A Full-Spectrum Evaluation of Sigma-1 Receptor (S1R) Positron Emission Tomography (PET) Radioligands from Binding Affinity to Clinical Imaging
Abstract
1. Introduction
2. Development of High Affinity and Selective S1R Radioligands
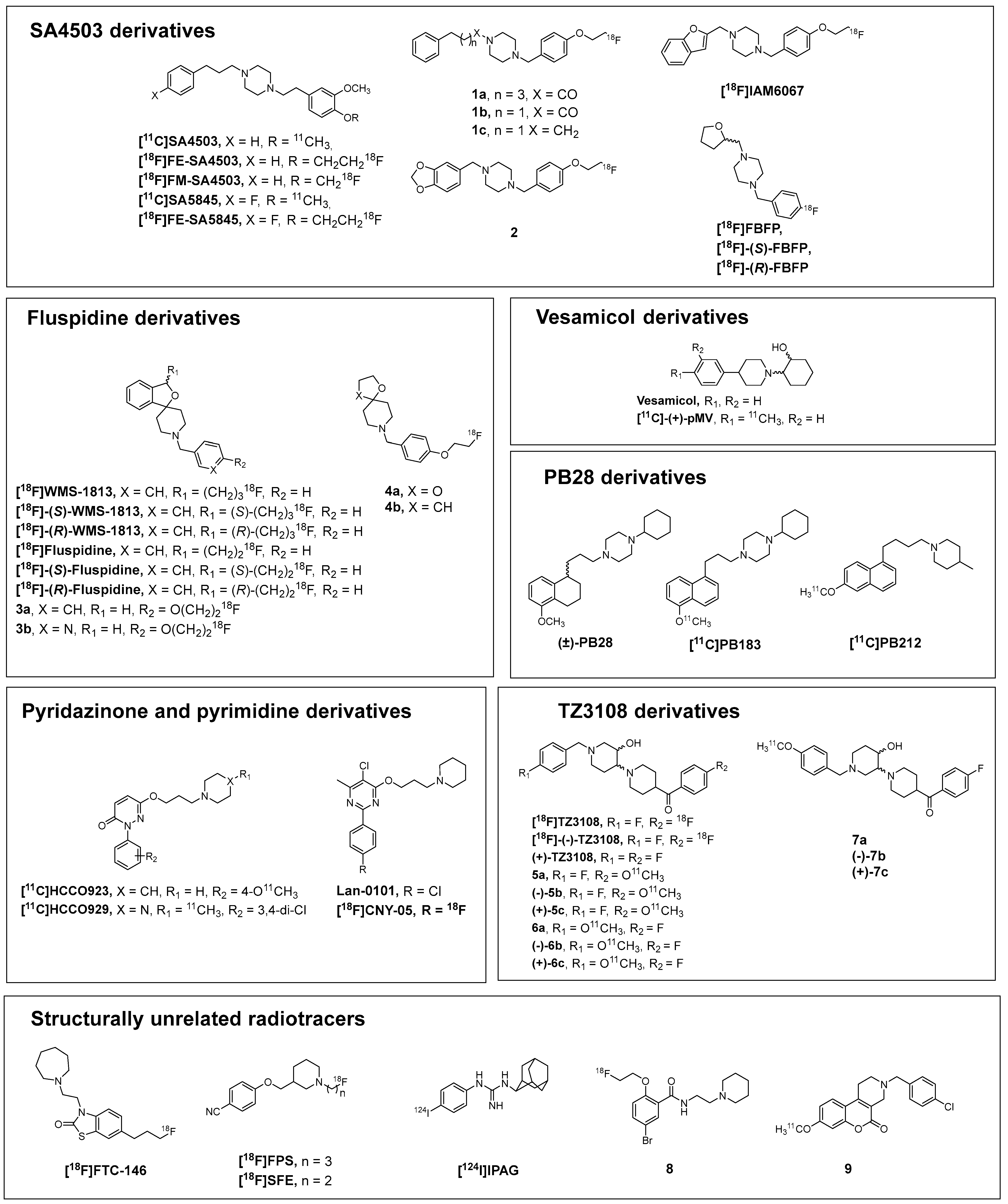
2.1. SA4503-Derivatives
2.2. Fluspidine Derivatives
2.3. Vesamicol Derivatives
2.4. PB28-Derivatives
2.5. Pyridazinone and Pyrimidine Derivatives
2.6. TZ3108-Derivatives
2.7. Structurally Unrelated Radiotracers
| Tracer | Ki S1R (nM) | Ki S2R (nM) | logD7.4 # | References |
|---|---|---|---|---|
| [11C]SA4503 | 4.6 | 63.1 | 2.52 # | [47] |
| [18F]FE-SA4503 | 8.0 | 113.2 | 2.70 # | [49] |
| [18F]FM-SA4503 | 6.4 | 250 | / | [51] |
| [11C]SA5845 | 33 | 9.5 | 2.60 # | [50] |
| [18F]FE-SA5845 | 3.10 | 6.8 | 2.70 # | [50] |
| 1a | 4.19 | 2000 | 3.56 | [52] |
| 1b | 1.19 | 2913 | 2.72 | [52] |
| 1c | 0.31 | 42.6 | 3.35 | [52] |
| 2 | 1.85 | 291 | 1.06 | [53] |
| [18F]IAM6067 | 2.6 | 486 | 3.35 | [54] |
| [18F]FBFP | 3.22 | 168 | 0.76 | [55] |
| [18F]-(S)-FBFP | 3.16 | 126 | 0.76 | [85] |
| [18F]-(R)-FBFP | 3.23 | 178 | 0.76 | [85] |
| [18F]WMS1813 | 1.4 | 837 | 3.56 | [57] |
| [18F]-(S)-WMS1813 | 0.59 | / | 3.56 | [60] |
| [18F]-(R)-WMS1813 | 1.8 | / | 3.56 | [60] |
| [18F]Fluspidine | 0.59 | 785 | 2.57 | [58] |
| [18F]-(S)-Fluspidine | 2.3 | 897 | 2.57 | [61] |
| [18F]-(R)-Fluspidine | 0.57 | 1650 | 2.57 | [61] |
| 3a | 0.79 | 277 | 2.41 | [62] |
| 3b | 2.30 | 327 | 2.58 | [63] |
| 4a | 5.4 | 164 | 0.8 | [64] |
| 4b | 8.62 | 378 | 1.6 | [65] |
| [18F]FTC-146 | 0.0025 | selectivity > 145,000 | 1.45 | [76] |
| [18F]FPS | 0.50 | 144 | 2.80 | [78] |
| [18F]SFE | 5.00 | 361 | 2.40 | [79] |
| [18F]TZ3108 | 0.48 | 1740 | 2.83 | [73] |
| [18F]-(-)-TZ3108 | 1.80 | 6960 | 2.83 | [74] |
| (+)-TZ3108 | 0.14 | 2390 | 2.83 | [74] |
| 5a | 2.5 | 2790 | / | [75] |
| (−)-5b | 30 | 3910 | / | [75] |
| (+)-5c | 0.82 | 6370 | / | [75] |
| 6a | 2.8 | 5520 | / | [75] |
| (−)-6b | 4.1 | >10,000 | / | [75] |
| (+)-6c | 0.73 | >10,000 | / | [75] |
| 7a | 23.7 | 502.6 | / | [75] |
| (−)-7b | 24.2 | 402.6 | / | [75] |
| (+)-7c | 14.3 | 408.8 | / | [75] |
| [11C]HCCO923 | 10.3 | 1146 | 0.89 | [70] |
| [11C]HCCO929 | 5.6 | 1528 | 1.73 | [70] |
| LAN-0101 | 1.06 | 1425 | / | [71] |
| [18F]CNY-05 | 0.04 | 4.72 | / | [72] |
| PB28 | 0.38 | 0.68 | / | [67] |
| [11C]PB183 | 0.50 | 6.50 | / | [68] |
| [11C]PB212 | 0.030 | 17.9 | 2.38 | [69] |
| (+)-Vesamicol | 31.5 | 330 | / | [66] |
| [11C]-(+)-pMV | 3.0 | 40.7 | / | [66] |
| [124I]IPAG | 9.0 | high selectivity * | / | [80] |
| 8 | 6.26 | 10.2 | 1.4 | [83] |
| 9 | / | / | 3.77 # | [84] |
3. Synthesis of S1R Radiotracers for PET
3.1. 11C Radiolabeling
3.2. 18F Radiolabeling
3.3. 124I Radiolabeling
4. Preclinical Biodistribution and Blocking Studies
5. In Vitro and Ex Vivo Autoradiographic Studies
6. In Vitro and In Vivo Metabolism
7. Kinetic Characterization of PET Radiotracers
8. Dosimetric Evaluation and Clinical Translation
9. Role of PET Imaging in the Assessment of Pathophysiological Processes
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal Structure of the Human Σ1 Receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.R.; Kruse, A.C. The Molecular Function of σ Receptors: Past, Present, and Future. Trends Pharmacol. Sci. 2019, 40, 636–654. [Google Scholar] [CrossRef]
- Lizama, B.N.; Kahle, J.; Catalano, S.M.; Caggiano, A.O.; Grundman, M.; Hamby, M.E. Sigma-2 Receptors—From Basic Biology to Therapeutic Target: A Focus on Age-Related Degenerative Diseases. Int. J. Mol. Sci. 2023, 24, 6251. [Google Scholar] [CrossRef]
- Fotakopoulos, G.; Gatos, C.; Georgakopoulou, V.E.; Christodoulidis, G.; Kagkouras, I.; Trakas, N.; Foroglou, N. Exploring the Role of Sigma Receptors in the Treatment of Cancer: A Narrative Review. Cureus 2024, 16, e70946. [Google Scholar] [CrossRef]
- Alon, A.; Lyu, J.; Braz, J.M.; Tummino, T.A.; Craik, V.; O’Meara, M.J.; Webb, C.M.; Radchenko, D.S.; Moroz, Y.S.; Huang, X.-P.; et al. Structures of the Σ2 Receptor Enable Docking for Bioactive Ligand Discovery. Nature 2021, 600, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Izzo, N.J.; Colom-Cadena, M.; Riad, A.A.; Xu, J.; Singh, M.; Abate, C.; Cahill, M.A.; Spires-Jones, T.L.; Bowen, W.D.; Mach, R.H.; et al. Proceedings from the Fourth International Symposium on σ-2 Receptors: Role in Health and Disease. eNeuro 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Sharma, N.; Patel, C.; Shenkman, M.; Kessel, A.; Ben-Tal, N.; Lederkremer, G.Z. The Sigma-1 Receptor Is an ER-Localized Type II Membrane Protein. J. Biol. Chem. 2021, 297, 101299. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.-P. Sigma-1 Receptor Chaperones at the ER- Mitochondrion Interface Regulate Ca2+ Signaling and Cell Survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Chu, U.B.; Ruoho, A.E. Biochemical Pharmacology of the Sigma-1 Receptor. Mol. Pharmacol. 2016, 89, 142–153. [Google Scholar] [CrossRef]
- Aishwarya, R.; Abdullah, C.S.; Morshed, M.; Remex, N.S.; Bhuiyan, M.S. Sigmar1’s Molecular, Cellular, and Biological Functions in Regulating Cellular Pathophysiology. Front. Physiol. 2021, 12, 705575. [Google Scholar] [CrossRef]
- Weng, T.-Y.; Tsai, S.-Y.A.; Su, T.-P. Roles of Sigma-1 Receptors on Mitochondrial Functions Relevant to Neurodegenerative Diseases. J. Biomed. Sci. 2017, 24, 74. [Google Scholar] [CrossRef]
- Kruse, A. Structural Insights into Sigma1 Function. In Sigma Proteins: Evolution of the Concept of Sigma Receptors; Kim, F.J., Pasternak, G.W., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2016; Volume 244, pp. 13–25. ISBN 978-3-319-65851-3. [Google Scholar]
- Su, T.-P.; Hayashi, T.; Maurice, T.; Buch, S.; Ruoho, A.E. The Sigma-1 Receptor Chaperone as an Inter-Organelle Signaling Modulator. Trends Pharmacol. Sci. 2010, 31, 557–566. [Google Scholar] [CrossRef]
- Alonso, G.; Phan, V.-L.; Guillemain, I.; Saunier, M.; Legrand, A.; Anoal, M.; Maurice, T. Immunocytochemical Localization of the Sigma1 Receptor in the Adult Rat Central Nervous System. Neuroscience 2000, 97, 155–170. [Google Scholar] [CrossRef]
- Siddiqui, T.; Bhatt, L.K. Targeting Sigma-1 Receptor: A Promising Strategy in the Treatment of Parkinson’s Disease. Neurochem. Res. 2023, 48, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Wang, J.; Li, N.; Li, G.; Ma, H.; Zhao, Y.; Li, Y. Sigma-1 Receptors in Depression: Mechanism and Therapeutic Development. Front. Pharmacol. 2022, 13, 925879. [Google Scholar] [CrossRef]
- Borbély, E.; Varga, V.; Szögi, T.; Schuster, I.; Bozsó, Z.; Penke, B.; Fülöp, L. Impact of Two Neuronal Sigma-1 Receptor Modulators, PRE084 and DMT, on Neurogenesis and Neuroinflammation in an Aβ1–42-Injected, Wild-Type Mouse Model of AD. Int. J. Mol. Sci. 2022, 23, 2514. [Google Scholar] [CrossRef] [PubMed]
- Freyssin, A.; Carles, A.; Guehairia, S.; Rubinstenn, G.; Maurice, T. Fluoroethylnormemantine (FENM) Shows Synergistic Protection in Combination with a Sigma-1 Receptor Agonist in a Mouse Model of Alzheimer’s Disease. Neuropharmacology 2024, 242, 109733. [Google Scholar] [CrossRef]
- Ruiz-Cantero, M.C.; González-Cano, R.; Tejada, M.Á.; Santos-Caballero, M.; Perazzoli, G.; Nieto, F.R.; Cobos, E.J. Sigma-1 Receptor: A Drug Target for the Modulation of Neuroimmune and Neuroglial Interactions during Chronic Pain. Pharmacol. Res. 2021, 163, 105339. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Pergolizzi, J.V. Bispecific Sigma1R-Antagonist/MOR-Agonist Compounds for Pain. Cureus 2024, 16, e59837. [Google Scholar] [CrossRef]
- Oflaz, F.E.; Koshenov, Z.; Hirtl, M.; Rost, R.; Malli, R.; Graier, W.F. Sigma-1 Receptor Modulation by Ligands Coordinates Cancer Cell Energy Metabolism. Biomolecules 2022, 12, 762. [Google Scholar] [CrossRef]
- Pontisso, I.; Combettes, L. Role of Sigma-1 Receptor in Calcium Modulation: Possible Involvement in Cancer. Genes 2021, 12, 139. [Google Scholar] [CrossRef]
- Megalizzi, V.; Le Mercier, M.; Decaestecker, C. Sigma Receptors and Their Ligands in Cancer Biology: Overview and New Perspectives for Cancer Therapy. Med. Res. Rev. 2012, 32, 410–427. [Google Scholar] [CrossRef]
- Abate, C.; Niso, M.; Abatematteo, F.S.; Contino, M.; Colabufo, N.A.; Berardi, F. PB28, the Sigma-1 and Sigma-2 Receptors Modulator With Potent Anti–SARS-CoV-2 Activity: A Review About Its Pharmacological Properties and Structure Affinity Relationships. Front. Pharmacol. 2020, 11, 589810. [Google Scholar] [CrossRef]
- Hashimoto, K. Repurposing of CNS Drugs to Treat COVID-19 Infection: Targeting the Sigma-1 Receptor. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Safety and Tolerability of Blarcamesine in Alzheimer’s Disease. Available online: https://clinicaltrials.gov/study/NCT04314934?term=NCT04314934&rank=1 (accessed on 28 October 2025).
- A Multi-Center, Open-Label Study Evaluating the Safety, Tolerability, and Efficacy of Pridopidine in Patients with Huntington’s Disease (Open PRIDE-HD). Available online: https://clinicaltrials.gov/study/NCT02494778 (accessed on 28 October 2025).
- A Phase 2, to Evaluating the Safety and Efficacy of Pridopidine vs. Placebo for Symptomatic Treatment in Patients with Huntington’s Disease. Available online: https://clinicaltrials.gov/study/NCT02006472 (accessed on 28 October 2025).
- Ishiwata, K.; Hashimoto, K. Sigma Receptor Ligands: Possible Application as Therapeutic Drugs and as Radiopharmaceuticals. CPD 2006, 12, 3857–3876. [Google Scholar] [CrossRef] [PubMed]
- Agha, H.; McCurdy, C.R. In Vitro and in Vivo Sigma 1 Receptor Imaging Studies in Different Disease States. RSC Med. Chem. 2021, 12, 154–177. [Google Scholar] [CrossRef] [PubMed]
- Van Waarde, A.; Rybczynska, A.A.; Ramakrishnan, N.K.; Ishiwata, K.; Elsinga, P.H.; Dierckx, R.A.J.O. Potential Applications for Sigma Receptor Ligands in Cancer Diagnosis and Therapy. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2703–2714. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Huang, Y. Imaging Sigma Receptors in the Brain: New Opportunities for Diagnosis of Alzheimer’s Disease and Therapeutic Development. Neurosci. Lett. 2019, 691, 3–10. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, U. Positron Emission Tomography: An Overview. J. Med. Phys. 2006, 31, 13. [Google Scholar] [CrossRef]
- Lameka, K.; Farwell, M.D.; Ichise, M. Positron Emission Tomography. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 135, pp. 209–227. ISBN 978-0-444-53485-9. [Google Scholar]
- Mastropasqua, F.; Luurtsema, G.; Filosa, C.; Colabufo, N.A. Designing a Small Molecule for PET Radiotracing: [18F]MC225 in Human Trials for Early Diagnosis in CNS Pathologies. Molecules 2025, 30, 3696. [Google Scholar] [CrossRef]
- Pichler, B.J.; Judenhofer, M.S.; Pfannenberg, C. Multimodal Imaging Approaches: PET/CT and PET/MRI. In Molecular Imaging I; Semmler, W., Schwaiger, M., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 185/1, pp. 109–132. ISBN 978-3-540-72717-0. [Google Scholar]
- Nerella, S.G.; Singh, P.; Sanam, T.; Digwal, C.S. PET Molecular Imaging in Drug Development: The Imaging and Chemistry Perspective. Front. Med. 2022, 9, 812270. [Google Scholar] [CrossRef]
- Almuhaideb, A.; Papathanasiou, N.; Bomanji, J. 18F-FDG PET/CT Imaging In Oncology. Ann. Saudi Med. 2011, 31, 3–13. [Google Scholar] [CrossRef]
- Hernández-Lozano, I.; Mairinger, S.; Filip, T.; Löbsch, M.; Stanek, J.; Kuntner, C.; Bauer, M.; Zeitlinger, M.; Hacker, M.; Helbich, T.H.; et al. Positron Emission Tomography-Based Pharmacokinetic Analysis To Assess Renal Transporter-Mediated Drug-Drug Interactions of Antimicrobial Drugs. Antimicrob. Agents Chemother. 2023, 67, e01493-22. [Google Scholar] [CrossRef]
- Weber, F.; Brust, P.; Laurini, E.; Pricl, S.; Wünsch, B. Fluorinated PET Tracers for Molecular Imaging of S1 Receptors in the Central Nervous System. In Sigma Receptors: Their Role in Disease and as Therapeutic Targets; Smith, S.B., Su, T.-P., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 964, pp. 31–48. ISBN 978-3-319-50172-7. [Google Scholar]
- Ludwig, F.-A.; Laurini, E.; Schmidt, J.; Pricl, S.; Deuther-Conrad, W.; Wünsch, B. [18F]Fluspidine—A PET Tracer for Imaging of σ1 Receptors in the Central Nervous System. Pharmaceuticals 2024, 17, 166. [Google Scholar] [CrossRef]
- Wang, T.; Sun, N.; Ma, Y.; Zhang, S. Recent Advances in the Development of Sigma Receptor (Radio)Ligands and Their Application in Tumors. ACS Pharmacol. Transl. Sci. 2025, 8, 951–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jia, H. The Sigma Receptors in Alzheimer’s Disease: New Potential Targets for Diagnosis and Therapy. Int. J. Mol. Sci. 2023, 24, 12025. [Google Scholar] [CrossRef] [PubMed]
- Pike, V.W. PET Radiotracers: Crossing the Blood–Brain Barrier and Surviving Metabolism. Trends Pharmacol. Sci. 2009, 30, 431–440. [Google Scholar] [CrossRef]
- Matsuno, K.; Nakazawa, M.; Okamoto, K.; Kawashima, Y.; Mita, S. Binding Properties of SA4503, a Novel and Selective Σ1 Receptor Agonist. Eur. J. Pharmacol. 1996, 306, 271–279. [Google Scholar] [CrossRef]
- Kawamura, K.; Ishiwata, K.; Tajima, H.; Ishii, S.-I.; Shimada, Y.; Matsuno, K.; Homma, Y.; Senda, M. Synthesis and in Vivo Evaluation of [11C]SA6298 as a PET Sigma1 Receptor Ligand. Nucl. Med. Biol. 1999, 26, 915–922. [Google Scholar] [CrossRef]
- Kawamura, K.; Ishiwata, K.; Tajima, H.; Ishii, S.-I.; Matsuno, K.; Homma, Y.; Senda, M. In Vivo Evaluation of [11C]SA4503 as a PET Ligand for Mapping CNS Sigma1 Receptors. Nucl. Med. Biol. 2000, 27, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R. Pharmacophore Identification for Sigma-1 (σ1) Receptor Binding: Application of the “Deconstruction-Reconstruction-Elaboration” Approach. Mini-Rev. Med. Chem. 2005, 5, 927–940. [Google Scholar] [CrossRef]
- Elsinga, P.H.; Kawamura, K.; Kobayashi, T.; Tsukada, H.; Senda, M.; Vaalburg, W.; Ishiwata, K. Synthesis and Evaluation of [18 F]Fluoroethyl SA4503 as a PET Ligand for the Sigma Receptor. Synapse 2002, 43, 259–267. [Google Scholar] [CrossRef]
- Lever, J.R.; Gustafson, J.L.; Xu, R.; Allmon, R.L.; Lever, S.Z. Σ1 and Σ2 Receptor Binding Affinity and Selectivity of SA4503 and Fluoroethyl SA4503. Synapse 2006, 59, 350–358. [Google Scholar] [CrossRef]
- Kawamura, K.; Tsukada, H.; Shiba, K.; Tsuji, C.; Harada, N.; Kimura, Y.; Ishiwata, K. Synthesis and Evaluation of Fluorine-18-Labeled SA4503 as a Selective Sigma1 Receptor Ligand for Positron Emission Tomography. Nucl. Med. Biol. 2007, 34, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, L.; Deuther-Conrad, W.; Chen, Y.; Zhang, X.; Zhang, J.; Huang, Y.; Brust, P.; Jia, H. 18F-Labeled Benzylpiperazine Derivatives as Highly Selective Ligands for Imaging σ1 Receptor with Positron Emission Tomography. Label. Comp. Radiopharm. 2019, 62, 425–437. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Deuther-Conrad, W.; Xie, F.; Chen, X.; Cui, M.-C.; Zhang, X.-J.; Zhang, J.-M.; Steinbach, J.; Brust, P.; et al. Synthesis and Biological Evaluation of 18F Labeled Fluoro-Oligo-Ethoxylated 4-Benzylpiperazine Derivatives for Sigma-1 Receptor Imaging. Bioorganic Med. Chem. 2013, 21, 215–222. [Google Scholar] [CrossRef]
- Moussa, I.A.; Banister, S.D.; Giboureau, N.; Meikle, S.R.; Kassiou, M. Synthesis and in Vivo Evaluation of [18F]N-(2-Benzofuranylmethyl)-N′-[4-(2-Fluoroethoxy)Benzyl]Piperazine, a Novel Σ1 Receptor PET Imaging Agent. Bioorganic Med. Chem. Lett. 2011, 21, 6820–6823. [Google Scholar] [CrossRef]
- He, Y.; Xie, F.; Ye, J.; Deuther-Conrad, W.; Cui, B.; Wang, L.; Lu, J.; Steinbach, J.; Brust, P.; Huang, Y.; et al. 1-(4-[18F]Fluorobenzyl)-4-[(Tetrahydrofuran-2-Yl)Methyl]Piperazine: A Novel Suitable Radioligand with Low Lipophilicity for Imaging σ1 Receptors in the Brain. J. Med. Chem. 2017, 60, 4161–4172. [Google Scholar] [CrossRef]
- Große Maestrup, E.; Wiese, C.; Schepmann, D.; Hiller, A.; Fischer, S.; Scheunemann, M.; Brust, P.; Wünsch, B. Synthesis of Spirocyclic σ1 Receptor Ligands as Potential PET Radiotracers, Structure–Affinity Relationships and in Vitro Metabolic Stability. Bioorganic Med. Chem. 2009, 17, 3630–3641. [Google Scholar] [CrossRef] [PubMed]
- Große Maestrup, E.; Fischer, S.; Wiese, C.; Schepmann, D.; Hiller, A.; Deuther-Conrad, W.; Steinbach, J.; Wünsch, B.; Brust, P. Evaluation of Spirocyclic 3-(3-Fluoropropyl)-2-Benzofurans as σ1 Receptor Ligands for Neuroimaging with Positron Emission Tomography. J. Med. Chem. 2009, 52, 6062–6072. [Google Scholar] [CrossRef] [PubMed]
- Maestrup, E.G.; Wiese, C.; Schepmann, D.; Brust, P.; Wünsch, B. Synthesis, Pharmacological Activity and Structure Affinity Relationships of Spirocyclic Σ1 Receptor Ligands with a (2-Fluoroethyl) Residue in 3-Position. Bioorganic Med. Chem. 2011, 19, 393–405. [Google Scholar] [CrossRef]
- Hellewell, S.B.; Bowen, W.D. A Sigma-like Binding Site in Rat Pheochromocytoma (PC12) Cells: Decreased Affinity for (+)-Benzomorphans and Lower Molecular Weight Suggest a Different Sigma Receptor Form from That of Guinea Pig Brain. Brain Res. 1990, 527, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.; Maestrup, E.G.; Schepmann, D.; Grimme, S.; Humpf, H.; Brust, P.; Wünsch, B. Enantioselective σ1 Receptor Binding and Biotransformation of the Spirocyclic PET Tracer 1′-benzyl-3-(3-fluoropropyl)-3 H-spiro[[2]Benzofuran-1,4′-piperidine]. Chirality 2011, 23, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Holl, K.; Falck, E.; Köhler, J.; Schepmann, D.; Humpf, H.; Brust, P.; Wünsch, B. Synthesis, Characterization, and Metabolism Studies of Fluspidine Enantiomers. ChemMedChem 2013, 8, 2047–2056. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zhang, J.; Deuther-Conrad, W.; Xie, F.; Zhang, X.; Liu, J.; Qiao, J.; Cui, M.; Steinbach, J.; et al. Synthesis and Evaluation of Novel18F-Labeled Spirocyclic Piperidine Derivatives as σ1Receptor Ligands for Positron Emission Tomography Imaging. J. Med. Chem. 2013, 56, 3478–3491. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Wang, X.; Zhang, J.-M.; Deuther-Conrad, W.; Zhang, X.-J.; Huang, Y.; Li, Y.; Ye, J.-J.; Cui, M.-C.; Steinbach, J.; et al. Synthesis and Evaluation of a 18F-Labeled Spirocyclic Piperidine Derivative as Promising Σ1 Receptor Imaging Agent. Bioorganic Med. Chem. 2014, 22, 5270–5278. [Google Scholar] [CrossRef]
- Xie, F.; Bergmann, R.; Kniess, T.; Deuther-Conrad, W.; Mamat, C.; Neuber, C.; Liu, B.; Steinbach, J.; Brust, P.; Pietzsch, J.; et al. 18F-Labeled 1,4-Dioxa-8-Azaspiro[4.5]Decane Derivative: Synthesis and Biological Evaluation of a σ1Receptor Radioligand with Low Lipophilicity as Potent Tumor Imaging Agent. J. Med. Chem. 2015, 58, 5395–5407. [Google Scholar] [CrossRef]
- Tian, J.; He, Y.; Deuther-Conrad, W.; Fu, H.; Xie, F.; Zhang, Y.; Wang, T.; Zhang, X.; Zhang, J.; Brust, P.; et al. Synthesis and Evaluation of New 1-Oxa-8-Azaspiro[4.5]Decane Derivatives as Candidate Radioligands for Sigma-1 Receptors. Bioorganic Med. Chem. 2020, 28, 115560. [Google Scholar] [CrossRef]
- Ishiwata, K.; Kawamura, K.; Yajima, K.; QingGeletu; Mori, H.; Shiba, K. Evaluation of (+)-p-[11C]Methylvesamicol for Mapping Sigma1 Receptors: A Comparison with [11C]SA4503. Nucl. Med. Biol. 2006, 33, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Abate, C.; Niso, M.; Lacivita, E.; Mosier, P.D.; Toscano, A.; Perrone, R. Analogues of σ Receptor Ligand 1-Cyclohexyl-4-[3-(5-Methoxy-1,2,3,4-Tetrahydronaphthalen-1-Yl)Propyl]Piperazine (PB28) with Added Polar Functionality and Reduced Lipophilicity for Potential Use as Positron Emission Tomography Radiotracers. J. Med. Chem. 2011, 54, 1022–1032. [Google Scholar] [CrossRef]
- Colabufo, N.A.; Abate, C.; Contino, M.; Inglese, C.; Niso, M.; Berardi, F.; Perrone, R. PB183, a Sigma Receptor Ligand, as a Potential PET Probe for the Imaging of Prostate Adenocarcinoma. Bioorganic Med. Chem. Lett. 2008, 18, 1990–1993. [Google Scholar] [CrossRef]
- Spinelli, F.; Haider, A.; Toscano, A.; Pati, M.L.; Keller, C.; Berardi, F.; Colabufo, N.A.; Abate, C.; Ametamey, S. Synthesis, Radiolabelling, and Evaluation of [11C]PB212 as a Radioligand for Imaging Sigma-1 Receptors Using PET. Am. J. Nucl. Med. Mol. imaging 2018, 8, 32–40. [Google Scholar] [PubMed]
- Lan, Y.; Bai, P.; Chen, Z.; Neelamegam, R.; Placzek, M.S.; Wang, H.; Fiedler, S.A.; Yang, J.; Yuan, G.; Qu, X.; et al. Novel Radioligands for Imaging Sigma-1 Receptor in Brain Using Positron Emission Tomography (PET). Acta Pharm. Sin. B 2019, 9, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Chen, Y.; Cao, X.; Zhang, J.; Wang, J.; Xu, X.; Qiu, Y.; Zhang, T.; Liu, X.; Liu, B.-F.; et al. Synthesis and Biological Evaluation of Novel Sigma-1 Receptor Antagonists Based on Pyrimidine Scaffold As Agents for Treating Neuropathic Pain. J. Med. Chem. 2014, 57, 10404–10423. [Google Scholar] [CrossRef]
- Bai, P.; Bagdasarian, F.A.; Xu, Y.; Wang, Y.; Wang, Y.; Gomm, A.; Zhou, Y.; Wu, R.; Wey, H.-Y.; Tanzi, R.E.; et al. Molecular Imaging of Alzheimer’s Disease-Related Sigma-1 Receptor in the Brain via a Novel Ru-Mediated Aromatic 18F-Deoxyfluorination Probe. J. Med. Chem. 2024, 67, 6207–6217. [Google Scholar] [CrossRef]
- Wang, W.; Cui, J.; Lu, X.; Padakanti, P.K.; Xu, J.; Parsons, S.M.; Luedtke, R.R.; Rath, N.P.; Tu, Z. Synthesis and in Vitro Biological Evaluation of Carbonyl Group-Containing Analogues for σ1 Receptors. J. Med. Chem. 2011, 54, 5362–5372. [Google Scholar] [CrossRef]
- Yue, X.; Jin, H.; Luo, Z.; Liu, H.; Zhang, X.; McSpadden, E.D.; Tian, L.; Flores, H.P.; Perlmutter, J.S.; Parsons, S.M.; et al. Chiral Resolution of Serial Potent and Selective Σ1 Ligands and Biological Evaluation of (−)-[18F]TZ3108 in Rodent and the Nonhuman Primate Brain. Bioorganic Med. Chem. 2017, 25, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Soda, A.K.; Huang, T.; Zhou, W.; Chen, H.; Jiang, H.; Jadhav, S.B.; Xing, Z.; Yu, Y.; Tian, L.; Wong, D.F.; et al. Synthesis and in Vivo Biological Characterization of Six Carbon-11 Sigma-1 Receptor Radiotracers in Rodent and Nonhuman Primate. Bioorganic Med. Chem. 2025, 126, 118218. [Google Scholar] [CrossRef]
- James, M.L.; Shen, B.; Zavaleta, C.L.; Nielsen, C.H.; Mesangeau, C.; Vuppala, P.K.; Chan, C.; Avery, B.A.; Fishback, J.A.; Matsumoto, R.R.; et al. New Positron Emission Tomography (PET) Radioligand for Imaging σ-1 Receptors in Living Subjects. J. Med. Chem. 2012, 55, 8272–8282. [Google Scholar] [CrossRef]
- Yous, S.; Wallez, V.; Belloir, M.; Caignard, D.H.; McCurdy, C.R.; Poupaert, J.H. Novel 2(3H)-Benzothiazolones as Highly Potent and Selective Sigma-1 Receptor Ligands. Med. Chem. Res. 2005, 14, 158–168. [Google Scholar] [CrossRef]
- Waterhouse, R.N.; Lee Collier, T. In Vivo Evaluation of [18F]1-(3-Fluoropropyl)-4-(4-Cyanophenoxymethyl)Piperidine: A Selective Sigma-1 Receptor Radioligand for PET. Nucl. Med. Biol. 1997, 24, 127–134. [Google Scholar] [CrossRef]
- Zhao, J.; Chang, R.; Carambot, P.; Waterhouse, R.N. Radiosynthesis and in Vivo Study of [18F]1-(2-fluoroethyl)-4-[(4-cyanophenoxy)Methyl]Piperidine: A Promising New Sigma-1 Receptor Ligand. Label. Comp. Radiopharm. 2005, 48, 547–555. [Google Scholar] [CrossRef]
- Gangangari, K.K.; Váradi, A.; Majumdar, S.; Larson, S.M.; Pasternak, G.W.; Pillarsetty, N.K. Imaging Sigma-1 Receptor (S1R) Expression Using Iodine-124-Labeled 1-(4-Iodophenyl)-3-(2-Adamantyl)Guanidine ([124I]IPAG). Mol. Imaging Biol. 2020, 22, 358–366. [Google Scholar] [CrossRef]
- Kimes, A.S.; Wilson, A.A.; Scheffel, U.; Campbell, B.G.; London, E.D. Radiosynthesis, Cerebral Distribution, and Binding of [125I]-1-(p-Iodophenyl)-3-(1-Adamantyl)Guanidine, a Ligand for .Sigma. Binding Sites. J. Med. Chem. 1992, 35, 4683–4689. [Google Scholar] [CrossRef]
- Itzhak, Y. (Ed.) Sigma Receptors; Neuroscience Perspectives; Academic Press: London, UK, 1994; ISBN 978-0-12-376350-1. [Google Scholar]
- Yang, D.; Comeau, A.; Bowen, W.D.; Mach, R.H.; Ross, B.D.; Hong, H.; Van Dort, M.E. Design and Investigation of a [18F]-Labeled Benzamide Derivative as a High Affinity Dual Sigma Receptor Subtype Radioligand for Prostate Tumor Imaging. Mol. Pharm. 2017, 14, 770–780. [Google Scholar] [CrossRef]
- Zhang, M.-R.; Haradahira, T.; Maeda, J.; Okauchi, T.; Kawabe, K.; Kida, T.; Suzuki, K.; Suhara, T. Synthesis and Evaluation of 3-(4-Chlorobenzyl)-8-[11C]Methoxy-1,2,3,4-Tetrahydrochromeno[3,4-c]Pyridin-5-One: A PET Tracer for Imaging Sigma1 Receptors. Nucl. Med. Biol. 2002, 29, 469–476. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Zhang, X.; Chen, L.; Zheng, M.; Zhang, J.; Brust, P.; Deuther-Conrad, W.; Huang, Y.; Jia, H. Synthesis and Characterization of the Two Enantiomers of a Chiral Sigma-1 Receptor Radioligand: (S)-(+)- and (R)-(-)-[18F]FBFP. Chin. Chem. Lett. 2022, 33, 3543–3548. [Google Scholar] [CrossRef]
- Dahl, K.; Halldin, C.; Schou, M. New Methodologies for the Preparation of Carbon-11 Labeled Radiopharmaceuticals. Clin. Transl. Imaging 2017, 5, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Haider, A.; Jeppesen, T.E.; Josephson, L.; Liang, S.H. Radiochemistry for Positron Emission Tomography. Nat. Commun. 2023, 14, 3257. [Google Scholar] [CrossRef] [PubMed]
- Pees, A.; Chassé, M.; Lindberg, A.; Vasdev, N. Recent Developments in Carbon-11 Chemistry and Applications for First-In-Human PET Studies. Molecules 2023, 28, 931. [Google Scholar] [CrossRef]
- Haveman, L.Y.; Vugts, D.J.; Windhorst, A.D. State of the art procedures towards reactive [18F] fluoride in PET tracer synthesis. EJNMMI Radiopharm. Chem. 2023, 8, 28. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Q.; Shi, H.; Cheng, D. Fluorine-18: Radiochemistry and Target-Specific PET Molecular Probes Design. Front. Chem. 2022, 10, 884517. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Goud, N.S.; Joshi, R.K.; Bharath, R.D.; Kumar, P. Fluorine-18: A Radionuclide with Diverse Range of Radiochemistry and Synthesis Strategies for Target Based PET Diagnosis. Eur. J. Med. Chem. 2020, 187, 111979. [Google Scholar] [CrossRef]
- Halder, R.; Ritter, T. 18F-Fluorination: Challenge and Opportunity for Organic Chemists. J. Org. Chem. 2021, 86, 13873–13884. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.; Stewart, M.; Littich, R.; Hoareau, R.; Scott, P. Radiosyntheses Using Fluorine-18: The Art and Science of Late Stage Fluorination. Curr. Top. Med. Chem. 2014, 14, 875–900. [Google Scholar] [CrossRef]
- Kroselj, M.; Socan, A.; Zaletel, K.; Dreger, T.; Knopp, R.; Gmeiner, T.; Kolenc Peitl, P. A Novel, Self-Shielded Modular Radiosynthesis System for Fully Automated Preparation of PET and Therapeutic Radiopharmaceuticals. Nucl. Med. Commun. 2016, 37, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Wiese, C.; Große Maestrup, E.; Hiller, A.; Deuther-Conrad, W.; Scheunemann, M.; Schepmann, D.; Steinbach, J.; Wünsch, B.; Brust, P. Molecular Imaging of σ Receptors: Synthesis and Evaluation of the Potent Σ1 Selective Radioligand [18F]Fluspidine. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 540–551. [Google Scholar] [CrossRef]
- Maisonial-Besset, A.; Funke, U.; Wenzel, B.; Fischer, S.; Holl, K.; Wünsch, B.; Steinbach, J.; Brust, P. Automation of the Radiosynthesis and Purification Procedures for [18F]Fluspidine Preparation, a New Radiotracer for Clinical Investigations in PET Imaging of σ1 Receptors in Brain. Appl. Radiat. Isot. 2014, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, M.; Wenzel, B.; Nikodemus, J.; Florea, A.; Hertel, F.; Kopka, K.; Vogg, A.T.J.; Kiessling, F.; Mottaghy, F.M. Improved Protocol for the Radiosynthesis of [18F]FTC-146: A Potent and Selective Sigma-1 Receptor Radioligand. Label. Comp. Radiopharm. 2023, 66, 116–125. [Google Scholar] [CrossRef]
- Schoultz, B.; Reed, B.; Marton, J.; Willoch, F.; Henriksen, G. A Fully Automated Radiosynthesis of [18F]Fluoroethyl-Diprenorphine on a Single Module by Use of SPE Cartridges for Preparation of High Quality 2-[18F]Fluoroethyl Tosylate. Molecules 2013, 18, 7271–7278. [Google Scholar] [CrossRef]
- Jin, H.; Fan, J.; Zhang, X.; Li, J.; Flores, H.P.; Perlmutter, J.S.; Parsons, S.M.; Tu, Z. Radiosynthesis and in Vivo Evaluation of a Novel σ1Selective PET Ligand. Med. Chem. Commun. 2014, 5, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, L.S.; Jentzen, W.; Stahl, A.; Bockisch, A.; Rosenbaum-Krumme, S.J. Clinical applications of 124I-PET/CT in patients with differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38 (Suppl. S1), 48–56. [Google Scholar] [CrossRef] [PubMed]
- Koehler, L.; Gagnon, K.; McQuarrie, S.; Wuest, F. Iodine-124: A Promising Positron Emitter for Organic PET Chemistry. Molecules 2010, 15, 2686–2718. [Google Scholar] [CrossRef]
- Matthews, P.M.; Rabiner, E.A.; Passchier, J.; Gunn, R.N. Positron Emission Tomography Molecular Imaging for Drug Development. Brit J. Clin. Pharma 2012, 73, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ishiwata, K.; Shimada, Y.; Kimura, Y.; Kobayashi, T.; Matsuno, K.; Homma, Y.; Senda, M. Preclinical Evaluation of [11C]SA4503: Radiation Dosimetry, in Vivo Selectivity and PET Imaging of Sigma1 Receptors in the Cat Brain. Ann. Nucl. Med. 2000, 14, 285–292. [Google Scholar] [CrossRef]
- Ishiwata, K.; Tsukada, H.; Kawamura, K.; Kimura, Y.; Nishiyama, S.; Kobayashi, T.; Matsuno, K.; Senda, M. Mapping of CNS Sigma1 Receptors in the Conscious Monkey: Preliminary PET Study with [11 C]SA4503. Synapse 2001, 40, 235–237. [Google Scholar] [CrossRef]
- Elsinga, P.H.; Tsukada, H.; Harada, N.; Kakiuchi, T.; Kawamura, K.; Vaalburg, W.; Kimura, Y.; Kobayashi, T.; Ishiwata, K. Evaluation of [18F]Fluorinated Sigma Receptor Ligands in the Conscious Monkey Brain. Synapse 2004, 52, 29–37. [Google Scholar] [CrossRef]
- Lepelletier, F.-X.; Vandesquille, M.; Asselin, M.-C.; Prenant, C.; Robinson, A.C.; Mann, D.M.A.; Green, M.; Barnett, E.; Banister, S.D.; Mottinelli, M.; et al. Evaluation of 18F-IAM6067 as a Sigma-1 Receptor PET Tracer for Neurodegeneration in Vivo in Rodents and in Human Tissue. Theranostics 2020, 10, 7938–7955. [Google Scholar] [CrossRef]
- Baum, E.; Cai, Z.; Bois, F.; Holden, D.; Lin, S.; Lara-Jaime, T.; Kapinos, M.; Chen, Y.; Deuther-Conrad, W.; Fischer, S.; et al. PET Imaging Evaluation of Four σ1 Radiotracers in Nonhuman Primates. J. Nucl. Med. 2017, 58, 982–988. [Google Scholar] [CrossRef]
- James, M.L.; Shen, B.; Nielsen, C.H.; Behera, D.; Buckmaster, C.L.; Mesangeau, C.; Zavaleta, C.; Vuppala, P.K.; Jamalapuram, S.; Avery, B.A.; et al. Evaluation of σ-1 Receptor Radiolig and 18F-FTC-146 in Rats and Squirrel Monkeys Using PET. J. Nucl. Med. 2014, 55, 147–153. [Google Scholar] [CrossRef]
- Waterhouse, R.N.; Stabin, M.G.; Page, J.G. Preclinical Acute Toxicity Studies and Rodent-Based Dosimetry Estimates of the Novel Sigma-1 Receptor Radiotracer [18F]FPS. Nucl. Med. Biol. 2003, 30, 555–563. [Google Scholar] [CrossRef]
- Waterhouse, R.N.; Chang, R.C.; Zhao, J.; Carambot, P.E. In Vivo Evaluation in Rats of [18F]1-(2-Fluoroethyl)-4-[(4-Cyanophenoxy)Methyl]Piperidine as a Potential Radiotracer for PET Assessment of CNS Sigma-1 Receptors. Nucl. Med. Biol. 2006, 33, 211–215. [Google Scholar] [CrossRef]
- Solon, E.G.; Schweitzer, A.; Stoeckli, M.; Prideaux, B. Autoradiography, MALDI-MS, and SIMS-MS imaging in pharmaceutical discovery and development. AAPS J. 2010, 12, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, M. Autoradiography with Positron Emitting Isotopes in Positron Emission Tomography Tracer Discovery. Mol. Imaging Biol. 2003, 5, 390–396. [Google Scholar] [CrossRef]
- Solon, E.G. Autoradiography Techniques and Quantification of Drug Distribution. Cell Tissue Res. 2015, 360, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.C.; Smith, C.B. Resolution, Sensitivity and Precision with Autoradiography and Small Animal Positron Emission Tomography: Implications for Functional Brain Imaging in Animal Research. Nucl. Med. Biol. 2005, 32, 719–725. [Google Scholar] [CrossRef]
- Amirrashedi, M.; Zaidi, H.; Ay, M.R. Advances in Preclinical PET Instrumentation. PET Clin. 2020, 15, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.K.; Padmanabhan, P.; Yang, C.-T.; Mishra, S.; Halldin, C.; Gulyás, B. Dealing with PET Radiometabolites. EJNMMI Res. 2020, 10, 109. [Google Scholar] [CrossRef]
- Lindberg, A.; Chassé, M.; Varlow, C.; Pees, A.; Vasdev, N. Strategies for designing novel positron emission tomography (PET) radiotracers to cross the blood–brain barrier. J. Label. Compd. Radiopharm. 2023, 66, 205–221. [Google Scholar] [CrossRef]
- Ramakrishnan, N.K.; Rybczynska, A.A.; Visser, A.K.D.; Marosi, K.; Nyakas, C.J.; Kwizera, C.; Sijbesma, J.W.A.; Elsinga, P.H.; Ishiwata, K.; Pruim, J.; et al. Small-Animal PET with a σ-Ligand, 11C-SA4503, Detects Spontaneous Pituitary Tumors in Aged Rats. J. Nucl. Med. 2013, 54, 1377–1383. [Google Scholar] [CrossRef][Green Version]
- Brust, P.; Deuther-Conrad, W.; Becker, G.; Patt, M.; Donat, C.K.; Stittsworth, S.; Fischer, S.; Hiller, A.; Wenzel, B.; Dukic-Stefanovic, S.; et al. Distinctive In Vivo Kinetics of the New σ1 Receptor Ligands (R)-(+)- and (S)-(–)-18F-Fluspidine in Porcine Brain. J. Nucl. Med. 2014, 55, 1730–1736. [Google Scholar] [CrossRef]
- Ludwig, F.-A.; Fischer, S.; Houska, R.; Hoepping, A.; Deuther-Conrad, W.; Schepmann, D.; Patt, M.; Meyer, P.M.; Hesse, S.; Becker, G.-A.; et al. In Vitro and in Vivo Human Metabolism of (S)-[18F]Fluspidine–A Radioligand for Imaging Σ1 Receptors With Positron Emission Tomography (PET). Front. Pharmacol. 2019, 10, 534. [Google Scholar] [CrossRef]
- Hjørnevik, T.; Cipriano, P.W.; Shen, B.; Park, J.H.; Gulaka, P.; Holley, D.; Gandhi, H.; Yoon, D.; Mittra, E.S.; Zaharchuk, G.; et al. Biodistribution and Radiation Dosimetry of 18F-FTC-146 in Humans. J. Nucl. Med. 2017, 58, 2004–2009. [Google Scholar] [CrossRef]
- Pantel, A.R.; Viswanath, V.; Muzi, M.; Doot, R.K.; Mankoff, D.A. Principles of Tracer Kinetic Analysis in Oncology, Part I: Principles and Overview of Methodology. J. Nucl. Med. 2022, 63, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou-Strauss, A.; Pan, L.; Sachpekidis, C. Kinetic Modeling and Parametric Imaging with Dynamic PET for Oncological Applications: General Considerations, Current Clinical Applications, and Future Perspectives. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 21–39. [Google Scholar] [CrossRef]
- Kimura, Y.; Naganawa, M.; Shidahara, M.; Ikoma, Y.; Watabe, H. PET Kinetic Analysis—Pitfalls and a Solution for the Logan Plot. Ann. Nucl. Med. 2007, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ye, W.; Brašić, J.R.; Crabb, A.H.; Hilton, J.; Wong, D.F. A Consistent and Efficient Graphical Analysis Method to Improve the Quantification of Reversible Tracer Binding in Radioligand Receptor Dynamic PET Studies. NeuroImage 2009, 44, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Sakata, M.; Kimura, Y.; Naganawa, M.; Oda, K.; Ishii, K.; Chihara, K.; Ishiwata, K. Mapping of Human Cerebral Sigma1 Receptors Using Positron Emission Tomography and [11C]SA4503. NeuroImage 2007, 35, 1–8. [Google Scholar] [CrossRef]
- Kawamura, K.; Kobayashi, T.; Matsuno, K.; Ishiwata, K. Different Brain Kinetics of Two Sigma1 Receptor Ligands, [3H](+)-pentazocine and [11C]SA4503, by P-glycoprotein Modulation. Synapse 2003, 48, 80–86. [Google Scholar] [CrossRef]
- Sakata, M.; Kimura, Y.; Naganawa, M.; Ishikawa, M.; Oda, K.; Ishii, K.; Hashimoto, K.; Chihara, K.; Ishiwata, K. Shortened Protocol in Practical [11C]SA4503-PET Studies for Sigma1 Receptor Quantification. Ann. Nucl. Med. 2008, 22, 143–146. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Preface, Executive Summary and Glossary. Ann. ICRP 2007, 37, 9–34. [Google Scholar] [CrossRef]
- Jackson, I.M.; Lee, S.J.; Sowa, A.R.; Rodnick, M.E.; Bruton, L.; Clark, M.; Preshlock, S.; Rothley, J.; Rogers, V.E.; Botti, L.E.; et al. Use of 55 PET Radiotracers under Approval of a Radioactive Drug Research Committee (RDRC). EJNMMI Radiopharm. Chem. 2020, 5, 24. [Google Scholar] [CrossRef]
- Ishiwata, K.; Ishii, K.; Kimura, Y.; Kawamura, K.; Oda, K.; Sasaki, T.; Sakata, M.; Senda, M. Successive Positron Emission Tomography Measurement of Cerebral Blood Flow and Neuroreceptors in the Human Brain: An 11C-SA4503 Study. Ann. Nucl. Med. 2008, 22, 411–416. [Google Scholar] [CrossRef]
- Kranz, M.; Sattler, B.; Wüst, N.; Deuther-Conrad, W.; Patt, M.; Meyer, P.; Fischer, S.; Donat, C.; Wünsch, B.; Hesse, S.; et al. Evaluation of the Enantiomer Specific Biokinetics and Radiation Doses of [18F]Fluspidine—A New Tracer in Clinical Translation for Imaging of Σ1 Receptors. Molecules 2016, 21, 1164. [Google Scholar] [CrossRef]
- Shen, B.; James, M.L.; Andrews, L.; Lau, C.; Chen, S.; Palner, M.; Miao, Z.; Arksey, N.C.; Shuhendler, A.J.; Scatliffe, S.; et al. Further Validation to Support Clinical Translation of [18F]FTC-146 for Imaging Sigma-1 Receptors. EJNMMI Res. 2015, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Park, J.H.; Hjørnevik, T.; Cipriano, P.W.; Yoon, D.; Gulaka, P.K.; Holly, D.; Behera, D.; Avery, B.A.; Gambhir, S.S.; et al. Radiosynthesis and First-In-Human PET/MRI Evaluation with Clinical-Grade [18F]FTC-146. Mol. Imaging Biol. 2017, 19, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R.N.; Zhao, J.; Stabin, M.G.; Ng, H.; Schindler-Horvat, J.; Chang, R.C.; Mirsalis, J.C. Preclinical Acute Toxicity Studies and Dosimetry Estimates of the Novel Sigma-1 Receptor Radiotracer, [18F]SFE. Mol. Imaging Biol. 2006, 8, 284–291. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ishiwata, K.; Ishii, K.; Kimura, Y.; Sakata, M.; Naganawa, M.; Oda, K.; Miyatake, R.; Fujisaki, M.; Shimizu, E.; et al. High Occupancy of Sigma-1 Receptors in the Human Brain after Single Oral Administration of Fluvoxamine: A Positron Emission Tomography Study Using [11C]SA4503. Biol. Psychiatry 2007, 62, 878–883. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sakata, M.; Ishii, K.; Kimura, Y.; Oda, K.; Toyohara, J.; Wu, J.; Ishiwata, K.; Iyo, M.; Hashimoto, K. High Occupancy of Σ1 Receptors in the Human Brain after Single Oral Administration of Donepezil: A Positron Emission Tomography Study Using [11C]SA4503. Int. J. Neuropsychopharm. 2009, 12, 1127. [Google Scholar] [CrossRef]
- Ramakrishnan, N.K.; Visser, A.K.D.; Schepers, M.; Luurtsema, G.; Nyakas, C.J.; Elsinga, P.H.; Ishiwata, K.; Dierckx, R.A.J.O.; Van Waarde, A. Dose-Dependent Sigma-1 Receptor Occupancy by Donepezil in Rat Brain Can Be Assessed with 11C-SA4503 and microPET. Psychopharmacology 2014, 231, 3997–4006. [Google Scholar] [CrossRef]
- Wang, W.-F.; Ishiwata, K.; Kiyosawa, M.; Kawamura, K.; Oda, K.; Kobayashi, T.; Matsuno, K.; Mochizuki, M. Visualization of Sigma1 Receptors in Eyes by Ex Vivo Autoradiography and in Vivo Positron Emission Tomography. Exp. Eye Res. 2002, 75, 723–730. [Google Scholar] [CrossRef]
- Toyohara, J.; Kobayashi, T.; Mita, S.; Ishiwata, K. Application of [11C]SA4503 to Selection of Novel Σ1 Selective Agonists. Nucl. Med. Biol. 2012, 39, 1117–1121. [Google Scholar] [CrossRef]
- Kortekaas, R.; Maguire, R.P.; Van Waarde, A.; Leenders, K.L.; Elsinga, P.H. Despite Irreversible Binding, PET Tracer [11C]-SA5845 Is Suitable for Imaging of Drug Competition at Sigma Receptors—The Cases of Ketamine and Haloperidol. Neurochem. Int. 2008, 53, 45–50. [Google Scholar] [CrossRef]
- Meyer, P.; Strauss, M.; Becker, G.; Hesse, S.; Bednasch, K.; Ettrich, B.; Zientek, F.; Rullmann, M.; Wilke, S.; Luthardt, J.; et al. Increased Sigma-1 Receptor (Sig-1R) Binding in the Brain of Unmedicated Patients with Acute Major Depressive Disorder (MDD) Using the Novel Sig-1R-Specific Radioligand (-)-[18F]Fluspidine and PET. J. Nucl. Med. 2018, 59, 551. [Google Scholar]
- Toussaint, M.; Deuther-Conrad, W.; Kranz, M.; Fischer, S.; Ludwig, F.-A.; Juratli, T.A.; Patt, M.; Wünsch, B.; Schackert, G.; Sabri, O.; et al. Sigma-1 Receptor Positron Emission Tomography: A New Molecular Imaging Approach Using (S)-(−)-[18F]Fluspidine in Glioblastoma. Molecules 2020, 25, 2170. [Google Scholar] [CrossRef]
- Grachev, I.D.; Meyer, P.M.; Becker, G.A.; Bronzel, M.; Marsteller, D.; Pastino, G.; Voges, O.; Rabinovich, L.; Knebel, H.; Zientek, F.; et al. Sigma-1 and Dopamine D2/D3 Receptor Occupancy of Pridopidine in Healthy Volunteers and Patients with Huntington Disease: A [18F] Fluspidine and [18F] Fallypride PET Study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1103–1115. [Google Scholar] [CrossRef]
- Shen, B.; Behera, D.; James, M.L.; Reyes, S.T.; Andrews, L.; Cipriano, P.W.; Klukinov, M.; Lutz, A.B.; Mavlyutov, T.; Rosenberg, J.; et al. Visualizing Nerve Injury in a Neuropathic Pain Model with [18F]FTC-146 PET/MRI. Theranostics 2017, 7, 2794–2805. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Fast, A.M.; Cipriano, P.; Shen, B.; Castillo, J.B.; McCurdy, C.R.; Mari Aparici, C.; Lum, D.; Biswal, S. Sigma-1 Receptor Changes Observed in Chronic Pelvic Pain Patients: A Pilot PET/MRI Study. Front. Pain Res. 2021, 2, 711748. [Google Scholar] [CrossRef]
- Reyes, S.T.; Deacon, R.M.J.; Guo, S.G.; Altimiras, F.J.; Castillo, J.B.; Van Der Wildt, B.; Morales, A.P.; Park, J.H.; Klamer, D.; Rosenberg, J.; et al. Effects of the Sigma-1 Receptor Agonist Blarcamesine in a Murine Model of Fragile X Syndrome: Neurobehavioral Phenotypes and Receptor Occupancy. Sci. Rep. 2021, 11, 17150. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R.N.; Chang, R.C.; Atuehene, N.; Collier, T.L. In Vitro and in Vivo Binding of Neuroactive Steroids to the Sigma-1 Receptor as Measured with the Positron Emission Tomography Radioligand [18F]FPS. Synapse 2007, 61, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Li, Z.; Peng, T.; Yang, J.; Bi, L.; Huang, G.; Qiu, Y.; Yang, M.; Ye, P.; Huang, M.; et al. Evaluation of [18F]F-TZ3108 for PET Imaging of Metabolic-Associated Fatty Liver Disease. Mol. Imaging Biol. 2022, 24, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yang, M.; Bi, L.; Ye, P.; Liu, Y.; He, P.; Huang, G.; Jin, H.; Xia, J. Quantitative Imaging Using [18F]F-TZ3108 to Assess Metabolic-Associated Fatty Liver Disease Progression and Low-Carbohydrate Diet Efficacy. Nucl. Med. Biol. 2025, 144–145, 108997. [Google Scholar] [CrossRef] [PubMed]
- Biodistribution and Pharmacokinetic Determination of the PET Radiopharmaceutical [18F]FTC-146 Using PET/MRI in Healthy (Asymptomatic) Volunteers and in Patients with CRPS and Sciatica. Available online: https://clinicaltrials.gov/study/NCT02753101?intr=FTC-146&rank=3 (accessed on 28 October 2025).
- 18F-FTC-146 PET/CT in Newly-Diagnosed Osteosarcoma. Available online: https://clinicaltrials.gov/study/NCT04365660?intr=FTC-146&rank=7 (accessed on 28 October 2025).

| Radioligand | Radiochemical Route | Radiochemical Yield | References |
|---|---|---|---|
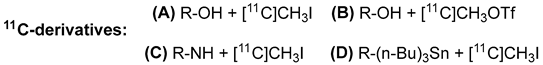 | |||
| [11C]SA4503 | A | 21–31% | [47] |
| [11C]PB-212 | A | 16–33% | [69] |
| (−)-5b | B | 20% | [75] |
| (+)-5c | B | 18% | [75] |
| (−)-6b | B | 20% | [75] |
| (+)-6c | B | 18% | [75] |
| (−)-7b | B | 17% | [75] |
| (+)-7c | B | 16% | [75] |
| [11C]HCCO923 | A | 6–15% | [70] |
| [11C]HCCO929 | C | 3–8% | [70] |
| [11C]-(+)-pMV | D | 8–19% | [66] |
| 9 | A | 32–59% | [84] |
| Radioligand | Radiochemical Route | Radiochemical Yield | References |
|---|---|---|---|
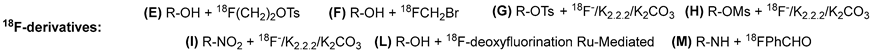 | |||
| [18F]FE-SA4503 | E | 4–7% | [49] |
| [18F]FM-SA4503 | F | 3–17% | [51] |
| [18F]WMS-1813 | G | 35–48% | [57] |
| [18F]Fluspidine | G | from 35–45% to 37 ± 8% | [96,97] |
| 3a | G | 35–60% | [62] |
| 3b | E | 8–10% | [63] |
| [18F]FTC-146 | G | from 4–7% to 41.7 ± 4.4% | [76,98] |
| [18F]FPS | H | 56–70% | [78] |
| [18F]SFE | H | 59 ± 8% | [79] |
| [18F]TZ3108 | I | 18–24% | [100] |
| [18F]CNY-05 | L | 25–40% | [72] |
| 4a | E | 15% | [64] |
| 4b | G | 12–35% | [65] |
| [18F]FBFP | M | 20–30% | [55] |
| 8 | E | 35–50% | [83] |
| [18F]IAM6067 | G | 18% | [54] |
| 1a-c | G | 42–55% | [52] |
| 2 | G | 30–50% | [53] |
| Radioligand | Radiochemical Route | Radiochemical Yield | Reference |
|---|---|---|---|
 | |||
| [124I]IPAG | N | 100% | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastropasqua, F.; Ludwig, F.-A.; Abate, C. A Full-Spectrum Evaluation of Sigma-1 Receptor (S1R) Positron Emission Tomography (PET) Radioligands from Binding Affinity to Clinical Imaging. Molecules 2025, 30, 4296. https://doi.org/10.3390/molecules30214296
Mastropasqua F, Ludwig F-A, Abate C. A Full-Spectrum Evaluation of Sigma-1 Receptor (S1R) Positron Emission Tomography (PET) Radioligands from Binding Affinity to Clinical Imaging. Molecules. 2025; 30(21):4296. https://doi.org/10.3390/molecules30214296
Chicago/Turabian StyleMastropasqua, Francesco, Friedrich-Alexander Ludwig, and Carmen Abate. 2025. "A Full-Spectrum Evaluation of Sigma-1 Receptor (S1R) Positron Emission Tomography (PET) Radioligands from Binding Affinity to Clinical Imaging" Molecules 30, no. 21: 4296. https://doi.org/10.3390/molecules30214296
APA StyleMastropasqua, F., Ludwig, F.-A., & Abate, C. (2025). A Full-Spectrum Evaluation of Sigma-1 Receptor (S1R) Positron Emission Tomography (PET) Radioligands from Binding Affinity to Clinical Imaging. Molecules, 30(21), 4296. https://doi.org/10.3390/molecules30214296







