Abstract
This paper explores and revisits in detail the formation and characterization of sugar-based aminonitriles, whose ultimate origin can be traced to the interaction of biomolecules with cyanide. Although the synthesis and spectroscopic data of 2-amino-aldononitriles were reported long ago, there are both contradictory and confusing results among the published data. We have now addressed this concern through an exhaustive structural elucidation of acylated 2-amino- and 2-alkyl(aryl)amino-2-deoxyaldonitriles using mass spectrometry and FT-IR, FT–Raman, and NMR spectroscopies. Several structures could be unambiguously determined through single-crystal X-ray diffraction, which allowed us to correct other misassignments. Moreover, this study unveils how steric and electronic effects influence the acylation outcome of the amino, (alkyl, aryl)amino, or acetamido group at C-2. The chirality at the latter, which was assigned tentatively through optical rotation correlation, and hence the preferential threo stereochemistry generated during the cyanohydrin synthesis of 2-amino-2-deoxy aldononitriles have now been established with confidence.
1. Introduction
α-Aminonitriles are important intermediates in the synthesis of α-amino acids, α-aminoamides, thiadiazoles, and imidazole derivatives [1]. The classical Strecker synthesis and Strecker-type reactions represent the most general methods of preparing α-aminonitriles [2,3,4,5], in which an aldehyde or ketone is reacted with an amine and hydrocyanic acid or a chemical equivalent, such as potassium cyanide in acid media or trimethylsilyl cyanide [6,7,8,9,10,11,12,13]. In a prebiotic context, α-aminonitriles can also be formed via electric discharge through a mixture of anhydrous methane and ammonia and hydrolyzed to amino acids, thereby presenting a credible hypothesis within the repertoire of chemical evolution [14,15]. Recent studies on prebiotic chemistry point to a cyanosulfidic scenario and disclosing a chemoselective, high-yielding α-aminonitrile ligation that leads to α-peptides under aqueous conditions [16]. α-Aminonitriles can be acetylated in water by thioacetate anions and this acylation activates the nitrile group of the newly formed α-acetamidonitrile just enough to promote a nucleophilic attack by H2S, not by water. The resulting thioacid then links to the α-aminonitriles to afford the corresponding peptides. Accordingly, short N-acyl peptide nitriles would have been plausible substrates during early evolution by interconnecting prebiotic α-aminonitrile synthesis with the formation of biological α-peptides.
On the other hand, peracylated aldononitriles (1) have been extensively studied with gas chromatography coupled to mass spectrometry [17,18,19,20,21,22,23,24,25,26] and used in various quantitative analyses of sugar and aminosugar mixtures [27,28,29,30,31,32,33,34,35,36,37,38]. Derivatization usually involves a base-catalyzed acetylation of the corresponding aldose oxime with acetic anhydride. The use of such derivatives provides some advantages over other sugar derivatives, mainly because it was believed that a single compound preserving the overall stereochemistry of the starting sugar is produced. However, the formation of significant amounts of peracetylated acyclic and cyclic oximes, both in furanoid and pyranoid structures, has raised questions regarding the quality of the method (Scheme 1) [39].
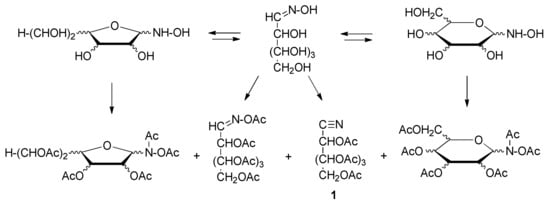
Scheme 1.
Landscape of acyclic and cyclic structures generated from aldoximes under acetylating conditions.
Despite the structural uncertainty, however, the procedure proves to be advantageous in biological analyses. Thus, for example, whole-cell hydrolysates of Bacteroides fragilis, of the genus Bacteroides Castellani and Chalmers 1919, and closely related genetical species B. vulgatus, B. ovatus, B. eggerthii, B. distasonis, B. uniformis, B. thetaiotaomicron, B. stercoris, B. merdae, and B. caccae were employed to determine, on the basis of chemical derivatization, characteristic carbohydrate patterns using capillary gas chromatography. Seven typical peaks for peracetylated aldononitriles could be selected from carbohydrate fingerprints of the reference strains to prepare a dichotomous identification key. Therefore, the classification of an unknown strain of Bacteroides fragilis group can be accomplished and requires only 4–5 h after primary isolation. As a result, other sophisticated techniques under anaerobic conditions can be avoided [40].
Also, the dehydration of per-O-acetylaldonamides leads to per-O-acetylaldononitriles [41], just like the radical fragmentation of sugar-derived β-hydroxy azides [42]. Conformational studies aided by molecular mechanics were conducted on peracylated aldononitriles, with the ultimate goal of performing a full stereochemistry elucidation of the aldose moiety starting from NMR spectroscopy [43,44,45]. In contrast, few data are available for 2-amino-2-desoxyaldononitriles (2) and their acyl derivatives (3). More recently, mass spectrometry of amino sugar aldononitrile acetate derivatives was used for isotope enrichment assessment of microbial residues in soil [35]. 2-Amino-2-deoxyaldononitriles and their N-alkyl and N-aryl derivatives are intermediates in the aminonitrile synthesis of 2-amino and 2-alkylamino-2-deoxyaldoses [46,47,48,49,50,51,52,53,54,55,56], and they are generally obtained by reacting an aldose with excess of ammonia or an amine in anhydrous methanol/ethanol with subsequent addition of anhydrous hydrogen cyanide. However, unexpected by-products like aminoamides 4 and iminolactones 5 are sometimes obtained (Scheme 2) [57,58,59,60].
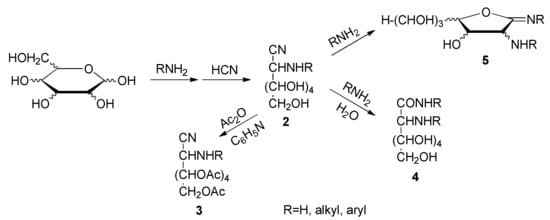
Scheme 2.
Compound set generated in the cyanohydrin synthesis and late derivatization of aminonitriles.
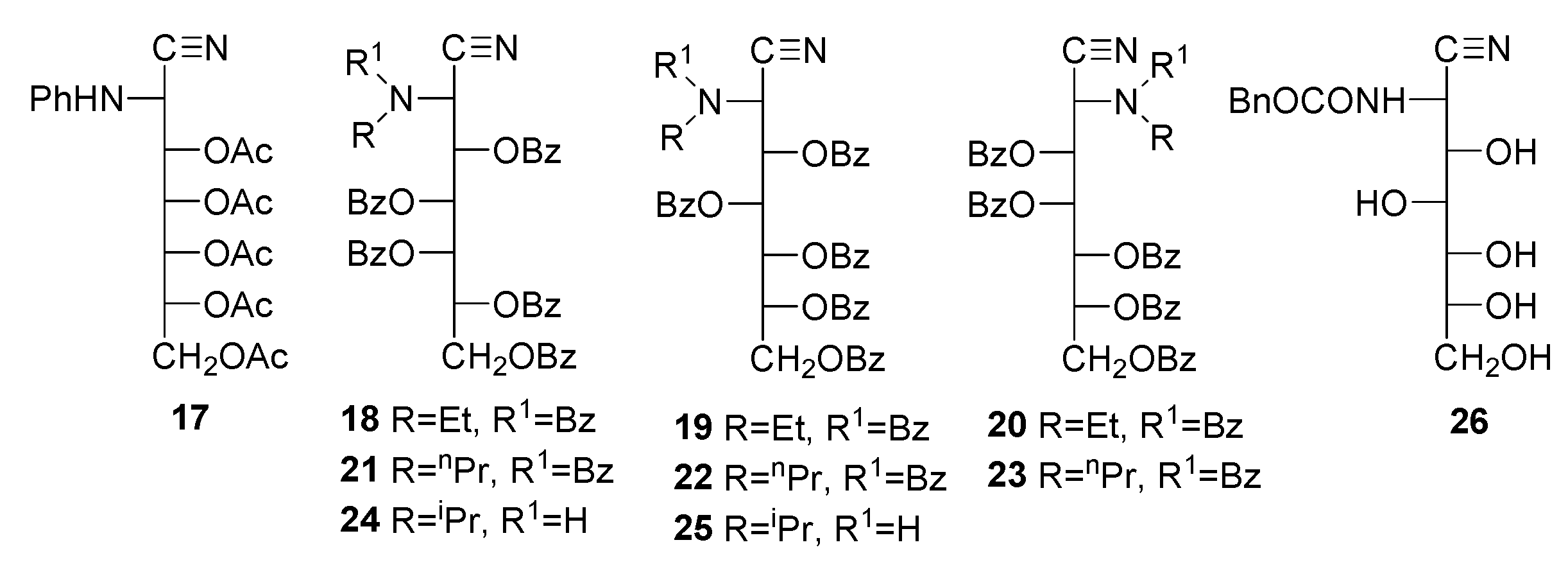
Peracyl-2-amino-2-deoxyaldononitriles can be generated through the acylation of 2-amino-2-deoxyaldononitriles [49,50,51,52,53,54,55,56] and oximes of 2-amino-2-deoxyaldosas [61]. Such substances have been used for characterizing 2-amino-2-deoxyaldoses and their N-alkyl and N-aryl derivatives, as well as synthons in the preparation of 1,2-diamino-1,2-dideoxyalditols [62], which are additional starting materials for the synthesis of polyhydroxyalkyl heterocycles. The penta-acetyl derivatives of 2-deoxy-2-methylamino-L-glucononitrile (6) and its l-mano epimer (7) were obtained through conventional acetylation with acetic anhydride and pyridine [63,64]. Similar routes have been applied to the enantiomeric pair of penta-acetyl derivatives 8 and 9 and tetra-acetate 10 [65,66]. Likewise, a wide variety of acetyl (8, 11–17) and benzoyl derivatives (18–25) have been reported, including the isolation of carbobenzoxyl aminonitrile 26 without hydroxyl protection [52].
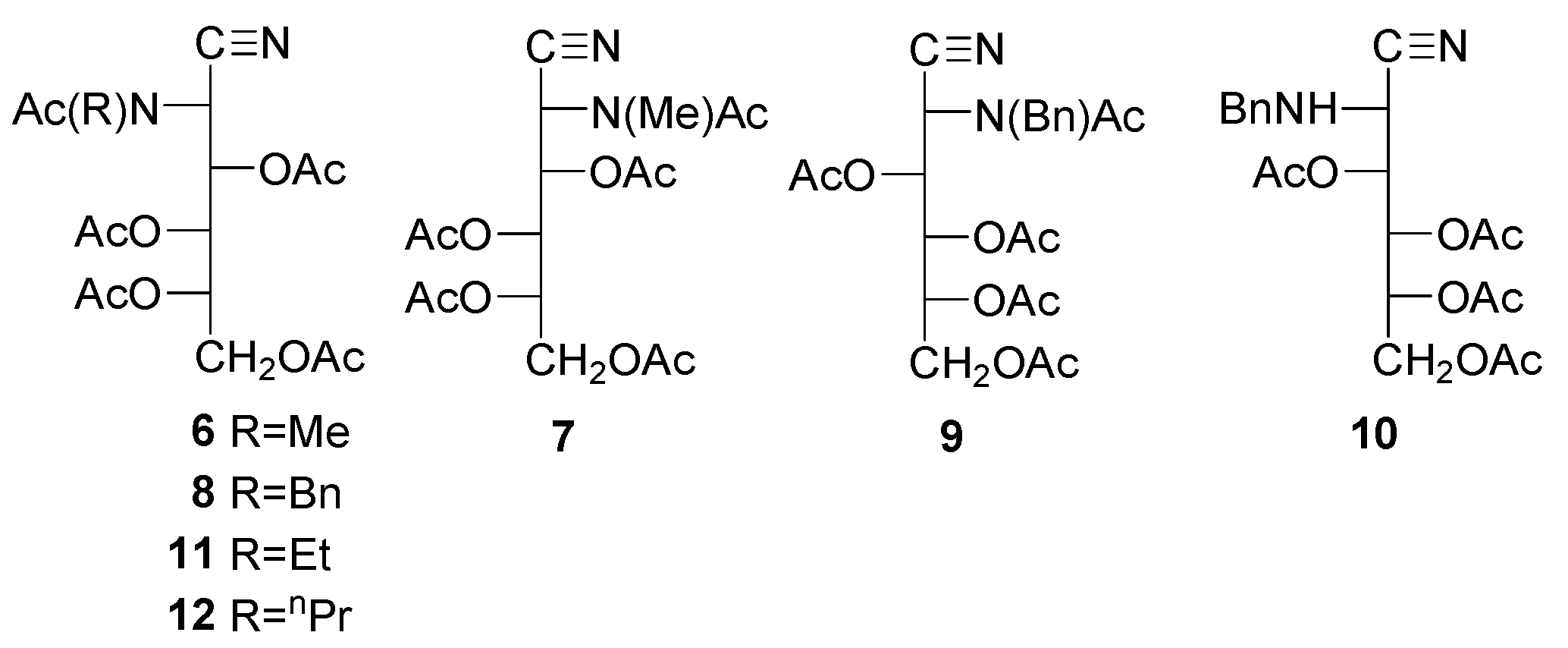
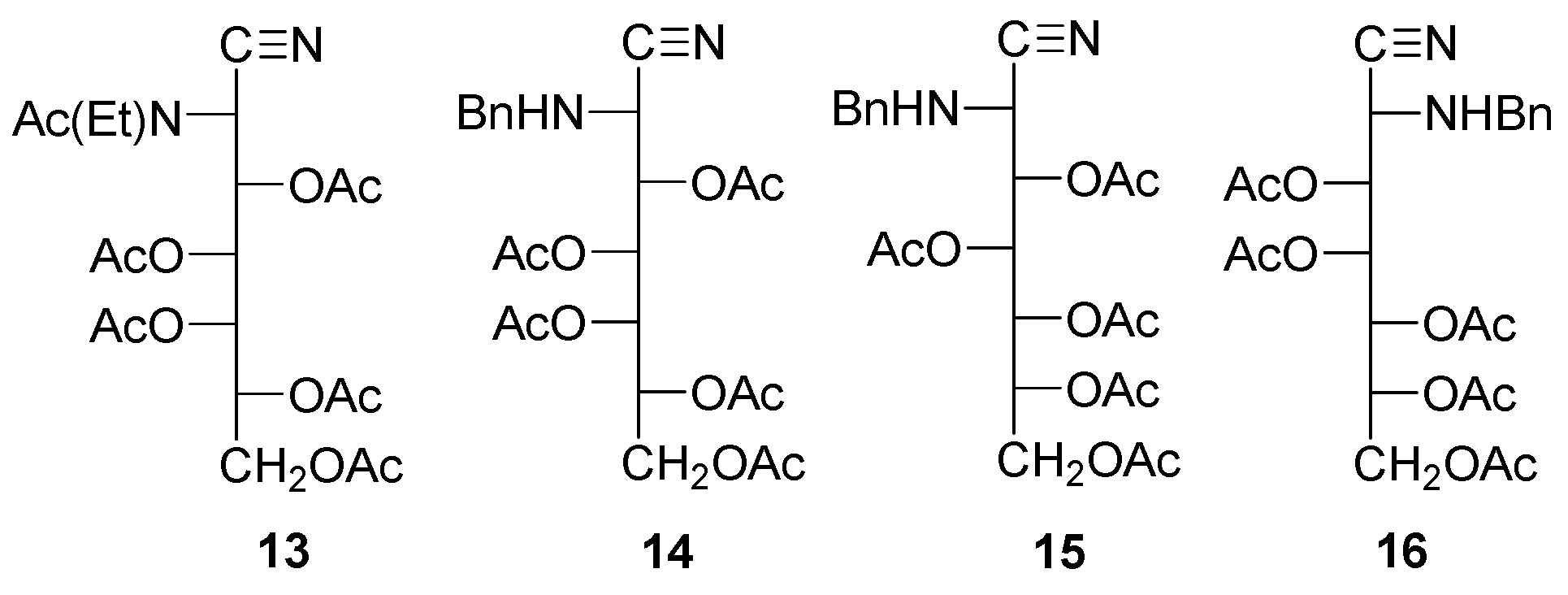
In general, however, spectral characterization is poor. Furthermore, data in the literature often indicate contradictory results. Thus, it has been reported that 2-amino-2-desoxialdononitriles bearing N-ethyl or N-propyl groups are acylated at the nitrogen atom and do not suffer acylation if the group is N-isopropyl or N-phenyl. For N-benzyl derivatives, the group can sometimes be acetylated (8,9) [65,66] or cannot (10, 14–16) [49,66].
In order to clarify these and other structural issues, the present work focuses on a series of representative sugar aminonitriles along with their acetyl and benzoyl derivatives. An extensive and compelling structural analysis has been performed, where single-crystal X-ray diffraction proved to be instrumental for determining the acetylation site and the stereochemical outcome.
2. Results and Discussion
2.1. Synthesis of Acylated Derivatives
The preparation of aminonitriles 27–36 was performed as described previously [49,50,51,52,53,54,55,56] using the classical cyanohydrin synthesis, i.e., the addition of liquid hydrogen cyanide to a glycosylamine formed through reaction of the corresponding monosaccharide with an excess of ammonia, alkyl, or aryl amines. In general, isolation of the glycosylamine derivative, rather than the one-pot protocol, leads to a clean and high-yielding synthesis. As mentioned above, this strategy avoids the formation of by-products like aminoamide 4 or iminolactone 5 [57]. The problem is associated primarily with the use of alkyl amines, while cleaner reactions occur when using aniline, probably due to its lower nucleophilicity [56].
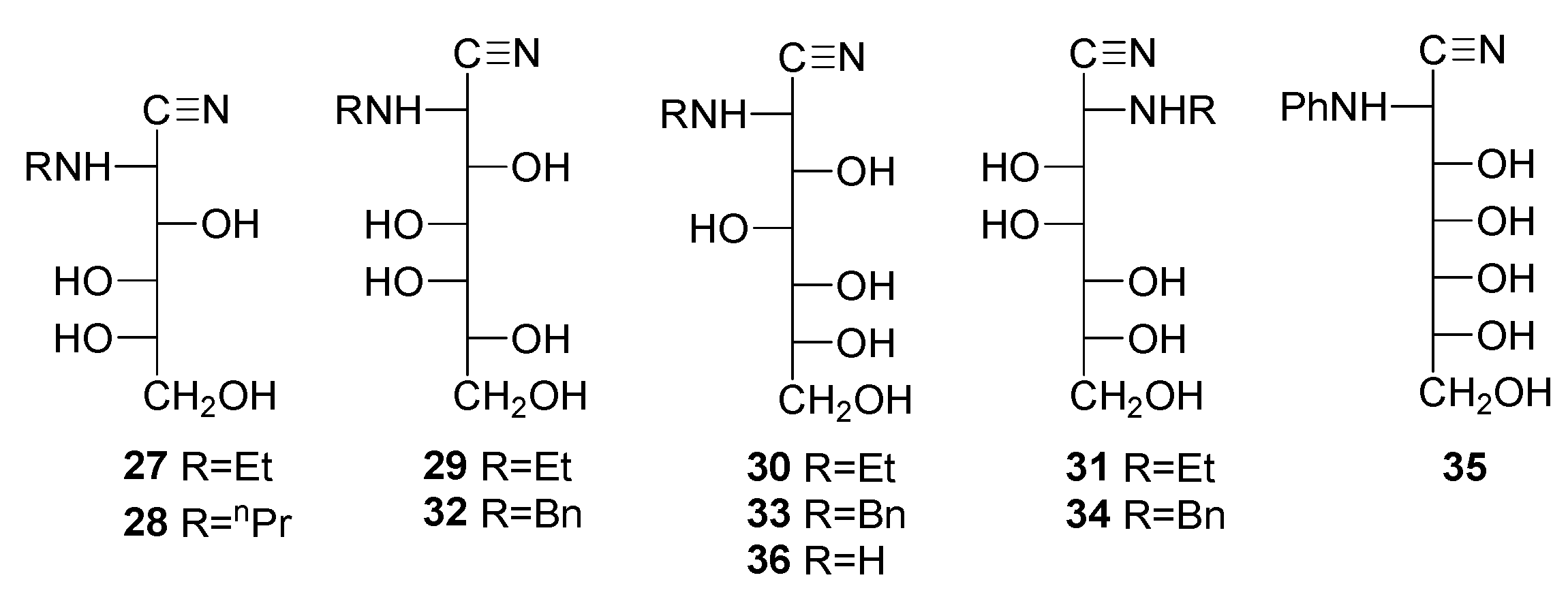
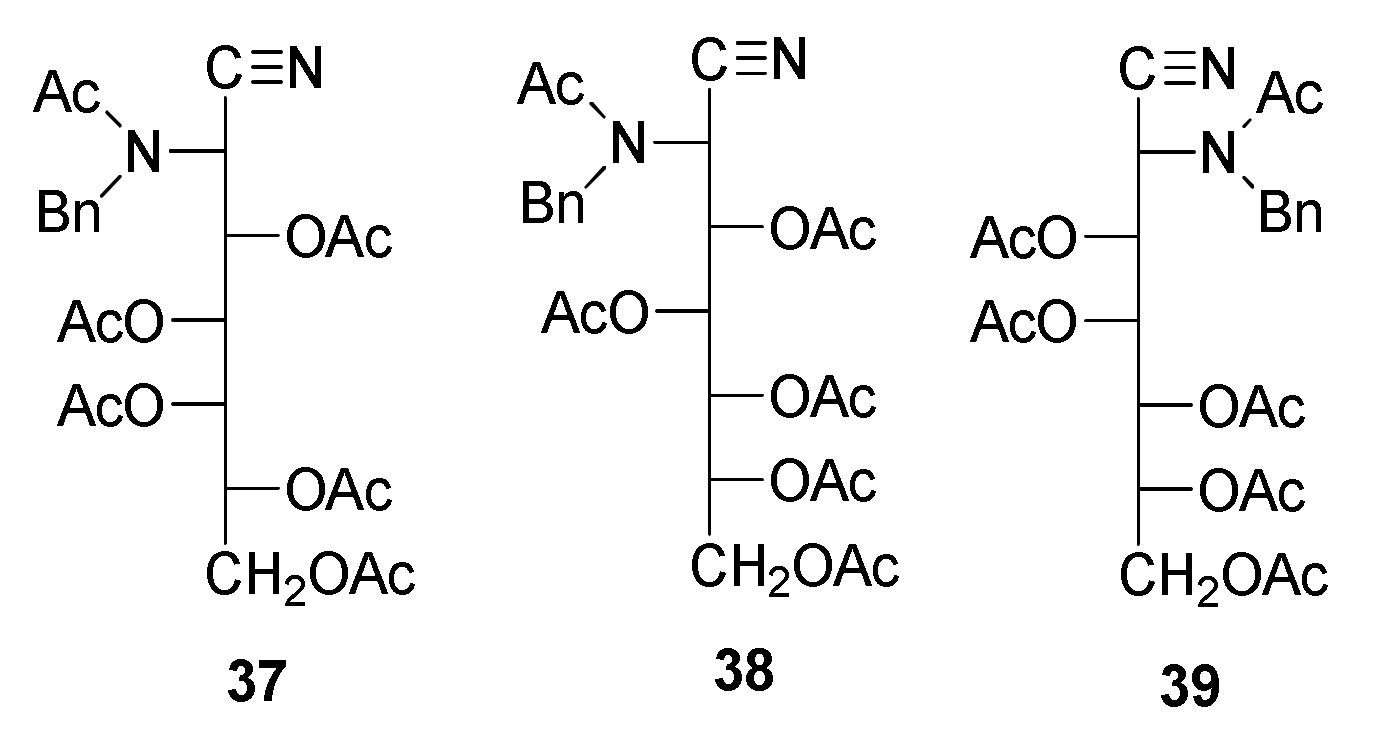
Acylation of the resulting aldonoaminonitriles was achieved using standard methods, namely the addition of acetic anhydride or benzoyl chloride to a solution/suspension of the aminonitrile in pyridine. Thus, peracetyl derivatives 11–13 could easily be obtained from 27–29, and perbenzoylated 18–20 from 29–31, respectively [49,50,51,52,53,54,55,56]. In contrast, the acetylation of 32 and 33 did not afford the described penta-acetyl derivatives 14 and 15 [49], and peracetyl 2-benzylamino-2-deoxyheptononitriles having d-glycero-l-gluco 37 and d-glycero-d-ido 38 configurations were obtained instead. Accordingly, the structure of the acetylated 2-benzylamino-2-deoxy-d-glycero-d-galacto-heptononitrile should be consistent with 39 rather than 16. The same reasoning applies to tetraacetate 10, which should be a penta-acetylated derivative.
Additionally, in contrast, it has been reported that acetylation of 2-deoxy-2-phenylamino-d-allo-heptononitrile (35) produces the penta-O-acetyl derivative 17. In order to confirm this assessment, we have prepared 2-deoxy-2-phenylamino-d-glucononitrile (41) through cyanohydrin synthesis from d-arabinose (40) and aniline (Scheme 3). Its conventional acetylation leads to tetraacetate 42 in a quantitative yield.
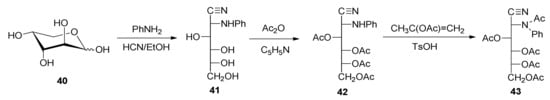
Scheme 3.
Cyanohydrin synthesis from d-arabinose and aniline followed by acetylation reactions.
However, the phenylamino group could be acetylated by heating 42 at reflux in isopropenyl acetate and using p-toluenesulfonic acid as catalyst, thereby obtaining 3,4,5,6-tetra-O-acetyl-2-(N-phenylacetamido)-2-deoxy-d-glucononitrile (43) in a high yield (82%). As a model compound, tetra-O-acetyl-2-acetamido-2-deoxy-d-glucononitrile (47) was also prepared through the dehydration under acetylating conditions of 2-amino-2-deoxy-d-glucose oxime (45), using a modification of the Restelli-Deulofeu method [61] (Scheme 4).
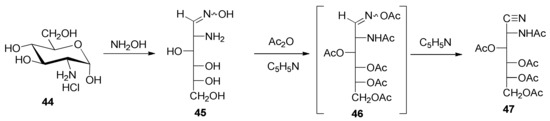
Scheme 4.
Synthesis of aminonitrile model 47 from oxime 45.
Analogously, penta-O-acetyl-2-acetamido-2-deoxy-d-glycero-l-gluco-heptononitrile (51) was generated through an oxime (49), which derives from 2-amino-2-deoxy-α-d-glycero-l-gluco-heptopyranose hydrochloride (48) [67] as a starting material (Scheme 5). It is worth pointing out that the amide character of the nitrogen atom at C-2 in both 47 and 51 prevents further acylation, at least under the conditions tested.
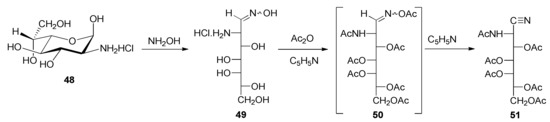
Scheme 5.
Synthetic strategy for producing peracetylated aminonitrile 51.
2.2. Structural Elucidation
All of the proposed structures are in agreement with their elemental analyses and spectral data, including FT-IR, FT–Raman, 1H and 13C NMR, and high-resolution mass spectra (HRMS), as well as X-ray diffractometry.
The oft-invoked diagnostic IR absorption of the cyano group has little value due to the extremely weak or even absent C≡N stretching vibrations [68]. It is well established that substitution of the alkyl group in alkyl nitriles by oxygen-containing functional groups results in a decrease in the intensity of the C≡N stretching band. Moreover, this effect can be enhanced further by increasing the number of such substituents and by attaching them to the carbon atom that bears the C≡N group [69,70]. In agreement with these observations, O-protected cyano carbohydrate derivatives do not generally exhibit the typical C≡N stretching vibration or it is extremely weak [68,71,72,73,74,75,76,77,78,79]. Also, α-acetamidoalkyl nitriles, such as N-(cyanomethyl)acetamide or ethyl acetamidocyanacetate, show weak or negligible C≡N stretching absorption bands [70]. Unlike IR spectroscopy, Raman spectra of substituted sugar cyanides exhibit strong C≡N stretching bands; however, this technique has not yet been applied to aldonoaminonitriles and their derivatives [80].
Since IR spectra of peracetylated α-aminonitriles do not provide clear-cut indications of the C≡N group in 2-amino-aldonitriles, its presence can be inferred from Raman (ṽC≡N ~2220 cm−1) or 13C NMR spectra (δC≡N ~115 ppm). Thus, for example, the signal at ~119 ppm demonstrates unequivocally the survival of the cyano group during the synthesis of 26. Tables S1–S3 collect a series of diagnostic signals, either stretching absorption bands or chemical shifts, for all of the compounds studied.
2.3. Peracetyl Alkylaminonitriles
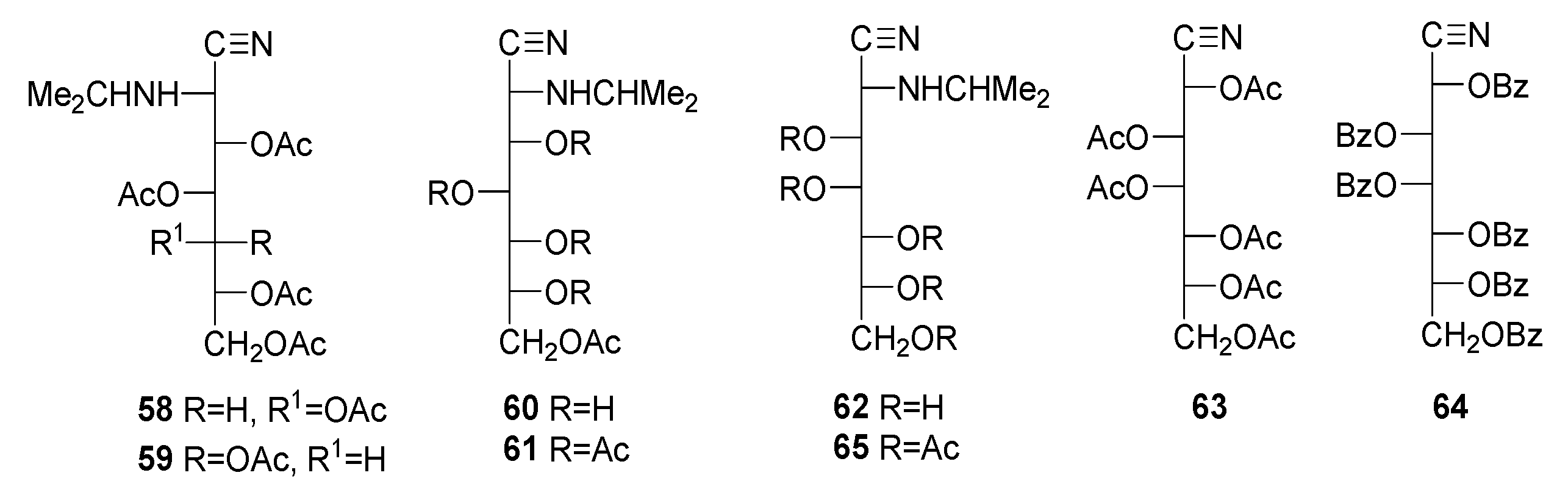
The determination of the molecular masses of 11 [52] and 13 [50] was performed by recording their HRMS (CI mode), which showed [M + H]+ and [M + NH4]+ peaks. Values of m/z = 415.1706 for 11 (C18H26N2O9, MW 414.1638) and m/z = 487.1904 (C21H30N2O11, MW 486.1850) for 13 were obtained. The spectroscopic data of compounds 11 and 13 are closely similar. Their IR spectra show typical absorptions of acetate and tertiary amide at ~1750 cm−1 and ~1650 cm−1, respectively [69]. The absorption bands of the C≡N group are very weak, but they are moderately intense in the corresponding Raman spectra. Signal integration and carbon counting in the 1H and 13C NMR spectra show the presence of five acetyl groups for 11 and 12, and six for 13 (four or five acetate groups and one acetamido group, respectively). The presence of a signal at ~115 ppm shows the presence of the C≡N group [81,82]. Finally, the structure of 13 could conclusively be demonstrated using single-crystal X-ray diffraction (Figure 1).
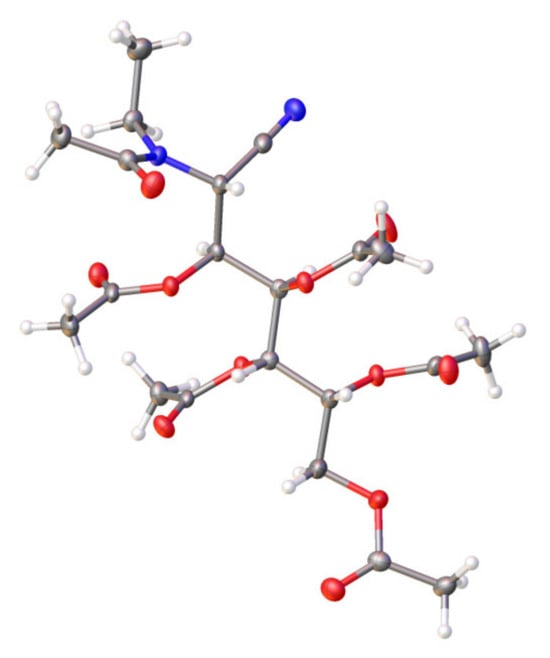
Figure 1.
X-ray diffraction structure of 13 recorded at 120 K. Thermal ellipsoids are drawn at 35% probability.
Along with the unequivocal constitution of the acylated aminonitriles, a second objective of this study involved confirming the stereochemistry at C-2 assigned to the parent aminonitriles. In previous work, the configuration at C-2 was tentatively based on the sign of optical rotations, in agreement with empirical rules for other acyclic compounds, such as monosaccharide nitriles, amides, hydrazides, and (alditol-1-yl)-heterocycles, although some exceptions have been found [65,78,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. Such rules establish a direct correlation between the sign and the absolute configuration at C-2, being positive for d-configured and negative for l-configured aminonitriles. Data from the literature evidence the general trend of such a correlation for sugar 2-aminonitriles [49,50,51,52,53,54,55,56,58,65,66,89,90,91,92,93,94,95,96,97,98]. The structure determined with X-ray diffraction for 13 shows that the stereochemistry at C-2 is R (Figure 1), thus confirming the stereochemistry at C-2 of the starting aminonitrile 29, whose configuration d-glycero-l-gluco has previously been assigned [50].
On the other hand, the stereochemistry of the amide and acetate groups in the solid state is Z-anti. In Table 1, some experimental parameters (crystal data) for the unit cell of 13 are collected and compared with those calculated in a vacuum and in chloroform (for theoretical calculations, see the next section). They have also been calculated in the solid state using the experimental data extracted from the crystallographic analyses.

Table 1.
Experimental and calculated geometrical parameters of 13.
The spectroscopic data of 12, bearing an N-n-propyl group, are similar to those of 11 and 13, and therefore consistent with acylation at the nitrogen atom. However, when the alkyl group is isopropyl, the nitrogen atom is not acylated (24, 25), probably due to steric hindrance [50]. This effect manifests itself even in the synthesis of N-isopropyl aminonitriles. Thus, the treatment of a solution of isopropylamine and d-galactose or d-glucose (or a solution of the previously isolated N-isopropyl glycosylamine, 52 or 53) with anhydrous hydrogen cyanide leads to the corresponding nitriles (54, 55) (Scheme 6) [50]. Their conventional benzoylation leads to 24 and 25, as previously described [50], whereas the corresponding acetylation produces the penta-O-acetyl heptononitriles 58 and 59. Because of the fact that the aminonitrile 55 obtained was impurified by its epimer (24%) with a d-glycero-d-gulo configuration (60), compound 59 was likewise obtained accompanied by its epimer 61 (21%). Although they do not show the characteristic absorption of the C≡N group in their IR spectra, the nitrile functionality agrees with the signal at ~118 ppm in the 13C NMR spectra.
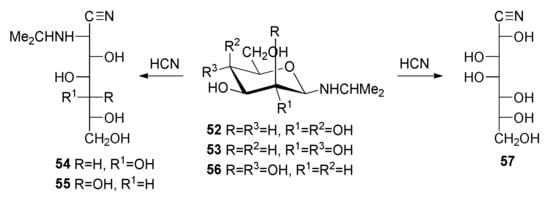
Scheme 6.
Cyanohydrin reactions of glycosylamines 52, 53, and 56.
The absence of acetylation at the nitrogen atom of 58 and 59 is supported by (a) the mass spectra showing the signal [M + H]+ at m/z 459 (C20H30N2O10) and not at m/z 501 (C22H32N2O11); (b) the presence of a weak signal at 3330 cm−1, corresponding to the NH stretching vibration, together with the absence of the amide band at ~1640 cm−1; (c) the proton and carbon signals in the NMR spectra indicating the presence of only five acetyl groups; and (d) the upfield chemical shift of H-2 with respect to the analogous proton of 13 (Δδ ~ 1.4 ppm).
However, when using isopropylamine and d-mannose as the starting aldoses, instead of aminonitrile 62, d-glycero-d-galacto-heptononitrile (57) was obtained in a low yield (17%). The latter increased up to 45% when starting from the previously isolated N-isopropyl-β-d-mannopyranosylamine (56). The resulting nitrile 57 shows the C≡N bond stretching absorption at ~2220 cm−1. Acetylation and benzoylation reactions afforded the corresponding peracetyl (63) and perbenzoyl (64) derivatives, neither showing a characteristic band that can be ascribed to the stretching vibration of the NH bond at ~3300 cm−1 or the first amide band at ~1650 cm−1, irrespective of spectra recorded in the solid state or chloroform solution.
The mass spectrum of the acetyl derivative shows a base peak at m/z 482.13, which could correspond to either the [M + H + Na]+ of 63 or [M + Na]+ of 65. In addition, 1H and 13C NMR spectra show the absence of the isopropyl group, and the proton count indicates the presence of six acetate groups. The number of carbon atoms attributable to the alkyl chain is seven, including the cyano group. Accordingly, nitrile functionality arises from the addition of HCN to d-mannose, leading to 57, whereas the O-protected acyl derivatives are 63 and 64, respectively. Such products have also been reported in the literature [99], and the stereochemistry at C-2 is consistent with the positive value of their optical rotations [49,50,51,52,53,54,55,56,65,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. Finally, the structures assigned to nitrile 57 and its acyl derivatives were supported by the unambiguous structure of 63 determined with X-ray diffraction. This result indicates that the configuration of the new chiral center at C-2 is R and confirms the stereochemistry results previously assigned to 57, 63, and 64. It is noteworthy that the conformation of the molecule is such that it can easily rotate 180° and still fit into the same average packing arrangement and a very similar volume of space (see Supplementary Materials). In the X-ray structure determination, this is seen as a disordered average structure that was modeled with the nitrile group partially occupied at either end of the chain. Figure 2 clearly shows the residual electron density peaks originating from the minor orientation of the molecule. The occupancy of the nitrile was refined to a 30/70 split (and fixed in the final cycles at this value)—meaning that 30% of the time, the molecule packs in the opposite orientation. It was not necessary to employ any geometrical or thermal parameter restraints.
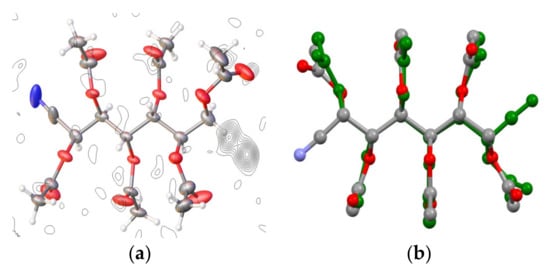
Figure 2.
(a) Single-crystal X-ray diffraction of 63 at 100 K. Thermal ellipsoids are drawn at 50% probability. The residual electron density map clearly shows the existence of a disordered nitrile position. (b) Overlay of a rotated molecule (green) with the original, showing how they fit into a very similar volume of space.
2.4. Peracetyl Benzylaminonitriles
Remarkably, the structures assigned to 14–16 and to the acetyl derivatives of 32–34 [49] cannot be distinguished from 37–39 through their elemental analyses, being essentially almost identical (C24H30N2O10:C, 56.90; H, 5.97; N, 5.53 versus C26H32N2O11:C, 56.93; H, 5.88; N, 5.11). This is probably one of the reasons accounting for the erroneous assignment of structures 14–16.
The steric, electronic, and conformational parameters [100,101,102,103] of different substituents carried by the nitrogen atom at C-2 are collected in Table 2. The A parameter for substituent X is given by Ax= −ΔG˚x, where ΔG˚x measures the variation of the free energy in the axial ⇄ equatorial equilibrium for cyclohexane carrying the X substituent. On the basis of such parameters, no clear-cut reason justifies the absence of acetylation at the nitrogen atom in compounds 32–34. The steric parameters for the benzyl group are similar to those of the n-propyl group, and there should be no steric hindrance to nitrogen acetylation. Moreover, the value of σ* does not explain this behavior, because even though the benzyl group is electron-withdrawing, σ* = 0.22 is lower than that of hydrogen (σ* = 0.49), which enables acetylation.

Table 2.
Steric, electronic, and conformational parameters for substituents at the nitrogen atom a.
To distinguish between the two types of structures, we performed a determination of the molecular mass of 37 (548.2006 for C26H32N2O11) using HRMS (CI mode). Peaks of [M + H]+ (m/z 549.2069) and [M + NH4]+ (m/z 566.2330) were detected, thus confirming the presence of the acetamido group. In the FT-IR spectra of 37 and 38, two carbonyl signals are observed for the acetate and amide groups at ~1750 cm−1 and ~1670 cm−1, respectively. The absorption of the C≡N group is almost nonexistent and even the Raman absorption, appearing at ~2250 cm−1, is weak. The 1H and 13C-NMR spectra confirm the amide structure of 37 due to, on one hand, the absence of a signal attributable to the NH group, and on the other, the integration of the acetamido group, together with signals showing the presence of five acetate groups. The methylene protons of the benzyl group are diastereotopic and appear as doublets, with a chemical shift difference of 0.23 ppm.
The structure of 37 determined with X-ray diffraction at 120 K is shown in Figure 3, and confirms definitively the acetylation at the nitrogen atom. In addition, the configuration at C-2 is R, which confirms in turn the stereochemistry at C-2 of the starting aminonitrile 32, an assignment made based on the negative sign of optical rotation [49]. The stereochemistry of the amide group is Z-syn-anti (see Scheme 6): that is, in the conformation adopted in the unit cell, the C=O amide group is not arranged parallel to the C2-H bond, as it is in the case of 13, but instead adopts an antiparallel arrangement.
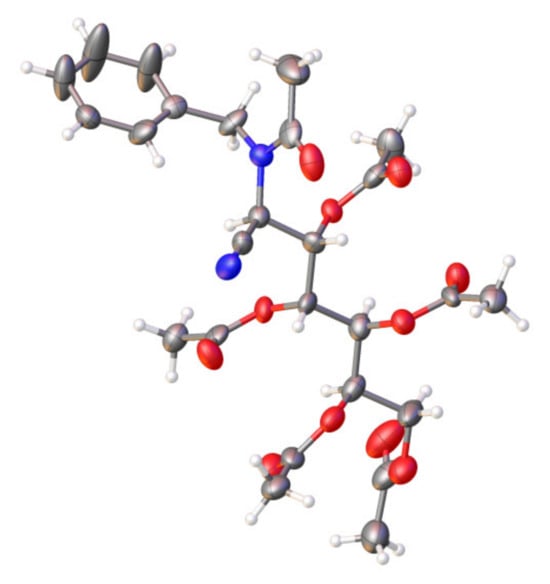
Figure 3.
X-ray diffraction structure of 37 at 120 K. Thermal ellipsoids are drawn at 35% probability.
Table 3 shows some experimental and calculated geometric parameters of 37, both in a vacuum and in chloroform, as well as in the solid state using the experimental data from the single-crystal X-ray analyses.

Table 3.
Experimental and calculated geometrical parameters of 37.
The 1H NMR spectrum of 38 is more complex than that of 37, and shows a duplicity of signals (Figure 4). This duplicity does not result from an epimeric nitrile at C-2, having a d-glycero-l-manno configuration, since its synthesis took place from pure 33. Initially, we thought of a mixture of two acetylated nitriles, i.e., both acylated and not acylated at the nitrogen atom. However, after successive trials and conducting the acetylation procedure at different temperatures or in the presence of sodium acetate or using 4-dimethylaminopyridine, the latter being known as an efficient nucleophilic catalyst in the acetylation of sterically hindered positions [104,105,106], no changes occurred. In addition, chromatographic analysis showed a single compound. We reasoned that the signal duplication should most likely be ascribed to a mixture of rotamers around the amide bond. Overall, the spectral patterns are similar to those observed for 37, nevertheless.
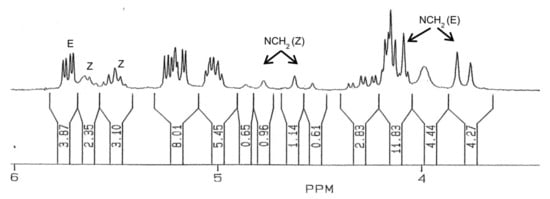
Figure 4.
1H NMR spectrum (magnified zone between ca. 4 and 6 ppm) of compound 38.
Scheme 7 (R = Me, R1 = Ph) shows the possible rotamers for the benzyl and tertiary acetamido groups [107], along with a visual description of the φ, ϕ, and ψ angles for conformational analyses. The amide-group stereochemistry of 37 in the solid state (Figure 3) is Z-syn-anti (φ = 0, ϕ = 0, ψ = 180), but the chemical shift of H-2 indicates that the most stable rotamer in the solution must be the Z-anti-syn (φ = 0, ϕ = 180, ψ = 0) conformer [107], since this is the disposition that generates less steric hindrance, being the only one actually detected in both the 1H and 13C NMR spectra.
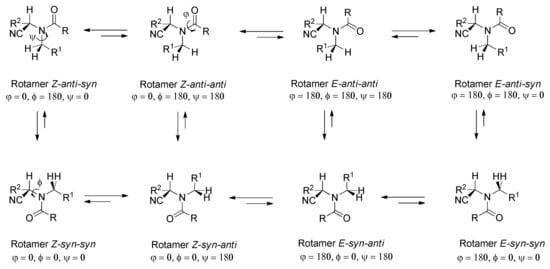
Scheme 7.
E/Z rotamers around N-alkyl and acyl amido moieties.
In stark contrast, compound 38 in the solution not only shows signals similar to those presented for 37, identifiable with the Z-anti-syn conformation (38Z, Table 3), but also another signal set emerges, which must correspond to the E-syn conformer (38E). In this conformation, the H-2 proton undergoes a strong shielding with respect to the chemical shift shown in the Z-anti-syn conformation (ΔδH-2 = δH-2Z − δH-2E ~ 1.6 ppm). This effect stems from two factors: namely the deshielding caused by the amide C=O group disappearing, and the H-2 proton residing in the shielding zone of the aromatic ring of the benzyl group (E-syn-anti). This behavior has already been described in tertiary amides, in which the CH proton adjacent to the amide group in the Z-anti orientation is shifted downfield, by ca. 1.4 ppm, relative to an E conformation [107,108]. Also, the existence of restricted rotation is evident through the signal shape, small and broad, of the C-2 and CH2 signals in the benzyl group of the Z-anti conformer.
2.5. Perbenzoyl Alkylaminonitriles
It is also interesting to ascertain whether an increase in size of the acylating agent, such as in the case of benzoylation, could prevent the acylation at the nitrogen atom in alkyl aminonitriles due to steric effects. Consequently, we have reassessed the structures of 18 and 20, to confirm whether, as described in [49], benzoylation at the nitrogen atom does actually occur.
Again, the determination of molecular mass peaks using HRMS shows that benzoylation has taken place; thus, for example, compound 18 shows [M + H]+ at m/z 859.2833 in agreement with the MW of 858.2789 for C51H42N2O11. The FT-IR spectra of 18 and 20 show benzamido group absorptions at ~1660 cm−1, which, together with the absence of absorptions above 3100 cm−1 (attributable to NH groups), demonstrate unequivocally the benzoylation reaction at the nitrogen atom. The 1H NMR spectrum of 18, recorded at 27 °C in Cl3CD, acetone-d6, or benzene-d5, shows broadened and unresolved signals corresponding to the chain protons, thus indicating restricted rotation around the amide bond. The same is observed when viewing the 1H NMR spectrum of its isomer 20, of a d-glycero-d-galacto configuration. This behavior also appears in the 13C NMR spectra, where the corresponding carbon signals of the ethyl group could not be identified. Improved results were obtained through longer acquisition pulses that allow for greater relaxation of the nuclei, yet poorly resolved signals remained.
1H NMR spectra recorded above ambient temperature lead to sharper signals with improved resolution, which split into two signal sets by lowering the temperature at −50 °C (Figure 5). At this temperature, the minor rotamer in Cl3CD, estimated from signal integration, amounts to 23%.
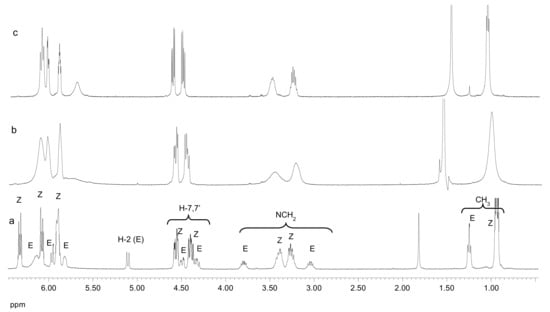
Figure 5.
1H NMR spectra of 18 in Cl3CD: (a) 240 K, (b) 300 K, and (c) 327 K.
The methylene protons of the ethyl group are diastereotopic. Interestingly, the minor rotamer shows a significant difference between the chemical shifts of both protons (0.76 ppm versus 0.11 ppm). This large difference and the appearance of a doublet signal at 5.11 ppm, attributable to H-2, represent diagnostic features used to assign the stereochemistry of each rotamer.
Scheme 7 (R = Ph, R1 = Me) depicts the geometric arrangement of the possible rotamers of 18. The more stable rotamer in the solution shows a Z-anti conformation, as occurs in the solid state for 13. In this disposition, the amide carbonyl lies parallel to the C2-H2 bond, whereas an E-anti-anti stereochemistry (φ = 180, ϕ = 180, ψ = 180) places the C=O parallel to one of the methylene hydrogens of the ethyl group, which causes different chemical shifts for the two methylene protons. At the same time, the aromatic ring is close to the H-2, which results in a strong shielding of ~0.77 ppm and shifts the signal at 5.11 ppm (Figure 6).
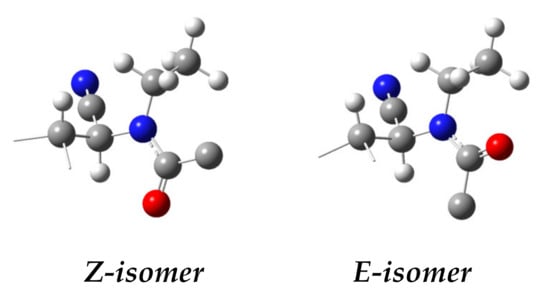
Figure 6.
Ball-and-stick models for the spatial arrangements of the Z and E isomers.
This structural feature has also been highlighted in secondary amides, -CONHCHR-, where the chemical shift of the N-CHR proton located in a Z-anti orientation to the NH proton consistently resonates ~0.8 ppm further downfield than the proton located in a gauche disposition [107], as well as in tertiary amides and amide bond rotamers of 5-acyl-6,7-dihydrothieno [3,2-c]pyridine derivatives [108].
In variable temperature experiments, the benzoylated derivative 20, having a d-glycero-d-galacto configuration, shows apparently a simpler thermal behavior with well-resolved NMR signals. At low temperatures, the appearance of multiple signals corresponding to a minor rotamer is observed, like in the case of 18. At 256 K, a small signal at ~5.35 ppm is detected, which could be assigned to the H-2 proton of that rotamer. However, no splitting is observed for the signals of the methylene protons of the ethyl group (Figure 7).
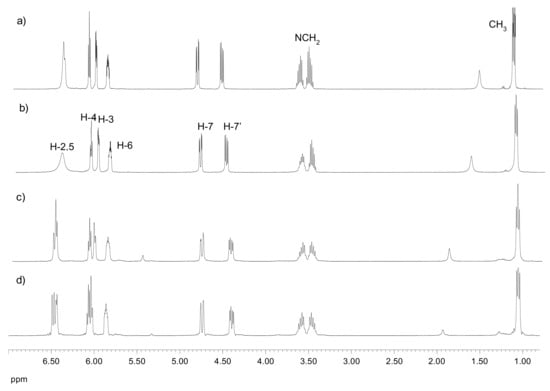
Figure 7.
1H NMR spectra of 20 recorded in Cl3CD: (a) 330 K, (b) 300 K, (c) 256 K, and (d) 235 K.
2.6. Peracetyl Phenylaminonitriles
Both the steric and the electronic parameters of the phenyl group (Table 2) explain why this group inhibits the acetylation at the nitrogen atom and enables the preparation of compounds 17 [56] and 42. To achieve the acetylation of the phenylamino group leading to 43, more drastic conditions must be used.
The presence of a sharp absorption peak at ~3350 cm−1, attributable to the stretching vibration of the NH bond, and the absence of the amide band at ~1660 cm−1, both in 17 and in 42, confirm that the phenylamino group cannot be acetylated under the aforementioned conditions. In contrast, in the spectrum of 43, the absorption of the NH disappears and the tertiary amide carbonyl appears at 1674 cm−1. The negligible absorption of the C≡N bond at 2200 cm−1 is noticeable as well. The absence or presence of acetylation at the nitrogen atom was evident anew through the determination of their molecular weights using mass spectrometry. Thus, 42 showed a [M + H]+ peak at m/z 421.1600, which corresponds to a molecular mass of 420.1533 for C20H24N2O8. However, compound 43 (462.1638 for C22H26N2O9) showed a [M + H]+ peak at m/z 463.1709, with a difference of 42 units with respect to that of 42, clearly corresponding to the presence of the acetyl group at the nitrogen atom.
The 1H NMR spectrum of 42 shows only four acetates and the NH signal resonates as a doublet at 4.59 ppm, which disappears upon exchanging with D2O. The large coupling constant with the H-2 signal (JNH,2 11.0 Hz) indicates that both protons adopt a relative antiperiplanar disposition. For compound 43, however, the NH signal has disappeared and five acetyl groups are observed. The acetylation at the nitrogen atom shifts the H-2 signal from 4.71 ppm to 5.72 ppm. This deshielding of ~1 ppm arises from the Z arrangement adopted by the acetamido group, whose carbonyl is placed parallel to the C2-H bond. In the 13C NMR spectra of 17, 42, and 43, the signals of nitrile functionality are clearly observed at ~115 ppm, whereas for 43, the signal of the methyl carbon of the acetamido group resonates at ~22.55 ppm.
Figure 8 shows the structure of 42, determined with X-ray diffraction at 120 K [83]. The crystal data confirm that there is no acetylation at the phenylamino group, probably due to both the steric effect created by the bulky phenyl group (Es ~ 2.6) and the decrease in nucleophilicity of the nitrogen atom (σ* = 0.60) when delocalizing its lone pair through the aromatic ring (see Table 2). In addition, the (S)-configuration at C-2 unequivocally allows us to assign the same stereochemistry to the C-2 atom of the starting nitrile 41. Table 4 collects the geometric data of 42, both experimental and calculated, which largely match each other.
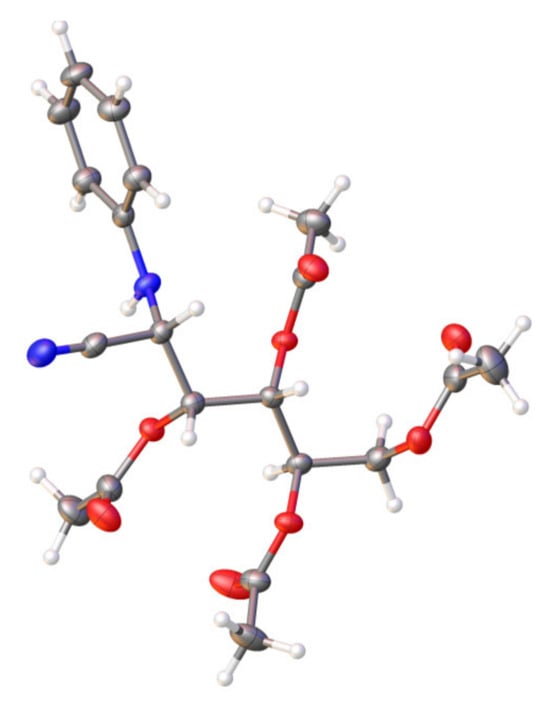
Figure 8.
Single-crystal X-ray structure for 42 at 120 K. Thermal ellipsoids are drawn at 35% probability.

Table 4.
Experimental and calculated geometrical parameters of 42.
2.7. Peracetyl Aminonitriles
Compound 47 shows a [M + H]+ peak at m/z 387.1406, in agreement with the molecular mass of 386.1325 for C16H22N2O9. For compounds 47 and 51, the NH absorption band of the secondary amide group appears at 3237 cm−1, with the first and second amide bands occurring at 1645 and 1541 cm−1, respectively. The stretching vibration of the C≡N group, generated by oxime dehydration, is hardly visible at ~2243 cm−1; however, the band is moderately intense in the Raman spectrum (also at 2243 cm−1). The 1H NMR spectra confirm likewise the structures of 47 and 51, and thus the NH signal appears as a doublet at ~6.7 ppm. In the 13C NMR spectra, the carbon signals of the acetamido group are clearly distinguishable from those of the acetate groups: for example, in the case of 47, such resonances appear at ~172 ppm and at ~22.9 ppm. As expected, the C≡N group resonates at ~116 ppm. Lastly, the unequivocal structure of compound 47 could be established with X-ray diffractometry (Figure 9) and shows the presence of the C≡N and acetamido groups at C-2.
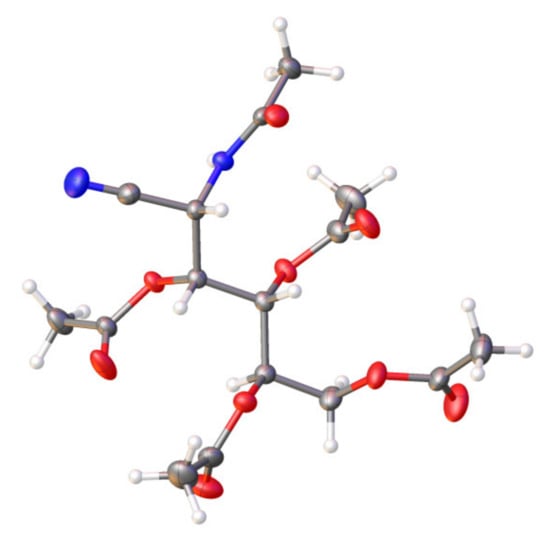
Figure 9.
Single-crystal structure for compound 47 at 120 K. Thermal ellipsoids are drawn at 35% probability.
Obviously, the stereochemistry at C-2 is S, the same configuration as that possessed by the parent oxime of d-glucosamine (45), since it is not affected during the elimination reaction from per-O-acetyl oxime 46, which leads in turn to the formation of the C≡N group (Scheme 4) [109]. On the other hand, the acetamido group adopts a Z-anti arrangement. Table 5 shows some experimental and calculated geometric parameters of 47.

Table 5.
Experimental and calculated geometrical parameters of 47.
2.8. Structural Assessment via Mass Spectrometry
Electron impact mass spectrometry provides valuable structural information on peracyl aminonitriles, which is usually lost in chemical ionization experiments. Accordingly, fragmentation patterns of some compounds were interrogated through EI-MS. In general, the [M+] peak is weak or does not appear at all for the per-O-acetyl-2-alkylacetamido-2-deoxyaldononitrile ion (66), while the second peak, appearing always, corresponds to the loss of acetic acid, [M+ − AcOH] (67), and then the fragmentation into the corresponding α,β-unsaturated 2-N-alkylacetamidonitrile (68), which is usually the basis peak (Scheme 8).
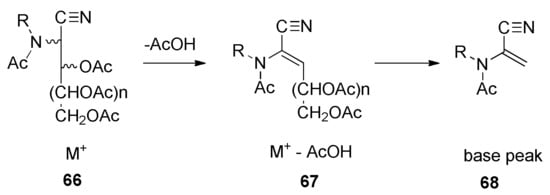
Scheme 8.
Typical EI-MS fragmentation patterns of per-O-acetyl-2-alkylaminonitriles.
When the nitrogen atom is not acylated (such as in 17 and 42), a molecular [M]+ peak is observed and the loss of hydrogen cyanide (m/z 27) usually leads to the base peak (70). For more basic nitrogen atoms (like in 58), a [M + H]+ peak (71) was recorded instead of [M]+. In this case, the base peak is [M + H-HCN]+ (72) (Scheme 9). Furthermore, the successive loss of acetic acid (m/z 60), radical acetate (m/z 59), ketene (m/z 42), and acetic anhydride (m/z 102) can also be typically detected in per-O-acetyl aldononitriles [110].
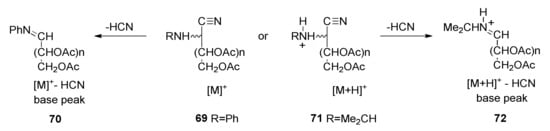
Scheme 9.
EI-MS fragmentation patterns of per-O-acetylated-2-alkylaminonitriles due to the loss of HCN.
2.9. Conformational Analyses
Theoretical analyses have been conducted to determine the most stable conformational dispositions in both the gas phase and in a solution, which have also been compared with those obtained in the solid state. To this end, DFT calculations at the M06-2X/6-311G(d,p) level [111] were employed and we simulated the bulk solvent effects (in CHCl3) with the SMD method [112].
As already mentioned, for the solid-state structures of 13, 37, 42, and 47, all of the acetamido and acetate groups adopt a Z stereochemistry (Scheme 6). Therefore, such a disposition was chosen as the starting point to obtain the computational estimations for the aforementioned compounds. Due to the small size and linear geometry of the C≡N group, these compounds do not adopt a P conformation with the chain fully extended in a zig-zag; they are more inclined towards the sickle conformation 1G+, which is reached through a 120° clockwise turn of C-1 (Scheme 10) [113,114]. In this way, the 1,3-diaxial interaction between the N-ethylacetamido group and the acetate at C-4 is eliminated. Table 6 shows the results obtained when calculating the relative stabilities of the 1G+ and 1G− conformations and the Z/E dispositions of the N-ethylacetamido group (letters A and B refer to different orientations of the substituted nitrogen with respect to the carbon chain, while stereochemical descriptors E and Z refer to the configuration adopted by the amide group).
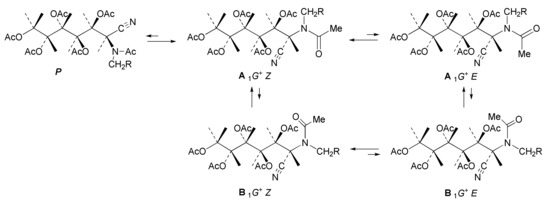
Scheme 10.
Conformational landscape for per-O-acylated aminonitriles.

Table 6.
Relative electronic and Gibbs energies a,b for the conformers of compound 13, along with their coupling constants determined in solution.
Figure 10 shows the calculated structures of the two most stable conformers. The most stable, both in the gas phase and in the presence of a solvent, is A 1G+ Z. The 1G+ chain conformation is supported by the coupling constants measured in the CDCl3 solution (Table 6). The high values of J2,3 and J4.5 (10.0 Hz) indicate an antiperiplanar arrangement between H-2 and H-3, and the same occurs with H-4 and H-5. In contrast, the low values of J3,4 and J5,6 (<2 Hz) confirm a gauche arrangement between such hydrogen atoms.
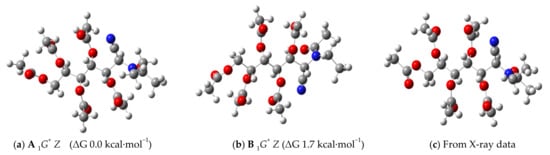
Figure 10.
Most stable conformers in solution (a,b) and in the solid state (c) for aminonitrile 13.
The geometric data of the A 1G+ Z conformation are included in Table 4 and are quite similar to those found in the solid state. For comparative purposes, Figure 10c shows the structure of the conformer determined with X-ray diffraction; it can be seen that the only appreciable difference with the most stable A 1G+ Z conformation is the arrangement adopted by the acetate group at the end of the chain (C-7). The conformational analysis performed for 37 is similar to that discussed for 13 (Scheme 10, R = Ph, Table 7).

Table 7.
Relative electronic and Gibbs energies a,b for the conformers of 37 and their coupling constants in solution.
The data gathered in Table 7 indicate that, like in the case with 13, the most stable conformer in the CDCl3 solution is A 1G+ Z, followed by B 1G+ Z. The former is practically identical with the conformer present in the crystalline unit cell (see the geometric data in Table 6): only the arrangement of the aromatic ring changes (Figure 11).
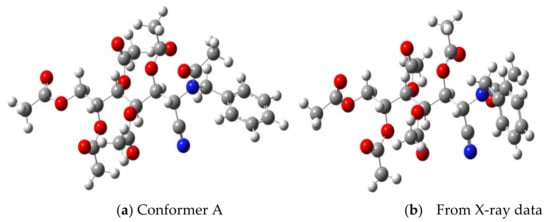
Figure 11.
Structure of conformer A 1G+Z (a) and X-ray structure (b) of 37.
When the conformational study was conducted on aminonitrile 42, similar results were obtained again (Table 8), not surprisingly as the absolute configurations of all carbon atoms (d-gluco chain) are mirror images of the first six stereogenic carbons of 13 and 37 (l-gluco chain).

Table 8.
Relative electronic and Gibbs energies for the conformers of 42 a,b.
The most stable conformer in solution is 1G+5G−, followed by 1G−5G−. Then, the conformer 5G− follows in stability, which is almost coincidental with the arrangement present in the solid-state unit cell (see the geometric data in Table 7 and Figure 12).
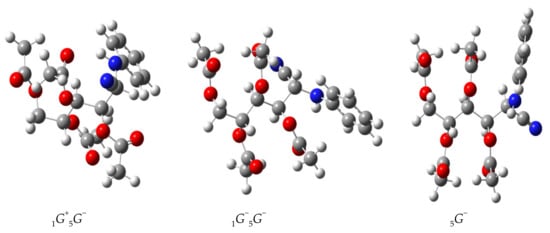
Figure 12.
Calculated structures of the most stable conformers of 42.
Finally, the results obtained in the conformational analysis of 47 agree well with those found for 42, since both share an identical stereochemical pattern (Table 9). Again, the most stable conformer in chloroform is 1G+5G−, while conformers 1G−5G− and 5G− exhibit practically identical stability. The third one represents the conformational disposition obtained in the unit cell (Table 8, Figure 13).

Table 9.
Relative electronic and Gibbs energies for the conformers of 47 a,b.
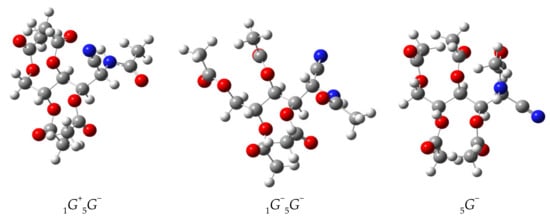
Figure 13.
Calculated structures of the most stable conformers of 47.
The experimental data confirm the conclusions reached through the above calculations. The high values of J2,NH (11.0 Hz) in both 42 and 47 are indicative of an antiperiplanar arrangement between the NH and H-2 atoms. In addition, the intermediate values of J2,3 (~6–7 Hz) being between those expected for anti (10 Hz) and syn (<2 Hz) arrangements evidence that there must be an equilibrium involving the 1G+5G− and 1G−5G− conformers in solution.
3. Conclusions
To summarize, this structural reinvestigation evidences that 2-amino-2-deoxyaldononitriles bearing unbranched alkyl groups at the α-carbon (methyl, ethyl, n-propyl, or benzyl groups) are acylated under standard conditions. However, the presence of an isopropyl group at the nitrogen atom impedes acetylation, most likely owing to a steric hindrance to the reagents being brought together. These steric effects are also disclosed in the addition of anhydrous hydrogen cyanide to N-isopropyl mannosylamine, leading to a nitrile derivative instead of the corresponding α-aminonitrile.
Acylation of the NH2 group prevents further acylation of the amidic nitrogen. In addition, when the nitrogen atom carries a phenyl group, lone-pair delocalization decreases the nucleophilicity of the former and also prevents the acylation reaction, although the reaction can be achieved under more drastic conditions.
Unambiguous structural elucidation via spectroscopic methods and X-ray diffraction has allowed us to assign the configuration at the C-2 atom of the starting 2-amino-2-deoxy-aldononitriles. This is the chiral center generated during cyanohydrin synthesis, whose chirality has previously only been assigned empirically through optical rotation correlations. Therefore, by justifying the empirical rule accounting for the major epimer of α-aminonitriles, the new chiral center generated here bears a 2,3-threo stereochemical relationship with the adjacent chiral center.
The tertiary amide group showed restricted rotation, and theoretical calculations predicted the most stable conformers observed. These results were compared with those obtained through coupling constants (3J) for the protons observed in solution.
4. Experimental Methodology
4.1. General Methods
All solvents and chemicals were purchased from commercial suppliers and used without further purification. Solvents were evaporated below 40 °C at pressures between 15 and 30 mm Hg. Melting points were measured on an Electrothermal 9100 apparatus in capillary tubes and were uncorrected. Optical rotations were determined on a Perkin-Elmer 141 polarimeter at different wavelengths (Na and Hg lamps) at 18 ± 2 °C, as specified. Microanalyses for C, H, and N were performed using a Leco CHNS-932 analyzer (Leco Corporation, St. Joseph, MI, USA). IR spectra were recorded on Perkin-Elmer 399 or FT-IR THERMO spectrophotometers in the range of 4000–600 cm−1 using KBr pellets (Merck, Darmstadt, Germany). Raman spectra were recorded in a Nicolet Almega XR apparatus (Thermo Fisher Scientific, Waltham, MA, USA). Analytical thin-layer chromatography (TLC) was performed on Aldrich Polygram Sil G/UV254 plates employing the following eluent systems: (a) ethyl ether–petroleum ether (3:1) and (b) ethyl ether–petroleum ether (1:1), with subsequent monitoring with UV light at 254 and 360 nm, and iodine vapors. 1H and 13C{1H} NMR spectra were recorded on Bruker spectrometers operating at 200/400/500 and 50/100/125 MHz, respectively, in different solvent systems. Chemical shifts are reported in parts per million (δ) downfield from tetramethylsilane (Me4Si, TMS) as an internal reference. Coupling constants (J values) are given in Hz and standard abbreviations are used to indicate spin multiplicities, namely s = singlet, bs = broad singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublet, ddd = doublet of doublet of doublet, sep = septuplet, and m = multiplet. Carbon chemical shifts in the decoupled 13C{1H} NMR spectra are reported relative to CHCl3 (δC 77.00 ppm, central line of triplet). Assignments were confirmed using homo- and hetero-nuclear double-resonance, DEPT (distortionless enhancement by polarization transfer), and 2D proton–proton (COSY) and proton–carbon (HMQC) correlations. EI–mass spectra were recorded with a Kratos MS-SORFA, using a direct insertion probe and heating between 30 °C and the melting point of the samples. High-resolution mass spectra, HRMS (ESI-TOF), were measured using ESI ionization techniques with the 6520 Accurate-Mass Q-TOF LC/MS equipment from Agilent Technologies (Santa Clara, CA, USA) at the Servicio de Apoyo a la Investigación (SAIUEX) of the University of Extremadura.
4.2. X-ray Data Collection and Structural Refinement
Air-stable, single crystals of 13, 37, 42, and 63 suitable for X-ray diffraction were mounted on a Nonius Kappa CCD diffractometer equipped with monochromated Mo-Kα radiation (λ = 0.71073 Å). Crystal data were collected using the software COLLECT Nonius [115] at low temperatures, usually 100.0 or 120.0 K, as specified in every case. Data reduction and cell refinements were carried out using DENZO [116]. Each structure was solved using SHELXS-97 [117] and refined with SHELXL-97 [118]. Crystallographic illustrations were prepared using the CAMERON software (X-LIB version) [119]. For compound 47, crystal data were acquired with a Bruker Kappa APEX-II diffractometer using Mo-Kα radiation (λ = 0.71073 Å) at 170.0 K, and refined with the above-mentioned protocols.
Crystal data have been deposited within the Cambridge Structural Database with the following registry numbers: CCDC: 2332391 (for 13), CCDC: 2332392 (for 37), CCDC: 2332393 (for 42), CCDC: 2334875 (for 47), and CCDC: 2332394 (for 63), which contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (accessed on 15 March 2024).
4.3. Computational Details
DFT calculations were carried out using the Gaussian09 suite of programs [120]. The M06-2X [111] hybrid functional was chosen for performing the geometry optimizations and frequency analyses, in conjunction with the 6-311G(d,p) basis set for all atoms [121]. Stationary points were confirmed via frequency analysis at the above-mentioned level of theory at 298.15 K. Solvent effects were modeled according to the geometry optimization of the corresponding gas-phase structures using the solvation model density (SMD) method [112].
4.4. Synthetic Procedures
All products described and fully characterized herein were obtained using methods reported in the literature or according to the procedure outlined in each case (11 [53], 12 [53], 13 [51], 17 [56], 18 [51], 20 [51], 26 [52], 32 [49], 33 [49], 45 [61], 47 [61], 48 [67], 54 [50], 55 [50], 57 [99], 63 [99], and 64 [99]). Caution: The preparation of carbohydrate nitriles involving cyanohydrin synthesis requires the in situ generation of liquid hydrogen cyanide. This is very poisonous, highly volatile, and should be handled with extreme caution and ensuring effective ventilation.
- 3,4,5,6-Tetra-O-acetyl-2-deoxy-2-(N-ethylacetamido)-l-glucononitrile (11) [53]. Colorless needles, m.p. 103–105 °C, [α]D20 −49.0°; [α]57820 −51.4°; [α]54620 −58.5°, [α]43620 −100.6°, [α]36520 −160.5° (c 1.0, chloroform); IR (KBr) ṽmax/cm−1 2239 (C≡N), 1755, 1649 (C=O), 1274, 1262, 1206 (C-O-C), 1057, 1029 (C-O); Raman ṽmax/cm−1 2239 (C≡N), 1736 (C=O); 1H NMR (500 MHz, CDCl3) δ 5.57 (m, 2H, H-3, H-4), 5.48 (d, J2,3 = 9.0 Hz, 1H, H-2), 5.11 (ddd, J5,6 = 3.0 Hz, J5,6′ = 5.0 Hz, J4,5 = 8.5 Hz, H-5), 4.27 (dd, J5,6 = 3.0 Hz, J6,6′ = 12.5 Hz, 1H, H-6), 4.07 (dd, J5,6′ = 5.0 Hz, J6,6′ = 12.5 Hz, 1H, H-6′), 3.41 (m, 2H, CH2), 2.20, 2.12, 2.08, 2.07, 2.06, 2.05 (s, 15H, CH3), 1.35 (t, J = 7.0 Hz, 3H, CH3); 13C{1H} NMR (125 MHz, CDCl3) δ 170.88, 170.53, 169.69, 169.46, 169.36 (C=O), 114.78 (C≡N), 68.20, 68.04, 67.85 (C-3, C-4, C-5), 61.56 (C-6), 45.34 (C-2), 42.86 (CH2), 21.09, 20.73, 20.65, 20.52, 20.33, 14.48 (CH3). HRMS-(ESI-TOF) m/z [M + H]+ calcd. for C18H27N2O9: 415.1717. Found: 415.1706. m/z [M + NH4]+ calcd. for C18H30N3O9: 432.1982. Found: 432.1969. m/z [M2 + Na]+ calcd. for C36H52N4O18Na: 851.3174. Found: 851.3133.
- 3,4,5,6-Tetra-O-acetyl-2-deoxy-2-(N-n-propylacetamido)-l-glucononitrile (12) [53]. Colorless needles, m.p. 115–116 °C, [α]D20 −48.0°; [α]57820 −48.9°; [α]54620 −55.5°, [α]43620 −95.6°, [α]36520 −154.2° (c 0.4, chloroform); IR (KBr) ṽmax/cm−1 2233 (C≡N), 1750 (C=O ester), 1655 (C=O amide), 1415, 1360, 1258 and 1210 (C-O-C), 1057, 1030 (C-O); 1H NMR (400 MHz, CDCl3) δ 5.60 (t, 2H, H-3, H-4), 5.34 (d, J2,3 = 8.8 Hz, 1H, H-2), 5.14 (m, J5,6 = 3.2 Hz, J5,6′ = 4.8 Hz, H-5), 4.27 (dd, J5,6 = 3.0 Hz, J6,6′ = 12.0 Hz, 1H, H-6), 4.07 (dd, J5,6′ = 3.0 Hz, J6,6′ = 12.0 Hz, 1H, H-6′), 3.25 (m, 2H, CH2), 2.21, 2.22, 2.08, 2.07, 2.05 (s, 15H, CH3), 0.97 (t, J = 7.2 Hz, 3H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 170.94, 170.54, 169.72, 169.44, 169.32 (C=O), 114.70 (C≡N), 68.15, 68.08, 67.87 (C-3, C-4, C-5), 61.57 (C-6), 45.94 (C-2), 50.21, 22.54 (CH2), 21.11, 20.74, 20.65, 20.52, 20.35, 11.19 (CH3).
- 3,4,5,6,7-Penta-O-acetyl-2-deoxy-2-(N-ethylacetamido)-d-glycero-l-gluco-heptononitrile (13) [51]. Colorless needles, m.p. 120–122 °C, [α]D20 −11.8°; [α]57820 −11.8°; [α]54620 −15.0°, [α]43620 −26.0° (c 0.5, chloroform); IR (KBr) ṽmax/cm−1 2255 (C≡N), 1749, 1662 (C=O), 1275, 1203 (C-O-C), 1067, 1036 (C-O); Raman ṽmax/cm−1 2254 (C≡N), 1743, 1661 (C=O); 1H-NMR (500 MHz, CDCl3) δ 5.57 (dd, J3,4 = 1.5 Hz, J2,3 = 10.0 Hz, 1H, H-3), 5.44 (dd, J3,4 = 1.5 Hz, J4,5 = 10.0 Hz, 1H, H-4), 5.38 (d, J2,3 = 10.0 Hz, 1H, H-2), 5.30 (dd, J5,6 = 2.0 Hz, J4,5 = 7.5 Hz, 1H, H-5), 5.23 (ddd, J5,6 = 1.5 Hz, J6,7 = 5.0 Hz, J6,7′ = 7.0 Hz, H-6), 4.28 (dd, J6,7 = 5.5 Hz, J7,7′ = 12.0 Hz, 1H, H-7), 3.85 (dd, J6,7′ = 7.0 Hz, J7,7′ = 11.5 Hz, 1H, H-7′), 3.39 (m, 2H, CH2), 2.21, 2.15, 2.14, 2.12, 2.06, 2.04 (s, 18H, CH3), 1.35 (t, J = 7.0 Hz, 3H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 170.70, 170.40, 170.32, 169.74, 169.61, 169.44 (C=O), 114.81 (C≡N), 67.76, 67.40, 67.36, 66.84 (C-3, C-4, C-5, C-6), 61.85 (C-7), 45.16 (C-2), 42.94 (CH2), 21.06, 20.73, 20.61, 20.57, 20.49, 20.38, 14.51 (CH3). HRMS-(ESI-TOF) m/z [M + H]+ calcd. for C21H31N2O11: 487.1928. Found: 487.1904. m/z [M + NH4]+ calcd. for C21H34N3O11: 504.2193. Found: 504.2158.
- 3,4,5,6,7-Penta-O-acetyl-2-deoxy-2-(N-phenylamino)-d-glycero-d-altro-heptononitrile (17) [56]. White solid, m.p. 100–102 °C, [α]D20 −43.6°; [α]57820 −45.9°; [α]54620 −55.2°, [α]43620 −103.2°, [α]36520 −183.6° (c 0.5, chloroform); IR (KBr) ṽmax/cm−1 3349 (NH), 2229 (C≡N), 1771, 1747 (C=O), 1509 (NH), 1252, 1231, 1213 (C-O-C), 1061 (C-O); 1H NMR (500 MHz, CDCl3) δ 7.26 (m, 2H, H-arom), 6.91 (t, 1H, H-arom), 6.70 (d, 2H, H-arom), 5.62 (dd, J2,3 = 4.0 Hz, J3,4 = 7.0 Hz, 1H, H-3), 5.46 (dd, J4,5 = 3.5 Hz, J3,4 = 7.0 Hz, 1H, H-4), 5.40 (dd, J4,5 = 3.5 Hz, J5,6 = 7.0 Hz, 1H, H-5), 5.30 (ddd, J6,7 = 3.0 Hz, J6,7′ = 6.0 Hz, J5,6 = 7.0 Hz, 1H, H-6), 4.52 (dd, J2,3 = 4.0 Hz, J2,NH = 11.0 Hz, 1H, H-2), 4.37 (dd, J6,7 = 3.0 Hz, J7,7′ = 12.5 Hz, 1H, H-7), 4.11 (d, J2,NH = 11.0 Hz, 1H, NH), 4.10 (dd, J6,7′ = 6.0 Hz, J7,7′ = 12.5 Hz, 1H, H-7′), 2.23, 2.12, 2.07, 2.06, 2.05 (s, 15H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 170.60, 170.01, 169.83, 169.53, 169.27, 169.11 (C=O), 143.96, 129.70, 120.91 (C-arom), 116.70 (C≡N), 114.46 (C-arom), 69.84, 69.65, 69.21 (C-3, C-4, C-5, C-6), 61.75 (C-7), 47.26 (C-2), 20.86, 20.67, 20.62 (CH3).
- 3,4,5,6,7-Penta-O-benzoyl-2-deoxy-2-(N-ethylbenzamido)-d-glycero-l-gluco-heptononitrile (18) [51]. White solid, m.p. 79–81 °C, [α]D20 −9.4°; [α]57820 −10.0°; [α]54620 −11.4°, [α]43620 −12.6° (c 0.5, chloroform) [Lit. [51] m. p. 80 °C, [α]D20 −23.9° (c 0.3, pyridine)]; IR (KBr) ṽmax/cm−1 1730, 1655 (C=O), 1259 (C-O-C), 1090, 1068, 1024 (C-O); Raman ṽmax/cm−1 2250 (C≡N), 1732, 1658 (C=O); 1H NMR (500 MHz, CDCl3) δ 8.10–7.00 (m, 30H, H-arom), 6.12 (bs, 2H, H-3, H-4), 6.04 (bs, 1H, H-5), 5.91 (s, 1H, H-6), 5.76 (bs, 1H, H-2), 4.60 (dd, J6,7 = 4.5 Hz, J7,7′ = 11.5 Hz, 1H, H-7), 4.48 (dd, J6,7′ = 7.0 Hz, J7,7′ = 11.5 Hz, 1H, H-7), 3.47 (bs, 1H, CH2), 3.26 (bs, 1H, CH2), 1.05 (bs, 3H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 171.90, 171.85, 165.85, 165.37, 165.23, 165.01(C=O),134.45, 133.98, 133.88, 133.77, 133.36, 133.01, 130.13, 130.08, 130.03, 1298.88, 129.69, 129.27, 128.72, 128.66, 128.47, 128.44, 128.24, 126.41 (C-arom), 114.87 (C≡N), 69.70, 69.17, 68.68 (C-3, C-4, C-5, C-6), 62.86 (C-7), 47.52 (C-2), 43.44 (CH2), 14.01 (CH3). HRMS-(ESI-TOF) m/z [M + H]+ calcd. for C51H43N2O11: 859.2867. Found: 859.2833. m/z [M + NH4]+ calcd. for C51H46N3O11: 876.3132. Found: 876.3097.
- 3,4,5,6,7-Penta-O-benzoyl-2-deoxy-2-(N-ethylbenzamido)-d-glycero-d-galacto-heptononitrile (20) [51]. White solid, m. p. 166–168 °C, [α]D20 +6.9° (c 0.5, pyridine) [Lit. [51] m. p. 170 °C, [α]D24 +5.8° (c 0.37, pyridine)]; IR (KBr) ṽmax/cm−1 2243 (C≡N), 1742, 1720, 1639 (C=O), 1264 (C-O-C), 1093, 1069, 1026 (C-O); Raman ṽmax/cm−1 2250 (C≡N), 1746, 1639 (C=O); [α]D22 +4.2°; [α]57822 +4.2°; [α]54622 +4.2°; [α]43622 +4.6° (c 0.5, chloroform); 1H-NMR (500 MHz, CDCl3) δ 8.06–7.88 (m, 10H, H-arom), 7.60–7.24 (m, 20H, H-arom), 6.40 (bs, 2H, H-2, H-5), 6.07 (t, J3,4 = 5.5 Hz, 1H, H-4), 5.98 (dd, J2,3 = 2.5 Hz, J3,4 = 5.5 Hz,1H, H-3), 5.85 (ddd, J6,7 = 3.0 Hz, J6,7′ = 5.0 Hz, J5,6 = 8.0 Hz, 1H, H-6), 4.79 (dd, J6,7 = 3.0 Hz, J7,7′ = 12.5 Hz, 1H, H-7), 4.48 (dd, J6,7′ = 5.0 Hz, J7,7′ = 12.5 Hz, 1H, H-7′), 3.61 (m, 1H, CH2), 3.48 (m, 1H, CH2), 1.10 (t, J = 7.5 Hz, 3H, CH3); 13C{1H} NMR (125 MHz, CDCl3) 171.95, 166.03, 165.52, 165.33, 165.27, 165.09 (C=O),134.95, 133.69, 133.62, 133.58, 133.23, 133.02, 130.11, 129.98, 129.90, 129.84, 129.42, 129.11, 128.97, 128.76, 128.63, 128.58, 128.52, 128.43, 128.30, 126.49 (C-arom), 114.80 (C≡N), 70.69, 69.43, 69.02, 68.37 (C-3, C-4, C-5, C-6), 62.61 (C-7), 47.38 (C-2), 43.50 (CH2), 15.05 (CH3). HRMS-(ESI-TOF) m/z [M + H]+ calcd. for C51H43N2O11: 859.2822. Found: 859.2826. m/z [M + NH4]+ calcd. for C51H46N3O11: 876.3132. Found: 876.3081.
- 2-(Benzyloxycarbonyl)amino-2-deoxy-d-glycero-d-ido-heptononitrile (26) [52]. M. p. 200–202 °C (dec.), [α]D22 −25.4° (c 0.5, pyridine); IR (KBr) ṽmax/cm−1 3440, 3400–3200 (OH), 3300 (NH), 2250 (C≡N), 1695 (C=O amide I), 1535 (C=O amide II), 1285 (C-O-C), 1085, 1050, 1040 (C-O); 13C{1H} NMR (50 MHz, DMSO-d6) δ 155.93 (C=O), 136.62, 128.52 (2C), 128.07, 127.94 (2C) (aromatics), 118.71 (C≡N), 72.13, 71.98, 71.38, 68.87, 66.17, 63.26 (C-7), 45.85 (C-2).
- 2-(N-Benzylamino)-2-deoxy-d-glycero-l-gluco-heptononitrile (32) [49]. M. p. 130–132 °C (dec.), [α]D22 −25.4° (c 0.5, pyridine); IR (KBr) ṽmax/cm−1 3500–3100, 3020, 2230 (C≡N), 1610, 1500, 1430, 1090, 1050, 760, 700 (C-O); 13C{1H} NMR (50 MHz, DMSO-d6) δ 138.95, 128.62, 128.49, 127.27 (C-arom), 119.76 (C≡N), 72.56, 71.30, 70.78, 69.52 (C-3, C-4, C-5, C-6), 63.71 (C-7), 51.50 (CH2), 50.87 (C-2).
- 2-(N-Benzylamino)-2-deoxy-d-glycero-d-ido-heptononitrile (33) [49]. M. p. 200–202 °C (dec.), [α]D22 −81.6° (c 0.5, pyridine); IR (KBr) ṽmax/cm−1 3400–3200, 2230 (C≡N), 1610, 1500, 740, 690; 13C{1H} NMR (50 MHz, DMSO-d6) δ 138.95, 128.62, 128.49, 127.27 (C-arom), 119.76 (C≡N), 72.56, 71.30, 70.78, 69.52 (C-3, C-4, C-5, C-6), 63.71 (C-7), 51.50 (CH2), 50.87 (C-2).
- 3,4,5,6,7-Penta-O-acetyl-2-(N-benzylacetamido)-2-deoxy-d-glycero-l-gluco-heptononitrile (37). A suspension of 2-benzylamino-2-deoxy-d-glycero-l-gluco-heptononitrile (32) [49] (2.1 g, 7.1 mmol) in pyridine (10 mL) was treated with acetic anhydride (10 mL) and the mixture was kept for 24 h at room temperature. After this time, it was poured into ice–water, and a white solid was separated by stirring and scraping. It was filtered, washed with cold water, and dried under a vacuum on silica gel. Compound 37 was obtained as a colorless solid (3.6 g, 92%). Crystallized from ethanol, it formed colorless needles. M. p. 135–137 °C, [α]D20 −11.2° (c 2.0, pyridine) (Lit. [49] for 14, m. p. 83–85 °C); IR (KBr) ṽmax/cm−1 2247 (C≡N), 1754, 1672 (C=O), 1250, 1215 (C-O-C), 1033 (C-O); 1H NMR (500 MHz, CDCl3) δ 7.41 (m, 2H, H-arom), 7.34 (m, 1H, H-arom), 7.24 (d, 2H, H-arom), 5.58 (d, J3,4 = J4,5 = 9.5 Hz, 1H, H-4), 5.53 (d, J2,3 = J3,4 = 9.5 Hz, 1H, H-3), 5.38 (d, J2,3 = 9.5 Hz, 1H, H-2), 5.32 (dd, J5,6 = 2.0 Hz, J4,5 = 9.5 Hz, 1H, H-5), 5.23 (t, J6,7 = 5.5 Hz, 1H, H-6), 4.67 (d, J = 7.5 Hz, 1H, CH2), 4.44 (d, J = 8.0 Hz, 1H, CH2), 4.29 (dd, J6,7 = 5.5 Hz, J7,7′ = 12.0 Hz, 1H, H-7), 3.86 (dd, J6,7′ = 7.5 Hz, J7,7′ = 12.0 Hz, 1H, H-7′), 2.19, 2.17, 2.12, 2.11, 2.05, 1.99 (s, 18H, CH3); 13C{1H} NMR (125 MHz, CDCl3) δ 171.62, 170.43, 170.37, 169.83, 169.79, 169.42 (C=O), 135.36, 129.18, 128.10, 126.00 (C-arom), 114.32 (C≡N), 67.89, 67.83, 67.45, 66.92 (C-3, C-4, C-5, C-6), 61.89 (C-7), 51.78 (CH2), 46.81 (C-2), 21.81, 20.75, 20.63, 20.50 (CH3). Anal. Calcd. for C26H32N2O11: C, 56.93; H, 5.88; N, 5.11. Found: C, 56.84; H, 5.90; N, 5.23. HRMS-(ESI-TOF) m/z [M + H]+ calcd. for C26H33N2O11: 549.2084. Found: 549.2069. m/z [M + NH4]+ calcd. for C26H36N3O11: 566.2350. Found: 566.2330.
- 3,4,5,6,7-Penta-O-acetyl-2-(N-benzylacetamido)-2-deoxy-d-glycero-d-ido-heptononitrile (38). A suspension of 2-benzylamino-2-deoxy-d-glycero-d-ido-heptononitrile (33) [49] (0.50 g, 1.7 mmol) in pyridine (3.0 mL) was treated with acetic anhydride (2.5 mL) and the mixture was kept for 24 h at room temperature. After this time, it was poured into ice–water, and a white solid was separated by stirring and scraping. It was filtered, washed with cold water, and dried under a vacuum on silica gel. Compound 38 was obtained as an amorphous, colorless, partially melted solid (0.81 g, 87%). M. p. 42–46 °C, [α]D20 −10.0° (c 1.0, pyridine) (Lit. [49] for 15, m. p. room temperature ~25 °C; [α]D20 −9.6° (c 1.27, pyridine)); IR (KBr) ṽmax/cm−1 2240 (C≡N), 1750 (C=O acetate),1670 (C=O amide), 1375, 1210 (C-O-C), 1050 (C-O); 1H NMR (200 MHz, CDCl3) δ 7.42–7.23 (m, 5H, H-arom), 5.73 (dd, J3,4 = 7.0 Hz, J4,5 = 3.1 Hz, 1H, H-4 E-syn), 5.68 (m, 1H, H-3 Z-syn), 5.62 (d, J2,3 = 5.0 Hz, 1H, H-2 Z-syn), 5.50 (m, 2H, H-4, H-5 Z-syn), 5.23 (dd, J2,3 = 3.9 Hz, J3,4 = 7.3 Hz, 1H, H-3 E-syn), 5.19 (dd, J4,5 = 3.1 Hz, J5,6 = 7.8 Hz, 1H, H-5 E-syn), 5.02 (m, 2H, H-6 E-syn and Z-syn), 4.82 (d, J = 17.6 Hz, 1H, PhCH2 Z-syn), 4.58 (d, J = 17.6 Hz, 1H, PhCH2 Z-syn), 4.32 (dd, J6,7 = 4.2 Hz, J7,7 = 12.3 Hz, 1H, H-7 E-syn), 4.17 (m, 2H, H-7, H-7′ Z-syn), 4.16 (dd, J6,7′ = 2.1 Hz, J7,7′ = 12.3 Hz, 1H, H-7′ E-syn), 4.08 (d, J = 12.8 Hz, 1H, PhCH2 E-syn), 3.99 (d, J2,3 = 3.9 Hz, 1H, H-2 E-syn), 3.80 (d, J = 13.0 Hz, 1H, PhCH2 E-syn), 2.15, 2.13, 2.11, 2.10, 2.08, 2.06, 2.04, 1.99 (s, 36H, CH3); 13C{1H} NMR (50 MHz, CDCl3) δ 172.02 (C=O Z-syn), 170.63 (Z-syn), 170.33 (E-syn), 170.14 (E-syn), 169.66 (Z-syn), 169.61 (E-syn), 169.42 (Z-syn), 169.20 (E-syn) (C=O), 137.52 (E-syn), 135.49 (Z-syn), 129.03 (Z-syn), 128.60 (E-syn), 128.30 (E-syn), 127.91 (Z-syn), 127.76 (Z-syn), 126.03 (Z-syn) (C-arom), 116.96 (C≡N E-syn), 114.41 (C≡N Z-syn), 70.15 (E-syn), 69.27 (Z-syn), 68.91 (Z-syn), 68.63 (Z-syn), 68.08 (E-syn), 68.00 (Z-syn), 67.93 (Z-syn), 67.76 (E-syn) (C-3, C-4, C-5, C-6), 61.22 (C-7 Z-syn), 61.11 (C-7 E-syn), 52.08 (CH2 Z-syn), 51.43 (CH2 E-syn), 49.35 (C-2 E-syn), 46.94 (C-2 Z-syn), 21.76 (CH3 Z-syn), 20.62, 20.54, 20.46, 20.37, 20.31 (CH3). Anal. Calcd. for C26H32N2O11: C, 56.93; H, 5.88; N, 5.11. Found: C, 56.72; H, 5.67; N, 5.33.
- 2-Deoxy-2-phenylamino-d-glucononitrile (41). To a suspension of d-arabinose (10.0 g, 66.6 mmol) in ethanol (30.0 mL) and water (3.0 mL), aniline (8.0 mL, 87.6 mmol) was added and the mixture was refluxed until dissolution. The reddish solution was concentrated in vacuo to a thick oil, which was treated with ethyl ether. The ethereal layer was decanted and the treatment was repeated twice. The residue was dissolved in hot ethanol and crystallized into N-phenyl-d-arabinopyranosylamine. The solid was dissolved by heating and then cooled rapidly to room temperature, and then anhydrous hydrogen cyanide (7–8 mL) was added. After one hour at room temperature, it was stored in a refrigerator overnight. The resulting solid was filtered, washed with cold ethanol and diethyl ether, and dried under a vacuum over silica gel (9.75 g, 58%). An additional yield of crystals was obtained from the mother liquors (2.17 g, 13%) (total yield: 11.92 g, 71%). White solid, m. p. 150–152 °C; [α]D18 +129.2°; [α]57818 +133.9°; [α]54618 +156.0° (c 0.5, pyridine); IR (KBr) ṽmax/cm−1 3500–3100 (OH, NH), 2229 (C≡N), 1603, 1499, 754, 693 (phenyl), 1142, 1103, 1053, 1034 (C-O, C-N); 13C{1H} NMR (125 MHz, DMSO-d6) δ 144.64 (C≡N), 70.78, 70.24, 68.97 (C-3, C-4, C-5), 63.36 (C-6), 52.86 (C-2).
- 3,4,5,6-Tetra-O-acetil-2-deoxy-2-phenylamino-d-glucononitrile (42). A suspension of 2-deoxy-2-phenylamino-d-glucononitrile (41) (0.50 g, 2.0 mmol) in pyridine (3.5 mL) was treated with acetic anhydride (2.8 mL) and the mixture was kept under agitation for 24 h at 0 °C. After this time, it was poured into ice–water and, by stirring and scraping, a white solid was separated. It was filtered, washed with cold water, and dried under a vacuum over silica gel (0.74 g, 89%). Crystallization from 96% ethanol gave rise to a white microcrystalline powder. M. p. 135–136 °C; [α]D22 +415.4°; [α]57822 +416.4°; [α]54622 +423.0°; [α]43622 +461.4° (c 0.5, chloroform); IR (KBr) ṽmax/cm−1 3347 (NH), 2239 (C≡N), 1763, 1743 (C=O), 1215 (C-O-C), 1081, 1050 (C-O); Raman ṽmax/cm−1 2236 (C≡N), 1737 (C=O); 1H NMR (500 MHz, CDCl3) δ 7.26 (m, 2H, H-arom), 6.91 (t, 1H, H-arom), 6.78 (d, 2H, H-arom), 5.57 (dd, J3,4 = 1.5 Hz, J4,5 = 9.0 Hz, 1H, H-4), 5.28 (ddd, J5,6 = 3.0 Hz, J5,6′ = 4.0 Hz, J4,5 = 9.0 Hz, 1H, H-5), 5.25 (dd, J3,4 = 2.0 Hz, J2,3 = 6.0 Hz, 1H, H-3), 4.72 (dd, J2,3 = 6.0 Hz, JNH,2 = 11.0 Hz, 1H, H-2), 4.59 (d, JNH,2 = 11.0 Hz, 1H, NH), 4.28 (dd, J5,6 = 2.5 Hz, J6,6′ = 12.5 Hz, 1H, H-6), 4.23 (dd, J5,6′ = 3.5 Hz, J6,6′ = 12.5 Hz, 1H, H-6′), 2.23, 2.19, 2.08, 2.05 (s, 12H, CH3); 13C{1H} NMR (125 MHz, CDCl3) δ 171.79, 170.52, 170.22, 169.63 (C=O), 144.08, 129.72, 121.02 (C-arom), 116.67 (C≡N), 114.87 (C-arom), 68.15, 67.67, 66.95 (C-3, C-4, C-5), 61.50 (C-6), 46.00 (C-2), 21.03, 20.70, 20.67, 20.58 (CH3). Anal. Calcd. for C20H24N2O8: C, 57.14; H, 5.75; N, 6.66. Found: C, 56.97; H, 6.06; N, 6.63. HRMS-(ESI-TOF) m/z [M + H]+ calcd. for C20H25N2O8: 421.1611. Found: 421.1600.
- 3,4,5,6-Tetra-O-acetyl-2-deoxy-2-(N-phenylacetamido)-d-glucononitrile (43). To a suspension of 3,4,5,6-tetra-O-acetyl-2-deoxy-2-phenylamino-d-glucononitrile (42) (0.2 g, 0.5 mmol) in isopropenyl acetate (5.0 mL), a catalytic amount of p-toluenesulfonic acid was added and the mixture was refluxed for 8 h. Upon cooling, a white solid was separated, which was filtered and dried under a vacuum over silica gel (0.18 g, 81%), and had an m. p. of 195–196 °C. [α]D22 −3.1°; [α]57822 −3.6°; [α]54622 +1.0°; [α]43622 +23.4° (c 0.5, chloroform); IR (KBr) ṽmax/cm−1 2237 (C≡N), 1765, 1744, 1673 (C=O), 1280, 1262, 1224 (C-O-C), 1065, 1036 (C-O); Raman ṽmax/cm−1 2243 (C≡N), 1751, 1671 (C=O); 1H NMR (500 MHz, CDCl3) δ 7.48 (m, 3H, H-arom), 7.33 (d, 2H, H-arom), 5.72 (d, J2,3 = 8.5 Hz, 1H, H-2), 5.67 (dd, J3,4 = 3.0 Hz, J2,3 = 8.5 Hz, 1H, H-4), 5.51 (dd, J3,4 = 3.0 Hz, J4,5 = 8.5 Hz, 1H, H-4), 5.09 (ddd, J5,6 = 3.5 Hz, J5,6′ = 5.5 Hz, J4,5 = 8.5 Hz, 1H, H-5), 4.26 (dd, J5,6 = 3.5 Hz, J6,6′ = 12.5 Hz, 1H, H-6), 4.04 (dd, J5,6′ = 5.5 Hz, J6,6′ = 12.5 Hz, 1H, H-6′), 2.15, 2.05, 1.84 (s, 15H, CH3); 13C{1H} NMR (125 MHz, CDCl3) δ 171.04, 170.53, 169.82, 169.63, 169.23 (C=O), 139.64, 130.20, 129.61, 128.93 (C-arom), 114.40 (C≡N), 68.90, 68.80, 68.48 (C-3, C-4, C-5), 61.55 (C-6), 49.14 (C-2), 22.54, 20.79, 20.67, 20.57, 20.54 (CH3). Anal. Calcd. for C22H27N2O9: C, 57.14; H, 5.62; N, 6.06. Found: C, 57.09; H, 5.76; N, 5.73. HRMS-(ESI-TOF) m/z [M + H]+ calcd. for C22H27N2O9: 463.1717. Found: 463.1709. m/z [M + NH4]+ calcd, for C22H30N3O9: 480.1982. Found: 480.1974.
- 2-Amino-2-deoxy-d-glucose Oxime Hydrochloride (45). This compound was synthesized following the procedure described by Restelli and Deulofeu [61]. IR (KBr) ṽmax/cm−1 3500–2400 (OH, NH+), 1610 (C=N), 1530, 1410, 1100, 1080, 1040, 1010 (C-O), 750; 1H NMR (200 MHz, DMSO-d6) δ 11.38 (s, 1H, NOH), 8.00 (bs, 2H), 5.31 (bs, 1H), 4.68 (bs, 1H), 4.52 (bs, 1H), (OH, NH), 7.35 (d, J2,3 = 5.6 Hz, 1H, H-1), 3.86 (m, 2H), 3.47 (m, 5H), 3.25 (d, 1H); 13C{1H} NMR (50 MHz, DMSO-d6) δ 144.64 (C=N), 70.78, 70.24, 68.97 (C-3, C-4, C-5), 63.36 (C-6), 52.86 (C-2).
- 2-Acetamido-3,4,5,6-tetra-O-acetyl-2-deoxy-d-glucononitrile (47). This compound was synthesized by modifying the Restelli and Deulofeu procedure [61]. A suspension of 45 (1.0 g, 4.2 mmol) in pyridine (6.0 mL) was treated with acetic anhydride (6.0 mL). The mixture was stirred for 24 h at room temperature. After this time, it was poured into ice–water and extracted with dichloromethane (2 × 50 mL), and the organic phase was washed successively with 1M hydrochloric acid (2 × 50 mL), saturated sodium bicarbonate solution (2 × 50 mL), and distilled water, and dried with anhydrous MgSO4. The solvent was removed in vacuo to dryness and the resulting residue was crystallized from ethanol. After being stored in the refrigerator for several hours, it was filtered, washed with cold ethanol, and dried under a vacuum over silica gel (0.77 g, 43%). Colorless prisms from ethanol, m. p. 126–129 °C; [α]D +18.0°; [α]578 +18.2°; [α]546 +21.4°; [α]436 +40.2° (c 0.5, chloroform) [Lit. [61] m. p. 126 °C; [α]D20 +20.5° (c 4.7, chloroform)]; IR (KBr) ṽmax/cm−1 3237 (NH), 2243 (C≡N), 1753, 1730, 1675, 1645 (C=O), 1541 (NH), 1252, 1211 (C-O-C), 1079, 1065, 1051 (C-O); Raman ṽmax/cm−1 2243 (C≡N), 1753, 1728, 1643 (C=O); 1H NMR (400 MHz, CDCl3) δ 6.69 (d, JNH,2 = 9.8 Hz, 1H, NH), 5.45 (dd, J2,3 = 6.8 Hz, JNH,2 = 9.6 Hz, 1H, H-2), 5.36 (dd, J3,4 = 1.8 Hz, J4,5 = 9.2 Hz, 1H, H-4), 5.19 (dt, J5,6 = 4.0 Hz, J5,6′ = 6.4 Hz, J4,5 = 9.2 Hz, 1H, H-5), 5.10 (dd, J3,4 = 1.6 Hz, J2,3 = 6.8 Hz, 1H, H-3), 4.24 (m, 1H, H-6), 4.23 (m, 1H, H-6′), 2.23, 2.15, 2.08, 2.07, 2.05 (s, 15H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ 172.31, 170.37, 169.54, 169.39, 169.20 (C=O), 115.77 (C≡N), 67.51, 67.30, 67.00 (C-3, C-4, C-5), 61.42 (C-6), 39.22 (C-2), 22.92, 21.03, 20.68, 20.61, 20.37 (CH3). HRMS-(ESI-TOF) m/z [M + H]+ calcd. for C16H23N2O9: 387.1404. Found: 387.1406. m/z [M + NH4]+ calcd. for C16H26N3O9: 404.1669. Found: 404.1675.
- 2-Amino-2-deoxy-d-glycero-l-gluco-heptose Oxime Hydrochloride (49). This compound was obtained in a similar way to that described for the preparation of 45: through the treatment of 48 [67] with hydroxylamine. Crystallized from aqueous ethanol, it formed colorless prisms, m. p. 157–160 °C (dec.). The product was characterized through its NMR spectra, which were almost coincidental with those of 45. 1H NMR (200 MHz, DMSO-d6) δ 11.44 (s, 1H, NOH), 8.16 (bs, 2H), 5.27 (bs, 1H), 4.56 (bs, 1H), 4.35 (bs, 1H), 3.72 (m, 1H) (OH, NH), 7.40 (d, J2,3 = 5.2 Hz, 1H, H-1), 3.92 (m, 2H), 3.50 (m, 6H); 13C{1H} NMR (50 MHz, DMSO-d6) δ 144.55 (C=N), 69.74, 69.28, 69.14, 68.97 (C-3, C-4, C-5, C-6), 63.20 (C-7), 52.98 (C-2).
- 2-Acetamido-3,4,5,6,7-penta-O-acetyl-2-deoxy-d-glycero-l-gluco-heptononitrile (51). 2-Amino-2-deoxy-d-glycero-l-gluco-heptose oxime hydrochloride (49) (0.15 g, 0.6 mmol) in pyridine (1.0 mL) was treated with acetic anhydride (1.1 mL). The mixture was stirred for 24 h at room temperature. After this time, it was poured into ice–water and a white solid was separated by stirring and scraping. It was filtered, washed with cold water, and dried under a vacuum over silica gel (11.6%). The aqueous phase was extracted with dichloromethane (3 × 10 mL) and the organic phase was washed successively with 1M hydrochloric acid (2 × 10 mL), saturated sodium bicarbonate solution (2 × 10 mL), and distilled water, and dried with anhydrous MgSO4. The solvent was removed in vacuo to dryness and the resulting residue was crystallized from ethanol. After being stored in the refrigerator for several hours, it was filtered, washed with cold ethanol, and dried under a vacuum over silica gel (8.1%) (total yield 19.7%). Crystallized from ethanol, it formed colorless prisms, m. p. 154–156 °C; [α]D +22.8°; [α]578 +24.0°; [α]546 +25.5°; [α]436 +40.6° (c 0.5, chloroform); IR (KBr) ṽmax/cm−1 3278 (NH), 2251 (C≡N), 1743, 1690, 1674 (C=O), 1274, 1222 (C-O-C), 1077, 1046, (C-O); Raman ṽmax/cm−1 2245 (C≡N), 1734, 1679 (C=O); 1H NMR (500 MHz, CDCl3) δ 6.67 (d, JNH,2 = 9.5 Hz, 1H, NH), 5.47 (dd, J2,3 = 6.5 Hz, JNH,2 = 10.0 Hz, 1H, H-2), 5.44 (dd, J3,4 = 2.0 Hz, J4,5 = 10.0 Hz, 1H, H-4), 5.39 (ddd, J5,6 = 2.0 Hz, J6,7 = 5.0 Hz, J6,7′ = 7.5 Hz, 1H, H-6), 5.26 (dd, J5,6 = 1.5 Hz, J4,5 = 10.0 Hz, 1H, H-5), 4.91 (dd, J3,4 = 1.5 Hz, J2,3 = 6.5 Hz, 1H, H-3), 4.26 (dd, J6,7 = 5.0 Hz, J7,7′ = 12.0 Hz, 1H, H-7), 3.85 (dd, J6,7′ = 7.5 Hz, J7,7′ = 11.5 Hz, 1H, H-7′), 2.23, 2.17, 2.10, 2.09, 2.07, 2.02 (s, 18H, CH3); 13C{1H} NMR (125 MHz, CDCl3) δ 173.01, 170.31, 170.06, 169.61, 169.49, 169.07 (C=O), 115.75 (C≡N), 67.50, 67.09, 66.71, 66.50 (C-3, C-4, C-5, C-6), 61.90 (C-7), 38.97 (C-2), 22.95, 21.13, 20.62, 20.57, 20.45 (CH3). HRMS-(ESI-TOF) m/z [M + H]+ calcd. for C19H27N2O11: 459.1615. Found: 459.1629.
- 2-Deoxy-2-(N-isopropylamino)-d-glycero-l-gluco-heptononitrile (54) [50]. White solid crystallized from ethanol, m. p. 130–132 °C (dec.); [α]D −13.0° (c 0.5, pyridine); IR (KBr) ṽmax/cm−1 3500–3200 (OH, NH), 2210 (C≡N), 1280, 1090, 1040, 1020 (C-O); 13C{1H} NMR (50 MHz, DMSO-d6) δ 120.99 (C≡N), 71.69, 70.73 (2C), 69.83 (C-3, C-4, C-5, C-6), 63.95 (C-7), 52.16 (CH, isopropyl), 47.82 (C-2), 23.90, 22.47 (CH3).
- 2-Deoxy-2-(N-isopropylamino)-d-glycero-d-ido-heptononitrile (55) and 2-deoxy-2- (N-isopropylamino)-d-glycero-d-gulo-heptononitrile (60) [50]. White solid crystallized from methanol, m. p. 132–134 °C (dec.). Spectroscopic data of 55: 13C{1H} NMR (50 MHz, DMSO-d6) δ 120.29 (C≡N), 72.75, 71.20, 70.04, 69.63 (C-3, C-4, C-5, C-6), 63.67 (C-7), 49.16 (C-2), 46.56 (CH, isopropyl), 23.40, 21.11 (CH3, isopropyl). Spectroscopic data of 60: 13C{1H} NMR (50 MHz, DMSO-d6) 119.98 (C≡N), 71.81, 71.33, 69.63, 69.23 (C-3, C-4, C-5, C-6), 63.45 (C-7), 50.90 (C-2), 46.25 (CH isopr.), 23.57, 21.49 (CH3, isopropyl).
- N-Isopropyl-β-d-mannopyranosylamine (56). To a suspension of d-mannose (5.0 g, 27.7 mmol) in methanol (50 mL), isopropylamine (5.2 mL, 60.0 mmol) was added and the mixture was stirred and heated until dissolution. The solvent was partially evaporated, which often results in crystal formation. When the latter did not occur, the mixture was concentrated and the residue was solidified by triturating it with diethyl ether. The white solid was filtered, washed with ether, and dried in vacuo (5.96 g, 97%). Crystallized from absolute ethanol–diethyl ether, it showed an m. p. of 108–109 °C (dec.); [α]D20 −21° (c 0.5, NH4OH-H2O 1:9); [α]D20 −17° (c 0.5, HCl 2.5 M). IR (KBr) ṽmax/cm−1 3500–3000 (OH), 3280 (NH), 1075, 1045, 1025 (C-O). Anal. Calcd. for C9H19NO5: C, 48.86; H, 8.64; N, 6.33. Found: C, 48.66; H, 8.50; N, 6.11.
- d-glycero-d-galacto-Heptononitrile (57) [99]. Method (a): To a suspension of d-mannose (5.4 g, 30.0 mmol) in ethanol (50 mL), isopropylamine (5.2 mL, 60.0 mmol) was added and the mixture was heated until dissolution. After cooling to room temperature, anhydrous hydrogen cyanide (3.0 mL, 80 mmol) was added. A white crystalline solid separated, which was filtered, washed with cold ethanol and diethyl ether, and dried in vacuo (1.1 g, 17%). Crystallized from methanol, it showed an m. p. of 112–113 °C (dec.). Method (b): To a solution of the N-isopropyl-β-d-mannopyranosylamine (8.0 g, 36.0 mmol) in ethanol (32 mL), anhydrous hydrogen cyanide (2.8 mL, 74.6 mmol) was added. The reaction mixture was kept overnight in the refrigerator. The resulting white crystalline solid was filtered, washed with cold ethanol and diethyl ether, dried in vacuo (3.4 g, 45%), and recrystallized from methanol. The white solid had an m. p. of 113–115 °C (dec.); [α]D20 +10.4° (c. 0.5, pyridine) [Lit. [99] m. p. 122 °C; [α]D20 +10.5° (c. 0.5, pyridine)]. IR (KBr) ṽmax/cm−1 3500–3100 (OH), 2220 (C≡N), 1400, 1070, 1000 (C-O); 13C{1H} NMR (125 MHz, DMSO-d6) δ 171.72, 170.26, 170.19, 170.04, 169.47 (C=O), 117.87 (C≡N), 68.45, 67.23, 67.18, 65.93 (C-3, C-4, C-5, C-6), 61.79 (C-7), 47.17 (C-2).
- 3,4,5,6,7-Penta-O-acetyl-2-deoxy-2-(N-isopropylamino)-d-glycero-l-gluco-heptononitrile (58). To a suspension of 2-deoxy-2-(N-isopropylamino)-d-glycero-l-gluco-heptononitrile (54) (0.25 g, 1.0 mmol) in pyridine (1.7 mL), acetic anhydride (1.4 mL) was added and the mixture was kept under stirring until dissolution, and then at 0 °C for 24 h. After this time, the solution was poured onto ice–water and a white solid was separated, which was filtered and washed with cold water (0.38 g, 64%). Crystallized from 96% ethanol, the white solid presented an m. p. of 125–127 °C; [α]D22 +17.2° (c 1.5, pyridine). IR (KBr) ṽmax/cm−1 3330 (NH), 2980, 1740 (C=O), 1365, 1210 (C-O-C), 1040 (C-O); 1H NMR (200 MHz, CDCl3) δ 5.42–5.34 (m, 3H, H-4, H-5, H-6), 4.90 (d, J2,3 = 6.0 Hz, J3,4 = 0 Hz, 1H, H-3), 4.27 (dd, J6,7 = 5.0 Hz, J7,7′ = 11.7 Hz, 1H, H-7), 3.90 (d, J2,3 = 6.0 Hz, 1H, H-2), 3.86 (dd, J6,7′ = 7.2 Hz, J7,7′ = 11.7 Hz, 1H, H-7′), 2.97 (sep, J = 6.2 Hz, 1H, CH isopropyl), 2.18, 2.17, 2.11, 2.09, 2.02 (s, 15H, CH3), 1.13 (d, J = 6.2 Hz, 3H, CH3 isopropyl), 1.01 (d, J = 6.2 Hz, 3H, CH3 isopropyl); 13C{1H} NMR (50 MHz, CDCl3) δ 171.72, 170.26, 170.19, 170.04, 169.47 (C=O), 117.87 (C≡N), 68.45, 67.23, 67.18, 65.93 (C-3, C-4, C-5, C-6), 61.79 (C-7), 47.17 (C-2), 23.24, 21.15, 20.95, 20.53, 20.45, 20.36, 20.28 (CH3). Anal. Calcd. for C20H30N2O10: C, 52.40; H, 6.60; N, 6.11. Found: C, 52.21; H, 6.45; N, 5.90.
- 3,4,5,6,7-Penta-O-acetyl-2-deoxy-2-(N-isopropylamino)-d-glycero-d-ido-heptononitrile (59) and 3,4,5,6,7-penta-O-acetyl-2-deoxy-2-(N-isopropylamino)-d-glycero-d-gulo- heptononitrile (61). White solid obtained from 55/60, using the procedure described for 58, as a mixture of 59 and 61 in a proportion of ~80:20. IR (KBr) ṽmax/cm−1 3330 (NH), 2980, 1740 (C=O), 1365, 1210 (C-O-C), 1040 (C-O). Spectroscopic data of 59: 1H NMR (200 MHz, CDCl3) δ 5.66 (dd, J3,4 = 6.7 Hz, J4,5 = 3.0 Hz, 1H, H-4), 5.37 (dd, J4,5 = 3.0 Hz, J5,6 = 7.7 Hz, 1H, H-5), 5.23 (dd, J2,3 = 3.8 Hz, J3,4 = 6.7 Hz, 1H, H-3), 4.99 (ddd, J5,6 = 7.7 Hz, J6,7 = 3.2 Hz, J6,7′ = 4.0 Hz, 1H, H-6), 4.23 (dd, J6,7 = 3.2 Hz, J7,7″ = 12.2 Hz, H-7), 4.13 (dd, J6,7′ = 4.0 Hz, J7,7′ = 12.2 Hz, 1H, H-7′), 4.10 (d, J2,3 = 3.8 Hz, 1H, H-2), 3.04 (sep, 1H, CH, isopropyl), 2.17, 2.14, 2.08, 2.05 (s, 15H, CH3, acetate), 1.17 (d, J = 6.2 Hz, 3H, CH3, isopropyl), 1.10 (d, J = 6.2 Hz, 3H, CH3, isopropyl); 13C{1H} NMR (50 MHz, CDCl3) δ 170.00, 169.93, 169.78, 169.24, 168.95 (C=O), 117.26 (C≡N), 70.30, 67.73 (3C) (C-3, C-4, C-5, C-6), 60.66 (C-7), 47.56 (C-2), 46.53 (CH, isopropyl), 23.15, 20.79 (CH3, isopropyl), 20.39, 20.22, 20.13, 20.03, 19.94 (CH3, acetate). Spectroscopic data of 61: 13C{1H} NMR (50 MHz, CDCl3) δ 170.00, 169.93, 169.88, 169.61, 168.87 (C=O), 117.32 (C≡N), 69.21, 69.12, 68.45, 68.34 (C-3, C-4, C-5, C-6), 60.98 (C-7), 49.14 (C-2), 48.73 (CH, isopropyl), 22.97, 20.71 (CH3, isopropyl), 20.79, 20.71, 20.57, 20.22, 20.03 (CH3, acetate).
- 2,3,4,5,6,7-Hexa-O-acetyl-d-glycero-d-galacto-heptononitrile (63) [99]. To a stirred solution of d-glycero-d-galacto-heptononitrile (57) (1.0 g, 4.1 mmol) in pyridine (5 mL), cooled externally with an ice bath, acetic anhydride (4.0 mL, 27.1 mmol) was added gradually. The reaction mixture was kept overnight in the refrigerator and, subsequently, it was poured onto a water–ice mixture. The resulting solid was filtered, washed with water, and dried under a vacuum over silica gel (1.65 g, 53%). Crystallized from acetone–water, it showed an m. p. of 163–165 °C; [α]D22 +28.7°; [α]578 +29.3°; [α]546 +32.7°; [α]436 +55.3° (c 0.3, chloroform) (Lit. [99] m. p. 124–125 °C). 1H NMR (500 MHz, CDCl3) δ 5.52 (dd, J3,4 = 10.0 Hz, J4,5 = 2.0 Hz, 1H, H-4), 5.50 (dd, J2,3 = 3.0 Hz, J3,4 = 10.0 Hz, 1H, H-3), 5.38 (d, J2,3 = 3.0 Hz, 1H, H-2), 5.38 (dd, J4,5 = 2.0 Hz, J5,6 = 9.0 Hz, 1H, H-5), 5.02 (ddd, J5,6 = 9.0 Hz, J6,7 = 3.0 Hz, J6,7′ = 5.0 Hz, 1H, H-6), 4.20 (dd, J6,7 = 3.0 Hz, J7,7″ = 12.5 Hz, H-7), 4.00 (dd, J6,7′ = 5.0 Hz, J7,7′ = 12.5 Hz, 1H, H-7′), 2.17 (s, 6H, CH3 acetate), 2.10, 2.07, 2.05, 2.04 (s, 12H, CH3, acetate); 13C{1H} NMR (50 MHz, CDCl3) δ 170.48, 169.80, 169.56, 169.02, 168.76 (C=O), 113.92 (C≡N), 67.81 (C-6), 66.99, 66.89 (C-3,C-5), 66.48 (C-4), 61.75 (C-7), 59.40 (C-2), 20.80, 20.62, 20.48, 20.45, 20.11 (CH3, acetate). HRMS-(ESI-TOF) m/z [M + NH4]+ calcd. for C19H29N2O12: 477.1720. Found: 477.1730.
- 2,3,4,5,6,7-Hexa-O-benzoyl-d-glycero-d-galacto-heptononitrile (64) [99]. To a stirred solution of d-glycero-d-galacto-heptononitrile (1.0 g, 4.1 mmol) in pyridine (5 mL), cooled externally with an ice bath, benzoyl chloride (4.0 mL, 27.1 mmol) was added gradually. The reaction mixture was stored overnight in the refrigerator and, subsequently, it was poured onto a water–ice mixture and extracted with dichloromethane (3 × 20 mL). The organic layer was washed twice with 3M sulfuric acid; twice with saturated sodium bicarbonate solution; and finally with distilled water. The organic phase was dried with anhydrous sodium sulfate, filtered through activated charcoal, and evaporated to dryness. The residue was evaporated repeatedly with ethanol. The resulting crystalline solid was dried under a vacuum over calcium chloride (1.65 g, 41%). Crystallized from acetone–water, it showed an m. p. of 165 °C; [α]D22 +30.4° (c 2.0, chloroform) [Lit. [99] m. p. 161–162 °C; [α]D22 +30.3° (c. 2, chloroform)]. IR (KBr) ṽmax/cm−1 1715, 1690 (C=O), 1590, 1570, 1435 (phenyl), 1240 (C-O-C), 1070, 1045 (C-O); 1H NMR (200 MHz, CDCl3) δ 8.04–7.87 (m, 12H, H-arom), 7.56–7.25 (m, 18H, H-arom), 6.31 (dd, J3,4 = 7.9 Hz, J4,5 = 2,1 Hz, 1H, H-4), 6.20 (d, J4,5 = 2.2 Hz, J5,6 = 7.8 Hz, 2H, H-5), 6.13 (dd, J2,3 = 4.2 Hz, J3,4= 7.8 Hz, 1H, H-3), 5.96 (d, J2,3 = 4.2 Hz, 1H, H-2), 5.79 (m, 1H, H-6), 4.85 (dd, J6,7 = 3.4 Hz, J7,7′ = 12.4 Hz, 1H, H-7), 4.45 (dd, J6,7′ = 5.4 Hz, J7,7′ = 12.4 Hz, 1H, H-7′); 13C{1H} NMR (50 MHz, CDCl3) δ 165.87, 165.21, 164.98, 164.81, 164.57, 164.18 (C=O),134.01, 133.79, 133.25, 133.05, 130.00, 129.91, 129.68, 129.27, 129.09, 128.73, 128.54, 128.49, 128.28, 127.42 (C-arom), 114.05 (C≡N), 69.39, 68.81, 68.22 (C-3,4,5), 62.35 (C-7), 60.67 (C-2).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29081796/s1: Crystal data, IR and NMR spectra, and computational data, as stated through the entire manuscript.
Author Contributions
E.M. and J.C.P. conceived the conceptual ideas and manuscript outline. J.C.P. and P.C. drafted the manuscript. M.E.L. performed the crystallographic and structural studies. E.M., P.C. and J.C.P. critically reviewed and edited the final content. All authors discussed and agreed with this contribution. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the financial support from the Junta de Extremadura and Fondo Europeo de Desarrollo Regional (Grant GR21039).
Informed Consent Statement
Not applicable.
Data Availability Statement
Crystallographic data have been deposited in the Cambridge Structural Database (CSD) and can be obtained free of charge at www.ccdc.cam.ac.uk/data_request/cif (accessed on 15 March 2024), or via email at data_request@ccdc.cam.ac.uk, or by contacting the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK. The corresponding registry numbers are indicated in the appropriate citations and correspond to the compounds mentioned throughout this manuscript.
Acknowledgments
We gratefully acknowledge the Servicio de Apoyo a la Investigación (SAIUEX) at the University of Extremadura for providing analytical and spectroscopic resources, and the computational facilities at the LUSITANIA Supercomputing Centre, supported by Cenits and the Computaex Foundation. This manuscript is dedicated to our long-standing mentor and friend, Juan A. Galbis, who pioneered this adventure with sugar aminonitriles, and to the memory of María Dolores Méndez-Cordero. Last, but not least, we thank R. Babiano and J. L. Jiménez for kindly providing samples of compounds 17 and 26, respectively.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grundke, C.; Vierengel, N.; Opatz, T. α-Aminonitriles: From Sustainable Preparation to Applications in Natural Product Synthesis. Chem. Rec. 2020, 20, 989–1016. [Google Scholar] [CrossRef] [PubMed]
- Strecker, A. Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Ann. Chem. Pharm. 1850, 75, 27–45. [Google Scholar] [CrossRef]
- Ullah, B.; Gupta, N.K.; Ke, Q.; Ullah, N.; Cai, X.; Liu, D. Organocatalytic Synthesis of α-Aminonitriles: A Review. Catalysts 2022, 12, 1149. [Google Scholar] [CrossRef]
- Kondo, Y.; Hirazawa, Y.; Kadota, T.; Yamada, K.; Morisaki, K.; Morimoto, H.; Ohshima, T. One-Pot Catalytic Synthesis of α-Tetrasubstituted Amino Acid Derivatives via In Situ Generation of N-Unsubstituted Ketimines. Org. Lett. 2022, 24, 6594–6598. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, V.K.; Galvis, C.E.P. Strecker reaction and α-amino nitriles: Recent advances in their chemistry, synthesis, and biological properties. Tetrahedron 2018, 74, 773–810. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Shen, X.; Zhang, X.; Deng, S.; Chen, Y. KOtBu-Mediated Reductive Cyanation of Tertiary Amides for Synthesis of α-Aminonitriles. J. Org. Chem. 2022, 87, 6321–6329. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Velazquez, J.M.; Banik, B.K. A truly green synthesis of α-aminonitriles via Strecker reaction. Org. Med. Chem. Lett. 2011, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Jarusiewicz, J.; Choe, Y.; Yoo, K.S.; Park, C.P.; Jung, K.W. Efficient three-component Strecker reaction of aldehydes/ketones via NHC-amidate palladium(II) complex catalysis. J. Org. Chem. 2009, 74, 2873–2876. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Nájera, C.; Sansano, J.M. Solvent-Free Synthesis of Racemic α-Aminonitriles. Synthesis 2007, 1230–1234. [Google Scholar] [CrossRef]
- Das, B.; Ramu, R.; Ravikanth, B.; Reddy, K.R. (Bromodimethyl)sulfonium Bromide Catalyzed One-Pot Synthesis of α-Aminonitriles. Synthesis 2006, 1419–1422. [Google Scholar] [CrossRef]
- Surendra, K.; Krishnaveni, N.S.; Mahesh, A.; Rao, K.R. Supramolecular Catalysis of Strecker Reaction in Water under Neutral Conditions in the Presence of β-Cyclodextrin. J. Org. Chem. 2006, 71, 2532–2534. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Kondo, K.; Aoyama, T. Construction of Tetrasubstituted Carbon by an Organocatalyst: Cyanation Reaction of Ketones and Ketimines Catalyzed by a Nucleophilic N-Heterocyclic Carbene. Synthesis 2006, 2649–2652. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Eeshwaraiah, B.; Srinivas, M. Montmorillonite KSF clay catalyzed one-pot synthesis of α-aminonitriles. Tetrahedron 2004, 60, 1767–1771. [Google Scholar] [CrossRef]
- Ponnamperuma, C.; Woeller, F. α-Aminonitriles formed by an electric discharge through a mixture of anhydrous methane and ammonia. Biosystems 1967, 1, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Chandha, M.S.; Molton, P.M.; Ponnamperuma, C. Aminonitriles: Possible role in chemical evolution. Orig. Life 1975, 6, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Canavelli, P.; Islam, S.; Powner, M.W. Peptide ligation by chemoselective aminonitrile coupling in water. Nature 2019, 571, 546–549. [Google Scholar] [CrossRef]
- Seymour, F.R.; Plattner, R.D.; Slodki, M.E. Gas-liquid chromatography-mass spectrometry of methylated and deuteriomethylated per-O-acetyl-aldononitriles from D-mannose. Carbohydr. Res. 1975, 44, 181–198. [Google Scholar] [CrossRef]
- Seymour, F.R.; Chen, E.C.M.; Bishop, S.H. Identification of aldoses by use of their peracetylated aldononitrile derivatives: A G.L.C-M.S. approach. Carbohydr. Res. 1979, 73, 19–45. [Google Scholar] [CrossRef]
- Seymour, F.R.; Chen, E.C.M.; Stouffer, J.E. Identification of ketoses by use of their peracetylated oxime derivatives: A G.L.C.-M.S. approach. Carbohydr. Res. 1980, 83, 201–242. [Google Scholar] [CrossRef]
- Seymour, F.R.; Unruh, S.L.; Nehlich, D.A. Quantitation of free sugars in plant tissue by g.l.c. of their peracetylated aldononitrile and ketoxime derivatives. Carbohydr. Res. 1989, 191, 175–189. [Google Scholar] [CrossRef]
- Dmitriev, B.A.; Backinowsky, L.V.; Chizhov, O.S.; Zolotarev, B.M.; Kochetkov, N.K. Gas-liquid chromatography and mass spectrometry of aldononitrile acetates and partially methylated aldononitrile acetates. Carbohydr. Res. 1971, 19, 432–435. [Google Scholar] [CrossRef]
- Li, B.W.; Cochran, T.W.; Vercellotti, J.R. Chemical-ionization mass spectra of per-O-acetyl-aldononitriles and methylated aldononitrile acetates. Carbohydr. Res. 1977, 59, 567–570. [Google Scholar] [CrossRef]
- Chen, C.C.; McGinnis, D. The use of 1-methylimidazole as a solvent and catalyst for the preparation of aldononitrile acetates of aldoses. Carbohydr. Res. 1981, 90, 127–130. [Google Scholar] [CrossRef]
- Mawhinney, T.P.; Peather, M.S.; Barbero, G.J.; Martinez, J.R. The rapid, quantitative determination of neutral sugars (as aldononitrile acetates) and amino sugars (as O-methyloxime acetates) in glycoproteins by gas-liquid chromatography. Anal. Biochem. 1980, 101, 112–117. [Google Scholar] [CrossRef]
- Calvey, E.M.; Taylor, L.T.; Palmer, J.K. Supercritical fluid chromatography with fourier transform infrared detection of peracetylated aldononitrile derivatives and byproducts from monosaccharides. J. Sep. Sci. 1988, 11, 739–741. [Google Scholar] [CrossRef]
- Calvey, E.M.; Roach, J.A.G.; Taylor, L.T.; Palmer, J.K. Supercritical fluid chromatography with Fourier transform infrared and mass spectrometric detection of peracetylated nitrogen derivatives of monosaccharides. J. Microcolumn Sep. 1989, 1, 294–301. [Google Scholar] [CrossRef]
- Varma, R.; Varma, R.S.; Wardi, A.H. Separation of aldononitrile acetates of neutral sugars by gas-liquid chromatography and its application to polysaccharides. J. Chromatogr. 1973, 77, 222–227. [Google Scholar] [CrossRef]
- Varma, R.; Varma, R.S.; Allen, W.S.; Wardi, A.H. Gas chromatographic determination of neutral sugars from glycoproteins and acid mucopolysaccharides as aldononitrile acetates. J. Chromatogr. 1973, 86, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Varma, R.S. Simultaneous determination of neutral sugars and hexosamines in glycoproteins and acid mucopolysaccharides (glycosaminoglycans) by gas-liquid chromatography. J. Chromatogr. A 1976, 128, 45–52. [Google Scholar] [CrossRef]
- Guerrant, G.O.; Moss, C.W. Determination of monosaccharides as aldononitrile, O-methyloxime, alditol, and cyclitol acetate derivatives by gas-chromatography. Anal. Chem. 1984, 56, 633–638. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- He, H.; Xie, H.; Zhang, X. A novel GC/MS technique to assess 15N and 13C incorporation into soil amino sugars. Soil Biol. Biochem. 2006, 38, 1083–1091. [Google Scholar] [CrossRef]
- Liang, C.; Pedersen, J.A.; Balser, T.C. Aminoglycoside antibiotics may interfere with microbial amino sugar analysis. J. Chromatogr. A 2009, 1216, 5296–5301. [Google Scholar] [CrossRef] [PubMed]
- Turrión, M.B.; Glaser, B.; Zech, W. Effects of deforestation on contents and distribution of amino sugars within particle-size fractions of mountain soils. Biol. Fertil. Soils 2002, 35, 49–53. [Google Scholar] [CrossRef]
- Liang, C.; Balser, T.C. Mass spectrometric characterization of amino sugar aldononitrile acetate derivatives used for isotope enrichment assessment of microbial residues. Soil Biol. Biochem. 2010, 42, 904–909. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Wei, L.; He, H.; Higbee, A.J.; Balser, T.C. Investigation of the molecular ion structure for aldononitrile acetate derivatized muramic acid. J. Microbiol. Methods 2011, 86, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Read, H.W.; Balser, T.C. GC-based Detection of Aldononitrile Acetate Derivatized Glucosamine and Muramic Acid for Microbial Residue Determination in Soil. J. Vis. Exp. 2012, 63, e3767. [Google Scholar]
- Garcia, A.D.; Leyva, V.; Bocková, J.; Pepino, R.L.; Meinert, C. Resolution and quantification of carbohydrates by enantioselective comprehensive two-dimensional gas chromatography. Talanta 2024, 271, 125728. [Google Scholar] [CrossRef] [PubMed]
- Niederer, I.B.; Manzardo, G.G.G.; Amadò, R. A reinvestigation of the derivatization of monosaccharides as aldononitrile peracetates. Carbohydr. Res. 1995, 278, 181–194. [Google Scholar] [CrossRef]
- Engelhard, E.; Mutters, R. Rapid differentiation of the species of the genus Bacteroides sensu strict by capillary gas chromatography of cellular carbohydrates. J. Appl. Bacteriol. 1991, 70, 216–220. [Google Scholar] [CrossRef]
- Bhattacharyya, N.K.; Jha, S.; Jha, S.; Bhutia, T.Y.; Adhikary, G. Dehydration of amides to nitriles: A review. Int. J. Chem. Eng. Appl. 2012, 4, 295–304. [Google Scholar]
- Hernández, R.; León, E.I.; Moreno, P.; Suárez, E. Radical Fragmentation of β-Hydroxy Azides. Synthesis of Chiral Nitriles. J. Org. Chem. 1997, 62, 8974–8975. [Google Scholar] [CrossRef]
- Castell, J.; Jaime, C.; López-Calahorra, F.; Santaló, N.; Velasco, D. Parameterization of cyano group MM2 constants in peracetylated aldononitriles. J. Org. Chem. 1988, 53, 5363–5366. [Google Scholar] [CrossRef]
- Velasco, D.; Castell, J.; López-Calahorra, F.; Jaime, C. Stereochemical elucidation of aldoses from the proton NMR spectrum of its peracetylated aldononitrile derivatives with the aid of MM2/3JHH calculations. J. Org. Chem. 1990, 55, 3526–3530. [Google Scholar] [CrossRef]
- López-Calahorra, F.; Velasco, D.; Castell, J.; Jaime, C. Conformational study of peracetylated aldononitriles. J. Org. Chem. 1990, 55, 3530–3536. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Q.; Thibaudeau, C.; Zhao, S.; Carmichael, I.; Serianni, A.S. [13C, 15N]2-Acetamido-2-deoxy-d-aldoses and their methyl glycosides: Synthesis and NMR investigations of J-couplings involving 1H, 13C, and 15N. J. Org. Chem. 2006, 71, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Horton, D. Monosaccharide Amino Sugars. In The Amino Sugars; Jeanloz, R.W., Ed.; Academic Press: New York, NY, USA, 1969; Volume IA, Chapter 1; pp. 18–23. [Google Scholar]
- Horton, D.; Wander, J.D. Amino Sugars. In The Carbohydrates; Pigman, W., Horton, D., Wander, J.D., Eds.; Academic Press: New York, NY, USA, 1980; Volume IB, Chapter 16; pp. 645–649. [Google Scholar]
- Galbis, J.A.; Fernández, J.I.; Areces, P. Síntesis de 2-alquilamino-2-desoxi-heptononitrilos: I. Síntesis de 2-bencilamino-2-desoxi-d-glicero-d-ido-heptononitrilo y 2-bencilamino-2-desoxi-d-glicero-d-galacto-heptononitrilo. An. Quim. 1976, 72, 820–822. [Google Scholar]
- Gómez, M.; Galbis, J.A.; Areces, P.; Fernández, J.I.; Ávalos, M.; Ramírez, J.M. Síntesis de 2-alquilamino-2-desoxi-heptononitrilos: II. Síntesis de 2-desoxi-2-propil (o isopropil)amino-heptononitrilos. An. Quim. 1978, 74, 633–636. [Google Scholar]
- Gómez, M.; Galbis, J.A.; Remón, J.I.; Jiménez, J.L. Síntesis de 2-alquilamino-2-desoxi-heptononitrilos: III. Síntesis de 2-desoxi-2-etilamino-heptononitrilos. An. Quim. 1978, 74, 651–653. [Google Scholar]
- Gómez, M.; Galbis, J.A.; Jiménez, J.L. Síntesis del 2-amino-2-desoxi-d-glicero-d-ido-heptononitrilo y de algunos de sus derivados. An. Quim. 1979, 75, 426–427. [Google Scholar]
- Galbis, J.A.; Palacios, J.C.; Jiménez, J.L.; Ávalos, M.; Fernández-Bolaños, J.M. Synthesis of 1-aryl-(glycofurano)imidazolidine-2-thiones from new 2-(alkylamino)-2-deoxyhexoses. Carbohydr. Res. 1984, 129, 131–141. [Google Scholar] [CrossRef]
- Galbis, J.A.; Palacios, J.C.; Jiménez, J.L.; Ávalos, M.; Fernández-Bolaños, J.M. 1-Aryl(glycofurano)imidazolidine-2-thiones derived from new 2-(alkylamino)-2-deoxyheptoses having d-glycero-d-galacto and d-glycero-d-talo configurations. Carbohydr. Res. 1984, 131, 71–82. [Google Scholar] [CrossRef]
- Galbis, J.A.; Jiménez, J.L.; Palacios, J.C.; Ávalos, M.; Fernández-Bolaños, J.M. 3-Alquil-1-aril(glicoheptofurano)- imidazolidina-2-tionas derivadas de nuevas 2-(alquilamino)-2-desoxi-heptosas de configuración D-glicero-L-gluco, D-glicero-L-mano, D-glicero-D-gulo y D-glicero-D-ido. An. Quim. 1986, 82, 11–17. [Google Scholar]
- Babiano, R.; Galbis, J.A. Preparación del hidrocloruro de 2-amino-2-desoxi-D-glicero-D-altro-heptosa a partir de D-alosa. An. Quim. 1986, 82, 92–95. [Google Scholar]
- Palacios, J.C.; Román, E.; Galbis, J.A. Limitations of the aminonitrile synthesis.New products from D-glucose, D-galactose, and D-mannose. Carbohydr. Res. 1985, 143, 117–128. [Google Scholar] [CrossRef]
- Kuhn, R.; Kirschenlohr, W. D-Galaktosamin aus D-Lyxose. Aminozucker-synthesen III. J. Liebigs Ann. Chem. 1956, 600, 126–134. [Google Scholar] [CrossRef]
- Kuhn, R.; Weiser, D.; Fischer, H. Aminozucker-Synthesen, XIX. Basen-katalysierte Umwandlungen der N-Phenyl-d-hexosaminsäurenitrile. Justus Liebigs Ann. Chem. 1959, 628, 207–239. [Google Scholar] [CrossRef]
- Kuhn, R.; Jochims, J.C. Aminozucker-Synthesen, XXV. Über die Epimerisierung N-substituierter 2-Amino-2-desoxy- hexonsäurenitrile. Chem. Ber. 1963, 96, 983–989. [Google Scholar] [CrossRef]
- Restelli de Labriola, E.; Deulofeu, V. Action of the Reagent Pyridine-Acetic Anhydride on d-α-Glucoheptose, d-Glucosamine and l-Fucose Oximes. J. Am. Chem. Soc. 1940, 62, 1611–1613. [Google Scholar] [CrossRef]
- Bueno, M.; Turmo, P.; Galbis, J.A. Synthesis of 1,2-diamino-1,2-dideoxy-D-glycero-L-manno-and -D-glycero-L-gluco-heptitol. Carbohydr. Res. 1991, 219, 241–246. [Google Scholar]
- Wolfrom, M.L.; Thompson, A.; Hooper, I.R. N-Methyl-L-glucosaminic Acid. J. Am. Chem. Soc. 1946, 68, 2343–2345. [Google Scholar] [CrossRef]
- Wolfrom, M.L.; Thompson, A. Derivatives of N-Methyl-L-glucosaminic Acid; N-Methyl-L-mannosaminic Acid. J. Am. Chem. Soc. 1947, 69, 1847–1849. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.; Kirschenlohr, W. 2-Amino-2-desoxy-zucker durch katalytische Halbhydrierung von Amino-, Arylamino-und Benzylamino-nitrilen; D-und L-Glucosamin. Aminozucker-synthesen II. Justus Liebigs Ann. Chem. 1956, 600, 115–125. [Google Scholar] [CrossRef]
- Kuhn, R.; Jochims, J.C. Aminozucker-Synthesen, XV Epimerisierung von N-Benzyl-glucosaminsäurenitril Darstellung von Mannosamin aus Arabinose. Justus Liebigs Ann. Chem. 1959, 628, 172–186. [Google Scholar] [CrossRef]
- Galbis, J.A.; Pinto, R.M.; Román, E.; Gómez, M. Síntesis de 2-amino-2-desoxi-heptosas por el método del aminonitrilo. An. Quim. 1979, 75, 387–391. [Google Scholar]
- Pauchert, C.J. The Aldrich Library of Infrared Spectra, 3rd ed.; Aldrich Chemical Company: Milwaukee, WI, USA, 1981; p. 518. [Google Scholar]
- Nakanishi, K.; Solomon, P.H. Infrared Absorption Spectroscopy, 2nd ed.; Holden-Day: San Francisco, CA, USA, 1977; pp. 42, 64. [Google Scholar]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules; Halsted Press: London, UK, 1975; Volume 1, pp. 294–298. [Google Scholar]
- Coxon, B.; Fletcher, H.G., Jr. Two Nitriles Derived from 2,3,4,6-Tetra-O-acetyl-α-D-glucopyranosyl Bromide. A 2-Cyano-2-methyl-1,3-dioxolane Derivative. J. Am. Chem. Soc. 1963, 85, 2637–2642. [Google Scholar] [CrossRef]
- Coxon, B.; Fletcher, H.G., Jr. The Structure of 2,3,4,6-Tetra-O-acetyl-β-D-galactopyranosyl Cyanide and Some Derivatives Therefrom. Synthesis of 1-Deoxy-D-galacto-heptulose. J. Am. Chem. Soc. 1964, 86, 922–926. [Google Scholar]
- Coxon, B. Studies of synthesis and conformation in the D-ribopyranose series. Tetrahedron 1966, 22, 2281–2302. [Google Scholar] [CrossRef]
- El Khadem, H.S.; El Ashry, E.S. Synthesis of a C-nucleoside analog of the antibiotic cordycepin. Carbohydr. Res. 1974, 32, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Acton, E.M.; Fujiwara, A.N.; Goodman, L.; Henry, D.W. Synthetic C-nucleosides: 3-(α-and β-D-arabinofuranosyl)pyrazolo[4,3-d]pyrimidine-5,7-diones. Carbohydr. Res. 1974, 33, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Huynh-Dinh, T.; Gouyette, C.; Igolen, J. Cyano-1 ceto-3 et -4 L-rhamnoses. Tetrahedron Lett. 1980, 21, 4499–4502. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Bemiller, J.N. Synthesis of 2,3-dideoxy-d-hex-2-enopyranosyl cyanides. Carbohydr. Res. 1982, 108, 229–235. [Google Scholar] [CrossRef]
- Deulofeu, V. The acylated nitriles of aldonic acids and their degradation. Adv. Carbohydr. Chem. 1949, 4, 119–151. [Google Scholar]
- Bourgeois, J.-M. Synthèse de sucres aminés ramifiés III. Synthèse et réactions de 1′α-amino-nitrile dérivé du di-O-isopropylidène-1,2:5,6-α-D-ribo-hexofurannosul-3-ose. Helv. Chim. Acta 1975, 58, 363–372. [Google Scholar] [CrossRef]
- Tu, A.T.; Lidde, W.K.; Lee, Y.C.; Myers, R.W. Characterisation of some anomeric pairs of per-O-acetylated aldohexopyranosyl cyanides by laser-Raman spectroscopy. Carbohydr. Res. 1983, 117, 291–297. [Google Scholar] [CrossRef]
- Myers, R.W.; Lee, Y.C. Synthesis and characterization of some anomeric pairs of per-O-acetylated aldohexopyranosyl cyanides (per-O-acetylated 2,6-anhydroheptononitriles). On the reaction of per-O-acetylaldohexopyranosyl bromides with mercuric cyanide in nitromethane. Carbohydr. Res. 1984, 132, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Affolter, C.; Herrera, A.; Martínez, R. Determinación Estructural de Compuestos Orgánicos; Springer-Verlag Ibérica: Madrid, Spain, 2001; p. 126. [Google Scholar]
- Deulofeu, V. A Rule for the Rotatory Direction of the Acetylated Aldonic Nitriles. Nature 1933, 131, 548. [Google Scholar] [CrossRef]
- El Khadem, H.S. (Ed.) Synthetic Methods for Carbohydrates; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1976; Volume 39, Chapter 5. [Google Scholar]
- El Khadem, H.S. A generalized, circular dichroism rule for nitrogen heterocycles attached to alditol residues. Carbohydr. Res. 1977, 59, 11–18. [Google Scholar] [CrossRef]
- El Khadem, H.S.; El Shafei, Z.M. A generalised rotation rule for heterocyclic and aromatic compounds. Tetrahedron Lett. 1963, 4, 1887–1889. [Google Scholar] [CrossRef]
- Avalos, M.; Babiano, R.; Cintas, P.; Clemente, F.R.; Gordillo, R.; Jiménez, J.L.; Palacios, J.C. Experimental and Theoretical Insights Regarding the Cycloaddition Reaction of Carbohydrate-Based 1,2-Diaza-1,3-butadienes and Acrylonitrile.A Model Case for the Behavior of Chiral Azoalkenes and Unsymmetric Olefins. J. Org. Chem. 2002, 67, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, E.; Cintas, P.; Palacios, J.C. Amphipathic 1,3-oxazolidines from N-alkyl glucamines and benzaldehydes: Stereochemical and mechanistic studies. New J. Chem. 2021, 45, 4365–4386. [Google Scholar] [CrossRef]
- Kuhn, R.; Kirschenlohr, W. Synthese von Aminozuckern durch Halbhydrierung von Amino-nitrilen. Angew. Chem. 1955, 67, 786. [Google Scholar] [CrossRef]
- Kuhn, R.; Kirschenlohr, W. Darstellung von N-Acetyl-lactosamin (4-β-D-Galaktopyranosyl-2-desoxy-2-acetamino- D-glucopyranose) aus Lactose. Aminozucker-synthesen IV. Justus Liebigs Ann. Chem. 1956, 600, 135–143. [Google Scholar] [CrossRef]
- Kuhn, R.; Bister, W. Aminozucker-Synthesen VIII. N-Substituierte D-und L-Glucosamine. Justus Liebigs Ann. Chem. 1957, 602, 217–227. [Google Scholar] [CrossRef]
- Kuhn, R.; Bister, W. Aminozucker-Synthesen XII. D-Idosamin und D-Gulosamin. Justus Liebigs Ann. Chem. 1958, 617, 92–108. [Google Scholar] [CrossRef]
- Kuhn, R.; Fischer, H. Aminozucker-Synthesen X. D-Talosamin. Justus Liebigs Ann. Chem. 1958, 612, 65–67. [Google Scholar] [CrossRef]
- Kuhn, R.; Fischer, H. Aminozucker-Synthesen XI. D-Altrosamin und D-Allosamin. Justus Liebigs Ann. Chem. 1958, 617, 88–91. [Google Scholar] [CrossRef]
- Kuhn, R.; Fischer, H. Aminozucker-Synthesen, XXII. Über D-Threosamin und D-Erythrosamin. Justus Liebigs Ann. Chem. 1961, 641, 152–160. [Google Scholar] [CrossRef]
- Kuhn, R.; Jochims, J.C. Aminozucker-Synthesen, XXI.N-[Fluorenyl-(9)]-und N-Diphenylmethyl-hexosaminsäurenitrile Eine neue Synthese von Allosamin und Talosamin. Justus Liebigs Ann. Chem. 1961, 641, 143–152. [Google Scholar] [CrossRef]
- Kuhn, R.; Baschang, G. Aminozucker-Synthesen, XVII Die vier Pentosamine (2-Amino-2-desoxy-pentosen) der d-Reihe. Justus Liebigs Ann. Chem. 1959, 628, 193–205. [Google Scholar] [CrossRef]
- Horton, D.; Liav, A. A synthesis of 2-amino-2,6-dideoxy-D-allose and -D-altrose hydrochlorides and their tetraacetyl derivatives. Carbohydr. Res. 1976, 47, 326–331. [Google Scholar] [CrossRef]
- Brigl, P.; Mühlschlegel, H.; Schinle, R. Kohlenhydrate, XI. Mitteil.: Über die al-Glucose. Ber. Dtsch. Chem. Ges. 1931, 64, 2921–2934. [Google Scholar] [CrossRef]
- Anslyn, E.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, USA, 2006; p. 455. [Google Scholar]
- Charton, M.; Charton, B.I. Steric effects.11. Substituents at sulfur. J. Org. Chem. 1978, 43, 1161–1165. [Google Scholar] [CrossRef]
- Charton, M.; Charton, B.I. Steric effects.13. Composition of the steric parameter as a function of alkyl branching. J. Org. Chem. 1978, 43, 3995–4001. [Google Scholar] [CrossRef]
- Taft, R.W. Steric Effects in Organic Chemistry; Newman, M.S., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1956; Chapter 13. [Google Scholar]
- Höfle, G.; Steglich, W.H.; Vorbrüggen, H. 4-Dialkylaminopyridines as Highly Active Acylation Catalysts. [New synthetic method (25)]. Angew. Chem. Int. Ed. Engl. 1978, 17, 569–583. [Google Scholar] [CrossRef]
- Berry, D.J.; DiGiovanna, C.V.; Metrick, S.S.; Murugan, R. Catalysis by 4-dialkylaminopyridines. Arkivoc 2001, 1, 201–226. [Google Scholar] [CrossRef]
- Xu, S.; Held, I.; Kempf, B.; Mayr, H.; Steglich, W.; Zipse, H. The DMAP-Catalyzed Acetylation of Alcohols-A Mechanistic Study (DMAP=4-(Dimethylamino)pyridine). Chem. Eur. J. 2005, 11, 4751–4757. [Google Scholar] [CrossRef] [PubMed]
- Avalos, M.; Babiano, R.; Barneto, J.L.; Cintas, P.; Clemente, F.R.; Jiménez, J.L.; Palacios, J.C. Conformation of Secondary Amides. A Predictive Algorithm That Correlates DFT-Calculated Structures and Experimental Proton Chemical Shifts. J. Org. Chem. 2003, 68, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Lanyon-Hogg, T.; Ritzefeld, M.; Masumoto, N.; Magee, A.I.; Rzepa, H.S.; Tate, E.W. Modulation of Amide Bond Rotamers in 5-Acyl-6,7-dihydrothieno[3,2-c]pyridines. J. Org. Chem. 2015, 80, 4370–4377. [Google Scholar] [CrossRef] [PubMed]
- Biermann, C.J.; McGinnis, G.D. Analysis of Carbohydrates by GLC and MS; CRC Press Inc.: Boca Raton, FL, USA, 1989; p. 11. [Google Scholar]
- Szafranek, J.; Pfaffenberger, C.D.; Horning, E.C.C. The mass spectra of some per-O-acetylaldononitriles. Carbohydr. Res. 1974, 38, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.; Wander, J.D. Conformation of acyclic derivatives of sugars. XI. Conformations of the D-aldopentose diethyl and diphenyl dithioacetals in solution. J. Org. Chem. 1974, 39, 1859–1863. [Google Scholar] [CrossRef]
- Sweeting, M.; Coxon, B.; Varma, R. Conformational analysis of peracetylated hexononitriles. Carbohydr. Res. 1979, 72, 43–55. [Google Scholar] [CrossRef]
- Hooft, R. COLLECT: Data Collection Software; Nonius BV: Delft, The Netherlands, 1998. [Google Scholar]
- Otwinowski, Z.; Minor, W. Macromolecular Crystallography Part A. In Methods in Enzymology; Carter, C.W., Jr., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Watkin, D.M.; Prout, C.K.; Pearce, L.J. CAMERON (X-LIB version). A Molecular Graphics Package; Chemical Crystallography Laboratory, University of Oxford: Oxford, UK, 1996. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Hehre, W.J.; Radom, L.; Schleyer, P.v.R.; Pople, J.A. Ab initio Molecular Orbital Theory; John Wiley & Sons, Inc.: New York, NY, USA, 1986. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).