Maillard Reaction-Derived S-Doped Carbon Dots Promotes Downregulation of PPARγ, C/EBPα, and SREBP-1 Genes In-Vitro
Abstract
1. Introduction
2. Result and Discussion
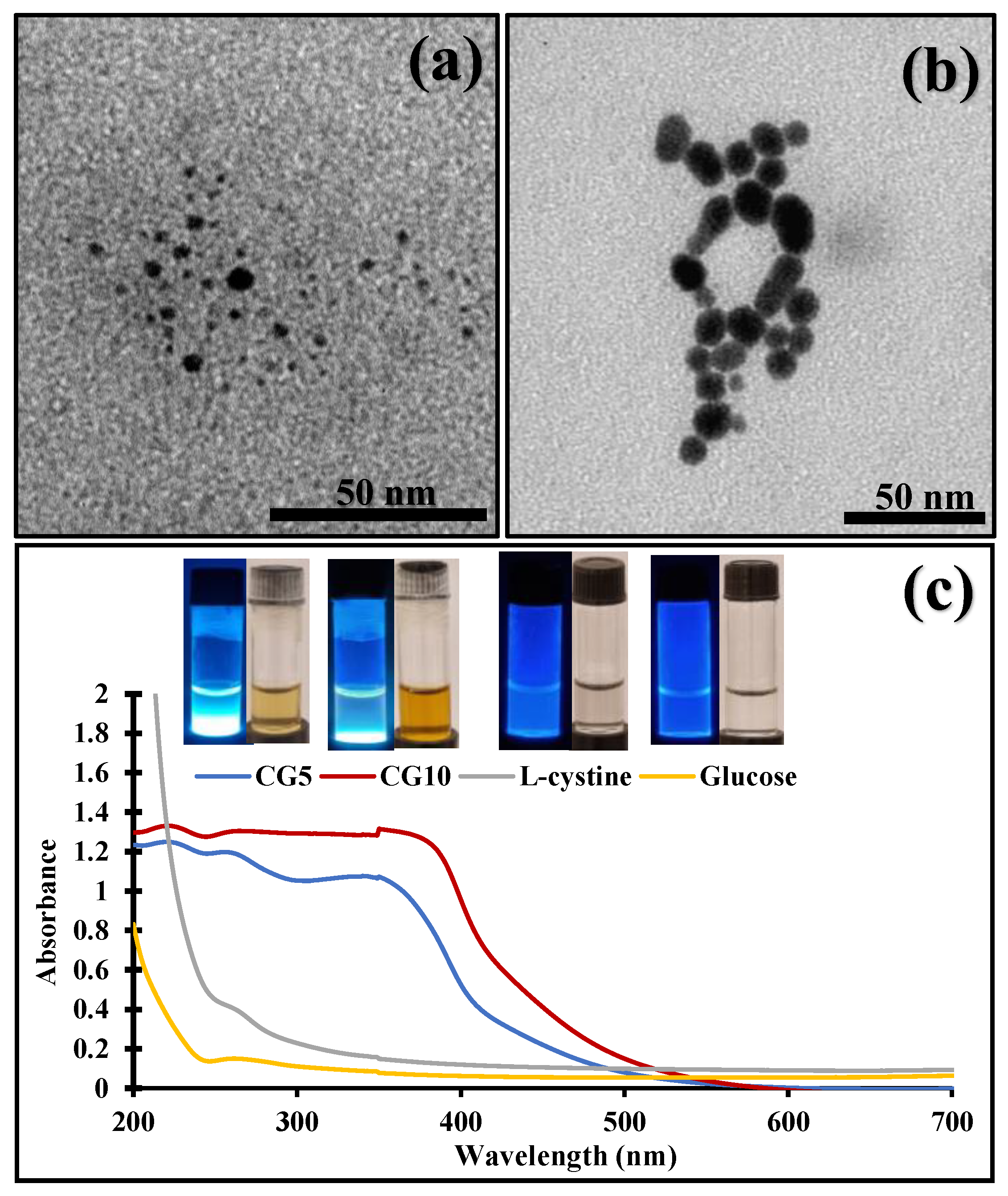
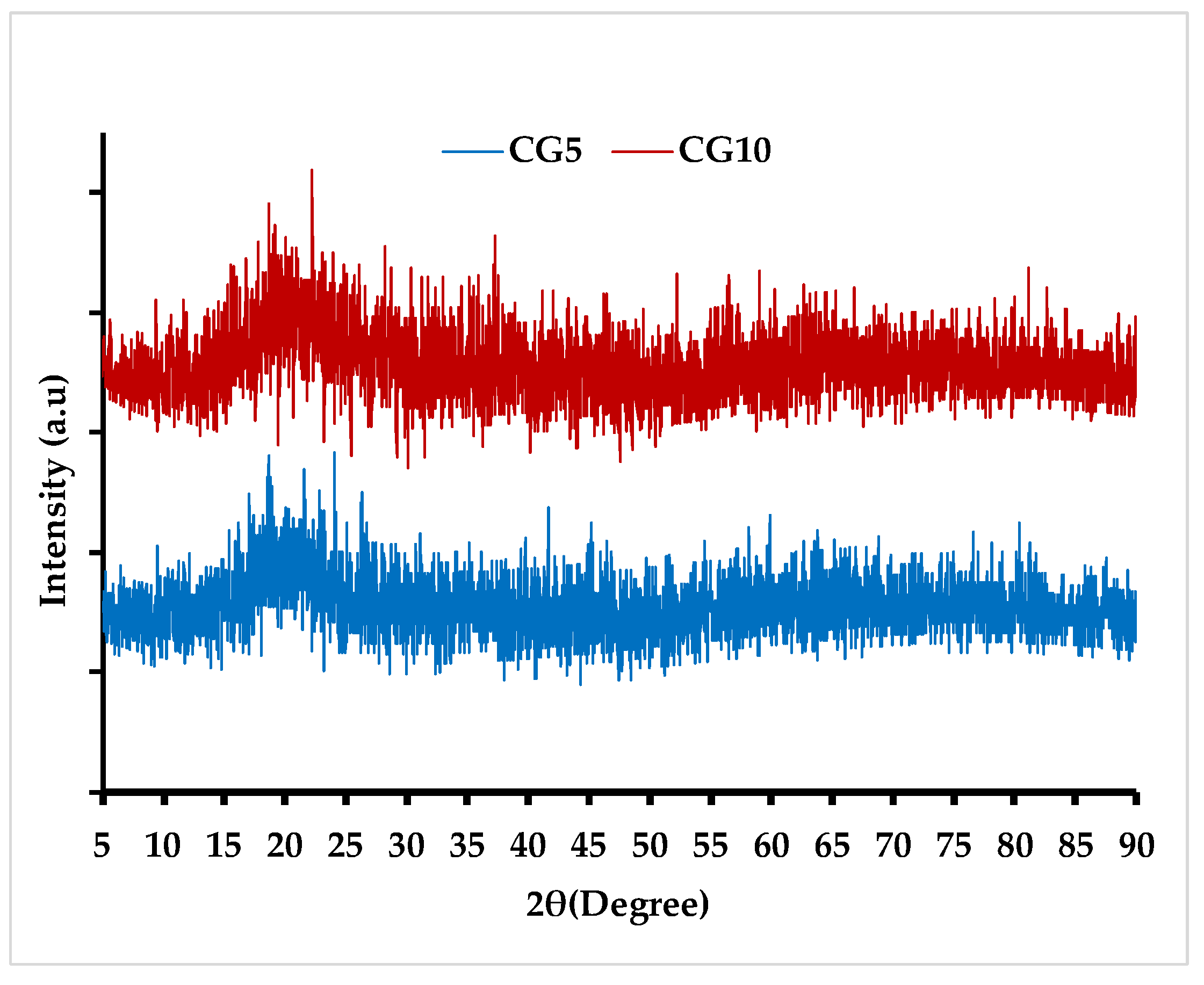
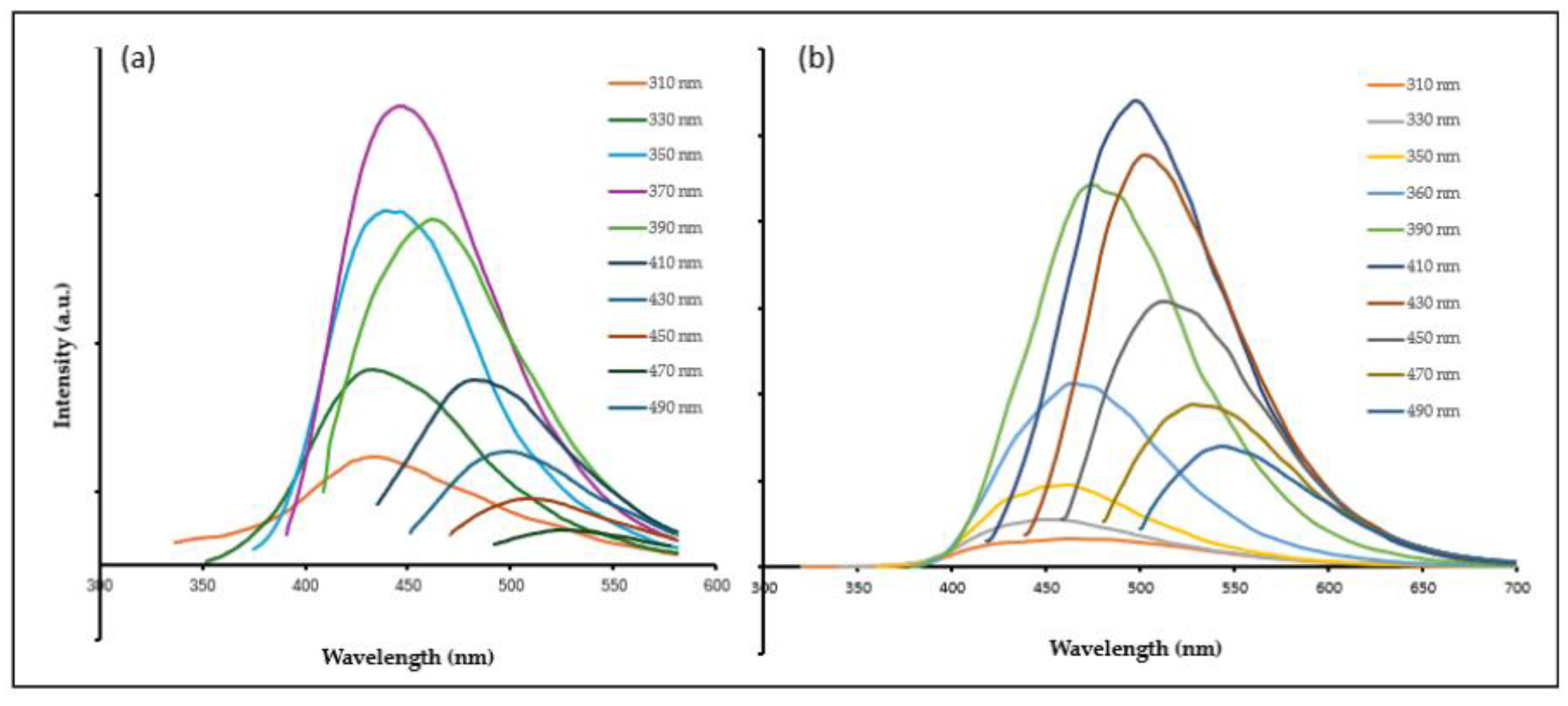
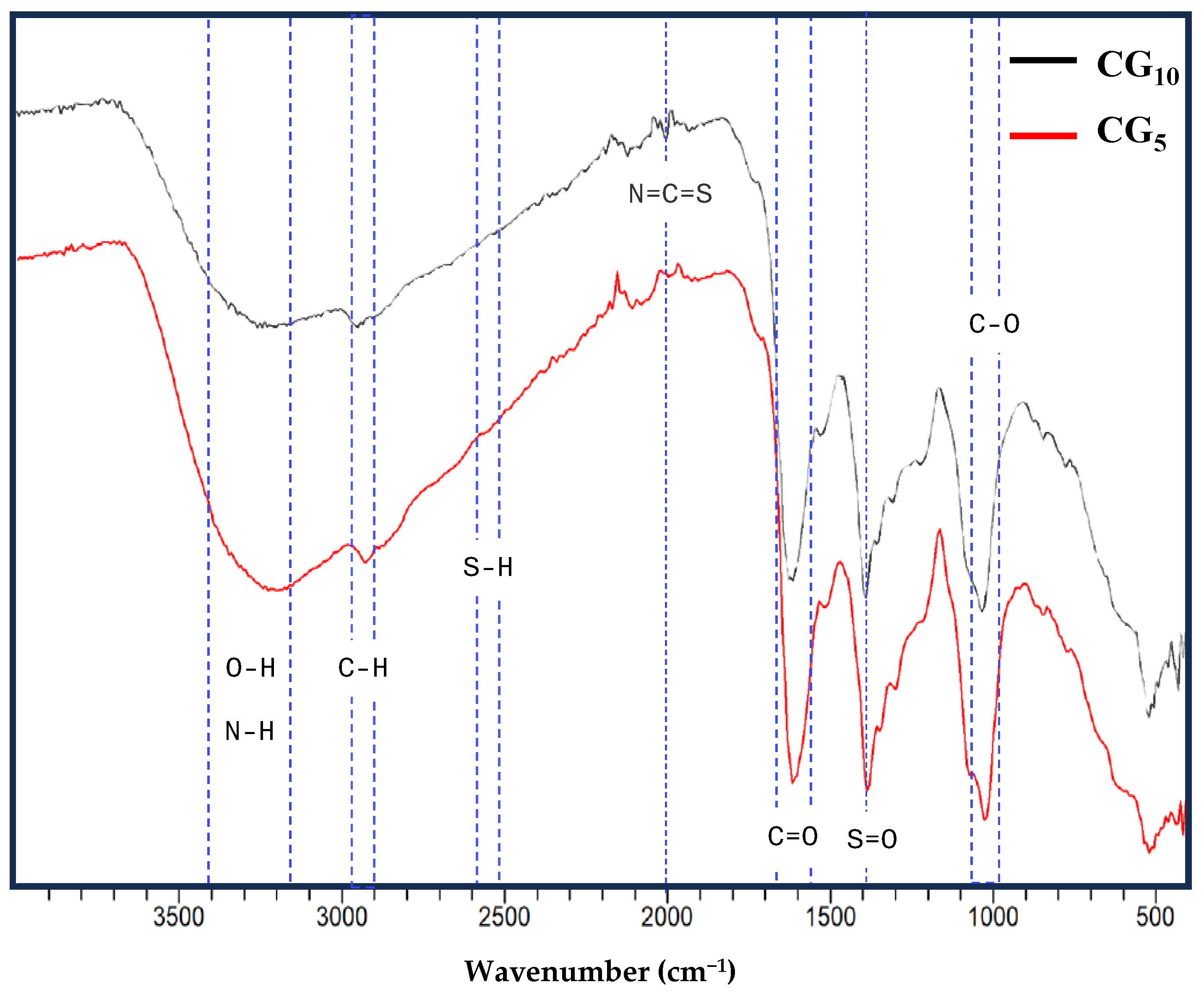
2.1. Synthesis and Physiochemical Characteristics
2.2. Radical Scavenging Ability
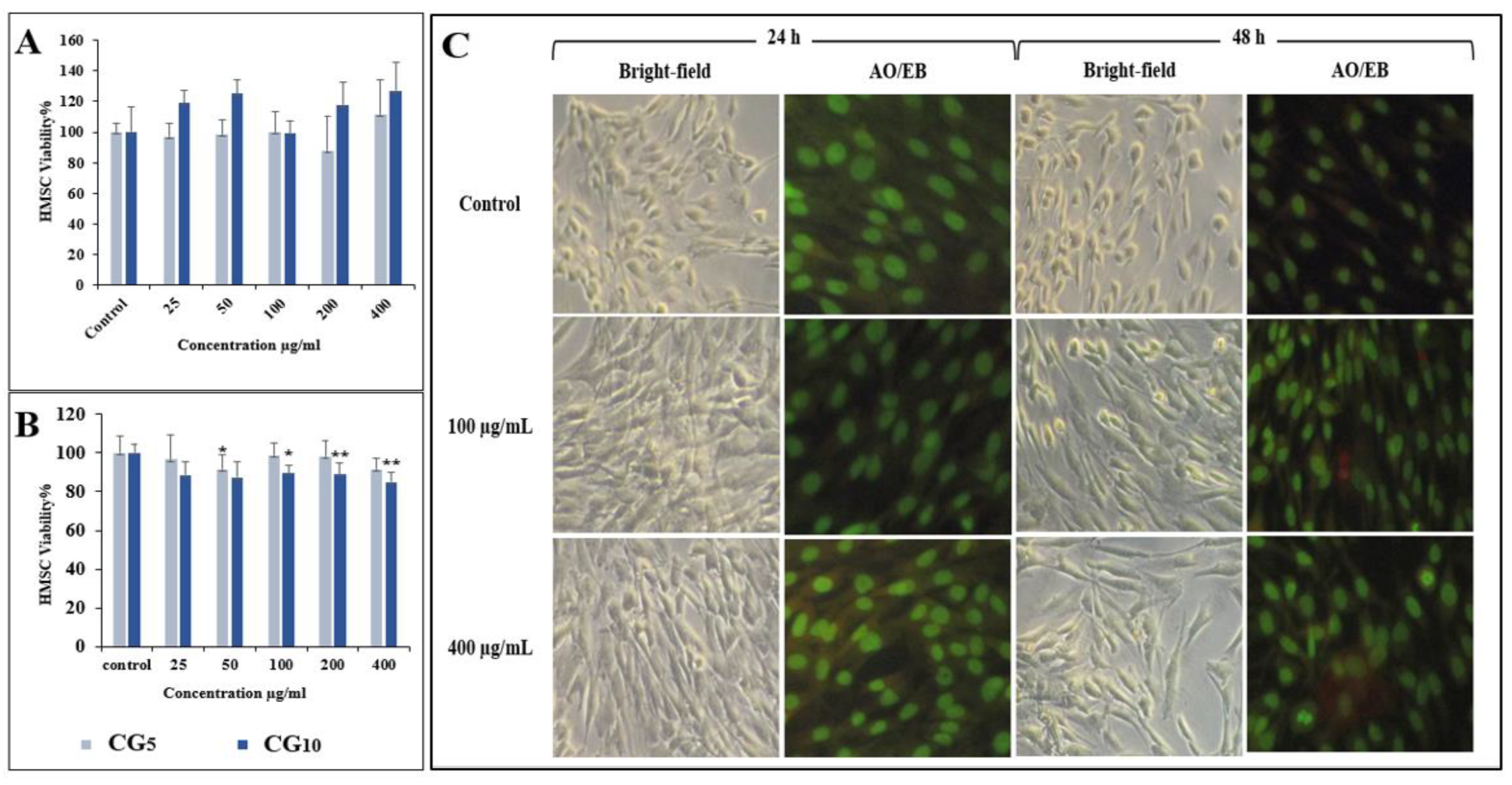
2.3. Cytotoxicity Assessment
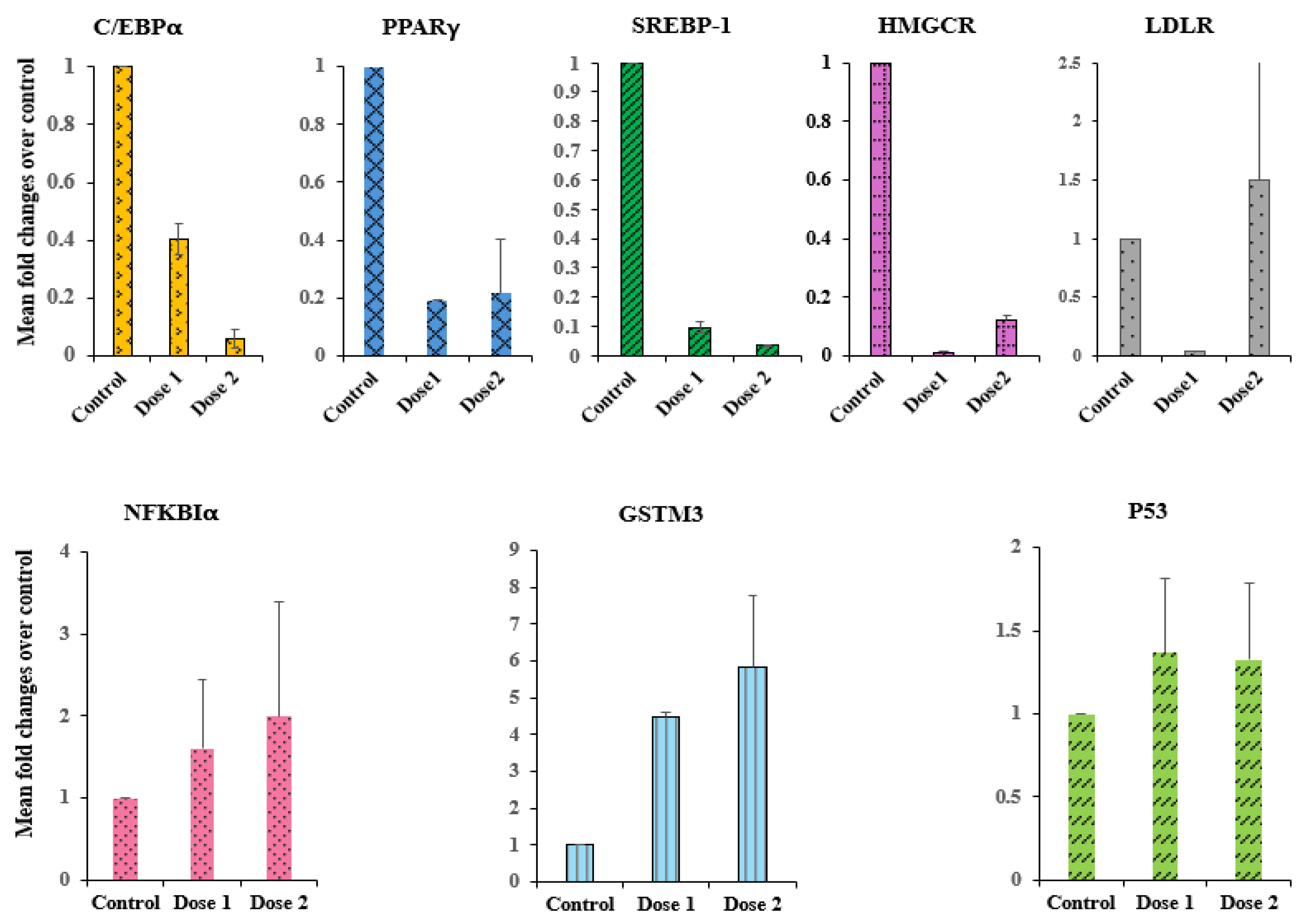
2.4. Gene Expression
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CDs via Maillard Reaction
3.3. Characterization of CDs
3.4. Free Radical Scavenging Activity
3.5. Cell Culture
3.6. Cell Viability Assay
3.7. Acridine Orange/Ethidium Bromide Staining
3.8. Assessment of Mitochondrial Membrane Potential (MMP)
3.9. LysoRed Staining
3.10. Hoechst Staining
3.11. ROS Assay
3.12. Gene Expression Analysis
3.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sk, M.P.; Jaiswal, A.; Paul, A.; Ghosh, S.S.; Chattopadhyay, A. Presence of amorphous carbon nanoparticles in food caramels. Sci. Rep. 2012, 2, 383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Na, X.; Lai, B.; Song, Y.; Wang, H.; Tan, M. Effects of fluorescent carbon dots from the baked lamb on energy and lipid metabolism. Food Chem. 2020, 338, 127832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shan, S.; Li, J.; Cao, L.; Lv, J.; Tan, M. Assessment of potential toxicity of foodborne fluorescent nanoparticles from roasted pork. Nanotoxicology 2019, 13, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, Y.; Wang, H.; Liu, S.; Song, L.; Li, S.; Tan, M. Carbon quantum dots from roasted Atlantic salmon (Salmo salar L.): Formation, biodistribution and cytotoxicity. Food Chem. 2019, 293, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Li, Y.; Wang, H.; Song, Y.; Cong, S.; Li, D.; Zhou, D.; Zhu, B.-W.; Tan, M. Physicochemical properties and cytotoxicity of carbon dots in grilled fish. New J. Chem. 2017, 41, 8490–8496. [Google Scholar] [CrossRef]
- Cong, S.; Wang, N.; Wang, K.; Wu, Y.; Li, D.; Song, Y.; Prakash, S.; Tan, M. Fluorescent nanoparticles in the popular pizza: Properties, biodistribution and cytotoxicity. Food Funct. 2019, 10, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, Y.; Na, X.; Bi, J.; Liu, S.; Zhang, L.; Tan, M. Fluorescent carbon dots in baked lamb: Formation, cytotoxicity and scavenging capability to free radicals. Food Chem. 2019, 286, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadi, A.M.; Periasamy, V.S.; Athinarayanan, J.; Al-Khalifa, A.S.; Alshatwi, A.A. Extraction of ultrafine carbon nanoparticles from samooli Bread and evaluation of their in vitro cytotoxicity in human mesenchymal stem cells. Process. Biochem. 2017, 52, 250–258. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, H.; Song, X.; Ma, X.; Wang, J.; Tan, M. Presence of photoluminescent carbon dots in Nescafe® original instant coffee: Applications to bioimaging. Talanta 2014, 127, 68–74. [Google Scholar] [CrossRef]
- Li, S.; Jiang, C.; Wang, H.; Cong, S.; Tan, M. Fluorescent nanoparticles present in Coca-Cola and Pepsi-Cola: Physiochemical properties, cytotoxicity, biodistribution and digestion studies. Nanotoxicology 2017, 12, 49–62. [Google Scholar] [CrossRef]
- Yang, L.; Ma, J.; Yang, B. Fluorescent Carbon Dots Derived from Soy Sauce for Picric Acid Detection and Cell Imaging. J. Fluoresc. 2023, 33, 1981–1993. [Google Scholar] [CrossRef]
- Cao, L.; Song, X.; Song, Y.; Bi, J.; Cong, S.; Yu, C.; Tan, M. Fluorescent nanoparticles from mature vinegar: Their properties and interaction with dopamine. Food Funct. 2017, 8, 4744–4751. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Y.; Zhang, X.; Liu, J.; Gong, P.; Su, Z.; Fan, L.; Li, G. Emerging Nanoparticles in Food: Sources, Application, and Safety. J. Agric. Food Chem. 2023, 71, 3564–3582. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Song, Y.; Tan, M. Toxicity Alleviation of Carbon Dots from Roast Beef after the Formation of Protein Coronas with Human Serum Albumin. J. Agric. Food Chem. 2020, 68, 9789–9795. [Google Scholar] [CrossRef]
- Wang, H.; Su, W.; Tan, M. Endogenous Fluorescence Carbon Dots Derived from Food Items. Innov. 2020, 1, 100009. [Google Scholar] [CrossRef]
- Neve, B.P.; Fruchart, J.-C.; Staels, B. Role of the Peroxisome Proliferator-Activated Receptors (PPAR) in Atherosclerosis. Biochem. Pharmacol. 2000, 60, 1245–1250. [Google Scholar] [CrossRef]
- Li, D.; Na, X.; Wang, H.; Xie, Y.; Cong, S.; Song, Y.; Xu, X.; Zhu, B.-W.; Tan, M. Fluorescent Carbon Dots Derived from Maillard Reaction Products: Their Properties, Biodistribution, Cytotoxicity, and Antioxidant Activity. J. Agric. Food Chem. 2018, 66, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.J.; Jiménez-Pérez, S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, J.; Shi, X.; Abdul, Q.; Jiang, Z. Characterization and antioxidant activity of products derived from xylose–bovine casein hydrolysate Maillard reaction: Impact of reaction time. Foods 2019, 8, 242. [Google Scholar] [CrossRef]
- Papaioannou, N.; Titirici, M.-M.; Sapelkin, A. Investigating the Effect of Reaction Time on Carbon Dot Formation, Structure, and Optical Properties. ACS Omega 2019, 4, 21658–21665. [Google Scholar] [CrossRef]
- Baccaro, L.F.; Conde, D.M.; Costa-Paiva, L.; Pinto-Neto, A.M. The epidemiology and management of postmenopausal osteoporosis: A viewpoint from Brazil. Clin. Interv. Aging 2015, 10, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Eskalen, H.; Çeşme, M.; Kerli, S.; Özğan, Ş. Green synthesis of water-soluble fluorescent carbon dots from rosemary leaves: Applications in food storage capacity, fingerprint detection, and antibacterial activity. J. Chem. Res. 2020, 45, 428–435. [Google Scholar] [CrossRef]
- Goryacheva, I.Y.; Sapelkin, A.V.; Sukhorukov, G.B. Carbon nanodots: Mechanisms of photoluminescence and principles of application. Trends Anal. Chem. 2017, 90, 27–37. [Google Scholar] [CrossRef]
- Hayase, F.; Kim, S.B.; Kato, H. Maillard Reaction Products Formed from D-Glucose and Glycine and the Formation Mechanisms of Amides as Major Components. Agric. Biol. Chem. 1985, 49, 2337–2341. [Google Scholar] [CrossRef]
- Billaud, C.; Maraschin, C.; Peyrat-Maillard, M.; Nicolas, J. Maillard reaction products derived from thiol compounds as inhibitors of enzymatic browning of fruits and vegetables: The structure-activity relationship. Ann. N. Y. Acad. Sci. 2005, 1043, 876–885. [Google Scholar] [CrossRef]
- Gu, F.; Kim, J.M.; Hayat, K.; Xia, S.; Feng, B.; Zhang, X. Characteristics and antioxidant activity of ultrafiltrated Maillard reaction products from a casein–glucose model system. Food Chem. 2009, 117, 48–54. [Google Scholar] [CrossRef]
- Lin, Q.; Li, M.; Xiong, L.; Qiu, L.; Bian, X.; Sun, C.; Sun, Q. Characterization and antioxidant activity of short linear glucan–lysine nanoparticles prepared by Maillard reaction. Food Hydrocoll. 2019, 92, 86–93. [Google Scholar] [CrossRef]
- Ruiz, R.; Jideonwo, V.; Ahn, M.; Surendran, S.; Tagliabracci, V.S.; Hou, Y.; Gamble, A.; Kerner, J.; Irimia-Dominguez, J.M.; Puchowicz, M.A.; et al. Sterol regulatory element-binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J. Biol. Chem. 2014, 289, 5510–5517. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, F. Regulation of SREBP-Mediated Gene Expression. Acta Biophys. Sin. 2012, 28, 287–294. [Google Scholar] [CrossRef]
- Sekiya, M.; Hiraishi, A.; Touyama, M.; Sakamoto, K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophys. Res. Commun. 2008, 375, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Soyal, S.M.; Nofziger, C.; Dossena, S.; Paulmichl, M.; Patsch, W. Targeting SREBPs for treatment of the metabolic syndrome. Trends Pharmacol. Sci. 2015, 36, 406–416. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Rieusset, J.; Touri, F.; Michalik, L.; Escher, P.; Desvergne, B.; Niesor, E.; Wahli, W. A new selective peroxisome proliferator-activated receptor γ antagonist with antiobesity and antidiabetic activity. Mol. Endocrinol. 2002, 16, 2628–2644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Majd, N.E.; Tabandeh, M.R.; Shahriari, A.; Soleimani, Z. Okra (Abelmoscus esculentus) Improved Islets Structure, and Down-Regulated PPARs Gene Expression in Pancreas of High-Fat Diet and Streptozotocin-Induced Diabetic Rats. Cell J. 2018, 20, 31–40. [Google Scholar]
- Wang, S.; Yang, J.; You, L.; Dai, M.; Zhao, Y. GSTM3 function and polymorphism in cancer: Emerging but promising. Cancer Manag. Res. 2020, 12, 10377–10388. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alatiah, K.A.; Alshatwi, A.A. Synthesis and cytocompatibility analysis of carbon nanodots derived from palmyra palm leaf for multicolor imaging applications. Sustain. Chem. Pharm. 2020, 18, 100334. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habelreeh, H.H.; Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Maillard Reaction-Derived S-Doped Carbon Dots Promotes Downregulation of PPARγ, C/EBPα, and SREBP-1 Genes In-Vitro. Molecules 2024, 29, 2008. https://doi.org/10.3390/molecules29092008
Habelreeh HH, Athinarayanan J, Periasamy VS, Alshatwi AA. Maillard Reaction-Derived S-Doped Carbon Dots Promotes Downregulation of PPARγ, C/EBPα, and SREBP-1 Genes In-Vitro. Molecules. 2024; 29(9):2008. https://doi.org/10.3390/molecules29092008
Chicago/Turabian StyleHabelreeh, Hanaa Hisham, Jegan Athinarayanan, Vaiyapuri Subbarayan Periasamy, and Ali A. Alshatwi. 2024. "Maillard Reaction-Derived S-Doped Carbon Dots Promotes Downregulation of PPARγ, C/EBPα, and SREBP-1 Genes In-Vitro" Molecules 29, no. 9: 2008. https://doi.org/10.3390/molecules29092008
APA StyleHabelreeh, H. H., Athinarayanan, J., Periasamy, V. S., & Alshatwi, A. A. (2024). Maillard Reaction-Derived S-Doped Carbon Dots Promotes Downregulation of PPARγ, C/EBPα, and SREBP-1 Genes In-Vitro. Molecules, 29(9), 2008. https://doi.org/10.3390/molecules29092008






