Near-Infrared Excited Mn4+- and Nd3+-Doped Y2SiO5 Luminescent Material with Flower-like Morphology for Plant-Centric Lighting Applications
Abstract
1. Introduction
2. Results and Discussion
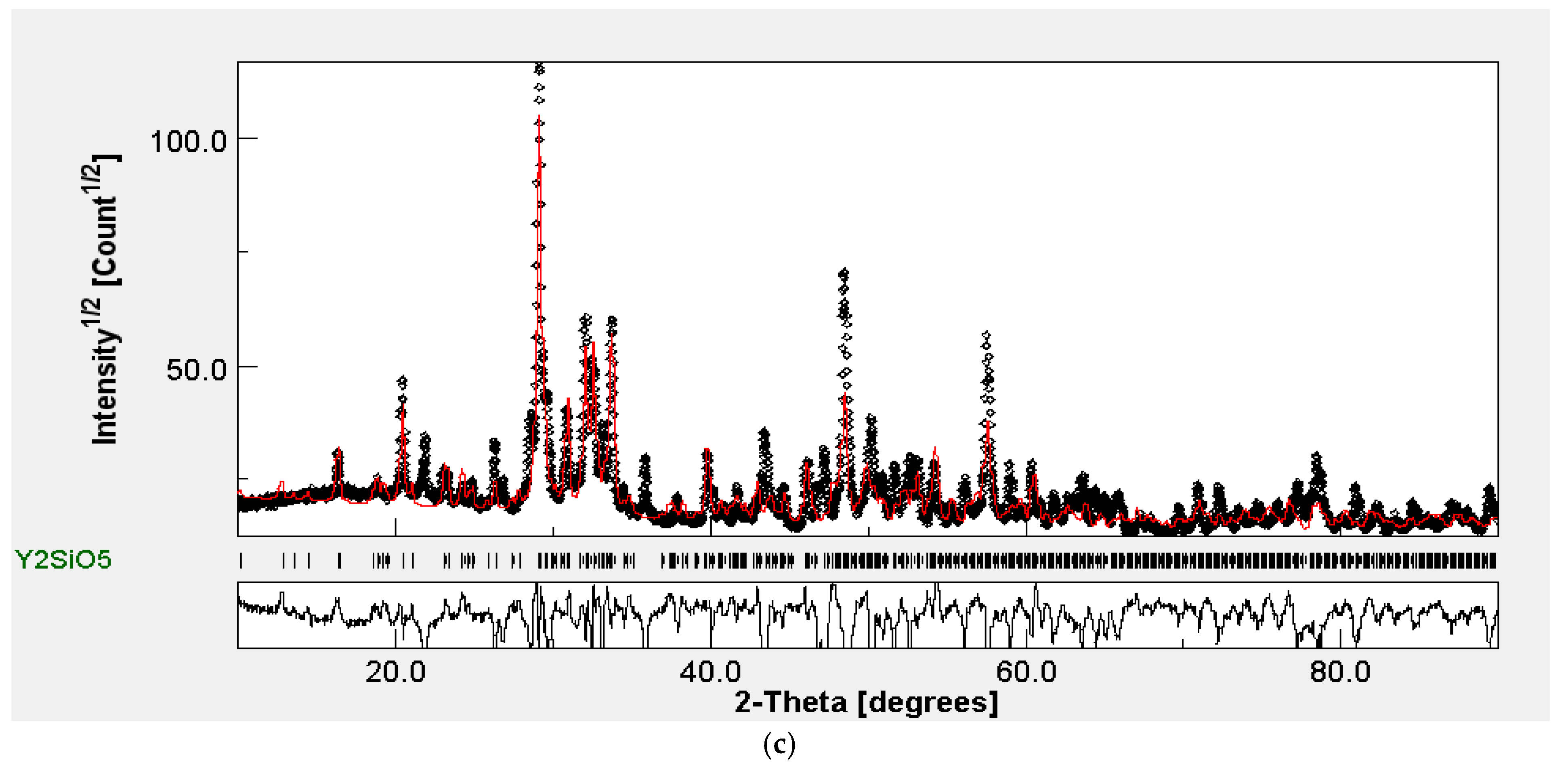
2.1. XRD Analysis
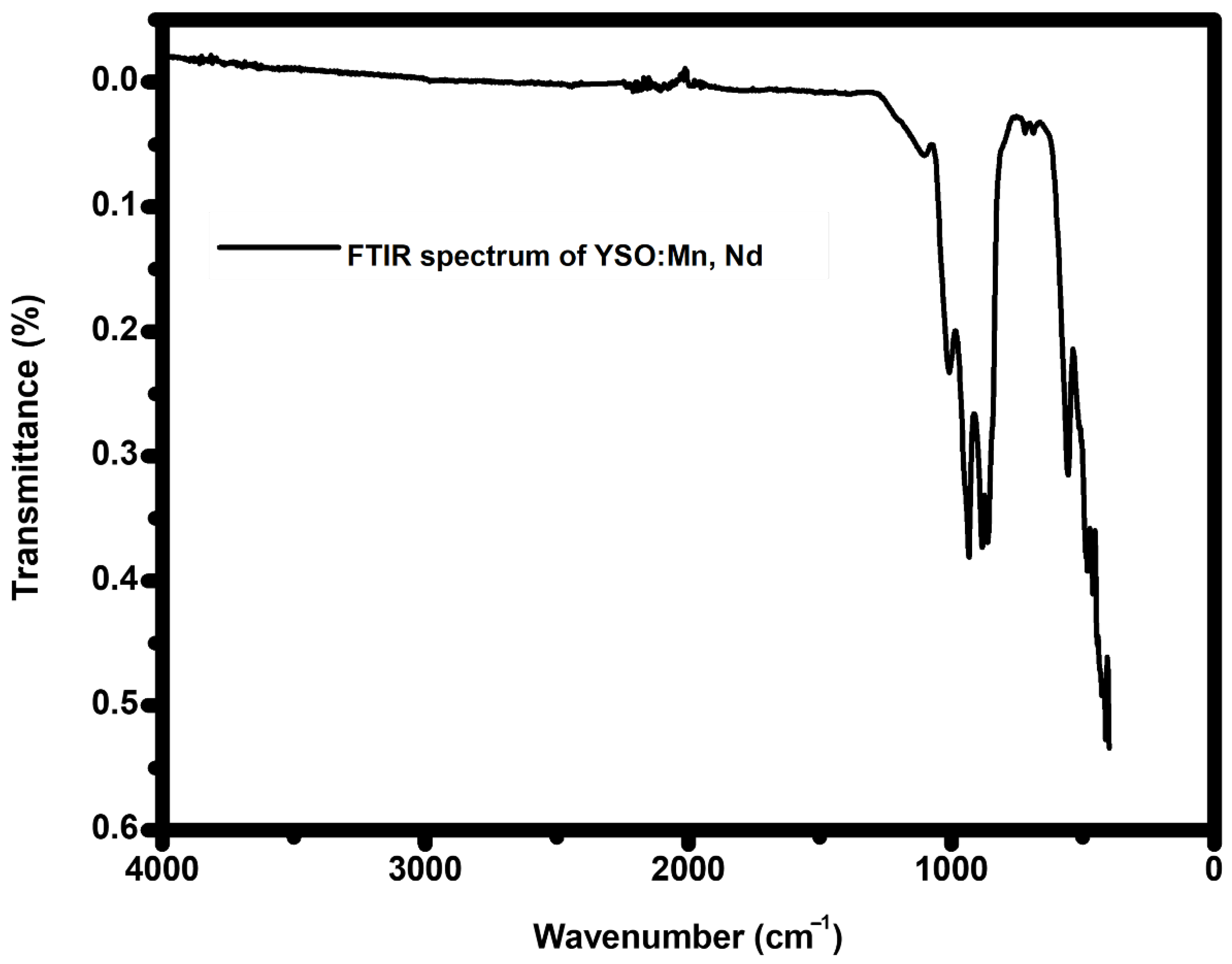
2.2. FTIR Study of Mn4+ and Nd3+Co-Doped Y2SiO5
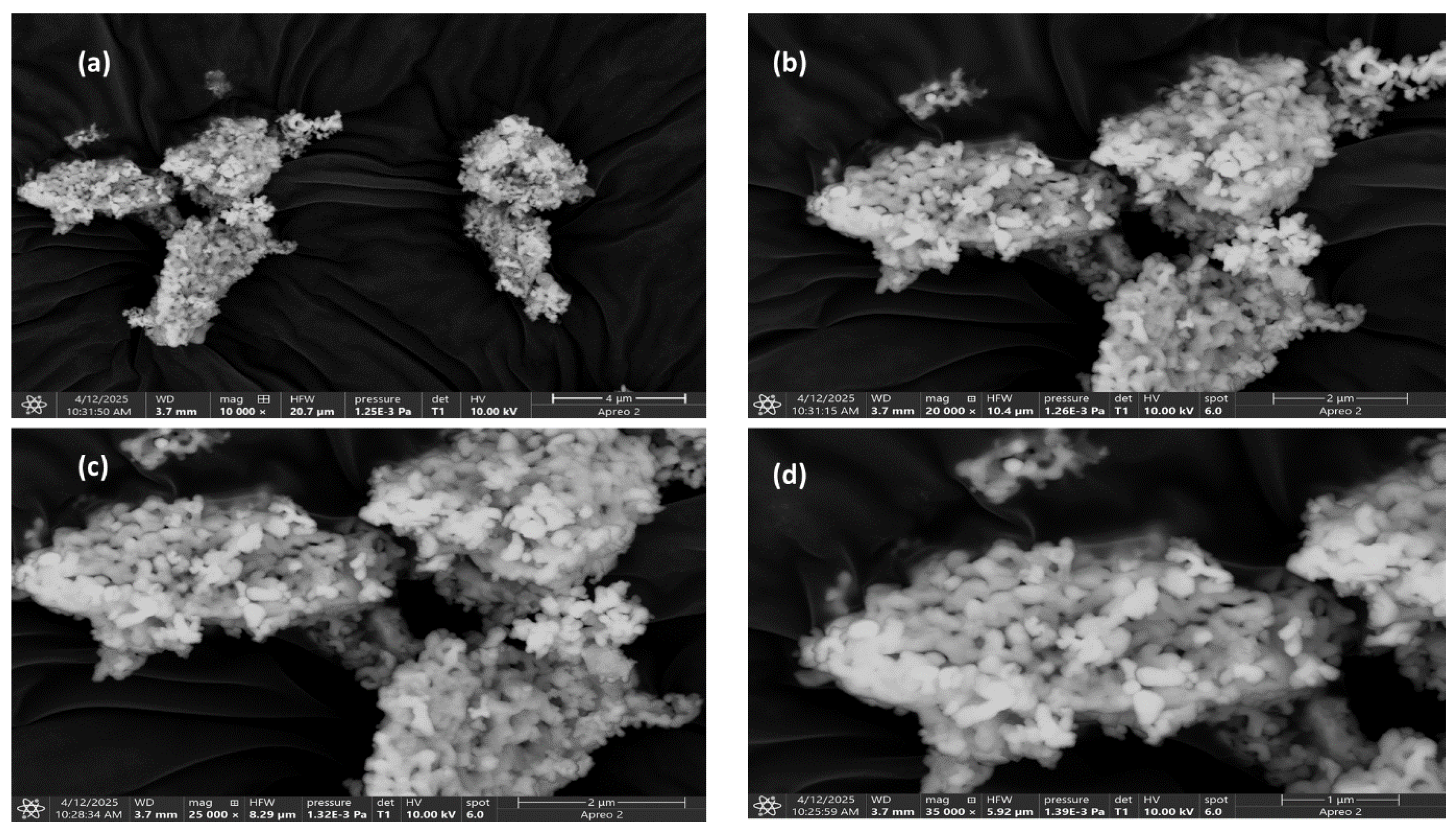
2.3. FEGSEM Study
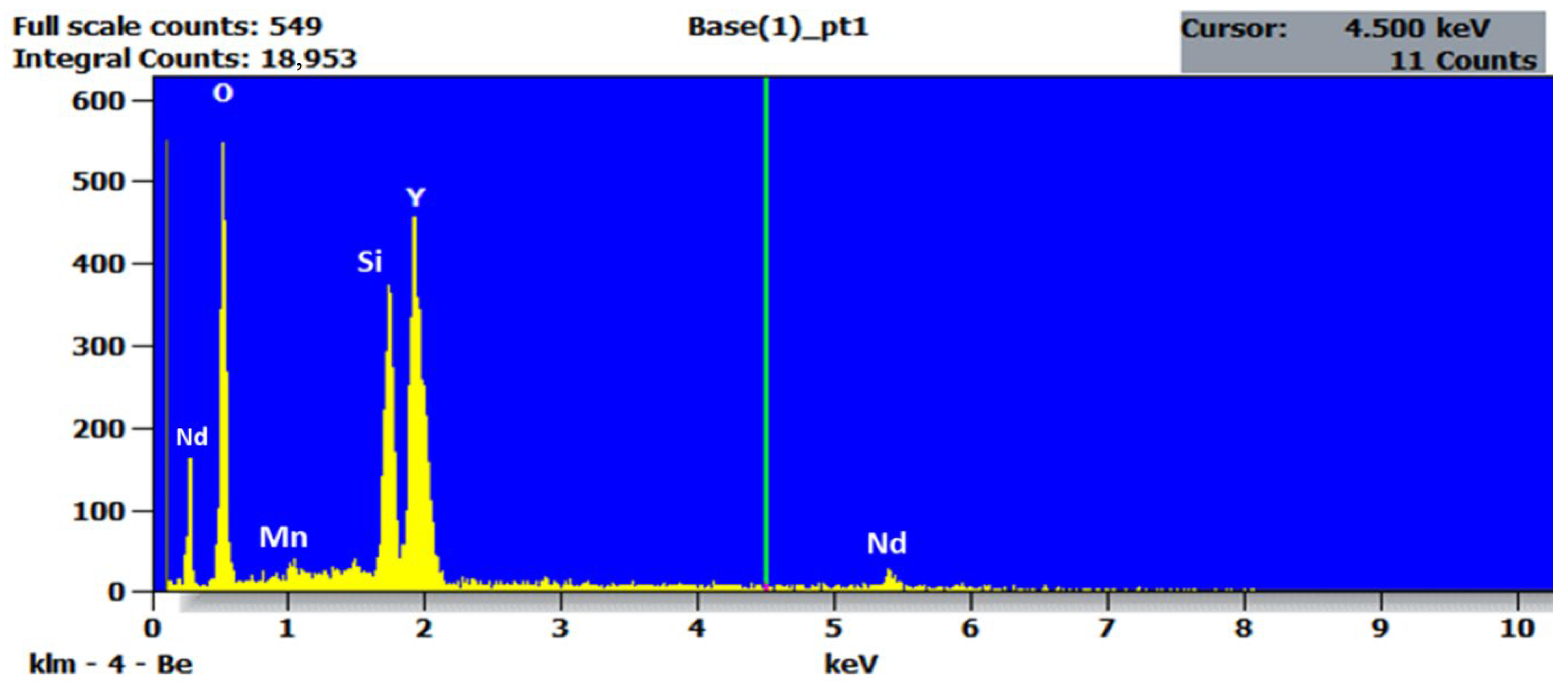
2.4. EDXS Analysis
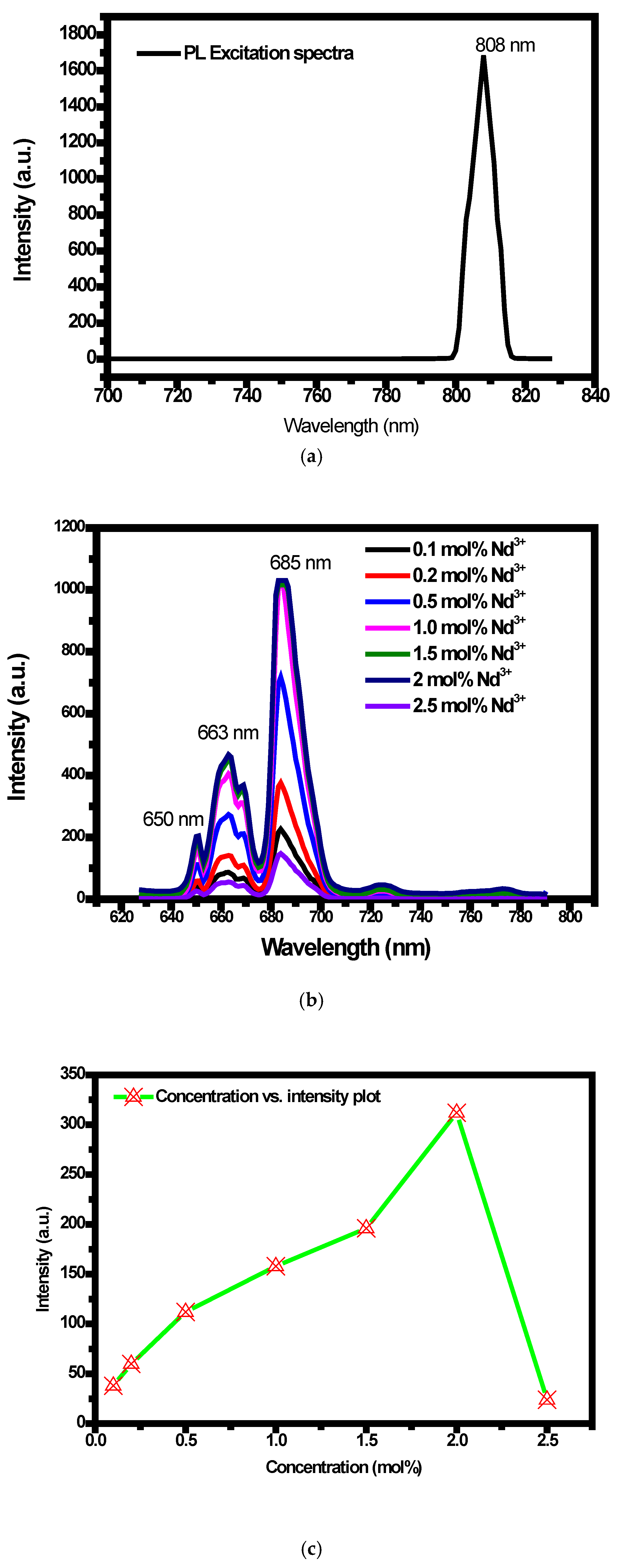
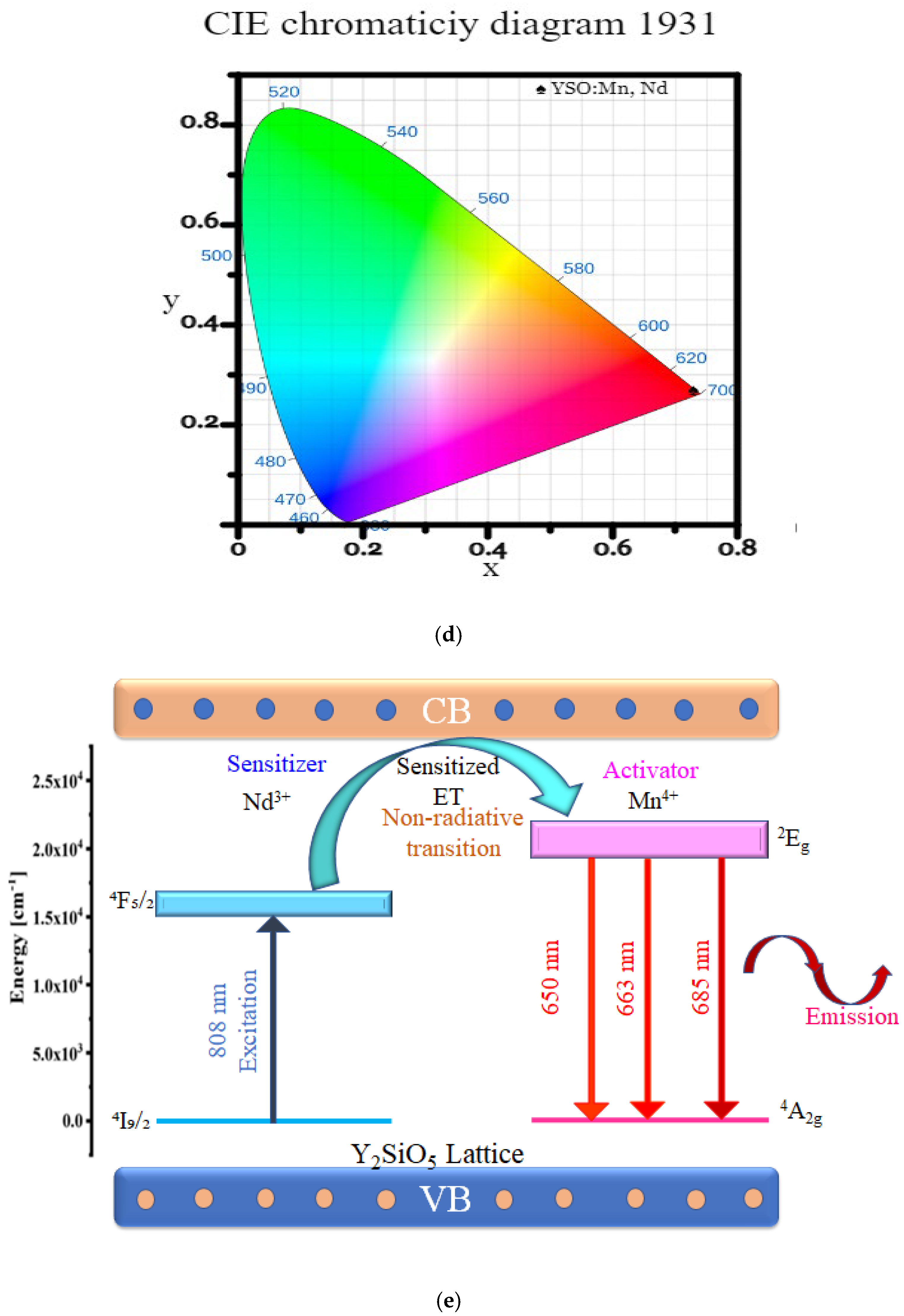
2.5. PL Emission and PLE Study of Y2SiO5:Mn4+ (1 Mol%), Nd3+ and Energy Transfer Mechanism
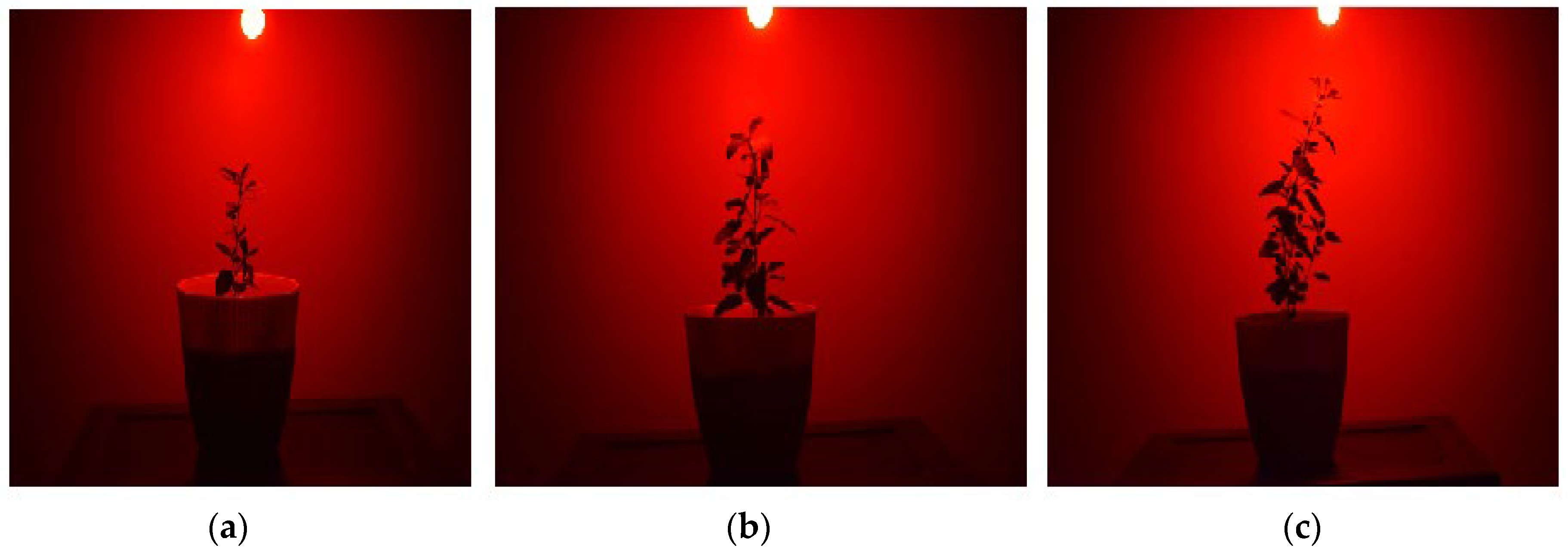
2.6. Real-World Application: Cultivation of Ocimum Tenuiflorum (Tulsi) Using Sunlight and Red LED Illumination
2.7. Plant Growth Application of a Fabricated Y2SiO5:Mn4+, Nd3+ Phosphor-LED: Cultivation of Ocimum Tenuiflorum
3. Experimental Procedure
3.1. Preparation of Y2SiO5:Mn4+, Nd3+ Phosphors
3.2. Characterization
3.3. Tulsi Growth Experiments
3.4. Fabrication of Red-Emitting pc-LEDs Based on Y2SiO5:Mn4+, Nd3+ Phosphors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qiu, L.; Zhang, W.; Xie, W.; Hu, Z.; Chen, J.; Feng, Z.; Luo, J.; Ye, Y.; Xiong, G. Thermally Stable Orange-Red BaLaLiWO6:Sm3+/Sr2+ Phosphors for Potential Application in Plant Growth. ECS J. Solid State Sci. Technol. 2025, 14, 026005. [Google Scholar] [CrossRef]
- Gong, W.; Luo, J.; Zhou, W.; Fan, J.; Sun, Z.; Zeng, S.; Pan, H.; Zhu, Z.; Yang, X.; Yu, Z.; et al. Thermal-stable blue-red dual-emitting Na2Mg2Si6O15:Eu2+, Mn2+ phosphor for plant growth lighting. J. Lumin. 2021, 239, 118372. [Google Scholar] [CrossRef]
- Fang, S.; Lang, T.; Cai, M.; Han, T. Light keys open locks of plant photoresponses: A review of phosphors for plant cultivation LEDs. J. Alloys Compd. 2022, 902, 163825. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Z.; Li, Y.; Jin, Z.; Ma, L.; Lu, R.; Zhang, X. Realization of plant growth lighting and temperature detecting based on novel Bi3+, Sm3+ and Mn4+ doped Ca2GdNbO6 double perovskite phosphors. Opt. Mater. 2023, 145, 114394. [Google Scholar] [CrossRef]
- Yang, C.; Liu, W.; You, Q.; Zhao, X.; Liu, S.; Xue, L.; Sun, J.; Jiang, X. Recent advances in light-conversion phosphors for plant growth and strategies for the modulation of photoluminescence properties. Nanomaterials 2023, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xia, M.; Zhou, C.; Kong, Z.; Molokeev, M.S.; Liu, L.; Wong, W.-Y.; Zhou, Z. Red shift properties, crystal field theory and nephelauxetic effect on Mn4+-doped SrMgAl10-yGayO17 red phosphor for plant growth LED light. Chem. Eng. J. 2020, 396, 125208. [Google Scholar] [CrossRef]
- Li, J.; Cheng, S.; Miao, X.; Ran, Y.; Huang, H.; Liu, R.; Deng, H.; Du, X.; Yu, R. Phonon sideband induced broadband far-red emission Li2GeTeO6:Mn4+ phosphors under blue-excitation for plant-cultivation LEDs, w-LEDs, and security ink. Appl. Mater. Today 2025, 42, 102598. [Google Scholar] [CrossRef]
- Yu, H.; Chan, J.; Devakumar, B.; Huang, X. Highly efficient far-red emitting Mn4+-activated Li3La3W2O12 phosphors for plant growth LED lighting. Mater. Today Chem. 2023, 30, 101584. [Google Scholar] [CrossRef]
- Tran, M.T.; Trung, D.Q.; Tu, N.; Anh, D.D.; Thu, L.T.H.; Du, N.V.; Quang, N.V.; Huyen, N.T.; Kien, N.D.T.; Viet, D.X.; et al. Single-phase far-red-emitting ZnAl2O4:Cr3+ phosphor for application in plant growth LEDs. J. Alloys Compd. 2021, 884, 161077. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, H.; Li, H.; Zhang, H.; Liu, C.; Zhao, R.; Yang, Z.; Tang, H.; Li, J.; Zhang, J.; et al. Manipulating alkali charge compensation to improve red fluorescence and thermostability in Ba5P6O20:Eu3+ phosphor. Appl. Mater. Today 2024, 37, 102095. [Google Scholar] [CrossRef]
- Wang, M.; Han, Z.; Huang, J.; Liao, J.; Sun, Y.; Huang, H.; Wen, H.R. NaLaMgWO6:Mn4+/Pr3+/Bi3+ bifunctional phosphors for optical thermometer and plant growth illumination matching phytochrome PR and PFR. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119915. [Google Scholar] [CrossRef]
- Nozue, K.; Devisetty, U.K.; Lekkala, S.; Mueller-Moulé, P.; Bak, A.; Casteel, C.L.; Maloof, J.N. Network analysis reveals a role for salicylic acid pathway components in shade avoidance. Plant Physiol. 2018, 178, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Yin, J.; Zhang, X.; Li, Y.; Su, L.; Zhou, Z.; Xia, M. A novel Cr3+-activated far-red titanate phosphor: Synthesis, luminescence enhancement and application prospect. Mater. Today Chem. 2022, 24, 100835. [Google Scholar] [CrossRef]
- Vaistij, F.E.; Barros-Galvão, T.; Cole, A.F.; Gilday, A.D.; He, Z.; Li, Y.; Harvey, D.; Larson, T.R.; Graham, I.A. MOTHER-OF-FT-AND-TFL1 represses seed germination under far-red light by modulating phytohormone responses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2018, 115, 8442–8447. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, C.; Yang, Z.; Li, T.; Zhao, J. Li2SrSiO4:Ce3+, Pr3+ phosphor with blue, red, and near-infrared emissions used for plant growth LED. J. Am. Ceram. Soc. 2016, 99, 218–225. [Google Scholar] [CrossRef]
- Cao, R.; Shi, Z.; Quan, G.; Chen, T.; Guo, S.; Hu, Z.; Liu, P. Preparation and luminescence properties of Li2MgZrO4:Mn4+ red phosphor for plant growth. J. Lumin. 2017, 188, 577–581. [Google Scholar] [CrossRef]
- Xiang, J.; Chang, J.; Chen, C.; Jin, S.; Chen, R.; Gao, R.; Jin, M.; Guo, C. Recent progress of inorganic phosphors in artificial plant cultivation LEDs. J. Mater. Chem. C 2025, 13, 1538–1556. [Google Scholar] [CrossRef]
- Ji, S.H.; Gururani, M.A.; Chun, S.C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef]
- Taikar, D.R.; Sonkusare, K.; Dhoble, S.J.; Yadav, R.S. Recent progress in Cr3+ doped phosphors for indoor plant cultivation LEDs: A Review. J. Mol. Struct. 2025, 1331, 141564. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Yeh, N.; Chung, J.P. High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Sustain. Energy Rev. 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Bargat, S.R.; Parauha, Y.R.; Shirbhate, N.S.; Mishra, G.; Dhoble, S.J. Novel red colour emitting Ca0.995Mg2(SO4)3:0.5Eu2+ phosphor under ultraviolet, blue, and green excitation for plant growth LEDs. Luminescence 2022, 37, 463–471. [Google Scholar] [CrossRef]
- Reyes, T.H.; Esparza, E.; Crestani, G.; Limonchi, F.; Cruz, R.; Salinas, N.; Scartazza, A.; Guglielminetti, L.; Cosio, E. Physiological responses of maca (Lepidium meyenii Walp) plants to UV radiation in its high-altitude mountain ecosystem. Sci. Rep. 2020, 10, 2654. [Google Scholar] [CrossRef]
- Deng, W.; Bates, J.A.; Wei, H.; Bartoschek, M.D.; Conradt, B.; Leonhardt, H. Tunable light and drug induced depletion of target proteins. Nat. Commun. 2020, 11, 304. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Tian, F.; Jia, C.; Li, C.; Li, Y. Transcriptomic profiling sheds light on the blue-light and red-light response of oyster mushroom (Pleurotus ostreatus). Amb Express 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Dhoble, S.J.; Priya, R.; Dhoble, N.S.; Pandey, O.P. Short review on recent progress in Mn4+-activated oxide phosphors for indoor plant light-emitting diodes. Luminescence 2021, 36, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Meng, Y.; Wen, L.; Huang, M.; Zhou, L.; Liao, L.; He, D. Double perovskite Ba2LaNbO6:Mn4+, Yb3+ phosphors: Potential application to plant-cultivation LEDs. Dye. Pigment. 2019, 160, 395–402. [Google Scholar] [CrossRef]
- Ahn, Y.D.; Bae, S.; Kang, S.J. Power controllable LED system with increased energy efficiency using multi-sensors for plant cultivation. Energies 2017, 10, 1607. [Google Scholar]
- Long, J.; Yuan, X.; Ma, C.; Du, M.; Ma, X.; Wen, Z.; Ma, R.; Wang, Y.; Cao, Y. Strongly enhanced luminescence of Sr4Al14O25:Mn4+ phosphor by co-doping B3+ and Na+ ions with red emission for plant growth LEDs. RSC Adv. 2018, 8, 1469–1476. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, H.; Zhang, X.; Zheng, Y.; Yuan, J.; Liu, H.; Liu, Y.; Lei, B.; Qiu, J. Ultrastable red-emitting phosphor-in-glass for superior high-power artificial plant growth LEDs. J. Mater. Chem. C 2018, 6, 1738–1745. [Google Scholar] [CrossRef]
- Xiang, J.; Zheng, J.; Zhou, Z.; Suo, H.; Zhao, X.; Zhou, X.; Zhang, N.; Molokeev, M.S.; Guo, C. Enhancement of red emission and site analysis in Eu2+ doped new-type structure Ba3CaK(PO4)3 for plant growth white LEDs. Chem. Eng. J. 2019, 356, 236–244. [Google Scholar]
- Zhou, Z.; Zheng, J.; Shi, R.; Zhang, N.; Chen, J.; Zhang, R.; Suo, H.; Goldys, E.M.; Guo, C. Ab initio site occupancy and far-red emission of Mn4+ in cubic-phase La(MgTi)1/2O3 for plant cultivation. ACS Appl. Mater. Interfaces 2017, 9, 6177–6185. [Google Scholar] [CrossRef]
- Liu, R.S. (Ed.) Phosphors, up Conversion Nano Particles, Quantum Dots and Their Applications; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1. [Google Scholar]
- Rahman, J.U.; Khan, S.; Jain, V.; Rajiv, A.; Dasi, S.; Fawy, K.F.; Jindal, P.K.; Sivaranjani, R. Exploring inorganic phosphors: Basics, types, fabrications and their luminescence properties for LED/WLED/displays. Rev. Inorg. Chem. 2025, 45, 55–76. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, W.; Lü, F.; Sui, Y.; Wang, J.; Xu, Z. Research of fluorescent properties of a new type of phosphor with Mn2+-doped Ca2SiO4. Sensors 2021, 21, 2788. [Google Scholar]
- Kumar, A.; Manam, J. Color tunable emission and temperature dependent photoluminescence properties of Eu3+ co-doped Gd2Zr2O7:Dy3+ phosphors. Opt. Mater. 2019, 96, 109373. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Q.; Xia, Z. Structural engineering of Eu2+-doped silicates phosphors for LED applications. Acc. Mater. Res. 2020, 1, 137–145. [Google Scholar]
- Nagaraj, R.; Raja, A.; Ranjith, S. Synthesis and luminescence properties of novel red-emitting Eu3+ ions doped silicate phosphors for photonic applications. J. Alloys Compd. 2020, 827, 154289. [Google Scholar] [CrossRef]
- Figueiredo, B.R.; Valente, A.A.; Lin, Z.; Silva, C.M. Photoluminescent porous and layered lanthanide silicates: A review. Microporous Mesoporous Mater. 2016, 234, 73–97. [Google Scholar]
- Richhariya, T.; Brahme, N.; Bisen, D.P.; Choubey, A.; Patle, Y.; Chandrawanshi, E. A comparative photoluminescence and Judd–Ofelt study on alumino silicate phosphors. J. Mater. Sci. Mater. Electron. 2020, 31, 13667–13679. [Google Scholar] [CrossRef]
- Verma, N.; Kaur, J.; Dubey, V.; Dubey, N.; Ram, T. Luminescence properties of Y2SiO5 phosphors: A review. Inorg. Chem. Commun. 2023, 147, 110234. [Google Scholar] [CrossRef]
- Parganiha, Y.; Kaur, J.; Dubey, V.; Chandrakar, D. Synthesis, characterization, thermoluminescence and optical studies of Eu3+ doped Y2SiO5 phosphor. Superlattices Microstruct. 2015, 77, 152–161. [Google Scholar] [CrossRef]
- Parganiha, Y.; Kaur, J.; Dubey, V.; Murthy, K.V.R. Near UV–blue emission from Ce doped Y2SiO5 phosphor. Mater. Sci. Semicond. Process. 2015, 31, 715–719. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, S.; Dubey, V.; Kshatri, D.S.; Patharia, P.; Dubey, N.; Rao, M.C. Synthesis, Structural, and Photoluminescence Studies of Tb3+ Activated Y2SiO5 Phosphor for Display Devices. J. Appl. Spectrosc. 2024, 91, 154–158. [Google Scholar] [CrossRef]
- Gowri, M.M.; Darshan, G.P.; Naik, Y.V.; Premkumar, H.B.; Kavyashree, D.; Sharma, S.C.; Nagabhushana, H. Phase dependent photoluminescence and thermoluminescence properties of Y2SiO5:Sm3+ nanophosphors and its advanced forensic applications. Opt. Mater. 2019, 96, 109282. [Google Scholar] [CrossRef]
- Xia, W.; Ye, Y.; Mao, Q.; Ding, Y.; Li, X.; Liu, M.; Zhong, J. Engineering efficient blue and far-red dual-emitting phosphor for plant growth. Mater. Today Chem. 2024, 36, 101958. [Google Scholar] [CrossRef]
- Deka, L.R.; Dubey, V. The great potential of inorganic phosphors in the growing field of plant cultivation: A review. Inorg. Chem. Commun. 2025, 180 Pt 2, 115058. [Google Scholar] [CrossRef]
- Adachi, S. Photoluminescence properties of Mn4+-activated oxide phosphors for use in white-LED applications: A review. J. Lumin. 2018, 202, 263–281. [Google Scholar] [CrossRef]
- Wu, J.; Mao, Y.; Wang, J.; Shi, S. Synthesis, structural characterization and intense far-red luminescence of Mn4+-activated SrLaMgTa1-yAlyO6 oxide phosphors. J. Lumin. 2025, 277, 120892. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, T.; Su, L.; Cheng, X.; Chen, T.; Guo, S.; Yu, X. Photoluminescence properties of Ba2LaSbO6:Mn4+ deep-red-emitting phosphor for plant growth LEDs. J. Lumin. 2019, 209, 1–7. [Google Scholar]
- Chen, Y.; Lin, X.; Luo, Z.; Huang, Y. Spectroscopic properties of Nd3+ ions in La2(WO4)3 crystal. Chem. Phys. Lett. 2003, 381, 598–604. [Google Scholar] [CrossRef]
- Han, S.; Deng, R.; Xie, X.; Liu, X. Enhancing luminescence in lanthanide-doped upconversion nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 11702–11715. [Google Scholar] [CrossRef]
- Daldosso, N.; Navarro-Urrios, D.; Melchiorri, M.; Pavesi, L.; Gourbilleau, F.; Carrada, M.; Rizk, R.; García, C.; Pellegrino, P.; Garrido, B.; et al. Absorption cross section and signal enhancement in Er-doped Si nanocluster rib-loaded waveguides. Appl. Phys. Lett. 2005, 86, 261103. [Google Scholar] [CrossRef]
- Devarajulu, G.; Kumar, B.K.; Babu, P.R.; Dhananjaya, M.; Bak, N.-H.; Pasupuleti, K.S.; Raju, B.D.P.; Kim, M.-D. Sensitization effect of Nd3+ ions on Yb3+/Nd3+ co-doped oxyfluoride glasses and study of their optical, fluorescence, and upconversion abilities for visible laser and NIR amplifier applications. Ceram. Int. 2022, 48, 24550–24559. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Found. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Zhu, Q.; Li, J.G. Cationic pair substitution in LaAlO3:Mn4+ for octahedral-tilting-dependent zero-phonon line. Inorg. Chem. Front. 2023, 10, 638–650. [Google Scholar] [CrossRef]
- Du, J.; De Clercq, O.Q.; Korthout, K.; Poelman, D. LaAlO3:Mn4+ as near-infrared emitting persistent luminescence phosphor for medical imaging: A charge compensation study. Materials 2017, 10, 1422. [Google Scholar] [CrossRef]
- Verma, N.; Michalska-Domańska, M.; Dubey, V.; Ram, T.; Kaur, J.; Dubey, N.; Aman, S.; Manners, O.; Saji, J. Investigation of Spectroscopic Parameters and Trap Parameters of Eu3+-Activated Y2SiO5 Phosphors for Display and Dosimetry Applications. Molecules 2024, 30, 108. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D. Structural and optical properties of green emitting Y2SiO5:Tb3+ and Gd2SiO5:Tb3+ nanoparticles for modern lighting applications. Rare Met. 2021, 40, 3289–3298. [Google Scholar] [CrossRef]
- Onutai, S.; Osugi, T.; Sone, T. Alumino-silicate structural formation during alkali-activation of metakaolin: In-situ and ex-situ ATR-FTIR studies. Materials 2023, 16, 985. [Google Scholar] [CrossRef]
- Wang, J.; Zou, B.; El-Sayed, M.A. Comparison between the polarized Fourier-transform infrared spectra of aged porous silicon and amorphous silicon dioxide films on Si (100) surface. J. Mol. Struct. 1999, 508, 87–96. [Google Scholar] [CrossRef]
- Moore, C.; Perova, T.S.; Kennedy, B.J.; Berwick, K.; Shaganov, I.I.; Moore, R.A. Study of structure and quality of different silicon oxides using FTIR and Raman microscopy. In Opto-Ireland 2002: Optics and Photonics Technologies and Applications; SPIE: Bellingham, WA, USA, 2003; Volume 4876, pp. 1247–1256. [Google Scholar]
- Singh, V.; Kummara, V.K.; Ravi, N.; Joo, J.B. Luminescence and electron spin resonance studies of narrow-band UVB emitting Gd3+ doped Y2SiO5 nanophosphors synthesized by sol-gel method. Optik 2021, 242, 167228. [Google Scholar] [CrossRef]
- Chu, H.A.; Sackett, H.; Babcock, G.T. Identification of a Mn− O− Mn cluster vibrational mode of the oxygen-evolving complex in photosystem II by low-frequency FTIR spectroscopy. Biochemistry 2000, 39, 14371–14376. [Google Scholar]
- El-Deen, L.S.; Al Salhi, M.S.; Elkholy, M.M. IR and UV spectral studies for rare earths-doped tellurite glasses. J. Alloys Compd. 2008, 465, 333–339. [Google Scholar] [CrossRef]
- Xia, M.; Gu, S.; Zhou, C.; Liu, L.; Zhong, Y.; Zhang, Y.; Zhou, Z. Enhanced photoluminescence and energy transfer performance of Y3Al4GaO12:Mn4+, Dy3+ phosphors for plant growth LED lights. RSC Adv. 2019, 9, 9244–9252. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Seto, T.; Liu, B.; Wang, Y.; Li, C.; Liu, Z.; Dong, H. Tremendous acceleration of plant growth by applying a new sunlight converter Sr4Al14− xGaxO25:Mn4+ breaking parity forbidden transition. Adv. Sci. 2023, 10, 2204418. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Pan, Y.; Jin, Y.; Lin, J. A review on the structural dependent optical properties and energy transfer of Mn4+ and multiple ion-codoped complex oxide phosphors. RSC Adv. 2021, 11, 760–779. [Google Scholar] [CrossRef] [PubMed]
- Golyeva, E.V.; Vaishlia, E.I.; Kurochkin, M.A.; Kolesnikov, E.Y.; Lähderanta, E.; Semencha, A.V.; Kolesnikov, I.E. Nd3+ concentration effect on luminescent properties of MgAl2O4 nanopowders synthesized by modified Pechini method. J. Solid State Chem. 2020, 289, 121486. [Google Scholar] [CrossRef]
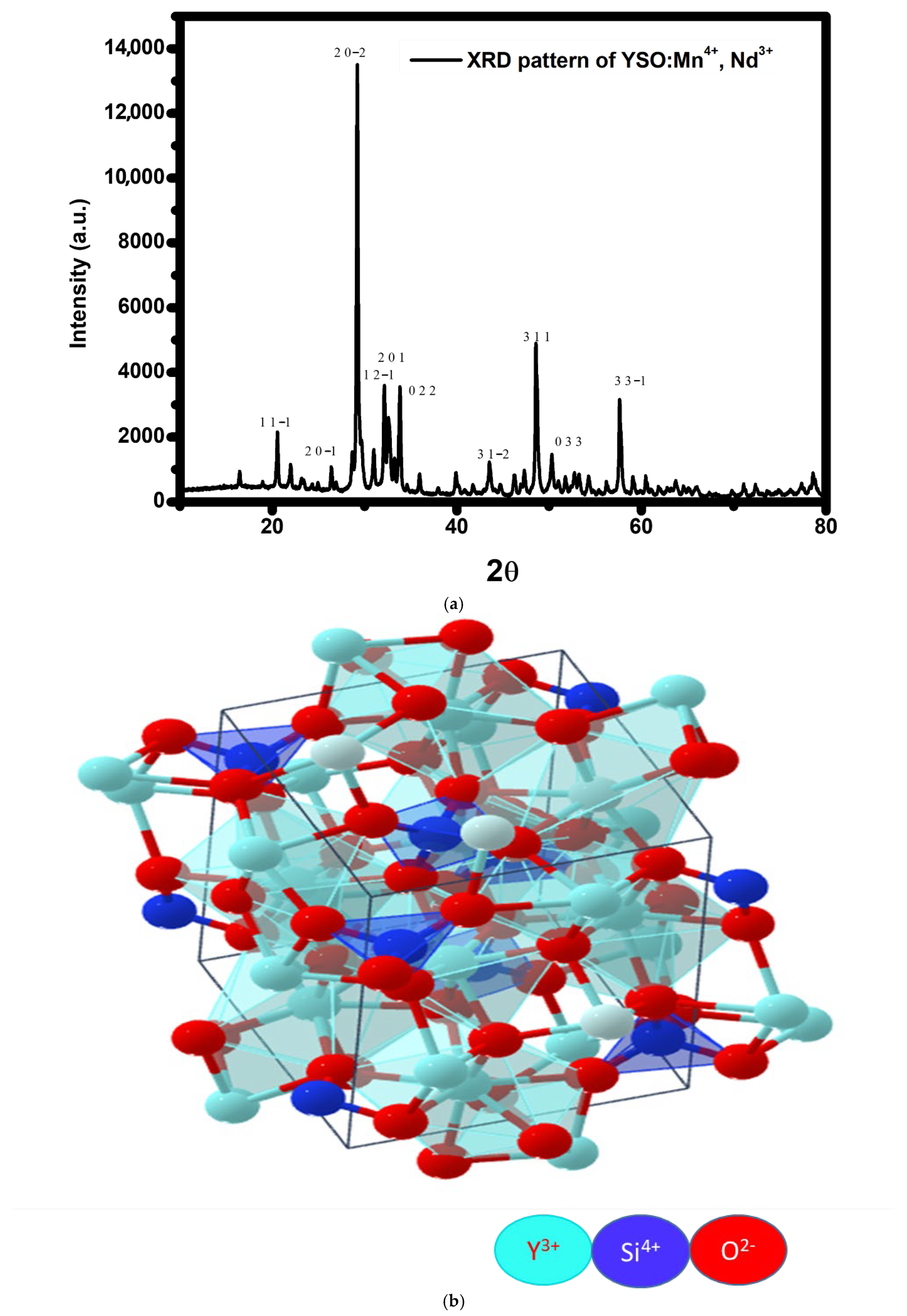


| Peak | B (degree) | B (rad) | 2θ | Θ (rad) | Cosθ | 0.9λ | B * Cosθ (rad) | D (nm) | hkl |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.22538 | 0.003932 | 20.5641 | 0.179365 | 0.983957 | 0.13865 | 0.003869 | 35.84036 | 11-1 |
| 2 | 0.19276 | 0.003363 | 26.42177 | 0.230457 | 0.973562 | 0.13865 | 0.003274 | 42.35292 | 20-1 |
| 3 | 0.22676 | 0.003956 | 29.22564 | 0.254913 | 0.967685 | 0.13865 | 0.003828 | 36.22124 | 20-2 |
| 4 | 0.29306 | 0.005112 | 30.99775 | 0.270369 | 0.963672 | 0.13865 | 0.004927 | 28.1435 | 12-1 |
| 5 | 0.98932 | 0.017258 | 32.42406 | 0.28281 | 0.960275 | 0.13865 | 0.016573 | 8.366263 | 201 |
| 6 | 0.26124 | 0.004557 | 33.83133 | 0.295084 | 0.956778 | 0.13865 | 0.00436 | 31.79899 | 022 |
| 7 | 0.2995 | 0.005225 | 43.56326 | 0.379968 | 0.928676 | 0.13865 | 0.004852 | 28.57609 | 31-2 |
| 8 | 0.30354 | 0.005295 | 48.62457 | 0.424114 | 0.911404 | 0.13865 | 0.004826 | 28.73011 | 311 |
| 9 | 0.34542 | 0.006026 | 50.29503 | 0.438684 | 0.905311 | 0.13865 | 0.005455 | 25.41667 | 033 |
| 10 | 0.30405 | 0.005304 | 57.69076 | 0.503192 | 0.876048 | 0.13865 | 0.004647 | 29.83947 | 33-1 |
| Observed Wavenumber (cm−1) | Assigned Vibration | Bond | Ref. No. |
|---|---|---|---|
| 1102 | Stretching | Si-O-Si | [60] |
| 1008 | Stretching | Si-O-Si | [61] |
| 932 | Stretching | Si-O | [62] |
| 883 | Asymmetric stretching | Si-O | [63] |
| 719 | Bending | Y-O | [63] |
| 686 | Asymmetric stretching | Si-O | [63] |
| 556 | Bending/symmetric/stretching | Y-O/Mn-O-Mn/Nd-O | [63,64,65] |
| Element | Molecular Weight (amu) | Weight Percentage (%) | Atomic Percentage (%) |
|---|---|---|---|
| Y | 88.906 | 60.8519 | 24.625 |
| Si | 28.086 | 9.66055 | 12.375 |
| O | 15.999 | 27.7933 | 62.5 |
| Mn | 54.938 | 0.19088 | 0.125 |
| Nd | 144.24 | 1.50343 | 0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deka, L.R.; Michalska-Domańska, M.; Mishra, S.; Kshatri, D.S.; Rao, M.C.; Verma, N.; Dubey, V. Near-Infrared Excited Mn4+- and Nd3+-Doped Y2SiO5 Luminescent Material with Flower-like Morphology for Plant-Centric Lighting Applications. Molecules 2025, 30, 4161. https://doi.org/10.3390/molecules30214161
Deka LR, Michalska-Domańska M, Mishra S, Kshatri DS, Rao MC, Verma N, Dubey V. Near-Infrared Excited Mn4+- and Nd3+-Doped Y2SiO5 Luminescent Material with Flower-like Morphology for Plant-Centric Lighting Applications. Molecules. 2025; 30(21):4161. https://doi.org/10.3390/molecules30214161
Chicago/Turabian StyleDeka, Liza Rani, Marta Michalska-Domańska, Shubhra Mishra, D. S. Kshatri, M. C. Rao, Neeraj Verma, and Vikas Dubey. 2025. "Near-Infrared Excited Mn4+- and Nd3+-Doped Y2SiO5 Luminescent Material with Flower-like Morphology for Plant-Centric Lighting Applications" Molecules 30, no. 21: 4161. https://doi.org/10.3390/molecules30214161
APA StyleDeka, L. R., Michalska-Domańska, M., Mishra, S., Kshatri, D. S., Rao, M. C., Verma, N., & Dubey, V. (2025). Near-Infrared Excited Mn4+- and Nd3+-Doped Y2SiO5 Luminescent Material with Flower-like Morphology for Plant-Centric Lighting Applications. Molecules, 30(21), 4161. https://doi.org/10.3390/molecules30214161








