Abstract
RNA triple helices are relatively understudied, including their interactions with small molecules. In this study, we evaluated eight previously reported triplex-binding molecules (TBMs) for their functional effects on the premature and mature MALAT1 triple helix. Based on UV thermal denaturation experiments, the TBMs berberine, coralyne, sanguinarine, berenil, and neomycin selectively stabilize the Hoogsteen interface of the MALAT1 triple helix. Moreover, fisetin, luteolin, and quercetin were more sensitive to nucleotide composition, whereas berberine, coralyne, sanguinarine, and berenil were more sensitive to changes in the length of the major-groove triple helix. Most TBMs could not outcompete MALAT1 triple helix-binding proteins, except for neomycin. Surface plasmon resonance experiments demonstrated that berberine and sanguinarine display relatively quick association and dissociation binding profiles. Treating human colorectal carcinoma cells with each of the TBMs reduced MALAT1 levels by ~20–60%. This study demonstrates that TBMs broadly recognize the premature and mature MALAT1 triple helix but exhibit subtle sensitivities, suggesting that TBMs can be designed to selectively bind triple helices based on nucleotide composition, length, and structural context.
1. Introduction
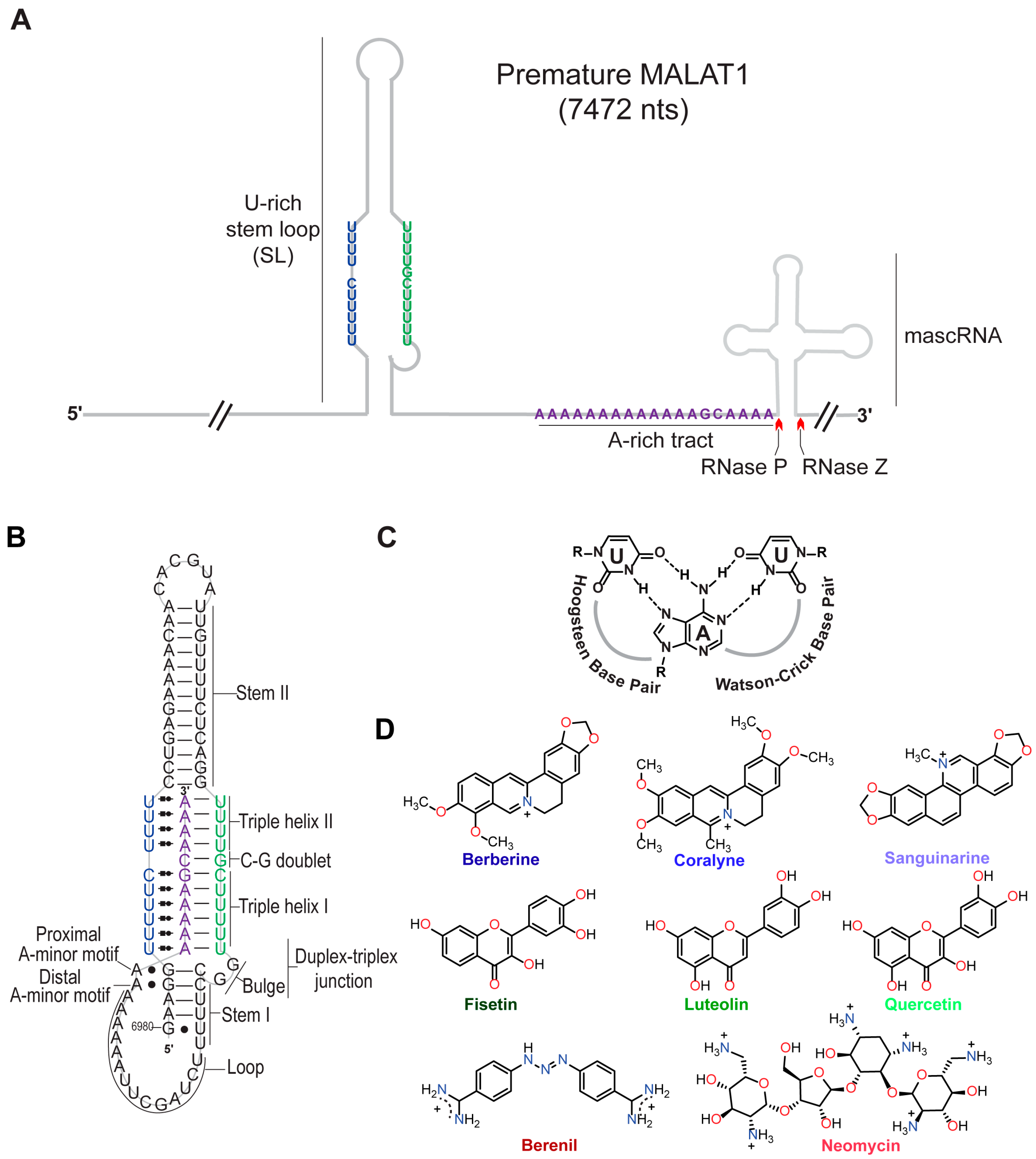
Technological advancements have established the importance of long noncoding RNAs (lncRNAs) in various biological functions, and their dysregulation is associated with various diseases, including cancer [1,2]. One lncRNA that promotes metastasis of various cancer types is metastasis-associated lung adenocarcinoma transcript 1 (MALAT1); therefore, MALAT1 has emerged as a drug target, most notably its 3′ end [2,3,4,5,6]. At its 3′ end, MALAT1 contains a U-rich stem loop (SL), a genomically encoded A-rich tract (A), and a tRNA-like structure called MALAT1-associated small cytoplasmic RNA (mascRNA) (Figure 1A) [7,8,9,10]. pre-mascRNA is excised by RNases P and Z, generating a mature form of MALAT1 that forms a blunt-ended triple helix (SL+A) involving the U-rich SL and A-rich tract (Figure 1A,B) [7,10,11]. This predominantly U•A-U-rich triple helix (whereby Hoogsteen and Watson-Crick interactions are represented by a dot (•) and solid line (-), respectively) protects MALAT1 from degradation (Figure 1B,C) [8,9,11]. The MALAT1 triple helix has one confirmed protein-binding partner: the N6-methyladenosine writer protein methyltransferase-like protein 16 (METTL16) [12,13]. In addition to protein binding, several exogenous small molecules, which we henceforth refer to as triplex-binding molecules (TBMs), bind to the MALAT1 triple helix: diphenylfuran (DPF) derivatives [14,15], imidazole derivatives [16], berenil and its derivatives [17], 1,2,3-triazole derivatives [18], and conjugated and aromatic heterocyclic compounds [19,20,21,22]. Although these studies have focused on the mature MALAT1 triple helix, it may be more therapeutically advantageous if a small molecule interfered with the formation of the triple helix by targeting a premature state such as the U-rich SL that likely forms co-transcriptionally or the premature 3′ end consisting of the SL+A+masc (Figure 1A). Like the mature MALAT1 triple helix, several aromatic/heteroaromatic small molecules have targeted a predicted triple helix at the 3′ end of the multiple endocrine neoplasia-β (MENβ) lncRNA: aurintricarboxylic acid, mitoxantrone emodin, GW5074, mitoxantrone, and rottlerin [23]. These TBMs have been explored primarily for therapeutic purposes, although we still do not have a solid understanding of the basic chemical and physical properties that TBMs leverage to preferentially bind to triple-helical RNA structures.
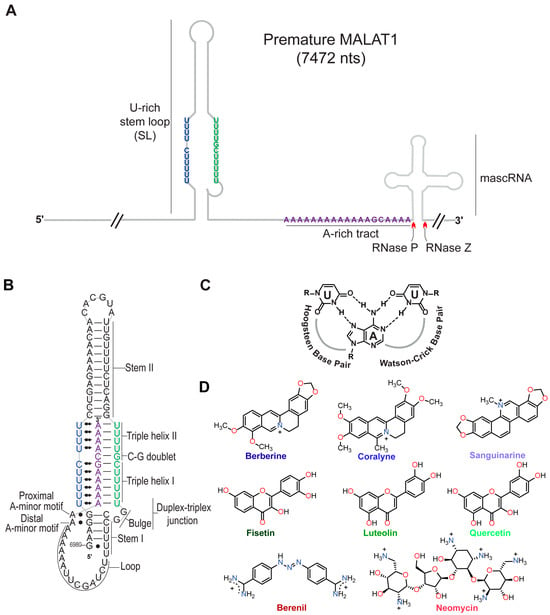
Figure 1.
Structures of the MALAT1 triple helix and eight triplex-binding molecules (TBMs). (A) Cartoon schematic depicts the arrangement of the U-rich stem loop (SL), A-rich tract (A), and mascRNA at the 3′ end of premature human MALAT1, which is 7472 nts long. Red carats denote cleavage sites of RNases P and Z. Schematic is not drawn to scale. (B) Shown is a schematic depicting the secondary and tertiary structure of the mature MALAT1 triple helix. The Hoogsteen and Watson-Crick interactions are represented by Leontis-Westhof notation ( ) and a solid line (−), respectively. (C) Chemical structure of a U•A-U base triple is shown with Hoogsteen and Watson-Crick base pairing denoted by dashed lines. (D) Chemical structures of the eight TBMs examined in this study. The TBMs that are alkaloids are represented by a shade of blue: dark blue, blue, and light blue represent berberine, coralyne, and sanguinarine, respectively. TBMs that are flavonoids are represented by a shade of green: dark green, green, and light green represent fisetin, luteolin, and quercetin, respectively. The colors dark red and red represent berenil and neomycin, respectively.
) and a solid line (−), respectively. (C) Chemical structure of a U•A-U base triple is shown with Hoogsteen and Watson-Crick base pairing denoted by dashed lines. (D) Chemical structures of the eight TBMs examined in this study. The TBMs that are alkaloids are represented by a shade of blue: dark blue, blue, and light blue represent berberine, coralyne, and sanguinarine, respectively. TBMs that are flavonoids are represented by a shade of green: dark green, green, and light green represent fisetin, luteolin, and quercetin, respectively. The colors dark red and red represent berenil and neomycin, respectively.
 ) and a solid line (−), respectively. (C) Chemical structure of a U•A-U base triple is shown with Hoogsteen and Watson-Crick base pairing denoted by dashed lines. (D) Chemical structures of the eight TBMs examined in this study. The TBMs that are alkaloids are represented by a shade of blue: dark blue, blue, and light blue represent berberine, coralyne, and sanguinarine, respectively. TBMs that are flavonoids are represented by a shade of green: dark green, green, and light green represent fisetin, luteolin, and quercetin, respectively. The colors dark red and red represent berenil and neomycin, respectively.
) and a solid line (−), respectively. (C) Chemical structure of a U•A-U base triple is shown with Hoogsteen and Watson-Crick base pairing denoted by dashed lines. (D) Chemical structures of the eight TBMs examined in this study. The TBMs that are alkaloids are represented by a shade of blue: dark blue, blue, and light blue represent berberine, coralyne, and sanguinarine, respectively. TBMs that are flavonoids are represented by a shade of green: dark green, green, and light green represent fisetin, luteolin, and quercetin, respectively. The colors dark red and red represent berenil and neomycin, respectively.
Previously, small molecules belonging to the class of alkaloids (e.g., berberine [24,25,26], coralyne [25,27], and sanguinarine [24]), flavonoids (e.g., fisetin [28], luteolin [29], and quercetin [20,30]), triazene (berenil) [17,31], and aminoglycosides (neomycin) [32] (Figure 1D) were characterized for their interactions with generic nucleic acid structures: DNA/RNA double helices, DNA/RNA triple helices, and DNA G-quadruplexes. In general, most TBMs (alkaloids, flavonoids, and neomycin) stabilize the Hoogsteen face by ~3–20 °C and the Watson-Crick face by less than 5 °C [24,25,26,28,29,30,32]. Most of these TBMs are either dietary supplements (e.g., berberine, fisetin, luteolin, and quercetin) or FDA-approved drugs for veterinary (berenil) or human (neomycin) medical treatment. Except for berenil [17] and quercetin [20], the other six compounds (Figure 1D) have not been tested for their ability to engage with the MALAT1 triple helix, and none have considered the premature form of the MALAT1 triple helix (SL+A+masc).
Herein, we sought to study the selectivity, binding trends, and cellular effect of eight TBMs with the MALAT1 triple helix (Figure 1B,D). Our thermal melting studies confirm that most TBMs preferentially stabilize Hoogsteen interactions, similar to what has been observed previously for poly(U•A-U) or poly(T•A-T) triple helices [24,25,26,27,28,29,30,31,32]. When the nucleotide composition and length of the major-groove triple helix are altered, most alkaloids stabilize, whereas most flavonoids destabilize Hoogsteen interactions. Only neomycin can effectively prevent proteins from binding to the MALAT1 triple helix in the presence of HCT116 cell lysate. Five TBMs demonstrate fast binding interactions, although berberine and sanguinarine are the fastest. Steady-state levels of premature and mature MALAT1 in HCT116 cells were reduced in the presence of the flavonoids, berenil, and neomycin. Our study demonstrates that these first-generation TBMs interact with the MALAT1 triple helix and could potentially target both the mature and premature forms of the MALAT1 triple helix.
2. Results
2.1. Most TBMs Differentially Interact with the MALAT1 Triple Helix When Its Nucleotide Composition and Length Are Varied
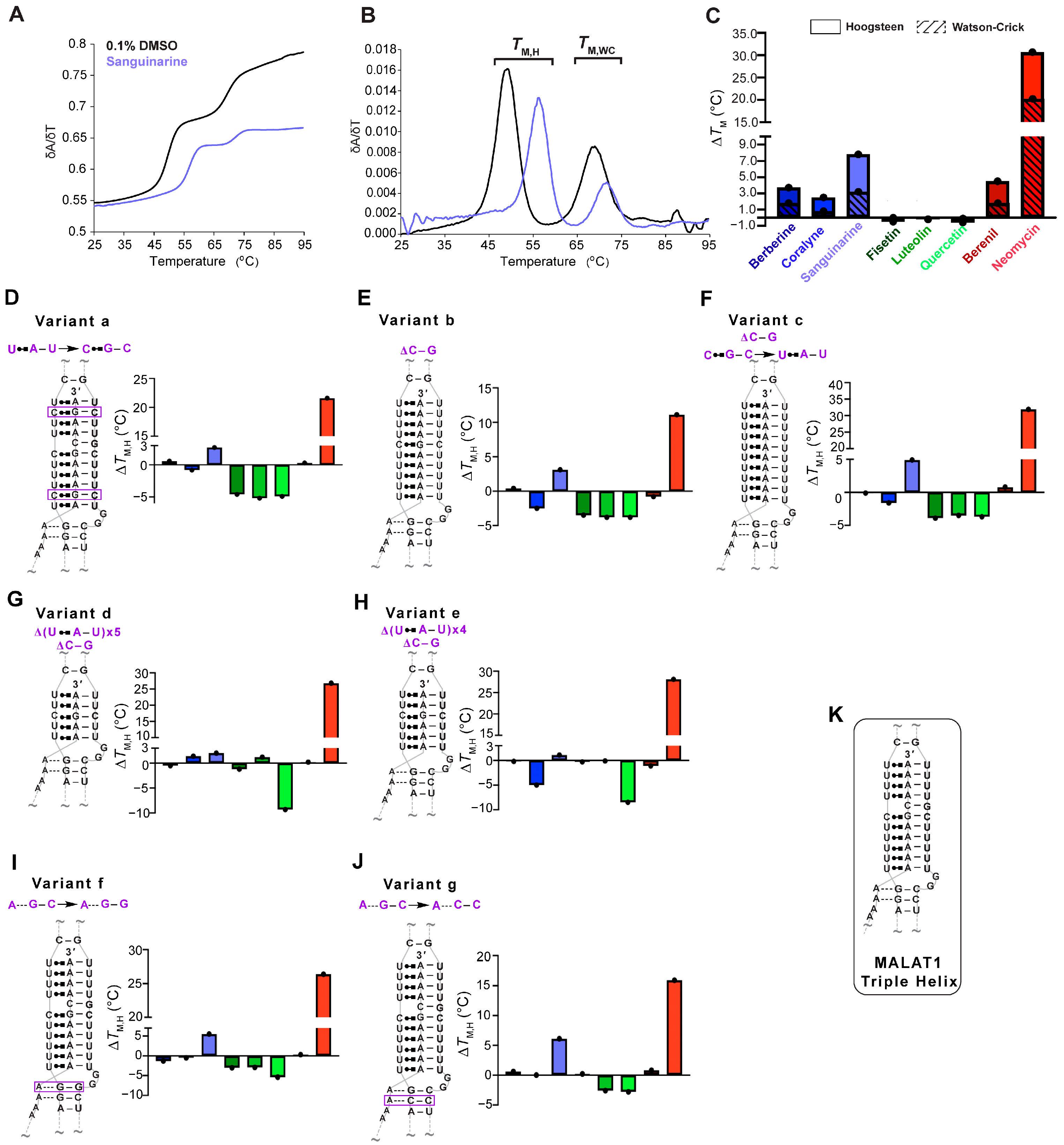
Our first objective was to determine if the eight TBMs preferentially target the major-groove triple helix of the MALAT1 triple helix as previously observed for poly(U•A-U) and poly(T•A-T) triple helices [24,25,26,28,29,30,31,32]. We employed UV thermal denaturation assays. As reported previously for the MALAT1 triple helix [9,14,33,34], its UV melting curve was biphasic, whereby the first derivative plot shows two distinct peaks: melting of primarily Hoogsteen interactions (TM,H), and likely the stem I and loop region, at 49.6 ± 0.4 °C and melting of the Watson-Crick interactions (TM,WC), particularly stem II, at 69 °C (Figure 1B and Figure 2A,B, Table 1). Because previous studies reported self-association of select TBMs, we next determined the UV melting profiles for each TBM at 10 µM in the absence of RNA (Figure S1A–C, File S1) [35,36,37,38,39]. Although peaks were observed, most were 0.3 to 6% of the peak heights that we determined for the MALAT1 triple helix in the absence of TBMs (Figure 1A,B and Figure S1D–F), except for quercetin having a shoulder peak at ~35 °C, which could be self-association of quercetin (Figure 1D and Figure S1D–F). These control assays establish that a positive or negative thermal shift (∆TM), respectively, indicates a net stabilization or destabilization of RNA structure in the presence of the TBM, likely induced by changes in non-covalent interactions between apo- and the TBM-bound MALAT1 triple helix states. All TBMs had a greater impact on ∆TM,H than ∆TM,WC; therefore, we focused on ∆TM,H (Figure 2C, Table 1). We note that ∆TM values greater than 2.0 °C are considered significant because they are generally two times the standard deviation. The greatest stabilization was observed for neomycin with a ∆TM,H of 30.7 °C (Figure 2C, Table 1). All alkaloids and berenil have ∆TM,H values of ~2.5 to 8 °C (Figure 2C, Table 1). In contrast, the flavonoids mildly destabilize the MALAT1 triple helix with ∆TM,H values of −0.2 °C to −0.6 °C (Figure 2C, Table 1). We also examined the flavonoids at 2% DMSO because that was previously used for quercetin [20]. There was no effect (i.e., ∆TM,H = 0.1 °C) on the MALAT1 triple helix stability at 2% DMSO (Figure S2). Overall, the ∆TM,H values ranged from approximately ~3–31 °C and were 2- to 3-fold greater than ∆TM,WC values. Thus, TBMs exert a greater thermal effect on interactions that stabilize the first melting transition, suggesting that TBMs likely alter Hoogsteen base pairs of the major-groove triple helix and/or base pairs in the stem I/loop region. To evaluate likely binding sites, we used AlphaFold 3 to predict the MALAT1 triple helix-TBM complexes using the MALAT1 triple helix crystal structure (PDB ID: 4plx) [11,40]. For all TBMs, the most favorable binding sites were in the regions that contribute to TM,H (i.e., duplex-triplex junction, triple helix I, and triple helix II) and not TM,WC (Figure 1B and Figure S3).
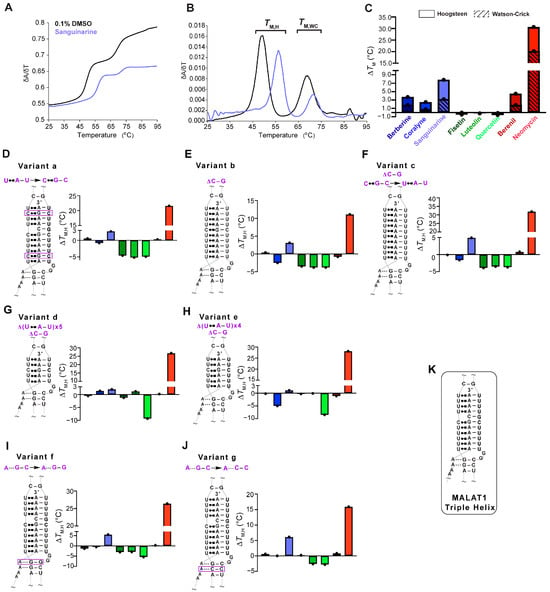
Figure 2.
UV thermal denaturation results for the WT MALAT1 triple helix and MALAT1 variant RNAs in the absence and presence of TBMs. Plots of normalized (A) UV absorbance and (B) first derivative with respect to temperature for the MALAT1 triple helix in the absence (black line) and presence of sanguinarine (light blue). Bar plots show changes in the Hoogsteen (solid color) and the Watson-Crick (striped) melting temperatures for TBMs binding to (C) WT MALAT1, (D–F) variants with changes in nucleotide composition variants a–c, (G,H) shorter triple helices variants d and e, and (I,J) disrupted A-minor interactions are variants f and g. Bar colors correspond to the TBM as defined in Figure legend 1C. All bar plots are the average ∆TM (n = 3), denoted by a single black dot. (K) A schematic is shown for the WT MALAT1 triple helix, focusing on the major-groove triple helix and A-minor motif. A tilde (~) denotes that peripheral RNA regions were present in the experiment but are not shown in the schematics for brevity. The Hoogsteen and Watson-Crick interactions are represented by Leontis-Westhof notation ( ) and a solid line (−), respectively. All RNAs used in this experiment are unimolecular, like the RNA shown in Figure 1B. All melting temperature values are compiled in Tables S1–S4. Raw and processed UV data are presented in File S1.
) and a solid line (−), respectively. All RNAs used in this experiment are unimolecular, like the RNA shown in Figure 1B. All melting temperature values are compiled in Tables S1–S4. Raw and processed UV data are presented in File S1.
 ) and a solid line (−), respectively. All RNAs used in this experiment are unimolecular, like the RNA shown in Figure 1B. All melting temperature values are compiled in Tables S1–S4. Raw and processed UV data are presented in File S1.
) and a solid line (−), respectively. All RNAs used in this experiment are unimolecular, like the RNA shown in Figure 1B. All melting temperature values are compiled in Tables S1–S4. Raw and processed UV data are presented in File S1.

Table 1.
UV melting temperatures obtained for the MALAT1 triple helix in the absence or presence of TBMs.
Next, we were interested in determining whether TBM binding to the wild type (WT) MALAT1 triple helix is sensitive to nucleotide composition and/or the length of the major-groove triple helix. To maintain biological relevance, the full-length MALAT1 triple helix (Figure 1B), including the peripheral regions stem I, II, and loops, was used, akin to other similar studies [14,17,20,23,41]. We first assessed nucleotide composition by replacing two U•A-U base triples with isosteric C+•G-C base triples (variant a, Figure 2D), deleting the C-G doublet (variant b, Figure 2E), and deleting the C-G doublet as well as replacing the C+•G-C base triple with U•A-U (variant c, Figure 2F) so that the triple helix is ten U•A-U base triples. For variants a–c, there were a few notable differences for the ∆TM,H values compared to WT: coralyne and all three flavonoids destabilized the MALAT1 variants by 0.8 to 5.2 °C, while berberine and berenil had almost no effect on ∆TM,H (Figure 2C–F, Tables S1 and S2). In general, coralyne and the flavonoids are sensitive to changes in nucleotide composition of the MALAT1 triple helix.
In addition to nucleotide composition, we also probed the length of the major-groove triple helix by shortening it to five (variant d) and six (variant e) base triples because most structurally validated naturally occurring RNA triple helices have only three to five base triples (Figure 2G,H) [42]. Most TBMs had no significant impact on ∆TM,H with two exceptions. One, quercetin induced a destabilization of ~9 °C and two, coralyne destabilized a six-base triple-long major-groove triple helix but not five (Figure 2G,H, Tables S3 and S4). To provide structural insights into why coralyne was sensitive to a one-base triple difference, we used FpocketR, a software tool that predicts binding pockets on a provided RNA target [43,44]. FpocketR showed different binding pockets along the duplex-triplex junction for both variants d and e; however, the pocket for variant e extends into stem I more than variant d (Figure S4), suggesting that coralyne binding may destabilize that part of the MALAT1 triple helix. Additionally, most TBMs were predicted to bind in triplex I or the duplex-triplex junctions (Figure 1B and Figure S3). These results suggest that TBMs have the ability to differentially recognize structural features of major-groove triple helices.
Lastly, we examined variants f and g (Figure 2I,J) that, respectively, disrupt the proximal and distal A•G-C minor-groove base triples, a location that might represent favorable binding pockets as previously identified for DPFp8 (Figure S5) [15] and by our computationally predicted MALAT1 triple helix-TBM complexes (Figure S3). For both variants, f and g, the alkaloids had no major effect except for sanguinarine, which increased TM,H by about 6 °C (Figure 2I,J). The flavonoids destabilized variants f and g by about 2.5 to 5.5 °C, except fisetin had no effect on variant g (Figure 2I,J, Table S4). These results suggest that fisetin may bind near the A-minor base triples, which is the predicted binding site in our MALAT1 triple helix-fisetin complex (Figure 2I,J, and Figure S3). In summary, our results show that flavonoids are more sensitive to nucleotide composition, alkaloids are more sensitive to changes in nucleotide length, berenil selectively stabilized only the WT MALAT1 triple helix, and neomycin hyperstabilized all MALAT1 triple helices. These results suggest that these TBMs have subtle tunability with respect to certain structural features.
2.2. Only Neomycin Prevents the Formation of a MALAT1 Triple Helix RNP Complex
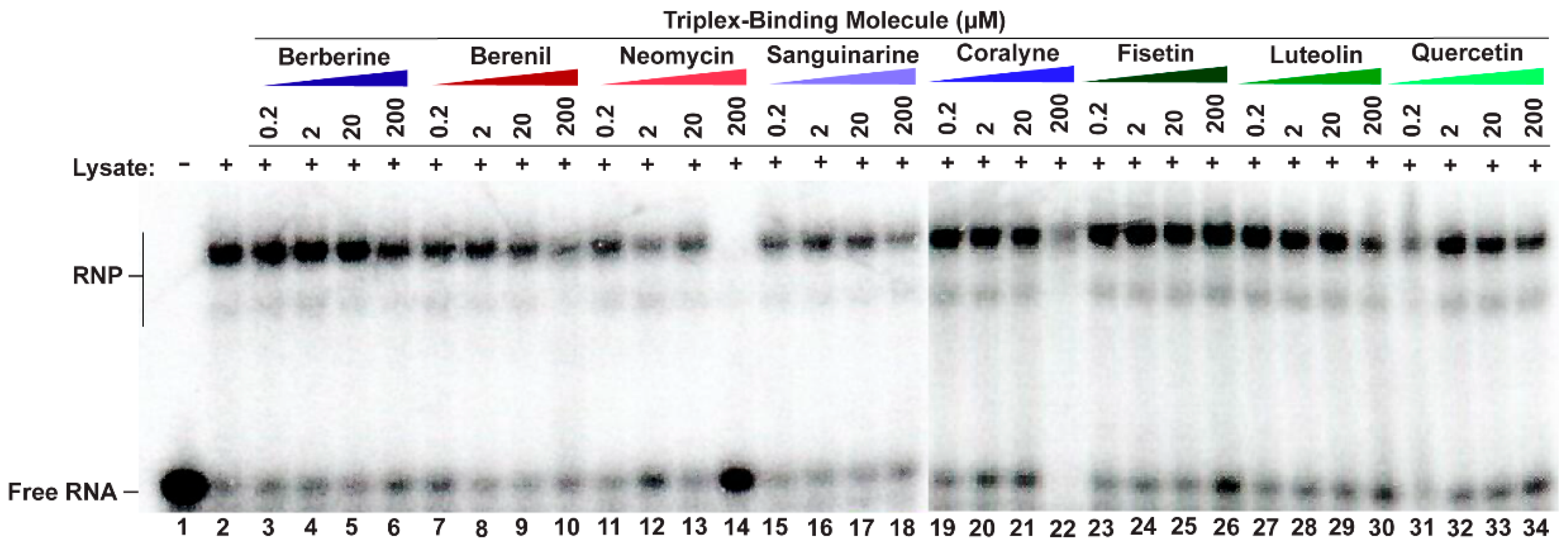
We next sought to probe each TBM’s ability to selectively target the MALAT1 triple helix in the presence of its protein-binding partners, such as METTL16 [12]. Although the exact binding interaction is unknown for the METTL16•MALAT1 triple helix complex, one would expect that the TBMs could prevent binding of METTL16 if they preferentially bind to a similar region of the major-groove triple helix region as does METTL16 [12]. Therefore, a competitive electrophoretic mobility shift assay (EMSA) was performed with increasing amounts of TBMs (0.2, 2, 20, and 200 µM) in the presence of whole-cell lysate extracted from HCT116 cells, a human colorectal carcinoma cell line. As observed previously using HEK293 cell lysate, ribonucleoprotein (RNP) complex formation also occurred in the presence of HCT116 cell lysate (Figure 3, lane 2) [12]. All TBMs, except for 200 µM neomycin (Figure 3, lane 14), did not reduce RNP formation (Figure 3, lanes 3–13, 15–34). This result is not too surprising considering these TBMs are known to bind multiple cellular targets, such as various proteins [45,46,47], ribosomes [48], microtubules [49,50], and G-quadruplexes [51,52,53,54] (Table S5). Therefore, it is possible that the TBMs are binding to these other cellular components instead. These TBMs cannot outcompete MALAT1 triple helix-binding proteins, such as METTL16 [12], suggesting either off-target binding by the TBMs or non-overlapping binding sites.
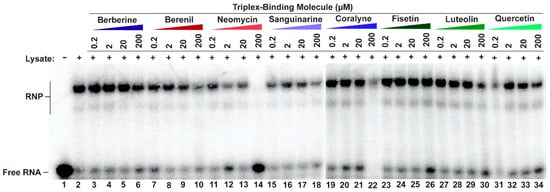
Figure 3.
Competitive EMSA showing most TBMs cannot disrupt RNP complex formation. A 5′-[32P]-radiolabeled MALAT1 triple helix was incubated in the presence of HCT116 cell lysate and increasing amounts of a competitor TBM. Please note that there are two native gel-shift images because not all samples could be loaded onto a single gel; the divide occurs between lanes 18 and 19.
2.3. Select TBMs Interact with RNAs Mimicking the Premature MALAT1 Triple Helix States
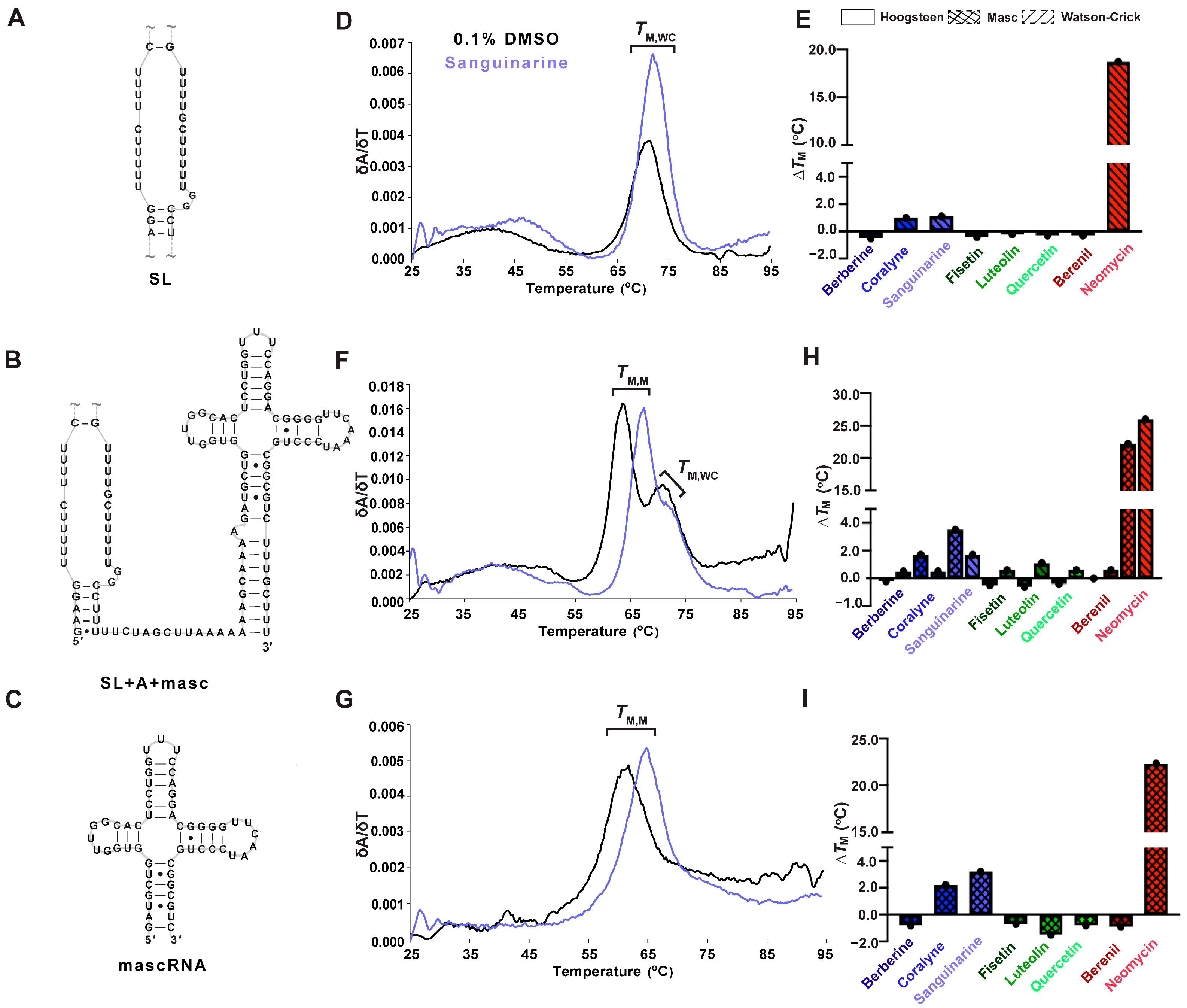
Because RNA folding occurs co-transcriptionally and the 3′ end of MALAT1 likely undergoes processing prior to formation of the mature MALAT1 triple helix (SL+A) [7,10,55], we were interested in determining whether TBMs could also target the premature MALAT1 RNAs (i.e., MALAT1 SL and MALAT1 SL+A-rich tract+mascRNA (SL+A+masc)) as well as mascRNA to monitor effects due to that unique structure [10] (Figure 4A–C, Table S6). The SL, which cannot form a triple helix but still contains structural motifs such as single mismatches and bulged loops like SL+A, exhibits a single-peak melting profile, i.e., only the TM,WC peak at 71.2 ± 0.3 °C (Figure 4A,D, Table S7). In general, the ∆TM,WC values were similar for both SL and SL+A in the presence of TBMs, except berberine, sanguinarine, and berenil exhibited about a 2 °C destabilization relative to SL+A (Table 1 and Table S8). This result suggests that the TBMs bind to a major-groove triple helix as opposed to an RNA lacking base triples (Figure 2C and Figure 4E, Tables S7 and S8).
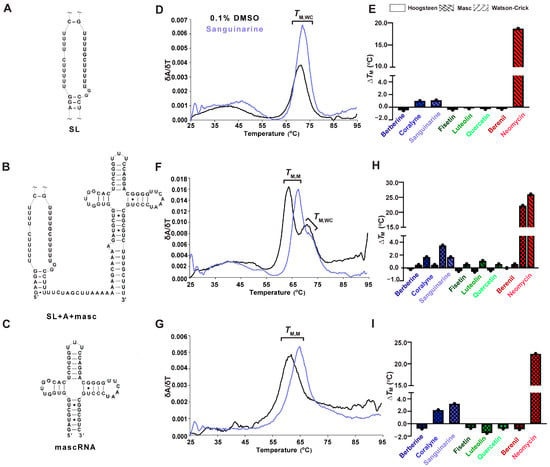
Figure 4.
UV thermal melting results of premature MALAT1 RNAs in the absence and presence of TBMs. For each RNA, a schematic denoting the MALAT1 (A) SL, (B) SL+A+mascRNA, and (C) mascRNA is on the left; (D,F,G) first derivative versus temperature plot is in the center; and (E,H,I) a bar plot showing change in melting temperatures (ΔTM) is shown for each TBM binding to the MALAT1 RNAs. All bar plots display the average ∆TM (n = 3), denoted by a single black dot. A tilde (~) denotes that peripheral RNA regions were present in the experiment but are not shown in the schematic for brevity. All RNAs used in this experiment are unimolecular, like the RNA shown in Figure 1B. All melting temperature values are compiled in Tables S7 and S8, and first derivative plots for all TBMs are presented in Figure S7. Raw and processed UV data are presented in File S1.
MALAT1 SL+A+masc results in two distinct melting transitions at 64.0 ± 0.3 and 70.1 ± 0.5 °C (Figure 4F, Table S7). The peak at 70.1 ± 0.5 °C is assigned to TM,WC because it is similar to TM,WC peaks observed for SL and SL+A RNAs (Figure 2B and Figure 4D,E, Table 1 and Table S7). We hypothesized that the other peak represents the melting of mascRNA, and indeed the melting temperature for pre-mascRNA (64.0 ± 0.1 °C, Figure S6) and mascRNA (61.6 ± 0.1 °C, Figure 4G, Table S7) corresponds to 64.0 ± 0.3 °C for SL+A+masc (Figure 4F, Table S7). Therefore, we assigned that peak as TM,M, where “M” represents mascRNA (Figure 4F,G). Please note that both SL and SL+A+masc have a broad transition present at ~48.5 ± 0.9 °C for no TBM added (Figure 4D,F). This peak may represent non-canonical U•U base pairs in the U-rich internal loop [56]. These short, broad peaks are interpreted as low-confidence because (i) their peak height is only 15% of WT peak heights and (ii) they are not reproducible in all replicates (Figure S7, Table S7 and File S1). We report the values in Table S7 but do not discuss them due to their uncertainty compared to TM,M and TM,WC.
The ∆TM,M values for SL+A+masc and mascRNA are mostly similar. Mild destabilization (−0.2 to −1.5 °C) is observed for berberine, all flavonoids, and berenil, whereas coralyne, sanguinarine, and neomycin stabilized RNAs by 1.7 to 23 °C (Figure 4H,I, Table S8). The ∆TM,WC values of SL+A+masc are comparable to SL+A and SL, except for luteolin, which stabilizes 1.1 °C (Figure 4E,H, Table S8). These results suggest that the flavonoids do not significantly stabilize or destabilize premature or mature MALAT1 RNAs. Additionally, berberine and berenil could be promising therapeutics for targeting mature MALAT1, as they alter the thermal stability for the SL+A at a higher magnitude compared to SL, SL+A+masc, and mascRNA.
2.4. Most TBMs Rapidly Associate with the Premature and Mature MALAT1 Triple Helix
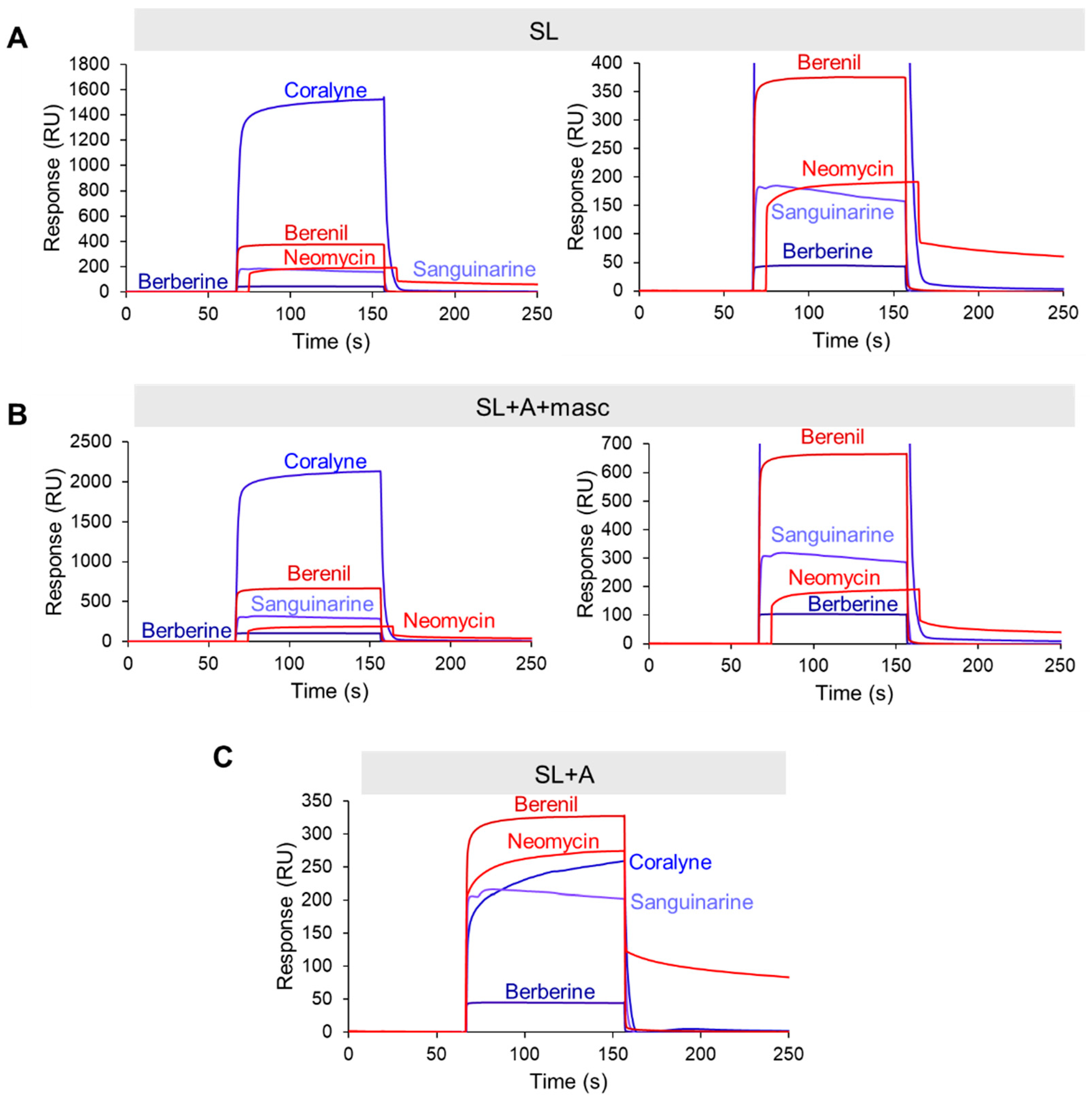
RNase P efficiently processes the 3′ end of MALAT1; therefore, any TBM targeting the precursor states would need to occur rapidly [10]. We employed surface plasmon resonance (SPR) to evaluate the relative binding trends of the TBMs engaging with the MALAT1 SL, SL+A+masc, and SL+A. We did not evaluate mascRNA, as the TBMs did not reveal a major impact on thermal stability (i.e., ∆TM < 3.2 °C, Table S8). To perform this experiment, we first modified each RNA by extending the 5′ end with a 24-nucleotide sequence that is complementary to a 3′-biotinylated DNA oligonucleotide (Table S6). This biotinylated DNA-RNA hybrid was immobilized onto the streptavidin-coated SPR sensor chip, followed by the injection of TBMs (0, 1, 10, and 100 µM) (Figure S8). Binding trends were inferred at 100 µM TBM concentration to ensure steady-state and saturation of the RNA. Our UV melting results revealed that the flavonoids did not alter the thermal stability of MALAT1 SL+A (Figure 2C) and SL+A+masc (Figure 4H); therefore, we did not examine these TBMs. We analyzed the SPR sensograms to qualitatively examine the relative association and dissociation slopes for MALAT1 SL, SL+A+masc, and SL+A (Figure 5A–C and Figure S9). Changes in the slope of the association phase were used to rank the TBMs on binding speed. Quick binding interactions were defined as steep slopes lacking curvature, while slow binding was gradual slopes with rounded curvature. Based on these criteria, the relative rankings from fastest to slowest are the same for all three RNAs: berberine = sanguinarine > berenil > coralyne > neomycin (Figure 5A–C and Figure S9). Coralyne displays an unusually large response, which may indicate self-association [38]. Dissociation slopes were relatively steep and similar for all TBMs, although neomycin did not fully dissociate after 100 s (Figure S9E). These results show that the five TBMs interact transiently with the three different RNAs, suggesting that it is possible for the TBMs to dual target the premature and mature forms of the MALAT1 triple helix.
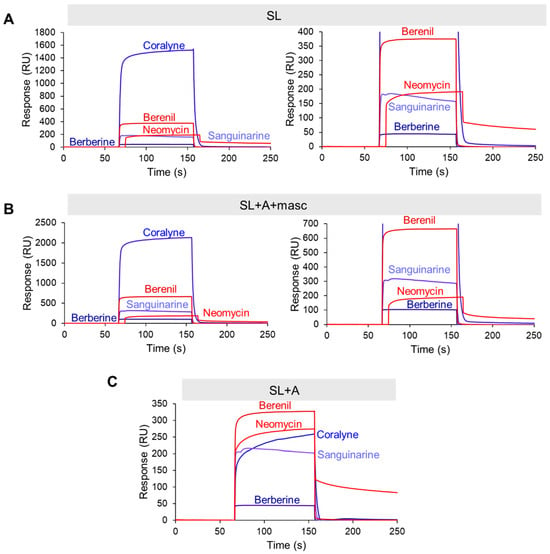
Figure 5.
SPR sensogram plots for TBMs binding to the premature and mature MALAT1 RNAs. Representative SPR sensograms are shown for (A) SL, (B) SL+A+masc, and (C) SL+A. Association step lasted 90 s, corresponding to the 59.1 s to 151.1 s time points on the plots. The concentration of TBM was 100 µM. Right panels for A and B show sensograms for SL and SL+A+masc with max y-axis values at 400 and 700 RU, respectively. All SPR sensograms are presented in Figures S8 and S9. All raw sensogram data are available in File S3.
2.5. TBMs Reduce MALAT1 Levels More Than MENβ in HCT116 Cells
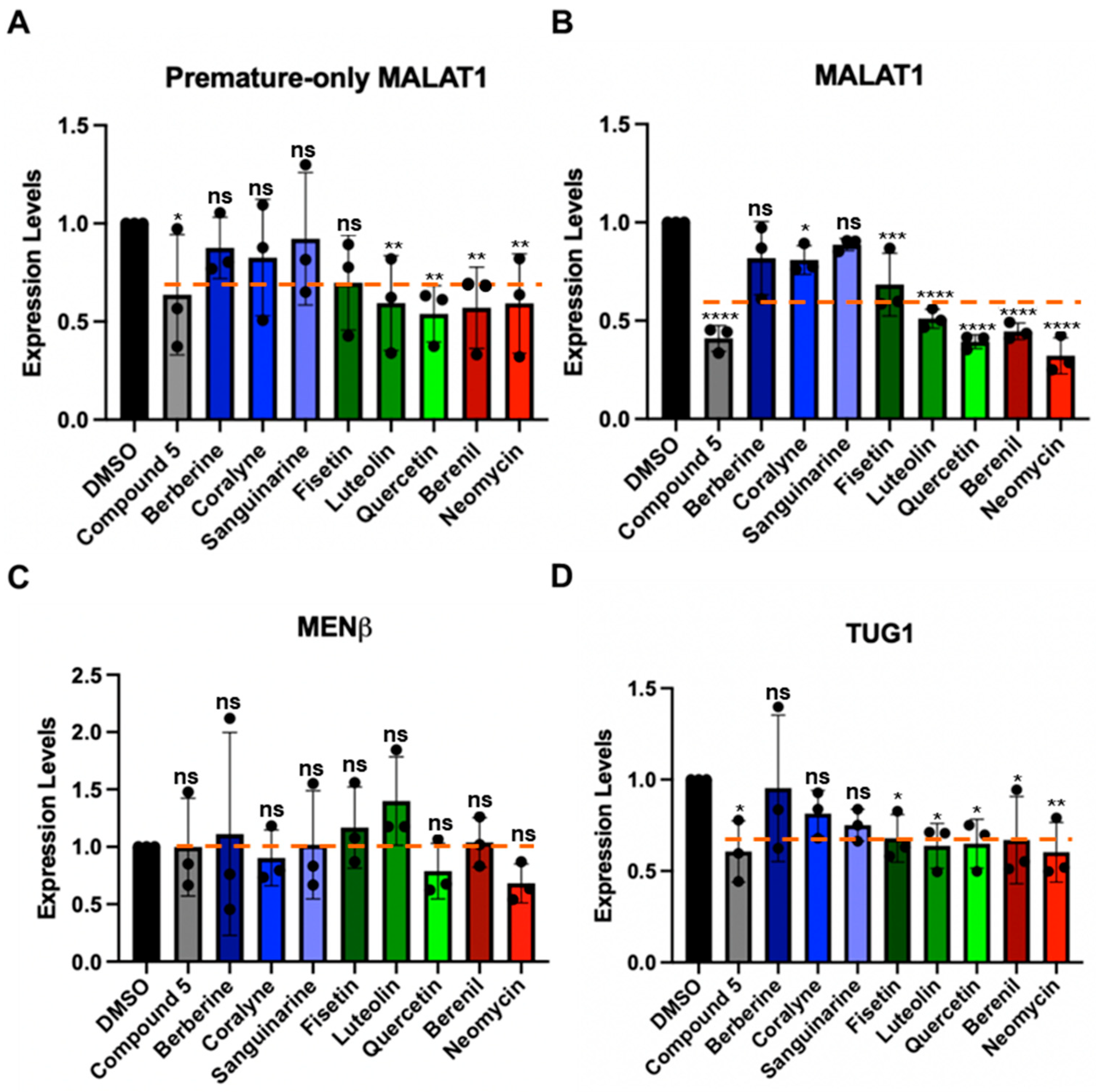
Although the TBMs interact with MALAT1 SL+A and SL+A+masc in vitro (Figure 4 and Figure 5), we also determined if they could alter the levels of mature and premature MALAT1 inside cells. HCT116 cells were treated with 1 µM of each TBM because previously 1 µM of compound 5 (Figure S5) and 1 µM of quercetin (Figure 1D) were shown to decrease MALAT1 levels by ~50% in the mouse mammary tumor virus-polyoma middle tumor-antigen (MMTV-PyMT) tumor organoid model and MCF7 breast cancer cell lines, respectively [16,20]. Furthermore, an MTT assay showed no measurable cytotoxic effects when HCT116 cells were treated with 1 µM TBM (Figure S10 and File S4) [16]. First, we used a primer pair that will detect only premature MALAT1 (Figure 6A) and a primer pair that detects both the premature and mature forms of MALAT1 (Figure 6B, Table S9). The positive control, compound 5 (Figure S5), reduced MALAT1 levels by 50%, as observed previously in MMTV-PyMT tumor organoids, and had a similar effect on lowering premature-only MALAT1 (Figure 6A,B, Table S10) [16]. Except for the three alkaloids, the levels of mature and premature MALAT1 (Figure 6A,B, Table S10) were reduced by ~30–70% (Figure 6B, Table S10). The alkaloids had a more modest decrease in approximately ~20% (Figure 6B, Table S10). Additionally, quercetin reduced MALAT1 levels 60% in HCT116 cells (Figure 6B, Table S10), which is similar to the levels measured for MCF7 cells [20].
We next tested whether the TBMs altered the expression of MENβ, an lncRNA that ends in a putative triple helix akin to that of the MALAT1 SL+A, and the intron 1-retained TUG1 lncRNA, which currently does not have any known triple-helical stability elements; its expression levels are similar to the MALAT1 and MENβ lncRNAs, and unspliced TUG1 localizes in the nucleus (Table S11) [57]. None of the TBMs significantly decrease MENβ compared to MALAT1 (Figure 6B,C), and the TBMs exhibit a selectivity factor of 1.0 to 2.6 for mature MALAT1 over MENβ (Table S12). All TBMs, except for berberine, decreased the levels of unspliced TUG1 (Figure 6D, Table S10). In general, the average percent decrease in unspliced TUG1 (30%) was comparable to or slightly less than the percent decrease in mature and premature (40%) and premature-only (30%) MALAT1 (Table S10). Additionally, the percent decrease in all three RNAs was greater than for MENβ (0%) (Table S10), demonstrating slightly more selective targeting of MALAT1 by TBMs yet revealing off-target effects. Overall, these results demonstrate that flavonoids, berenil, and neomycin affect the expression of other lncRNA and premature-only MALAT1. However, there is still a larger effect on MALAT1, providing evidence that, amongst the TBMs tested herein, the flavonoids and berenil are the most promising scaffolds for selective targeting of the MALAT1 triple helix.
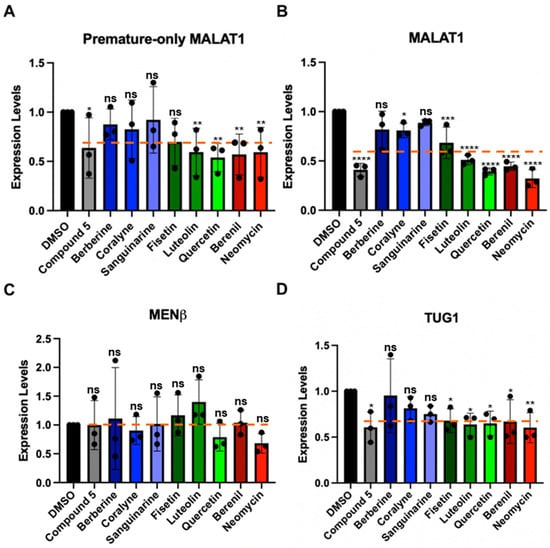
Figure 6.
Effect of TBMs on the expression levels of various RNAs in HCT116 cells. RT-qPCR results show changes in expression for (A) premature-only MALAT1, (B) premature and mature MALAT1, (C) MENβ, and (D) TUG1 (unspliced Intron 1) when HCT116 cells were treated for 48 h with 1 μM of each TBM (berberine, coralyne, sanguinarine, fisetin, luteolin, quercetin, berenil, and neomycin) and with a previously studied TBM: compound 5 (Figure S5) [16]. The expression values were first normalized with respect to the geometric mean of four housekeepers: 18s rRNA, beta-actin (β-actin), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and U6 snRNA. The resulting values were then normalized with respect to the DMSO-treated sample set at an arbitrary value of 1 [58]. Results are averages ± standard deviation of biological triplicates (n = 3). p-values were calculated from the average 2−∆∆CT using a two-way ANOVA test, comparing each TBM to the DMSO control: **** p-value < 0.0001, *** p-value < 0.0002, ** p-value < 0.0021, * p-value < 0.0332, ns p-value < 0.1234. The software used for statistical analysis was GraphPad Prism 10 (RRID:SCR_002798). An orange dashed line indicates average expression change for each RNA target across all TBMs. Raw and processed RT-qPCR data, including PCR efficiencies (90.1–98.7%), are presented in File S4.
Figure 6.
Effect of TBMs on the expression levels of various RNAs in HCT116 cells. RT-qPCR results show changes in expression for (A) premature-only MALAT1, (B) premature and mature MALAT1, (C) MENβ, and (D) TUG1 (unspliced Intron 1) when HCT116 cells were treated for 48 h with 1 μM of each TBM (berberine, coralyne, sanguinarine, fisetin, luteolin, quercetin, berenil, and neomycin) and with a previously studied TBM: compound 5 (Figure S5) [16]. The expression values were first normalized with respect to the geometric mean of four housekeepers: 18s rRNA, beta-actin (β-actin), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and U6 snRNA. The resulting values were then normalized with respect to the DMSO-treated sample set at an arbitrary value of 1 [58]. Results are averages ± standard deviation of biological triplicates (n = 3). p-values were calculated from the average 2−∆∆CT using a two-way ANOVA test, comparing each TBM to the DMSO control: **** p-value < 0.0001, *** p-value < 0.0002, ** p-value < 0.0021, * p-value < 0.0332, ns p-value < 0.1234. The software used for statistical analysis was GraphPad Prism 10 (RRID:SCR_002798). An orange dashed line indicates average expression change for each RNA target across all TBMs. Raw and processed RT-qPCR data, including PCR efficiencies (90.1–98.7%), are presented in File S4.
3. Discussion
MALAT1 has garnered tremendous research interest as a diagnostic biomarker and as a therapeutic target, particularly with the triple helix being a major drug target [5,14,15,16,17,18,19,20,21,22,34,59]. However, the molecular details of TBMs interacting with triple helices are comparatively less understood than small molecules binding to double-stranded DNA/RNA. Several factors influence how a small molecule recognizes RNA versus a protein, such as a high electronegative surface potential and a lack of structural diversity due to only four dominant nucleobases and a less pronounced binding pocket [60]. In general, small molecules that bind to RNA share the following features: heteroatom-rich aromatic rings, numerous hydrogen bond donors and acceptors, large polar surface area, low octanol-water partition coefficient, and 3D rod-like shape [60]. Thus far, the following small molecules have been investigated for their ability to interact with the MALAT1 triple helix: DPF derivatives [14,15], an imidazole-derived compound [16], a benzimidazole-derived compound [16], 1,2,3-triazole-derived compounds [18], an aromatic sulfonamide like MTC07 [19], berenil derivatives [17], and quercetin [20] (Figure 1D and Figure S5). Previous studies involving DPF and berenil derivatives demonstrated that small molecules with a 3D-rod shape, rather than sphere or disc shapes, prefer to bind to the MALAT1 triple helix and require 8–10 base triples for 1:1 binding [14,15,17,61]. In our study, we expanded this collection of small molecules with diverse classes of previously reported TBMs such as the alkaloids with polycyclic aromatic rings (berberine, coralyne, and sanguinarine); flavonoids rich in hydrogen bond donor and acceptor sites (fisetin, luteolin, and quercetin); triazene (berenil) with a 3D rod shape; and aminoglycoside (neomycin) with hydrogen bond donor and acceptor groups attached to aliphatic rings (Figure 1D) [24,25,26,28,29,30,31,32]. We determined that (i) berberine and berenil predominately stabilize only the WT MALAT1 SL+A (Figure 2 and Figure 4), (ii) alkaloids display greater sensitivity to the length of the MALAT1 triple helix, whereas base composition impacts the flavonoids (Figure 2D–H), (iii) most TBMs have a selectivity factor for MALAT1 over MENβ greater than 2, demonstrating selective targeting of the MALAT1 triple helix over the similar MENβ triple helix (Table S10), and (iv) neomycin shows mostly non-specific behavior with all MALAT1 RNAs (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6).
The alkaloid TBMs berberine and sanguinarine are naturally occurring, and coralyne is a synthetic analogue of berberine (Figure 1D). All three alkaloids preferentially stabilize the interactions that contribute to the Hoogsteen melting temperature of a poly(U•A-U) triple helix by ~3–17 °C and in the much shorter U•A-U triple helix of MALAT1 by ~2.5–7.8 °C (Figure 2C and Figure S11) [24,25,26]. Amongst all TBMs tested herein, the alkaloids are an attractive scaffold because of (i) sensitivity to length and nucleotide composition of the triple helix, suggesting fewer off-target effects (Figure 2), and (ii) their fast association and increased binding response for berberine and sanguinarine to the MALAT1 SL+A+masc and SL+A, suggesting the potential to dual-target the premature and mature forms of the MALAT1 triple helix (Figure 5). Their major disadvantage is their mild decrease in MALAT1 levels in HCT116 cells (Figure 6A,B), although their poor performance could be due to other factors.
All three flavonoids examined herein are naturally occurring and readily available as antioxidant dietary supplements (Table S5) [62,63,64]. The three flavonoid TBMs show selective stabilization for Hoogsteen melting (~14–17 °C) (Figure S11) over Watson-Crick (<5 °C) in the presence of poly(U•A-U) triple helices and/or poly(A-U) duplexes [28,29,30]. In contrast, all three flavonoids mildly destabilized Hoogsteen melting of the various MALAT1 triple helices, including the all U•A-U triple helix variant c, with ΔTM,H and ΔTM,WC values of approximately −3.7 °C (Figure 2C,F and Figure S11, Table S2). These different trends may be due to structural context because, unlike poly(U•A-U) triple helices, the major-groove triple helix of MALAT1 and variant c is flanked by various structures: double-stranded RNA, a GG bulge, and an A-minor motif (Figure 1B) [11,65]. The flavonoids are an interesting scaffold, as they demonstrate unique sensitivity to changes in nucleotide composition and A-minor disruptions compared to base triple length, which the flavonoids did not have a significant impact on the TM,H for variant d-e (Figure 2D–J). FpocketR analysis shows unique binding pockets predominately in the duplex-triplex junction, where all three flavonoids are predicted to bind in our computational binding analysis (Figures S3 and S4). Quercetin was shown to bind along the major groove in silico, which is stabilized via electrostatic and hydrogen bond interactions with the MALAT1 triple helix [20]. Other studies reported intercalative binding interactions with poly(U•A-U), resulting in the stabilization of the RNA construct [29]. In this study, we observed destabilization for the poly(U•A-U) variant c as well as variants with increased C+•G-C base triples (Figure 2D–F). Unlike the poly(U•A-U) triple helices, MALAT1 is surrounded by duplexes and unstructured loops, which could explain the difference in binding trends. Alterations to the MALAT1 triple helix composition could modify base stacking and electrostatic interactions, possibly influencing the binding mode of the flavonoids and disruption of the RNA structure. Unlike the alkaloids in their iminium state [66], the flavonoids are not charged at neutral pH [29], which could influence the binding interactions when nucleotide composition is altered.
Berenil (diminazene aceturate) is one of the earliest known compounds tested for binding to poly (U•A-U or T•A-T) triple helices [31]. For the MALAT1 triple helix, berenil increases Hoogsteen melting more than Watson-Crick and does not alter the thermal stability of MALAT1 triple helix variants nor the premature forms of the MALAT1 triple helix (Figure 2 and Figure 4). Berenil has an established equilibrium dissociation constant of ~1.4 to 34.1 µM for poly(U•A-U) triple helices [17,31]. Differential scanning calorimetry (DSC) showed that binding of berenil is enthalpically driven for DNA/RNA triple helices but not the corresponding duplexes; a similar outcome was reported for berenil derivatives bound to the MALAT1 triple helix based on isothermal titration calorimetry results [17,31]. Our study, along with Zafferani et al., who used berenil-derived compounds to target the MALAT1 triple helix [17], shows that berenil alone, although more selective than most TBMs, is not sufficient to achieve selective binding to the MALAT1 triple helix but requires incorporating rod-like properties through chemical modifications.
Neomycin is an aminoglycoside antibiotic that binds in a pocket composed of noncanonical base pairs and nucleotide bulges of the A-site in Escherichia coli 16s rRNA [67]. Neomycin selectively increases Hoogsteen melting of 22-base triple U•A-U and T•A-T triple helices by approximately 4–25 °C (Figure S11), which is similar to our observed ∆TM,H of ~31 °C for the MALAT1 triple helix (Figure 2 and Figure 4) [32]. Neomycin appears to have nonspecific interactions likely influenced by its positive charge (Figure 1D). Additionally, neomycin thermally stabilizes the Watson-Crick interface and has similar binding responses for premature and mature MALAT1 triple helixes (Figure 4H,I and Figure 5). Our study confirms the suspicion of others in the field: that aminoglycosides like neomycin are a poor choice to specifically target the MALAT1 triple helix [14,68].
Among all small molecules known to bind the MALAT1 triple helix (Figure 1D and Figure S5), the tightest binders are 1,2,3-triazole-derived compounds 3, 7, 10, and 15 (KD = 13 nM to 655 nM) [18] = diphenylfuran derivatives (EC50 = 27 nM to 1.6 µM) [14,15] > compound 5 (KD = 2.3 µM) [16] > DMZ-M1 (KD = 5.0 µM) [17] > compound 16 (KD = 6.1 µM) [16] > quercetin (KD = 0.5 µM) [20] > MTC07 (KD = 400 µM) [19]. Although test-tube assays are informative, cell-based assays are needed to determine if small molecules can decrease MALAT1 levels, which is the desired outcome to treat most MALAT1-upregulated human diseases, particularly cancer. Six of the eight TBMs have been explored previously to treat cancer: berberine [69], coralyne [70], sanguinarine [71], fisetin [72], luteolin [62], quercetin [73], and berenil [74] (Table S5). Only neomycin reduced RNP formation in the competitive EMSAs (Figure 3), and most TBMs decreased premature and mature MALAT1 levels in treated HCT116 cells (Figure 6A,B). The maturation of the MALAT1 triple helix via RNase P cleavage is vital for the stabilization and the accumulation of MALAT1 [10]. This brings to question whether TBMs could recognize the MALAT1 SL+A+masc prior to RNase P cleavage, disrupting maturation and the accumulation of MALAT1. Our in vitro thermal denaturation and SPR assays suggest that most TBMs prefer binding to the MALAT1 triple helix and the precursor SL+A+masc rather than the U-rich SL (Figure 4 and Figure 5). Importantly, TBM binding is relatively rapid, suggesting berenil could decrease maturation efficiency via RNase P (Figure 5). However, cell-based assays showed berenil reducing mature and premature MALAT1 levels similarly (Figure 6A,B). Importantly, MENβ levels were not significantly reduced even though this lncRNA terminates in a triple helix that is predicted to be highly similar to that of MALAT1 (Figure 6C) [8,9]. Thus, it is not clear if TBMs reduce MALAT1 levels via direct interference with the triple helix or by other mechanisms. Additionally, we cannot rule out that the TBMs could be interacting with the peripheral structural motifs surrounding the MALAT1 triple helix.
To date, there are six TBMs whose selectivity factor for reducing MALAT1, but not MENβ, is at least two: luteolin (2.6), berenil (2.3), neomycin (2.1), compound 5 (~2.0–2.2) [16], compound 16 (~2.1) [16], and quercetin (~2.0) (Table S12) [20]. Comparing expression levels for only MALAT1 and MENβ provides an inadequate assessment of selectivity. Compound 5, despite demonstrating selective targeting of MALAT1 over MENβ in MMTV-PyMT cells, also reduced unspliced TUG1 levels (Figure 6D, Table S10). No RNA-seq studies have been performed on human cells treated with any of the eight TBMs studied herein, but most TBMs appear to be promiscuous given their widespread pharmacological effects and disparate biological targets (Table S5). Additionally, TBM dose is important because compound 1a for DMPK mRNA displays higher selectivity at higher doses [75]. In theory, developing a MALAT1-specific TBM should be achievable based on our results presented herein and published literature. One such example of a small molecule specifically targeting an RNA is Risdiplam, an SMN2 splicing modulator compound, which causes minimal off-target effects on pre-mRNA splicing [76]. One important consideration is the cellular localization of small molecules inside cells. A recent study [77] showed that small molecules like berberine are enriched in the mitochondria, yet MALAT1 is predominantly in nuclear speckles [6] (Tables S5 and S11) [78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128]. Other studies show that sanguinarine [97], fisetin [129], and quercetin [130] are localized in the nucleus, but we did not observe any correlation between the percent decrease in nuclear lncRNAs like premature or mature MALAT1 [6], MENβ [131], and unspliced TUG1 [57] and TBM localization, likely because the net effect is a culmination of multiple factors such as cellular uptake of TBM, cellular localization of TBM, metabolism of TBM, free TBM concentration, RNA recognition, and off-target effects such as interactions with other cellular components that could impact RNA expression (Figure 6, Table S5). Our study demonstrates that TBMs reduced MALAT1 levels more than other nuclear lncRNAs, but further work is needed to improve TBM specificity, cellular localization, and selectivity of the MALAT1 lncRNA over other RNA types.
In summary, this study expands our characterization-knowledge base of TBMs that interact with the MALAT1 triple helix. In addition to compound 5, DPFp8, berenil, and its derivatives (Figure 1D and Figure S5), TBMs like alkaloids (with extended aromatic π systems) and flavonoids (with rich hydrogen bond donor and acceptor sites) may represent a suitable scaffold but would benefit greatly from a 3D structure of the MALAT1 triple helix in complex with a TBM. Other classes that remain underexplored include ruthenium and other metal complexes [132], polyamines [133], electrophilic covalent inhibitors [134], and TBMs conjugated to oligonucleotides [135]. Another intriguing possibility would be synthetic cyclic mismatch-binding ligands that bind to RNA via pseudo-canonical base pairs [136]. Additionally, human health and medicine, small molecules that bind to double-stranded RNA, such as psoralen [137], and G-quadruplexes, such as QUMA-1 [138], represent research tools that have expanded our knowledge of the biological and cellular roles of non-canonical structures of nucleic acids. A TBM with exquisite specificity for triple helices could do the same.
4. Materials and Methods
4.1. Preparation of RNA
DNA oligonucleotides were chemically synthesized by Sigma-Aldrich (St. Louis, MO, USA); these oligonucleotides were used in PCR reactions to generate the DNA template needed for in vitro transcription reactions. Wild-type MALAT1 triple helix, extended MALAT1 triple helix, and MALAT1 triple helix variants (Table S6) were generated via in vitro transcription using homemade T7 RNA polymerase as previously described [9]. RNAs were gel purified, treated with phenol-chloroform (Thermo Fisher Scientific, Waltham, MA, USA), and precipitated using isopropanol (JT Baker, Radnor, PA, USA). The final RNA concentrations were measured at room temperature using a NanoDropTMOneC (Thermo Fisher Scientific, Waltham, MA, USA) to determine absorbance at 260 nm. For the competitive EMSAs, the 5′ end of the MALAT1 triple helix was dephosphorylated using calf intestinal alkaline phosphatase (CIP) (New England Biolabs, Ipswich, MA, USA) and radiolabeled using γ-[32P]ATP (~7000 Ci/mmol, PerkinElmer, Shelton, CT, USA) and T4 PNK (New England Biolabs, Ipswich, MA, USA) per the manufacturer’s protocol. G25 microspin columns (GE Healthcare, Chicago, IL, USA) were used to remove excess γ-[32P]ATP.
4.2. Triplex-Binding Molecules
All triplex-binding molecules (TBMs) were purchased from various commercial vendors with >90% purity and used as such. Berberine, berenil, compound 5 (i.e., MALAT1-IN-5), coralyne, neomycin, quercetin, and sanguinarine were purchased from Sigma-Aldrich (St. Louis, MO, USA); fisetin was purchased from PhytoLab (GmbH & Co., Vestenbergsgreuth, Germany); and luteolin was acquired from Indofine chemicals (Hillsborough, NJ, USA). Water-soluble compounds (i.e., berberine, berenil, coralyne, and neomycin) were dissolved in deionized autoclaved water. Stock solutions for fisetin, luteolin, quercetin, sanguinarine, and compound 5 were made by dissolving molecules in 100% DMSO (AmericanBio, Canton, MA, USA) and further diluted with deionized water until the final DMSO concentration was 0.1%. Unless stated otherwise, 0.1% DMSO was maintained in all the samples irrespective of solubility.
4.3. UV Thermal Denaturation Assay
A Cary 3500 Multicell UV-Vis Spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) was used to conduct all the UV thermal denaturation assays along with quartz cuvettes (Starna Cells, Inc., Atascadero, CA, USA) having an optical path length of 1 cm. For each sample, the total RNA concentration (with or without TBMs) was maintained at 0.5 μM. TBM concentration was 10 µM (i.e., RNA:TBM stoichiometry was 1:20). All RNA absorbances (0.3–2.1, see File S1) were measured within the linear range (<6) of the Cary 3500 Multicell UV-Vis Spectrophotometer [139]. RNA samples were prepared in low ionic UV buffer (25 mM sodium cacodylate pH 7.0 (Sigma-Aldrich, St. Louis, MO, USA), 50 mM KCl (Sigma-Aldrich, St. Louis, MO, USA), 0.1 mM MgCl2 (Sigma-Aldrich, St. Louis, MO, USA), and 0.1% DMSO) and were folded by heating (25 °C to 95 °C) and cooling (95 °C to 25 °C) at a ramp rate of 5 °C/min. TBMs were added (maintaining the final DMSO concentration at 0.1% except when 2% DMSO was used for flavonoids) soon after the folding step and incubated for an additional 30 min at 25 °C. Absorbance was measured at 260 nm, which is the same wavelength used for previous MALAT1 triple helix thermal denaturation assays [9,14,20,33,59,65,140]. Absorbance was recorded at 0.3 °C intervals from 25 °C to 95 °C at a ramp rate of 0.8 °C/min. All the melting curves were buffer subtracted, and the melting temperatures were extrapolated from the peak maxima of first derivatives of the melting curves (δA/δT) that were smoothed over 1.2 °C using the Savitzky-Golay method. Raw and processed data are provided in File S1.
4.4. Predicting RNA-Ligand Complexes and FpocketR
RNA-ligand complex predictions for TBMs bound to the MALAT1 triple helix crystal structure (PDB ID: 4plx) (nts 1-76) were generated via AlphaFold3 (AlphaFold3 inference pipeline v3.0.1) [40]. Inputs were the 4plx RNA FASTA and TBM smiles, which are included in File S2. The PDB complex output files were analyzed using PyMOL (v. 4.6.0, PyMOL. Retrieved from http://www.pymol.org/pymol; accessed on 23 September 2025).
FpocketR software (version 1.3.4) was used to compute potential binding pockets of the MALAT1 variants d-e (sequences are provided in Table S7) [43,44]. The MALAT1 triple helix variants d-e were generated using AlphaFold 3 via the AlphaFold 3 (AlphaFold3 inference pipeline v3.0.1) and exported as PDBs. The input sequences are those shown in Figure 2G,H. These RNAs were then used as the input for FpocketR software (version 1.3.4). Visual presentation of these binding pockets was completed using PyMOL (v. 4.6.0).
4.5. SPR
This SPR protocol was adopted from previously published methods [141,142,143]. All SPR experiments were conducted using a BiacoreTM T200 (Cytiva Life Sciences, Marlborough, MA, USA) surface plasmon resonance system with a high-affinity streptavidin (SA) sensor chip (Cytiva Life Sciences, Marlborough, MA, USA) to immobilize biotinylated molecules for SPR interaction analysis. The MALAT1 SL, MALAT1 SL+A+masc, and MALAT1 SL+A have a 5′-24-nucleotide extension (Table S6) that was annealed to a complementary 3′-biotinylated DNA oligonucleotide (5′-TTCACAGTGGCTAAGTTCCGC-3′ (Sigma-Aldrich, St. Louis, MO, USA)). RNA was folded using a high-ionic SPR buffer (10 mM Tris pH 7.5 (Thermo Fisher Scientific, Waltham, MA, USA), 1 mM MgCl2, 152.6 mM NaCl (Sigma-Aldrich, St. Louis, MO, USA)). Before immobilization, the SA sensor chips were activated using 50 mM NaOH and 1 M NaCl, followed by priming the sensor chip with the high-ionic SPR running buffer. For the immobilization, 25 nM of the extended RNA and 25 nM of the 3′-biotinylated oligonucleotide (1:1 stoichiometry) were folded by heating to 95 °C for 5 min, snap cooling on ice for 10 min, followed by incubation at room temperature for 1.5 h. After attaining the required baseline stabilization, the annealed biotinylated DNA-RNA hybrid was immobilized onto the streptavidin chip with a flow rate of 1 μL/min for about 100 min. The SPR running buffer was the high-ionic SPR buffer supplemented with 0.005% Tween-20 (AmericanBio, Inc., Canton, MA, USA) and 3% DMSO. After the immobilization step, varying concentrations of TBMs (0, 1, 10, and 100 μM, from low to high concentration to avoid carryover artifacts from high TBM concentrations) (Figure S8) were injected with a 90-s association step and varying dissociation times (350 to 1000 s) dependent on test injections at a flow rate of 50 μL/min. A reference subtraction method was used for each TBM injection, which corresponds to the subtraction from the empty reference flow cell. Raw and processed data are in File S3.
4.6. Culturing HCT116 Cells
Human colorectal carcinoma (HCT116) cells (RRID:CVCL_0291) were grown in complete McCoy’s 5A (modified) media (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA), 2 mM glutamate, and 1× penicillin-streptomycin (Thermo Fisher-Scientific, Waltham, MA, USA) at 37 °C with 5% CO2.
4.7. Preparation of Native HCT116 Whole-Cell Lysate
HCT116 cells (3 × 100-mm tissue culture plates) were harvested at ~90% confluency. After trypsinization, cells were centrifuged at 1200 rpm for 5 min. Cell pellets were resuspended in 500 µL native lysate buffer (50 mM Tris pH 7 at room temperature, 100 mM KCl, 0.2 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA), 1 mM MgCl2, 10% glycerol (AmericanBio., Canton, MA, USA), 1 mM DTT, 1× EDTA-free protease inhibitor cocktail (Sigma-Aldrich, Roche Diagnostics GmbH, Mannheim, Germany), and 1 mM PMSF (AmericanBio, Canton, MA, USA)). Cells were sonicated 3 times for 7 s with 30-s intervals on ice. Lysed cells were then centrifuged at 4 °C at maximum RPM for 10 min. A BCA assay (ThermoFisher Scientific, Waltham, MA, USA) was used to determine the total protein concentration.
4.8. Competitive EMSA
All the samples were prepared in 1× EMSA buffer (25 mM HEPES pH 7.5 at room temperature (Sigma-Aldrich, St. Louis, MO, USA), 50 mM NaCl, 100 mM KCl, 1 mM MgCl2, 1 mM TCEP (Gold Biotechnology, St. Louis, MO, USA), 7% glycerol, 0.1% DMSO, 1 mg/mL yeast tRNA, and 2 nM MALAT1 SL+A). The 5′-[32P] radiolabeled MALAT1 SL+A was folded by heating at 95 °C (5 min) and snap cooling on ice (10 min), followed by equilibration at room temperature for 1 h. To the folded RNA, increasing amounts of TBMs (0–200 μM) were added and incubated at room temperature for 30 min. Then, cell lysate was added (∼1 μg/μL unless indicated otherwise) and incubated for an additional 30 min at room temperature. Using a 5% native polyacrylamide gel (19:1 acrylamide:bisacrylamide, 40 mM Tris-borate pH 8.3, 1 mM MgCl2), the samples were loaded and electrophoresed with running buffer (40 mM Tris-borate pH 8.3, 1 mM MgCl2) at 130 V for ∼3 h at room temperature. After wrapping the gel in a transparent plastic wrap, it was exposed to a Phosphorimager screen overnight. The screens were scanned using an Amersham Typhoon IP Phosphorimager 1.0.0. (GE Healthcare, Chicago, IL, USA).
4.9. Treatment of HCT116 Cells with TBMs and RT-qPCR
HCT116 cells were plated at a seeding density of 1.0 × 105 cells/well in a 24-well plate and were grown to ~70% confluency. Each biological replicate was plated from different starting cells, and the same treatment was conducted for each replicate. After 48 h, cells were treated with 0.1% DMSO (AmericanBio, Canton, MA, USA) as a control or 1 µM of each TBM. Final DMSO was 0.1% for all compounds. After 48 h of treatment, cells were washed using 1× PBS (Sigma-Aldrich, St. Louis, MO, USA) and detached by applying 1 mL TRIzol (Life Technologies, Carlsbad, CA, USA) to each well. RNA was then extracted per the manufacturer’s recommended protocol. RNA was resuspended in 20 µL RNase-free water. RNA concentration was obtained at a 1:10 dilution using a NanoDropTM One/OneC Microvolume UV-Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). RNA quality control was examining rRNA bands via a 1% agarose gel. DNase treatment and cDNA synthesis were performed using the iScriptTM gDNA Clear cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) per the manufacturer’s instructions. Previously published primer pairs were used for premature and mature MALAT1 [12], MENβ [12], TUG1 [57], 18s rRNA [144], β-actin [145], U6 snRNA [146], and glyceraldyhde-3-phosphare dehydrogenase (GAPDH) [147] (Table S9) (Sigma-Aldrich, St. Louis, MO, USA). No template control, no reverse transcriptase control, and no standard curves were included. The amount of cDNA dilution used for all samples was determined based on Ct values for GAPDH at serial 10-fold dilutions. SsoAdvanced Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA), 500 nM primer pairs (Sigma-Aldrich, St. Louis, MO, USA), and cDNA template (20 µL total volume) were combined in a 96-well plate, inserted into a Bio-Rad CFX96 Touch Real-Time PCR or Bio-Rad CFX Opus 96 (Bio-Rad, Hercules, CA, USA), and subjected to the following cycling conditions: activation: 98 °C for 3 min; denaturation/annealing: 98 °C for 5 s, 59.4 °C for 30 s (35 cycles); extension: 72 °C for 2 min). The 2−ΔΔCT values [148] for premature-only MALAT1, premature and mature MALAT1, MENβ, and unspliced TUG1 RNAs were calculated using individual ΔCT values after normalization to the geometric mean of the four housekeepers, 18s rRNA, β-Actin, GAPDH, and U6 snRNA using GraphPad Prism 10. (RRID:SCR_002798) [58]. p-values were calculated from the average 2−∆∆CT using a two-way ANOVA test, comparing each TBM to the DMSO control: **** p-value < 0.0001, *** p-value < 0.0002, ** p-value < 0.0021, * p-value < 0.0332, ns p-value < 0.1234. Raw and processed data, including PCR efficiencies (90.1–98.7%), for RT-qPCR experiments are in File S4.
4.10. MTT Assay
HCT116 cells were plated at a seeding density of 5000 cells/well in a 96-well plate. Following 48 h, cells were treated with 1 µM TBM in technical triplicates. After a 48-h treatment, media was removed, and fresh media (100 µL) and 5 mg/mL stock of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (10 µL) were added to each well (total volume 110 µL). After a 4-h incubation with MTT, 10% sodium dodecyl sulfate (SDS) (90 µL) and 100% DMSO (10 µL) were combined and added to each well (total volume 100 µL) and incubated at 37 °C overnight. After incubation, absorbance of each well is measured at a 590 nm wavelength using a Synergy H1 Microplate Reader (Agilent BioTek). Measurements are background subtracted using wells that contain only a mixture of 0.2 mg/mL MTT (0.2 mg/mL), 4.3% SDS, and 4.8% DMSO in media. After background subtraction, sample measurements are normalized to the control wells (cells + media). Data is an average of biological replicates (n = 3). Raw and processed data for the MTT assay are in File S4.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30214277/s1, Figure S1: UV thermal melting results for the TBMs. Figure S2: UV thermal assays performed using 2% DMSO for flavonoids. Figure S3: Prediction of MALAT1 triple helix-TBM complex. Figure S4: FpocketR analysis of the MALAT1 triple helix variants d and e. Figure S5: Chemical structures of previously reported TBMs binding to the MALAT1 triple helix. Figure S6: UV thermal melting results for the pre-mascRNA. Figure S7: UV thermal melting results for the MALAT1 RNA in the absence and presence of TBMs. Figure S8: SPR sensogram plots comparing how each TBM binds with the three different RNAs at all concentrations. Figure S9: SPR sensogram plots comparing how each TBM interacts with the three different RNAs. Figure S10: MTT cell viability assay for TBM-treated HCT116 cells. Figure S11: Bar plot showing ΔTM,H values for the MALAT1 triple helix (solid color) and the poly(U•A-U) triple helix (light gray) in the presence of TBMs. Table S1: TM values for the MALAT1 triple helix variants a–c in the absence or presence of TBMs. Table S2: ΔTM values for the MALAT1 triple helix variants a–c in the absence or presence of TBMs. Table S3: TM values for the MALAT1 triple helix variants d–g in the absence or presence of TBMs. Table S4: ΔTM values for the MALAT1 triple helix variants d–g in the absence or presence of TBMs. Table S5: Summary of characteristics for each TBM and its subcellular localization. Table S6: In vitro transcribed RNAs used in this study. Table S7: Average TM values for the premature and mature MALAT1 RNAs in the absence or presence of TBMs. Table S8: ΔTM values for the premature and mature MALAT1 RNAs in the absence or presence of TBMs. Table S9: Sequences of primers used for RT-qPCR experiments. Table S10: Average 2−∆∆CT values and standard deviation for all lncRNA targets. Table S11: Summary of lncRNA expression levels and cellular localization. Table S12: Selectivity factor of each TBM for reducing MALAT1 over MENβ. File S1: Raw and processed data from UV thermal denaturation experiments. File S2: FASTA and SMILES inputs for AlphaFold 3 complex prediction. File S3: Raw and processed data from SPR experiments. File S4: Raw and processed data from MTT and RT-qPCR experiments.
Author Contributions
Conceptualization, J.A.B.; Formal analysis, M.M.M. and J.Y.; Funding acquisition, J.A.B.; Investigation, M.M.M., K.M.S. and J.Y.; Methodology, M.M.M., K.M.S. and J.Y.; Supervision, J.A.B.; Visualization, M.M.M. and K.M.S.; Writing—original draft, M.M.M., K.M.S. and J.A.B.; Writing—review and editing, All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by startup funds from the University of Notre Dame, the Clare Boothe Luce Program of the Henry Luce Foundation and the National Institutes of Health grant R35GM133696. M.M.M. was supported through the Chemistry-Biochemistry-Biology Interface (CBBI) Training Program at the University of Notre Dame from the National Institutes of Health Grant [5T32GM075762-15, 1T32GM145773-01]. This work was supported by the American Heart Association grant 23PRE1011061/M.M.G./2023. M.M.M. is an Arthur J. Schmitt Presidential Leadership Fellow at the University of Notre Dame. Research conducted by J.Y. was made possible in part by support from the College of Science Summer Undergraduate Research Fellowship (COS-SURF), University of Notre Dame.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful for Jacob Hulewicz who compiled a list of TBMs from literature, Gowthami Mahendran who prepared native HCT116 cell lysate, Mika Schievelbein for aiding in preparation of select RNAs (i.e., pre-mascRNA, mascRNA, and MALAT1 SL+A+masc RNA), and Nikhil Vijai for guidance on computational analysis. We thank the Biophysics Instrumentation Core Facility for use of multiple instruments: Azure c400 Bioanalytical Imaging System, Amersham Typhoon IP Phosphorimager, and BiacoreTM T200. SPR analyses were performed at the Notre Dame Biophysics Instrumentation Core Facility with support of NIH grant S10OD028553. We would like to thank Notre Dame’s Center for Research Computing, most especially Dodi Heryadi for his assistance and installation of AlphaFold 3 and FpocketR software. We thank Patricia Clark’s laboratory for the use of ultra-sonication system to lyse HCT116 cells and the Agilent Biotek Synergy H1 Microplate Reader. We thank Bonnie Huge, Matthew Champion, and Norman Dovichi for the use of their Bio-Rad CFX Opus 96 and Bio-Rad CFX96 Touch Real-Time PCR instrumentation and guidance on RT-qPCR experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DPF | diphenyl furan |
| DSC | differential scanning calorimetry |
| EMSA | electrophoretic mobility shift assay |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| HCT116 | human colorectal carcinoma cell line |
| lncRNA | long non-coding RNA |
| mascRNA | MALAT1-associated small cytoplasmic RNA |
| MALAT1 | metastasis-associated lung adenocarcinoma transcript 1 |
| MALAT1 SL | MALAT1 U-rich stem loop |
| MALAT1 SL+A+masc | MALAT1 U-rich stem loop, A-rich tract, and mascRNA |
| MALAT1 SL+A | MALAT1 U-rich stem loop and A-rich tract |
| MENβ | multiple endocrine neoplasia-β |
| METTL16 | methyltransferase-like protein 16 |
| MMTV-PyMT | mouse mammary tumor virus-polyoma middle tumor-antigen |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| RNP | ribonucleoprotein |
| RT-qPCR | quantitative reverse transcription polymerase chain reaction |
| SDS | sodium dodecyl sulfate |
| SPR | surface plasmon resonance |
| TBM | triplex-binding molecule |
| TUG1 | taurine-upregulated gene |
| TM | melting temperature |
| ∆TM | change in melting temperature or thermal shift |
| WT | wild type |
References
- Chen, L.-L.; Kim, V.N. Small and Long Non-Coding RNAs: Past, Present, and Future. Cell 2024, 187, 6451–6485. [Google Scholar] [CrossRef]
- Watmuff, H.; Crawford, A.; Eusse, B.; Jones, A.N. Structure–Function-Guided Drug Development Efforts to Target lncRNAs. Trends Pharmacol. Sci. 2025, 46, 703–721. [Google Scholar] [CrossRef]
- Gutschner, T.; Hämmerle, M.; Eißmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Groß, M.; et al. The Noncoding RNA MALAT1 Is a Critical Regulator of the Metastasis Phenotype of Lung Cancer Cells. Cancer Res. 2013, 73, 1180–1189. [Google Scholar] [CrossRef]
- Arun, G.; Diermeier, S.; Akerman, M.; Chang, K.-C.; Wilkinson, J.E.; Hearn, S.; Kim, Y.; MacLeod, A.R.; Krainer, A.R.; Norton, L.; et al. Differentiation of Mammary Tumors and Reduction in Metastasis upon Malat1 lncRNA Loss. Genes Dev. 2016, 30, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Goyal, B.; Yadav, S.R.M.; Awasthee, N.; Gupta, S.; Kunnumakkara, A.B.; Gupta, S.C. Diagnostic, Prognostic, and Therapeutic Significance of Long Non-Coding RNA MALAT1 in Cancer. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2021, 1875, 188502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Ahmed, M.; Li, Y.; Liao, J.C.; Wong, P.K. Long Noncoding RNA MALAT1 Is Dynamically Regulated in Leader Cells during Collective Cancer Invasion. Proc. Natl. Acad. Sci. USA 2023, 120, e2305410120. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, J.E.; Freier, S.M.; Spector, D.L. 3′ End Processing of a Long Nuclear-Retained Noncoding RNA Yields a tRNA-like Cytoplasmic RNA. Cell 2008, 135, 919–932. [Google Scholar] [CrossRef]
- Wilusz, J.E.; JnBaptiste, C.K.; Lu, L.Y.; Kuhn, C.-D.; Joshua-Tor, L.; Sharp, P.A. A Triple Helix Stabilizes the 3′ Ends of Long Noncoding RNAs That Lack Poly(A) Tails. Genes Dev. 2012, 26, 2392–2407. [Google Scholar] [CrossRef]
- Brown, J.A.; Valenstein, M.L.; Yario, T.A.; Tycowski, K.T.; Steitz, J.A. Formation of Triple-Helical Structures by the 3′-End Sequences of MALAT1 and MENβ Noncoding RNAs. Proc. Natl. Acad. Sci. USA 2012, 109, 19202–19207. [Google Scholar] [CrossRef]
- Torabi, S.-F.; DeGregorio, S.J.; Steitz, J.A. tRNA-like Leader-Trailer Interaction Promotes 3′-End Maturation of MALAT1. RNA 2021, 27, 1140–1147. [Google Scholar] [CrossRef]
- Brown, J.A.; Bulkley, D.; Wang, J.; Valenstein, M.L.; Yario, T.A.; Steitz, T.A.; Steitz, J.A. Structural Insights into the Stabilization of MALAT1 Noncoding RNA by a Bipartite Triple Helix. Nat. Struct. Mol. Biol. 2014, 21, 633–640. [Google Scholar] [CrossRef]
- Brown, J.A.; Kinzig, C.G.; DeGregorio, S.J.; Steitz, J.A. Methyltransferase-like Protein 16 Binds the 3′-Terminal Triple Helix of MALAT1 Long Noncoding RNA. Proc. Natl. Acad. Sci. USA 2016, 113, 14013–14018. [Google Scholar] [CrossRef]
- Warda, A.S.; Kretschmer, J.; Hackert, P.; Lenz, C.; Urlaub, H.; Höbartner, C.; Sloan, K.E.; Bohnsack, M.T. Human METTL16 Is a N6-Methyladenosine (m6A) Methyltransferase That Targets Pre-mRNAs and Various Non-Coding RNAs. EMBO Rep. 2017, 18, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Donlic, A.; Morgan, B.S.; Xu, J.L.; Liu, A.; Roble, C.; Hargrove, A.E. Discovery of Small Molecule Ligands for MALAT1 by Tuning an RNA-Binding Scaffold. Angew. Chem. Int. Ed. 2018, 57, 13242–13247. [Google Scholar] [CrossRef] [PubMed]
- Donlic, A.; Zafferani, M.; Padroni, G.; Puri, M.; Hargrove, A.E. Regulation of MALAT1 Triple Helix Stability and in Vitro Degradation by Diphenylfurans. Nucleic Acids Res. 2020, 48, 7653–7664. [Google Scholar] [CrossRef] [PubMed]
- Abulwerdi, F.A.; Xu, W.; Ageeli, A.A.; Yonkunas, M.J.; Arun, G.; Nam, H.; Schneekloth, J.S.; Dayie, T.K.; Spector, D.; Baird, N.; et al. Selective Small-Molecule Targeting of a Triple Helix Encoded by the Long Noncoding RNA, MALAT1. ACS Chem. Biol. 2019, 14, 223–235. [Google Scholar] [CrossRef]
- Zafferani, M.; Martyr, J.G.; Muralidharan, D.; Montalvan, N.I.; Cai, Z.; Hargrove, A.E. Multiassay Profiling of a Focused Small Molecule Library Reveals Predictive Bidirectional Modulation of the lncRNA MALAT1 Triplex Stability In Vitro. ACS Chem. Biol. 2022, 17, 2437–2447. [Google Scholar] [CrossRef]
- Pernak, M.; Fleurisson, C.; Delorme, C.; Moumné, R.; Benedetti, E.; Micouin, L.; Azoulay, S.; Foricher, Y.; Duca, M. Development of Comprehensive Screening and Assessment Assays for Small-Molecule Ligands of MALAT1 lncRNA. ACS Chem. Biol. 2025, 20, 1068–1076. [Google Scholar] [CrossRef]
- François-Moutal, L.; Miranda, V.G.; Mollasalehi, N.; Gokhale, V.; Khanna, M. In Silico Targeting of the Long Noncoding RNA MALAT1. ACS Med. Chem. Lett. 2021, 12, 915–921. [Google Scholar] [CrossRef]
- Rakheja, I.; Ansari, A.H.; Ray, A.; Joshi, D.C.; Maiti, S. Small Molecule Quercetin Binds MALAT1 Triplex and Modulates Its Cellular Function. Mol. Ther. Nucleic Acids 2022, 30, 241–256. [Google Scholar] [CrossRef]
- Rocca, R.; Polerà, N.; Juli, G.; Grillone, K.; Maruca, A.; Di Martino, M.T.; Artese, A.; Amato, J.; Pagano, B.; Randazzo, A.; et al. Hit Identification of Novel Small Molecules Interfering with MALAT1 Triplex by a Structure-Based Virtual Screening. Arch. Pharm. 2023, 356, 2300134. [Google Scholar] [CrossRef]
- Zablowsky, N.; Farack, L.; Rofall, S.; Kramer, J.; Meyer, H.; Nguyen, D.; Ulrich, A.K.C.; Bader, B.; Steigemann, P. High Throughput FISH Screening Identifies Small Molecules That Modulate Oncogenic lncRNA MALAT1 via GSK3B and hnRNPs. Non-Coding RNA 2023, 9, 2. [Google Scholar] [CrossRef]
- An, H.; Elvers, K.T.; Gillespie, J.A.; Jones, K.; Atack, J.R.; Grubisha, O.; Shelkovnikova, T.A. A Toolkit for the Identification of NEAT1_2/Paraspeckle Modulators. Nucleic Acids Res. 2022, 50, e119. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kumar, G.S.; Ray, A.; Maiti, M. Spectroscopic and Thermodynamic Studies on the Binding of Sanguinarine and Berberine to Triple and Double Helical DNA and RNA Structures. J. Biomol. Struct. Dyn. 2003, 20, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Kumar, G.S. Interaction of Isoquinoline Alkaloids with an RNA Triplex: Structural and Thermodynamic Studies of Berberine, Palmatine, and Coralyne Binding to Poly(U).Poly(A)*Poly(U). J. Phys. Chem. B 2009, 113, 13410–13420. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Das, S.; Hossain, M.; Haq, L.; Suresh Kumar, G. Biophysical Characterization of the Strong Stabilization of the RNA Triplex Poly(U)•poly(A)*poly(U) by 9-O-(ω-Amino) Alkyl Ether Berberine Analogs. PLoS ONE 2012, 7, e37939. [Google Scholar] [CrossRef]
- Moraru-Allen, A. Coralyne Has a Preference for Intercalation between TA.T Triples in Intramolecular DNA Triple Helices. Nucleic Acids Res. 1997, 25, 1890–1896. [Google Scholar] [CrossRef]
- Bhuiya, S.; Haque, L.; Goswami, R.; Das, S. Multispectroscopic and Theoretical Exploration of the Comparative Binding Aspects of Bioflavonoid Fisetin with Triple- and Double-Helical Forms of RNA. J. Phys. Chem. B 2017, 121, 11037–11052. [Google Scholar] [CrossRef]
- Tiwari, R.; Haque, L.; Bhuiya, S.; Das, S. Third Strand Stabilization of Poly(U)·poly(A)* Poly(U) Triplex by the Naturally Occurring Flavone Luteolin: A Multi-Spectroscopic Approach. Int. J. Biol. Macromol. 2017, 103, 692–700. [Google Scholar] [CrossRef]
- Pradhan, A.B.; Bhuiya, S.; Haque, L.; Das, S. Role of Hydroxyl Groups in the B-Ring of Flavonoids in Stabilization of the Hoogsteen Paired Third Strand of Poly(U).Poly(A)*Poly(U) Triplex. Arch. Biochem. Biophys. 2018, 637, 9–20. [Google Scholar] [CrossRef]
- Pilch, D.S.; Kirolos, M.A.; Breslauer, K.J. Berenil Binding to Higher Ordered Nucleic Acid Structures: Complexation with a DNA and RNA Triple Helix. Biochemistry 1995, 34, 16107–16124. [Google Scholar] [CrossRef]
- Arya, D.P.; Coffee, R.L.; Willis, B.; Abramovitch, A.I. Aminoglycoside−Nucleic Acid Interactions: Remarkable Stabilization of DNA and RNA Triple Helices by Neomycin. J. Am. Chem. Soc. 2001, 123, 5385–5395. [Google Scholar] [CrossRef]
- Ageeli, A.A.; McGovern-Gooch, K.R.; Kaminska, M.M.; Baird, N.J. Finely Tuned Conformational Dynamics Regulate the Protective Function of the lncRNA MALAT1 Triple Helix. Nucleic Acids Res. 2019, 47, 1468–1481. [Google Scholar] [CrossRef]
- Miao, S.; Bhunia, D.; Devari, S.; Liang, Y.; Munyaradzi, O.; Rundell, S.; Bong, D. Bifacial PNAs Destabilize MALAT1 by 3′ A-Tail Displacement from the U-Rich Internal Loop. ACS Chem. Biol. 2021, 16, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, S.; Bellucci, M.C.; Mondelli, R. Mode of Binding of the Cytotoxic Alkaloid Berberine with the Double Helix Oligonucleotide d(AAGAATTCTT)2. Bioorganic Med. Chem. 2003, 11, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Bessi, I.; Bazzicalupi, C.; Richter, C.; Jonker, H.R.A.; Saxena, K.; Sissi, C.; Chioccioli, M.; Bianco, S.; Bilia, A.R.; Schwalbe, H.; et al. Spectroscopic, Molecular Modeling, and NMR-Spectroscopic Investigation of the Binding Mode of the Natural Alkaloids Berberine and Sanguinarine to Human Telomeric G-Quadruplex DNA. ACS Chem. Biol. 2012, 7, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Le, V.; Kalia, D.; Nakayama, S.; Mikek, C.; Lewis, E.A.; Sintim, H.O. Diminazene or Berenil, a Classic Duplex Minor Groove Binder, Binds to G-Quadruplexes with Low Nanomolar Dissociation Constants and the Amidine Groups Are Also Critical for G-Quadruplex Binding. Mol. Biosyst. 2014, 10, 2724–2734. [Google Scholar] [CrossRef]
- Kaushik, S.; Kaushik, M.; Barthwal, R.; Kukreti, S. Self-Association of Coralyne: An Ordered Thermal Destacking. Results Chem. 2020, 2, 100043. [Google Scholar] [CrossRef]
- Deogratias, G.; Shadrack, D.M.; Munissi, J.J.E.; Kinunda, G.A.; Jacob, F.R.; Mtei, R.P.; Masalu, R.J.; Mwakyula, I.; Kiruri, L.W.; Nyandoro, S.S. Hydrophobic π-π Stacking Interactions and Hydrogen Bonds Drive Self-Aggregation of Luteolin in Water. J. Mol. Graph. Model. 2022, 116, 108243. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Swain, M.; Ageeli, A.A.; Kasprzak, W.K.; Li, M.; Miller, J.T.; Sztuba-Solinska, J.; Schneekloth, J.S.; Koirala, D.; Piccirili, J.; Fraboni, A.J.; et al. Dynamic Bulge Nucleotides in the KSHV PAN ENE Triple Helix Provide a Unique Binding Platform for Small Molecule Ligands. Nucleic Acids Res. 2021, 49, 13179–13193. [Google Scholar] [CrossRef]
- Brown, J.A. Unraveling the Structure and Biological Functions of RNA Triple Helices. WIREs RNA 2020, 11, e1598. [Google Scholar] [CrossRef]
- Veenbaas, S.D.; Koehn, J.T.; Irving, P.S.; Lama, N.N.; Weeks, K.M. Ligand-Binding Pockets in RNA and Where to Find Them. Proc. Natl. Acad. Sci. USA 2025, 122, e2422346122. [Google Scholar] [CrossRef]
- Veenbaas, S.D.; Felder, S.; Weeks, K.M. fpocketR: A Platform for Identification and Analysis of Ligand-Binding Pockets in RNA. bioRxiv 2025. [Google Scholar] [CrossRef]
- Hossain, M.; Khan, A.Y.; Kumar, G.S. Interaction of the Anticancer Plant Alkaloid Sanguinarine with Bovine Serum Albumin. PLoS ONE 2011, 6, e18333. [Google Scholar] [CrossRef]
- Jash, C.; Kumar, G.S. Binding of Alkaloids Berberine, Palmatine and Coralyne to Lysozyme: A Combined Structural and Thermodynamic Study. RSC Adv. 2014, 4, 12514–12525. [Google Scholar] [CrossRef]
- Chu, M.; Chen, X.; Wang, J.; Guo, L.; Wang, Q.; Gao, Z.; Kang, J.; Zhang, M.; Feng, J.; Guo, Q.; et al. Polypharmacology of Berberine Based on Multi-Target Binding Motifs. Front. Pharmacol. 2018, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, S.N.; Pfister, P.; Bruell, C.; Sander, P.; François, B.; Westhof, E.; Böttger, E.C. Binding of Neomycin-Class Aminoglycoside Antibiotics to Mutant Ribosomes with Alterations in the A Site of 16S rRNA. Antimicrob. Agents Chemother. 2006, 50, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Sechi, M.; Mukhtar, H. Dietary Flavonoid Fisetin Binds to β-Tubulin and Disrupts Microtubule Dynamics in Prostate Cancer Cells. Cancer Lett. 2015, 367, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Salmela, A.-L.; Pouwels, J.; Varis, A.; Kukkonen, A.M.; Toivonen, P.; Halonen, P.K.; Perälä, M.; Kallioniemi, O.; Gorbsky, G.J.; Kallio, M.J. Dietary Flavonoid Fisetin Induces a Forced Exit from Mitosis by Targeting the Mitotic Spindle Checkpoint. Carcinogenesis 2009, 30, 1032–1040. [Google Scholar] [CrossRef]
- Wen, L.-N.; Xie, M.-X. Competitive Binding Assay for G-Quadruplex DNA and Sanguinarine Based on Room Temperature Phosphorescence of Mn-Doped ZnS Quantum Dots. J. Photochem. Photobiol. A Chem. 2014, 279, 24–31. [Google Scholar] [CrossRef]
- Padmapriya, K.; Barthwal, R. Binding of the Alkaloid Coralyne to Parallel G-Quadruplex DNA [d(TTGGGGT)]4 Studied by Multi-Spectroscopic Techniques. Biophys. Chem. 2016, 219, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dickerhoff, J.; Brundridge, N.; McLuckey, S.A.; Yang, D. Berberine Molecular Recognition of the Parallel MYC G-Quadruplex in Solution. J. Med. Chem. 2021, 64, 16205–16212. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cong, Y.; Qi, Y.; Zhang, J.Z.H. Binding of Berberine Derivates to G-Quadruplex: Insight from a Computational Study. Phys. Chem. Chem. Phys. 2023, 25, 10741–10748. [Google Scholar] [CrossRef]
- Schärfen, L.; Neugebauer, K.M. Transcription Regulation Through Nascent RNA Folding. J. Mol. Biol. 2021, 433, 166975. [Google Scholar] [CrossRef]
- Yonkunas, M.J.; Baird, N.J. A Highly Ordered, Nonprotective MALAT1 ENE Structure Is Adopted Prior to Triplex Formation. RNA 2019, 25, 975–984. [Google Scholar] [CrossRef]
- Dumbović, G.; Braunschweig, U.; Langner, H.K.; Smallegan, M.; Biayna, J.; Hass, E.P.; Jastrzebska, K.; Blencowe, B.; Cech, T.R.; Caruthers, M.H.; et al. Nuclear Compartmentalization of TERT mRNA and TUG1 lncRNA Is Driven by Intron Retention. Nat. Commun. 2021, 12, 3308. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Shivakumar, K.M.; Mahendran, G.; Brown, J.A. Locked Nucleic Acid Oligonucleotides Facilitate RNA•LNA-RNA Triple-Helix Formation and Reduce MALAT1 Levels. Int. J. Mol. Sci. 2024, 25, 1630. [Google Scholar] [CrossRef]
- Childs-Disney, J.L.; Yang, X.; Gibaut, Q.M.R.; Tong, Y.; Batey, R.T.; Disney, M.D. Targeting RNA Structures with Small Molecules. Nat. Rev. Drug Discov. 2022, 21, 736–762. [Google Scholar] [CrossRef]
- Nguyen, B.; Hamelberg, D.; Bailly, C.; Colson, P.; Stanek, J.; Brun, R.; Neidle, S.; Wilson, W.D. Characterization of a Novel DNA Minor-Groove Complex. Biophys. J. 2004, 86, 1028–1041. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A Dietary Antioxidant for Health Promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Baird, N. Direct Stacking of Peripheral Duplexes Significantly Stabilizes RNA Triple Helices. Biophys. J. 2024, 123, 364a. [Google Scholar] [CrossRef]
- Maiti, M.; Kumar, G.S. Molecular Aspects on the Interaction of Protoberberine, Benzophenanthridine, and Aristolochia Group of Alkaloids with Nucleic Acid Structures and Biological Perspectives. Med. Res. Rev. 2007, 27, 649–695. [Google Scholar] [CrossRef]
- Fourmy, D.; Recht, M.I.; Puglisi, J.D. Binding of Neomycin-Class Aminoglycoside Antibiotics to the A-Site of 16 s rRNA1. J. Mol. Biol. 1998, 277, 347–362. [Google Scholar] [CrossRef]
- Xi, H.; Arya, D.P. Recognition of Triple Helical Nucleic Acids by Aminoglycosides. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 327–338. [Google Scholar] [CrossRef]
- La, X.; Zhang, L.; Li, Z.; Yang, P.; Wang, Y. Berberine-Induced Autophagic Cell Death by Elevating GRP78 Levels in Cancer Cells. Oncotarget 2017, 8, 20909–20924. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Gupta, P.; Bandyopadhyay, S.K.; Patro, B.S.; Chattopadhyay, S. Coralyne, a Protoberberine Alkaloid, Causes Robust Photosenstization of Cancer Cells through ATR-P38 MAPK-BAX and JAK2-STAT1-BAX Pathways. Chem.-Biol. Interact. 2018, 285, 27–39. [Google Scholar] [CrossRef]
- Ahmad, N.; Gupta, S.; Husain, M.M.; Heiskanen, K.M.; Mukhtar, H. Differential Antiproliferative and Apoptotic Response of Sanguinarine for Cancer Cells versus Normal Cells. Clin. Cancer Res. 2000, 6, 1524–1528. [Google Scholar]
- Zhou, C.; Huang, Y.; Nie, S.; Zhou, S.; Gao, X.; Chen, G. Biological Effects and Mechanisms of Fisetin in Cancer: A Promising Anti-Cancer Agent. Eur. J. Med. Res. 2023, 28, 297. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The Potential Anti-Cancer Effects of Quercetin on Blood, Prostate and Lung Cancers: An Update. Front. Immunol. 2023, 14, 1077531. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Bielawska, A.; Szymanowski, W.; Gabryel-Porowska, H.; Czarnomysy, R.; Bielawski, K. Mechanism of Anticancer Action of Novel Berenil Complex of Platinum(II) Combined with Anti-MUC1 in MCF-7 Breast Cancer Cells. Oncol. Lett. 2018, 15, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Gibaut, Q.M.R.; Bush, J.A.; Tong, Y.; Baisden, J.T.; Taghavi, A.; Olafson, H.; Yao, X.; Childs-Disney, J.L.; Wang, E.T.; Disney, M.D. Transcriptome-Wide Studies of RNA-Targeted Small Molecules Provide a Simple and Selective r(CUG)Exp Degrader in Myotonic Dystrophy. ACS Cent. Sci. 2023, 9, 1342–1353. [Google Scholar] [CrossRef]
- Ratni, H.; Ebeling, M.; Baird, J.; Bendels, S.; Bylund, J.; Chen, K.S.; Denk, N.; Feng, Z.; Green, L.; Guerard, M.; et al. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61, 6501–6517. [Google Scholar] [CrossRef]
- Kilgore, H.R.; Mikhael, P.G.; Overholt, K.J.; Boija, A.; Hannett, N.M.; Van Dongen, C.; Lee, T.I.; Chang, Y.-T.; Barzilay, R.; Young, R.A. Distinct Chemical Environments in Biomolecular Condensates. Nat. Chem. Biol. 2024, 20, 291–301. [Google Scholar] [CrossRef]
- Yang, W.; Soares, J.; Greninger, P.; Edelman, E.J.; Lightfoot, H.; Forbes, S.; Bindal, N.; Beare, D.; Smith, J.A.; Thompson, I.R.; et al. Genomics of Drug Sensitivity in Cancer (GDSC): A Resource for Therapeutic Biomarker Discovery in Cancer Cells. Nucleic Acids Res. 2013, 41, D955–D961. [Google Scholar] [CrossRef]
- Kwok, Z.H.; Roche, V.; Chew, X.H.; Fadieieva, A.; Tay, Y. A Non-Canonical Tumor Suppressive Role for the Long Non-Coding RNA MALAT1 in Colon and Breast Cancers. Int. J. Cancer 2018, 143, 668–678. [Google Scholar] [CrossRef]
- Shen, X.; Ye, Z.; Wu, W.; Zhao, K.; Cheng, G.; Xu, L.; Gan, L.; Wu, Y.; Yang, Z. LncRNA NEAT1 Facilitates the Progression of Colorectal Cancer via the KDM5A/Cul4A and Wnt Signaling Pathway. Int. J. Oncol. 2021, 59, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Yu, X.; Su, S.; Wu, B.; Su, Y.; Guo, L. Long Non-Coding RNA NEAT1 Promotes Colorectal Cancer Progression via Interacting with SIRT1. Sci. Rep. 2025, 15, 5673. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Ma, Y.; Liu, Y.; Xue, Y.; Li, Y.; Gao, X.; Wang, Y.; Chu, M. Advance in Identified Targets of Berberine. Front. Pharmacol. 2025, 16. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Huang, G.; Gu, C.; Liu, Y.; Yang, J.; Fei, J. Discovery of Berberine That Targetedly Induces Autophagic Degradation of Both BCR-ABL and BCR-ABL T315I through Recruiting LRSAM1 for Overcoming Imatinib Resistance. Clin. Cancer Res. 2020, 26, 4040–4053. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cao, S.-J.; Li, C.-Y.; Zhang, Q.; Zhang, B.-L.; Qiu, F.; Kang, N. Berberine Directly Targets AKR1B10 Protein to Modulate Lipid and Glucose Metabolism Disorders in NAFLD. J. Ethnopharmacol. 2024, 332, 118354. [Google Scholar] [CrossRef]
- Ruan, H.; Zhan, Y.Y.; Hou, J.; Xu, B.; Chen, B.; Tian, Y.; Wu, D.; Zhao, Y.; Zhang, Y.; Chen, X.; et al. Berberine Binds RXRα to Suppress β-Catenin Signaling in Colon Cancer Cells. Oncogene 2017, 36, 6906–6918. [Google Scholar] [CrossRef]
- Qi, F.; Zhang, M.; Yang, G.; Wang, W.; Hu, Y.; Shen, Y.; Wan, J.; Li, J.; Liu, G.; Deng, Y. Identification of TIGAR, a Direct Proteomic Target Associated with the Hypoglycemic Effect of Berberine. Fitoterapia 2025, 180, 106332. [Google Scholar] [CrossRef]
- Serafim, T.L.; Oliveira, P.J.; Sardao, V.A.; Perkins, E.; Parke, D.; Holy, J. Different Concentrations of Berberine Result in Distinct Cellular Localization Patterns and Cell Cycle Effects in a Melanoma Cell Line. Cancer Chemother. Pharmacol. 2008, 61, 1007–1018. [Google Scholar] [CrossRef]
- Jin, M.; Ji, X.; Stoika, R.; Liu, K.; Wang, L.; Song, Y. Synthesis of a Novel Fluorescent Berberine Derivative Convenient for Its Subcellular Localization Study. Bioorganic Chem. 2020, 101, 104021. [Google Scholar] [CrossRef]
- Węgierek-Ciuk, A.; Arabski, M.; Ciepluch, K.; Brzóska, K.; Lisowska, H.; Czerwińska, M.; Stępkowski, T.; Lis, K.; Lankoff, A. Coralyne Radiosensitizes A549 Cells by Upregulation of CDKN1A Expression to Attenuate Radiation Induced G2/M Block of the Cell Cycle. Int. J. Mol. Sci. 2021, 22, 5791. [Google Scholar] [CrossRef]
- Basu, A.; Suresh Kumar, G. Coralyne Induced Self-Structure in Polyadenylic Acid: Thermodynamics of the Structural Reorganization. J. Chem. Thermodyn. 2016, 101, 221–226. [Google Scholar] [CrossRef]
- Vempati, R.K.; Malla, R.R. Coralyne Targets the Catalytic Domain of MMP9: An In Silico and In Vitro Investigation. Crit. Rev. Oncog. 2025, 30. [Google Scholar] [CrossRef] [PubMed]
- Gatto, B.; Sanders, M.M.; Yu, C.; Wu, H.Y.; Makhey, D.; LaVoie, E.J.; Liu, L.F. Identification of Topoisomerase I as the Cytotoxic Target of the Protoberberine Alkaloid Coralyne. Cancer Res. 1996, 56, 2795–2800. [Google Scholar] [PubMed]
- Basu, P.; Kumar, G.S. Sanguinarine and Its Role in Chronic Diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 155–172. ISBN 978-3-319-41334-1. [Google Scholar]
- Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Liu, L. Sanguinaria Canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses. Int. J. Mol. Sci. 2016, 17, 1414. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Lin, S.; Chen, K.; Yin, S.; Peng, H.; Cai, N.; Ma, W.; Songyang, Z.; Huang, Y. Natural Product Library Screens Identify Sanguinarine Chloride as a Potent Inhibitor of Telomerase Expression and Activity. Cells 2022, 11, 1485. [Google Scholar] [CrossRef]
- Li, X.; You, Q. Sanguinarine Identified as a Natural Dual Inhibitor of AURKA and CDK2 through Network Pharmacology and Bioinformatics Approaches. Sci. Rep. 2024, 14, 29608. [Google Scholar] [CrossRef]
- Holy, J.; Lamont, G.; Perkins, E. Disruption of Nucleocytoplasmic Trafficking of Cyclin D1 and Topoisomerase II by Sanguinarine. BMC Cell Biol. 2006, 7, 13. [Google Scholar] [CrossRef]
- Sengupta, B.; Banerjee, A.; Sengupta, P.K. Interactions of the Plant Flavonoid Fisetin with Macromolecular Targets: Insights from Fluorescence Spectroscopic Studies. J. Photochem. Photobiol. B 2005, 80, 79–86. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Chakraborty, S.; Sengupta, P.K.; Bhowmik, S. Exploring the Interactions of the Dietary Plant Flavonoids Fisetin and Naringenin with G-Quadruplex and Duplex DNA, Showing Contrasting Binding Behavior: Spectroscopic and Molecular Modeling Approaches. J. Phys. Chem. B 2016, 120, 8942–8952. [Google Scholar] [CrossRef]
- Velazhahan, V.; Glaza, P.; Herrera, A.I.; Prakash, O.; Zolkiewski, M.; Geisbrecht, B.V.; Schrick, K. Dietary Flavonoid Fisetin Binds Human SUMO1 and Blocks Sumoylation of P53. PLoS ONE 2020, 15, e0234468. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a Flavonoid, as an Anticancer Agent: A Review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Sharma, S.; Mandal, S.; Goswami, A.; Mukhopadhyay, S.; Majumder, H.K. Luteolin, an Emerging Anti-Cancer Flavonoid, Poisons Eukaryotic DNA Topoisomerase I. Biochem. J. 2002, 366, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Li, G.; Ivanov, D.N.; Wang, Z.; Velasco, M.X.; Hernández, G.; Kaundal, S.; Villarreal, J.; Gupta, Y.K.; Qiao, M.; et al. Luteolin Inhibits Musashi1 Binding to RNA and Disrupts Cancer Phenotypes in Glioblastoma Cells. RNA Biol. 2018, 15, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Munafò, F.; Donati, E.; Brindani, N.; Ottonello, G.; Armirotti, A.; De Vivo, M. Quercetin and Luteolin Are Single-Digit Micromolar Inhibitors of the SARS-CoV-2 RNA-Dependent RNA Polymerase. Sci. Rep. 2022, 12, 10571. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An Insight into Anticancer, Antioxidant, Antimicrobial, Antidiabetic and Anti-Inflammatory Effects of Quercetin: A Review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef]
- Primikyri, A.; Chatziathanasiadou, M.V.; Karali, E.; Kostaras, E.; Mantzaris, M.D.; Hatzimichael, E.; Shin, J.-S.; Chi, S.-W.; Briasoulis, E.; Kolettas, E.; et al. Direct Binding of Bcl-2 Family Proteins by Quercetin Triggers Its pro-Apoptotic Activity. ACS Chem. Biol. 2014, 9, 2737–2741. [Google Scholar] [CrossRef]
- Ko, C.-C.; Chen, Y.-J.; Chen, C.-T.; Liu, Y.-C.; Cheng, F.-C.; Hsu, K.-C.; Chow, L.-P. Chemical Proteomics Identifies Heterogeneous Nuclear Ribonucleoprotein (HnRNP) A1 as the Molecular Target of Quercetin in Its Anti-Cancer Effects in PC-3 Cells *. J. Biol. Chem. 2014, 289, 22078–22089. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.-G.; Byun, S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Kobayashi, M.; Kamatari, Y.O.; Mitsunaga, T.; Yamauchi, K. Development of Flavonoid Probes and the Binding Mode of the Target Protein and Quercetin Derivatives. Bioorg. Med. Chem. 2022, 68, 116854. [Google Scholar] [CrossRef]
- Gonzalez, O.; Fontanes, V.; Raychaudhuri, S.; Loo, R.; Loo, J.; Arumugaswami, V.; Sun, R.; Dasgupta, A.; French, S.W. The Heat Shock Protein Inhibitor Quercetin Attenuates Hepatitis C Virus Production. Hepatol. Baltim. Md 2009, 50, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, S.; Uzonna, J.E. Diminazene Aceturate (Berenil), a New Use for an Old Compound? Int. Immunopharmacol. 2014, 21, 342–345. [Google Scholar] [CrossRef]
- Mikek, C.G.; West, S.J.; Gwin, J.C.; Dayal, N.; Sintim, H.O.; Lewis, E.A. Berenil Binds Tightly to Parallel and Mixed Parallel/Antiparallel G-Quadruplex Motifs with Varied Thermodynamic Signatures. ACS Omega 2018, 3, 11582–11591. [Google Scholar] [CrossRef]
- Reddy, B.S.P.; Sondhi, S.M.; Lown, J.W. Synthetic DNA Minor Groove-Binding Drugs. Pharmacol. Ther. 1999, 84, 1–111. [Google Scholar] [CrossRef]
- Pilch, D.S.; Kirolos, M.A.; Liu, X.; Plum, G.E.; Breslauer, K.J. Berenil [1,3-Bis(4’-Amidinophenyl)Triazene] Binding to DNA Duplexes and to a RNA Duplex: Evidence for Both Intercalative and Minor Groove Binding Properties. Biochemistry 1995, 34, 9962–9976. [Google Scholar] [CrossRef]
- Pilch, D.S.; Breslauer, K.J. Ligand-Induced Formation of Nucleic Acid Triple Helices. Proc. Natl. Acad. Sci. USA 1994, 91, 9332–9336. [Google Scholar] [CrossRef]
- Portugal, J. Berenil Acts as a Poison of Eukaryotic Topoisomerase II. FEBS Lett. 1994, 344, 136–138. [Google Scholar] [CrossRef]
- Xi, H.; Gray, D.; Kumar, S.; Arya, D.P. Molecular Recognition of Single-Stranded RNA: Neomycin Binding to Poly(A). FEBS Lett. 2009, 583, 2269–2275. [Google Scholar] [CrossRef]
- Mao, T.; Kim, J.; Peña-Hernández, M.A.; Valle, G.; Moriyama, M.; Luyten, S.; Ott, I.M.; Gomez-Calvo, M.L.; Gehlhausen, J.R.; Baker, E.; et al. Intranasal Neomycin Evokes Broad-Spectrum Antiviral Immunity in the Upper Respiratory Tract. Proc. Natl. Acad. Sci. USA 2024, 121, e2319566121. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, N.; Andreasen, K.F.; Arora, Y.; Xue, L.; Arya, D.P. Surface Dependent Dual Recognition of a G-Quadruplex DNA With Neomycin-Intercalator Conjugates. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.N.; Xi, H.; Arya, D.P. Molecular Recognition of a DNA:RNA Hybrid: Sub-Nanomolar Binding by a Neomycin–Methidium Conjugate. Bioorg. Med. Chem. Lett. 2008, 18, 4142–4145. [Google Scholar] [CrossRef]
- Wallis, M.G.; von Ahsen, U.; Schroeder, R.; Famulok, M. A Novel RNA Motif for Neomycin Recognition. Chem. Biol. 1995, 2, 543–552. [Google Scholar] [CrossRef]
- Herrmann, E.; Gierschik, P.; Jakobs, K.H. Neomycin Induces High-Affinity Agonist Binding of G-Protein-Coupled Receptors. Eur. J. Biochem. 1989, 185, 677–683. [Google Scholar] [CrossRef]
- Gabev, E.; Kasianowicz, J.; Abbott, T.; McLaughlin, S. Binding of Neomycin to Phosphatidylinositol 4,5-Bisphosphate (PIP2). Biochim. Biophys. Acta 1989, 979, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Faber, C.; Sticht, H.; Schweimer, K.; Rösch, P. Structural Rearrangements of HIV-1 Tat-Responsive RNA upon Binding of Neomycin B*. J. Biol. Chem. 2000, 275, 20660–20666. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huber, P.W.; Cui, M.; Czarnik, A.W.; Mei, H.-Y. Binding of Neomycin to the TAR Element of HIV-1 RNA Induces Dissociation of Tat Protein by an Allosteric Mechanism. Biochemistry 1998, 37, 5549–5557. [Google Scholar] [CrossRef]
- McFarland, A.W., Jr.; Fernando, L.P.; Kellish, P.; Story, S.P.; Schober, G.B.; Kumar, S.; Gong, C.; King, A.; Gong, X.; Leutou, A.S.; et al. Nucleic Acid Specificity, Cellular Localization and Reduced Toxicities of Thiazole Orange-Neomycin Conjugates. ChemistryOpen 2025, 14, e202400189. [Google Scholar] [CrossRef] [PubMed]
- Kammerud, S.C.; Metge, B.J.; Elhamamsy, A.R.; Weeks, S.E.; Alsheikh, H.A.; Mattheyses, A.L.; Shevde, L.A.; Samant, R.S. Novel Role of the Dietary Flavonoid Fisetin in Suppressing rRNA Biogenesis. Lab. Investig. 2021, 101, 1439–1448. [Google Scholar] [CrossRef]
- Notas, G.; Nifli, A.-P.; Kampa, M.; Pelekanou, V.; Alexaki, V.-I.; Theodoropoulos, P.; Vercauteren, J.; Castanas, E. Quercetin Accumulates in Nuclear Structures and Triggers Specific Gene Expression in Epithelial Cells. J. Nutr. Biochem. 2012, 23, 656–666. [Google Scholar] [CrossRef]
- Sasaki, Y.T.F.; Ideue, T.; Sano, M.; Mituyama, T.; Hirose, T. MENε/β Noncoding RNAs Are Essential for Structural Integrity of Nuclear Paraspeckles. Proc. Natl. Acad. Sci. USA 2009, 106, 2525–2530. [Google Scholar] [CrossRef]
- Peng, X.; Liu, X.; Li, J.; Tan, L. RNA-Binding of Ru(II) Complexes [Ru(Phen)2(7-OCH3-Dppz)]2+ and [Ru(Phen)2(7-NO2-Dppz)]2+: The Former Serves as a Molecular “Light Switch” for Poly(A)•poly(U). J. Inorg. Biochem. 2022, 237, 111991. [Google Scholar] [CrossRef]
- Lightfoot, H.L.; Hall, J. Endogenous Polyamine Function—The RNA Perspective. Nucleic Acids Res. 2014, 42, 11275–11290. [Google Scholar] [CrossRef] [PubMed]
- Bereiter, R.; Flemmich, L.; Nykiel, K.; Heel, S.; Geley, S.; Hanisch, M.; Eichler, C.; Breuker, K.; Lusser, A.; Micura, R. Engineering Covalent Small Molecule–RNA Complexes in Living Cells. Nat. Chem. Biol. 2025, 21, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; El-Sagheer, A.H.; Brown, T. Fluorogenic Thiazole Orange TOTFO Probes Stabilise Parallel DNA Triplexes at pH 7 and above. Chem. Sci. 2018, 9, 7681–7687. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Błaszczyk, L.; Rypniewski, W.; Falschlunger, C.; Micura, R.; Murata, A.; Dohno, C.; Nakatani, K.; Kiliszek, A. Structural Insights into Synthetic Ligands Targeting A–A Pairs in Disease-Related CAG RNA Repeats. Nucleic Acids Res. 2019, 47, 10906–10913. [Google Scholar] [CrossRef]
- Lu, Z.; Gong, J.; Zhang, Q.C. PARIS: Psoralen Analysis of RNA Interactions and Structures with High Throughput and Resolution. Methods Mol. Biol. 2018, 1649, 59–84. [Google Scholar] [CrossRef]
- Chen, X.-C.; Chen, S.-B.; Dai, J.; Yuan, J.-H.; Ou, T.-M.; Huang, Z.-S.; Tan, J.-H. Tracking the Dynamic Folding and Unfolding of RNA G-Quadruplexes in Live Cells. Angew. Chem. Int. Ed. 2018, 57, 4702–4706. [Google Scholar] [CrossRef]
- Agilent.com. Available online: https://www.agilent.com/cs/library/technicaloverviews/public/te-cary-3500-uv-vis-biomolecules-5994-7043en-agilent.pdf (accessed on 25 October 2025).
- Schievelbein, M.J.; Resende, C.; Glennon, M.M.; Kerosky, M.; Brown, J.A. Global RNA Modifications to the MALAT1 Triple Helix Differentially Affect Thermostability and Weaken Binding to METTL16. J. Biol. Chem. 2024, 300, 105548. [Google Scholar] [CrossRef]
- Vo, T.; Paul, A.; Kumar, A.; Boykin, D.W.; Wilson, W.D. Biosensor-Surface Plasmon Resonance: A Strategy to Help Establish a New Generation RNA-Specific Small Molecules. Methods 2019, 167, 15–27. [Google Scholar] [CrossRef]
- Menichelli, E.; Lam, B.J.; Wang, Y.; Wang, V.S.; Shaffer, J.; Tjhung, K.F.; Bursulaya, B.; Nguyen, T.N.; Vo, T.; Alper, P.B.; et al. Discovery of Small Molecules That Target a Tertiary-Structured RNA. Proc. Natl. Acad. Sci. USA 2022, 119, e2213117119. [Google Scholar] [CrossRef]
- Arney, J.W.; Weeks, K.M. RNA–Ligand Interactions Quantified by Surface Plasmon Resonance with Reference Subtraction. Biochemistry 2022, 61, 1625–1632. [Google Scholar] [CrossRef]
- Nakagawa, S.; Ip, J.Y.; Shioi, G.; Tripathi, V.; Zong, X.; Hirose, T.; Prasanth, K.V. Malat1 Is Not an Essential Component of Nuclear Speckles in Mice. RNA 2012, 18, 1487–1499. [Google Scholar] [CrossRef]
- Zhang, N.; Lan, R.; Chen, Y.; Hu, J. Identification of KDM4C as a Gene Conferring Drug Resistance in Multiple Myeloma. Open Life Sci. 2024, 19, 20220848. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Zhou, J.; Xu, J.; Peng, C.; Ye, F.; Shen, Y.; Lu, W.; Wan, X.; Xie, X. miR-375 Is Down-Regulated in Squamous Cervical Cancer and Inhibits Cell Migration and Invasion via Targeting Transcription Factor SP1. Am. J. Pathol. 2011, 179, 2580–2588. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The Nuclear-Retained Noncoding RNA MALAT1 Regulates Alternative Splicing by Modulating SR Splicing Factor Phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).