An Innovative Use of the QuEChERs Method and LC-MS/MS Technique for Fast and Simple Determination of Quinolizidine Alkaloids in Leguminous Plants
Abstract
1. Introduction
2. Results and Discussion
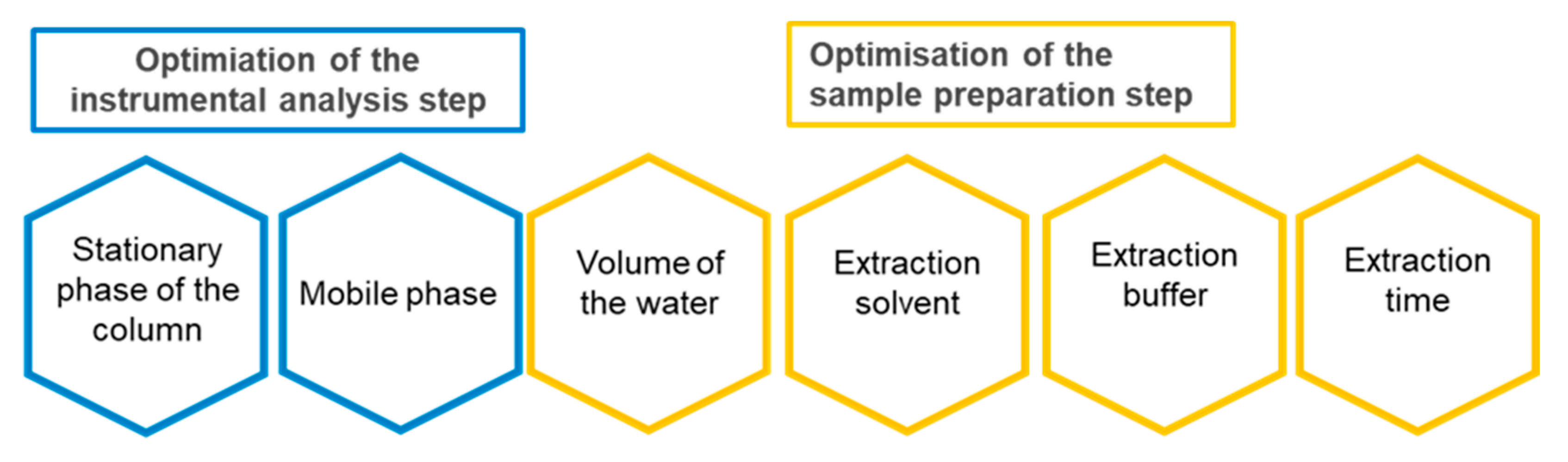
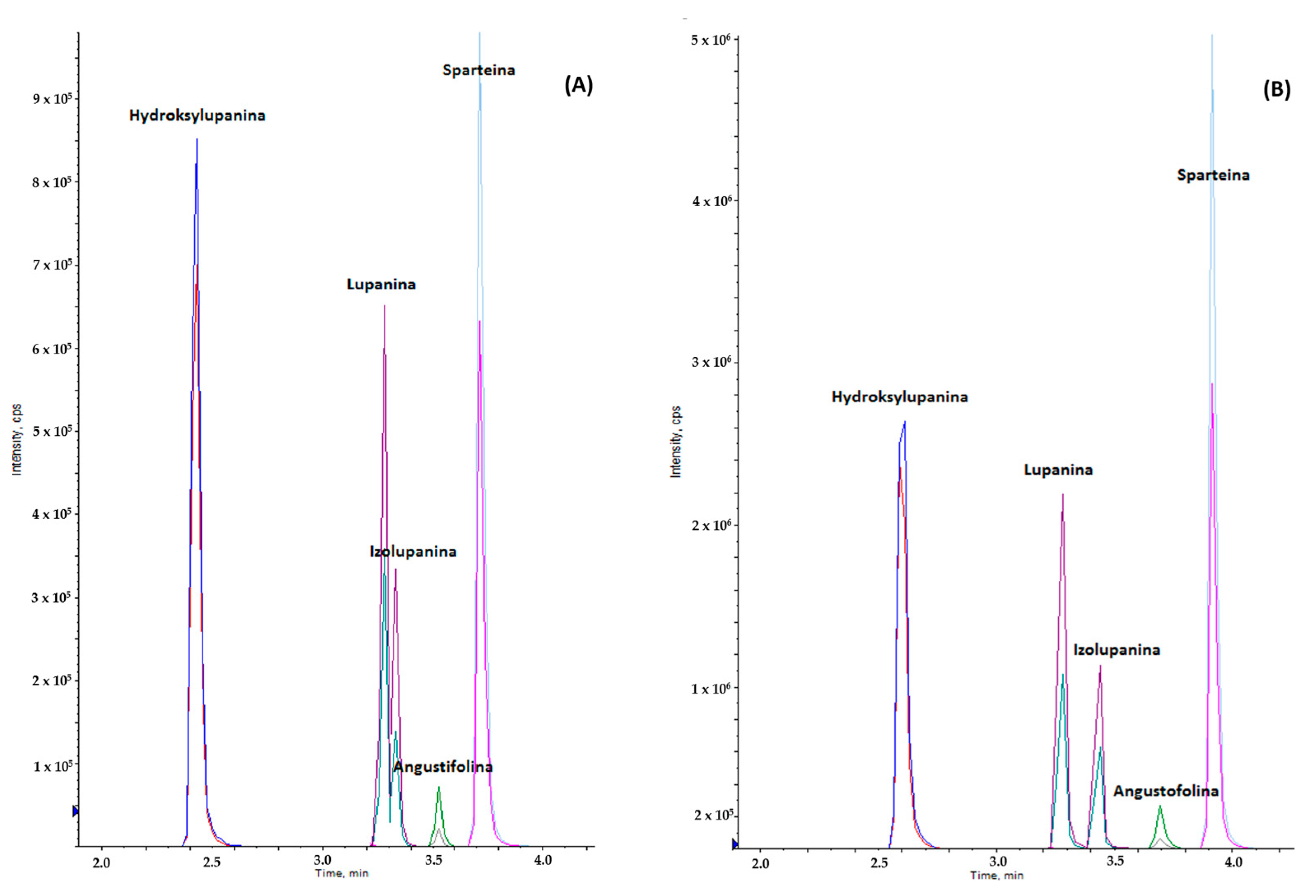
2.1. QuEChERS Method and LC-MS/MS Techniques Development
2.2. Method Validation Results
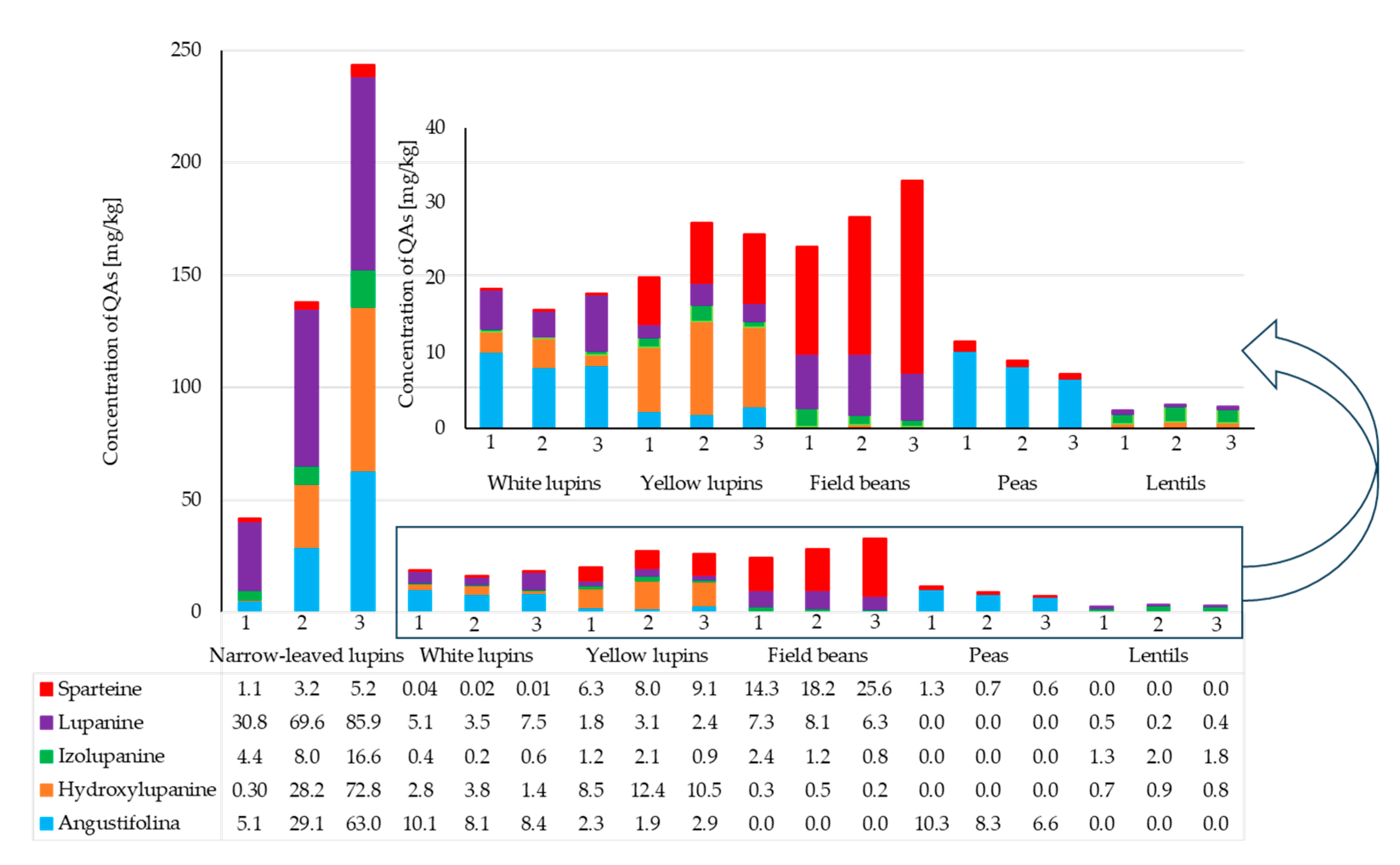
2.3. Analysis of QAs in Leguminous Plants
3. Materials and Methods
3.1. Materials
3.2. Chemicals and Reagents
3.3. Standards
3.4. Sample Preparation
3.5. LC–MS/MS Conditions
3.6. Method Validation
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOAC | Association of Official Analytical Chemists |
| C18 | Octadecylsilane |
| DEV | Deviation of the Back-Calculated Concentration |
| EU | European Union |
| FID | Flame Ionization Detector |
| GC-MS | Gas Chromatography with Mass Spectrometry |
| LC-MS/MS | Liquid Chromatography Tandem Mass Spectrometry |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MgSO4 | Magnesium sulfate |
| ME | Matrix Effect |
| MRM | Multiple Reaction Monitoring |
| ML | Maximum Level |
| Na2HC6H5O7*1.5H2O | Disodium hydrogen citrate sesquehydrate |
| Na3C6H5O7*2H2O | Trisodium citrate dihydrate |
| NaCl | Sodium chloride |
| NaOH | Sodium hydroxide |
| PAs | Pyrrolizidine alkaloids |
| PAH | Polycyclic Aromatic Hydrocarbons |
| R | Recovery |
| S/N | Signal/Noise |
| SPE | Solid Phase Extraction |
| QuEChERS | Quick, Easy, Cheap, Effective, Rugged, and Safe |
| QAs | Quinolizidine Alkaloids |
| UHPLC-QTOF-MS | Ultra-High-Performance Liquid Chromatography-triple/time-of-flight Mass Spectrometry |
| TAs | Tropane alkaloids |
| U | Uncertainty |
References
- Dell’Olmo, E.; Tiberini, A.; Sigillo, L. Leguminous Seedborne Pathogens: Seed Health and Sustainable Crop Management. Plants 2023, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R. Legume Seeds as an Important Component of Human Diet. Foods 2020, 9, 1812. [Google Scholar] [CrossRef] [PubMed]
- Nwaji, A.R.; Arieri, O.; Anyang, A.S.; Nguedia, K.; Abiade, E.B.; Forcados, G.E.; Oladipo, O.O.; Makama, S.; Elisha, I.L.; Ozele, N.; et al. Natural toxins and One Health: A review. Sci. One Health 2022, 1, 100013. [Google Scholar] [CrossRef]
- Kishimoto, S.; Sato, M.; Tsunematsu, Y.; Watanabe, K. Evaluation of Biosynthetic Pathway and Engineered Biosynthesis of Alkaloids. Molecules 2016, 21, 1078. [Google Scholar] [CrossRef]
- Eugelio, F.; Palmieri, S.; Fanti, F.; Messuri, L.; Pepe, A.; Compagnone, D.; Sergi, M. Development of an HPLC-MS/MS Method for the Determination of Alkaloids in Lupins. Molecules 2023, 28, 1531. [Google Scholar] [CrossRef] [PubMed]
- Adaszyńska, M.; Swarcewicz, M. Secondary plant metabolites as antimicrobial agents. Wiad. Chem. 2013, 67, 303–319. [Google Scholar]
- Kaczyński, P.; Łozowicka, B. A novel approach for fast and simple determination pyrrolizidine alkaloids in herbs by ultrasound-assisted dispersive solid phase extraction method coupled to liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 187, 113351. [Google Scholar] [CrossRef]
- Jankowska, M.; Łozowicka, B. Natural and synthetic toxic substances occurring in agricultural plants and their products. Prog. Plant Prot. 2021, 61, 24–30. [Google Scholar] [CrossRef]
- Wink, M. Quinolizidine and pyrrolizidine alkaloid chemical ecology—A mini-review on their similarities and differences. J. Chem. Ecol. 2019, 45, 109–115. [Google Scholar] [CrossRef]
- Namdar, D.; Mulder, P.P.J.; Ben-Simchon, E.; Hacham, Y.; Basheer, L.; Cohen, O.; Sternberg, M.; Shelef, O. New Analytical Approach to Quinolizidine Alkaloids and Their Assumed Biosynthesis Pathways in Lupin Seeds. Toxins 2024, 16, 163. [Google Scholar] [CrossRef]
- Commission Regulation (EU). 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Hwang, I.M.; Lee, H.W.; Lee, H.M.; Yang, J.S.; Seo, H.Y.; Chung, Y.J.; Kim, S.H. Rapid and Simultaneous Quantification of Five Quinolizidine Alkaloids in Lupinus angustifolius L. and Its Processed Foods by UPLC-MS/MS. ACS Omega 2020, 5, 20825–20830. [Google Scholar] [CrossRef] [PubMed]
- Otterbach, S.L.; Yang, T.; Kato, L.; Janfelt, C.; Geu-Flores, F. Quinolizidine alkaloids are transported to seeds of bitter narrow-leafed lupin. J. Exp. Bot. 2019, 70, 5799–5808. [Google Scholar] [CrossRef] [PubMed]
- Khedr, T.; Juhász, A.; Singh, K.B.; Foley, R.; Nye-Wood, M.G.; Colgrave, M.L. Development and validation of a rapid and sensitive LC-MS/MS approach for alkaloid testing in different Lupinus species. J. Food Compos. Anal. 2023, 121, 105391. [Google Scholar] [CrossRef]
- Roman, L.; Tsochatzis, E.; Tarin, K.; Röndahl, E.M.; Ottosen, C.-O.; Corredig, M. Compositional Attributes of Blue Lupin (Lupinus angustifolius) Seeds for Selection of High-Protein Cultivars. J. Agric. Food Chem. 2023, 71, 17308–17320. [Google Scholar] [CrossRef]
- Cortés-Avendaño, P.; Tarvainen, M.; Suomela, J.P.; Glorio-Paulet, P.; Yang, B.; Repo-Carrasco-Valencia, R. Profile and Content of Residual Alkaloids in Ten Ecotypes of Lupinus mutabilis Sweet after Aqueous Debittering Process. Plant Foods Hum. Nutr. 2020, 75, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Święcicki, W.; Czepiel, K.; Wilczura, P.; Barzyk, P.; Kaczmarek, Z.; Kroc, M. Chromatographic Fingerprinting of the Old World Lupins Seed Alkaloids: A Supplemental Tool in Species Discrimination. Plants 2019, 8, 548. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive soildphase extraction for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Łozowicka, B.; Kaczyński, P.; Jankowska, M.; Rutkowska, E.; Iwaniuk, P.; Konecki, R.; Rogowska, W.; Zhagyparova, A.; Absatarova, D.; Łuniewski, S.; et al. Evaluation of Broad-Spectrum Pesticides Based on Unified Multi-Analytical Procedure in Fruits and Vegetables for Acute Health Risk Assessment. Foods 2025, 14, 2528. [Google Scholar] [CrossRef]
- Hrynko, I.; Kaczyński, P.; Łozowicka, B. A global study of pesticides in bees: QuEChERS as a sample preparation methodology for their analysis—Critical review and perspective. Sci. Total Environ. 2021, 792, 148385. [Google Scholar] [CrossRef]
- García-Vara, M.; Postigo, C.; Palma, P.; Bleda, M.J.; de Alda, M.L. QuEChERS-based analytical methods developed for LC-MS/MS multiresidue determination of pesticides in representative crop fatty matrices: Olives and sunflower seeds. Food Chem. 2022, 386, 132558. [Google Scholar] [CrossRef]
- Hakami, R.A.; Aqel, A.; Ghfar, A.A.; ALOthman, Z.A.; Badjah-Hadj-Ahmed, A.-Y. Development of QuEChERS extraction method for the determination of pesticide residues in cereals using DART-ToF-MS and GC-MS techniques. Correlation and quantification study. J. Compos. Anal. 2021, 98, 103822. [Google Scholar] [CrossRef]
- Kaczyński, P.; Iwaniuk, P.; Jankowska, M.; Orywal, K.; Socha, K.; Perkowski, M.; Ali Farhan, J.; Łozowicka, B. Pesticide residues in common and herbal teas combined with risk assessment and transfer to the infusiom. Chemosphere 2024, 367, 143550. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.-Y.; Wang, X.-D.; Lv, Y.-F.; Wei, N. Application of QuEChERS for Analysis of Contaminants in Dairy Products: A Review. J. Food Prot. 2025, 88, 100453. [Google Scholar] [CrossRef]
- Piechowicz, B.; Kuliga, A.; Kobylarz, D.; Koziorowska, A.; Zaręba, L.; Podbielska, M.; Piechowicz, I.; and Sadło, S. A case study on the occurrence of pyrimethanil, cyprodinil and cyflufenamid residues in soil and on apple leaves, blossoms and pollen, and their transfer by worker bees to the hive. J. Plant Prot. Res. 2022, 62, 176–188. [Google Scholar] [CrossRef]
- Sadighara, P.; Basaran, B.; Afshar, A.; Nazmara, S. Optimization of clean-up in QuEChERS method for extraction of mycotoxins in food samples: A systematic review. Microchem. J. 2024, 197, 109711. [Google Scholar] [CrossRef]
- Ingegno, M.; Zianni, R.; Della Rovere, I.; Chiappinelli, A.; Nardelli, V.; Casamassima, F.D.; Calitri, A.; Quinto, M.; Nardiello, D.; Iammarino, M. Development of a highly sensitive method based on QuEChERS and GC–MS/MS for the determination of polycyclic aromatic hydrocarbons in infant foods. Front. Nutr. 2024, 11, 1403541. [Google Scholar] [CrossRef] [PubMed]
- Rivai, S.N.; Kristianto, S.; Putri, R.E.; Fatiqin, A.; Abidin, M.H.Z. Modification of the QuEChERS Method for Drug Analysis in Biological Sample: A Review. J. Multidiscip. Appl. Nat. Sci. 2025, 5, 523–535. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Wang, X.; Guo, W.; Jia, W.; Zhang, F. An analytical strategy for discovering structural analogues of alkaloids in plant food using characteristic structural fragments extraction by high resolution orbitrap mass spectrometry. LWT-Food Sci. Technol. 2022, 154, 112329. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)—A powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef]
- AOAC International. AOAC Official Method 2007.01, Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Gas Chromatography/Mass Spectrometry and Liquid Chromatography/Tandem Mass Spectrometry First Action 2007; AOAC International: Rockville, MD, USA, 2007; pp. 1–9. Available online: https://nucleus.iaea.org/sites/fcris/Shared%20Documents/SOP/AOAC_2007_01.pdf (accessed on 25 August 2025).
- EN 15662:2018-06; Foods of Plant origin—Multimethod for the Determination of Pesticide Residues Using GC and/or LC-based Analysis Following Acetonitrile Extraction/Partioning and Clean-up by Dispersive SPE–Modular QuEChERS-Method. Polish Committee for Standardization: Warsaw, Poland, 2018; pp. 1–82.
- Čajka, T.; Sandy, C.; Bachanova, V.; Drabova, L.; Kalachova, K.; Pulkrabova, J.; Hajšlová, J. Streamlining sample preparation and gas chromatography-tandem mass spectrometry analysis of multiple pesticide residues in tea. Anal. Chim. Acta 2012, 743, 51–60. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- García-Valcárcel, A.I.; Martínez-Ferrer, M.T.; Campos-Rivela, J.M.; Guil, M.D.H. Analysis of pesticide residues in honeybee (Apis mellifera L.) and in corbicular pollen. Exposure in citrus orchard with an integrated pest management system. Talanta 2019, 204, 153–162. [Google Scholar] [CrossRef]
- SANTE. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. SANTE/11312/2021, 2021, 1–51. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 25 August 2025).
- Remane, D.; Meyer, M.R.; Wissenbach, D.K.; Maurer, H.H. Ion suppression and enhancement effects of co-eluting analytes in multi-analyte approaches: Systematic investigation using ultra-high-performance liquid chromatography/mass spectrometry with atmospheric-pressure chemical ionization or electrospray ionization. Rapid Commun. Mass Spectrom. 2010, 24, 3103–3108. [Google Scholar]
- Kroc, M.; Rybiński, W.; Wilczura, P.; Kamel, K.; Kaczmarek, Z.; Barzyk, P.; Święcicki, W. Quantitative and qualitative analysis of alkaloids composition in the seeds of a white lupin (Lupinus albus L.) collection. Genet. Resour. Crop Evol. 2017, 64, 1853–1860. [Google Scholar] [CrossRef]
- Romeo, F.V.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and antimicrobial activity of alkaloid extracts from seeds of different genotypes of Lupinus spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef]
- Rutkowska, E.; Kaczyński, P.; Iwaniuk, P.; Łozowicka, B.; Hrynko, I.; Jankowska, M.; Konecki, R.; Rogowska, W.; Rusiłowska, J.; Pietkun, M.; et al. An extensive pesticide residue study in minor polish vegetables based on critical consumer diets. Food Control 2025, 176, 111383. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
| QA (1) | 0.01 (mg/kg) | 0.1 (mg/kg) | 1.0 (mg/kg) | 10.0 (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R (2) (%) (RSD) (3) | U (4) (%) | ME (5) (%) | R (%) (RSD) | U (%) | ME (%) | R (%) (RSD) | U (%) | ME (%) | R (%) (RSD) | U (%) | ME (%) | |
| Narrow-leaved lupins | ||||||||||||
| A (6) | 84 (9) | 26 | 1 | 83 (7) | 18 | −4 | 91 (4) | 14 | 5 | 88 (2) | 14 | 3 |
| H (7) | 79 (3) | 20 | 14 | 77 (3) | 28 | −18 | 86 (2) | 18 | 12 | 86 (15) | 15 | 11 |
| I (8) | 72 (3) | 17 | −6 | 72 (7) | 25 | −9 | 82 (6) | 11 | −11 | 89 (7) | 7 | −13 |
| L (9) | 115 (3) | 16 | 12 | 98 (14) | 18 | −10 | 104 (9) | 12 | −11 | 95 (4) | 7 | −6 |
| S (10) | 78 (4) | 17 | −10 | 71 (6) | 17 | −8 | 79 (4) | 10 | −11 | 74 (3) | 1 | −6 |
| White lupins | ||||||||||||
| A | 106 (4) | 22 | −19 | 104 (8) | 19 | −17 | 101 (3) | 16 | −15 | 106 (10) | 9 | −13 |
| H | 71 (4) | 22 | −14 | 73 (4) | 16 | −14 | 72 (1) | 12 | −14 | 79 (11) | 23 | −18 |
| I | 103 (2) | 27 | −14 | 94 (3) | 21 | −19 | 100 (5) | 12 | −13 | 99 (12) | 6 | −16 |
| L | 106 (3) | 10 | 12 | 104 (5) | 18 | −10 | 101 (3) | 12 | 10 | 105 (3) | 5 | −7 |
| S | 114 (2) | 18 | −13 | 104 (2) | 18 | −9 | 103 (6) | 13 | −9 | 99 (5) | 9 | −10 |
| Yellow lupins | ||||||||||||
| A | 112 (6) | 19 | −16 | 106 (8) | 20 | −15 | 107 (3) | 14 | −10 | 101 (6) | 9 | −12 |
| H | 92 (2) | 20 | −18 | 83 (3) | 17 | −15 | 85 (5) | 14 | −12 | 84 (2) | 7 | −11 |
| I | 79 (8) | 22 | −14 | 81 (9) | 27 | −12 | 80 (8) | 20 | −13 | 70 (8) | 12 | −13 |
| L | 74 (6) | 20 | 13 | 78 (7) | 23 | −19 | 80 (8) | 19 | −17 | 72 (7) | 15 | −17 |
| S | 86 (7) | 25 | −8 | 84 (6) | 28 | −12 | 88 (6) | 18 | −7 | 93 (5) | 14 | −7 |
| Peas | ||||||||||||
| A | 83 (5) | 20 | −16 | 86 (7) | 17 | −9 | 87 (7) | 16 | −11 | 86 (8) | 17 | −11 |
| H | 84 (15) | 28 | −5 | 84 (5) | 22 | −13 | 98 (5) | 21 | −18 | 75 (8) | 12 | −20 |
| I | 79 (15) | 27 | 9 | 84 (9) | 24 | −17 | 91 (8) | 18 | 7 | 79 (5) | 10 | −7 |
| L | 72 (11) | 23 | −12 | 76 (7) | 28 | −14 | 78 (8) | 14 | −3 | 86 (6) | 15 | −3 |
| S | 73 (12) | 25 | −6 | 78 (8) | 28 | −20 | 77 (6) | 23 | −6 | 70 (9) | 20 | −6 |
| Field beans | ||||||||||||
| A | 92 (14) | 25 | −15 | 91 (5) | 24 | −19 | 87 (4) | 17 | −9 | 89 (8) | 18 | −9 |
| H | 77 (15) | 27 | −13 | 81 (8) | 26 | −6 | 84 (7) | 13 | −11 | 83 (4) | 17 | −11 |
| I | 77 (10) | 23 | −7 | 79 (3) | 23 | −9 | 84 (8) | 18 | −6 | 85 (8) | 13 | −6 |
| L | 76 (9) | 19 | −9 | 76 (5) | 24 | −14 | 74 (4) | 17 | −4 | 87 (9) | 15 | −12 |
| S | 71 (14) | 20 | −12 | 76 (9) | 29 | −13 | 77 (3) | 22 | −13 | 79 (8) | 19 | −19 |
| Lentils | ||||||||||||
| A | 85 (14) | 22 | −16 | 94 (8) | 19 | −7 | 90 (5) | 24 | −8 | 74 (10) | 16 | −12 |
| H | 82 (15) | 27 | −4 | 89 (6) | 22 | −12 | 96 (6) | 18 | −16 | 91 (7) | 17 | −9 |
| I | 71 (8) | 23 | −19 | 76 (7) | 25 | −7 | 83 (5) | 18 | −5 | 86 (6) | 16 | −13 |
| L | 74 (14) | 24 | −15 | 80 (6) | 24 | −12 | 87 (3) | 14 | −9 | 70 (9) | 14 | −17 |
| S | 76 (12) | 26 | −6 | 76 (9) | 27 | −6 | 84 (4) | 16 | −12 | 89 (8) | 19 | −14 |
| QA (CAS Number) | Chemical Structure | Molecular Formula | Molecular Weight (g/mol) |
|---|---|---|---|
| (+)-Angustifoline (550-43-6) | 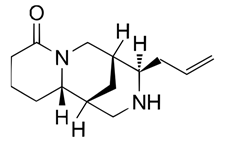 | C14H22N2O | 234.34 |
| (+)-13α-Hydroxylupanine (15358-48-2) | 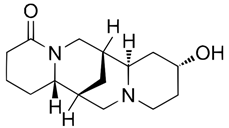 | C15H24N2O2 | 264.36 |
| α-Isolupanine (486-87-3) | 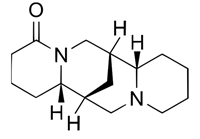 | C15H24N2O | 248.36 |
| (+)-Lupanine (7400-11-5) | 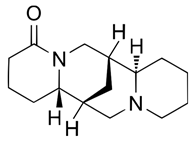 | C15H24N2O | 248.36 |
| (−)-Sparteine (90-39-1) | 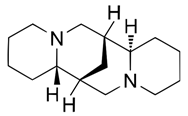 | C15H26N2 | 234.39 |
| Quinolizidine Alkaloids | Retention Time (min) | Precursor Ion (m/z) | DP (1)/EP (2) (V) | Product Ion (m/z) | CE (V) (3) | CXP (V) (4) |
|---|---|---|---|---|---|---|
| Angustifolin | 3.70 | 235.1 | 23/12 | 193/112.1 * | 27/37 | 6/6 |
| Hydroksylupanine | 2.60 | 265 | 15/10 | 247.2/148.1 * | 36/50 | 10/10 |
| Izolupanine | 3.55 | 248.9 | 21/11 | 136.1/114 * | 41/38 | 4/8 |
| Lupanine | 3.30 | 248.9 | 21/11 | 136.1/114 * | 41/38 | 4/8 |
| Sparteine | 4.95 | 235 | 32/10 | 98.1/233.1 * | 49/38 | 8/8 |
| Parameter | Criteria | How/What |
|---|---|---|
| Recovery | 70–120% (30–140% in routine analyses) | Four spiking level (LOQ, 10 × LOQ, 100 × LOQ, 1000 × LOQ) in five replications. |
| RSD 1 | ≤20% | 5 replications at each level (4 level). |
| Linearity | % DEV 6 = (Cmeasured − Ctrue) × 100/Ctrue) −20 ≤ DEV ≤ 20% | Matrix-matched calibration at 7 concentration levels (0.01, 0.05, 0.1, 0.5, 1.0, 5.0 and 10.0 µg/mL). |
| LOQ 2 | S 7/N 8 ≥ 10 | The lowest amount of the analyte in a sample that can be determined with acceptable precision and accuracy. |
| LOD 3 | S/N ≥ 3 | The lowest amount of analyte that can be detected but not necessarily quantified. |
| ME 4 | % ME = (slopematrix/slopesolvent − 1) × 100% –20% ≤ ME ≤20% | ME was estimated by comparing the slopes of seven points on matrix-matched and solvent calibration curves. |
| U 5 | U ≤ 50% | U was calculated based on validation data using a “top-down” model with coverage factor k = 2 at a 95% confidence level. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowska, E. An Innovative Use of the QuEChERs Method and LC-MS/MS Technique for Fast and Simple Determination of Quinolizidine Alkaloids in Leguminous Plants. Molecules 2025, 30, 4085. https://doi.org/10.3390/molecules30204085
Rutkowska E. An Innovative Use of the QuEChERs Method and LC-MS/MS Technique for Fast and Simple Determination of Quinolizidine Alkaloids in Leguminous Plants. Molecules. 2025; 30(20):4085. https://doi.org/10.3390/molecules30204085
Chicago/Turabian StyleRutkowska, Ewa. 2025. "An Innovative Use of the QuEChERs Method and LC-MS/MS Technique for Fast and Simple Determination of Quinolizidine Alkaloids in Leguminous Plants" Molecules 30, no. 20: 4085. https://doi.org/10.3390/molecules30204085
APA StyleRutkowska, E. (2025). An Innovative Use of the QuEChERs Method and LC-MS/MS Technique for Fast and Simple Determination of Quinolizidine Alkaloids in Leguminous Plants. Molecules, 30(20), 4085. https://doi.org/10.3390/molecules30204085






