Unless otherwise stated, all commercially available reagents were obtained from InnoChem (Beijing, China) and used without further purification. All reactions were carried out in a 10 mL tube under magnetic stirring and monitored by TLC, GC-MS and LC-MS. HRMS analyses of the compounds were conducted using an ultrahigh-resolution electrospray ionization time-of-flight mass spectrometer (Waters Xevo G2-XS QTOF, Waters, Medford, MA, USA) and an electron impact ionization time-of-flight mass spectrometer(Q Exactive™ GC Orbitrap™ GC-MS/MS, Thermo Fisher Scientific, Waltham, MA, USA). TLC was performed using 0.25 mm silica plates, and flash column chromatography was carried out with 200−300 mesh silica gel supplied by Qingdao Haiyang Chemical Co., Ltd. (Qingdao, China). 1H NMR, 13C NMR and 19F NMR spectrum were recorded on Bruker Ultrashield TM 300 MHz instruments (Bruker Corporation, Billerica, MA, USA) and were calibrated using residual undeuterated solvent (TMS δ 0.00 ppm, CDCl3 δ 7.26 ppm, 1H NMR; CDCl3 δ 77.16 ppm 13C NMR). Data were reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, td = triplet of doublets, qd = quartet of doublets, m = multiplet), coupling constants (Hz) and integration.
Experimental Procedure
Synthesis of silyl enol ether [
21]. Under anhydrous and oxygen-free conditions, a 10 mL glass vial was charged with acetophenone (0.6 mmol, 1.0 equiv.), trimethylchlorosilane (0.72 mmol, 1.2 equiv.) and triethylamine (1.44 mmol, 2.4 equiv.), followed by the slow addition of a NaI solution in acetonitrile (0.6 mL, 0.72 mmol, 1.2 equiv., 1.2 mmol/mL) at room temperature. The mixture was stirred at 25 °C under N
2 conditions and monitored by TLC until the reaction was complete. The reaction was completed in approximately 40 min. The reaction mixture was filtered and washed with ethyl acetate, followed by removal of the solvent under reduced pressure at room temperature to afford the desired product.
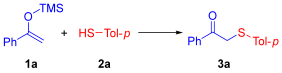
Synthesis of β-Keto Sulfides. In a typical experiment procedure, a 10 mL glass vial was charged with 1-Phenyl-1-trimethylsiloxyethylene derived from acetophenone (0.6 mmol, 1.0 equiv.), thiophenol (1.2 mmol, 2 equiv.) and MeOH (6 mL). The mixture was stirred at 25 °C under ambient air conditions and monitored by TLC, GC-MS and LC-MS until the reaction was complete. H2O was added, and the aqueous phase was extracted with EA (3 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure to afford the crude product. The residue was purified by column chromatography on silica gel using a mixture of EA and n-hexane (1:19, v/v) as the eluent to yield the desired products.
Synthesis of 3-methyl-2-phenyl-2,3-dihydrobenzo[
b][
1,
4]oxathiine (Compound
G) [
16].
F (0.4 mmol, 1.0 equiv.) in DCM (0.05 M) under N
2 was cooled to 0 °C and treated dropwise with TFA (4 mmol, 10 equiv.) followed by Et
3SiH (1.6 mmol, 4 equiv.). The mixture was stirred at 0–25 °C until TLC showed complete consumption of starting material (2–10 h), then poured into ice-cold saturated NaHCO
3, extracted with DCM, washed with brine, dried over Na
2SO
4, and purified by flash chromatography (EA/n-hexanes, 1:5) to afford 3-methyl-2-phenyl-2,3-dihydrobenzo[
b][
1,
4]oxathiine in 88% yield.
Synthesis of
β-hydroxy sulfide (H) [
19]. NaBH
4 (1.0 mmol) was added to a cooled solution (0 °C) of
3-2a (0.5 mmol) in MeOH/DCM (4/1, 10 mL). The reaction mixture was stirred at room temperature for 0.5 h and then concentrated to remove the solvent. After that, the reaction was quenched by the addition of saturated aqueous NH
4Cl (20.0 mL), and the resulting mixture was extracted with ethyl acetate (10.0 mL × 3). The combined organic layers were washed with brine, dried over Na
2SO
4, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel using (EA/n-hexanes, 1:8) as the eluent to afford the
β-hydroxy sulfide.
Synthesis of benzothiophene (I) [
14]. Polyphosphoric acid (2 mL) was heated to 90 °C and
3-2a (0.5 mmol) was added under mechanical stirring, while maintaining the temperature below 94 °C. The mixture was stirred at 90 °C for 3 h, allowed to cool to 70 °C, and the resultant viscous liquid was poured into rapidly stirring ice-cold water (50 mL). The resulting precipitate was collected by filtration and dried in air overnight. The solid was slurried in acetone under reflux for 1 h, the mixture was cooled to room temperature, and the solids were filtered off. The resulting solid was washed with acetone and dried in vacuo to afford benzothiophene.
Synthesis of phenethyl p henyl sulfane (J) [
18]. AlCl
3 (2 mmol) was added at room temperature under an inert atmosphere to a solution of dry THF (5 mL) containing compound
3a (0.5 mmol). After brief stirring, LiAlH
4 (0.5 mmol) was introduced in one portion, and the mixture was stirred for 10 min until TLC indicated the complete disappearance of the starting sulfide. The reaction was quenched by slow addition of saturated aqueous Na
2CO
3 (5 mL). The aqueous layer was extracted with Et
2O (3 × 5 mL), the combined organic phases were washed with brine, dried over Na
2SO
4, and the solvent was evaporated to afford the sulfide in 84% yield.
Synthesis of
β-keto sulfone (
K) [
17]. 60% m-CPBA (2 equiv.) was added portion-wise to a 0 °C solution of the sulfide (0.5 mmol) in DCM (2.5 mL). After stirring at room temperature for 6 h, the reaction mixture was monitored by TLC until the reaction was complete. The mixture was then diluted with hexanes, filtered, washed with aqueous Na
2SO
3 and water, and dried over MgSO
4. The residue was purified by column chromatography on silica gel using EA and n-hexane (1:5) as the eluent to provide the
β-keto sulfone.
1-phenyl-1-trimethylsiloxyethylene (1a). White oil. 98% yield, 1H NMR (300 MHz, CDCl3) δ 7.56–7.49 (m, 2H), 7.28–7.17 (m, 3H), 4.84 (d, J = 1.7 Hz, 1H), 4.36 (d, J = 1.7 Hz, 1H), 0.20 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 155.78, 137.63, 128.34, 128.19, 125.33, 91.19, 0.21.
1-(p-tolyl)-2-(p-tolylthio)ethan-1-one (3-1a). 85% yield, yellow solid. (m.p. 55–57 °C). 1H NMR (300 MHz, CDCl3) δ 7.85–7.76 (m, 2H), 7.30–7.24 (m, 2H), 7.22 (d, J = 8.1 Hz, 2H), 7.06 (d, J = 8.0 Hz, 2H), 4.16 (s, 2H), 2.38 (s, 3H), 2.28 (s, 3H).13C NMR (75 MHz, CDCl3) δ 193.81, 144.26, 137.29, 132.86, 131.25, 131.06, 129.82, 129.32, 128.80, 41.70, 21.69, 21.08. HRMS (EI): m/z calculated for C16H16OS+[M]+: 256.0916, found: 256.0914
1-(4-methoxyphenyl)-2-(p-tolylthio)ethan-1-one (3-1b). 83% yield, yellow solid. (m.p. 58–61 °C). 1H NMR (300 MHz, CDCl3) δ 7.94–7.88 (m, 2H), 7.30 (d, J = 8.2 Hz, 2H), 7.08 (d, J = 8.4 Hz, 2H), 6.94–6.89 (m, 2H), 4.17 (s, 2H), 3.85 (s, 3H), 2.30 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 192.85, 163.70, 137.29, 131.22, 131.16, 131.04, 129.84, 128.36, 113.81, 55.51, 41.56, 21.10. HRMS (EI): m/z calculated for C16H16O2S+[M]+: 272.0866, found: 272.0863.
1-(4-(t-butyl)phenyl)-2-(p-tolylthio)ethan-1-one (3-1c). 74% yield, yellow solid. (m.p. 48–50 °C). 1H NMR (300 MHz, CDCl3) δ 7.95–7.87 (m, 2H), 7.52–7.44 (m, 2H), 7.32 (d, J = 8.2 Hz, 2H), 7.10 (d, J = 7.7 Hz, 2H), 4.22 (s, 2H), 2.32 (s, 3H), 1.36 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 193.76, 157.13, 137.29, 132.81, 131.28, 131.11, 129.84, 128.69, 125.61, 41.69, 35.15, 31.08, 21.12. HRMS (EI): m/z calculated for C19H22OS+[M]+: 298.1386, found: 298.1383.
1-(4-fluorophenyl)-2-(p-tolylthio)ethanone (3-1d). 75% yield, yellow solid. (m.p. 68–72 °C). 1H NMR (300 MHz, CDCl3) δ 7.99–7.88 (m, 2H), 7.27 (d, J = 8.1 Hz, 2H), 7.09 (td, J = 8.4, 2.0 Hz, 4H), 4.15 (s, 2H), 2.29 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 192.69, 165.83 (d, J = 255.4 Hz), 137.67, 131.78 (d, J = 3.0 Hz), 131.56, 131.45 (d, J = 9.4 Hz), 130.61, 129.93, 115.77 (d, J = 22.0 Hz), 41.66, 21.11. 19F NMR (282 MHz, CDCl3) δ −104.33 (tt, J = 8.6, 5.4 Hz). HRMS (EI): m/z calculated for C15H13FOS+[M]+: 260.0666, found: 260.0664.
1-(4-chlorophenyl)-2-(p-tolylthio)ethan-1-one (3-1e). 71% yield, yellow solid. (m.p. 107–109 °C). 1H NMR (300 MHz, CDCl3) δ 7.90–7.82 (m, 2H), 7.45–7.39 (m, 2H), 7.29–7.25 (m, 2H), 7.09 (d, J = 7.7 Hz, 2H), 4.15 (s, 2H), 2.31 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 193.12, 139.94, 137.94, 133.77, 131.85, 130.48, 130.28, 130.05, 129.07, 41.80, 21.24. HRMS (EI): m/z calculated for C15H13ClOS+[M]+: 276.0370, found: 276.0368.
1-(4-bromophenyl)-2-(p-tolylthio)ethan-1-one (3-1f). 86% yield, yellow solid. (m.p. 110–114 °C). 1H NMR (300 MHz, CDCl3) δ 7.81–7.72 (m, 2H), 7.60–7.53 (m, 2H), 7.26 (d, J = 8.2 Hz, 2H), 7.08 (d, J = 7.9 Hz, 2H), 4.13 (s, 2H), 2.30 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 193.22, 137.83, 134.09, 131.98, 131.73, 130.41, 130.30, 129.99, 128.63, 41.67, 21.19. HRMS (EI): m/z calculated for C15H13BrOS+[M]+: 319.9865, found: 319.9861.
1-(o-tolyl)-2-(p-tolylthio)ethan-1-one (3-1g). 75% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.56 (d, J = 7.9 Hz, 1H), 7.37–7.31 (m, 1H), 7.26–7.17 (m, 4H), 7.05 (d, J = 8.0 Hz, 2H), 4.15 (s, 2H), 2.39 (s, 3H), 2.28 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 197.78, 139.13, 137.21, 136.12, 132.06, 131.68, 131.06, 130.99, 129.81, 128.80, 125.58, 44.08, 21.25, 21.09. HRMS (EI): m/z calculated for C16H16OS+[M]+: 256.0916, found: 256.0914.
1-(m-tolyl)-2-(p-tolylthio)ethan-1-one (3-1h). 73% yield, white solid. (m.p. 72–75 °C). 1H NMR (300 MHz, CDCl3) δ 7.74 (d, J = 6.0 Hz, 2H), 7.41–7.29 (m, 4H), 7.13–7.06 (m, 2H), 4.21 (s, 2H), 2.39 (s, 3H), 2.32 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 194.36, 138.44, 137.41, 135.42, 134.19, 131.44, 131.02, 129.85, 129.20, 128.51, 125.92, 41.88, 21.34, 21.10. HRMS (EI): m/z calculated for C16H16OS+[M]+: 256.0916, found: 256.0915.
(S)-1-phenyl-2-(p-tolylthio)propan-1-one (3-1i). 89% yield. yellow oil. 1H NMR (300 MHz, CDCl3) δ 8.00–7.90 (m, 2H), 7.57–7.49 (m, 1H), 7.47–7.38 (m, 2H), 7.25–7.19 (m, 2H), 7.10–7.02 (m, 2H), 4.55 (q, J = 6.8 Hz, 1H), 2.30 (s, 3H), 1.49 (d, J = 6.8 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 196.16, 139.05, 135.82, 135.24, 133.03, 129.77, 128.70, 128.60, 127.76, 46.19, 21.27, 16.88. HRMS (ESI): m/z calculated for C16H16NaOS+[M+Na]+: 279.0814, found: 279.0810.
1-phenyl-2-(p-tolylthio)hexan-1-one (3-1j). 53% yield. yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.89–7.78 (m, 2H), 7.48–7.40 (m, 1H), 7.37–7.30 (m, 2H), 7.12 (d, J = 8.1 Hz, 2H), 6.97 (d, J = 8.0 Hz, 2H), 4.28 (t, J = 7.2 Hz, 1H), 2.21 (s, 3H), 1.93–1.66 (m, 2H), 1.41–1.20 (m, 4H), 0.79 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 195.90, 138.95, 136.44, 135.13, 132.95, 129.75, 128.59, 128.09, 51.57, 30.56, 29.54, 22.62, 21.27, 14.03. HRMS (ESI): m/z calculated for C19H22NaOS+[M+Na]+: 321.1284, found: 321.1293.
1-(naphthalen-2-yl)-2-(p-tolylthio)ethan-1-one (3-1k). 86% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 8.39 (s, 1H), 8.01 (dd, J = 8.7, 1.8 Hz, 1H), 7.91–7.84 (m, 3H), 7.63–7.52 (m, 2H), 7.38–7.31 (m, 2H), 7.10 (d, J = 7.7 Hz, 2H), 4.33 (s, 2H), 2.31 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 194.17, 137.55, 135.61, 132.69, 132.37, 131.61, 130.95, 130.59, 129.90, 129.63, 128.68, 128.50, 127.77, 126.81, 124.22, 41.92, 21.11. HRMS (EI): m/z calculated for C19H16OS+[M]+: 292.096, found: 292.0914.
1-(p-tolylthio)butan-2-one (3-1l). 35% yield, white solid. (m.p. 30–35 °C). 1H NMR (300 MHz, CDCl3) δ 7.27–7.23 (m, 2H), 7.10 (d, J = 7.8 Hz, 2H), 3.63 (s, 2H), 2.61 (q, J = 7.3 Hz, 2H), 2.31 (s, 3H), 1.04 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 206.56, 137.33, 131.12, 130.57, 130.06, 44.41, 34.02, 21.19, 8.00. HRMS (EI): m/z calculated for C11H14OS+[M]+: 194.0760, found: 194.0757.
1-phenyl-2-(phenylthio)ethan-1-one (
3-2a) [
19]. 88% yield, yellow oil.
1H NMR (300 MHz, CDCl
3) δ 8.02–7.92 (m, 2H), 7.64–7.56 (m, 1H), 7.52–7.39 (m, 4H), 4.31 (s, 2H).
13C NMR (75 MHz, CDCl
3) δ 194.19, 135.46, 134.85, 133.62, 130.61, 129.19, 128.81, 127.23, 41.33. HRMS (EI):
m/
z calculated for C
14H
12OS
+[M]
+: 228.0603, found: 228.0601.
1-phenyl-2-(
p-tolylthio)ethanone (
3-2b) [
22]. 91% yield, yellow oil.
1H NMR (300 MHz, CDCl
3) δ 7.98–7.91 (m, 2H), 7.61–7.53 (m, 1H), 7.45 (dd,
J = 8.3, 6.8 Hz, 2H), 7.34–7.27 (m, 2H), 7.10 (d,
J = 8.0 Hz, 2H), 4.22 (s, 2H), 2.32 (s, 3H).
13C NMR (75 MHz, CDCl
3) δ 194.17, 137.47, 135.38, 133.41, 131.44, 130.88, 129.88, 128.71, 128.65, 41.80, 21.12. HRMS (EI):
m/
z calculated for C
15H
14OS
+[M]
+: 242.0760, found: 242.0758.
2-((4-methoxyphenyl)thio)-1-phenylethan-1-one (
3-2c) [
23]. 87% yield, yellow oil. 1H NMR (300 MHz, CDCl
3) δ 7.98–7.91 (m, 2H), 7.61–7.53 (m, 1H), 7.44 (d,
J = 7.1 Hz, 2H), 7.31 (d,
J = 8.2 Hz, 2H), 7.10 (d,
J = 8.0 Hz, 2H), 4.22 (s, 2H), 2.32 (s, 3H).
13C NMR (75 MHz, CDCl
3) δ 194.29, 159.67, 135.39, 134.58, 13 3.32, 128.69, 128.61, 124.51, 114.68, 55.27, 42.76. HRMS (EI):
m/
z calculated for C15H14O2S
+[M]
+: 258.0709, found: 258.0708.
2-((4-(t-butyl)phenyl)thio)-1-phenylethan-1-one (3-2d). 86% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.98–7.91 (m, 2H), 7.60–7.53 (m, 1H), 7.48–7.41 (m, 2H), 7.38–7.29 (m, 4H), 4.26 (s, 2H), 1.31 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 194.26, 150.46, 135.44, 133.41, 131.16, 130.82, 128.70, 128.65, 126.15, 41.62, 34.53, 31.26. HRMS (EI): m/z calculated for C18H20OS+[M]+: 284.1229, found: 284.1227.
2-((4-fluorophenyl)thio)-1-phenylethan-1-one (
3-2e) [
24]. 73% yield, yellow oil.
1H NMR (300 MHz, CDCl
3) δ 7.99–7.84 (m, 2H), 7.61–7.51 (m, 1H), 7.49–7.30 (m, 4H), 7.05–6.86 (m, 2H), 4.19 (s, 2H).
13C NMR (75 MHz, CDCl
3) δ 193.96, 162.40 (d,
J = 247.7 Hz), 135.26, 133.85 (d,
J = 8.2 Hz), 133.55, 129.46 (d,
J = 3.4 Hz), 128.70 (d,
J = 3.4 Hz), 116.22 (d,
J = 22.0 Hz), 42.07.
19F NM R (282 MHz, CDCl
3) δ −113.62 (tt,
J = 8.7, 5.2 Hz). HRMS (EI):
m/
z calculated for C
14H
11FOS
+[M]
+: 246.0509, found: 246.0516.
2-((2-chlorophenyl)thio)-1-phenylethanone (
3-2f) [
23]. 69% yield, yellow solid. (m.p. 82–84 °C).
1H NMR (300 MHz, CDCl
3) δ 7.97–7.88 (m, 2H), 7.61–7.53 (m, 1H), 7.45 (t,
J = 7.5 Hz, 2H), 7.32–7.26 (m, 2H), 7.25–7.18 (m, 2H), 4.24 (s, 2H).
13C NMR (75 MHz, CDCl3) δ 193.74, 135.18, 133.65, 133.24, 133.18, 131.84, 129.20, 128.77, 128.67, 41.19. HRMS (EI):
m/
z calculated for C
14H
11ClOS
+[M]
+: 262.0214, found: 262.0211.
2-((4-bromophenyl)thio)-1-phenylethan-1-one (
3-2g) [
24]. 65% yield, white solid. (m.p. 85–88 °C).
1H NMR (300 MHz, CDCl
3) δ 7.96–7.87 (m, 2H), 7.58 (t,
J = 7.4 Hz, 1H), 7.49–7.35 (m, 4H), 7.22 (d,
J = 8.5 Hz, 2H), 4.25 (s, 2H).
13C NMR (75 MHz, CDCl
3) δ 193.74, 135.19, 133.98, 133.71, 132.15, 131.95, 128.81, 128.71, 121.16, 41.04. HRMS (EI):
m/
z calculated for C
14H
11BrOS
+[M]
+: 305.9708, found: 305.9707.
1-phenyl-2-(o-tolylthio)ethan-1-one (3-2h). 82% yield, yellow solid. (m.p. 57–60 °C). 1H NMR (300 MHz, CDCl3) δ 8.00–7.92 (m, 2H), 7.62–7.54 (m, 1H), 7.46 (t, J = 7.6 Hz, 2H), 7.39–7.32 (m, 1H), 7.21–7.10 (m, 3H), 4.25 (s, 2H), 2.39 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 194.02, 138.62, 135.32, 133.94, 133.43, 130.27, 130.14, 128.63, 128.62, 126.99, 126.62, 40.44, 20.43. HRMS (EI): m/z calculated for C15H14OS+[M]+: 242.0760, found: 242.0758.
1-phenyl-2-(m-tolylthio)ethan-1-one (3-2i). 73% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.99–7.91 (m, 2H), 7.61–7.54 (m, 1H), 7.46 (t, J = 7.5 Hz, 2H), 7.24–7.13 (m, 3H), 7.04 (d, J = 6.8 Hz, 1H), 4.28 (s, 2H), 2.31 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 194.10, 138.82, 135.36, 134.50, 133.45, 131.01, 128.91, 128.66, 127.93, 127.34, 41.17, 21.30. HRMS (EI): m/z calculated for C15H14OS+[M]+: 242.0760, found: 242.0757.
2-((2-isopropylphenyl)thio)-1-phenylethan-1-one (3-2j). 75% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.92 (d, J = 7.0 Hz, 2H), 7.55 (t, J = 7.4 Hz, 1H), 7.47–7.36 (m, 3H), 7.27–7.19 (m, 2H), 7.16–7.07 (m, 1H), 4.22 (s, 2H), 3.47 (p, J = 6.9 Hz, 1H), 1.16 (d, J = 6.9 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 194.20, 149.66, 135.47, 133.49, 132.87, 131.51, 128.75, 128.69, 127.82, 126.55, 125.80, 41.75, 30.42, 23.59. HRMS (EI): m/z calculated for C17H18OS+[M]+: 270.1073, found: 270.1072.
2-((2-chlorophenyl)thio)-1-phenylethan-1-one (
3-2k) [
25]. 67% yield, yellow solid. (m.p. 84–87 °C).
1H NMR (300 MHz, CDCl
3) δ 8.00–7.92 (m, 2H), 7.61–7.56 (m, 1H), 7.46 (t,
J = 7.5 Hz, 2H), 7.40–7.36 (m, 2H), 7.22–7.12 (m, 2H), 4.33 (s, 2H).
13C NMR (75 MHz, CDCl
3) δ 193.79, 135.36, 134.75, 133.99, 133.74, 130.81, 129.92, 128.83, 128.75, 127.99, 127.41, 39.79. HRMS (EI):
m/
z calculated for C
14H
11ClOS
+[M]
+: 262.0214, found: 262.0210.
2-((3,4-dimethoxyphenyl)thio)-1-phenylethanone (3-2l). 85% yield, yellow solid. 1H NMR (300 MHz, CDCl3) δ 7.85–7.77 (m, 2H), 7.50–7.z42 (m, 1H), 7.34 (t, J = 7.5 Hz, 2H), 6.90 (dd, J = 8.3, 2.1 Hz, 1H), 6.83 (d, J = 2.1 Hz, 1H), 6.67 (d, J = 8.3 Hz, 1H), 4.06 (s, 2H), 3.74 (s, 3H), 3.70 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 194.39, 149.14, 148.89, 135.38, 133.33, 128.67, 128.58, 125.63, 124.87, 115.85, 111.46, 55.83, 42.59. HRMS (EI): m/z calculated for C16H16O3S+[M]+: 288.0815, found: 288.0812.
2-(naphthalen-2-ylthio)-1-phenylethan-1-one (
3-2m) [
23]. 77% yield, yellow solid. (m.p. 50–53 °C).
1H NMR (300 MHz, CDCl
3) δ 7.99–7.95 (m, 2H), 7.84–7.72 (m, 4H), 7.61–7.56 (m, 1H), 7.50–7.43 (m, 5H), 4.38 (s, 2H).
13C NMR (75 MHz, CDCl
3) δ 194.14, 135.48, 133.76, 133.66, 132.29, 132.27, 128.92, 128.83, 128.79, 128.05, 127.82, 127.45, 126.72, 126.25, 41.23. HRMS (EI):
m/
z calculated for C
18H
14OS
+[M]
+: 278.0760, found: 278.0758.
1-phenyl-2-(thiophen-2-ylthio)ethanone (3-2n). 45% yield, purple oil. 1H NMR (300 MHz, CDCl3) δ 7.94–7.87 (m, 2H), 7.61–7.55 (m, 1H), 7.49–7.43 (m, 2H), 7.37 (dd, J = 5.4, 1.3 Hz, 1H), 7.12 (dd, J = 3.6, 1.3 Hz, 1H), 6.95 (dd, J = 5.4, 3.6 Hz, 1H), 4.17 (s, 2H). 13C NMR (75 MHz, CDCl3) δ 194.00, 135.46, 135.44, 133.59, 132.21, 130.73, 128.78, 128.75, 127.80, 45.36. HRMS (EI): m/z calculated for C12H10OS2+[M]+: 234.0168, found: 234.0166.
2-((1s, 3s)-adamantan-1-ylthio)-1-phenylethanone (3-2o). 30% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.92–7.86 (m, 2H), 7.52–7.45 (m, 1H), 7.39 (dd, J = 8.3, 6.7 Hz, 2H), 3.79 (s, 2H), 2.00–1.94 (m, 3H), 1.82 (d, J = 3.0 Hz, 6H), 1.60 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 196.68, 135.69, 133.35, 128.89, 128.68, 46.00, 43.30, 36.22, 33.18, 29.79. HRMS (ESI): m/z calculated for C18H22NaOS+[M+Na]+: 309.1284, found: 309.129.
butyl 2-((2-oxo-2-phenylethyl)thio)acetate (3-2p). 35% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.99–7.93 (m, 2H), 7.61–7.54 (m, 1H), 7.49–7.43 (m, 2H), 4.11 (t, J = 6.7 Hz, 2H), 4.03 (s, 2H), 3.32 (s, 2H), 1.64–1.55 (m, 2H), 1.40–1.30 (m, 2H), 0.91 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 194.10, 170.04, 135.40, 133.64, 128.82, 128.70, 65.48, 37.78, 33.44, 30.60, 19.14, 13.77. HRMS (EI): m/z calculated for C14H18O3S+[M]+: 266.0971, found: 266.0969.
2,2′-(1,2-phenylenebis(sulfanediyl))bis(1-phenylethan-1-one) (3-2q). 36% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.95–7.87 (m, 4H), 7.55 (t, J = 7.4 Hz, 2H), 7.45–7.33 (m, 6H), 7.12–7.15 (m, 2H), 4.28 (s, 4H). 13C NMR (75 MHz, CDCl3) δ 194.07, 136.18, 135.40, 133.56, 131.14, 128.73, 128.70, 127.79, 40.27. HRMS (ESI): m/z calculated for C22H18NaO2S2+[M+Na]+: 401.0640, found: 401.0631.
1-(4-methoxyphenyl)-2-((3-methoxyphenyl)thio)ethan-1-one (D). 82% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 8.00–7.87 (m, 2H), 7.18 (t, J = 7.9 Hz, 1H), 6.91 (d, J = 9.1 Hz, 4H), 6.73 (dd, J = 8.3, 1.6 Hz, 1H), 4.25 (s, 2H), 3.85 (s, 3H), 3.75 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 192.73, 163.83, 159.82, 136.49, 131.06, 129.88, 128.34, 121.97, 115.11, 113.89, 112.67, 55.55, 55.28, 40.74. HRMS (EI): m/z calculated for C16H16O3S+[M]+: 288.0815, found: 288.0812.
6-methoxy-2-(4-methoxyphenyl)benzo[b]thiophene (E). 87% yield, white solid. (m.p. 163–165 °C). 1H NMR (300 MHz, CDCl3) δ 7.66–7.54 (m, 3H), 7.37–7.26 (m, 2H), 7.01–6.89 (m, 3H), 3.88 (s, 3H), 3.85 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 159.59, 157.30, 141.64, 140.74, 135.03, 127.55, 127.40, 124.03, 117.89, 114.49, 114.44, 105.00, 55.76, 55.53. HRMS (EI): m/z calculated for C15H14O2S+[M]+: 270.0709, found: 270.0707.
2-((2-hydroxyphenyl)thio)-1-phenylpropan-1-one (F). 75% yield, yellow oil. 1H NMR (300 MHz, CDCl3) δ 7.98–7.82 (m, 2H), 7.60–7.51 (m, 1H), 7.43 (t, J = 7.5 Hz, 2H), 7.35–7.22 (m, 2H), 7.05 (s, 1H), 6.94 (dd, J = 8.2, 1.4 Hz, 1H), 6.81 (td, J = 7.5, 1.3 Hz, 1H), 4.62 (q, J = 7.0 Hz, 1H), 1.48 (d, J = 7.0 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 197.33, 158.22, 137.71, 135.04, 133.62, 132.31, 128.82, 128.72, 120.67, 115.47, 115.32, 47.24, 17.32. HRMS (EI): m/z calculated for C15H14OS+[M]+: 258.0709, found: 258.0706.
3-methyl-2-phenyl-2,3-dihydrobenzo[
b][
1,
4]oxathiine (
G). 88% yield, yellow oil.
1H NMR (300 MHz, CDCl
3) δ 7.50–7.32 (m, 5H), 7.16–6.90 (m, 4H), 5.50 (s, 1H), 3.43 (q,
J = 7.1 Hz, 1H), 1.26 (d,
J = 6.9 Hz, 3H).
13C NMR (75 MHz, CDCl
3) δ 151.49, 139.38, 128.49, 127.92, 127.85, 125.91, 125.64, 121.99, 118.53, 116.90, 76.74, 38.26, 15.20. HRMS (EI):
m/
z calculated for C
15H
14OS
+[M]
+: 242.0760, found: 242.0756.
phenyl-2-(phenylthio)ethan-1-ol (
H) [
19]. 88% yield, yellow oil.
1H NMR (300 MHz, CDCl
3) δ 7.42–7.19 (m, 10H), 4.69 (dd,
J = 9.4, 3.6 Hz, 1H), 3.29 (dd,
J = 13.8, 3.6 Hz, 1H), 3.07 (dd,
J = 13.8, 9.4 Hz, 1H).
13C NMR (75 MHz, CDCl3) δ 142.22, 134.98, 130.21, 129.21, 128.63, 128.06, 126.82, 125.94, 71.72, 43.96. HRMS (ESI):
m/
z calculated for C
14H
14NaOS
+ [M+Na]
+: 253.0658, found: 253.0658.
2-phenylbenzo[b]thiophene (I). 83% yield, white solid. (m.p. 166–170 °C). 1H NMR (300 MHz, CDCl3) δ 7.90–7.67 (m, 4H), 7.56 (s, 1H), 7.48–7.29 (m, 5H). 13C NMR (75 MHz, CDCl3) δ 144.38, 140.83, 139.63, 134.43, 129.09, 128.41, 126.64, 124.65, 124.46, 123.70, 122.41, 119.59. HRMS (EI): m/z calculated for C14H10S+[M]+: 210.0498, found: 210.0496.
phenethyl(phenyl)sulfane (J). 84% yield, white oil. 1H NMR (300 MHz, CDCl3) δ 7.41–7.09 (m, 10H), 3.15 (t, J = 7.8 Hz, 2H), 2.90 (t, J = 7.9 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 140.28, 136.45, 129.22, 129.02, 128.60, 126.54, 126.04, 35.69, 35.12. HRMS (EI): m/z calculated for C14H14S+[M]+: 214.0811, found: 214.0812.
1-phenyl-2-(phenylsulfonyl)ethan-1-one (K). 75% yield, yellow solid. (m.p. 91–95 °C). 1H NMR (300 MHz, CDCl3) δ 7.90–7.86 (m, 4H), 7.59–7.35 (m, 6H), 4.75 (s, 2H). 13C NMR (75 MHz, CDCl3) δ 188.01, 138.68, 135.54, 134.24, 134.11, 129.10, 128.72, 128.36, 63.10. HRMS (ESI): m/z calculated for C14H12NaO3S+[M+Na]+: 283.0399, found: 283.0400.