Systematic Review of Chemical Compounds with Immunomodulatory Action Isolated from African Medicinal Plants
Abstract
1. Introduction
2. Results
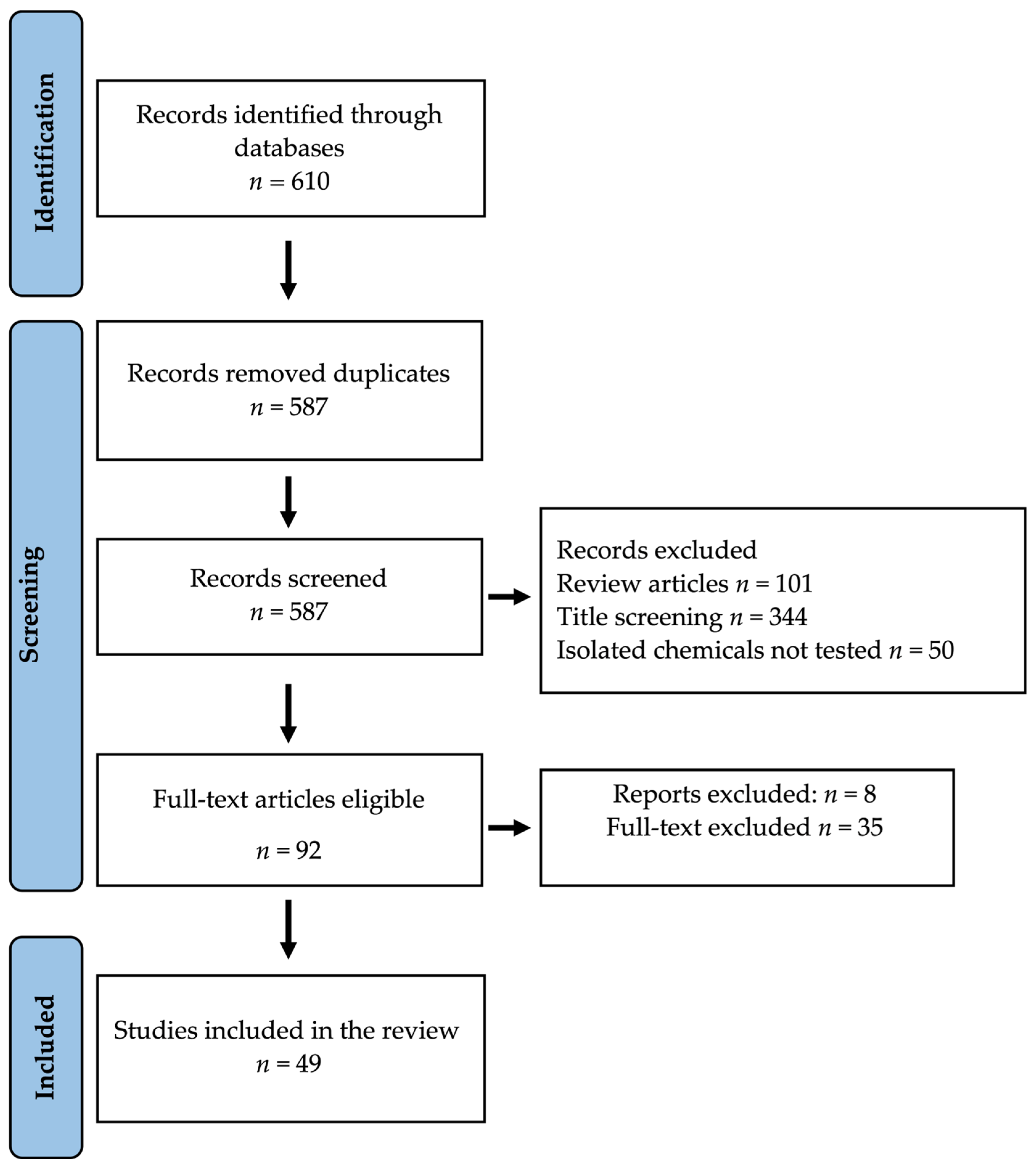
2.1. Database Search Results
2.2. African Medicinal Plants Used for Immunomodulation
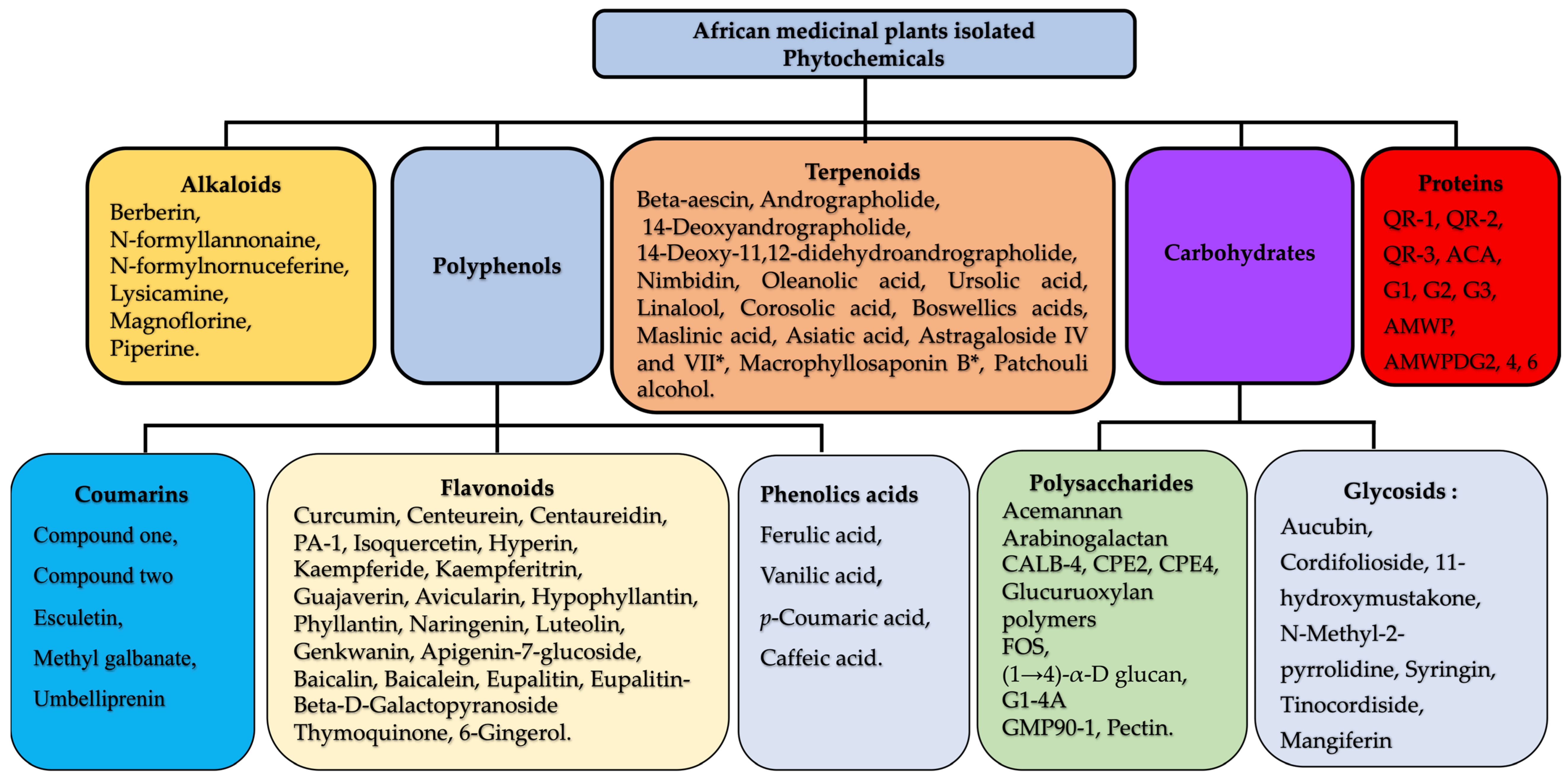
2.3. Chemistry of Plant-Derived Immunomodulators
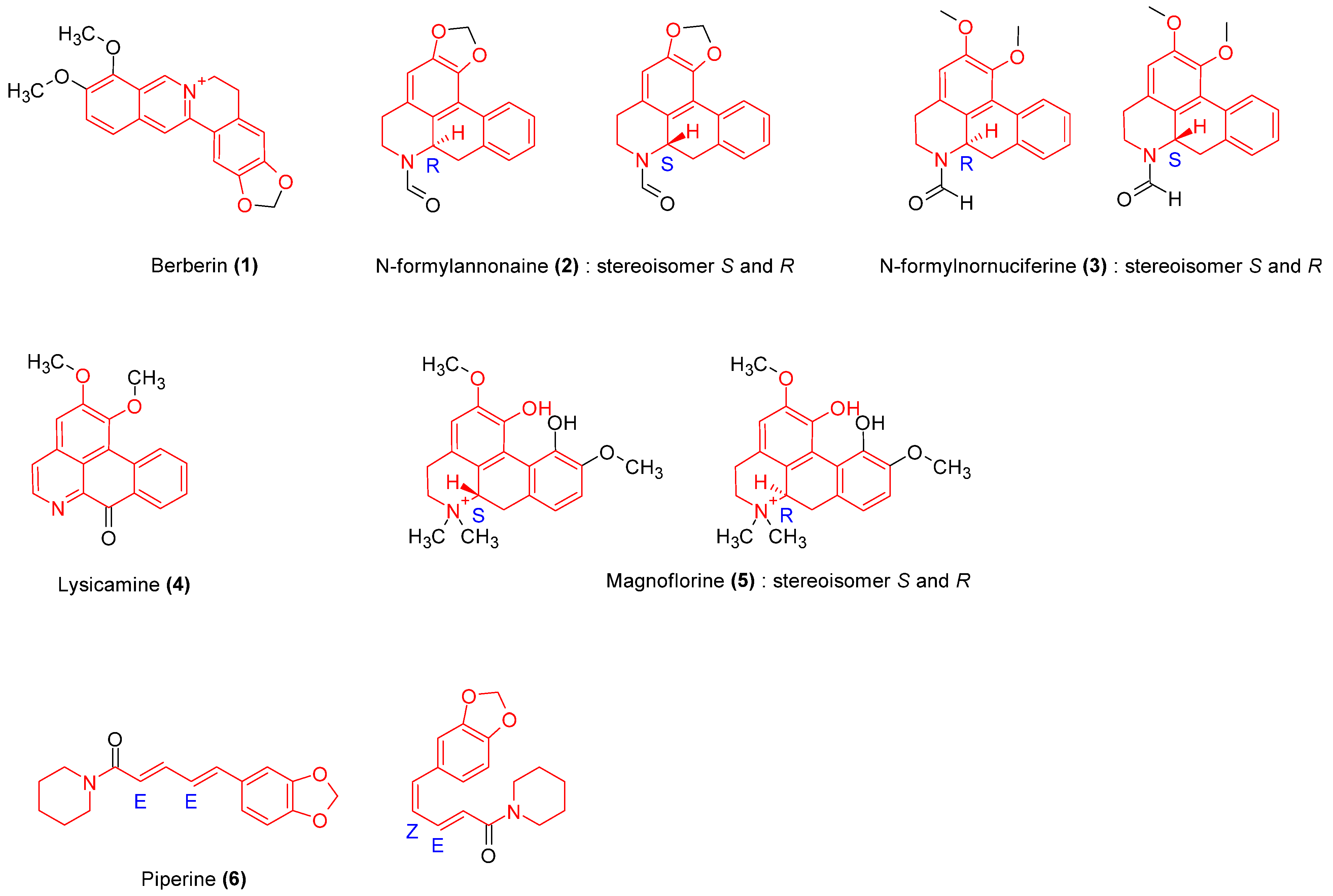
2.3.1. Alkaloids
| Isolated Molecules (n°) | Models | Pharmacodynamic Parameters ED50/IC50 | Biological Effects | Cellular Effect | References |
|---|---|---|---|---|---|
| 1 | In vitro mouse primary splenocyte assay | 6.6 µM | No significant effect on cell viability at 0.8, 1.6, and 3.3 μM | Downregulates splenocytes cytokines (IL2, 4, 10, TNFα) expression. | [14] |
| 2 | Mouse macrophage RAW 264.7 viability, chemotactic, phagocytic assay, ROS, NO, PGE2, and cytokine production, monocyte chemoattractant Protein-1 (MCP-1) production. | nd | Isolated compounds 2, 3, 4, and 5 at concentrations above 25 μg/mL showed toxic effects on macrophages’ viability (<90%). Stimulation of cell migration. Increase in macrophage migration. Stimulation of cell migration, strong enhancement of macrophage phagocytic activity (81.01% compound 5). Augmentation of ROS and NO generation. | Significantly stimulates PGE2 production, enhances the MCP-1 level. Significantly increases IL-1β, IL6, and TNFα production. | [17,18] |
| 3 | nd | ||||
| 4 | nd | ||||
| 5 | 23.8 mM | ||||
| 6 | In vivo hematological assay, in vitro Dalton’s lymphoma ascites (DLA), Ehrlich ascites carcinoma (EAC) cells assay, L929 cells | nd | Increase in white cell count (138.9%), stimulation of stem cell proliferation, enhancement of the number of plaque-forming cells (71.4%) Cytotoxicity on DLA, EAC at 200 μg/mL, and L929 at 50 μg/mL. | Enhancement of the antibody production. | [21] |
2.3.2. Polysaccharides
| Sources | Extraction Method | Molecular Weight (kDa) | Monosaccharide Composition | Active Substance | Biological Activity | References |
|---|---|---|---|---|---|---|
| Allium cepa | Hot water | 1.8 × 102 | D-galactose: 6-O-Me-D-galactose: 3-O-acetyl-D-methyl galacturonate: D-methyl galacturonate1:1:1:1 | Pectin | Enhancement of NO production in macrophage, stimulation of splenocyte and thymocyte proliferation. | [57] |
| Hot ethanol | FOS: monosaccharide to hexasaccharide | Increase in splenocytes/thymocytes proliferation (~3-fold), macrophage phagocytic activity, NO production (~2.5-fold). | [58] | |||
| Moringa oleifera | Distilled water | 70 | Gluc. | (1→4)-α-D glucan | Increase in macrophage phagocytic activity, and in the number and percentage of globulin. | [74] |
| Garcinia mangostana L. | Water extraction | 5.3 | Ara., Gal., Rham. | GMP90-1 = arabinofurane | Enhancement of phagocytic activity (28.0%; 40.3% at 100 and 200 μg/mL, respectively), increase in NO secretion (2.2, 3.9, and 10.3 times at the concentrations of 50, 100, and 200, respectively), IL1β (38.42% at 200 μg/mL), IL6 (4.6, 5.1, and 8.5 times at 50, 100, and 200, respectively), TNFα (5.6, 41.7, and 200.1% at 50, 100, and 200 μg/mL, respectively). | [26] |
| Aloe vera | Distilled water | - | Man, Gluc, Gal. 62.9:13.1:0.6 | Heteroglycan or acemannan | Increase in splenocyte proliferation (5.7 and 7.1% after 24 and 48 h, respectively). Increase in IL-1 and TNFα secretion in irradiated mice (2.34 and 1.32~fold, respectively). | [52] |
| Echinaneae purpurea L. | Water | Diploid, tetraploid | CPE2, CPE4 | Stimulation of lymphocyte proliferation and cytokine secretion. | [24] | |
| Gal, Ara | Arabinogalactane | [79,80] | ||||
| Fructus aurantii | Cold water, hot water | 3.14 × 102 | Man, Rha, GlcUA, GalUA, Gal, Ara 16.3:4.0:2.9:3.4:21.7:41.7 | Pectic polysaccharide CALB-4 | Promotion of PBMC proliferation. Upregulation of NO production. Affects TNFα, IL1β, IL6, and IL8 secretion. Increases of proIL-1 expression. | [25] |
| Siraitia grosvenorii | Hot water | Gluc, Gal. Ara. Rham 5.8:0.77:0.38:0.12 | Promotion of B and T lymphocyte proliferation. Increase in thymus index. Increase in IL-2 and decrease in IL-1. | [27] | ||
| T. cordifolia | Acetone extract | G1-4A | Upregulation of TNFα, IL1β, IL6, IL10, IL12, and IFNγ expression. Enhancement of NO level. | [20] | ||
| Tamarindus indica | Fresh water | Gal., Man., Gluc. | Increase in phagocytic activity. Inhibition of PHA-induced lymphocyte proliferation and leukocyte migration by 63–70.% | [72] | ||
| Salvia officinalis L. | Ethanol-water | 10,000 < Mw > 50,000 | Rham. Ara., Xyl., Man., Gluc., Gal., UA. | Arabinogalactans (A), Pectins (B), Glucurunoxylan polymers (D). | Polysaccharides-induced thymocyte proliferation. | [73] |
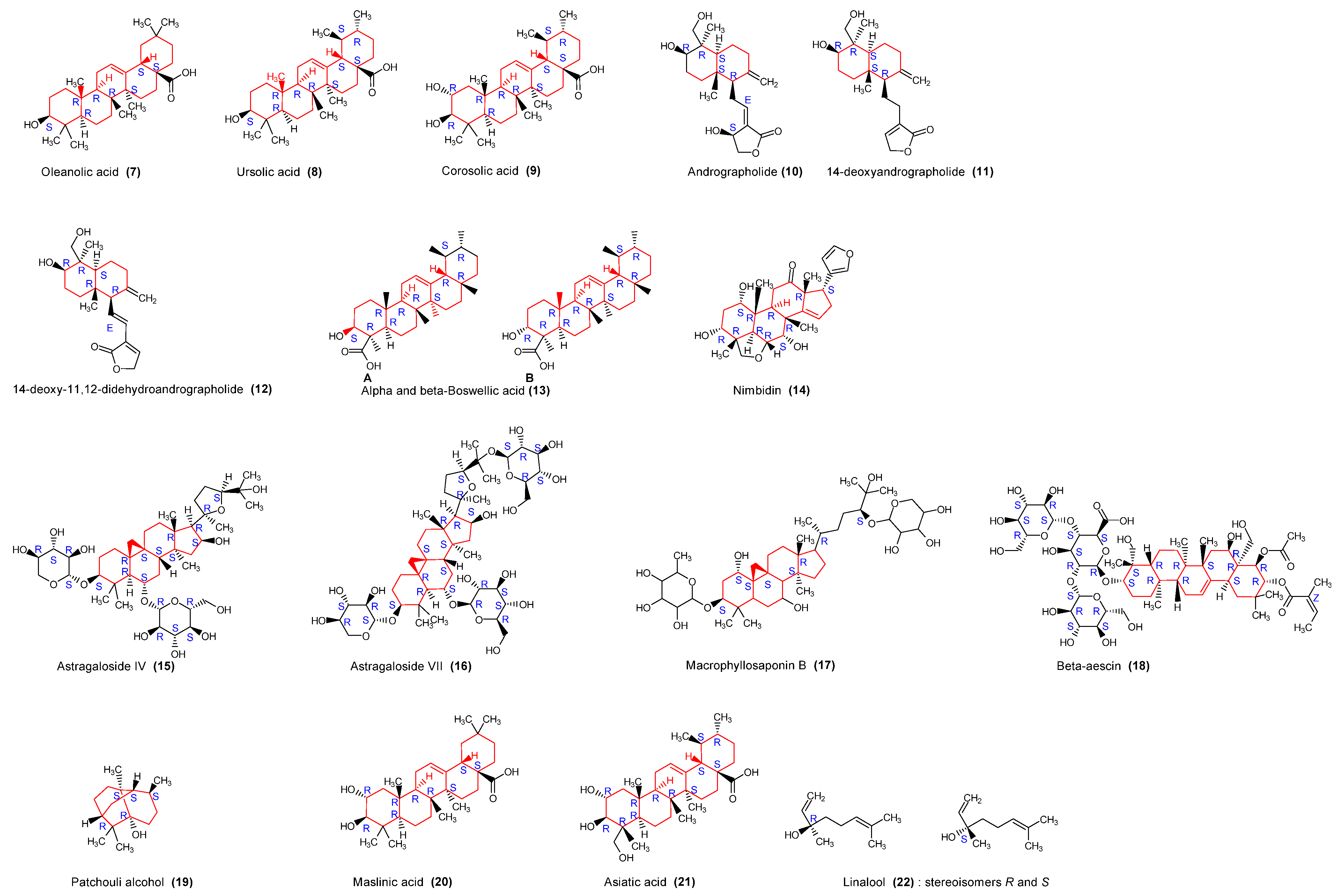
2.3.3. Triterpenoids
2.3.4. Polyphenols
| Isolated Molecules (n°) | Models | Pharmacodynamic Parameters | Biological Effects | Cellular Effect | References | |
|---|---|---|---|---|---|---|
| ED50 | IC50 | |||||
| 23 | Mouse macrophage, lymphocytes (PBMCs) proliferation assay, natural killer cytotoxicity assay | nd | nd | No effect on cell viability. Inhibition of lymphocyte proliferation. Enhancement of NK cytotoxicity | Inhibition of PHA-induced IL2 release, weak inhibition of TNFα production in PBMC | [38] |
| Splenocytes assay | 3.5 μg/mL | Inhibition of splenocyte proliferation | Inhibition IL-2 synthesis | [39] | ||
| 18 | PBMC cell proliferation assay | 1.5 mg/mL | Suppression of lymphocyte proliferation | [36] | ||
| 24, 25 | IFNγ promoter-driven luciferase reporter and T cells assay. | 75 0.9 mg/mL | Modulation of IFNγ transcription | [35] | ||
| 27, 28, 29 | in vitro RAW 264.7 macrophage proliferation assay | nd | nd | Increase in macrophages’ proliferation (by 1.53-fold for compound 27 and 1.43-fold for compound 28). No significant increase was observed for compound 29. | [37] | |
| 26 | Proliferation of murine splenocytes, macrophages, and human PBMCs. NO production, lysosomal enzyme activity, and neutral red uptake assay | nd | Increase in cell viability in the absence of LPS (macrophages 23%, splenocytes 17%, human PBMCs 24%). Increase in lysosome activity (57%) in a concentration-dependent manner. Lack of effect on neutral red uptake. Stimulation of NK cell activity (11%) | No effect on NO release. | [41] | |
| 30, 31 | HKLs | nd | nd | Modulation affects the viability of HKLs. No effect on cell viability Increase in lysozyme activity | [46] | |
| 41, 43, 44 | In vitro mouse splenocyte proliferation assay, NK cell activity, cytotoxicity T lymphocyte activity, lysosomal enzyme activity | nd | nd | Induction of splenocyte proliferation in the presence or absence of mitogen. Enhancement of NK activity. Inhibition of lysosomal function in a dose-dependent manner | Reduction of NO production (from 53.37 μM to 22.33 μM for compound 44; 20.66 μM for compound 43; and 28.64 μM compound 41) | [50] |
| 32–38 | Human PBMCs assay | nd nd | nd nd | Stimulation of PBMC proliferation | Stimulation of IFNγ secretion | [46] |
| 42 | Human PBMCs assay | nd | nd | Inhibition of cell proliferation | Inhibition of IL2 secretion. Inhibition of NO release | [71] |
| 39, 40 | Human PBMCs assay, RAW cells assay | nd | nd | Inhibition of cell proliferation (n°39). Inhibition of lymphocyte proliferation. No effect on NK cytotoxicity | ||
2.3.5. Coumarins
| Isolated Molecules (n°) | Models | Pharmacodynamic Parameters | Biological Effects | Cellular Effect | References | |
|---|---|---|---|---|---|---|
| ED50 | IC50 | |||||
| 47 | Murine macrophages and lymphocytes assay | nd | nd | No effect on macrophage viability. Enhancement of endocytic activity induced by LPS on macrophages at concentrations of 80 and 120 mM. Increase in mutagenic-induced cell proliferation. Induction of LAK activity of splenic lymphocytes. | Enhances NO production and iNOS gene expression | [53] |
| 48, 49 | Murine splenocytes assay | nd | nd | No effect on cell viability for tested concentrations (0.5–15 μM). Compound 48 at concentration >0.5 μM decreased splenocytes stimulation index. Compound 49 decreased cell proliferation at lowest dose. Suppression of PHA-induced cell proliferation. | Significantly augments IL4 secretion. Inhibits IFNγ production. Inhibits NO production by stimulated macrophages. Compound 48 increases PGE2 release; however, compound 49 inhibits it. | [50] |
2.3.6. Other Molecules: Glycosides
| Isolated Molecules (n°) | Models | Pharmacodynamic Parameters | Biological Effects | Cellular Effect | References | |
|---|---|---|---|---|---|---|
| ED50 | IC50 | |||||
| 50 | Murine RAW 264.7 cell viability assay Chemotaxis assay Phagocytosis assay NO, ROS, PGE2 production Monocyte chemoattractant Protein-1 production Cytokine production | nd | nd | Toxicity effect above 25 μg/mL. Reduction of cell chemotactic and phagocytosis activities. Diminution of MCP-1 production (IC50 = 48.3) | Reduction of NO production. Inhibition of PGE2 production (IC50 = 12.08 μM). Decrease in IL1β, IL6, and TNFα production. | [17] |
| 51, 52, 53, 54 | PMN cells viability assay Phagocytosis assay ROS, NO production assay | nd | nd | Increase in phagocytosis activity | Dose-dependent increase in NO and superoxide production | [18] |
| 55 | Human mononuclear cells assay Lymphocytes transformations test | nd | nd | Stimulation of PBMC proliferation | Enhancement of IFN-γ production | [63] |
2.3.7. Proteins
| Sources | Extraction Method | Isolated Proteins | Molecular Weight (kDa) | Biological Effects | References |
|---|---|---|---|---|---|
| Allium sativum | QR-1, QR-2, QR3 (7:28:1) | 13 | Mitogenic activity on human PBMC, murine splenocytes and thymocytes. QR-1 and QR-2 showed hemagglutination and mannose-binding activities. | [54] | |
| Allium cepa | Dialysis-D-mannose chromatography | ACA: Allium cepa Agglutinin | 12 | ACA at 0.1 μg/well and 0.01 μg/well enhance thymocyte proliferation by ~4- and 3.5-fold, respectively, with a marginal effect on B cells proliferation (~1.3-fold at 0.01 μg/well), significantly increased cytokine production (TNFα, IL12), and IFN-γ and IL2 expression. ACA induced an ~8-fold increase in NO production by rat peritoneal cells at 12 and 24 h. ACA (0.01–10 μg/well) significantly enhanced IL12 (~3-fold) and TNFα (~2–3-fold) release. The phagocytosis activity is enhanced by 2-fold by ACA (0.1; 1; 10 μg). | [59] |
| Tinospora cordifolia | Chromatography | G1, G2, G3 | 10–80 | The proteins at a concentration range of 1–10 μg/mL showed mitogenic activity (3-fold) in murine splenocytes at 1–10 μg/mL and ~5–7-fold in thymocytes. They induced NO release by macrophages and enhanced macrophage phagocytosis activity. | [19] |
| Astragalus membranaceus, | Alkali extraction | AMWP (16 aa) AMWPDG2 (16 aa), AMWPDG4 (15 aa), AMWPDG6 (15 aa) | - 406.115 268.795 342.281 | All proteins contain seven essential amino acids: Thr, Val., Met., Ile., Leu., Phe., and Lys. Proteins at 50 μg/mL significantly promoted in murine peritoneal macrophage phagocytosis activity, secretion of immunomodulatory factors like NO (AMWPDG2 > AMWPDG4 = AMWPDG6) and H2O2 (AMWPDG2 > AMWPDG6 > AMWPDG4) and inflammatory cytokines (TNFa and IL6) | [61] |
2.4. Mechanism of Action of Plant-Derived Immunomodulators
| Compounds N° | Phytochemical Group | Cellular Model | Receptor | Transduction Pathway | Mechanism of Action | Cellular Actions | References |
|---|---|---|---|---|---|---|---|
| 5 | Alkaloids | Macrophages (U937) | TLR4 | MAPKs, PI3K-Akt | Augmentation of Akt phosphorylation, induction of JNK, ERK, and p38 phosphorylation | Enhancement of upregulation of TNFα, IL1β, PGE2, COX-2 | [92] |
| G1-4A | Polysaccharides | Macrophages | TLR4/MyD88 | MAPKs | Activation of JNK, ERK, and p38 phosphorylation | Upregulation of the expression of TNFα, IL6, IL12, IL10 | [20] |
| 8 | Triterpenoids | Macrophages | TLR4 | TLR4- MyD88 | Blocking TLR4/MyD88 | Decrease in TNF-α, IL-1β et IL-6 release | [94] |
| 7 | Triterpenoids | THP1 cells | TLR3 | MAPKs | Inhibition of IκB phosphorylation and NF-κB translocation | [89] | |
| 15 | Terpenoids saponins | Macrophages | MAPKs/NFκB | Increase in the phosphorylation of p65, p38, JNK, and ERK, and a decrease in their protein expression | Increase in IL1β, IL6, TNFα, and inducible nitric oxide synthase | [91] | |
| 23 | Flavonoids | Dendritic cells | MAPKs/NFκB | Suppression of MAPKs and p65 activation | Reduction of inducible NO synthase and IL-12 | [93] | |
| 27 | Flavonoids | Macrophages | TLR4 | MAPKs | Suppression of phosphorylation of proteins p50/p65 | Increase in TNFα, IL1β, iNOS | [95] |
| 55 | Glycosides | 3T3-L1 adipocytes | NF-κB | Suppression of ERK phosphorylation and IκBα degradation | Inhibiting TNFα production | [96] | |
| 56 | Glycosides | Mouse primary hepatocytes | MAPKs | Inhibiting the activation of c-JNK and ERK ½ | [97] |
3. Discussion and Perspectives
3.1. Alkaloids
3.2. Polysaccharides and Proteins
3.3. Terpenoids
3.4. Polyphenols
3.5. Glycosides
4. Methods
4.1. Search Strategy
4.2. Data Extraction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholson, L.B. The Immune System. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef]
- Mohandas, S.; Jagannathan, P.; Henrich, T.J.; Sherif, Z.A.; Bime, C.; Quinlan, E.; Portman, M.A.; Gennaro, M.; Rehman, J. RECOVER Mechanistic Pathways Task Force Immune Mechanisms Underlying COVID-19 Pathology and Post-Acute Sequelae of SARS-CoV-2 Infection (PASC). Elife 2023, 12, e86014. [Google Scholar] [CrossRef]
- Nielsen, D.G. The Relationship of Interacting Immunological Components in Dengue Pathogenesis. Virol. J. 2009, 6, 211. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Pathogenesis of Autoimmune Disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, Z.A.; Charles, M.; Pratap, P.; Naeem, A.; Siddiqui, Z.; Naqvi, N.; Srivastava, S. Cytokine Storm and Mucus Hypersecretion in COVID-19: Review of Mechanisms. J. Inflamm. Res. 2021, 14, 175–189. [Google Scholar] [CrossRef]
- Castro, C.; Gourley, M. Diagnostic Testing and Interpretation of Tests for Autoimmunity. J. Allergy Clin. Immunol. 2010, 125, S238–S247. [Google Scholar] [CrossRef]
- Mohamed, S.I.A.; Jantan, I.; Haque, M.A. Naturally Occurring Immunomodulators with Antitumor Activity: An Insight on Their Mechanisms of Action. Int. Immunopharmacol. 2017, 50, 291–304. [Google Scholar] [CrossRef]
- Shantilal, S.; Vaghela, J.S.; Sisodia, S.S. Review on Immunomodulation and Immunomodulatory Activity of Some Medicinal Plant. Eur. J. Biomed. 2018, 5, 163–174. [Google Scholar]
- Zhao, Z.; Zheng, L.; Chen, W.; Weng, W.; Song, J.; Ji, J. Delivery Strategies of Cancer Immunotherapy: Recent Advances and Future Perspectives. J. Hematol. Oncol. 2019, 12, 126. [Google Scholar] [CrossRef]
- Cox, E.; Verdonck, F.; Vanrompay, D.; Goddeeris, B. Adjuvants Modulating Mucosal Immune Responses or Directing Systemic Responses towards the Mucosa. Vet. Res. 2006, 37, 511–539. [Google Scholar] [CrossRef]
- Lutsiak, M.E.C.; Kwon, G.S.; Samuel, J. Biodegradable Nanoparticle Delivery of a Th2-Biased Peptide for Induction of Th1 Immune Responses. J. Pharm. Pharmacol. 2006, 58, 739–747. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Li, Q.; Yang, Y.; Zhao, G.; Wang, B.; Wu, D. Immunostimulatory Activity of Water-Extractable Polysaccharides from Cistanche Deserticola as a Plant Adjuvant In Vitro and In Vivo. PLoS ONE 2018, 13, e0191356. [Google Scholar] [CrossRef]
- Bdallah, E.; Koko, W. Medicinal Plants of Antimicrobial and Immunomodulating Properties. Antimicrob. Res. Nov. Bioknowl. Educ. Programs 2017, 6, 127–139. [Google Scholar]
- Lin, W.-C.; Lin, J.-Y. Berberine Down-Regulates the Th1/Th2 Cytokine Gene Expression Ratio in Mouse Primary Splenocytes in the Absence or Presence of Lipopolysaccharide in a Preventive Manner. Int. Immunopharmacol. 2011, 11, 1984–1990. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, J.-C.; Hsiang, C.-Y.; Wu, S.-L.; Wu, H.-C.; Ho, T.-Y. Berberine Suppresses Inflammatory Agents-Induced Interleukin-1β and Tumor Necrosis Factor-α Productions via the Inhibition of IκB Degradation in Human Lung Cells. Pharmacol. Res. 2007, 56, 193–201. [Google Scholar] [CrossRef]
- Okon, E.; Kukula-Koch, W.; Halasa, M.; Jarzab, A.; Baran, M.; Dmoszynska-Graniczka, M.; Angelis, A.; Kalpoutzakis, E.; Guz, M.; Stepulak, A.; et al. Magnoflorine—Isolation and the Anticancer Potential against NCI-H1299 Lung, MDA-MB-468 Breast, T98G Glioma, and TE671 Rhabdomyosarcoma Cancer Cells. Biomolecules 2020, 10, 1532. [Google Scholar] [CrossRef]
- Ahmad, W.; Jantan, I.; Kumolosasi, E.; Haque, M.A.; Bukhari, S.N.A. Immunomodulatory Effects of Tinospora crispa Extract and Its Major Compounds on the Immune Functions of RAW 264.7 Macrophages. Int. Immunopharmacol. 2018, 60, 141–151. [Google Scholar] [CrossRef]
- Sharma, U.; Bala, M.; Kumar, N.; Singh, B.; Munshi, R.K.; Bhalerao, S. Immunomodulatory Active Compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012, 141, 918–926. [Google Scholar] [CrossRef]
- Aranha, I.; Clement, F.; Venkatesh, Y.P. Immunostimulatory Properties of the Major Protein from the Stem of the Ayurvedic Medicinal Herb, Guduchi (Tinospora cordifolia). J. Ethnopharmacol. 2012, 139, 366–372. [Google Scholar] [CrossRef]
- Gupta, P.K.; Rajan, M.G.R.; Kulkarni, S. Activation of Murine Macrophages by G1-4A, a Polysaccharide from Tinospora cordifolia, in TLR4/MyD88 Dependent Manner. Int. Immunopharmacol. 2017, 50, 168–177. [Google Scholar] [CrossRef]
- Sunila, E.S.; Kuttan, G. Immunomodulatory and Antitumor Activity of Piper longum Linn. and Piperine. J. Ethnopharmacol. 2004, 90, 339–346. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; El-Zamarany, E.A.; Salem, M.L.; El-Bahrawy, H.A.; Al-Ashmawy, G.M. In Vitro and In Vivo Studies of the Immunomodulatory Effect of Echinacea Purpurea on Dendritic Cells. J. Genet. Eng. Biotechnol. 2015, 13, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.M.; Pokorny, A.J.; Rhule, A.; Wenner, C.A.; Kandhi, V.; Cech, N.B.; Shepherd, D.M. Echinacea Purpurea Extracts Modulate Murine Dendritic Cell Fate and Function. Food Chem. Toxicol. 2010, 48, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, K.; Liu, C.; Peng, P.; Bai, M.; Sun, J.; Li, Q.; Yang, Z.; Yang, Y.; Wu, H. A Comparison of the Immunostimulatory Effects of Polysaccharides from Tetraploid and Diploid Echinacea Purpurea. BioMed Res. Int. 2018, 2018, e8628531. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Yang, Y.; Xing, N.; Wang, Y.; Wang, Q.; Kuang, H. Structural Characterization and Immunomodulatory Activity of a Pectic Polysaccharide (CALB-4) from Fructus Aurantii. Int. J. Biol. Macromol. 2018, 116, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Wang, X.; An, L.; Bao, J.; Zhang, J.; Cui, J.; Li, Y.; Jin, D.-Q.; Tuerhong, M.; et al. Isolation, Structural Elucidation, and Immunoregulation Properties of an Arabinofuranan from the Rinds of Garcinia mangostana. Carbohydr. Polym. 2020, 246, 116567. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Ting Jun, H.; Chun Ni, L. Immunomodulatory and Antioxidant Activity of a Siraitia Grosvenorii Polysaccharide in Mice. Afr. J. Biotechnol. 2011, 10, 10045–10053. [Google Scholar] [CrossRef]
- Michelini, F.M.; Alché, L.E.; Bueno, C.A. Virucidal, Antiviral and Immunomodulatory Activities of β-Escin and Aesculus Hippocastanum Extract. J. Pharm. Pharmacol. 2018, 70, 1561–1571. [Google Scholar] [CrossRef]
- Low, M.; Khoo, C.S.; Münch, G.; Govindaraghavan, S.; Sucher, N.J. An In Vitro Study of Anti-Inflammatory Activity of Standardised Andrographis paniculata Extracts and Pure Andrographolide. BMC Complement. Altern. Med. 2015, 15, 18. [Google Scholar] [CrossRef]
- Naik, S.R.; Hule, A. Evaluation of Immunomodulatory Activity of an Extract of Andrographolides from Andographis paniculata. Planta Med. 2009, 75, 785–791. [Google Scholar] [CrossRef]
- Kaur, G.; Sarwar Alam, M.; Athar, M. Nimbidin Suppresses Functions of Macrophages and Neutrophils: Relevance to Its Antiinflammatory Mechanisms. Phytother. Res. 2004, 18, 419–424. [Google Scholar] [CrossRef]
- Vaghasiya, J.; Datani, M.; Nandkumar, K.; Malaviya, S.; Jivani, N. Comparative Evaluation of Alcoholic and Aqueous Extracts of Ocimum Sanctum for Immunomodulatory Activity. Int. J. Pharm. Biol. Res. 2010, 1, 25e9. [Google Scholar]
- Sharma, M.L.; Kaul, A.; Khajuria, A.; Singh, S.; Singh, G.B. Immunomodulatory Activity of Boswellic Acids (Pentacyclic Triterpene Acids) from Boswellia serrata. Phytother. Res. 1996, 10, 107–112. [Google Scholar] [CrossRef]
- Liao, J.; Wu, D.; Peng, S.; Xie, J.; Li, Y.; Su, J.; Chen, J.; Su, Z. Immunomodulatory Potential of Patchouli Alcohol Isolated from Pogostemon Cablin (Blanco) Benth (Lamiaceae) in Mice. Trop. J. Pharm. Res 2013, 12, 559–565. [Google Scholar] [CrossRef]
- Chang, S.-L.; Chiang, Y.-M.; Chang, C.L.-T.; Yeh, H.-H.; Shyur, L.-F.; Kuo, Y.-H.; Wu, T.-K.; Yang, W.-C. Flavonoids, Centaurein and Centaureidin, from Bidens pilosa, Stimulate IFN-γ Expression. J. Ethnopharmacol. 2007, 112, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.L.; Ibrahim, T.; Lucchetti, L.; da Silva, A.J.; Gonçalves de Moraes, V.L. Immunosuppressive and Anti-Inflammatory Effects of Methanolic Extract and the Polyacetylene Isolated from Bidens pilosa L. Immunopharmacology 1999, 43, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, M.I.S.; Kamal, A.M.; Rauf, A.; Mubarak, M.S.; Hadda, T.B. Bioassay-Guided Isolation and POM Analyses of a New Immunomodulatory Polyphenolic Constituent from Callistemon viridiflorus. Nat. Prod. Res. 2016, 30, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory Effects of Curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef]
- Ranjan, D.; Chen, C.; Johnston, T.D.; Jeon, H.; Nagabhushan, M. Curcumin Inhibits Mitogen Stimulated Lymphocyte Proliferation, NFkappaB Activation, and IL-2 Signaling. J. Surg. Res. 2004, 121, 171–177. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Ortiz-Sánchez, E.; Domínguez, F.; Arana-Argáez, V.; Juárez-Vázquez, M.d.C.; Chávez, M.; Carranza-Álvarez, C.; Gaspar-Ramírez, O.; Espinosa-Reyes, G.; López-Toledo, G.; et al. Antitumor and Immunomodulatory Effects of Justicia spicigera Schltdl (Acanthaceae). J. Ethnopharmacol. 2012, 141, 888–894. [Google Scholar] [CrossRef]
- Del Carmen Juárez-Vázquez, M.; Josabad Alonso-Castro, A.; García-Carrancá, A. Kaempferitrin Induces Immunostimulatory Effects In Vitro. J. Ethnopharmacol. 2013, 148, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, H.; Jantan, I.; Haque, M.A.; Kumolosasi, E. Anti-Inflammatory Effects of Phyllanthus amarus Schum. & Thonn. through Inhibition of NF-κB, MAPK, and PI3K-Akt Signaling Pathways in LPS-Induced Human Macrophages. BMC Complement. Altern. Med. 2018, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Kassuya, C.A.L.; Leite, D.F.P.; de Melo, L.V.; Rehder, V.L.G.; Calixto, J.B. Anti-Inflammatory Properties of Extracts, Fractions and Lignans Isolated from Phyllanthus amarus. Planta Med. 2005, 71, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Ilangkovan, M.; Yuandani; Mohamad, H.F. Correlation between the Major Components of Phyllanthus amarus and Phyllanthus urinaria and Their Inhibitory Effects on Phagocytic Activity of Human Neutrophils. BMC Complement. Altern. Med. 2014, 14, 429. [Google Scholar] [CrossRef]
- Shabbir, A.; Butt, H.; Shahzad, M.; Arshad, H.; Waheed, I. Immunostimulatory Effect of Methanolic Leaves Extract of Psidium guajava (Guava) on Humoral and Cell-Mediated Immunity in Mice. J. Anim. Plant Sci. 2016, 26, 1492–1500. [Google Scholar]
- Nhu, T.Q.; Dam, N.P.; Bich Hang, B.T.; Bach, L.T.; Thanh Huong, D.T.; Buu Hue, B.T.; Scippo, M.-L.; Phuong, N.T.; Quetin-Leclercq, J.; Kestemont, P. Immunomodulatory Potential of Extracts, Fractions and Pure Compounds from Phyllanthus amarus and Psidium guajava on Striped Catfish (Pangasianodon hypophthalmus) Head Kidney Leukocytes. Fish. Shellfish. Immunol. 2020, 104, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Ben Sghaier, M.; Krifa, M.; Mensi, R.; Bhouri, W.; Ghedira, K.; Chekir-Ghedira, L. In Vitro and In Vivo Immunomodulatory and Anti-Ulcerogenic Activities of Teucrium ramosissimum Extracts. J. Immunotoxicol. 2011, 8, 288–297. [Google Scholar] [CrossRef]
- Nasr-Bouzaiene, N.; Sassi, A.; Bedoui, A.; Krifa, M.; Chekir-Ghedira, L.; Ghedira, K. Immunomodulatory and Cellular Antioxidant Activities of Pure Compounds from Teucrium ramosissimum Desf. Tumour Biol. 2016, 37, 7703–7712. [Google Scholar] [CrossRef] [PubMed]
- Askari, V.R.; Alavinezhad, A.; Rahimi, V.B.; Rezaee, S.A.; Boskabady, M.H. Immuno-Modulatory Effects of Methanolic Extract of Ferula szowitsiana on Isolated Human Th1/Th2/Treg Cytokines Levels, and Their Genes Expression and Nitric Oxide Production. Cytokine 2021, 138, 155387. [Google Scholar] [CrossRef]
- Zamani Taghizadeh Rabe, S.; Iranshahi, M.; Mahmoudi, M. In Vitro Anti-Inflammatory and Immunomodulatory Properties of Umbelliprenin and Methyl Galbanate. J. Immunotoxicol. 2016, 13, 209–216. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Song, Y.; Nie, L.; Wang, L.; Liu, B.; Shen, P.; Liu, Y. Isolation, Structure Elucidation, Antioxidative and Immunomodulatory Properties of Two Novel Dihydrocoumarins from Aloe Vera. Bioorg. Med. Chem. Lett. 2006, 16, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tiku, A.B. Immunomodulatory Potential of Acemannan (Polysaccharide from Aloe Vera) against Radiation Induced Mortality in Swiss Albino Mice. Food Agric. Immunol. 2016, 27, 72–86. [Google Scholar] [CrossRef]
- Leung, K.; Leung, P.; Kong, L.; Leung, P. Immunomodulatory Effects of Esculetin (6,7-Dihydroxycoumarin) on Murine Lymphocytes and Peritoneal Macrophages. Cell Mol. Immunol. 2005, 2, 181–188. [Google Scholar] [PubMed]
- Clement, F.; Pramod, S.N.; Venkatesh, Y.P. Identity of the Immunomodulatory Proteins from Garlic (Allium sativum) with the Major Garlic Lectins or Agglutinins. Int. Immunopharmacol. 2010, 10, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, P.M.; Prashanth, K.V.H.; Venkatesh, Y.P. Isolation, Structural Elucidation and Immunomodulatory Activity of Fructans from Aged Garlic Extract. Phytochemistry 2011, 72, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, P.M.; Venkatesh, Y.P. Identification of the Protein Components Displaying Immunomodulatory Activity in Aged Garlic Extract. J. Ethnopharmacol. 2009, 124, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Patra, P.; Sen, I.K.; Bhanja, S.K.; Nandi, A.K.; Samanta, S.; Das, D.; Devi, K.S.P.; Maiti, T.K.; Islam, S.S. Pectic Polysaccharide from Immature Onion Stick (Allium cepa): Structural and Immunological Investigation. Carbohydr. Polym. 2013, 92, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Prashanth, K.V.H.; Venkatesh, Y.P. Structural Analyses and Immunomodulatory Properties of Fructo-Oligosaccharides from Onion (Allium cepa). Carbohydr. Polym. 2015, 117, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.K.; Venkatesh, Y.P. Characterization of Onion Lectin (Allium cepa Agglutinin) as an Immunomodulatory Protein Inducing Th1-Type Immune Response In Vitro. Int. Immunopharmacol. 2015, 26, 304–313. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. The Role of Astragalus membranaceus as Immunomodulator in Poultry. World’s Poult. Sci. J. 2019, 75, 43–54. [Google Scholar] [CrossRef]
- Huang, H.; Luo, S.-H.; Huang, D.-C.; Cheng, S.-J.; Cao, C.-J.; Chen, G.-T. Immunomodulatory Activities of Proteins from Astragalus membranaceus Waste. J. Sci. Food Agric. 2019, 99, 4174–4181. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, Q.; Zhang, L.; Wang, R.; Xu, X.; Hu, X. Astragalus membranaceus Treatment Protects Raw264.7 Cells from Influenza Virus by Regulating G1 Phase and the TLR3-Mediated Signaling Pathway. Evid.-Based Complement. Altern. Med. 2019, 2019, 2971604. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-C.; Ng, L.T.; Chiang, W.; Chang, M.-Y.; Lin, C.-C. Immunomodulatory Activities of Flavonoids, Monoterpenoids, Triterpenoids, Iridoid Glycosides and Phenolic Compounds of Plantago Species. Planta Med. 2003, 69, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Nie, S.; Jiang, L.; Xie, M. A Novel Polysaccharide from the Seeds of Plantago Asiatica L. Induces Dendritic Cells Maturation through Toll-like Receptor 4. Int. Immunopharmacol. 2014, 18, 236–243. [Google Scholar] [CrossRef] [PubMed]
- García, D.; Leiro, J.; Delgado, R.; Sanmartín, M.L.; Ubeira, F.M. Mangifera Indica L. Extract (Vimang) and Mangiferin Modulate Mouse Humoral Immune Responses. Phytother. Res. 2003, 17, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Jayakumar, T.; Nishigaki, I.; Ekambaram, G.; Nishigaki, Y.; Vetriselvi, J.; Sakthisekaran, D. Immunomodulatory Effect of Mangiferin in Experimental Animals with Benzo(a)Pyrene-Induced Lung Carcinogenesis. Int. J. Biomed. Sci. 2013, 9, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Gholamnezhad, Z.; Rafatpanah, H.; Sadeghnia, H.R.; Boskabady, M.H. Immunomodulatory and Cytotoxic Effects of Nigella sativa and Thymoquinone on Rat Splenocytes. Food Chem. Toxicol. 2015, 86, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Deng, Y.; Xie, Q. The Modulatory Effects of the Volatile Oil of Ginger on the Cellular Immune Response In Vitro and In Vivo in Mice. J. Ethnopharmacol. 2006, 105, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Schmidt, G.; Lopes Romero, A.; Sartoretto, J.; Caparroz-Assef, S.; Bersani-Amado, C.; Cuman, R. Immunomodulatory Activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. Essential Oils: Evidence for Humor- and Cell-Mediated Responses. J. Pharm. Pharmacol. 2009, 61, 961–967. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Lee, K.-C.; Chen, S.-Y.; Chang, H.-H. 6-Gingerol Inhibits ROS and iNOS through the Suppression of PKC-α and NF-κB Pathways in Lipopolysaccharide-Stimulated Mouse Macrophages. Bioch. Biophys. Res. Commun. 2009, 382, 134–139. [Google Scholar] [CrossRef]
- Pandey, R.; Maurya, R.; Singh, G.; Sathiamoorthy, B.; Naik, S. Immunosuppressive Properties of Flavonoids Isolated from Boerhaavia diffusa Linn. Int. Immunopharmacol. 2005, 5, 541–553. [Google Scholar] [CrossRef]
- Sreelekha, T.T.; Vijayakumar, T.; Ankanthil, R.; Vijayan, K.K.; Nair, M.K. Immunomodulatory Effects of a Polysaccharide from Tamarindus Indica. Anti-Cancer Drugs 1993, 4, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Capek, P.; Hrıbalová, V.; Švandová, E.; Ebringerová, A.; Sasinková, V.; Masarová, J. Characterization of Immunomodulatory Polysaccharides from Salvia officinalis L. Int. J. Biol. Macromol. 2003, 33, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Chakraborty, I.; Pramanik, M.; Rout, D.; Islam, S. Structural Studies of an Immunoenhancing Polysaccharide Isolated from Mature Pods (Fruits) of Moringa oleifera (Sajina). Med. Chem. Res. 2004, 13, 390–400. [Google Scholar] [CrossRef]
- Sadeghalvad, M.; Mohammadi-Motlagh, H.-R.; Rezaei, N. Structure and Function of the Immune System. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 24–38. ISBN 978-0-323-90303-5. [Google Scholar]
- Bafna, A.; Mishra, S. Antioxidant and Immunomodulatory Activity of the Alkaloidal Fraction of Cissampelos pareira Linn. Sci. Pharm. 2010, 78, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. Dendritic Cells. Encycl. Cell Biol. 2016, 741–749. [Google Scholar] [CrossRef]
- Parker, G.A.; Papenfuss, T.L. Immune System. In Atlas of Histology of the Juvenile Rat; Parker, G.A., Picut, C.A., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 293–347. ISBN 978-0-12-802682-3. [Google Scholar]
- Luettig, B.; Steinmuller, C.; Gifford, G.E.; Wagner, H.; Lohmann-Matthes, M.-L. Macrophage Activation by the Polysaccharide Arabinogalactan Isolated From Plant Cell Cultures of Echinacea Purpurea. J. Natl. Cancer Inst. 1989, 81, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Steinmüller, C.; Roesler, J.; Gröttrup, E.; Franke, G.; Wagner, H.; Lohmann-Matthes, M.L. Polysaccharides Isolated from Plant Cell Cultures of Echinacea Purpurea Enhance the Resistance of Immunosuppressed Mice against Systemic Infections with Candida Albicans and Listeria Monocytogenes. Int. J. Immunopharmacol. 1993, 15, 605–614. [Google Scholar] [CrossRef]
- Miranda, R.d.S.; Jesus, B.d.S.M.; Silva Luiz, S.R.; Viana, C.B.; Adão Malafaia, C.R.; Figueiredo, F.d.S.; Carvalho, T.d.S.C.; Silva, M.L.; Londero, V.S.; Costa-Silva, T.A.; et al. Antiinflammatory Activity of Natural Triterpenes—An Overview from 2006 to 2021. Phytother. Res. 2022, 36, 1459–1506. [Google Scholar] [CrossRef]
- Renda, G.; Gökkaya, İ.; Şöhretoğlu, D. Immunomodulatory Properties of Triterpenes. Phytochem. Rev. 2022, 21, 537–563. [Google Scholar] [CrossRef]
- Nalbantsoy, A.; Nesil, T.; Erden, S.; Calış, I.; Bedir, E. Adjuvant Effects of Astragalus saponins Macrophyllosaponin B and Astragaloside VII. J. Ethnopharmacol. 2011, 134, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Li, X.-Y.; Song, C.-Q.; Hu, Z.-B. Effect of Astragaloside IV on T, B Lymphocyte Proliferation and Peritoneal Macrophage Function in Mice. Acta Pharmacol. Sin. 2002, 23, 263–266. [Google Scholar] [PubMed]
- Xin, W.; Zhang, L.; Sun, F.; Jiang, N.; Fan, H.; Wang, T.; Li, Z.; He, J.; Fu, F. Escin Exerts Synergistic Anti-Inflammatory Effects with Low Doses of Glucocorticoids In Vivo and In Vitro. Phytomedicine 2011, 18, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Singh, V.K.; Agarwal, S.S.; Maurya, R.; Srimal, R.C. Antilymphoproliferative Activity of Ethanolic Extract of Boerhaavia diffusa Roots. Exp. Mol. Pathol. 2002, 72, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Jang, H.-J.; Kim, M.H.; Lee, S.; Lee, S.W.; Lee, S.-J.; Rho, M.-C. Oleanolic Acid Acetate Exerts Anti-Inflammatory Activity via IKKα/β Suppression in TLR3-Mediated NF-κB Activation. Molecules 2019, 24, 4002. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, H.; Sui, Z.; Jing, F.; Quan, X.; Zhao, W.; Liu, G. Ursolic Acid Exhibits Anti-Inflammatory Effects through Blocking TLR4-MyD88 Pathway Mediated by Autophagy. Cytokine 2019, 123, 154726. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Lee, D.-S.; Kim, D.-C.; Yoon, C.-S.; Ko, W.; Oh, H.; Kim, Y.-C. Anti-Inflammatory Effects and Mechanisms of Action of Coussaric and Betulinic Acids Isolated from Diospyros Kaki in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules 2016, 21, 1206. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, T.; Hao, N.; Tao, H.; Zou, S.; Li, M.; Ming, P.; Ding, H.; Dong, J.; Feng, S.; et al. Immune Regulation Mechanism of Astragaloside IV on RAW264.7 Cells through Activating the NF-κB/MAPK Signaling Pathway. Int. Immunopharmacol. 2017, 49, 38–49. [Google Scholar] [CrossRef]
- Haque, M.A.; Jantan, I.; Harikrishnan, H.; Abdul Wahab, S.M. Magnoflorine Enhances LPS-Activated Pro-Inflammatory Responses via MyD88-Dependent Pathways in U937 Macrophages. Planta Med. 2018, 84, 1255–1264. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Kim, K.-H.; Lee, S.-H.; Yoon, M.-S.; Lee, H.-J.; Moon, D.-O.; Lee, C.-M.; Ahn, S.-C.; Park, Y.C.; Park, Y.-M. Curcumin Inhibits Immunostimulatory Function of Dendritic Cells: MAPKs and Translocation of NF-Kappa B as Potential Targets. J. Immunol. 2005, 174, 8116–8124. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-J.; Song, J.; Kim, H.-R.; Hwang, K.-A. Oleanolic Acid Regulates NF-κB Signaling by Suppressing MafK Expression in RAW 264.7 Cells. BMB Rep 2014, 47, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Miao, L.; Zhang, H.; Tan, L.; Zhao, Y.; Tu, Y.; Prieto, M.A.; Simal-Gandara, J.; Chen, L.; He, C.; et al. Anti-Inflammatory Activity of Flavonols via Inhibiting MAPK and NF-κB Signaling Pathways in RAW264.7 Macrophages. Curr. Res. Food Sci. 2022, 5, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S. Aucubin, a Naturally Occurring Iridoid Glycoside Inhibits TNF-α-Induced Inflammatory Responses through Suppression of NF-κB Activation in 3T3-L1 Adipocytes. Cytokine 2013, 62, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.B.; Sinha, K.; Sil, P.C. Mangiferin, a Natural Xanthone, Protects Murine Liver in Pb(II) Induced Hepatic Damage and Cell Death via MAP Kinase, NF-κB and Mitochondria Dependent Pathways. PLoS One 2013, 8, e56894. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Kumari, S.; Ojha, S.; Arya, D.S. Protective Effect of Mangiferin on Myocardial Ischemia-Reperfusion Injury in Streptozotocin-Induced Diabetic Rats: Role of AGE-RAGE/MAPK Pathways. Sci. Rep. 2017, 7, 42027. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis Vulgaris and Berberine: An Update Review. Phytother. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The Metabolism of Berberine and Its Contribution to the Pharmacological Effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zheng, X.; Vong, C.T.; Zhao, J.; Xiao, J.; Wang, Y.; Zhong, Z. Combined Effects of Berberine and Evodiamine on Colorectal Cancer Cells and Cardiomyocytes In Vitro. Eur. J. Pharmacol. 2020, 875, 173031. [Google Scholar] [CrossRef]
- Gao, X.; Wang, J.; Li, M.; Wang, J.; Lv, J.; Zhang, L.; Sun, C.; Ji, J.; Yang, W.; Zhao, Z.; et al. Berberine Attenuates XRCC1-mediated Base Excision Repair and Sensitizes Breast Cancer Cells to the Chemotherapeutic Drugs. J. Cell. Mol. Med. 2019, 23, 6797–6804. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Y.; Shen, H.; Xu, W.; Li, H. Berberine Sensitizes Ovarian Cancer Cells to Cisplatin through miR-21/PDCD4 Axis. Acta Biochim. Biophys. Sin. (Shanghai) 2013, 45, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, A.; Iapichino, A.; Cura, F.; Scapoli, L.; Carinci, F.; Mandrone, M.; Martinelli, M. Pre-Treatment with Berberine Enhances Effect of 5-Fluorouracil and Cisplatin in HEP2 Laryngeal Cancer Cell Line. J. Biol. Regul. Homeost. Agents 2018, 32, 167–177. [Google Scholar]
- Pandey, A.; Vishnoi, K.; Mahata, S.; Tripathi, S.C.; Misra, S.P.; Misra, V.; Mehrotra, R.; Dwivedi, M.; Bharti, A.C. Berberine and Curcumin Target Survivin and STAT3 in Gastric Cancer Cells and Synergize Actions of Standard Chemotherapeutic 5-Fluorouracil. Nutr. Cancer 2015, 67, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Plazas, E.; Avila M, M.C.; Muñoz, D.R.; Cuca, S.L.E. Natural Isoquinoline Alkaloids: Pharmacological Features and Multi-Target Potential for Complex Diseases. Pharmacol. Res. 2022, 177, 106126. [Google Scholar] [CrossRef]
- Cecil, C.E.; Davis, J.M.; Cech, N.B.; Laster, S.M. Inhibition of H1N1 Influenza A Virus Growth and Induction of Inflammatory Mediators by the Isoquinoline Alkaloid Berberine and Extracts of Goldenseal (Hydrastis canadensis). Int. Immunopharmacol. 2011, 11, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.W.; Cheng, Y.-W.; Lin, S.-S.; Lai, Y.-Y.; Lin, L.-Y.; Chou, M.-Y.; Chou, M.-C.; Yang, C.-C. Anti-Herpes Simplex Virus Effects of Berberine from Coptidis rhizoma, a Major Component of a Chinese Herbal Medicine, Ching-Wei-San. Arch. Virol. 2010, 155, 1933–1941. [Google Scholar] [CrossRef]
- Varghese, F.S.; van Woudenbergh, E.; Overheul, G.J.; Eleveld, M.J.; Kurver, L.; van Heerbeek, N.; van Laarhoven, A.; Miesen, P.; den Hartog, G.; de Jonge, M.I.; et al. Berberine and Obatoclax Inhibit SARS-CoV-2 Replication in Primary Human Nasal Epithelial Cells In Vitro. Viruses 2021, 13, 282. [Google Scholar] [CrossRef]
- Bodiwala, H.S.; Sabde, S.; Mitra, D.; Bhutani, K.K.; Singh, I.P. Synthesis of 9-Substituted Derivatives of Berberine as Anti-HIV Agents. Eur. J. Med. Chem. 2011, 46, 1045–1049. [Google Scholar] [CrossRef]
- Luganini, A.; Mercorelli, B.; Messa, L.; Palù, G.; Gribaudo, G.; Loregian, A. The Isoquinoline Alkaloid Berberine Inhibits Human Cytomegalovirus Replication by Interfering with the Viral Immediate Early-2 (IE2) Protein Transactivating Activity. Antiviral Res. 2019, 164, 52–60. [Google Scholar] [CrossRef]
- Shin, H.-B.; Choi, M.-S.; Yi, C.-M.; Lee, J.; Kim, N.-J.; Inn, K.-S. Inhibition of Respiratory Syncytial Virus Replication and Virus-Induced P38 Kinase Activity by Berberine. Int. Immunopharmacol. 2015, 27, 65–68. [Google Scholar] [CrossRef]
- Pradeep, C.R.; Kuttan, G. Piperine Is a Potent Inhibitor of Nuclear Factor-κB (NF-κB), c-Fos, CREB, ATF-2 and Proinflammatory Cytokine Gene Expression in B16F-10 Melanoma Cells. Int. Immunopharmacol. 2004, 4, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Yang, R.; Lin, R.; Yang, S. Piperine Functions as a Tumor Suppressor for Human Ovarian Tumor Growth via Activation of JNK/P38 MAPK-Mediated Intrinsic Apoptotic Pathway. Biosci. Rep. 2018, 38, BSR20180503. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M.; Ericsson, A.C. Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Front. Immunol. 2021, 12, 620510. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Zohora, F.; Saboor-Yaraghi, A.A.; Aliyu, M.; Zohora, F.; Saboor-Yaraghi, A.A. Spleen in Innate and Adaptive Immunity Regulation. AIMS Allergy Immunol. 2021, 5, 1–17. [Google Scholar] [CrossRef]
- Ayatollahi, A.M.; Ghanadian, M.; Afsharypour, S.; Abdella, O.M.; Mirzai, M.; Askari, G. Pentacyclic Triterpenes in Euphorbia microsciadia with Their T-Cell Proliferation Activity. Iran. J. Pharm. Res. 2011, 10, 287–294. [Google Scholar] [PubMed]
- Mioc, M.; Milan, A.; Malița, D.; Mioc, A.; Prodea, A.; Racoviceanu, R.; Ghiulai, R.; Cristea, A.; Căruntu, F.; Șoica, C. Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part I). Int. J. Mol. Sci. 2022, 23, 7740. [Google Scholar] [CrossRef] [PubMed]
- Salinas, F.M.; Vázquez, L.; Gentilini, M.V.; O´Donohoe, A.; Regueira, E.; Nabaes Jodar, M.S.; Viegas, M.; Michelini, F.M.; Hermida, G.; Alché, L.E.; et al. Aesculus Hippocastanum L. Seed Extract Shows Virucidal and Antiviral Activities against Respiratory Syncytial Virus (RSV) and Reduces Lung Inflammation In Vivo. Antiviral Res. 2019, 164, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Cavalcanti, E.; Vadrucci, E.; Delvecchio, F.R.; Addabbo, F.; Bettini, S.; Liou, R.; Monsurrò, V.; Huang, A.Y.-C.; Pizarro, T.T.; Santino, A.; et al. Administration of Reconstituted Polyphenol Oil Bodies Efficiently Suppresses Dendritic Cell Inflammatory Pathways and Acute Intestinal Inflammation. PLOS ONE 2014, 9, e88898. [Google Scholar] [CrossRef]
- Li, P.; Hu, J.; Shi, B.; Tie, J. Baicalein Enhanced Cisplatin Sensitivity of Gastric Cancer Cells by Inducing Cell Apoptosis and Autophagy via Akt/mTOR and Nrf2/Keap 1 Pathway. Biochem. Biophys. Res. Commun. 2020, 531, 320–327. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Huang, L.; Yang, F.; Liu, A.; Zhang, J. Combination of Lapatinib and Luteolin Enhances the Therapeutic Efficacy of Lapatinib on Human Breast Cancer through the FOXO3a/NQO1 Pathway. Biochem. Biophys. Res. Commun. 2020, 531, 364–371. [Google Scholar] [CrossRef]
- Bahrami, A.; Fereidouni, M.; Pirro, M.; Bianconi, V.; Sahebkar, A. Modulation of Regulatory T Cells by Natural Products in Cancer. Cancer Lett. 2019, 459, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Du, Y.; Lin, X.; Qian, Y.; Zhou, T.; Huang, Z. CD4+CD25+ Regulatory T Cells in Tumor Immunity. Int. Immunopharmacol. 2016, 34, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.C.; Amedei, A.; Aquilano, K.; Azmi, A.S.; Benencia, F.; Bhakta, D.; Bilsland, A.E.; Boosani, C.S.; Chen, S.; Ciriolo, M.R.; et al. Cancer Prevention and Therapy through the Modulation of the Tumor Microenvironment. Semin. Cancer Biol. 2015, 35, S199–S223. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Zhang, Z.; Xu, B.; Zhao, S.; Ding, Y.; Wu, X.; Wu, R.; Lv, Y.; Dong, J. Baicalein and Baicalin Promote Antitumor Immunity by Suppressing PD-L1 Expression in Hepatocellular Carcinoma Cells. Int. Immunopharmacol. 2019, 75, 105824. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of Polyphenols and Its Anticancer Properties in Biomedical Research: A Narrative Review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Meylan, E.; Dooley, A.L.; Feldser, D.M.; Shen, L.; Turk, E.; Ouyang, C.; Jacks, T. Requirement for NF-κB Signalling in a Mouse Model of Lung Adenocarcinoma. Nature 2009, 462, 104–107. [Google Scholar] [CrossRef]
- Xia, Y.; Yeddula, N.; Leblanc, M.; Ke, E.; Zhang, Y.; Oldfield, E.; Shaw, R.J.; Verma, I.M. Reduced Cell Proliferation by IKK2 Depletion in a Mouse Lung-Cancer Model. Nat. Cell Biol. 2012, 14, 257–265. [Google Scholar] [CrossRef]
- Bassères, D.S.; Ebbs, A.; Levantini, E.; Baldwin, A.S. Requirement of the NF-kappaB Subunit P65/RelA for K-Ras-Induced Lung Tumorigenesis. Cancer Res. 2010, 70, 3537–3546. [Google Scholar] [CrossRef]
- Keutgens, A.; Robert, I.; Viatour, P.; Chariot, A. Deregulated NF-kappaB Activity in Haematological Malignancies. Biochem. Pharmacol. 2006, 72, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zheng, D.; Fung, G.; Deng, H.; Chen, L.; Liang, J.; Jiang, Y.; Hu, Y. Mangiferin Suppressed Advanced Glycation End Products (AGEs) through NF-κB Deactivation and Displayed Anti-Inflammatory Effects in Streptozotocin and High Fat Diet-Diabetic Cardiomyopathy Rats. Can. J. Physiol. Pharmacol. 2016, 94, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Lal, J.; Gupta, P.K. Immunotherapeutic Effects of Mangiferin Mediated by the Inhibition of Oxidative Stress to Activated Lymphocytes, Neutrophils and Macrophages. Toxicology 2005, 215, 57–68. [Google Scholar] [CrossRef]



 : activation or stimulation by phytochemical;
: activation or stimulation by phytochemical;  : inhibition or reduction by phytochemical; →: transduction way or activation.
: inhibition or reduction by phytochemical; →: transduction way or activation.
 : activation or stimulation by phytochemical;
: activation or stimulation by phytochemical;  : inhibition or reduction by phytochemical; →: transduction way or activation.
: inhibition or reduction by phytochemical; →: transduction way or activation.
| Species and Families of Plants | Parts of Plant Used | Solvent | Chemical Groups | Isolated Molecules | Other Biological Activity | Reference |
|---|---|---|---|---|---|---|
| Cissampelos pareira L., Menispermaceae | Roots | Methanol | Alkaloids | Berberine (1), Tetrandrine. | Antioxidant, antibacterial | [14,15] |
| Tinospora crispa, Menispermaceae | Stem | Ethanol | Alkaloids, glycosides, terpenoids | N-formyllannonaine (2), N-formylnornuceferine (3), Lysicamine (4) Magnoflorine (5) Syringin (50) 1-Octacosanol. | Anti-inflammatory, antioxidant | [16,17] |
| Tinospora cordifolia (Wild) Hook. F. & Thomson, Menispermaceae | Stem | Methanol, n-hexane, chloroform, ethyl acetate and n-butanol | Alkaloids, glycosides, proteins | 11-hydroxymustakone (51), N-methyl-2-pyrrolidone (52), N-formylannonaine (2) Cordifolioside (53), Tinocordiside (54) Syringin (50) | [18,19,20] | |
| Piper longum Linn. Piperaceae | Fruits | Methanol | Alkaloids | Piperine (6) | Anti-inflammatory, anti-infectious, antitumor, analgesic | [21] |
| Echinacea purpura, Echinaceae | Whole plant, Root | Methanol, ethanol, aqueous | Polysaccharides, flavonoids | Polysaccharides, alkyl amides, Arabinogalactans, Caffeic acid(34) | Antioxidant, anti-inflammatory | [22,23,24] |
| Fructus aurantii, Rutaceae | Fruit | Polysaccharides | Pectic polysaccharide: CALB-4 | Anti-carcinogenic, antimicrobial | [25] | |
| Garcinia mangostana L., Guttiferae | Bark | Methanol | Polysaccharides | Arabinofuran (GMP90-1) | Antioxidant, anti-inflammatory, antimicrobial | [26] |
| Siraitia grosvenorii, Cucurbitaceae | Whole plant | Aqueous | Polysaccharides | Polysaccharides | Antioxidant, anti-inflammatory | [27] |
| Aesculus hippocastanum, Hippocastanaceae | Seed | Alcoholic | Saponins triterpenoides | β-aescin (18) | Antiviral | [28] |
| Andrographis paniculata, Acanthaceae | Whole plant | Methanol-water | Terpenoids | Andrographolide (10) 14-deoxyandrographolide (11); 14-deoxy-11,12-didehydroandrographolide (12), | Anticancer, Anti-inflammatory | [29,30] |
| Azadirachta indica, Meliaceae | Oil | Terpenoids | Nimbidin (14) | Anti-inflammatory, anti-arthritic | [31] | |
| Ocimum sanctum Lamiaceae | Whole plant | Alcoholic, aqueous | Terpenoids, essential oils, phenols, flavonoids | Eugenol, Carvacrol, Oleanolic acid (7), Ursolic acid (8), | Anti-inflammatory, antiallergic | [32] |
| Boswellia serrata Roxb. Burseraceae | Oleogum resin | Terpenoids | Boswellic acids (13) | Anti-inflammatory | [33] | |
| Pogostemon cablin Benth. Lamiaceae | Aerial parts | Ethanol aqueous | Terpenoids | PA: Patchouli alcoholic (19) | Antioxidant, Antimicrobial | [34] |
| Biden Pilosa, Asteraceae | Whole plant | n-butanol | Flavonoids | Polyacetylene 2-O-β-D-glucosyltrideca-11E-en-3,5,7,9-tetrayn-1,2-diol (PA-1), Centaurein (24), Centaureidin (25) | Anti-inflammatory, antihyperglycemic | [35,36] |
| Callistenom viridiflorus, Myrtaceae | Leaves | Ethanol | Phenols, flavonoids | Apigenin 4′-O-β-d-glucopyranosyl- (1″’ → 4″)-O-β-d-glucopyranoside, Kaempferide (28), Isoquercetin (27), Hyperin (29) | Anti-inflammatory, analgesic, antibacterial, antifungal. | [37] |
| Curcuma longa, Zingiberaceae | Rhizome | Flavonoids | Curcumin (23) | Anti-inflammatory, antimutagenic | [38,39] | |
| Justicia spicigera Schltdl. Acanthaceae | Leaves | Ethanol | Flavonoids | Kaempferitrin (26) | Antioxidant, antitumor | [40,41] |
| Phyllantus amarus, Euphorbiaceae | Leaves | Ethanol, fractions: ethyl acetate, dichloromethane | Flavonoids, lignan | Corosolic acid (9), Oleanolic acid (7), Phyllanthin, Hypophyllanthin (30) | Anti-inflammatory, antiviral, antimutagenic. | [42,43,44] |
| Psidium guajava, Myrtaceae | Leaves | Ethanol | Flavonoids, glycosides, phenolic compounds, terpenoids | Ellagic acid, Hyperin (29), Isoquercitin (27), Guajaverin (31), Avicularin (32), Asiatic acid (21), Maslinic acid (20), Corosolic acid (9), Oleanolic acid (7), Ursolic acid (8) | Antiallergic, antitumoral, anti-inflammatory, analgesic, antimicrobial | [45,46] |
| Teucrium ramosissimum Desf., Lamiaceae | Aerials parts | Chloroform | Flavonoids | Apigenin-7-glucoside (44), Genkwanin (43) Naringenin (41) | Antioxidant, anti-inflammatory | [47,48] |
| Ferula szowitsiana, Apiaceae | Roots | Methanol | Coumarins terpenoids | Methyl galbanate (49), Umbelliprenin (48) | Anti-inflammatory, antioxidant | [49,50] |
| Aloe vera, Liliaceae | Whole roots | Chloroform | Coumarins, flavonoids, phenolics, carbohydrates, lignans | Esculetin (6,7-dihydrocoumarin) (47) Acemannann | Anti-inflammatory, antioxidant | [51,52,53] |
| Allium sativum, Alliaceae | Bulbs | PBS | Proteins | Proteins (QR-1, QR-2, QR-3), Fructans, proteins (QA-1, QA-2, QA-3) | Anti-inflammatory, antioxidant, antimicrobial, antitumor | [54,55,56] |
| Allium cepa, Alliaceae | Bulbs | Ethanol | Proteins, polysaccharides, lectins | Pectin, FOS (fructo-oligosaccharides), Agglutinin | Antimicrobial | [57,58,59] |
| Astragalus membranaceus, Fabaceae | Waste | Alkali solvent | Proteins, saponins, alkaloids, polysaccharides, glucosides | Proteins: AMWPDG2, AMWPDG4, AMWPDG6, Astragaloside IV (15), Astragaloside VII (16), Macrophyllosaponin B (17) | Immunoadjuvants | [60,61,62] |
| Plantago sp. (P. major, P. asiatica) Plantiginaceae. | Leaves | Aqueous | Flavonoids, phenols, terpenoids, iridoids, | Aucubin (55), Chlorogenic acid (35), Ferulic acid (36), p-Coumaric acid (37), Vanillic acid (38), Luteolin (42), Ursolic acid (8), Oleanolic acid (7), Baicalein (33), Baicalin (33′). | Anticancer, antimicrobial, anti-inflammatory, antioxidant | [63,64] |
| Mangifera indica L. Anacardiaceae | Leaves | - | Xanthone glucoside | Mangiferin (56) | Antioxidant, antitumoral | [65,66] |
| Nigella sativa L. Ranunculaceae | Seeds | Ethanolic | Volatile oil | Thymoquinone (46) | Anti-inflammatory, antioxidant. | [67] |
| Zingiber officinale Zingiberaceae | Dried ginger | Distilled water | Volatile oil, polyphenols | 6-Gingerol (45) | Antibacterial, anti-inflammatory, antitumoral | [68,69,70] |
| Boerhavia diffusa Nyctaginaceae | Leaves | Hexane, chloroform, ethanol | Flavonoids | Eupalitin (BdI) (39), Eupalitin-3-O-β-D-galactopyranoside (BdII) (40) | Anti-inflammatory | [71] |
| Tamarindus indica Leguminoseae | Seeds | Water | Polysaccharides | Polysaccharides | Antitumoral | [72] |
| Salvia officinalis L. Lamiaceae | Arial parts | Methanol-chloroform | Polysaccharides, proteins | Arabinogalactans (A), Pectins (B), Glucurunoxylan polymers (D). | Anti-inflammatory | [73] |
| Moringa oleifera, Moringaceae | Mature pods | Aqueous | Polysaccharides | (1→4)-α-D glucan | Anti-inflammatory | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiema, W.A.; Ouédraogo, M.; Ouédraogo, W.P.; Fofana, S.; Ouédraogo, B.H.A.; Delma, T.E.; Amadé, B.; Abdoulaye, G.M.; Sawadogo, A.S.; Ouédraogo, R.; et al. Systematic Review of Chemical Compounds with Immunomodulatory Action Isolated from African Medicinal Plants. Molecules 2024, 29, 2010. https://doi.org/10.3390/molecules29092010
Nikiema WA, Ouédraogo M, Ouédraogo WP, Fofana S, Ouédraogo BHA, Delma TE, Amadé B, Abdoulaye GM, Sawadogo AS, Ouédraogo R, et al. Systematic Review of Chemical Compounds with Immunomodulatory Action Isolated from African Medicinal Plants. Molecules. 2024; 29(9):2010. https://doi.org/10.3390/molecules29092010
Chicago/Turabian StyleNikiema, Wendwaoga Arsène, Moussa Ouédraogo, Windbedma Prisca Ouédraogo, Souleymane Fofana, Boris Honoré Amadou Ouédraogo, Talwendpanga Edwige Delma, Belem Amadé, Gambo Moustapha Abdoulaye, Aimé Serge Sawadogo, Raogo Ouédraogo, and et al. 2024. "Systematic Review of Chemical Compounds with Immunomodulatory Action Isolated from African Medicinal Plants" Molecules 29, no. 9: 2010. https://doi.org/10.3390/molecules29092010
APA StyleNikiema, W. A., Ouédraogo, M., Ouédraogo, W. P., Fofana, S., Ouédraogo, B. H. A., Delma, T. E., Amadé, B., Abdoulaye, G. M., Sawadogo, A. S., Ouédraogo, R., & Semde, R. (2024). Systematic Review of Chemical Compounds with Immunomodulatory Action Isolated from African Medicinal Plants. Molecules, 29(9), 2010. https://doi.org/10.3390/molecules29092010









