The Chelating Abilities of Tertiary Amines with N-O-Donors Towards Cu(II) Ions and the Catalytic Properties of the Resulting Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. Coordination Studies
Potentiometric Studies
2.2. Catalytic Ability
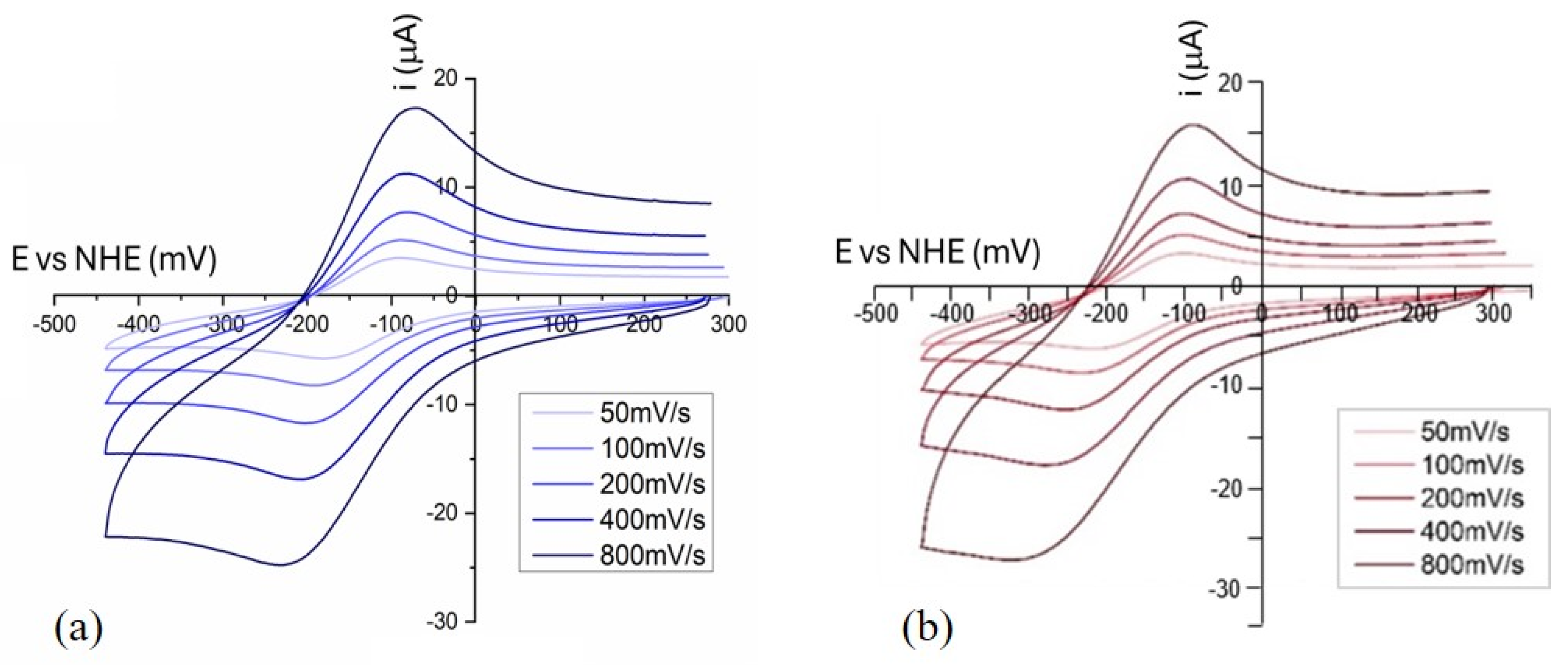
2.2.1. Cyclic Voltammetry
2.2.2. SOD Activity
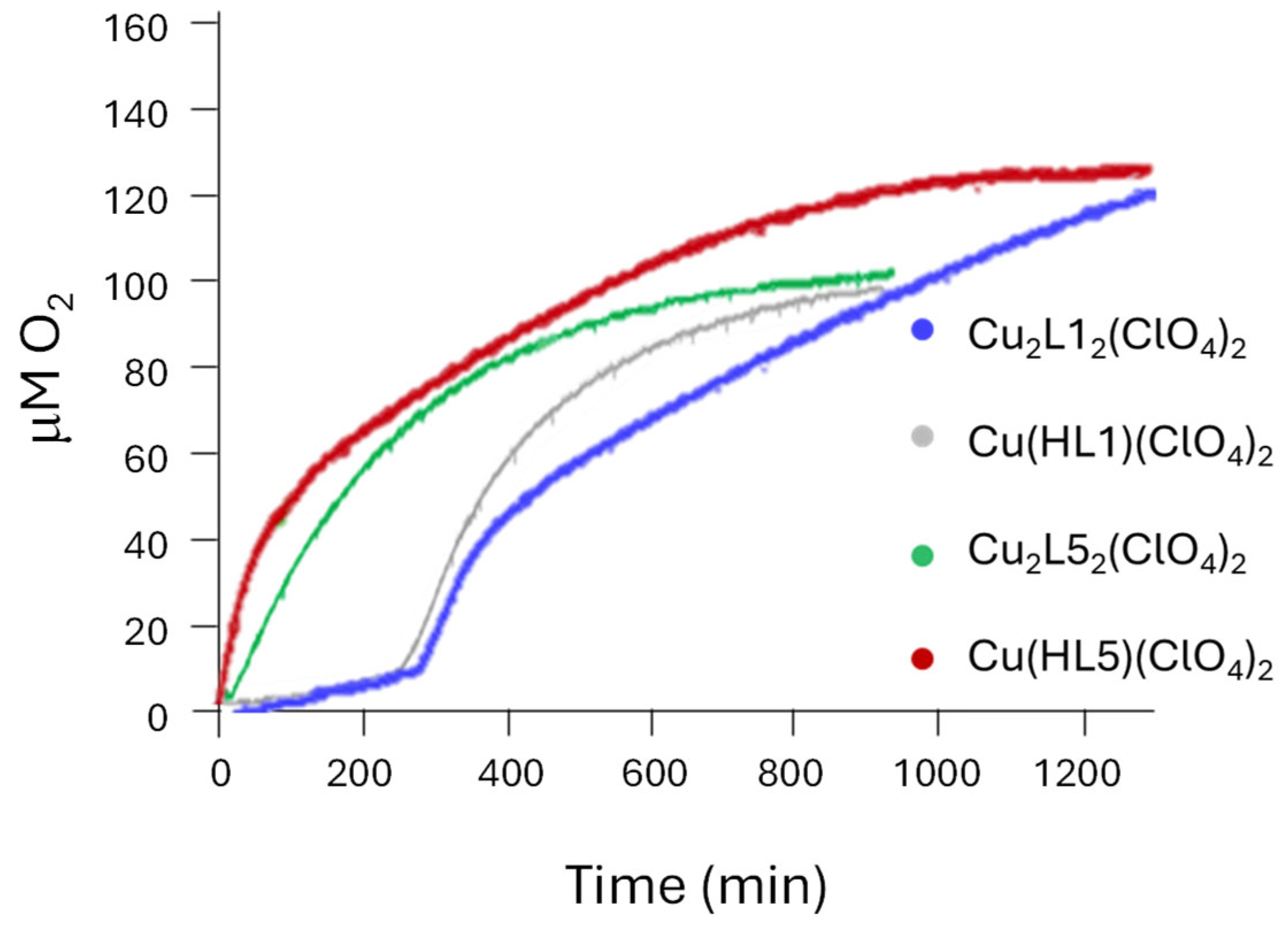
2.2.3. CAT Activity
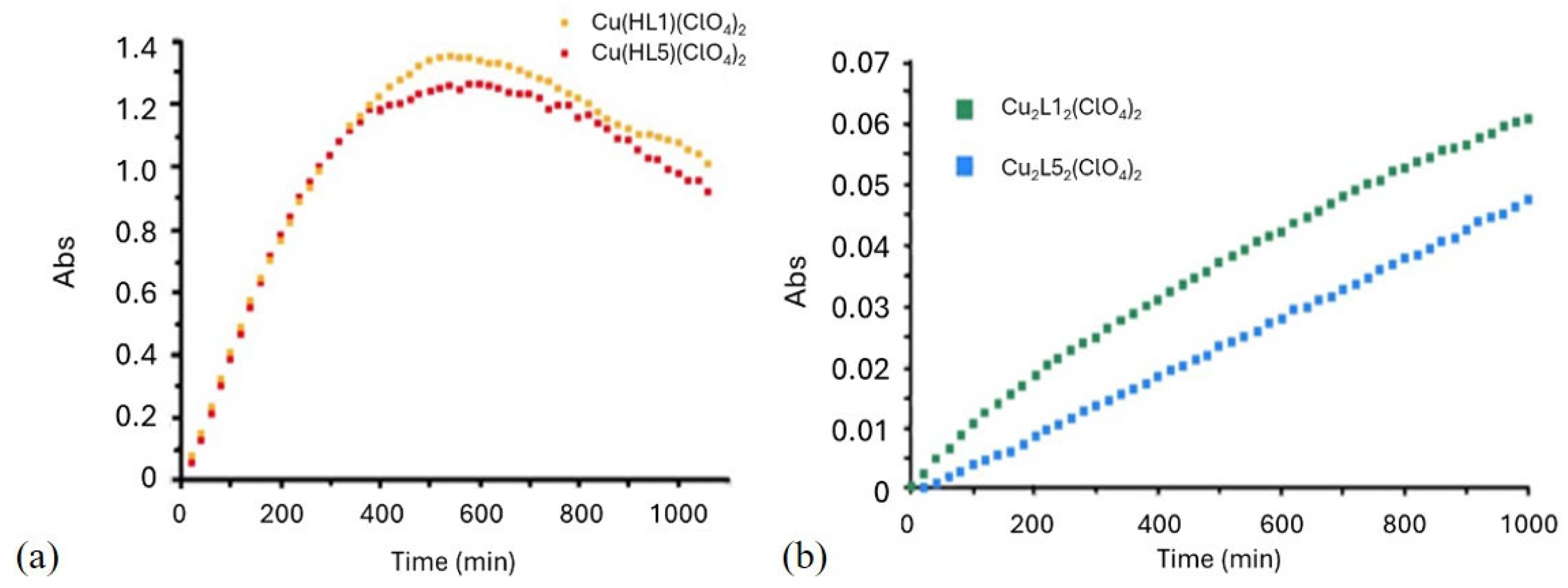
2.2.4. Peroxidase Activity
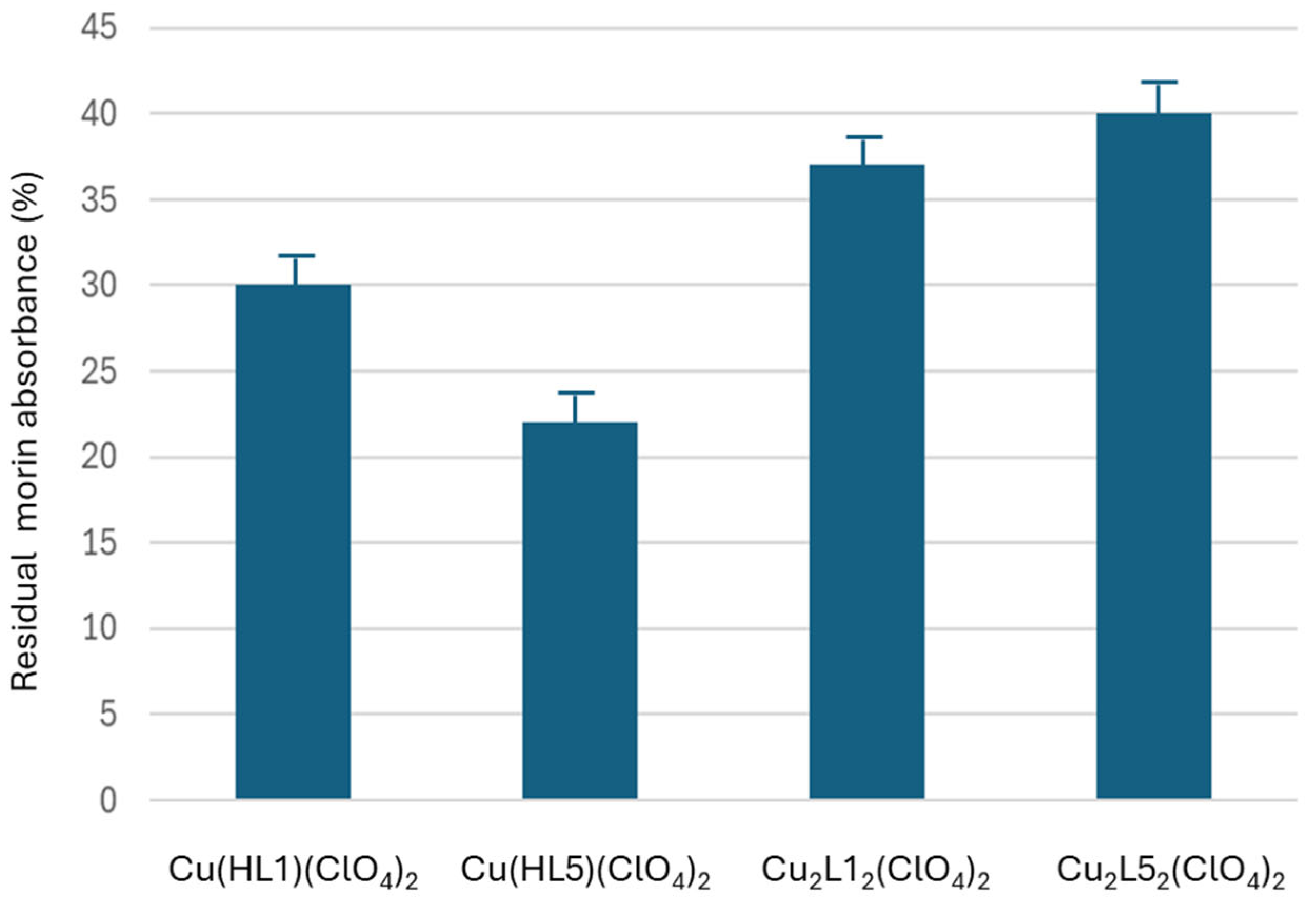
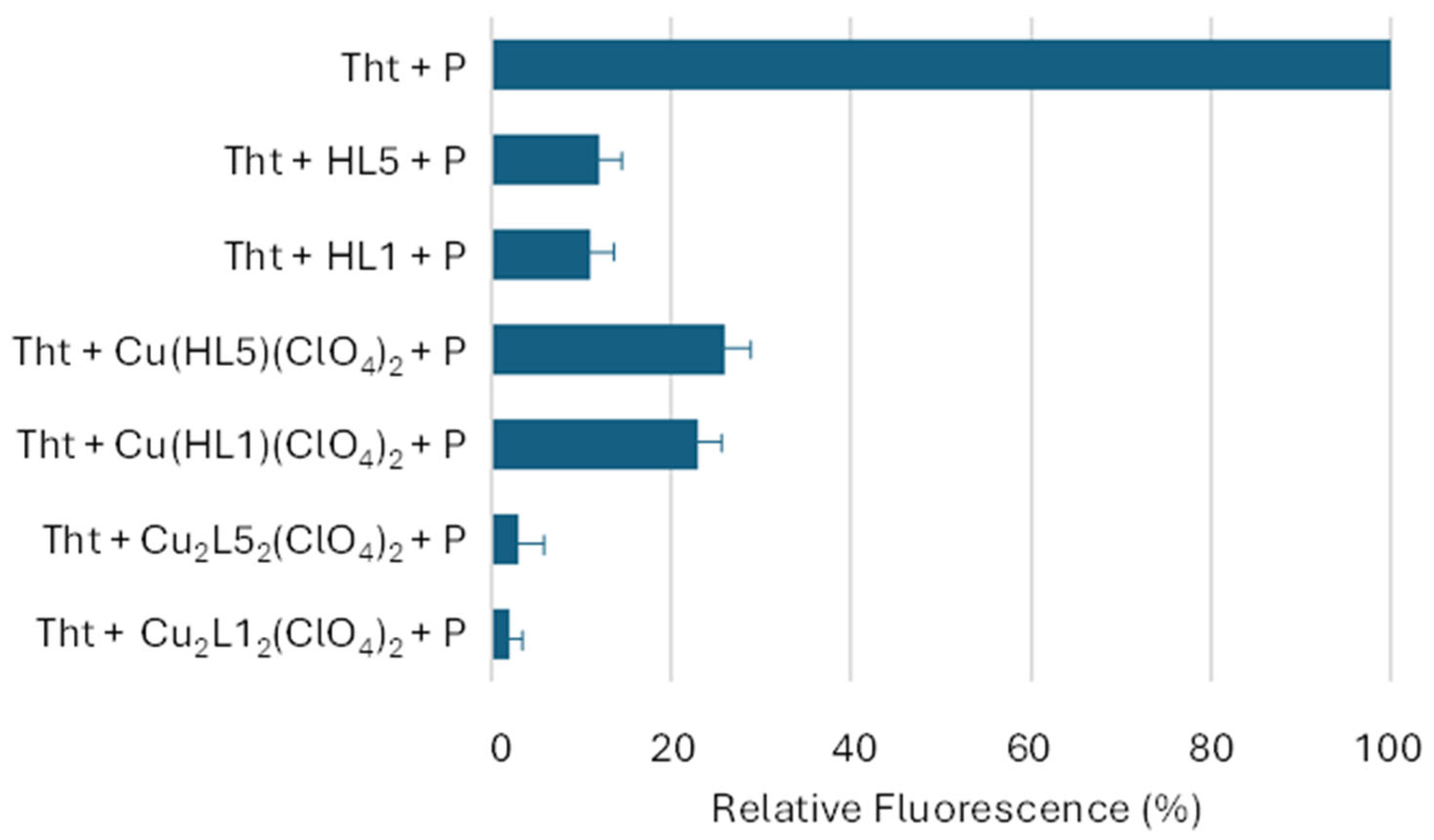
2.3. Antiaggregation Test
3. Materials and Methods
3.1. Reagents
3.2. Synthesis
3.2.1. Synthesis of the Mononuclear Complexes
3.2.2. Synthesis of the Dinuclear Complexes
3.3. Instrumentation and Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Perry, J.J.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 2010, 1804, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, O.J. Manganese complexes displaying superoxide dismutase activity: A balance between different factors. Bioorg. Chem. 2011, 39, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef] [PubMed]
- López, M.B.; Oterino, M.B.; González, J.M. The Structural Biology of Catalase Evolution. Subcell Biochem. 2024, 104, 33–47. [Google Scholar]
- Shank, M.; Barynin, V.; Dismukes, G.C. Protein coordination to manganese determines the high catalytic rate of dimanganese catalases. Comparison to functional catalase mimics. Biochemistry 1994, 33, 15433. [Google Scholar] [CrossRef]
- Pruteanu, L.L.; Bailey, D.S.; Grădinaru, A.C.; Jäntschi, L. The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants 2023, 12, 860. [Google Scholar] [CrossRef]
- Haberle, I.B.; Clair, D.S.; Vujaskovic, Z.; Salvemini, D.; Tovmasyan, A.; Spasojevic, I.; Miriyala, S. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta 2012, 1822, 794–814. [Google Scholar]
- Soll, M.; Goldshtein, H.; Rotkopf, R.; Russek-Blum, N.; Gross, Z. A Synthetic SOD/Catalase Mimic Compound for the Treatment of ALS. Antioxidants 2021, 10, 827. [Google Scholar] [CrossRef]
- Squarcina, A.; Sorarù, A.; Rigodanza, F.; Carraro, M.; Brancatelli, G.; Carofiglio, T.; Geremia, S.; Larosa, V.; Morosinotto, T.; Bonchio, M. Merged Heme and Non-Heme Manganese Cofactors for a Dual Antioxidant Surveillance in Photosynthetic Organisms. ACS Catal. 2017, 7, 1971–1976. [Google Scholar] [CrossRef]
- Vincent, A.; Thauvin, M.; Quévrain, E.; Mathieu, E.; Layani, S.; Seksik, P.; Batinic-Haberle, I.; Vriz, S.; Policar, C.; Delsuc, N. Evaluation of the Compounds Commonly Known as Superoxide Dismutase and Catalase Mimics in Cellular Models. J. Inorg. Biochem. 2021, 219, 111431. [Google Scholar] [CrossRef]
- Davies, K.M.; Mercer, J.F.B.; Chen, J.N.; Double, K.L. Copper dyshomoeostasis in Parkinson’s disease: Implications for pathogenesis and indications for novel therapeutics. Clin. Sci. 2016, 130, 565–574. [Google Scholar] [CrossRef]
- Scott, E.L.; Orvig, C. Medicinal inorganic chemistry approaches to passivation and removal of aberrant metal ions in disease. Chem. Rev. 2009, 109, 4885–4910. [Google Scholar] [CrossRef]
- Ramadan, A.M.E.J. Synthesis, characterization, and DNA-binding studies of mixed ligand copper(II) complexes with 2,2′-bipyridine and some bio-relevant ligands. Coord. Chem. 2012, 65, 1417–1433. [Google Scholar] [CrossRef]
- Menezes, L.B.; Segat, B.B.; Tolentino, H.; Pires, D.C.; Mattos, L.M.M.; Hottum, H.M.; Pereira, M.D.; Latini, A.; Horn, A., Jr.; Fernandes, C. ROS Scavenging of SOD/CAT Mimics Probed by EPR and Reduction of Lipid Peroxidation in S. cerevisiae and Mouse Liver, under Severe Hydroxyl Radical Stress Condition. J. Inorg. Biochem. 2023, 239, 112062. [Google Scholar] [CrossRef] [PubMed]
- Ceolin, J.; Siqueira, J.D.; Martins, F.M.; Piquini, P.C.; Iglesias, B.A.; Back, D.F.; de Oliveira, G.M. Oxazolidine Copper Complexes: Synthesis, Characterization and Superoxide Dismutase Activity of Copper(II) Complexes with Oxazolidine Ligands Derived from Hydroxyquinoline Carboxaldehyde. Appl. Organomet. Chem. 2018, 32, e4218. [Google Scholar] [CrossRef]
- Diószegi, R.; Bonczidai-Kelemen, D.; Bényei, A.C.; May, N.V.; Fábián, I.; Lihi, N. Copper(II) Complexes of Pyridine-2,6-dicarboxamide Ligands with High SOD Activity. Inorg. Chem. 2022, 61, 2319–2332. [Google Scholar] [CrossRef]
- Siqueira, J.D.; de Pellegrin, S.F.; dos Santos, S.S.; Iglesias, B.A.; Piquini, P.C.; Arantes, L.P.; Soares, F.A.; Chaves, O.A.; Neves, A.; Back, D.F. SOD Activity of New Copper(II) Complexes with Ligands Derived from Pyridoxal and Toxicity in Caenorhabditis elegans. J. Inorg. Biochem. 2020, 204, 110950. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Fernandes, C.; Melo, K.V.; Ferreira, S.S.; Lessa, J.A.; Franco, R.W.A.; Schenk, G.; Pereira, M.D.; Horn, A., Jr. Iron, copper, and manganese complexes with in vitro superoxide dismutase and/or catalase activities that keep Saccharomyces cerevisiae cells alive under severe oxidative stress. Free. Radic. Biol. Med. 2015, 80, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Day, B.J. Catalase and Glutathione Peroxidase Mimics. Biochem. Pharmacol. 2009, 77, 285–296. [Google Scholar] [CrossRef]
- Eckshtain, M.; Zilbermann, I.; Mahammed, A.; Saltsman, I.; Okun, Z.; Maimon, E.; Cohen, H.; Meyersteinc, D.; Gross, Z. Superoxide Dismutase Activity of Corrole Metal Complexes. Dalton Trans. 2009, 1822, 7879–7882. [Google Scholar] [CrossRef]
- Costa, R.O.; Ferreira, S.S.; Pereira, C.A.; Harmer, J.R.; Noble, C.J.; Schenk, G.; Franco, R.W.A.; Resende, J.A.L.C.; Comba, P.; Roberts, A.E. A New Mixed-Valence Mn(II)Mn(III) Compound With Catalase and Superoxide Dismutase Activities. Front. Chem. 2018, 6, 491. [Google Scholar] [CrossRef]
- Puentes-Díaz, N.; Chaparro, D.; Morales-Morales, D.; Flores-Gaspar, A.; Alí-Torres, J. Role of Metal Cations of Copper, Iron, and Aluminum and Multifunctional Ligands in Alzheimer’s Disease: Experimental and Computational Insights. ACS Omega 2023, 8, 4508–4526. [Google Scholar] [CrossRef]
- Budimir, A. Metal Ions, Alzheimer’s Disease and Chelation Therapy. Acta Pharm. 2011, 61, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Chand, K.; Chaves, S. Recent progress in repositioning Alzheimer’s disease drugs based on a multitarget strategy. Future Med. Chem. 2016, 8, 2113–2142. [Google Scholar] [CrossRef] [PubMed]
- Kenche, V.B.; Barnham, K.J. Alzheimer’s disease & metals: Therapeutic opportunities. Br. J. Pharmacol. 2011, 163, 211–219. [Google Scholar]
- Zhang, Q.; Hu, X.; Wang, W.; Yuan, Z. Study of a Bifunctional Aβ Aggregation Inhibitor with the Abilities of Antiamyloid-β and Copper Chelation. Biomacromolecules 2016, 17, 564–574. [Google Scholar] [CrossRef]
- Cendron, A.; Chianese, M.; Zarzycki, K.; Ruzza, P.; Honisch, C.; Brasuń, J.; Carraro, M. Chelating Properties of N6O-Donors Toward Cu(II) Ions: Speciation in Aqueous Environments and Catalytic Activity of the Dinuclear Complexes. Molecules 2024, 29, 5708. [Google Scholar] [CrossRef] [PubMed]
- Reddig, N.; Pursche, D.; Kloskowski, M.; Slinn, C.; Baldeau, S.M.; Rompel, A. Tuning the Catalase Activity of Dinuclear Manganese Complexes by Utilizing Different Substituted Tripodal Ligands. Eur. J. Inorg. Chem. 2004, 2004, 879–887. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Perez, L.R.; Franz, K.J. Minding metals: Tailoring multifunctional chelating agents for neurodegenerative disease. Dalton Trans. 2010, 39, 2177–2187. [Google Scholar] [CrossRef]
- Ágoston, C.G.; Miskolczy, Z.; Nagy, Z.; Sóvágó, I. The effect of ring size of fused chelates on the stability constants and spectroscopic properties of nickel(II) and palladium(II) complexes of peptides. Polyhedron 2003, 22, 2607–2615. [Google Scholar] [CrossRef]
- Hosseini-Monfared, H.; Soleymani-Babadi, S.; Sadighian, S.S.; Pazio, A.; Wozniak, K.; Siczek, M.; Mayer, P. Syntheses, structures and catalytic activities of dinuclear copper complexes with tetradentate diaminebis(phenolate) ligands. Transit. Met. Chem. 2015, 40, 255–267. [Google Scholar] [CrossRef]
- Mandal, B.; Majee, M.C.; Rakshit, T.; Banerjee, S.; Mitra, P.; Mandal, D. Synthesis, structure, DFT study and catechol oxidase activity of Cu(II) complex with sterically constrained phenol based ligand. J. Mol. Struct. 2019, 1193, 265–273. [Google Scholar] [CrossRef]
- Kafentzi, M.C.; Papadakis, R.; Gennarini, F.; Kochem, A.; Iranzo, O.; Le Mest, Y.; Le Poul, N.; Tron, T.; Faure, B.; Simaan, A.J.; et al. Electrochemical Water Oxidation and Stereoselective Oxygen Atom Transfer Mediated by a Copper Complex. Chem. Eur. J. 2018, 24, 5213–5224. [Google Scholar] [CrossRef]
- Osterbrink, J.; Dos Santos, F.; Kaifer, E.; Himmel, H.J. Redox Reactivity Control Through Electromerism. Eur. J. Inorg. Chem. 2024, 2024, e202400070. [Google Scholar] [CrossRef]
- Jitsukawa, K.; Harata, M.; Arii, H.; Sakurai, H.; Masuda, H. SOD activities of the copper complexes with tripodal polypyridylamine ligands having a hydrogen bonding site. Inorg. Chem. Acta 2001, 324, 108–116. [Google Scholar] [CrossRef]
- Signorella, S.; Bruno, M.; Frattini, G.; Palopoli, C.M.; Moreno, D.M. The relative impact of ligand flexibility and redox potential on the activity of Cu superoxide dismutase mimics. Dalton Trans. 2025, 54, 1477–9226. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide Radical: An Endogenous Toxicant. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Abuhijleh, A.L.; Khalaf, J. Copper (II) complexes of the anti-inflammatory drug naproxen and 3-pyridylmethanol as auxiliary ligand. Characterization, superoxide dismutase and catecholase-mimetic activities. Eur. J. Med. Chem. 2010, 45, 3811–3817. [Google Scholar] [CrossRef]
- Beloglazkina, K.E.; Barskaya, E.S.; Majouga, A.G.; Zyk, N.V. The first tris(imidazolylbenzothiazole) copper(II) complex. Mendeleev Commun. 2015, 25, 148–149. [Google Scholar] [CrossRef]
- Durackova, Z.; Mendiola, M.A.; Sevilla, M.T.; Valent, A. Thiohydrazone copper(II) complexes. The relationship between redox properties and superoxide dismutase mimetic activity. Bioelectrochem. Bioenerg. 1999, 48, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Saczewski, F.; Dziemidowicz-Borys, E.; Bednarski, P.J.; Grunert, R.; Gdaniec, G.; Tabin, P. Synthesis, crystal structure and biological activities of copper(II) complexes with chelating bidentate 2-sbubstituted benzimidazole ligands. J. Inorg. Biochem. 2006, 100, 1389–1398. [Google Scholar] [CrossRef]
- Pap, J.S.; Kripli, B.; Bors, I.; Bogáth, D.; Giorgi, M.; Kaizer, J.; Speier, G.J. Stability and superoxide dismutase activity of copper(II) complexes of N,N′-disubstituted ethylenediamine ligands. Inorg. Biochem. 2012, 117, 60–70. [Google Scholar] [CrossRef]
- Batianic-Haberle, I.; Reboucas, J.S.; Spasojević, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef]
- Daier, V.A.; Rivière, E.; Mallet-Ladeira, S.; Moreno, D.M.; Hureau, C.; Signorella, S.R. Synthesis, characterization and activity of imidazolate-bridged and Schiff-base dinuclear complexes as models of Cu, Zn-SOD. J. Inorg. Biochem. 2016, 163, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Squarcina, A.; Santoro, A.; Hickey, N.; De Zorzi, R.; Carraro, M.; Geremia, S.; Bonchio, M. Neutralization of Reactive Oxygen Species at Dinuclear Cu(II)-Cores: Tuning the Antioxidant Manifold in Water by Ligand Design. ACS Catal. 2020, 10, 7295–7306. [Google Scholar] [CrossRef]
- Martins, D.; English, A.M. Catalase activity is stimulated by H2O2 in rich culture medium and is required for H2O2 resistance and adaptation in yeast. Redox Biol. 2014, 2, 308–313. [Google Scholar] [CrossRef]
- Gao, J.; Martell, A.E.; Reibenspies, J.H. Synthesis and Metal Ion Uptake Studies of Silica Gel-Immobilized Schiff Base Derivatives and Catalytic Behaviors of their Cu(II) Complexes. Inorg. Chem. Acta 2003, 346, 32–42. [Google Scholar] [CrossRef]
- Coulibaly, K.; Thauvin, M.; Melenbacher, A.; Testard, C.; Trigoni, E.; Vincent, A.; Stillman, M.J.; Vriz, S.; Policar, C.; Delsuc, N. A di-Copper Peptidyl Complex Mimics the Activity of Catalase, a Key Antioxidant Metalloenzyme. Inorg. Chem. 2021, 60, 9309–9319. [Google Scholar] [CrossRef]
- Ben Hadj Hammouda, Y.; Coulibaly, K.; Bathily, A.; Teoh Sook Han, M.; Policar, C.; Delsuc, N. Improvement of Peptidyl Copper Complexes Mimicking Catalase: A Subtle Balance between Thermodynamic Stability and Resistance towards H2O2 Degradation. Molecules 2022, 27, 5476. [Google Scholar] [CrossRef] [PubMed]
- Hureau, C.; Faller, P. Aβ-mediated ROS production by Cu ions: Structural insights, mechanisms and relevance to Alzheimer’s disease. Biochimie 2009, 91, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Cassagnes, L.E.; Hervé, V.; Nepveu, F.; Hureau, C.; Faller, P.; Collin, F. The Catalytically Active Copper-Amyloid-Beta State: Coordination Site Responsible for Reactive Oxygen Species Production. Angew. Chem. Int. Ed. 2013, 52, 11110–11113. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; La Penna, G. Metal ions and intrinsically disordered proteins and peptides: From Cu/Zn amyloid-β to general principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef]
- Vicario, J.; Eelkema, R.; Browne, W.R.; Meetsma, A.; La Crois, R.M.; Feringa, B.L. Catalytic molecular motors: Fuelling autonomous movement by a surface bound synthetic manganese catalase. Chem. Commun. 2005, 31, 3936–3938. [Google Scholar] [CrossRef]
- Kani, Y.; Ohba, S.; Ito, S.; Nishida, Y. Redetermination of bis{[(2-hydroxyphenylmethyl)bis(2-pyridylmethyl)aminato]copper(II)} diperchlorate. Acta Crystallogr. Sect. C 2000, 56, e201. [Google Scholar]
- Irving, H.M.; Miles, M.G.; Pettit, L.D. A Study of Some Problems in Determining the Stoicheiometric Proton Dissociation Constants of Complexes by Potentiometric Titrations Using a Glass Electrode. Anal. Chim. Acta 1967, 38, 475–488. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. SUPERQUAD: An Improved General Program for Computation of Formation Constants from Potentiometric Data. J. Chem. Soc. Dalton Trans. 1985, 6, 1195–1200. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of Equilibria in Solution. Determination of Equilibrium Constants with the HYPERQUAD Suite of Programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Available online: http://avogadro.cc/ (accessed on 25 July 2025).
- Sheldrick, G.M. Crystal structure solution with ShelXT. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Müller, P. Practical suggestions for better crystal structures. Crystallogr. Rev. 2009, 15, 57–83. [Google Scholar] [CrossRef]
- Müller, P.; Herbst-Irmer, R.; Spek, A.L.; Schneider, T.R.; Sawaya, M.R. Crystal Structure Refinement: A Crystallographer’s Guide to SHELXL; Müller, P., Ed.; IUCR Texts on Crystallography; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Hamman, J.N.; Rolff, M.; Tuczek, F. Monooxygenation of an appended phenol in a model system of tyrosinase: Implications on the enzymatic reaction mechanism. Dalton Trans. 2015, 44, 3251–3258. [Google Scholar] [CrossRef]
- Connor, G.P.; Mayer, K.J.; Tribble, C.S.; McNamara, W.R. Hydrogen evolution catalyzed by an iron polypyridyl complex in aqueous solutions. Inorg. Chem. 2014, 53, 5408–5410. [Google Scholar] [CrossRef] [PubMed]
- Wendt, F.; Rolff, M.; Thimm, W.; Nather, C.; Tuczek, F. A Small-molecule Model System of Galactose Oxidase: Geometry, Reactivity, and Electronic Structure. Z. Anorg. Allg. Chem. 2013, 639, 2502–2509. [Google Scholar] [CrossRef]


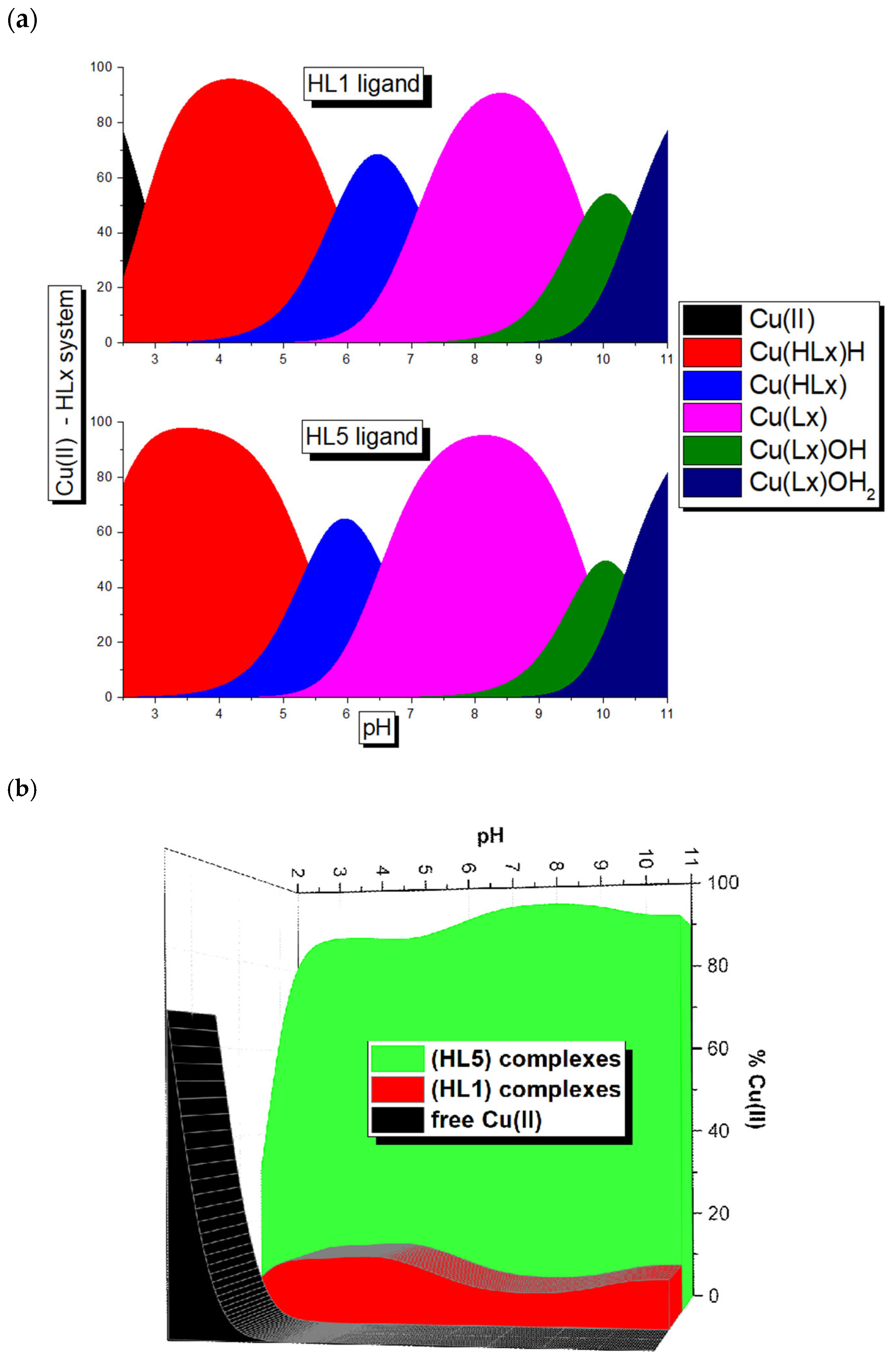
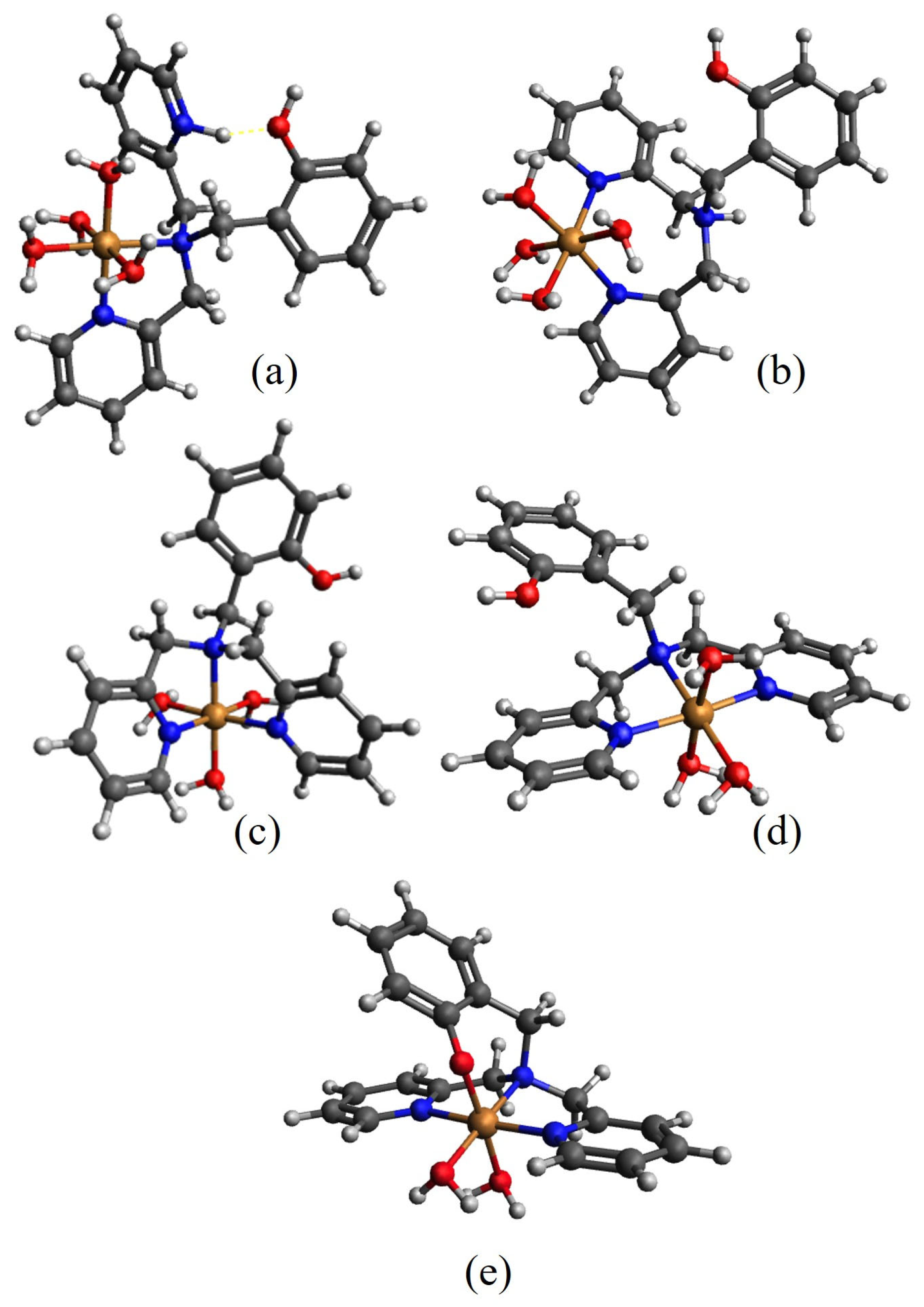
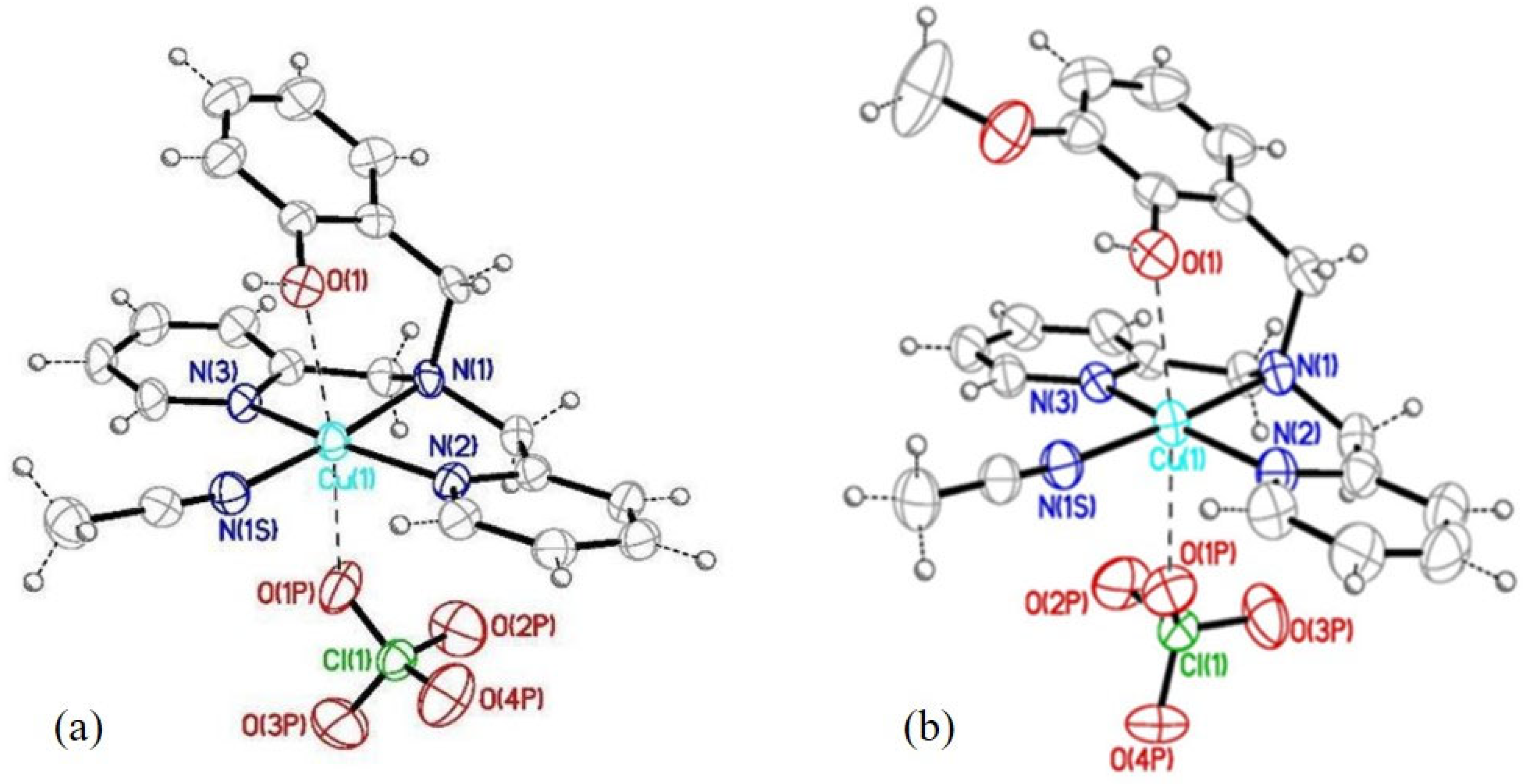

| Equilibrium Reaction | logβi HL1 | logβi HL5 | Equilibrium Reaction | logKi HL1 | logKi HL5 |
|---|---|---|---|---|---|
Lx− + H+  HLx HLx | 9.99 ± 0.01 | 9.52 ± 0.01 | Lx− + H+  HLx HLx | 9.99 | 9.52 |
Lx− + 2H+  [(HLx)H]+ [(HLx)H]+ | 16.29 ± 0.02 | 15.77 ± 0.02 | HLx + H+  [(HLx)H]+ [(HLx)H]+ | 6.30 | 6.25 |
Lx− + 3H+  [(HLx)H2]2+ [(HLx)H2]2+ | 21.53 ± 0.02 | 20.71 ± 0.02 | [(HLx)H]+ +H+  [(HLx)H2]2+ [(HLx)H2]2+ | 5.24 | 4.94 |
Lx− + 4H+  [(HLx)H3]3+ [(HLx)H3]3+ | 25.24 ± 0.02 | 24.43 ± 0.02 | [(HLx)H2]2+ +H+  [(HLx)H3]3+ [(HLx)H3]3+ | 3.71 | 3.72 |
| Species | logβi x = 1 | logβi x = 5 | pKi x = 1 | pKi x = 5 |
|---|---|---|---|---|
| [Cu(HLx)H]3+ | 22.67 ± 0.06 | 23.43 ± 0.08 | ||
| [Cu(HLx)]2+ | 16.84 ± 0.08 | 18.04 ± 0.10 | 5.83 | 5.39 |
| [Cu(Lx)]+ | 9.73 ± 0.08 | 11.51 ± 0.10 | 7.11 | 6.53 |
| [Cu(Lx)(OH)] | 0.03 ± 0.09 | 1.77 ± 0.11 | 9.70 | 9.74 |
| [Cu(Lx)(OH)2]− | −10.42 ± 0.09 | −8.56 ± 0.11 | 10.45 | 10.33 |
| Complexes | Epc (V) | Epa (V) | E1/2 (V) | ΔE (V) | │ia/ic│ |
|---|---|---|---|---|---|
| Cu(HL1)(ClO4)2 | −0.177 | −0.092 | −0.135 | −0.085 | 0.61 |
| Cu + HL1 (in situ) | −0.223 | −0.143 | −0.183 | −0.080 | 1.35 |
| Cu(HL5)(ClO4)2 | −0.202 | −0.096 | −0.149 | −0.106 | 0.52 |
| Cu + HL5 (in situ) | −0.216 | −0.134 | −0.175 | −0.082 | 1.44 |
| Cu2L12(ClO4)2 | −0.229 | −0.150 | −0.190 | −0.079 | 1.04 |
| Cu2L52(ClO4)2 | −0.219 | −0.138 | −0.179 | −0.081 | 0.90 |
| Complexes | IC50 (μM) | Log (kcat) | Ref. |
|---|---|---|---|
| Native Cu-ZnSOD | 0.04 a | 9.30 | [40,41] |
| CuSO4 | 72.2 b | ~6 | [42] |
| Cu(HL1)(ClO4)2 | 1.41 b | 6.27 h | This work |
| Cu2(L1)2(ClO4)2 | 1.99 b | 6.13 h | This work |
| Cu(HL5)(ClO4)2 | 1.04 b | 6.41 h | This work |
| Cu2(L5)2(ClO4)2 | 1.28 b | 6.31 h | This work |
| Cu(dib)Cl2 c | 0.09 a | - | [43] |
| [Cu(BPMPA)]+ d | 1.04 b | 7.09 | [44] |
| Cu(TAAP)(NO3)2 e | 0.55 a | 7.26 | [8] |
| Cu2(tppen)Cl4 f | 0.54 b | - | [45] |
| [Cu2(L-RNH2)(H2O)2](ClO4)3 | 0.66 | 6.59 | [28] |
| [CuZn(salpn)Cl2 | 3.2 a | - | [46] |
| [Cu(py2bn)]2+ | 0.078 a | - | [38] |
| Cu2L32 g | 0.072 b | 7.55 | [47] |
| Complexes | Induction Time (min) | Rmax (μM/min) | Time (min) | O2 (mmol/L) | Ref. |
|---|---|---|---|---|---|
| Cu(HL1)(ClO4)2 (a) | 250 | 0.25 | 950 | 7.6 | This work (e) |
| Cu(HL5)(ClO4)2 (a) | - | 0.80 | 1300 | 9.8 | This work (e) |
| Cu2(L1)2(ClO4)2 (a) | 200 | 0.20 | 1300 | 9.2 | This work (e) |
| Cu2(L5)2(ClO4)2 (a) | - | 0.36 | 950 | 7.7 | This work (e) |
| Cu2L12 (b) | 10 | 52 | 240 | 5.0 | [47] |
| Cu2L22 (b) | - | 264 | 240 | 5.4 | [47] |
| Cu2L32 (b) | - | 258 | 240 | 5.4 | [47] |
| [Cu2(L-CH3)](ClO4)3 (c) | - | 45 | 180 | 6.5 | [28] |
| [Cu2(L-RNH2)](ClO4)3 (c) | - | 55 | 180 | 6.9 | [28] |
| [Cu2(L-RCOOH)](ClO4)3 (c) | 30 | 44 | 180 | 7.9 | [28] |
| (CATm1)Cu2 (d) | - | 273 | 3 | 0.3 | [50,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zonzin, M.; Chianese, M.; Squarcina, A.; Dereje, D.M.; Campofelice, A.; Da Fermo, A.; Belluti, F.; Marino, N.; Dębicki, F.; Kotynia, A.; et al. The Chelating Abilities of Tertiary Amines with N-O-Donors Towards Cu(II) Ions and the Catalytic Properties of the Resulting Complexes. Molecules 2025, 30, 3419. https://doi.org/10.3390/molecules30163419
Zonzin M, Chianese M, Squarcina A, Dereje DM, Campofelice A, Da Fermo A, Belluti F, Marino N, Dębicki F, Kotynia A, et al. The Chelating Abilities of Tertiary Amines with N-O-Donors Towards Cu(II) Ions and the Catalytic Properties of the Resulting Complexes. Molecules. 2025; 30(16):3419. https://doi.org/10.3390/molecules30163419
Chicago/Turabian StyleZonzin, Martina, Martina Chianese, Andrea Squarcina, Degnet Melese Dereje, Ambra Campofelice, Alessia Da Fermo, Federica Belluti, Nadia Marino, Filip Dębicki, Aleksandra Kotynia, and et al. 2025. "The Chelating Abilities of Tertiary Amines with N-O-Donors Towards Cu(II) Ions and the Catalytic Properties of the Resulting Complexes" Molecules 30, no. 16: 3419. https://doi.org/10.3390/molecules30163419
APA StyleZonzin, M., Chianese, M., Squarcina, A., Dereje, D. M., Campofelice, A., Da Fermo, A., Belluti, F., Marino, N., Dębicki, F., Kotynia, A., Marciniak, A., Brasuń, J., & Carraro, M. (2025). The Chelating Abilities of Tertiary Amines with N-O-Donors Towards Cu(II) Ions and the Catalytic Properties of the Resulting Complexes. Molecules, 30(16), 3419. https://doi.org/10.3390/molecules30163419








