Abstract
Metathesis reactions of Ag[C(CN)3] with anhydrous YbCl3 dissolved in water combined with stoichiometric amounts of the alkali-metal salts A[C(CN)3] (A = Na or K) yield the non-isotypic tetragonal compounds NaYb[C(CN)3]4 (P4/nnc with a = 1188.37(9) pm, c = 1232.41(9) pm) and KYb[C(CN)3]4 (P4/nbm with a = 1179.26(9) pm, c = 668.73(5) pm). Both crystal structures contain a three-dimensional framework (Niggli formula: {(Yb[C(CN)3]8/2)−}) with Yb3+ in square antiprismatic coordination of terminal nitrogen atoms (d(Yb–N) = 241–242 pm) from eight planar star-shaped tricyanomethanide anions [C(CN)3]−. The Na+ or K+ cations occupy vacancies, which provide them with a tetrahedral coordination sphere of nitrogen (d(Na–N) = 239 pm vs. d(K–N) = 276 pm) from four [C(CN)3]− anions. This difference results from secondary contacts with the central carbon atoms (d(Na–C) = 361 pm vs. d(K–C) = 367 pm) of four different [C(CN)3]− units, which do not contribute to NaYb[C(CN)3]4, but effectuate a lot in the case of KYb[C(CN)3]4. The Raman spectrum recorded for NaYb[C(CN)3]4 corroborates the presence of a pseudo-D3h-symmetric tricyanomethanide anion [C(CN)3]− and the absence of water.
1. Introduction
The tricyanomethanide anion [C(CN)3]−, as one of the bulkiest pseudo-halides, was discovered by Schmidtmann [1], but only scant research has been performed or reported for decades in spite of the chemical stability of this moiety. Therefore, some interesting features of this special anion were only recognized recently. It appears as a planar, star-shaped molecular entity (usually exhibiting D3h point symmetry or something rather close to it), so its π-bonding system has to be interpreted as a delocalized 8π-electron, seven-center bond arrangement with a sp2-hybridized carbon atom in the center allowing for a participating electron pair in the p-orbital to carry a negative charge. According to Figure 1 (bottom), the electronic delocalization, a situation often addressed as “Y aromaticity” [2], also takes a negative charge in all three of the terminal nitrogen atoms as equally contributing mesomeric resonance structures. Upon protonation, this very stable [C(CN)3]− anion is disturbed so much that the corresponding strong Brønsted acid H[C(CN)3] not only exhibits a pKa value of −5 [3], but the synthesis and final proof for the tricyanomethane form HC(CN)3, which is a thermodynamically more stable tautomer as compared to the dicyanoketenimine form (NC)2C(CNH) (Figure 1, top), was achieved only recently [4,5]. This is despite the fact that the central carbon atom would have to change its hybridization from sp2 to sp3, as the resulting small amounts of the dicyanoketenimine tautomer reveal themselves to be very prone to oligomerization at ambient temperatures in solution. On the other hand, this dicyanoketenimine form becomes much favored when H+ is replaced with the “big organometallic proton” [(H3C)3Si]+ [5].
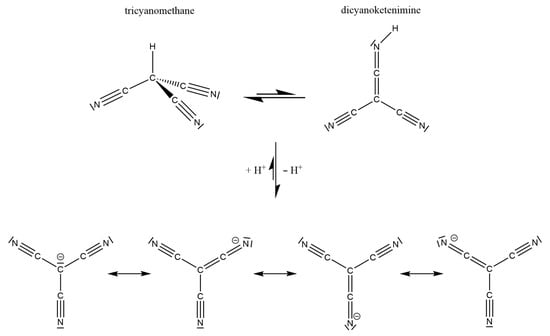
Figure 1.
The four mesomeric resonance structures of the triangular planar tricyanomethanide anion [C(CN)3]− (bottom) and its tautomeric protonation products tricyanomethane (top, left) and dicyanoketenimine (top, right), inspired by [5].
The anion [C(CN)3]− usually coordinates with its terminal nitrogen atoms, especially with hard cations according to the Pearson HSAB (hard and soft acids and bases) concept [6], but a real contribution at the lone-pair electrons of the central carbon atom occurs with soft, polarizable, low-charged cations (e.g., Ag+ in Ag[C(CN)3] [7,8,9]), particularly when they also have some lone-pair activity. The unique crystal structure of Tl[C(CN)3] [10] with Tl+ as a lone-pair cation (6s2 versus 6sp or 6p2) compared to those with the alkali metals A[C(CN)3] (A = Li–Cs) [11,12,13,14,15] may serve as the best example. Even for the isotypic crystal structures of Ca[C(CN)3]2 [16,17] and Cd[C(CN)3]2 [18], there are some interesting central carbon-atom contributions.
Compounds with the trivalent rare-earth metals (RE) come as hydrates from aqueous solution, for instance those with the compositions RE[C(CN)3]3(H2O)2 (RE = Y and Tb–Lu), RE[C(CN)3]3(H2O)3 (RE = Sc), RE[C(CN)3]3(H2O)4 (RE = La–Nd, Sm–Tb) and RE[C(CN)3]3(H2O)5 (RE = La–Nd) [14,19]. Together with alkali metals, water-poor hydrates are formed again, e.g., KLa[(C(CN)3]4(H2O) [20] and KRE[C(CN)3]4(H2O) (RE = La–Nd, Sm–Gd) [14], but only a few representatives can be obtained in anhydrous form, namely KRE[C(CN)3]4 (RE = Tb–Lu) [14].
Since lanthanoid compounds always have potential as phosphor materials but water-containing ones often suffer from vibrational luminescence quenching, we were aiming at their pseudo-ternary anhydrous tricyanomethanides. The unique [C(CN)3]− ligands with their conjugated 4c–5e π-system might even serve as an antenna for necessary energy-transfer processes. Moreover, the empirical formula KLn[C(CN)3]4 with trivalent lanthanoids (Ln3+), which typically shows 4f–4f transitions, could mimic a scenario for a Eu2+-containing one formula unit (Eu[C(CN)3]2), where divalent europium should be able to replace both monovalent K+ and trivalent Ln3+ due to their similar ionic radii; much more efficient luminescence based on 4f–5d transitions leaps into view.
2. Methods and Experimental
2.1. Source of Material
Ag[C(CN)3] was synthesized by blending an aqueous solution containing 1.13 g (10 mmol) of Na[C(CN)3] (>98%, TCI, Eschborn, Germany) with an aqueous solution of 2 g (12 mmol) Ag[NO3] (≥99%, Sigma-Aldrich, St. Louis, MO, USA). The resulting off-white precipitate was washed with deionized water and dried using an aspirator. A total of 340 mg (3 mmol) of Ag[C(CN)3] was added to 5 mL of demineralized water containing 245 mg (1 mmol) YbCl3 (>98%, ChemPur, Karlsruhe, Germany). To this preparation, 1 mmol of either Na[C(CN)3] (113 mg) or K[C(CN)3] (129.1 mg, 96%, powder, Alfa Aesar, Ward Hill, MA, USA) was added. After stirring the respective mixture for six hours, the resulting solution was filtered to remove the precipitated AgCl and the surplus Ag[C(CN)3]. The water was allowed to evaporate under ambient temperatures and atmospheric conditions. After a few days, colorless and transparent irregular crystals of the two title compounds were identified as the sole products in a quantitative yield considering the employed amount of YbCl3. Without the addition of A[C(CN)3] (A = Na or K), Yb[C(CN)3]3 crystallizes as dihydrate [14] (Yb[C(CN)3]3(H2O)2: orthorhombic, P212121; a = 1007.15(7) pm, b = 1171.57(8) pm, c = 1412.80(9) pm) [21]. However, when Na[C(CN)3] reacts with Ag[NO3] in aqueous slurry to form Ag[C(CN)3], traces of Na[C(CN)3] always remain, leading to the formation of NaYb[C(CN)3]4 as a by-product during the synthesis of Yb[C(CN)3](H2O)2. The latter can be completely converted to NaYb[C(CN)3]4 in a phase-pure manner with an excess of Na[C(CN)3] (Figure 2), while the product of the attempted synthesis of KYb[C(CN)3]4 always contains a share of NaYb[C(CN)3]4 (Figure 3). What is remarkable here, at first glance, is that no mixed crystals are formed in which the alkali-metal position is occupied by both sodium and potassium, but instead, despite the very similar crystal structures, both phases occur separately. On the other hand, this is in accord with the Shannon radii for Na+ (99 pm for C.N. = 4) and K+ (137 pm for C.N. = 4), so this might not come as a surprise [22].
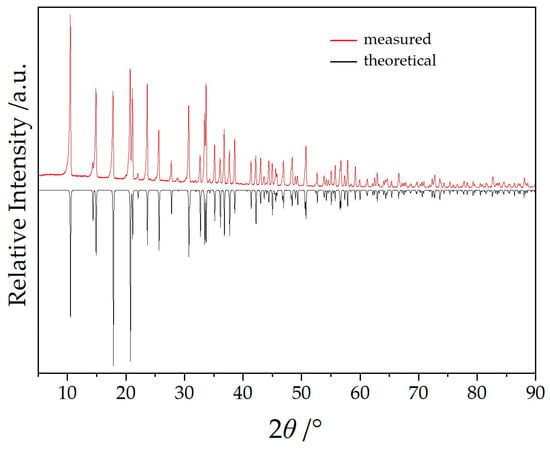
Figure 2.
Powder X-ray diffractogram of phase-pure NaYb[C(CN)3]4.
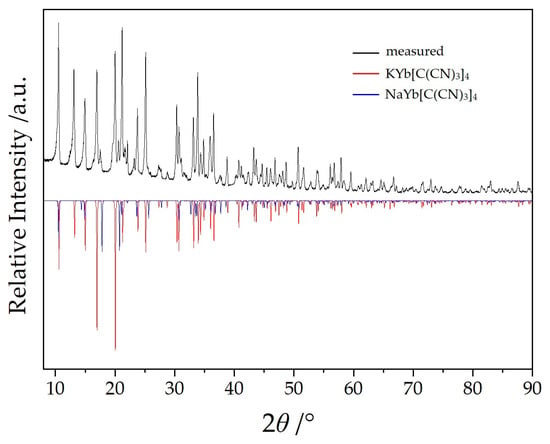
Figure 3.
Powder X-ray diffractogram of KYb[C(CN)3]4 with NaYb[C(CN)3]4 as minority phase.
2.2. Crystallographic Studies
Single-crystal selection took place with the help of a polarization microscope and suitable crystals were sealed into a thin-walled glass capillary. Intensity data sets for NaYb[C(CN)3] were collected with a Stoe Stadivari diffractometer (Ag-Kα radiation: λ = 56.08 pm) and for KYb[C(CN)3] with a Stoe Ipds (Mo-Kα radiation: λ = 71.07 pm). The crystallographic data are listed in Table 1 and fractional atomic coordinates and equivalent isotropic displacement coefficients are shown in Table 2. The powder samples of NaYb[C(CN)3]4 (Figure 2) and KYb[C(CN)3]4 (Figure 3) were measured in reflection geometry on a Rigaku SmartLab X-ray powder diffractometer using Cu-Kα1 radiation.

Table 1.
Crystallographic data for NaYb[C(CN)3]4 and KYb[C(CN)3]4 with their determination.

Table 2.
Atomic positions and equivalent isotropic displacement parameters for AYb[C(CN)3]4 (A = Na and K).
2.3. Raman Spectroscopy
Raman spectroscopy was performed with the single crystal used for the X-ray measurements on a XploRA spectrometer (Horiba, Kyoto, Japan) with 25 mW, excitation line at λ = 638 nm (red LASER).
2.4. Thermal Analysis
The TG/DTA curves of NaYb[C(CN)3]4 and KYb[C(CN)3]4 were recorded using a Netzsch STA409 with corundum crucibles in an argon atmosphere and a heating rate of 10 °C/min−1.
3. Results and Discussion
3.1. Crystal Structures of NaYb[C(CN)3]4 and KYb[C(CN)3]4
NaYb[C(CN)3]4 crystallizes in the tetragonal space group P4/nnc (no. 126, origin choice 2 with origin at , 1/4, 1/4, 1/4 away from 422) with the lattice parameters a = 1188.37 (9) and c = 1232.41 (9) pm (c/a = 1.037), whereas KYb[C(CN)3]4 prefers P4/nbm (no. 125, origin choice 2 with origin at 2/m, 1/4, 1/4, 0 away from 422) with the parameters a = 1179.26 (9) and c = 668.73 (5) pm (c/a = 0.567 = 0.5 × 1.134). Thus, for both structures, the centrosymmetric setting was chosen, since they share the same structural elements and have a surprising number of similarities. They both contain a single crystallographically independent star-shaped [C(CN)3]− anion (Figure 4) with almost identical C–C (139–142 pm for NaYb[C(CN)3]4 and 141 pm for KYb[C(CN)3]4) and C–N distances (114–115 pm) for both (Table 3). Each anion has contact with one A+ and two Yb3+ cations, which graft to its three terminal nitrogen atoms (Figure 2). The Yb–N–C angles of 171° for NaYb[C(CN)3]4 and 172° for KYb[C(CN)3]4 deviate less from linearity (180°) than the Na–N–C angle of 155° and the K–N–C angle of 158°.
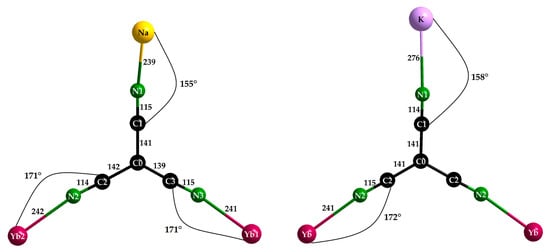
Figure 4.
Molecular structure and coordination behavior of the [C(CN)3]− anions in NaYb[C(CN)3]4 (left) and KYb[C(CN)3]4 (right); all distances are in pm and all angles are in °.

Table 3.
Selected interatomic distances (d/pm) for NaYb[C(CN)3]4 (left) and KYb[C(CN)3]4 (right).
Interestingly, in NaYb[C(CN)3]4 there are two different positions for the Yb3+ cations, whereas there is only one in KYb[C(CN)3]4. For magnetically interesting interactions, the shortest Yb3+···Yb3+ contacts (616 and 669 pm, respectively) seem to be much too long. However, the Yb3+ environment with eight nitrogen atoms from eight different [C(CN)3]− anions as a square antiprism is almost identical (site symmetry 422 in all three cases), and even the Yb–N distances appear equal within the error tolerance at 241–242 pm (Figure 5 and Table 3). These square antiprisms [YbN8] form a network that can be described using the Niggli formula {(Yb[C(CN)3]8/2)−}, with the A+ cations embedded in suitable vacancies apt to provide them [AN4] tetrahedra as first coordination spheres (Figure 6).
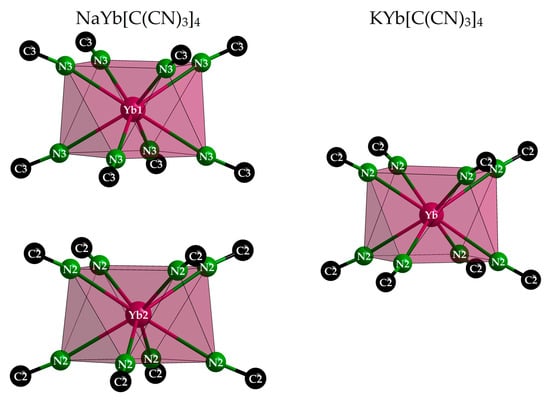
Figure 5.
Square antiprisms [YbN8] with 422 symmetry in the tetragonal crystal structures of NaYb[C(CN)3]4 (left) and KYb[C(CN)3]4 (right). The [C(CN)3]− anions are reduced to their Nn≡Cn parts for clarity reasons.
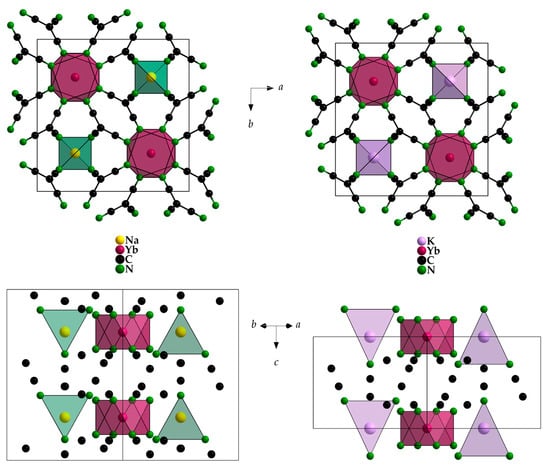
Figure 6.
Unit-cell content of NaYb[C(CN)3]4 (left) and KYb[C(CN)3]4 (right) with views along [001] (top) and [110] (bottom).
Both alkali-metal cations surround each other tetrahedrally with four [C(CN)3]− anions and bonding takes place via the terminal nitrogen atoms again. Not surprisingly, the N–A distances of d(N–Na) = 239 pm and d(N–K) = 276 pm are clearly different due to the individual ionic radii of the two alkali metals (ri(Na+) = 100 pm, ri(K+) = 150 pm) [22]. This becomes more interesting with a look at the second coordination sphere (Figure 7). In each case, four further [C(CN)3]− anions interact with the alkali-metal cations via their central C0 atoms and also form a tetrahedron around the central particles, whereby these distances are almost the same in both compounds, with a difference of 6 pm with d(Na···C0) = 4 × 361 pm or d(K···C0) = 4 × 367 pm, while there is a difference of 27 pm in their first nitrogen coordination spheres. ECoN calculations for the Effective Coordination Number [23] of both alkali-metal cations (A+) have been carried out with the following restrictions: (a) the radii for the cyanide part (Cn≡Nn) of each tricyanomethanide anion were fixed at 95 pm for Cn and Nn because of the 190 pm ionic radius of a cyanide anion ([CN]−) [24], (b) the radii of the involved cations were fixed at 100 pm for both Na+ and Yb3+, but at 150 pm for K+ [24], (c) the target was to reach an ECoN close to one for the N1 contribution for Na+ and K+, respectively. Astoundingly, these targets could be reached with ECoN (from N1) = 1.02 versus ECoN (from C0) = 0.01 for NaYb[C(CN)3]4 for r(C0) = 120 pm with no contribution from C0 for NaYb[C(CN)3]4, as well as ECoN (from N1) = 1.01 and ECoN (from C0) = 0.99) in the case of KYb[C(CN)3]4 for r(C0) = 175 pm under otherwise identical conditions. These meaningless numbers just suggest that in this case (for K+) the whole (C0)C3-star (Figure 7) acts as a ligand with its full 4c–5e π-system, what Srinivasan, Tragl and Meyer have called “a shielded potassium ion” in their KLa[C(CN)3]4 · H2O structure [20]. Despite a space-group change from P4/n for this hydrate (a ≈ 1238 pm, c ≈ 657 pm, c/a ≈ 0.53) to P4/nbm for KYb[C(CN)3]4 (a ≈ 1179 pm, c ≈ 669 pm, c/a ≈ 0.57) occurring, the crystal structure basically remains the same. Only the capped square antiprism around La3+ (C.N. = 9, 8 × N + 1 × O from H2O) turns into an uncapped one around Yb3+ (C.N. = 8, 8 × N), which is accompanied with an expected decrease in the a-axis due to the lanthanoid contraction, as well as an unexpected decrease in the c-axis, which is well reflected by the dissimilar c/a-values. A comparison of these naïve assessments for the NaYb[C(CN)3]4-KYb[C(CN)3]4 pair leads to the impressive result that Na+ (site symmetry: 222) is satisfied with its fourfold nitrogen coordination, while K+ (site symmetry: 2m) reaches a comfortable 4 + 4 coordination with almost equal parts of N1 (4×) and C0 (4×), as examples of structure-intrinsic agostic sp2-C-p2 recognition.
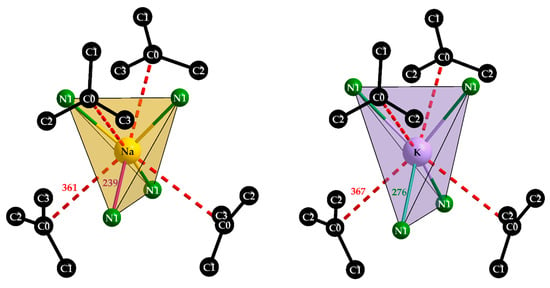
Figure 7.
The tetrahedral environment [NaN4] and [KN4] of the alkali-metal cations Na+ (left) and K+ (right) with their secondary contacts to the carbon centers (C0) of the next unbonded [C(CN)3]− units.
The program POLYNATOR [25] verifies the shape of the polyhedra as disphenoids for Na+ and K+ on the one hand and, on the other hand, as square antiprisms for Yb3+ in all cases. Heterocubane-like arrangements for C.N. = 4 + 4 (N1)4(C0)4 around K+ with 2m symmetry and Na+ with 222 symmetry complete the shielded coordination spheres [20] for both alkali-metal cations. Noteworthy to mention is that C0 exhibits not even the shortest contact with Na+ with 361 pm, since C1 comes even closer (346 pm), in contrast to the case of K+ (367 pm to C0 vs. 384 pm to C1).
The comparison with the A–N distances in Na[C(CN)3] (235–264 pm for C.N.(Na+) = 6) [12] and K[C(CN)3] (284–294 pm for C.N.(K+) = 7) [12], where no agostic sp2-C-p2 assistance is necessary to reach high coordination numbers, reveals larger values than those for both NaYb[C(CN)3]4 (239 pm, 4×) and KYb[C(CN)3]4 (276 pm, 4×), supporting the idea that there is some need for them in the latter pseudo-ternaries, at least for the potassium compound. The Yb–N distances in Yb[C(CN)3]3(H2O)2 (239–246 pm, 6×) [14,22] agree quite well with those (241–242 pm for C.N.(Yb3+) = 8) in both NaYb[C(CN)3]4 and KYb[C(CN)3]4, especially if one keeps in mind that two extra Yb–O contacts (227 and 228 pm) with oxygen atoms of water molecules during hydration come on top to realize C.N.(Yb3+) = 8 in the dihydrate as well.
Neglecting the light atoms carbon and nitrogen, a smaller tetragonal cell with a’ = 841.08 (7) pm and c’ = 616.54 (5) pm was found utilizing Mo-Kα radiation from a κ-CCD diffractometer from Bruker-Nonius for NaYb[C(CN)3]4. A subsequent refinement in the space group P4/mmm (no. 123) yields only one position for the Yb3+ and one for the Na+ cations, too. The light atoms carbon and nitrogen were also found and refined, but the [C(CN)3]− units coincide in this too-small unit cell, which is related via a 1/ relationship to the a- and b-axes and a halved c-axis with the real one, and would have to be treated to induce under-occupation of these sites to avoid an eightfold coordination for Na+ instead of a fourfold one and a formal condensation of the discrete [C(CN)3]− anions to fictive “(NC)2NC–CN(CN)2” units with bizarre interatomic N–C and C–C distances. Ag-Kα radiation of a STADIVARI STOE diffractometer was used to solve this problem, which led to an undoubtedly correct unit cell with ·a’ = ·b’ and 2·c’.
The similarity of several atomic coordinates in Table 2 (e.g., x/a(C2) ≈ y/b(C3), x/a(C3) ≈ y/b(C2), z/c(C2) ≈ −z/c(C3) and x/a(N2) ≈ x/a(N3); y/b(N2) ≈ y/b(N3); z/c for N2 ≈ z/c + 1/2 for N3) implies some higher space-group symmetry for the crystal structure of NaYb[C(CN)3]4, despite being P4/nnc already. Coding like P4/n2/n2/c → P4/n2/b2/m (2c’ = c) represents a minimal non-isomorphous supergroup of P4/nbm, and the couple NaYb[C(CN)3]4/KYb[C(CN)3]4 fulfills this requirement perfectly. Higher symmetry would either presuppose body-centering, with the inverse chloroscheelite-type arrangement of LiGdCl4 [26] with C.N.(Li+) = 4 and C.N.(Gd3+) = 8 standing for this analogy, in spite of the adoption of a low-Laue-symmetry space group (I41/a11, no. 88), or, with preservation of the high Laue symmetry, Y[PS4] [27], which could serve as an example according to PYS4 even in the highest tetragonal space group (no. 142), namely I41/a2/c2/d, which still offers two independent Y3+ cations in sulfur atoms with eightfold coordination from discrete [PS4]3− tetrahedra (Y1 in 8a with 11 symmetry and Y2 in 8b with 222 symmetry) [27].
3.2. Raman Spectrum of NaYb[C(CN)3]4
The frequencies and intensities obtained from the well-resolved Raman spectrum of NaY[C(CN)3]4 (Figure 8 and Table 4) agree well with those of other salt-like tricyanomethanides with high point symmetry, e.g., Rb[C(CN)3] and Cs[C(CN)3], indicating the presence of the tricyanomethanide anion [C(CN)3]−. The splitting degree of the ν(C≡N) stretching mode can be taken as a good indicator for the bonding situation or the point symmetry of the tricyanomethanide anion—the higher the number of different ν(C≡N) stretching modes, the larger the differences in the ‘branches‘ in terms of bonding and coordination patterns exhibiting deviations from the ideal D3h point symmetry. The good resolution of the spectrum even allows us to identify the weak band at 2159 cm−1 as the ν(13C≡14N) stretching mode [28].
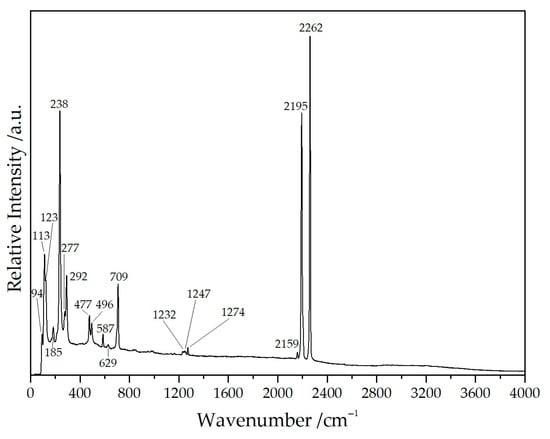
Figure 8.
Raman spectrum of NaYb[C(CN)3]4 recorded with an excitation energy of λ = 638 nm.

Table 4.
Raman data for NaYb[C(CN)3]4 and Cs[C(CN)3] [15] as reference; wavenumbers are given in cm−1 (intensity coding: vs = very strong, s = strong, m = medium, w = weak, vw = very weak, sh = shoulder).
Moreover, the flat region between 2400 and 4000 cm−1 proves the absence of any crystal-water molecules, which are present in Yb[C(CN)3]3(H2O)2 [14,21].
3.3. Thermal Analysis of NaYb[C(CN)3]4 and KYb[C(CN)3]4
As can be seen in Figure 9, NaYb[C(CN)3]4 started to decompose at approximately 400 °C, leaving a grayish-black residue behind. Low quality powder X-ray diffractograms revealed this residue to be almost amorphous, but its black color indicated the formation of carbon. A very similar picture emerges in Figure 10, where a mixture of both compounds (KYb[C(CN)3]4 and NaYb[C(CN)3]4) was used for thermal treatment. Here, the decomposition began at 404 °C and, in addition, the mass steadily decreased, since parts of the decomposition products transitioned into the gas phase (cyanogen, (CN)2). We therefore assume the following decomposition reaction:
as we could detect at least the strongest reflections of alkali-metal cyanides (A[CN] [29,30]) and ytterbium(III) carbodiimide (Yb2[CN2]3) [31].
2 AYb[C(CN)3]4 → 2 A[CN] + Yb2[CN2]3 + 11 C + 8 (CN)2 (A = Na and K),
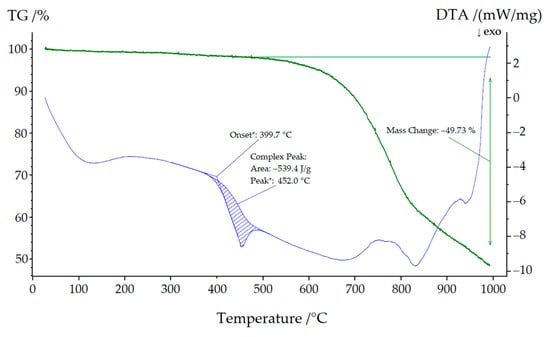
Figure 9.
Thermal analysis of NaYb[C(CN)3]4.
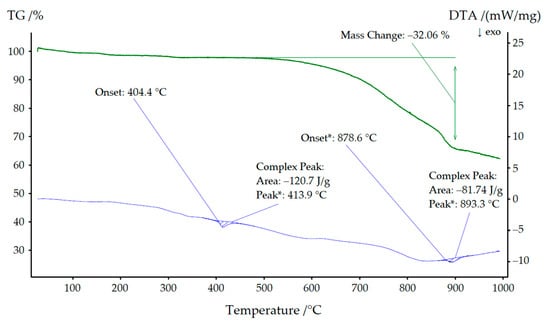
Figure 10.
Thermal analysis of the KYb[C(CN)3]4 with NaYb[C(CN)3]4 as minority phase.
For KEu[C(CN)3]4(H2O) and KTb[C(CN)3]4, the decomposition temperatures were significantly lower (534 °C and 501 °C, respectively) [14].
Similarly, a very weak signal was detectable at 350 °C in both thermograms. A powder X-ray diffractogram of the sodium compound was of very poor quality but nevertheless showed lattice parameters (a ≈ 1188 pm and c ≈ 616 pm), which suggests that an irreversible phase transformation into the corresponding KYb[C(CN)3]4 structure occurred.
4. Conclusions and Outlook
By using the well-established Ag[C(CN)3] metathesis method, NaYb[C(CN)3]4 and KYb[C(CN)3]4 were obtained from aqueous brine. Their crystal structures are similar, but not isotypic, showing Yb3+ in square antiprismatic, and the alkali-metal cation A+ in disphenoidal, nitrogen coordination. Owing to the fact that the crystal structures of both NaYb[C(CN)3]4 and KYb[C(CN)3]4 show two completely different coordination geometries for the A+ and Yb3+ cations, it seems improbable that they could serve as plausible structural environments for a potential Eu[C(CN)3]2 arrangement describing europium(II) tricyanomethanide, where Eu2+ would need to replace both A+ and Yb3+ simultaneously. The idea to aim at anhydrous rather than hydrated pseudo-ternary ALn[C(CN)3]4 compounds seems to be proven by our preliminary finding that tetragonal NaEu[C(CN)3]4 · H2O (space group: P4/n) shows no luminescence at all because of quenching processes, while tetragonal NaTb[C(CN)3]4 (space group: P4/nbm) shows a strong green one. We will report on this in a separate publication in the near future.
Author Contributions
Conceptualization, R.J.C.L., C.W. and T.S.; methodology, R.J.C.L., G.M., R.U.S., A.I.A.E., F.L. and O.R.; software, R.J.C.L., G.M., R.U.S., A.I.A.E., F.L. and O.R.; validation, R.J.C.L., G.M., R.U.S., A.I.A.E., F.L. and O.R.; formal analysis, R.J.C.L., G.M., R.U.S., A.I.A.E., F.L. and O.R.; investigation, R.J.C.L., G.M., R.U.S., A.I.A.E., F.L. and O.R.; resources, C.W. and T.S.; data curation, R.J.C.L., C.W. and T.S.; writing—original draft preparation, R.J.C.L., C.W. and T.S.; writing—review and editing, R.J.C.L., C.W. and T.S.; visualization, R.J.C.L.; supervision, C.W. and T.S.; project administration, C.W. and T.S.; funding acquisition, C.W. and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available from the authors upon request.
Acknowledgments
We want to thank Bertold Rasche and Rainer Niewa (Institute for Inorganic Chemistry, University of Stuttgart) for their miscellaneous support.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Schmidtmann, H. Über einige Derivate des Malonitrils. Ber. Chem. Ges. 1896, 29, 1168–1175. [Google Scholar] [CrossRef]
- Dworkin, A.; Naumann, P.; Seigfred, C.; Karty, J.M.; Mo, J. Y-aromaticity: Why is the trimethylenemethane dication more stable than the butadienyl dication? J. Org. Chem. 2005, 70, 7605–7616. [Google Scholar] [CrossRef] [PubMed]
- Golub, A.M. (Ed.) Chemie der Pseudohalogenide; Dr. Alfred Hüthig Verlag: Heidelberg, Germany, 1979. [Google Scholar]
- Soltner, T.; Häusler, J.; Kornath, A.J. The Existence of Tricyanomethane. Angew. Chem. Int. Ed. 2015, 54, 13775–13776. [Google Scholar] [CrossRef]
- Surkau, J.; Bresien, J.; Michalik, D.; Schulz, A. Tricyanomethane or Dicyanoketenimine—Silylation makes the Difference. Angew. Chem. Int. Ed. 2024, 63, e202413565. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical Hardness; Wiley-VCH: Weinheim, Germany; New York, NY, USA, 1997. [Google Scholar]
- Reckeweg, O.; Schulz, A.; DiSalvo, F.J. Synthesis, single-crystal structure determination and Raman spectrum of Cu[C(CN)3]2 · 2 NH3. Z. Naturforsch. 2015, 70b, 177–181. [Google Scholar] [CrossRef]
- Konnert, J.; Britton, D. The crystal structure of AgC(CN)3. Inorg. Chem. 1966, 5, 1193–1196. [Google Scholar] [CrossRef]
- Batten, S.R.; Hoskins, B.F.; Robson, R. Structures of [Ag(tcm)], [Ag(tcm)](phz)1/2 and [Ag(tcm)](pyz) (Tcm = Tricyanomethanide, C(CN)3−, Phz = Phenazine, Pyz = Pyrazine). New J. Chem. 1998, 22, 173–175. [Google Scholar] [CrossRef]
- Reckeweg, O.; Lissner, F.; Heitkämper, J.; Kästner, J.; Schleid, T. The unexpected crystal structure of thallium(I) tricyanomethanide Tl[C(CN)3]. Z. Naturforsch. 2022, 77b, 237–243. [Google Scholar] [CrossRef]
- Reckeweg, O.; Conrad, M.; Schulz, A.; DiSalvo, F.J.; Schleid, T. Synthesis, vibrational spectra and single crystal structure determination of lithiumtricyanomethanide Li[C(CN)3]. Z. Naturforsch. 2018, 73b, 225–229. [Google Scholar] [CrossRef]
- Reckeweg, O.; Thiebes, Y.; Niewa, R. Revisiting Na[C(CN)3]—Refinement of the crystal structure from X-ray powder diffraction data, the Raman and IR spectra. Z. Naturforsch. 2025, 80b, 49–54. [Google Scholar] [CrossRef]
- Reckeweg, O.; DiSalvo, F.J. Two lithium tricyanomethanide compounds: Syntheses and single-crystal structure determination of LiK[C(CN)3]2 and Li[C(CN)3] · 1/2 (H3C)2CO. Z. Naturforsch. 2015, 70b, 191–196. [Google Scholar] [CrossRef]
- Montana, G. Neue Alkalimetall- und Lanthanidtricyanomethanide—Darstellung, Struktur, Spektroskopie. Ph.D. Thesis, University of Siegen, Siegen, Germany, 2011. [Google Scholar]
- Reckeweg, O.; Schulz, A.; DiSalvo, F.J. Syntheses, single-crystal structure determination, and Raman spectra of Rb[C(CN)3] and Cs[C(CN)3]. Z. Naturforsch. 2015, 70b, 151–154. [Google Scholar] [CrossRef]
- Bessler, K.E.; Gatto, C.C.; Romualdo, L.L.; Ellena, J.A.; de Sales, M.J.A. Pseudohalides of Main Group Metals Tricyanomethanides—The Crystal Structures of Calcium and Barium Tricyanomethaniode. Main. Group. Met. Chem. 2007, 30, 135–142. [Google Scholar] [CrossRef]
- Bessler, K.E.; Gatto, C.C.; Romualdo, L.L.; Ellena, J.A.; de Sales, M.J.A. Pseudohalides of Main Group Metals Tricyanomethanides—Crystal Structures and Thermal Behavior of Alkaline Earth Tricyanomethanides. Z. Naturforsch. 2018, 73b, 225–229. [Google Scholar]
- Majumdar, D.; Philip, J.E.; Tüzün, B.; Roy, S. Synthesis, characterization, crystallographic aspects, Fukui function, and photodynamic antifungal chemotherapy investigation of Cd(II)-tricyanomethanide coordination polymer: Insights from DFT. Inorg. Chem. Commun. 2023, 155, 111057. [Google Scholar] [CrossRef]
- Reckeweg, O.; Locke, R.J.C.; Lissner, F.; Schleid, T. Group III-Element Tricyanomethanide Hydrates: Synthesis and Crystal Structures of Sc[C(CN)3]3(H2O)3, Y[C(CN)3]3(H2O)2 and La[C(CN)3]3(H2O)4. Z. Naturforsch. 2025, 80b, 249–258. [Google Scholar] [CrossRef]
- Srinivasan, R.; Tragl, S.; Meyer, H.-J. Synthesis and Crystal Structure of KLa[C(CN)3]4 · H2O Containing a Shielded Potassium Ion. Z. Anorg. Allg. Chem. 2005, 631, 1075–1078. [Google Scholar] [CrossRef]
- Locke, R.J.C.; Reckeweg, O.; Schleid, T. On the Dihydrates of the Heavy Lanthanoid Tristricyanomethanides Ln[C(CN)3]3 · 2 H2O (Ln = Tb − Lu). Z. Naturforsch. 2026, 81b. to be submitted. [Google Scholar]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1975, A32, 751–767. [Google Scholar] [CrossRef]
- Hoppe, R. Effective coordination numbers (ECoN) and mean fictive ionic radii (MEFIR). Z. Kristallogr. 1979, 150, 23–52. [Google Scholar] [CrossRef]
- Morris, D.F.C. Crystal radius of the cyanide ion. Acta Crystallogr. 1961, 14, 547–548. [Google Scholar] [CrossRef]
- Link, L.; Niewa, R. Polynator: A tool to identify and quantitatively evaluate polyhedra and other shapes in crystal structures. J. Appl. Crystallogr. 2023, 56, 1855–1864. [Google Scholar] [CrossRef]
- Meyer, G. GdLiCl4, ein inverser Chloroscheelit. Z. Anorg. Allg. Chem. 1984, 511, 193–200. [Google Scholar] [CrossRef]
- Komm, T.; Schleid, T. Y[PS4]: An Unusual ABX4 Compound with a PtS-Related Crystal Structure. Z. Anorg. Allg. Chem. 2004, 630, 1544–1546. [Google Scholar] [CrossRef]
- Weidlein, J.; Müller, U.; Dehnicke, K. Schwingungsfrequenzen I—Hauptgruppenelemente, 1st ed.; Auflage, Georg-Thieme-Verlag: Stuttgart, Germany; New York, NY, USA, 1981. [Google Scholar]
- Verweel, H.J.; Bijvoet, J.M. Die Kristallstruktur des NaCN. Z. Kristallogr. 1938, 100, 201–207. [Google Scholar] [CrossRef]
- Bijvoet, J.M.; Lely, J.A. Eine orthorhombische Modifikation des Kaliumcyanids. Rec. Trav. Chim. Pays-Bas 1940, 59, 908–912. [Google Scholar] [CrossRef]
- Reckeweg, O.; Schleid, T.; DiSalvo, F.J. Synthesis, crystal structure and optical spectra of Yb2[CN2]3. Z. Naturforsch. 2007, 62b, 658–662. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).