Trifolirhizin: A Phytochemical with Multiple Pharmacological Properties
Abstract
1. Introduction
2. Electronic Literature Search Strategy
3. Toxicity Studies
4. Pharmacokinetics Studies
5. Pharmacological Activities of Trifolirhizin
5.1. Antibacterial Activity of Trifolirhizin
5.2. Hepatoprotective Activity of Trifolirhizin
5.3. Antiplatelet Aggregation Effect of Trifolirhizin
5.4. Estrogenic Effect of Trifolirhizin
5.5. Antiasthma Activity of Trifolirhizin
5.6. Anti-Inflammatory Effect of Trifolirhizin
5.6.1. In Vitro Anti-Inflammatory Effect of Trifolirhizin
5.6.2. In Vivo Anti-Inflammatory Effect of Trifolirhizin
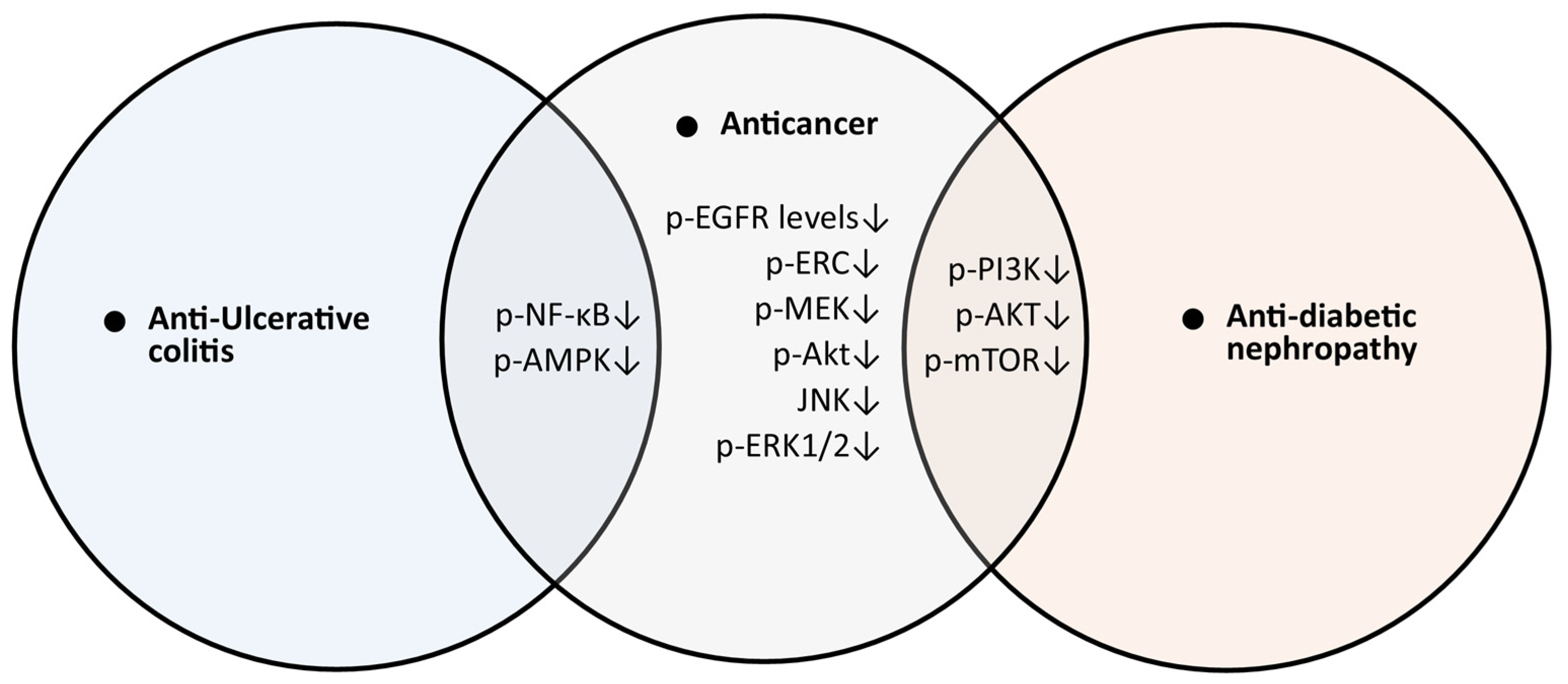
5.7. Anti-Ulcerative Colitis Activity of Trifolirhizin
5.8. Protective Effect of Trifolirhizin on Bone
5.8.1. In Vitro Protective Effect of Trifolirhizin on Bone
5.8.2. In Vivo Protective Effect of Trifolirhizin on Bone
5.9. Skin-Whitening and Tyrosinase Inhibition Activity
5.10. Anti-Diabetic Nephropathy
5.11. Anticancer Effects of Trifolirhizin
5.11.1. In Vitro Anticancer Activities of Trifolirhizin
5.11.2. In Vivo Anticancer Activities of Trifolirhizin
5.12. Wound-Healing Effects of Trifolirhizin
6. Gaps and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liang, B.; Srivastava, K.; Zeng, J.; Zhan, J.; Brown, L.; Sampson, H.; Goldfarb, J.; Emala, C.; Li, X.-M. The Sophora flavescens flavonoid compound trifolirhizin inhibits acetylcholine induced airway smooth muscle contraction. Phytochemistry 2013, 95, 259–267. [Google Scholar] [CrossRef]
- Aratanechemuge, Y.; Hibasami, H.; Katsuzaki, H.; Imai, K.; Komiya, T. Induction of apoptosis by maackiain and trifolirhizin (maackiain glycoside) isolated from sanzukon (Sophora Subprostrate Chen et T. Chen) in human promyelotic leukemia HL-60 cells. Oncol. Rep. 2004, 12, 1183–1188. [Google Scholar] [CrossRef]
- Lu, X.; Ma, J.; Qiu, H.; Yang, L.; Cao, L.; Shen, J. Anti-proliferation effects of trifolirhizin on MKN45 cells and possible mechanism. Oncol. Rep. 2016, 36, 2785–2792. [Google Scholar] [CrossRef]
- Sun, D.; Tao, W.; Zhang, F.; Shen, W.; Tan, J.; Li, L.; Meng, Q.; Chen, Y.; Yang, Y.; Cheng, H. Trifolirhizin induces autophagy-dependent apoptosis in colon cancer via AMPK/mTOR signaling. Signal Transduct. Target. Ther. 2020, 5, 174. [Google Scholar] [CrossRef]
- Zhou, H.; Lutterodt, H.; Cheng, Z.; Yu, L. Anti-inflammatory and antiproliferative activities of trifolirhizin, a flavonoid from Sophora flavescens roots. J. Agric. Food Chem. 2009, 57, 4580–4585. [Google Scholar] [CrossRef]
- Zhu, X.F.; Sun, Z.L.; Ma, J.; Hu, B.; Yu, M.C.; Liu, X.J.; Yang, P.; Xu, Y.; Ju, D.; Mu, Q. Synergistic anticancer effect of flavonoids from Sophora alopecuroides with Sorafenib against hepatocellular carcinoma. Phytother. Res. 2023, 37, 592–610. [Google Scholar] [CrossRef]
- Jiang, X.; Yin, H.; Su, W.; Quan, H.; Yuan, X.; Feng, X.; Li, P.; He, Y.; Xiao, J.; Li, R. Trifolirhizin inhibits proliferation, migration and invasion in nasopharyngeal carcinoma cells via PI3K/Akt signaling pathway suppression. Biochem. Biophys. Res. Commun. 2023, 667, 111–119. [Google Scholar] [CrossRef]
- Jaiswal, V.; Lee, H.-J. The Bioactivity and Phytochemicals of Muscari comosum (Leopoldia comosa), a Plant of Multiple Pharmacological Activities. Int. J. Mol. Sci. 2024, 25, 2592. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Lee, H.-J. Pharmacological Properties of Shionone: Potential Anti-Inflammatory Phytochemical against Different Diseases. Molecules 2023, 29, 189. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Song, D.; Xu, M.; Gan, K.; Wang, C.; Chen, L.; Huang, Q.; Chen, J.; Su, Y.; Xu, J. Trifolirhizin reduces osteoclast formation and prevents inflammatory osteolysis by inhibiting RANKL-induced activation of NF-κB and MAPK signaling pathways and ROS. Phytother. Res. 2024, 38, 4650–4666. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, Z.; Ye, J.; Wei, J.; Chen, S.; Zhou, W.; Bi, Y.; Zhou, Z.; Xie, G.; Yuan, G. Trifolirhizin protects ovariectomy-induced bone loss in mice by inhibiting osteoclast differentiation and bone resorption. Heliyon 2024, 10, e34250. [Google Scholar] [CrossRef]
- Yun, H.-M.; Cho, M.H.; Jeong, H.; Kim, S.H.; Jeong, Y.H.; Park, K.-R. Osteogenic Activities of Trifolirhizin as a Bioactive Compound for the Differentiation of Osteogenic Cells. Int. J. Mol. Sci. 2023, 24, 17103. [Google Scholar] [CrossRef]
- Xin, Y.; Xu, X.; Yang, X.; Chen, Y.; Zhu, D. Trifolirhizin relieves renal injury in a diabetic nephropathy model by inducing autophagy and inhibiting oxidative stress through the regulation of PI3K/AKT/mTOR pathway. Trop. J. Pharm. Res. 2022, 21, 2107–2113. [Google Scholar] [CrossRef]
- Jang, S.M.; Bae, S.H.; Choi, W.-K.; Park, J.B.; Kim, D.; Min, J.S.; Yoo, H.; Kang, M.; Ryu, K.H.; Bae, S.K. Pharmacokinetic properties of trifolirhizin, (–)-maackiain, (–)-sophoranone and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran after intravenous and oral administration of Sophora tonkinensis extract in rats. Xenobiotica 2015, 45, 1092–1104. [Google Scholar] [CrossRef]
- Ni, K.-h.; Huang, X.-c.; Wang, C.-x.; Ye, T.-t.; Hu, G.-x.; Zhou, M.-t. Determination of trifolirhizin in rat plasma by UPLC: Application to a pharmacokinetic study. J. Chromatogr. B 2015, 990, 181–184. [Google Scholar] [CrossRef]
- Yoo, H.; Ryu, K.H.; Bae, S.K.; Kim, J. Simultaneous determination of trifolirhizin, (–)-maackiain, (–)-sophoranone, and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran from Sophora tonkinensis in rat plasma by liquid chromatography with tandem mass spectrometry and its application to a pharmacokinetic study. J. Sep. Sci. 2014, 37, 3235–3244. [Google Scholar]
- Hyun, S.K.; Lee, W.-H.; Jeong, D.M.; Kim, Y.; Choi, J.S. Inhibitory effects of kurarinol, kuraridinol, and trifolirhizin from Sophora flavescens on tyrosinase and melanin synthesis. Biol. Pharm. Bull. 2008, 31, 154–158. [Google Scholar] [CrossRef]
- Öz, B.E.; İşcan, G.S.; Akkol, E.K.; Süntar, İ.; Acıkara, Ö.B. Isoflavonoids as wound healing agents from Ononidis Radix. J. Ethnopharmacol. 2018, 211, 384–393. [Google Scholar]
- Wang, Y.; Fang, Y. Trifolirhizin targets PTK6 to induce autophagy and exerts antitumor effects in nasopharyngeal carcinoma. Drug Dev. Res. 2024, 85, e70000. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Lee, H.-J. Conservation and evolution of antigenic determinants of SARS-CoV-2: An insight for immune escape and vaccine design. Front. Immunol. 2022, 13, 832106. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.-G.; Jeong, J.-Y.; Yum, S.-H.; Hwang, Y.-J. Inhibitory Effects of Selected Medicinal Plants on Bacterial Growth of Methicillin-Resistant Staphylococcus aureus. Molecules 2022, 27, 7780. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-Y.; Jung, I.-G.; Yum, S.-H.; Hwang, Y.-J. In vitro synergistic inhibitory effects of plant extract combinations on bacterial growth of methicillin-resistant Staphylococcus aureus. Pharmaceuticals 2023, 16, 1491. [Google Scholar] [CrossRef]
- Kang, M.H.; Lee, J.H.; Cho, S.Y.; Choi, J.S.; Kim, Y.; Kang, S.S.; Jeong, C.S. Antigastritic and anti Helicobacter pylori of trifolirhizin from Sophora radix. Korean J. Pharmacogn. 2006, 37, 266–271. [Google Scholar]
- Alotaibi, B.S. Targeting Filamenting temperature-sensitive mutant Z (FtsZ) with bioactive phytoconstituents: An emerging strategy for antibacterial therapy. PLoS ONE 2023, 18, e0290852. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Lee, H.H.; Ro, H.; Jung, J.Y.; Chang, J.H.; Chung, W.; Kim, A.J. The Fatty Liver Index’s Association with Incident Chronic Kidney Disease in Korean Middle-Aged Adults: A Community-Based Cohort Study. J. Clin. Med. 2024, 13, 1616. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S. Preliminary pharmacological study of the pterocarpans macckian and trifolirhizin isolated from the roots of Ononis vaginalis. Pak J Pharm Sci 2010, 23, 182–187. [Google Scholar] [PubMed]
- Woldemariam, T.Z.; Fell, A.F.; Linley, P.A.; Bibby, M.C.; Phillips, R.M. Evaluation of the anti-tumour action and acute toxicity of kosins from Hagenia abyssinica. J. Pharm. Biomed. Anal. 1992, 10, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.; Ji, S. Trifolirhizin regulates the balance of Th17/Treg cells and inflammation in the ulcerative colitis mice through inhibiting the TXNIP-mediated activation of NLRP3 inflammasome. Clin. Exp. Pharmacol. Physiol. 2022, 49, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Lei, P.; Yi, L.; Yupei, W.; Rui, G.; Xin, L.; Huimin, X.; Qing, H. Trifolirhizin mitigates ovalbumin-induced lung inflammation and tissue damage in neonatal rats via inhibition of the NF-κB signaling pathway. Trop. J. Pharm. Res. 2020, 19, 2303–2308. [Google Scholar]
- Lee, D.-H.; Kwak, H.J.; Shin, Y.; Kim, S.J.; Lee, G.H.; Park, I.-H.; Kim, S.H.; Kang, K.S. Elucidation of Phytochemicals Affecting Platelet Responsiveness in Dangguisu-san: Active Ingredient Prediction and Experimental Research Using Network Pharmacology. Plants 2023, 12, 1120. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Koklesova, L.; Samec, M.; Sokol, J.; Samuel, S.M.; Kudela, E.; Biringer, K.; Bugos, O.; Pec, M.; et al. Antithrombotic and antiplatelet effects of plant-derived compounds: A great utility potential for primary, secondary, and tertiary care in the framework of 3P medicine. EPMA J. 2022, 13, 407–431. [Google Scholar] [CrossRef]
- García-Marcos, L.; Chiang, C.-Y.; Asher, M.I.; Marks, G.B.; El Sony, A.; Masekela, R.; Bissell, K.; Ellwood, E.; Ellwood, P.; Pearce, N.; et al. Asthma management and control in children, adolescents, and adults in 25 countries: A Global Asthma Network Phase I cross-sectional study. Lancet Glob. Health 2023, 11, e218–e228. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, K.-S.; Lee, W.-Y.; Kim, C.-E.; Lee, S. Integrative Approach to Identifying System-Level Mechanisms of Chung-Sang-Bo-Ha-Hwan’s Influence on Respiratory Tract Diseases: A Network Pharmacological Analysis with Experimental Validation. Plants 2023, 12, 3024. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Bachheti, A.; Rai, N.; Tadesse, M.G.; Nasser Singab, A.; Chaubey, K.K.; Bachheti, R.K. Anti-inflammatory activity of phytochemicals from medicinal plants and their nanoparticles: A review. Curr. Res. Biotechnol. 2023, 6, 100152. [Google Scholar] [CrossRef]
- Jo, H.-G.; Baek, C.Y.; Lee, J.; Hwang, Y.; Baek, E.; Song, A.; Song, H.S.; Lee, D. Inhibitory Effects of Reynoutria japonica Houtt. on Pain and Cartilage Breakdown in Osteoarthritis Based on Its Multifaceted Anti-Inflammatory Activity: An In Vivo and In Vitro Approach. Int. J. Mol. Sci. 2024, 25, 10647. [Google Scholar] [CrossRef]
- He, M.T.; Park, G.; Park, D.H.; Choi, M.; Ku, S.; Go, S.H.; Lee, Y.G.; Song, S.J.; Ahn, C.-W.; Jang, Y.P.; et al. So Shiho Tang Reduces Inflammation in Lipopolysaccharide-Induced RAW 264.7 Macrophages and Dextran Sodium Sulfate-Induced Colitis Mice. Biomolecules 2024, 14, 451. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Kim, H.-Y.; Jang, J.-T.; Hong, S. Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation. Molecules 2023, 28, 8099. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Lee, H.-J. Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases. Life 2023, 13, 555. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Jun, D.-S.; Lee, D.-K.; Baik, J.-M. Clinical Characteristics of Elderly People with Osteoporotic Vertebral Compression Fracture Based on a 12-Year Single-Center Experience in Korea. Geriatrics 2022, 7, 123. [Google Scholar] [CrossRef]
- Kim, G.H.; Lee, Y.H.; Yoo, A.Y.; Amna, S.; Park, J.K. Evaluation of the effect of molecular weight change of konjac glucomannan on antioxidant and tyrosinase activities. Macromol. Res. 2024, 32, 401–413. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic nephropathy: Challenges in pathogenesis, diagnosis, and treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Jung, I.; Nam, S.; Park, S.Y.; Yu, J.H.; Seo, J.A.; Lee, D.H.; Kim, N.H. Association of succinate and adenosine nucleotide metabolic pathways with diabetic kidney disease in patients with type 2 diabetes mellitus. Diabetes Metab. J. 2024, 48, 1126–1134. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.Y.; Lee, S.; Kang, K.S. In vitro studies to assess the α-glucosidase inhibitory activity and insulin secretion effect of isorhamnetin 3-o-glucoside and quercetin 3-o-glucoside isolated from Salicornia herbacea. Processes 2021, 9, 483. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, D.; Seo, Y.-H.; Ryu, S.-M.; Lee, A.-Y.; Moon, B.-C.; Kim, W.-J.; Kang, K.-S.; Lee, J. Chemical Constituents from the Roots of Angelica reflexa That Improve Glucose-Stimulated Insulin Secretion by Regulating Pancreatic β-Cell Metabolism. Pharmaceutics 2023, 15, 1239. [Google Scholar] [CrossRef]
- Jaiswal, V.; Lee, H.-J. Antioxidant activity of Urtica dioica: An important property contributing to multiple biological activities. Antioxidants 2022, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Nah, S.-Y.; Park, I.-H.; Shin, M.-S.; Kang, K.S. Gintonin Isolated from Ginseng Inhibits the Epithelial—Mesenchymal Transition Induced by TGF-β in A549 Lung Cancer Cells. Plants 2023, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, Y.H.; Lee, K.H.; Lee, B.S.; Alishir, A.; Ko, Y.-J.; Kang, K.S.; Kim, K.H. Aviculin Isolated from Lespedeza cuneata Induce Apoptosis in Breast Cancer Cells through Mitochondria-Mediated Caspase Activation Pathway. Molecules 2020, 25, 1708. [Google Scholar] [CrossRef]
- Lee, D.; Yu, J.S.; Ha, J.W.; Lee, S.R.; Lee, B.S.; Kim, J.-C.; Kim, J.K.; Kang, K.S.; Kim, K.H. Antitumor Potential of Withanolide Glycosides from Ashwagandha (Withania somnifera) on Apoptosis of Human Hepatocellular Carcinoma Cells and Tube Formation in Human Umbilical Vein Endothelial Cells. Antioxidants 2022, 11, 1761. [Google Scholar] [CrossRef]
- Umer, M.; Mohib, Y.; Atif, M.; Nazim, M. Skeletal metastasis in renal cell carcinoma: A review. Ann. Med. Surg. 2018, 27, 9–16. [Google Scholar] [CrossRef]
- Xu, Z.; Jia, K.; Wang, H.; Gao, F.; Zhao, S.; Li, F.; Hao, J. METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial–mesenchymal transition of renal tubular cells in diabetic kidney disease. Cell Death Dis. 2021, 12, 32. [Google Scholar] [CrossRef]
- Razia, S.; Park, H.; Shin, E.; Shim, K.-S.; Cho, E.; Kang, M.C.; Kim, S.Y. Synergistic effect of Aloe vera flower and Aloe gel on cutaneous wound healing targeting MFAP4 and its associated signaling pathway: In-vitro study. J. Ethnopharmacol. 2022, 290, 115096. [Google Scholar] [CrossRef]
- Park, D.H.; Park, J.Y.; Shin, M.-S.; Hwang, G.S. Wound Healing Effect of 20(S)-Protopanaxadiol of Ginseng Involves VEGF-ERK Pathways in HUVECs and Diabetic Mice. Processes 2023, 11, 692. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Cai, P.; Xia, Y.; Yi, C.; Shang, A.; Akanyibah, F.A.; Mao, F. The emerging role of the gut microbiota and its application in inflammatory bowel disease. Biomed. Pharmacother. 2024, 179, 117302. [Google Scholar] [CrossRef]
- Omolekan, T.O.; Chamcheu, J.C.; Buerger, C.; Huang, S. PI3K/AKT/mTOR Signaling Network in Human Health and Diseases. Cells 2024, 13, 1500. [Google Scholar] [CrossRef]

| Sr. No. | Activity | Model and Dose | Method | Result | Ref |
|---|---|---|---|---|---|
| 1 | Anti-inflammatory activity | LPS-induced inflammation in the J774A.1 macrophage of the mouse | QRTPCR to study the mRNA expression of tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) | TNF-α ↓ and IL-6 ↓ | [8] |
| ELISAQRTPCR to study the mRNA expression of tumor necrosis factor (TNF-α) | TNF-α ↓ | ||||
| WB to study the protein expression of COX-2 | COX-2 ↓ | ||||
| 2 | Wound-healing | Inhibition assay of enzymes at 50 and 100 μg/mL | Enzymes hyaluronidase, collagenase, and elastase enzymes | Percentage inhibition (at 100 μg/mL) was 28.45 ± 1.96, 27.84 ± 0.72, and 21.53 ± 1.66 for hyaluronidase ↓, collagenase ↓, and elastase enzymes ↓, respectively | [21] |
| 3 | Skin-whitening | Treated with different concentrations (1–500 μg/mL) | Tyrosinase inhibition activity | IC50: 506.77 ± 4.94 μM | [20] |
| B16 melanoma cells treated with different concentrations (12.5, 25, and 50 μM) | B16 cells induced with IBMX | IC50: 36 μM | |||
| 4 | Protect bone loss | MC3T3-E1 cells were used to study osteogenesis in the study | ALP activity and staining assays | ALP ↑ | [15] |
| RT-qPCR | ColI ↑, bone sialoprotein Bsp ↑, and Alp ↑ | ||||
| Detection of F-actin polymerization in the cells; phalloidin staining | F-actin polymerization ↑ | ||||
| Cell migration through Matrigel-coated membranes | Cell migration ↑ | ||||
| ARS staining to study mineralization by calcium deposition | Mineralization ↑ | ||||
| WB | RUNX2 ↑, β-catenin ↑, p-Smad1/5/8 ↑, p-JNK ↑, p-GSK3β ↑, and p-β-catenin ↓ | ||||
| Marker in the immunofluorescence assay | The accumulation of RUNX2 ↑ (in the nucleus) | ||||
| BMM extracted from the bone marrow cavity of the femur and tibia of C57BL/6J and RANKL used as a stimulator Dose: 10, 20, and 40 μM | Cell staining to detect TRAP activity | Significant reduction in the formation of multinucleated osteoclasts was observed at all doses (10, 20, and 40 μM) used in the study | [14] | ||
| Western blot analysis | NFATc1 ↓ | ||||
| RT-qPCR | ACP5 ↓, ATc1 ↓, DC-STAMP ↓, MMP9 ↓, V-ATPase-D2 ↓, and CTSK ↓ were found to be downregulated in a dose-dependent manner in the treatment groups | ||||
| Bone resorption assay | In the bone resorption analysis, trifolirhizin treatment (at 20 and 40 μM) significantly decreased absorption area | ||||
| 5 | Anticancer | Human leukemia cells (HL-60 cells) | Viable cell number estimated by the trypan blue dye exclusion method | Proliferation of HL-60 cells ↓ | [5] |
| Analysis of morphological changes through microscopic observation | Apoptotic bodies and fragmentation of genomic DNA were observed in the treated cells | ||||
| A2780 ovarian cancer cells (5–250 μM) | MTT assay | Proliferation of A2780 ovarian cancer cells ↓ (at 50 μM or more) | [8] | ||
| H23 lung cancer cells (5–250 μM) | MTT assay | Proliferation of H23 lung cancer cells ↓ (at 250 μM) | |||
| MKN45 cells was 20, 30, and 40 μg/mL | MTT assay | Proliferation of MKN45 cells ↓ (IC50: 33.27 ± 2.06 μg/mL) | [6] | ||
| Flow cytometry Hoechst staining TUNEL staining and Annexin V/PI assay | Increased the number of apoptotic cells, cell shrinkage, nuclear fragmentation, and chromatin compaction of apoptosis accumulation in the G2/M phase in MKN45 cells | ||||
| The JC-10 assay | Normal cells ↓ and apoptotic cells ↑ (20–40 μg/mL trifolirhizin treatment) | ||||
| Western blot analysis | p-EGFR levels ↓, cyclin B ↓, Cdc2 ↓, p-ERC ↓, p-MEK ↓, p-P38 ↑, P53 ↑, c-Myc ↑, caspase-9 ↑, and caspase-3 ↑ | ||||
| HCT116 and SW620 cells | Immuno-blotting assay of two autophagy marker proteins, LC-3 and p62/SQSTM-1 | LC-3B-I ↑ and LC-3B-II ↑ | [7] | ||
| CCK-8 assay | Cell viability ↓ | ||||
| Transmission electron microscopy | Autophagic vacuole ↑ | ||||
| AdmCherry-GFP-LC3B fluorescent assay | Autophagosomes ↑ and autophagolysosomes ↑ | ||||
| Flow cytometry analysis and TUNEL staining | Long-term growth of both cell lines ↓ | ||||
| Western blot | Cleaved caspase-3 ↑, cleaved caspase-8 ↑, cleaved poly ADP-ribose polymerase ↑, p-AMPK ↑, and p-mTOR ↓ | ||||
| Human nasopharyngeal carcinoma cell lines (6–10 B and HK1) | CCK-8 assays | Cell viability of 6–10 B cells ↓ (IC50: 83.67 ± 1.70 μmol/L at 72 h) Cell viability of HK1cells ↓ (IC50: 33.21 ± 1.40 μmol/L at 72 h) | [10] | ||
| Flow cytometry analysis through PI staining | Nasopharyngeal cancer cells at G0/G1 phase | ||||
| Scratch assay to study cell migration | 6–10 B and HK1 cell migration ↓ | ||||
| Transwell assays | Invaded cell numbers ↓ in both 6–10 B and HK1 cells | ||||
| RNA-seq analysis followed by volcano plot and KEGG pathway analysis | The PI/Akt pathway was among the enriched pathways by the differentially expressed genes | ||||
| WB | Phosphorylation of both PI3K and Akt proteins ↓ (in both 6–10 B and HK1 cells) | ||||
| MHCC97H, MHCC97L, and HepG2 cells (12.5, 25, 50, and 100 μg/mL) | MTT assay | IC50: 143.1 μg/mL (alone) IC50: 1.5 ± 0.06 0.7 ± 0.17 μg/M (in combination with SF (100 μg/mL)) IC50: 104.2 μg/mL (alone) IC50: 0.7 ± 0.17 μg/M (in combination with SF (100 μg/mL)) IC50: >60 μg/mL (alone) IC50: 8.4 ± 0.54 μg/M (in combination with SF (100 μg/mL)) | [9] | ||
| Microscopy using DAPI Annexin-V FITC/PI analysis using flow cytometry (50.0 μg/mL, 22.4 μM) and SF (2.0 μM) | Nucleus fragmentation ↑ and apoptotic body formation ↑ Percentage of apoptotic cells significantly increased by 5.0-fold for SF-induced apoptosis in comparison to the single treatment of SF | ||||
| Cell cycle assay | Similarly, the combination of compound 17 with SF arrested MHCC97H cells in the G1 phase | ||||
| JC-1 assay | ΔΨm ↓ | ||||
| WB | Cyclin D1 ↓ and cyclin B1 ↓, leaved- caspase-3 ↑ cleaved caspase-9 ↑, Bax/Bcl2 ↑, JNK ↓, P38 ↓, p-ERK1/2 ↓, and p-AKT ↓ | ||||
| Nasopharyngeal carcinoma cell lines (C666-1) (0, 0.005, 0.01, 0.02, 0.04, 0.08, 0.16, 0.4, 0.8, and 2 mg/mL) | CCK-8 assay and EdU staining | Viability of C666-1 cells ↓ | [22] | ||
| TUNEL staining | Apoptosis of C666-1 cells ↑ | ||||
| WB | PTK6 ↓, LC3-II/I ↑, Beclin1 ↑, p62 ↓, Ki67 ↓, and PCNA ↓ | ||||
| RT-qPCR | PTK6 ↓ | ||||
| Immunofluorescence (IF) | LC3 accumulation ↑, levels of LC3-II/I ↑, and Beclin1 ↑ |
| Sr. No. | Activity | Animal Model and Dose | Method | Results | Reference |
|---|---|---|---|---|---|
| 1 | Hepatoprotective activity | Wistar rats and 7.5 mg/kg (20.7 μmol/kg) 5 days before CCl4 administration and continued until the end of the experiment. | Carbon tetrachloride (CCl4)-induced liver toxicity, and the levels of liver enzymes in serum were studied | SGOT ↓, SGPT ↓, ALP ↓, and total bilirubin ↓ | [31] |
| Non-protein sulfhydryl groups in the liver (wet weight) | Non-protein sulfhydryl groups ↑ | ||||
| 2 | Antiplatelet aggregation activity | Blood was collected from Wistar rats via cardiac puncture; 400 and 800 μg/mL concentrations were used in the study. | Aggregation of platelets was caused by ADP | Aggregation of platelets ↓ | [31] |
| 3 | Estrogenic activity | Female Wistar rat model; 20 mg/kg body weight. | Weights of the uteri in control and treated animals to the whole animal weight were also calculated (positive control 17β-estradiol) | Uterine weight ↑ | [31] |
| 4 | Anti-inflammatory activity | Wistar rat paw edema as a model and 4.5 mg/kg dose. | Carrageenan was used to induce edema; indomethacin was used as a positive drug | Paw edema ↓ # (35%) | [31] |
| 5 | Protect against bone loss | Wild-type (WT) C57BL/6J mice; ovariectomy was executed 10 and 20 mg/kg by intraperitoneal injection for 6 weeks. | μCT scanning analysis of tibia and histological assessments | Bone loss ↓ Number of TRAP-positive osteoclasts ↓ | [14] |
| Male C57BL/6J mice; 5 and 10 mg/kg injected periosteum in the midline of the skull on days 2, 4, 6, and 8. | LPS-induced osteolysis of cranial cap bone analyzed through μCT scanning | Bone loss ↓, BV/TV ↑, and bone destruction area ↓ | [13] | ||
| Histological analysis | Number of TRAP-positive osteoclasts ↓, IL-1β-positive area in of cranial cap bone ↓ | ||||
| 6 | Anticancer | BALB/C nude mice; dose: 1–3 mg/kg for 21 days of treatment. | Intraperitoneal injection of MKN45 cells to induce tumors in mice | Tumor weight ↓ | [32] |
| Immunohistochemistry | Ki67-positive cells ↓ and cleaved caspase-3-positive cells ↑ | ||||
| BALB/C mice; dose: 10 mg/kg once every three days for 21 days. | Xenograft tumor studies | Size of tumor ↓ | [7] | ||
| Hematoxylin and eosin (H&E) and TUNEL staining | Tumor tissue showed the damage of tumor cells | ||||
| WB to study expression | Cleaved caspase-3 ↑, cleaved caspase-8 ↑, p-AMPK ↑, p-mTOR ↓, Atg5 ↑, and Atg7 ↑ | ||||
| Male nude mice; dose: administered every other day for 14 days at a dose of 40 mg/kg. | Xenograft tumor model 6–10 B and HK1 cells injected subcutaneously into the right axilla of nude mice | Tumor size and weight ↓ | [10] | ||
| HE staining | Tumor tissue showed damage to tumor cells | ||||
| 7 | Anti-UC | C57BL/6 mice; dose: intraperitoneally injected trifolirhizin (12.5, 25, 50 mg/kg) for one time. | Mice received DSS treatment for 7 days with 1.5% DSS in the drinking water | Body weight ↑ and length of the colon ↑ | [33] |
| Quantitative reverse transcription (qRT)-polymerase chain reaction (PCR). | TNF-α ↓, IL-6 ↓, IL-1β ↓, NLRP3 ↓, caspase 1 ↓, and ASC ↓ | ||||
| ELISA | The protein expression of TNF-α ↓, IL-6 ↓, IL-1β ↓, IL-17 ↓, IL-10 ↑, IgM ↑, IgA ↓, and IgG ↓ (colon tissue) | ||||
| WB | p-nuclear factor (NF)-κB/NF-κB ↓, RORγt protein ↓, Foxp3 ↑, NLRP3 ↓, caspase 1 ↓ and ASC ↓, IL-1β ↓, p-AMPK/AMPK ↓, and TXNIP ↑ | ||||
| Flow cytometry analysis of cells from mesenteric lymph nodes and the spleen | Th17 (CD4+ IL17+) cells ↓ Treg (CD4+ CD25+ Foxp3+) cells ↑ | ||||
| Immunofluorescence staining | NLRP3 expression ↓ | ||||
| 8 | Anti-diabetic nephropathy | Male db/db mice; trifolirhizin (0, 12.5, 25 and 50 mg/kg); 3 weeks. | Histological analysis of renal tissues was performed by H & E staining | Body and renal weight ↓, fasting blood glucose ↓, renal injury ↓ | [16] |
| TUNEL staining of renal tissues | Apoptosis ↓ | ||||
| ELISA | BUN ↓, creatinine ↓ MDA ↓, and SOD ↑ | ||||
| WB of renal tissues | LC3II ↑, Beclin1 ↑, p62 ↓, p-PI3K/PI3K ↓, p-AKT/AKT ↓, and p-mTOR/mTOR ↓ | ||||
| DHE staining of renal tissues | ROS ↓ | ||||
| 9 | Antiasthma | Six-week-old BALB/c asthmatic mouse model induced by albumin sensitization and challenge. | ASM contraction in tracheal rings was induced by acetylcholine | ASM contraction ↓ | [4] |
| Neonatal pups of SD rats; asthmatic mouse model induced by albumin sensitization. | Histopathology | Tissue damage ↓, aggregation of inflammatory cells ↓, edema in pulmonary tissues ↓, and histological scores ↓ | [34] | ||
| Immunohistochemistry | Muc5AC ↓ and Muc5B ↓ genes (the lungs) TNF-α ↓ and ICAM-1 ↓, IL-4 ↓, IL-5 ↓, and IL-13 ↓ (in BALF) | ||||
| WB | IκBα protein expression ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, V.; Lee, H.-J. Trifolirhizin: A Phytochemical with Multiple Pharmacological Properties. Molecules 2025, 30, 383. https://doi.org/10.3390/molecules30020383
Jaiswal V, Lee H-J. Trifolirhizin: A Phytochemical with Multiple Pharmacological Properties. Molecules. 2025; 30(2):383. https://doi.org/10.3390/molecules30020383
Chicago/Turabian StyleJaiswal, Varun, and Hae-Jeung Lee. 2025. "Trifolirhizin: A Phytochemical with Multiple Pharmacological Properties" Molecules 30, no. 2: 383. https://doi.org/10.3390/molecules30020383
APA StyleJaiswal, V., & Lee, H.-J. (2025). Trifolirhizin: A Phytochemical with Multiple Pharmacological Properties. Molecules, 30(2), 383. https://doi.org/10.3390/molecules30020383









