Proton and Metal Dication Affinities of Tetracyclic Imidazo[4,5-b]Pyridine-Based Molecules: Insights from Mass Spectrometry and DFT Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Mass Spectrometry
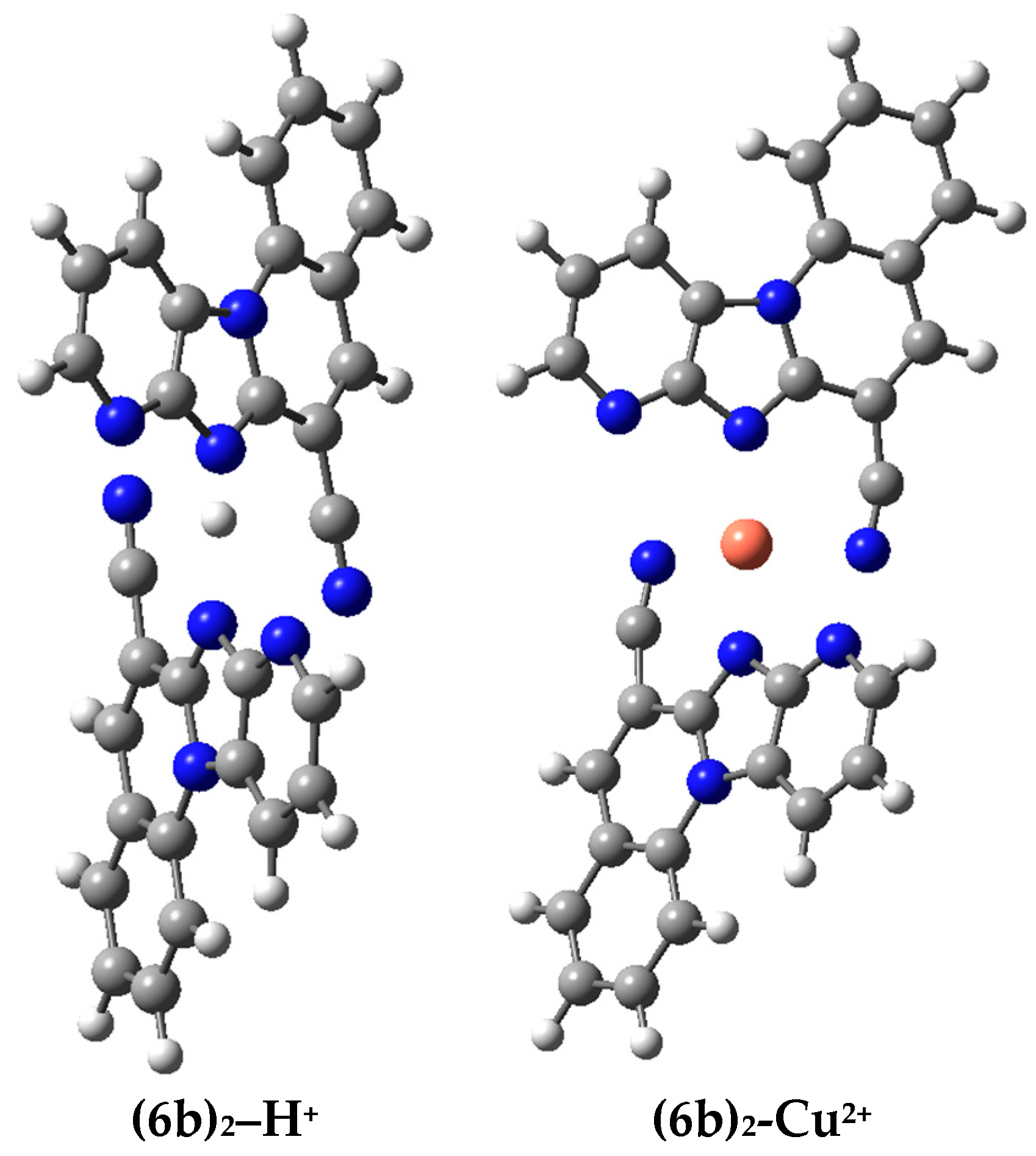
2.2. Computational DFT Analysis
2.2.1. Gas-Phase Basicities and Affinities for a Proton
2.2.2. Metal Dication Affinities
3. Materials and Methods
3.1. Mass Spectrometry Measurements
3.2. Computational Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarmoni, K.; Misbahi, K.; Ferrières, V. Imidazo[4,5-b]Pyridines: From Kinase Inhibitors to more Diversified Biological Properties. Curr. Med. Chem. 2023, 31, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Peytam, F.; Emamgholipour, Z.; Mousavi, A.; Moradi, M.; Foroumadi, R.; Firoozpour, L.; Divsalar, F.; Safavi, M.; Foroumadi, A. Imidazopyridine-based kinase inhibitors as potential anticancer agents: A review. Bioorg. Chem. 2023, 140, 106831. [Google Scholar] [CrossRef]
- Krause, M.; Foks, H.; Gobis, K. Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives. Molecules 2017, 22, 399. [Google Scholar] [CrossRef]
- Marinescu, M.; Popa, C.-V. Pyridine Compounds with Antimicrobial and Antiviral Activities. Int. J. Mol. Sci. 2022, 23, 5659. [Google Scholar] [CrossRef]
- Shelke, R.N.; Pansare, D.N.; Pawar, C.D.; Deshmukh, A.K.; Pawar, R.P.; Bembalkar, S.R. Synthesis of 3H-imidazo[4,5-b]pyridine with evaluation of their anticancer and antimicrobial activity. Eur. J. Chem. 2017, 8, 25–32. [Google Scholar] [CrossRef]
- Devi, N.; Singh, D.; Rawal, R.K.; Bariwal, J.; Singh, V. Medicinal Attributes of Imidazo[1,2-a]pyridine Derivatives: An Update. Curr. Top. Med. Chem. 2016, 16, 2963–2994. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thornton, J.M. Metal ions in biological catalysis: From enzyme databases to general principles. J. Biol. Inorg. Chem. 2008, 13, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc and Human Disease. In Interrelations Between Essential Metal Ions and Human Diseases; Metal Ions in Life Sciences; Sigel, A., Sigel, H., Sigel, R., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 13. [Google Scholar] [CrossRef]
- Helmann, J.D. Metals in Motion: Understanding Labile Metal Pools in Bacteria. Biochemistry 2025, 64, 329–345. [Google Scholar] [CrossRef]
- Chaudhran, P.A.; Sharma, A. Progress in the Development of Imidazopyridine-Based Fluorescent Probes for Diverse Applications. Crit. Rev. Anal. Chem. 2024, 54, 2148–2165. [Google Scholar] [CrossRef]
- O’HAir, R.A.J. The 3D quadrupole ion trap mass spectrometer as a complete chemical laboratory for fundamental gas-phase studies of metal mediated chemistry. Chem. Commun. 2006, 2006, 1469–1481. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Lončar, B.; Perin, N.; Mioč, M.; Boček, I.; Grgić, L.; Kralj, M.; Tomić, S.; Stojković, M.R.; Hranjec, M. Novel amino substituted tetracyclic imidazo[4,5-b]pyridine derivatives: Design, synthesis, antiproliferative activity and DNA/RNA binding study. Eur. J. Med. Chem. 2021, 217, 113342. [Google Scholar] [CrossRef]
- Perin, N.; Lončar, B.; Kadić, M.; Kralj, M.; Starčević, K.; Carvalho, R.A.; Jarak, I.; Hranjec, M. Design, Synthesis, Antitumor Activity and NMR-Based Metabolomics of Novel Amino Substituted Tetracyclic Imidazo[4,5-b]Pyridine Derivatives. ChemMedChem 2024, 19, e202300633. [Google Scholar] [CrossRef] [PubMed]
- McIndoe, J.S.; Vikse, K.L. Assigning the ESI mass spectra of organometallic and coordination compounds. J. Mass Spectrom. 2019, 54, 466–479. [Google Scholar] [CrossRef]
- Gianelli, L.; Amendola, V.; Fabbrizzi, L.; Pallavicini, P.; Mellerio, G.G. Investigation of reduction of Cu(II) complexes in positive-ion mode electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 2347–2353. [Google Scholar] [CrossRef]
- Lavanant, H.; Virelizier, H.; Hoppilliard, Y. Reduction of copper(II) complexes by electron capture in an electrospray ionization source. J. Am. Soc. Mass Spectrom. 1998, 9, 1217–1221. [Google Scholar] [CrossRef]
- Frański, R.; Klonowska-Wieszczycka, K.; Borowiak-Resterna, A.; Olszanowski, A. Influence of solvent and counter ion on copper complexes with N-alkyl-pyridine-2-carboxamides as studied by electrospray ionization mass spectrometry. Eur. J. Mass Spectrom. 2006, 12, 311–316. [Google Scholar] [CrossRef]
- Tshepelevitsh, S.; Kütt, A.; Lõkov, M.; Kaljurand, I.; Saame, J.; Heering, A.; Plieger, P.G.; Vianello, R.; Leito, I. On the Basicity of Organic Bases in Different Media. Eur. J. Org. Chem. 2019, 2019, 6735–6748. [Google Scholar] [CrossRef]
- Hunter, E.P.L.; Lias, S.G. Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update. J. Phys. Chem. Ref. Data 1998, 27, 413–656. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Maksić, Z.B.; Kovačević, B.; Vianello, R. Advances in Determining the Absolute Proton Affinities of Neutral Organic Molecules in the Gas Phase and Their Interpretation: A Theoretical Account. Chem. Rev. 2012, 112, 5240–5270. [Google Scholar] [CrossRef] [PubMed]
- Schwamm, R.J.; Vianello, R.; Maršavelski, A.; Garcia, M.A.; Claramunt, R.M.; Alkorta, I.; Saame, J.; Leito, I.; Fitchett, C.M.; Edwards, A.J.; et al. 15N NMR Spectroscopy, X-ray and Neutron Diffraction, Quantum-Chemical Calculations, and UV/vis-Spectrophotometric Titrations as Complementary Techniques for the Analysis of Pyridine-Supported Bicyclic Guanidine Superbases. J. Org. Chem. 2016, 81, 7612–7625. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chattaraj, P.K. Conceptual density functional theory based electronic structure principles. Chem. Sci. 2021, 12, 6264–6279. [Google Scholar] [CrossRef]
- Boughlala, Z.; Guerra, C.F.; Bickelhaupt, F.M. Alkali Metal Cation versus Proton and Methyl Cation Affinities: Structure and Bonding Mechanism. ChemistryOpen 2016, 5, 247–253. [Google Scholar] [CrossRef]
- Xu, J.; Koopal, L.K.; Fang, L.; Xiong, J.; Tan, W. Proton and Copper Binding to Humic Acids Analyzed by XAFS Spectroscopy and Isothermal Titration Calorimetry. Environ. Sci. Technol. 2018, 52, 4099–4107. [Google Scholar] [CrossRef] [PubMed]
- Boček, I.; Hranjec, M.; Vianello, R. Imidazo[4,5-b]pyridine derived iminocoumarins as potential pH probes: Synthesis, spectroscopic and computational studies of their protonation equilibria. J. Mol. Liq. 2022, 355, 118982. [Google Scholar] [CrossRef]
- Cole, R.B. (Ed.) Electrospray and MALDI Mass Spectrometry: Fundamentals, Instrumentation, Practicalities, and Biological Applications; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Di Marco, V.B.; Bombi, G.G. Electrospray Mass Spectrometry (ESI-MS) in the Study of Metal-Ligand Solution Equilibria. Mass Spectrom. Rev. 2006, 25, 347–379. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2013. Available online: https://webbook.nist.gov/ (accessed on 18 May 2025). [CrossRef]
- Glušič, M.; Stare, J.; Grdadolnik, J.; Vianello, R. Binding of cadmium dication to glutathione facilitates cysteine –SH deprotonation: A computational DFT study. J. Inorg. Biochem. 2013, 119, 90–94. [Google Scholar] [CrossRef]
- Pantalon Juraj, N.; Tandarić, T.; Tadić, V.; Perić, B.; Moreth, D.; Schatzschneider, U.; Brozović, A.; Vianello, R.; Kirin, S.I. Tuning the coordination properties of chiral pseudopeptide bis(2-picolyl)amine and iminodiacetamide ligands in Zn(II) and Cu(II) complexes. Dalton Trans. 2022, 51, 17008–17021. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Bagherinejad, A.; Kayanian, J.; Vianello, R. An experimental and computational study of new spiro-barbituric acid pyrazoline scaffolds: Restricted rotation vs. annular tautomerism. New J. Chem. 2022, 46, 7242–7252. [Google Scholar] [CrossRef]
- Vrban, L.; Vianello, R. Prominent neuroprotective potential of indole-2-N-methylpropargylamine: High affinity and irreversible inhibition efficiency towards monoamine oxidase B revealed by computational scaffold analysis. Pharmaceuticals 2024, 17, 1292. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01, Fox Gaussian, Inc.: Wallingford, CT, USA, 2016.
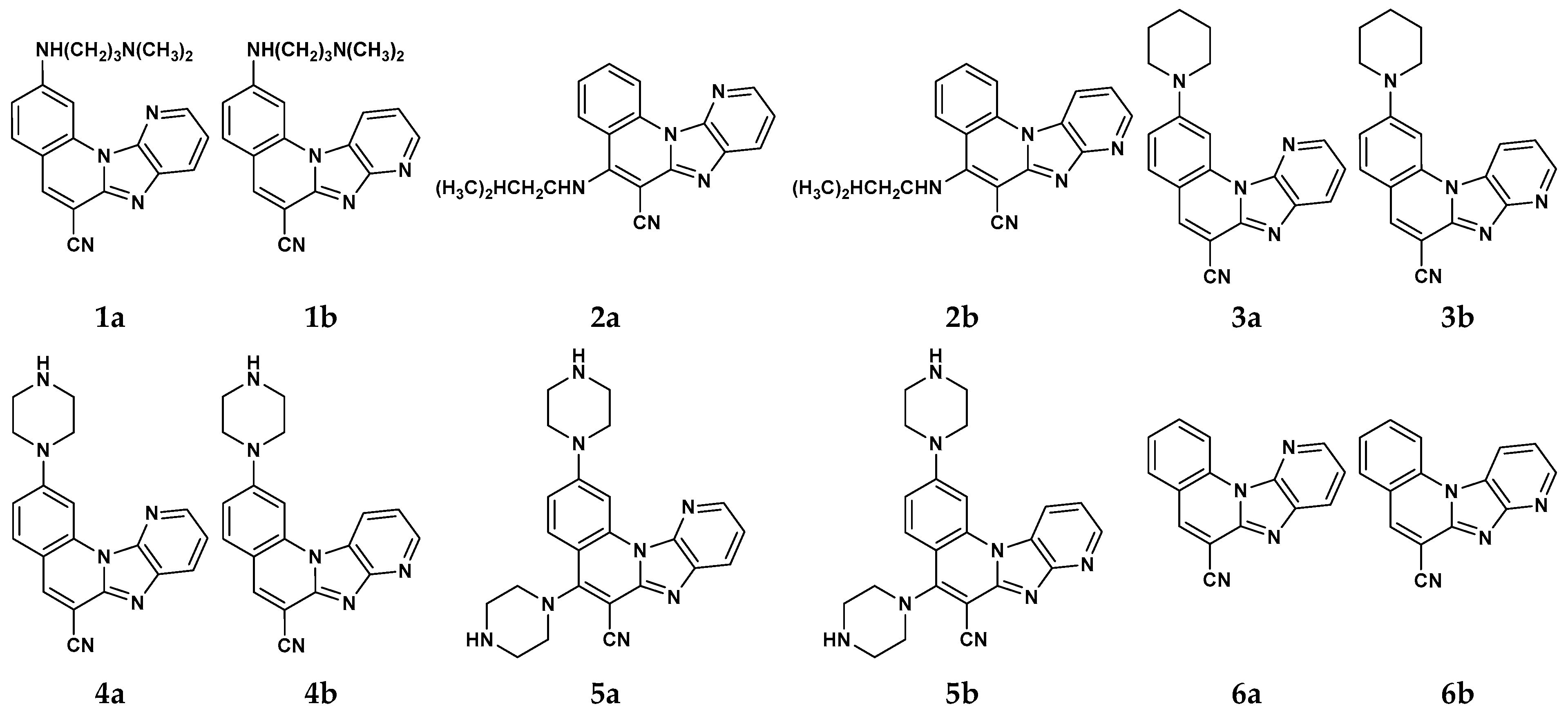
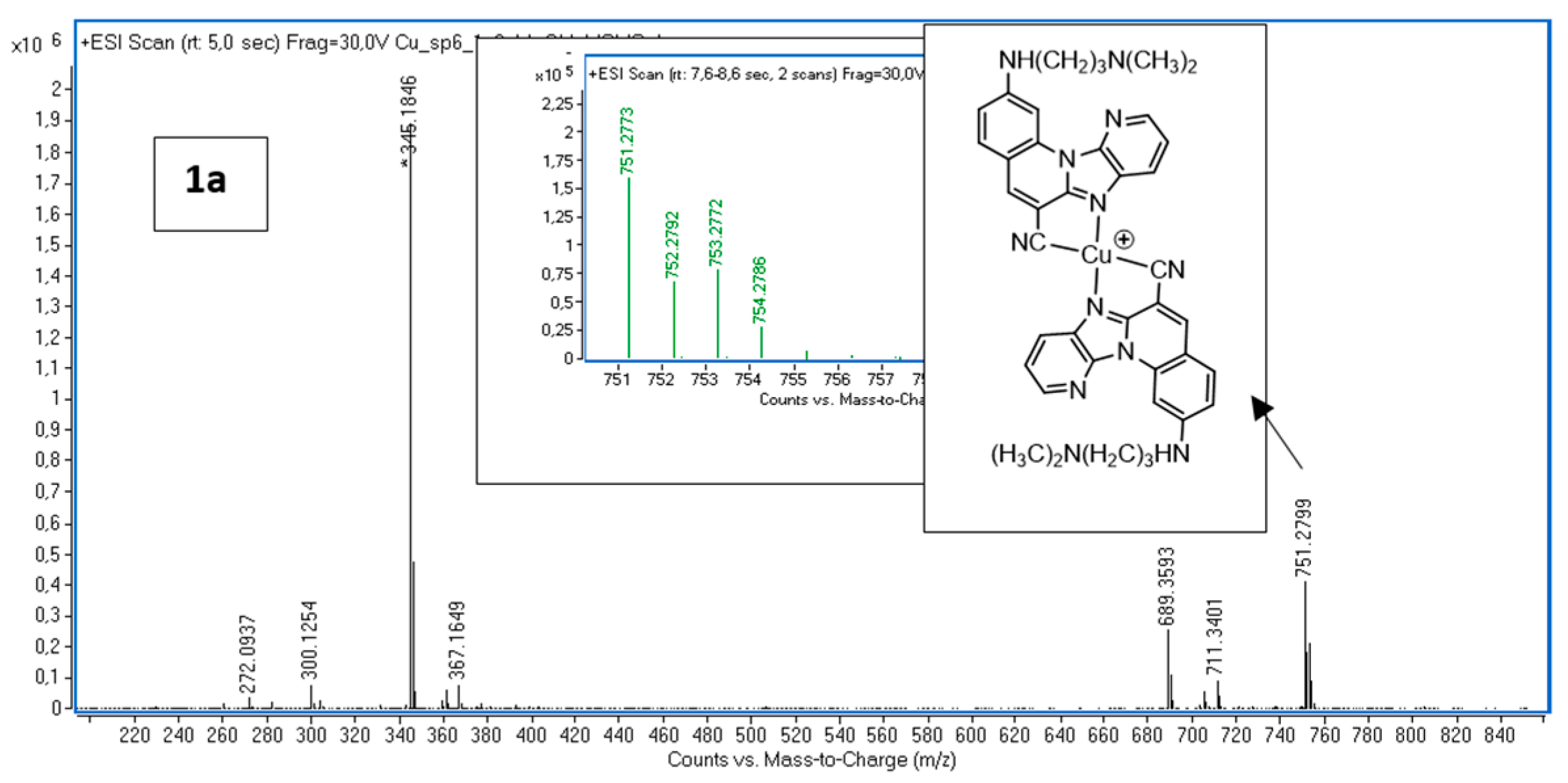
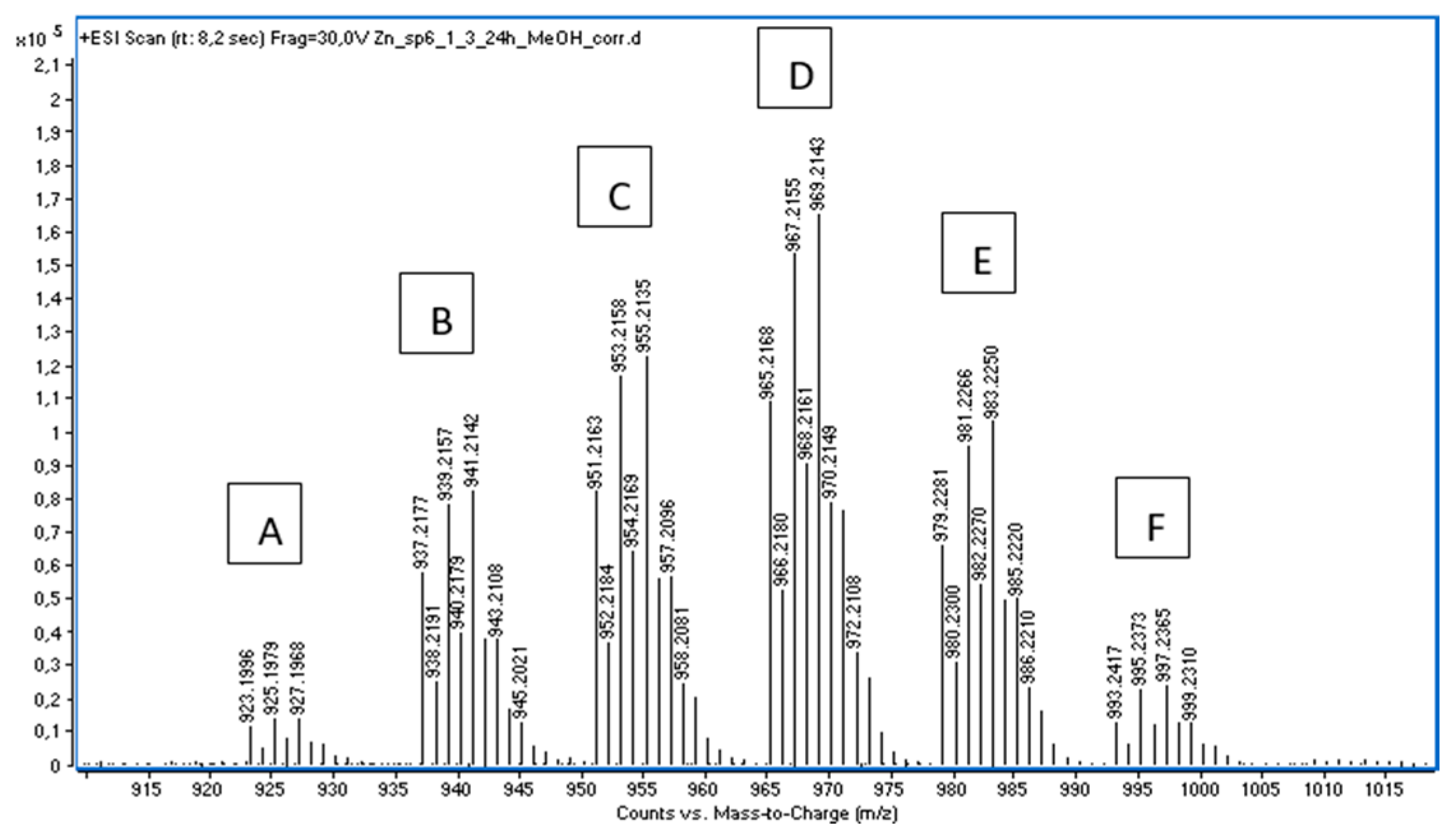
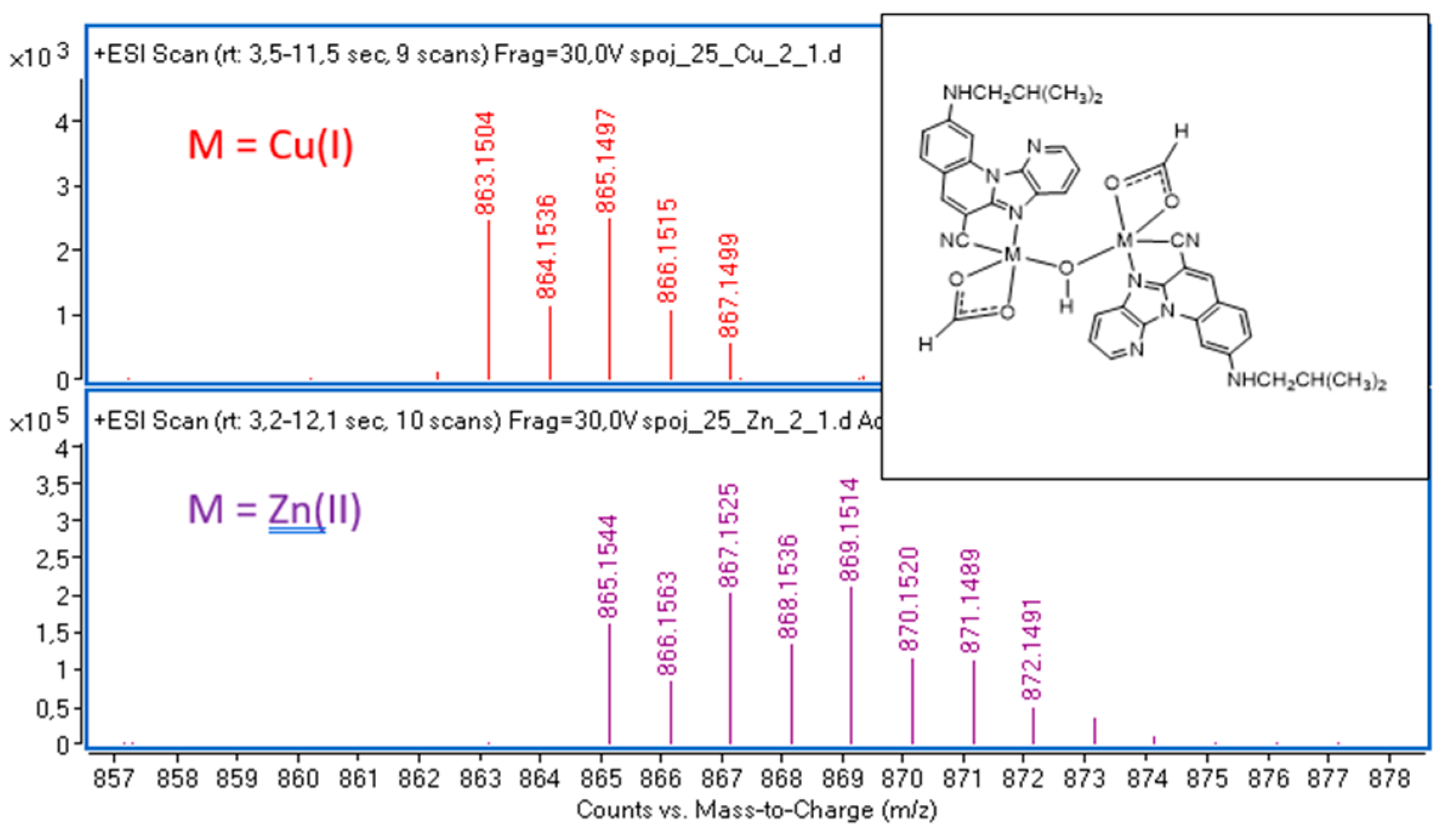
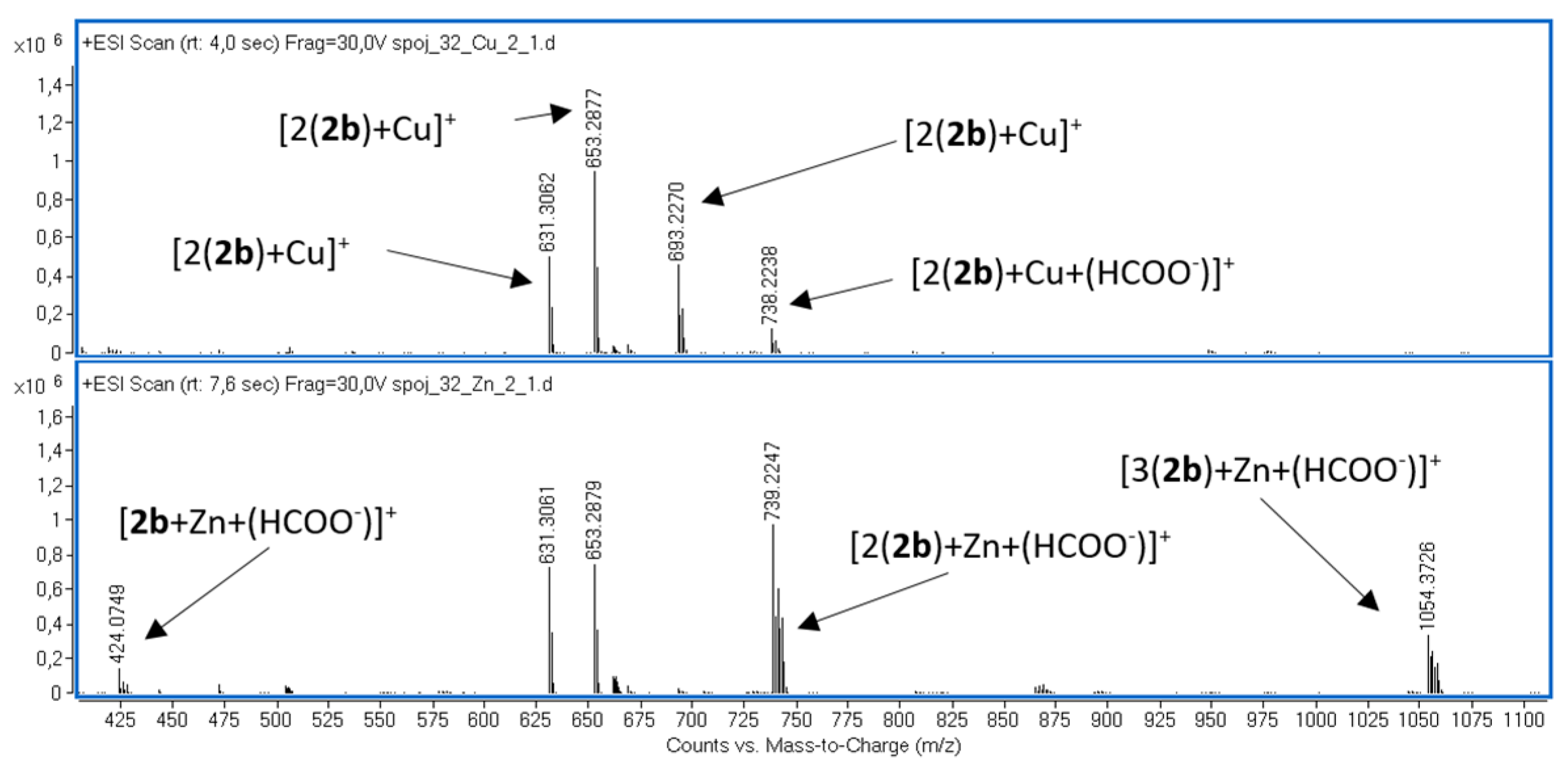
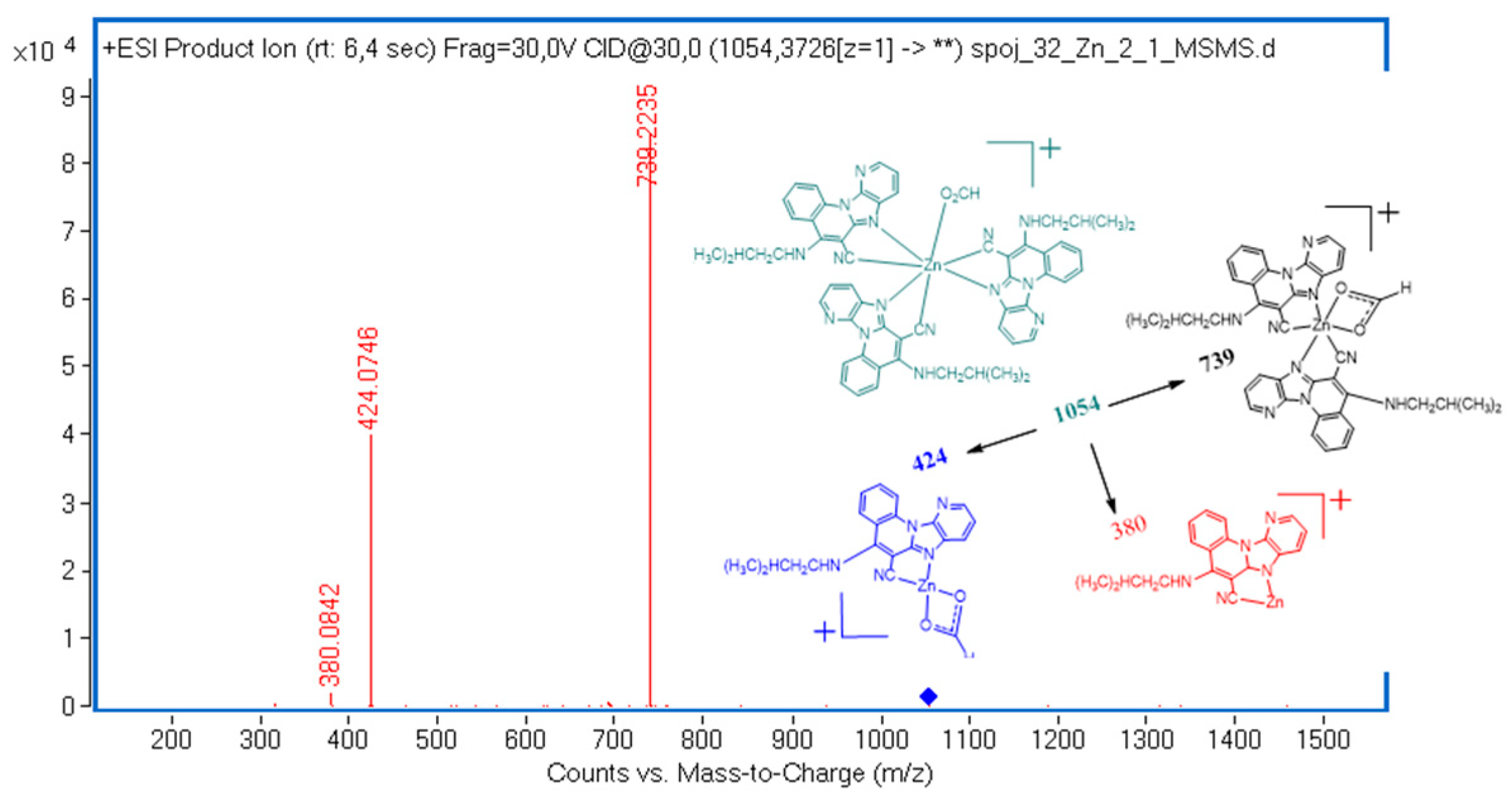
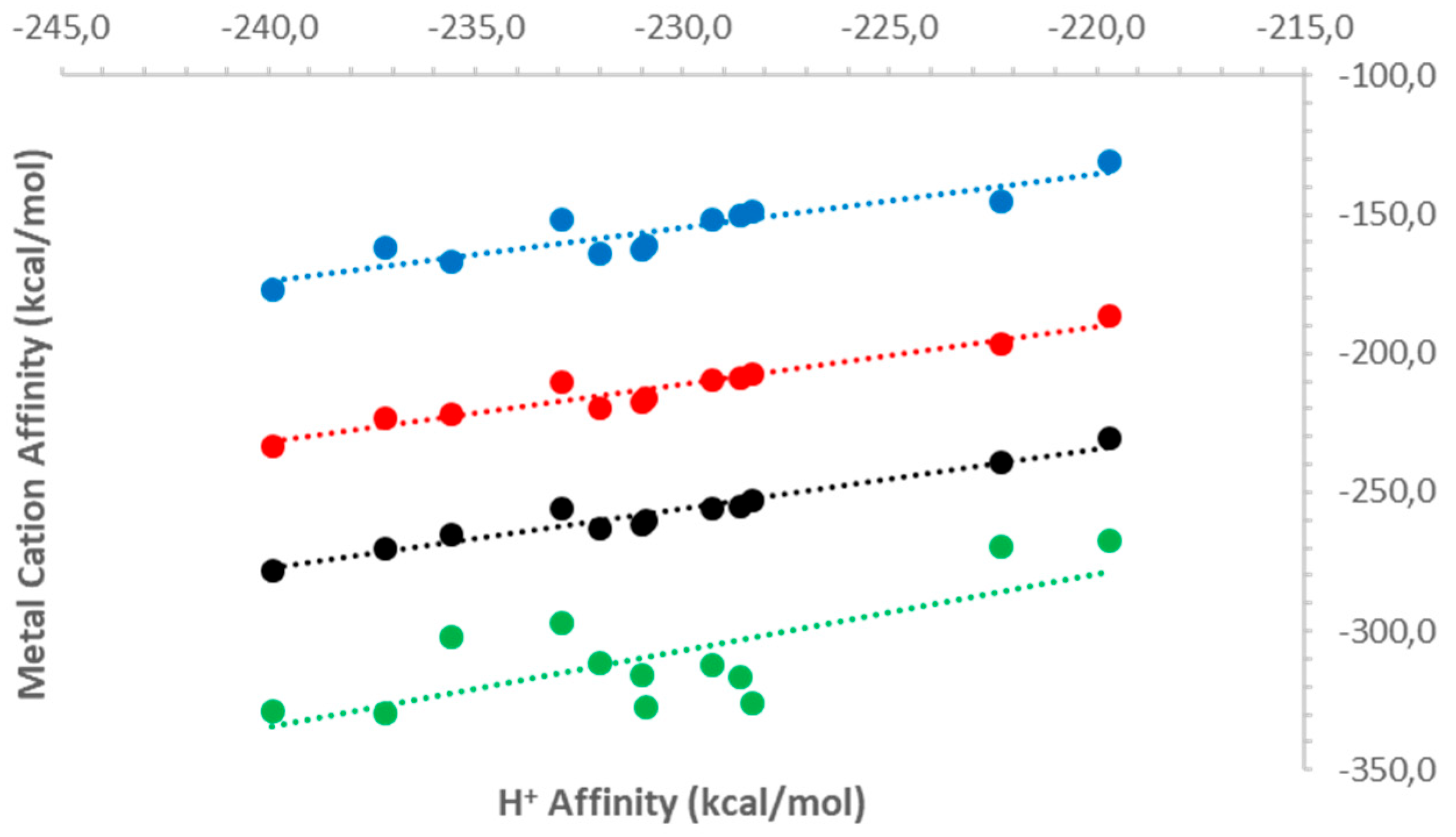
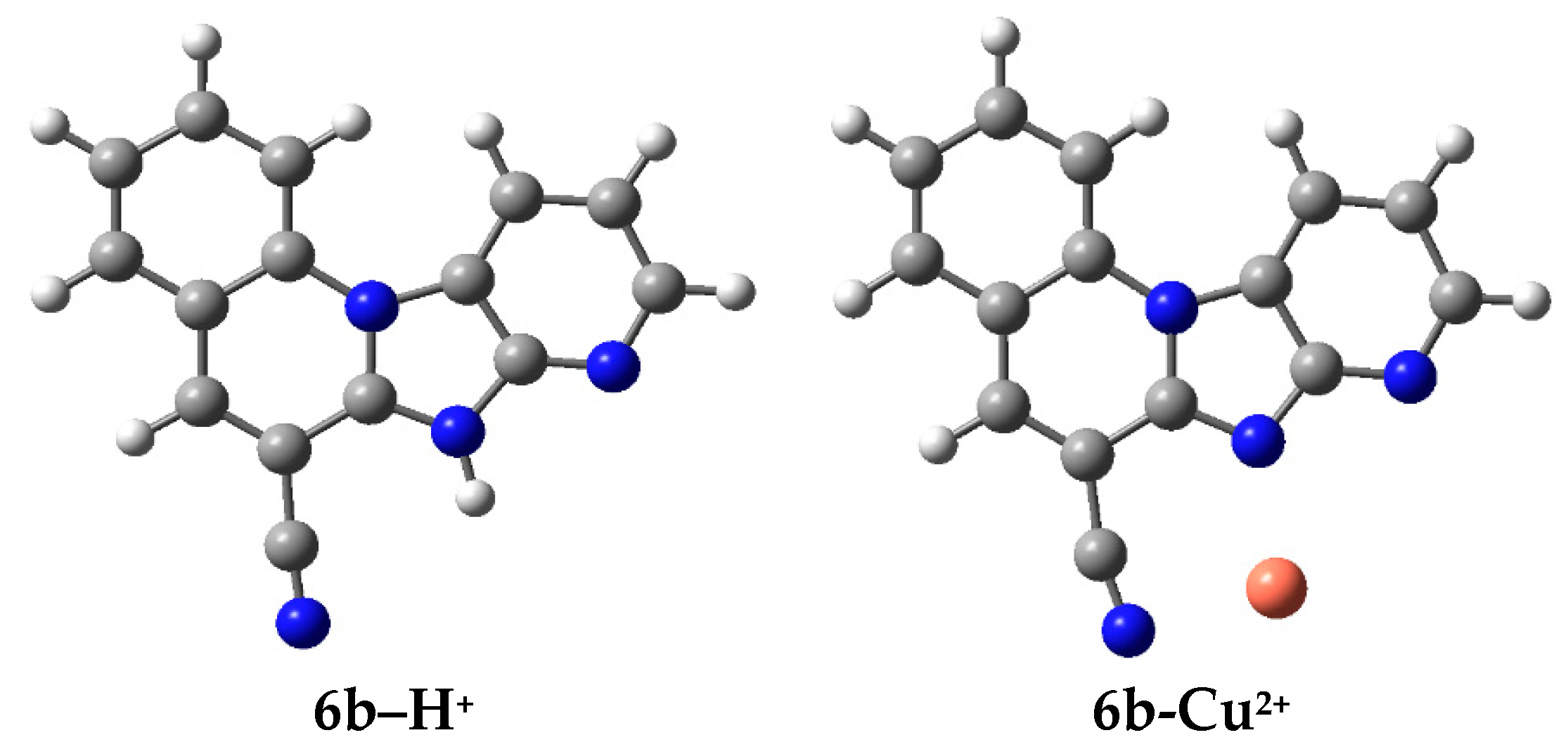
| Sign | m/z | Assignation |
|---|---|---|
| A | 923.1996 | 2(1a) + 2 Zn2+ + 2(HCOO−) + (OH−) |
| B | 937.2177 | 2(1a) + 2 Zn2+ + (HCOO−) + (CH3COO−) + (OH−) |
| C | 951.2163 | 2(1a) + 2 Zn2+ + 3(HCOO−) |
| D | 956.2168 | 2(1a) + 2 Zn2+ + 2(HCOO−) + (CH3COO−) |
| E | 979.2281 | 2(1a) + 2 Zn2+ + (HCOO−) + 2(CH3COO−) |
| F | 993.2417 | 2(1a) + 2 Zn2+ + 3(CH3COO−) |
| Ligand | H+ | Ca2+ | Mg2+ | Zn2+ | Cu2+ | Ligand | H+ | Ca2+ | Mg2+ | Zn2+ | Cu2+ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | −228.3 | −148.7 | −207.1 | −253.0 | −326.1 | 1b | −230.9 | −161.4 | −216.3 | −260.0 | −327.4 |
| 2a | −232.9 | −151.4 | −210.1 | −256.0 | −296.9 | 2b | −235.6 | −166.8 | −221.5 | −265.1 | −301.9 |
| 3a | −229.3 | −151.4 | −209.9 | −256.2 | −312.0 | 3b | −232.0 | −164.2 | −219.5 | −263.4 | −311.8 |
| 4a | −228.6 | −150.2 | −209.1 | −255.3 | −316.6 | 4b | −231.0 | −162.4 | −217.7 | −261.7 | −316.2 |
| 5a | −237.2 | −162.1 | −223.3 | −270.0 | −329.8 | 5b | −239.9 | −176.7 | −233.4 | −277.9 | −329.1 |
| 6a | −219.7 | −130.7 | −186.5 | −230.8 | −267.3 | 6b | −222.3 | −145.1 | −196.9 | −239.0 | −269.5 |
| System | H+ | Zn2+ | Cu2+ | System | H+ | Zn2+ | Cu2+ |
|---|---|---|---|---|---|---|---|
| 1a | −245.6 [−17.3] | −362.0 [−109.0] | −376.4 [−49.6] | 1b | −250.6 [−19.7] | −374.5 [−114.6] | −381.3 [−53.9] |
| 2a | −249.2 [−16.3] | −369.3 [−113.3] | −363.7 [−66.8] | 2b | −254.1 [−18.6] | −384.3 [−119.2] | −392.8 [−92.4] |
| 3a | −247.1 [−17.4] | −366.4 [−109.8] | −375.1 [−62.8] | 3b | −252.2 [−20.2] | −378.0 [−114.5] | −378.7 [−67.0] |
| 4a | −245.1 [−16.5] | −364.9 [−109.6] | −372.8 [−56.2] | 4b | −249.8 [−18.4] | −376.4 [−114.3] | −376.9 [−61.2] |
| 5a | −252.8 [−15.3] | −383.1 [−112.9] | −385.8 [−55.6] | 5b | −258.7 [−18.3] | −396.0 [−117.7] | −389.9 [−60.3] |
| 6a | −236.4 [−16.6] | −336.5 [−105.7] | −330.1 [−62.7] | 6b | −241.8 [−19.5] | −351.1 [−112.1] | −360.1 [−90.6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrban, L.; Martinac, I.A.; Hranjec, M.; Pocrnić, M.; Galić, N.; Kobetić, R.; Vianello, R. Proton and Metal Dication Affinities of Tetracyclic Imidazo[4,5-b]Pyridine-Based Molecules: Insights from Mass Spectrometry and DFT Analysis. Molecules 2025, 30, 2684. https://doi.org/10.3390/molecules30132684
Vrban L, Martinac IA, Hranjec M, Pocrnić M, Galić N, Kobetić R, Vianello R. Proton and Metal Dication Affinities of Tetracyclic Imidazo[4,5-b]Pyridine-Based Molecules: Insights from Mass Spectrometry and DFT Analysis. Molecules. 2025; 30(13):2684. https://doi.org/10.3390/molecules30132684
Chicago/Turabian StyleVrban, Lucija, Ingrid Ana Martinac, Marijana Hranjec, Marijana Pocrnić, Nives Galić, Renata Kobetić, and Robert Vianello. 2025. "Proton and Metal Dication Affinities of Tetracyclic Imidazo[4,5-b]Pyridine-Based Molecules: Insights from Mass Spectrometry and DFT Analysis" Molecules 30, no. 13: 2684. https://doi.org/10.3390/molecules30132684
APA StyleVrban, L., Martinac, I. A., Hranjec, M., Pocrnić, M., Galić, N., Kobetić, R., & Vianello, R. (2025). Proton and Metal Dication Affinities of Tetracyclic Imidazo[4,5-b]Pyridine-Based Molecules: Insights from Mass Spectrometry and DFT Analysis. Molecules, 30(13), 2684. https://doi.org/10.3390/molecules30132684








