Structure, Ecotoxicity, Redox and Bactericidal Activity of Cu-Containing Nanocrystalline Ferrites
Abstract
1. Introduction
2. Results and Discussion
2.1. Magnetic Properties
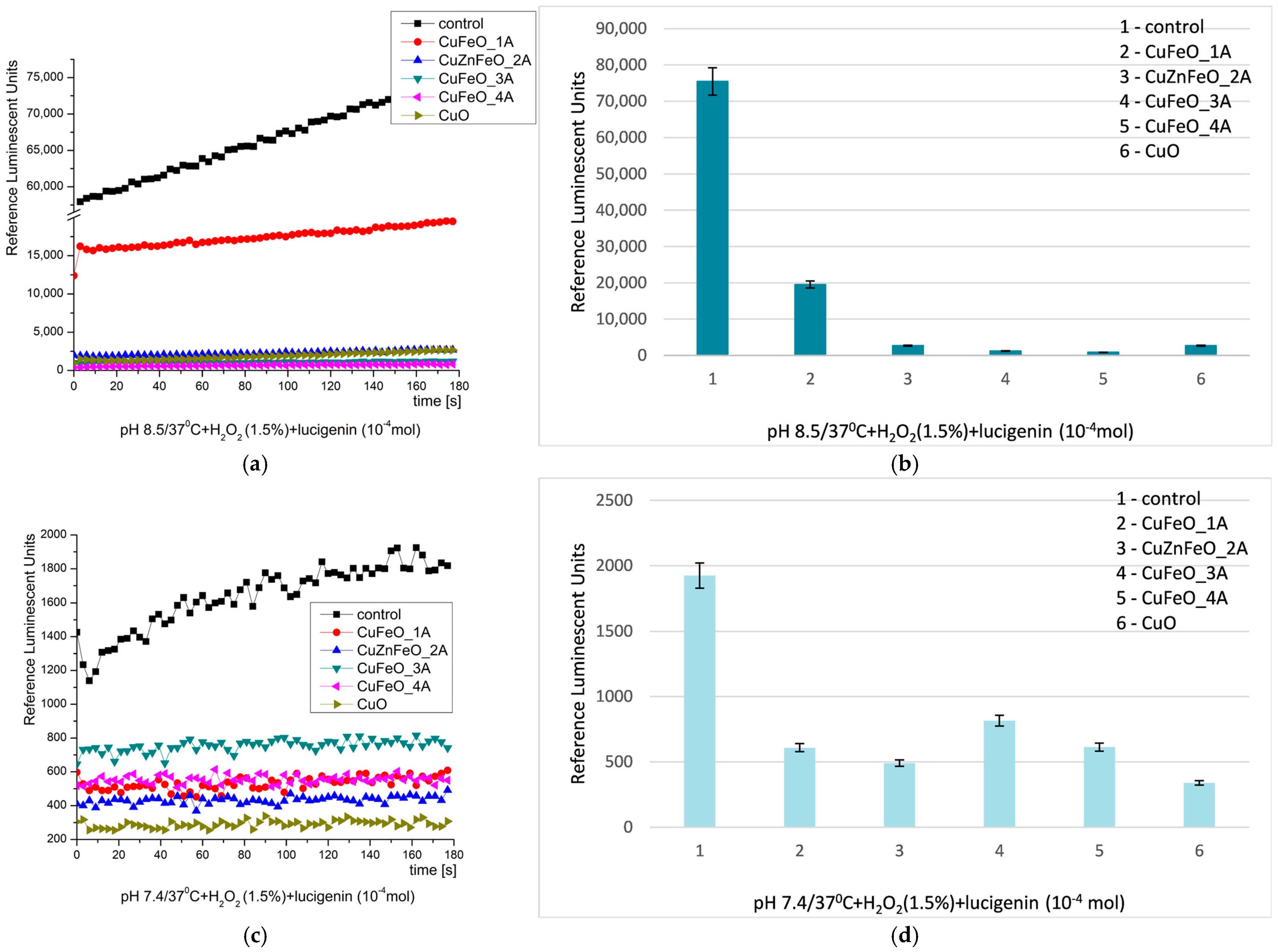
2.2. Redox Activities
2.2.1. System I
- (1)
- Fe2+ + H2O2 → Fe3+ + ·OH + −OH
- (2)
- Fe3+ + H2O2 → Fe2+ + ·OOH + H+
2.2.2. System II
2.2.3. System III
2.3. Antimicrobial Activities
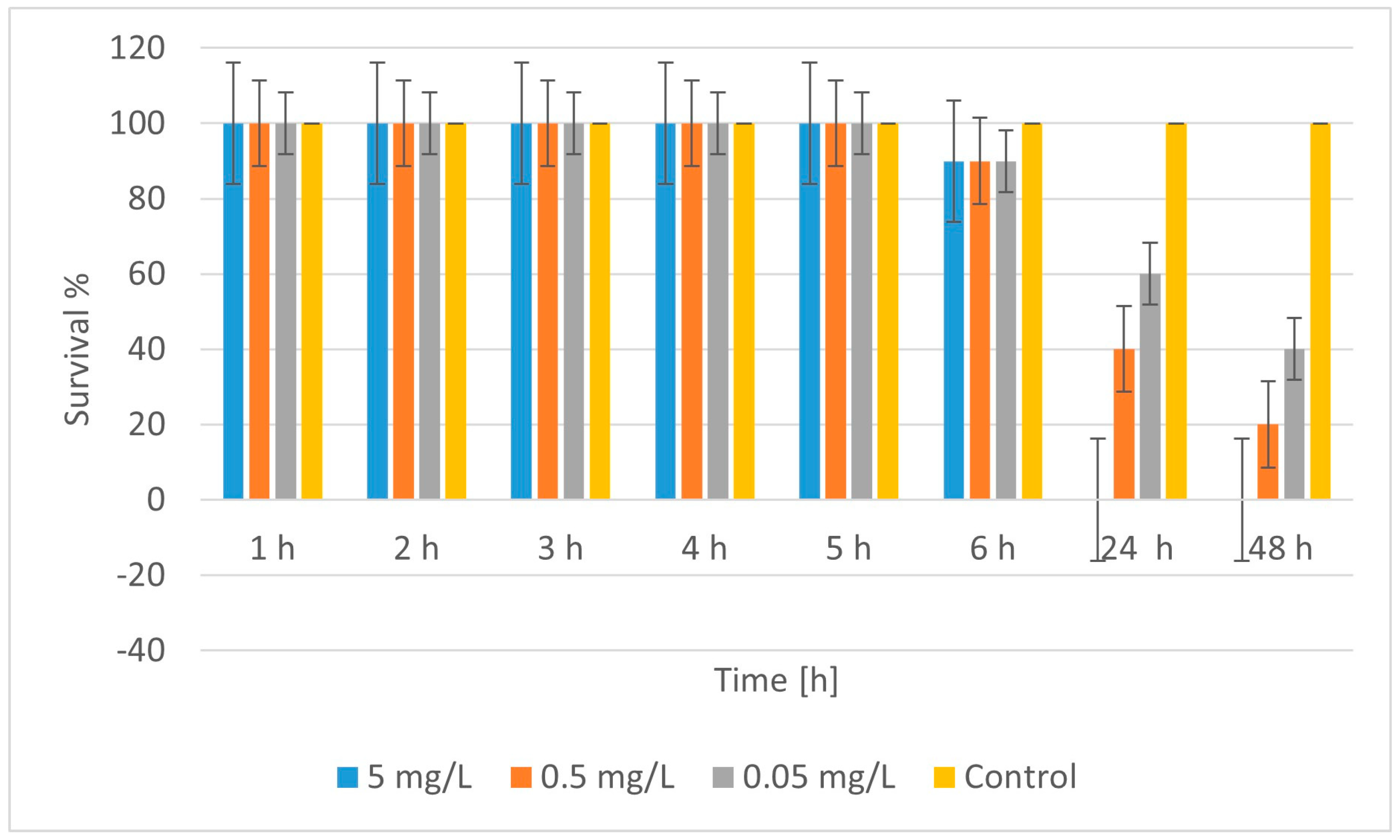
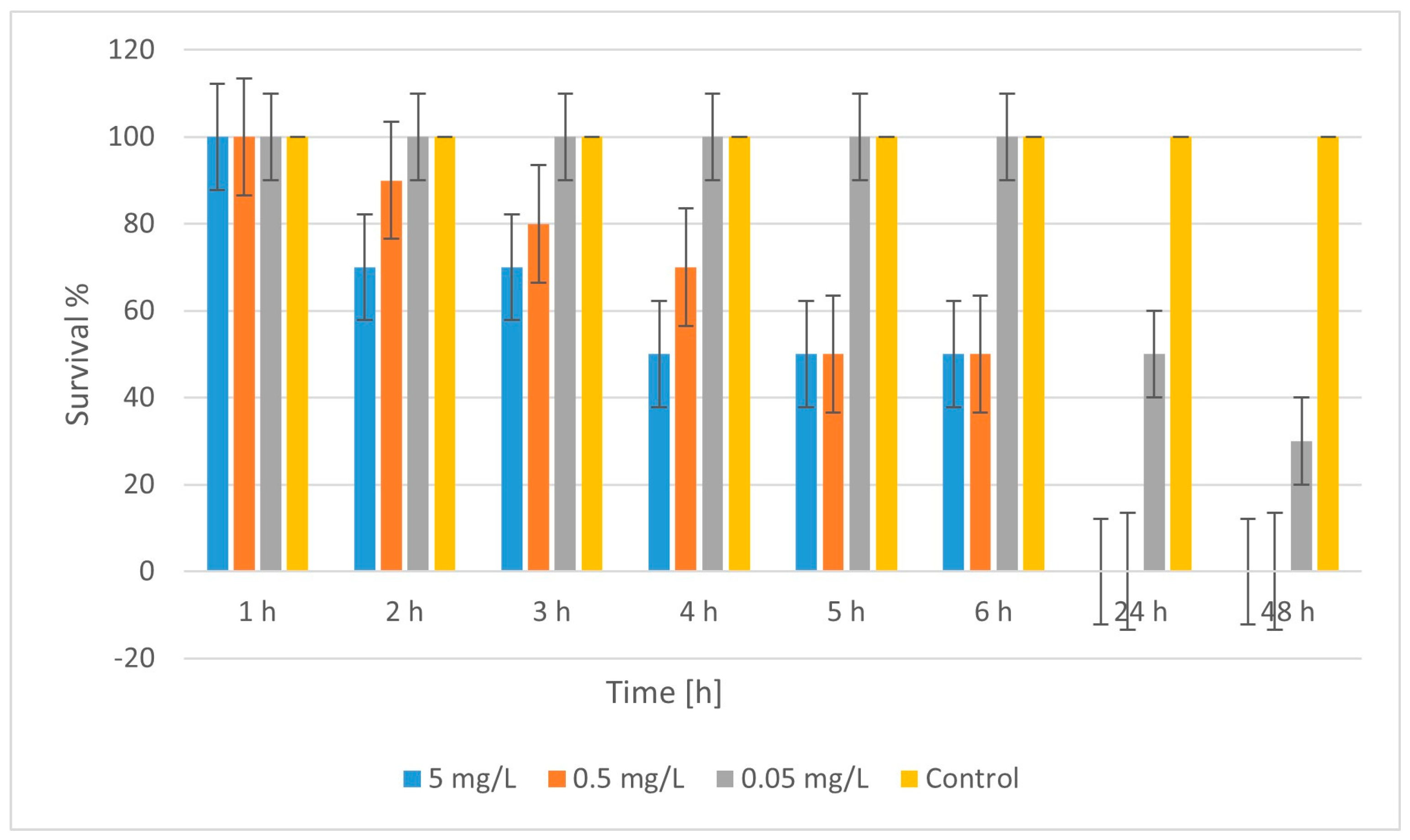
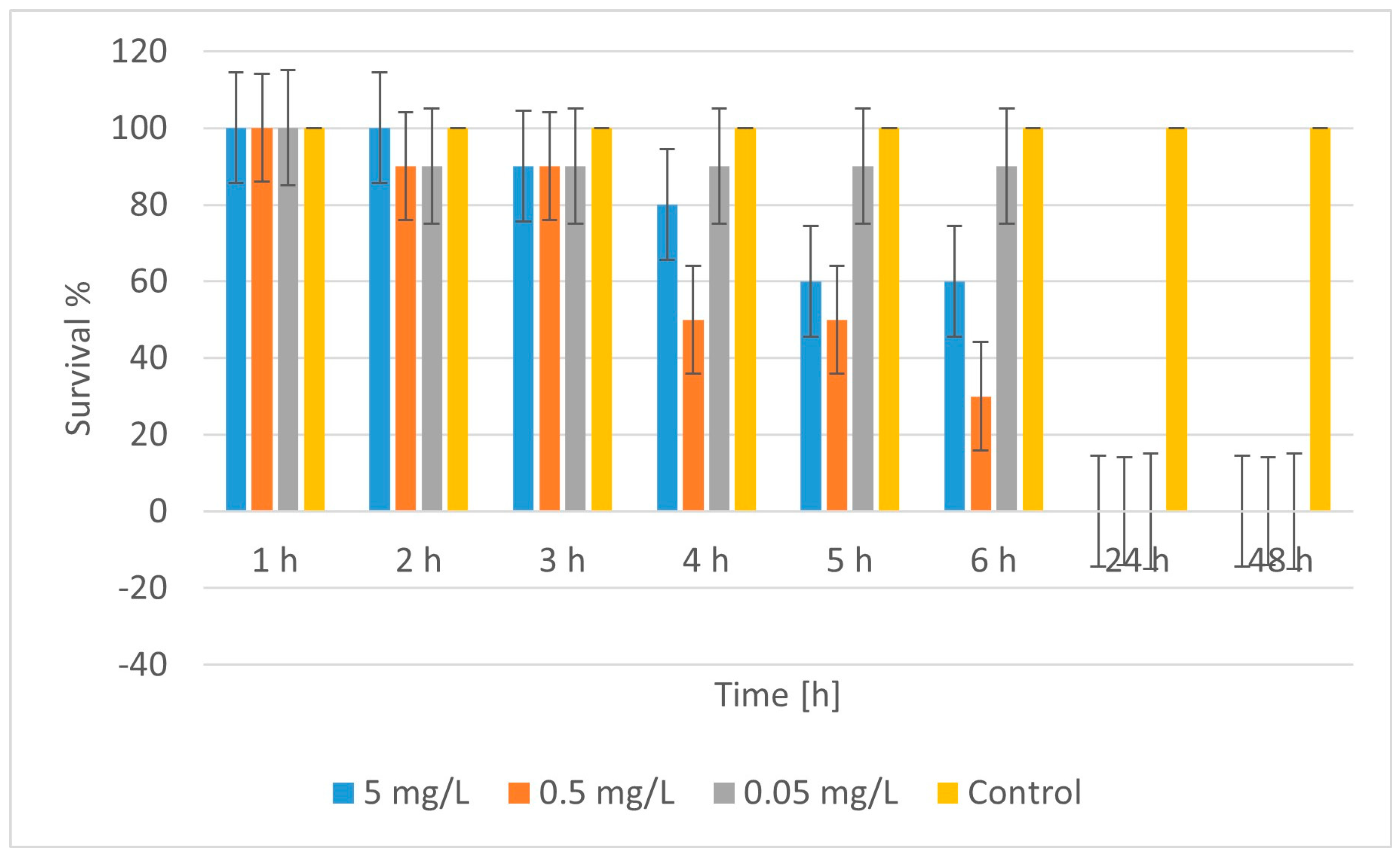
2.4. Ecotoxicity
- LC50 ≤ 1 mg/L → Very toxic;
- 1 < LC50 ≤ 10 mg/L → Toxic;
- LC50 > 10 mg/L → Slightly toxic;
- LC50 not reached → Slightly or nontoxic.
3. Materials
4. Methods
4.1. Nanocrystalline Materials’ Preparation and Characterization
4.2. Chemiluminescent Assay
4.3. Antimicrobial Activity Tests
4.4. Environmental Toxicity by Testing the Lethality of Daphnia Magna
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arcos, D. Nanomaterials in Biomedicine 2022. Int. J. Mol. Sci. 2023, 24, 9026. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M.; Rahdar, A. Functional Nanomaterials in Biomedicine Current Uses and Potential Applications. ChemMedChem 2022, 17, 202200142. [Google Scholar] [CrossRef] [PubMed]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine How nanomaterials are transforming drug delivery, bio-imaging, and diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Mohana, S.; Sumathi, S. Agaricus Bisporus Mediated Synthesis of Cobalt Ferrite, Copper Ferrite and Zinc Ferrite Nanoparticles for Hyperthermia Treatment and Drug Delivery. J. Clust. Sci. 2024, 35, 129–142. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Rah, B.; Al-Omari, I.A.; Kamzin, A.S.; Khurshid, H.; Muhammad, J.S.; Obaidat, I.M.; Issa, B. Evaluation of Antiproliferative Properties of CoMnZn-Fe2O4 Ferrite Nanoparticles in Colorectal Cancer Cells. Pharmaceuticals 2024, 17, 327. [Google Scholar] [CrossRef]
- Racca, L.; Cauda, V. Remotely Activated Nanoparticles for Anticancer Therapy. Nano-Micro Lett. 2021, 13, 11. [Google Scholar] [CrossRef]
- Han, J.; Chen, Y.; Xiang, X.; Wang, T.; Shen, J.; Zhang, N.; Liang, C.; Liu, X.; Ma, X. A Comparative Analysis of the Antibacterial Spectrum of Ultrasmall Manganese Ferrite Nanozymes with Varied Surface Modifications. ACS Appl. Mater. Interfaces 2024, 16, 14385–14404. [Google Scholar] [CrossRef]
- Haghniaz, R.; Rabbani, A.; Vajhadin, F.; Khan, T.; Kousar, R.; Khan, A.R.; Montazerian, H.; Iqbal, J.; Libanori, A.; Kim, H.-J.; et al. Anti-bacterial and wound healing-promoting effects of zinc ferrite nanoparticles. J. Nanobiotechnol. 2021, 19, 38. [Google Scholar] [CrossRef]
- Rabbani, A.; Haghniaz, R.; Khan, T.; Khan, R.; Khalid, A.; Naz, S.S.; Ul-Islam, M.; Vajhadin, F.; Wahid, F. Development of bactericidal spinel ferrite nanoparticles with effective biocompatibility for potential wound healing applications. RSC Adv. 2021, 11, 1773–1782. [Google Scholar] [CrossRef]
- Mendes, C.; Thirupathi, A.; Corrêa, M.E.A.B.; Gu, Y.; Silveira, P.C.L. The Use of Metallic Nanoparticles in Wound Healing: New Perspectives. Int. J. Mol. Sci. 2022, 23, 15376. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.H.K.; Yun, Y.S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Sukoviene, A.; Ali, S.; Jagminas, A.; Ramanavicius, S. Magnetic Cobalt and Other Types of Ferrite Nanoparticles: Synthesis Aspects and Novel Strategies for Application in Wastewater Treatment (Review). Appl. Sci. 2025, 15, 857. [Google Scholar] [CrossRef]
- Garg, V.K.; Sharma, V.K.; Kuzmann, E. Purification of Water by Ferrites—Mini Review. In Ferrites and Ferrates: Chemistry and Applications in Sustainable Energy and Environmental Remediation; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2016; Volume 1238, Chapter 5; pp. 137–143. [Google Scholar]
- Mikhailovsky, V.L.; Radovenchik, V.M. Water and Wastewater Treatment Using Ferrites. In Chemical Water and Wastewater Treatment IV; Hahn, H.H., Hoffmann, E., Ødegaard, H., Eds.; Springer: Berlin, Germany, 1996. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.M.; Bandala, E.R. Use of Ferrate and Ferrites for Water Disinfection. In Ferrites and Ferrates: Chemistry and Applications in Sustainable Energy and Environmental Remediation; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2016; Volume 1238, Chapter 6; pp. 145–159. [Google Scholar]
- Rmeid, S.; Aridi, A.; Habanjar, K.; Rahman, E.M.A.; Khalil, W.F.; El-Subruiti, G.M.; Awad, R. Removal of radioactive Co(II) and Sr(II) using (Co0.5Zn0.5)Fe2O4 nanoparticles co-doped with barium and antimony. Ceram. Int. 2025, 51, 4593–4612. [Google Scholar] [CrossRef]
- Naik, P.R.; Rajashekara, V.A.; Mudhulu, S.; Channegowda, M. Facile synthesis, characterisation and application of zinc ferrite in removal of uranium from water by adsorption. J. Contamin. Hydrol. 2025, 273, 104583. [Google Scholar] [CrossRef]
- Shehzad, K.; Zhu, H.; Ahmad, M.; Yusuf, K.; Butt, M.T.; Rahmat, M.; Xu, W.; Liu, J.; Xu, Y. Facile synthesis of novel zinc ferrite nanostructures (ZFN) for enhanced adsorption of highly mobile and toxic As(III) from aqueous solutions. J. Water Proc. Eng. 2024, 63, 105464. [Google Scholar] [CrossRef]
- Carrese, B.; Sanità, G.; Lamberti, A. Nanoparticles Design for Theranostic Approach in Cancer Disease. Cancers 2022, 14, 4654. [Google Scholar] [CrossRef]
- Chen, F.; Ehlerding, E.B.; Cai, W. Theranostic Nanoparticles. J. Nucl. Med. 2014, 55, 1919–1922. [Google Scholar] [CrossRef]
- Revia, R.A.; Stephen, Z.R.; Zhang, M. Theranostic Nanoparticles for RNA-Based Cancer Treatment. Acc. Chem. Res. 2019, 52, 1496–1506. [Google Scholar] [CrossRef]
- World Health Organization. 10 Threats to Global Health in 2018. 2018. Available online: https://medium.com/who/10-threats-toglobal-health-in-2018-232daf0bbef3 (accessed on 5 June 2025).
- Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2023, 12, 116. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Ellah, N.H.A.; Zanetti, S.; Donadu, M.G. Nanotechnology as a promising approach to combat multidrug resistant bacteria: A comprehensive review and future perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Klasen, H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef]
- Dutta, P.; Wang, B. Zeolite-supported silver as antimicrobial agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar] [CrossRef]
- Pavlova, E.L.; Nenova, E.P.; Yocheva, L.D.; Ivanova, I.A.; Georgiev, P.A. Antimicrobial and Oxidative Activities of Different Levels of Silver-Exchanged Zeolites X and ZSM-5 and Their Ecotoxicity. Pharmaceuticals 2024, 17, 1586. [Google Scholar] [CrossRef]
- Mahmoudi, M. The need for robust characterization of nanomaterials for nanomedicine applications. Nat. Commun. 2021, 12, 5246. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.-I.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef]
- Lipovsky, A.; Tzitrinovich, Z.; Friedmann, H.; Applerot, G.; Gedanken, A.; Lubart, R. EPR study of visible light-induced ROS generation by nanoparticles of ZnO. J. Phys. Chem. C 2009, 113, 15997–16001. [Google Scholar] [CrossRef]
- Leung, Y.H.; Chan, C.M.N.; Ng, A.M.C.; Chan, H.T.; Chiang, M.W.L.; Djurišić, A.B.; Ng, Y.H.; Jim, W.Y.; Guo, M.Y.; Leung, F.C.C.; et al. Antibacterial activity of ZnO nanoparticles with a modified surface under ambient illumination. Nanotechnology 2012, 23, 475703. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Le Guével, X.; Fleury, J.; Hanssen, E.; Nguyen, T.H.P.; Juodkazis, S.; Bryant, G.; Crawford, R.J.; Stoodley, P.; et al. Antibacterial Action of Nanoparticles by Lethal Stretching of Bacterial Cell Membranes. Adv. Mater. 2020, 32, 2005679. [Google Scholar] [CrossRef]
- Chen, K.L.; Bothun, G.D. Nanoparticles Meet Cell Membranes: Probing Nonspecific Interactions using Model Membranes, Environ. Sci. Technol. 2014, 48, 873–880. [Google Scholar] [CrossRef]
- Bhabra, G.; Sood, A.; Fisher, B.; Cartwright, L.; Saunders, M.; Evans, W.H.; Surprenant, A.; Lopez-Castejon, G.; Mann, S.; Davis, S.A.; et al. Nanoparticles can cause DNA damage across a cellular barrier. Nat. Nanotechnol. 2009, 4, 876–883. [Google Scholar] [CrossRef]
- Gakis, G.P.; Aviziotis, I.G.; Charitidis, C. Metal and metal oxide nanoparticle toxicity: Moving towards a more holistic structure–activity approach. Environ. Sci. Nano 2023, 10, 761–780. [Google Scholar] [CrossRef]
- Hankiewicz, J.; Stoll, J.; Stroud, J.; Davidson, J.; Livesey, K.; Tvrdy, K.; Roshko, A.; Russek, S.; Stupic, K.; Bilski, P.; et al. Nano-sized ferrite particles for magnetic resonance imaging thermometry. J. Magn. Magn. Mater 2019, 469, 550–557. [Google Scholar] [CrossRef]
- Wen, L.; Fu, X.; Zhang, H.; Ye, P.; Fu, H.; Zhou, Z.; Sun, R.; Xu, T.; Fu, C.; Zhu, C.; et al. Tailoring Zinc Ferrite Nanoparticle Surface Coating for Macrophage-Affinity Magnetic Resonance Imaging of Atherosclerosis. ACS Appl. Mater. Interf. 2024, 16, 13496–13508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Liu, J.; Deng, Z.; Zhang, X.; Alifu, N.; Zhang, X.; Yu, Z.; Liu, Y.; Lan, Z.; et al. Recent advances in functionalized ferrite nanoparticles: From fundamentals to magnetic hyperthermia cancer therapy. Colloids Surf. B Biointerfaces 2024, 254, 113754. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Oza, G.; Ángeles-Pascual, A.; González, M.M.; Antaño-López, R.; Vera, A.; Leija, L.; Reguera, E.; Arriaga, L.G.; Hernández, J.M.H.; et al. Synthesis, Characterization and Magnetic Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics. Molecules 2020, 25, 4428. [Google Scholar] [CrossRef]
- Hastings, J.M.; Corliss, L.M. Neutron Diffraction Studies of Zinc Ferrite and Nickel Ferrite. Rev. Mod. Phys. 1953, 25, 114–119. [Google Scholar] [CrossRef]
- Andersen, H.L.; Saura-Múzquiz, M.; Granados-Miralles, C.; Canévet, E.; Lock, N.; Christensen, M. Crystalline and magnetic structure–property relationship in spinel ferrite nanoparticles. Nanoscale 2018, 10, 14902–14914. [Google Scholar] [CrossRef]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef]
- Scholefield, M.; Church, S.J.; Xu, J.; Patassini, S.; Roncaroli, F.; Hooper, N.M.; Unwin, R.D.; Cooper, G.J.S. Widespread Decreases in Cerebral Copper Are Common to Parkinsons Disease Dementia and Alzheimer’s Disease Dementia. Front. Aging Neurosci. 2021, 13, 641222. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M.; Javed, R. Javed, Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Appl. Microbiol. Biotechnol. 2023, 107, 1039–1061. [Google Scholar] [CrossRef]
- Havryliuk, O.; Rathee, G.; Blair, J.; Hovorukha, V.; Tashyrev, O.; Morató, J.; Pérez, L.M.; Tzanov, T. Unveiling the Potential of CuO and Cu2O Nanoparticles against Novel Copper-Resistant Pseudomonas Strains- An In-Depth Comparison. Nanomaterials 2024, 14, 1644. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, S.; Kim, Y.; Ryu, Y.B. Antimicrobial Activity of Morphology-Controlled Cu2O Nanoparticles- Oxidation Stability under Humid and Thermal Conditions. Materials 2024, 17, 261. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Q.; Li, Y.; Liu, B.; Miao, Y. Recent Advances in the Biomedical Applications of Copper Nanomaterial-Mediated Cuproptosis. Adv. NanoBiomed Res. 2024, 4, 2400018. [Google Scholar] [CrossRef]
- Wozniak-Budych, M.J.; Staszak, K.; Staszak, M. Copper and Copper-Based Nanoparticles in Medicine—Perspectives and Challenges. Molecules 2023, 28, 6687. [Google Scholar] [CrossRef]
- Tang, X.-X.; Manthiram, A.; Goodenough, J.B. Copper Ferrite Revisited. J. Sol. St. Chem. 1989, 79, 250–262. [Google Scholar] [CrossRef]
- Balagurov, A.M.; Bobrikov, I.A.; Maschenko, M.S.; Sangaa, D.; Simkin, V.G. Structural Phase Transition in CuFe2O4 Spinel. Crystallogr. Rep. 2013, 58, 710–717. [Google Scholar] [CrossRef]
- Zhuravlev, V.; Minin, R.; Itin, V.; Lilenko, I.Y. Structural parameters and magnetic properties of copper ferrite nanopowders obtained by the sol-gel combustion. J. Alloys Compd. 2017, 692, 705–712. [Google Scholar] [CrossRef]
- Calvo-de la Rosa, J.; Segarra, M.R. Influence of the Synthesis Route in Obtaining the Cubic or Tetragonal Copper Ferrite Phases. Inorg. Chem. 2020, 59, 8775–8788. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Patron, L.; Cizmas, C.-B. Tetragonal copper ferrite obtained by self-propagating combustion. J. All. Compd. 2008, 460, 627–631. [Google Scholar] [CrossRef]
- Dhyani, R.; Srivastava, R.C.; Rawat, P.S.; Dixit, G. Structural and elastic properties of tetragonal nano-structured copper ferrite. Int. J. Mater. Res. 2022, 113, 884–892. [Google Scholar] [CrossRef]
- Caddeo, F.; Loche, D.; Casula, M.F.; Corrias, A. Evidence of a cubic iron sub-lattice in t-CuFe2O4 demonstrated by X-ray Absorption Fine Structure. Sci. Rep. 2018, 8, 797. [Google Scholar] [CrossRef] [PubMed]
- Samavati, A.; Ismail, A.F. Antibacterial properties of copper-substituted cobalt ferrite nanoparticles synthesized by co-precipitation method0. Particuology 2017, 30, 158–163. [Google Scholar] [CrossRef]
- Atia, T.A.; Altimari, P.; Moscardini, E.; Pettiti, I.; Toro, L.; Pagnanelli, F. Synthesis and characterization of copper ferrite magnetic nanoparticles by hydrothermal route. Chem. Eng. Trans. 2016, 47, 151–156. [Google Scholar]
- Kahkesh, K.H.; Baghbantaraghdari, Z.; Jamaledin, D.; Moghaddam, F.D.; Kaneko, N.; Ghovvati, M. Synthesis, Characterization, Antioxidant and Antibacterial Activities of Zinc Ferrite and Copper Ferrite Nanoparticles. Mater. Chem. Horiz. 2023, 2, 49–56. [Google Scholar]
- Gu, Y.; Xiao, F.; Luo, L.; Zhou, X.; Zhou, X.; Li, J.; Li, Z. Bacterial Disinfection by CuFe2O4 Nanoparticles Enhanced by NH2OH: A Mechanistic Study. Nanomaterials 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montelongo, J.H.; Hernandez-Rangel, R.; Campos-Avelar, I.; Betancourt, I.; Quintero, L.H.; Cano, M.E.; Zapien, J.A.; Medina-Ramirez, I.E. Antimicrobial activity of CuFe2O4 and CuFe2O4/ZnO: Squaring colorimetric and traditional microbiology assays with atomic force- and holotomography-microscopies. Ceram. Int. 2025, 51, 2452–2466. [Google Scholar] [CrossRef]
- Wang, J.; Qiang, R.; Miao, Q.; Hu, R.; Chen, H.; Guo, S.; Liu, Z. Synthesis and potent antibacterial activity of nano-CuFe2O4/MoS2@Ag composite under visible light. Appl. Surf. Sci. 2025, 684, 161908. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.; Li, F.; Xiao, Y.; Zhang, Y.; Bu, T.; Jia, P.; Zhe, T.; Wang, L. Multifunctional Magnetic Copper Ferrite Nanoparticles as Fenton like Reaction and Near-Infrared Photothermal antibacterial Therapy. ACS Appl. Mater. Interfaces 2019, 11, 31649–31660. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef]
- Pavlova, E.L.; Toshkovska, R.D.; Doncheva, T.E.; Ivanova, I.A. Ivanova, Prooxidant and antimicrobic effects of iron and titanium oxide nanoparticles and thalicarpine. Arch. Microbiol. 2020, 202, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Taufiq, A.; Sunaryono, S.; Putra, E.G.R.; Pratapa, S.; Darminto, D. Nano-structural studies on Fe3O4 particles dispersing in a magnetic fluid using X-ray diffractometry and small-angle neutron scattering. Mater. Sci. Forum 2015, 827, 213–218. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, B.P.; Ningthoujam, R.S. Confirmation of highly stable 10 nm sized Fe3O4 nanoparticle formation at room temperature and understanding of heat generation under AC magnetic fields for potential application in hyperthermia. AIP Adv. 2020, 10, 105033. [Google Scholar] [CrossRef]
- Safitri, I.; Wibowo, Y.G.; Rosarina, D. Synthesis and characterization of magnetite (Fe3O4) nanoparticles from iron sand in Batanghari Beach. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012020. [Google Scholar] [CrossRef]
- Philip, J.; Gnanaprakash, G.; Panneerselvam, G.; Antony, M.P.; Jayakumar, T.; Raj, B. Effect of thermal annealing under vacuum on the crystal structure, size, and magnetic properties of ZnFe2O4 nanoparticles. J. Appl. Phys. 2007, 102, 054305. [Google Scholar] [CrossRef]
- Kang, M.J.; An, N.H.; Kang, Y.S. Magnetic and Photochemical Properties of Cu Doped Hematite Nanocrystal. Mater. Sci. Forum 2017, 893, 136–143. [Google Scholar] [CrossRef]
- Ahmadi-Arpanah, A.; Meleki-Ghaleh, H.; Dargahi, Z.; Khademi-Azandehi, P.; Mirzaei, G.; Beygi-Khosrowshahi, Y.; Siadati, M.H. The photocatalytic antibacterial behavior of Cu-doped nanocrystalline hematite prepared by mechanical alloying. Appl. Nanosci. 2021, 11, 11817–11832. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Gadri, A.; Ammar, S. Structural, optical and morphological characterization of Cu-doped α-Fe2O3 nanoparticles synthesized through co-precipitation technique. J. Mol. Struct. 2017, 1148, 276–281. [Google Scholar] [CrossRef]
- Krehula, S.; Ristić, M.; Petrović, Ž.; Krehula, L.K.; Mitar, I.; Musić, S. Effects of Cu doping on the microstructural, thermal, optical and photocatalytic properties of α-FeOOH and α-Fe2O3 1D nanoparticles. J. All. Comp. 2019, 802, 290–300. [Google Scholar] [CrossRef]
- Shull, C.G.; Strauser, W.A.; Wollan, E.O. Neutron Diffraction by Paramagnetic and Antiferromagnetic Substances. Phys. Rev. 1951, 83, 333–345. [Google Scholar] [CrossRef]
- Pavlova, E.L.; Ivanova, I.A.; Staneva, A.D.; Kostadinova, A.S.; Kichukova, D.G.; Yocheva, L.D. Prooxidant, antioxidant and biological activity of nanocomposites of reduced graphene oxide, silver, copper and their combinations. Chem. Pap. 2022, 76, 6789–6800. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854, ISSN 0006-291X. [Google Scholar] [CrossRef]
- Hancock, J.T.; Desikan, R.; Neill, S. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001, 29 Pt 2, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Mazurenko, J.; Sijo, A.K.; Kaykan, L.; Kotsyubynsky, V.; Gondek, Ł.; Zywczak, A.; Marzec, M.; Vyshnevskyi, O. Synthesis and Characterization of Copper Ferrite Nanoparticles for Efficient Photocatalytic Degradation of Organic Dyes. J. Nanotechnol. 2025, 2025, 8899491. [Google Scholar] [CrossRef]
- Díez-García, M.I.; Lana-Villarreal, T.; Gómez, R. Study of Copper Ferrite as a Novel Photocathode for Water Reduction: Improving Its Photoactivity by Electrochemical Pretreatment. ChemSusChem 2016, 9, 1504–1512. [Google Scholar] [CrossRef]
- Liu, H.; Di Valentin, C. Band Gap in Magnetite above Verwey Temperature Induced by Symmetry Breaking. J. Phys. Chem. C 2017, 121, 25736–25742. [Google Scholar] [CrossRef] [PubMed]
- Sielska, A.; Skuza, L. Copper Nanoparticles in Aquatic Environment: Release Routes and Oxidative Stress-Mediated Mechanisms of Toxicity to Fish in Various Life Stages and Future Risks. Curr. Issues Mol. Biol. 2025, 47, 472. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Vijver, M.G.; Chen, G.; Peijnenburg, W.J. Toxicity and Accumulation of Cu and ZnO Nanoparticles in Daphnia magna. Environ. Sci. Technol. 2015, 49, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Yang, H.; Xia, J.; Zong, Y.; Liu, J. Toxicity of Cu and Cr nanoparticles to Daphnia magna. Water Air Soil Pollut. 2017, 228, 18. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD Publishing: Paris, France, 2004. [Google Scholar]
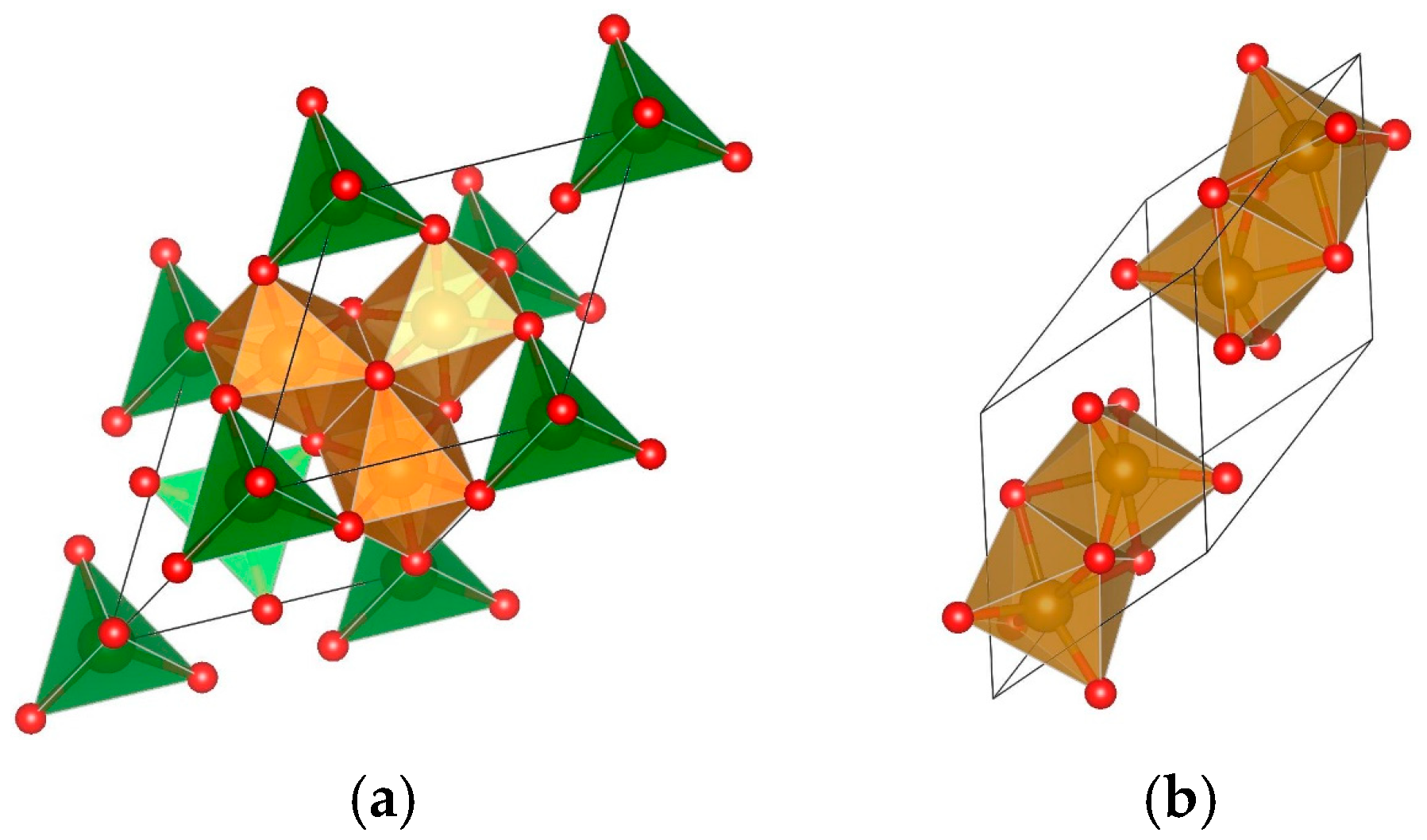
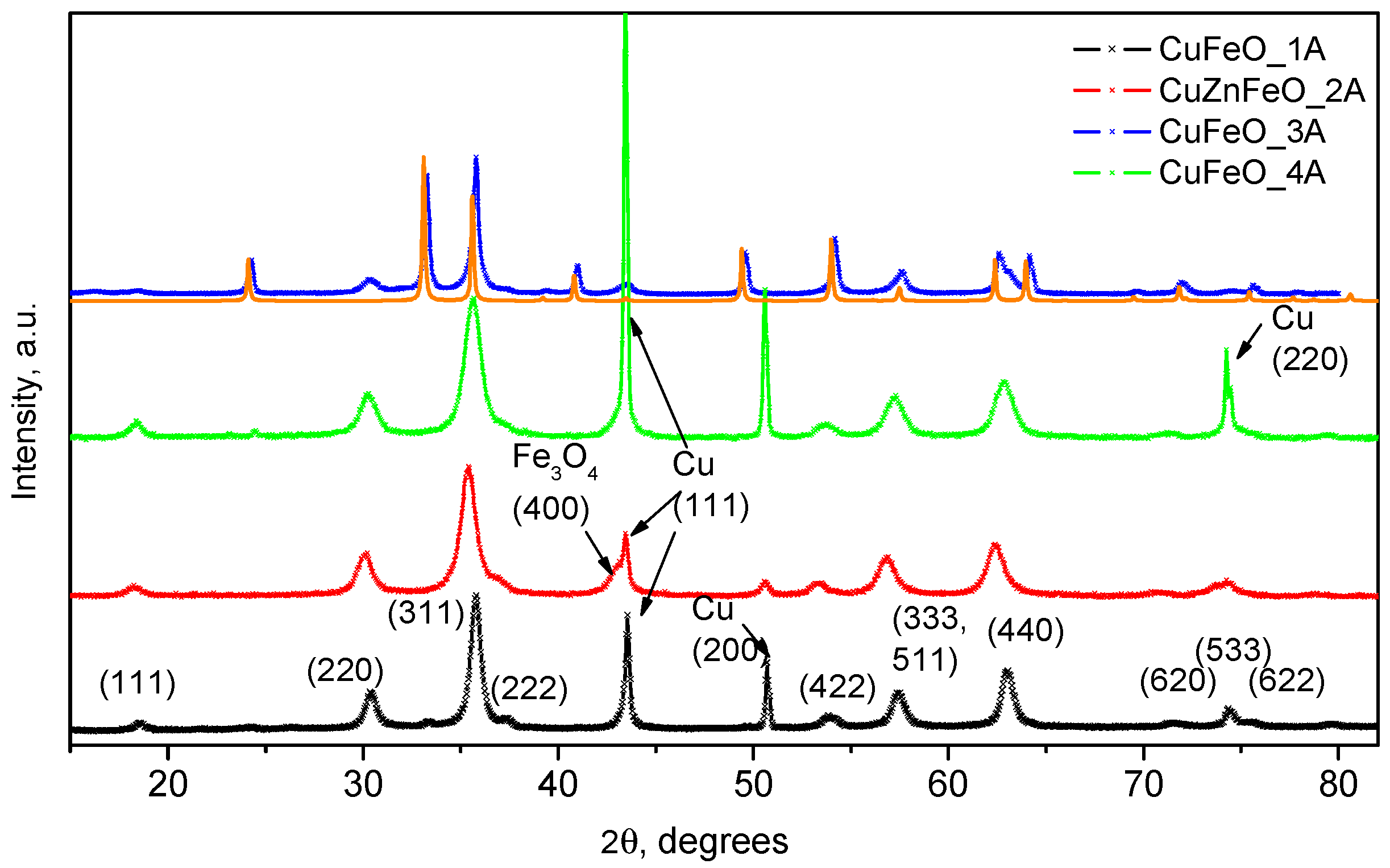
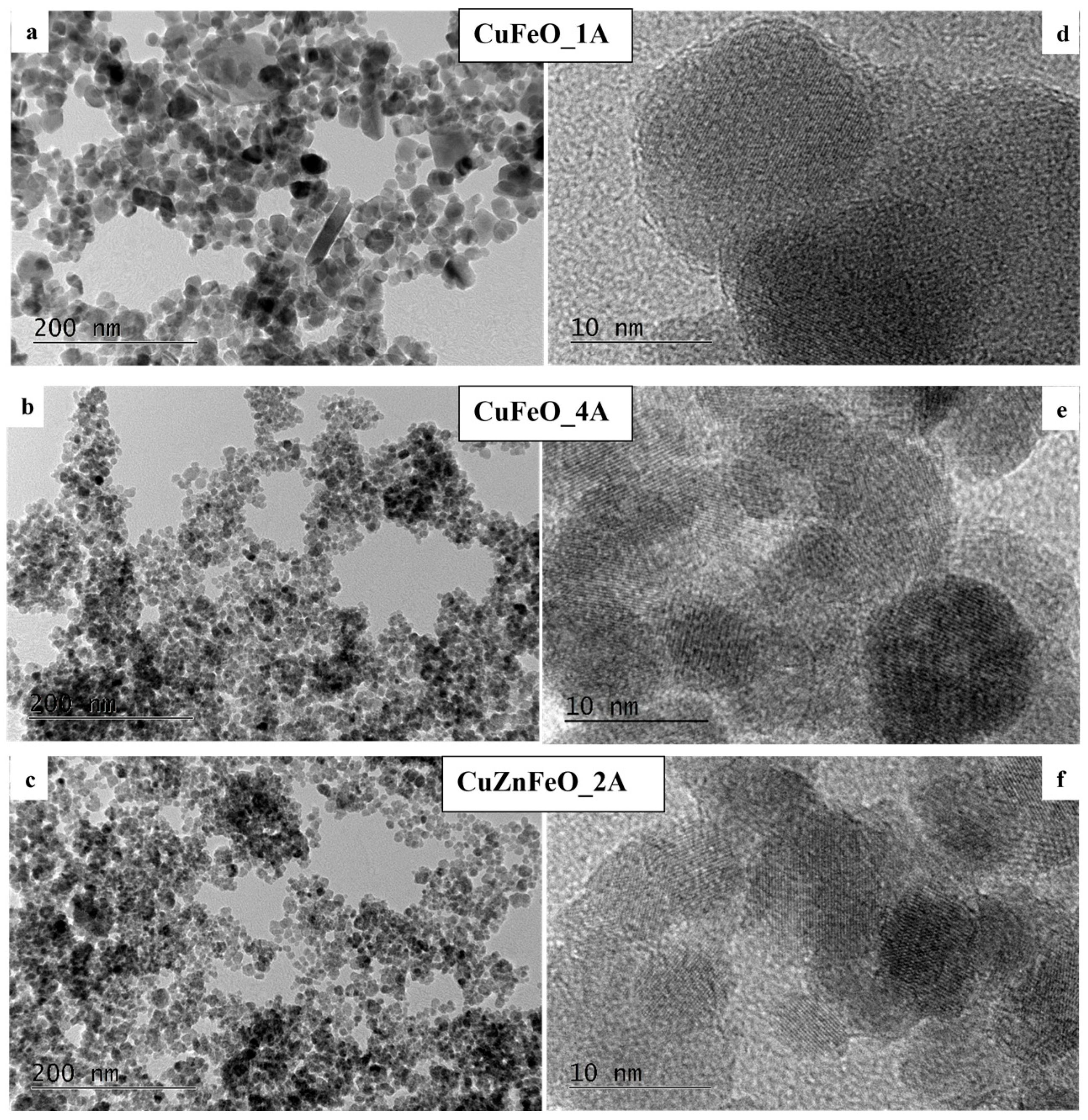






| Sample | Phase | Latt. Par., [Å] | Cry. Size, [nm] | Mmax, [emu/g] | HC, Oe |
|---|---|---|---|---|---|
| CuFeO_1A | Magnetite + Cu | 8.36 (7) | 15 | 56 | 89 |
| CuZnFeO_2A | Magnetite + Cu | 8.428 (5) | 9 | 27 | 0 |
| CuFeO_3A | Hematite + Magnetite | 5.03 (0), 13.76 (4), 8.34 (6) | n.d. | 22 | 16 |
| CuFeO_4A | Magnetite + Cu | 8.37 (7) | 11 | 46 | 0 |
| SYSTEM/ROS | pH, 37 °C | OBSERVATION | MOST ACTIVE MATERIALS | EFFECT (FOLDS) | TYPE OF EFFECT |
|---|---|---|---|---|---|
| Fenton’s system (·OH, ·OOH, H2O2, −OH) | 8.5 (optimal) | all materials showed definitive inhibitory activity | CuFeO_3A CuFeO_4A | 128× 113× | strong inhibition |
| Fenton’s system (·OH, ·OOH, H2O2, −OH) | 7.4 (physiological) | all materials suppressed oxidation | CuFeO_1A CuZnFeO_2A | 4.5× 4.2× | moderate inhibition |
| H2O2 system | 8.5 (optimal) | all materials inhibited oxidation | CuFeO_4A | 88× | strong inhibition |
| H2O2 system | 7.4 (physiological) | well-distinguished suppression of the oxidation | CuO | ~6× | clear inhibition |
| O2·− radicals | 8.5 (optimal) | strong inhibition observed | CuFeO_1A | 8.8× | strong inhibition |
| O2·− radicals | 7.4 (physiological) | oxidation suppression converted into prooxidant effect; only CuFeO_4A showed inhibition | CuFeO_1A (prooxidant), CuFeO_4A (inhibitory) | CuFeO_1A > 2.4× CuFeO_4A < 2× | prooxidant/weak inhibition |
| Active Material | 10 mg/mL | 5 mg/mL | 1.0 mg/mL | 0.1 mg/mL |
|---|---|---|---|---|
| nCuO | BC | BC | MBC | MIC |
| CuFeO_1A | MBC | MIC | No Effect | No Effect |
| CuZnFeO_2A | MBC | MIC | No Effect | No Effect |
| CuFeO_3A | MBC | MIC | No Effect | No Effect |
| CuFeO_4A | MBC | MIC | No Effect | No Effect |
| Active Material | 10 mg/mL | 5 mg/mL | 1.0 mg/mL | 0.1 mg/mL |
|---|---|---|---|---|
| nCuO | BC | BC | MBC | MIC |
| CuFeO_1A | No Effect | No Effect | No Effect | No Effect |
| CuZnFeO_2A | MBC | MIC | No Effect | No Effect |
| CuFeO_3A | No Effect | No Effect | No Effect | No Effect |
| CuFeO_4A | MBC | MIC | No Effect | No Effect |
| No. | Material | LC50 (48 h) [mg/L] | Toxicity Class * |
|---|---|---|---|
| 1 | CuFeO_1A | ~5.0 | Moderately toxic |
| 2 | CuZnFeO_2A | ~0.042 | Highly toxic |
| 2 | CuFeO_3A | ~0.036 | Highly toxic |
| 5 | CuFeO_4A | <0.05 (100% lethality) | Highly toxic |
| 6 | nCuO | >0.0125 (n.d. LC50) | Nontoxic/weakly toxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadimov, T.R.; Nenova, E.P.; Pavlova, E.L.; Ivanova, I.A.; Georgieva, M.T.; Georgiev, P.A. Structure, Ecotoxicity, Redox and Bactericidal Activity of Cu-Containing Nanocrystalline Ferrites. Molecules 2025, 30, 4454. https://doi.org/10.3390/molecules30224454
Karadimov TR, Nenova EP, Pavlova EL, Ivanova IA, Georgieva MT, Georgiev PA. Structure, Ecotoxicity, Redox and Bactericidal Activity of Cu-Containing Nanocrystalline Ferrites. Molecules. 2025; 30(22):4454. https://doi.org/10.3390/molecules30224454
Chicago/Turabian StyleKaradimov, Todor R., Elena P. Nenova, Elitsa L. Pavlova, Iliana A. Ivanova, Milena T. Georgieva, and Peter A. Georgiev. 2025. "Structure, Ecotoxicity, Redox and Bactericidal Activity of Cu-Containing Nanocrystalline Ferrites" Molecules 30, no. 22: 4454. https://doi.org/10.3390/molecules30224454
APA StyleKaradimov, T. R., Nenova, E. P., Pavlova, E. L., Ivanova, I. A., Georgieva, M. T., & Georgiev, P. A. (2025). Structure, Ecotoxicity, Redox and Bactericidal Activity of Cu-Containing Nanocrystalline Ferrites. Molecules, 30(22), 4454. https://doi.org/10.3390/molecules30224454







