Redox Potential (E0′) of the β-Chain 93Cys of HbS Measured with the Equilibrium Technique in a Heterozygous Sickle Cell Carrier Subject
Abstract
1. Introduction
2. Results
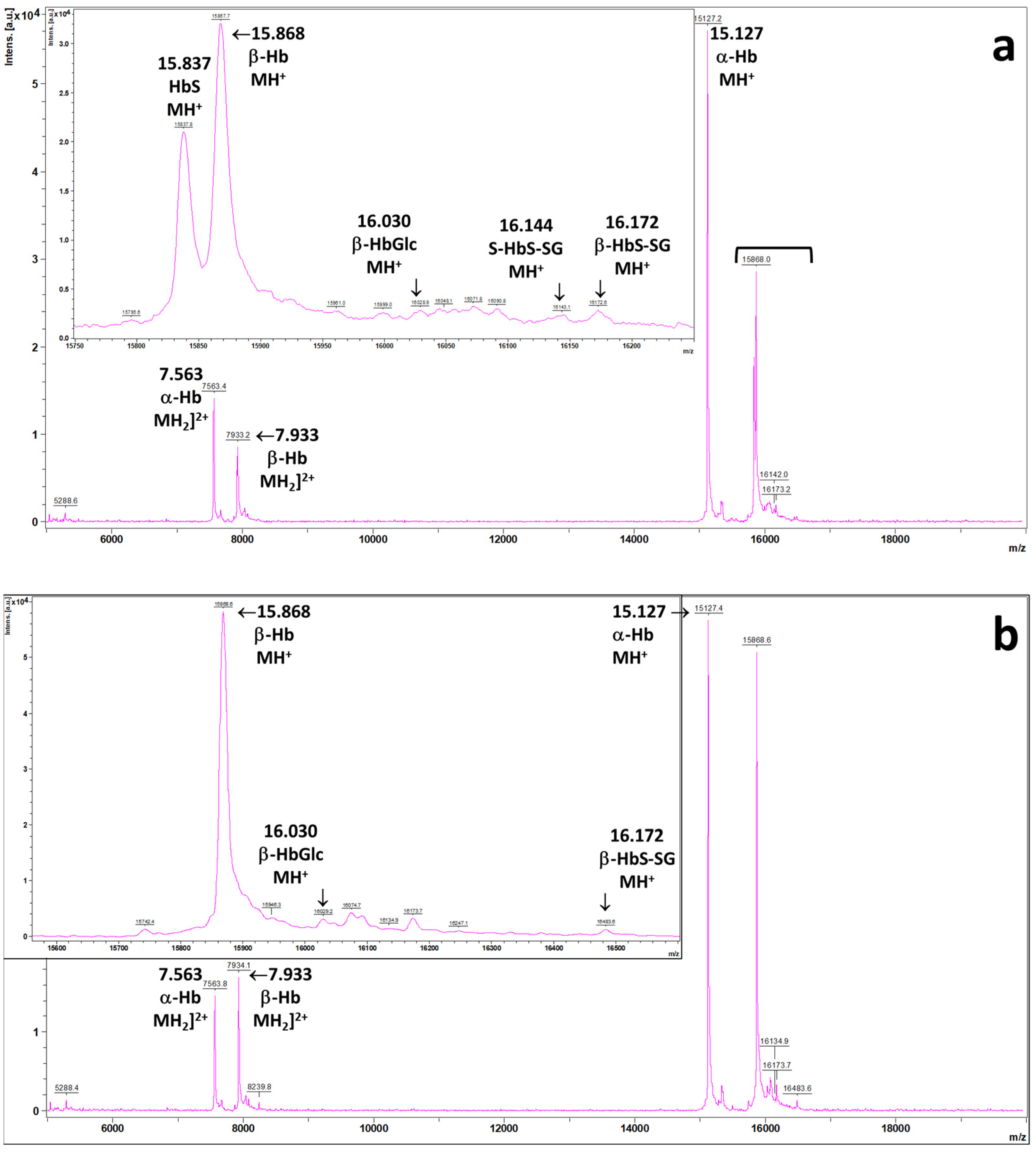
2.1. MALDI Mass Spectra of the Patient’s Hemolysates
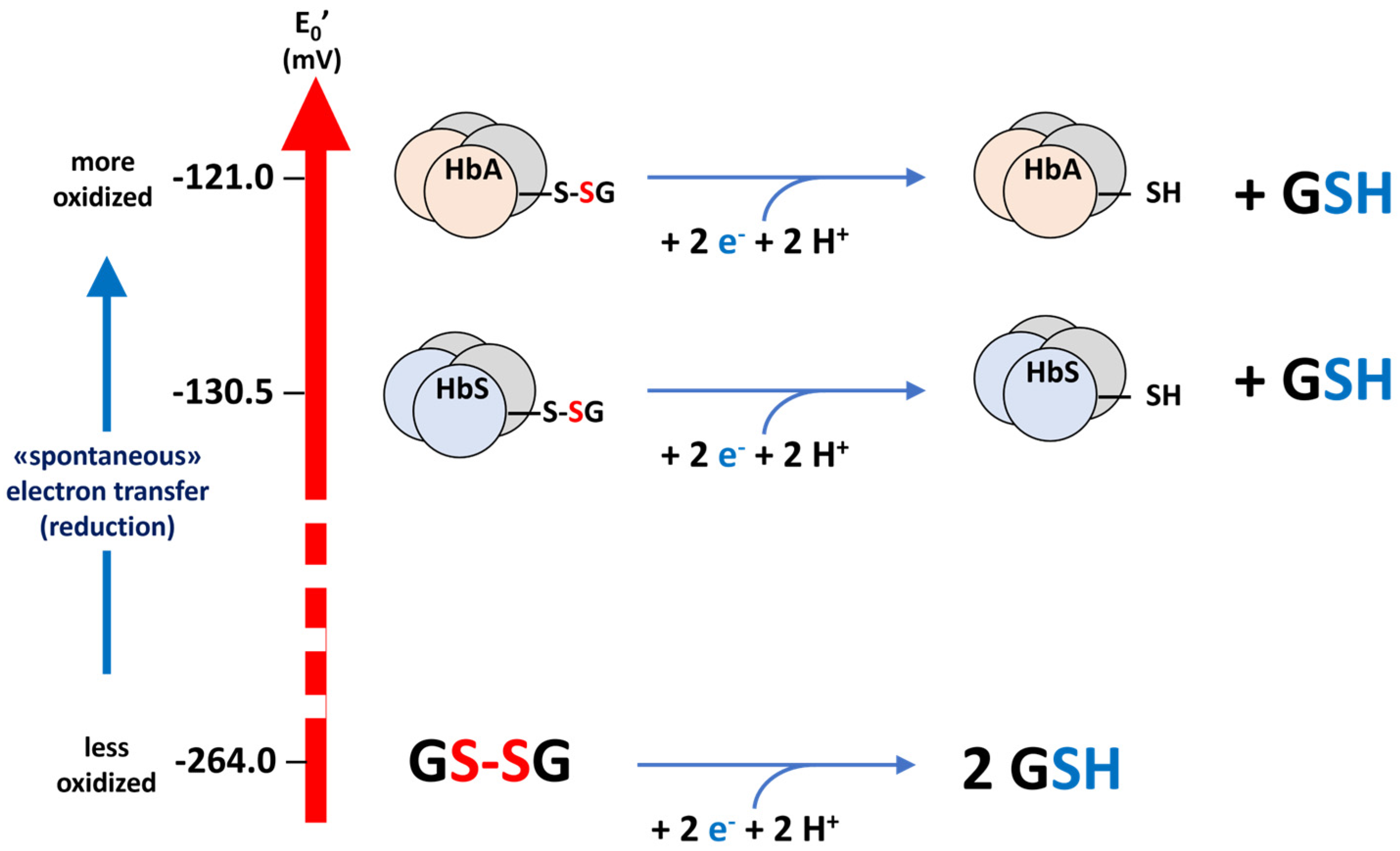
2.2. Calculation of the Value of E0 for the 93Cys of HbS
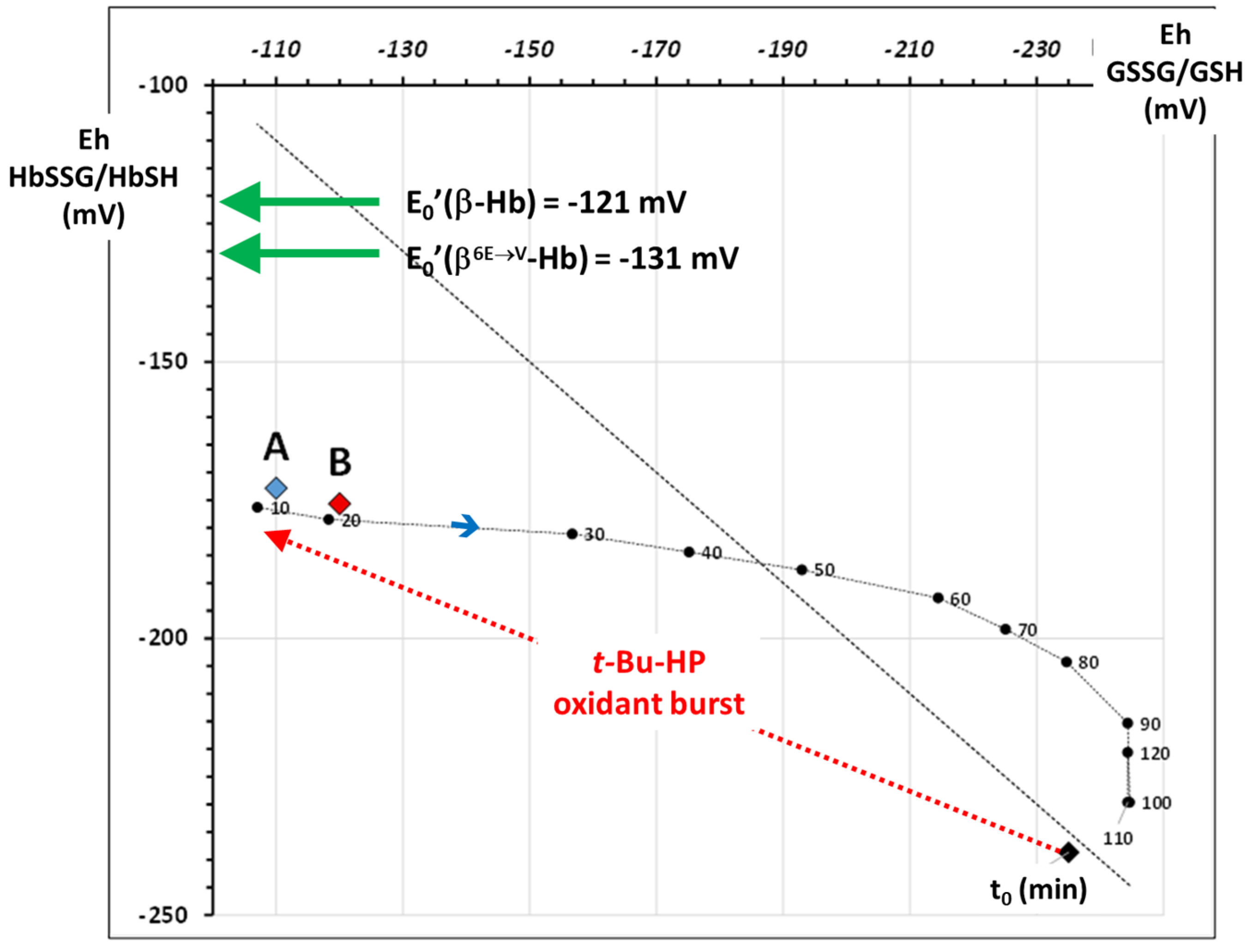
2.3. Application: The Oxidative Stress Level of the Patient Compared to That of In Vitro Oxidatively Challenged Wild-Type Healthy RBCs
3. Discussion
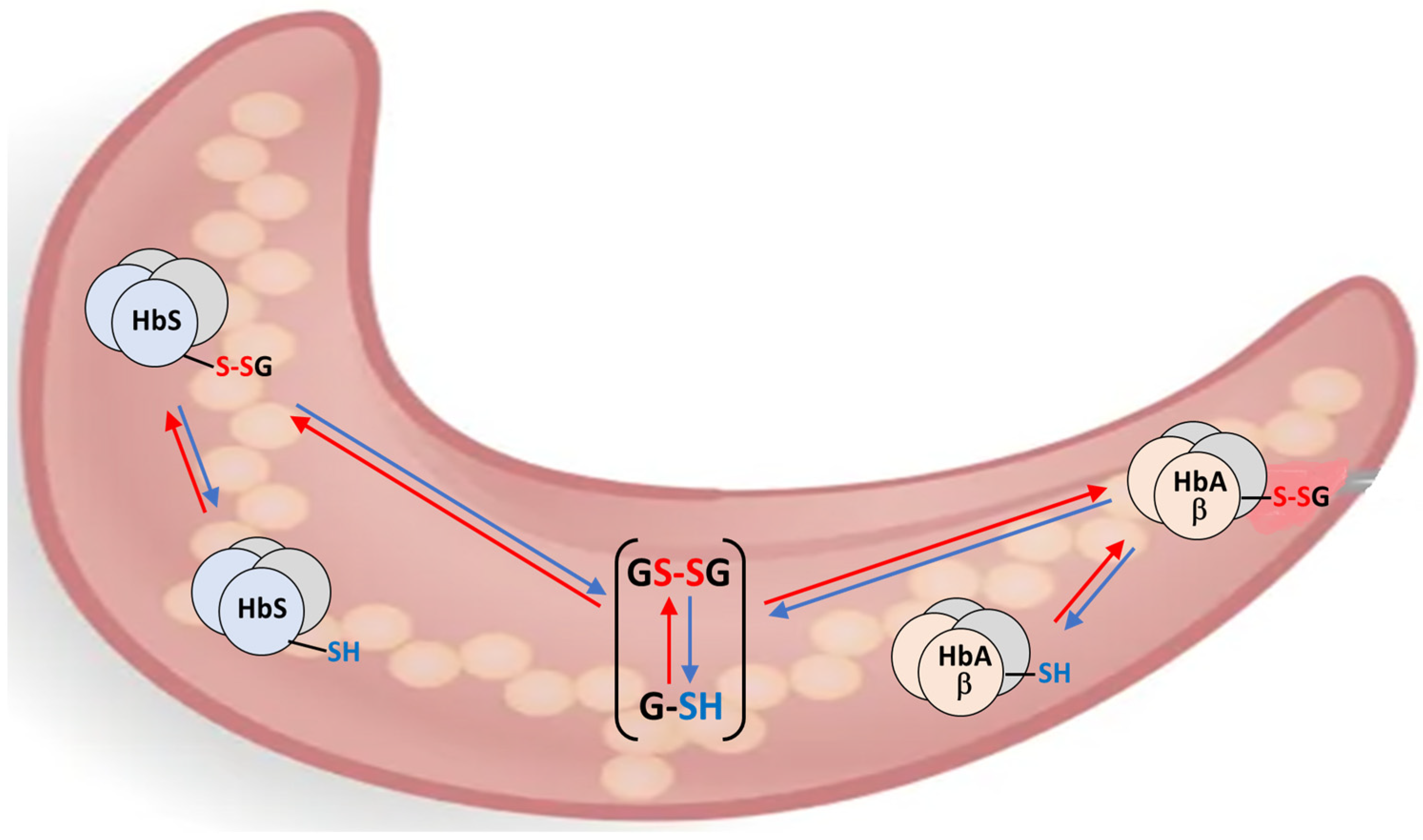
3.1. The “Thermodynamic”—“Kinetic” Electrochemical Model of Thiol Oxidative Stress in RBCs
3.2. The S-Variant of Hemoglobin and Oxidative Stress in RBCs in Malaria Infection
4. Materials and Methods
4.1. Patient History
4.2. Blood Samples
4.3. Measurement of Glutathionyl–Hemoglobin
4.4. Calculation of the Molecular Masses of Hemoglobin Proteins and Variants
4.5. Data Analysis
4.6. Calculation of the E0 Electrochemical Potential of the HbS Glutathionyl–Hemoglobin
=E0β6Glu→Val Hb) + RT/nF ln [β6Glu→Val Hb-SSG]/[β6Glu→Val Hb-SH]
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| βHb | wild-type (6Glu) β-chain of human hemoglobin |
| β6Glu→Val Hb | 6Glu > Val point-mutated β-chain of human hemoglobin |
| E0′ | Standard electrochemical reduction potential (at “near-physiological” pH 7) |
| Eh | Concentration-dependent electrochemical reduction potential |
| GSH | Glutathione, “reduced” thiol form |
| GSSG | Glutathione, “oxidized” disulfide form |
| Prot-SSG | Glutathionylated form of generic protein |
| HbA | Main wild-type form of adult human hemoglobin α2β2 |
| HbS | Main mutated form of sickle cell hemoglobin α2(β6E→V)2 |
| HbA-SH | Main wild-type form of adult human hemoglobin α2β2 thiol form |
| HbS-SH | Main mutated form of sickle cell hemoglobin α2(β6E→V)2 thiol form |
| HbA-SSG | Glutathionyl–hemoglobin A, glutathionylated at β-93Cys |
| HbS-SSG | Glutathionyl–hemoglobin S, glutathionylated at β-93Cys |
| HbA1c | Glycated hemoglobin A |
| β-HbGlc | Glycated hemoglobin A (β-chain) |
| β6Glu→Val HbGlc | Glycated hemoglobin S |
| RBC | Red blood cell(s) |
| SCD | Sickle Cell Disease |
Appendix A. Characterization of the Main Proteins in the RBC Samples by MALDI-ToF Mass Spectrometry
| MH+ | Samp_1 | Samp_2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Type | Protein | PTM 1 | Molecular Formula | m/z calc | m/z | Δ_m/z 2 | m/z | Δ_m/z 2 |
| wt | α-Hb | - | C685 H1071 N187 O194 S3 | 15,127.5 | 15,127.4 | 0.4 | 15,127.0 | 0.9 |
| wt | α-Hb | Glc | C691 H1081 N187 O199 S3 | 15,289.7 | 15,289.1 | 0.5 | 15,288.5 | 1.6 |
| wt | β-Hb | - | C724 H1119 N195 O201 S3 | 15,868.4 | 15,868.2 | 0.5 | 15,867.9 | 0.7 |
| wt | β-Hb | Glc | C730 H1129 N195 O206 S3 | 16,030.5 | 16,044.1 | 3.8 | 16,043.8 | 1.8 |
| wt | β-Hb | SSG | C734 H1134 N198 O207 S4 | 16,173.6 | 16,172.9 | 0.6 | 16,173.0 | 6.0 |
| β6Glu→Val | β-Hb | - | C724 H1121 N195 O199 S3 | 15,838.4 | 15,838.4 | 0.5 | 15,838.2 | 0.8 |
| β6Glu→Val | β-Hb | Glc | C730 H1131 N195 O204 S3 | 16,000.5 | 15,999.5 | 1.4 | 15,999.1 | 6.4 |
| β6Glu→Val | β-Hb | SSG | C734 H1136 N198 O205 S4 | 16,143.7 | 16,143.4 | 2.6 | 16,141.0 | 3.0 |
| Repl | βHb 1 | β6Glu→Val βHb 1 | βHb 1 | β6Glu→Val βHb 1 |
|---|---|---|---|---|
| Samp1 | Samp2 | |||
| Repl_1 | 61.1 | 38.9 | 61.2 | 38.8 |
| Repl_2 | 60.3 | 39.7 | 60.6 | 39.4 |
| Repl_3 | 60.2 | 39.8 | 61.6 | 38.4 |
| Repl_4 | 60.4 | 39.6 | 61.1 | 38.9 |
| M | 60.5 | 39.5 | 61.2 | 38.8 |
| DS | 0.3 | 0.3 | 0.3 | 0.3 |
| CV% | 0.7 | 1.1 | 0.6 | 1.0 |
| Samp | A | Samp | B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicates | Replicates | |||||||||||
| Species | 1 | 2 | 3 | 4 | M | SD | 1 | 2 | 3 | 4 | M | SD |
| β-HbSH | 55.8 | 55.6 | 54.9 | 55.5 | 55.5 | 0.4 | 56.6 | 56.1 | 56.4 | 56.4 | 56.4 | 0.2 |
| β-HbGlc | 3.7 | 3.2 | 3.6 | 3.3 | 3.4 | 0.2 | 3.3 | 3.3 | 4.2 | 3.6 | 3.6 | 0.4 |
| β-HbSSG | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 | 0.0 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 0.0 |
| β6Glu→Val-HbSH | 35.5 | 36.6 | 36.3 | 36.4 | 36.2 | 0.5 | 35.8 | 36.4 | 35.2 | 35.9 | 35.8 | 0.5 |
| β6Glu→Val-HbGlc | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | ||||
| β6Glu→Val-HbSSG | 2.3 | 1.9 | 2.5 | 2.1 | 2.2 | 0.3 | 2.1 | 2.1 | 2.0 | 2.0 | 2.0 | 0.0 |
| ∑ all HbSSG | 4.9 | 0.2 | 4.2 | 0.0 | ||||||||
Appendix B. Calculation of the E0 of S-Hb with the Equilibrium Method
| Samp | A | B | |||||||||||
| Hb (g/dL)a | 11.9 | 13.9 | |||||||||||
| Ht %a | 35.6% | 42.6% | |||||||||||
| [Hb] mM | 5.02 | 4.90 | |||||||||||
| Species | 1 | 2 | 3 | 4 | M | SD | 1 | 2 | 3 | 4 | M | SD | |
| β-HbA-SH | 55.8% | 55.6% | 54.9% | 55.5% | 55.5% | 0.4% | 56.6% | 56.1% | 56.4% | 56.4% | 56.4% | 0.2% | |
| β-HbGlc | 3.7% | 3.2% | 3.6% | 3.3% | 3.4% | 0.2% | 3.3% | 3.3% | 4.2% | 3.6% | 3.6% | 0.4% | |
| β-HbSSG | 2.7% | 2.7% | 2.7% | 2.7% | 2.7% | 0.0% | 2.2% | 2.2% | 2.2% | 2.2% | 2.2% | 0.0% | |
| β6Glu→Val HbA-SH | 35.5% | 36.6% | 36.3% | 36.4% | 36.2% | 0.5% | 35.8% | 36.4% | 35.2% | 35.9% | 35.8% | 0.5% | |
| β6Glu→Val HbGlc | <0.5% | <0.5% | <0.5% | <0.5% | <0.5% | <0.5% | <0.5% | <0.5% | |||||
| β6Glu→Val HbS-SSG | 2.3% | 1.9% | 2.5% | 2.1% | 2.2% | 0.3% | 2.1% | 2.1% | 2.0% | 2.0% | 2.0% | 0.05% | |
| β-HbSH | mM | 2.80 | 2.79 | 2.76 | 2.78 | 2.77 | 2.75 | 2.76 | 2.76 | ||||
| β-HbGlc | mM | 0.18 | 0.16 | 0.18 | 0.17 | 0.16 | 0.16 | 0.21 | 0.17 | ||||
| β-HbSSG | mM | 0.14 | 0.14 | 0.13 | 0.14 | 0.11 | 0.11 | 0.11 | 0.11 | ||||
| β6Glu→Val-HbSH | mM | 1.78 | 1.84 | 1.82 | 1.83 | 1.75 | 1.78 | 1.72 | 1.76 | ||||
| β6Glu→Val-HbGlc | mM | ||||||||||||
| β6Glu→Val HbSSG | mM | 0.12 | 0.10 | 0.13 | 0.11 | 0.10 | 0.10 | 0.10 | 0.10 | ||||
| Nernst calculation | |||||||||||||
| RT/nF (mV) | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | |||||
| E0_β-Hb (mV) | mV | −121 | −121 | −121 | −121 | −121 | −121 | −121 | −121 | ||||
| β _[OX]/[RED]^2 | 0.0174 | 0.0174 | 0.0177 | 0.0175 | 0.0139 | 0.0143 | 0.0142 | 0.0138 | |||||
| β6Glu→Val [OX]/[RED]^2 | 0.0372 | 0.0284 | 0.0378 | 0.0316 | 0.0333 | 0.0319 | 0.0331 | 0.0316 | |||||
| β_logRat | −1.758 | −1.760 | −1.752 | −1.758 | −1.857 | −1.846 | −1.848 | −1.860 | |||||
| β6Glu→Val _logRat | −1.430 | −1.547 | −1.422 | −1.501 | −1.477 | −1.496 | −1.480 | −1.501 | |||||
| A_Eh (mV) | −173 | −173 | −173 | −173 | −172.8 | ±0.1 | −176 | −175 | −176 | −176 | −175.7 | ±0.2 | |
| β6Glu→Val _Eh-E0 (mV) | −42 | −46 | −42 | −44 | −43.5 | ±1.8 | −44 | −44 | −44 | −44 | −43.9 | ±0.3 | |
| E0′β6Glu→Val-Hb (mV) | mV | −130.7 | −127.3 | −130.7 | −128.6 | −129.3 | ±1.7 | −132.2 | −131.3 | −131.9 | −131.6 | −131.7 | ±0.4 |
References
- Pauling, L.; Itano, H.A.; Singer, S.J.; Wells, I.C. Sickle Cell Anemia, a Molecular Disease. Science 1949, 110, 543–548. [Google Scholar] [CrossRef]
- Ingram, V.M. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature 1956, 178, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Ingram, V.M. Gene mutations in human haemoglobin: The chemical difference between normal and sickle cell haemoglobin. Nature 1957, 180, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Garel, M.C.; Domenget, C.; Caburi-Martin, J.; Prehu, C.; Galacteros, F.; Beuzard, Y. Covalent binding of glutathione to hemoglobin. I. Inhibition of hemoglobin S polymerization. J. Biol. Chem. 1986, 261, 14704–14709. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Mitra, A.; Mitra, G.; Maity, D.; Bhat, V.; Pal, D.; Ross, C.; Kurpad, A.V.; Mandal, A.K. Molecular insights of inhibition in sickle hemoglobin polymerization upon glutathionylation: Hydrogen/deuterium exchange mass spectrometry and molecular dynamics simulation-based approach. Biochem. J. 2018, 475, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Mitra, A.; Das, R. Sickle Cell Hemoglobin. Subcell. Biochem. 2020, 94, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.M.; Rets, A.V. Advances in Hemoglobinopathies and Thalassemia Evaluation. Clin. Lab. Med. 2024, 44, 441–453. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Haematology. Sickle cell disease: A neglected health priority. Lancet Haematol. 2025, 12, e769. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Orrico, F.; Villar, S.F.; López, A.C.; Silva, N.; Donzé, M.; Thomson, L.; Denicola, A. Oxidants and Antioxidants in the Redox Biochemistry of Human Red Blood Cells. ACS Omega 2022, 8, 147–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daraghmeh, D.N.; Karaman, R. The Redox Process in Red Blood Cells: Balancing Oxidants and Antioxidants. Antioxidants 2024, 14, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spinelli, S.; Marino, A.; Remigante, A.; Morabito, R. Redox Homeostasis in Red Blood Cells: From Molecular Mechanisms to Antioxidant Strategies. Curr. Issues Mol. Biol. 2025, 47, 655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fenk, S.; Melnikova, E.V.; Anashkina, A.A.; Poluektov, Y.M.; Zaripov, P.I.; Mitkevich, V.A.; Tkachev, Y.V.; Kaestner, L.; Minetti, G.; Mairbäurl, H.; et al. Hemoglobin is an oxygen-dependent glutathione buffer adapting the intracellular reduced glutathione levels to oxygen availability. Redox Biol. 2022, 58, 102535. [Google Scholar] [CrossRef] [PubMed]
- Kuleshova, I.D.; Zaripov, P.I.; Poluektov, Y.M.; Anashkina, A.A.; Kaluzhny, D.N.; Parshina, E.Y.; Maksimov, G.V.; Mitkevich, V.A.; Makarov, A.A.; Petrushanko, I.Y. Changes in Hemoglobin Properties in Complex with Glutathione and after Glutathionylation. Int. J. Mol. Sci. 2023, 24, 13557. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.; Panusa, A.; Yeum, K.-J.; Carini, M.; Aldini, G. Hemoglobin Glutathionylation Can Occur Through Cysteine Sulfenic Acid Intermediate: Electrospray Ionization LTQ-Orbitrap Hybrid Mass Spectrometry Studies. J. Chromatogr. B 2009, 877, 3456–3461. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Dalle-Donne, I.; Giustarini, D.; Gagliano, N.; Portinaro, N.; Colombo, R.; Rossi, R.; Milzani, A.D.G. Cellular Redox Potential and Hemoglobin S-Glutathionylation in Human and Rat Erythrocytes: A Comparative Study. Blood Cells Mol. Dis. 2010, 44, 133–139. [Google Scholar] [CrossRef]
- Minetti, M.; Pietraforte, D.; Carbone, V.; Salzano, A.M.; Scorza, G.; Marino, G. Scavenging of Peroxynitrite by Oxyhemoglobin and Identification of Modified Globin Residues. Biochemistry 2000, 39, 6689–6697. [Google Scholar] [CrossRef]
- Böhmer, A.; Pich, A.; Schmidt, M.; Haghiki, A.; Tsikas, D. Evidence by chromatography and mass spectrometry that inorganic nitrite induces S-glutathionylation of hemoglobin in human red blood cells. J. Chromatogr. B 2016, 1019, 72–82. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Pokidova, O.V.; Sanina, N.A.; Topunov, A.F. Effects of Nitrosyl Iron Complexes with Thiol, Phosphate, and Thiosulfate Ligands on Hemoglobin. Int. J. Mol. Sci. 2024, 25, 7194. [Google Scholar] [CrossRef] [PubMed]
- Van ‘t Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic. Biol. Med. 2013, 65, 742–749. [Google Scholar]
- Mieyal, J.J.; Starke, D.W.; Gravina, S.A.; Dothey, C.; Chung, J.S. Thioltransferase in Human Red Blood Cells: Purification and Properties. Biochemistry 1991, 30, 6088–6097. [Google Scholar] [CrossRef]
- Scirè, A.; Casari, G.; Romaldi, B.; de Bari, L.; Antognelli, C.; Armeni, T. Glutathionyl Hemoglobin and Its Emerging Role as a Clinical Biomarker of Chronic Oxidative Stress. Antioxidants 2023, 12, 1976. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Key, A.; Haiman, Z.; Palsson, B.O.; D’Alessandro, A. Modeling Red Blood Cell Metabolism in the Omics Era. Metabolites 2023, 13, 1145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spinelli, S.; Marino, A.; Morabito, R.; Remigante, A. Interplay Between Metabolic Pathways and Increased Oxidative Stress in Human Red Blood Cells. Cells 2024, 13, 2026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Alessandro, A. Red blood cell metabolism: A window on systems health towards clinical metabolomics. Curr. Opin. Hematol. 2025, 32, 111–119. [Google Scholar] [CrossRef]
- Haiman, Z.B.; Key, A.; D’Alessandro, A.; Palsson, B.O. RBC-GEM: A genome-scale metabolic model for systems biology of the human red blood cell. PLoS Comput. Biol. 2025, 21, e1012109. [Google Scholar] [CrossRef] [PubMed]
- Thom, G.G.; Kallanagowdar, C.; Somjee, S.S.; Velez, M.C.; Yu, L.C.; Hempe, J.M. Characterization of S-glutathionyl hemoglobin in homozygous sickle cell disease. J. Pediatr. Hematol. Oncol. 2009, 31, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, M.; Mitra, A.; Maity, D.; Pal, D.; Mandal, A.K. Structural analysis of glutathionyl hemoglobin using native mass spectrometry. J. Struct. Biol. 2019, 208, 107386. [Google Scholar] [CrossRef] [PubMed]
- Rabenstein, D.L.; Millis, K.K. Nuclear magnetic resonance study of the thioltransferase-catalyzed glutathione/glutathione disulfide interchange reaction. Biochim. Biophys. Acta 1995, 1249, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.M.; Pitton, M.; Caneva, E.; Pappini, M.; Colombi, A. Thiol-disulfide redox equilibria of glutathione metaboloma compounds investigated by tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2008, 22, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.M. The Redox Potential of the β-93-Cysteine Thiol Group in Human Hemoglobin Estimated from In Vitro Oxidant Challenge Experiments. Molecules 2021, 26, 2528. [Google Scholar] [CrossRef] [PubMed]
- Duca, L.; Ottolenghi, S.; Coppola, S.; Rinaldo, R.; Dei Cas, M.; Rubino, F.M.; Paroni, R.; Samaja, M.; Chiumello, D.A.; Motta, I. Differential Redox State and Iron Regulation in Chronic Obstructive Pulmonary Disease, Acute Respiratory Distress Syndrome and Coronavirus Disease 2019. Antioxidants 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Houston, C.T.; Reilly, J.P. Rapid Analysis of Hemoglobin from Whole Human Blood by Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 1435–1439. [Google Scholar] [CrossRef]
- Ehrmann, D.C.; Rose, K.; Calcutt, M.W.; Beller, A.B.; Hill, S.; Rogers, T.J.; Steele, S.D.; Hachey, D.L.; Aschner, J.L. Glutathionylated γG and γA subunits of hemoglobin F: A novel post-translational modification found in extremely premature infants by LC-MS and nanoLC-MS/MS. J. Mass Spectrom. 2014, 49, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, G.; Sun, D.; Lin, W.; Yan, T.; Wu, Y.; Wu, M.; Chen, J.; Zou, S.; Xie, W.; et al. MALDI-TOF-MS for Rapid Screening and Typing of β-Globin Variant and β-Thalassemia through Direct Measurements of Intact Globin Chains. Clin. Chem. 2022, 68, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Lanzkron, S.; Strouse, J.J.; Wilson, R.; Beach, M.C.; Haywood, C.; Park, H.; Witkop, C.; Bass, E.B.; Segal, J.B. Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Ann. Intern. Med. 2008, 148, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Muralidharan, M.; Srivastava, D.; Das, R.; Bhat, V.; Mandal, A.K. Assessment of Cysteine Reactivity of Human Hemoglobin at Its Residue Level: A Mass Spectrometry-Based Approach. Hemoglobin 2017, 41, 300–305. [Google Scholar] [CrossRef]
- Bulaj, G.; Kortemme, T.; Goldenberg, D.P. Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 1998, 37, 8965–8972. [Google Scholar] [CrossRef] [PubMed]
- Martinovich, G.G.; Cherenkevich, S.N.; Sauer, H. Intracellular redox state: Towards quantitative description. Eur. Biophys. J. 2005, 34, 937–942. [Google Scholar] [CrossRef]
- Kemp, M.; Go, Y.-M.; Jones, D.P. Nonequilibrium Thermodynamics of Thiol/Disulfide Redox Systems: A Perspective on Redox Systems Biology. Free Radic. Biol. Med. 2008, 44, 921–937. [Google Scholar] [CrossRef]
- Khazim, K.; Giustarini, D.; Rossi, R.; Verkaik, D.; Cornell, J.E.; Cunningham, S.E.; Mohammad, M.; Trochta, K.; Lorenzo, C.; Folli, F.; et al. Glutathione redox potential is low and glutathionylated and cysteinylated hemoglobin levels are elevated in maintenance hemodialysis patients. Transl. Res. 2013, 162, 16–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alayash, A.I. βCysteine 93 in human hemoglobin: A gateway to oxidative stability in health and disease. Lab. Investig. 2021, 101, 4–11. [Google Scholar] [CrossRef]
- Anashkina, A.A.; Simonenko, S.Y.; Orlov, Y.L.; Petrushanko, I.Y. Glutathione Non-Covalent Binding Sites on Hemoglobin and Major Glutathionylation Target betaCys93 Are Conservative among Both Hypoxia-Sensitive and Hypoxia-Tolerant Mammal Species. Int. J. Mol. Sci. 2024, 25, 53. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Kassa, T.; Strader, M.B.; Soman, J.; Olson, J.S.; Alayash, A.I. Substitutions in the β subunits of sickle-cell hemoglobin improve oxidative stability and increase the delay time of sickle-cell fiber formation. J. Biol. Chem. 2019, 294, 4145–4159. [Google Scholar] [CrossRef]
- Sae-Lee, W.; McCafferty, C.L.; Verbeke, E.J.; Havugimana, P.C.; Papoulas, O.; McWhite, C.D.; Houser, J.R.; Vanuytsel, K.; Murphy, G.J.; Drew, K.; et al. The protein organization of a red blood cell. Cell Rep. 2022, 40, 111103. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox Environment of the Cell as Viewed Through the Redox State of the Glutathione Disulfide/Glutathione Couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar]
- Jones, D.P. Redox Potential of [GSH]/[GSSG] Couple: Assay and Biological Significance. Methods Enzym. 2002, 348, 93–112. [Google Scholar]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Blanco, R.A.; Ziegler, T.R.; Carlson, B.A.; Cheng, P.Y.; Park, Y.; Cotsonis, G.A.; Accardi, C.J.; Jones, D.P. Diurnal variation in glutathione and cysteine redox states in human plasma. Am. J. Clin. Nutr. 2007, 86, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Berndt, C.; Lillig, C.H.; Flohé, L. Redox regulation by glutathione needs enzymes. Front. Pharmacol. 2014, 5, 168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flohé, L. The Fairytale of the GSSG/GSH Redox Potential. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3139–3142. [Google Scholar] [CrossRef]
- Niwa, T. Protein glutathionylation and oxidative stress. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 855, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tamma, G.; Valenti, G. Evaluating the Oxidative Stress in Renal Diseases: What Is the Role for S-Glutathionylation? Antioxid. Redox Signal. 2016, 25, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.M.; Della Noce, C.; Vigna, L.; De Giuseppe, R.; Novembrino, C.; De Liso, F.; Maiavacca, R.; Patrini, L.; Riboldi, L.; Bamonti, F. Measurement of Glutathionylated Haemoglobin by MAL-DI-ToF Mass Spectrometry as a Biomarker of Oxidative Stress in Heavy Smokers and in Occupational Obese Subjects. Int. J. Anal. Mass Spectrom. Chromatogr. 2013, 1, 22–30. [Google Scholar] [CrossRef][Green Version]
- Primavera, A.; Fustinoni, S.; Biroccio, A.; Ballerini, S.; Urbani, A.; Bernardini, S.; Federici, G.; Capucci, E.; Manno, M.; Bello, M.L. Glutathione Transferases and Glutathionylated Hemoglobin in Workers Exposed to Low Doses of 1,3-Butadiene. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3004–3012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laval, G.; Peyrégne, S.; Zidane, N.; Harmant, C.; Renaud, F.; Patin, E.; Prugnolle, F.; Quintana-Murci, L. Recent Adaptive Acquisition by African Rainforest Hunter-Gatherers of the Late Pleistocene Sickle-Cell Mutation Suggests Past Differences in Malaria Exposure. Am. J. Hum. Genet. 2019, 104, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.; Mendis, K.N. Evolutionary and historical aspects of the burden of malaria. Clin. Microbiol. Rev. 2002, 15, 564–594, Erratum in Clin. Microbiol. Rev. 2003, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Esoh, K.; Wonkam, A. Evolutionary history of sickle-cell mutation: Implications for global genetic medicine. Hum. Mol. Genet. 2021, 30, R119–R128. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Castillo, A.; Contreras-Puentes, N.; Alvear-Sedán, C.; Moneriz-Pretell, C.; Rodríguez-Cavallo, E.; Mendez-Cuadro, D. Sickle Cell Trait Induces Oxidative Damage on Plasmodium falciparum Proteome at Erythrocyte Stages. Int. J. Mol. Sci. 2019, 20, 5769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flohé, L.; Hecht, H.J.; Steinert, P. Glutathione and trypanothione in parasitic hydroperoxide metabolism. Free Radic. Biol. Med. 1999, 27, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Gizi, A.; Papassotiriou, I.; Apostolakou, F.; Lazaropoulou, C.; Papastamataki, M.; Kanavaki, I.; Kalotychou, V.; Goussetis, E.; Kattamis, A.; Rombos, I.; et al. Assessment of oxidative stress in patients with sickle cell disease: The glutathione system and the oxidant-antioxidant status. Blood Cells Mol. Dis. 2011, 46, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Mitra, G.; Muralidharan, M.; Narayanan, S.; Pinto, J.; Srinivasan, K.; Mandal, A.K. Glutathionylation induced structural changes in oxy human hemoglobin analyzed by backbone amide hydrogen/deuterium exchange and MALDI-mass spectrometry. Bioconjug. Chem. 2012, 23, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Renó, C.O.; Barbosa, A.R.; de Carvalho, S.S.; Pinheiro, M.B.; Rios, D.R.; Cortes, V.F.; Barbosa, L.A.; Santos, H.L. Oxidative stress assessment in sickle cell anemia patients treated with hydroxyurea. Ann. Hematol. 2020, 99, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Renó, C.O.; Maia, G.A.S.; Nogueira, L.S.; de Barros Pinheiro, M.; Rios, D.R.A.; Cortes, V.F.; de Oliveira Barbosa, L.A.; de Lima Santos, H. Biochemical Evaluation of the Effects of Hydroxyurea in Vitro on Red Blood Cells. Antioxidants 2021, 10, 1599. [Google Scholar] [CrossRef]
- Bolarinwa, A.B.; Oduwole, O.; Okebe, J.; Ogbenna, A.A.; Otokiti, O.E.; Olatinwo, A.T. Antioxidant supplementation for sickle cell disease. Cochrane Database Syst. Rev. 2024, 2024, CD013590. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.G.; Ricci, O., Jr.; de Almeida, E.A.; Bonini-Domingos, C.R. Potential utility of melatonin as an antioxidant therapy in the management of sickle cell anemia. J. Pineal Res. 2015, 58, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Rubino, F.M.; Caroli, D.; Bondesan, A.; Mai, S.; Cella, S.G.; Centofanti, L.; Paroni, R.; Sartorio, A. Effects of Melatonin on Exercise-Induced Oxidative Stress in Adults with Obesity Undergoing a Multidisciplinary Body Weight Reduction Program. J. Clin. Med. 2024, 13, 5216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohemeng, A.; Boadu, I. The role of nutrition in the pathophysiology and management of sickle cell disease among children: A review of literature. Crit. Rev. Food Sci. Nutr. 2018, 58, 2299–2305. [Google Scholar] [CrossRef]
- Kalpatthi, R.; Novelli, E.M. Measuring success: Utility of biomarkers in sickle cell disease clinical trials and care. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 482–492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orsi, B.C.; Gorski, D.; Krul, N.E.; Wiens, A.; Brito, M.; Tonin, F.S.; Pontarolo, R. The effects of nutritional supplementation for children and adolescents with sickle cell disease: A systematic review and meta-analyses. Clin. Nutr. 2025, 47, 157–168. [Google Scholar] [CrossRef]
- Rubino, F.M.; Ottolenghi, S.; Brizzolari, A.; Maioli, C.; Samaja, M.; Paroni, R. Enhanced-Precision Measurement of Glutathionyl Hemoglobin by MALDI-ToF MS. Molecules 2023, 28, 497. [Google Scholar] [CrossRef] [PubMed]
- Loos, M.; Gerber, C.; Corona, F.; Hollender, J.; Singer, H. Accelerated Isotope Fine Structure Calculation Using Pruned Transition Trees. Anal. Chem. 2015, 87, 5738–5744. [Google Scholar] [CrossRef] [PubMed]
- Gravina, S.A.; Mieyal, J.J. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry 1993, 32, 3368–3376. [Google Scholar] [CrossRef]
- Mieyal, J.J.; Gallogly, M.M.; Qanungo, S.; Sabens, E.A.; Shelton, M.D. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid. Redox Signal. 2008, 10, 1941–1988. [Google Scholar] [CrossRef]
- Lang, L.; Reinert, P.; Diaz, C.; Deponte, M. The dithiol mechanism of class I glutaredoxins promotes specificity for glutathione as a reducing agent. Redox Biol. 2024, 78, 103410. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubino, F.M.; Sadikovic, A.; Morano, C.; Dei Cas, M.; Bignotto, M.; Ottolenghi, S.; Mondoni, M.; Chiumello, D.; Samaja, M.; Paroni, R. Redox Potential (E0′) of the β-Chain 93Cys of HbS Measured with the Equilibrium Technique in a Heterozygous Sickle Cell Carrier Subject. Molecules 2025, 30, 4342. https://doi.org/10.3390/molecules30224342
Rubino FM, Sadikovic A, Morano C, Dei Cas M, Bignotto M, Ottolenghi S, Mondoni M, Chiumello D, Samaja M, Paroni R. Redox Potential (E0′) of the β-Chain 93Cys of HbS Measured with the Equilibrium Technique in a Heterozygous Sickle Cell Carrier Subject. Molecules. 2025; 30(22):4342. https://doi.org/10.3390/molecules30224342
Chicago/Turabian StyleRubino, Federico Maria, Aldijana Sadikovic, Camillo Morano, Michele Dei Cas, Monica Bignotto, Sara Ottolenghi, Michele Mondoni, Davide Chiumello, Michele Samaja, and Rita Paroni. 2025. "Redox Potential (E0′) of the β-Chain 93Cys of HbS Measured with the Equilibrium Technique in a Heterozygous Sickle Cell Carrier Subject" Molecules 30, no. 22: 4342. https://doi.org/10.3390/molecules30224342
APA StyleRubino, F. M., Sadikovic, A., Morano, C., Dei Cas, M., Bignotto, M., Ottolenghi, S., Mondoni, M., Chiumello, D., Samaja, M., & Paroni, R. (2025). Redox Potential (E0′) of the β-Chain 93Cys of HbS Measured with the Equilibrium Technique in a Heterozygous Sickle Cell Carrier Subject. Molecules, 30(22), 4342. https://doi.org/10.3390/molecules30224342










