Therapeutic Potentials of the Seaweed-Derived Compounds for Alzheimer’s Disease
Abstract
1. Introduction
2. Molecular and Cellular Mechanisms Underlying Cognitive Performance in AD
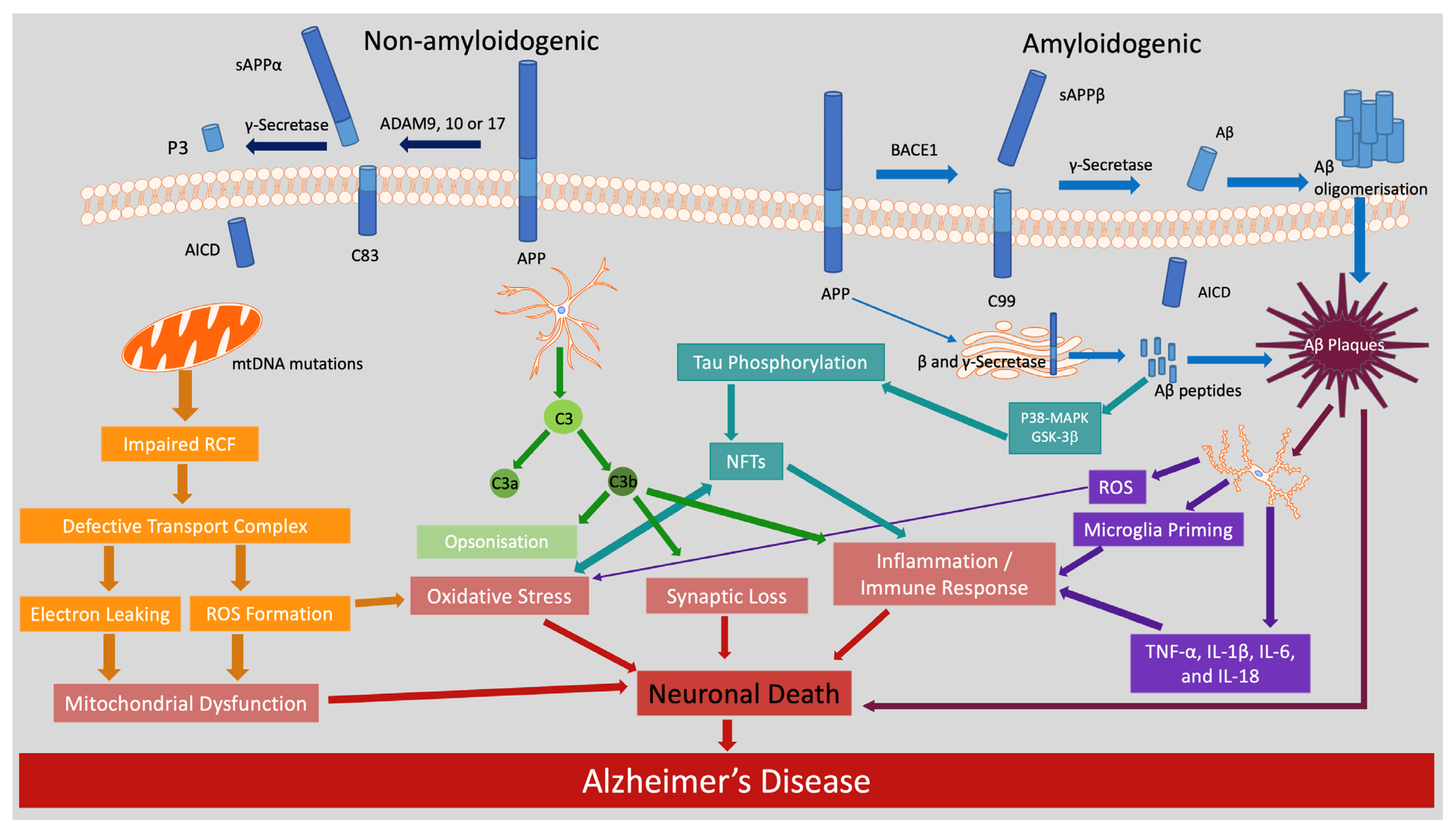
2.1. Amyloid and Non-Amyloidogenic Pathways in AD
2.2. Tau Phosphorylation and Neurofibrillary Tangles
2.3. Oxidative Stress and Mitochondrial Dysfunction
2.4. Neuroinflammation
2.5. Additional Pathways
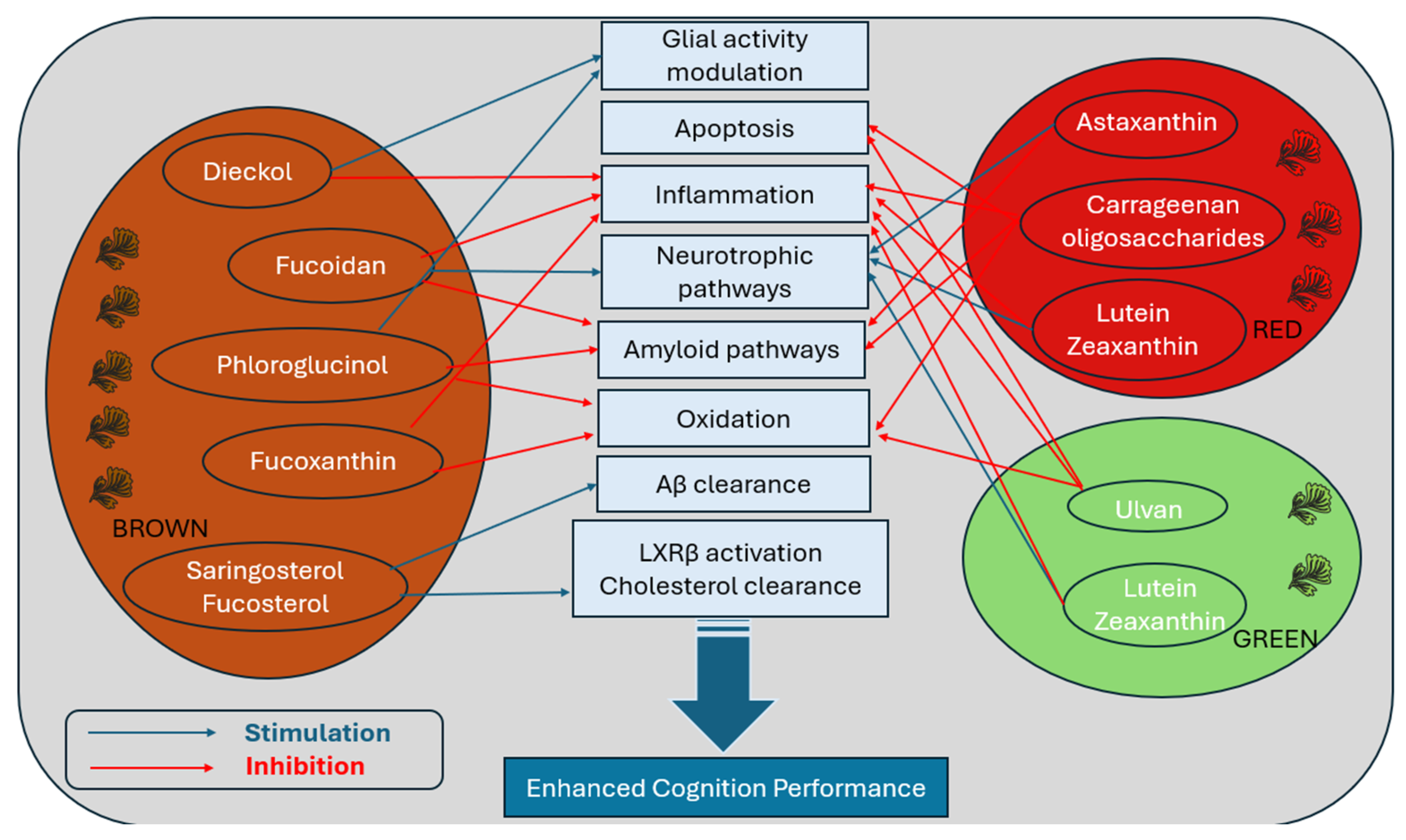
3. Therapeutic Potential of Seaweed Compounds in Modulating Brain Ageing Pathways
3.1. Phaeophyceae (Brown Seaweed)
3.2. Rhodophyta (Red Seaweed)
3.3. Chlorophyta (Green Seaweed)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| AChEIs | Acetylcholinesterase inhibitors |
| ADAM10 | a disintegrin and metalloproteinase domain-containing protein 10 |
| AICD | APP intracellular domain |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AOC | Antioxidant capacity |
| APP | Amyloid precursor protein |
| ARIAs | amyloid-related imaging abnormalities |
| ARIA-E | vasogenic oedema |
| BACE1 | β-site APP-cleaving enzyme 1 |
| BDNF | brain-derived neurotrophic factor |
| COX-2 | cyclooxygenase-2 |
| CREB | Cyclic AMP-responsive element-binding protein |
| CTNF | ciliary neurotrophic factor |
| DLPFC | dorsolateral prefrontal cortex |
| EGCG | epigallocatechin gallate |
| GFAP | Glial fibrillary acidic protein |
| GABA | Gamma-aminobutyric acid |
| GMLT | Groton maze learning test |
| GSK3β | Glycogen synthase kinase-3 beta |
| Iba-1 | allograft inflammatory factor 1 |
| ICV | intracerebral ventricular |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| IOE | I. Okamurae extracts |
| JNK | c-Jun N-terminus kinase |
| KOS | κ-carrageenan oligosaccharides |
| LPS | lipopolysaccharide |
| LTP | long-term potentiation |
| LXR | liver X receptor |
| MAB | monoclonal antibodies |
| MAPK | mitogen-activated protein kinase |
| MAPK/ERK | mitogen-activated protein kinase/extracellular signal-regulated kinase |
| MCI | Mild cognitive impairment |
| MDA | malondialdehyde |
| MPOD | macular pigment optic density |
| mtDNA | Mitochondrial DNA |
| NF-κB | nuclear factor kappa-light-chain-enhance of activated B cells |
| NFTs | Neurofibrillary tangles |
| NO | nitric oxide |
| OLGs | oligodendrocyte lineage cells |
| OLT | object location test |
| OPCs | oligodendrocyte precursor cells |
| ORT | object recognition test |
| PARP | poly (ADP-ribose) polymerase |
| PI3K/Akt | phosphatidylinositol 3-kinase/protein kinase B |
| PLOOH | phospholipid hydroperoxide |
| PT | Phaeodactylum tricornutum |
| P38-MAPK | P38 mitogen-activated protein kinase |
| RCF | Respiratory chain function |
| RCTs | Randomised controlled trials |
| ROS | Reactive oxygen species |
| sAPP | Soluble |
| TNF | tumour necrosis factor |
| 4-HNE | 4-hydroxynoneal |
| 5-HT | serotonin |
| 6-OHDA | 6-hydroxydopamine |
| α7 nAChRs | α7 subtype of nicotinic acetylcholine receptors |
References
- World Health Organisation. Dementia. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 6 June 2025).
- Gardner, R.C.; Valcour, V.; Yaffe, K. Dementia in the oldest old: A multi-factorial and growing public health issue. Alzheimer’s Res. Ther. 2013, 5, 27. [Google Scholar] [CrossRef]
- Welberry, H.J.; Brodaty, H.; Hsu, B.; Barbieri, S.; Jorm, L.R. Measuring dementia incidence within a cohort of 267,153 older Australians using routinely collected linked administrative data. Sci. Rep. 2020, 10, 8781. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Emmady, P.D.; Schoo, C.; Tadi, P. Major Neurocognitive Disorder (Dementia). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557444/ (accessed on 24 June 2025).
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 64, 1077–1083. [Google Scholar] [CrossRef]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.S.; Choi, D.K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef] [PubMed]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef] [PubMed]
- Latham, A.S.; Moreno, J.A.; Geer, C.E. Biological agents and the aging brain: Glial inflammation and neurotoxic signaling. Front. Aging 2023, 4, 1244149. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed]
- Lye, S.; Aust, C.E.; Griffiths, L.R.; Fernandez, F. Exploring New Avenues for Modifying Course of Progression of Alzheimer’s Disease: The Rise of Natural Medicine. J. Neurol. Sci. 2021, 422, 117332. [Google Scholar] [CrossRef]
- Castellani, R.J.; Jamshidi, P.; Plascencia-Villa, G.; Perry, G. The Amyloid Cascade Hypothesis: A Conclusion in Search of Support. Am. J. Pathol. 2024, 195, 1988–1997. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturia, Z.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Lyo, M.; Namba, H.; Fukushi, K.; Shinotoh, H.; Nagatsuka, S.; Suhara, T.; Sudo, Y.; Suzuki, K.; Irie, T. Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer’s disease. Lancet 1997, 349, 1805–1809. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981, 10, 122–126. [Google Scholar] [CrossRef]
- Muth, K.; Schönmeyer, R.; Matura, S.; Haenschel, C.; Schröder, J.; Pantel, J. Mild Cognitive Impairment in the Elderly is Associated with Volume Loss of the Cholinergic Basal Forebrain Region. Biol. Psychiatry 2010, 67, 588–591. [Google Scholar] [CrossRef]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A.; et al. Vitamin E and Donepezil for the Treatment of Mild Cognitive Impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef]
- Feldman, H.H.; Ferris, S.; Winblad, B.; Sfikas, N.; Mancione, L.; He, Y.; Tekin, S.; Burns, A.; Cummings, J.; del Ser, T.; et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: The InDDEx study. Lancet Neurol. 2007, 6, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Bernabei, R.; Bullock, R.; Jentoft, A.J.C.; Frölich, L.; Hock, C.; Raivio, M.; Triau, E.; Vandewoude, M.; Wimo, A.; et al. Safety and efficacy of galantamine (Reminyl) in severe Alzheimer’s disease (the SERAD study): A randomised, placebo-controlled, double-blind trial. Lancet Neurol. 2009, 8, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Delrieu, J.; Piau, A.; Caillaud, C.; Voisin, T.; Vellas, B. Managing Cognitive Dysfunction through the Continuum of Alzheimer’s Disease. CNS Drugs 2011, 25, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Kim, S.; Nam, Y.; Park, Y.H.; Shin, S.M.; Moon, M. Second-generation anti-amyloid monoclonal antibodies for Alzheimer’s disease: Current landscape and future perspectives. Transl. Neurodegener. 2025, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, R.; Nixon, J.; Love, B.L.; Yunusa, I. Impacts of FDA Approval and Medicare Restriction on Antiamyloid Therapies for Alzheimer’s Disease: Patient Outcomes, Healthcare Costs, and Drug Development. Lancet Reg. Health–Am. 2023, 20, 100467. [Google Scholar] [CrossRef]
- Jannat, K.; Balakrishnan, R.; Han, J.H.; Yu, Y.J.; Kim, G.W.; Choi, D.K. The Neuropharmacological Evaluation of Seaweed: A Potential Therapeutic Source. Cells 2023, 12, 2652. [Google Scholar] [CrossRef]
- Lin, Y.; Im, H.; Diem, L.T.; Ham, S. Characterizing the structural and thermodynamic properties of Aβ42 and Aβ40. Biochem. Biophys. Res. Commun. 2019, 510, 442–448. [Google Scholar] [CrossRef]
- Meisl, G.; Yang, X.; Hellstrand, E.; Frohm, B.; Kirkegaard, J.B.; Cohen, S.I.A.; Dobson, C.M.; Linse, S.; Knowles, T.P.J. Differences in nucleation behavior underlie the contrasting aggregation kinetics of the Aβ40 and Aβ42 peptides. Proc. Natl. Acad. Sci. USA 2014, 111, 9384–9389. [Google Scholar] [CrossRef]
- Gu, L.; Guo, Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Haessler, A.; Gier, S.; Jung, N.; Windbergs, M. The Aβ42:Aβ40 ratio modulates aggregation in beta-amyloid oligomers and drives metabolic changes and cellular dysfunction. Front. Cell Neurosci. 2024, 18, 1516093. [Google Scholar] [CrossRef]
- Pauwels, K.; Williams, T.L.; Morris, K.L.; Jonckheere, W.; Vandersteen, A.; Kelly, G.; Schymkowitz, J.; Rousseau, F.; Pastore, A.; Serpell, L.C.; et al. Structural Basis for Increased Toxicity of Pathological Aβ42:Aβ40 Ratios in Alzheimer Disease. J. Biol. Chem. 2012, 287, 5650–5660. [Google Scholar] [CrossRef]
- Jensen, L.E.; Bultynck, G.; Luyten, T.; Amijee, H.; Bootman, M.D.; Roderick, H.L. Alzheimer’s disease-associated peptide Aβ42 mobilizes ER Ca2+ via InsP3R-dependent and -independent mechanisms. Front. Mol. Neurosci. 2013, 6, 36. [Google Scholar] [CrossRef]
- Li, S.; Selkoe, D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer brain. J. Neurochem. 2020, 154, 583–597. [Google Scholar] [CrossRef]
- Marsh, J.; Alifragis, P. Synaptic dysfunction in Alzheimer’s disease: The effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention. Neural Regen. Res. 2018, 13, 616–623. [Google Scholar] [CrossRef]
- Shankar, G.M.; Walsh, D.M. Alzheimer’s disease: Synaptic dysfunction and Aβ. Mol. Neurodegener. 2009, 4, 48. [Google Scholar] [CrossRef]
- Abanto, J.T.; Dwivedi, A.K.; Imbimbo, B.P.; Espay, A.J. Association between amyloid-β42 levels and neuropsychiatric symptoms in Alzheimer’s disease trials. Brain Commun. 2025, 7, fcaf089. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, Z.; Huang, Q.; Lei, Y.; Liu, H.; Xu, P. The Role of Oligodendrocyte Lineage Cells in the Pathogenesis of Alzheimer’s Disease. Neurochem. Res. 2025, 50, 72. [Google Scholar] [CrossRef]
- Ziar, R.; Tesar, P.J.; Clayton, B.L.L. Astrocyte and oligodendrocyte pathology in Alzheimer’s disease. Neurotherapeutics 2025, 22, e00540. [Google Scholar] [PubMed]
- Sasmita, A.O.; Depp, C.; Nazarenko, T.; Sun, T.; Siems, S.B.; Ong, E.C.; Nkeh, Y.B.; Böhler, C.; Yu, X.; Bues, B.; et al. Oligodendrocytes produce amyloid-β and contribute to plaque formation alongside neurons in Alzheimer’s disease model mice. Nat. Neurosci. 2024, 27, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Spieth, L.; Simons, M. Remember oligodendrocytes: Uncovering their overlooked role in Alzheimer’s disease. PLoS Biol. 2024, 22, e3002798. [Google Scholar] [CrossRef]
- Elsworthy, R.J.; Dunleavy, C.; Whitham, M.; Aldred, S. Exercise for the prevention of Alzheimer’s disease: Multiple pathways to promote non-amyloidogenic AβPP processing. Aging Health Res. 2022, 2, 100093. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Jabir, M.S.; Al-Gareeb, A.I.; Albuhadily, A.K.; Albukhaty, S.; Sulaiman, G.M.; Batiha, G.E. Evaluation and targeting of amyloid precursor protein (APP)/amyloid beta (Aβ) axis in amyloidogenic and non-amyloidogenic pathways: A time outside the tunnel. Ageing Res. Rev. 2023, 92, 102119. [Google Scholar] [CrossRef] [PubMed]
- Chasseigneaux, S.; Allinquant, B. Functions of Aβ, sAPPα and sAPPβ: Similarities and differences. J. Neurochem. 2012, 120 (Suppl. 1), 99–108. [Google Scholar] [CrossRef]
- Habib, A.; Sawmiller, D.; Tan, J. Restoring sAPPα functions as a potential treatment for Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 973–991. [Google Scholar] [CrossRef] [PubMed]
- Hefter, D.; Draguhn, A. APP as a Protective Factor in Acute Neuronal Insults. Front. Mol. Neurosci. 2017, 10, 22. [Google Scholar] [CrossRef]
- Mockett, B.G.; Ryan, M.M. The therapeutic potential of the neuroactive peptides of soluble amyloid precursor protein-alpha in Alzheimer’s disease and related neurological disorders. Semin. Cell Dev. Biol. 2023, 139, 93–101. [Google Scholar] [CrossRef]
- Dar, N.J.; Glazner, G.W. Deciphering the neuroprotective and neurogenic potential of soluble amyloid precursor protein alpha (sAPPα). Cell. Mol. Life Sci. 2020, 77, 2315–2330. [Google Scholar] [CrossRef]
- Dineley, K.T.; Bell, K.A.; Bui, D.; Sweatt, J.D. β-Amyloid Peptide Activates α7 Nicotinic Acetylcholine Receptors Expressed in Xenopus Oocytes *. J. Biol. Chem. 2002, 277, 25056–25061. [Google Scholar] [CrossRef]
- Sharma, K.; Pradhan, S.; Duffy, L.K.; Yeasmin, S.; Bhattarai, N.; Schulte, M.K. Role of Receptors in Relation to Plaques and Tangles in Alzheimer’s Disease Pathology. Int. J. Mol. Sci. 2021, 22, 12987. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Jones, O.D.; Peppercorn, K.; Ohline, S.M.; Tate, W.P.; Abraham, W.C. Secreted amyloid precursor protein-alpha can restore novel object location memory and hippocampal LTP in aged rats. Neurobiol. Learn. Mem. 2017, 138, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Hullinger, R.; Puglielli, L. Molecular and cellular aspects of age-related cognitive decline and Alzheimer’s disease. Behav. Brain Res. 2017, 322 Pt B, 191–205. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Ye, H.; Han, Y.; Li, P.; Su, Z.; Huang, Y. The Role of Post-Translational Modifications on the Structure and Function of Tau Protein. J. Mol. Neurosci. 2022, 72, 1557–1571. [Google Scholar] [CrossRef]
- Malafaia, D.; Albuquerque, H.M.T.; Silva, A.M.S. Amyloid-β and tau aggregation dual-inhibitors: A synthetic and structure-activity relationship focused review. Eur. J. Med. Chem. 2021, 214, 113209. [Google Scholar] [CrossRef]
- Rodriguez Camargo, D.C.; Sileikis, E.; Chia, S.; Axell, E.; Bernfur, K.; Cataldi, R.L.; Cohen, S.I.A.; Miesl, G.; Habchi, J.; Knowles, T.P.J.; et al. Proliferation of Tau 304–380 Fragment Aggregates through Autocatalytic Secondary Nucleation. ACS Chem. Neurosci. 2021, 12, 4406–4415. [Google Scholar] [CrossRef]
- Thacker, D.; Barghouth, M.; Bless, M.; Zhang, E.; Linse, S. Direct observation of secondary nucleation along the fibril surface of the amyloid β 42 peptide. Proc. Natl. Acad. Sci. USA 2023, 120, e2220664120. [Google Scholar] [CrossRef]
- Nam, Y.; Shin, S.J.; Kumar, V.; Won, J.; Kim, S.; Moon, M. Dual modulation of amyloid beta and tau aggregation and dissociation in Alzheimer’s disease: A comprehensive review of the characteristics and therapeutic strategies. Transl. Neurodegener. 2025, 14, 15. [Google Scholar] [CrossRef]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; D’Adamio, L.; Grassi, C.; Devanand, D.P.; Honig, L.S.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimer’s Dis. JAD 2018, 64 (Suppl 1), S611–S631. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Buchholz, S.; Zempel, H. The six brain-specific TAU isoforms and their role in Alzheimer’s disease and related neurodegenerative dementia syndromes. Alzheimer’s Dement. 2024, 20, 3606–3628. [Google Scholar] [CrossRef]
- Park, S.A.; Ahn, S.I.; Gallo, J.M. Tau mis-splicing in the pathogenesis of neurodegenerative disorders. BMB Rep. 2016, 49, 405–413. [Google Scholar] [CrossRef]
- Zhang, C.C.; Xing, A.; Tan, M.S.; Tan, L.; Yu, J.T. The Role of MAPT in Neurodegenerative Diseases: Genetics, Mechanisms and Therapy. Mol. Neurobiol. 2016, 53, 4893–4904. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Huang, L.; Lei, F.; Li, T.; Luo, Y.; Zeng, M.; Wang, Z. The Role and Pathogenesis of Tau Protein in Alzheimer’s Disease. Biomolecules 2025, 15, 824. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, H.; Cai, Q.; Tai, H.C. The Enigma of Tau Protein Aggregation: Mechanistic Insights and Future Challenges. Int. J. Mol. Sci. 2024, 25, 4969. [Google Scholar] [CrossRef]
- Sayas, C.L.; Ávila, J. GSK-3 and Tau: A Key Duet in Alzheimer’s Disease. Cells 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; de Barreda, E.G.; Fuster-Matanzo, A.; Lucas, J.J.; Avila, J. GSK3: A possible link between beta amyloid peptide and tau protein. Exp. Neurol. 2010, 223, 322–325. [Google Scholar] [CrossRef]
- Almasoudi, S.H.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Eliwa, D.; Alexiou, A.; Papadakis, M.; Batiha, G.E. Role of mitogen-activated protein kinase inhibitors in Alzheimer’s disease: Rouge of brain kinases. Brain Res. Bull. 2025, 224, 111296. [Google Scholar] [CrossRef]
- Son, S.H.; Lee, N.R.; Gee, M.S.; Song, C.W.; Lee, S.J.; Lee, S.K.; Lee, Y.; Kim, H.J.; Lee, J.K.; Inn, K.S.; et al. Chemical Knockdown of Phosphorylated p38 Mitogen-Activated Protein Kinase (MAPK) as a Novel Approach for the Treatment of Alzheimer′s Disease. ACS Cent. Sci. 2023, 9, 417–426. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Zou, J.; Gao, H.; Shao, Z.; Li, C.; Lei, P. Protein kinases in neurodegenerative diseases: Current understandings and implications for drug discovery. Signal Transduct. Target. Ther. 2025, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Neddens, J.; Temmel, M.; Flunkert, S.; Kerschbaumer, B.; Hoeller, C.; Loeffler, T.; Niederkofler, V.; Daum, G.; Attems, J.; Hutter-Paier, B. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Iqbal, K. Hyperphosphorylation of Microtubule-Associated Protein Tau: A Promising Therapeutic Target for Alzheimer Disease. Curr. Med. Chem. 2008, 15, 2321–2328. [Google Scholar] [CrossRef]
- Hallinan, G.I.; Vargas-Caballero, M.; West, J.; Deinhardt, K. Tau Misfolding Efficiently Propagates between Individual Intact Hippocampal Neurons. J. Neurosci. 2019, 39, 9623–9632. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants 2023, 12, 131. [Google Scholar] [CrossRef]
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, R.E.; Helwig, M.; Bae, E.J.; Aboutalebi, H.; Lee, S.J.; Ulusoy, A.; Di Monte, D.A. Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular α-synuclein transfer. J. Clin. Investig. 2019, 129, 3738–3753. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar]
- Sastry, P.S.; Rao, K.S. Apoptosis and the Nervous System. J. Neurochem. 2000, 74, 1–20. [Google Scholar] [CrossRef]
- Millecamps, S.; Julien, J.P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 161–176. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Lim, J.L.; van der Pol, S.M.A.; Baron, W.; McCord, J.M.; de Vries, H.E.; van Horssen, J. Protandim Protects Oligodendrocytes against an Oxidative Insult. Antioxidants 2016, 5, 30. [Google Scholar] [CrossRef]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Brickman, A.M. White matter changes in Alzheimer’s disease: A focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef]
- Bona, D.; Scapagnini, G.; Candore, G.; Castiglia, L.; Colonna-Romano, G.; Duro, G.; Nuzzo, D.; Lemolo, F.; Lio, D.; Pellicano, M.; et al. Immune-Inflammatory Responses and Oxidative Stress in Alzheimers Disease: Therapeutic Implications. Curr. Pharm. Des. 2010, 16, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Wu, C.; Parker, E.; Liu, T.C.Y.; Duan, R.; Yang, L. Microglia and Astrocytes in Alzheimer’s Disease: Significance and Summary of Recent Advances. Aging Dis. 2024, 15, 1537–1564. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, H.J.; Vidyadhara, D.J.; Mahadevan, A.; Philip, M.; Parmar, S.K.; Manohari, S.G.; Shankar, S.K.; Raju, T.R.; Alladi, P.A. Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol. Aging 2015, 36, 3321–3333. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Bronzetti, E.; Sabbatini, M.; Vega, J.A. Astrocyte changes in aging cerebral cortex and hippocampus: A quantitative immunohistochemical study. Microsc. Res. Tech. 1998, 43, 29–33. [Google Scholar] [CrossRef]
- Cerbai, F.; Lana, D.; Nosi, D.; Petkova-Kirova, P.; Zecchi, S.; Brothers, H.M.; Wenk, G.L.; Giovannini, M.G. The Neuron-Astrocyte-Microglia Triad in Normal Brain Ageing and in a Model of Neuroinflammation in the Rat Hippocampus. PLoS ONE 2012, 7, e45250. [Google Scholar] [CrossRef]
- Kanaan, N.M.; Kordower, J.H.; Collier, T.J. Age-related changes in glial cells of dopamine midbrain subregions in rhesus monkeys. Neurobiol. Aging 2010, 31, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Robillard, K.N.; Lee, K.M.; Chiu, K.B.; MacLean, A.G. Glial Cell Morphological and Density Changes Through the Lifespan of Rhesus Macaques. Brain Behav. Immun. 2016, 55, 60–69. [Google Scholar] [CrossRef]
- Mansour, H.; Chamberlain, C.G.; Weible, I.I.M.W.; Hughes, S.; Chu, Y.; Chan-Ling, T. Aging-related changes in astrocytes in the rat retina: Imbalance between cell proliferation and cell death reduces astrocyte availability. Aging Cell. 2008, 7, 526–540. [Google Scholar] [CrossRef]
- Jinno, S. Regional and laminar differences in antigen profiles and spatial distributions of astrocytes in the mouse hippocampus, with reference to aging. Neuroscience 2011, 180, 41–52. [Google Scholar] [CrossRef]
- Xiaoli, W.; Yun, X.; Fang, W.; Lihua, T.; Zhilong, L.; Honglian, L.; Shenhong, L. Aging-related changes of microglia and astrocytes in hypothalamus after intraperitoneal injection of hypertonic saline in rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2006, 26, 231–234. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, M.M.; Erikson, G.A.; Shokhirev, M.N.; Allen, N.J. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep. 2018, 22, 269–285. [Google Scholar] [CrossRef]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The Complement System: An Unexpected Role in Synaptic Pruning During Development and Disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Litvinchuk, A.; Chiang, A.C.A.; Aithmitti, N.; Jankowsky, J.L.; Zheng, H. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. J. Neurosci. 2016, 36, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Wu, T.; Tsai, M.C.; Graykowski, D.; Gandham, V.D.; Rose, C.M.; Bakalarski, C.E.; Ngu, H.; Wang, Y.; Pandey, S.; et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2022, 2, 837–850. [Google Scholar] [CrossRef]
- Chen, Y.; Chu, J.M.T.; Wong, G.T.C.; Chang, R.C.C. Complement C3 from Astrocytes Plays Significant Roles in Sustained Activation of Microglia and Cognitive Dysfunctions Triggered by Systemic Inflammation After Laparotomy in Adult Male Mice. J. Neuroimmune Pharmacol. 2024, 19, 8. [Google Scholar] [CrossRef]
- Gomez-Arboledas, A.; Acharya, M.M.; Tenner, A.J. The Role of Complement in Synaptic Pruning and Neurodegeneration. ImmunoTargets Ther. 2021, 10, 373–386. [Google Scholar] [CrossRef]
- Choi, J.H.; Sim, S.E.; Kim, J.I.; Choi, D.I.; Oh, J.; Ye, S.; Lee, J.; Kim, T.H.; Ko, H.G.; Lim, C.S.; et al. Interregional synaptic maps among engram cells underlie memory formation. Science 2018, 360, 430–435. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia Emerge as Central Players in Brain Disease—ProQuest. 2017. Available online: https://www.proquest.com/docview/1936797057?pq-origsite=primo&accountid=8194&sourcetype=Scholarly%20Journals (accessed on 26 June 2025).
- Streit, W.J.; Sammons, N.W.; Kuhns, A.J.; Sparks, D.L. Dystrophic microglia in the aging human brain. Glia 2004, 45, 208–212. [Google Scholar] [CrossRef]
- Harry, G.J.; Kraft, A.D. Neuroinflammation and Microglia: Considerations and approaches for neurotoxicity assessment. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1265–1277. [Google Scholar] [CrossRef]
- Fan, Z.; Brooks, D.J.; Okello, A.; Edison, P. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 2017, 140, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Okello, A.; Edison, P.; Archer, H.A.; Turkheimer, F.E.; Kennedy, J.; Bullock, R.; Walker, Z.; Kennedy, A.; Fox, N.; Rossor, M.; et al. Microglial activation and amyloid deposition in mild cognitive impairment. Neurology 2009, 72, 56–62. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, C. Microglia activation linking amyloid-β drive tau spatial propagation in Alzheimer’s disease. Front. Neurosci. 2022, 16, 951128. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Yamasaki, T.R.; Laferla, F.M. Microglia as a Potential Bridge between the Amyloid β-Peptide and Tau. Ann. N. Y. Acad. Sci. 2004, 1035, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Sharma, K.P.; Lecours, C.; Sánchez, M.G.; El Hajj, H.; Milior, G.; Olmos-Alonso, A.; Gómez-Nicola, D.; Luheshi, G.; Vallières, L.; et al. Dark microglia: A new phenotype predominantly associated with pathological states. Glia 2016, 64, 826–839. [Google Scholar] [CrossRef]
- Streit, W.J.; Xue, Q.S.; Tischer, J.; Bechmann, I. Microglial pathology. Acta Neuropathol. Commun. 2014, 2, 142. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Rauti, R.; Cellot, G.; D’Andrea, P.; Colliva, A.; Scaini, D.; Tongiorgi, E.; Ballerini, L. BDNF impact on synaptic dynamics: Extra or intracellular long-term release differently regulates cultured hippocampal synapses. Mol. Brain 2020, 13, 43. [Google Scholar] [CrossRef]
- Alqahtani, S.M.; Al-kuraishy, H.M.; Al Gareeb, A.I.; Albuhadily, A.K.; Alexiou, A.; Papadakis, M.; Hemeda, L.R.; Feheem, S.A.; El-Saber Batiha, G. Unlocking Alzheimer’s Disease: The Role of BDNF Signaling in Neuropathology and Treatment. Neuromol. Med. 2025, 27, 36. [Google Scholar] [CrossRef]
- Laske, C.; Stellos, K.; Hoffmann, N.; Stransky, E.; Straten, G.; Eschweiler, G.W.; Leyhe, T. Higher BDNF serum levels predict slower cognitive decline in Alzheimer’s disease patients. Int. J. Neuropsychopharmacol. 2011, 14, 399–404. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Xue, B.; Waseem, S.M.A.; Zhu, Z.; Alshahrani, M.A.; Nazam, N.; Anjum, F.; Habib, A.H.; Rafeeq, M.M.; Nazam, F.; Sharma, M. Brain-Derived Neurotrophic Factor: A Connecting Link Between Nutrition, Lifestyle, and Alzheimer’s Disease. Front. Neurosci. 2022, 16, 925991. [Google Scholar] [CrossRef]
- Shen, R.; Ardianto, C.; Celia, C.; Sidharta, V.M.; Sasmita, P.K.; Satriotomo, I.; Turana, Y. Brain-derived neurotrophic factor interplay with oxidative stress: Neuropathology approach in potential biomarker of Alzheimer’s disease. Dement Neuropsychol. 2023, 17, e20230012. [Google Scholar] [CrossRef]
- Ye, X.; Tai, W.; Zhang, D. The early events of Alzheimer’s disease pathology: From mitochondrial dysfunction to BDNF axonal transport deficits. Neurobiol. Aging 2012, 33, 1122.e1–1122.e10. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Buchman, A.S.; Yu, L.; Boyle, P.A.; Schneider, J.A.; De Jager, P.L.; Bennett, D.A. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 2016, 86, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2023, 9, e12385. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, C.A.; Tomoaia-Cotisel, M.; Sevastre-Berghian, A.; Tomoaia, G.; Mocanu, A.; Pal-Racz, C.; Toma, V.A.; Roman, I.; Ujica, M.A.; Pop, L.C. A Review on Current Aspects of Curcumin-Based Effects in Relation to Neurodegenerative, Neuroinflammatory and Cerebrovascular Diseases. Molecules 2025, 30, 43. [Google Scholar] [CrossRef] [PubMed]
- Azargoonjahromi, A.; Abutalebian, F. Unraveling the therapeutic efficacy of resveratrol in Alzheimer’s disease: An umbrella review of systematic evidence. Nutr. Metab. 2024, 21, 15. [Google Scholar] [CrossRef]
- Fernandes, L.; Cardim-Pires, T.R.; Foguel, D.; Palhano, F.L. Green Tea Polyphenol Epigallocatechin-Gallate in Amyloid Aggregation and Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 718188. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Lee, J.Y.; Wong, C.Y.; Koh, R.Y.; Lim, C.L.; Kok, Y.Y.; Chye, S.M. Natural Bioactive Compounds from Macroalgae and Microalgae for the Treatment of Alzheimer’s Disease: A Review. Yale J. Biol. Med. 2024, 97, 205–224. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef]
- Ciancia, M.; Fernández, P.V.; Leliaert, F. Diversity of Sulfated Polysaccharides from Cell Walls of Coenocytic Green Algae and Their Structural Relationships in View of Green Algal Evolution. Front. Plant Sci. 2020, 11, 554585. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Liyanage, N.M.; Nagahawatta, D.P.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Jayawrdhana, H.H.A.C.K.; Kim, J.I.; Jeon, Y.J. Sulfated Polysaccharides from Seaweeds: A Promising Strategy for Combatting Viral Diseases—A Review. Mar. Drugs 2023, 21, 461. [Google Scholar] [CrossRef] [PubMed]
- Aluta, U.P.; Aderolu, A.Z.; Ishola, I.O.; Alyassin, M.; Morris, G.A.; Olajide, O.A. Chemical characterisation of sulfated polysaccharides from the red seaweed Centroceras clavulatum and their in vitro immunostimulatory and antioxidant properties. Food Hydrocoll. Health 2023, 3, 100135. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. From the ocean to the brain: Harnessing the power of marine algae for neuroprotection and therapeutic advances. Explor. Neuroprot. Ther. 2023, 3, 409–428. [Google Scholar] [CrossRef]
- Myung, C.S.; Shin, H.C.; Bao, H.Y.; Yeo, S.J.; Lee, B.H.; Kang, J.S. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Youn, K.; Kim, D.H.; Ahn, M.R.; Yoon, E.; Kim, O.Y.; Jun, M. Anti-Neuroinflammatory Property of Phlorotannins from Ecklonia cava on Aβ25-35-Induced Damage in PC12 Cells. Mar. Drugs 2018, 17, 7. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Lee, S.H. Ameliorating Activity of Ishige okamurae on the Amyloid Beta-Induced Cognitive Deficits and Neurotoxicity through Regulating ERK, p38 MAPK, and JNK Signaling in Alzheimer’s Disease-Like Mice Model. Mol. Nutr. Food Res. 2020, 64, e1901220. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Duan, L.; Li, X.; Yang, W.; Huang, T.; Kong, M.; Guan, F.; Ma, S. Fucoidan ameliorates LPS-induced neuronal cell damage and cognitive impairment in mice. Int. J. Biol. Macromol. 2022, 222, 759–771. [Google Scholar] [CrossRef]
- Maury, J.; Delbrut, A.; Villard, V.; Pradelles, R. A Standardized Extract of Microalgae Phaeodactylum tricornutum (Mi136) Inhibit D-Gal Induced Cognitive Dysfunction in Mice. Mar. Drugs 2024, 22, 99. [Google Scholar] [CrossRef]
- Yoo, C.; Maury, J.; Gonzalez, D.E.; Ko, J.; Xing, D.; Jenkins, V.; Dickerson, B.; Leonard, M.; Estes, L.; Johnson, S.; et al. Effects of Supplementation with a Microalgae Extract from Phaeodactylum tricornutum Containing Fucoxanthin on Cognition and Markers of Health in Older Individuals with Perceptions of Cognitive Decline. Nutrients 2024, 16, 2999. [Google Scholar] [CrossRef]
- Martens, N.; Zhan, N.; Yam, S.C.; Leijten, F.P.J.; Palumbo, M.; Caspers, M.; Tiane, A.; Friedrichs, S.; Li, Y.; van Vark-van der Zee, L.; et al. Supplementation of Seaweed Extracts to the Diet Reduces Symptoms of Alzheimer’s Disease in the APPswePS1ΔE9 Mouse Model. Nutrients 2024, 16, 1614. [Google Scholar] [CrossRef] [PubMed]
- Crowe-White, K.M.; Phillips, T.A.; Ellis, A.C. Lycopene and cognitive function. J. Nutr. Sci. 2019, 8, e20. [Google Scholar] [CrossRef]
- Yang, E.J.; Mahmood, U.; Kim, H.; Choi, M.; Choi, Y.; Lee, J.P.; Cho, J.Y.; Hyun, J.W.; Kim, Y.S.; Chang, M.J.; et al. Phloroglucinol ameliorates cognitive impairments by reducing the amyloid β peptide burden and pro-inflammatory cytokines in the hippocampus of 5XFAD mice. Free Radic. Biol. Med. 2018, 126, 221–234. [Google Scholar] [CrossRef]
- Yang, E.J.; Ahn, S.; Ryu, J.; Choi, M.S.; Choi, S.; Chong, Y.H.; Hyun, J.W.; Chang, M.J.; Kim, H.S. Phloroglucinol Attenuates the Cognitive Deficits of the 5XFAD Mouse Model of Alzheimer’s Disease. PLoS ONE 2015, 10, e0135686. [Google Scholar] [CrossRef] [PubMed]
- Martens, N.; Schepers, M.; Zhan, N.; Leijten, F.; Voortman, G.; Tiane, A.; Rombaut, B.; Poisquet, J.; van de Sande, N.; Kerksiek, A.; et al. 24(S)-Saringosterol Prevents Cognitive Decline in a Mouse Model for Alzheimer’s Disease. Mar. Drugs 2021, 19, 190. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xue, M.; Li, J.; Ma, Y.; Wang, Y.; Zhang, H.; Liang, H. Fucoidan Improves D-Galactose-Induced Cognitive Dysfunction by Promoting Mitochondrial Biogenesis and Maintaining Gut Microbiome Homeostasis. Nutrients 2024, 16, 1512. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. The Effects of Lutein and Zeaxanthin Supplementation on Cognitive Function in Adults with Self-Reported Mild Cognitive Complaints: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Nutr. 2022, 9, 843512. [Google Scholar] [CrossRef]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: A randomised, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Burdeos, G.C.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiol. Behav. 2019, 211, 112650. [Google Scholar] [CrossRef]
- Kumari, K.N.; Jeyabalan, S.; Rajangam, J.; Gopinathan, N.; Ramakrishnan, S.R.; Reddy, V.J. Neuroprotective Potential of Total Extract of Ulva Lactuca: An In vitro study. Res. J. Pharm. Technol. 2023, 16, 5948–5953. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, D.; Qi, R.; Chen, Y.; Sheng, B.; Zhang, X. Association between Intake of Edible Mushrooms and Algae and the Risk of Cognitive Impairment in Chinese Older Adults. Nutrients 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Han, J.M.; Shin, Y.N.; Park, Y.S.; Shin, Y.R.; Park, S.W.; Roy, V.C.; Lee, H.J.; Kumagai, H.; Kishimura, H.; et al. Exploring Bioactive Compounds in Brown Seaweeds Using Subcritical Water: A Comprehensive Analysis. Mar. Drugs 2023, 21, 328. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Clerici, M.T.P.S. Brown algae and their multiple applications as functional ingredient in food production. Food Res. Int. 2023, 167, 112655. [Google Scholar] [CrossRef]
- Nho, J.A.; Shin, Y.S.; Jeong, H.R.; Cho, S.; Heo, H.J.; Kim, G.H.; Kim, D.O. Neuroprotective Effects of Phlorotannin-Rich Extract from Brown Seaweed Ecklonia cava on Neuronal PC-12 and SH-SY5Y Cells with Oxidative Stress. J. Microbiol. Biotechnol. 2020, 30, 359–367. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kwon, O.I.; Hwang, H.J.; Shin, H.C.; Yang, S. Therapeutic effects of phlorotannins in the treatment of neurodegenerative disorders. Front. Mol. Neurosci. 2023, 16, 1193590. [Google Scholar] [CrossRef]
- Cui, Y.; Park, J.Y.; Wu, J.; Lee, J.H.; Yang, Y.S.; Kang, M.S.; Jung, S.C.; Park, J.M.; Yoo, E.S.; Kim, S.H.; et al. Dieckol Attenuates Microglia-mediated Neuronal Cell Death via ERK, Akt and NADPH Oxidase-mediated Pathways. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2015, 19, 219–228. [Google Scholar] [CrossRef]
- Cui, Y.; Amarsanaa, K.; Lee, J.H.; Rhim, J.K.; Kwon, J.M.; Kim, S.H.; Park, J.M.; Jung, S.M.; Eun, S.Y. Neuroprotective mechanisms of dieckol against glutamate toxicity through reactive oxygen species scavenging and nuclear factor-like 2/heme oxygenase-1 pathway. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2019, 23, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Kim, K.J.; Park, H.; Lee, M.G.; Cho, S.; Choi, S.I.; Heo, H.J.; Kim, D.O.; Kim, G.H. Effects of Ecklonia cava Extract on Neuronal Damage and Apoptosis in PC-12 Cells against Oxidative Stress. J. Microbiol. Biotechnol. 2021, 31, 584–591. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, N.; Youn, K.; Jo, M.R.; Kim, H.R.; Lee, D.S.; Ho, C.T.; Jun, M. Dieckol Ameliorates Aβ Production via PI3K/Akt/GSK-3β Regulated APP Processing in SweAPP N2a Cell. Mar. Drugs 2021, 19, 152. [Google Scholar] [CrossRef]
- Gao, Y.; Li, C.; Yin, J.; Shen, J.; Wang, H.; Wu, Y.; Jin, H. Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive impairment induced by infusion of Aβ peptide in rats. Environ. Toxicol. Pharmacol. 2012, 33, 304–311. [Google Scholar] [CrossRef]
- Jin, W.; Lu, C.; Zhu, Y.; Zhao, J.; Zhang, W.; Wang, L.; Linhardt, R.J.; Wang, C.; Zhang, F. Fucoidans inhibited tau interaction and cellular uptake. Carbohydr. Polym. 2023, 299, 120176. [Google Scholar] [CrossRef]
- Bauer, S.; Jin, W.; Zhang, F.; Linhardt, R.J. The Application of Seaweed Polysaccharides and Their Derived Products with Potential for the Treatment of Alzheimer’s Disease. Mar. Drugs 2021, 19, 89. [Google Scholar] [CrossRef]
- Gueven, N.; Spring, K.J.; Holmes, S.; Ahuja, K.; Eri, R.; Park, A.Y.; Fitton, H.J. Micro RNA Expression after Ingestion of Fucoidan: A Clinical Study. Mar. Drugs 2020, 18, 143. [Google Scholar] [CrossRef]
- So, M.J.; Cho, E.J. Phloroglucinol Attenuates Free Radical-induced Oxidative Stress. Prev. Nutr. Food Sci. 2014, 19, 129–135. [Google Scholar] [CrossRef]
- Li, N.; Gao, X.; Zheng, L.; Huang, Q.; Zeng, F.; Chen, H.; Farag, M.A.; Zhao, C. Advances in fucoxanthin chemistry and management of neurodegenerative diseases. Phytomedicine 2022, 105, 154352. [Google Scholar] [CrossRef] [PubMed]
- Goodbody, E.; Maury, J.; Doolan, A.; DunnGalvin, G.; Kakilla, C.; Pradelles, R.; Dinan, T.G. Promising Benefits of Six-Month Phaeodactylum tricornutum Microalgae Supplementation on Cognitive Function and Inflammation in Healthy Older Adults with Age-associated Memory Impairment. Front. Aging. 2025, 6, 1540115. [Google Scholar] [CrossRef] [PubMed]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef] [PubMed]
- Martens, N.; Zhan, N.; Voortman, G.; Leijten, F.P.J.; van Rheenen, C.; van Leerdam, S.; Geng, X.; Huybrechts, M.; Liu, H.; Jonker, J.W.; et al. Activation of Liver X Receptors and Peroxisome Proliferator-Activated Receptors by Lipid Extracts of Brown Seaweeds: A Potential Application in Alzheimer’s Disease? Nutrients 2023, 15, 3004. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Niu, Z.; Wang, B.; Zhao, S.; Sun, C.; Wu, Y.; Li, Y.; Ying, H.; Liu, H. Saringosterol from Sargassum fusiforme Modulates Cholesterol Metabolism and Alleviates Atherosclerosis in ApoE-Deficient Mice. Mar. Drugs 2021, 19, 485. [Google Scholar] [CrossRef]
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red Seaweed Pigments from a Biotechnological Perspective. Phycology 2022, 2, 1–29. [Google Scholar] [CrossRef]
- Yanshin, N.; Kushnareva, A.; Lemesheva, V.; Birkemeyer, C.; Tarakhovskaya, E. Chemical Composition and Potential Practical Application of 15 Red Algal Species from the White Sea Coast (the Arctic Ocean). Molecules 2021, 26, 2489. [Google Scholar] [CrossRef]
- Ismail, Z.; Ahmad, W.I.W.; Hamjah, S.H.; Astina, I.K. The Impact of Population Ageing: A Review. Iran. J. Public Health 2021, 50, 2451–2460. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization, antioxidant properties, cholinesterase inhibitory and anti-amyloidogenic activities of sulfated polysaccharides from some seaweeds. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100182. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordiero, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Li, X. κ-carrageenan-derived pentasaccharide attenuates Aβ25-35-induced apoptosis in SH-SY5Y cells via suppression of the JNK signaling pathway. Mol. Med. Rep. 2017, 15, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kang, S.; Moon, N.R.; Shin, B.K.; Park, S. Zeaxanthin and Lutein Ameliorate Alzheimer’s Disease-like Pathology: Modulation of Insulin Resistance, Neuroinflammation, and Acetylcholinesterase Activity in an Amyloid-β Rat Model. Int. J. Mol. Sci. 2024, 25, 9828. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Li, H.; Yu, H.; Xi, Y. The Protective Effects of Zeaxanthin on Amyloid-β Peptide 1–42-Induced Impairment of Learning and Memory Ability in Rats. Front. Behav. Neurosci. 2022, 16, 912896. [Google Scholar] [CrossRef]
- Nazari, L.; Komaki, S.; Salehi, I.; Raoufi, S.; Golipoor, Z.; Kourosh-Arami, M.; Komaki, A. Investigation of the protective effects of lutein on memory and learning using behavioral methods in a male rat model of Alzheimer’s disease. J. Funct. Foods 2022, 99, 105319. [Google Scholar] [CrossRef]
- Johnson, E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly12345. Am. J. Clin. Nutr. 2012, 96, 1161S–1165S. [Google Scholar] [CrossRef]
- Sun, D.; Wu, S.; Li, X.; Ge, B.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. The Structure, Functions and Potential Medicinal Effects of Chlorophylls Derived from Microalgae. Mar. Drugs 2024, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Yang, Q.; Jiang, Y.; Fu, S.; Shen, Z.; Zong, W.; Xia, Z.; Zhan, Z.; Jiang, X. Protective Effects of Ulva lactuca Polysaccharide Extract on Oxidative Stress and Kidney Injury Induced by D-Galactose in Mice. Mar. Drugs 2021, 19, 539. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Rodríguez-Coello, A.; Latire, T.; Bourgougnon, N.; Torres, M.D.; Buján, M.; Muíños, A.; Muíños, A.; Meijide-Faílde, R.; Blanco, F.J.; et al. Anti-inflammatory potential of ulvan. Int. J. Biol. Macromol. 2023, 253, 126936. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Ki, J.S. Immunomodulatory, Antioxidant, Anticancer, and Pharmacokinetic Activity of Ulvan, a Seaweed-Derived Sulfated Polysaccharide: An Updated Comprehensive Review. Mar. Drugs 2023, 21, 300. [Google Scholar] [CrossRef]
- Ambati, R.R.; Siew Moi, P.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Hongo, N.; Takamura, Y.; Nishimaru, H.; Matsumoto, J.; Tobe, K.; Saito, T.; Saido, T.C.; Nishijo, H. Astaxanthin Ameliorated Parvalbumin-Positive Neuron Deficits and Alzheimer’s Disease-Related Pathological Progression in the Hippocampus of AppNL-G-F/NL-G-F Mice. Front Pharmacol. 2020, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, C.; Yang, M.; Li, A.; Fan, C.; Gan, D.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Astaxanthin Improved the Cognitive Deficits in APP/PS1 Transgenic Mice Via Selective Activation of mTOR. J. Neuroimmune Pharmacol. 2021, 16, 609–619. [Google Scholar] [CrossRef]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.J.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a Potential Neuroprotective Agent for Neurological Diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef]
- Grimmig, B.; Kim, S.H.; Nash, K.; Bickford, P.C.; Douglas Shytle, R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience 2017, 39, 19–32. [Google Scholar] [CrossRef]
- Liu, N.; Lyu, X.; Zhang, X.; Zhang, F.; Chen, Y.; Li, G. Astaxanthin attenuates cognitive deficits in Alzheimer’s disease models by reducing oxidative stress via the SIRT1/PGC-1α signaling pathway. Cell Biosci. 2023, 13, 173. [Google Scholar] [CrossRef]
- Queen, C.J.J.; Sparks, S.A.; Marchant, D.C.; McNaughton, L.R. The Effects of Astaxanthin on Cognitive Function and Neurodegeneration in Humans: A Critical Review. Nutrients 2024, 16, 826. [Google Scholar] [CrossRef]
- Hayashi, M.; Ishibashi, T.; Maoka, T. Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on cognitive function in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2018, 62, 195–205. [Google Scholar] [CrossRef]
- Allaert, F.A.; Demais, H.; Collén, P.N. A randomized controlled double-blind clinical trial comparing versus placebo the effect of an edible algal extract (Ulva Lactuca) on the component of depression in healthy volunteers with anhedonia. BMC Psychiatry 2018, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Haskell-Ramsay, C.F.; Jackson, P.A.; Dodd, F.L.; Forster, J.S.; Bérubé, J.; Levinton, C.; Kennedy, D.O. Acute Post-Prandial Cognitive Effects of Brown Seaweed Extract in Humans. Nutrients 2018, 10, 85. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Shin, H.C.; Rosenfeld, C.; Guttendorf, R.J.; Wade, S.B.; Park, Y.J.; Kim, J.H.; Kim, S.H.; Lee, B.H.; Hwang, H.J. A Pharmacokinetic and Bioavailability Study of Ecklonia cava Phlorotannins Following Intravenous and Oral Administration in Sprague–Dawley Rats. Mar. Drugs 2024, 22, 500. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Kong, Q.; You, L.; Zhong, S.; Hileuskaya, K. Polysaccharides from brown seaweed: Physicochemical properties, absorption in the intestine, and beneficial effects on intestinal barrier. Food Front. 2023, 4, 1547–1560. [Google Scholar] [CrossRef]
- Tan, J.; Song, Y.; Wang, J.; Wu, N.; Yue, Y.; Zhang, Q. Pharmacokinetics of fucoidan and low molecular weight fucoidan from Saccharina japonica after oral administration to mice. J. Oceanol. Limnol. 2023, 41, 1900–1909. [Google Scholar] [CrossRef]
- You, L.; Gong, Y.; Li, L.; Hu, X.; Brennan, C.; Kulikouskaya, V. Beneficial effects of three brown seaweed polysaccharides on gut microbiota and their structural characteristics: An overview. Int. J. Food Sci. Technol. 2020, 55, 1199–1206. [Google Scholar] [CrossRef]
- Komersová, A.; Svoboda, R.; Skalická, B.; Bartoš, M.; Šnejdrová, E.; Mužíková, J.; Matzick, K. Matrix Tablets Based on Chitosan–Carrageenan Polyelectrolyte Complex: Unique Matrices for Drug Targeting in the Intestine. Pharmaceuticals 2022, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, K.T.; Hwang, S.; Choi, K.Y.; Park, Y.J.; Choi, J.H.; Truong, T.Q.; Kim, S.M. Nanoencapsulation enhances the bioavailability of fucoxanthin in microalga Phaeodactylum tricornutum extract. Food Chem. 2023, 403, 134348. [Google Scholar] [CrossRef] [PubMed]
- Ramos-de-la-Peña, A.M.; Contreras-Esquivel, J.C.; Aguilar, O.; González-Valdez, J. Structural and bioactive roles of fucoidan in nanogel delivery systems. A review. Carbohydr. Polym. Technol. Appl. 2022, 4, 100235. [Google Scholar] [CrossRef]
- Portela, M.; Silva, A.; Carpena, M.; Grosso, C.; Barroso, M.F.; Oliveira, A.I.; Martins, C.; Ribeiro, C.; Prieto, M.A. Phytosomes-Based Nanocarriers Enhanced with Seaweed Extracts: Overcoming the Blood–Brain Barrier. Eng. Proc. 2025, 87, 75. [Google Scholar] [CrossRef]
- Han, M.; Yi, B.; Song, R.; Wang, D.; Huang, N.; Ma, Y.; Zhao, L.; Liu, S.; Zhang, H.; Xu, R.; et al. Fucoidan-derived carbon dots as nanopenetrants of blood-brain barrier for Parkinson’s disease treatment. J. Colloid. Interface Sci. 2025, 680, 516–527. [Google Scholar] [CrossRef]
| Compound | Chemical Structure | Algal Source | Mechanism | Tested Model | Main Findings | Reference |
|---|---|---|---|---|---|---|
| Dieckol | 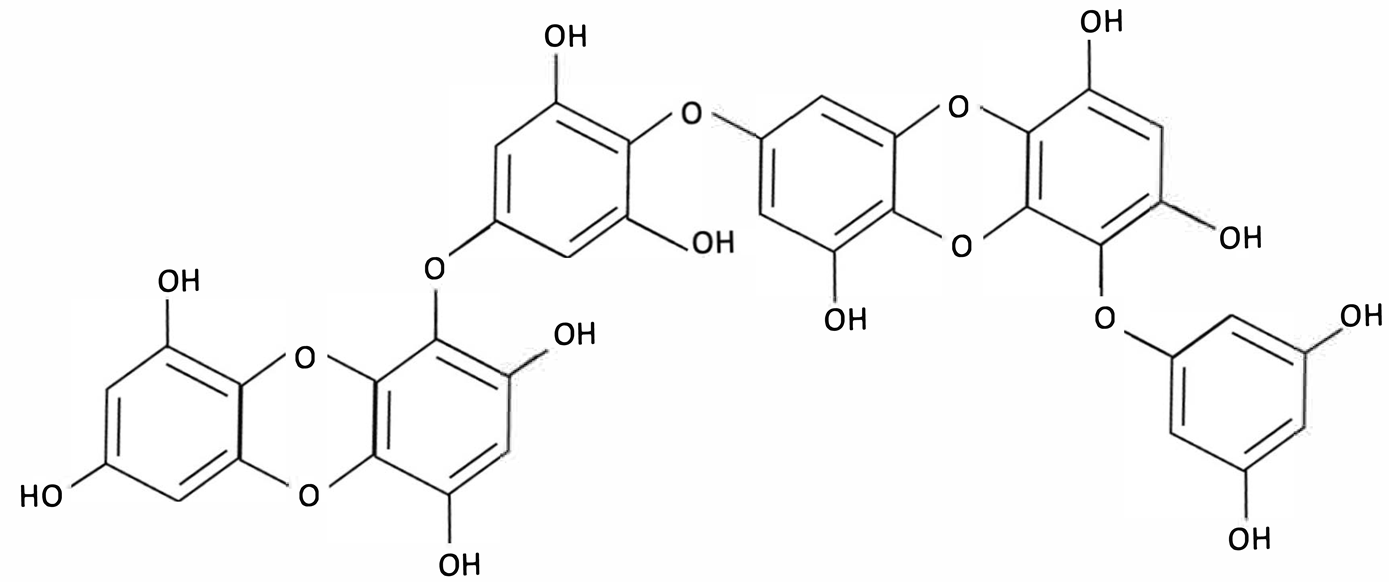 | Ecklonia cava, Brown Seaweed | AChE inhibition, Neurotransmitter modulation | Male ICR mice received oral dieckol (1 or 10 mg/kg/day) for 7 days. Cognitive performance was assessed via passive avoidance test; brain neurotransmitter levels and AChE activity were measured. | Significantly improved memory in ethanol-treated mice, restored hippocampal 5-HT and glutamate levels, reduced elevated GABA and norepinephrine levels, increased brain ACh and inhibited AChE activity (IC50 ≈ 17.5 µM). | [148] |
| Dieckol |  | Ecklonia cava, Brown Seaweed | Antioxidant, Anti-inflammatory, Antiapoptotic, NF-κB and MAPK signalling | In vitro, using PC12 cells treated with Aβ(25–35) simulating AD-like neurotoxicity. Phlorotannins were administered at varying concentrations 1 h before Aβ. | Dieckol significantly restored PC12 cell viability, reduced Aβ25–35–induced oxidative stress, inflammation, and apoptosis, and modulated NF-κB and MAPK signalling pathways. | [149] |
| Diphlorethohydroxycarmalol (DPHC) | 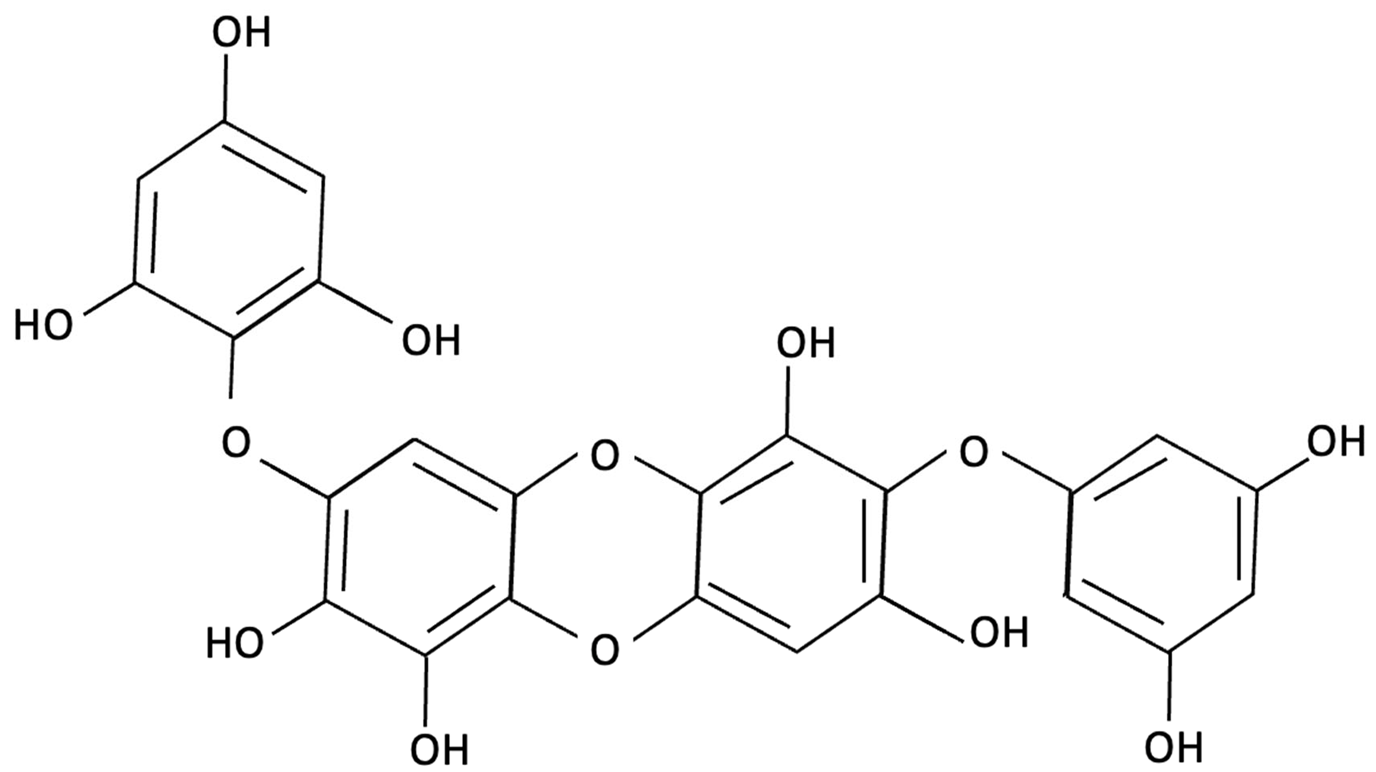 | Ishige okamurae, Brown Algae | Anti-inflammatory, Neuroprotective | AD-like cognitive impairment induced in male C57BL/6 mice via ICV injection of Aβ25–35. In vitro, PC12 neuronal cells used for further investigation of molecular mechanisms related to oxidative stress, apoptosis and MAPK pathway activation. | Significantly attenuated Aβ(25–35)–induced cognitive impairment, improved maze test performance. IOE reduced neuronal apoptosis, cleaved caspase-3 and PARP, suppressed neuroinflammation (iNOS, COX-2), and decreased ROS overproduction. Both in vivo and PC12 cell models, IOE reversed abnormal phosphorylation of ERK, p38 MAPK, and JNK. | [150] |
| Fucoidan | 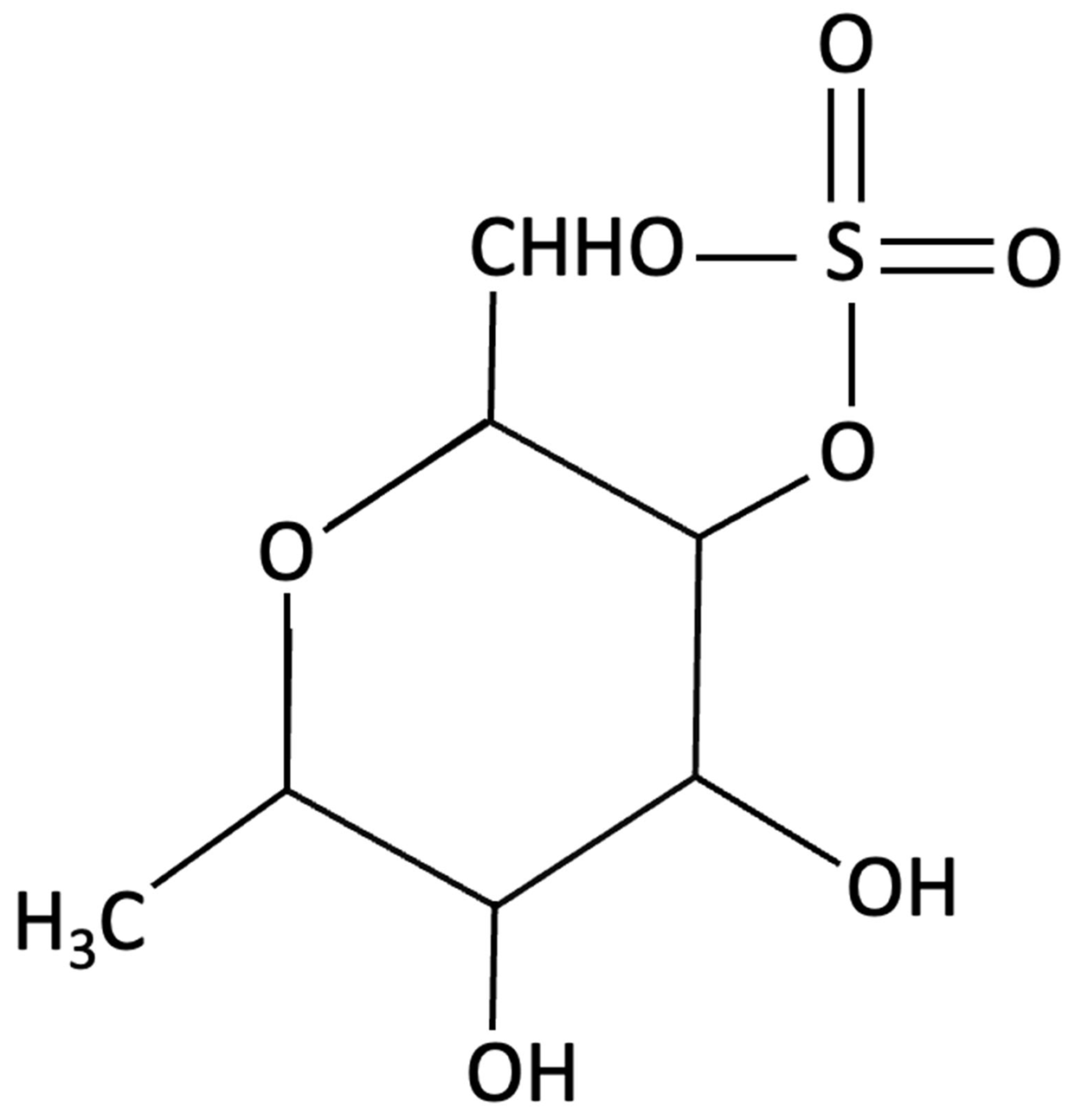 | Fucus vesiculosi, Brown Algae | Neuroprotective | Male C57BL/6 mice treated with LPS and oral fucoidan (10 mg/kg) daily for three weeks. | Attenuated LPS-induced cognitive impairment by reducing neuroinflammation, oxidative stress, AChE activity. Enhancing BDNF expression and neurogenesis. | [151] |
| Fucoxanthin | 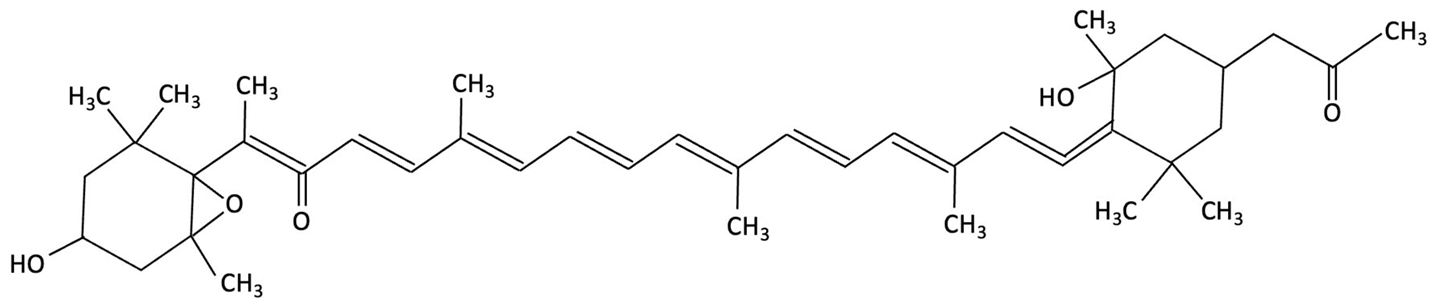 | Phaeodactylum tricornutum, Brown Algae | Antioxidant, Anti-inflammatory | D-galactose-induced ageing mouse model using male Swiss mice (n = 72), treated with PT extract for 79 days. Cognitive performance assessed via Y-maze, Morris Water Maze, and Passive Avoidance tests. | Significantly reversed induced cognitive impairment in Y-maze, Morris Water Maze and Passive Avoidance tests, reduced hippocampal lipid peroxidation, and decreased elevated TNF-α and IL-6 levels in brain and plasma, particularly at higher doses. | [152] |
| Fucoxanthin | 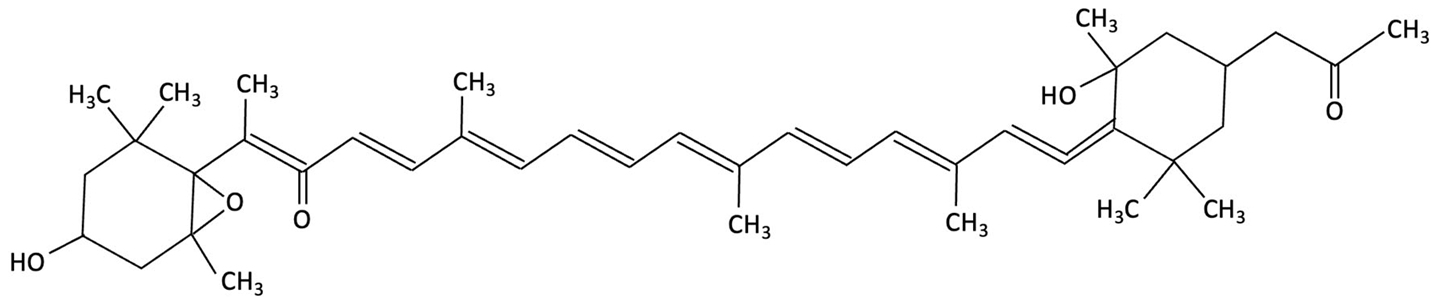 | Phaeodactylum tricornutum, Brown Algae | Antioxidant, Anti-inflammatory | 12-week double-blind, randomised, placebo-controlled clinical trial involving older adults (55–75 years) with age-associated memory impairment. Assessing 8.8 mg/day supplementation on cognitive performance and inflammatory biomarkers | Improved working and episodic memory, attention, vigilance and executive function. Inflammatory cytokines showed minimal changes, a slight increase in IL-1β and stable TNF-α and IL-6 levels. | [153] |
| Fucosterol and Saringosterol | 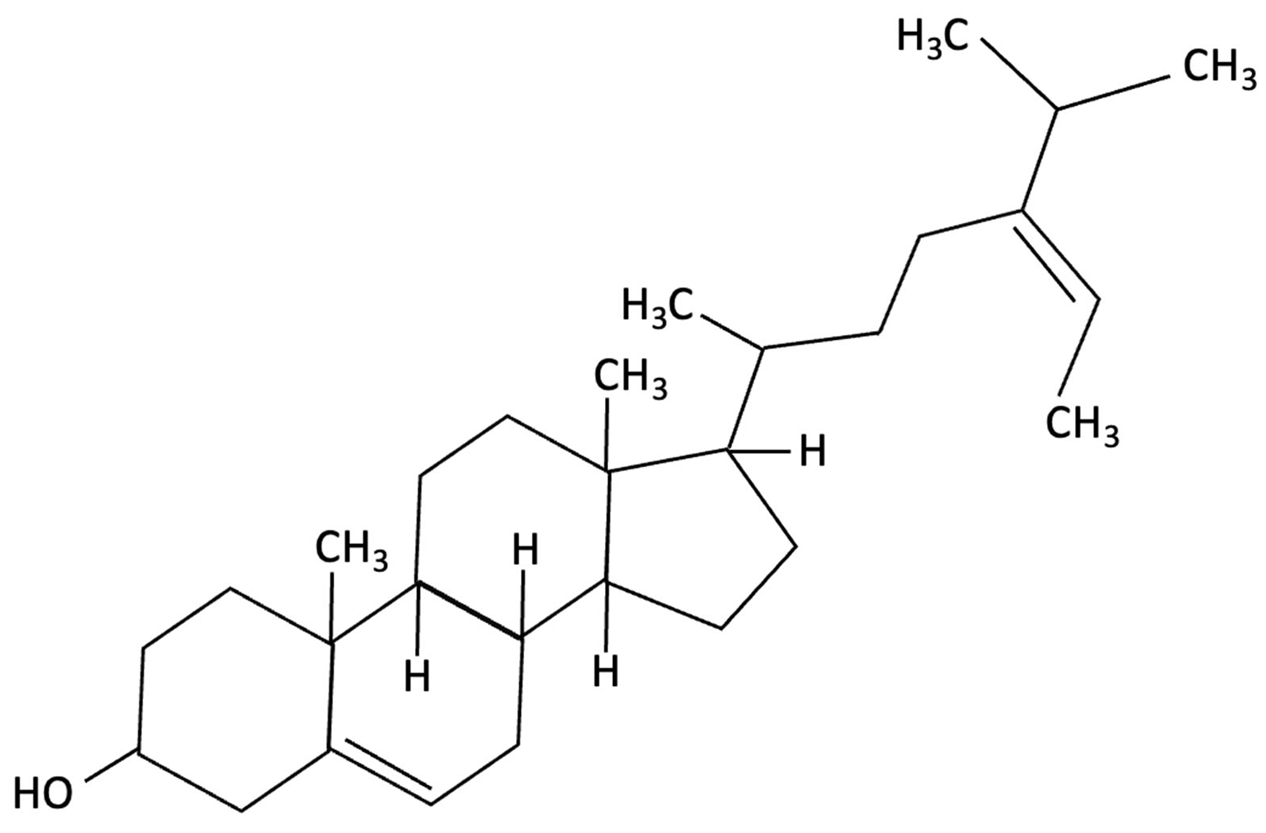 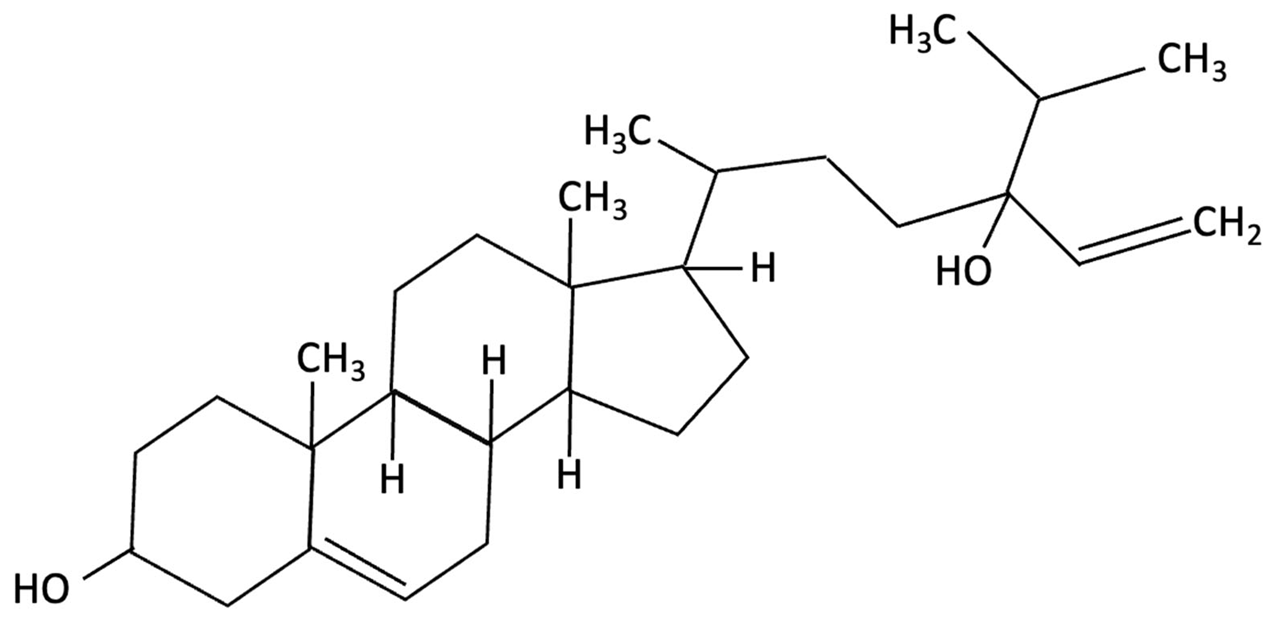 | Himanthalia elongate, Sargassum fusiforme, Brown Seaweed | Anti-inflammatory | In vitro testing using LXR luciferase reporter assays and in vivo via 12-week dietary supplementation in APPswePS1ΔE9 mice. | Significantly prevented cognitive decline in APPswePS1ΔE9 mice across object, spatial, and working memory tasks. Both H. elongata and S. fusiforme extracts reduced cortical GFAP expression, suggesting attenuation of astrocyte activation. | [154] |
| Lycopene | 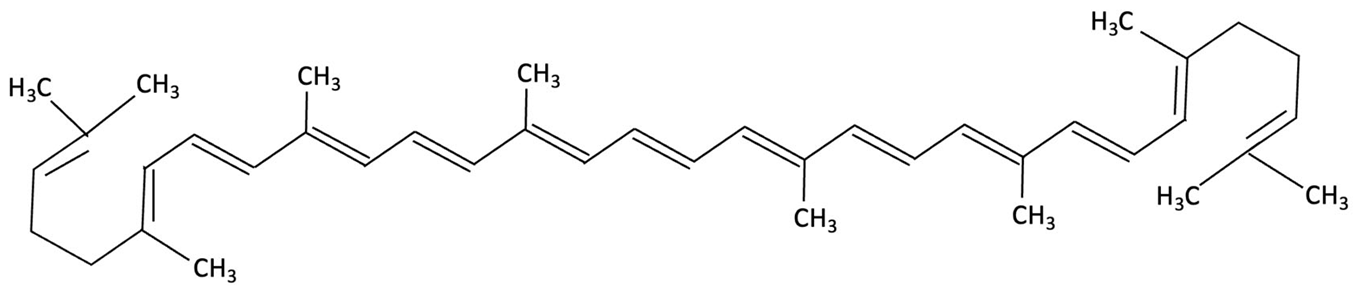 | Dictyota spiralis, Brown Seaweed | Neuroprotective | Male C57Bl/6J mice (3-month) administered lycopene-supplemented diet (0.3% w/w) for five weeks. 9 days of LPS induction of neuroinflammation. | Alleviated LPS-induced amyloidogenesis and memory loss, through inhibited microglial activation, reduced inflammatory mediators and enhances antioxidant enzymes, partly via modulating MAPK, NF-κB, PI3K/Akt, and Keap1/Nrf2 pathways. | [155] |
| Phloroglucinol | 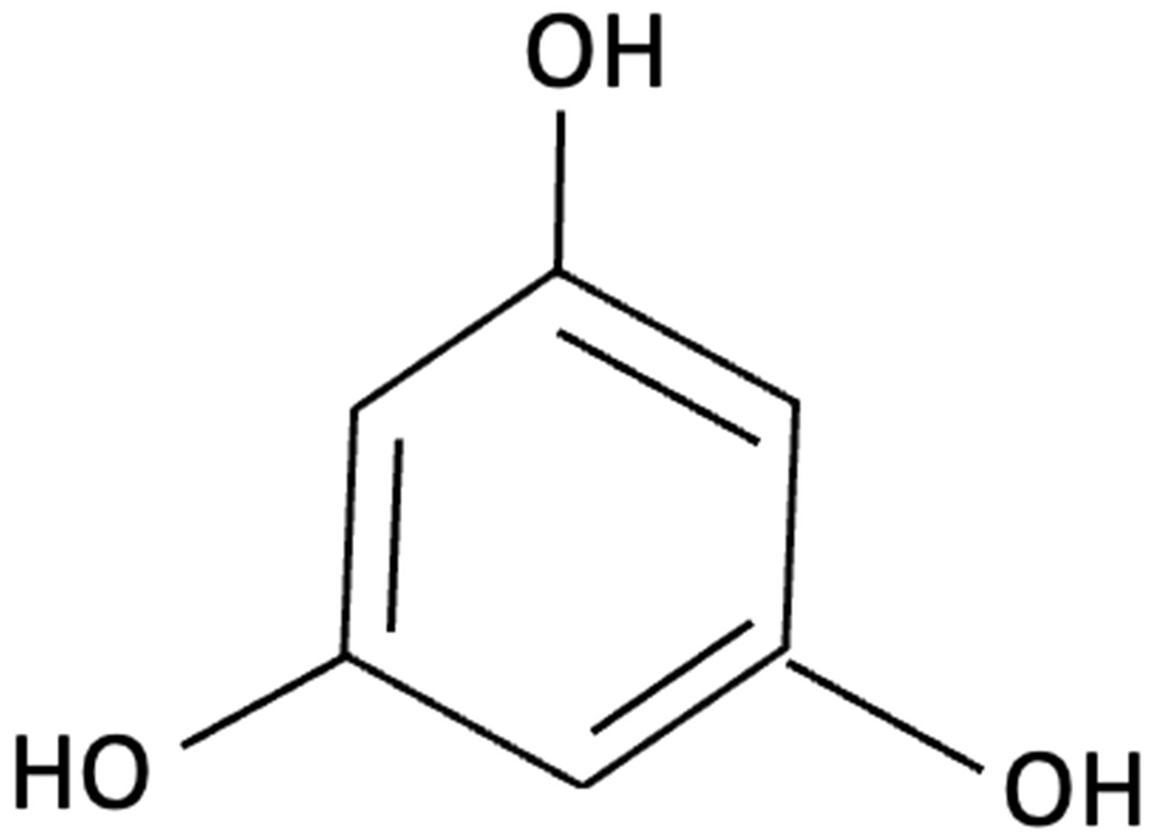 | Ecklonia cava, family Laminariaceae, Brown Seaweed | Antioxidant, Anti-inflammatory, Aβ metabolism | 5XFAD transgenic mouse model of Alzheimer’s disease used to assess the neuroprotective effects of orally administered phloroglucinol (100 mg/kg/day) over 2 months. | Significantly improved cognitive performance in 5XFAD mice (T-maze and Y-maze), reduced Aβ protein levels and plaque burden, lowered oxidative stress (↓4-HNE), and suppressed glial activation (↓GFAP, ↓Iba-1). Additionally, decreased pro-inflammatory cytokines (TNF-α, IL-6), reduced BACE1 expression, and restored dendritic spine density and mature spine morphology in the hippocampus. | [156] |
| Phloroglucinol | 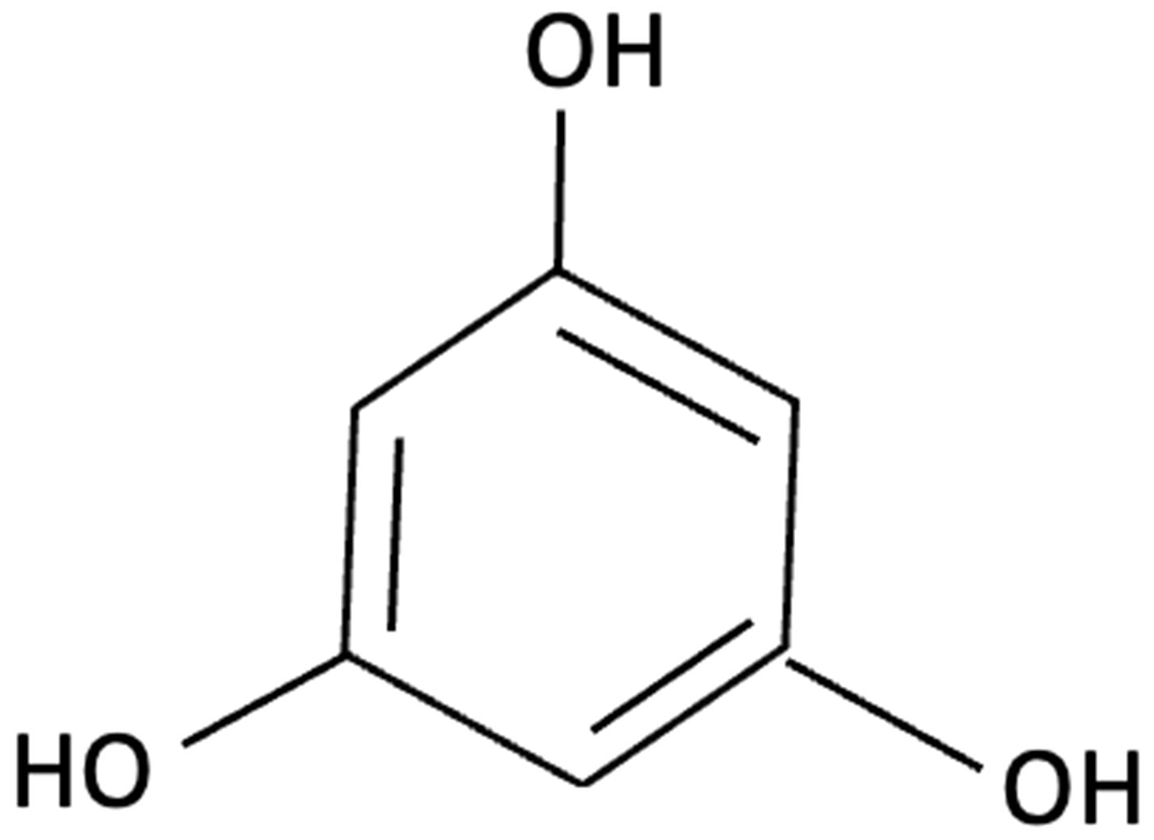 | Brown Seaweed | Neuroprotective | In vitro assays using HT-22 and primary hippocampal neurons were conducted against Aβ1–42-induced cytotoxicity, oxidative stress, and synaptic damage, supported by in vivo stereotaxic hippocampal injection and behavioural testing in 5XFAD mice. | Phloroglucinol significantly reduced Aβ-induced ROS accumulation and synaptic loss in vitro and improved spatial learning and working memory in 5XFAD mice. | [157] |
| PFF-A (Phlorotannin-s) | 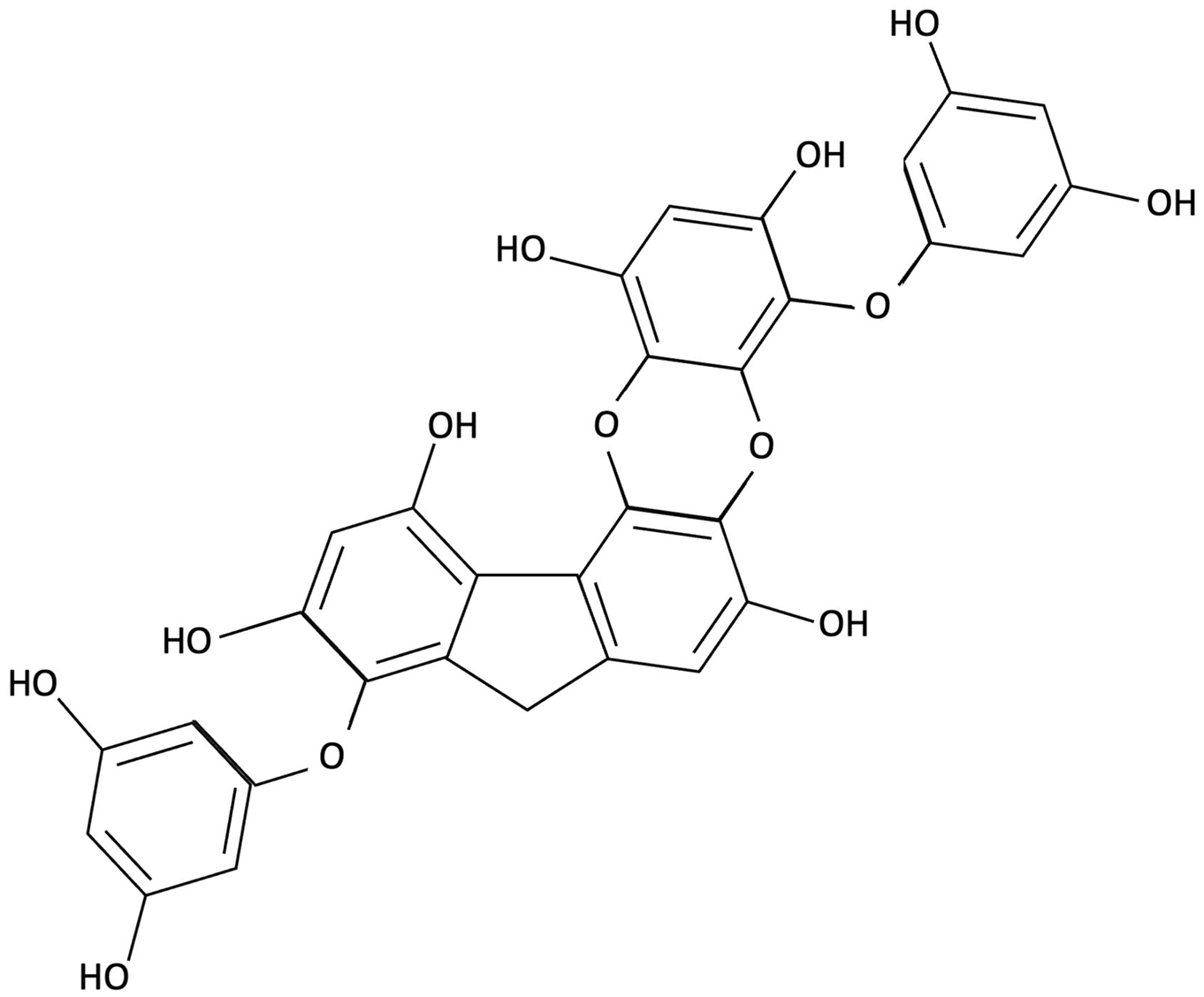 | Ecklonia cava, Brown Seaweed | AChE inhibition, Neurotransmitter modulation | Male ICR mice received oral PFF (0.2 or 2 mg/kg/day) for 7 days. Memory was evaluated using passive avoidance; neurotransmitter analysis and AChE inhibition were also conducted. | Improved cognitive performance in memory-impaired mice, normalised hippocampal norepinephrine and glutamate, decreased GABA, increased 5-HT and Ach and inhibited AChE (IC50 ≈ 27.4 µM). | [148] |
| Saringosterol | 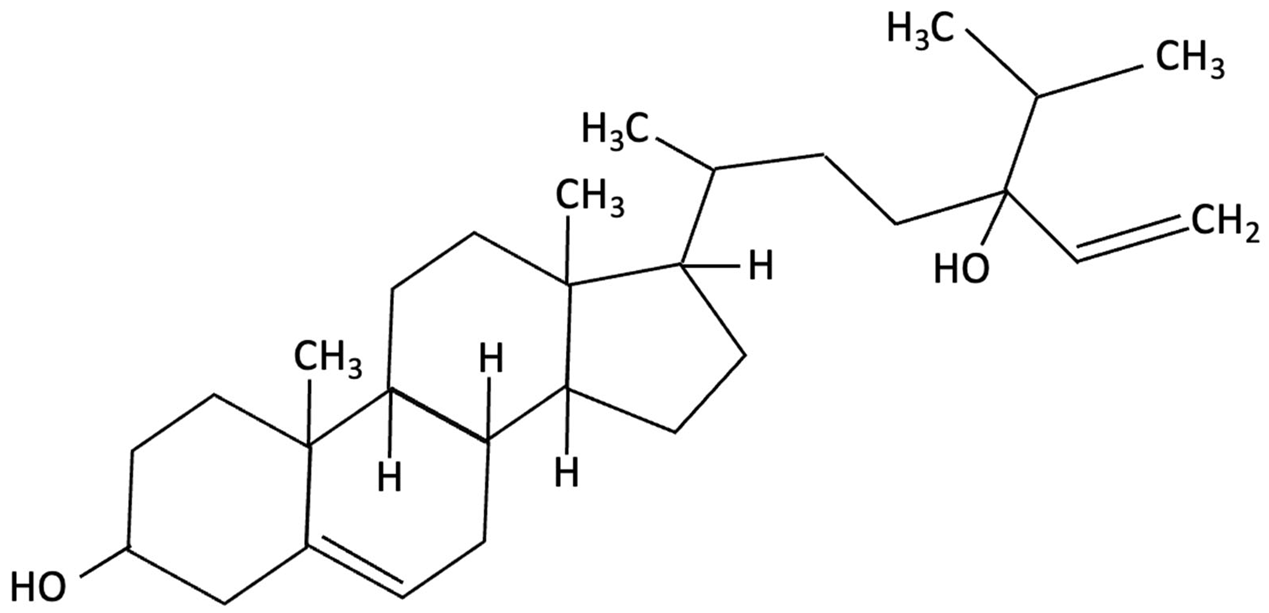 | Sargassum fusiforme, Brown Seaweed | Anti-inflammatory | Male APPswePS1ΔE9 and WT mice received daily oral gavage of 24(S)-saringosterol (0.5 mg/25 g) for 10 weeks to assess cognitive effects. | Significantly prevented cognitive decline in APPswePS1ΔE9 mice, improving spatial and object memory (OLT and ORT), likely via LXR-mediated microglial modulation. | [158] |
| k-carrageenan | 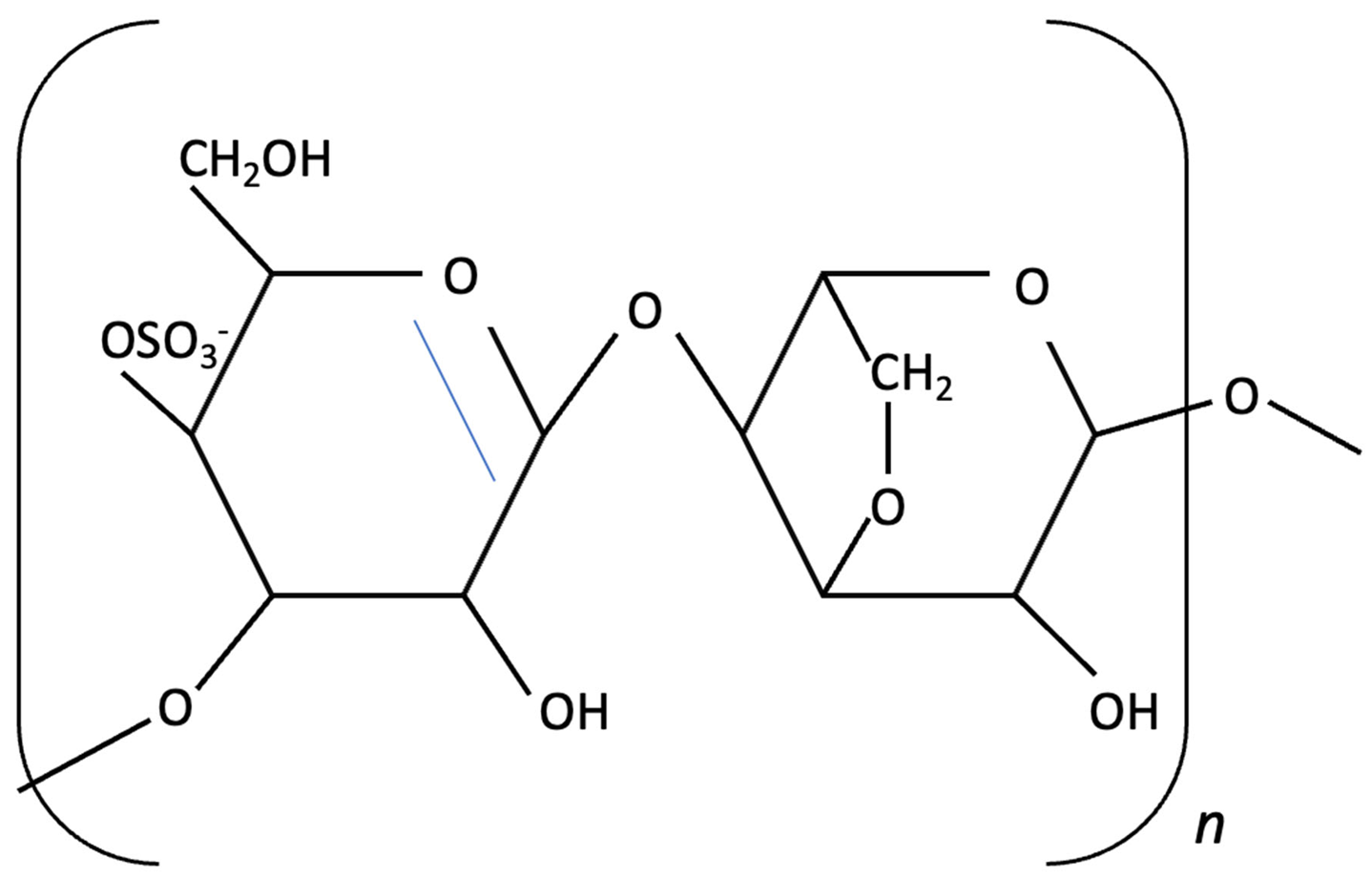 | Kappaphycus alvarezii, Red Algae | Anti-inflammatory, Neuroprotective | In vitro LPS stimulated neuroinflammation in Murine microglial N9 cell. Treated with KOS or desulphated derivatives (DSK) | Attenuated neuroinflammation by reducing NO, TNF-, and IL-10 release, inhibited microglial over proliferation and preserved resting microglial morphology. | [159] |
| Lutein and Zeaxanthin |  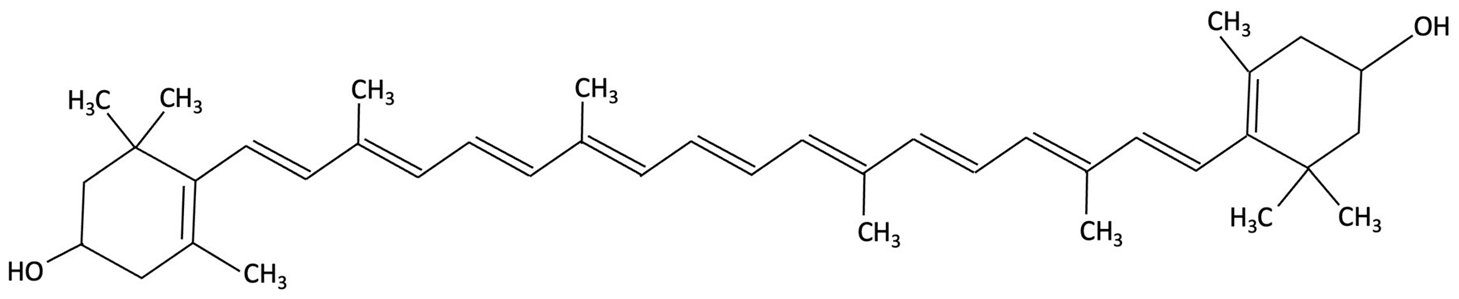 | Porphyra (Nori), Red Seaweed | Neuroprotective | 6-month randomised, double-blind, placebo-controlled trial in adults (40–75 years) assessing daily supplementation of lutein (10 mg) and zeaxanthin (2 mg). | Improvements in visual episodic memory compared to placebo and visual learning. | [160] |
| Astaxanthin (Ax-Hp) | 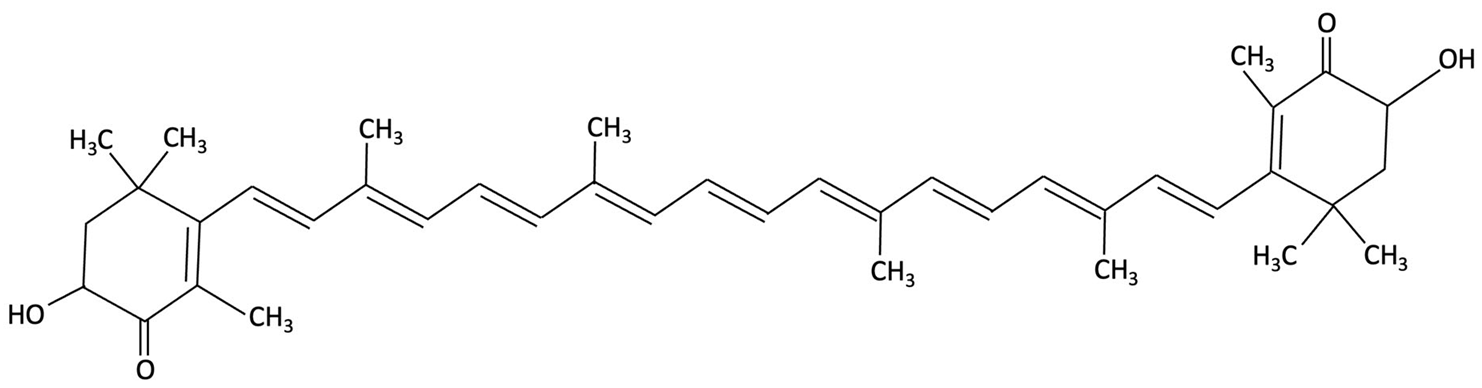 | Haematococcus pluvialis, Green Algae | Antioxidant, Anti-inflammatory | A 12-week randomised, double-blind, placebo-controlled trial in healthy middle-aged adults (aged 45–64 years) with age-related subjective cognitive complaints. | High-dose supplementation improved one CogHealth task, response time and accuracy, with trends in three others. GMLT total errors significantly decreased by week 4, suggesting cognitive benefits. | [161] |
| Astaxanthin (Ax-Hp) |  | Haematococcus pluvialis, Green Algae | Antioxidant | 12-week randomised, double-blind, placebo-controlled trial (n = 30) healthy adults (50–69 years), receiving 0 mg, 6 mg or 12 mg/day. | Significantly increased erythrocyte Ax-Hp levels and reduced PLOOH, indicating antioxidant activity. | [162] |
| Macular Xanthophylls | Chlorella and Dunaliella species, Green Algae | Antioxidant, Anti-inflammatory, Neurotrophic Modulation | 6-month randomised, double-blind, placebo-controlled trial in healthy young adults (18–25 years), assessing dose-dependent effects of macular xanthophyll supplementation (13 mg or 27 mg/day) on cognitive and biochemical outcomes. | Supplementation improved composite and verbal memory, attention and processing speeds. BDNF, AOC, MPOD, lutein and zeaxanthin increased; IL-1β decreased. Cognitive gains correlated with BDNF and MPOD changes. | [163] | |
| Ulvan | 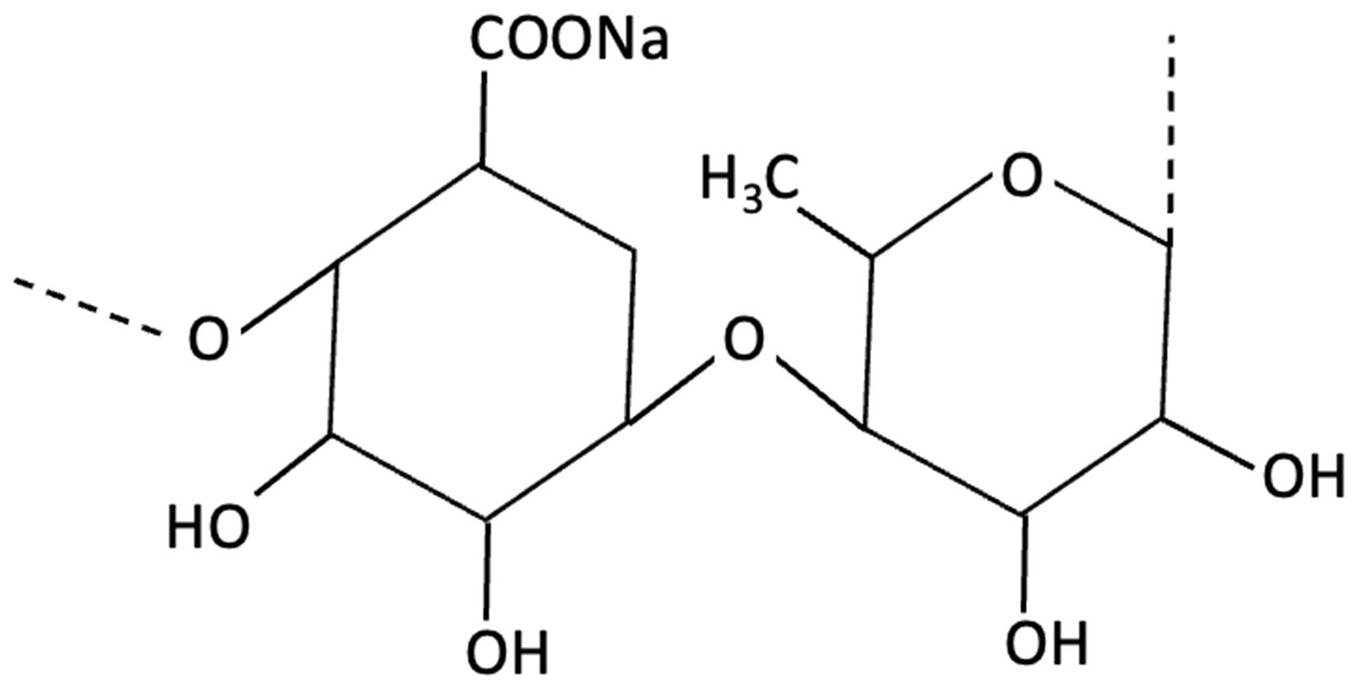 | Ulva lactuca, Green Seaweed | Neuroprotective | In vitro study using SH-SY5Y neuroblastoma cells of Ulva against BPA-induced toxicity. | Strong antioxidant, anticholinesterase and neuroprotective effects by restoring cell viability, inhibiting capase-3 activation. | [164] |
| Mixed Compounds, not explicitly recorded | Mixed Edible Algae | Not directly assessed | Cross-sectional analysis of 2018 CLHLS data from older Chinese adults (≥65 years), examining associations between edible mushroom/algae intake and cognitive impairment. | In the fully adjusted model, cognitive impairment risk was reduced by 29% (OR: 0.710) for daily intake and 25.3% (OR: 0.747) for occasional intake. | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ward, K.; Cole, M.H.; Griffiths, L.R.; Sutherland, H.G.; Winberg, P.; Meyer, B.J.; Fernandez, F. Therapeutic Potentials of the Seaweed-Derived Compounds for Alzheimer’s Disease. Molecules 2025, 30, 4456. https://doi.org/10.3390/molecules30224456
Ward K, Cole MH, Griffiths LR, Sutherland HG, Winberg P, Meyer BJ, Fernandez F. Therapeutic Potentials of the Seaweed-Derived Compounds for Alzheimer’s Disease. Molecules. 2025; 30(22):4456. https://doi.org/10.3390/molecules30224456
Chicago/Turabian StyleWard, Keanie, Michael H. Cole, Lyn R. Griffiths, Heidi G. Sutherland, Pia Winberg, Barbara J. Meyer, and Francesca Fernandez. 2025. "Therapeutic Potentials of the Seaweed-Derived Compounds for Alzheimer’s Disease" Molecules 30, no. 22: 4456. https://doi.org/10.3390/molecules30224456
APA StyleWard, K., Cole, M. H., Griffiths, L. R., Sutherland, H. G., Winberg, P., Meyer, B. J., & Fernandez, F. (2025). Therapeutic Potentials of the Seaweed-Derived Compounds for Alzheimer’s Disease. Molecules, 30(22), 4456. https://doi.org/10.3390/molecules30224456








