Amide-Based Anti-Wear/Extreme-Pressure Additives for Silica-Thickened Greases: Structure and Wear Resistance
Abstract
1. Introduction
2. Results and Discussion
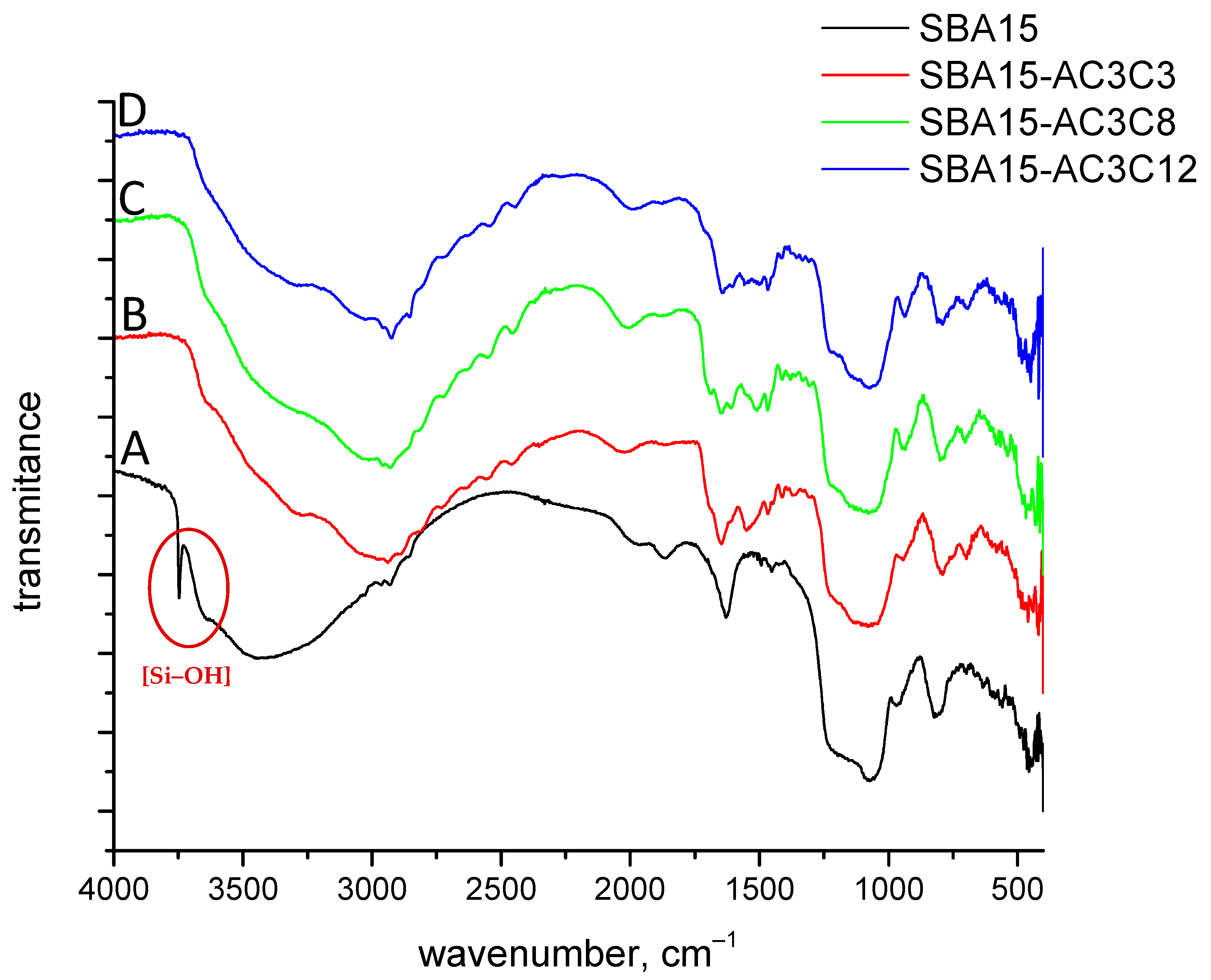
2.1. Characterization of Free Amides and SBA-15-Grafted Compounds
2.2. Characterization of Mixtures of PAO Base Oil and Investigated Additives
2.3. Analysis of Silica-Thickened Greases Containing Free Amides
3. Materials and Methods
3.1. Analytical Methods
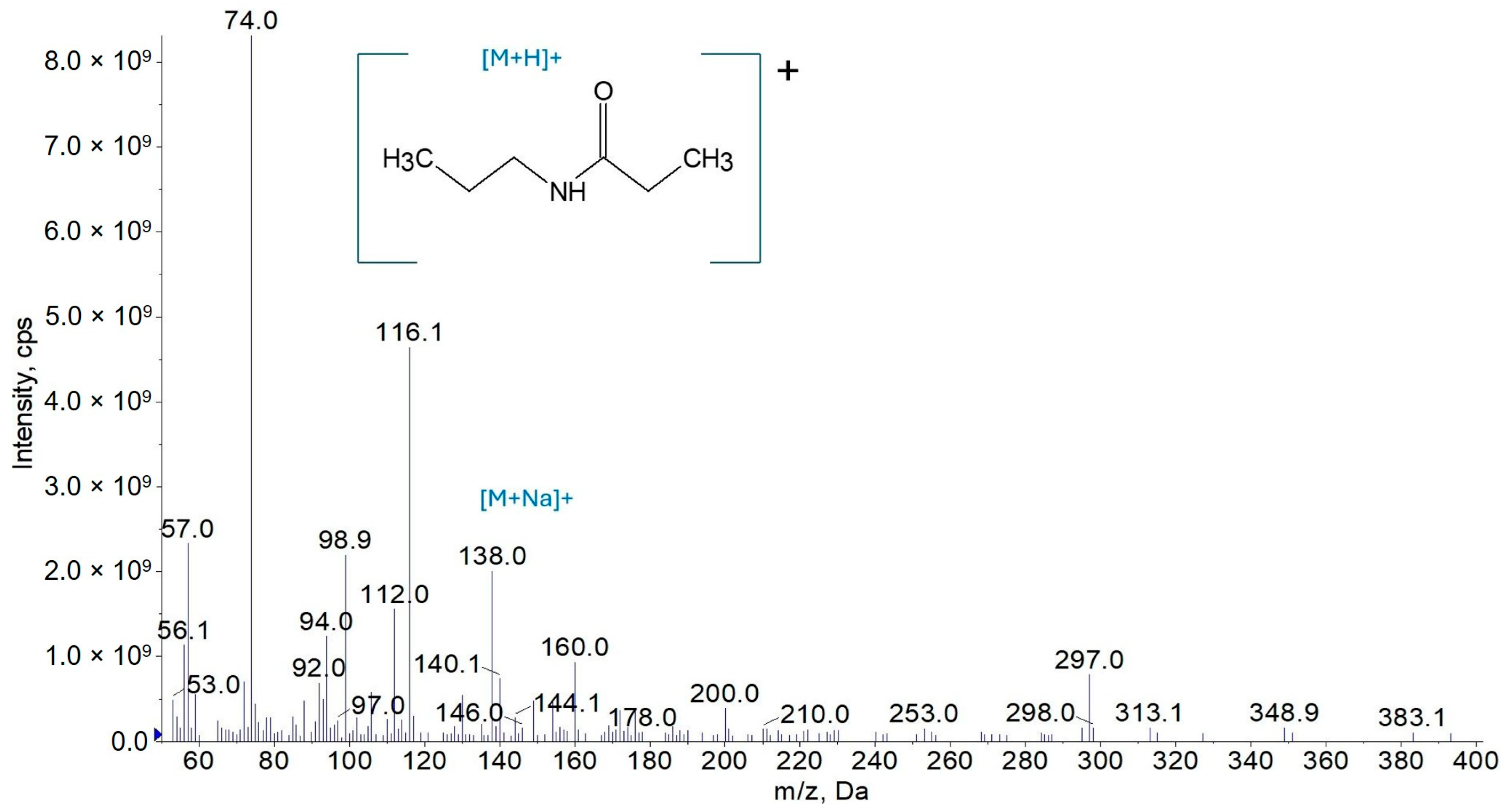
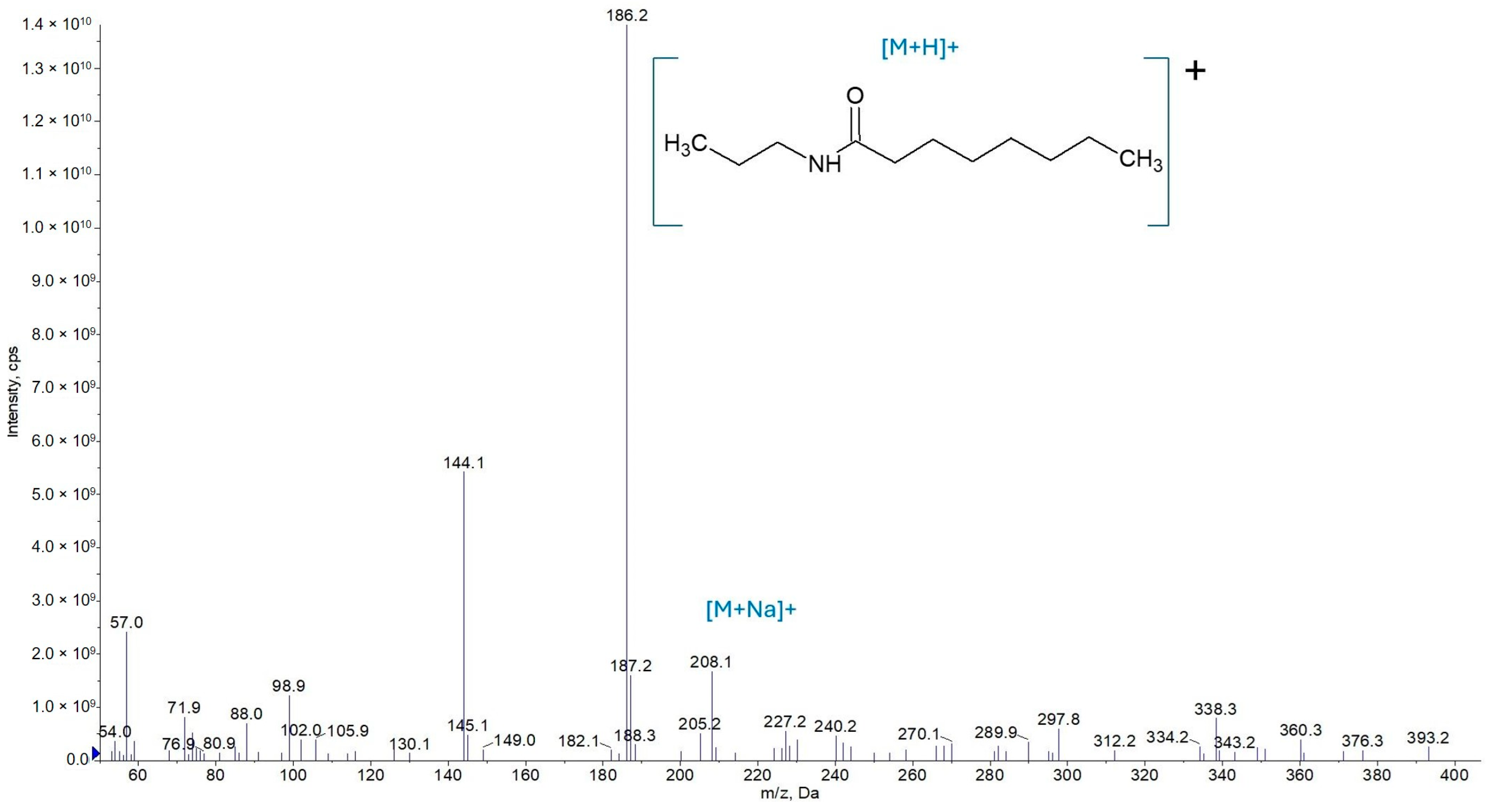
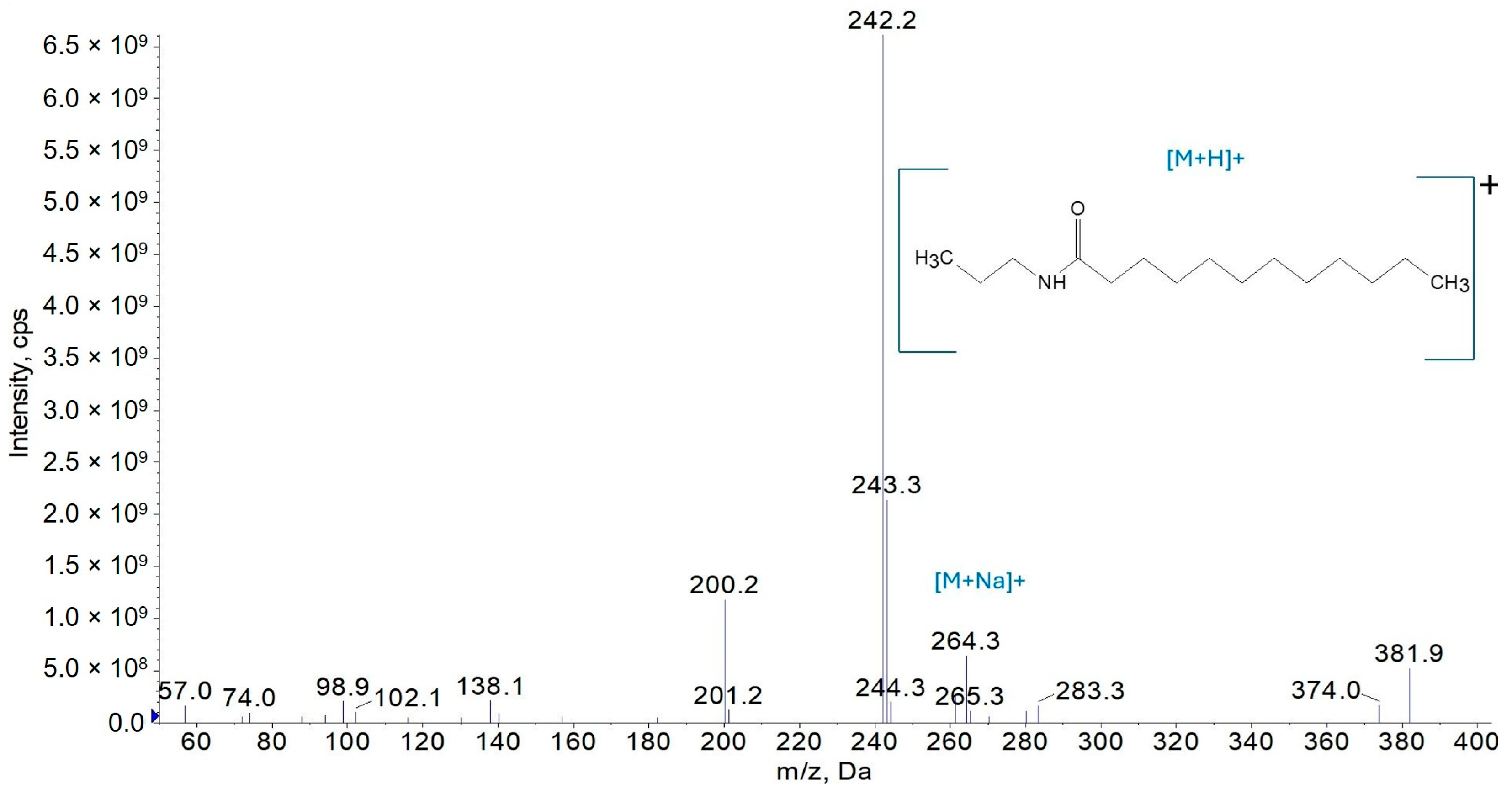
3.1.1. Mass Spectrometry
3.1.2. FT-IR Spectroscopy
3.1.3. Tribological Tests
3.2. Synthesis Procedures
3.2.1. Synthesis of N-Propylpropanamide (AC3C3)
3.2.2. Synthesis of N-Propyloctanamide (AC3C8)
3.2.3. Synthesis of N-Propyldodecanamide (AC3C12)
3.2.4. Synthesis of SBA-15 Nanosilica
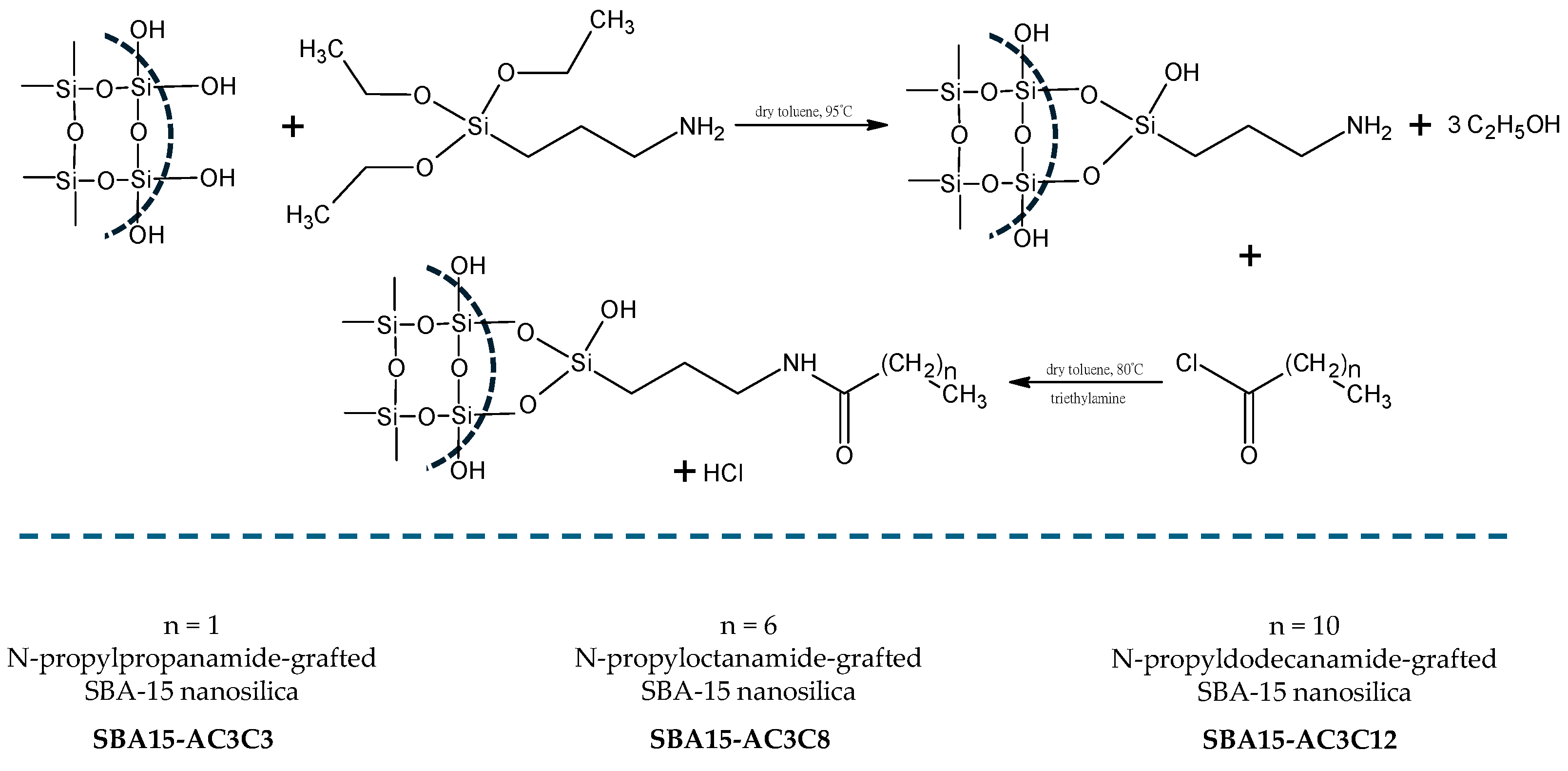
3.2.5. Synthesis of N-Propylpropanamide-Grafted SBA-15 Nanosilica (SBA15-AC3C3)
3.2.6. Synthesis of N-Propyloctanamide-Grafted SBA-15 Nanosilica (SBA15-AC3C8)
3.2.7. Synthesis of N-Propyldodecanamide-Grafted SBA-15 Nanosilica (SBA15-AC3C12)
3.3. Preparation of Mixtures of PAO Base Oil and Investigated Additives
3.4. Preparation of Greases
4. Conclusions
- AC3C8 provided the best wear protection, reducing the diameter of wear scars by approximately 25%.
- Grafting the amide compound onto SBA-15 silica nanoparticles improved the anti-wear properties of the lubricant by approximately a further 30%. To our knowledge, this is the first publication to use SBA-15 as a lubricant additive.
- SBA-15 grafted with AC3C8 amide resulted in better anti-wear properties than similarly modified spherical commercial silica. Unfortunately, both commercial silica and SBA-15 led to sedimentation during their storage in PAO-100 oil. This aspect of their application still needs to be improved. Stabilizing the dispersion of functionalized silica may depend on various factors, including the homogenization technique used and the conditions it was carried out under, the chemical structure of the ligands, and even the grafting conditions [63].
- Due to the sedimentation of the silica, free-amide AC3C8 was used for the real tests, which showed that using it at 3%w/w generated the best anti-wear properties. We have shown that the properties of this sample are better than those obtained with the commercial additive (Additive 1). The developed additive can be used wherever certified lubricants are required, such as in the food production industry. In this industry, a health quality certification is required for the finished lubricant when used in both food and non-food areas.
- AC3C8 showed a synergistic effect with the commercial additive, which contained butylated triphenyl phosphate as an anti-wear agent. The mechanism behind this synergy is unknown and should be studied further using SEM/EDS. The aim of these analyses will be the evaluation of the tribofilm created and include an elemental analysis.
- We assume that the anti-wear and extreme-pressure performance of AC3C8 depends on its polar amide bond and, therefore, its ability to adsorb onto the triboparts under boundary conditions, while the length of its carbon chain (C8) makes it properly soluble in PAO oil.
- The mechanism behind the performance improvement of the lubricant containing AC3C8 grafted onto SBA-15 nanosilica is probably related to its morphology. Similarly shaped, elongated nanoparticles, such as carbon nanotubes, have been successfully used in lubricants, resulting in improved anti-wear performance. Previous studies suggest that these carbon nanotubes are able to behave as (nano)rolling bearings, as well as a mending agent, filling in the cracks in the triboparts [57].
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mousavi, S.B.; Pourpasha, H.; Heris, S.Z. High-Temperature Lubricity and Physicochemical Behaviors of Synthesized Cu/TiO2/MnO2-Doped GO Nanocomposite in High-Viscosity Index Synthetic Biodegradable PAO Oil. Int. Commun. Heat Mass Transf. 2024, 156, 107642. [Google Scholar] [CrossRef]
- Braun, J. Additives. In Lubricants and Lubrication; Wiley-VCH: Weinheim, Germany, 2017; pp. 117–152. ISBN 978-3-527-64556-5. [Google Scholar]
- Bovington, C.H. Friction, Wear and the Role of Additives in Controlling Them. In Chemistry and Technology of Lubricants; Mortier, R.M., Fox, M.F., Orszulik, S.T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 77–105. ISBN 978-1-4020-8662-5. [Google Scholar]
- Ratoi, M.; Niste, V.B.; Alghawel, H.; Suen, Y.F.; Nelson, K. The Impact of Organic Friction Modifiers on Engine Oil Tribofilms. RSC Adv. 2014, 4, 4278–4285. [Google Scholar] [CrossRef]
- Tysoe, W. On Stress-Induced Tribochemical Reaction Rates. Tribol. Lett. 2017, 65, 48. [Google Scholar] [CrossRef]
- Syed, J.; Hakkim, N.L.; Nebhani, L.; Gosvami, N.N. Enhancing Tribological Properties of Lubricated Contacts via Synergistic Interactions of Green Silica Nanoparticles and ZDDP. Tribol. Int. 2024, 197, 109829. [Google Scholar] [CrossRef]
- Hewstone, R.K. Environmental Health Aspects of Lubricant Additives. Sci. Total Environ. 1994, 156, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jiang, J.C.; Aswath, P.B. Mechanism of Wear at Extreme Load and Boundary Conditions with Ashless Anti-Wear Additives: Analysis of Wear Surfaces and Wear Debris. Wear 2011, 270, 181–194. [Google Scholar] [CrossRef]
- Lundgren, S.M.; Persson, K.; Mueller, G.; Kronberg, B.; Clarke, J.; Chtaib, M.; Claesson, P.M. Unsaturated Fatty Acids in Alkane Solution: Adsorption to Steel Surfaces. Langmuir 2007, 23, 10598–10602. [Google Scholar] [CrossRef] [PubMed]
- Prutton, C.F.; Frey, D.R.; Turnbull, D.; Dlouhy, G. Corrosion of Metals by Organic Acids in Hydrocarbon Solvents. Ind. Eng. Chem. 1945, 37, 90–100. [Google Scholar] [CrossRef]
- Khalkar, S.; Bhowmick, D.; Pratap, A. Synthesis and Effect of Fatty Acid Amides as Friction Modifiers in Petroleum Base Stock. J. Oleo Sci. 2013, 62, 901–904. [Google Scholar] [CrossRef]
- Xu, X.; Yang, F.; Yang, H.; Zhao, Y.; Sun, X.; Tang, Y. Preparation and Tribological Behaviors of Sulfur- and Phosphorus-Free Organic Friction Modifier of Amide–Ester Type. Lubricants 2024, 12, 196. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Yasuda, K.; Koga, N.; Zhang, H. Adsorption Behavior of TEMPO-Based Organic Friction Modifiers during Sliding between Iron Oxide Surfaces: A Molecular Dynamics Study. Langmuir 2022, 38, 3170–3179. [Google Scholar] [CrossRef]
- Hou, J.; Tsukamoto, M.; Hor, S.; Chen, X.; Yang, J.; Zhang, H.; Koga, N.; Yasuda, K.; Fukuzawa, K.; Itoh, S.; et al. Molecules with a TEMPO-Based Head Group as High-Performance Organic Friction Modifiers. Friction 2023, 11, 316–332. [Google Scholar] [CrossRef]
- Hu, M.; Ma, R.; Zhang, S.; Han, Y.; Zhao, J.; Zhang, M.; Li, W.; Liu, H. Preparation and Tribological Study of Novel Amide-Based Organic Friction Modifiers. Tribol. Int. 2024, 194, 109465. [Google Scholar] [CrossRef]
- Drabik, J.; Korasiak, K.; Chrobak, J.; Woch, J.; Brzeźniak, N.; Barszcz, W.; Kozdrach, R.; Iłowska, J. Amide/Amino-Based Functional Additives for Lubricants: Structure, Antimicrobial Activity and Wear Resistance. Molecules 2024, 29, 122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, S.; Shi, Q.; Ge, X.; Wang, W. Graphene-Family Lubricant Additives: Recent Developments and Future Perspectives. Lubricants 2022, 10, 215. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Y.; Wang, L.; Li, W. Multilayer Graphene as a Lubricating Additive in Bentone Grease. Tribol. Lett. 2014, 55, 455–464. [Google Scholar] [CrossRef]
- Ismail, N.A.; Zulkifli, N.W.M.; Chowdhury, Z.Z.; Johan, M.R. Functionalization of Graphene-Based Materials: Effective Approach for Enhancement of Tribological Performance as Lubricant Additives. Diam. Relat. Mater. 2021, 115, 108357. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Li, X.; Jin, L.; Su, G.; Mo, Y.; Li, L.; Zhu, H.; Tian, Y. Research Progress in Application of 2D Materials in Liquid-Phase Lubrication System. Materials 2018, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Uzoma, P.C.; Hu, H.; Khadem, M.; Penkov, O.V. Tribology of 2D Nanomaterials: A Review. Coatings 2020, 10, 897. [Google Scholar] [CrossRef]
- Bhagavathi Kandy, S.; Simon, G.P.; Cheng, W.; Zank, J.; Saito, K.; Bhattacharyya, A.R. Effect of Organic Modification on Multiwalled Carbon Nanotube Dispersions in Highly Concentrated Emulsions. ACS Omega 2019, 4, 6647–6659. [Google Scholar] [CrossRef]
- Kumar, N.; Goyal, P. Experimental Study of Carbon Nanotubes to Enhance Tribological Characteristics of Lubricating Engine Oil SAE10W40. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1225, 012052. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, L.; Yan, M.; He, C.; Zhang, J.; Mou, J.; Wu, D.; Ren, Y. Strategies for Improving Friction Behavior Based on Carbon Nanotube Additive Materials. Tribol. Int. 2022, 176, 107875. [Google Scholar] [CrossRef]
- Tarasov, S.; Kolubaev, A.; Belyaev, S.; Lerner, M.; Tepper, F. Study of Friction Reduction by Nanocopper Additives to Motor Oil. Wear 2002, 252, 63–69. [Google Scholar] [CrossRef]
- Sia, S.Y.; Sarhan, A.A.D. Morphology Investigation of Worn Bearing Surfaces Using SiO2 Nanolubrication System. Int. J. Adv. Manuf. Technol. 2014, 70, 1063–1071. [Google Scholar] [CrossRef]
- Sia, S.Y.; Bassyony, E.Z.; Sarhan, A.A.D. Development of SiO2 Nanolubrication System to Be Used in Sliding Bearings. Int. J. Adv. Manuf. Technol. 2014, 71, 1277–1284. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Qin, B.; Xing, D.; Guo, Y.; Fan, R. Investigation of the Mending Effect and Mechanism of Copper Nano-Particles on a Tribologically Stressed Surface. Tribol. Lett. 2004, 17, 961–966. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic Amorphous Silica Nanoparticles: Toxicity, Biomedical and Environmental Implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Kasaai, M.R. Nanosized Particles of Silica and Its Derivatives for Applications in Various Branches of Food and Nutrition Sectors. J. Nanotechnol. 2015, 2015, 852394. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the Real World: Redeveloping the Nanomaterial Consumer Products Inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef]
- Kashefi, M.H.; Saedodin, S.; Rostamian, S.H. Effect of Silica Nano-Additive on Flash Point, Pour Point, Rheological and Tribological Properties of Lubricating Engine Oil: An Experimental Study. J. Therm. Anal. Calorim. 2022, 147, 4073–4086. [Google Scholar] [CrossRef]
- Sui, T.; Song, B.; Zhang, F.; Yang, Q. Effect of Particle Size and Ligand on the Tribological Properties of Amino Functionalized Hairy Silica Nanoparticles as an Additive to Polyalphaolefin. J. Nanomater. 2015, 2015, 492401. [Google Scholar] [CrossRef]
- Guo, P.; Chen, L.; Wang, J.; Geng, Z.; Lu, Z.; Zhang, G. Enhanced Tribological Performance of Aminated Nano-Silica Modified Graphene Oxide as Water-Based Lubricant Additive. ACS Appl. Nano Mater. 2018, 1, 6444–6453. [Google Scholar] [CrossRef]
- López, T.D.-F.; González, A.F.; Del Reguero, Á.; Matos, M.; Díaz-García, M.E.; Badía-Laíño, R. Engineered Silica Nanoparticles as Additives in Lubricant Oils. Sci. Technol. Adv. Mater. 2015, 16, 055005. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Fan, S.; Song, N.; Zhang, Y.; Zhang, S. Surface Regulation of Silicon Dioxide Nanoparticle and Its Tribological Properties as Additive in Base Oils with Different Polarity. Tribol. Int. 2024, 191, 109105. [Google Scholar] [CrossRef]
- Yao, J.; Fan, S.; Song, N.; Gao, C.; Zhang, Y.; Zhang, S. Extending Applicability of Amino-Functionalized Silica Nanoparticle as Poly-Alpha-Olefin Additive for Different Metal–Metal Sliding Pairs via Secondary Surface-Capping by Polyisobutylene Succinic Anhydride. Lubr. Sci. 2024, 36, 561–569. [Google Scholar] [CrossRef]
- Wright, R.A.E.; Wang, K.; Qu, J.; Zhao, B. Oil-Soluble Polymer Brush Grafted Nanoparticles as Effective Lubricant Additives for Friction and Wear Reduction. Angew. Chem. Int. Ed. 2016, 55, 8656–8660. [Google Scholar] [CrossRef]
- Sui, T.; Song, B.; Wen, Y.; Zhang, F. Bifunctional Hairy Silica Nanoparticles as High-Performance Additives for Lubricant. Sci. Rep. 2016, 6, 22696. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Li, P.; Aljabri, A.; Li, H.; Liu, G.; Xie, Z.; Li, T. Investigation on the Tribological Performance of Functionalized Nanoscale Silica as an Amphiphilic Lubricant Additive. J. Mater. Res. Technol. 2021, 15, 5507–5515. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Lu, H.; Xu, L. Surfactant-Modified Silica Nanoparticles-Stabilized Magnetic Polydimethylsiloxane-in-Water Pickering Emulsions for Lubrication and Anticorrosion. ACS Sustain. Chem. Eng. 2022, 10, 10816–10826. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Yang, T.; Su, H.; Wang, X.; Zhang, S.; Lou, W. Rheological Behaviors and Tribological Properties of Nano-Silica Grease: A Study Compared with Lithium Grease and Polyurea Grease. Tribol. Int. 2023, 186, 108657. [Google Scholar] [CrossRef]
- Kharabati, S.; Saedodin, S.; Rostamian, S.H. Experimental Investigation of Thermal and Rheological Behavior of Silica/Soybean Oil Nano Lubricant in Low-Temperature Performance of Internal Combustion Engine. Energy Sources Part Recovery Util. Environ. Eff. 2025, 47, 5493–5507. [Google Scholar] [CrossRef]
- Wang, A.-E.; Chang, Z.; Liu, Y.-P.; Huang, P.-Q. Mild N-Deacylation of Secondary Amides by Alkylation with Organocerium Reagents. Chin. Chem. Lett. 2015, 26, 1055–1058. [Google Scholar] [CrossRef]
- Hirai, T.; Kato, D.; Mai, B.K.; Katayama, S.; Akiyama, S.; Nagae, H.; Himo, F.; Mashima, K. Esterification of Tertiary Amides: Remarkable Additive Effects of Potassium Alkoxides for Generating Hetero Manganese–Potassium Dinuclear Active Species. Chem. -Eur. J. 2020, 26, 10735–10742. [Google Scholar] [CrossRef]
- Liang, B.; Zhu, P.; Gu, J.; Yuan, W.; Xiao, B.; Hu, H.; Rao, M. Advancing Adsorption and Separation with Modified SBA-15: A Comprehensive Review and Future Perspectives. Molecules 2024, 29, 3543. [Google Scholar] [CrossRef]
- Aldosari, O.F.; Alhumaimess, M.S.; Betiha, M.A.; Ahmed, E.A.; Alhaidari, L.M.; Altwala, A.; Hassan, H.M.A. Characterization and Catalytic Performance of Al-SBA-15 Catalyst Fabricated Using Ionic Liquids with High Aluminum Content. Catalysts 2023, 13, 1395. [Google Scholar] [CrossRef]
- Caravaggio, G.; Nossova, L.; Turnbull, M. Effect of Zirconia on Pd–Pt Supported SBA-15 Catalysts for the Oxidation of Methane. Catalysts 2023, 13, 0926. [Google Scholar] [CrossRef]
- Szymańska, K.; Bryjak, J.; Jarzębski, A.B. Immobilization of Invertase on Mesoporous Silicas to Obtain Hyper Active Biocatalysts. Top. Catal. 2009, 52, 1030–1036. [Google Scholar] [CrossRef]
- Zniszczoł, A.; Szymańska, K.; Kocurek, J.; Bryjak, J.; Walczak, K.; Jarzębski, A. Kinetics of Enantiomerically Enriched Synthesis of Solketal Esters Using Native and SBA-15 Supported P. Fluorescens Lipase. Chem. Process Eng. 2017, 38, 209–215. [Google Scholar] [CrossRef][Green Version]
- Wawrzyńczak, A.; Nowak, I.; Feliczak-Guzik, A. SBA-15- and SBA-16-Functionalized Silicas as New Carriers of Niacinamide. Int. J. Mol. Sci. 2023, 24, 17567. [Google Scholar] [CrossRef]
- Nurwita, A.; Stawicka, K.; Trejda, M. SBA-15 Type Mesoporous Silica Modified with Vanadium as a Catalyst for Oxidative and Extractive Oxidative Desulfurization Processes. Materials 2024, 17, 4041. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.; Roy, R.; Younis, H.; AlNahyan, M.; Younes, H. Carbon Nanomaterial-Based Lubricants: Review of Recent Developments. Lubricants 2022, 10, 281. [Google Scholar] [CrossRef]
- Wang, S.; Ren, G.; Li, W.; Wang, B.; Wei, F.; Liang, Z.; Chen, D. A Green Modification Technology of Carbon Nanotubes toward Enhancing the Tribological Properties of Aqueous-Based Lubricants. Tribol. Int. 2023, 180, 108268. [Google Scholar] [CrossRef]
- Zhang, F.-Z.; Liu, X.-B.; Yang, C.-M.; Chen, G.-D.; Meng, Y.; Zhou, H.-B.; Zhang, S.-H. Insights into Robust Carbon Nanotubes in Tribology: From Nano to Macro. Mater. Today 2024, 74, 203–234. [Google Scholar] [CrossRef]
- Opia, A.C.; Abdollah, M.F.B.; Mamah, S.C.; Hamid, M.K.B.A.; Syahrullail, S.; Audu, I.A.; Johnson, C.; Basiron, J. Lubricity Effectiveness of Bio-Lubricant Modified with Multi-Wall Carbon Nanotube and Organic Polymer. Wear 2023, 528–529, 204974. [Google Scholar] [CrossRef]
- Ye, X.; E, S.; Fan, M. The Influences of Functionalized Carbon Nanotubes as Lubricating Additives: Length and Diameter. Diam. Relat. Mater. 2019, 100, 107548. [Google Scholar] [CrossRef]
- Zimnoch Dos Santos, J.H.; Brentano Capeletti, L. Fourier Transform Infrared and Raman Characterization of Silica-Based Materials. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; Stauffer, M.T., Ed.; IntechOpen: Rijeka, Croatia, 2016; ISBN 978-953-51-2681-2. [Google Scholar]
- Liu, H.; Kaya, H.; Lin, Y.-T.; Ogrinc, A.; Kim, S.H. Vibrational Spectroscopy Analysis of Silica and Silicate Glass Networks. J. Am. Ceram. Soc. 2022, 105, 2355–2384. [Google Scholar] [CrossRef]
- Al Rashid, A.; Al-Maslamani, N.A.; Abutaha, A.; Hossain, M.; Koç, M. Cobalt Iron Oxide (CoFe2O4) Reinforced Polyvinyl Alcohol (PVA) Based Magnetoactive Polymer Nanocomposites for Remote Actuation. Mater. Sci. Eng. B 2025, 311, 117838. [Google Scholar] [CrossRef]
- Panicker, C.Y.; Vanghese, H.T.; Thansani, T. Spectroscopic Studies and Hartree-Fock Ab Initio Calculations of a Substituted Amide of Pyrazine-2-Carboxylic Acid-C_{16}H_{18}ClN_3O. Turk. J. Chem. 2009, 33, 633–646. [Google Scholar] [CrossRef]
- Porubská, M.; Szöllős, O.; Kóňová, A.; Janigová, I.; Jašková, M.; Jomová, K.; Chodák, I. FTIR Spectroscopy Study of Polyamide-6 Irradiated by Electron and Proton Beams. Polym. Degrad. Stab. 2012, 97, 523–531. [Google Scholar] [CrossRef]
- Sui, T.; Ding, M.; Ji, C.; Yan, S.; Wei, J.; Wang, A.; Zhao, F.; Fei, J. Dispersibility and Rheological Behavior of Functionalized Silica Nanoparticles as Lubricant Additives. Ceram. Int. 2018, 44, 18438–18443. [Google Scholar] [CrossRef]
- Vengudusamy, B.; Grafl, A.; Novotny-Farkas, F.; Schimmel, T.; Adam, K. Tribological Behaviour of Antiwear Additives Used in Hydraulic Applications: Synergistic or Antagonistic with Other Surface-Active Additives? Tribol. Int. 2013, 67, 199–210. [Google Scholar] [CrossRef]
- Pavelko, G.F. Synergism and Antagonism of Anti-Wear Additives as a Method of Confirming the Mechanism of Their Action. J. Frict. Wear 2023, 44, 53–57. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, C.; Dong, Y.; Reddyhoff, T. Research on Mutual Synergy of Antiwear Additives in Lithium Complex Grease. Tribol. Trans. 2016, 59, 330–339. [Google Scholar] [CrossRef]
- Eickworth, J.; Aydin, E.; Dienwiebel, M.; Rühle, T.; Wilke, P.; Umbach, T.R. Synergistic Effects of Antiwear and Friction Modifier Additives. Ind. Lubr. Tribol. 2020, 72, 1019–1025. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Wang, J.; Yao, X.; Xiong, X.; Huang, H. Synergistic Tribological Performance of Phosphorus- and Sulfur-Based Extreme Pressure and Anti-Wear Additives. Lubricants 2025, 13, 55. [Google Scholar] [CrossRef]
- Minami, I.; Mimura, K. Synergistic Effect of Antiwear Additives and Antioxidants in Vegetable Oil. J. Synth. Lubr. 2004, 21, 193–205. [Google Scholar] [CrossRef]
- Acharya, B.; Avva, K.S.; Thapa, B.; Pardue, T.N.; Krim, J. Synergistic Effect of Nanodiamond and Phosphate Ester Anti-Wear Additive Blends. Lubricants 2018, 6, 56. [Google Scholar] [CrossRef]
- WTWT-94/MPS-025; Badanie Właściwości Przeciwzużyciowych Olejów i Smarów Plastycznych. Metodyka Badawcza—Materiałów Pędnych i Smarów. Air Force Institute of Technology: Warszawa, Poland, 1994.
- PN-C-04147:1976; Przetwory Naftowe—Badanie Właściwości Smarnych Olejów i Smarów. Polish Committee for Standardization: Warszawa, Poland, 1976.
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]



| Line | Model Dispersion | Wear Diameter, d, mm Mean ± SD | Significance of Wear Diameter Reduction, (T)rue/(F)alse * | Goz40, N·mm−2 Mean ± SD | Visual Appearance |
|---|---|---|---|---|---|
| 1 | PAO-100 | 0.46 ± 0.01 | reference | 950 ± 20 | transparent liquid |
| 2 | PAO-100 + 0.5%w/w AC3C3 | 0.44 ± 0.03 | F | 1100 ± 200 | cloudy liquid |
| 3 | PAO-100 + 0.5%w/w AC3C8 | 0.34 ± 0.02 | T | 1800 ± 200 | transparent liquid |
| 4 | PAO-100 + 0.5%w/w AC3C12 | 0.36 ± 0.03 | T | 1500 ± 200 | cloudy liquid |
| 5 | PAO-100 + 0.5%w/w SBA15 | 0.52 ± 0.02 | T | 750 ± 50 | sedimentation observed |
| 6 | PAO-100 + 0.5%w/w SBA15-AC3C3 | 0.36 ± 0.02 | T | 1600 ± 100 | sedimentation observed |
| 7 | PAO-100 + 0.5%w/w SBA15-AC3C8 | 0.22 ± 0.01 | T | 4200 ± 300 | sedimentation observed |
| 8 | PAO-100 + 0.5%w/w SBA15-AC3C12 | 0.28 ± 0.01 | T | 2600 ± 200 | sedimentation observed |
| 9 | PAO-100 + 0.5%w/w (spherical) silica **-AC3C8 | 0.35 ± 0.02 | T | 1700 ± 200 | sedimentation observed |
| Line | Grease | Wear Diameter, d, mm Mean ± SD | Significance of Wear Diameter Reduction, (T)rue/(F)alse * | Goz40, N·mm−2 Mean ± SD | Welding Load, N |
|---|---|---|---|---|---|
| 1 | PAO-100 + Aerosil® (base grease) | 0.59 ± 0.01 | reference | 590 ± 20 | 3090.1 |
| 2 | base grease + 1.0%w/w AC3C8 | 0.55 ± 0.01 | T | 680 ± 20 | 3090.1 |
| 3 | base grease + 3.0%w/w AC3C8 | 0.46 ± 0.01 | T | 960 ± 40 | 3924.0 |
| 4 | base grease + 5.0%w/w AC3C8 | 0.61 ± 0.01 | F | 550 ± 20 | 3924.0 |
| 5 | base grease + 2.0%w/w Additive-1 ** | 0.55 ± 0.01 | T | 670 ± 20 | 3924.0 |
| 6 | base grease + 2.0%w/w Additive-1 ** + 3.0%w/w AC3C8 | 0.32 ± 0.01 | T | 2000 ± 100 | 7848.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drabik, J.; Korasiak, K.; Chrobak, J.; Kozdrach, R.; Woch, J.; Cyl, M.; Zarębska, M.; Kaźmierczak, B.; Iłowska, J.; Szymańska, K. Amide-Based Anti-Wear/Extreme-Pressure Additives for Silica-Thickened Greases: Structure and Wear Resistance. Molecules 2025, 30, 2492. https://doi.org/10.3390/molecules30122492
Drabik J, Korasiak K, Chrobak J, Kozdrach R, Woch J, Cyl M, Zarębska M, Kaźmierczak B, Iłowska J, Szymańska K. Amide-Based Anti-Wear/Extreme-Pressure Additives for Silica-Thickened Greases: Structure and Wear Resistance. Molecules. 2025; 30(12):2492. https://doi.org/10.3390/molecules30122492
Chicago/Turabian StyleDrabik, Jolanta, Kamil Korasiak, Justyna Chrobak, Rafał Kozdrach, Julia Woch, Michał Cyl, Magdalena Zarębska, Bernadetta Kaźmierczak, Jolanta Iłowska, and Katarzyna Szymańska. 2025. "Amide-Based Anti-Wear/Extreme-Pressure Additives for Silica-Thickened Greases: Structure and Wear Resistance" Molecules 30, no. 12: 2492. https://doi.org/10.3390/molecules30122492
APA StyleDrabik, J., Korasiak, K., Chrobak, J., Kozdrach, R., Woch, J., Cyl, M., Zarębska, M., Kaźmierczak, B., Iłowska, J., & Szymańska, K. (2025). Amide-Based Anti-Wear/Extreme-Pressure Additives for Silica-Thickened Greases: Structure and Wear Resistance. Molecules, 30(12), 2492. https://doi.org/10.3390/molecules30122492









