Standardization of Germinated Oat Extracts and Their Neuroprotective Effects Against Aβ1-42 Induced Cytotoxicity in SH-SY5Y Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation for Germinated Oat Extracts (GOEs)
2.3. Qualitative and Quantitative Analysis of Avenanthramides (AVNs) in Daeyang Germinated Oat Extracts (GOEs) by HPLC-UV
Analytical Method Validation
2.4. Identification of Phenolic Acids in Daeyang Germinated Oat Extracts (GOEs)
2.5. Human Neuroblastoma Cell (SH-SY5Y) Culture
2.6. Preparation and Aggregation of Aβ1-42 Peptide
2.7. Cell Viability Using an MTT Assay
2.8. Measurement of Reactive Oxygen Species (ROS) Induced by Aβ1-42
2.9. Sample Preparation for Reverse Transcription Polymerase Chain Reaction
2.10. RNA Isolation and Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
2.11. Statistical Analysis
3. Results and Discussion
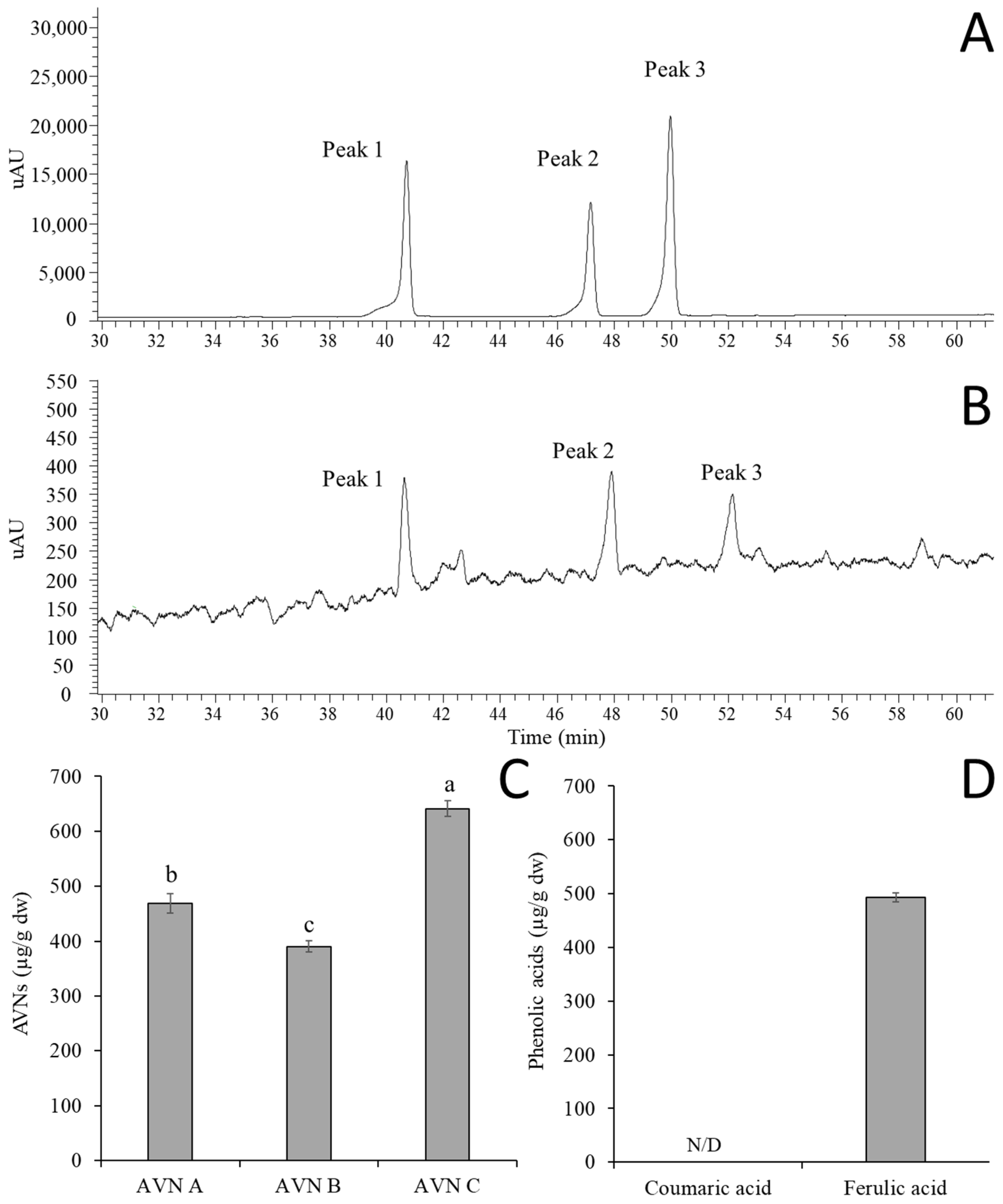
3.1. Bioanalytical Methods Validation of AVN A, B, and C by HPLC-UV
3.2. Standardization of GOEs by Quantifying AVN and Phenolic Acids
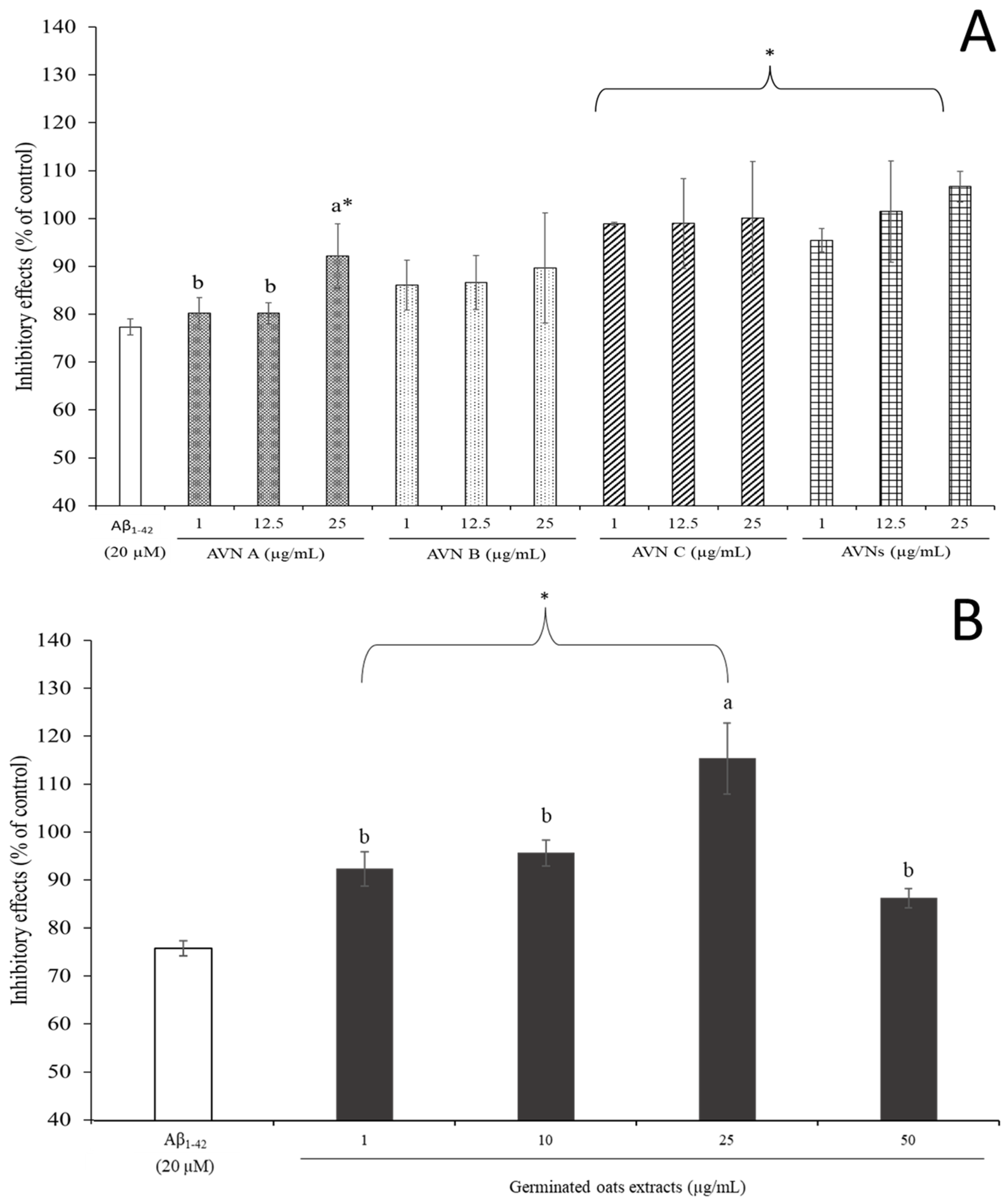
3.3. Effects of AVNs and GOEs on Aβ1-42-Induced Cytotoxicity of SH-SY5Y Cells
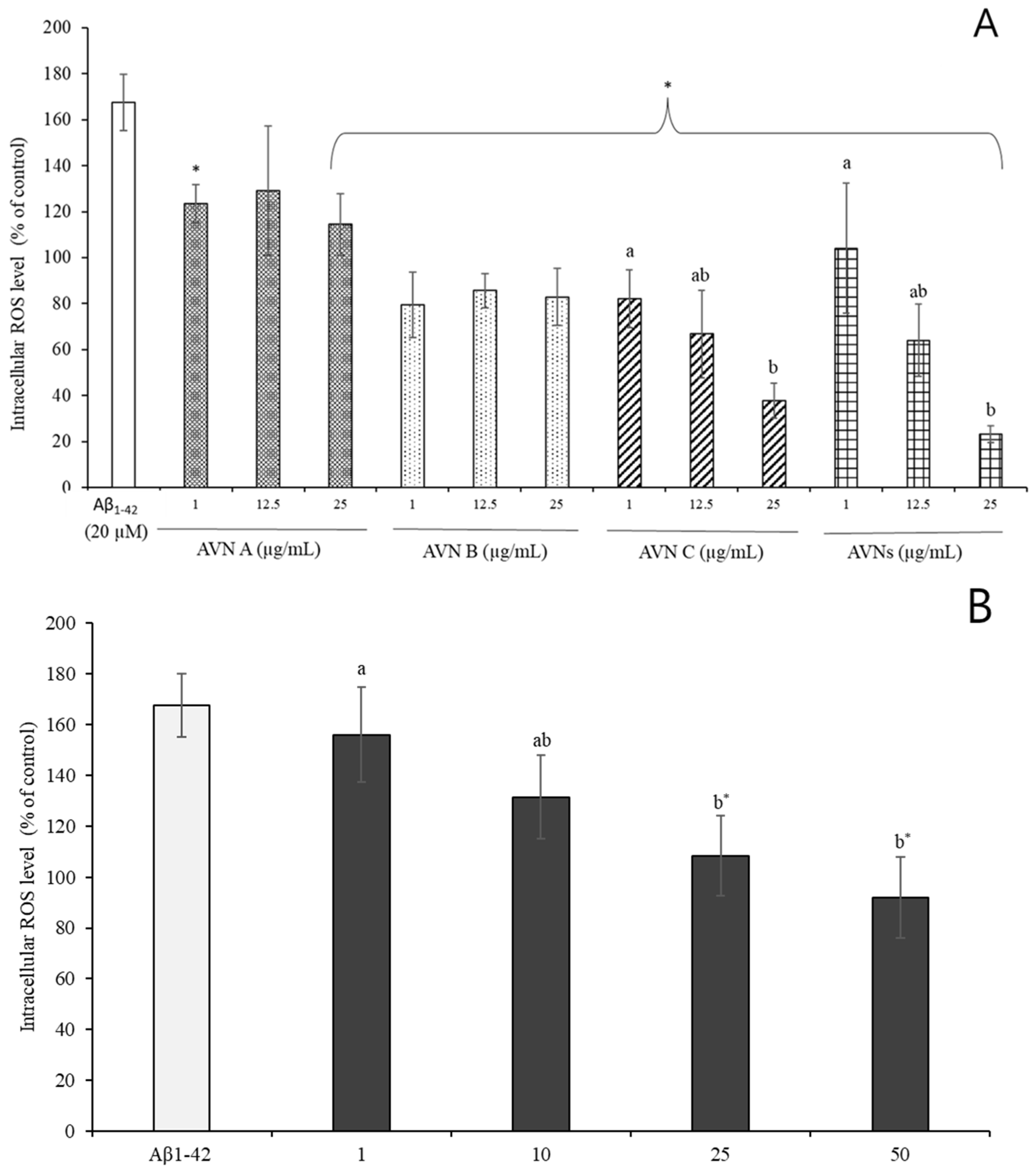
3.4. Effects of SH-SY5Y Cell by AVNs and GOEs from Aβ1-42-Induced Oxidative Stress
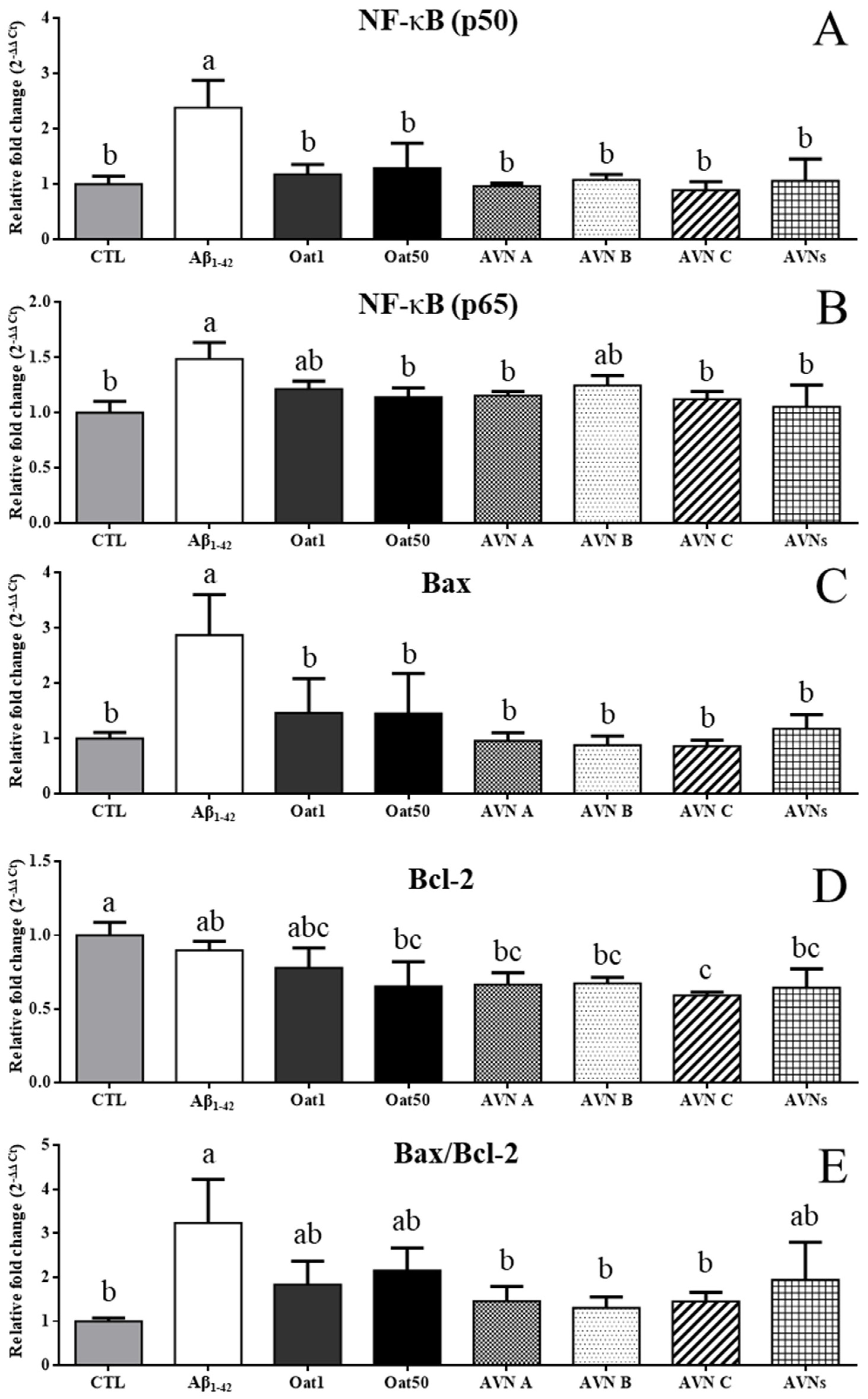
3.5. Effects of AVNs and GOEs Against Aβ1-42-Induced Inflammation and Apoptosis of SH-SY5Y Cell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uwishema, O.; Mahmoud, A.; Sun, J.; Correia, I.F.S.; Bejjani, N.; Alwan, M.; Nicholas, A.; Oluyemisi, A.; Dost, B. Is Alzheimer’s disease an infectious neurological disease? A review of the literature. Brain Behav. 2022, 12, e2728. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, L.J.; Zhang, L.J. Neuroinflammation, Gut Microbiome, and Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8243–8250. [Google Scholar] [CrossRef]
- Peng, Y.; Tao, H.X.; Wang, S.P.; Xiao, J.B.; Wang, Y.T.; Su, H.X. Dietary intervention with edible medicinal plants and derived products for prevention of Alzheimer’s disease: A compendium of time-tested strategy. J. Funct. Foods 2021, 81, 104463. [Google Scholar] [CrossRef]
- Garbuz, D.; Zatsepina, O.; Evgen’ev, M. Beta amyloid, tau protein, and neuroinflammation: An attempt to integrate different hypotheses of Alzheimer’s disease pathogenesis. Mol. Biol. 2021, 55, 670–682. [Google Scholar] [CrossRef]
- Cosentino, S.A.; Stern, Y.; Sokolov, E.; Scarmeas, N.; Manly, J.J.; Tang, M.X.; Schupf, N.; Mayeux, R.P. Plasma ss-amyloid and cognitive decline. Arch. Neurol. 2010, 67, 1485–1490. [Google Scholar] [CrossRef]
- Nedaei, H.; Rezaei-Ghaleh, N.; Giller, K.; Becker, S.; Karami, L.; Moosavi-Movahedi, A.A.; Griesinger, C.; Saboury, A.A. The calcium-free form of atorvastatin inhibits amyloid-beta(1–42) aggregation in vitro. J. Biol. Chem. 2022, 298, 101662. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, S.B.; Nam, S.Y.; Oh, K.W.; Hong, J.T. Inflammation and Alzheimer’s disease. Arch. Pharm. Res. 2010, 33, 1539–1556. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Gao, Y.; Sun, J.-Y.; Meng, X.-L.; Yang, D.; Fan, L.-H.; Xiang, L.; Wang, P. Traditional Chinese medicine: Role in reducing β-amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction of Alzheimer’s disease. Front. Pharmacol. 2020, 11, 497. [Google Scholar] [CrossRef]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and Alzheimer’s disease: The role of antioxidants in prevention and treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Suematsu, N.; Hosoda, M.; Fujimori, K. Protective effects of quercetin against hydrogen peroxide-induced apoptosis in human neuronal SH-SY5Y cells. Neurosci. Lett. 2011, 504, 223–227. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Lee, S.; Cho, E.J. Quercetin and quercetin-3-β-d-glucoside improve cognitive and memory function in Alzheimer’s disease mouse. Appl. Biol. Chem. 2016, 59, 721–728. [Google Scholar] [CrossRef]
- Chen, C.Y.; Milbury, P.E.; Collins, F.W.; Blumberg, J.B. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J. Nutr. 2007, 137, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Biel, W.; Bobko, K.; Maciorowski, R. Chemical composition and nutritive value of husked and naked oats grain. J. Cereal. Sci. 2009, 49, 413–418. [Google Scholar] [CrossRef]
- Hitayezu, R.; Baakdah, M.M.; Kinnin, J.; Henderson, K.; Tsopmo, A. Antioxidant activity, avenanthramide and phenolic acid contents of oat milling fractions. J. Cereal. Sci. 2015, 63, 35–40. [Google Scholar] [CrossRef]
- Boz, H. Phenolic Amides (Avenanthramides) in Oats—A review. Czech. J. Food Sci. 2015, 33, 399–404. [Google Scholar] [CrossRef]
- Matsukawa, T.; Isobe, T.; Ishihara, A.; Iwamura, H. Occurrence of avenanthramides and hydroxycinnamoyl-CoA:hydroxyanthranilate N-hydroxycinnamoyltransferase activity in oat seeds. Z. Naturforsch. C J. Biosci. 2000, 55, 30–36. [Google Scholar] [CrossRef]
- Meydani, M. Potential health benefits of avenanthramides of oats. Nutr. Rev. 2009, 67, 731–735. [Google Scholar] [CrossRef]
- Pridal, A.A.; Bottger, W.; Ross, A.B. Analysis of avenanthramides in oat products and estimation of avenanthramide intake in humans. Food Chem. 2018, 253, 93–100. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Mangos and their bioactive components: Adding variety to the fruit plate for health. Food Funct. 2017, 8, 3010–3032. [Google Scholar] [CrossRef]
- Xu, F.; Mu, J.; Teng, Y.; Zhang, X.; Sundaram, K.; Sriwastva, M.K.; Kumar, A.; Lei, C.; Zhang, L.; Liu, Q.M. Restoring Oat Nanoparticles Mediated Brain Memory Function of Mice Fed Alcohol by Sorting Inflammatory Dectin-1 Complex Into Microglial Exosomes. Small 2022, 18, 2105385. [Google Scholar] [CrossRef]
- Kennedy, D.O. Polyphenols and the human brain: Plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv. Nutr. 2014, 5, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Peng, S.; Song, Z.; Bai, F.; Li, X.; Fang, J. Oat polyphenol avenanthramide-2c confers protection from oxidative stress by regulating the Nrf2-ARE signaling pathway in PC12 cells. Arch. Biochem. Biophys. 2021, 706, 108857. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Lee, H.J.; Yang, J.Y.; Shin, H.L.; Choi, S.W.; Kim, J.K.; Seo, W.D.; Kim, E.H. The Potential Neuroprotective Effects of Extracts from Oat Seedlings against Alzheimer’s Disease. Nutrients 2022, 14, 4103. [Google Scholar] [CrossRef]
- Skoglund, M.; Peterson, D.M.; Andersson, R.; Nilsson, J.; Dimberg, L.H. Avenanthramide content and related enzyme activities in oats as affected by steeping and germination. J. Cereal. Sci. 2008, 48, 294–303. [Google Scholar] [CrossRef]
- Kaukovirta-Norja, A.; Wilhelmson, A.; Poutanen, K. Germination: A means to improve the functionality of oat. Agric. Food Sci. 2004, 13, 100–112. [Google Scholar] [CrossRef]
- Tian, B.; Xie, B.; Shi, J.; Wu, J.; Cai, Y.; Xu, T.; Xue, S.; Deng, Q. Physicochemical changes of oat seeds during germination. Food Chem. 2010, 119, 1195–1200. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Milbury, P.E.; Kwak, H.-K.; Collins, F.W.; Samuel, P.; Blumberg, J.B. Avenanthramides and phenolic acids from oats are bioavailable and act synergistically with vitamin C to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2004, 134, 1459–1466. [Google Scholar] [CrossRef]
- Lee, A.Y.; Choi, J.M.; Lee, M.H.; Lee, J.; Lee, S.; Cho, E.J. Protective effects of perilla oil and alpha linolenic acid on SH-SY5Y neuronal cell death induced by hydrogen peroxide. Nutr. Res. Pract. 2018, 12, 93–100. [Google Scholar] [CrossRef]
- Woolman, M.; Liu, K. Simplified Analysis and Expanded Profiles of Avenanthramides in Oat Grains. Foods 2022, 11, 560. [Google Scholar] [CrossRef]
- Sereia, A.L.; de Oliveira, M.T.; Baranoski, A.; Marques, L.L.M.; Ribeiro, F.M.; Isolani, R.G.; de Medeiros, D.C.; Chierrito, D.; Lazarin-Bidoia, D.; Zielinski, A.A.F.; et al. In vitro evaluation of the protective effects of plant extracts against amyloid-beta peptide-induced toxicity in human neuroblastoma SH-SY5Y cells. PLoS ONE 2019, 14, e0212089. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, X.; Yang, T.; Liu, C.; Wang, P. Alcohol Dehydrogenase 1B Suppresses beta-Amyloid-Induced Neuron Apoptosis. Front. Aging Neurosci. 2019, 11, 135. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. Vitr. 2013, 27, 954–963. [Google Scholar] [CrossRef]
- Miyagawa, H.; Ishihara, A.; Nishimoto, T.; Ueno, T.; Mayama, S. Induction of avenanthramides in oat leaves inoculated with crown rust fungus, Puccinia coronata f. sp. avenae. Biosci. Biotechnol. Biochem. 1995, 59, 2305–2306. [Google Scholar] [CrossRef]
- Nkhata Malunga, L.; Ames, N.; Mitchell Fetch, J.; Netticadan, T.; Joseph Thandapilly, S. Genotypic and environmental variations in phenolic acid and avenanthramide content of Canadian oat (Avena sativa). Food Chem. 2022, 388, 132904. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Wang, M.; Son, Y.; Yang, E.J.; Kang, M.S.; Kim, H.J.; Kim, H.S.; Jo, J. Oat Extract Avenanthramide-C Reverses Hippocampal Long-Term Potentiation Decline in Tg2576 Mice. Molecules 2021, 26, 6105. [Google Scholar] [CrossRef] [PubMed]
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated grains--sources of bioactive compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef]
- Ramasamy, V.S.; Samidurai, M.; Park, H.J.; Wang, M.; Park, R.Y.; Yu, S.Y.; Kang, H.K.; Hong, S.; Choi, W.S.; Lee, Y.Y.; et al. Avenanthramide-C Restores Impaired Plasticity and Cognition in Alzheimer’s Disease Model Mice. Mol. Neurobiol. 2020, 57, 315–330. [Google Scholar] [CrossRef]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Feng, L.; Zhou, Y.; Carew, J.S.; Hileman, E.O.; Plunkett, W.; Keating, M.J.; Huang, P. Inhibition of mitochondrial respiration: A novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J. Biol. Chem. 2003, 278, 37832–37839. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Yan, P.; Situ, B.; Mou, Y.; Liu, P. Cryptotanshinione inhibits beta-amyloid aggregation and protects damage from beta-amyloid in SH-SY5Y cells. Neurochem. Res. 2012, 37, 622–628. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Baez-Jurado, E.; Guio-Vega, G.; Hidalgo-Lanussa, O.; González, J.; Echeverria, V.; Ashraf, G.M.; Sahebkar, A.; Barreto, G.E. Mitochondrial Neuroglobin Is Necessary for Protection Induced by Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cells in Astrocytic Cells Subjected to Scratch and Metabolic Injury. Mol. Neurobiol. 2019, 56, 5167–5187. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Xu, T.; Niu, C.; Zhang, X.; Dong, M. β-Ecdysterone protects SH-SY5Y cells against β-amyloid-induced apoptosis via c-Jun N-terminal kinase- and Akt-associated complementary pathways. Lab. Investig. 2018, 98, 489–499. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42, S125–S152. [Google Scholar] [CrossRef]
- Mancuso, M.; Coppedè, F.; Murri, L.; Siciliano, G. Mitochondrial Cascade Hypothesis Alzheimer’s disease: Myth or reality? Antioxid. Redox Signal. 2007, 9, 1631–1646. [Google Scholar] [CrossRef]
- Bosetti, F.; Brizzi, F.; Barogi, S.; Mancuso, M.; Siciliano, G.; Tendi, E.A.; Murri, L.; Rapoport, S.I.; Solaini, G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol. Aging 2002, 23, 371–376. [Google Scholar] [CrossRef]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Yuan, X.; Ou, Y.; Zhu, X.; Cheng, Z.; Zhang, P.; Wu, X.; Meng, Y.; Zhang, L. The Relationship between the Bcl-2/Bax Proteins and the Mitochondria-Mediated Apoptosis Pathway in the Differentiation of Adipose-Derived Stromal Cells into Neurons. PLoS ONE 2016, 11, e0163327. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, X.; Wang, S.; Ou, Y.; Zheng, X.; Wang, Q. The relationship between mitochondrial fusion/fission and apoptosis in the process of adipose-derived stromal cells differentiation into astrocytes. Neurosci. Lett. 2014, 575, 19–24. [Google Scholar] [CrossRef]
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| NF-κB (p50) | GCAGCACTACTTCTTGACCACC | TCTGCTCCTGAGCATTGACGTC |
| NF-κB (p65) | TGAACCGAAACTCTGGCAGCTG | CATCAGCTTGCGAAAAGGAGCC |
| BCL-2 | ATCGCCCTGTGGATGACTGAGT | GCCAGGAGAAATCAAACAGAGGC |
| BAX | TCAGGATGCGTCCACCAAGAAG | TGTGTCCACGGCGGCAATCATC |
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
| Parameters | AVN A | AVN B | AVN C |
|---|---|---|---|
| Linearity | |||
| Concentration range (µg/mL) | 1–50 | 1–50 | 1–50 |
| Regression Equation | y = 55608x − 22077 | y = 45200x − 14845 | y = 35929x − 19394 |
| Correlation coefficient, r2 | 0.9997 | 0.9998 | 1 |
| Accuracy and Precision | |||
| Accuracy (%) | 98.82–100.47 | 100.17–102.70 | 99.70–101.59 |
| Precision (CV%) | 1.40–4.51 | 2.65–4.85 | 0.91–2.30 |
| Sensitivity | |||
| Limit of detection (LOD, µg/mL) | 0.733 | 0.616 | 0.610 |
| Limit of quantification (LOQ, µg/mL) | 2.223 | 1.866 | 1.848 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-Y.; Na, I.-S.; Kim, J.-E.; Song, J.-G.; Han, C.-E.; Kim, H.-W.; Shim, S.-M. Standardization of Germinated Oat Extracts and Their Neuroprotective Effects Against Aβ1-42 Induced Cytotoxicity in SH-SY5Y Cells. Molecules 2025, 30, 3291. https://doi.org/10.3390/molecules30153291
Lee Y-Y, Na I-S, Kim J-E, Song J-G, Han C-E, Kim H-W, Shim S-M. Standardization of Germinated Oat Extracts and Their Neuroprotective Effects Against Aβ1-42 Induced Cytotoxicity in SH-SY5Y Cells. Molecules. 2025; 30(15):3291. https://doi.org/10.3390/molecules30153291
Chicago/Turabian StyleLee, Yu-Young, In-Su Na, Jeong-Eun Kim, Jae-Gwang Song, Chae-Eun Han, Hyung-Wook Kim, and Soon-Mi Shim. 2025. "Standardization of Germinated Oat Extracts and Their Neuroprotective Effects Against Aβ1-42 Induced Cytotoxicity in SH-SY5Y Cells" Molecules 30, no. 15: 3291. https://doi.org/10.3390/molecules30153291
APA StyleLee, Y.-Y., Na, I.-S., Kim, J.-E., Song, J.-G., Han, C.-E., Kim, H.-W., & Shim, S.-M. (2025). Standardization of Germinated Oat Extracts and Their Neuroprotective Effects Against Aβ1-42 Induced Cytotoxicity in SH-SY5Y Cells. Molecules, 30(15), 3291. https://doi.org/10.3390/molecules30153291







