-silicon via magnesiothermic reactions. Ceram. Int. 2019, 45, 21618–21624. [Google Scholar] [CrossRef]
- Muñoz, J.L.T.; Carranza, B.H.; Cortés, Y.P.C.; Rodríguez, L.A.A. Comprehensive characterization of high Andean sugarcane production systems (Saccharum officinarum) for panela production in Colombia. Rev. Ceres 2024, 71, e71036. [Google Scholar] [CrossRef]
- Sales, A.; Lima, S.A. Use of Brazilian sugarcane bagasse ash in concrete as sand replacement. Waste Manag. 2010, 30, 1114–1122. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0956053X10000681?via%3Dihub (accessed on 12 October 2025). [CrossRef] [PubMed]
- Joshaghani, A.; Moeini, M.A. Evaluating the effects of sugar cane bagasse ash (SCBA) and nanosilica on the mechanical and durability properties of mortar. Constr. Build. Mater. 2017, 152, 818–831. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0950061817313776?via%3Dihub (accessed on 12 October 2025). [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Biriș, S.-Ș. Sustainable Valorization of Waste and By-Products from Sugarcane Processing. Sustainability 2022, 14, 11089. Available online: https://www.mdpi.com/2071-1050/14/17/11089/htm (accessed on 12 October 2025). [CrossRef]
- Norsuraya, S.; Fazlena, H.; Norhasyimi, R. Sugarcane Bagasse as a Renewable Source of Silica to Synthesize Santa Barbara Amorphous-15 (SBA-15). Procedia Eng. 2016, 148, 839–846. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Aly, A.A.; Bräse, S. Recent progress in the applications of silica-based nanoparticles. RSC Adv. 2022, 12, 13706. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9073631/ (accessed on 9 November 2024). [CrossRef]
- Nakashima, H.; Omae, K.; Sakai, T.; Yamazaki, K.; Sakurai, H. Acute and subchronic inhalation toxicity of tetraethoxysilane (TEOS) in mice. Arch. Toxicol. 1994, 68, 277–283. Available online: https://link.springer.com/article/10.1007/s002040050069 (accessed on 12 October 2025). [CrossRef]
- Nakashima, H. Time course of effects of tetraethoxysilane (TEOS) on the kidney and blood silicon concentration in mice. Arch. Toxicol. 1994, 69, 59–64. Available online: https://link.springer.com/article/10.1007/s002040050138 (accessed on 12 October 2025). [CrossRef] [PubMed]
- Cáceres-Zambrano, J.; Ramírez-Gil, J.G.; Barrios, D. Validating Technologies and Evaluating the Technological Level in Avocado Production Systems: A Value Chain Approach. Agronomy 2022, 12, 3130. Available online: https://www.mdpi.com/2073-4395/12/12/3130/htm (accessed on 9 November 2025). [CrossRef]
- Martins, R.; Fernandes De Oliveira, S.; Martinho, H.; Santos, L.D.; Fernandes, S.S.; Buranelo Egea, M. Exploring Plant Agro-Industrial By-Products as a Source of Fibrous Food Ingredients: A Review of Extraction Methods and Technological Properties. Food Sci. 2025, 90, e70408. [Google Scholar] [CrossRef]
- Moreno Choconta, L.N. Aprovechamiento del Bagazo de la Caña Procedente de la Industria Panelera, a Través del Tratamiento Hidrotermal. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2024. Available online: https://repositorio.unal.edu.co/handle/unal/86262 (accessed on 7 November 2025).
- Mod, B.; Baskar, A.V.; Bahadur, R.; Tavakkoli, E.; Van Zwieten, L.; Singh, G.; Vinu, A. From cane to nano: Advanced nanomaterials derived from sugarcane products with insights into their synthesis and applications. Sci. Technol. Adv. Mater. 2024, 25, 2393568. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11376298/ (accessed on 9 November 2025). [CrossRef]
- UN Office for Partnerships. Sustainable Development Goals—Briefing Book 2023; United Nations: New York, NY, USA, 2023. [Google Scholar]
- Berton-Carabin, C.C.; Schroën, K. Pickering emulsions for food applications: Background, trends, and challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. Available online: https://pubmed.ncbi.nlm.nih.gov/25705932/ (accessed on 20 April 2025). [CrossRef]
- Heidari, F.; Jafari, S.M.; Ziaiifar, A.M.; Anton, N. Preparation of Pickering Emulsions Stabilized by Modified Silica Nanoparticles via the Taguchi Approach. Pharmaceutics 2022, 14, 1561. Available online: https://www.mdpi.com/1999-4923/14/8/1561/htm (accessed on 10 June 2024). [CrossRef]
- Zhao, H.; Yang, Y.; Chen, Y.; Li, J.; Wang, L.; Li, C. A review of multiple Pickering emulsions: Solid stabilization, preparation, particle effect, and application. Chem. Eng. Sci. 2022, 248, 117085. [Google Scholar] [CrossRef]
- Björkegren, S.; Nordstierna, L.; Törncrona, A.; Palmqvist, A. Hydrophilic and hydrophobic modifications of colloidal silica particles for Pickering emulsions. J. Colloid Interface Sci. 2017, 487, 250–257. Available online: https://www.sciencedirect.com/science/article/abs/pii/S002197971630786X?via%3Dihub (accessed on 12 October 2025). [CrossRef]
- Sadeghpour, A.; Pirolt, F.; Glatter, O. Submicrometer-sized pickering emulsions stabilized by silica nanoparticles with adsorbed oleic acid. Langmuir 2013, 29, 6004–6012. [Google Scholar] [CrossRef] [PubMed]
- Premaratne, W.A.P.J.; Priyadarshana, W.M.G.I.; Gunawardena, S.H.P.; De Alwis, A.A.P. Synthesis of nanosilica from paddy husk ash and their surface functionalization. J. Sci. Univ. Kelaniya 2013, 8, 33–48. [Google Scholar] [CrossRef]
- Future Market Insights. Oleic Acid Industry Outlook from 2024 to 2034 [Internet]. 2023. Available online: https://www.futuremarketinsights.com/reports/oleic-acid-market (accessed on 26 September 2024).
- Li, Z.; Zhu, Y. Surface-modification of SiO2 nanoparticles with oleic acid. Appl. Surf. Sci. 2003, 211, 315–320. [Google Scholar] [CrossRef]
- Nistor, C.L.; Ianchis, R.; Ghiurea, M.; Nicolae, C.A.; Spataru, C.I.; Culita, D.C.; Pandele Cusu, J.; Fruth, V.; Oancea, F.; Donescu, D. Aqueous Dispersions of Silica Stabilized with Oleic Acid Obtained by Green Chemistry. Nanomaterials 2016, 6, 9. Available online: https://www.mdpi.com/2079-4991/6/1/9/htm (accessed on 12 October 2025). [CrossRef]
- Mohd, N.K.; Wee, N.N.A.N.; Azmi, A.A. Green synthesis of silica nanoparticles using sugarcane bagasse. AIP Conf. Proc. 2017, 1885, 020123. [Google Scholar]
- Rovani, S.; Santos, J.J.; Corio, P.; Fungaro, D.A. An alternative and simple method for the preparation of bare silica nanoparticles using sugarcane waste ash, an abundant and despised residue in the Brazilian industry. J. Braz. Chem. Soc. 2019, 30, 1524–1533. [Google Scholar] [CrossRef]
- Bortolotto Teixeira, L.; Guzi de Moraes, E.; Paolinelli Shinhe, G.; Falk, G.; Novaes de Oliveira, A.P. Obtaining Biogenic Silica from Sugarcane Bagasse and Leaf Ash. Waste Biomass Valoriz. 2021, 12, 3205–3221. [Google Scholar] [CrossRef]
- Ni’mah, Y.L.; Muhaiminah, Z.H.; Suprapto, S. Synthesis of Silica Nanoparticles from Sugarcane Bagasse by Sol-Gel Method. Nano Part. 2023, 4, 4–8. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An overview of pickering emulsions: Solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017, 8, 235054. Available online: www.frontiersin.org (accessed on 10 June 2024). [CrossRef]
- Santamaría, E.; Maestro, A.; Vilchez, S.; González, C. Study of nanoemulsions using carvacrol/MCT-(Oleic acid-potassium oleate)/ Tween 80 ®- water system by low energy method. Heliyon 2023, 9, e16967. Available online: https://pubmed.ncbi.nlm.nih.gov/37332948/ (accessed on 10 November 2025). [CrossRef]
- Oba, N.; Sugimura, H.; Umehara, Y.; Yoshida, M.; Kimura, T.; Yamaguchi, T. Evaluation of an oleic acid water-in-oil-in-water-type multiple emulsion as potential drug carrier via the enteral route. Lipids 1992, 27, 701–705. Available online: https://pubmed.ncbi.nlm.nih.gov/1487968/ (accessed on 12 October 2025). [CrossRef]
- González-González, A.; Sánchez-Arribas, N.; Santini, E.; Rodríguez-Villafuerte, J.L.; Carbone, C.; Ravera, F.; Ortega, F.; Liggieri, L.; Rubio, R.G.; Guzmán, E. Effects of Oil Phase on the Inversion of Pickering Emulsions Stabilized by Palmitic Acid Decorated Silica Nanoparticles. Colloids Interfaces 2022, 6, 27. Available online: https://www.mdpi.com/2504-5377/6/2/27/htm (accessed on 16 October 2023). [CrossRef]
- Lin, Z.; Zhang, Z.; Li, Y.; Deng, Y. Magnetic nano-Fe3O4 stabilized Pickering emulsion liquid membrane for selective extraction and separation. Chem. Eng. J. 2016, 288, 305–311. [Google Scholar] [CrossRef]
- Oyola-Oliveras, M. Understanding the Surface Interaction Between Silica and Oleic Acid. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2005. Available online: https://etd.ohiolink.edu/acprod/odb_etd/ws/send_file/send?accession=osu1730298184582362&disposition=inline#page=75.08 (accessed on 10 November 2025).
- Liu, W.; Chen, S. An investigation of the tribological behaviour of surface-modified ZnS nanoparticles in liquid paraffin. Wear 2000, 238, 120–124. [Google Scholar] [CrossRef]
- Capek, I. Degradation of kinetically-stable o/w emulsions. Adv. Colloid Interface Sci. 2004, 107, 125–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; He, Y.; Xu, X.; Zhao, X. Oil density and viscosity affect emulsion stability and destabilization mechanism. J. Food Eng. 2024, 366, 111864. [Google Scholar] [CrossRef]
- Tan, H.; Han, L.; Yang, C. Effect of oil type and β-carotene incorporation on the properties of gelatin nanoparticle-stabilized pickering emulsions. LWT 2021, 141, 110903. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, F.; Ma, C.; Yuan, F.; Gao, Y. Effect of carrier oils on the physicochemical properties of orange oil beverage emulsions. Food Res. Int. 2015, 74, 260–268. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, Y.; Chen, L.; Jiang, H.; Tian, C.; Fu, M.; Lyu, C. Effect of hazelnut protein oxidation on O/W emulsion stability, interfacial properties and lipid oxidation based on hazelnut protein oxidation. J. Stored Prod. Res. 2025, 111, 102534. Available online: https://www.sciencedirect.com/science/article/pii/S0022474X24002911?via%3Dihub#bib32 (accessed on 10 November 2025). [CrossRef]
- Gao, J.; Bu, X.; Zhou, S.; Wang, X.; Bilal, M.; Hassan, F.U.; Hassanzadeh, A.; Xie, G.; Chelgani, S.C. Pickering emulsion prepared by nano-silica particles—A comparative study for exploring the effect of various mechanical methods. Ultrason. Sonochem. 2022, 83, 105928. [Google Scholar] [CrossRef]
- Yi, J.; Ning, J.; Zhu, Z.; Cui, L.; Decker, E.A.; McClements, D.J. Impact of interfacial composition on co-oxidation of lipids and proteins in oil-in-water emulsions: Competitive displacement of casein by surfactants. Food Hydrocoll. 2019, 87, 20–28. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0268005X18306672 (accessed on 23 April 2025). [CrossRef]
- McDonald, C. The solubility of dodecane in water-amide mixtures. J. Pharm. Pharmacol. 1970, 22, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xu, A.; Zhang, T.; Hong, L. Tailoring the Wettability of Colloidal Particles for Pickering Emulsions via Surface Modification and Roughness. Front. Chem. 2018, 6, 225. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6018170/ (accessed on 13 April 2024). [CrossRef] [PubMed]
- de Carvalho-Guimarães, F.B.; Correa, K.L.; de Souza, T.P.; Rodríguez Amado, J.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. A Review of Pickering Emulsions: Perspectives and Applications. Pharmaceuticals 2022, 15, 1413. Available online: https://www.mdpi.com/1424-8247/15/11/1413/htm (accessed on 10 June 2024). [CrossRef]
- Gómez Hoyos, C.; Botero, L.D.; Flórez-Caro, A.; Velásquez-Cock, J.A.; Zuluaga, R. Nanocellulose from Cocoa Shell in Pickering Emulsions of Cocoa Butter in Water: Effect of Isolation and Concentration on Its Stability and Rheological Properties. Polymers 2023, 15, 4157. Available online: https://www.mdpi.com/2073-4360/15/20/4157/htm (accessed on 11 March 2024). [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. Available online: https://www.nature.com/articles/nmeth.2019 (accessed on 15 October 2025). [CrossRef]
- Mikulcová, V.; Bordes, R.; Kašpárková, V. On the preparation and antibacterial activity of emulsions stabilized with nanocellulose particles. Food Hydrocoll. 2016, 61, 780–792. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Sun, X.; Fan, F. Development and Characterization of Pickering Emulsion Stabilized by Walnut Protein Isolate Nanoparticles. Molecules 2023, 28, 5434. [Google Scholar] [CrossRef]
- Mugabi, J.; Igura, N.; Shimoda, M. Effect of process parameters on oil-in-water emulsion droplet size and distribution in swirl flow membrane emulsification. J. Chem. Eng. Jpn. 2018, 51, 229–236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).