Boron-Centered Compounds: Exploring the Optical Properties of Spiro Derivatives with Imidazo[1,5-a]Pyridines
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Optical Properties
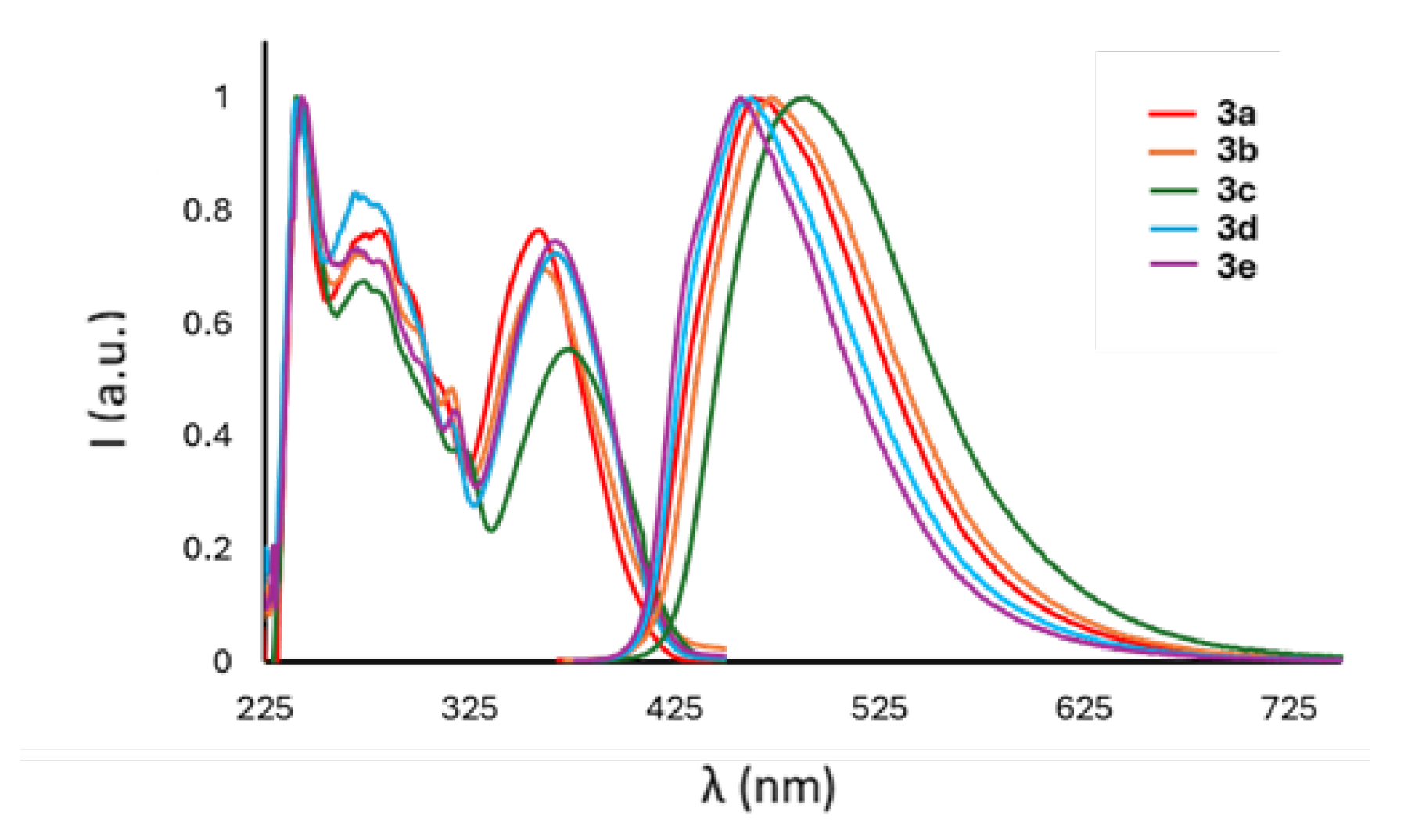
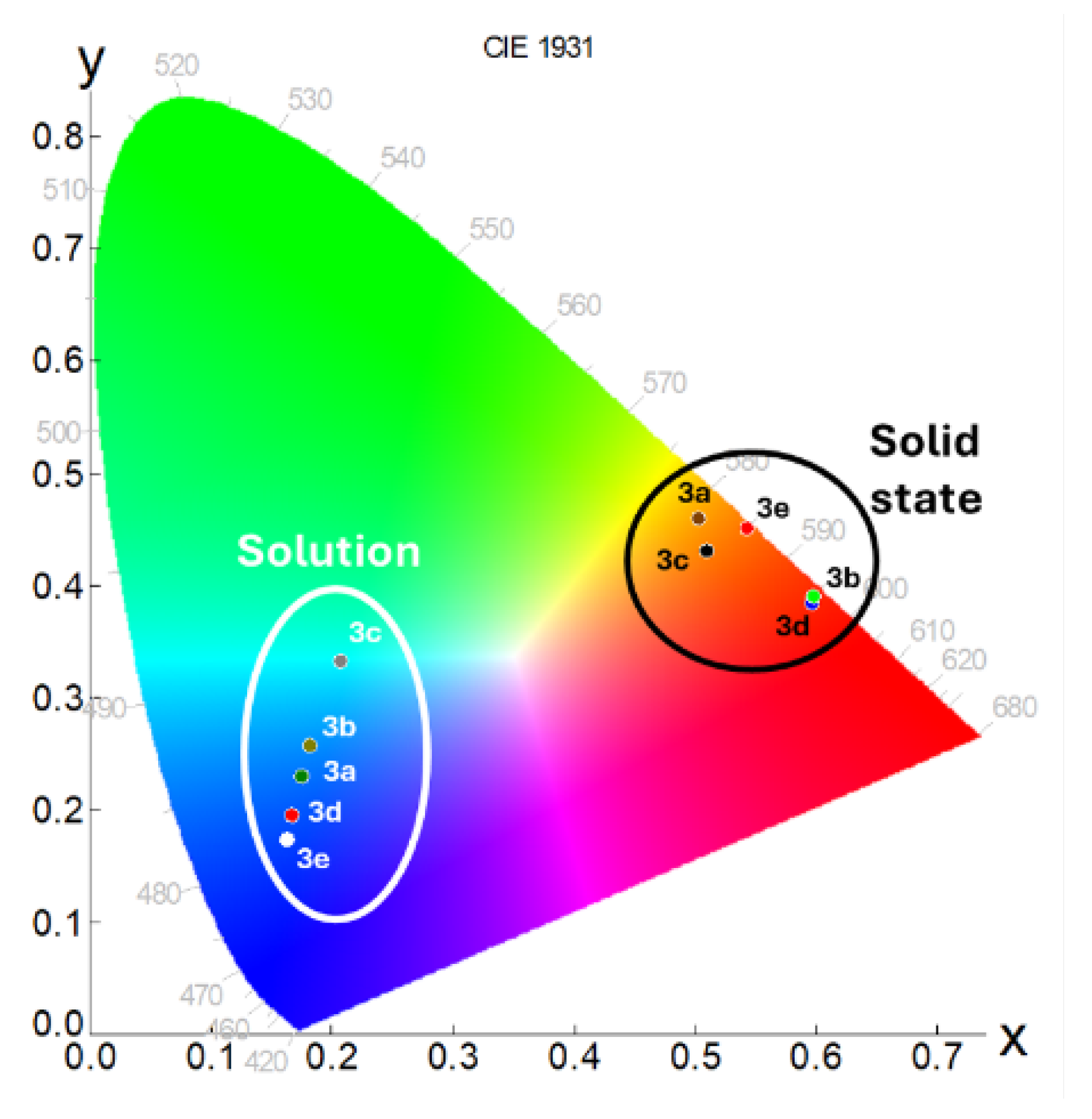
2.2.1. Analysis in Solution
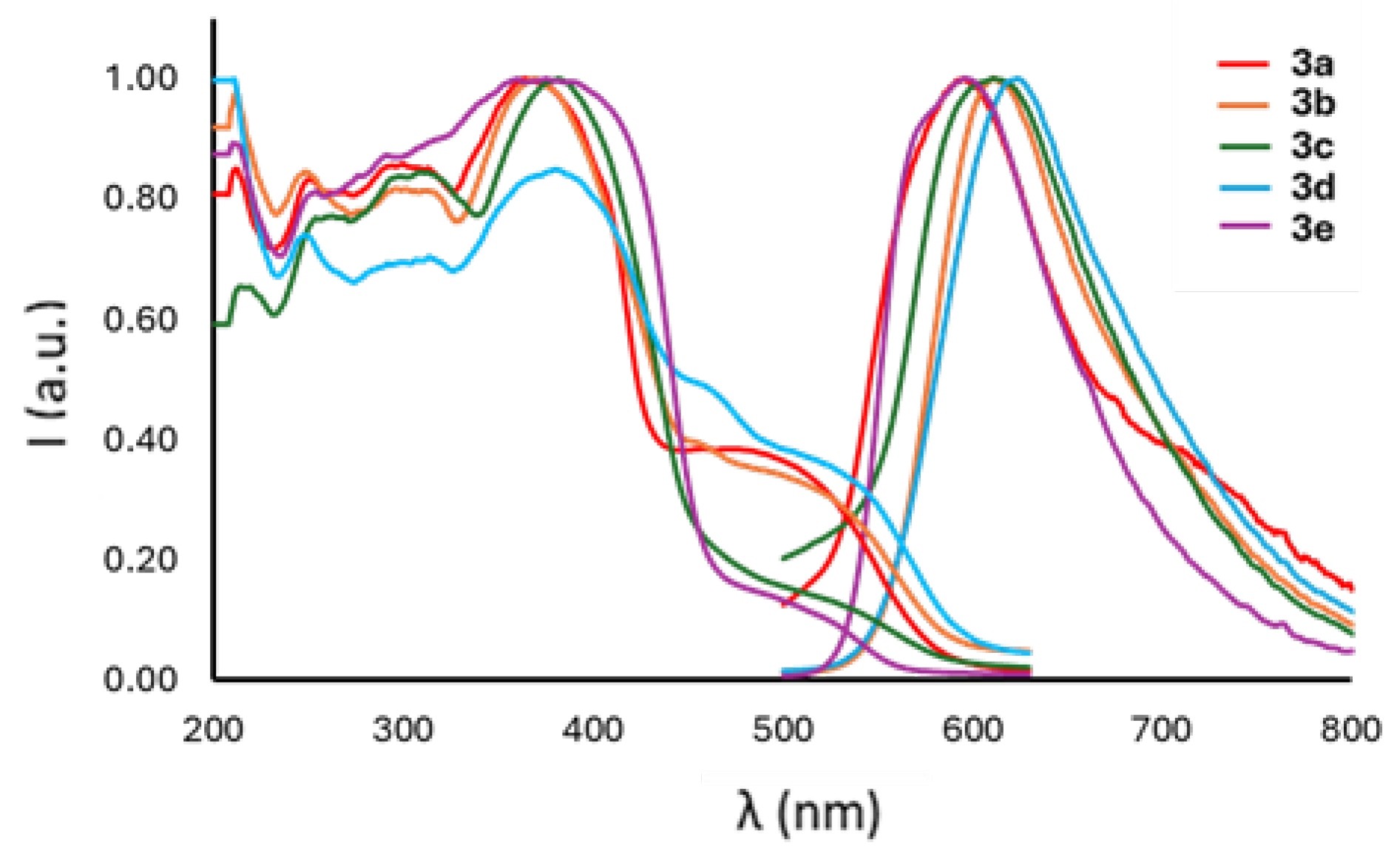
2.2.2. Solid State Analysis
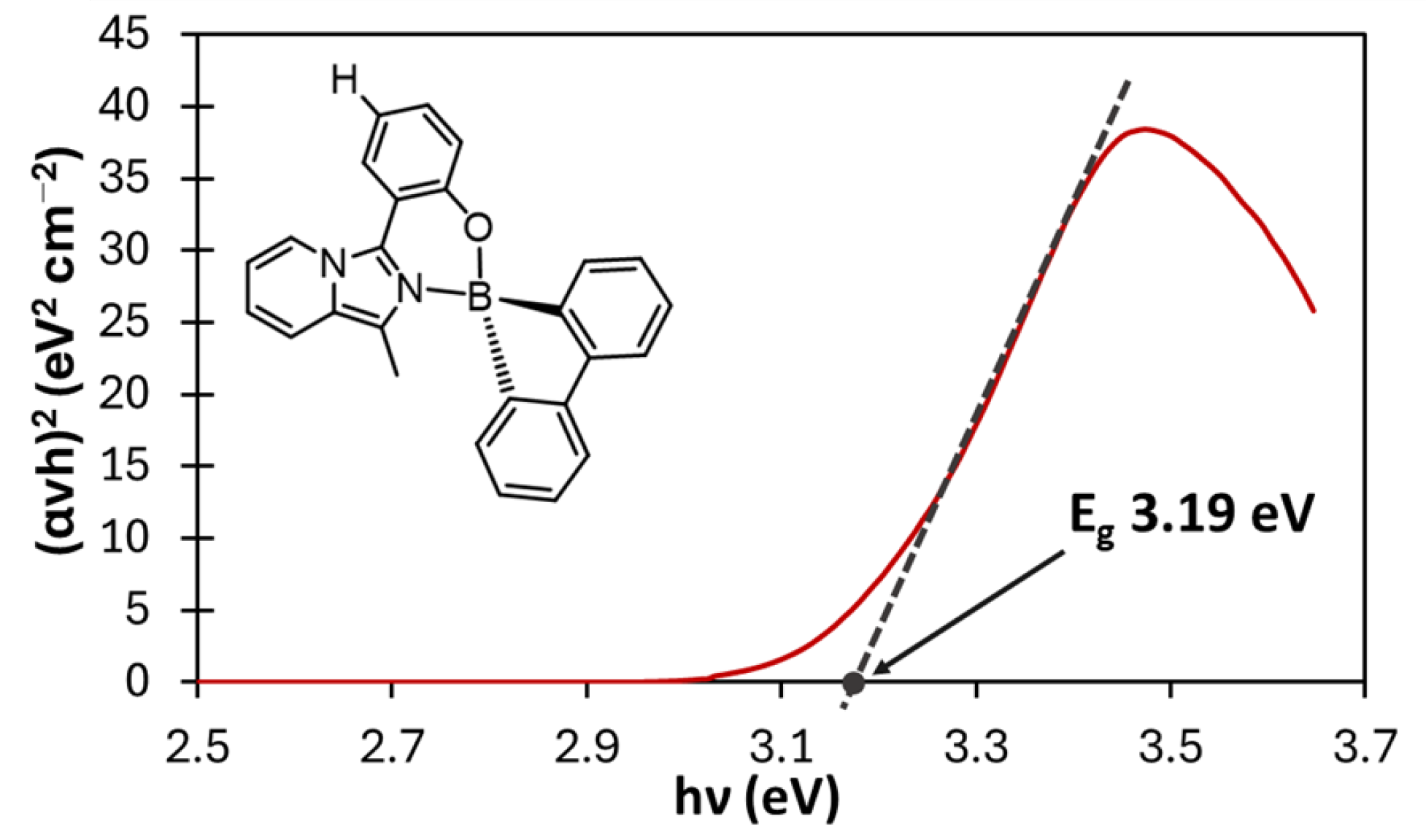
2.2.3. UV-Vis Optical Gap
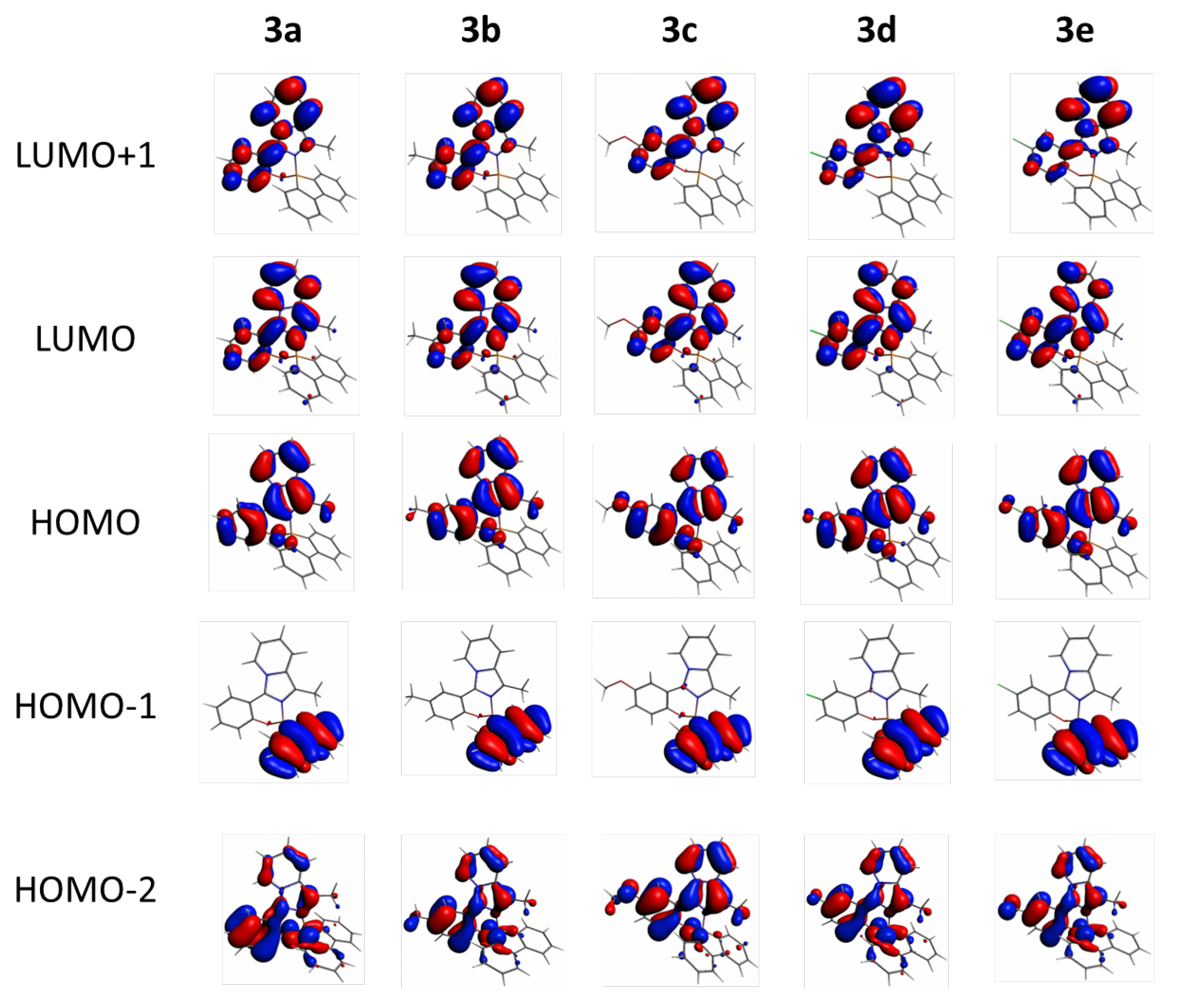
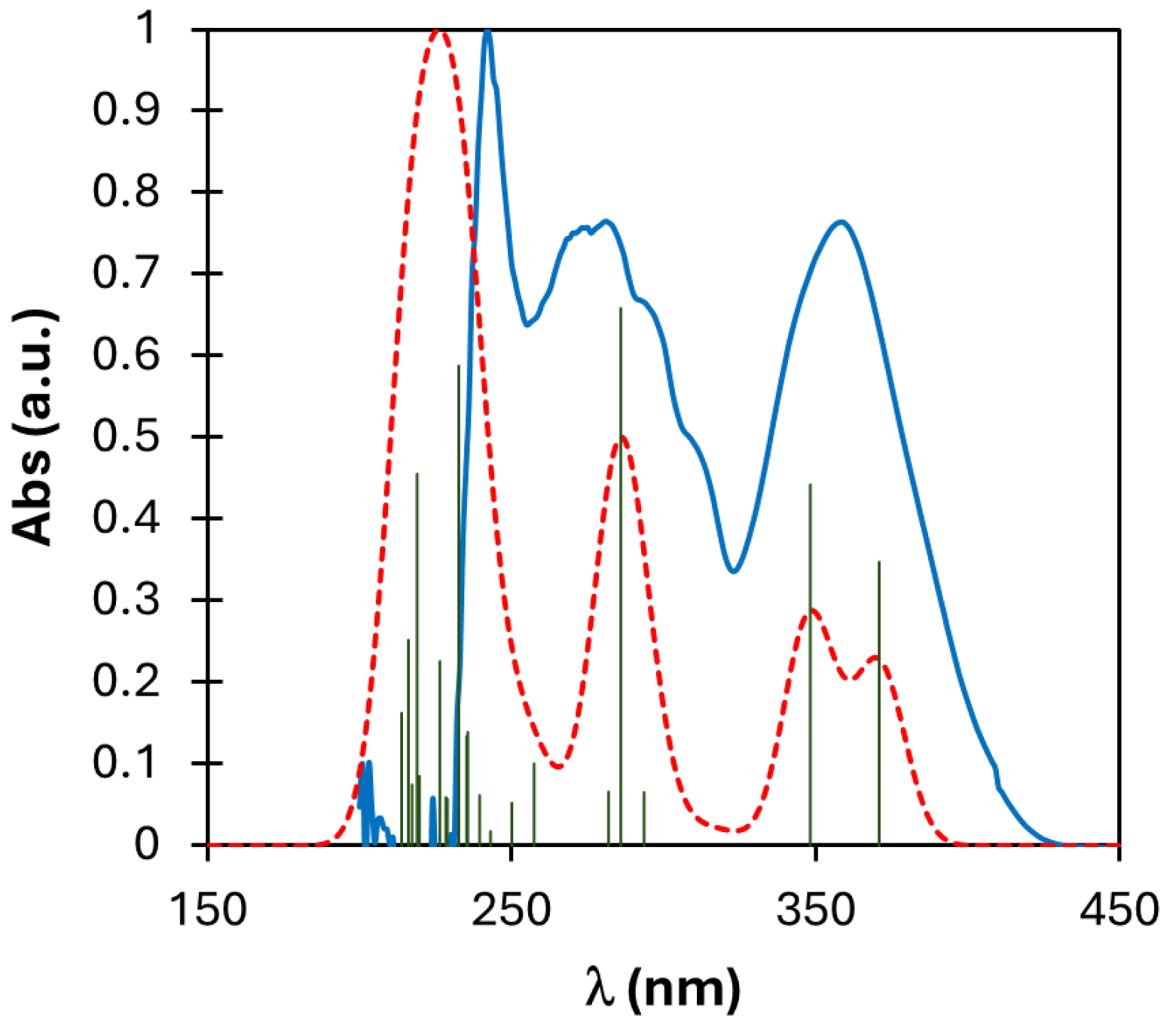
2.3. DFT Calculations
3. Experimental Section
3.1. Materials and Methods
3.2. General Procedure for the Synthesis of Boron Compounds 3a–e
3.3. X-Ray Characterization
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, I.; Kumar, S.; Tsai, Y.-F.; Gautam, P.; Shahnawaz; Kesavan, K.; Lin, J.-T.; Khai, L.; Chou, K.-H.; Choudhury, A.; et al. Status and Challenges of Blue OLEDs: A Review. Nanomaterials 2023, 13, 2521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yang, C. Blue fluorescent emitters: Design tactics and applications in organic light-emitting diodes. Chem. Soc. Rev. 2013, 42, 4963. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Stimuli and shape responsive ‘boron-containing’ luminescent organic materials. J. Mater. Chem. C 2016, 4, 2647. [Google Scholar] [CrossRef]
- Ito, S.; Gon, M.; Tanaka, K.; Chujo, Y. Recent developments in stimuli-responsive luminescent polymers composed of boron compounds. Polym. Chem. 2021, 12, 6372. [Google Scholar] [CrossRef]
- Tanaka, K.; Gon, M.; Ito, S.; Ochi, J.; Chujo, Y. Recent progresses in the mechanistic studies of aggregation-induced emission-active boron complexes and clusters. Coord. Chem. Rev. 2022, 472, 214779. [Google Scholar] [CrossRef]
- Qi, Y.; Ding, N.; Wang, Z.; Xu, L.; Fang, Y. Mechanochromic wide-spectrum luminescence based on a monoboron complex. ACS Appl. Mater. Interfaces 2019, 11, 8676. [Google Scholar] [CrossRef]
- Entwistle, C.D.; Marder, T.B. Boron chemistry lights the way: Optical properties of molecular and polymeric systems. Angew. Chem. Int. Ed. 2002, 41, 2927. [Google Scholar] [CrossRef]
- Bumagina, N.A.; Antina, E.V.; Ksenofontov, A.A.; Antina, L.A.; Kalyagin, A.A.; Berezin, M.B. Basic structural modifications for improving the practical properties of BODIPY. Coord. Chem. Rev. 2022, 469, 214684. [Google Scholar] [CrossRef]
- Kuznetsova, R.T.; Askenova, Y.V.; Prokopenko, A.A.; Bashkirtsev, D.E.; Tel’minov, E.N.; Arabei, S.M.; Pavich, T.A.; Solovyov, K.N.; Antina, E.V. Spectral-luminescent, photochemical, and lasing characteristics of boron dipyrromethene difluoro (III) derivatives in liquid and solid-state media. Russ. Phys. J. 2016, 59, 568. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Wang, C.; Huang, S.; Guo, J.; Wang, Y. Construction of full-color-tunable and strongly emissive materials by functionalizing a boron-chelate four-ring-fused π-conjugated core. J. Mater. Chem. 2012, 22, 4319. [Google Scholar] [CrossRef]
- Kim, H.J.; Godumala, M.; Kim, K.S.; Yoon, J.; Kim, C.Y.; Park, H.; Kwon, J.H.; Cho, J.M.; Choi, D.H. Color-Tunable Boron-Based Emitters Exhibiting Aggregation-Induced Emission and Thermally Activated Delayed Fluorescence for Efficient Solution-Processable Nondoped Deep-Blue to Sky-Blue OLEDs. Adv. Opt. Mater. 2020, 8, 1902175. [Google Scholar] [CrossRef]
- Huang, F.; Fan, X.-C.; Cheng, Y.-C.; Xie, Y.; Luo, S.; Zhang, T.; Wu, H.; Xiong, X.; Yu, J.; Zhang, D.-D.; et al. Multiple Resonance Organoboron OLED Emitters with High Efficiency and High Color Purity via Managing Long-and Short-Range Charge-Transfer Excitations. Adv. Opt. Mater. 2023, 11, 2202950. [Google Scholar] [CrossRef]
- Fung, T.H.-C.; Wong, C.-L.; Tang, W.-K.; Leung, M.-Y.; Low, K.-H.; Yam, V.W.-W. Photochromic dithienylethene-containing four-coordinate boron (iii) compounds with a spirocyclic scaffold. Chem. Commun. 2022, 58, 4231. [Google Scholar] [CrossRef]
- Zhang, Z.; Zang, H.; Jiao, C.; Ye, K.; Zhang, H.; Zhang, J.; Wang, Y. 2-(2-Hydroxyphenyl) benzimidazole-based four-coordinate boron-containing materials with highly efficient deep-blue photoluminescence and electroluminescence. Inorg. Chem. 2015, 54, 2652. [Google Scholar] [CrossRef]
- Urban, M.; Durka, K.; Górka, P.; Wiosna-Sałyga, G.; Nawara, K.; Jankowski, P.; Luliński, S. The effect of locking π-conjugation in organoboron moieties in the structures of luminescent tetracoordinate boron complexes. Dalton Trans. 2019, 48, 8642. [Google Scholar] [CrossRef]
- Stanoppi, M.; Lorbach, A. Boron-based donor-spiro-acceptor compounds exhibiting thermally activated delayed fluorescence (TADF). Dalton Trans. 2018, 47, 10394. [Google Scholar] [CrossRef] [PubMed]
- Nandi, P.R.; Sudhakar, P.; Kalluvettukuzhy, N.K.; Thilagar, P. Triarylborane-appended anils and boranils: Solid-state emission, mechanofluorochromism, and phosphorescence. Chem. Eur. J. 2020, 26, 16306. [Google Scholar] [CrossRef]
- Volpi, G.; Rabezzana, R. Imidazo [1,5-a]pyridine derivatives: Useful, luminescent and versatile scaffolds for different applications. New J. Chem. 2021, 45, 5737. [Google Scholar] [CrossRef]
- Volpi, G.; Laurenti, E.; Rabezzana, R. Imidazopyridine Family: Versatile and Promising Heterocyclic Skeletons for Different Applications. Molecules 2024, 29, 2668. [Google Scholar] [CrossRef]
- Ge, Y.Q.; Li, F.R.; Zhang, Y.J.; Bi, Y.S.; Cao, X.Q.; Duan, G.Y.; Wang, J.W.; Liu, Z.L. Synthesis, crystal structure, optical properties and antibacterial evaluation of novel imidazo[1,5-a]pyridine derivatives bearing a hydrazone moiety. Luminescence 2013, 29, 293. [Google Scholar] [CrossRef]
- Nirogi, R.; Mohammed, A.R.; Shinde, A.K.; Bagaraju, N.; Gagginapalli, S.R.; Ravella, S.R.; Kota, L.; Bhyrapuneni, G.; Muddana, N.R.; Benade, V.; et al. Synthesis and SAR of Imidazo[1,5-a]pyridine derivatives as 5-HT4 receptor partial agonists for the treatment of cognitive disorders associated with Alzheimer’s disease. Eur. J. Med. Chem. 2015, 103, 289. [Google Scholar] [CrossRef]
- Kamal, A.; Rao, A.V.S.; Lakshma Nayak, V.; Reddy, N.V.; Swapna, K.; Ramakrishna, G.; Alvala, M. Synthesis and biological evaluation of imidazo[1,5-a]pyridine-benzimidazole hybrids as inhibitors of both tubulin polymerization and PI3K/Akt pathway. Org. Biomol. Chem. 2014, 12, 9864. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.R.; Darapaneni, C.M.; Patil, R.D.; Kumari, H. Recent synthetic methodologies for imidazo[1,5-a]pyridines and related heterocycles. Org. Biomol. Chem. 2022, 20, 3440. [Google Scholar]
- Volpi, G. Luminescent Imidazo[1,5-a]pyridine Scaffold: Synthetic Heterocyclization Strategies-Overview and Promising Applications. Asian J. Org. Chem. 2022, 11, e202200171. [Google Scholar] [CrossRef]
- Colombo, G.; Ardizzoia, G.A.; Brenna, S. Imidazo[1,5-a]pyridine-based derivatives as highly fluorescent dyes. Inorganica Chim. Acta 2022, 535, 120849. [Google Scholar] [CrossRef]
- Kong, X.; Zhao, J.; Yang, L.; Wang, F.; Sun, Z. A novel 2-(2-aminophenyl) imidazo[1,5-a]pyridine-based fluorescent probe for rapid detection of phosgene. Anal. Bioanal. Chem. 2024, 416, 329. [Google Scholar] [CrossRef]
- Cerrato, V.; Volpi, G.; Priola, E.; Giordana, A.; Garino, C.; Rabezzana, R.; Diana, E. Mono-, Bis-, and Tris-Chelate Zn(II) Complexes with Imidazo[1,5-a]pyridine: Luminescence and Structural Dependence. Molecules 2023, 28, 3703. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Liu, C.; Zhang, P.; Qin, K.; Ge, Y. An Imidazo [1, 5-a] pyridine Benzopyrylium-Based NIR Fluorescent Probe with Ultra-Large Stokes Shifts for Monitoring SO2. Molecules 2023, 28, 515. [Google Scholar] [CrossRef]
- Albrecht, G.; Rössiger, C.; Herr, J.M.; Locke, H.; Yanagi, H.; Göttlich, R.; Schlettwein, D. Optimization of the Substitution Pattern of 1,3-Disubstituted Imidazo[1,5-a]Pyridines and -Quinolines for Electro-Optical Applications. Phys. Status Solidi B 2020, 257, 1900677. [Google Scholar] [CrossRef]
- Hutt, J.T.; Jo, J.; Olasz, A.; Chen, C.-H.; Lee, D.; Aron, Z.D. Fluorescence Switching of Imidazo[1,5-a]pyridinium Ions: pH-Sensors with Dual Emission Pathways. Org. Lett. 2012, 14, 3162. [Google Scholar] [CrossRef]
- Volpi, G.; Lace, B.; Garino, C.; Priola, E.; Artuso, E.; Cerreia Vioglio, P.; Barolo, C.; Fin, A.; Genre, A.; Prandi, C. New substituted imidazo[1,5-a]pyridine and imidazo[5,1-a]isoquinoline derivatives and their application in fluorescence cell imaging. Dye. Pigment. 2018, 157, 298. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.; Hou, P. A novel imidazo[1, 5-a]pyridine-based fluorescent probe with a large Stokes shift for imaging hydrogen sulfide. Sens. Actuators B Chem. 2018, 256, 1086. [Google Scholar] [CrossRef]
- Ge, Y.; Ji, R.; Shen, S.; Cao, X.; Li, F. A ratiometric fluorescent probe for sensing Cu2+ based on new imidazo[1,5-a]pyridine fluorescent dye. Sens. Actuators B Chem. 2017, 245, 875. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Brenna, S.; Therrien, B. The Adaptable Coordination Chemistry of 6-Chloro-2-(quinolin-2-yl)-2,4-dihydro-1H-benzo[d][1,3]oxazine Towards Zinc(II) and Mercury(II). Eur. J. Inorg. Chem. 2010, 21, 3365. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Brenna, S.; Bea, M.; Therrien, B. A Quantitative Description of the σ-Donor and π-Acceptor Properties of Substituted Phenanthrolines. Eur. J. Inorg. Chem. 2016, 23, 3829. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Brenna, S.; Civati, F.; Colombo, V.; Sironi, A. A phosphorescent copper(I) coordination polymer with sodium 3,5-dimethyl-4-sulfonate pyrazolate. CrystEngComm 2017, 19, 6020. [Google Scholar] [CrossRef]
- Marchesi, A.; Brenna, S.; Ardizzoia, G.A. Synthesis and emissive properties of a series of tetrahydro(imidazo[1,5-a]pyrid-3-yl) phenols: A new class of large Stokes shift organic dyes. Dye. Pigment. 2019, 161, 457. [Google Scholar] [CrossRef]
- Colombo, G.; Romeo, A.; Ardizzoia, G.A.; Furrer, J.; Therrien, B.; Brenna, S. Boron difluoride functionalized (tetrahydroimidazo[1,5-a]pyridin-3-yl) phenols: Highly fluorescent blue emissive materials. Dye. Pigment. 2020, 182, 108636. [Google Scholar] [CrossRef]
- Colombo, G.; Ardizzoia, G.A.; Furrer, J.; Therrien, B.; Brenna, S. Driving the Emission Towards Blue by Controlling the HOMO-LUMO Energy Gap in BF2-Functionalized 2-(Imidazo[1,5-a]pyridin-3-yl)phenols. Chem. Eur. J. 2021, 27, 12380. [Google Scholar] [CrossRef]
- Colombo, G.; Cinco, A.; Ardizzoia, G.A.; Brenna, S. Long-Alkyl Chain Functionalized Imidazo[1,5-a]pyridine Derivatives as Blue Emissive Dyes. Colorants 2023, 2, 179. [Google Scholar] [CrossRef]
- Colombo, G.; Cinco, A.; Furrer, J.; Therrien, B.; Brenna, S.; Ardizzoia, G.A. Luminescent blue emissive bis(alkynyl) borane compounds with a N,O-coordinated ligand. Dyes Pigment. 2023, 220, 111722. [Google Scholar] [CrossRef]
- Yagishita, F.; Hoshi, K.; Mukai, S.; Kinouchi, T.; Katayama, T.; Yoshida, Y.; Minagawa, K.; Furube, A.; Imada, Y. Effect of Phenolic Substituent Position in Boron Complexes of Imidazo[1,5-a]pyridine. Asian J. Org. Chem. 2022, 11, e202200040. [Google Scholar] [CrossRef]
- Hoshi, K.; Itaya, M.; Tahara, K.; Matsumoto, A.; Tabata, A.; Nagamune, H.; Yoshida, Y.; Hase, E.; Minamikawa, T.; Yasui, T.; et al. Two-photon excitable boron complex based on tridentate imidazo[1,5-a]pyridine ligand for heavy-atom-free mitochondria-targeted photodynamic therapy. RSC Adv. 2021, 11, 26403. [Google Scholar] [CrossRef] [PubMed]
- Nemytov, A.I.; Utepova, A.I.; Belskaya, N.P.; Eltyshev, A.K.; Yalunina, V.A.; Chupakhin, N.O.; Slepukhin, P.A. Four-coordinate boron luminophores with N,O-heterobiaryl chelating ligands. Synthesis, structure and photoluminescent properties. Dye. Pigment. 2023, 220, 11726. [Google Scholar] [CrossRef]
- Berger, C.J.; He, G.; Merten, C.; McDonald, R.; Ferguson, M.J.; Rivard, E. Synthesis and Luminescent Properties of Lewis Base-Appended Borafluorenes. Inorg. Chem. 2014, 53, 1475. [Google Scholar] [CrossRef]
- Goze, C.; Ulrich, G.; Ziessel, R. Tetrahedral boron chemistry for the preparation of highly efficient “cascatelle” devices. J. Org. Chem. 2007, 72, 313. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- Fialho, C.B.; Cruz, T.F.C.; Rodrigues, A.I.; Calhorda, M.J.; Vieira Ferreira, L.F.; Pander, P.; Dias, F.B.; Morgado, J.; Maçanita, A.L.; Gomes, P.T. 9-Borafluoren-9-yl and diphenylboron tetracoordinate complexes of F- and Cl-substituted 8-quinolinolato ligands: Synthesis, molecular and electronic structures, fluorescence and application in OLED devices. Dalton Trans. 2023, 52, 4933. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, X.; Mellerup, S.K.; Kozin, I.; Wang, S. Spiro-BODIPYs with a diaryl chelate: Impact on aggregation and luminescence. J. Org. Chem. 2017, 82, 13481. [Google Scholar] [CrossRef]
- Marek-Urban, P.H.; Urban, M.; Wiklińska, M.; Paplińska, K.; Woźniak, K.; Blacha-Grzechnik, A.; Durka, K. Heavy-Atom Free spiro Organoboron Complexes As Triplet Excited States Photosensitizers for Singlet Oxygen Activation. J. Org. Chem. 2021, 86, 12714. [Google Scholar] [CrossRef]
- Urban, M.; Wrochna, K.; Marek-Urban, P.H.; Natkowski, D.R.; Woźniak, K.; Pander, P.; Monkman, A.P.; Durka, K.; Luliński, S. Strongly fluorescent spiro-type tetracoordinate complexes of dibenzo[b,e][1,4]thiaborinine dioxide with functionalized 2-(benzo[d]heterazol-2-yl)phenolate ligands displaying TADF. J. Mater. Chem. C 2025, 13, 146. [Google Scholar] [CrossRef]
- Chandra Garain, B.; Samanta, P.K.; Pati, S.K. Intersystem Crossing in Boron-Based Donor–Spiro–Acceptor Organic Chromophore: A Detailed Theoretical Study. Dalton Trans. 2021, 125, 6674. [Google Scholar]
- Klein, J.; Kampermann, L.; Mockenhaupt, B.; Behrens, M.; Strunk, J.; Bacher, G. Limitations of the Tauc plot method. Adv. Funct. Mater. 2023, 33, 2304523. [Google Scholar] [CrossRef]
- Zanatta, A.R. Revisiting the optical bandgap of semiconductors and the proposal of a unified methodology to its determination. Sci. Rep. 2019, 9, 11225. [Google Scholar] [CrossRef]
- Aderne, R.E.; Borges, B.G.A.L.; Ávila, H.C.; von Kieseritzky, F.; Hellberg, J.; Koehler, M.; Cremona, M.; Roman, L.S.; Araujo, C.M.; Rocco, M.L.M.; et al. On the energy gap determination of organic optoelectronic materials: The case of porphyrin derivatives. Mater. Adv. 2022, 3, 1791. [Google Scholar] [CrossRef]
- Djohan, N.; Estrada, R.; Sari, D.; Dahrul, M.; Kurniawan, A.; Iskandar, J.; Hardhienata, H.; Irzaman. The effect of annealing temperature variation on the optical properties test of LiTaO3 thin films based on Tauc Plot method for satellite technology. IOP Conf. Ser. Earth Environ. Sci. 2017, 54, 012093. [Google Scholar] [CrossRef]
- Jubu, P.R.; Obasaeki, O.S.; Nathan-Abutu, A.; Yam, F.K.; Yusof, Y.; Ochang, M.B. Dispensability of the conventional Tauc’s plot for accurate bandgap determination from UV–vis optical diffuse reflectance data. Results Opt. 2022, 9, 100273. [Google Scholar] [CrossRef]
- Bredas, J.-L. Mind the gap! Mater. Horiz. 2014, 1, 17. [Google Scholar] [CrossRef]
- Dittmer, A.; Izsák, R.; Neese, F.; Maganas, D. Accurate band gap predictions of semiconductors in the framework of the similarity transformed equation of motion coupled cluster theory. Inorg. Chem. 2019, 58, 9303. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, A. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226. [Google Scholar] [CrossRef]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comp. Chem. 2021, 22, 931. [Google Scholar] [CrossRef]
- Fonseca Guerra, C.; Snijders, J.G.; Te Velde, G.; Baerends, E.J. Towards an order-N DFT method. Theor. Chem. Acc. 1998, 99, 391. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ziegler, T.; Autschbach, J.; Bashford, D.; Bérces, A.; Bickelhaupt, F.M.; Bo, C.; Boerrigter, P.M.; Cavallo, L.; Chong, D.P.; et al. ADF2014, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: https://www.scm.com (accessed on 7 June 2025).
- Chong, D.P. Augmenting basis set for time-dependent density functional theory calculation of excitation energies: Slater-type orbitals for hydrogen to krypton. Mol. Phys. 2005, 103, 749. [Google Scholar] [CrossRef]
- Klamt, A.; Schürmann, G.J. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799. [Google Scholar] [CrossRef]
- Klamt, A.; Jonas, V. Treatment of the outlying charge in continuum solvation models. J. Chem. Phys. 1996, 105, 9972. [Google Scholar] [CrossRef]
- Pye, C.C.; Ziegler, T. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package. Theor. Chem. Acc. 1999, 101, 396. [Google Scholar] [CrossRef]

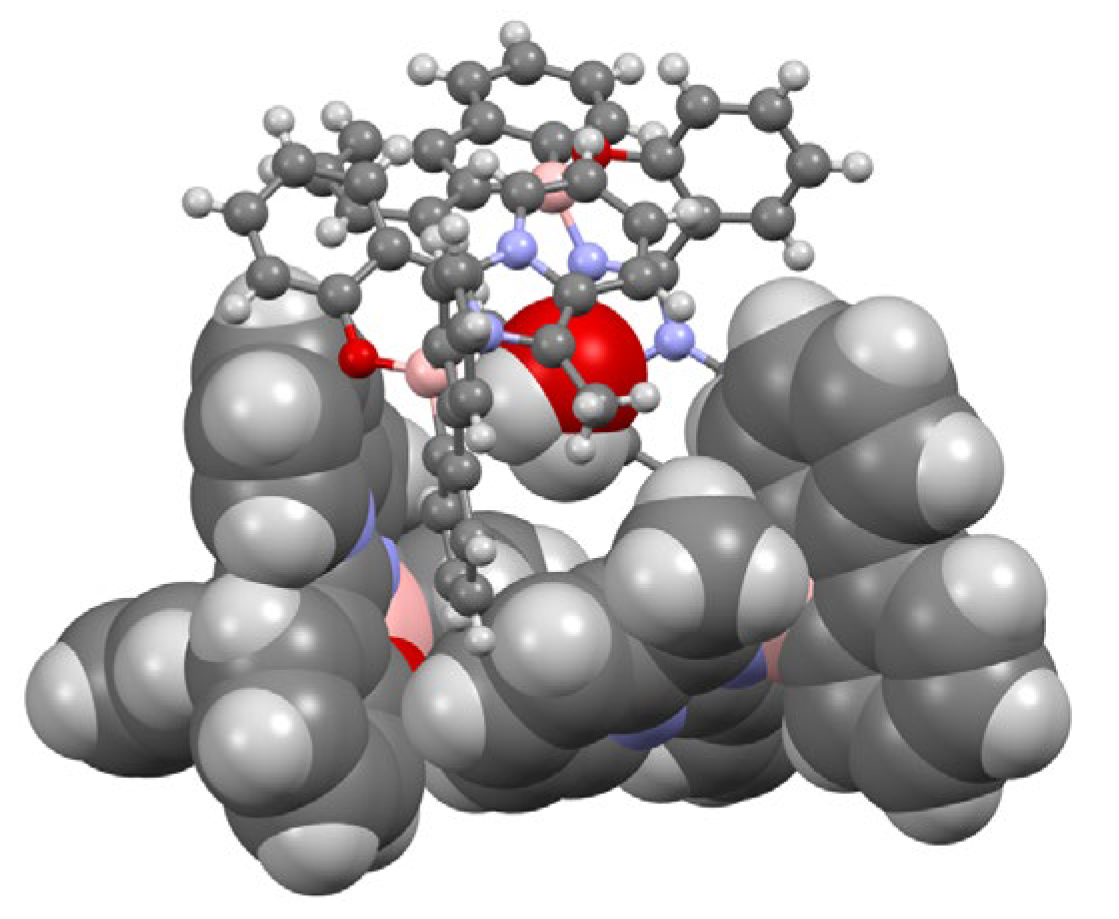
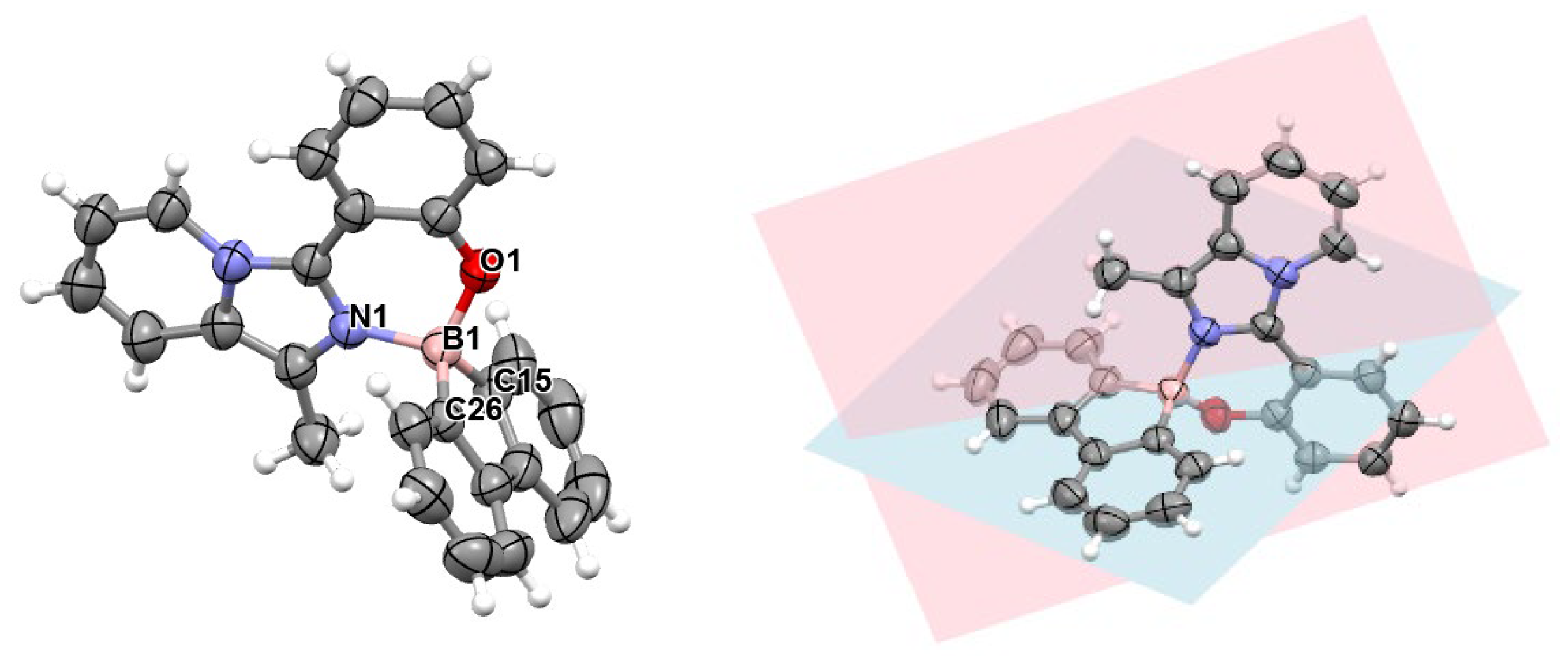
| Distances (Å) | Angles (°) | ||
|---|---|---|---|
| N(1)-B(1)-O(1) | 105.8 (1) | ||
| B(1)-N(1) | 1.592 (2) | N(1)-B(1)-C(15) | 115.3 (1) |
| B(1)-O(1) | 1.484 (2) | N(1)-B(1)-C(26) | 110.4 (1) |
| B(1)-C(15) | 1.611 (2) | O(1)-B(1)-C(15) | 110.4 (1) |
| B(1)-C(26) | 1.615 (2) | O(1)-B(1)-C(26) | 114.8 (1) |
| C(15)-B(1)-C(26) | 100.5 (1) |
| R | σp | λabs (nm) | λexc (nm) | λem (nm) | Stokes Shift (cm−1) | Stokes Shift (eV) | ΦPL | τ (ns) | kr (107 s−1) | knr (108 s−1) | ε (M−1 cm−1) | Optical Band Gap (eV) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3a | H | 0 | 358 | 357 | 463 | 6533 | 0.81 | 0.20 | 3.2 | 6.3 | 2.5 | 15,523 | 3.19 |
| 3b | Me | −0.17 | 361 | 364 | 472 | 6613 | 0.82 | 0.12 | 2.7 | 4.4 | 3.3 | 15,077 | 3.13 |
| 3c | OMe | −0.27 | 373 | 372 | 489 | 7017 | 0.87 | 0.13 | 2.8 | 4.6 | 3.1 | 10,165 | 3.01 |
| 3d | F | 0.06 | 367 | 367 | 462 | 5726 | 0.71 | 0.22 | 3.3 | 6.7 | 2.4 | 15,049 | 3.07 |
| 3e | Cl | 0.23 | 366 | 369 | 457 | 5484 | 0.68 | 0.23 | 3.1 | 7.4 | 2.5 | 16,499 | 3.06 |
| R | σp | λabs (nm) | λexc (nm) | λem (nm) | Stokes Shift (cm−1) | Stokes Shift (eV) | ΦPL | τ (ns) | |
|---|---|---|---|---|---|---|---|---|---|
| 3a | H | 0 | 367 | 416 | 595 | 10,441 | 1.29 | <0.05 | 2.09 (80.52%) 3.66 (19.48%) |
| 3b | Me | −0.17 | 368 | 532 | 612 | 10,834 | 1.34 | <0.05 | 2.16 (52.52%) 3.80 (47.48%) |
| 3c | OMe | −0.27 | 387 | 529 | 611 | 9473 | 1.17 | <0.05 | 3.06 (81.69%) 6.54 (18.31%) |
| 3d | F | 0.06 | 385 | 533 | 624 | 9948 | 1.23 | <0.05 | 1.68 (57.51%) 3.56 (42.49%) |
| 3e | Cl | 0.23 | 381 | 502 | 596 | 9468 | 1.17 | <0.05 | 0.46 (96.13%) 2.35 (3.87%) |
| 3a | 3b | 3c | 3d | 3e | |
|---|---|---|---|---|---|
| LUMO+1 | −1.40 | −1.39 | −1.42 | −1.48 | −1.49 |
| LUMO | −1.60 | −1.59 | −1.62 | −1.70 | −1.71 |
| HOMO | −5.83 | −5.76 | −5.72 | −5.85 | −5.88 |
| HOMO-1 | −6.19 | −6.18 | −6.18 | −6.21 | −6.21 |
| HOMO-2 | −6.70 | −6.58 | −6.36 | −6.68 | −6.69 |
| 3a | HOMO → LUMO | 86.3% |
| HOMO-1 → LUMO | 5.2% | |
| HOMO-2 → LUMO | 4.9% | |
| HOMO → LUMO+1 | 1.7% | |
| 3b | HOMO → LUMO | 89.0% |
| HOMO-2 → LUMO | 5.2% | |
| HOMO → LUMO+1 | 2.5% | |
| HOMO-1 → LUMO | 1.5% | |
| 3c | HOMO → LUMO | 88.4% |
| HOMO → LUMO+1 | 5.7% | |
| HOMO-2 → LUMO | 4.0% | |
| 3d | HOMO → LUMO | 79.0% |
| HOMO → LUMO+1 | 8.7% | |
| HOMO-1 → LUMO | 6.3% | |
| HOMO-2 → LUMO | 3.4% | |
| HOMO-2 → LUMO+1 | 1.6% | |
| 3e | HOMO → LUMO | 81.0% |
| HOMO → LUMO+1 | 6.8% | |
| HOMO-1 → LUMO | 5.8% | |
| HOMO-2 → LUMO | 4.0% | |
| HOMO-2 → LUMO+1 | 1.5% |
| SP 1 | Relaxed 2 | Relaxed 2 | |
|---|---|---|---|
| Optical Eg | PBE0 (IP/EA) | PBE0 (IP/EA) | PBE-D3(BJ) (IP/EA) |
| 3.19 | 3.82 | 3.46 | 3.15 |
| error % | 19.7 | 8.5 | −1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinco, A.; Ardizzoia, G.A.; Brenna, S.; Therrien, B.; Colombo, G. Boron-Centered Compounds: Exploring the Optical Properties of Spiro Derivatives with Imidazo[1,5-a]Pyridines. Molecules 2025, 30, 2552. https://doi.org/10.3390/molecules30122552
Cinco A, Ardizzoia GA, Brenna S, Therrien B, Colombo G. Boron-Centered Compounds: Exploring the Optical Properties of Spiro Derivatives with Imidazo[1,5-a]Pyridines. Molecules. 2025; 30(12):2552. https://doi.org/10.3390/molecules30122552
Chicago/Turabian StyleCinco, Anita, G. Attilio Ardizzoia, Stefano Brenna, Bruno Therrien, and Gioele Colombo. 2025. "Boron-Centered Compounds: Exploring the Optical Properties of Spiro Derivatives with Imidazo[1,5-a]Pyridines" Molecules 30, no. 12: 2552. https://doi.org/10.3390/molecules30122552
APA StyleCinco, A., Ardizzoia, G. A., Brenna, S., Therrien, B., & Colombo, G. (2025). Boron-Centered Compounds: Exploring the Optical Properties of Spiro Derivatives with Imidazo[1,5-a]Pyridines. Molecules, 30(12), 2552. https://doi.org/10.3390/molecules30122552










