Upcycling of Waste PVC into CaCO3/KOH-Modified Porous Carbon for Supercapacitor Applications
Abstract
1. Introduction
2. Results and Discussion
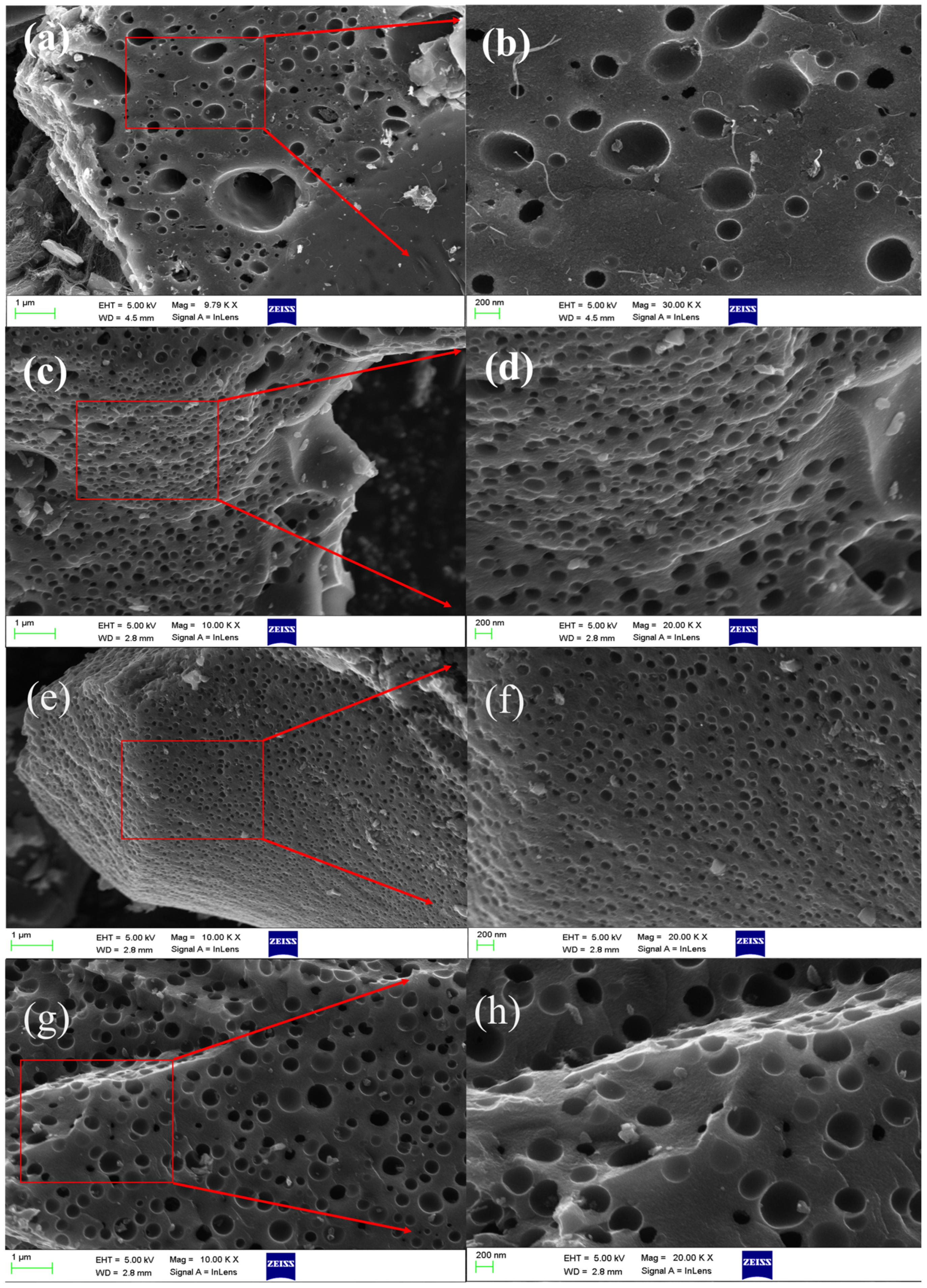
2.1. Influence of Different Activation Methods on the Microstructure of Porous Carbon Materials
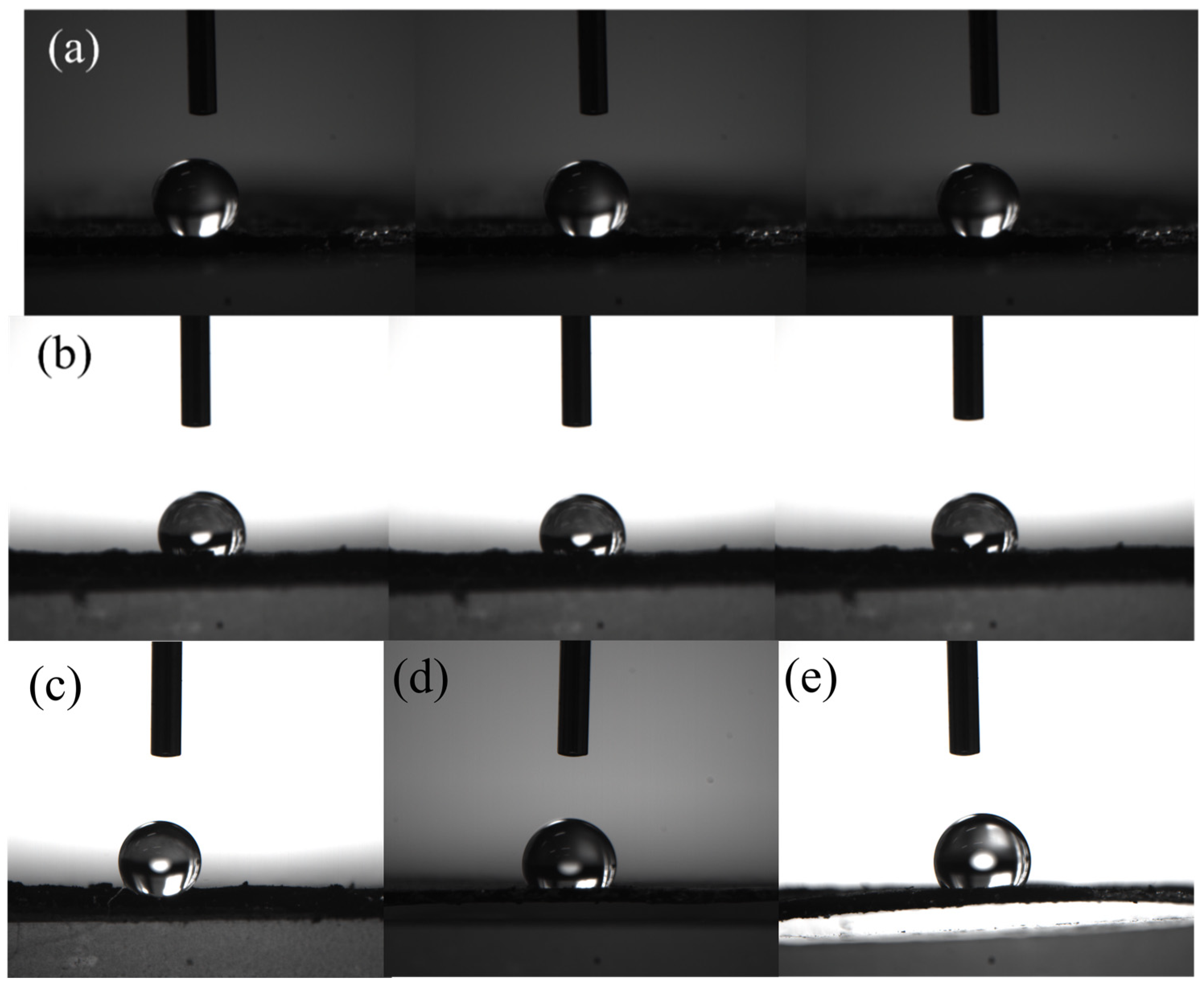
2.2. Effect of the Two Activation Methods on the Textural Properties of Porous Carbon Materials
2.3. Effect of Activation Method on the Chemical Composition of Porous Carbon Materials
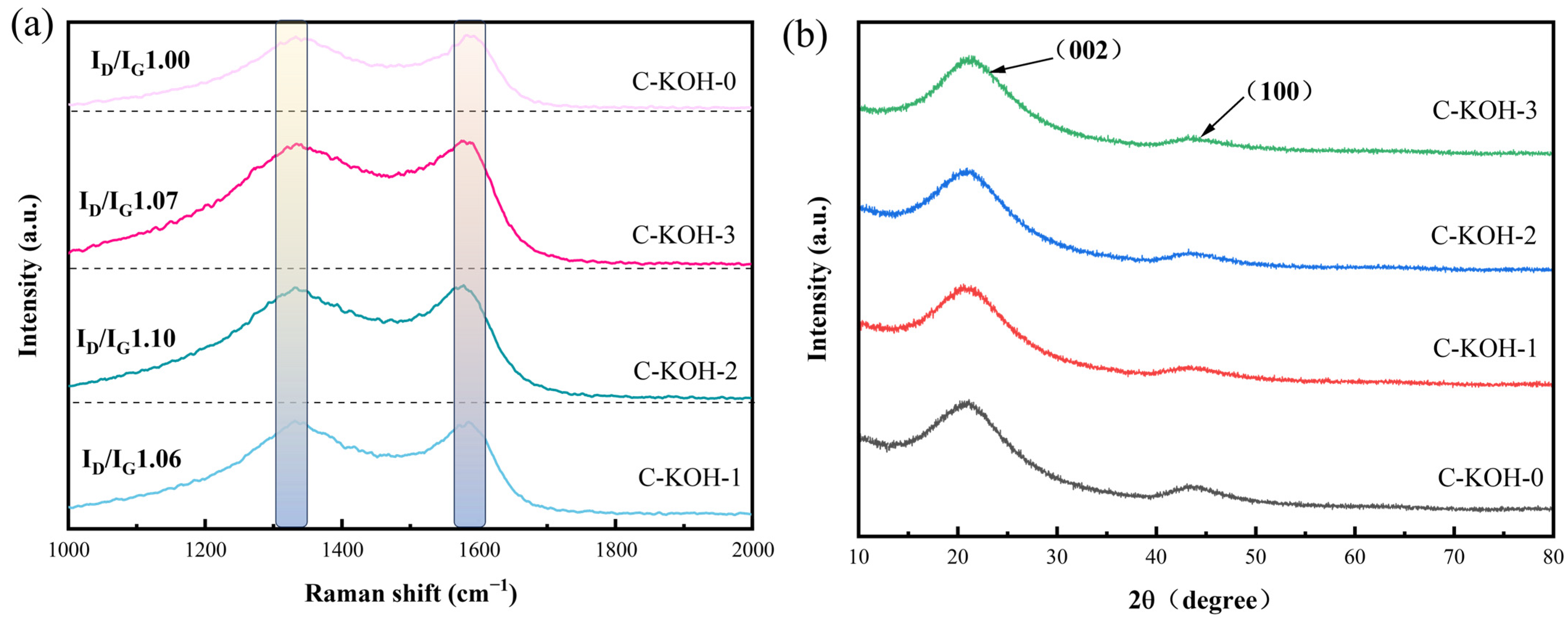
2.4. Characterization of Graphitization Degree and Crystalline Structure of Porous Carbon Materials
3. Electrochemical Performance Evaluation of Carbon Materials Prepared by Different Activation Methods
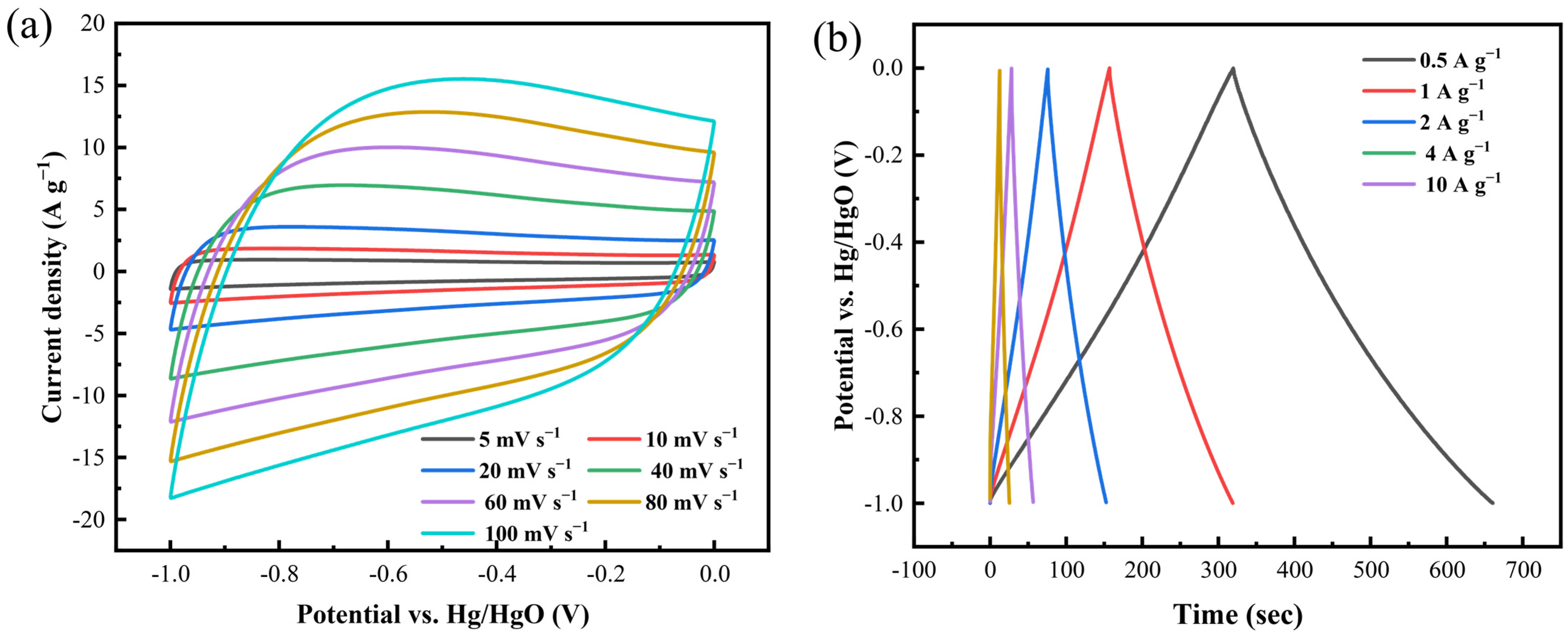
3.1. Electrochemical Properties of CaCO3 Activation
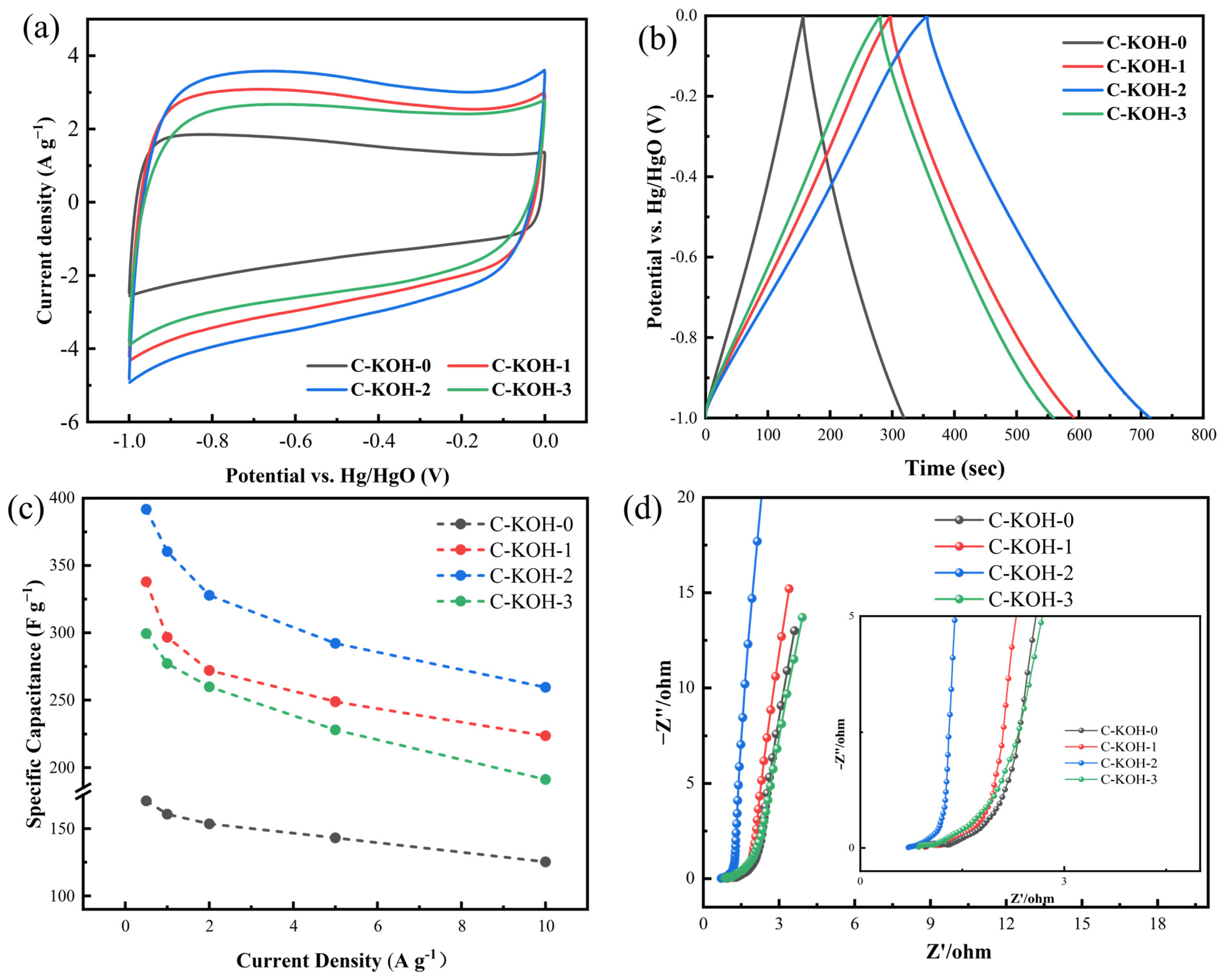
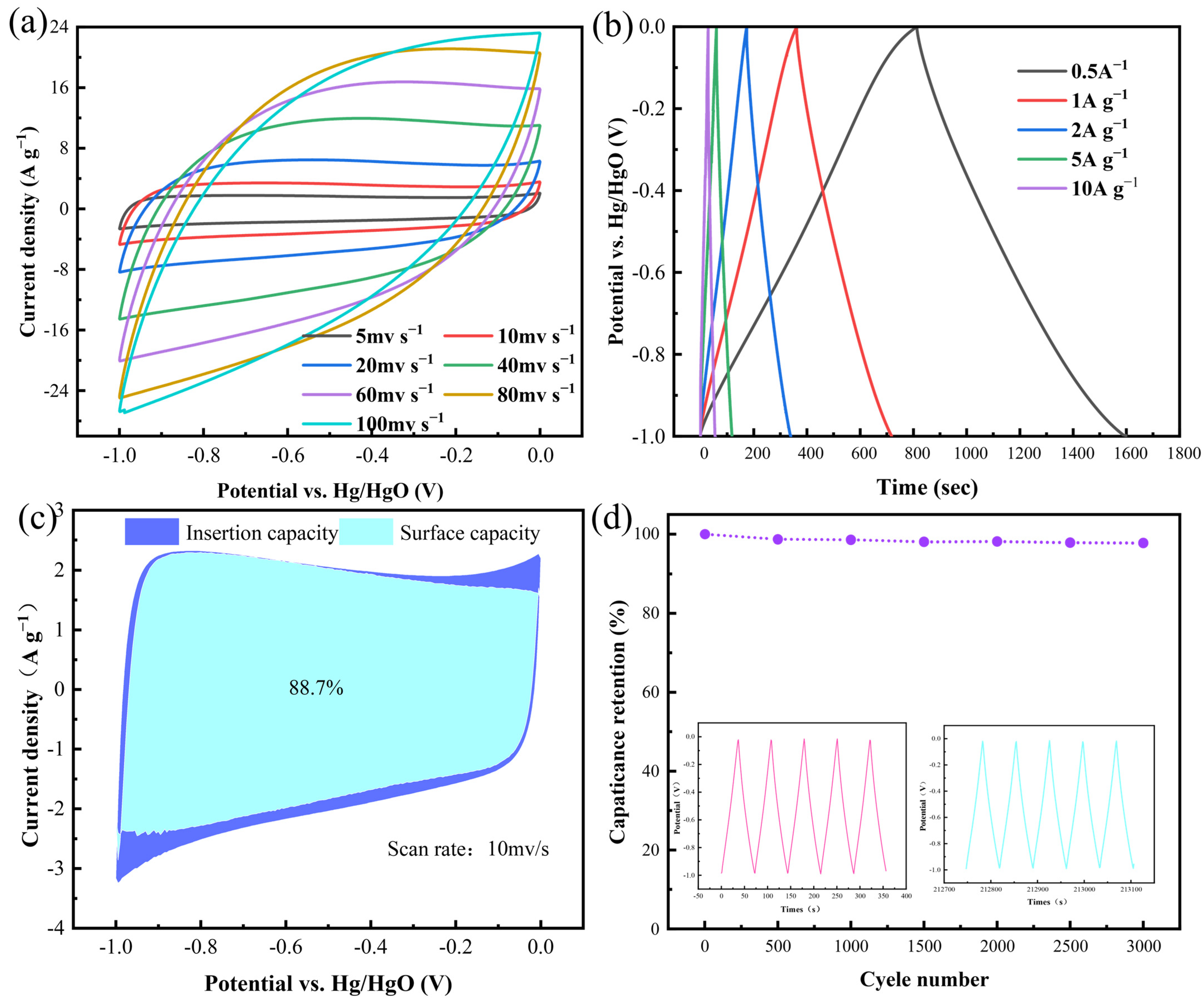
3.2. Synergistic-Activated Carbon Electrochemical Testing
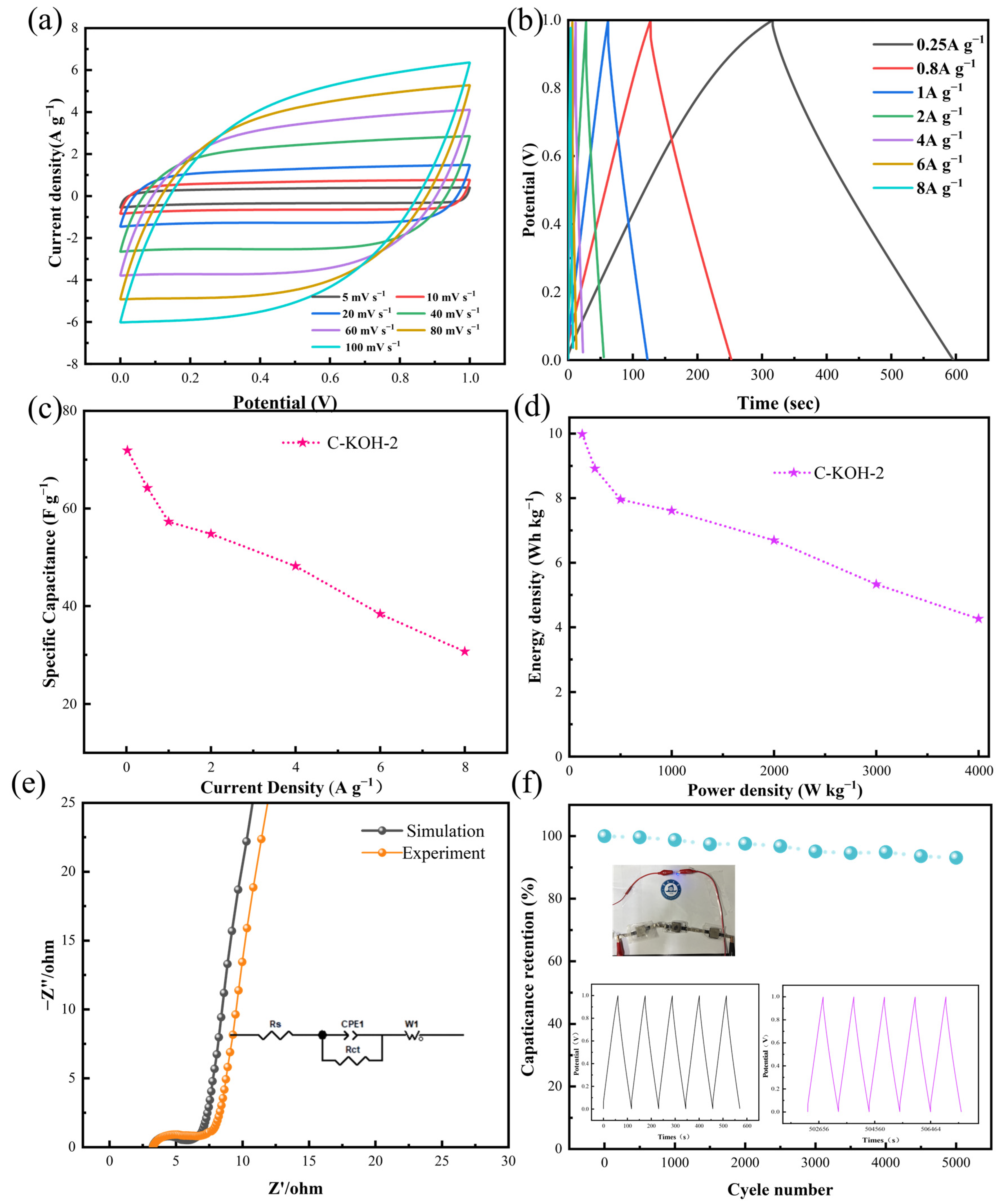
3.3. Electrochemical Performance Analysis of Symmetric Supercapacitors
4. Materials and Methods
4.1. Materials Used in This Experiment
4.2. Material Preparation
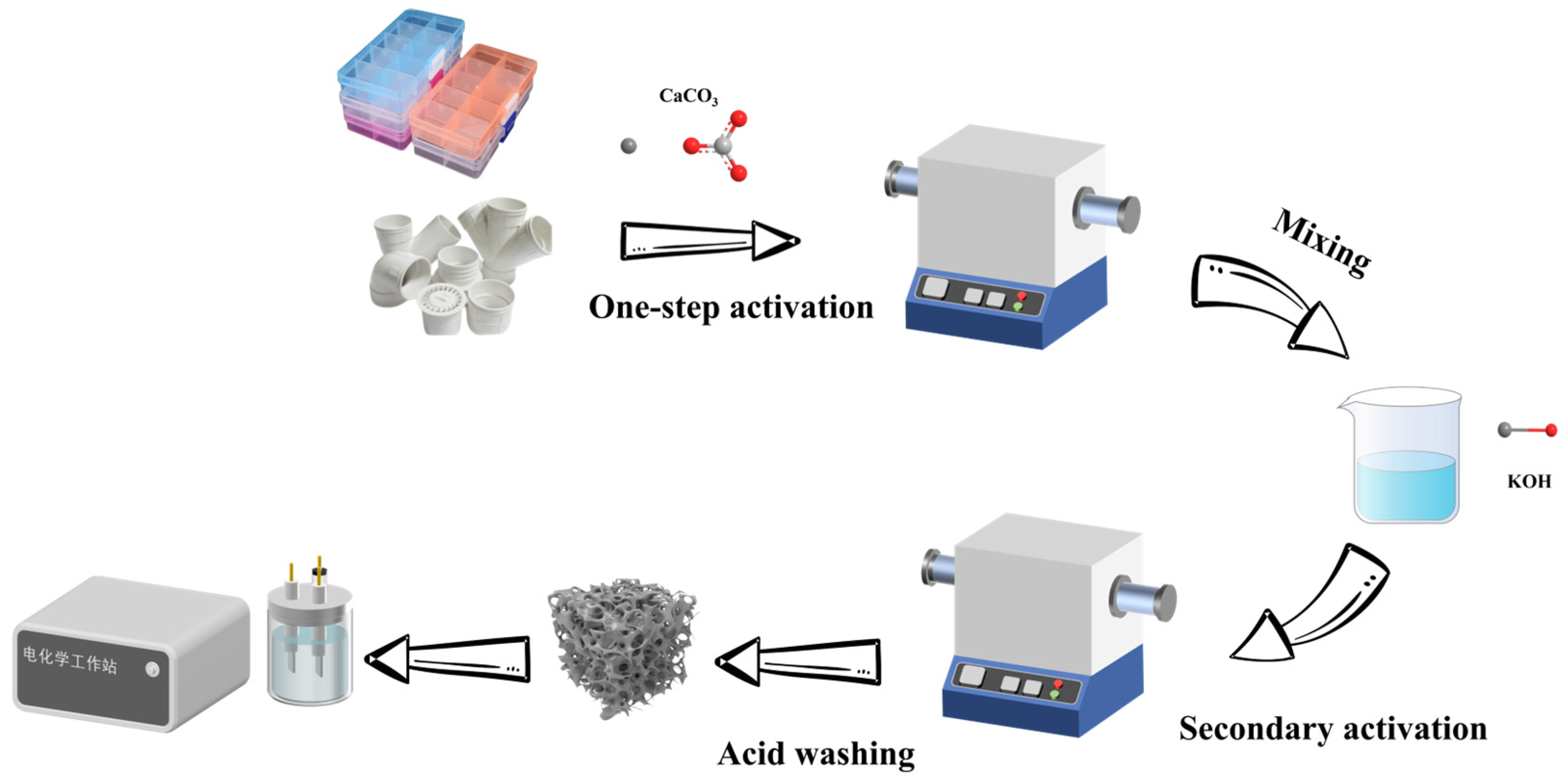
4.2.1. Preparation of Porous Carbon Materials Derived from PVC as the Carbon Source
4.2.2. Material Characterization
4.2.3. Electrochemical Performance Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Zhao, C.; Yao, S.; Li, C.; An, Y.; Zhao, S.; Sun, X.; Wang, K.; Zhang, X.; Ma, Y. Recent advances in transition metal oxides as anode materials for high-performance lithium-ion capacitors. Chem. Eng. J. 2024, 497, 154535. [Google Scholar] [CrossRef]
- Du, X.; Lin, Z.; Wang, X.; Zhang, K.; Hu, H.; Dai, S. Electrode Materials, Structural Design, and Storage Mechanisms in Hybrid Supercapacitors. Molecules 2023, 28, 6432. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2021, 21, 801–825. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Liu, Y.; Gong, X. Electrochemical Energy Storage Devices—Batteries, Supercapacitors, and Battery–Supercapacitor Hybrid Devices. ACS Appl. Electron. Mater. 2025, 7, 2233–2270. [Google Scholar] [CrossRef]
- Zhang, L.; He, Z.; Hou, J.; Gao, R.; Kong, L. Tuning pore structure of polymer-derived carbon materials for supercapacitor electrode materials using microscopic phase separation engineering. J. Energy Storage 2023, 73, 109028. [Google Scholar] [CrossRef]
- Haladkar, S.; Alegaonkar, P. Preparation and performance evaluation of Carbon-Nano-Sphere for electrode double layer capacitor. Appl. Surf. Sci. 2018, 449, 500–506. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Owens, G.; Xu, H. Porous carbon-based thermally conductive materials: Fabrication, functions and applications. Chin. J. Struct. Chem. 2023, 42, 100006. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S.; Tian, N.; Yan, F.; You, C.; Yang, Y. Carbon foams: 3D porous carbon materials holding immense potential. J. Mater. Chem. A 2020, 8, 23699–23723. [Google Scholar] [CrossRef]
- Tang, J.; Guo, Z.; Kong, X.; Wong, S.I.; Wong, N.H.; Sunarso, J.; Xing, W.; Zhou, J.; Zhao, Y.; Zhuo, S. Soybean meal-derived heteroatoms-doped porous carbons for supercapacitor electrodes. Mater. Chem. Phys. 2022, 284, 126055. [Google Scholar] [CrossRef]
- Do, T.H.; Nguyen, V.T.; Nguyen, T.N.; Ha, X.L.; Nguyen, Q.D.; Tran, T.K.N. Synthesis of Porous Carbon Nanomaterials from Vietnamese Coal: Fabrication and Energy Storage Investigations. Appl. Sci. 2024, 14, 965. [Google Scholar] [CrossRef]
- Saeed, M.; Shahzad, U.; Fazle Rabbee, M.; Manzar, R.; Al-Humaidi, J.Y.; Siddique, A.; Sheikh, T.A.; Althomali, R.H.; Qamar, T.; Rahman, M.M. Potential Development of Porous Carbon Composites Generated from the Biomass for Energy Storage Applications. Chemistry 2024, 19, e202400394. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, X.; Ni, R.; Wang, H.; Li, Y.; Lu, H.; Zheng, L. CoSe2@ carbon composite with open, superlarge voids for improved supercapacitor performance. Diam. Relat. Mater. 2024, 142, 110808. [Google Scholar] [CrossRef]
- Lin, X.; Yin, S.; Jiang, J.; Li, X. B/N/O/Zn doped porous carbon materials for supercapacitor with high performance. J. Electroanal. Chem. 2022, 918, 116498. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Guo, W.; Tian, K.; Zhang, J.; Zhang, B.; Li, X.; Wang, H. Activation-induced bowl-shaped nitrogen and oxygen dual-doped carbon material and its excellent supercapacitance. J. Mater. Sci. Technol. 2023, 160, 1–8. [Google Scholar] [CrossRef]
- Chen, T.; Wu, J.; Zhang, X.; Han, X.; Liu, S.; Yang, J. Pseudocapacitive contribution in sulfur-doped porous carbon nanosheets enables high-performance sodium-ion storage. Carbon 2024, 227, 119276. [Google Scholar] [CrossRef]
- Feng, W.; Feng, N.; Liu, W.; Cui, Y.; Chen, C.; Dong, T.; Liu, S.; Deng, W.; Wang, H.; Jin, Y. Liquid-State Templates for Constructing B, N, Co-Doping Porous Carbons with a Boosting of Potassium-Ion Storage Performance. Adv. Energy Mater. 2021, 11, 2003215. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Shi, Z.; Sui, Y.; Zhang, X.; Wu, L. Cassia seed-derived N-P double-doped porous carbon as an efficient sulfur host material for high-performance Li-S batteries. Adv. Powder Technol. 2024, 35, 104556. [Google Scholar] [CrossRef]
- He, T.; Yu, Z.; Yue, W.; Zhang, X.; Zhang, Y.; Ma, X. Two-step pyrolysis of mango branch for the preparation of B/N/O co-doped porous carbon materials. J. Anal. Appl. Pyrolysis 2024, 178, 106411. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X. A new approach to transforming PVC waste into energy via combined hydrothermal carbonization and fast pyrolysis. Energy 2017, 141, 1156–1165. [Google Scholar] [CrossRef]
- Yang, W.; Xie, Y.; Xu, S.; Wu, G.; Wang, Y. Upcycling of polyvinyl chloride to porous carbon for high-performance electromagnetic wave absorption materials. Chem. Eng. J. 2024, 496, 154054. [Google Scholar] [CrossRef]
- Li, W.; Bai, Z.; Zhang, T.; Jia, Y.; Hou, Y.; Chen, J.; Guo, Z.; Kong, L.; Bai, J.; Li, W. Comparative study on pyrolysis behaviors and chlorine release of pure PVC polymer and commercial PVC plastics. Fuel 2023, 340, 127555. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, Y.; Wang, C. Porous carbon derived from waste plastics for energy and environmental application: A review. J. Environ. Chem. Eng. 2025, 13, 115368. [Google Scholar] [CrossRef]
- Wang, K.; Guo, C.; Li, J.; Wang, K.; Cao, X.; Liang, S.; Wang, J. High value-added conversion and functional recycling of waste polyethylene terephthalate (PET) plastics: A comprehensive review. J. Environ. Chem. Eng. 2024, 12, 113539. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Hou, X.; Feng, X.; Jiang, K.; Wang, M. Structured carbon for electromagnetic shielding and microwave absorption from carbonization of waste Polymer: A review. Chem. Eng. J. 2024, 496, 154013. [Google Scholar] [CrossRef]
- Tito, E.; dos Passos, J.S.; Rombolà, A.G.; Torri, C.; Bensaid, S.; Pirone, R.; Biller, P. Sequential hydrothermal dechlorination and liquefaction of PVC. Energy Convers. Manag. 2024, 304, 118228. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Guo, X. Dechlorination of PVC at low temperature by solvothermal treatment with alkaline additives. Process Saf. Environ. Prot. 2024, 183, 945–951. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Duan, H.; Li, Y.; Xu, J.; Huang, Y.; Liu, B.; Zhang, T. Polyvinyl Chloride-Derived Carbon Spheres for CO2 Adsorption. ChemSusChem 2020, 13, 6426–6432. [Google Scholar] [CrossRef]
- Wei, S.; Qiu, Y.; Sun, X.; Wang, X.; Li, H.; Lan, G.; Liu, J.; Li, Y. Sustainable Nanoporous Carbon Catalysts Derived from Melamine Assisted Cross-Linking of Poly(vinyl chloride) Waste for Acetylene Hydrochlorination. ACS Sustain. Chem. Eng. 2022, 10, 10476–10485. [Google Scholar] [CrossRef]
- Xie, M.; Xiao, R.; Yu, Y.; Zhang, Y.; Du, C.; Wan, L.; Chen, J. Superhydrophilicity and ultrahigh-rate supercapacitor performances enabled by mesoporous carbon doped with conjugated hydroxyl. J. Energy Storage 2021, 43, 103296. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, T.; Lu, M.; Qiu, X.; Zhang, W. Sustainable lignin-derived hierarchical mesoporous carbon synthesized by a renewable nano-calcium carbonate hard template method and its utilization in zinc ion hybrid supercapacitors. Electronic supplementary information (ESI) available. Green Chem. 2024, 26, 5441–5451. [Google Scholar] [CrossRef]
- Ahi, Z.; Roshanravan, B.; Younesi, H.; Bahramifar, N. Highly Porous Carbon Materials Prepared Through KOH-Assisted CO2-Activation of Carbonized Waste Tires for Removal of Cr(VI) and Cd(II) from Water. ChemistrySelect 2024, 9, e202304215. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, S.; He, S.; Wu, C. Direct air capture of CO2 by KOH-activated bamboo biochar. J. Energy Inst. 2022, 105, 399–405. [Google Scholar] [CrossRef]
- Acosta, R.; Nabarlatz, D.; Sánchez-Sánchez, A.; Jagiello, J.; Gadonneix, P.; Celzard, A.; Fierro, V. Adsorption of Bisphenol A on KOH-activated tyre pyrolysis char. J. Environ. Chem. Eng. 2018, 6, 823–833. [Google Scholar] [CrossRef]
- Cui, X.; Jiang, Y.; He, Z.; Liu, Z.; Yang, X.; Wan, J.; Liu, Y.; Ma, F. Preparation of tank-like resin-derived porous carbon sphere for supercapacitor: The influence of KOH activator and activation temperature on structure and performance. Diam. Relat. Mater. 2023, 136, 110054. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, R.; Wu, W.; Guo, B.; Wang, P.; Ma, H. Study of the Correlation Between the Doped-Oxygen Species and the Supercapacitive Performance of TiC–CDC Carbon-Based Material. Nano 2019, 14, 1950142. [Google Scholar] [CrossRef]
- Cui, J.; Labbe, M.; Chung, H.J.; Ivey, D.G. Low-temperature tolerant poly(acrylic acid) (PAA) gel polymer electrolytes for rechargeable zinc–air batteries. J. Mater. Chem. A 2023, 11, 13971–13983. [Google Scholar] [CrossRef]
- Qian, L.; Chen, C.; Lv, Y.; Li, J.; Cao, X.; Ren, H.; Hassan, M.L. Preparation and electrochemical application of porous carbon materials derived from extraction residue of Ganoderma lucidum. Biomass Bioenergy 2022, 166, 106593. [Google Scholar] [CrossRef]
- Li, J.; Han, K.; Wang, D.; Teng, Z.; Cao, Y.; Qi, J.; Li, M.; Wang, M. Fabrication of high performance structural N-doped hierarchical porous carbon for supercapacitors. Carbon 2020, 164, 42–50. [Google Scholar] [CrossRef]
- Yan, W.; Meng, Z.; Zou, M.; Miao, H.; Ma, F.; Yu, R.; Qiu, W.; Liu, X.Y.; Lin, N. Neutralization reaction in synthesis of carbon materials for supercapacitors. Chem. Eng. J. 2020, 381, 122547. [Google Scholar] [CrossRef]
- Yue, W.; Yu, Z.; Man, Y.; Zhang, X.; Li, J.; Liu, H.; Ma, X. Synthesis of natural oxygen-doped bamboo-derived hierarchical micro-mesoporous composite carbon materials using a green activation strategy. J. Energy Storage 2024, 90, 111871. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, Y.; Huang, Y.; Li, N.; Hu, Y.; Zhao, C. Soybean root-derived N, O co-doped hierarchical porous carbon for supercapacitors. Appl. Surf. Sci. 2021, 555, 149726. [Google Scholar] [CrossRef]
- Wang, J.; Tu, J.; Lei, H.; Zhu, H. The effect of graphitization degree of carbonaceous material on the electrochemical performance for aluminum-ion batteries. RSC Adv. 2019, 9, 38990–38997. [Google Scholar] [CrossRef]
- Eftekhari, A. Surface Diffusion and Adsorption in Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 3692–3701. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Lin, H.; Vogel, F.; Li, W.; Cao, L.; Lin, Z.; Zhang, P. Low-density polyethylene-derived carbon nanotubes from express packaging bags waste as electrode material for supercapacitors. J. Ind. Eng. Chem. 2023, 119, 633–646. [Google Scholar] [CrossRef]
- Surya, K.; Michael, M.S. Novel interconnected hierarchical porous carbon electrodes derived from bio-waste of corn husk for supercapacitor applications. J. Electroanal. Chem. 2020, 878, 114674. [Google Scholar] [CrossRef]



| Samples | SBET (m2 g−1) | Vtotal (cm3 g−1) | VMicro (cm3 g−1) | VMeso+Macro (cm3 g−1) | Dap (nm) |
|---|---|---|---|---|---|
| C-KOH-1 | 1269 | 0.66 | 0.59 | 0.07 | 2.35 |
| C-KOH-2 | 1729 | 0.96 | 0.88 | 0.08 | 2.22 |
| C-KOH-3 | 1453 | 0.78 | 0.71 | 0.07 | 2.14 |
| C-KOH-0 | 326 | 0.21 | 0.12 | 0.09 | 3.67 |
| Element | C | O |
|---|---|---|
| C-KOH-1 | 92.41 | 6.35 |
| C-KOH-2 | 92.11 | 7.37 |
| C-KOH-3 | 90.89 | 8.27 |
| C-KOH-0 | 94.10 | 5.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.; Liu, L.; Zhang, P.; Lin, Z. Upcycling of Waste PVC into CaCO3/KOH-Modified Porous Carbon for Supercapacitor Applications. Molecules 2025, 30, 3420. https://doi.org/10.3390/molecules30163420
Cai W, Liu L, Zhang P, Lin Z. Upcycling of Waste PVC into CaCO3/KOH-Modified Porous Carbon for Supercapacitor Applications. Molecules. 2025; 30(16):3420. https://doi.org/10.3390/molecules30163420
Chicago/Turabian StyleCai, Wenbo, Le Liu, Peng Zhang, and Zhidan Lin. 2025. "Upcycling of Waste PVC into CaCO3/KOH-Modified Porous Carbon for Supercapacitor Applications" Molecules 30, no. 16: 3420. https://doi.org/10.3390/molecules30163420
APA StyleCai, W., Liu, L., Zhang, P., & Lin, Z. (2025). Upcycling of Waste PVC into CaCO3/KOH-Modified Porous Carbon for Supercapacitor Applications. Molecules, 30(16), 3420. https://doi.org/10.3390/molecules30163420







