Abstract
This review summarizes the recent developments in titanium suboxide (TSO) doping and the application of doped materials in pollutant degradation and electrochemistry. Doping is mainly limited to transition and rare-earth metals, with some exceptions, of similar ionic radii and charge, that can replace Ti ions in TSO without too much disturbance to the lattice. Consequently, doping is limited to below 10 at%, which predominantly induces oxygen vacancy formation. Doping mechanisms are weighted, and their effect on conductivity, stability, and catalytic activity is overviewed. High-temperature H2 reduction of TiO2 is still the dominant preparation method, with carbothermal reduction and Ti reduction gaining ground due to safety and energy concerns. Doping predominantly increases the conductivity 2–5 times, while the stability can be both improved or worsened, depending on the size and charge of the doping ion. Electrochemical oxidation, at positive overpotentials, of per- and polyfluoroalkyl substances (PFAS), antibiotics, and other water pollutants, is the main avenue of application. Doping almost exclusively leads to complete selected pollutant degradation and improvement of the pristine TSO, which is summarized in detail. New niche applications of peroxide, hydrogen, and chlorine production are also viable on doped TSO and are touched upon. Complementing experimental results are theoretical calculations, and we give an overview of density functional theory (DFT) results of transition metal-doped TSOs, identifying active centers, degradation trends, and potential new doping candidates.
1. Introduction
Titanium oxides represent a versatile class of inorganic materials with a wide range of applications. Among them, titanium dioxide (TiO2) is the most thermodynamically stable phase with distinguished chemical stability, biocompatibility, abundance, and low cost [1,2,3]. TiO2 finds applications in diverse fields, such as reducing the toxicity of dyes and pharmaceutical drugs [4], wastewater treatment [5,6], sensing [7], agriculture [8], air purification [9], and solar cells [10]. Owing to its semiconducting nature, TiO2 is extensively utilized in photocatalysis [11,12].
Under reductive conditions or controlled thermal treatment in reducing atmospheres, titanium dioxide can be transformed into a series of oxygen-deficient, non-stoichiometric titanium suboxides known as Magnéli phases (MPs), with the general chemical formula TinO2n−1, where n typically ranges from 3 to 10 [13,14,15,16]. These suboxides, often commercially recognized under the trade name Ebonex® [17], have gained researchers’ attention for their unique physicochemical properties [18,19].
While stoichiometric TiO2 is a wide-bandgap semiconductor with extremely low electrical conductivity in ambient conditions (10−13 to 10−14 S/cm), Magnéli phases exhibit semi-metallic or metallic-like behavior [15,20]. These oxygen-deficient titanium suboxides (TSOs) possess significantly enhanced electrical conductivity, often comparable to that of carbon, and display excellent chemical and thermal stability, making them highly attractive for a range of energy and environmental applications. The value of n strongly influences the electrical conductivity of Magnéli phases, reaching its maximum for n = 3–5, while higher values are associated with a progressive reduction in conductivity [15,21]. Among these, Ti4O7 stands out due to its exceptionally high electrical conductivity (reaching approximately 1.6 × 103 S/cm at ambient temperature), comparable to graphite (∼1000 S/cm) [13]. Consequently, Magnéli phases, especially Ti4O7, are being increasingly investigated as electrode materials for electrochemical applications due to their superior conductivity and enhanced thermal and chemical stability [15].
Reduced titanium oxides have been investigated for use in various applications, including electrode materials [18,22,23], thermoelectric devices [24,25], and photocatalysts [16].
The crystal structure of the Ti–O Magnéli phase is primarily composed of Ti–O octahedra similar to those in rutile TiO2, featuring regularly arranged planar oxygen vacancies occurring every n layers of Ti–O octahedra as a result of missing oxygen atoms [26].
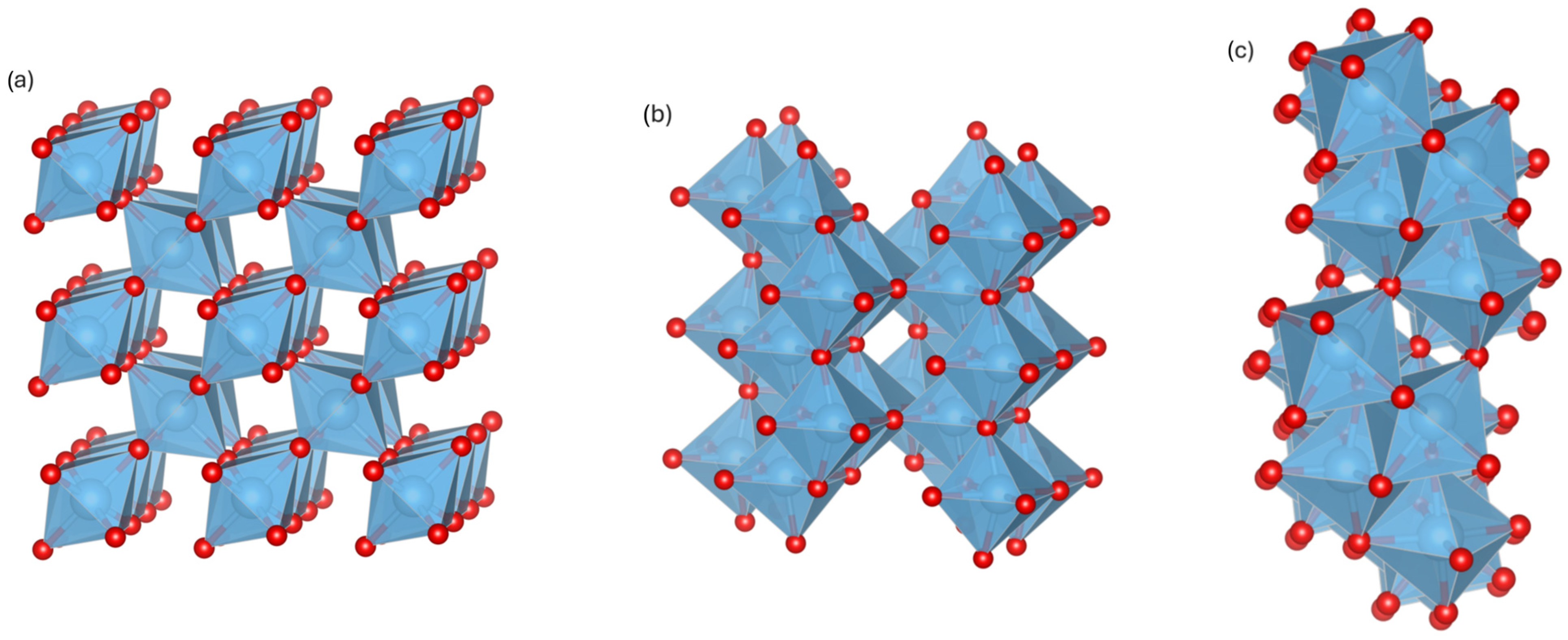
Magnéli and co-workers used XRD to study transition-metal oxides (Ti, V, Mo, W) and confirmed the homologous series TinO2n−1 (n = 2, 3, …), all derived from the rutile structure. Wadsley later introduced the concept of crystallographic shear (CS) structures, formed by block division, shear translation, and removal of overlaps. For TinO2n−1 (n = 2–10), the CS plane and vector were identified as (121) and in rutile structure, while for n > 10, Bursill et al. [27] reported a shift to the (132) rutile plane [24]. As the n value in MPs decreases, the interspacing between the CS plane contracts, leading to an increase in oxygen-vacancy density and an increase in the number of Ti3+ centers. The vacancy-derived Ti(d) defect states are located close to the conduction-band minimum and serve as intrinsic dopants, facilitating electron delocalization across the CS plane structure and thus improving the conductivity of MPs with lower n [28]. Half of the oxygen vacancies corresponds to the concentration of delocalized carriers with vacancy density (and n) directly affecting electrical conductivity [29]. Ti4O7 is the first member and possesses the highest electrical conductivity among the MP family of titanium suboxides. For example, Ti3O5 does not classify as an MP because of the lack of ordered shear planes in its crystal structure, preventing it from obtaining the same extensive vacancy-band conduction. Experiments and DFT+U studies on reduced Ti-suboxides demonstrate that the formation of Magnéli phases with an intermediate average Ti valence (3+/4+), particularly Ti4O7 with equal Ti3+/Ti4+ (Ti3.5+), results in fully delocalized d-electrons and metallic-like transport [20]. The crystal structure of Ti4O7 belongs to the triclinic P-1 space group. It can be described as rutile blocks four octahedra wide, separated by crystallographic shear planes. These blocks are shifted relative to one another, creating chains of edge-sharing TiO6 octahedra (Figure 1) [30].

Figure 1.
Crystal structure of original rutile (a) TiO2, (b) Magnéli Ti3O5, and (c) Magnéli Ti4O7. Red: oxygen atoms; blue: titanium atoms.
Magnéli phase TSOs can be synthesized either by heating TiO2 with metallic titanium in an inert atmosphere according to the following reaction:
or by reducing TiO2 at elevated temperatures using a reducing agent such as hydrogen gas, following the reaction
nTiO2 + H2 → TinO2n−1 + H2O
Upon reduction, rutile TiO2 undergoes a stepwise transformation through a series of MP TSOs, progressing as follows: TiO2 (tetragonal) → Ti9O17 (triclinic) → Ti8O15 (triclinic) → Ti6O11 (triclinic) → Ti5O9 (triclinic) → Ti4O7 (triclinic) → Ti3O5 (monoclinic) → Ti2O3 (trigonal) [31].
TiO2 is commonly used as the primary precursor due to its availability and low cost. The reduction typically requires temperatures above 1273 K and a reducing atmosphere, usually hydrogen [13,15]. The heat treatment induces defects in TiO2’s lattice, including oxygen vacancies, which alter the titanium-to-oxygen ratio, resulting in the formation of different suboxide phases [32]. Ti4O7 can be synthesized using a molten salt method, yielding rod-like particles as suggested by [26]. A bulk MP prepared by spark plasma sintering (SPS) shows high conductivity (over 950 S/cm), significantly higher than MPs from conventional routes. Another SPS route combined with sol-gel synthesis is applied for the preparation of MP nanocomposites (TinO2n−1), both as pristine nano-MPs (n = 3, 4, 5, 6, 8) with up to 300 m2/g, low resistivity, and thermal conductivity [33]. Zhang et al. [34] densified Ti4O7 powders with particle sizes of 1–2 μm nearly to full density at 900 °C under a 40 MPa axial pressure using SPS. However, even under vacuum during SPS, the Ti4O7 completely oxidized to Ti5O9. Yu et al. report the densification of Magnéli phase titanium suboxide powders using Flash SPS, aiming to preserve the original Ti4O7 phase and achieve a uniform microstructure in the sintered samples [35]. Hydrothermal reactor surface areas have a substantial influence on hydrothermal synthesis. Low-temperature (only 363 K) triclinic MP Ti6O11 was obtained with hierarchical morphology [36]. Additionally, Magnéli phases were obtained via hot-pressed sintering of single-phase submicron Ti4O7 powders [37], hot-pressed sintering TiO2 and Ti powder under a vacuum [38], and through high-pressure torsion [39].
MP materials are highly valued for their exceptional chemical inertness, durability, and resistance to corrosion in aggressive environments. For example, Ebonex® exhibits remarkable stability, with a projected half-life of 50 years in 4 M sulfuric acid at ambient temperature [15,40]. The rutile-type surface of MP ceramics serves as an advanced support for various catalytic coatings [15], while the addition of transition metals can lead to improved catalytic performance.
Doping is a powerful strategy for tailoring the properties of TinO2n−1 phases for specific applications. The incorporation of different dopants has been shown to alter the electrical and thermal properties, thereby enhancing photocatalytic, thermoelectric, and electrocatalytic performance. However, a dopant selection is often limited to size and favors high solubility of solutes in metal alloys. Dopants with high solubility reduce the risk of forming secondary phases, which are difficult to detect at low concentrations. Studies suggest that the low electronegativity difference between solute and host with similar coordination number and the atomic radii within ±15% in similar crystal structures should be followed, while relaxing the electronegativity constraint to not more than ±0.4 on the Pauling scale [41]. This refinement of potential dopant lists provides a basis for studying their influence on the transformations between TinO2n−1 and TiO2. However, although Pauling’s rules provide a useful framework for describing MP structures, their predictive capability may be limited. George et al. [42] reported that only a small fraction of metal oxides conform to these rules, indicating that more quantitative predictors of crystal stability should be taken into account.
Successful doping resulting in enhanced materials does not warrant better application performance; thus, doping methods are still under consideration by researchers, especially those oriented toward sustainable water treatment solutions. The emergence of Magnéli doping and its application prompted us to review this emerging niche briefly. Recently, several reviews on the Magnéli phase topic have been published [42,43,44,45]. However, this paper provides a review of the enhancements of doped Magnéli phases for environmental and energy applications.
2. Synthesis of Doped Magnéli Phases
The preparation of doped Magnéli phases requires controlled replacement of the dopant element into the lattice with concurrent oxygen vacancy formation. Traditionally, this is performed at high temperatures (1173–1473 K) using hydrogen, carbon, or titanium as reducing agents, as the most reliable approach for achieving homogeneous dopant distribution and stable phase formation. These conditions facilitate the diffusion of dopant ions into Ti sites and the generation of oxygen vacancies necessary for charge compensation. Doping is often achieved using a transition metal precursor, while recent studies investigated rare-earth dopants for MP structures (e.g., La, Ce, Nd, and Sm). These dopants influence the electronic structure and defect chemistry in MPs, as an alternative route for tuning their activity. Additionally, recent studies have demonstrated that solution-based methods, including solvothermal and hydrothermal reduction, can yield partially reduced TiO2 structures that evolve into doped Magnéli-like phases upon mild post-annealing. Such approaches enable better control of particle morphology, dopant valence states, and local defect concentration. However, achieving complete phase transformation and avoiding dopant segregation remain key challenges for low-temperature synthesis. A combined strategy that integrates low-temperature dopant incorporation with subsequent controlled annealing may therefore represent a promising route toward scalable, compositionally tunable Magnéli materials.
An elegant selection of dopants relies on their ionic radius, electronegativity, thermodynamic properties, and electronic environment. Metals with a coordination number of 6, a trivalent oxidation state, and a corundum-like crystal structure are good candidates for doping. Thus, Fe, V, and Cr, with ionic radius 60–75 pm (near the value for Ti (III/IV), 61–74 pm) and electronegativity 1.9–1.6, were the starting point for experimental studies. The substitution of Ti by other transition-metal dopants such as V or Ce is also governed by the balance between ionic radius compatibility and oxidation state stability. Vanadium, with a comparable ionic radius (V4+ = 72 pm) compared to Ti4+ (74 pm), can occupy Ti sites with minimal lattice distortion while promoting localized Ti3+ formation to preserve charge neutrality. In contrast, Fe3+ (60 pm) substitution induces more apparent lattice strain and facilitates the evolution of oxygen vacancies, acting as electron donors and enhancing electrical conductivity. Ce incorporation, due to its flexible Ce3+/Ce4+ redox couple and larger ionic radius (102 pm for Ce3+, 101 pm for Ce4+), introduces significant local distortion but contributes additional oxygen vacancy pathways through redox exchange. The dopant effects modify the Ti3+/Ti4+ ratio and the density of defect states near the Fermi level, which in turn influence charge transport and surface reactivity. Consequently, the electrochemical performance of doped Magnéli phases can be directly correlated to the interaction between dopant-induced lattice distortions, oxygen vacancy formation mechanisms, and changes in Ti valence distribution.
To resolve a mechanism for thermal oxidation and investigate the influence of doping, the thermal oxidation behavior in air of Ti4O7 doped with V, Cr, and Fe was investigated by English and Wilkinson [41] using thermogravimetry. Materials were prepared by high-temperature H2 reduction of dopant-containing TiO2. V- and Fe-doping improved the thermal stability of Ti4O7, while diffusion reaction models described the solid-state kinetics of oxidation. The estimated material lifetimes show a decrease with Cr doping, although the amount was kept at 2% despite its optimal radius and crystal structure. The MP electrodes were also synthesized by mechanically activating rutile with Ti and Nb, V, and Fe additives, followed by sintering in reducing or inert atmospheres up to 1353 K [21]. Mixed V/Ti Vn−xTixO2n−1 Magnéli phases were synthesized under vacuum, starting from commercial V, V2O5, and Ti2O3, as powders and single crystals grown by chemical vapor transport [46]. Ti incorporation minimally affects the vanadium oxide structure but suppresses its metal–insulator transitions. The structure and properties of the obtained material, such as the presence of crystalline phases and thermoelectric characteristics, will depend on the doping level. A series of reduced Ti1−xNbxO2−δ materials was synthesized by conventional solid-state reduction in a 10% H2 below 1480 K [47]. Compositions up to x = 0.01 contained mixed anorthic phases (TinO2n−1, 8 ≤ n ≤ 10) with additional Ti10O18 reflections, whereas the x = 0.02 sample showed rutile TiO2, and higher Nb concentrations (x = 0.04 and 0.08) stabilized the rutile phase exclusively. The optimum thermoelectric response was obtained at x = 0.01, with a ZT value of 0.023 at 380 K, which is almost 2.6 times higher compared to the undoped composition.
Several other elements have also been investigated as dopants. For example, Ga(III) and Mn(IV), but they exhibited drawbacks in terms of stability and feasibility [41,48].
Gardos tested Cu, Fe, Co, and Ni ions as dopants, introduced in the form of their stable oxides through a simple ball-milling, hot-pressing, and annealing process, but only the (Ti + Cu)O1.80 model mixture yielded the desired reaction. Hot-pressing anatase/CuO powders with subsequent heating in a 3% H2 atmosphere resulted in a portion of Cu entering the rutile-transformed lattice to form a titanium–copper crystallographic shear-induced MP [49]. The alloying effect of ZrO2 on the microstructure of MP was also studied. Magnéli contains periodic shear planes in its rutile matrix, and ZrO2 has a rutile structure—6 mol% of dopant enables homogeneous Zr distribution [50].
Ti4O7 powders with varying Ce doping levels can be synthesized via a wet-chemical route combined with hydrogen reduction. Ce is incorporated into the lattice exclusively as Ce3+, with a solubility limit below 9 at%. Electrical conductivity decreases with Ce doping due to reduced electronic states near the Fermi level [51]. Sn-doped MP was prepared by polymerization–calcination and surface-loading Sn onto MP via grinding–calcination. Doping induced higher conductivity, lattice distortion, and increased oxygen vacancies/Ti3+, while surface doping favors the 4e− pathway due to surface Sn–Ti interactions [52]. Table 1 summarizes the applications or the alterations in the structural and thermal characteristics of doped MP.

Table 1.
Summary of doped MP and their applications or alterations in structural/thermal features.
3. Electrocatalytic Applications
Due to their intrinsic conductivity and relative stability, doped Magnéli phases have found numerous applications in (electro)catalysis, particularly in pollutant degradation and other electrochemical fields.
Industrial wastewater often contains contaminants that necessitate pretreatment before being released into biological treatment systems and natural water bodies. Magnéli phase titanium oxides were doped with V, Fe, and Cr to assess their suitability for the electrochemical oxidation of industrial wastewater [56]. Conductivity increased slightly upon doping from 120 S/cm to between 300 and 380 S/cm. The V effect corresponds to one seen for Fe-doping, which outperforms Cr-dopingand pristine MP. Additionally, V and Fe improved the thermal stability of MP in air. However, Cr doping of MP decreased its oxidative stability, while V doping improved it and was therefore chosen for the testing of industrial wastewater degradation. V-doped MP achieved complete pollutant degradation and diminished chemical oxygen demand, which was attributed to its greater electrochemically active surface area and improved anodic stability, compared to pristine MP.
Pharmaceuticals often resist degradation, and electrochemical methods with suitable electrode materials are investigated in this area. Tao et al. [57] prepared Sm-doped Ti4O7 for anodic decomposition of sulfamethazine with three times higher degradation efficacy over pristine Ti4O7 and 91% overall degradation. Doping with Sm shifted the electro-generation of the hydroxyl radical to a more positive potential, increasing the electro-oxidation efficacy and effectiveness. Sn doping of Ti4O7 was prepared by Jia et al. [59] via high-temperature H2 reduction for wastewater antibiotic (tetracycline) degradation. At low dopant concentration (<1%), a tetracycline degradation of 92% was achieved after 2 h, an improvement upon 71% from the pristine Magnéli phase, and complete removal after 3 h. Kao [68] conducted La, Al, and Co doping of Ti4O7 for the degradation of pharmaceutics. Among the four studied drugs (cyclophosphamide, carbamazepine, sulfamethoxazole, and ibuprofen), cobalt-doping proved to be the most effective for almost complete sulfamethoxazole removal within 60 min. However, leaching of Co was evidenced, and the lifetime of the electrode was limited to 30 h of operation.
Jing et al. [58] successfully prepared Co-doped TSO via a simple ball milling procedure with no oxide impurities. The material was proven to completely remove sulfamethoxazole in wastewater at high effluent fluxes with a large rate constant. Strong Co-TSO interaction is cited as the reason for its stability, and Co proclivity towards forming OH-radical and 1O2 as the reason for the high rate constant.
Chen et al. [60] simultaneously doped MP with Ce and composited with carbon black (CB) in an effort to achieve monocycline degradation within wastewater. Complete removal was achieved within 20 min, but the electrode efficiency dropped to 98.5% after only five cycles. OH-radical and 1O2 were identified as the dominant species responsible for monocycline degradation, similar to [58].
Another persistent class of compounds is per- and polyfluoroalkyl substances (PFAS). Recently, Sui et al. [55] performed solid-state Ce and Nb Ti4O7 doping for the degradation of perfluorooctane sulfonate (PFOS) through electrochemical oxidation. Nb doping was put forward for PFOS degradation due to its lower charge transfer resistance and higher effective electrochemical surface area (EESA). Ce doping led to lower EESA and lower activity for PFOS degradation, but when normalized with respect to EESA, it also showed improvement compared to pristine Ti4O7. Listed recent studies point to doped-TSO being predominantly used for pollutant degradation: PFAS/PFOS, antibiotics, pesticides, microbe inactivation, and overall (waste)water purification. The main mechanism includes OH-radical and 1O2 generation at higher potentials, which can directly, or in an indirect way, degrade target molecules. Shifting the oxygen evolution potential to more positive values is the main goal, as it improves the degradation process efficacy.
Other niche applications, including bacteria inactivation and H2 and Cl2 production towards health and safety, are also being considered.
Zhang et al. [63] 3D printed Nd-, Sm-, and Pr-doped TSO and tested it for E. coli inactivation. Printed reactive electrochemical membranes increased hydroxyl radical yield by 50–450% compared to pure Ti4O7.
Wierzbicka et al. [53] prepared a mixed-phase TiO2-based catalyst with 32% anatase, 11% rutile, and 57% Magnéli phases loaded with Pt for H2 evolution. Higher H2 production, between 50–100 times that of plain anatase loaded with a similar amount of Pt, was reported, which was attributed to a multijunction of various Magnéli titania species and Pt.
The production of peroxide as a disinfectant, with H2 and Cl2 as desired base chemicals, is another avenue of doped-TSO application that is gaining attention due to doped-TSO’s concurrent activity and stability. Sun et al. [52] successfully incorporated Sn into the bulk of TSO and utilized it for the selective reduction of O2 to peroxide. The onset potential for peroxide evolution was close to theoretical in 0.1 M KOH, thanks to the abundance of oxygen defects resulting from Sn incorporation with a selectivity of 96 %. Lee et al. [62] incorporated Ru inside TSO, forming surface Ru-O4 motifs via a wet impregnation and mild annealing for the chlorine evolution reaction. Low-level Ru doping (0.13 wt%) provided outstanding activity and selectivity toward the chlorine evolution reaction due to direct and selective Cl− adsorption on Ru-O4 motifs.
4. Theoretical Insights
Doped MPs have been treated by density functional theory (DFT), with results indicating that doping with transition metals or non-metals can further tune their electronic structure, conductivity, and catalytic activity. Detailed analysis of how dopant incorporation modifies the local environment around crystallographic shear planes, alters the distribution of oxygen vacancies, and influences the density of states near the Fermi level is still lacking. Such insights would be of great significance for optimizing doped MPs.
The work by Yuan et al. provides guidelines for designing high-performance doped Ti4O7. Namely, by using DFT and machine learning, the stability and electronic structure of doped (Ti, M)4O7 (M = Sc, Y, La, Ce, Zr, Hf, V, Nb, Ta, Cr, Mo, W) were examined. Although doped MP are thermodynamically stable, Y, La, and Ce introduce significant lattice distortion, while Zr, Nb, Mo, and W support stability, stronger bonding, and minimal distortion. The rigidity modulus and solid entropy of dopants, within the doping site, are key factors controlling stability. Moreover, low-concentration Ce doping experimentally confirmed the predicted distortions [69].
The previously mentioned atomically dispersed Co catalysts incorporated into Magnéli phase Ti4O7 ceramic membranes achieved uniform Co dispersion (Co2+/Co3+) while retaining high conductivity and stability. DFT calculations confirmed the role of Co active sites and strong metal–support interactions in generating ·OH and 1O2 radicals. This approach demonstrated energy consumption orders of magnitude lower than typical electrochemical treatments [58].
Sui et al. [55] conducted density functional theory (DFT) calculations to acquire theoretical insights into the impact of doping on the calculated adsorption energies of PFOS and the C–S bond length in PFOS on both modified and unmodified Ti4O7 anodes. Researchers used the Vienna Ab initio Simulation Package (VASP) to perform the calculations with pristine and Ti4O7 with 18 distinct elements incorporated (Ag, Al, Au, Bi, Co, Cr, Cu, Fe, Mn, Mo, Ni, W, Pt, Ce, Y, Ta, Zr, and Nb) to evaluate the dopants’ effect on MPs’ catalytic activity. An MP with five dopants (Ce, Y, Ta, Zr, and Nb) exhibits enhanced performance relative to undoped anodes (reduced adsorption energies and an increase in C–S bond length in PFOS). DFT studies have also revealed the implications of doping on the band structure of Ti4O7. For example, Wei et al. [51] investigated Ce-doping in Ti4O7 and predicted that the introduction of Ce3+ ions modified the electronic structure and increased the electrical conductivity, showing how first-principles calculations can predict the functional capabilities of doped Ti4O7 in practical applications. These calculations predict changes in the band gap and electronic interactions, critical in optimizing Ti4O7 for use as an electrocatalyst.
The importance of theoretical insights is outlined in the context of determining the optimal doping levels and types. The DFT approach allows for the prediction of how variations in dopant concentration can influence the robustness and efficiency of Ti4O7 in different environments, guiding experimental synthesis and optimization routes. The missing part in the current literature is the application of theoretical calculations (DFT and molecular dynamics), together with machine learning algorithms, on interactions between doped MPs and different pollutants. This type of calculation is crucial for understanding adsorption and/or degradation processes.
5. Perspectives and Outlook
The versatility of doped MP titanium suboxides places them at the forefront of future electrode materials in electrochemical applications. So far, their reported effectiveness in the degradation of emerging and persistent pollutants, such as PFAS and pharmaceuticals, warrants further scrutiny. Dopant-supported modulation of stability, conductivity, electronic structure, oxygen evolution potential, and reactive radical generation extends their use from environmental to energy-related applications.
Several research directions deem attention: (i) broadening the scope of contaminants to pesticides, dyes, and other persistent pollutants that would place TSOs as electrodes of choice for water treatment; (ii) conductivity modulation geared towards electrocatalytic processes, and energy conversion and storage, including the oxygen reduction reaction and 2e− pathways for hydrogen peroxide production; (iii) modification of electronic structure for photocatalytic reactions; (iv) preparation of composites with solid supports for targeted applications; and (v) rational dopant selection, supported by density functional theory (DFT), can help establish favorable adsorption energies and reaction kinetics for both environmental and energy applications.
The prospects for future doped TSO applications rely on advances in material science to prevent dopant leaching. Under electrochemical operating conditions, partial leaching of dopants may occur due to redox cycling and local reorganization near the surface. This may alter the Ti3+/Ti4+ equilibrium and lead to lower conductivity and activity. The incorporation of dopants within composite frameworks overcomes these drawbacks by forming stronger bonds and thereby stabilizing the structure. Other strategies for surface modification aim towards thin coatings or the formation of composite materials with carbons or conductive polymers that stabilize dopant sites without compromising electronic structure. Prevention of leaching, coupled with controlled doping, provides the performance and stability necessary for targeted applications.
Furthermore, future studies should enable efficient substitution without loss in stability and porosity and simplify synthesis methods. The combination of computational and characterization methods is essential to model bond cleavage, pollutant oxidation, and electrokinetic intermediates.
Whenever there is a rush over novel and underinvestigated materials, researchers tend to unnecessarily overcomplicate synthesis and applications. Here, we might have a class of materials to actually suit several processes, from environmental and energy-related ones, justifying the rising number of studies, both theoretical and experimental. Look at the periodic table; a lot of research is still ahead.
6. Conclusions
Doping TSO has proven to be a viable strategy for producing materials with preserved intrinsic high conductivity and stability. The level of foreign atom incorporation is limited to <10% at. with higher doping levels, inducing irreversible changes to the original lattice and phase separation and resulting in lower activity. High-temperature reduction with H2, carbon, or Ti is the dominant route for producing pristine and doped TSOs with desirable conductivity and stability. Complete electrochemical oxidation of a myriad of pollutants is viable, including PFAS/PFOS, antibiotics, microbes, pharmaceutics, wastewater, etc. As this niche is developing, around 15 doped TSOs have been made experimentally so far, with DFT predicting sufficient stability for 11 more to be prepared.
Author Contributions
Conceptualization, M.M.-R. and N.G.; methodology, V.V.; software, B.N.V.; validation, N.G. and D.B.-B.; formal analysis, V.V., A.J., N.R.M. and M.R.; investigation, N.R.M. and V.V.; data curation, A.J. and M.R.; writing—original draft preparation, N.G., V.V. and M.M.-R.; writing—review and editing, A.J., M.M.-R. and D.B.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (grant numbers 451-03-137/2025-03/200146, 451-03-136/2025-03/200146, 451-03-137/2025-03/200111, and 451-03-136/2025-03/200026) and supported by the Science Fund of the Republic of Serbia, GRANT No 17990, Advanced electrochemical treatment of PFAS contaminated water: Novel Materials and Mechanisms—ALTER.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. Shape-Controlled Activation of Peroxymonosulfate by Single Crystal α-Mn2O3 for Catalytic Phenol Degradation in Aqueous Solution. Appl. Catal. B 2014, 154–155, 246–251. [Google Scholar] [CrossRef]
- Kitada, A.; Hasegawa, G.; Kobayashi, Y.; Kanamori, K.; Nakanishi, K.; Kageyama, H. Selective Preparation of Macroporous Monoliths of Conductive Titanium Oxides TinO2n−1 (n = 2, 3, 4, 6). J. Am. Chem. Soc. 2012, 134, 10894–10898. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-Ordering Electrochemistry: A Review on Growth and Functionality of TiO2 Nanotubes and Other Self-Aligned MOx Structures. Chem. Commun. 2009, 45, 2791–2808. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Cumbal, L. Light Harvesting Titanium Nanocatalyst for Remediation of Methyl Orange. Int. J. Chem. Mol. Eng. 2014, 8, 196–199. [Google Scholar] [CrossRef]
- Theron, J.; Walker, J.A.; Cloete, T.E. Nanotechnology and Water Treatment: Applications and Emerging Opportunities. Crit. Rev. Microbiol. 2008, 34, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Quétel, C.R.; Vassileva, E.; Petrov, I.; Chakarova, K.; Hadjiivanov, K.I. First Results on Fe Solid-Phase Extraction from Coastal Seawater Using Anatase TiO2 Nano-Particles. Anal. Bioanal. Chem. 2010, 396, 2349–2361. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influences of Nano-Anatase TiO2 on the Nitrogen Metabolism of Growing Spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef]
- Slimen, H.; Ochiai, T.; Nakata, K.; Murakami, T.; Houas, A.; Morito, Y.; Fujishima, A. Photocatalytic Decomposition of Cigarette Smoke Using a TiO2-Impregnated Titanium Mesh Filter. Ind. Eng. Chem. Res. 2012, 51, 587–590. [Google Scholar] [CrossRef]
- Mbonyiryivuze, A.; Zongo, S.; Diallo, A.; Bertrand, S.; Minani, E.; Lal Yadav, L.; Mwakikunga, B.; Dhlamini, S.M.; Maaza, M.; Yadav, L.L. Titanium Dioxide Nanoparticles Biosynthesis for Dye Sensitized Solar Cells Application: Review. Phys. Mater. Chem. 2015, 3, 12–17. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar Photocatalysis: Materials, Reactors, Some Commercial, and Pre-Industrialized Applications. A Comprehensive Approach. Appl. Catal. B 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Smith, J.R.; Walsh, F.C.; Clarke, R.L. Electrodes Based on Magnéli Phase Titanium Oxides: The Properties and Applications of Ebonex® Materials. J. Appl. Electrochem. 1998, 28, 1021–1033. [Google Scholar] [CrossRef]
- Miller-Folk, R.R.; Noftle, R.E.; Pletcher, D. Electron Transfer Reactions at Ebonex Ceramic Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1989, 274, 257–261. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wills, R.G.A. The Continuing Development of Magnéli Phase Titanium Sub-Oxides and Ebonex® Electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Toyoda, M.; Yano, T.; Tryba, B.; Mozia, S.; Tsumura, T.; Inagaki, M. Preparation of Carbon-Coated Magneli Phases TinO2n−1 and Their Photocatalytic Activity under Visible Light. Appl. Catal. B 2009, 88, 160–164. [Google Scholar] [CrossRef]
- Ellis, K.; Hill, A.; Hill, J.; Loyns, A.; Partington, T. The Performance of Ebonex® Electrodes in Bipolar Lead-Acid Batteries. J. Power Sources 2004, 136, 366–371. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-Enhanced Redox Chemistry of Polysulphides on a Metallic and Polar Host for Lithium-Sulphur Batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef]
- Rashkova, V.; Kitova, S.; Vitanov, T. Electrocatalytic Behavior of Thin Co–Te–O Films in Oxygen Evolution and Reduction Reactions. Electrochim. Acta 2007, 52, 3794–3803. [Google Scholar] [CrossRef]
- Arif, A.F.; Balgis, R.; Ogi, T.; Iskandar, F.; Kinoshita, A.; Nakamura, K.; Okuyama, K. Highly Conductive Nano-Sized Magnéli Phases Titanium Oxide (TiOx). Sci. Rep. 2017, 7, 3646. [Google Scholar] [CrossRef]
- Gusev, A.A.; Avvakumov, E.G.; Medvedev, A.Z.H.; Masliy, A.I. Ceramic Electrodes Based on Magneli Phases of Titanium Oxides. Sci. Sinter. 2007, 39, 51–57. [Google Scholar] [CrossRef]
- Ioroi, T.; Senoh, H.; Yamazaki, S.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Stability of Corrosion-Resistant Magnéli-Phase Ti4O7-Supported PEMFC Catalysts at High Potentials. J. Electrochem. Soc. 2008, 155, B321. [Google Scholar] [CrossRef]
- Tao, X.; Wang, J.; Ying, Z.; Cai, Q.; Zheng, G.; Gan, Y.; Huang, H.; Xia, Y.; Liang, C.; Zhang, W.; et al. Strong Sulfur Binding with Conducting Magnéli-Phase TinO2n−1 Nanomaterials for Improving Lithium–Sulfur Batteries. Nano Lett. 2014, 14, 5288–5294. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Tanaka, K.; Inui, H. Thermoelectric Properties and Crystallographic Shear Structures in Titanium Oxides of the Magnèli Phases. J. Appl. Phys. 2010, 108, 083703. [Google Scholar] [CrossRef]
- Tsuyumoto, I.; Hosono, T.; Murata, M. Thermoelectric Power in Nonstoichiometric Orthorhombic Titanium Oxides. J. Am. Ceram. Soc. 2006, 89, 2301–2303. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Ye, J.; Qiu, W. Synthesis, Microstructural Characterization, and Electrochemical Performance of Novel Rod-like Ti4O7 Powders. J. Alloys Compd. 2017, 704, 18–25. [Google Scholar] [CrossRef]
- Bursill, L.A.; Hyde, B.G. Crystal Structures in the {l32} CS Family of Higher Titanium Oxides TinO2n−1. Acta Crystallogr. B 1971, 27, 210–215. [Google Scholar] [CrossRef]
- Padilha, A.C.M.; Osorio-Guillén, J.M.; Rocha, A.R.; Dalpian, G.M. TinO2n−1 Magnéli Phases Studied Using Density Functional Theory. Phys. Rev. B 2014, 90, 035213. [Google Scholar] [CrossRef]
- Sensoy, M.G.; Carpick, R.W.; Srolovitz, D.J.; Rappe, A.M. Manipulating the Electrical Properties of Conductive Substoichiometric Titanium Oxides. Phys. Rev. B 2024, 109, 064106. [Google Scholar] [CrossRef]
- Le Page, Y.; Strobel, P. Structural Chemistry of Magnéli Phases TinO2n−1 (4 ≤ n ≤ 9). I. Cell and Structure Comparisons. J. Solid State Chem. 1982, 43, 314–319. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ye, J. Investigation of Fabrication of Ti4O7 by Carbothermal Reduction in Argon Atmosphere and Vacuum. J. Mater. Sci. Mater. Electron. 2016, 27, 3683–3692. [Google Scholar] [CrossRef]
- Malik, H.; Sarkar, S.; Mohanty, S.; Carlson, K. Modelling and Synthesis of Magnéli Phases in Ordered Titanium Oxide Nanotubes with Preserved Morphology. Sci. Rep. 2020, 10, 8050. [Google Scholar] [CrossRef]
- Portehault, D.; Maneeratana, V.; Candolfi, C.; Oeschler, N.; Veremchuk, I.; Grin, Y.; Sanchez, C.; Antonietti, M. Facile General Route toward Tunable Magnéli Nanostructures and Their Use As Thermoelectric Metal Oxide/Carbon Nanocomposites. ACS Nano 2011, 5, 9052–9061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Ye, J.; Zhu, R. Fabrication of Ti4O7 Electrodes by Spark Plasma Sintering. Mater. Lett. 2014, 114, 34–36. [Google Scholar] [CrossRef]
- Yu, M.; Saunders, T.; Grasso, S.; Mahajan, A.; Zhang, H.; Reece, M.J. Magnéli Phase Titanium Suboxides by Flash Spark Plasma Sintering. Scr. Mater. 2018, 146, 241–245. [Google Scholar] [CrossRef]
- Fahem, M.Q.; Hassan, T.A.A. Magnéli Phase Titanium Sub-Oxide Production Using a Hydrothermal Process. Karbala Int. J. Mod. Sci. 2022, 8, 651–656. [Google Scholar] [CrossRef]
- Liu, H.J.; Luo, M.Q.; Yang, L.X.; Zeng, C.L.; Fu, C. A High Strength and Conductivity Bulk Magnéli Phase Ti4O7 with Superior Electrochemical Performance. Ceram. Int. 2022, 48, 25538–25546. [Google Scholar] [CrossRef]
- Geng, X.; Xia, Y.; Liang, H.; Yao, D.; Zeng, Y.-P. Fabrication of High-Performance Magnéli Phase Ti4O7 Ceramics by in-Situ Hot-Pressed Sintering in a Single Step. Mater. Today Commun. 2023, 37, 107058. [Google Scholar] [CrossRef]
- Mito, M.; Mokutani, N.; Tang, Y.; Matsumoto, K.; Tajiri, T.; Horita, Z. Achieving High-Tc Superconductivity in Magnéli Phase Based on Ti Oxides: Prediction by Machine Learning and Material Synthesis by High-Pressure Torsion Processing. J. Mater. Sci. 2024, 59, 5981–5994. [Google Scholar] [CrossRef]
- Kao, W.; Patel, P.; Haberichter, S.L. Formation Enhancement of a Lead/Acid Battery Positive Plate by Barium Metaplumbate and Ebonex®. J. Electrochem. Soc. 1997, 144, 1907–1911. [Google Scholar] [CrossRef]
- English, J.T.; Wilkinson, D.P. The Thermal-Oxidation Behavior of Pristine and Doped Magnéli Phase Titanium Oxides. ECS J. Solid State Sci. Technol. 2021, 10, 034004. [Google Scholar] [CrossRef]
- George, J.; Waroquiers, D.; Di Stefano, D.; Petretto, G.; Rignanese, G.-M.; Hautier, G. The Limited Predictive Power of the Pauling Rules. Angew. Chem. Int. Ed. 2020, 59, 7569. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, H.; Wang, Y. A Review: Synthesis and Applications of Titanium Sub-Oxides. Materials 2023, 16, 6874. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, T.; Jia, H.; Li, J.; Liu, B. Preparation and Electrochemical Applications of Magnéli Phase Titanium Suboxides: A Review. Chem. Eur. J. 2024, 30, e202402188. [Google Scholar] [CrossRef]
- Kumar, A.; Barbhuiya, N.H.; Singh, S.P. Magnéli Phase Titanium Sub-Oxides Synthesis, Fabrication and Its Application for Environmental Remediation: Current Status and Prospect. Chemosphere 2022, 307, 135878. [Google Scholar] [CrossRef]
- Calestani, D.; Licci, F.; Kopnin, E.; Calestani, G.; Gauzzi, A.; Bolzoni, F.; Besagni, T.; Boffa, V.; Marezio, M. Preparation and Characterization of Powders and Crystals of Vn−xTixO2n−1 Magneli Oxides. Cryst. Res. Technol. 2005, 40, 1067–1071. [Google Scholar] [CrossRef]
- Yuan, Z.; Gong, J.; Xu, S.; Li, Z.; Tang, G. Investigation of the Thermoelectric Properties of Reduced Nb-Doped TiO2-Ceramics. J. Alloys Compd. 2017, 710, 778–783. [Google Scholar] [CrossRef]
- English, J.T.; Wilkinson, D.P. The Thermal-Oxidation Behavior of Pristine and Doped Magnéli Phase Titanium Oxides. ECS Trans. 2020, 98, 11–22. [Google Scholar] [CrossRef]
- Gardos, M.N. Magnéli Phases of Anion-Deficient Rutile as Lubricious Oxides. Part II. Tribological Behavior of Cu-Doped Polycrystalline Rutile (TinO2n−1). Tribol. Lett. 2000, 8, 79–96. [Google Scholar] [CrossRef]
- Teramoto, T.; Takai, Y.; Hashiguchi, H.; Okunishi, E.; Tanaka, K. Distribution of Alloying Quadrivalent Zirconium in TiO2−x Magnèli Phase. Mater. Trans. 2019, 60, 2199–2203. [Google Scholar] [CrossRef]
- Wei, W.; Jin, N.; Ye, J. Structural and Electronic Properties of Ce-doped Ti4O7 Based on Experiments and First-principles Calculations. J. Am. Ceram. Soc. 2023, 106, 7556–7566. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, Y.; Dai, L.; Zheng, Y.; Zhang, H.; Wang, Y. Sn Bulk Phase Doping and Surface Modification on Ti4O7 for Oxygen Reduction to Hydrogen Peroxide. Chem. Eur. J. 2024, 30, e202303602. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, E.; Domaschke, M.; Denisov, N.; Fehn, D.; Hwang, I.; Kaufmann, M.; Kunstmann, B.; Schmidt, J.; Meyer, K.; Peukert, W.; et al. Magnéli Phases Doped with Pt for Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2019, 2, 8399–8404. [Google Scholar] [CrossRef]
- Lin, H.; Xiao, R.; Xie, R.; Yang, L.; Tang, C.; Wang, R.; Chen, J.; Lv, S.; Huang, Q. Defect Engineering on a Ti4O7 Electrode by Ce3+ Doping for the Efficient Electrooxidation of Perfluorooctanesulfonate. Environ. Sci. Technol. 2021, 55, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Zhu, X.; Li, L.; Wang, Y.; Li, G.; Dong, S.; Wang, Y.; Lin, H.; Li, K.; Huang, Q. Robust Titanium Suboxide Anodes Doped by Sintering Enhance PFOS Degradation in Water. Chemosphere 2025, 379, 144438. [Google Scholar] [CrossRef]
- English, J.T.; Wilkinson, D.P. Vanadium-Doped Ti4O7 Porous Transport Layers for Efficient Electrochemical Oxidation of Industrial Wastewater Contaminants. J. Electrochem. Soc. 2023, 170, 083501. [Google Scholar] [CrossRef]
- Tao, N.; Tian, Y.; Wang, J.; Teng, J. Enhanced Electrochemical Removal of Sulfamethazine on Sm-Doped Ti4O7 Anode: Mechanisms and Toxicity Evaluation. J. Hazard. Mater. Adv. 2025, 17, 100607. [Google Scholar] [CrossRef]
- Jing, J.; Li, S.; Wu, H.; Li, S.; Zhang, Y.; Song, G.; Li, W.; Liang, R.; Zhou, M. Energy-Efficient Wastewater Treatment Using Enhanced Fenton-like Electroactive Ceramic Membrane with Atomic-Level Engineered Cobalt on Magnéli Phase Supports. Appl. Catal. B Environ. Energy 2025, 377, 125484. [Google Scholar] [CrossRef]
- Jia, H.; Jiang, W.; Chen, T.; Yang, W.; Lu, W.; Ma, D.; Lang, A.; Li, J.; Liu, B. Magnéli-Phase Sn-Ti4O7-Coated Electrodes via Plasma Electrolytic Oxidation: Enhanced Charge Transfer and Electrochemical Degradation of Tetracycline. J. Environ. Chem. Eng. 2025, 13, 117719. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Lei, C.; Li, W.; Yang, Z.; Zhou, Q. Research on Carbon Black and Cerium Co-Doped Ti4O7-CB-Ce Electrocatalytic Oxidation of Tetracycline-Based Antibiotics. Environ. Sci. Pollut. Res. 2024, 31, 44983–44994. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, D.; Li, L.; Zhang, Y.; Chen, S.; Wu, Q.; Wang, P.; Zhang, C.; Sun, J. Insights into the Electrooxidation of Florfenicol by a Highly Active La-Doped Ti4O7 Anode. Sep. Purif. Technol. 2022, 291, 120904. [Google Scholar] [CrossRef]
- Lee, W.; Choung, S.; Kim, S.; Hong, J.; Kim, D.; Tarpeh, W.A.; Han, J.W.; Cho, K. Atomically Dispersed Ru-doped Ti4O7 Electrocatalysts for Chlorine Evolution Reaction with a Universal Activity. Small 2024, 20, 2401248. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Wang, P.; Bu, Z.; Wang, B.; Shi, H.; Wang, H.; Zhang, W.; Gao, S.; Huang, Q. 3D Printed Lanthanide-Doped Ti4O7 Reactive Membrane for Efficient Electrochemical Disinfection and Degradation of Antibiotic Resistance Genes. Chem. Eng. J. 2024, 502, 157829. [Google Scholar] [CrossRef]
- Misal, S.N.; Lin, M.-H.; Mehraeen, S.; Chaplin, B.P. Modeling Electrochemical Oxidation and Reduction of Sulfamethoxazole Using Electrocatalytic Reactive Electrochemical Membranes. J. Hazard. Mater. 2020, 384, 121420. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Xia, Y.; Zhu, M.; Zhao, J.; Yao, D.; Zeng, Y. Effect of Al2O3 and Ta2O5 on Mechanical and Electrical Properties of Ti4O7 Ceramics. Adv. Eng. Mater. 2025, 27, 2500876. [Google Scholar] [CrossRef]
- Yuan, T.; Wei, W.; Wang, Y.; Jin, N.; Ye, J. Effect of V-Doping on Structure and Electrical Conductivity of Magnéli Phase Ti4O7. J. Alloys Compd. 2024, 978, 173492. [Google Scholar] [CrossRef]
- Yuan, T.; Wei, W.; Yun, Y.; Jin, N.; Ye, J. The Formation of V-Doped Ti4O7 Hollow Magnéli Phase with Improved Electrical Conductivity and Thermal Stability. Ceram. Int. 2023, 49, 40668–40675. [Google Scholar] [CrossRef]
- Kao, H.-Y. The Development of M3+-Doped Mesoporous Ti4O7 Electrode for Pharmaceutical Degradation in Fresh Human Urine. Master’s Thesis, University of Washington, Washington, DC, USA, 2022. [Google Scholar]
- Yuan, T.; Jin, N.; Cheng, W.; Yun, Y.; Tian, X.; Wang, L.; Ye, J. Insight into the Stability in Cation Substitution of Magnéli Phase Ti4O7. Appl. Phys. Lett. 2022, 121, 214101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).