2.1. Sc Atom
As the first step of this work, the Sc atom was studied aiming to test the accuracy of three multireference approaches on its excited states. Specifically, the MRCI/aug-cc-pV5Z-DK (MRCI
Sc), MRCI/aug-cc-pwCV5Z-DK (C-MRCI
Sc), and MRCI+Q/aug-cc-pwCV5Z-DK (C-MRCI+Q
Sc) calculations were performed to obtain the excitation energies of all
J states of the first six electronic states of Sc (
Table 1). The ground electronic state of the Sc atom is a
2D ([Ar]3d
14s
2) and its two
J terms (i.e.,
J = 3/2 and 5/2) are energetically separated by 168 cm
−1 [
19]. In agreement with the experiment, all our theoretical approaches predicted a
2D
3/2 ground state for the Sc atom (
Table 1). The MRCI
Sc and C-MRCI
Sc predicted excitation energies for the
2D
5/2 are in good agreement with the experiment (i.e., 182, 193, and 168 cm
−1, respectively). Among the three approaches, C-MRCI+Q
Sc predicted the highest
2D
3/2 →
2D
5/2 excitation energy (i.e., 221 cm
−1). According to experiment, the first two excited electronic states of the Sc atom (i.e.,
4F and
2F) lie at 11,520–11,678 and 14,926–15,042 cm
−1, respectively [
19]. All our calculations overestimated the excitation energies of the
J = 3/2, 5/2, 7/2, and 9/2 of
4F and
J = 5/2 and 7/2 of
2F, similar to the excitation energy of
2D
5/2 (
Table 1). Specifically, the largest discrepancies with respect to the experiment (for the
J states of
4F and
2F) were provided by the MRCI
Sc (~2850 cm
−1), whereas the overestimations by both C-MRCI
Sc and C-MRCI+Q
Sc levels are ~700–800 cm
−1. Both MRCI
Sc and C-MRCI
Sc underestimated the excitation energies of
4F
o by ~1100 and ~300 cm
−1 while the corresponding C-MRCI+Q
Sc excitation energies are in reasonable agreement with the experiment (the discrepancies are only 74–90 cm
−1). Similarly, the MRCI
Sc substantially underestimated the excitation energies of the next two excited electronic states,
4D° and
2D°, of the Sc atom (by 1173–1413 cm
−1). The excitation energies predicted by both C-MRCI
Sc and C-MRCI+Q
Sc for these two states only differ by 9–172 cm
−1 with respect to the experiment. Overall, both C-MRCI
Sc and C-MRCI+Q
Sc representations of the excited states of the Sc atom are significantly better compared to the representation of MRCI
Sc, which highlights the importance of the core electron correlation for obtaining accurate excitation energies. Furthermore, the +Q correction was found to improve the predictions of the higher excited states of the Sc atom. Specifically, the C-MRCI+Q excitation energies of the Sc atom for all
J states of
2F,
4F
o,
4D°, and
2D° (except for the
J = 3/2 and
J = 5/2 of
4D°) are in better agreement with the experiment compared to the C-MRCI (
Table 1).
2.2. ScH+
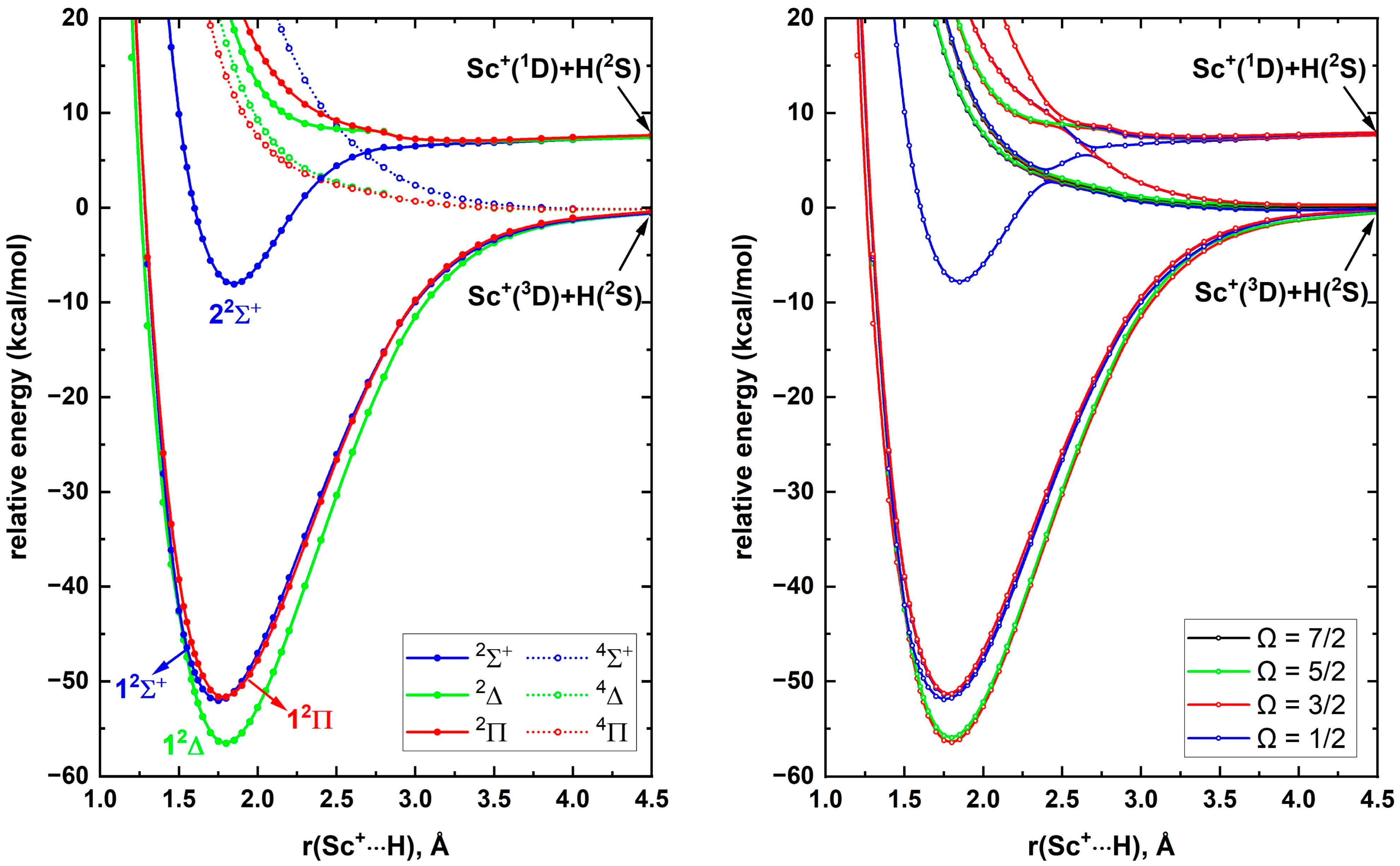
To study ScH
+, the reactions between a few low-lying electronic states of Sc
+ and the ground state of H atom were considered. Specifically, the Sc
+(
3D; 3d
14s
1)+H(
2S), Sc
+(
1D; 3d
14s
1)+H(
2S), Sc
+(
3F; 3d
2)+H(
2S), and Sc
+(
1D; 3d
2)+H(
2S) interactions were investigated. These combinations produce
4,2[Σ
+, Π, Δ],
2[Σ
+, Π, Δ],
4,2[Σ
–, Π, Δ, Φ], and
2[Σ
+, Π, Δ] molecular states of ScH
+, respectively, and their PECs are given in
Figure 1. All the quartet-spin PECs resulting from the ground state fragments [i.e., Sc
+(
3D; 3d
14s
1)+H(
2S)] are repulsive in nature. On the other hand, the doublet-spin PECs of the same reactants are strongly attractive and create the first three electronic states of ScH
+ (i.e., 1
2Δ, 1
2Σ
+, and 1
2Π). According to
Figure 1, the first two excited states of ScH
+ (i.e., 1
2Σ
+ and 1
2Π) lie ~4 kcal/mol above the 1
2Δ ground state. Recall that the core electron correlation significantly improved the excitation energies of the Sc atom (
Section 2.1) and similarly the higher electron correlation effects may be necessary for identifying the exact ordering of the closely lying 1
2Σ
+ and 1
2Π states of ScH
+. Indeed, we discuss such electron correlation effects on the excited states and a few other properties of ScH
+ in detail later in this section. The Sc
+(
1D; 3d
14s
1)+H(
2S) interaction produces only one stable potential energy minimum (i.e., 2
2Σ
+), which is the third excited state of ScH
+. The Sc
+(
3F; 3d
2)+H(
2S) interaction does not produce strongly attractive PECs except for a few PECs with shallow minima (i.e.,
2Π,
4Σ
-,
2Δ, and
4Φ) around 2.2–2.7 Å.
The first four electronic states of ScH
+ (i.e., 1
2Δ, 1
2Σ
+, 1
2Π, and 2
2Σ
+) bear single-reference electron configurations (ESI
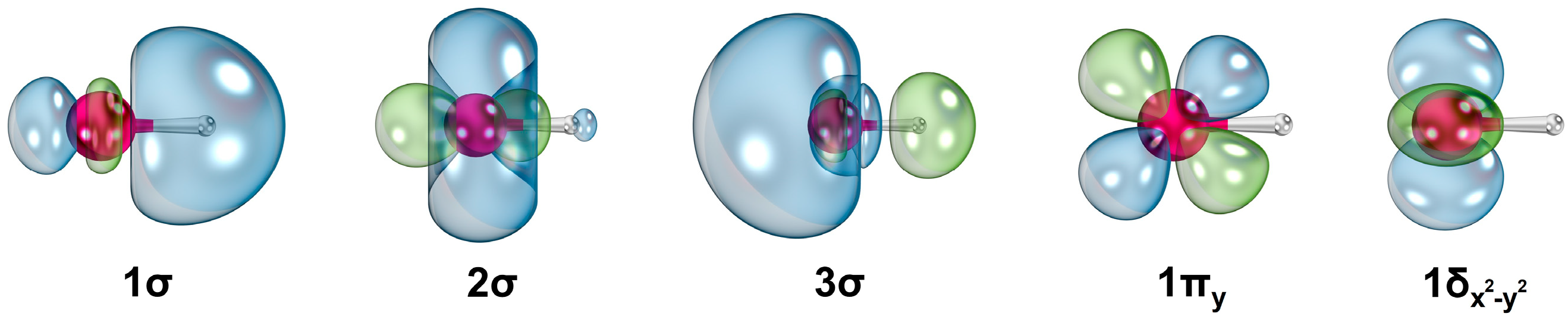
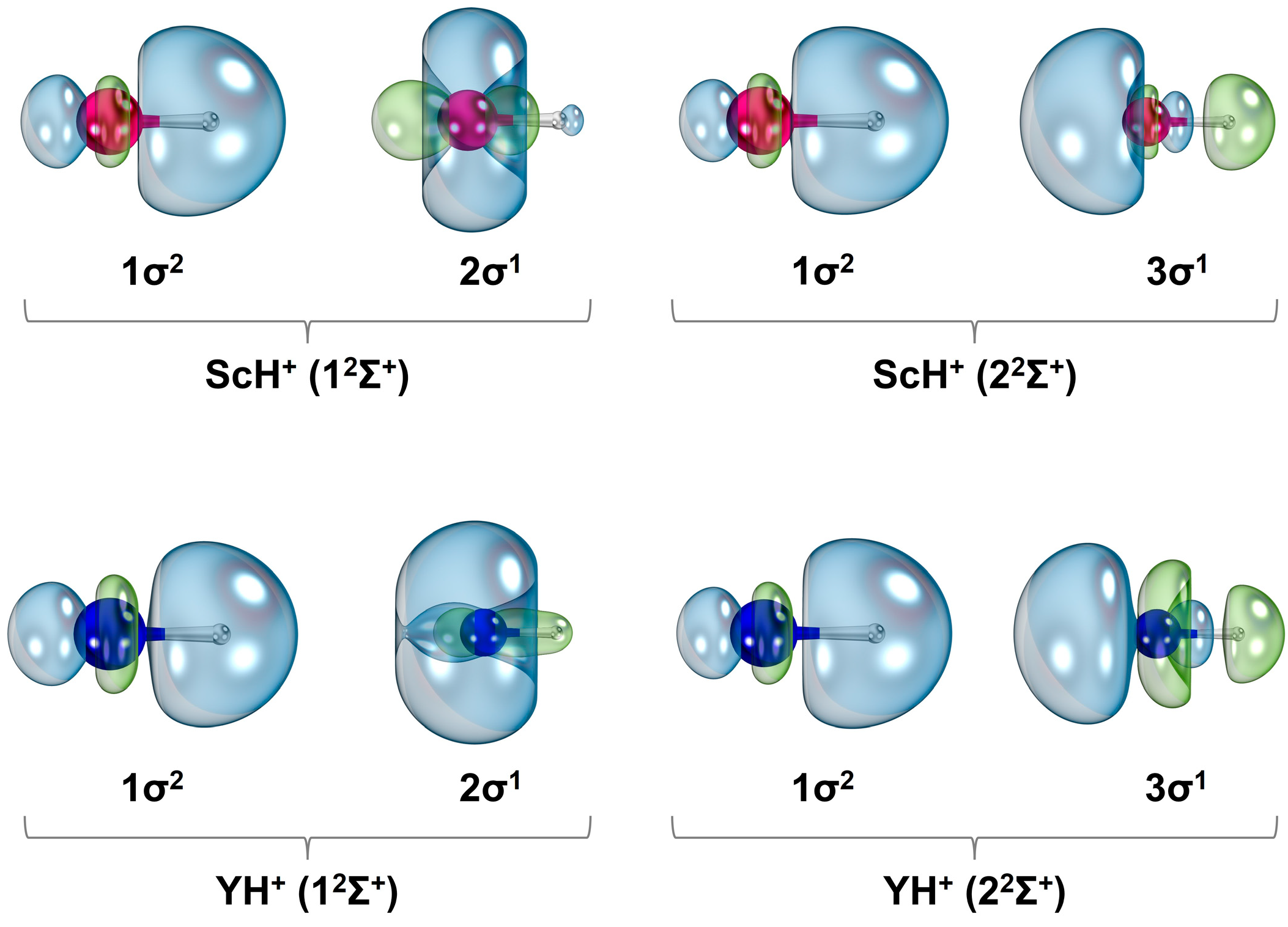
Table S1). Several state average molecular orbitals, which are plotted by including these four states of ScH
+ at the CASSCF level, are given in
Figure 2. The 1σ, 2σ, 3σ, 1π, and 1δ orbitals chiefly correlate to the 1s of H (with minor
and 4s of Sc),
(with some 4s) of Sc, 4s of Sc (with a small contribution from 1s of H), 3d
yz/3d
xz of Sc, and
/3d
xy of Sc, respectively. Based on the contours of the molecular orbitals (
Figure 2), the 1σ
21δ
1 electron configuration of 1
2Δ translates to an approximate ionic Sc
2+H
− structure. According to the NBO population analysis, the 1
2Δ of ScH
+ carries Sc
+1.68H
−0.68 charge localization with Sc[4s
0.163d
1.16]H[1s
1.66] electron population. The next three electronic states of ScH
+ (i.e., 1
2Σ
+, 1
2Π, and 2
2Σ
+) bear 1σ
22σ
1, 1σ
21π
1, and 1σ
23σ
1 equilibrium electron configurations with Sc
+1.57H
−0.57, Sc
+1.69H
−0.69, and Sc
+1.82H
−0.82 NBO charge distributions, respectively. Their NBO electron populations are Sc[4s
0.583d
0.84]H[1s
1.55], Sc[4s
0.193d
1.11]H[1s
1.65], and Sc[4s
0.473d
0.59]H[1s
1.73], respectively.
For the sake of textual brevity, from now on, the notations AXZ- [for, aug-cc-pVXZ-DK (of H) cc-pVXZ-DK (of Sc), aug-cc-pVXZ (of H) cc-pVXZ-PP (of Y), aug-cc-pVXZ-DK (of H) cc-pVXZ-DK3 (of La)] and AXZ-C- [for, aug-cc-pVXZ-DK (of H) cc-pwCVXZ-DK (of Sc), aug-cc-pVXZ (of H) cc-pwCVXZ-PP (of Y), aug-cc-pVXZ-DK (of H) cc-pwCVXZ-DK3 (of La)] (X = T, Q, 5) are used throughout the paper to denote the basis set combinations. All our theoretical approaches predicted a 1
2Δ ground electronic state for the ScH
+ (
Table 2). Recall that according to our A5Z-MRCI potential energy profile (
Figure 1), 1
2Π is slightly more stable than 1
2Σ
+. However, A5Z-MRCI calculations built on top of the CASSCF wave functions created by including only 1
2Δ, 1
2Σ
+, 1
2Π, and 2
2Σ
+ states of ScH
+ predicted 1
2Σ
+ to be more stable than 1
2Π (
Table 2). Indeed, all MRCI and MRCI+Q approaches listed in
Table 2 predicted 1
2Σ
+ to be 369–388 cm
−1 more stabilized compared to 1
2Π. Utilization of higher levels of theories such as A5Z-CCSD(T), A5Z-C-CCSD(T), and A5Z-fcFCI decreased the energy difference between 1
2Σ
+ and 1
2Π to ~150 cm
−1 (
Table 2). According to our largest theory that does not account spin–orbit effects, CBS-fcFCI-δcore [i.e., E
δcore = E
A5Z-C-CCSD(T) − E
A5Z-CCSD(T)], this energy difference is only 154 cm
−1 (i.e., the T
e values of 1
2Σ
+ and 1
2Π are 1386 and 1540 cm
−1, respectively). Under all our MRCI and MRCI+Q predictions, the 2
2Σ
+ rests at ~15,250 cm
−1 (
Table 2). The core electron correlation is vital for gaining accurate predictions of r
e values of the first-row transition metal diatomic species [
21,
22,
23,
24]. According to the findings of this study, the 3s
23p
6 electron correlation decreased the r
e by 0.03–0.04 Å while simultaneously increasing the ω
e by 33–52 cm
−1 [compare C-CCSD(T) and CCSD(T) r
e and ω
e values listed in
Table 2]. The ω
ex
e, B
e, α
e, and
e values predicted under different levels for the first four electronic states of ScH
+ are also given in
Table 2.
At the AQZ-MRCI level, ScH
+(1
2Δ) carries a D
0 of 54.46 kcal/mol. This value is in good agreement with the experimental D
0 of ScH
+ reported by the Armentrout group (i.e., 55.35 ± 2.31 kcal/mol) [
25]. The application of the Davidson correction (i.e., AQZ-MRCI+Q) slightly increased the D
0 of ScH
+(1
2Δ) to 54.54 kcal/mol. The improvement of the basis set from AQZ to A5Z increased the D
0 by 0.45 kcal/mol for both MRCI and MRCI+Q (
Table 2). Both A5Z-CCSD(T) and AQZ-CCSD(T) D
0 values of ScH
+(1
2Δ) are slightly lower compared to the A5Z-MRCI and A5Z-MRCI+Q D
0 values. As expected, the AQZ-fcFCI and A5Z-fcFCI D
0 values are closer to the experimental values than the D
0 of AQZ-CCSD(T) and A5Z-CCSD(T), clearly due to the better representation of electron correlation at fcFCI compared to CCSD(T). The 3s
23p
6 core electron correlation of Sc slightly increased the D
0 (by ~0.6 kcal/mol). The CBS extrapolation increased the D
0 by 0.17 kcal/mol compared to the A5Z-fcFCI D
0 of ScH
+ (
Table 2). The inclusion of the core electron correlation correction (i.e., δcore) to the CBS-fcFCI provided us with a D
0 of 55.55 kcal/mol (CBS-fcFCI-δcore) for ScH
+ (1
2Δ).
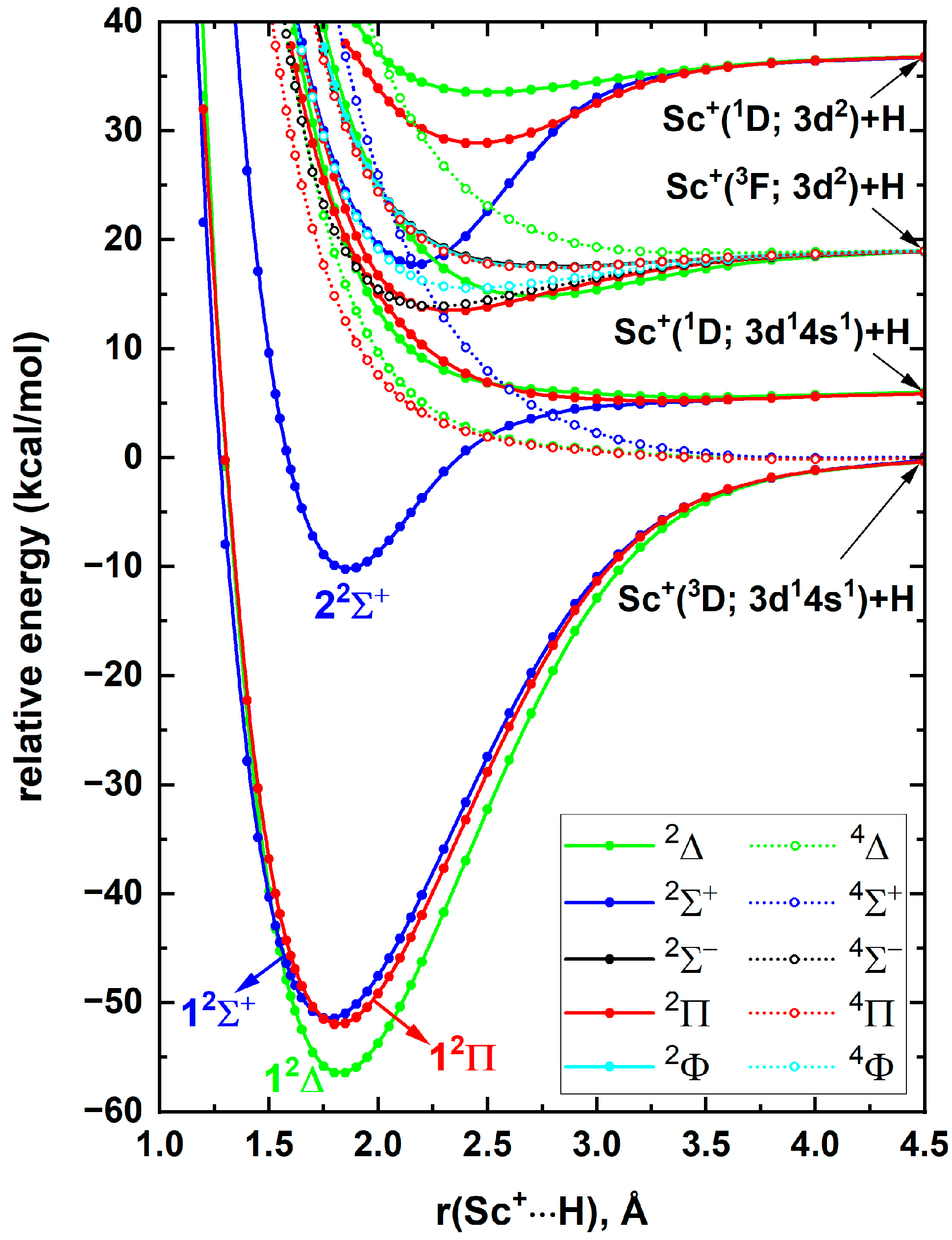
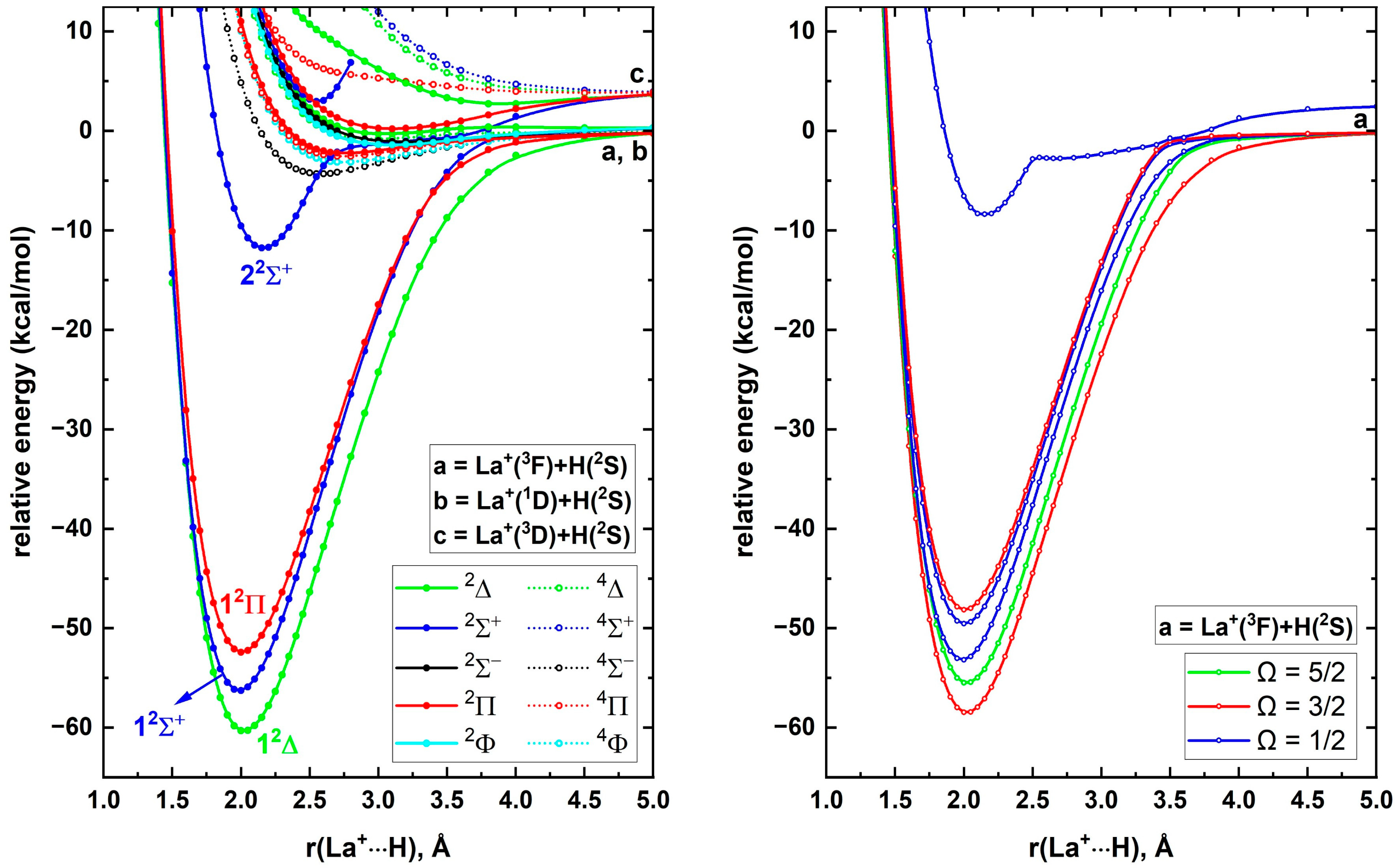
Generally, the spin–orbit coupling effects of the early first-row transition metal-based diatomic species are minor, and similarly we do not expect the spin–orbit coupling of ScH
+ to be significant. However, full spin–orbit coupling curves are useful for gaining insight on true dissociations of the states of diatomic species and constructing accurate radiative models. Aiming to understand the spin–orbit curves and the corresponding avoided crossings, full spin–orbit curves of ScH
+ were produced at the A5Z-C-MRCI level and are given in
Figure 3 (right panel).
Figure 3 (left panel) illustrates the spin–free PECs of ScH
+ under the same method. Observe that the spin–free A5Z-C-MRCI approach indeed predicted 1
2Σ
+ to be more stable than 1
2Π. The 1
2Δ, 1
2Σ
+, 1
2Π, and 2
2Σ
+ electronic states of ScH
+ split into Ω = 3/2, 5/2, Ω = 1/2, Ω = 3/2, 1/2, and Ω = 1/2 spin–orbit states of ScH
+. As expected, the spin–orbit coupling is minor for ScH
+ at all internuclear distances (compare the spin–orbit curves and parent spin–free PECs of ScH
+ in
Figure 3). The spin–orbit splitting of Sc
+(
3D) is minor. Specifically, experimentally the T
e of the
J states of Sc
+(
3D) are 0.00, 67.72, and 177.76 cm
−1, respectively [
19]. Hence, the Ω states resulting from the Sc
+(
3D)+H(
2S) nearly degenerate at the bond dissociation limit (
Figure 3, right panel). The experimental excitation energy of Sc
+(
1D
2) is 2540.95 cm
−1. According to our spin–orbit energy profile (
Figure 3, right panel) this excitation energy is ~7.9 kcal/mol (or 2763 cm
−1), which is higher compared to the corresponding experimental value by 222 cm
−1. This discrepancy could be a result of the size-extensivity issues of MRCI, and the findings may be further improved by the application of MRCI+Q that partially corrects the size-extensivity errors of MRCI. The spin–orbit ground state of ScH
+ is a 1
2Δ
3/2 with a D
0 of 54.17 kcal/mol (at A5Z-C-MRCI), which is only 0.10 kcal/mol lower than the spin–orbit effect disregarded D
0 of 1
2Δ obtained at the same approach. We arrived at our best D
0 estimate of ScH
+ by introducing this spin–orbit correction to the CBS-fcFCI-δcore D
0 of ScH
+ [i.e., CBS-fcFCI-δcore-δSO]. Indeed, the CBS-fcFCI-δcore-δSO D
0 of ScH
+ is in excellent agreement with the experimental value of the Armentrout group (i.e., 55.45 vs. 55.35 ± 2.31 kcal/mol) (
Table 3). The Ω = 5/2 component of 1
2Δ lies 175 cm
−1 above the 1
2Δ
3/2 (ESI
Table S2). The next two spin–orbit states of ScH
+ (Ω = 1/2 at 1582 cm
−1 and Ω = 1/2 at 1752 cm
−1) bear 80% 1
2Σ
+ + 20% 1
2Π and 80% 1
2Π + 20% 1
2Σ
+ ΛS compositions, respectively. The proceeding two spin–orbit states of ScH
+ (Ω = 3/2 at 1779 cm
−1 and Ω = 1/2 at 17,006 cm
−1) dominantly correlate to the 1
2Π and 2
2Σ
+ (ESI
Table S2). We observed several avoided crossings for the higher energy PECs (ESI
Figure S1). The most obvious example is the Ω = 1/2 of 2
2Σ
+, which dissociates to the spin–orbit products of Sc
+(
3D)+H(
2S) (
Figure 3 right panel and ESI
Figure S1). Our final spectroscopic predictions obtained combining the CBS-fcFCI-δcore and MRCI spin–orbit effects [i.e., CBS-fcFCI-δcore-δSO] are listed in
Table 3. Specifically, we are reporting T
e, r
e, ω
e, ω
ex
e, B
e, α
e,
e, and D
0 values of the first five spin–orbit states of ScH
+ at the composite CBS-fcFCI-δcore-δSO level to assist future experimental studies of ScH
+.
Table 2.
Adiabatic excitation energy (Te, cm−1), bond length (re, Å), harmonic vibrational frequency (ωe, cm−1), anharmonicity (ωexe, cm−1), equilibrium rotational constant (Be, cm−1), anharmonic correction to the rotational constant (αe, cm−1), centrifugal distortion constant (e, cm−1), and the bond dissociation energies with respect to the Sc+(3D; 3d14s1)+H(2S) fragments (D0, kcal/mol) of the first four electronic states of ScH+ a.
Table 2.
Adiabatic excitation energy (Te, cm−1), bond length (re, Å), harmonic vibrational frequency (ωe, cm−1), anharmonicity (ωexe, cm−1), equilibrium rotational constant (Be, cm−1), anharmonic correction to the rotational constant (αe, cm−1), centrifugal distortion constant (e, cm−1), and the bond dissociation energies with respect to the Sc+(3D; 3d14s1)+H(2S) fragments (D0, kcal/mol) of the first four electronic states of ScH+ a.
| State | Method | Te | re | ωe | ωexe | Be | αe ×10−4 | e ×10−6 | D0 |
|---|
| 12Δ | CBS-fcFCI-δcore | … | 1.791 | 1657 | 22.3 | 5.333 | 1074 | 220 | 55.55 |
| CBS-fcFCI | … | 1.827 | 1621 | 21.1 | 5.125 | 1013 | 205 | 54.98 |
| A5Z-fcFCI | … | 1.827 | 1620 | 21.1 | 5.122 | 1013 | 205 | 54.81 |
| AQZ-fcFCI | … | 1.828 | 1619 | 21.1 | 5.118 | 1016 | 205 | 54.75 |
| A5Z-C-CCSD(T) | … | 1.791 | 1657 | 22.1 | 5.330 | 1072 | 221 | 55.26 |
| AQZ-C-CCSD(T) | … | 1.793 | 1653 | 22.0 | 5.320 | 1082 | 221 | 54.96 |
| A5Z-CCSD(T) | … | 1.827 | 1621 | 21.2 | 5.122 | 1019 | 205 | 54.69 |
| AQZ-CCSD(T) | … | 1.828 | 1620 | 21.3 | 5.118 | 1015 | 204 | 54.63 |
| A5Z-MRCI+Q | … | 1.826 | 1617 | 21.2 | 5.129 | 1076 | 207 | 54.99 |
| A5Z-MRCI | … | 1.826 | 1616 | 21.3 | 5.128 | 1022 | 207 | 54.91 |
| AQZ-MRCI+Q | … | 1.827 | 1616 | 21.3 | 5.126 | 1020 | 206 | 54.54 |
| AQZ-MRCI | … | 1.827 | 1616 | 21.5 | 5.125 | 1023 | 206 | 54.46 |
| CCSD(T) [26] | … | 1.79 | | | | | | 50.10 |
| MCPF [16] | … | 1.830 | 1568 | | | | | 56.00 |
| MP2 [26] | … | 1.78 | | | | | | 43.00 |
| MCSCF+1+2 [14] | … | 1.822 | 1595 | | | | | 52.7 |
| Experiment [25] | … | | | | | | | 55.35 ± 2.31 |
| 12Σ+ | CBS-fcFCI-δcore | 1386 | 1.745 | 1648 | 26.5 | 5.615 | 1292 | 261 | 51.58 |
| CBS-fcFCI | 1295 | 1.775 | 1598 | 24.6 | 5.429 | 1305 | 251 | 51.31 |
| A5Z-fcFCI | 1296 | 1.775 | 1595 | 24.5 | 5.427 | 1298 | 251 | 51.14 |
| AQZ-fcFCI | 1299 | 1.776 | 1592 | 24.4 | 5.424 | 1295 | 251 | 51.08 |
| A5Z-C-CCSD(T) | 1406 | 1.745 | 1648 | 27.9 | 5.617 | 1336 | 261 | 51.24 |
| AQZ-C-CCSD(T) | 1334 | 1.745 | 1647 | 26.5 | 5.614 | 1299 | 261 | 51.18 |
| A5Z-CCSD(T) | 1317 | 1.774 | 1597 | 24.4 | 5.432 | 1310 | 251 | 50.96 |
| AQZ-CCSD(T) | 1321 | 1.775 | 1595 | 25.1 | 5.429 | 1305 | 252 | 50.85 |
| A5Z-MRCI+Q | 1026 | 1.774 | 1606 | 32.5 | 5.433 | 1832 | 249 | 52.06 |
| A5Z-MRCI | 1046 | 1.775 | 1595 | 26.3 | 5.431 | 1824 | 252 | 51.92 |
| AQZ-MRCI+Q | 1036 | 1.775 | 1601 | 31.9 | 5.429 | 1822 | 250 | 51.59 |
| AQZ-MRCI | 1056 | 1.775 | 1593 | 30.5 | 5.426 | 1814 | 252 | 51.45 |
| MCPF [16] | 1700 | 1.788 | 1571 | | | | | |
| MCSCF+1+2 [14] | | 1.776 | 1532 | | | | | |
| 12Π | CBS-fcFCI-δcore | 1540 | 1.780 | 1626 | 22.5 | 5.397 | 1135 | 238 | 51.14 |
| CBS-fcFCI | 1432 | 1.817 | 1590 | 22.7 | 5.180 | 1073 | 220 | 50.93 |
| A5Z-fcFCI | 1439 | 1.818 | 1590 | 22.5 | 5.176 | 1074 | 220 | 50.74 |
| AQZ-fcFCI | 1447 | 1.819 | 1587 | 22.0 | 5.171 | 1077 | 220 | 50.66 |
| A5Z-C-CCSD(T) | 1565 | 1.781 | 1624 | 22.3 | 5.392 | 1153 | 238 | 50.78 |
| AQZ-C-CCSD(T) | 1562 | 1.783 | 1622 | 22.7 | 5.381 | 1127 | 237 | 50.50 |
| A5Z-CCSD(T) | 1456 | 1.818 | 1590 | 21.7 | 5.177 | 1080 | 220 | 50.53 |
| AQZ-CCSD(T) | 1465 | 1.819 | 1588 | 21.9 | 5.171 | 1075 | 219 | 50.49 |
| A5Z-MRCI+Q | 1414 | 1.816 | 1587 | 21.9 | 5.185 | 1086 | 222 | 51.00 |
| A5Z-MRCI | 1417 | 1.817 | 1586 | 21.5 | 5.183 | 1074 | 221 | 50.91 |
| AQZ-MRCI+Q | 1423 | 1.817 | 1585 | 21.7 | 5.180 | 1071 | 221 | 50.53 |
| AQZ-MRCI | 1425 | 1.817 | 1584 | 21.7 | 5.178 | 1086 | 221 | 50.44 |
| MCSCF+1+2 [14] | | 1.816 | 1560 | | | | | |
| 22Σ+ | A5Z-MRCI+Q | 15,245 | 1.861 | 1555 | 35.5 | 4.937 | 1263 | 199 | 11.50 |
| A5Z-MRCI | 15,265 | 1.861 | 1554 | 34.9 | 4.938 | 1274 | 199 | 11.36 |
| AQZ-MRCI+Q | 15,253 | 1.862 | 1551 | 34.5 | 4.935 | 1283 | 200 | 11.03 |
| AQZ-MRCI | 15,273 | 1.861 | 1554 | 34.4 | 4.936 | 1250 | 199 | 10.89 |
Figure 3.
A5Z-C-MRCI spin–free PECs (left panel) and spin–orbit curves (right panel) of ScH+ as a function of Sc+···H distance [r(Sc+···H), Å]. In the left plot the relative energies are referenced to the Sc+(3D) + H(2S) fragments placed at 200 Å, which is set to 0 kcal/mol, whereas, in the right plot the relative energies are with respect to the lowest energy spin–orbit state at 200 Å.
Figure 3.
A5Z-C-MRCI spin–free PECs (left panel) and spin–orbit curves (right panel) of ScH+ as a function of Sc+···H distance [r(Sc+···H), Å]. In the left plot the relative energies are referenced to the Sc+(3D) + H(2S) fragments placed at 200 Å, which is set to 0 kcal/mol, whereas, in the right plot the relative energies are with respect to the lowest energy spin–orbit state at 200 Å.
Table 3.
CBS-fcFCI-δcore-δSO adiabatic excitation energy (Te, cm−1), bond length (re, Å), harmonic vibrational frequency (ωe, cm−1), anharmonicity (ωexe, cm−1), equilibrium rotational constant (Be, cm−1), anharmonic correction to the rotational constant (αe, cm−1), centrifugal distortion constant (e, cm−1), and bond energies (D0, kcal/mol) of the first five spin–orbit states of ScH+.
Table 3.
CBS-fcFCI-δcore-δSO adiabatic excitation energy (Te, cm−1), bond length (re, Å), harmonic vibrational frequency (ωe, cm−1), anharmonicity (ωexe, cm−1), equilibrium rotational constant (Be, cm−1), anharmonic correction to the rotational constant (αe, cm−1), centrifugal distortion constant (e, cm−1), and bond energies (D0, kcal/mol) of the first five spin–orbit states of ScH+.
| State | Te | re | ωe | ωexe | Be | αe ×10−4 | e ×10−6 | D0 |
|---|
| 12Δ3/2 | … | 1.791 | 1657 | 23.3 | 5.323 | 1047 | 223 | 55.45 |
| 12Δ5/2 | 175 | 1.791 | 1658 | 23.8 | 5.324 | 1025 | 219 | 54.95 |
| 12Σ+1/2 | 1397 | 1.751 | 1518 | 24.6 | 5.578 | 1839 | 249 | 51.61 |
| 12Π1/2 | 1602 | 1.771 | 1611 | 23.8 | 5.425 | 1509 | 240 | 50.91 |
| 12Π3/2 | 1624 | 1.780 | 1648 | 24.9 | 5.446 | 1144 | 235 | 50.70 |
2.3. YH+
The ground state of Y
+ is a
1S with 5s
2 valence electron configuration in contrast to Sc
+ which bears a
3D (3d
14s
1) ground electronic state [
19]. Interestingly, the corresponding
1S (4s
2) state of Sc
+ is its fourth excited electronic state lying at 11,736.36 cm
−1. However, the first three excited states of Y
+ (
3D; 4d
15s
1,
1D; 4d
15s
1, and
3F; 4d
2) and the first three electronic states of Sc
+ (
3D; 3d
14s
1,
1D; 3d
14s
1, and
3F; 3d
2) are similar [
19]. Since the atomic spectra of Sc
+ and Y
+ are somewhat different, we expect the electronic spectra of YH
+ and ScH
+ to differ as well.
For the calculations of YH
+, the AXZ [aug-cc-pVXZ (of H) cc-pVXZ-PP (of Y)] and AXZ-C [aug-cc-pVXZ (of H) cc-pwCVXZ-PP (of Y)] (X = Q, 5) basis sets were used with the energy-consistent relativistic pseudopotential (ECP28). It should be noted that the utilization of such ECPs for second row-transition metal species is common, especially to avoid convergence issues caused by inner electrons and to minimize the computational cost [
27,
28,
29,
30,
31,
32,
33]. To investigate the electronic spectrum of YH
+, here we have considered the Y
+(
1S)+H(
2S), Y
+(
3D)+H(
2S), and Y
+(
1D)+H(
2S) reactions that produce
2Σ
+,
4,2[Σ
+, Π, Δ], and
2[Σ
+, Π, Δ] molecular states of YH
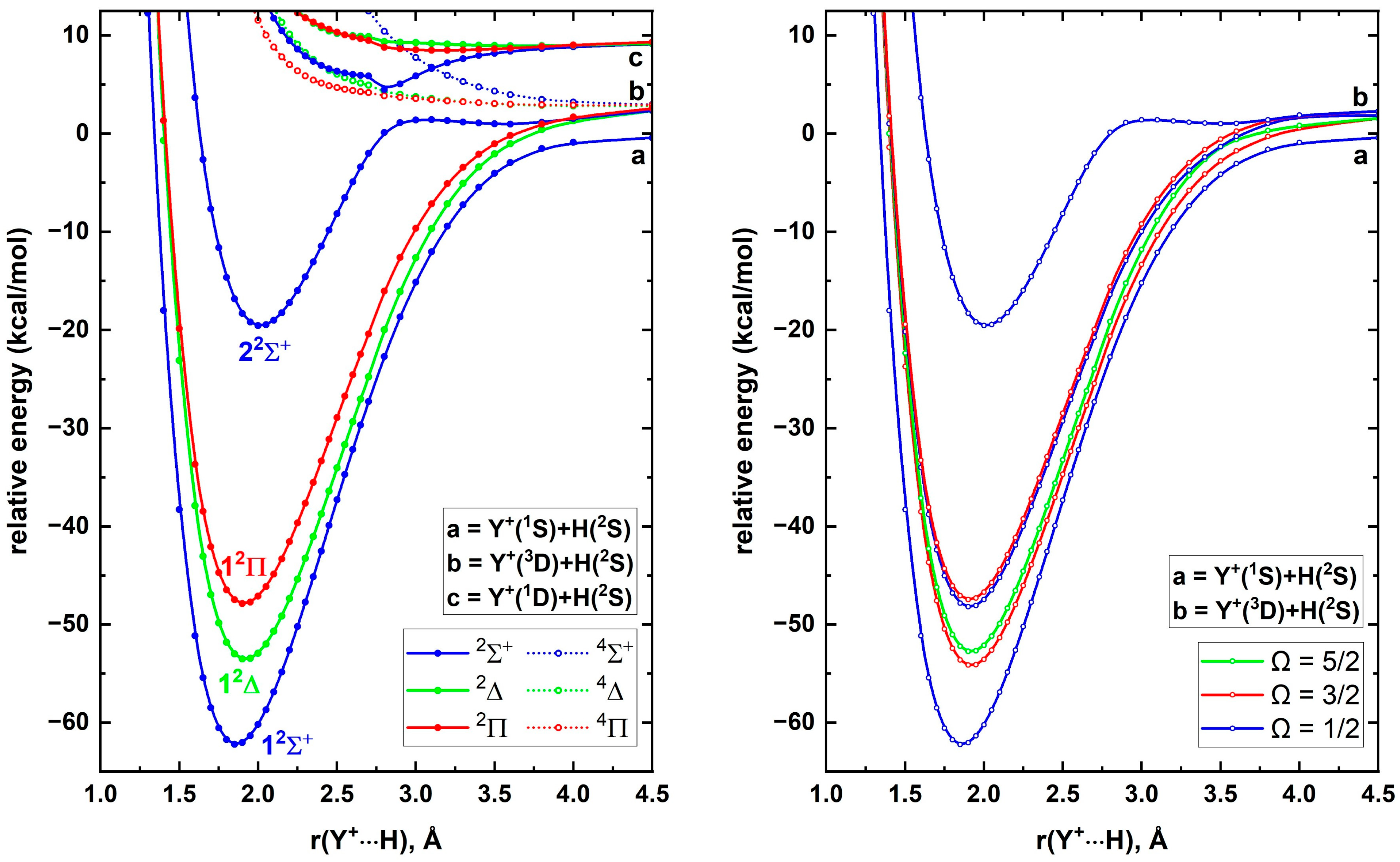
+. The A5Z-C-MRCI PECs of these electronic states of YH
+ are given in
Figure 4 (left panel). The ground state of YH
+ is a 1
2Σ
+ originating from the Y
+(
1S)+H(
2S) ground state fragments. All doublet-spin states arising from the Y
+(
3D)+H(
2S) are attractive while its quartet molecular states are repulsive, similar to the PECs of the Sc
+(
3D)+H(
2S) case (
Figure 1). The first and second excited states of YH
+ (i.e., 1
2Δ and 1
2Π) smoothly dissociate to Y
+(
3D)+H(
2S). The 2
2Σ
+ of the same fragments undergoes an avoided crossing (around 2.6 Å) with the
2Σ
+ PEC coming from the Y
+(
1D)+H(
2S). Recall that the 2
2Σ
+ of ScH
+ originated from the Sc
+(
1D; 3d
14s
1)+H(
2S), which further confirms the true origin (considering the avoided crossing) of YH
+(2
2Σ
+) as Y
+(
1D; 4d
15s
1)+H(
2S). Overall, the first four electronic states of YH
+ (1
2Σ
+, 1
2Δ, 1
2Π, and 2
2Σ
+) are bound with respect to the ground state fragments similar to the ScH
+ case. As we expected earlier, the electronic spectra of ScH
+ and YH
+ are different, with the first two electronic states of ScH
+ being 1
2Δ and 1
2Σ
+ while they are opposite in order for YH
+ (i.e., 1
2Σ
+ and 1
2Δ).
The 1
2Σ
+, 1
2Δ, 1
2Π, and 2
2Σ
+ electronic states of YH
+ bear similar single-reference electron configurations and NBO charges to those of ScH
+ (i.e., 1
2Σ
+: 1σ
22σ
1, 1
2Δ: 1σ
21δ
1, 1
2Π: 1σ
21π
1, and 2
2Σ
+: 1σ
23σ
1) (ESI
Tables S1 and S4). The shapes of the CASSCF state average molecular orbitals resulting from these four states of YH
+ are qualitatively similar to those of ScH
+ (
Figure 2). The NBO electron populations of 1
2Δ and 1
2Π of ScH
+ and those of the YH
+ are similar to each other. On the contrary, the electron populations of YH
+(1
2Σ
+, 2
2Σ
+) and ScH
+(1
2Σ
+, 2
2Σ
+) are slightly different (i.e., Y[5s
0.944d
0.43]H[1s
1.58] and Sc[4s
0.583d
0.84]H[1s
1.55] for 1
2Σ
+ and Y[5s
0.244d
0.79]H[1s
1.77] and Sc[4s
0.473d
0.59]H[1s
1.73] for 2
2Σ
+). This substantial difference between the electron population on the Y and Sc atoms is elucidated by their state-specific equilibrium Hartree–Fock (HF) molecular orbitals (
Figure 5). Observe that the 2σ of YH
+(1
2Σ
+) is dominated by the 5s of Y, whereas 2σ of ScH
+(1
2Σ
+) is a clear hybrid of
and 4s of Sc (
Figure 5). On the other hand, the 3σ of ScH
+(2
2Σ
+) bears more s orbital character compared to the s orbital character of the 3σ of YH
+(2
2Σ
+) (
Figure 5).
Similar to the ScH
+ case, several theoretical approaches were utilized to determine the spectroscopic parameters of 1
2Σ
+, 1
2Δ, 1
2Π, and 2
2Σ
+ of YH
+ (
Table 4). Our CBS-fcFCI-δcore estimated that the r
e, ω
e, and ω
ex
e of the ground state of YH
+ are 1.862 Å, 1688 cm
−1, and 20.5 cm
−1, respectively. The core electron correlation disregarded calculations predicting that the r
e and ω
e of 1
2Σ
+ are closer to the corresponding MCPF values reported by Pettersson et al. [
16]. In all cases, the core electron correlation was found to shorten the r
e while increasing the ω
e values [compare A5Z-CCSD(T) and A5Z-C-CCSD(T) values in
Table 4]. All our core electron correlation accounted D
0 (of 1
2Σ
+) predictions are within the error bars of the experimental D
0 reported by Sievers et al. (i.e., 61.11 ± 1.84 kcal/mol), which further substantiates the importance of the core electron correlation for gaining accurate predictions of transition metal diatomic species [
34]. Our CBS-fcFCI-δcore theoretical approach provided the best D
0 (i.e., 60.54 kcal/mol), which is only 0.57 kcal/mol larger than the experimental D
0 of YH
+ (
Table 4) [
34].
The spin–orbit states resulting from the first four electronic states of YH
+ are illustrated in
Figure 4 (right panel). There is no spin–orbit splitting for the 1
2Σ
+ state of YH
+. However, the spin–orbit splitting of both 1
2Δ and 1
2Π is substantial, especially compared to the splitting of both the 1
2Δ and 1
2Π of ScH
+. Specifically, the Ω = 3/2 and Ω = 5/2 of 1
2Δ and Ω = 1/2 and Ω = 3/2 of 1
2Π of YH
+ are separated by 492 and 263 cm
−1 (ESI
Table S5), while those splits of ScH
+ are 175 and 27 cm
−1, respectively. At equilibrium distances, the spin–orbit states of YH
+ do not carry significant mixings (ESI
Table S5), hence their spectroscopic parameters are either same or nearly identical to the spectroscopic parameters of corresponding electronic states (
Table 4 and ESI
Table S5). Similar to the ScH
+ case, the CBS-fcFCI-δcore-δSO spectroscopic parameters of YH
+ are provided for five spin–orbit states (
Table 5).
2.4. LaH+
Having obtained a better understanding of the electronic structures of ScH
+ and YH
+, next we shifted our attention to LaH
+. The ground state of La
+ is a
3F with 5d
2 valence electron configuration. Sc
+ and Y
+ populated similar
3F(
nd
2) in their second and third excited states (at 4802–4988 cm
−1 and 8003–8744 cm
−1, respectively) [
19]. The first two excited states of La
+ (i.e.,
1D; 5d
2 and
3D; 5d
16s
1) lie very close to its ground state (at 1394.46 and 1895.15–3250.35 cm
−1, respectively) with large spin–orbit splittings [
19]. To study LaH
+, as the first step, the reactions of the first three electronic states of La
+ with H(
2S) were studied. The AQZ-C-MRCI PECs of the
2,4[Σ
–, Π, Δ, Φ],
2[Σ
+, Π, Δ], and
2,4[Σ
+, Π, Δ] molecular states originating from La
+(
3F)+H(
2S), La
+(
1D)+H(
2S), and La
+(
3D)+H(
2S) are given in
Figure 6 (left panel). The ground electronic state of LaH
+ is 1
2Δ, which smoothly dissociates to La
+(
3F)+H(
2S). Similarly, the second excited state of LaH
+ (i.e., 1
2Π) is a product of the same fragments. The first excited state of LaH
+ (i.e., 1
2Σ
+) dissociates to La
+(
1D)+H(
2S), but displays an avoided crossing at ~3.75 Å with a
2Σ
+ PEC resulting from La
+(
3D)+H(
2S). Indeed, this
2Σ
+ of La
+(
3D)+H(
2S) turned out to be the third excited state of LaH
+ (i.e., 2
2Σ
+) (
Figure 6, left panel). Several doublet and quartet spin PECs of the selected La
+ + H reactions produce shallow minima that are bound with respect to the ground state fragments. Specifically, the fifth state of LaH
+ is such a state (i.e.,
4Σ
-), with a 2.6 Å r
e and a 4.32 kcal/mol binding energy with respect to ground state fragments. We observed another
2Σ
+ state stabilized energetically in between La
+(
1D)+H(
2S) and La
+(
3D)+H(
2S) asymptotes, which are plotted up to 2.8 Å (
Figure 6, left panel). Overall, upon considering the first four electronic states, the third and fourth states of all LaH
+, YH
+, and ScH
+ are 1
2Π and 2
2Σ
+. While the ordering of the first two states of LaH
+ and ScH
+ is identical (1
2Δ and 1
2Σ
+), the order is swapped in the YH
+ case to 1
2Σ
+ and 1
2Δ.
The charge distributions and electron populations of 1
2Δ, 1
2Σ
+, 1
2Π, and 2
2Σ
+ of LaH
+ and YH
+ are approximately similar to each other (compare the NBO findings listed in ESI
Tables S4 and S6). The spectroscopic constants of LaH
+ calculated under various quantum chemical approaches are given in
Table 6. In line with the findings for ScH
+ and YH
+, core electron correlation was found to shorten the r
e values and increase the ω
e values of LaH
+ (
Table 2,
Table 4, and
Table 6). Our CBS-fcFCI-δcore r
e, ω
e, and ω
ex
e values of LaH
+ are 2.011 Å, 1521 cm
−1, and 17.0 cm
−1, respectively. Elkind et al.’s experimentally measured D
0 of LaH
+ is 57.19 ± 2.08 kcal/mol [
17]. The CBS extrapolation increased the D
0 of LaH
+ (1
2Δ) by 0.51 kcal/mol, which is larger compared to the CBS effect of the ScH
+ (1
2Δ) and YH
+ (1
2Σ
+) which are only 0.17 and 0.18 kcal/mol, respectively. Our largest level of theory without the spin–orbit coupling effects (i.e., CBS-fcFCI-δcore) predicted a D
0 of 64.19 kcal/mol for LaH
+ (1
2Δ) with respect to ground state fragments.
Since the spin–orbit coupling effects are dominant for lanthanides, next we evaluated the spin–orbit coupling of LaH
+. The spin–orbit matrix of LaH
+ was constructed by including all the electronic states given in the left panel of
Figure 6. Due to complexity, only the spin–orbit curves arising from the PECs of the first four electronic states of LaH
+ are illustrated in the right panel of
Figure 6. The ground spin–orbit state of LaH
+ is an Ω = 3/2 with a dominant 1
2Δ character (i.e., 98% 1
2Δ + 2% 1
2Π) (ESI
Table S7). The spin–orbit effect included AQZ-C-MRCI D
0 of LaH
+ is 56.36 kcal/mol, which is in excellent agreement with Elkind et al.’s experimental D
0 [
17]. The spin–orbit coupling disregarded AQZ-C-MRCI D
0 of LaH
+ is 58.21 kcal/mol, hence we can identify a 1.85 kcal/mol spin–orbit effect for the D
0 of LaH
+. If we incorporate this spin–orbit correction into our CBS-fcFCI-δcore D
0 of LaH
+ (1
2Δ) then the D
0 of LaH
+ would be 62.34 kcal/mol, which is 3.07 kcal/mol larger than the upper bound of the experimental D
0 reported by Elkind et al.’s D
0 (
Table 7). Large spin–orbit splittings were observed for the 1
2Δ (Ω = 3/2 and Ω = 5/2) and 1
2Π (Ω = 1/2 and Ω = 3/2) of LaH
+ (
Figure 6, right panel and ESI
Table S7). Specifically, the aforementioned Ω states of the 1
2Δ and 1
2Π are energetically separated by 1042 and 488 cm
−1, respectively (ESI
Table S7). Compared to the corresponding splittings of YH
+, they are 550 and 225 cm
−1 larger, respectively. Among the studied Ω states, the third and fourth spin–orbit states of LaH
+ (i.e., Ω = 1/2 at 1857 cm
−1 and Ω = 1/2 at 3125 cm
−1) carry the largest ΛS mixings (i.e., 93% 1
2Σ
+ + 7% 1
2Π and 93% 1
2Π + 7% 1
2Σ
+, respectively).