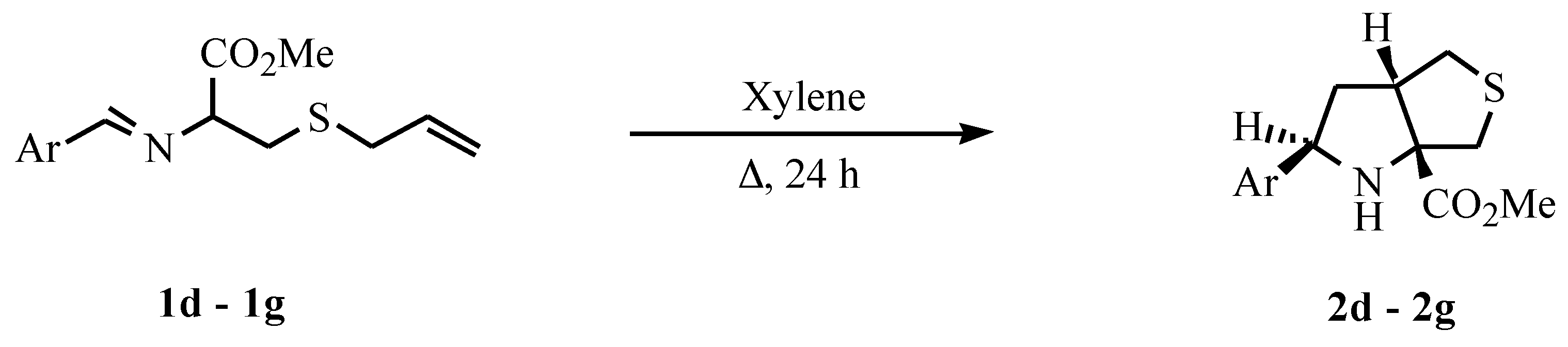General procedure for the preparation of imines
S-Allylcysteine methyl ester hydrochloride [
1] (20 mmol) was treated with a solution of NaOMe (from 20 mmol of Na in 30 ml of dry MeOH). The aldehyde (20 mmol) was added and the resulting mixture was stirred at room temperature for 24 hours. The solvent was evaporated and the residue dissolved in CH
2Cl
2 and washed with water. The organic solvent was dried (MgSO
4) and evaporated off. The crude imines were used directly for the cycloaddition (except for
1f) as distillation resulted in cyclization and/or in decomposition.
Methyl N-(1-naphthylidene)-S-allylcysteine (1d)
An oil. Yield 97%. 1H NMR δ 3.00 (1H, dd, J 8.2 and 13.7), 3.16 (2H, d, J 7.3), 3.20 (1H, dd, J 5.2 and 13.7), 3.78 (3H, s, CO2CH3), 4.21 (1H, dd, J 5.2 and 8.2, CHCO2CH3), 5.08-5.15 (2H, m, CH=CH2), 5.69-5.81 (1H, m, CH=CH2), 7.47-7.61 (3H, m, ArH), 7.85-7.96 (4H, m, ArH) and 8.95 (1H, s, CH=N). MS m/z 313 (M+, 5%), 254 (36), 186 (4), 127 (20) and 169 (100).
Methyl N-phenylidene-S-allylcysteine (1e)
An oil. Yield 75%. 1H NMR δ 2.92 (1H, dd, J 7.9 and 13.8), 3.08-3.15 (3H, m), 3.74 (3H, s, CO2CH3), 4.13 (1H, dd, J 5.46 and 7.95, CHCO2CH3), 5.07-5.13 (2H, m, CH=CH2), 5.68-5.82 (1H, m, CH=CH2), 7.37-7.44 (3H, m, ArH), 7.77-7.81 (2H, m, ArH) and 8.31 (1H, s, CH=N). MS m/z 278 (M+, 1%), 205 (2), 201 (3), 190 (100), 174 (11) and 142 (2).
Methyl N-(4-dimethylaminophenylidene)-S-allylcysteine (1f)
Yield 63%. M.p. 74-76°C (from ethyl ether-petroleum ether 40/60). Analysis: Found C, 63.11; H, 7.14; N, 9.08; C16H22N2O2S requires C, 62.72; H, 7.24; N, 9.14%. 1H NMR δ 2.88 (1H, dd, J 7.7 and 13.8), 3.01 (6H, s, NMe2), 3.06-3.16 (3H, m), 3.75 (3H, s, CO2CH3), 4.06 (1H, dd, J 5.8 and 7.7, CHCO2CH3), 5.07-5.14 (2H, m, CH=CH2), 5.69-5.83 (1H, m, CH=CH2), 6.68 (2H, d, J 8.9, ArH), 7.65 (2H, d, J 8.9, ArH) and 8.17 (1H, s, CH=N). MS m/z 306 (M+, 29%), 265 (15), 247 (13), 219 (100) and 83 (64).
Methyl N-(2-methoxyphenylidene)-S-allylcysteine (1g)
An oil. Yield 70%. 1H NMR δ 2.77 (1H, dd, J 7.7 and 13.8), 2.93-3.07 (3H, m), 3.48 (3H, s), 3.66 (3H, s), 4.03 (1H, dd, J 5.7 and 7.7, CHCO2CH3), 4.94-4.97 (2H, m, CH=CH2), 5.55-5.73 (1H, m, CH=CH2), 6.67-6.85 (2H, m, ArH), 7.18-7.27 (2H, m, ArH) and 8.63 (1H, s, CH=N). MS m/z 293 (M+, 20%), 278 (30), 262 (9), 186 (50) 127 (82) and 117 (100).
General procedure for the intramolecular cycloaddition
A solution of the imine 1 (2 mmol) in xylene (10 ml) was heated under reflux, under nitrogen, for 24 hours. The solvent was evaporated and the crude product was purified by crystallization (2d, 2f and 2g crystallization from ethyl ether-petroleum ether 40/60) or by flash chromatography (2e).
Methyl 2-(1-naphthyl)tetrahydro-1H-thieno[3,4-b]pyrrole-6a(6H)-carboxylate (2d)
Yield 51%. M.p. 122-123°C (from ethyl ether-petroleum ether 40/60). Analysis: Found C, 68.54; H, 6.04; N, 4.10; C18H19NO2S requires C, 68.98; H, 6.11; N, 4.47%. 1H NMR δ 2.16-2.34 (2H, m, Hb), 2.65 (1H, brs, NH), 2.80 (1H, dd, J 4.1 and 11.7, Hd), 2.95 (1H, d, J 12.2, Hf), 3.24 (1H, dd, J 7.4 and 11.7, He), 3.39 (1H, d, J 12.2, Hg), 3.34-3.41 (1H, m, Hc), 3.63 (3H, s, OCH3), 5.29 (1H, t, J 7.5, Ha), 7.38-7.54 (3H, m, ArH), 7.60 (1H, d, J 7.1, ArH), 7.72 (1H, d, J 8.2, ArH), 7.83 (1H, dd, J 5.7 and 1.5, ArH) and 8.16 (1H, d, J 8.2, ArH). 13C NMR δ 38.89, 39.76, 44.32, 52.16, 52.49, 58.32, 79.41, 122.02, 123.38, 125.26, 125.42, 125.93, 127.54, 128.65, 131.31, 133.67, 138.18 and 175.15. MS m/z 313 (M+, 33%), 254 (43), 206 (28), 169 (100) and 127 (23).
Methyl 2-phenyltetrahydro-1H-thieno[3,4-b]pyrrole-6a(6H)-carboxylate (2e)
An oil. Yield 37%. 1H NMR δ 2.03-2.10 (2H, m, Hb), 2.65 (1H, brs, NH), 2.71 (1H, dd, J 2.7 and 11.5, Hd), 2.90 (1H, d, J 12.2, Hf), 3.18 (1H, dd, J 9.7 and 11.5, He), 3.26-3.33 (1H, m, Hc), 3.40 (1H, d, J 12.2, Hg), 3.73 (3H, s, OCH3), 4.53 (1H, t, J 6.8, Ha) and 7.37-7.44 (5H, m, ArH); m/z 278 (M+, 1%), 263 (10), 231 (1), 201 (3), 190 (100), 130 (89) and 103 (74).
Methyl 2-(4-dimethylaminophenyl)tetrahydro-1H-thieno[3,4-b]pyrrole-6a(6H)-carboxylate (2f)
Yield 71%. M.p. 80-81°C (from ethyl ether-petroleum ether 40/60). Analysis: Found C, 62.64; H, 7.24; N, 8.80; S, 10.85. C16H22N2O2S requires C, 62.72; H, 7.24; N, 9.14; S, 10.46%). 1H NMR δ 2.00-2.04 (2H, m, Hb), 2.65 (1H, brs, NH), 2.72 (1H, dd, J 5.3 and 11.4, Hd), 2.88 (1H, d, J 12, Hf), 2.88 (6H, s, 2xCH3), 3.15 (1H, dd, J 7.8 and 11.4, He), 3.24-3.29 (1H, m, Hc), 3.39 (1H, d, J 12, Hg), 3.73 (3H, s, OCH3), 4.42 (1H, t, J 7.8, Ha), 6.65 (2H, d, J 8.6, ArH) and 7.21 (2H, d, J 8.6, ArH); m/z 306 (M+, 30%), 233 (100) and 162 (58).
Methyl 2-(2-methoxyphenyl)tetrahydro-1H-thieno[3,4-b]pyrrole-6a(6H)-carboxylate (2g)
Yield 67%. M.p. 114-117°C (from ethyl ether-petroleum ether 40/60). Analysis: Found C, 61.47; H, 6.43; N, 4.22; S, 10.40 C15H19NO3S requires C, 61.41; H, 6.33; N, 4.79; S, 10.93%. 1H NMR δ 2.07-2.21 (2H, m, Hb), 2.75 (1H, dd, J 4.4 and 11.7, Hd), 2.88 (1H, d, J 12.2, Hf), 2.96 (1H, brs, NH), 3.19 (1H, dd, J 7.5 and 11.7, He), 3.30 (1H, d, J 12.2, Hg), 3.33-3.39 (1H, m, Hc), 3.64 (3H, s, CO2CH3), 3.83 (3H, s), 4.76 (1H, t, J 6.8, Ha), 6.82-6.93 (2H, m, ArH) ,7.18-7.24 (1H, m, ArH) and 7.31 (1H, dd, J 1.4 and 7.5, ArH). MS m/z 293 (M+, 4%), 234 (98), 220 (60), 186 (37), 149 (100) and 134 (94).