Abstract
This study systematically investigated the dissolution equilibrium of lithium carbonate (Li2CO3) in mixed Na2CO3-NaCl aqueous solutions through isothermal dissolution experiments spanning 283.15–353.15 K. Precise solubility determinations were conducted using a gravimetric analysis under controlled thermodynamic conditions. The obtained solubility data were successfully correlated with the Extended Debye–Hückel (E-DH) model, yielding residual standard deviations below 0.09, which validates the model’s applicability in this ternary system. Both experimental observations and theoretical predictions confirmed that increasing the salt molality enhances the synergistic suppression of the Li2CO3 solubility through combined common-ion and salt effects. The thermodynamic analysis revealed the dissolution process to be exothermic (ΔHd < 0), and entropy change dominates (ξS ≈ 78%), with negative entropy changes (ΔSd < 0) indicating predominant hydration ordering effects. These mechanistic insights establish critical thermodynamic benchmarks for optimizing lithium carbonate precipitation processes in brine lithium extraction operations.
1. Introduction
Lithium, a naturally occurring trace element, is widely distributed in geological formations, including igneous rocks, soils, brine deposits, seawater, and biological systems, across plant and animal organisms. Based on incomplete statistical data, current global lithium reserves are estimated at approximately 13 million metric tons (in lithium metal equivalent). Brine lithium resources are primarily concentrated in Bolivia, Chile, China, and the United States [1]. Li2CO3, a commercially vital compound in lithium salt production, functions both as a flux agent and performance-enhancing additive in glass and ceramic manufacturing. This inorganic salt also serves as a fundamental precursor for synthesizing specialized lithium compounds, including lithium tantalate (LiTaO3) and lithium niobate (LiNbO3) monocrystals—materials essential for manufacturing surface acoustic wave elements with optimized elastic properties [2,3]. Recent technological developments have revealed significant potential for high-purity lithium salts in emerging applications, particularly within new energy systems, advanced materials, and high-tech industries. These compounds have emerged as critical components in electric vehicle technologies, demonstrating a growing importance in sustainable transportation solutions.
Contemporary lithium extraction primarily focuses on pegmatite-type lithium minerals and lithium-enriched brine deposits, containing lithium-bearing minerals such as spodumene, petalite, lepidolite, and amblygonite. Prior to the 1990s, lithium production predominantly relied on solid mineral processing, a method constrained by high energy consumption, challenging tailings/wastewater management, and significant environmental impacts. Since 1996, salt lake brine extraction has emerged as the dominant global lithium production method, accounting for 80% of the worldwide Li2CO3 output. Major lithium-bearing salt lakes with proven reserves exceeding one million metric tons include Salar de Uyuni, Salar de Hombre Muerto, Salar de Atacama, and the Zabuye Salt Lake [4,5]. China possesses the world’s second-largest lithium resource reserves after Bolivia, with brine deposits constituting approximately 79% of its total lithium reserves. The Qinghai–Tibet Plateau hosts China’s primary salt lake lithium resources, which demonstrate comparable proven reserves to global counterparts and substantial economic potential, forming a critical foundation for national lithium industry development [6]. Notably, the Qaidam Basin in Qinghai contains brine lithium reserves of 15.201 million metric tons (as LiCl equivalent) [7].
Conventional brine lithium extraction techniques include precipitation, calcination-leaching, selective membrane separation, adsorption, and solvent extraction methods [7,8,9,10,11,12,13]. The industrial process involving LiCl production from brine followed by reaction crystallization with Na2CO3 to precipitate Li2CO3 faces persistent challenges: a low lithium yield, product purity limitations, a poor crystal morphology, and severe particle agglomeration. The Li2CO3 precipitation process represents a critical yet underdeveloped stage in the lithium production chain, combining purification/enrichment with precipitation. While purification techniques receive substantial research attention, precipitation technology remains relatively primitive.
Song et al. [14] systematically investigated Li2CO3 solubility and supersolubility in aqueous systems through a thermodynamic analysis. Wang et al. [15] extended this work to LiCl-NaCl-KCl-Na2SO4 mixed solutions, examining salt effects on Li2CO3’s metastable zone. Cheng et al. [16] determined the solubility of lithium carbonate (Li2CO3) in Na-K-Cl systems and employed the Pitzer model to fit its solubility in these solutions. Gisele Azimi et al. [17] carried out an in-depth study on the crystallization process of lithium carbonate; when mixing lithium sulfate and a sodium carbonate solution, the sodium sulfate was removed by freezing, the recovery of lithium was 90%, and the purity of the lithium carbonate was 99.0%.
Although significant progress has been made in lithium carbonate preparation using sodium carbonate precipitation, the resulting mother liquor system retains a mixed solution of NaCl and Na2CO3. Notably, the lithium loss during the carbonation precipitation process reaches up to 15%, highlighting the technical bottleneck that underscores the necessity for systematic investigation into the solubility mechanisms within the lithium carbonate precipitation system. However, fundamental studies on the solubility of Li2CO3 in Na2CO3-NaCl aqueous solutions remain critically lacking. This deficiency in phase equilibrium data directly impedes the optimization of lithium recovery efficiency in precipitation processes, despite its pivotal role in advancing industrial-scale lithium extraction. This study systematically examines the solubility of Li2CO3 in mixed Na2CO3-NaCl aqueous solutions across a temperature range of 278.15–358.15 K. Experimental solubility data were correlated using the Extended Debye–Hückel equation with subsequent thermodynamic parameter derivation through established thermodynamic modeling. The combined experimental and theoretical investigation aims to elucidate the dissolution behavior of Li2CO3 in mixed Na2CO3-NaCl aqueous solutions, while establishing fundamental data to optimize Li2CO3 precipitation processes.
2. Experimental Section
2.1. Instruments and Reagents
The main chemical reagents and instruments used in the experiments are listed in Table 1 and Table 2, respectively. All experimental solutions were prepared through direct dissolution of analytical-grade reagents with strict quality control. Secondary deionized water (DDW) with conductivity < 1 × 10−4 S·m−1 served as the solvent system. Prior to application, the DDW was decarbonized by sequential heating and boiling processes, attaining a stable pH value of 6.60.

Table 1.
Reagent description table.

Table 2.
Experimental apparatus.
2.2. Experimental Method
This study employed the isothermal dissolution method [18] to determine lithium carbonate (Li2CO3) solubility. Sodium carbonate (Na2CO3) and sodium chloride (NaCl) mixed solutions of varying molalities were separately prepared. Each 150 mL aliquot was transferred to a polyethylene conical flask containing 5 g Li2CO3 excess solid. The sealed flasks were immersed in a thermostated magnetic stirring bath, maintaining constant temperature (±0.05 °C) with continuous agitation throughout the experiment. Following five-day equilibration with daily sampling verification, supernatants were syringe-filtered through 0.22 μm microporous membranes (25 mm diameter) to eliminate residual particulates. Filtered aliquots were quantitatively transferred to 10 mL polyethylene vials and diluted to 250 mL with deionized water for subsequent analysis. Equilibrium was confirmed when consecutive daily measurements showed <0.5% variation in ionic molalities, at which point the final stabilized values were recorded as solubility data. Solid phases were immediately collected via vacuum filtration to minimize atmospheric exposure. Phase composition of equilibrium solids was verified by X-ray diffraction (XRD) analysis. Powder XRD patterns were collected on a diffractometer using Cu Kα radiation (Kα1 = 1.540598 Å and Kα2 = 1.544426, Ratio K-α2/K-α1 = 0.5) over a 2θ range of 5–80°, with a step size of 0.02° and a counting time of 38 s per step.
2.3. Analytical Methods
Lithium ion (Li+) molalities were analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES). Carbonate (CO32−) molalities were analyzed via standardized hydrochloric acid titration. Chloride (Cl−) molalities were analyzed through mercurimetric titration with Hg(NO3)2 standard solution, employing diphenylcarbazide–bromophenol blue mixed indicator system [19].
3. Results and Discussion
3.1. Solubility of Li2CO3 in Mixed Solution
To validate the experimental accuracy of Li2CO3 solubility determinations, the molal solubility in deionized water was experimentally measured (Table 3). The comparative analysis revealed relative deviations (RDs) below 0.0243 versus the literature values, demonstrating an excellent agreement between experimental and published solubility data.

Table 3.
Solubility of Li2CO3 in pure water (p = 77.3 KPa) a.
The solubility of Li2CO3 in mixed Na2CO3-NaCl aqueous solutions was experimentally determined across the temperature range of 283.15–353.15 K, with the comprehensive dataset tabulated in Table 4. Figure 1 displays the X-ray diffraction (XRD) patterns characterizing the equilibrated solid phases. The XRD analysis confirmed the absence of Na2CO3 and NaCl crystalline phases in the equilibrium solids. This observation establishes that the measured molality of Li2CO3 in the liquid phase directly represents its thermodynamic solubility under the experimental conditions.

Table 4.
Solubility of Li2CO3 in mixed solutions of Na2CO3 and NaCl at 283.15–353.15 K and pressure of p = 77.3 kPa a.
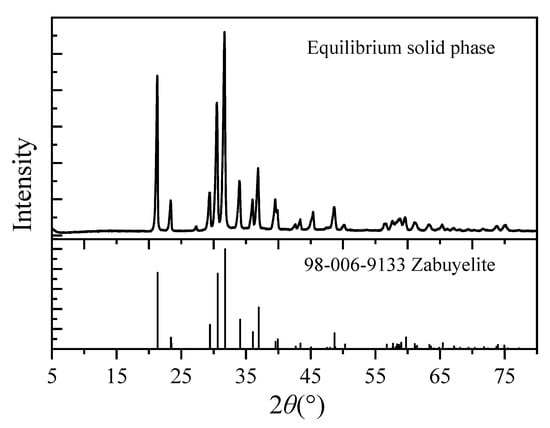
Figure 1.
X-ray diffraction pattern of the equilibrium solid phase (Zabuyelite is a natural lithium carbonate mineral).
To systematically investigate the influence of the main components in the lithium precipitation mother liquor, Na2CO3 and NaCl, on the solubility of Li2CO3, we fixed Na2CO3 at 0.5 mol·kg−1 and NaCl at 1.0 mol·kg−1 based on the ionic concentrations in the mother liquor, respectively, while varying the concentration of the other salt. We plotted the Li2CO3 solubility values as a function of the temperature and the molality of the two salts, as shown in Figure 2. The solubility of Li2CO3 in the mixed electrolyte system exhibits a clear negative correlation with the temperature, which is consistent with the inverse relationship observed in pure water systems. As the molality of either constituent salt increases, the solubility gradually decreases. This dissolution behavior results from the combined effects of (i) the common-ion effect primarily attributed to the carbonate ions from Na2CO3 and (ii) the non-common-ion salt effect arising from collective ionic interactions in the mixed electrolyte system.

Figure 2.
Solubility of Li2CO3 in mixed solutions of Na2CO3 and NaCl at different temperatures.
To isolate the individual effects of Na2CO3 and NaCl on the Li2CO3 solubility, Figure 3 illustrates the solubility variations under controlled conditions with incremental salt additions. Experimental data demonstrate systematic decreases in the Li2CO3 solubility with increasing concentrations of both salts, albeit through distinct mechanisms. As shown in Figure 3a, at a fixed Na2CO3 concentration (0.5 mol·kg−1), the Li2CO3 solubility decreases monotonically with the rising NaCl concentration. This behavior originates from a non-specific salting-out effect induced by Na+ and Cl−: Na+ compresses the ionic atmosphere surrounding CO32− through electrostatic screening, significantly reducing the activity coefficient of CO32− (γCO32−). Simultaneously, Cl− enhances the solution polarity, promoting Li+ hydration and decreasing the free Li+ concentration. The temperature elevation intensifies this suppression: the reduced dielectric constant (ε) of water strengthens the electrostatic attraction between Na+ and CO32−, further inhibiting the dissolution equilibrium. Figure 3b reveals that at a fixed NaCl concentration (1.0 mol·kg−1), the increasing Na2CO3 concentration also reduces the solubility but with a progressively diminishing rate. This non-linear response stems from competing ion interaction mechanisms: (i) the common-ion effect (CO32−) directly suppresses Li2CO3 dissociation, while high Na+ concentrations compete with Li+ for CO32− binding, forming NaCO3− ion pairs and reducing free carbonate, and (ii) at elevated Na2CO3 concentrations, a high CO32− density promotes Li+-CO32− ion pair formation (LiCO3−), generating a salting-in effect. Concurrently, the dielectric constant reduction due to the increased ionic strength enhances the Li+-CO32− electrostatic attraction, partially offsetting the common-ion suppression. It is particularly noteworthy that the temperature exerts a dominant influence: Li2CO3 maintains an inverse solubility–temperature dependence across all salt concentrations, which is consistent with its behavior in pure water. Elevated temperatures primarily facilitate ion association by weakening hydration shells while simultaneously reducing εr to intensify interionic electrostatic forces (inhibiting dissolution). These mechanistic insights reveal limitations in industrial precipitation processes: the competitive shielding of CO32− by Na+ and the saturation of ion association effects prevent the linear enhancement of the lithium recovery efficiency with excessive Na2CO3 additions.
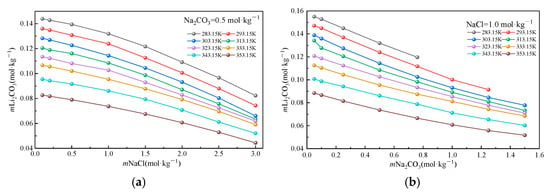
Figure 3.
Li2CO3 solubility in a mixed solution. (a): a mixed solution of Na2CO3 = 0.5 mol·kg−1; (b) a mixed solution of NaCl = 1.0 mol·kg−1.
3.2. Solubility Model
The dissolution equilibrium of Li2CO3 in mixed Na2CO3-NaCl aqueous solutions under isothermal conditions is governed by two competing mechanisms: the common-ion (homoionic) effect and ionic strength-mediated salt effects. The common-ion effect modifies the dissolution equilibrium through solubility product (Ksp) constraints, while the salt effect operates via the ionic strength-dependent modulation of activity coefficients for Li+ and CO32− ions in the solution. Following the classical solubility product theory, the dissolution equilibrium for Li2CO3 in this ternary system can be formally expressed as
Using experimental solubility data of Li2CO3 in pure water, Cheng et al. [16] derived new parameters for the Li+-CO32− ion pair within the Pitzer activity coefficient model, enabling the successful prediction of Li2CO3 solubility in NaCl, KCl, LiCl, and NaCl-KCl mixed solutions. While such ion-specific models provide accurate predictions, they require extensive parametrization for complex systems. To develop a more streamlined approach for quantifying concentration-dependent solubility variations across mixed electrolytes, we established reference solubility products (Ksp(T)) for Li2CO3 in defined baseline solutions (0.5 mol·kg−1 Na2CO3 + 1.0 mol·kg−1 NaCl). These reference values were subsequently incorporated into an Extended Debye–Hückel (E-DH) framework to construct a predictive solubility model for multi-component systems [20].
where I denotes the ionic strength of the solution at the dissolution equilibrium, mol·kg−1; A denotes the Debye–Hückel limiting slope, which is calculated by listing A in Table 5 at different temperatures; Bα is used as a model parameter for the theoretical term (long-range force-acting term); and C and D denote the model parameter for the short-range force correction term.

Table 5.
Parameters and standard deviations of Debye–Hückel extension model at different temperatures.
Based on the solubility model equation (Equation (2)), multivariate regression analyses were performed to determine the solubility product coefficients (β) at various temperatures. This computational approach enabled the derivation of thermodynamic parameters for Li2CO3 in a mixed electrolyte system containing 0.5 mol·kg−1 Na2CO3 and 1.0 mol·kg−1 NaCl across the studied temperature range. The optimized parameters are summarized in Table 5. To evaluate the model’s accuracy, both the relative deviation (ε, Equation (5)) and standard deviation (δ, Equation (6)) were employed as statistical metrics, with the corresponding computational results presented in Table 5.
The Li2CO3 molalities in the mixed solution were calculated according to the model parameters, respectively, and the deviation ε relative to the total ionic strength in the mixed solution is plotted in Figure 4. As can be seen in Figure 4, the relative deviations of all the experimental values of the solubility of Li2CO3 from the model-calculated values are within ±0.1, which suggests that the solubility model equations can better represent the solubility properties of Li2CO3 in the Na2CO3-NaCl mixed solution.
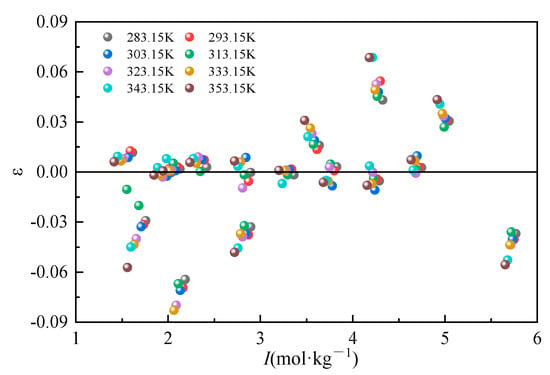
Figure 4.
Relative deviation of calculated and experimental values of Li2CO3 solubility in the mixed system.
3.3. Thermodynamics of Dissolution
The solubility of Li2CO3 in mixed Na2CO3-NaCl aqueous solutions is expressed in terms of the molar fraction x, as in Equation (7):
where mi is the mass of Li2CO3, Na2CO3, NaCl, and H2O, in grams, respectively; and Mi is the molar mass of Li2CO3, Na2CO3, NaCl, and H2O, expressed in g·mol−1, respectively.
Using enthalpies of formation for solid Li2CO3 and aqueous Li+ and CO32− ions from the literature, Lee et al. [21] calculated the enthalpy of dissolution (ΔHd) for Li2CO3 to be −4.3 kJ·mol−1 based on a 1 mol/kg standard state via a thermodynamic cycle approach. While such indirect calculations based on formation enthalpies provide valuable insights, the apparent enthalpy of the dissolution can also be directly determined from experimental solubility data using the temperature dependence expressed by the Van’t Hoff equation [22]. This fundamental thermodynamic relationship, derived from activity coefficient relationships under ideal solution approximations, establishes a linear correlation between the natural logarithm of the solute mole fraction (ln x) and the reciprocal absolute temperature (1/T), as expressed by Equation (8):
where R = 8.314 J·mol−1·K−1 (universal gas constant); ΔHd= dissolution enthalpy (J·mol−1); ΔSd = dissolution entropy (J·mol−1·K−1); and T= system temperature (K).
The Gibbs energy change (ΔrG) calculated here represents the apparent standard Gibbs energy of the dissolution for lithium carbonate (Li2CO3) in mixed electrolytes. This macroscopic thermodynamic property is derived directly from solubility measurements using the relation ΔrG = –RTln(Ksp). It should be noted that this approach provides the net thermodynamic driving force of the dissolution equilibrium. The values reported are apparent properties, reflecting the overall thermodynamics of the system under the given experimental conditions, without incorporating molecular-scale solvation studies. Although microscopic analyses—such as solvation energetics (e.g., ΔGsolv)—could offer further mechanistic interpretation, they are not prerequisites for establishing phase equilibrium thermodynamics.
Within the experimental temperature range, ΔHd and ΔSd are assumed to be temperature-independent based on the thermodynamic convention for limited temperature intervals. Using Equations (8) and (9), we determined the apparent molar dissolution enthalpy (ΔHd) and entropy (ΔSd) for Li2CO3 across various mixed solutions. Figure 5 presents these apparent thermodynamic parameters (ΔHd, ΔSd, ΔGd) characterizing the Li2CO3 dissolution in mixed Na2CO3-NaCl aqueous solutions, where the corresponding Gibbs free energy change (ΔGd) was calculated at the mean experimental temperature. The negative ΔHd values (ΔHd < 0) confirm the exothermic nature of the dissolution, which is consistent with the observed inverse solubility–temperature dependence. NaCl and Na2CO3 exhibit antagonistic modulations on the dissolution enthalpy: (a) at the fixed Na2CO3 (0.5 mol·kg−1), ΔHd decreases (becomes more negative) with the increasing NaCl concentration; (b) conversely, at the fixed NaCl (1.0 mol·kg−1), ΔHd increases (becomes less negative) progressively with the Na2CO3 concentration. This antagonistic effect suggests that NaCl enhances the temperature sensitivity of the solubility, whereas Na2CO3 mitigates thermal effects. The calculated ΔHd for the dissolution in the NaCl-Na2CO3 system ranges from approximately −5.8 to −6.4 kJ·mol−1. This value is significantly more negative than the standard dissolution enthalpy of −4.3 kJ·mol−1 reported in the literature [21]. The observed difference arises primarily because the dissolution medium (NaCl-Na2CO3 brine) constitutes a non-ideal solution, where significant deviations from ideal behavior occur due to ionic interactions and altered activity coefficients.

Figure 5.
Enthalpy of dissolution (ΔHd), entropy of dissolution (ΔSd), and entropy of dissolution (ΔGd) of Li2CO3. (a): a mixed solution of Na2CO3 = 0.5 mol·kg−1; (b) a mixed solution of NaCl = 1.0 mol·kg−1.
The red dashed trendline in Figure 5 delineates the molality-dependent dissolution entropy (ΔSd) evolution. Crystalline dissolution comprises two sequential processes: (i) lattice disintegration into constituent ions and (ii) subsequent ion hydration. The first stage generates increased ionic disorder, yielding positive sublimation entropy (ΔsubSθm > 0). The subsequent hydration stage induces hydration structuring around ions, producing negative hydration entropy (ΔhydSθm < 0). The net dissolution entropy (ΔsSθm) is therefore the following summation: ΔsSmθ = ΔsubSθm + ΔhSθm [23,24]. The observed ΔSd < 0 indicates an overall entropy reduction during dissolution, signifying the dominance of the hydration entropy over sublimation effects. The ionic hydration process requires the destruction of the original ionic hydration in the solution and the establishment of a new mixed ionic hydration structure. The dissolution entropy (ΔSd) exhibits an inverse relationship with the salt molality across both NaCl and Na2CO3 concentration gradients. The progressive salt addition depletes free water molecules available for hydration. The enhanced ion association generates ordered ionic assemblies, which amplify solution structuring effects and thereby intensify negative ΔSd magnitudes at elevated ionic strengths.
The concentration dependence of the Gibbs free energy change (ΔGd) for Li2CO3 dissolution was quantitatively evaluated using the Gibbs–Helmholtz relationship (Equation (9)). Figure 5 reveals positive ΔGd values (ΔGd > 0) across all experimental conditions, confirming the thermodynamically unfavorable nature of the Li2CO3 dissolution. The progressive increase in ΔGd with rising salt molalities demonstrates that the concomitant addition of both salts produces synergistic solubility suppression in industrial lithium precipitation systems.
The entropic contribution to the dissolution spontaneity was quantified through the dimensionless parameter: [25,26]. As shown in Figure 6, calculated ξS values range from 0.781 to 0.797 across experimental conditions, exhibiting a positive correlation with the Na2CO3 molality. This confirms entropy-driven dominance in the Li2CO3 dissolution. The combined evidence of negative dissolution entropy (ΔSd < 0) and a high entropic contribution (ξS ≈ 0.79) reveals that hydration structure reorganization governs dissolution thermodynamics. Specifically, Li+ ions exhibit exceptional hydration energy compared to other alkali cations, which intensifies water ordering effects and thereby suppresses the Li2CO3 solubility relative to its alkali metal analogs. This finding is consistent with the work of Lee et al. [21], who calculated thermodynamic dissolution parameters for M2CO3 (M = Li, Na, K) and demonstrated that dissolution entropy is the primary factor governing the solubility differences among these alkali carbonates.

Figure 6.
Li2CO3 dissolved entropy drive percentage versus salt molality.
4. Conclusions
Through isothermal dissolution equilibrium studies of Li2CO3 in lithium precipitation mother liquors across 283.15–353.15 K, with the precise determination of the equilibrium solubility, we derive the following conclusions:
- (1)
- Concentration dependence: Li2CO3 solubility exhibits an inverse proportionality to both Na2CO3 and NaCl molalities. Notably, Na2CO3 manifests dual behaviors: while its common-ion effect dominates the solubility reduction, a concurrent weak salting-in effect partially offsets this suppression. NaCl exerts unidirectional salting-out effects through ionic strength modulation.
- (2)
- Model validation: The Extended Debye–Hückel (E-DH) model demonstrates a strong predictive capability, yielding residual standard deviations < 0.09 across all isotherms, confirming its applicability to industrial lithium precipitation systems.
- (3)
- Thermodynamic analysis: The systematic evaluation of dissolution parameters reveals the apparent enthalpy of the dissolution (ΔHd < 0), with positive ΔGd values confirming non-spontaneous dissolution behavior. Negative ΔSd values indicate enhanced solution ordering, with the entropy-driven percentage (ξS) maintaining at 78% across all salt concentrations. The predominance of entropic control suggests that dissolution dynamics are governed by the hydration structure reorganization rather than enthalpic interactions.
Investigating the thermodynamic properties of lithium carbonate dissolution in lithium precipitation mother liquors can provide critical insights for optimizing industrial processes to enhance lithium recovery yields during carbonation crystallization.
Author Contributions
Methodology, H.G.; Writing—original draft, H.G.; Visualization, H.G.; Supervision, M.W.; Funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Key R&D and Transformation of Qinghai Province, China. (No. 2022-GX-102).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Marcinov, V.; Klimko, J.; Takáčová, Z.; Pirošková, J.; Miškufová, A.; Sommerfeld, M.; Dertmann, C.; Friedrich, B.; Oráč, D. Lithium Production and Recovery Methods: Overview of Lithium Losses. Metals 2023, 13, 1213. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Urbańska, W.; Janicka, A.; Zawiślak, M.; Matla, J. The Necessity of Recycling of Waste Li-Ion Batteries Used in Electric Vehicles as Objects Posing a Threat to Human Health and the Environment. Recycling 2021, 6, 35. [Google Scholar] [CrossRef]
- Wang, Y.Q. A brief discussion the preparation of high-purity lithium carbonate. Xinjiang Nonfer. Met. 2009, 32, 92–93. (In Chinese) [Google Scholar]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of Spent Lithium-Ion Batteries in View of Lithium Recovery: A Critical Review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Fitch, S.D.; Bartlett, P.N.; Garcia-Araez, N. LiFePO4 Battery Material for the Production of Lithium from Brines: Effect of Brine Composition and Benefits of Dilution. ChemSusChem 2022, 15, e202102182. [Google Scholar] [CrossRef]
- Ma, P.H. Comprehensive utilization of salt lake resources. Adv. Earth Sci. 2000, 15, 365–375. (In Chinese) [Google Scholar]
- Wang, B.C. Lithium-bearing brine resources status and its progress of development technology in China. Ind. Miner. Process 2000, 29, 13–15. (In Chinese) [Google Scholar]
- Wang, Y.; Ni, Y.; Sun, X.T.; Zou, Z.D.; Li, Y.T.; Zhou, Y.Q.; Ge, F. Patent analysis of lithium extraction technology from brine. J. Salt Lake Res. 2018, 26, 82–87. (In Chinese) [Google Scholar]
- Zhu, J.Q.; Xu, B.J.; Song, X.W.; Chen, B. Process of extracting lithium technology. Met. Mine 2018, 62–69. [Google Scholar]
- Luo, A.M.; Chen, F.; Li, H.G.; Yang, J.Y. Research progress on lithium extraction from lake brine. Ind. Miner. Process 2018, 47, 66–72. (In Chinese) [Google Scholar]
- Liu, D.F.; Sun, S.Y.; Yu, J.G. Research and development on technique of lithium recovery from salt lake brine. CIESC J. 2018, 69, 141–155. (In Chinese) [Google Scholar]
- Zhao, X.Y. Review on new techniques for lithium extraction from seawater and brine. J. Chem. Eng. Chin. Univ. 2017, 31, 497–508. [Google Scholar]
- Chen, Y.C.; Qin, Z.H.; Li, B.J. Sino-Swiss cooperation to tap with “hsu technique” the lithium resource from the salt lakes in Qinghai province of China. Geol. Chem. Min. 1998, 20, 49–50. [Google Scholar]
- Song, C.B.; Li, R.C. Measurement and thermodynamic analysis of the solubility and super-solubility of lithium carbonate in water. Chem. Ind. Eng. Prog. 2016, 35, 2350–2354. [Google Scholar]
- Wang, H.; Du, B.; Wang, M. Study of the solubility, supersolubility and metastable zone width of Li2CO3 in the LiCl–NaCl–KCl–Na2SO4 system from 293.15 to 353.15 K. J. Chem. Eng. Data 2018, 63, 1429–1434. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Cheng, F. Solubility of Li2CO3 in Na-K-Li-Cl brines from 20 to 90 °C. J. Chem. Thermodyn. 2013, 67, 74–82. [Google Scholar]
- Liu, H.; Azimi, G. Process analysis and study of factors affecting the lithium carbonate crystallization from sulfate media during lithium extraction. Hydrometallurgy 2021, 199, 105532. [Google Scholar] [CrossRef]
- Ge, H.; Wang, H.; Wang, M. Solubility and Thermodynamics of Lithium Carbonate in Sodium Carbonate Solution. CIESC J. 2019, 70, 4123–4130. [Google Scholar]
- Qinghai Institute of Salt Lakes, Chinese Academy of Sciences. Analytical Methods of Brines and Salts, 2nd ed.; Science Press: Beijing, China, 1998; pp. 59–67. [Google Scholar]
- Speight, J.G. Lange’s Handbook of Chemistry, 16th ed.; McGraw Hill: New York, NY, USA, 2005. [Google Scholar]
- Lee, T.B.; McKee, M.L. Dissolution Thermochemistry of Alkali Metal Dianion Salts (M2X1, M = Li+, Na+, and K+ with X = CO32−, SO42−, C8H82−, and B12H122−). Inorg. Chem. 2011, 50, 11412–11422. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xie, Z.; Xu, J.; Qin, Y.; Du, Y.; Du, S.; Gong, J. Experimental and modeling studies on the solubility of d-pantolactone in four pure solvents and ethanol-water mixtures. J. Chem. Eng. Data 2015, 60, 870–875. [Google Scholar] [CrossRef]
- Qin, F.H.; Qiu, H.Y.; Xiao, B.Y.; Mi, Y.; Huang, Z.Y. Investigation into the thermodynamic properties of nano-silver halides based on the principle of dissolution thermodynamics. Chem. J. Chin. Univ. 2018, 39, 2214–2220. [Google Scholar]
- Zhang, W.J.; Zhang, C.J. Thermodynamic Theoretical Study on the Dissolution of Substances in Water. Chem. Teach. Learn. 2018, 25–27. [Google Scholar]
- Sousa, J.; Fonseca, I. Solubility of hydrofluorocarbons in halobenzene solvents. J. Chem. Eng. Data 2014, 59, 3605–3609. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.; Wu, B.; Zhang, Y.; Tang, H.; Li, Q. Determination and modeling of the solubility of 2,4-dimethoxybenzoic acid in six pure and isopropanol + ethyl acetate mixed organic solvents at temperatures from (288.15 to 323.15) K. J. Chem. Eng. Data 2015, 60, 1098–1105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).