Abstract
The increasing popularity of edible insects as a sustainable food source necessitates stringent safety measures to monitor pesticide contamination. This study aimed to assess and enhance a QuEChERS-based extraction method coupled with gas chromatography–tandem mass spectrometry (GC-MS/MS) for the quantification of pesticide residues in edible insects (bamboo caterpillars, house crickets, silkworm pupae, giant water bugs, and grasshoppers) by combining multiple individual insect specimens into a single, homogenized sample—five replicates were tested. The method was optimized by evaluating various extraction parameters and showed strong linearity for all 47 target pesticides, with correlation coefficients (R2) ranging from 0.9940 to 0.9999. The limits of detection (LODs) varied between 1 and 10 µg/kg, while the limits of quantification (LOQs) ranged from 10 to 15 µg/kg. Recovery studies conducted at three fortification levels (10, 100, and 500 µg/kg) revealed recoveries ranging from 64.54% to 122.12%, that over 97.87% of the pesticides exhibited satisfactory recoveries within the range of 70–120%, and relative standard deviations (RSDs) below 20%, between 1.86% and 6.02%. Matrix effects (%MEs) range from −33.01% to 24.04%, and to those that experienced no effect. More than 94% of the analytes showed minimal ion suppression or enhancement. These results conform to the SANTE guidelines for monitoring pesticide residues in edible insects, enhancing food safety standards and safeguarding consumer protection.
1. Introduction
The global shift towards sustainability and eco-friendly food options has generated increasing interest in edible insects as a viable alternative protein source [1]. These insects provide a rich source of high-quality protein and essential fatty acids, vitamins, and minerals. Their production is notably more resource-efficient than that of conventional livestock, requiring significantly less land, water, and feed, which contributes to a more sustainable food system [2]. In numerous regions, especially across Asia, Africa, and Latin America, consuming insects has a long-standing history. In Thailand, the consumption of insects is deeply ingrained in cultural and culinary practices. Commonly consumed species include house crickets (Acheta domesticus), grasshoppers (Melanoplus foedus), silkworm (Bombyx mori) pupae, bamboo caterpillars (Omphisa fuscidentalis), and diving beetle (Cybister limbatus) [3].
While edible insects offer potential benefits for food security and nutrition, they also pose food safety concerns due to the risk of pesticide contamination. Insects are exposed to pesticides both directly from treated areas and indirectly through their contaminated diet [4]. Furthermore, specimens gathered from their natural environments might be influenced by agricultural runoffs or the use of pesticides within their ecosystems [5]. Several studies have reported the presence of pesticide residues in edible insect samples, specifically in food products made from A. domesticus collected in Vietnam, with some residues belonging to pesticides that are either banned or restricted in the country [6]. Previous studies identified nine agrochemicals in six species of edible insects, including insecticides, herbicides, and fungicides, with some species exceeding maximum residue limits for certain chemicals. Notably, the Nigerian cricket (Brachytrupes membranaceus) contained detectable residues of several pesticides currently in use, although the levels of most residues remained below the established maximum residue limit of 0.01 mg/kg, indicating compliance with safety standards [7]. Moreover, a study analyzing various species of edible insects, including mealworms (Tenebrio molitor), crickets, silkworm pupae, and grasshoppers, found that some samples contained high microbial counts, particularly those that were raw or inadequately processed. Chemical analysis also revealed the presence of heavy metals, including lead, cadmium, and arsenic, although the levels remained within Canadian safety limits. Mycotoxins and pesticide residues were either undetectable or present at very low concentrations [8]. The study of pesticides in edible insects is complicated by the elevated levels of fat and protein in the matrix [9]. Chromatographic techniques for quantifying pesticide residue concentrations often necessitate comprehensive lipid removal before sample introduction into the system [10]. Consequently, the cleanup protocol must be refined to eliminate sample-derived matrices efficiently. Various extraction methods have been developed for this purpose, including solid-phase extraction (SPE), liquid–liquid extraction (LLE), solid-phase microextraction (SPME), and the QuEChERS method, which stands for quick, easy, cheap, effective, rugged, and safe [11].
The QuEChERS method is widely recognized for its versatility and effectiveness in extracting pesticide residues from various food matrices. Its advantages include high recovery rates, reduced solvent consumption, and suitability for high-throughput workflows. This method typically involves extraction with acetonitrile and a salt mixture to induce phase separation, followed by cleanup using dispersive solid-phase extraction (dSPE) with sorbents such as primary secondary amine (PSA) and anhydrous magnesium sulfate (MgSO4). Additional sorbents, such as C18 or graphitized carbon black (GCB), are sometimes employed to enhance cleanup, particularly for complex matrices [12,13,14,15]. Given the variability in fat content and matrix complexity among edible insect species, adapting and optimizing the QuEChERS protocol is essential to ensure accurate and reliable pesticide residue analysis.
Pesticide residue extraction methods are often analyzed using chromatographic techniques, such as gas chromatography–mass spectrometry (GC-MS/MS) or liquid chromatography–mass spectrometry (LC-MS/MS). Recently, liquid chromatography paired with quadrupole time-of-flight mass spectrometry (LC-QTOF/MS) has emerged as a powerful approach for both non-targeted and targeted pesticide screening in complex food matrices, owing to its enhanced resolution, accuracy, and sensitivity [16]. Studies have demonstrated the successful application of QuEChERS, combined with LC-MS/MS or GC-MS/MS, to detect pesticides in high-fat or complex matrices, such as rice, vegetable oils, and mealworms [17,18]. Previous studies using liquid chromatography–tandem mass spectrometry (LC-MS/MS) were developed to analyze 353 pesticides in Tenebrio molitor larvae. The method achieved limits of quantitation ≤10 μg/kg, with over 90% recovery and negligible matrix effects for most pesticide analyses [19].
The purpose of this study is to develop and validate an optimized QuEChERS-based extraction method combined with GC-MS/MS for multiresidue pesticide analysis in edible insects. The method was applied to real insect samples to assess potential pesticide contamination, supporting food safety monitoring efforts for insects, which are increasingly recognized as sustainable future food sources.
2. Results and Discussion
2.1. Optimization of Sample Extraction Procedure
The extraction of pesticide residues from edible insect matrices presents a significant analytical challenge due to their high protein and lipid content. In particular, lipids are highly soluble in organic solvents, which facilitates their co-extraction. To ensure accurate analysis, it is essential to purify the sample using appropriate pretreatment procedures prior to gas chromatography (GC) analysis [17,19]. The QuEChERS method was modified to use the optimal solvent/sample ratio. A standard mixed solution of 47 selected pesticides (100 μg/mL) was spiked into edible insect samples (2.5, 5.0, and 10.0 g). Each sample was transferred to a 50 mL centrifuge tube, to which varying amounts of acetonitrile (ACN) (5.0, 10.0, and 15.0 mL) were added, followed by 5 mL of water. The mixtures were then agitated for 5 min. Subsequently, a QuEChERS extraction package comprising 6 g of magnesium sulfate (MgSO4) and 1.5 g of sodium citrate (Na3C6H5O7) was incorporated to enhance phase separation and optimize cleanup efficacy.
The number of pesticides extracted increased significantly with the addition of higher volumes of ACN across all sample sizes, as illustrated in Figure 1. In the 2.5 g sample, the number of detectable pesticides increased markedly from 21 (extracted with 5 mL of ACN) to 45 (with 15 mL of ACN), indicating that extraction efficiency improves with increased solvent volume. The 5.0 g and 10.0 g samples exhibited a similar trend, though their overall recovery rates were lower. Using a larger volume of solvent relative to the sample size is especially critical for smaller samples, as it facilitates the separation of lipophilic pesticide residues into the extraction solvent [20,21,22]. This study demonstrated that increasing the volume of ACN significantly enhanced the extraction efficiency of lipophilic pesticides. Similar to the findings of Shin et al. (2020) [19], who demonstrated that a solvent-to-sample ratio of 3:1 or greater substantially improved the recovery of lipophilic pesticides in mealworms, this study found that increasing the volume of acetonitrile led to a marked increase in the number of detectable analytes, especially in small sample sizes.
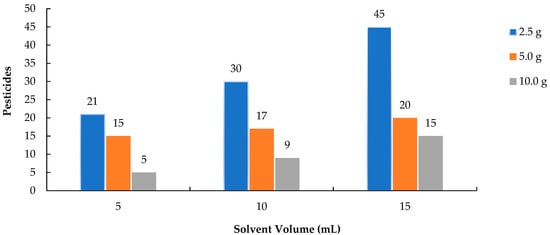
Figure 1.
Number of pesticides for extractions with different amounts analyzed by GC-MS/MS.
The extraction of lipophilic (fat-loving) pesticides was effectively enhanced by using a greater volume of acetonitrile (ACN) in this study. This observation aligns with the findings of Lee et al. [23], who demonstrated that using a higher solvent-to-sample ratio facilitates more efficient migration of pesticides from complex insect matrices into the organic layer. A larger volume of ACN promotes efficient partitioning, thereby improving analyte transfer, as the adipose layer in the insects acts as a reservoir for lipophilic compounds.
This study utilized freeze-drying (lyophilization) as a sample preparation technique to enhance analytical precision and maintain the integrity of pesticide residues in edible insect matrices. Freeze-drying efficiently eliminates water content without applying heat, thus reducing the likelihood of thermal degradation or enzymatic modification of target analytes, which is particularly crucial for thermolabile or pH-sensitive pesticides [20]. Moreover, dry samples offer enhanced control over sample weight and solvent ratios, which is crucial for method optimization. Utilizing smaller sample sizes (e.g., 2.5–5.0 g) is especially beneficial for insects, which frequently produce less biomass and possess a higher fat content that may hinder extraction efficiency [19]. Our study confirms that lyophilized insect samples, when adequately rehydrated, provide consistent analyte recoveries and facilitate accurate control over sample-to-solvent ratios [17]. In this study, 5 mL of water was added to all extraction mixtures to hydrate the freeze-dried samples, demonstrating that the addition of water improves the partitioning efficiency of pesticides during QuEChERS extraction by swelling the matrix and enhancing analyte desorption [20,24]. Similarly, studies on food matrices with a low moisture content showed that insufficient hydration can lead to poor recovery due to the inadequate extraction of target compounds trapped within the matrix [25]. The consistent recovery of most pesticides (70–120%) indicates that the water proportion used was sufficient to optimize extraction without excessively diluting the solvent system. However, slight variations in recovery were observed for some lipophilic pesticides, which could be influenced by the balance between solvent volume and water content. Excessive water may reduce the extraction efficiency of non-polar pesticides by increasing the polarity of the extraction environment, thus hindering their transfer into the organic phase.
The quality of the extract was also enhanced by the addition of MgSO4 and Na3 Citrate, which facilitated phase separation and effectively removed residual water. This improvement is essential when dealing with insect matrices that are high in fat and susceptible to emulsification. These findings are consistent with recent food safety studies that employed QuEChERS-based extraction protocols [10,26].
2.2. Optimization of MRM Condition for GC–MS/MS Analysis
To optimize pesticide detection, collision energy (CE) tests were conducted to identify the two most suitable ion transitions—primary and secondary—from precursor to product ions for multiple reaction monitoring (MRM). Quantification was performed by GC-MS/MS using the external standard method. The Agilent Mass Hunter software (version 10.2) was used to determine the peak area of each analyte’s primary ion transition.
Precursor ions were identified through full-scan analysis for each pesticide, and their retention times (RTs) were determined under the specified gas chromatography (GC) conditions. Product ion scans were then carried out at varying collision energies (0–50 eV) using the selected precursor ions. The optimal MRM conditions were established by evaluating the sensitivity of the resulting product ions at varying CE levels. For confirmation, two product ions per precursor, along with their ion ratios and retention times, were selected. The qualitative and quantitative ions were selected based on their signal intensity and minimal interference from surrounding ions or baseline noise, as detailed in Table 1, and Figure 2 shows the mass spectra of the representative pesticide standards obtained under collision-induced dissociation (CID) conditions, including the ion ratios for dichlorvos and β-BHC.

Table 1.
The MRM Conditions of the primary and secondary transitions of a precursor to product ions for pesticides.
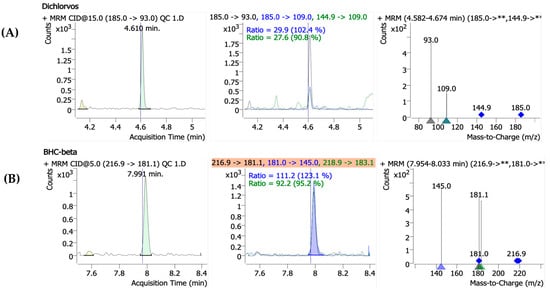
Figure 2.
The representative mass spectra of the selected pesticide standards obtained under collision-induced dissociation (CID) conditions. (A) Dichlorvos** The protonated molecular ion [M+H]+ at m/z 185.0 yields fragment ions at m/z 93.0 and 109.0. (B) BHC-beta** The precursor ion [M+H]+ at m/z 216.9 generates a fragment ion at m/z 181.1, while another ion at m/z 218.9 fragments into m/z 183.1.
2.3. Method Validations
2.3.1. Linearity, Selectivity, Limit of Detection, and Limit of Quantification
The selectivity of the analytical method was evaluated by comparing chromatograms of the recovery samples with those of standard solutions and blank samples. All 47 pesticide compounds demonstrated consistent retention times and m/z values under the established method using matrix-matched standards, with no interfering peaks observed. These results confirm the high selectivity and effective separation achieved by the developed analytical technique. The limits of detection (LODs) for individual pesticides ranged from 1 to 5 μg/kg, while the limits of quantification (LOQs) ranged from 10 to 15 μg/kg. Linearity, which refers to the method’s ability to produce results directly proportional to the analyte concentration within a specified range, was assessed using matrix-matched calibration curves. The GC–MS/MS results, summarized in Table 2 and Table 3, include correlation coefficients (R2), y-intercepts, and calibration curve parameters. The R2 values ranged from 0.9940 to 0.9999, and the linearity of the method was evaluated by assessing the percentage deviation of the back-calculated concentration (BBC) from the nominal concentration at each calibration level. The % deviations of the BBC at each calibration level were calculated and reported following the criteria specified in Table 2, which were used according to the SANTE guidelines [24]. The results demonstrated that the majority of the pesticide compounds (47 analytes) had acceptable limits across all levels, indicating excellent linearity. Most compounds exhibited deviation values below ±30%, while, at mid- to high levels (10–500 µg/kg), deviations generally fell within ±20%. These findings confirm that the analytical method demonstrates excellent linearity for quantitative analysis.

Table 2.
The linearity of the analytical method based on the % deviation of back-calculated concentrations (BBCs) in edible insect samples (n = 5).

Table 3.
Limit of detection (LOD), limit of quantification (LOQ), recovery, precision, and matrix effect in pooled edible insect samples by GC-MS/MS (n = 5).
2.3.2. Precision and Accuracy
The accuracy and precision of the target compounds in the developed method were assessed based on average recovery and relative standard deviation (RSD) from five trials (n = 5). Three spiking concentrations—low, medium, and high—were selected based on the linear range of the target compounds. For a linear range of 5 to 500 μg/kg, the spiking levels were set at 10 μg/kg (low), 100 μg/kg (medium), and 500 μg/kg (high). Recovery rates ranged from 70% to 120%, with RSD values below 20%, indicating the method’s effectiveness. In the recovery test using GC–MS/MS for edible insects, all pesticides exhibited recovery within the acceptable range of 70–120%. Additionally, RSD values were confirmed to be under 20% for all pesticides, as shown in Table 3.
In our study, acetonitrile was selected as the extraction solvent for the multiresidue analysis of pesticides in edible insect samples. In addition, to achieve better efficiency in the extraction and recovery of multiresidue pesticides from the samples, the QuEChERS procedure was employed in our study, as all recovery rates fell within the range considered effective and promising. The QuEChERS procedure yielded extraction recoveries ranging from 64.54% to 122.12%, with RSDs between 1.86% and 6.02%. On the other hand, the QuEChERS procedure may have caused strong salting-out effects, as the mean recoveries of methamidophos (122.12%) treated at a level of 100 μg/kg were observed to be higher than those of other pesticides [19,27]. However, such salting-out effects were not observed in our study. Therefore, the recoveries of multiresidue pesticides were generally between 70 and 120% with RSDs below 20%, indicating that the proposed method in this study was feasible to analyze 47 pesticides in samples.
The validated method developed in this study demonstrates performance metrics comparable to and, in some aspects, exceeding those reported in the recent literature. The technique achieved strong linearity (R2 = 0.9940–0.9999) across all 47 target pesticides, which aligns closely with the other studies in Table 4, and Shin et al. (2020) [19] and Labu et al. (2022) [6], both of which reported R2 values > 0.995. The LOQ range (10–15 µg/kg) in this study is slightly higher than the 5 µg/kg commonly reported in several other investigations, such as those by Kim et al. (2020) [17] and Kolakowski et al. (2021) [7], yet remains within acceptable sensitivity limits for regulatory purposes. In terms of recovery, this study showed results from 64.54% to 122.12%, with over 97.87% of the analytes falling within the 70–120% range defined by SANTE. And, relative standard deviations (RSDs) in this study were consistently below 6.02%, indicating excellent repeatability.
Moreover, the relative response of each analyte varied according to its molecular structure and fragmentation pattern. Nevertheless, the sensitivity of the developed GC-MS/MS method was sufficient to screen GC-amenable pesticides at a fortification level of 10 μg/kg using a 2.5 g sample and 15 mL of solvent for extraction. Minimal matrix interference was observed under these conditions, as demonstrated by the comparison of total ion chromatograms between blank edible insect samples and those fortified at 100 and 500 μg/kg (Figure 3). These results confirm the method’s suitability for analyzing trace levels of pesticides in complex, high-fat matrices.
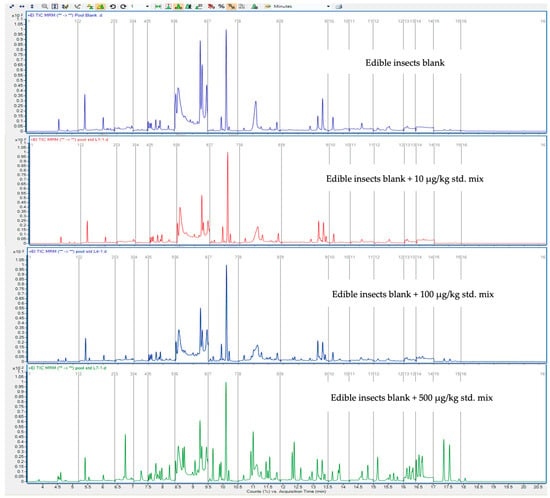
Figure 3.
Reconstructed GC-MS/MS chromatograms of edible insects blank and edible insects blank fortified at 10, 100, and 500 μg/kg in 20 min analyses of 47 target compounds with 1 μL injection volume.
2.3.3. Matrix Effect
The matrix effect provided information about the matrix effects. Depending on the decrease/increase in the percentage of the slope, different matrix effects could be observed: a signal suppression or enhancement effect between −20% and 0% and between 0% and +20% was considered to be mild; a medium effect was supposed when the slope values were between −50% and −20% or +20% and +50%; and a strong effect of signal suppression or enhancement was supposed when values were below −50% or above +50%. These distributions depended not only on the matrix effect but also on the combination of the compound matrix. Pesticides with %ME values within the acceptable range of −20% to +20% experience minimal matrix effects and are less affected by matrix interferences, ensuring more reliable quantification [27,28,29].
Matrix effects (%MEs) observed in the analysis of pesticide residues in edible insect samples varied widely among the compounds, ranging from −33.01% to 24.04%. For instance, fenobucarb and prothiofos exhibited high matrix suppression effects at −33.00% and −33.01%. While omethoate was at +24.24% and endosulfan at +23.70%, respectively, more than 95.74% of the pesticides showed a soft matrix effect (Table 2) with negligible effects in the tested range [29]. Moreover, our findings are in agreement with the study by Łozowicka et al. (2017), who emphasized that modified QuEChERS protocols incorporating buffering salts and increased solvent volumes effectively reduce matrix effects during GC-MS/MS analysis in complex matrices such as soil or insects [10]. However, most of the pesticides in the current investigation were unaffected by the matrix, likely due to the edible insect matrices being effectively eliminated. The extraction procedure effectively removed numerous proteins and lipids that significantly impaired the matrix effect. The dilution procedure used to prepare the sample can also be helpful. Between the extraction and partitioning stages, 5–0 times more solvent was utilized than in traditional QuEChERS procedures [10,30]. Dilution reduced the concentration of the sample matrices to a point where the signal remained unaffected. However, using this method, a small percentage of pesticides (4.25%) exhibited a moderate matrix effect (Table 3). Therefore, for accurate quantification, a matrix-matched calibration procedure ought to be employed.

Table 4.
Previous studies’ analytical methods for determining pesticide residues in edible insects.
Table 4.
Previous studies’ analytical methods for determining pesticide residues in edible insects.
| Matrix | Instrument | No. Analysis | RSD (%) | Recovery (%) | LOQ (µg/kg) | Linearity (r2) | Matrix Effect (ME %) | Reference |
|---|---|---|---|---|---|---|---|---|
| Mealworms | LC-MS/MS | 353 | 5–15 | 75–115 | 5 | >0.995 | −20 to +25 | [19] |
| Mealworms | GC-MS/MS, LC-MS/MS | 300 | 5–12 | 80–115 | 5 | >0.993 | −15 to +20 | [17] |
| Edible insects | GC-MS/MS, LC-MS/MS | - | 5–20 | 75–120 | 5 | >0.990 | −18 to +22 | [7] |
| Edible insects (6 species) | LC-MS/MS | 374 | 6–15 | 75–110 | 5 | >0.995 | −15 to +25 | [6] |
| Mealworm larvae | GC-MS/MS | 247 | 0–19.9 | 70–120 | 50 | ≥0.990 | Not specified | [31] |
3. Materials and Methods
3.1. Chemicals and Materials
Standard pesticide mixtures with a purity of at least 99% were obtained from Dr. Ehrenstorfer (Augsburg, Germany). The combinations comprised a total of 47 chemicals. A diverse array of chemical classes was incorporated—organophosphates (OPs), carbamates (CBs), pyrethroids (PYs), and organochlorines (OCs). Pesticides commonly used in agricultural activities particularly relevant to crops and associated with edible insect habitats were prepared at a concentration of 1000 µg/mL in acetonitrile. For standard fortification, a composite pesticide stock solution was established with various concentrations of standard curve pesticides ranging from 5 to 500 μg/L. A standard mix was used to fortify 2.5 g of pooled edible insect blank by adding 100 μL of the mix to the sample prior to extraction. HPLC-grade acetonitrile, ethyl acetate, and water were obtained from J.T. Baker (Avantor, Radnor Township, PA, USA). They were used for GC/MS and solvent extraction. QuEChERS original packet 50 mL centrifuge tubes filled with 4 g of MgSO4, 1 g of NaCl, 1 g of Na3C6H5O7·2H2O, and 0.5 g of Na2HC6H5O7·1.5H2O from Agilent Technologies, Santa Clara, CA, USA. Additionally, dispersive cleanup tubes (2 mL) were sourced, containing 150 mg of MgSO4, 50 mg of PSA sorbent, bulk carbograph, and 50 mg of end-capped C-18 sorbent, also from Agilent Technologies, USA. For GC-MS/MS, helium (99.999%) and nitrogen (99.999%) served as the carrier gas and collision gas, respectively.
3.2. Sample Selection
In June and July 2023, edible insects were gathered from a local market in Chiang Mai, including bamboo caterpillars, house crickets, silkworm pupae, giant water bugs (Lethocerus indicus), and grasshoppers. A total of 200 g of edible insect samples were collected and freeze-dried at −50 °C for at least 72 h using an SP VirTis Genesis Pilot Freeze Dryer and the Genesis 25 L Pilot Lyophilizer (Westminster, CO, USA). After freeze-drying, the samples were ground into a fine powder. A sample blank was produced by combining multiple individual insect specimens into a single, homogenized unit in studies that involve consumable insects. The non-fortified sample was also prepared to create a blank matrix for matrix-matched standards. Each sample, weighing 2.5 g, was placed in a 50 mL centrifuge tube (Corning Inc., Wujiang, China) and stored at −20 °C until analysis.
3.3. Sample Extraction
Pesticide residues in the edible insect samples were extracted using the EN QuEChERS method [26], with slight modifications. Prior to extraction, the samples were brought to room temperature and spiked with an appropriate standard mixture to achieve final concentrations of 10, 100, and 500 μg/kg. A non-fortified (blank) sample was also prepared to serve as a blank matrix for matrix-matched standards. For the extraction, 5 mL of purified water and 15 mL of acetonitrile were added to each sample tube and mixed thoroughly. The tubes were then tightly capped and shaken for 5 min using a SPEX 2000 Geno Grinder (Digital Multi-Tube Vortex Mixer, Model VXMTDG, OHAUS, Parsippany, Morris County, NJ, USA) at 2500 rpm. Following this, a QuEChERS extraction packet containing 4 g of MgSO4, 1 g of NaCl, 1 g of Na3C6H5O7·2H2O, and 0.5 g of Na2HC6H5O7·1.5H2O was added. The contents were shaken again for 5 min at the same speed and then centrifuged at 2000 rpm for 5 min.
Next, approximately 1 mL of the upper (organic) layer of the extract was transferred into a 2 mL dispersive solid-phase extraction (SPE) tube containing 150 mg of MgSO4, 50 mg of PSA sorbent, bulk carbograph, and 50 mg of end-capped C18 sorbent. The tube was capped, vortexed for 1 min, and centrifuged at 1300 rpm for 3 min. The resulting upper layer was filtered through a 0.45 µm syringe filter and transferred to sample vials for analysis. Finally, 1 μL was injected into a Gas Chromatography Triple Quadrupole Mass Spectrometer (GC-MS/MS) using an autosampler.
3.4. GC-MS/MS Instrument Conditions
GC-MS/MS analysis was performed using an Agilent 8890 Gas Chromatograph, equipped with a 7693A autosampler and a 7000E triple quadrupole mass spectrometer, operated through Mass Hunter Software (Qualitative Analysis version B.12.0.430.0 and Quantitative Analysis version B.12.1.938.3) installed on a dedicated workstation for data acquisition and processing (Agilent Technologies, Inc., Santa Clara, CA, USA). The GC and backflush method parameters, including the tandem mass spectrometry and MRM (multiple reaction monitoring) mode settings, are detailed in Table 5 and Table 6. Analyte separation was achieved using two HP-5 ms Ultra Inert capillary columns from Agilent (0.25 mm internal diameter × 30 m length, 0.25 μm film thickness), connected via a backflush union.

Table 5.
The GC and backflush method conditions.

Table 6.
The tandem mass spectrometer and MRM mode condition.
3.5. Method Validation
3.5.1. Linearity and Sensitivity
The linearity of all compounds in both the solvent and mixed standard solutions was evaluated by plotting the peak area against standard concentrations of 5, 10, 25, 50, 100, 250, and 500 µg/kg. Calibration curves were constructed using a 1/x weighting factor, excluding the origin. The coefficient of determination (R2) was calculated to assess the accuracy of the calibration model, with an acceptance criterion of R2 ≥ 0.9900. Moreover, the linearity of the method was evaluated by assessing the percentage deviation of the back-calculated concentration (BBC) from the nominal concentration at each calibration level. According to the criteria, the deviation should not exceed ±20% at all concentration levels. Instrument sensitivity was assessed by determining the limit of detection (LOD) and the limit of quantification (LOQ). The LOD was defined as the lowest concentration at which a pesticide signal could be reliably distinguished from the background noise at the corresponding retention time. Following the SANTE/11312/2021 guidelines [24], signal-to-noise (S/N) ratios of 3 and 10 were used to define the LOD and LOQ, respectively.
3.5.2. Precision and Accuracy
The accuracy of the method was evaluated through recovery studies, while precision was assessed based on the relative standard deviation (RSD) [24]. For this purpose, a mixture of standard solutions was spiked into a pooled blank sample of edible insects. Recovery and precision were determined at three fortification levels—10, 100, and 500 µg/kg (n = 3 for each level)—using matrix-matched standards. The precision of the method was further confirmed by calculating RSD of the analyte responses across replicates.
3.5.3. Matrix Effect
The matrix effect (ME) of each analyte was assessed using a mass spectrometry-based analytical method [24]. To determine the ME of the pesticides in each sample, the linear regression slope of the matrix-matched calibration curve was compared to the linear regression slope of the standard solution calibration without the matrix using the following Equation (1).
4. Conclusions
A thorough multiresidue analytical technique was devised and validated using GC-MS/MS for the simultaneous identification of 47 pesticides in high-fat edible insect matrices. The refined QuEChERS extraction technique, utilizing acetonitrile and dispersive solid-phase extraction with MgSO4, PSA, and C18, facilitated the efficient elimination of lipids and moisture while maintaining analyte integrity. Method validation was performed in accordance with SANTE guidelines. Most pesticides exhibited adequate recovery rates (70–120%) and relative standard deviations of less than 5% overall. Despite several chemicals demonstrating reduced recovery at the lowest concentration level, the overall technique performance stayed within acceptable regulatory parameters. Matrix-matched calibration proficiently mitigated matrix effects, guaranteeing precise quantification. This established technology is suitable for regular pesticide monitoring in consumable insects, supporting food safety oversight and regulatory compliance.
Author Contributions
Conceptualization, S.H.; methodology, A.W. and N.S.; validation, P.T., K.S., S.K. and U.J.; formal analysis, P.T.; investigation, C.J., B.C. and S.H.; data curation, B.C. and S.H.; writing—original draft preparation, P.T.; writing review and editing, A.W., B.C. and S.H.; visualization, N.S. and S.H.; supervision, S.H.; project administration, P.T.; funding acquisition, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the School of Health Sciences Research, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand, No. 015/2566, and this research was also supported by National Research Foundation of Korea (NRF 2018R1A6A1A03024862), and a TA/RA Scholarship from Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
All laboratory facilities were supported by Research Institute for Health Sciences, Chiang Mai University (RIHES, CMU), and the authors would like to thank Chiang Mai University for facilitating this study.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Puwastien, P.; Attig, G. Edible insects in Thailand: An unconventional protein source? Ecol. Food Nutr. Ecol. Food Nutr. 1997, 36, 133–149. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Klunder, H.; Wolkers-Rooijackers, J.C.M.; Korpela, J.; Nout, M.J. Microbiological aspects of processing and storage of edible insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Hoang, H. Consumer acceptability of alternative foods: A study of processed cricket-based foods in Vietnam. IOP Conf. Ser. Earth Environ. Sci. 2023, 1155, 012025. [Google Scholar] [CrossRef]
- Labu, S.; Subramanian, S.; Cheseto, X.; Akite, P.; Kasangaki, P.; Chemurot, M.; Tanga, C.M.; Salifu, D.; Egonyu, J.P. Agrochemical Contaminants in Six Species of Edible Insects from Uganda and Kenya. Curr. Res. Insect Sci. 2022, 2, 100049. [Google Scholar] [CrossRef]
- Kolakowski, B.M.; Johaniuk, K.; Zhang, H.; Yamamoto, E. Analysis of Microbiological and Chemical Hazards in Edible Insects Available to Canadian Consumers. J. Food Prot. 2021, 84, 1575–1581. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef]
- Lee, J.; Kim, L.; Shin, Y.; Lee, J.; Lee, J.; Kim, E.; Moon, J.K.; Kim, J.H. Rapid and Simultaneous Analysis of 360 Pesticides in Brown Rice, Spinach, Orange, and Potato Using Microbore GC-MS/MS. J. Agric. Food Chem. 2017, 65, 3387–3395. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2019, 86, 412–431. [Google Scholar] [CrossRef]
- Polgár, L.; Kmellár, B.; Garcia-Reyes, J.F.; Fodor, P. Comprehensive Evaluation of the Clean-up Step in the QuEChERS Procedure for the Multi-Residue Determination of Pesticides in Different Vegetable Oils Using LC-MS/MS. Anal. Methods 2012, 4, 1142–1148. [Google Scholar] [CrossRef]
- Hou, R.; Jiao, W.; Xiao, Y.; Guo, J.; Lv, Y.; Tan, H.; Hu, J.; Wan, X. Novel use of PVPP in a modified QuEChERS extraction method for UPLC-MS/MS analysis of neonicotinoid insecticides in tea matrices. Anal. Methods 2015, 7, 5521–5529. [Google Scholar] [CrossRef]
- Li, J.; Dong, F.; Xu, J.; Liu, X.; Li, Y.; Shan, W.; Zheng, Y. Enantioselective determination of triazole fungicide simeconazole in vegetables, fruits, and cereals using modified QuEChERS (quick, easy, cheap, effective, rugged and safe) coupled to gas chromatography/tandem mass spectrometry. Anal. Chim. Acta 2011, 702, 127–135. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Qin, D.; Jiang, S.; Liu, F. Application of Graphitized Carbon Black to the QuEChERS Method for Pesticide Multiresidue Analysis in Spinach. J. AOAC Int. 2009, 92, 538–547. [Google Scholar] [CrossRef]
- Rajski, Ł.; Lozano, A.; Uclés, A.; Ferrer, C.; Fernández-Alba, A.R. Determination of pesticide residues in high oil vegetal commodities by using various multi-residue methods and clean-ups followed by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2013, 1304, 109–120. [Google Scholar] [CrossRef]
- Hou, X.; Han, M.; Dai, X.; Yang, X.; Yi, S. A multi-residue method for the determination of 124 pesticides in rice by modified QuEChERS extraction and gas chromatography–tandem mass spectrometry. Food Chem. 2013, 138, 1198–1205. [Google Scholar] [CrossRef]
- Kim, L.; Baek, S.; Son, K.; Kim, E.; Noh, H.H.; Kim, D.; Oh, M.-s.; Moon, B.-c.; Ro, J.-H. Optimization of a Simplified and Effective Analytical Method of Pesticide Residues in Mealworms (Tenebrio molitor Larvae) Combined with GC–MS/MS and LC–MS/MS. Molecules 2020, 25, 3518. [Google Scholar] [CrossRef]
- Lehotay, S.; Mastovska, K.; Lightfield, A. Use of Buffering Other Means to Improve Results of Problematic Pesticides in a Fast Easy Method for Residue Analysis of Fruits Vegetables. J. AOAC Int. 2005, 88, 615–629. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, C.; Baek, S.; Kim, L.; Son, K.-A.; Lee, H.-D.; Kim, D.; Kim, J.-H.; Noh, H. Liquid Chromatography-Tandem Mass Spectrometry for the Simultaneous Analysis of 353 Pesticides in the Edible Insect Tenebrio molitor Larvae (Mealworms). Molecules 2020, 25, 5866. [Google Scholar] [CrossRef]
- Lehotay, S. QuEChERS Sample Preparation Approach for Mass Spectrometric Analysis of Pesticide Residues in Foods. Methods Mol. Biol. 2011, 747, 65–91. [Google Scholar] [CrossRef]
- Li, J.; Sun, M.; Chang, Q.; Hu, X.; Kang, J.; Fan, C. Determination of Pesticide Residues in Teas via QuEChERS Combined with Dispersive Liquid–Liquid Microextraction Followed by Gas Chromatography–Tandem Mass Spectrometry. Chromatographia 2017, 80, 1447–1458. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, X.; Wei, H.; Liu, D.; Xia, G.; Yang, X. Dispersive Liquid-Liquid Microextraction Combined with Gas Chromatography-Mass Spectrometry for the Determination of Multiple Pesticides in Celery. Food Anal. Methods 2016, 9, 2133–2141. [Google Scholar] [CrossRef]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Env. Sci. Pollut. Res. Int. 2017, 24, 7124–7138. [Google Scholar] [CrossRef]
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed (SANTE/11312/2021). Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 17 April 2025).
- Marchi, I.; Viette, V.; Badoud, F.; Fathi, M.; Saugy, M.; Rudaz, S.; Veuthey, J.-L. Characterization and classification of matrix effects in biological samples analyses. J. Chromatogr. A 2010, 1217, 4071–4078. [Google Scholar] [CrossRef]
- EN 15662:2018; Foods of Plant Origin—Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE—QuEChERS-Method. CEN: Brussels, Belgium, 2018. Available online: https://www.en-standard.eu/bs-en-15662-2018-foods-of-plant-origin-multimethod-for-the-determination-of-pesticide-residues-using-gc-and-lc-based-analysis-following-acetonitrile-extraction-partitioning-and-clean-up-by-dispersive-spe-modular-quechers-method/ (accessed on 17 April 2025).
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass. Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef]
- Chawla, S.; Patel, H.; Gor, N.; Vaghela, K.; Solanki, P.; Shah, P. Evaluation of Matrix Effects in Multiresidue Analysis of Pesticide Residues in Vegetables and Spices by LC-MS/MS. J. AOAC Int. 2017, 100, 616–623. [Google Scholar] [CrossRef]
- Ferrer, C.; Lozano, A.; Agüera, A.; Girón, A.J.; Fernández-Alba, A.R. Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J. Chromatogr. A 2011, 1218, 7634–7639. [Google Scholar] [CrossRef]
- Mauer, L.; Forny, L.; Meunier, V.; Taylor, L. Optimizing the Quality of Food Powder Products: The Challenges of Moisture-Mediated Phase Transformations. Annu. Rev. Food Sci. Technol. 2019, 10, 457–478. [Google Scholar] [CrossRef]
- Noh, H.H.; Kim, C.J.; Kim, S.-H.; Eun, H.-R.; Shin, Y.; Jeong, W.T. GC–MS/MS–based multiresidue pesticide analysis in mealworm (Tenebrio molitor) larvae: Optimization of standard QuEChERS-based method to minimize matrix effects. Food Chem. X 2025, 27, 102386. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).