Effects of Echinacea purpurea and Alkylamides on Respiratory Virus Replication and IL-8 Expression In Vitro
Abstract
1. Introduction
2. Results
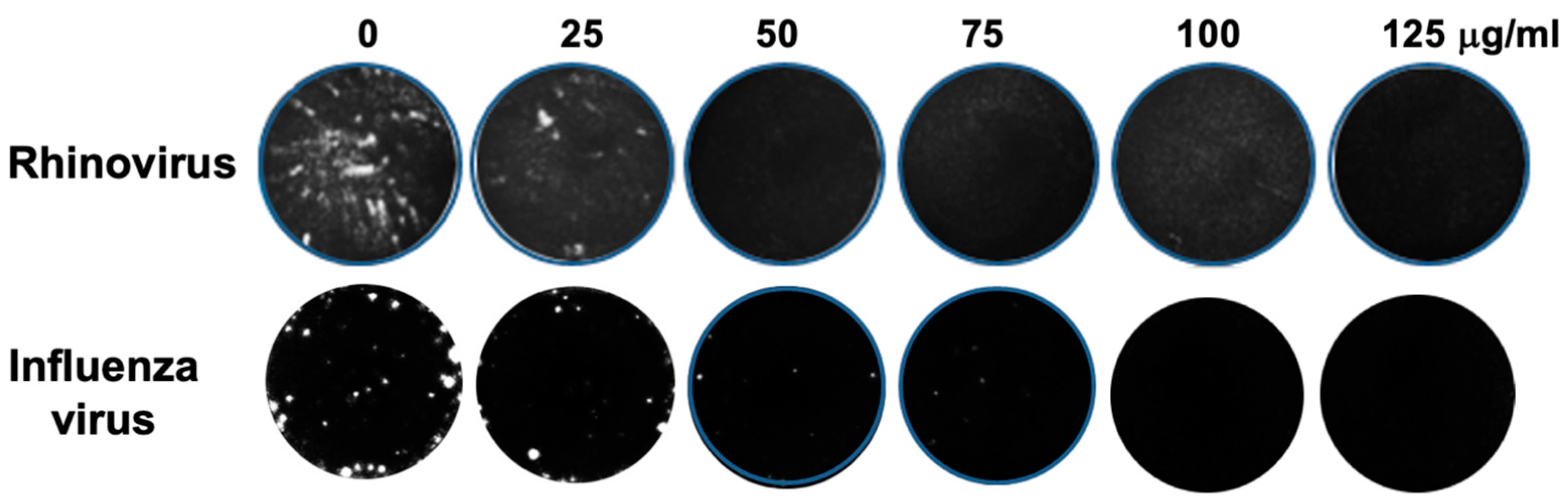
2.1. Effect of E. purpurea Extract on Rhinovirus and Influenza Virus Replication
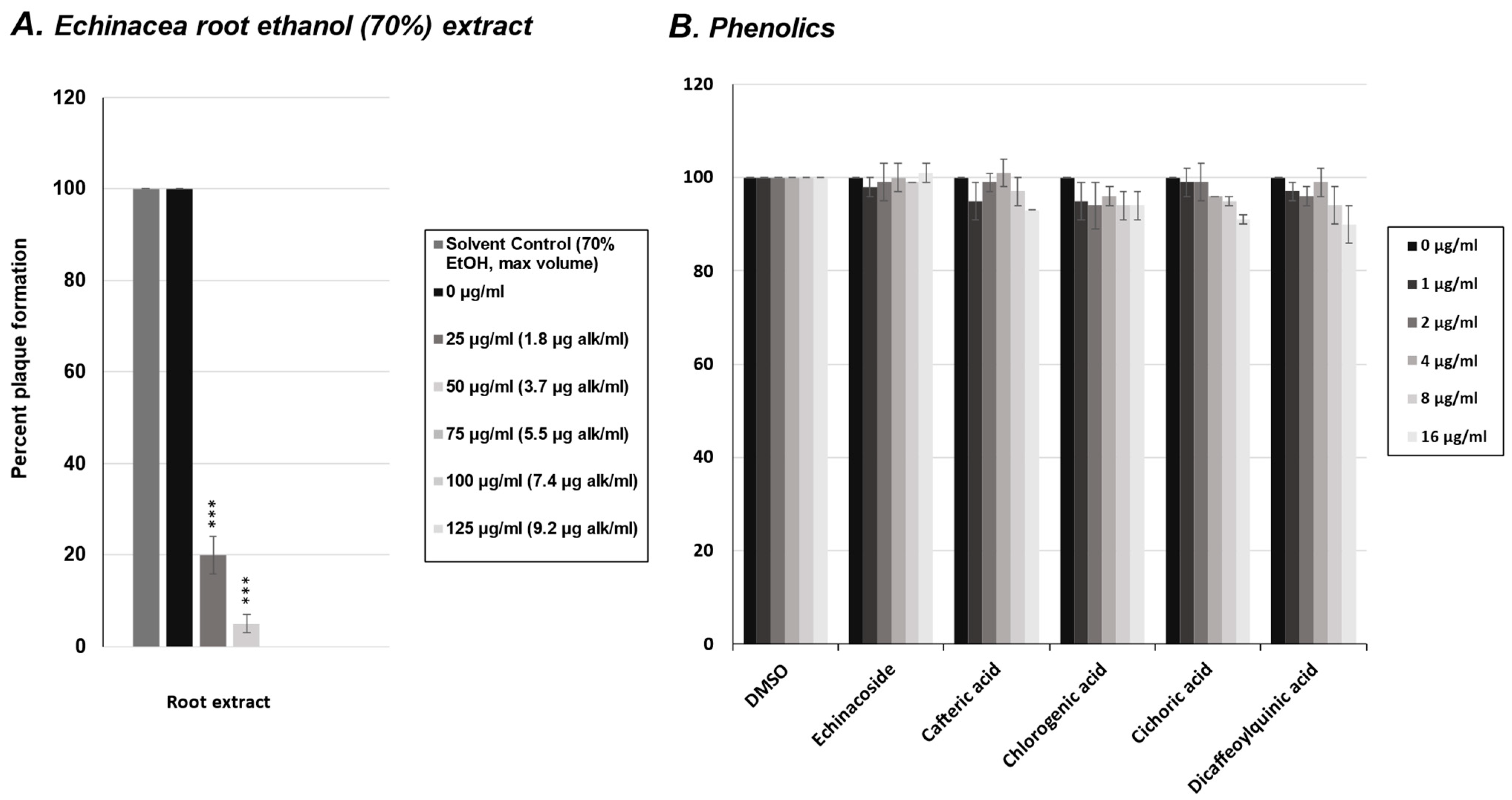
2.2. Effect of Phenolics from E. purpurea on Rhinovirus Replication
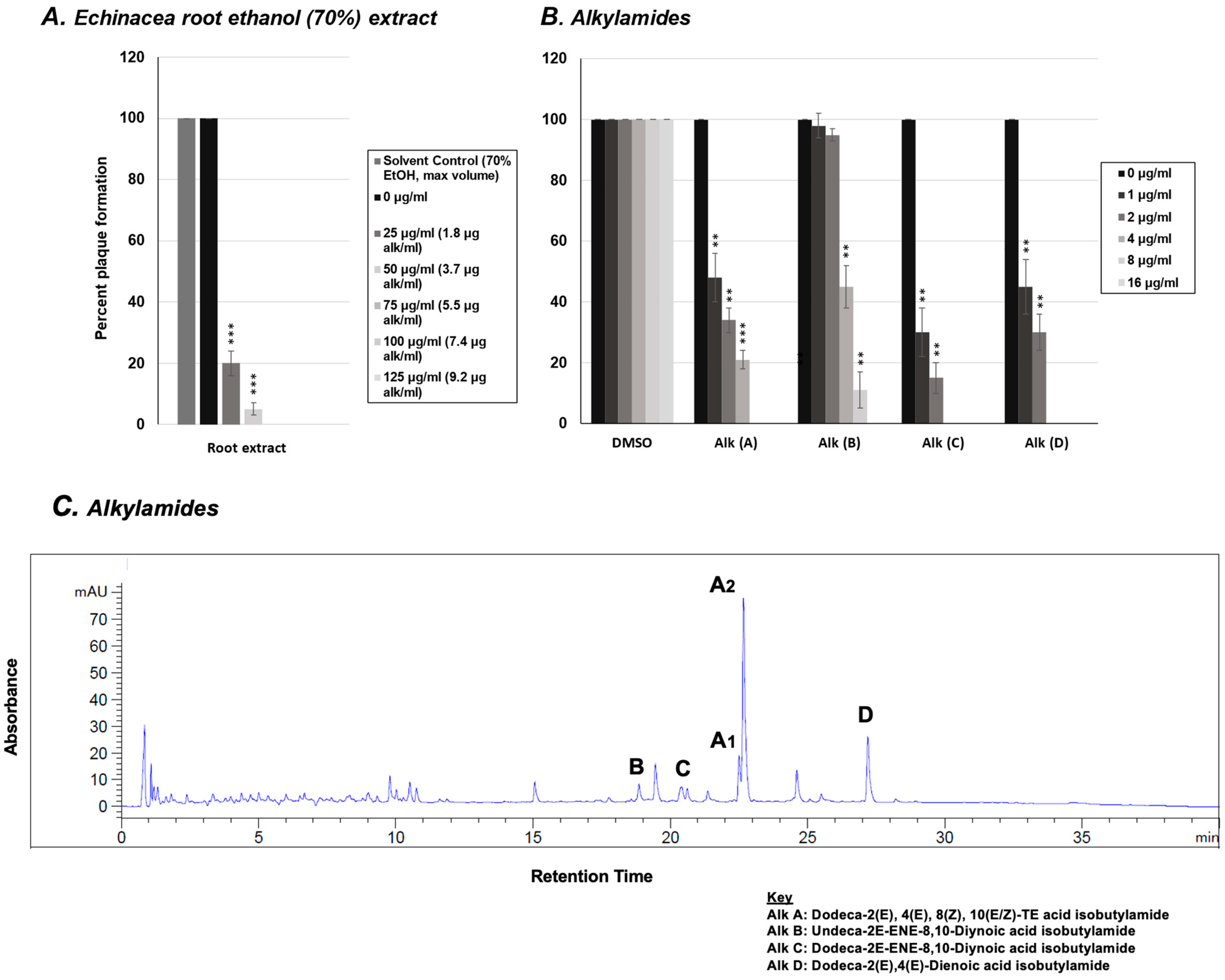
2.3. Effect of Alkylamides from E. purpurea on Rhinovirus Replication
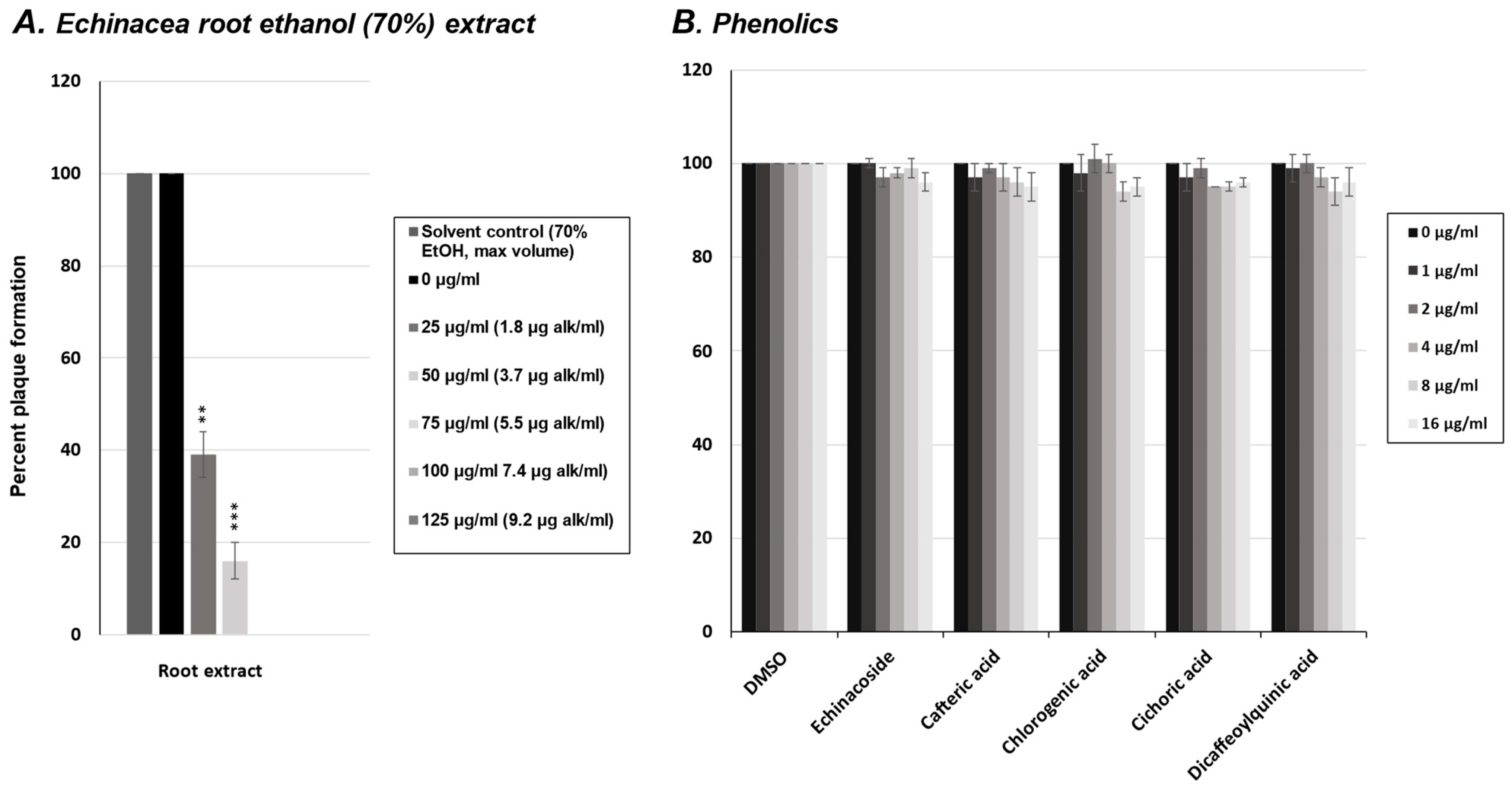
2.4. Effect of Phenolics from E. purpurea on Influenza Virus Replication
2.5. Effect of Alkylamides from E. purpurea on Influenza Virus Replication
2.6. Regulation of LPS-Induced IL-8 Secretion with E. purpurea Extract and Alkylamides
2.7. Selectivity Index Analysis of E. purpurea Extracts and Alkylamides
3. Discussion
4. Materials and Methods
- 0.01 ≤ p < 0.05: Significant (p < 0.05);
- 0.001 ≤ p < 0.01: Highly significant (p < 0.01);
- p < 0.001: Extremely significant (p < 0.001).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moerman, D.E. Ethnobotany in Native North America. In Encyclopaedia of the History of Science, Technology, and Medicine in Non-Western Cultures; Springer: Dordrecht, The Netherlands, 2014; pp. 1–10. ISBN 9789400739345. [Google Scholar]
- Barrett, B. Medicinal Properties of Echinacea: A Critical Review. Phytomedicine 2003, 10, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Flannery, M.A. From Rudbeckia to Echinacea: The Emergence of the Purple Cone Flower in Modern Therapeutics. Pharm. Hist. 1999, 41, 52–59. [Google Scholar]
- Nadaf, M.; Joharchi, M.R.; Amiri, M.S. Ethnomedicinal Uses Plants Treatment Nervous Disorders Herbal Markets Bojnord, North Khorasan Province, Iran. Iran. J. Phytomedicine 2018, 9, 153–163. [Google Scholar] [CrossRef]
- Cao, C.; Kindscher, K. The Medicinal Chemistry of Echinacea Species. In Echinacea; Springer International Publishing: Cham, Switzerland, 2016; pp. 127–145. ISBN 9783319181554. [Google Scholar]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and Pharmacological Properties. A Review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the Use of Complementary Health Approaches among Adults: United States, 2002–2012. Natl. Health Stat. Report. 2015, 79, 1–16. [Google Scholar]
- Parsons, J.L.; Cameron, S.I.; Harris, C.S.; Smith, M.L. Echinacea Biotechnology: Advances, Commercialization and Future Considerations. Pharm. Biol. 2018, 56, 485–494. [Google Scholar] [CrossRef]
- Li, T.S.C. Echinacea: Cultivation and Medicinal Value. Horttechnology 1998, 8, 122–129. [Google Scholar] [CrossRef]
- Market Data Forecast Echinacea Extract Market Size, Share, Growth 2024 to 2029. Available online: https://www.marketdataforecast.com/market-reports/echinacea-extract-market (accessed on 15 September 2024).
- Billah, M.; Hosen, B.; Khan, F.; Niaz, K. Echinacea Book: Nonvitamin Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2019; pp. 205–210. [Google Scholar]
- CDC. About Common Cold. Available online: https://www.cdc.gov/common-cold/about/index.html (accessed on 7 October 2024).
- Spector, S.L. The Common Cold: Current Therapy and Natural History. J. Allergy Clin. Immunol. 1995, 95, 1133–1138. [Google Scholar] [CrossRef]
- Worrall, G. Common Cold. Can. Fam. Physician 2011, 57, 1289–1290. [Google Scholar]
- CDC. About Influenza. Available online: https://www.cdc.gov/flu/about/index.html (accessed on 7 October 2024).
- Vos, L.M.; Bruyndonckx, R.; Zuithoff, N.P.A.; Little, P.; Oosterheert, J.J.; Broekhuizen, B.D.L.; Lammens, C.; Loens, K.; Viveen, M.; Butler, C.C.; et al. Lower Respiratory Tract Infection in the Community: Associations between Viral Aetiology and Illness Course. Clin. Microbiol. Infect. 2021, 27, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.G. Clinical Features of Influenza. Semin. Respir. Infect. 1992, 7, 26–37. [Google Scholar]
- Cate, T.R. Clinical Manifestations and Consequences Influenza. Am. J. Med. 1987, 82, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, G.L. The Common Cold. Prim. Care 1996, 23, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Fendrick, A.M.; Monto, A.S.; Nightengale, B.; Sarnes, M. The Economic Burden of Non-Influenza-Related Viral Respiratory Tract Infection in the United States. Arch. Intern. Med. 2003, 163, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Monto, A.S. Epidemiology of Viral Respiratory Infections. Am. J. Med. 2002, 112, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.J.; Puhakka, T.; Ruuskanen, O.; Leinonen, M.; Saikku, P.; Kimpimäki, M.; Blomqvist, S.; Hyypiä, T.; Arstila, P. Viruses and Bacteria in the Etiology of the Common Cold. J. Clin. Microbiol. 1998, 36, 539–542. [Google Scholar] [CrossRef]
- Sharma, M.; Anderson, S.A.; Schoop, R.; Hudson, J.B. Induction of Multiple Pro-Inflammatory Cytokines by Respiratory Viruses and Reversal by Standardized Echinacea, a Potent Antiviral Herbal Extract. Antivir. Res. 2009, 83, 165–170. [Google Scholar] [CrossRef]
- Gern, J.E.; Martin, M.S.; Anklam, K.A.; Shen, K.; Roberg, K.A.; Carlson-Dakes, K.T.; Adler, K.; Gilbertson-White, S.; Hamilton, R.; Shult, P.A.; et al. Relationships among Specific Viral Pathogens, Virus-Induced Interleukin-8, and Respiratory Symptoms in Infancy. Pediatr. Allergy Immunol. 2002, 13, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Gwaltney, J.M. Clinical Significance and Pathogenesis of Viral Respiratory Infections. Am. J. Med. 2002, 112, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Van Reeth, K. Cytokines in the Pathogenesis of Influenza. Vet. Microbiol. 2000, 74, 109–116. [Google Scholar] [CrossRef]
- Thiel, V.; Weber, F. Interferon and Cytokine Responses to SARS-Coronavirus Infection. Cytokine Growth Factor Rev. 2008, 19, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.H.; Lam, G.K.L.; Shin, T.; Samson, S.I.; Greenberg, D.P.; Chit, A. Efficacy and Effectiveness of High-Dose Influenza Vaccine in Older Adults by Circulating Strain and Antigenic Match: An Updated Systematic Review and Meta-Analysis. Vaccine 2021, A24–A35. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.Y.; Wan, X.-F. Antigenic Characterization of Influenza and SARS-CoV-2 Viruses. Anal. Bioanal. Chem. 2022, 414, 2841–2881. [Google Scholar] [CrossRef] [PubMed]
- Monto, S.S.; Gravenstein, M. Clinical Signs Symptoms Predicting Influenza Infection Arch Intern Med. Arch. Intern. Med. 2000, 160, 3243–3247. [Google Scholar] [CrossRef]
- Li, J.-H.; Wu, C.-C.; Tseng, Y.-J.; Han, S.-T.; Pekosz, A.; Rothman, R.; Chen, K.-F. Applying Symptom Dynamics to Accurately Predict Influenza Virus Infection: An International Multicenter Influenza-like Illness Surveillance Study. Influenza Other Respi. Viruses 2023, 17, e13081. [Google Scholar] [CrossRef] [PubMed]
- Berlanda Scorza, F.; Tsvetnitsky, V.; Donnelly, J.J. Universal Influenza Vaccines: Shifting to Better Vaccines. Vaccine 2016, 34, 2926–2933. [Google Scholar] [CrossRef]
- Jefferson, T.; Jones, M.; Doshi, P.; Spencer, E.; Onakpoya, I.; Heneghan, C. Oseltamivir Influenza Adults Children: Systematic Review Clinical Study Reports Summary Regulatory Comments. BMJ 2014, 348, 2545. [Google Scholar] [CrossRef] [PubMed]
- Aoki, F.Y.; Macleod, M.D.; Paggiaro, P. IMPACT Study Group Early Administration Oral Oseltamivir Increases Benefits Influenza Treatment. J. Antimicrob. Chemother. 2003, 51, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Pleschka, S.; Stein, M.; Schoop, R.; Hudson, J.B. Anti-Viral Properties and Mode of Action of Standardized Echinacea Purpurea Extract against Highly Pathogenic Avian Influenza Virus (H5N1, H7N7) and Swine-Origin H1N1 (S-OIV). Virol. J. 2009, 6, 197. [Google Scholar] [CrossRef]
- Whitley, R.J.; Boucher, C.A.; Lina, B.; Nguyen-Van-Tam, J.S.; Osterhaus, A.; Schutten, M.; Monto, A.S. Global Assessment of Resistance to Neuraminidase Inhibitors, 2008-2011: The Influenza Resistance Information Study (IRIS). Clin. Infect. Dis. 2013, 56, 1197–1205. [Google Scholar] [CrossRef]
- Karsch-Völk, M.; Barrett, B.; Kiefer, D.; Bauer, R.; Ardjomand-Woelkart, K.; Linde, K. Echinacea for Preventing and Treating the Common Cold. Cochrane Database Syst. Rev. 2014, 2014, CD000530. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Cunningham, R. Echinacea for the Prevention and Treatment of Upper Respiratory Tract Infections: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2019, 44, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gancitano, G.; Mucci, N.; Stange, R.; Ogal, M.; Vimalanathan, S.; Sreya, M.; Booker, A.; Hadj-Cherif, B.; Albrich, W.C.; Woelkart-Ardjomand, K.; et al. Echinacea Reduces Antibiotics by Preventing Respiratory Infections: A Meta-Analysis (ERA-PRIMA). Antibiotics 2024, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Aucoin, M.; Cardozo, V.; McLaren, M.D.; Garber, A.; Remy, D.; Baker, J.; Gratton, A.; Kala, M.A.; Monteiro, S.; Warder, C.; et al. A Systematic Review on the Effects of Echinacea Supplementation on Cytokine Levels: Is There a Role in COVID-19? Metabol. Open 2021, 11, 100115. [Google Scholar] [CrossRef] [PubMed]
- Schapowal, A.; Klein, P.; Johnston, S.L. Echinacea Reduces the Risk of Recurrent Respiratory Tract Infections and Complications: A Meta-Analysis of Randomized Controlled Trials. Adv. Ther. 2015, 32, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Palat, C.T., III; Chien, S.H.; Chang, Z.G.; Kennedy, D.T. Evaluation of Echinacea for Treatment of the Common Cold. Pharmacotherapy 2000, 20, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sander, S.; White, C.M.; Rinaldi, M.; Coleman, C.I. Evaluation of Echinacea for the Prevention and Treatment of the Common Cold: A Meta-Analysis. Lancet Infect. Dis. 2007, 7, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; Del Mar Contreras, M.; Soltani-Nejad, A.; et al. Echinacea Plants as Antioxidant and Antibacterial Agents: From Traditional Medicine to Biotechnological Applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Ardjomand-Woelkart, K.; Bauer, R. Review and Assessment of Medicinal Safety Data of Orally Used Echinacea Preparations. Planta Med. 2016, 82, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, R.; Bächler, A.; Feldhaus, S.; Lang, G.; Klein, P.; Schoop, R. Safety and Dose-Dependent Effects of Echinacea for the Treatment of Acute Cold Episodes in Children: A Multicenter, Randomized, Open-Label Clinical Trial. Children 2020, 7, 292. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A Review of Their Chemistry, Pharmacology and Clinical Properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Sharma, C.; Goyal, S.N.; Kumar, S.; Jha, N.K.; Ojha, S. Can Echinacea Be a Potential Candidate to Target Immunity, Inflammation, and Infection—The Trinity of Coronavirus Disease 2019. Heliyon 2021, 7, e05990. [Google Scholar] [CrossRef] [PubMed]
- Woelkart, K.; Bauer, R. The Role of Alkamides as an Active Principle of Echinacea. Planta Med. 2007, 73, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Kakouri, E.; Talebi, M.; Tarantilis, P.A. Echinacea spp.: The Cold-Fighter Herbal Remedy? Pharmacol. Res. Mod. Chin. Med. 2024, 10, 100397. [Google Scholar] [CrossRef]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, Phytochemistry and Analysis Methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; van Klink, J.W.; Burgess, E.J.; Parmenter, G.A. Alkamide Levels in Echinacea purpurea: A Rapid Analytical Method Revealing Differences among Roots, Rhizomes, Stems, Leaves and Flowers. Planta Med. 1997, 63, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.S. Use of Echinacea in Medicine. Biochem. Pharmacol. 2000, 60, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Caram-Salas, N.L.; Li, W.; Wang, L.; Arnason, J.T.; Harris, C.S. Interactions of Echinacea spp. Root Extracts and Alkylamides with the Endocannabinoid System and Peripheral Inflammatory Pain. Front. Pharmacol. 2021, 12, 651292. [Google Scholar] [CrossRef]
- Chicca, A.; Raduner, S.; Pellati, F.; Strompen, T.; Altmann, K.-H.; Schoop, R.; Gertsch, J. Synergistic Immunomopharmacological Effects of N-Alkylamides in Echinacea purpurea Herbal Extracts. Int. Immunopharmacol. 2009, 9, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Gulledge, T.V.; Collette, N.M.; Mackey, E.; Johnstone, S.E.; Moazami, Y.; Todd, D.A.; Moeser, A.J.; Pierce, J.G.; Cech, N.B.; Laster, S.M. Mast Cell Degranulation and Calcium Influx Are Inhibited by an Echinacea Purpurea Extract and the Alkylamide Dodeca-2E,4E-Dienoic Acid Isobutylamide. J. Ethnopharmacol. 2018, 212, 166–174. [Google Scholar] [CrossRef]
- Hinz, B.; Woelkart, K.; Bauer, R. Alkamides from Echinacea Inhibit Cyclooxygenase-2 Activity in Human Neuroglioma Cells. Biochem. Biophys. Res. Commun. 2007, 360, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Kariman, K.; Mousavi, M.; Rengel, Z. Echinacea: Bioactive Compounds and Agronomy. Plants 2024, 13, 1235. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Rezaei, K.A.; Temelli, F.; Ooraikul, B. Supercritical Fluid Extraction of Alkylamides from Echinacea Angustifolia. J. Agric. Food Chem. 2002, 50, 3947–3953. [Google Scholar] [CrossRef]
- Petrova, O.M.; Petkova, N.; Denev, P. Phytochemical Characterization Purple Coneflower Roots (Echinacea purpurea (L.) Moench.) Extracts. Molecules 2023, 28, 3956. [Google Scholar] [CrossRef]
- Binns, S.E.; Livesey, J.F.; Arnason, J.T.; Baum, B.R. Phytochemical Variation Echinacea from Roots and Flowerheads of Wild and Cultivated Populations. J. Agric. Food Chem. 2002, 50, 3673–3687. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R. Phytomedicines Europe: Chemistry Biological Activity. In Echinacea: Biological effects active principals; Lawson, L.D., Bauer, R., Eds.; American Chemical Society: Washington, DC, USA, 1998; pp. 140–157. [Google Scholar]
- Matthias, A.; Banbury, L.; Bone, K.M.; Leach, D.N.; Lehmann, R.P. Echinacea alkylamides Modulate Induced Immune Responses in T-Cells. Fitoterapia 2008, 79, 53–58. [Google Scholar] [CrossRef]
- Hudson, J.; Vimalanathan, S.; Kang, L.; Amiguet, V.T.; Livesey, J.; Arnason, J.T. Characterization of Antiviral Activities In Echinacea. Root Preparations. Pharm. Biol. 2005, 43, 790–796. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Kang, L.; Amiguet, V.T.; Livesey, J.; Arnason, J.T.; Hudson, J. Echinacea purpurea. Aerial Parts Contain Multiple Antiviral Compounds. Pharm. Biol. 2005, 43, 740–745. [Google Scholar] [CrossRef]
- Moazami, Y.; Gulledge, T.V.; Laster, S.M.; Pierce, J.G. Synthesis and Biological Evaluation of a Series of Fatty Acid Amides from Echinacea. Bioorg. Med. Chem. Lett. 2015, 25, 3091–3094. [Google Scholar] [CrossRef] [PubMed]
- Hensel, A.; Bauer, R.; Heinrich, M.; Spiegler, V.; Kayser, O.; Hempel, G.; Kraft, K. Challenges at the Time of COVID-19: Opportunities and Innovations in Antivirals from Nature. Planta Med. 2020, 86, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Woelkart, K.; Xu, W.; Pei, Y.; Makriyannis, A.; Picone, R.P.; Bauer, R. The Endocannabinoid System as a Target for Alkamides from Echinacea angustifolia Roots. Planta Med. 2005, 71, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Raduner, S.; Majewska, A.; Chen, J.-Z.; Xie, X.-Q.; Hamon, J.; Faller, B.; Altmann, K.-H.; Gertsch, J. Alkylamides from Echinacea Are a New Class of Cannabinomimetics. Cannabinoid Type 2 Receptor-Dependent and -Independent Immunomodulatory Effects. J. Biol. Chem. 2006, 281, 14192–14206. [Google Scholar] [CrossRef] [PubMed]
- Matthias, A.; Penman, K.G.; Matovic, N.J.; Bone, K.M.; De Voss, J.J.; Lehmann, R.P. Bioavailability of Echinacea Constituents: Caco-2 Monolayers and Pharmacokinetics of the Alkylamides and Caffeic Acid Conjugates. Molecules 2005, 10, 1242–1251. [Google Scholar] [CrossRef]
- Woelkart, K.; Marth, E.; Suter, A.; Schoop, R.; Raggam, R.B.; Koidl, C.; Kleinhappl, B.; Bauer, R. Bioavailability and Pharmacokinetics of Echinacea purpurea Preparations and Their Interaction with the Immune System. Int. J. Clin. Pharmacol. Ther. 2006, 44, 401–408. [Google Scholar] [CrossRef]
- Sasagawa, M.; Cech, N.B.; Gray, D.E.; Elmer, G.W.; Wenner, C.A. Echinacea alkylamides Inhibit Interleukin-2 Production by Jurkat T Cells. Int. Immunopharmacol. 2006, 6, 1214–1221. [Google Scholar] [CrossRef]
- Clifford, L.J.; Nair, M.G.; Rana, J.; Dewitt, D.L. Bioactivity of Alkamides Isolated from Echinacea purpurea (L.) Moench. Phytomedicine 2002, 9, 249–253. [Google Scholar] [CrossRef]
- Vieira, S.F.; Gonçalves, V.; Llaguno, C.P. Bioactivity Echinacea purpurea Extracts Modulate Production Inflammatory Mediators. Int. J. Mol. Sci. 2022, 23, 13616. [Google Scholar] [CrossRef]
- Kabganian, R.; Carrier, D.J.; Sokansanj, S. Drying Echinacea angustifolia Roots. J. Herbs. Spices. Med. Plants 2002, 10, 11–18. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Stuart, D.L. Alkylamide and Cichoric Acid Levels in Echinacea purpurea Grown in Australia. Food Chem. 1999, 67, 385–388. [Google Scholar] [CrossRef]
- Mudge, E.; Lopes-Lutz, D.; Brown, P.; Schieber, A. Analysis of Alkylamides in Echinacea Plant Materials and Dietary Supplements by Ultrafast Liquid Chromatography with Diode Array and Mass Spectrometric Detection. J. Agric. Food Chem. 2011, 59, 8086–8094. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Remiger, P. TLC and HPLC Analysis of Alkamides in Echinacea Drugs1,2. Planta Med. 1989, 55, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Zhang, S.C.; Sung, J.M. Caffeoyl Phenols and Alkamides of Cultivated Echinacea purpurea and Echinacea atrorubens var. Paradoxa. Pharm. Biol. 2009, 47, 835–840. [Google Scholar] [CrossRef]
- Spelman, K.; Wetschler, M.H.; Cech, N.B. Comparison of Alkylamide Yield in Ethanolic Extracts Prepared from Fresh versus Dry Echinacea purpurea Utilizing HPLC-ESI-MS. J. Pharm. Biomed. Anal. 2009, 49, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Jbilo, O.; Derocq, J.M.; Segui, M.; Le Fur, G.; Casellas, P. Stimulation of Peripheral Cannabinoid Receptor CB2 Induces MCP-1 and IL-8 Gene Expression in Human Promyelocytic Cell Line HL60. FEBS Lett. 1999, 448, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kishimoto, S.; Oka, S.; Gokoh, M. Biochemistry, Pharmacology and Physiology of 2-Arachidonoylglycerol, an Endogenous Cannabinoid Receptor Ligand. Prog. Lipid Res. 2006, 45, 405–446. [Google Scholar] [CrossRef] [PubMed]
- American Herbal Pharmacopoeia. Available online: https://www.herbal-ahp.org (accessed on 7 October 2024).
- Rogers, K.L.; Grice, I.D.; Mitchell, C.J.; Griffiths, L.R. High Performance Liquid Chromatography Determined Alkamide Levels in Australian-Grown Echinacea spp. Aust. J. Exp. Agric. 1998, 38, 403. [Google Scholar] [CrossRef]
- Livesey, J.; Awang, D.; Aranson, J.T.; Letchamo, W.; Barrett, M.; Penyroyal, G. Effect of temperature on stability of marker constituents in Echinacea purpurea root formulations. Phytomedicine 1999, 6, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Murphy, P.A. Alkamide Stability in Echinacea purpurea Extracts with and without Phenolic Acids in Dry Films and in Solution. J. Agric. Food Chem. 2007, 55, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.N.; Chan, M.; Paley, L.; Betz, J.M. Determination of Major Phenolic Compounds in Echinacea spp. Raw Materials and Finished Products by High-Performance Liquid Chromatography with Ultraviolet Detection: Single-Laboratory Validation Matrix Extension. J. AOAC Int. 2011, 94, 1400–1410. [Google Scholar] [CrossRef]
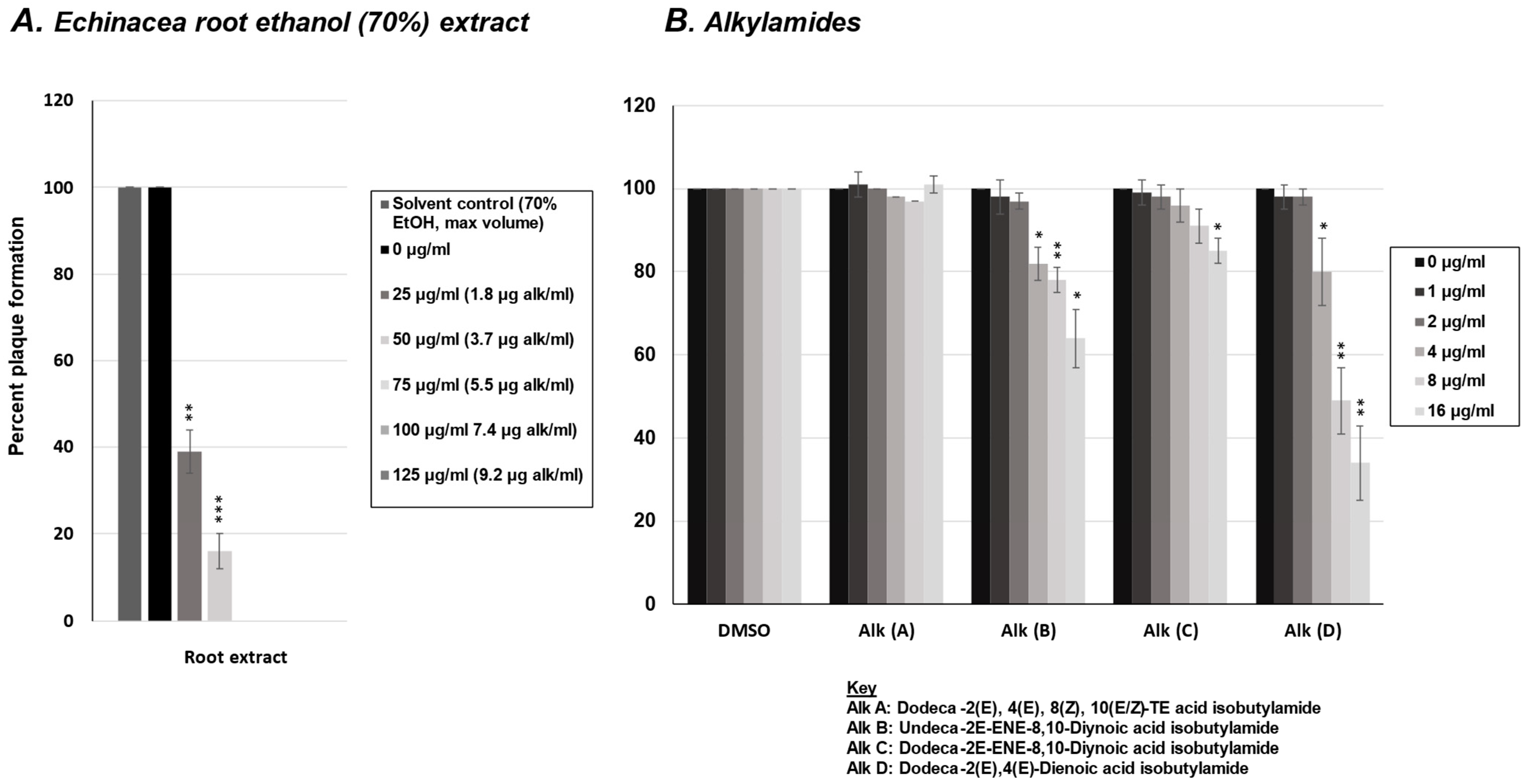
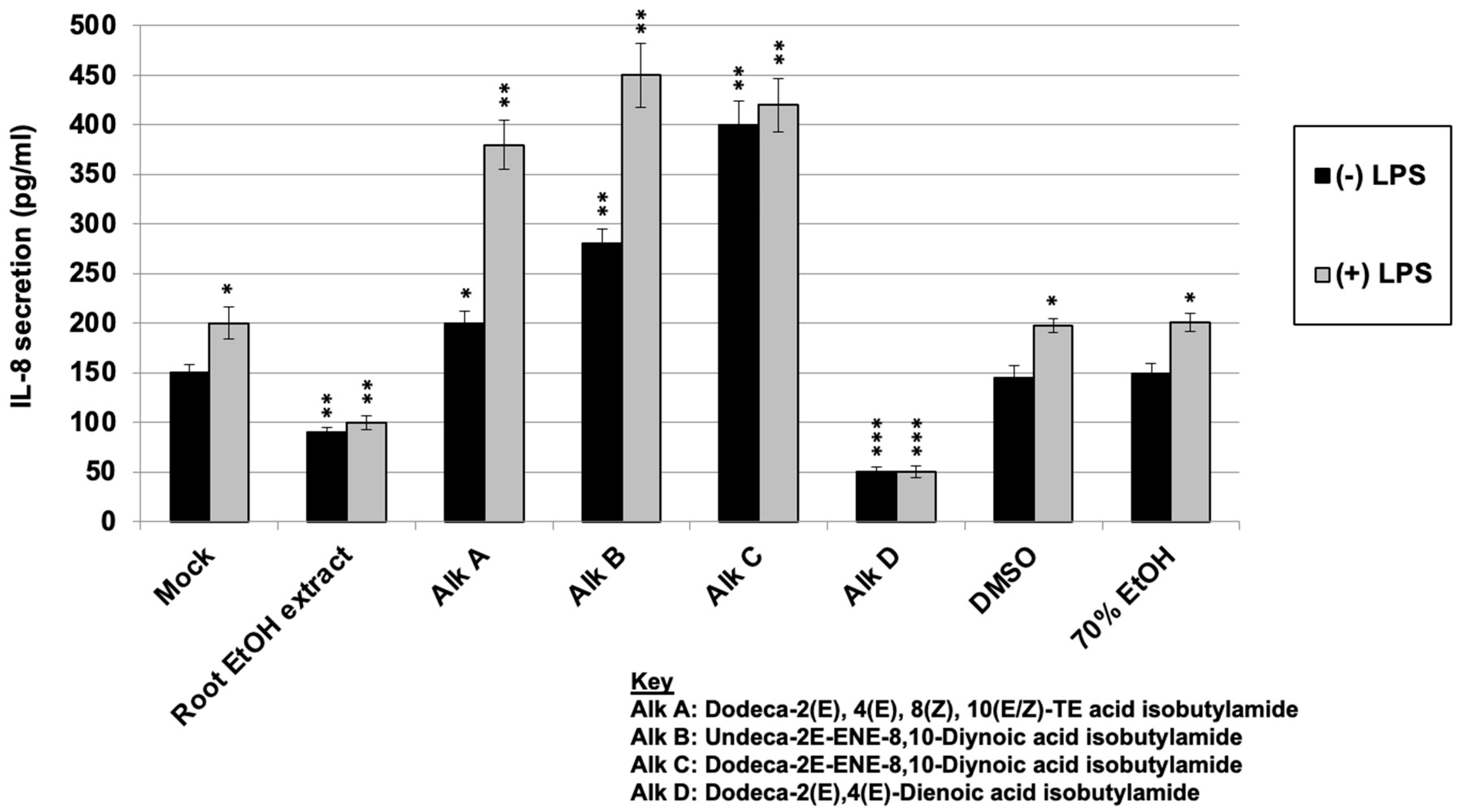
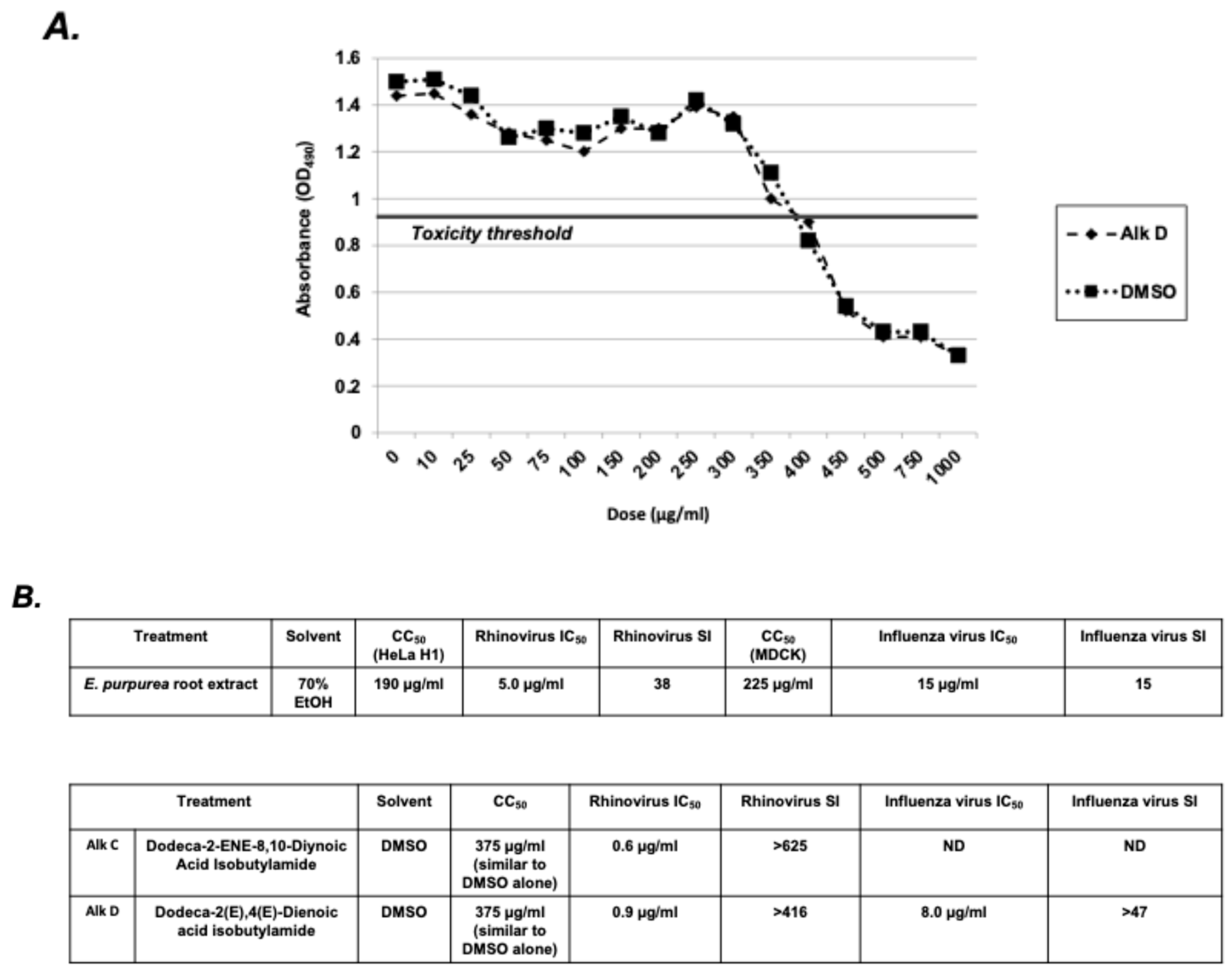
| Sample | Alkylamide | Anti-Rhinovirus Activity | Anti-Influenza Virus Activity | Inhibition of IL-8 Secretion |
|---|---|---|---|---|
| Alk A | Dodeca-2(E), 4(E), 8(Z), 10(E/Z)-TE acid isobutylamide | +++ | − | − |
| Alk B | Undeca-2E-ENE-8,10-Diynoic acid isobutylamide | ++ | + | − |
| Alk C | Dodeca-2E-ENE-8,10-Diynoic acid isobutylamide | ++++ | − | − |
| Alk D | Dodeca-2(E),4(E)-Dienoic acid isobutylamide | ++++ | ++ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchalski, K.; Gerstel, J.A.; Jimoh, A.; Shokoohinia, Y.; Langland, J. Effects of Echinacea purpurea and Alkylamides on Respiratory Virus Replication and IL-8 Expression In Vitro. Molecules 2025, 30, 386. https://doi.org/10.3390/molecules30020386
Puchalski K, Gerstel JA, Jimoh A, Shokoohinia Y, Langland J. Effects of Echinacea purpurea and Alkylamides on Respiratory Virus Replication and IL-8 Expression In Vitro. Molecules. 2025; 30(2):386. https://doi.org/10.3390/molecules30020386
Chicago/Turabian StylePuchalski, Keely, Johanne A. Gerstel, Abiola Jimoh, Yalda Shokoohinia, and Jeffrey Langland. 2025. "Effects of Echinacea purpurea and Alkylamides on Respiratory Virus Replication and IL-8 Expression In Vitro" Molecules 30, no. 2: 386. https://doi.org/10.3390/molecules30020386
APA StylePuchalski, K., Gerstel, J. A., Jimoh, A., Shokoohinia, Y., & Langland, J. (2025). Effects of Echinacea purpurea and Alkylamides on Respiratory Virus Replication and IL-8 Expression In Vitro. Molecules, 30(2), 386. https://doi.org/10.3390/molecules30020386








