Ionic Liquid-Based Centrifuge-Less Cloud Point Extraction of a Copper(II)–4-Nitrocatechol Complex and Its Analytical Application
Abstract
1. Introduction
2. Results and Discussion
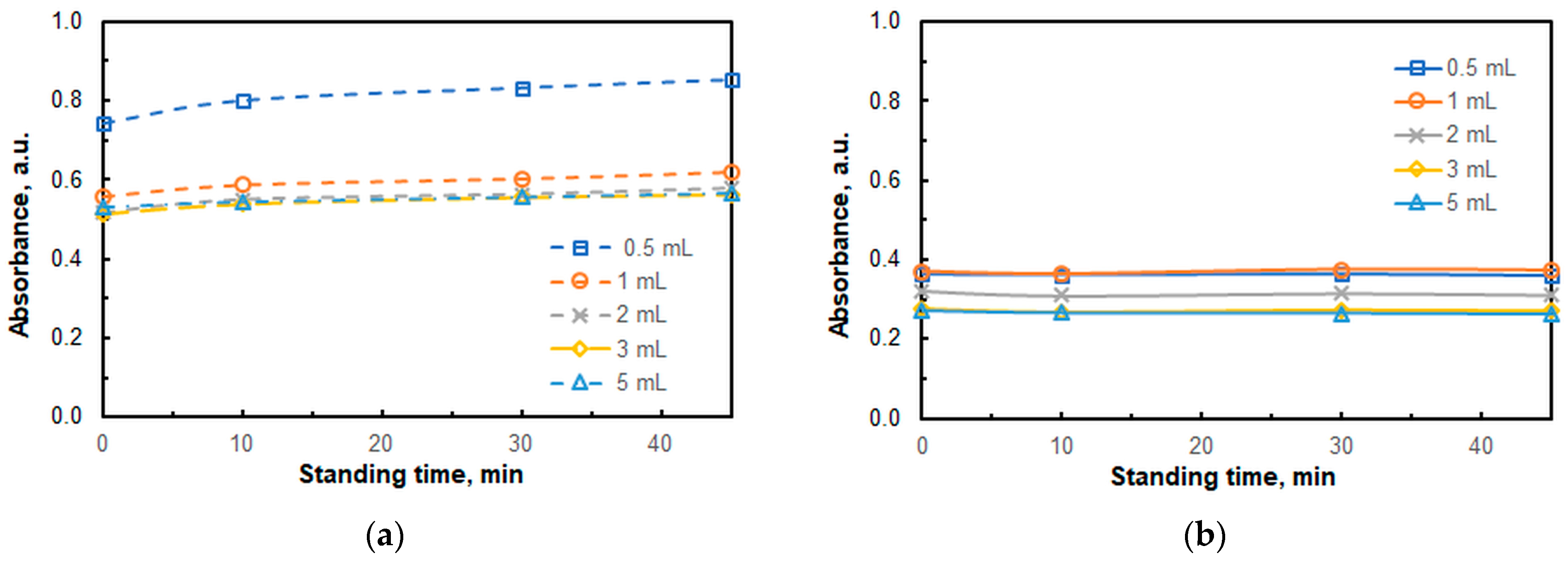
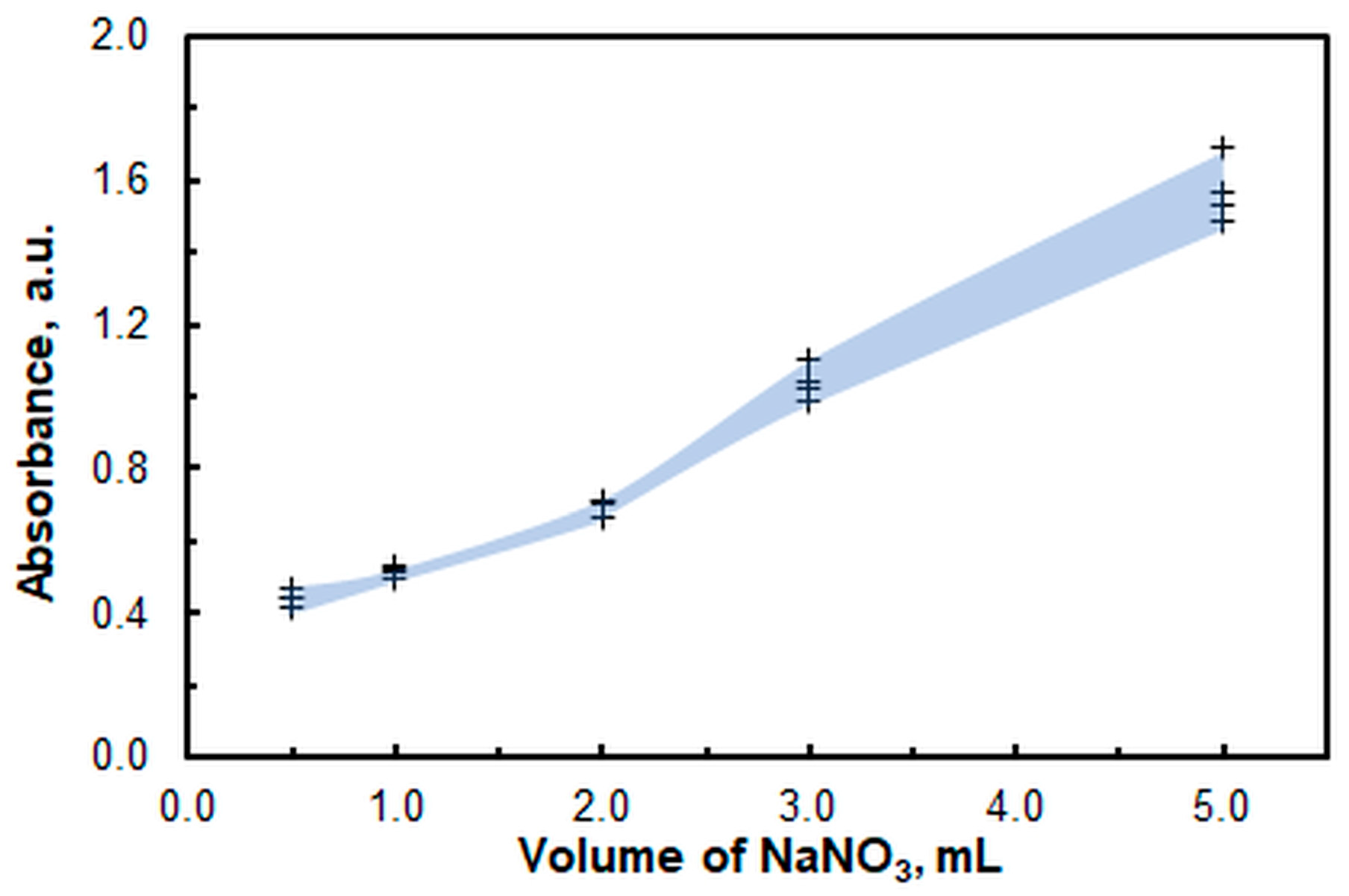
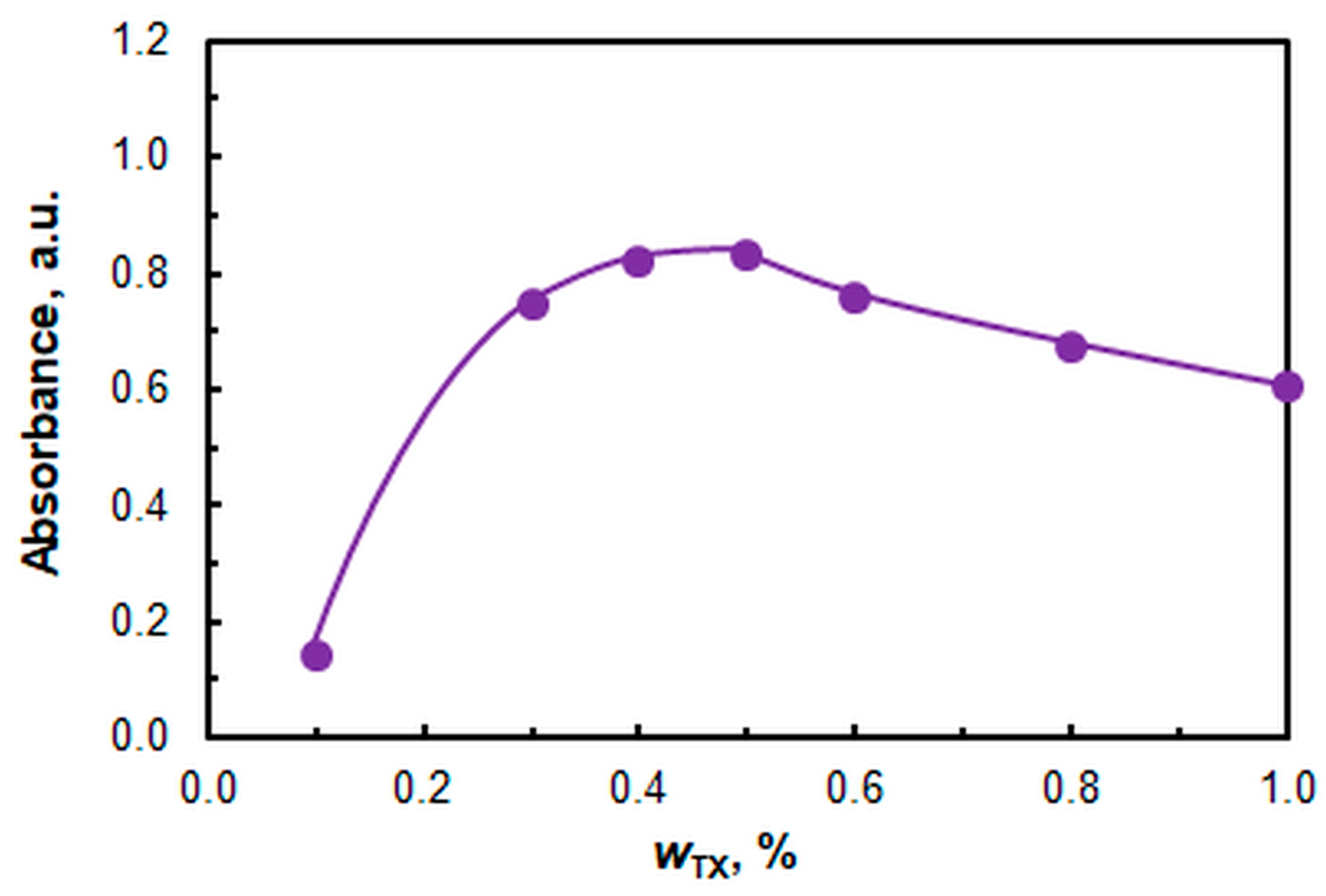
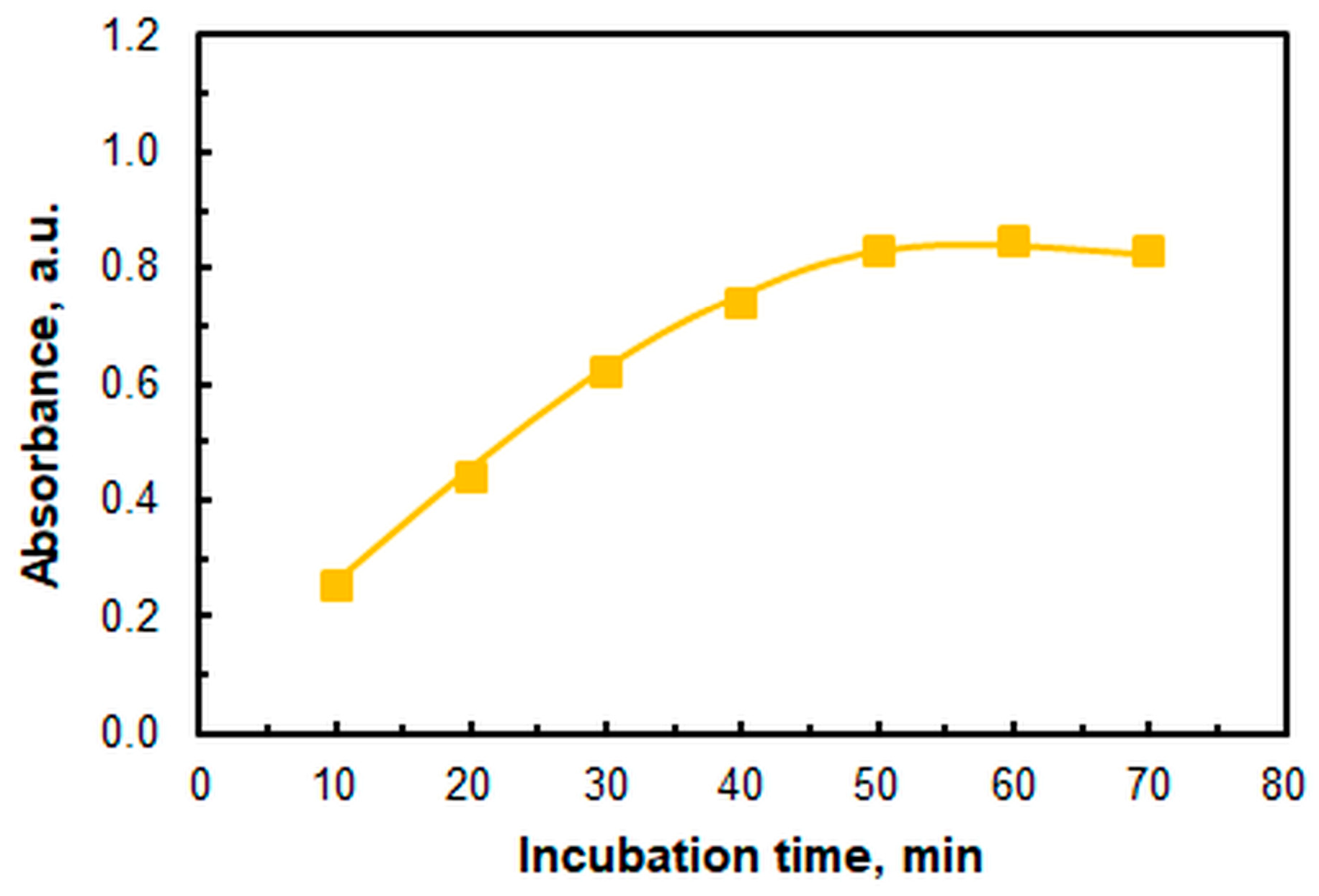
2.1. Optimal Operating Conditions
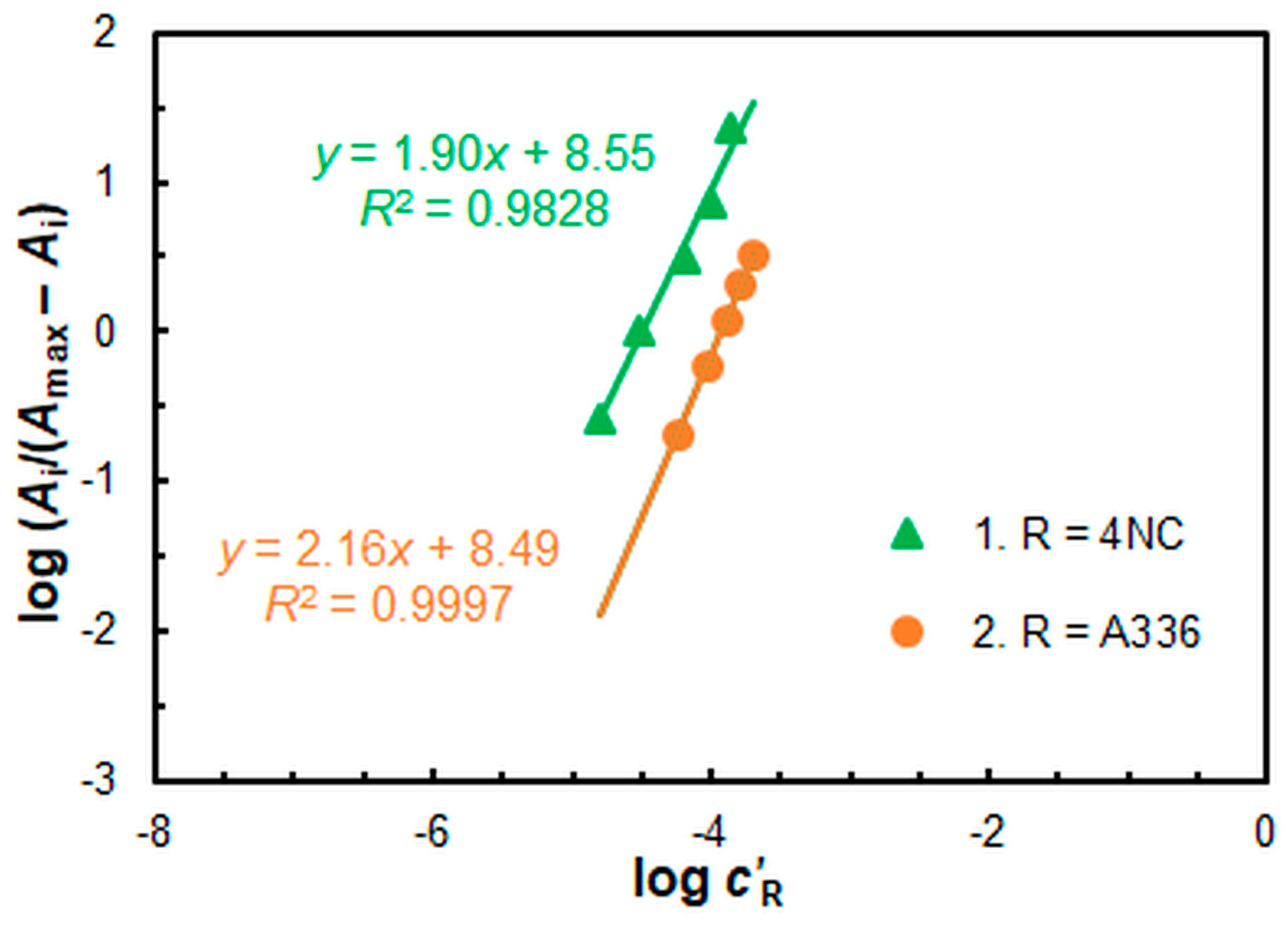
2.2. Formula and Extraction Constant
2.3. Analytical Characteristics
2.4. Effects of Diverse Ions and Analytical Applications
2.5. Comparisons with Existing Methods and Evaluations with Modern Assessment Tools
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Instrumentation
3.3. Samples and Sample Preparation
3.4. Optimization Procedure
3.5. Recommended Analytical Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Potential environmental pollution from copper metallurgy and methods of management. Environ. Res. 2021, 197, 111050. [Google Scholar] [CrossRef]
- Oorts, K. Copper. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 367–394. [Google Scholar] [CrossRef]
- IMA Database of Mineral Properties. Available online: https://rruff.info/ima/ (accessed on 28 June 2025).
- Chatterjee, K.K. Uses of Metals and Metallic Minerals; New Age International (P) Ltd.: New Delhi, India, 2007; pp. 205–209. [Google Scholar]
- Chen, L.; Zhou, M.; Wang, J.; Zhang, Z.; Duan, C.; Wang, X.; Zhao, S.; Bai, X.; Li, Z.; Li, Z.; et al. A global meta-analysis of heavy metal(loid)s pollution in soils near copper mines: Evaluation of pollution level and probabilistic health risks. Sci. Total Environ. 2022, 835, 155441. [Google Scholar] [CrossRef]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J.; Grybos, M.; Lens, P.N.L.; Guibaud, G. Copper metallurgical slags—Current knowledge and fate: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2424–2488. [Google Scholar] [CrossRef]
- Collins, J.F. Chapter Nine—Copper nutrition and biochemistry and human (patho)physiology. In Advances in Food and Nutrition Research; Eskin, N.A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 96, pp. 311–364. [Google Scholar] [CrossRef]
- Witt, B.; Schaumlöffel, D.; Schwerdtle, T. Subcellular localization of copper—Cellular bioimaging with focus on neurological disorders. Int. J. Mol. Sci. 2020, 21, 2341. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products. Nutrition and Allergies, Scientific opinion on dietary reference values for copper. EFSA J. 2015, 13, 4253. [Google Scholar] [CrossRef]
- Altarelli, M.; Ben-Hamouda, N.; Schneider, A.; Berger, M.M. Copper deficiency: Causes, manifestations, and treatment. Nutr. Clin. Pract. 2019, 34, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.; Dringen, R.; Mercer, J.F.B. Copper: Effects of deficiency and overload. In Interrelations Between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 359–387. [Google Scholar] [CrossRef]
- Topuz, B. Simultaneous spectrometric determination of Cu(II), Co(II), and Ni(II) in pharmaceutical and environmental samples with XAD-4/DMMDTC solid-phase extraction system. Biol. Trace Elem. Res. 2020, 194, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.B.; Souri, E. Third derivative spectrophotometric method for simultaneous determination of copper and nickel using 6-(2-naphthyl)-2, 3-dihydro-1,2,4- triazine-3-thione. J. Chem. 2011, 8, 905139. [Google Scholar] [CrossRef]
- Shaikh, A.B.; Barache, U.B.; Anuse, M.A.; Gaikwad, S.H. 4-(4’-Nitrobenzylideneimino)-3-methyl-5-mercapto-1,2,4-triazole, A new chromogenic reagent for extractive spectrophotometric determination of copper (II) in pharmaceutical and alloy samples. S. Afr. J. Chem. 2016, 69, 157–165. [Google Scholar] [CrossRef]
- Barache, U.B.; Shaikh, A.B.; Lokhande, T.N.; Kamble, G.S.; Anuse, M.A.; Gaikwad, S.H. An efficient, cost effective, sensing behaviour liquid-liquid extraction and spectrophotometric determination of copper(II) incorporated with 4-(4′-chlorobenzylideneimino)-3-methyl-5-mercapto-1, 2, 4-triazole: Analysis of food samples, leafy vegetables, fertilizers and environmental samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 443–453. [Google Scholar] [CrossRef]
- Wannas, F.A.; Azooz, E.A.; Shalan Al-Mushhdi, A.A.A.A.-H.; Naguib, I.A. Ionic liquid-based cloud point extraction for spectrophotometric determination of copper in water and food samples using a novel imidazole derivative. J. Food Compos. Anal. 2024, 135, 106638. [Google Scholar] [CrossRef]
- AbdulKareem, E.A.; Al-Murshedi, A.Y.M.; Ridha, R.K.; Azooz, E.A. Optimization and greenness assessments of the supramolecular solvent-assisted cloud point extraction method for copper spectrophotometric determination in water and food samples. Green Anal. Chem. 2024, 11, 100181. [Google Scholar] [CrossRef]
- Snigur, D.; Duboviy, V.; Barbalat, D.; Zhukovetska, O.; Chebotarev, A.; Bevziuk, K. A rapid room-temperature cloud point extraction for spectrophotometric determination of copper (II) with 6,7-dihydroxy-2,4-diphenylbenzopyrylium chloride. Anal. Sci. 2022, 38, 949–954. [Google Scholar] [CrossRef]
- Gouda, A.A.; Amin, A.S. Cloud-point extraction, preconcentration and spectrophotometric determination of trace quantities of copper in food, water and biological samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 88–96. [Google Scholar] [CrossRef]
- Ghali, A.A.; Hussein, A.S. Cloud point extraction for the separation, preconcentration and spectrophotometric determination of trace copper (II) in herbal plants samples using new synthesized reagent. J. Glob. Pharma Technol. 2019, 11, 817–826. [Google Scholar]
- Mussa, Y.O.; Ghali, A.A.; Hussei, A.S. Cloud point extraction, preconcentration and spectrophotometric determination of Co(II) and Cu(II) using 15-crown-5. Indian J. Forensic Med. Toxicol. 2020, 14, 1146–1152. [Google Scholar] [CrossRef]
- Wen, X.; Ye, L.; Deng, Q.; Peng, L. Investigation of analytical performance for rapidly synergistic cloud point extraction of trace amounts of copper combined with spectrophotometric determination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 259–264. [Google Scholar] [CrossRef]
- Snigur, D.; Chebotarev, A.; Dubovyi, V.; Barbalat, D.; Klochkova, A. Room temperature cloud point extraction: An application to preconcentration and spectrophotometric determination of copper(II). J. Serb. Chem. Soc. 2020, 85, 89–96. [Google Scholar] [CrossRef]
- Racheva, P.V.; Milcheva, N.P.; Genc, F.; Gavazov, K.B. A centrifuge-less cloud point extraction-spectrophotometric determination of copper(II) using 6-hexyl-4-(2-thiazolylazo)resorcinol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120106. [Google Scholar] [CrossRef]
- Ghasemi, E.; Kaykhaii, M. Determination of zinc, copper, and mercury in water samples by using novel micro cloud point extraction and UV-Vis spectrophotometry. Eurasian J. Anal. Chem. 2017, 12, 313–324. [Google Scholar] [CrossRef]
- Zengin, H.B.; Marsan, H.; Gürkan, R. Selective extraction of Cu+ and Cu2+ ions from mushroom and lichen samples prior to analysis by micro-volume UV-Vis spectrophotometry: Application of a novel poly (SMIm)-Tris-Fe3O4 nanocomposite. J. Food Compos. Anal. 2020, 91, 103539. [Google Scholar] [CrossRef]
- Temel, N.K.; Gürkan, R. Application of ultrasound-assisted cloud-point extraction and spectrophotometry for preconcentration and determination of trace amounts of copper(II) in beverages. J. Anal. Chem. 2019, 74, 1174–1183. [Google Scholar] [CrossRef]
- Hassan, M.K.; Khudhair, A.F. Analysis and cloud point extraction of trace copper (II) in urine of occupational workers. Asian J. Chem. 2019, 31, 353–359. [Google Scholar] [CrossRef]
- Stefanova-Bahchevanska, T.; Ahmedov, R.S.; Zaruba, S.; Andruch, V.; Gavazov, K.B.; Delchev, V.B.; Dospatliev, L.K. A cloud-point extraction-chromogenic system for copper(II) based on 1-(2-thiazolylazo)-2-naphthol. Bulg. Chem. Commun. 2018, 50, 38–43. [Google Scholar]
- Liang, P.; Yang, J. Cloud point extraction preconcentration and spectrophotometric determination of copper in food and water samples using amino acid as the complexing agent. J. Food Compos. Anal. 2010, 23, 95–99. [Google Scholar] [CrossRef]
- Silva, S.G.; Oliveira, P.V.; Rocha, F.R. A green analytical procedure for determination of copper and iron in plant materials after cloud point extraction. J. Braz. Chem. Soc. 2010, 21, 234–239. [Google Scholar] [CrossRef]
- Yang, S.; Fang, X.; Duan, L.; Yang, S.; Lei, Z.; Wen, X. Comparison of ultrasound-assisted cloud point extraction and ultrasound-assisted dispersive liquid liquid microextraction for copper coupled with spectrophotometric determination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 148, 72–77. [Google Scholar] [CrossRef]
- Altantawy, H.A.; Mortada, W.I.; Abdel-Latif, E.; El-Reash, Y.G.A. Cloud point extraction of copper using 4-(2-chloroacetamido)-salicylic acid as a complexing agent. Egypt. J. Chem. 2021, 64, 25–32. [Google Scholar] [CrossRef]
- Abbas, A.S.; Al-Khafaji, Y.; Al-Khafaji, H.A.H. Cloud point extraction as a procedure of separation and pre-concentration for cupper(II) determination using spectrofluorometric techniques. J. Pharm. Sci. Res. 2018, 10, 1748–1752. [Google Scholar]
- Akl, M.A.; AL-Rabasi, A.; Molouk, A.F. Cloud point extraction and FAAS determination of copper(II) at trace level in environmental samples using N-benzamido-N’-benzoylthiocarbamide and CTAB. Egypt. J. Chem. 2021, 64, 313–322. [Google Scholar] [CrossRef]
- Snigur, D.V.; Dubovyi, V.P.; Chebotarev, A.N. Atomic-absorption determination of copper(II) in water samples after its cloud-point extraction preconcentration. Mosc. Univ. Chem. Bull. 2020, 75, 328–332. [Google Scholar] [CrossRef]
- Yeliz, C.; Bişgin, A.T.; Sürme, Y.; Uçan, M.; Narin, İ. Micelle Mediated Extraction and Flame Atomic Absorption Spectrometric determination of trace amounts of copper in different mushroom species. J. Anal. Chem. 2020, 75, 1131–1136. [Google Scholar] [CrossRef]
- Aswathi, M.; Mathai, S.; Joseph, S.C.; Biju, V.M. Room temperature ionic liquid, cetyl pyridinium naphthenate, supported cloud point extractive separation and ultra trace determination of copper in blood and environmental samples. Sep. Sci. Technol. 2017, 52, 2540–2546. [Google Scholar] [CrossRef]
- Karadaş, C. A novel cloud point extraction method for separation and preconcentration of cadmium and copper from natural waters and their determination by flame atomic absorption spectrometry. Water Qual. Res. J. Can. 2017, 52, 178–186. [Google Scholar] [CrossRef]
- Yıldız, D.; Demir, M. Flame atomic absorption determination of copper in environmental water with cloud point extraction using Isonitrosoacetophenone 2-aminobenzoylhydrazone. J. Anal. Chem. 2019, 74, 437–443. [Google Scholar] [CrossRef]
- Snigur, D.; Vitaliy, D.; Michael, A.; Kateryna, B.; Viacheslav, M.; Dmytro, B.; Zhukovetska, O. A novel fast room-temperature cloud point extraction coupled to graphite furnace atomic absorption spectroscopy for copper(II) traces determination. Int. J. Environ. Anal. Chem. 2024, 104, 5254–5263. [Google Scholar] [CrossRef]
- Souza, V.S.; Teixeira, L.S.G.; Santos, Q.O.; Gomes, I.S.; Bezerra, M.A. Determination of copper and cadmium in petroleum produced formation water by electrothermal atomic absorption spectrometry after cloud point extraction. J. Braz. Chem. Soc. 2020, 31, 1186–1193. [Google Scholar] [CrossRef]
- Nekouei, F.; Nekouei, S. Solid phase-assisted determination of trace copper(II) and iron(III) in food and water samples by cloud point extraction: Response surface methodology optimization. Chiang Mai J. Sci. 2018, 45, 1960–1974. [Google Scholar]
- Procházková, S.; Halko, R. Determination of copper in human urine by cloud point extraction and flame atomic absorption spectrometry. Anal. Lett. 2016, 49, 1656–1668. [Google Scholar] [CrossRef]
- Miura, J.; Ishii, H.; Watanabe, H. Extraction and separation of nickel chelate of 1-(2-thiazolylazo)-2-naphthol in nonionic surfactant solution. Bunseki Kagaku 1976, 25, 808–809. [Google Scholar] [CrossRef]
- Yamini, Y.; Feizi, N.; Moradi, M. Chapter 7—Surfactant-Based Extraction Systems. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 209–239. [Google Scholar] [CrossRef]
- Mortada, W.I. Recent developments and applications of cloud point extraction: A critical review. Microchem. J. 2020, 157, 105055. [Google Scholar] [CrossRef]
- Halko, R.; Hagarová, I.; Andruch, V. Innovative approaches in cloud-point extraction. J. Chromatogr. A 2023, 1701, 464053. [Google Scholar] [CrossRef]
- Snigur, D.; Azooz, E.A.; Zhukovetska, O.; Guzenko, O.; Mortada, W. Recent innovations in cloud point extraction towards a more efficient and environmentally friendly procedure. Trac-Trends Anal. Chem. 2023, 164, 117113. [Google Scholar] [CrossRef]
- Semysim, F.A.; Shabaa, G.J.; Azooz, E.A.; Snigur, D. Alternative green solvents in cloud point extraction methods: Recent developments, challenges, and greenness evaluation. Trends Environ. Anal. Chem. 2025, 45, e00250. [Google Scholar] [CrossRef]
- Divarova, V.; Gajdošová, A.; Racheva, P.; Gavazov, K. An ionic liquid-assisted mixed micelle-mediated centrifuge-less cloud point extraction spectrophotometric method for the determination of molybdenum(VI). Int. J. Mol. Sci. 2025, 26, 4597. [Google Scholar] [CrossRef]
- Gajdošová, A.; Racheva, P.; Kiradzhiyska, D.; Divarova, V.; Saravanska, A.; Šandrejová, J.; Gavazov, K. Centrifuge-less mixed micelle-mediated cloud point extraction-spectrophotometric determination of vanadium using 4-nitrocatechol and cetylpyridinium chloride. Int. J. Mol. Sci. 2025, 26, 5808. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich Safety Data Sheet: 4-Nitrocatechol. Available online: https://www.sigmaaldrich.com/BG/en/sds/aldrich/n15553?userType=anonymous (accessed on 28 June 2025).
- Alempijević, S.B.; Vidović, K.; Vukosav, P.; Frka, S.; Kroflič, A.; Mihaljević, I.; Grgić, I.; Strmečki, S. Integrating voltammetry in ecotoxicology: Cu(II)-nitrocatechol complexes formation as a driver of Cu(II) and nitrocatechol toxicity in aquatic systems. Electrochim. Acta 2025, 522, 145938. [Google Scholar] [CrossRef]
- Häkkinen, P. Formation of copper(II) and zinc(II) complexes of 1,2-dihydroxy-4-nitrobenzene in aqueous solution. Finn. Chem. Lett. 1984, 9–12. [Google Scholar]
- Gavazov, K.B. Nitroderivatives of catechol: From synthesis to application. Acta Chim. Slov. 2012, 59, 1–17. [Google Scholar]
- Sai Krishna, D.; Noorbasha, N.M.; Jai Kumar, S. A new sequential and simultaneous speciation analysis of thallium in coal effluents by graphite furnace atomic absorption spectrometry after a novel ligandless mixed micelle cloud point extraction. Int. J. Environ. Anal. Chem. 2020, 100, 1079–1093. [Google Scholar] [CrossRef]
- Jameson, R.F.; Wilson, M.F. Thermodynamics of the interactions of catechol with transition metals. Part III. The effect of 4-chloro- and 4-nitro-substitution on proton and metal catechol complex formation. Dalton Trans. 1972, 2617–2621. [Google Scholar] [CrossRef]
- Triton™ X-114. Available online: https://www.sigmaaldrich.com/BG/en/product/sial/x114?srsltid=AfmBOorNeNd-XYYwrMeloEmk2Ao3KyEv4boAEWyCarCUhMe0GWjMRuDD (accessed on 28 June 2025).
- Mikkola, J.-P.; Virtanen, P.; Sjoholm, R. Aliquat 336—A versatile and affordable cation source for an entirely new family of hydrophobic ionic liquids. Green Chem. 2006, 8, 250–255. [Google Scholar] [CrossRef]
- Gavazov, K.B.; Racheva, P.V.; Saravanska, A.D.; Genc, F.; Delchev, V.B. Mono- and binuclear complexes in a centrifuge-less cloud-point extraction system for the spectrophotometric determination of zinc(II). Molecules 2024, 29, 4511. [Google Scholar] [CrossRef]
- Zhiming, Z.; Dongsten, M.; Cunxiao, Y. Mobile equilibrium method for determining composition and stability constant of coordination compounds of the form MmRn. J. Rare Earths 1997, 15, 216–219. [Google Scholar]
- Asmus, E. Eine neue Methode zur Ermittlung der Zusammensetzung schwacher Komplexe. Fresenius’ J. Anal. Chem. 1960, 178, 104–116. [Google Scholar] [CrossRef]
- Holme, A.; Langmyhr, F.J. A modified and a new straight-line method for determining the composition of weak complexes of the form AmBn. Anal. Chim. Acta 1966, 36, 383–391. [Google Scholar] [CrossRef]
- Racheva, P.V.; Saravanska, A.D.; Toncheva, G.K.; Kiradzhiyska, D.D.; Milcheva, N.P.; Divarova, V.V.; Pencheva, I.P.; Stojnova, K.T.; Delchev, V.B.; Gavazov, K.B. A Semi-Micro Extraction Spectrophotometric Determination of Iron Using 4-Nitrocatechol and Xylometazoline Hydrochloride. Molecules 2025, 30, 899. [Google Scholar] [CrossRef] [PubMed]
- Divarova, V.V.; Stojnova, K.T.; Radkovska, I.D.; Saravanska, A.D.; Toncheva, G.K.; Delchev, V.B.; Gavazov, K.B. Extraction system for the spectrophotometric determination of tungsten(VI) with 4-nitrocatechol and benzalkonium chloride. Acta Chim. Slov. 2024, 71, 519–527. [Google Scholar] [CrossRef]
- Murakami, Y.; Tokunaga, M. Stability order in metal chelate compounds. III. 4-nitro-and 4-chlorocatechol complexes. Bull. Chem. Soc. Jpn. 1964, 37, 1562–1563. [Google Scholar] [CrossRef]
- Biligo 2 Copper Plantis®. Available online: https://fitness1.bg/minerali/artesania-agricola/biligo-2-copper-plantis-0.858-mg/52842 (accessed on 28 June 2025).
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Mansour, F.R.; Bedair, A.; Locatelli, M. Click Analytical Chemistry Index as a novel concept and framework, supported with open source software to assess analytical methods. Adv. Sample Prep. 2025, 14, 100164. [Google Scholar] [CrossRef]
- Marczenko, Z.; Balcerzak, M. Separation, Preconcentration and Spectrophotometry in Inorganic Analysis; Elsevier: Amsterdam, The Netherlands, 2000; p. 178. [Google Scholar]





| Parameter | Optimal Value |
|---|---|
| Wavelength, nm | 451 |
| pH | 6.0 |
| Volume of the buffer, mL | 1.0 |
| Mass fraction of TX (wTX), % | 0.5 |
| Concentration of A336 (cA336), M | 3.0 × 10−4 |
| Concentration of 4NC (c4NC), M | 1.5 × 10−4 |
| Concentration of NaNO3 (cNaNO3), M | 2.6 × 10−2 |
| Incubation time at 60 °C (tinc), min | 50 |
| Refrigeration time (−20 °C), min | 50 |
| Test tube volume, mL | 50 |
| Mass * of the diluted SRP, g | 5.00 |
| Method | Log Kex * |
|---|---|
| Mobile equilibrium method | 7.85 ± 0.08 (n = 5) |
| Holme–Langmyhr method | 7.93 ± 0.11 (n = 4) |
| Ion | Added Salt Formula | Ion:CuII Mass Ratio | CuII Found, μg | R, % |
|---|---|---|---|---|
| AlIII | Al2(SO4)3·18H2O | 20 a | 16.0 | 105 |
| Br− | NaBr | 2000 b | 14.5 | 95.1 |
| CaII | Ca(NO3)2 | 500 | 14.6 | 95.6 |
| CdII | Cd(NO3)2·4H2O | 250 | 14.7 | 96.5 |
| Cl− | NaCl | 2000 b | 14.9 | 97.6 |
| CoII | Co(NO3)2·6H2O | 200 | 15.1 | 99.3 |
| CrIII | Cr2(SO4)3 | 0.1 | 16.2 | 106 |
| F− | NaF | 1000 | 15.9 | 104 |
| FeIII | NH4Fe(SO4)2·12H2O | 0.5 | 46.3 | 303 |
| HgI | Hg2(NO3)2 | 2 | 14.6 | 95.4 |
| I− | KI | 1000 | 15.3 | 100 |
| LiI | LiCl | 2000 b | 15.8 | 104 |
| MgII | MgSO4·7H2O | 2000 b | 15.6 | 102 |
| MnII | MnSO4·5H2O | 250 b | 15.3 | 100 |
| NiII | NiSO4·6H2O | 250 | 15.0 | 98.6 |
| PbII | Pb(NO3)2 | 2 | 16.0 | 105 |
| ReVII | NH4ReO4 | 250 | 14.7 | 96.2 |
| VV | NH4VO3 | 0.2 | 23.0 | 148 |
| WVI | Na2WO4·2H2O | 1 | 17.8 | 117 |
| ZnII | ZnSO4·7H2O | 100 | 15.2 | 98.9 |
| Alloy | Ingredients [4] | Copper Found, % | RSD, % | R, % |
|---|---|---|---|---|
| Constantan | 55.0% Cu, 45.0% Ni | 54.6 | 3.8 | 99.3 |
| German silver (#1) | 55.0% Cu, 18.0% Ni, 27.0% Zn | 54.5 | 3.6 | 99.1 |
| German silver (#2) | 62.0% Cu, 10.0% Ni, 28.0% Zn | 63.6 | 2.7 | 102.5 |
| Die-casting brass | 60.0% Cu, 39.5% Zn; 0.5% Al | 58.6 | 2.3 | 97.7 |
| Manganin | 86.0% Cu,12.0% Mn, 2.0% Ni | 85.9 | 2.3 | 99.9 |
| Cupro-nickel (#1) | 68.0% Cu, 30.0% Zn; 1.0% Fe; 1.0% Mn | 69. 8 | 2.0 | 102.6 |
| Cupro-nickel (#2) | 88.0% Cu, 10.0% Zn; 1.5% Fe; 0.5% Mn | 87.7 | 1.0 | 99.7 |
| Reagent(s) | Extraction Technique | Extractant(s) | pH | λmax, nm | ε·10−4, M−1 cm−1 | Linear Range, ng mL−1 | LOD, ng mL−1 | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 15-Crown-5 | CPE | TX-114 | 4 | 252 | 1.02 | 200–7000 | 100 | Spinach, tomato sauce, green tea, and black tea | [21] |
| ATAP | CPE | TX-114 | 4.5 | 608 | 43.7 | 4.0–115 | 1.20 | Food, water, and biological samples | [19] |
| BIDP + OMIAFP6 | IL-CPE | TX-100 | 9.0 | 610 | N. R. | 0.3–600 | 0.1 | Water, fruit, and vegetable samples | [16] |
| BMPH | SUPRAS-CPE | TX-114 | 2 | 570 | 6.3 | 0.2–700.0 | 0.1 | Food and drinking water | [17] |
| BrPAA | CPE | TX-114 | 8 | 512 | 2.32 | 7–1500 | 5.9 | Herbal plants | [20] |
| DDTC | RS-CPE | TX-100 | 12 | 435 | N. R. | Up to 50 | 0.4 | Water samples, defatted milk powder, tea | [22] |
| DHMPhB | RT-CPE | TX-100 | 4.5 | 540 | N. R. | 20–950 | 6 | Water samples | [23] |
| HTAR | CL-CPE | TX-114 | 5.9 | 535 | 25.4 | 4.5–254 | 1.34 | Water samples, saline solution for infusion | [24] |
| Isoleucine | CPE | TX-100 | 9.0 | 230 | N. R. | 10–1000 | 5 | Food and water samples | [30] |
| PAR | M-CPE | TX-114 | 8.0 | 515 | N. R. | 20–100 | 9.8 | Tap water | [25] |
| PG + ST | UA-CPE | TX-114 | 5.5 | 532 | N. R. | 2–300 | 0.6 | Beverages | [27] |
| Poly (SMIm)-Tris-Fe3O4 | UA-CPE | TX-114 (CuI) CTAB (CuII) | 7.0 5.0 | 347 | N. R. | 0.3−150 (CuI) 10−350 (CuII) | 0.093 3.03 | Lichen and mushroom samples | [26] |
| SAO | CPE | TX-114 | 4.2 | 380 | N. R. | 500−16,000 | 103 | Urine | [28] |
| 4NC+ A336 | IL-MM-CL-CPE | TX-114 | 6.0 | 451 | 8.9 | 32–763 | 9.7 | Synthetic mixtures and pharmaceutical samples | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiradzhiyska, D.; Milcheva, N.; Ruzmanova, M.; Genç, F.; Racheva, P.; Gavazov, K. Ionic Liquid-Based Centrifuge-Less Cloud Point Extraction of a Copper(II)–4-Nitrocatechol Complex and Its Analytical Application. Molecules 2025, 30, 3287. https://doi.org/10.3390/molecules30153287
Kiradzhiyska D, Milcheva N, Ruzmanova M, Genç F, Racheva P, Gavazov K. Ionic Liquid-Based Centrifuge-Less Cloud Point Extraction of a Copper(II)–4-Nitrocatechol Complex and Its Analytical Application. Molecules. 2025; 30(15):3287. https://doi.org/10.3390/molecules30153287
Chicago/Turabian StyleKiradzhiyska, Denitsa, Nikolina Milcheva, Miglena Ruzmanova, Fatma Genç, Petya Racheva, and Kiril Gavazov. 2025. "Ionic Liquid-Based Centrifuge-Less Cloud Point Extraction of a Copper(II)–4-Nitrocatechol Complex and Its Analytical Application" Molecules 30, no. 15: 3287. https://doi.org/10.3390/molecules30153287
APA StyleKiradzhiyska, D., Milcheva, N., Ruzmanova, M., Genç, F., Racheva, P., & Gavazov, K. (2025). Ionic Liquid-Based Centrifuge-Less Cloud Point Extraction of a Copper(II)–4-Nitrocatechol Complex and Its Analytical Application. Molecules, 30(15), 3287. https://doi.org/10.3390/molecules30153287









