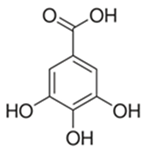
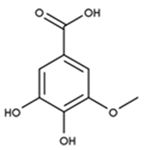
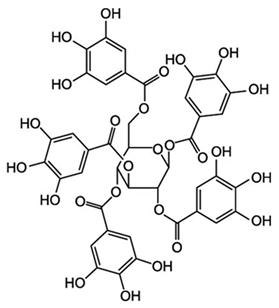
Abstract
This study evaluates the corrosion-inhibiting effects of the methanolic (P1) and the hydroalcoholic (P2) extracts of the Rhus typhina L. leaves on carbon steel (OL37) in 1 M HCl. Extracts were prepared with microwave-assisted extraction and characterized using HPLC and LC-MS. Electrochemical methods (OCP, EIS, PDP) and surface analyses (SEM, EDX) assessed the performance of both extracts. The results showed that the P1 and P2 extracts significantly reduced corrosion rates by forming protective layers on the metal surface, with inhibition efficiencies exceeding 90%, at 1000 ppm concentration, for P1 (93%), for P2 at 800 ppm (91%) and 1000 ppm (94%). The P2 extract demonstrated superior long-term performance, maintaining protection after 96 h of immersion. The extracts function as mixed-type inhibitors, affecting both anodic and cathodic reactions, with physicochemical adsorption demonstrated by the Langmuir isotherm. Overall, the Rhus typhina leaf extracts, particularly the P2 extract, offer a promising, eco-friendly approach to corrosion prevention in acidic environments.
Keywords:
corrosion inhibitors; plant extract; Rhus typhina L.; OL37; HPLC; LC-MS; electrochemical methods 1. Introduction
The uses of carbon steels are numerous, and include kitchen appliances and structural elements. Considering its mechanical qualities and affordability, carbon steel is the material chosen for pipeline construction, and is widely utilized in the oil and gas sector [1]. Because carbon steel is vulnerable to corrode during industrial processes (Figure 1), substantial levels of corrosion inhibition are required for operations to be both safe and economical [2] Corrosion is defined as the degradation of the metallic materials caused by adverse interactions with the surrounding environment, leading to the loss of their physical and chemical properties [3,4,5,6]. Corrosion leads to global economic losses ranging from USD 700 billion to USD 1 trillion annually, and poses serious safety risks due to structural degradation [7,8]. In many industries, such as oil and gas, construction, marine, aircraft, petrochemical, military, and ceramics, corrosion is a major concern.
Figure 1.
Diagram representation of the industries that use carbon steel.
Corrosion rates are especially high in acidic conditions in fields that rely extensively on mild steel infrastructure, resulting in yearly losses estimated to be billions of dollars (USD). Preventing acid–metal contact is therefore the highest priority. By minimizing the direct interaction between metals and acids, organic inhibitors, when they are applied, create a barrier that prevents corrosion [9,10]. An inhibitor is a chemical or natural material that can reduce or stop corrosion in corrosive environments when added in a limited quantity (1 to 15,000 ppm) [11]. An inhibitor can reduce corrosion by affecting the components of a corrosion cell, causing anodic and cathodic resistance, and diffusion inhibition [12].
Corrosion inhibitors are widely used for their simplicity, cost-effectiveness, adaptability, and superior performance in reducing corrosion across diverse environments [13,14]. Currently, various types of corrosion inhibitors have proven effective in preventing corrosion under different conditions, providing significant economic benefits [15]. Conventional inhibitors (imidazolines, mercury salts, phosphates, and polyphosphates) are costly and toxic for the environment, whereas natural alternatives are more environmentally friendly and cost-effective.
Green chemistry is gaining interest for environmental protection and human health [13]. Until now, considerably research has been invested into designing scalable green corrosion inhibitors, including ionic liquids [16,17,18], natural polymers [19,20,21], plant extracts [1,22,23,24], and amino acids [25,26,27].
Plant extracts, rich in flavonoids, alkaloids, triterpenes, sterols, and fatty acids, are favored as green corrosion inhibitors due to their abundant sources and lower environmental impact. They are extensively utilized in the machinery, chemical, energy, oil and gas extraction, and refining industries [13]. Plant-derived alternatives tend to perform best at higher concentrations [15]. Plant extracts used as corrosion inhibitors offer numerous advantages, such as availability, affordability, non-toxicity, and biodegradability. These green inhibitors present a sustainable and cost-effective alternative to conventional methods, primarily due to their low toxicity and environmentally friendly nature [28,29]. The use of plant extracts as corrosion inhibitors dates back to 1930, when Chelidonium majus and other species were employed in pickling baths [30]. More recently, Eddy et al. [31] emphasized the application of vegetable and fruit extracts as effective green inhibitors, particularly under acidic conditions.
Various plant extracts have been discovered to be just as useful as classical corrosion inhibitors, and can be utilized as green corrosion inhibitors. They have a lot of active adsorption centers [32], which can effectively prevent corrosion and build a protective film with the metal surface [15,33]. The effectiveness of using these organic inhibitors to prevent corrosion in carbon steel has been acknowledged in a number of published studies. A considerable number of these studies have investigated the inhibition mechanism and efficiency of organic inhibitors in a carbon steel, 1 M HCl solution system [34]. Numerous scientific studies have investigated the corrosion-inhibiting effect of several natural plant extracts [35,36,37,38,39,40,41,42,43,44,45] as corrosion inhibitors. The most recent examples of various plant part extracts acting as green corrosion inhibitors on different metal surfaces and in diverse corrosive environments are shown in Table 1.

Table 1.
The latest examples of plant part extracts acting as green corrosion inhibitors on various metal surfaces and in diverse corrosive environments.
Rhus typhina L. (staghorn sumac), a species native to Eastern North America, is known for its wide range of pharmacological properties attributed to various parts of the plant [55,56]. The leaves have been reported to possess antioxidative, anticarcinogenic, antiviral, and antimicrobial properties [57]. The fruit has been reported to show significant biological potential, including antioxidative, antimicrobial, anti-inflammatory, and cytotoxic effects against selected cancer cell lines [58]. The wood has been reported to exhibit both antioxidative and antitumorigenic effects [59]. Phytochemical analyses of the Rhus typhina extracts have identified a rich profile of bioactive compounds, including phenolic acids (gallic acid and tannic acid are notably present in the leaves and bark) [60], flavonoids (compounds such as quercetin, kaempferol, and myricetin have been detected in the leaves) [61], hydrolyzable tannins, and organic acids (the fruit contains organic acids, contributing to their astringent and acidic taste, as well as malic, citric, and tartaric acids) [55,62].
The chemical composition of the Rhus typhina L. stem, including gallic acid, tryptophan, scopolin, methyl gallate, fustin, quercetin, rutin [63]. These constituents are largely responsible for the plant’s reported antioxidant, antimicrobial, anti-inflammatory, and cytotoxic activities. All these findings suggest that R. typhina may serve as a valuable source of natural bioactive compounds for different applications. Rhus typhina L. is a valuable plant that deserves further scientific study because of its many applications and therapeutic qualities. This species of sumac is also used in the food industry, in the making of fuels, cosmetics, and insecticides, as well as in the paper industry [64].
However, studies on their potential use as eco-friendly corrosion inhibitors have not yet been published; by electrochemical evaluation, only the weight loss measurement method was used to evaluate the corrosion inhibition efficacy of the Rhus typhina L. leaf extracts [65]. Răuță et al. [65] showed that the Rhus typhina L. leaf extracts, prepared in a 1:1 methanol–water mixture, exhibited excellent corrosion inhibition on mild steel, with efficiencies exceeding 99.9%. Extracts obtained from the summer leaves were more effective than those from the autumn leaves, as indicated by lower mass loss (0.0667 g vs. 0.0897 g), while the untreated control sample showed severe corrosion with a weight loss of 0.5823 g. Based on these findings, summer-collected leaves were selected for the present study.
According to [64], most of the active components are found in the leaves, which is one more reason why we selected this part of the plant for the present study.
This work focused on evaluating the environmental corrosion inhibitors from the Rhus typhina L. leaves extract (RTLE) on OL37 in 1 M HCl environments. High-performance liquid chromatography (HPLC) and liquid chromatography–mass spectrometry (LC-MS), were used to characterize the leaves extract. Open circuit potential (OCP), electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization (PDP) were among the techniques used to assess the corrosion inhibition capacity of the RTLE. Surface morphological features were also examined using the scanning electron microscope (SEM), which is equipped with energy dispersive X-ray spectroscopy (EDX).
2. Results and Discussion
2.1. Quantitative and Qualitative Evaluation of Used Extract
The alcoholic and hydroalcoholic leaf extracts of the Rhus typhina L. composition were evaluated using the HPLC-DAD and LC-MS techniques (Table 2 and Figure 2). Beside a large number of compounds, like phenolic acids, there are three major compounds which mainly account for anticorrosion effects. Further, in this study, it will be noted as the P1 extract, for the methanolic extract of the Rhus typhina L. leaves, and as the P2 extract, for the hydroalcoholic (methanol and water (1:1 v/v) extract of the Rhus typhina L. leaves.

Table 2.
Concentration of some compounds found in the RTLE.
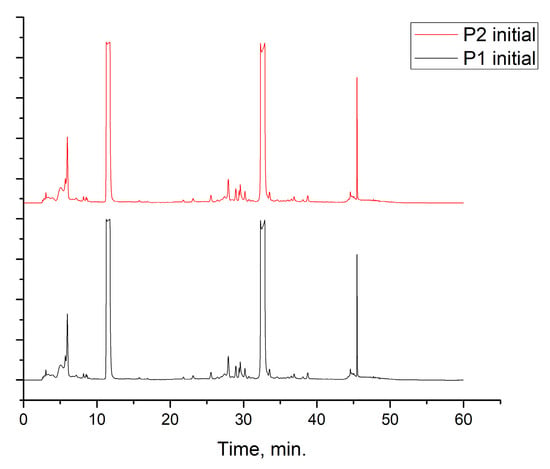
Figure 2.
Chromatogram of the Rhus typhina L. leaf extracts.
The RTLE contain high quantities of phenolic acids and tannins. Not all compounds present in the extract can be identified and quantified even with the tandem HPLC-DAD/LC-MS. Only the major compounds were evaluated and quantified. Initial extracts are too concentrated to be used directly, thus, some dilutions were made in order to better asses the influence on natural compound concentrations. In Figure 3, the chromatograms of 1000, 800, and 500 ppm dilutions were presented before and after the electrochemical process. Initial extracts (Figure 2) were diluted as follow: for obtaining 1000 to 20 ppm solutions we take aliquots of each extracts (1000 to 20 mg) which were diluted to 1 L with 1 M HCl solution.
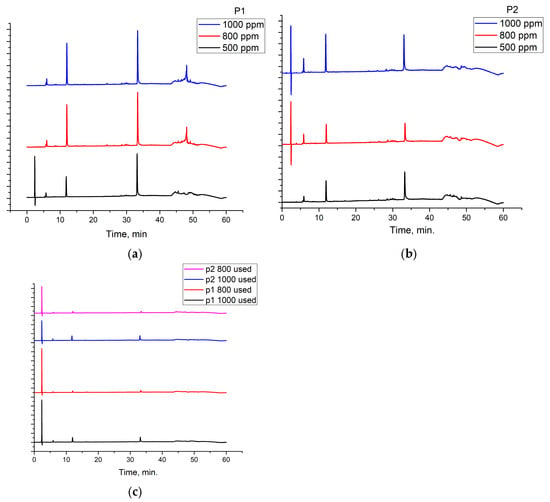
Figure 3.
Chromatograms for the RTLE diluted in 1 M HCl solution used in the electrochemical process: (a,b) before and (c) after the corrosion inhibition.
It can be observed that, in these chromatograms the working solution presents three major peaks which can be identified using the LC-MS technique (Table 3, Supplementary Materials).

Table 3.
Major compounds found in the RTLE involved in the anticorrosion process.
Also, chromatograms for spent solutions (Figure 3c) show a significant reduction of the major compounds’ peak areas as a result of intense adsorption on the metal surface. The adsorption efficiency (Table 4) range is between 34 and 77% depending on the compound and the type of extract. The adsorption efficiency is significant taking into account that, in fact in this process, a competition between these compounds occur on the metal surface. For lower concentrations (presented in the Supplementary Materials), the adsorption efficiency reached 100% for all three compounds.

Table 4.
Adsorption efficiency of natural compounds present in extract on metal surface.
The adsorption efficiency was calculated as follows:
where —represents the peak area of each major compound found in the diluted extracts before corrosion process and —represents the peak area of each major compound found in the diluted extracts after corrosion process. The peak area was used as a replacement for concentrations due to the absence of analytical standards for the two major compounds involved in the corrosion process.
Gallic acid and tannin compounds present an important anticorrosion effect due to the presence of numerous hydroxyl and carbonyl moieties. These groups contain oxygen atoms which are able to donate the lone pair of electrons in an interaction with the metal surface. Also, tannin molecules contain double bonds that are able to bind to electrons in the free orbitals of the metal atoms. Moreover, tannins feature large molecules which can cover the metal surface in a large proportion.
2.2. Electrochemical
2.2.1. Potentiodynamic Polarization Technique
Figure 4A,B shows the polarization curves of the OL37 electrode in 1 M HCl solution with and without different inhibitor concentrations, where the calomel electrode was used as a reference electrode for the analysis.
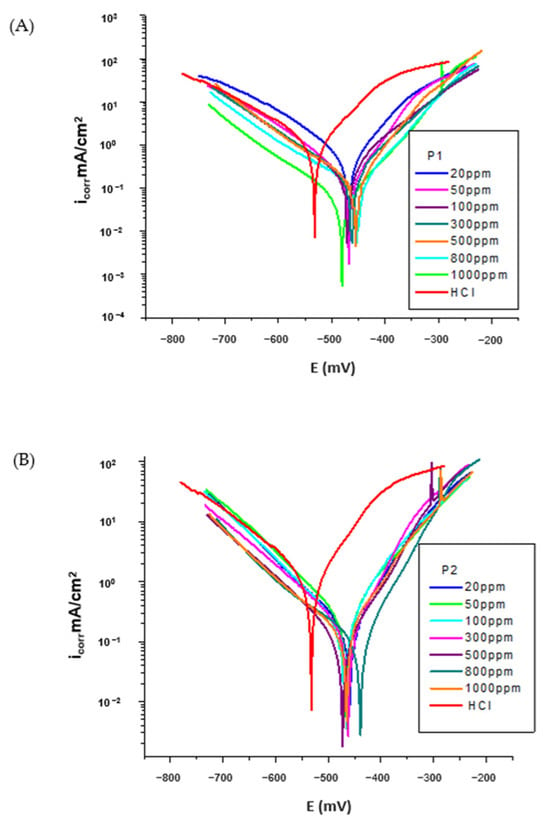
Figure 4.
Polarization curve for OL37 in 1 M HCl at different concentrations of (A) P1 inhibitor at 25 °C and (B) P2 inhibitor at 25 °C.
It was determined that both the cathodic and anodic polarization curves showed a lower current density in the presence of inhibitors than those determined in non-inhibitor solutions. This behavior showed that the corrosion inhibitors significantly suppressed both the cathodic and anodic reactions of the electrochemical processes, with a pronounced influence on the anodic reaction.
Also, in Figure 4A,B, it is observed that both the anodic process of metal dissolution and the cathodic process of hydrogen evolution were prevented by the addition of these environmentally friendly inhibitors in the aggressive medium (HCl).
2.2.2. Corrosion Kinetic Parameters
As shown in the Tafel curves (Figure 4) and the corresponding data presented in Table 5 and Table 6, the corrosion kinetic parameters are categorized into measured and derived values. The experimentally measured parameters include the polarization resistance (Rp), corrosion potential (Ecorr), the anodic and cathodic Tafel slopes (ba and bc), and the corrosion current density (icorr). Based on these, the corrosion rates were subsequently calculated and expressed in terms of Rmpy, penetration rate (Pmm/year), and (Kg). For each measurement the Tafel plot was carefully inspected, and the linear region closest to Ecorr was selected and the linear curve was fitted (by the least squares method) using the VoltaMaster 4 software (v 7.09). The corrosion inhibition efficiency was calculated using Equation (1). The experimental results showed that the addition of these environmentally friendly inhibitors shifted the corrosion potential (Ecorr) to more positive values (increasing the corrosion potential (Ecorr) from −531 mV without inhibitor to −488 mV in the presence of the P1 extract and to −424 mV for the P2 extract, at 1000 ppm). The reduction of the inhibited anodic Tafel slopes (ba) shows the influence of the RTLE on the anodic dissolution process of the metal. This can be attributed to the adsorption of inhibitor molecules on the surface of OL37 and the prevention of corrosion by blocking the anodic dissolution process of the metal.
where is the corrosion current density at 0 ppm, without the RTLE and is the corrosion current density with the RTLE (20–1000 ppm).

Table 5.
Kinetic corrosion parameters of the OL37 carbon steel in 1 M HCl, and at different concentrations of the P1 inhibitor at 25 °C.

Table 6.
Corrosion kinetic parameters of the OL37 carbon steel in 1 M HCl and at different concentrations of the P2 inhibitor at 25 °C.
The electrochemical data shows that the corrosion rate of the OL37 carbon steel in 1 M HCl solution significantly decreases with the addition of the green corrosion inhibitor (sample P1 RTLE). In the absence of the inhibitor (0 ppm), the corrosion current density (icorr) was high, at 0.855 µA·cm−2, and the polarization resistance (Rp) was very low (20 Ω·cm−2), indicating severe corrosion. As the concentration of the inhibitor increased from 20 ppm to 1000 ppm, icorr progressively decreased to 0.059 mA·cm−2, at 1000 ppm P1, while Rp rose suddenly to 243 Ω·cm−2, confirming improved surface protection.
At all concentrations, the corrosion rate, determined by different measures (Pmm/year, g/m²·h), dramatically decreased. The shift in Ecorr is less than 85 mV, indicating that the compound acts as a mixed-type inhibitor with a more pronounced effect on the anodic reaction. Changes in the Tafel slopes (ba and bc) further support that both anodic dissolution and cathodic hydrogen evolution were suppressed, confirming the inhibitor’s dual-action mechanism.
In the absence of the inhibitor, the corrosion current density was highest at 0.855 mA/cm2, indicating active corrosion. The low polarization resistance (20 Ω·cm−2) confirms the high corrosion rate (399 mpy). This establishes the aggressive corrosive nature of 1 M HCl toward the OL37 steel, and serves as the baseline for comparison. The addition of the P2 RTLE inhibitor significantly reduces icorr and Rmpy, and increases Rp, demonstrating its corrosion-inhibiting action. For instance, at 20 ppm, icorr dropped to 0.183 mA/cm2, representing a 79% inhibition efficiency. This trend continues up to 1000 ppm, where icorr reached a minimum of 0.055 mA/cm2, and Rp peaked at 236 Ω·cm−2, corresponding to the maximum inhibition efficiency of 94%.
With increasing concentrations of the P2 extract, the corrosion potential showed slight shifts towards more positive values (from −531 mV at 0 ppm to −464 mV at 20 ppm and −440 mV at 800 ppm). However, these shifts are less than 85 mV, indicating that the P2 extract acts as a mixed-type inhibitor—affecting both anodic and cathodic reactions.
The anodic (ba) and cathodic (bc) Tafel slopes show variations but no systematic trend, suggesting that the inhibition mechanism is not purely controlled by changes in the activation energy but rather by surface coverage and adsorption of the inhibitor molecules on both the anodic and cathodic sites.
By comparative investigation of the inhibition efficiency and corrosion rate (expressed in Rmpy, P, and Kg) for all inhibitors, under the same conditions, it is evident that these potential P1 and P2 inhibitors have excellent efficiency for corrosion protection of the OL37 electrode in 1 M HCl.
2.2.3. Immersion Time
The influence of increasing the immersion period 0–144 h on the corrosion inhibition of the organic P1 and P2 compounds on the corrosion of OL37 in 1 M HCl was analyzed by potentiodynamic polarization. The influence of the protective efficacy of these inhibitors by immersion periods is presented in Figure 5A,B and Table 7 and Table 8.
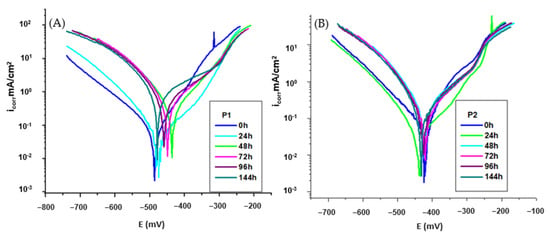
Figure 5.
Polarization curve for OL37 in 1 M HCl at different immersion time at 25 °C for (A) P1 inhibitor and (B) P2 inhibitor.

Table 7.
Kinetic parameters of OL37 in 1 M HCl at different immersion time of the P1 extract at 25 °C.

Table 8.
Kinetic parameters of OL37 in 1 M HCl at different immersion time of the P2 extract at 25 °C.
Despite this, after 96 h of immersion, the inhibition efficiency of the P2 extract remains at 88% (Figure 5B and Table 7) demonstrating that the P2 extract is a highly effective inhibitor over extended exposure periods. It presented a non-degradable behavior after 96 h of immersion in the aggressive medium, further verifying its excellent protective capacity. By contrast, as shown in Figure 5A and Table 6, the inhibition efficiency of the P1 extract drops to 53% after the same immersion period, indicating that the P1 extract is significantly less effective as a long-term corrosion inhibitor.
2.2.4. Electrochemical Impedance Spectroscopy
The corrosion-inhibiting effect of the OL37 steel with environmentally friendly inhibitors was also studied by electrochemical impedance spectroscopy (EIS). The EIS tests present information about the protective properties of these green inhibitors on the OL37 electrodes in the corrosive-HCl environment. Nyquist plots for OL37 uninhibited and in the presence of different inhibitor concentrations are shown in Figure 6.
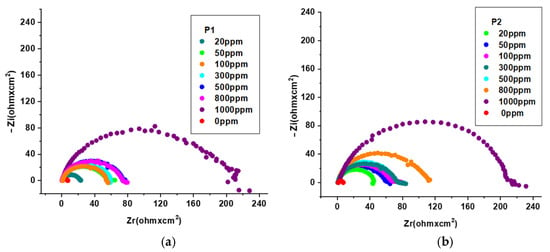
Figure 6.
Nyquist plot for OL37 in 1 M HCl at different inhibitor concentrations at 25 °C for (a) P1 and (b) P2.
Figure 6a,b shows that the diameters of the capacitance loops in the presence of the P1 and P2 extracts are larger than those in the absence, assuming that these inhibitors have excellent protective properties on the OL37 electrode in the 1 M HCl medium.
It is evident from the Nyquist plots that the impedance response of the carbon steel was significantly modified by the addition of the P1 and P2 inhibitors, implying that the protective layer obtained was confirmed by the addition of these two RTLEs.
The Bode plots shown in Figure 7 are consistent with the Nyquist plots (Figure 6). It is evident that, without the inhibitor, the OL37 electrode denotes a time constant suitable for a phase angle of 35°; that is, it exhibits capacitive behavior.
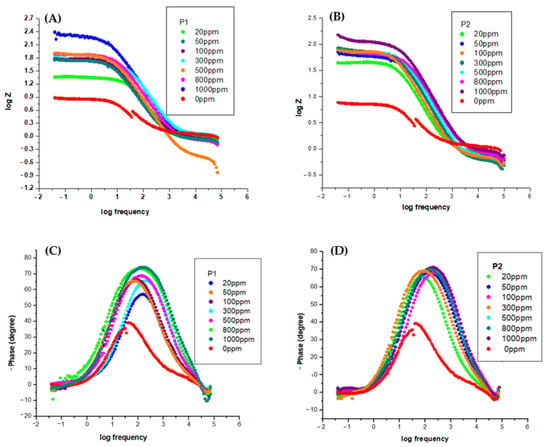
Figure 7.
Bode plot for OL37 in 1 M HCl at different inhibitor concentrations at 25 °C for P1 (A,C) and P2 (B,D).
From Figure 7, it can be seen that the presence of the P1 and P2 RTLE on the “phase angle versus logarithm of frequency” plot shows a very well established maximum attributed to a phase angle of about 75°. Therefore, in this case, the electrode has a higher capacitive behavior in agreement with the Nyquist plots and the experimental data obtained by potentiodynamic polarization.
The analysis of the experimental data was determined by matching the results (splicing the experimental results) to obtain a suitable equivalent circuit shown in Figure 8.
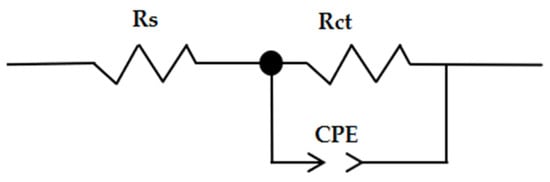
Figure 8.
The equivalent circuit.
The various impedance characteristics, such as solution resistance (Rs), charge transfer resistance (Rct), and double layer capacitance (Cdl), were calculated at the temperature of 25 °C; the results are shown in Table 9 and Table 10.

Table 9.
Electrochemical–EIS parameters of the OL37 carbon steel in 1 M HCl and at different concentrations of the P1 inhibitor at 25 °C.

Table 10.
Electrochemical-EIS parameters of the OL37 carbon steel in 1 M HCl and at different concentrations of the P2 inhibitor at 25 °C.
Electrochemical impedance spectroscopy (EIS) was employed to further evaluate the corrosion inhibition performance of the P1 and P2 inhibitors on the OL37 carbon steel in 1 M HCl at 25 °C. The Nyquist plots were fitted using an equivalent circuit model; the extracted parameters are summarized in Table 9 and Table 10. In the absence of inhibitors, the steel exhibited a low charge transfer resistance (Rct) of 6.32 Ω·cm2, indicative of high corrosion activity. Upon the addition of inhibitors, a substantial increase in Rct was observed, reflecting enhanced corrosion protection. Specifically, the Rct increased to 214 Ω·cm2 for the P1 extract and 223 Ω·cm2 for the P2 extract at a concentration of 1000 ppm, confirming the superior performance of the P2 extract in forming a more resistive protective layer.
Simultaneously, the double-layer capacitance, represented by the constant phase element (Q-Yo), decreased significantly with the increasing inhibitor concentration, suggesting the adsorption of the inhibitor molecules on the metal surface and a consequent reduction in the active surface area available for the charge transfer. The phase shift factor (Q-n) increased from 0.74 in the uninhibited solution to values between 0.88 and 0.90 in the presence of the inhibitors, indicating a transition towards a more homogeneous and capacitive surface.
The EIS results show that the charge transfers resistance (Rct) increased and the double layer capacitance (Cdl) decreased with the concentration of the inhibitor. As a consequence of the increase of the Rct values with the inhibitor concentration, the inhibition effectiveness increases significantly, which demonstrates that the inhibitor exhibits good protective ability on the OL37 electrode corrosion.
Therefore, under these circumstances, the inhibited electrodes have good capacitive behavior according to the Nyquist data and the determinations carried out by the potentiodynamic polarization technique. The increase in the impedance modulus (Zmod) indicates an important protective capacitance, and it is evident that the Zmod increases when the inhibitor film is enhanced. Higher Zmod leads to higher protective efficacy.
The effect of increasing the immersion period (0–144 h) on the corrosion inhibition performance of the environmentally friendly P1 and P2 inhibitors (at 1000 ppm) on the OL37 steel in 1 M HCl was evaluated using the EIS. The results illustrating the protective properties of these inhibitors over time are presented in Figure 9 and Table 11 and Table 12.
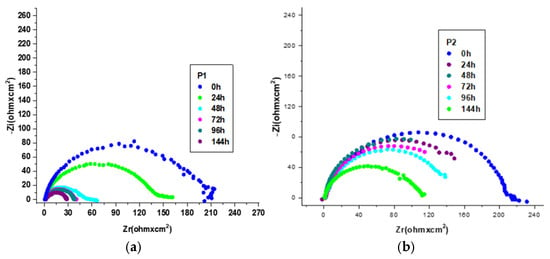
Figure 9.
Nyquist plot for OL37 in 1 M HCl at 1000 ppm inhibitor concentrations at different immersion times at 25 °C for (a) P1 and (b) P2.

Table 11.
Electrochemical–EIS parameters of the OL37 carbon steel in 1 M HCl with 1000 ppm concentrations of the P1 inhibitor at different immersion times at 25 °C.

Table 12.
Electrochemical-EIS parameters of the OL37 carbon steel in 1 M HCl with 1000ppm concentrations of the P2 inhibitor at different immersion times at 25 °C.
As shown in Figure 9, the size of the capacitance loops in the presence of the P1 and P2 inhibitors decreases slightly with increasing immersion time. Moreover, between 0 and 144 h of immersion, a slight reduction in the protective efficiency of the inhibitor film is observed, likely due to the diffusion of corrosive chloride ions (Cl−) through the inhibited surface. The EIS results (Figure 9 and Table 11 and Table 12) confirm the ability of the environmentally friendly P1 and P2 inhibitors to impede the migration of aggressive chloride ions (Cl−) to the metal surface over extended immersion periods. After 96 h of immersion, the inhibition performance of the P2 extract remains stable, demonstrating its effectiveness and durability as a long-term corrosion inhibitor. Its non-degradable behavior in the corrosive medium further confirms its good protective capacity. By contrast, as shown in Figure 9 and Table 11, the inhibition performance of the P1 extract begins to decline after 72 h of immersion, indicating that the P1 extract is significantly less effective for long-term corrosion protection.
2.2.5. Adsorption Isotherm
The adsorption isotherm elucidates the interaction between the organic inhibitor and the OL37 surface, with its protective efficiency attributed to adsorption. The inhibitor’s adsorption is considered the primary corrosion prevention mechanism [66]. To assess the influence of the inhibitor concentration, the data were fitted to the Langmuir isotherm (Figure 10), which showed an excellent correlation with surface coverage, confirming monolayer adsorption behavior.
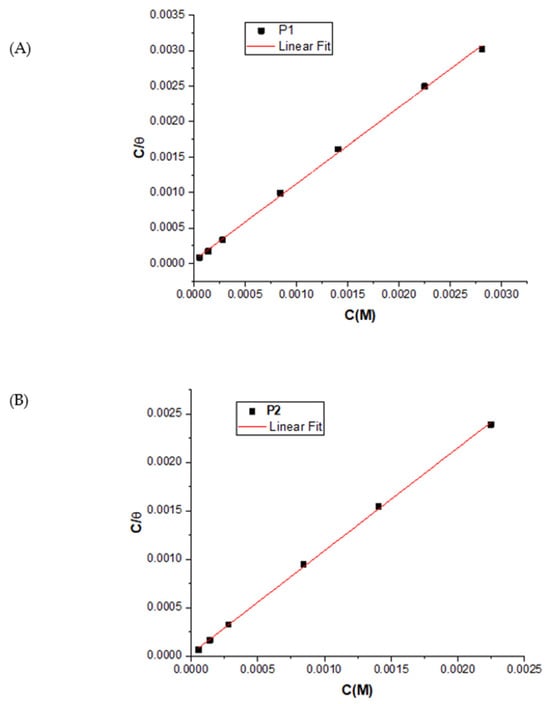
Figure 10.
Langmuir plot for P1 (A) and (B) P2 on OL37 in 1 M HCl.
The Langmuir adsorption isotherm is used to describe the inhibitor’s adsorption mechanism, and is expressed as follows:
where C is the inhibitor concentration, θ is the surface coverage, and K is the adsorption equilibrium constant. Surface coverage (θ) is calculated from electrochemical measurements using the following relation:
where icorr and iinh represent the corrosion current densities in 1 M HCl without and with the inhibitor, respectively.
θ = (icorr − iinh)/icorr
All correlation coefficients (R2 > 0.99), such as P1 (R2 = 0.9986) and P2 (R2 = 0.9996), confirm that the corrosion inhibition is primarily due to the adsorption of the inhibitors onto the substrate. The initial step in the corrosion inhibition process of the metal substrate in 1 M HCl involves the interaction between the inhibitor molecules and the metal surface, as follows:
In this study, linear plots of Cinh/θ versus Cinh with a slope close to 1 (slope for the P1 extract is 1.07, and for the P2 extract is 1.05) confirm that the adsorption of the green inhibitors follows the Langmuir isotherm. The adsorption equilibrium constant (Kads), calculated from the intercept (Table 13), reflects the strength of the adsorption. High Kads values indicate strong surface interaction, effective corrosion inhibition by the P1 and P2 extracts in 1 M HCl, and significant modification of the metal–solution interface, enhancing the corrosion resistance of the OL37 steel.

Table 13.
The values of Kads and for the P1 and P2 extracts on OL37 in 1 M HCl.
The adsorption equilibrium constant (Kads) is related to the standard free energy of adsorption () through the following relationship:
where 55.5 mol/L is the molar concentration of water substituted with 1000 g/L to meet the unit of Kads, R is the universal gas constant, and T is the thermodynamic temperature of the medium.
The negative values indicate that the adsorption of the green inhibitors is spontaneous, with stronger interactions between the inhibitor molecules and the substrate. The values around −20 kJ/mol suggest physical adsorption (electrostatic interaction), while values near −40 kJ/mol or lower indicate chemisorption, where charge sharing or coordination bonds form between the OL37 substrate and the inhibitor molecules (Table 13).
2.2.6. The Reaction Mechanism
Presented below is a detailed overview of the chemical reactions involved in the corrosion process of carbon steel (which contains 99.293% Fe) in acidic environments (in HCl), and how this process can be inhibited by green inhibitors via chemisorption mechanisms, with a schematic representation in Figure 11. The reaction mechanism has been adapted from [34,67,68], and is written below:
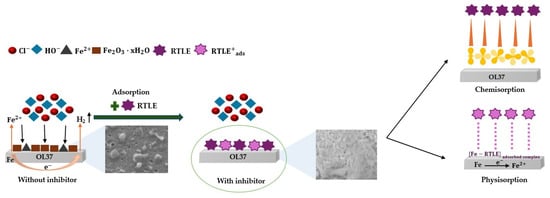
Figure 11.
Schematic representation of the metal surface with adsorption of the main phytochemical constituents from the RTLE as the GCI on the OL37 carbon steel surface, forming a protective film; without the RTLE as the GCI, the surface corrodes.
General Corrosion Mechanism:
Anodic process: metal dissolution, iron oxidation
- (a)
- Without Inhibitor (Acidic medium):
- (b)
- In the presence of ions:
The acidic medium accelerates the anodic reaction, increasing the corrosion rate.
Cathodic process: reduction of oxygen, hydrogen evolution mechanism
Over time, dehydrates and transforms into the following:
Corrosion Inhibition Mechanism (with RTLE—inhibitor):
2.3. SEM and EDX Analysis
Figure 12 displays a scanning electron microscopy (SEM) image illustrating the freshly polished carbon steel (Figure 12A), carbon steel with corrosion products in HCl without inhibitor (Figure 12B), with corrosion inhibitor P1 (Figure 12C) and P2 (Figure 12D). The surface of the corroded carbon steel is irregular and has more imperfections than the carbon steel, which is inhibited, as can be seen visually. Rhus typhina L. successfully protects the carbon steel in acidic solutions including HCl.
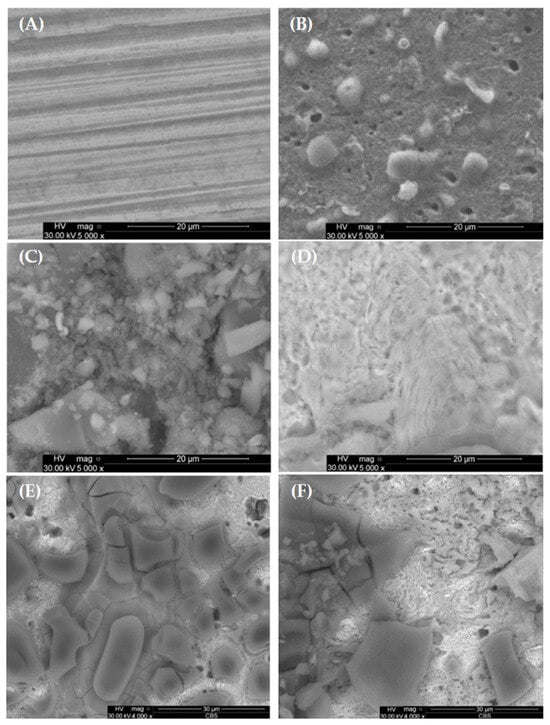
Figure 12.
SEM morphology of carbon steel surface: (A) freshly polished carbon steel; (B) corrosion products in HCl without RTLE; (C) with RTLE, P1 1000 ppm; (D) with RTLE P2 1000 ppm; (E) RTLE, P1 1000 ppm after 96 h immersion time; (F) RTLE P2, 1000 ppm after 96 h immersion time.
A protective layer that forms on the carbon steel plate’s surface can significantly slow down the rate of corrosion, creating a protected metal surface with less surface roughness. The complete surface shown in Figure 12C,D was subjected to the EDX analysis, and Figure 12 and Figure 13 compiles and summarizes the results.
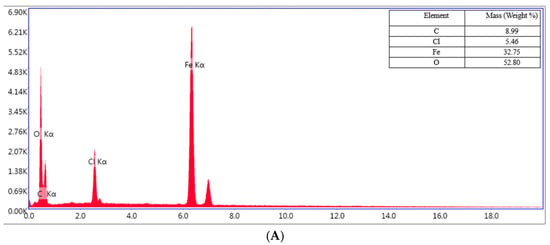
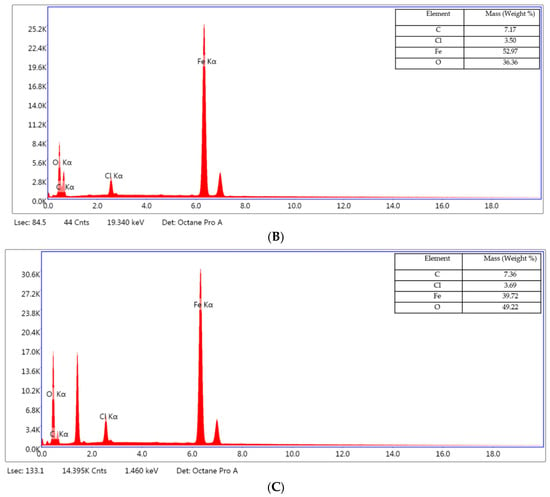
Figure 13.
EDS spectrum for the OL37 uninhibited electrode (A), and protected electrodes with 1000 ppm of P1 (B) and P2 (C).
After 96 h of immersion, the SEM analysis (Figure 12E,F—where E is the OL37 electrode surface with the RTLE, P1, 1000 ppm after 96 h immersion time, and F is the OL37 electrode surface with the RTLE, P2, 1000 ppm after 96 h immersion time) shows that the surface treated with the P2 inhibitor exhibits significantly less surface degradation compared to the surface protected with the P1 inhibitor. This finding, in agreement with the electrochemical results, confirms that the P2 inhibitor maintains a more effective and stable corrosion inhibition over time in acidic conditions than the P1 inhibitor.
The EDX spectrum of the OL37 carbon steel sample immersed in the uninhibited solution reveals characteristic peaks corresponding to a significant presence of chloride (Cl) and oxygen (O) elements (Figure 13A). These elements are associated with the formation of corrosion products on the steel surface. However, upon the addition of the eco-friendly P1 and P2 inhibitors, the concentrations of these elements are reduced, indicating the inhibitors’ protective efficacy against corrosive attack (Figure 13B,C). The inhibitors function by limiting chloride ion adsorption and preventing iron oxidation at the metal surface. In the present study, it is evident that, in the presence of hydrochloric acid, elevated levels of chlorine and oxygen accelerate the corrosion process. Conversely, in the presence of the P1 and P2 inhibitors (extracted from the RTLE), a marked reduction in these elements is observed. This effect is attributed to the formation of a protective film on the steel surface which mitigates corrosion.
3. Materials and Methods
3.1. Extract Preparation and Leaves Extraction
The leaves were collected from the local domestic areas in the summer season (Figure 14). The leaves were non-chemically cleaned, left at room temperature under controlled environmental conditions. The dried vegetal material was evenly shred before the extraction processes began in order to obtain pieces smaller than 2 mm (as verified through screening). Schematic representation of the extraction process, characterization and application of the RTLE as green corrosion inhibitors is illustrated in Figure 15.

Figure 14.
Rhus typhina L. plant (left) and the plant material for the extract (right).

Figure 15.
Schematic representation of the extraction process, characterization and application of the RTLE as green corrosion inhibitors for OL37.
The method used to obtain the natural extracts was a microwave-assisted method that involved heating the vegetal material and the solvent for 60 min at 100 °C with an Ethos Easy Advanced Microwave Digestion System (Milestone S.r.l,, Sorisole, Italy) using an 800 W microwave power. The solvents for the extraction were methanol and ultra-pure water.
The ratio of vegetable material to solvent was kept at 1:10 (w/v), and the solvent included the following:
- Methanol, 100%, noted as P1;
- Hydroalcoholic mixture of methanol and water (1:1 v/v), noted as P2.
Reagent-quality methanol was utilized (Sigma Aldrich, St. Louis, MO, USA), and laboratory-sourced bi-distilled water (GFL 2102 water still, GFL, Burgwedel, Germany) was used for all tests.
3.2. High-Performance Liquid Chromatography (HPLC) Extracts Characterization
For the HPLC mobile phase, we used trifluoroacetic acid, 0.1% (Sigma Aldrich), acetonitrile (Sigma Aldrich), and distilled water. An L-3000 HPLC system (Rigol Technologies Inc., Beijing, China) with a diode-array detector (HPLC-DAD) and a Kinetex EVO C18, 150 × 4.6 mm, 5 µm particle size, Kinetex EVO C18 column (Phenomenex, Torrance, CA, USA) was used to quantify the compounds contained in the extracts. A two-solvent system made up the mobile phase, and a gradient mode elution was used. An amount of 0.1% trifluoroacetic acid (TFA) in water (I) and 0.1% TFA in acetonitrile (II) were the solvents utilized. The following was the elution gradient: 2–100% solvent (II) for 60 min at 35 °C with a 0.6 mL/min elution flow rate. In accordance with the literature, the analysis was carried out at five distinct wavelengths (255, 280, 325, and 355 nm). The injection volume was 10 μL. The LC-MS conditions were as follows: Column Thermo Accord C18 (150 × 4.1 mm, 2.1 μm); Pump operating conditions: gradient; Mobile phase A: water with 0.1% formic acid; Mobile phase B: methanol with 0.1% formic acid and 4 mM ammonium formate; Flow rate: 0.5 mL/min; column temperature: 30 °C; injection volume: 5 μL. Mass spectrometry conditions were as follows: Ion Source: Heated electrospray (HESI-II); Ion Mode: Positive; Capillary Temperature: 280 °C; Vaporizer Temperature: 295 °C; Spray Voltage: 2200 V; Sheath Gas: 32 arbitrary units; Aux Gas: 7 arbitrary units; Scan Type: Full MS scan; Mass Range: m/z 120–1000; Mass Resolution: 70,000
Stock solutions with the reference chemicals from several classes: The following substances were prepared to have a concentration of 1000 µg/mL: phenolic acids (gallic acid); flavonoids (catechin, epicatechin, hyperoside, naringin, naringenin); hydroxycinnamic acids and derivatives (caffeic acid, p-coumaric acid); chlorogenic acids (chlorogenic acid). The concentrations utilized for calibration curves ranged from 10 to 400 µg/mL. At each retention time, the identification and quantification of the compound was accomplished by a comparison with the standard spectra.
3.3. Electrochemical Studies
The working electrode for the corrosion investigation was OL37. The structure of OL37 is represented in Table 14.

Table 14.
OL37 elemental composition.
A standard three-electrode cell setup was used for the tests, with a disk electrode serving as the operational working electrode (Figure 16). A calomel electrode acted as the reference, while a platinum plate served as the opposing electrode next to it. To obtain a mirror-like quality, the working samples (carbon steel) were mechanically polished using sandpapers with different grits (600–4000). After being washed with benzene to remove any oily residue, the electrodes were rinsed with bi-distilled water, allowed to dry at room temperature, and then put into the electrochemical cell.
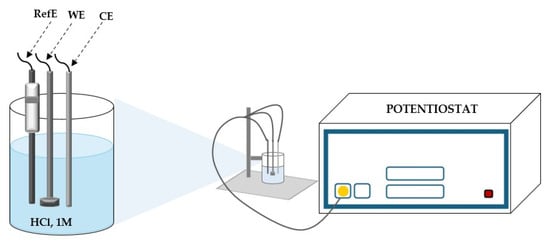
Figure 16.
Diagram of the electrochemical cell, in 1 M HCl (RefE = reference electrode; WE = working electrode OL37; CE = platinum sheet counter electrode).
The corrosive medium was 1 M HCl, achieved by diluting AG 36% HCl (from Merck, Rahway, NJ, USA) with bi-distilled water.
The corrosion inhibition efficiency of the P1 and P2 compounds was investigated for the OL37 carbon steel immersed in the 1 M HCl solution. Electrochemical measurements were carried out using a PGZ 301 potentiostat/galvanostat controlled by VoltaMaster 4 software.
The efficacy of these inhibitors was investigated by potentiostatic and potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). The evaluation of the inhibitor performance was primarily based on the polarization studies and EIS analysis. Experimental impedance determinations were performed at open-circuit potential-OCP in the frequency range from 100 KHz to 40 mHz with an AC wave of ± 10 mV (peak-to-peak).
The results underscore the effectiveness of the corrosion inhibitors as a reliable method for mitigating metal degradation in aggressive media, by suppressing anodic, cathodic, or both electrochemical reactions involved in the corrosion process.
Examination of the Tafel polarization curves was carried out by plotting the potential from cathodic to anodic potential in the potential range of ±250 mV versus OCP, at a sweep rate of 2 mVs−1 for the working OL37 electrode.
For the electrochemical analysis, dilutions were made from the stock extract solution (solvent + natural compounds, P1 and P2), resulting in the following concentrations for each sample: 20, 50, 100, 300, 500, 800, and 1000 ppm.
3.4. Surface Analysis
An extremely effective method for examining the modifications brought on by corrosion on the electrode surface is surface analysis. Consequently, energy dispersive X-ray spectroscopy (EDX) and scanning electron microscopy were used to examine the surface of the OL37 samples. The electron microscopy images were obtained using a Quanta Inspect F50 (FEI Company, Eindhoven, The Netherlands) equipped with a field emission gun (FEG) with a 1.2 nm resolution, and an energy dispersive X-ray spectrometer (EDX) with an MnK resolution of 133 eV Kα.
4. Conclusions
This study demonstrates that the methanolic (P1) and hydroalcoholic (P2) extracts of the Rhus typhina L. leaves are effective green inhibitors for the corrosion of the OL37 carbon steel in 1 M HCl solution. Electrochemical analyses, potentiodynamic polarization, and electrochemical impedance spectroscopy showed that both extracts significantly reduced corrosion rates, with efficiencies reaching 93% for the P1 extract and 94% for the P2 extract at 1000 ppm. These results correlate with increases in polarization resistance and reductions in corrosion current density, confirming a concentration-dependent protective effect.
The observed shift in corrosion potential and surface coverage values suggests that both extracts act as mixed-type inhibitors, with a stronger influence on anodic processes. HPLC profiling identified a variety of phytochemicals, including gallic, caffeic, ferulic, and sinapic acids, compounds known for their antioxidant and metal-chelating properties. These constituents likely adsorb onto the steel surface via a combined physisorption–chemisorption mechanism, forming a barrier that inhibits electron transfer and ion penetration.
Surface analyses (SEM/EDX) confirmed the formation of a continuous, compact protective layer, with the P2 sample showing superior long-term stability and lower morphological degradation after 96 h of immersion. These findings were further supported by Langmuir isotherm modeling, which indicates monolayer adsorption and suggests strong interactions between the inhibitors and the metal substrate.
Overall, the Rhus typhina L. leaf extracts, particularly the P2 extract, demonstrate high potential as sustainable, environmentally friendly alternatives to conventional corrosion inhibitors. Future research should explore their performance under extended immersion times, elevated temperatures, and in other corrosive media, such as sulfuric acid or saline environments. Additionally, expanding the application to other metal substrates and integrating advanced techniques, such as XPS and AFM, would provide deeper insight into the adsorption mechanisms involved.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30122660/s1.
Author Contributions
Conceptualization, F.B., S.-M.A. and E.M.; methodology, D.-I.R. and F.B.; software, F.B., R.-D.T. and S.-M.A.; validation, S.-M.A. and E.M.; formal analysis, D.-I.R., F.B. and E.M.; investigation, D.-I.R., F.B., R.-D.T. and S.-M.A.; data curation, D.-I.R. and E.M.; writing—original draft preparation, D.-I.R.; writing—review and editing, D.-I.R., F.B., R.-D.T., S.-M.A. and E.M.; visualization, E.M.; supervision, E.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RTLE | Rhus typhina L. leaves extract |
| HPLC-DAD | High-performance liquid chromatography with diode-array detection |
| LC-MS | Liquid chromatography–mass spectrometry |
| SEM | Scanning electron microscope |
| EDX | Energy dispersive X-ray analysis |
| GCI | Green corrosion inhibitors |
| OCP | Open circuit potential |
| EIS | Electrochemical impedance spectroscopy |
| PDP | Potentiodynamic polarization |
| XPS | X-ray photoelectron spectroscopy |
| AFM | Atomic force microscopy |
| RefE | reference electrode |
| WE | working electrode OL7 |
| CE | platinum sheet counter electrode. |
References
- Fazal, B.R.; Becker, T.; Kinsella, B.; Lepkova, K. A review of plant extracts as green corrosion inhibitors for CO2 corrosion of carbon steel. npj Mater. Degrad. 2022, 6, 5. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Lavanya, M.; Machado, A.A. Surfactants as biodegradable sustainable inhibitors for corrosion control in diverse media and conditions: A comprehensive review. Sci. Total Environ. 2024, 908, 168407. [Google Scholar] [CrossRef]
- Brantley, W.; Berzins, D.; Iijima, M.; Tufekçi, E.; Cai, Z. 1-Structure/property relationships in orthodontic alloys. In Orthodontic Applications of Biomaterials; Eliades, T., Brantley, W.A., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–38. [Google Scholar]
- Cragnolino, G.A. 2-Corrosion fundamentals and characterization techniques. In Techniques for Corrosion Monitoring; Yang, L., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 6–45. [Google Scholar]
- George Coman, A.B.; Nicolae Costea, M.; Sohaciu, M.; Matei, E.; Predescu, A.; Ciuca, S.; Predescu, C. Corrosion Behavior In Aqueous Environments Of Martensitic Stainless Steels Used In Hydropower Turbine Blades. UPB Sci. Bull. Ser. B 2024, 86, 247–257. [Google Scholar]
- Lazorenko, G.; Kasprzhitskii, A.; Nazdracheva, T.J.C.; Materials, B. Anti-corrosion coatings for protection of steel railway structures exposed to atmospheric environments: A review. Constr. Build. Mater. 2021, 288, 123115. [Google Scholar] [CrossRef]
- Manoj, M.; Kumaravel, A.; Mangalam, R.; Prabunathan, P.; Hariharan, A.; Alagar, M. Exploration of high corrosion resistance property of less hazardous pyrazolidine-based benzoxazines in comparison with bisphenol-F derivatives. J. Coat. Technol. Res. 2020, 17, 921–935. [Google Scholar] [CrossRef]
- Mahraz, M.A.; Salim, R.; Loukili, E.H.; Laftouhi, A.; Haddou, S.; Elrherabi, A.; Bouhrim, M.; Herqash, R.N.; Shahat, A.A.; Eto, B.; et al. Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments–electrochemical, EDX, DFT, and Monte Carlo studies. Open Life Sci. 2025, 20, 20221050. [Google Scholar] [CrossRef]
- El Caid, Z.A.; Left, D.B.; Zertoubi, M. An exploratory assessment supported by experimental and modeling approaches for dinitrophenylhydrazine compound as a potent corrosion inhibitor for carbon steel in sulfuric acid solution. J. Mol. Struct. 2024, 1300, 137218. [Google Scholar] [CrossRef]
- Palanisamy, G. Corrosion inhibitors. In Corrosion Inhibitors; IntechOpen: London, UK, 2019. [Google Scholar]
- Sheydaei, M. The Use of Plant Extracts as Green Corrosion Inhibitors: A Review. Surfaces 2024, 7, 380–403. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wang, C.; Li, Y.; Wu, Y. Exploring the potential of plant extracts as corrosion inhibitors: A comprehensive review. Prog. Org. Coat. 2025, 198, 108915. [Google Scholar] [CrossRef]
- Abdallah, M.; Altass, H.; Al-Gorair, A.S.; Al-Fahemi, J.H.; Jahdaly, B.; Soliman, K. Natural nutmeg oil as a green corrosion inhibitor for carbon steel in 1.0 M HCl solution: Chemical, electrochemical, and computational methods. J. Mol. Liq. 2021, 323, 115036. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, X. Synthesis and evaluation of new type plant extraction corrosion inhibitor. Geoenergy Sci. Eng. 2025, 245, 213495. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Fatyeyeva, K. Ionic liquids as green and sustainable steel corrosion inhibitors: Recent developments. Chem. Eng. J. 2021, 425, 131480. [Google Scholar] [CrossRef]
- Zunita, M.; Kevin, Y. Ionic liquids as corrosion inhibitor: From research and development to commercialization. Results Eng. 2022, 15, 100562. [Google Scholar] [CrossRef]
- Rizvi, M. Characterization, Mechanism, Applications. Nat. Polym. Corros. Inhibitors 2021, 411–434. [Google Scholar]
- Korniy, S.; Zin, I.; Danyliak, M.-O.; Rizun, Y.Y. Eco-friendly metal corrosion inhibitors based on natural polymers (A review). Mater. Sci. 2023, 58, 567–578. [Google Scholar] [CrossRef]
- Ouakki, M.; Aribou, Z.; Dahmani, K.; Saber, I.; Galai, M.; Srhir, B.; Cherkaoui, M.; Verma, C. Natural polymers and their derivatives as corrosion inhibitors. In Phytochemistry in Corrosion Science; CRC Press: Boca Raton, FL, USA, 2024; pp. 76–98. [Google Scholar]
- Alrefaee, S.H.; Rhee, K.Y.; Verma, C.; Quraishi, M.; Ebenso, E.E. Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: Recent advancements. J. Mol. Liq. 2021, 321, 114666. [Google Scholar] [CrossRef]
- Răuță, D.-I.; Matei, E.; Avramescu, S.-M. Recent Development of Corrosion Inhibitors: Types, Mechanisms, Electrochemical Behavior, Efficiency, and Environmental Impact. Technologies 2025, 13, 103. [Google Scholar] [CrossRef]
- Badawi, A.; Fahim, I. A critical review on green corrosion inhibitors based on plant extracts: Advances and potential presence in the market. Int. J. Corros. Scale Inhib. 2021, 10, 1385–1406. [Google Scholar]
- Kumar, D.; Jain, N.; Jain, V.; Rai, B. Amino acids as copper corrosion inhibitors: A density functional theory approach. Appl. Surf. Sci. 2020, 514, 145905. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Rasul, H.H.; Mamad, D.M.; Azeez, Y.H.; Omer, R.A.; Omer, K.A.J.C.; Chemistry, T. Theoretical investigation on corrosion inhibition efficiency of some amino acid compounds. Comput. Theor. Chem. 2023, 1225, 114177. [Google Scholar] [CrossRef]
- Megahed, M.M.; AbdElRhiem, E.; Atta, W.; Ghany, N.A.; Abdelbar, M. Investigation and evaluation of the efficiency of palm kernel oil extract for corrosion inhibition of brass artifacts. Sci. Rep. 2025, 15, 4473. [Google Scholar] [CrossRef]
- Benzidia, B.; Hammouch, H.; Dermaj, A.; Benassaoui, H.; Abbout, S.; Hajjaji, N. Investigation of green corrosion inhibitor based on Aloe vera (L.) Burm. F. for the protection of bronze B66 in 3% NaCl. Anal. Bioanal. Electrochem. 2019, 11, 165–177. [Google Scholar]
- Zouarhi, M.J.E. Bibliographical synthesis on the corrosion and protection of archaeological iron by green inhibitors. Electrochem 2023, 4, 103–122. [Google Scholar] [CrossRef]
- Eddy, N.O.; Ibok, U.J.; Garg, R.; Garg, R.; Iqbal, A.; Amin, M.; Mustafa, F.; Egilmez, M.; Galal, A.M. A Brief Review on Fruit and Vegetable Extracts as Corrosion Inhibitors in Acidic Environments. Molecules 2022, 27, 2991. [Google Scholar] [CrossRef]
- Lukova, P.; Katsarov, P.; Pilicheva, B. Application of Starch, Cellulose, and Their Derivatives in the Development of Microparticle Drug-Delivery Systems. Polymers 2023, 15, 3615. [Google Scholar] [CrossRef]
- Huang, L.; Chen, W.-Q.; Wang, S.-S.; Zhao, Q.; Li, H.-J.; Wu, Y.-C. Starch, cellulose and plant extracts as green inhibitors of metal corrosion: A review. Environ. Chem. Lett. 2022, 20, 3235–3264. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef]
- Royani, A.; Hanafi, M.; Haldhar, R.; Manaf, A. Evaluation of Morinda citrifolia extract as sustainable inhibitor for mild steel in saline environment. J. Eng. Res. 2024, 12, 321–327. [Google Scholar] [CrossRef]
- Royani, A.; Verma, C.; Mujawar, M.; Alfantazi, A.; Aigbodion, V.; Hanafi, M.; Manaf, A. Enhancing the Corrosion Inhibition Performance of Tinospora Cordifolia Extract Using Different Fractions of Methanol Solvent on Carbon Steel Corrosion in A Seawater- Simulated Solution. Appl. Surf. Sci. Adv. 2023, 18, 100465. [Google Scholar] [CrossRef]
- Royani, A.; Hanafi, M.; Mujawar, M.; Priyotomo, G.; Aigbodion, V.; Musabikha, S.; Manaf, A. Unveiling green corrosion inhibitor of Aloe vera extracts for API 5L steel in seawater environment. Sci. Rep. 2024, 14, 14085. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahjani, M.; Hosseini, M.; Safari, R.; Moshrefi, R.; Shiri, H.M. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid. Interface Sci. 2017, 490, 444–451. [Google Scholar] [CrossRef]
- Boudalia, M.; Fernández-Domene, R.M.; Tabyaoui, M.; Bellaouchou, A.; Guenbour, A.; Garcia-Anton, J. Green approach to corrosion inhibition of stainless steel in phosphoric acid of Artemesia herba albamedium using plant extract. J. Mater. Res. Technol. 2019, 8, 5763–5773. [Google Scholar] [CrossRef]
- Kellal, R.; Left, D.B.; Azzi, M.; Zertoubi, M.J. Insight on the corrosion inhibition performance of Glebionis coronaria plant extract in various acidic mediums. J. Appl. Electrochem. 2023, 53, 811–832. [Google Scholar] [CrossRef]
- Fouda, A.E.-A.S.; El-Dossoki, F.I.; Atia, M.F.; Abd El Aziz, F.M.; El-Hossiany, A. Contribution to the corrosion inhibition of aluminum by aqueous extract of Moringa oleifera in 2 M HCl. Discov. Chem. Eng. 2025, 5, 1. [Google Scholar] [CrossRef]
- Fouda, A.; Mohamed, O.; Elabbasy, H.J. Ferula hermonis plant extract as safe corrosion inhibitor for zinc in hydrochloric acid solution. J. Bio- Tribo-Corros. 2021, 7, 135. [Google Scholar] [CrossRef]
- Abdelaziz, S.; Benamira, M.; Messaadia, L.; Boughoues, Y.; Lahmar, H.; Boudjerda, A.J.; Physicochemical, S.A.; Aspects, E. Green corrosion inhibition of mild steel in HCl medium using leaves extract of Arbutus unedo L. plant: An experimental and computational approach. Colloids Surf. A Physicochem. Eng. Aspects 2021, 619, 126496. [Google Scholar] [CrossRef]
- Fouda, A.; El-Awady, G.; El Behairy, W. Prosopis juliflora plant extract as potential corrosion inhibitor for low-carbon steel in 1 M HCl solution. J. Bio- Tribo-Corrosion 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Tan, B.; Xiang, B.; Zhang, S.; Qiang, Y.; Xu, L.; Chen, S.; He, J. Papaya leaves extract as a novel eco-friendly corrosion inhibitor for Cu in H2SO4 medium. J. Colloid. Interface Sci. 2021, 582, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Gapsari, F.; Setyarini, P.H.; Anam, K.; Hadisaputra, S.; Hidayatullah, S.; Purnami; Sulaiman, A.M.; Lai, C.W. Efficacy of Andrographis paniculata leaf extract as a green corrosion inhibitor for mild steel in concentrated sulfuric acid: Experimental and computational insights. Results Surf. Interfaces 2025, 18, 100361. [Google Scholar] [CrossRef]
- Huang, M.; Wang, R.; Xiong, J.; Liu, C.; Wang, J.; Wang, Q. Inhibition effect and adsorption behavior of Michelia alba leaf extract as corrosion inhibitors for Cu in 0.5 M H2SO4. J. Dispers. Sci. Technol. 2025, 1–18. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, S.; Sihmar, A.; Dahiya, H.; Rani, J.; Kumar, S.; Abdi, G.; Abbasi Tarighat, M. A sustainable and green method for controlling acidic corrosion on mild steel using leaves of Araucaria heterophylla. Sci. Rep. 2025, 15, 2225. [Google Scholar] [CrossRef]
- Nguyen, K.D.H.; Manh, T.D.; Nguyen, L.T.P.; Vu, D.T.; Duong Ngo, K.L. Syzygium polyanthum (Wight) Walp. leaf extract as a sustainable corrosion inhibitor for carbon steel in hydrochloric acidic environment. J. Ind. Eng. Chem. 2025, 143, 468–487. [Google Scholar] [CrossRef]
- El-Asri, A.; Rguiti, M.M.; Jmiai, A.; Lin, Y.; El Issami, S. Natal Plum leaf extract as sustainable corrosion inhibitor for Brass in HNO3 medium: Integrated experimental analysis and computational electronic/atomic-scale simulation. J. Taiwan. Inst. Chem. Eng. 2025, 170, 106038. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, B.; He, Y.; Lai, C.; Li, X.; Wei, Y.; Wang, W.; Yao, B.; Wen, X.; Deng, C. Experimental and theoretical insights into Ramie (Boehmeria nivea (L.) Gaudich) leaves extract as an eco-friendly corrosion inhibitor for mild steel in 1.0 M HCl. Surf. Interfaces 2025, 62, 106155. [Google Scholar] [CrossRef]
- Carmona-Hernandez, A.; Barreda-Serrano, M.C.; Saldarriaga-Noreña, H.A.; López-Sesenes, R.; González-Rodríguez, J.G.; Mejía-Sánchez, E.; Ramírez-Cano, J.A.; Orozco-Cruz, R.; Galván-Martínez, R. Insight into the Corrosion Inhibition Performance of Pistia stratiotes Leaf Extract as a Novel Eco-Friendly Corrosion Inhibitor for Mild Steel in 1 M HCl Solution. Molecules 2024, 29, 5243. [Google Scholar] [CrossRef]
- Derfouf, H.; Messaoudi, B.; Attar, T.; Guezzoul, M.H.; Basirun, W.J. Experimental and theoretical study of Atriplex halimus L. leaves extract as an ecologic corrosion inhibitor for XC38 carbon steel in an HCl environment. J. Mol. Liq. 2025, 433, 127785. [Google Scholar] [CrossRef]
- Mamudu, U.; Santos, J.H.; Umoren, S.A.; Alnarabiji, M.S.; Lim, R.C. Investigations of corrosion inhibition of ethanolic extract of Dillenia suffruticosa leaves as a green corrosion inhibitor of mild steel in hydrochloric acid medium. Corros. Commun. 2024, 15, 52–62. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Chemical composition and biological activity of staghorn sumac (Rhus typhina). Food Chem. 2017, 237, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jin, S.; Feng, J.; Yang, T.; Zhai, M.; Sun, G. High performance liquid chromatography fingerprint combined with two-dimensional quantum fingerprint and antioxidant activity to comprehensively evaluate Rhus typhina L. branches quality. Microchem. J. 2025, 209, 112771. [Google Scholar] [CrossRef]
- Antov, G.; Vilhelmova-Ilieva, N.; Nikolova, M.; Nikolova, I.; Simeonova, L.; Grozdanov, P.; Krasteva, M.; Vladimirova, A.; Gospodinova, Z. In vitro evaluation of anticancer, antiviral, and antioxidant properties of an aqueous methanolic extract of Rhus typhina L. leaves. Int. Food Res. J. 2023, 30, 774–782. [Google Scholar] [CrossRef]
- Kossah, R.; Zhang, H.; Chen, W. Antimicrobial and antioxidant activities of Chinese sumac (Rhus typhina L.) fruit extract. Food Control. 2011, 22, 128–132. [Google Scholar] [CrossRef]
- Arlandini, E.; Gelmini, F.; Testa, C.; Angioletti, S.; Beretta, G. GC-MS analysis and biological activity of hydroalcholic extracts and essential oils of Rhus typhina L. wood (anacardiaceae) in comparison with leaves and fruits. Nat. Prod. Res. 2021, 35, 4764–4768. [Google Scholar] [CrossRef]
- McCune, L.M. Traditional Medicinal Plants of Indigenous Peoples of Canada and Their Antioxidant Activity in Relation to Treatment of Diabetes. In Bioactive Food as Dietary Interventions for Diabetes; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2013; Chapter 22; pp. 221–234. [Google Scholar]
- Zannou, O.; Oussou, K.F.; Chabi, I.B.; Alamou, F.; Awad, N.M.H.; Miassi, Y.E.; Loké, F.C.V.; Abdoulaye, A.; Pashazadeh, H.; Redha, A.A.; et al. Phytochemical and nutritional properties of sumac (Rhus coriaria): A potential ingredient for developing functional foods. J. Future Foods 2025, 5, 21–35. [Google Scholar] [CrossRef]
- Demchik, S.; Rajangam, A.S.; Hall, J.; Singsaas, E. Fatty Acids, Carbohydrates and total proteins of wild sumac (Rhus typhina L.) drupes from the upper Midwest of the United States. Am. J. Essent. Oils Nat. Prod. 2015, 3, 30–34. [Google Scholar]
- Liu, T.; Li, Z.; Li, R.; Cui, Y.; Zhao, Y.; Yu, Z. Composition analysis and antioxidant activities of the Rhus typhina L. stem. J. Pharm. Anal. 2019, 9, 332–338. [Google Scholar] [CrossRef]
- Cocîrlea, M.; Oancea, S. Rhus typhina–from environmental threat to industry development opportunity. Curr. Trends Nat. Sci. 2023, 12, 305–320. [Google Scholar] [CrossRef]
- Răuță, D.L.G.; Marius, E.; Avramescu, S. Corrosion inhibition of mild steel by rhus typhina leaf extract in hcl solutions. UPB Sci. Bull. Ser. B 2024, 86, 12. [Google Scholar]
- Branzoi, F.; Băran, A.; Mihai, M.A.; Zaki, M.Y. The Inhibition Action of Some Brij-Type Nonionic Surfactants on the Corrosion OLC 45 in Various Aggressive Environments. Materials 2024, 17, 1378. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tian, H.; Zou, F.; Li, W.; Qiang, Y.; Hou, B. Turning Waste into Treasure: Invasive Plant Ambrosia trifida L Leaves as a High-Efficiency Inhibitor for Steel in Simulated Pickling Solutions. Materials 2024, 17, 3758. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Lgaz, H.; Verma, D.; Ebenso, E.E.; Bahadur, I.; Quraishi, M. Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase: A review. J. Mol. Liq. 2018, 260, 99–120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).