Abstract
An efficient dearomative (3 + 2) cycloaddition of para-quinamines and 2-nitrobenzofurans has been developed. This reaction proceeds smoothly under mild conditions and affords a series of benzofuro[3,2-b]indol-3-one derivatives in good to excellent yields (up to 98%) with perfect diastereoselectivities (all cases > 20:1 dr). The scale-up synthesis and versatile derivatizations demonstrate the potential synthetic application of the protocol. A plausible reaction mechanism is also proposed to account for the observed reaction process. This work represents the first instance of the N-triggered dearomative (3 + 2) cycloaddition of 2-nitrobenzofurans.
1. Introduction
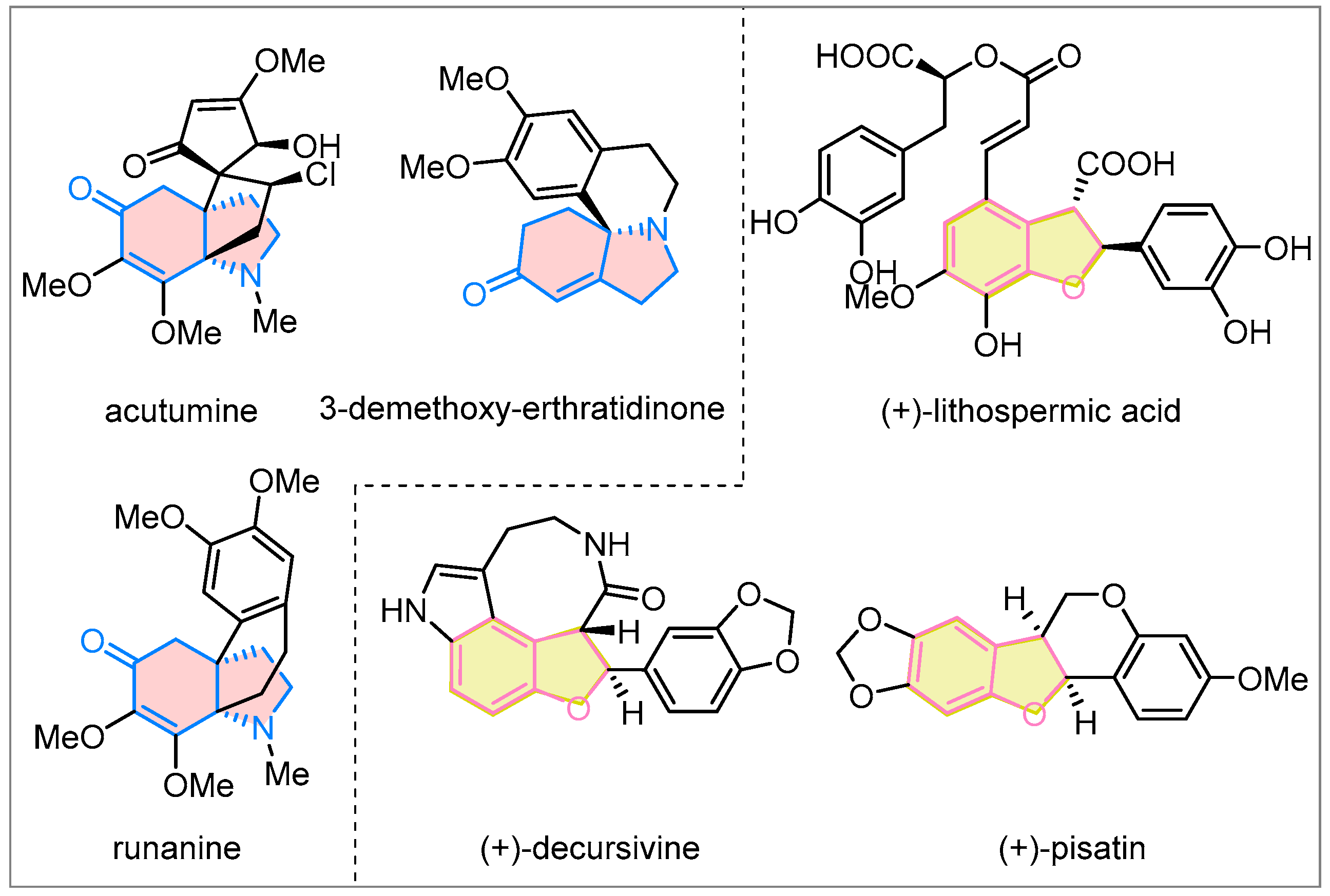
Polycyclic frameworks are not only commonly found in biologically active molecules and natural products [1,2,3,4,5], but they also have the potential to provide structural foundations for fragment-based drug discovery [6,7,8,9,10]. The ring-fusion strategy has been one of the most powerful methods for efficiently constructing polycyclic frameworks [11,12,13,14,15,16]. Hydroindoline-5-one and 2,3-dihydrobenzofuran are abundant bioactive core structures (Figure 1) [17,18,19,20,21,22]. In fact, most natural products and their analogs containing hydroindoline-5-one and 2,3-dihydrobenzofuran cores exhibit promising biological properties, such as selective T-cell cytotoxicity and antimalarial, anti-HIV, and antifungal activity [20,23,24,25]. In this context, developing effective and innovative approaches for fusing these two frameworks to construct structurally diverse polycyclic compounds is appealing and highly desirable.
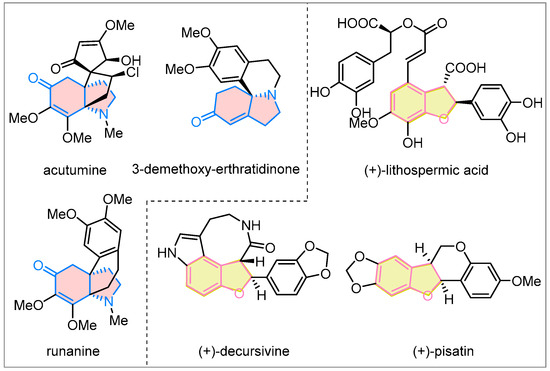
Figure 1.
Natural products containing hydroindoline-5-one or 2,3-dihydrobenzofuran cores.
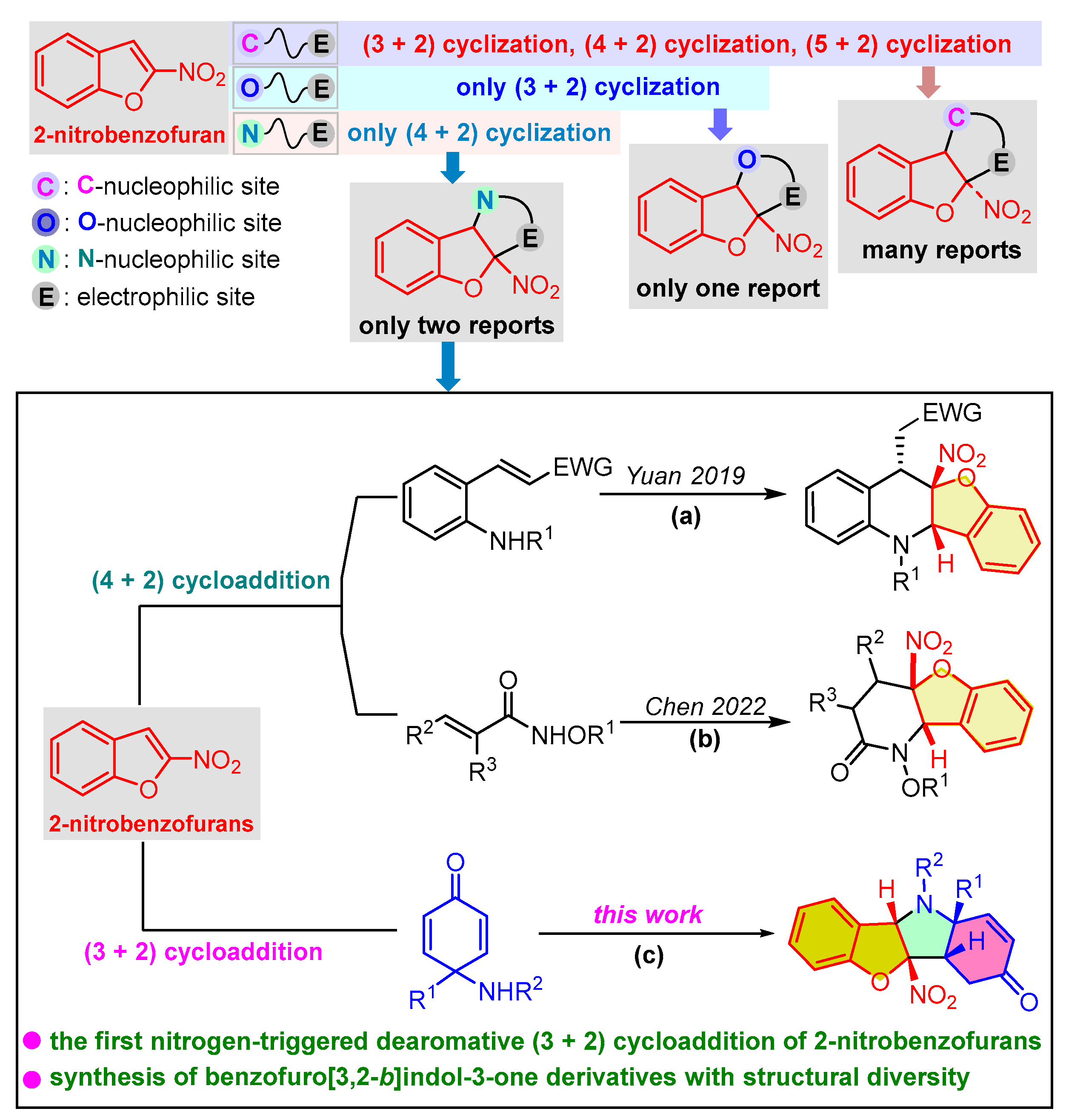
In recent years, extensive research on the dearomative cyclization reaction of 2-nitrobenzofuran has revealed the significant potential of this strategy in the construction of polycyclic compounds containing a 2,3-dihydrobenzofuran core (Scheme 1, top) [26,27,28]. Among the numerous transformations with respect to the dearomative cyclization reaction of 2-nitrobenzofurans, the carbon nucleophile-triggered dearomative (3 + 2), (4 + 2), and (5 + 2) cyclization reactions have been extensively studied [29,30,31,32,33,34,35,36]. In contrast, there have been limited reports on oxygen or nitrogen nucleophile-triggered dearomative cyclization reactions so far. You and co-workers reported on the only reaction of oxygen nucleophile-triggered dearomative (3 + 2) cyclization between 2-nitrobenzofurans and epoxybutenes for the straightforward construction of tetrahydrofurobenzofurans with a chiral palladium catalyst [37]. In addition, there are two reports so far on dearomative cyclization reactions of 2-nitrobenzofurans triggered by nitrogen nucleophiles [38,39]. One is the asymmetric dearomative (4 + 2) cycloaddition reaction reported by our group, involving 2-nitrobenzofurans and 2-aminochalcones to construct tetrahydrobenzofuro[3,2-b]quinolines by using a chiral squaramide catalyst (Scheme 1a) [38]. The other is the base-catalyzed (4 + 2) cycloaddition reaction between 2-nitrobenzofurans and N-alkoxyacrylamides to access [3,2-b]benzofuropyridinones (Scheme 1b) [39]. These precedents have demonstrated the great potential of heteroatomic nucleophile-driven dearomative cyclization reaction of 2-nitrobenzofurans in the construction of complex polyheterocyclic compounds. On the other hand, much attention has been paid to the application of para-quinamines as three-atom building blocks for the cascade reaction to construct nitrogen-containing heterocycles with structural diversity [40,41,42,43,44,45,46,47,48]. Para-quinamines are often used as N-nucleophiles to undergo (3 + 2) cycloaddition with two-atom reaction partners, thus leading to the formation of hydroindoline-5-one scaffolds [49,50,51,52,53]. Therefore, we speculated that conducting the dearomative (3 + 2) cycloaddition reaction between para-quinamines and 2-nitrobenzofurans would lead to the fusion of hydroindoline-5-one and 2,3-dihydrobenzofuran. As part of our ongoing research on the dearomatization of electron-deficient heteroarenes [54,55,56,57], herein, we will report the first nitrogen nucleophile-triggered dearomative (3 + 2) cycloaddition reaction of 2-nitrobenzofurans by using para-quinamines to construct benzofuro[3,2-b]indol-3-one skeletons (Scheme 1c).
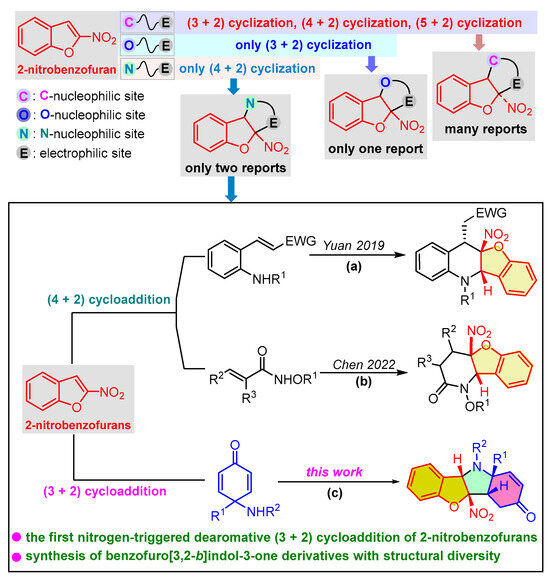
Scheme 1.
Profile of dearomative cyclization reactions of 2-nitrobenzofurans [38,39].
2. Results and Discussion
In the early stage of the experiment, we chose potassium tert-butoxide (tBuOK) as the base to verify the possibility of the (3 + 2) cycloaddition reaction between para-quinamine 1a and 2-nitrobenzofuran 2a at 65 °C with acetonitrile as the solvent. To our delight, we found that the reaction proceeded smoothly under these conditions and produced the cycloadduct 3aa in 51% isolated yield with >20:1 dr value (Table 1, entry 1). With this promising result in hand, we continued our investigations on other types of bases, and the outcomes indicated that inorganic bases have an advantage over organic bases for the (3 + 2) cycloaddition transformation (Table 1, entries 2–4, 7, and 8 vs. 5 and 6). Among them, K2CO3 serving as a base could produce a better result (Table 1, entry 4). Afterward, we further examined the influence of the solvent on the reaction. When acetonitrile was changed to ethyl acetate, tetrahydrofuran, methyl tertiary butyl ether, alcohol, acetone, or ethyl acetate, the reaction did not show a positive influence on improving the yield of 3aa (Table 1, entries 9–15). Subsequently, the ratio of the starting materials 1a to 2a was also thoroughly investigated (Table 1, entries 16 and 17). Adjusting the ratio of 1a/2a from 1:1.2 to 1.2:1 could elevate the yield of product 3aa to 88% (Table 1, entry 16). Moreover, by changing the ratio of 1a/2a to 1.5:1, the dearomative (3 + 2) cycloaddition reaction could proceed smoothly and furnish product 3aa at a 95% yield (Table 1, entry 17). Conducting the reaction at 80 °C did not lead to an increase in the yield of 3aa (Table 1, entry 18). Delightfully, reducing the amount of K2CO3 from 2.0 equiv to 1.0 equiv had no negative impact on the yield of product 3aa (Table 1, entry 19). We also examined strong bases, including NaH and LiHMDS, and found that the reaction time was significantly reduced. However, the isolated yield of product 3aa did not show any further improvement.

Table 1.
Optimization of reaction conditions [a].
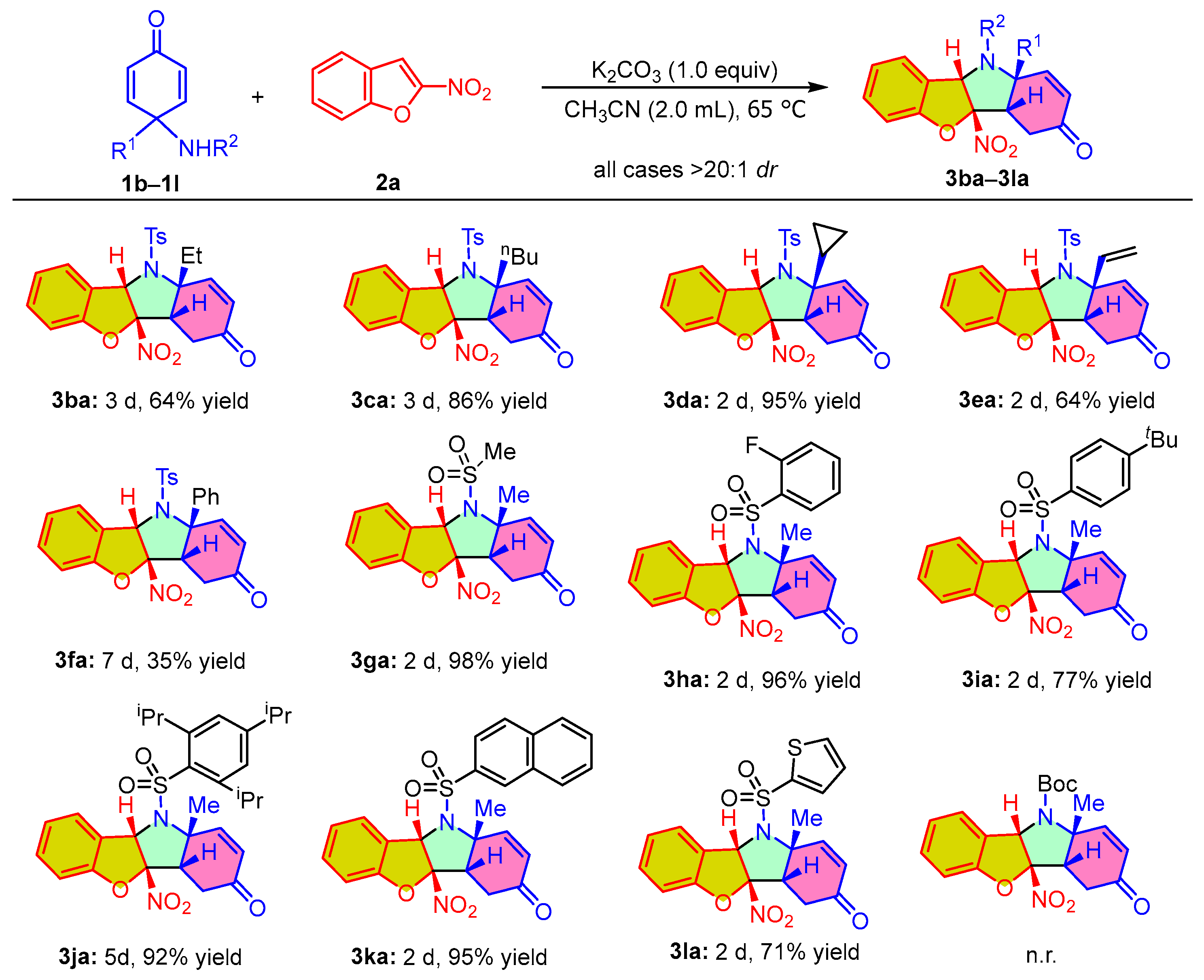
After successfully obtaining the optimal conditions of the dearomative (3 + 2) cycloaddition reaction, we next investigated the substrate adaptability of this protocol by using various para-quinamines 1 to react with 2-nitrobenzofuran 2a. As shown in Scheme 2, the reactions showed good adaptability with para-quinamines and furnished a series of benzofuro[3,2-b]indol-3-one derivatives in excellent diastereoselectivities (all cases > 20:1 dr). Substrates 1b–1d equipped with a linear or cyclic alkyl group (Et, tBu, or cyclopropyl) could smoothly react with 2a under the standard conditions, producing the corresponding cycloadducts 3ba–3da in good to high yields. Para-quinamine 1e bearing a vinyl substituent also could smoothly react with 2-nitrobenzofuran 2a and produce product 3ea at 64% yield. However, when installing a phenyl group into the para-quinamine, the reactivity of the reaction significantly decreased. Even extending the reaction time to seven days, the corresponding product 3fa could be obtained only at 35% yield. On the other hand, we also investigated the influence of the substituents attached to the nitrogen atom of para-quinamine on the dearomative (3 + 2) cycloaddition reaction. It was found that the methanesulfonyl-substituted para-quinamine was compatible with the developed cycloaddition reaction, leading to the formation of product 3ga at 98% yield. In addition, we also found that the developed protocol exhibited tolerance towards para-quinamine 1h–1j bearing various substituted phenylsulfonyl groups, allowing for the successful synthesis of the desired cycloadducts 3ha–3ja at 77–96% yield. Moreover, introducing the naphthalenesulfonyl group or heteroaromatic cyclic sulfonyl group into para-quinamines did not significantly affect the dearomative (3 + 2) cycloaddition reaction, as exemplified by the formation of products 3ka and 3la at 95% and 71% yield, respectively. When we replaced the sulfonyl substituent with the Boc group, the corresponding dearomative (3 + 2) cycloaddition reaction did not occur under the standard conditions, which indicated that the proton attached to the nitrogen is not sufficiently acidic to be deprotonated by K2CO3.

Scheme 2.
Scope study on the dearomative (3 + 2) cycloaddition reaction of various para-quinamines 1 with 2-nitrobenzofuran 2a. Reaction conditions: unless otherwise noted, the reactions were carried out with 1 (0.15 mmol), 2a (0.10 mmol), and K2CO3 (1.0 equiv) in 2.0 mL of acetonitrile at 65 °C for the specified reaction time. The dr values were determined by 1H NMR analysis. The yields refer to the isolated yield of product. n.r. = no reaction.
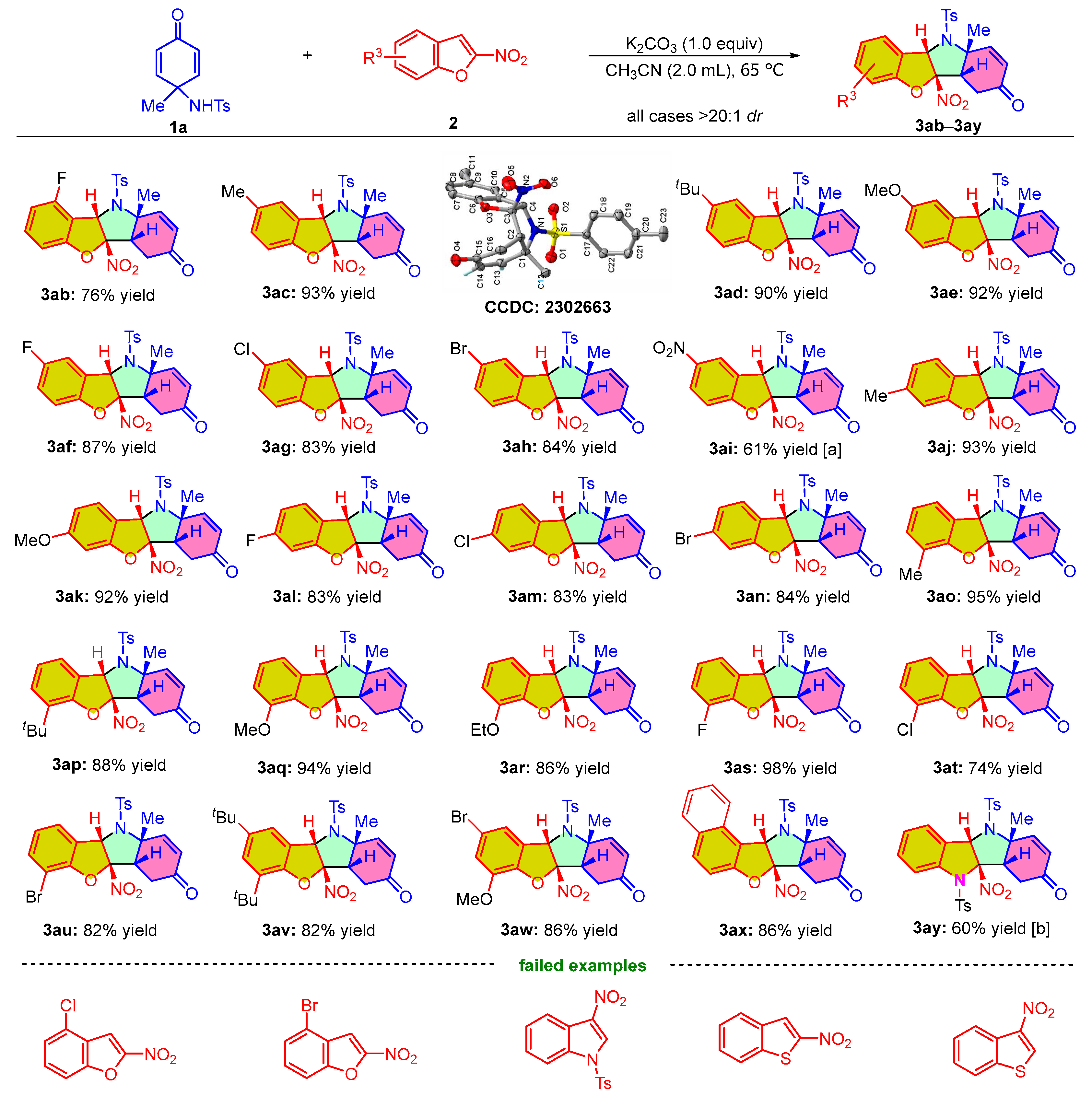
The scope of substrates with respect to 2-nitrobenzofurans 2 for the developed protocol was next tested. As shown in Scheme 3, all the reactions that occurred were able to produce polyheterocyclic compounds with excellent diastereoselectivities (>20:1 dr). 4-fluoro-2-nitrobenzofuran 1b could smoothly react with para-quinamine 2a to produce the corresponding product 3ab at 76% yield. However, when the fluorine atom was replaced with a bromine or chlorine atom, perhaps due to the increasing steric hindrance limiting the transformation, the reactions with the substrates did not occur under standard conditions. We also examined the different substituents at C5-position of the 2-nitrobenzofurans and found that substrates 2c–2e with electron-donating groups (Me-, tBu-, MeO-) exhibited better performance for providing the corresponding products 3ac–3ae at 90–93% yield. In contrast, 2-nitrobenzofurans 2f–2i bearing an electron-withdrawing substituent (F-, Cl-, Br-, NO2-) at C5-position could furnish the cycloaddition products 3af–3ai at 61–87% isolated yield. As to the reaction of the C6-substituted 2-nitrobenzofurans with 1a, regardless of the electron-donating (Me-, MeO-) or electron-withdrawing substitution (F-, Cl-, Br-), it was found that the transformations proceeded smoothly and generated their respective cycloadducts 3aj–3an at 83–93% yield. Likewise, upon incorporating an electron-donating (Me-, tBu-, MeO-, EtO-) or electron-withdrawing substituent (F-, Cl-, Br-) at C7-position, the corresponding 2-nitrobenzofurans 2ao–2au were also applicable to the dearomative (3 + 2) cycloaddition reaction, affording cyclicadducts 3ao–3au at 74–98% yield. Moreover, the reactions between 2-nitrobenzofurans bearing two substituents and para-quinamine 1a proceeded smoothly and gave rise to the desired products 3av and 3aw at 82% and 86% yield, respectively. In addition, 2-nitronaphtho[2,1-b]furan 2x was added to the (3 + 2) cycloaddition reaction to generate product 3ax at 86% yield. However, the dearomative (3 + 2) cycloaddition reaction of 2-nitroindole 2y and 1a required Cs2CO3 as the base to initiate the reaction, delivering the corresponding product 3ay at 60% yield. Unfortunately, the dearomative (3 + 2) cycloaddition reactions with 3-nitroindole and 2- and 3-nitrobenzo[b]thiophene as substrates did not proceed under the developed conditions. Ultimately, we performed single crystal growth and X-ray diffraction analysis to verify the structure and relative configuration of product 3ac (CCDC 2302663 contains the supplementary crystallographic data for this paper. For details, see the Supporting Information).
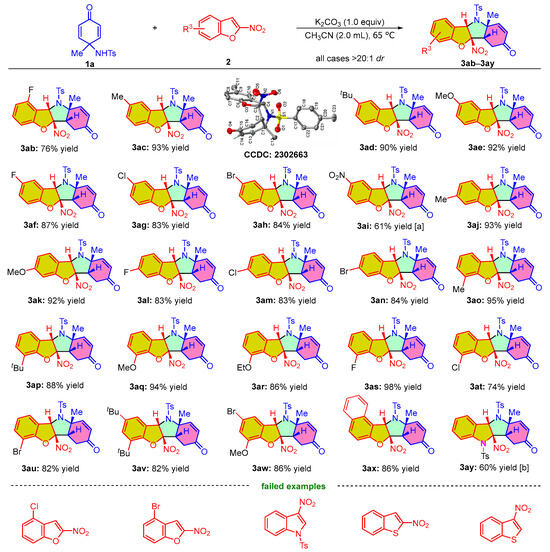
Scheme 3.
Substrate scope on the dearomative (3 + 2) cycloaddition reaction of various 2-nitrobenzofurans 2 with para-quinamines 1a. Reaction conditions: unless otherwise noted, the reactions were carried out with 1a (0.15 mmol), 2 (0.10 mmol), and K2CO3 (1.0 equiv) in 2.0 mL of acetonitrile at 65 °C for 2 days. The dr values were determined by 1H NMR analysis. The yields refer to the isolated yield of product. [a] The reaction was carried out for 3 days. [b] The reaction was carried out with 1a (0.15 mmol), 2y (0.10 mmol), and Cs2CO3 (1.0 equiv) in 2.0 mL of acetonitrile at 25 °C for 12 h.
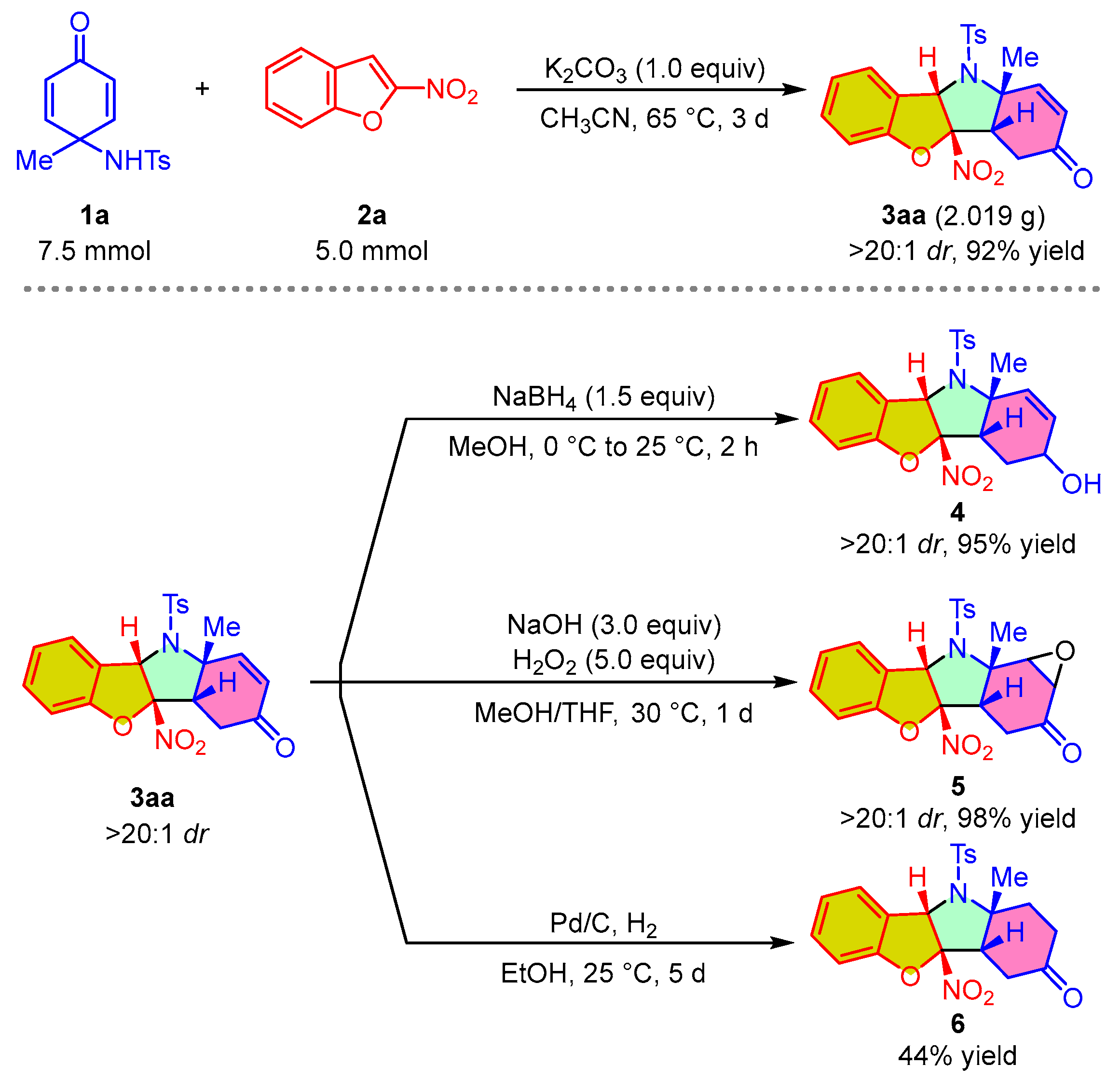
To demonstrate the practicality and scalability of this methodology, we performed scale-up preparation and diverse transformations of product 3aa. As shown in Scheme 4, the dearomative (3 + 2) cycloaddition reaction of 1a and 2a was performed on a preparative scale of 5.0 mmol under standard reaction conditions to generate product 3aa at 92% isolated yield with >20:1 dr. The treatment of compound 3aa with sodium borohydride in methanol at room temperature resulted in the selective reduction of the carbonyl group in 3aa, leading to the formation of product 4 at 95% yield with >20:1 dr. In addition, treating 3aa with H2O2 as an oxidizing agent under alkaline conditions led to the efficient epoxidation of the double bond, affording product 5 at 95% yield with no loss of diastereoselectivity. The conversion of compound 3aa into 6 was achieved with the combination of Pd/C and H2 in ethyl alcohol at room temperature, but only a moderate yield.

Scheme 4.
Scale-up synthesis and synthetic transformations of 3aa.
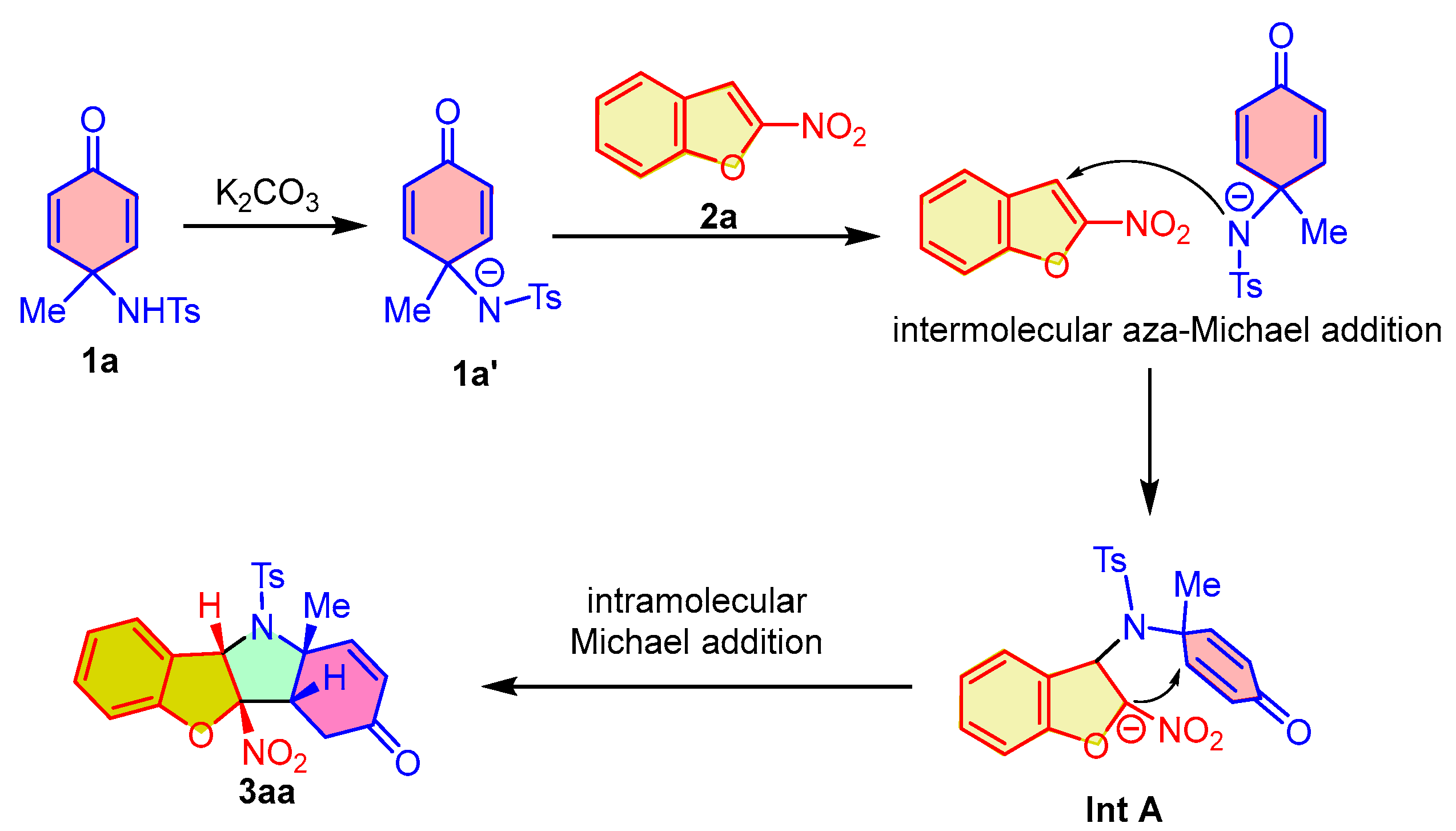
In order to further provide a more comprehensive explanation for the dearomative (3 + 2) cycloaddition reaction, we propose a possible reaction mechanism according to the experimental results. As outlined in Scheme 5, using the reaction between para-quinamine 1a and 2-nitrobenzofuran 2a as an example, under the promotion of an inorganic base, the in situ-generated intermediate 1a′ from 1a undergoes an intermolecular aza-Michael reaction by attacking the C3-position of 2-nitrobenzofuran 2a, which leads to the dearomatization of 2-nitrobenzofuran and the formation of intermediate Int A. Subsequently, an intramolecular Michael addition of intermediate Int A occurs and gives rise to the fusion of hydroindoline-5-one and 2,3-dihydrobenzofuran, thus producing the polycyclic benzofuro[3,2-b]indol-3-one 3aa.
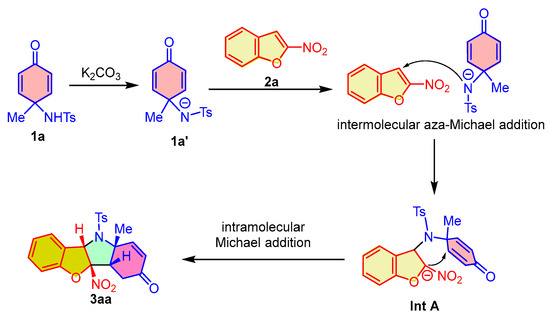
Scheme 5.
Reaction mechanism for the dearomative (3 + 2) cycloaddition of para-quinamines and 2-nitrobenzofurans.
3. Materials and Methods
3.1. General Information
Reagents were purchased from commercial sources and were used as received unless mentioned otherwise. 2-nitrobenzofurans were prepared according to the procedure described by Ackermann and co-workers [58]. Para-quinamines were prepared according to the procedure described by Ackermann and co-workers [45,59]. Flash column chromatography was performed over silica gel (H, purchased from Qingdao Ocean Chemical Co., Ltd. Qingdao, China). Analytical thin-layer chromatography (TLC) was performed on silica gel HSGF254 glass plates (purchased from Yantai Xinuo Chemical Co., Ltd. Yantai, China) containing a 254 nm fluorescent indicator. Proton nuclear magnetic resonance (1H NMR) spectra were measured on a Bruker AVANCE NEO 400 MHz spectrometer (Billerica, MA, USA) in ambient temperature at 400 MHz. Proton chemical shifts are reported in parts per million (δ scale) and are referenced using tetramethylsilane (TMS) as an internal standard or residual protium in the NMR solvent (CDCl3: δ 7.26 (CHCl3) or DMSO-d6: δ 2.50 (CD2HSOCD3)). Data are reported as follows: chemical shift [multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = doublet of doublets, td = triplet of doublets, brs = broad singlet), coupling constant(s) (Hz), integration]. Carbon-13 nuclear magnetic resonance (13C NMR) spectra were also measured on a Bruker AVANCE NEO 400 MHz spectrometer in ambient temperature of 13C at 101MHz. Carbon chemical shifts are reported in parts per million (δ scale), and referenced using the carbon resonances of the solvent (δ 77.16 (CDCl3) or δ 39.52 (DMSO-d6)). 1H NMR and 13C NMR spectra were recorded in CDCl3 or DMSO-d6. 1H NMR chemical shifts are reported in ppm employed as the internal standard (CDCl3 at 7.26 ppm, DMSO-d6 at 2.50 ppm). The melting points of products were recorded on a Büchi Melting Point B-545 and temperatures were not corrected. High-resolution mass spectra (HRMS) were recorded by an Agilent 6545 LC/Q-TOF mass spectrometer by using an electrospray ionization (ESI) source analyzed by quadrupole time-of-flight (Q-TOF). Mass error of HRMS data is maintained below 5 ppm.
3.2. General Experimental Procedures for Synthesis of Compounds 3 (Scheme 2 and Scheme 3)
- 10a-Methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3aa): white solid; 41.6 mg, 95% yield; m.p. 203.1–204.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 8.3 Hz, 2H), 7.73 (d, J = 7.5 Hz, 1H), 7.49 (d, J = 8.2 Hz, 2H), 7.38 (m, 1H), 7.16 (m, 1H), 6.92 (d, J = 8.1 Hz, 1H), 6.58 (s, 1H), 6.38 (d, J = 11.7 Hz, 1H), 5.50 (d, J = 10.3 Hz, 1H), 3.59 (d, J = 6.0 Hz, 1H), 2.88 (dd, J = 18.1, 6.2 Hz, 1H), 2.58 (d, J = 18.1 Hz, 1H), 2.44 (s, 3H), 1.44 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.3, 158.1, 148.3, 143.9, 140.1, 131.5, 130.1, 127.6, 127.3, 126.7, 125.1, 123.9, 119.8, 110.1, 71.0, 64.5, 52.6, 32.8, 22.2, 21.0; HRMS (ESI) m/z [M + H]+ Calcd for C22H21N2O6S: 441.1115; found 441.1116.
- 10a-Ethyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ba): white solid; 29.1 mg, 64% yield; m.p. 192.5–193.2 °C; 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 7.6 Hz, 1H), 7.85 (d, J = 7.9 Hz, 2H), 7.38 (d, J = 7.9 Hz, 2H), 7.33 (m, 1H), 7.11 (m, 1H), 6.87 (d, J = 8.2 Hz, 1H), 6.56 (d, J = 10.5 Hz, 1H), 6.24 (s, 1H), 5.67 (d, J = 10.5 Hz, 1H), 3.55 (d, J = 6.2 Hz, 1H), 2.87 (d, J = 18.3 Hz, 1H), 2.57 (dd, J = 18.3, 6.4 Hz, 1H), 2.48 (s, 3H), 2.06 (m, 1H), 1.85 (m, 1H), 0.69 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 193.2, 158.6, 147.4, 144.4, 140.4, 131.8, 130.2, 129.2, 128.7, 127.1, 124.5, 124.3, 119.8, 110.5, 72.4, 68.4, 51.3, 34.2, 29.0, 21.8, 8.9; HRMS (ESI) m/z: [M + Na]+ Calcd for C23H22N2O6SNa: 477.1091, found 477.1064.
- 10a-Butyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ca): white solid; 41.6 mg, 86% yield; m.p. 182.8–183.6 °C; 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 7.6 Hz, 1H), 7.85 (d, J = 7.9 Hz, 2H), 7.38 (d, J = 7.9 Hz, 2H), 7.33 (m, 1H), 7.11 (m, 1H), 6.87 (d, J = 8.2 Hz, 1H), 6.56 (d, J = 10.5 Hz, 1H), 6.24 (s, 1H), 5.67 (d, J = 10.5 Hz, 1H), 3.55 (d, J = 6.2 Hz, 1H), 2.87 (d, J = 18.3 Hz, 1H), 2.57 (dd, J = 18.3, 6.4 Hz, 1H), 2.48 (s, 3H), 2.06 (m, 1H), 1.85 (m, 1H), 0.69 (t, J = 7.4 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 193.2, 158.5, 147.6, 144.4, 140.3, 131.8, 130.1, 128.8, 128.7, 127.1, 124.5, 124.3, 119.7, 110.4, 72.3, 68.0, 51.5, 36.0, 34.1, 26.6, 22.9, 21.7, 13.6; HRMS (ESI) m/z: [M + H]+ Calcd for C25H27N2O6S: 483.1584, found 483.1587.
- 10a-Cyclopropyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3da): white solid; 44.2 mg, 95% yield; m.p. 231.5–232.3 °C; 1H NMR (400 MHz, CDCl3) δ 7.92 (m, 3H), 7.34 (m, 3H), 7.12 (m, 1H), 6.86 (d, J = 8.1 Hz, 1H), 6.32 (s, 1H), 6.28 (d, J = 10.5 Hz, 1H), 5.67 (d, J = 10.5 Hz, 1H), 3.65 (d, J = 5.8 Hz, 1H), 2.82 (d, J = 18.1 Hz, 1H), 2.66 (dd, J = 18.2, 6.2 Hz, 1H), 2.46 (s, 3H), 0.58 (t, J = 6.9 Hz, 2H), 0.48 (m, 1H), 0.28 (m, 1H), 0.17–0.09 (m, 1H); 13C{1H} NMR (101 MHz, CDCl3) δ 193.3, 158.4, 144.3, 142.3, 140.5, 131.8, 131.6, 129.9, 128.4, 127.1, 124.7, 124.3, 119.6, 110.4, 72.5, 67.8, 55.3, 33.6, 21.7, 16.5, 3.4, 2.1; HRMS (ESI) m/z: [M + H]+ Calcd for C24H23N2O6S: 467.1271, found 467.1272.
- 4b-Nitro-10-tosyl-10a-vinyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ea): white solid; 29.0 mg, 64% yield; m.p. 194.9–195.7 °C; 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 7.6 Hz, 1H), 7.83 (d, J = 7.9 Hz, 2H), 7.36 (m, 3H), 7.14 (m, 1H), 6.89 (d, J = 8.2 Hz, 1H), 6.55 (d, J = 10.4 Hz, 1H), 6.25 (s, 1H), 5.79 (d, J = 10.4 Hz, 1H), 5.52 (dd, J = 17.2, 10.4 Hz, 1H), 5.30 (d, J = 10.4 Hz, 1H), 5.20 (d, J = 17.3 Hz, 1H), 3.51 (d, J = 5.4 Hz, 1H), 2.76 (d, J = 17.8 Hz, 1H), 2.48 (s, 3H), 2.43 (dd, J = 17.9, 6.0 Hz, 1H); 13C{1H} NMR (101 MHz, CDCl3) δ 193.2, 158.5, 144.5, 144.3, 139.7, 134.3, 131.9, 130.5, 130.0, 128.5, 127.5, 124.4, 124.3, 121.3, 119.6, 110.6, 72.0, 68.1, 53.1, 32.3, 21.8; HRMS (ESI) m/z: [M + H]+ Calcd for C23H21N2O6S: 453.1115, found 453.1116.
- 4b-Nitro-10a-phenyl-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3fa): white solid; 17.7 mg, 35% yield; m.p. 245.3–245.8 °C; 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 7.6 Hz, 1H), 7.37 (m, 1H), 7.28 (d, J = 10.9 Hz, 1H), 7.26–7.07 (m, 5H), 6.98 (m, 6H), 6.36 (s, 1H), 5.86 (d, J = 10.5 Hz, 1H), 4.08 (d, J = 4.4 Hz, 1H), 2.73 (d, J = 17.8 Hz, 1H), 2.40 (s, 3H), 2.19 (dd, J = 17.8, 5.7 Hz, 1H); 13C{1H} NMR (101 MHz, CDCl3) δ 193.2, 158.9, 146.1, 143.6, 138.9, 133.8, 131.9, 130.1, 129.4, 129.3, 129.0, 128.1, 127.2, 124.3, 124.3, 118.9, 110.4, 73.2, 68.5, 56.6, 32.6, 21.6; HRMS (ESI) m/z: [M + H]+ Calcd for C27H23N2O6S: 503.1271, found 503.1275.
- 10a-Methyl-10-(methylsulfonyl)-4b-nitro-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ga): white solid; 35.8 mg, 98% yield; m.p. 186.1–186.5 °C; 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 7.6 Hz, 1H), 7.32 (m, 1H), 7.09 (m, 1H), 6.86 (d, J = 8.2 Hz, 1H), 6.47 (d, J = 10.4 Hz, 1H), 5.94 (s, 1H), 5.59 (d, J = 10.4 Hz, 1H), 3.61 (d, J = 5.7 Hz, 1H), 3.26 (s, 3H), 2.92 (d, J = 18.0 Hz, 1H), 2.71 (dd, J = 18.0, 6.1 Hz, 1H), 1.93 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.5, 158.4, 148.3, 131.9, 128.1, 128.0, 124.3, 123.7, 119.8, 110.6, 71.3, 64.8, 54.1, 44.5, 33.1, 23.9; HRMS (ESI) m/z: [M + H]+ Calcd for C16H17N2O6S: 365.0802, found 365.0806.
- 10-((2-Fluorophenyl)sulfonyl)-10a-methyl-4b-nitro-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ha): white solid; 42.8 mg, 96% yield; m.p. 224.9–225.6 °C; 1H NMR (400 MHz, CDCl3) δ 8.00 (m, 1H), 7.88 (d, J = 7.6 Hz, 1H), 7.68 (m, 1H), 7.40–7.30 (m, 3H), 7.13 (m, 1H), 6.87 (d, J = 8.2 Hz, 1H), 6.61 (d, J = 10.4 Hz, 1H), 6.34 (s, 1H), 5.61 (d, J = 10.4 Hz, 1H), 3.57 (d, J = 5.8 Hz, 1H), 2.89 (d, J = 18.1 Hz, 1H), 2.60 (dd, J = 18.1, 6.2 Hz, 1H), 1.47 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.7, 158.9 (d, JCF = 255.4 Hz), 158.6, 148.2, 135.8 (d, JCF = 8.7 Hz), 131.9, 130.9 (d, JCF = 13.5 Hz), 129.9, 128.2 (d, JCF = 29.3 Hz), 125.0 (d, JCF = 3.7 Hz), 124.3, 124.3, 119.6, 117.6 (d, JCF = 21.4 Hz), 110.5, 71.2 (d, JCF = 7.2 Hz), 64.6, 52.8, 32.9, 23.3; HRMS(ESI) m/z: [M + H]+ Calcd for C21H18FN2O6S:445.0864, found 445.0865.
- 10-((4-(tert-Butyl)phenyl)sulfonyl)-10a-methyl-4b-nitro-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ia): white solid; 37.3 mg, 77% yield; m.p. 239.7–240.5 °C; 1H NMR (400 MHz, CDCl3) δ 7.90 (m, 3H), 7.60 (d, J = 8.1 Hz, 2H), 7.34 (m, 1H), 7.13 (m, 1H), 6.87 (d, J = 8.2 Hz, 1H), 6.54 (d, J = 10.4 Hz, 1H), 6.27 (s, 1H), 5.56 (d, J = 10.4 Hz, 1H), 3.45 (d, J = 5.6 Hz, 1H), 2.86 (d, J = 18.1 Hz, 1H), 2.58 (dd, J = 18.1, 6.1 Hz, 1H), 1.50 (s, 3H), 1.38 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.7, 158.4, 157.3, 148.6, 140.2, 131.8, 128.2, 127.8, 126.6, 126.6, 124.3, 124.3, 119.8, 110.5, 72.3, 64.5, 53.8, 35.4, 32.9, 31.2, 23.0; HRMS (ESI) m/z: [M + H]+ Calcd for C25H27N2O6S: 483.1584, found 483.1591.
- 10a-Methyl-4b-nitro-10-((2,4,6-triisopropylphenyl)sulfonyl)-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ja): white solid; 51.0 mg, 92% yield; m.p. 204.7–205.6 °C; 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 7.6 Hz, 1H), 7.32 (m, 1H), 7.24 (s, 2H), 7.10 (m, 1H), 6.99 (d, J = 10.5 Hz, 1H), 6.83 (d, J = 8.2 Hz, 1H), 6.24 (s, 1H), 5.59 (d, J = 10.5 Hz, 1H), 4.16 (m, 2H), 3.44 (d, J = 5.5 Hz, 1H), 2.93 (dd, J = 15.9, 9.1 Hz, 2H), 2.61 (dd, J = 18.2, 6.0 Hz, 1H), 1.32 (d, J = 6.7 Hz, 12H), 1.28 (d, J = 6.9 Hz, 6H), 1.24 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 193.0, 158.7, 154.3, 151.0, 147.5, 134.2, 131.7, 128.6, 128.5, 124.4, 124.1, 123.7, 119.2, 110.3, 71.1, 64.1, 53.1, 34.2, 32.8, 29.6, 25.2, 24.4, 23.9, 23.6, 23.5; HRMS (ESI) m/z: [M + H]+ Calcd for C30H37N2O6S: 553.2367, found 553.2369.
- 10a-Methyl-10-(naphthalen-2-ylsulfonyl)-4b-nitro-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ka): white solid; 45.4 mg, 95% yield; m.p. 167.9–168.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.57 (s, 1H), 8.07 (d, J = 8.7 Hz, 1H), 8.02 (d, J = 7.8 Hz, 1H), 7.95 (m, 3H), 7.69 (m, 2H), 7.36 (m, 1H), 7.16 (m, 1H), 6.89 (d, J = 8.2 Hz, 1H), 6.58 (d, J = 10.4 Hz, 1H), 6.38 (s, 1H), 5.57 (d, J = 10.4 Hz, 1H), 3.43 (d, J = 5.7 Hz, 1H), 2.86 (d, J = 18.1 Hz, 1H), 2.55 (dd, J = 18.1, 6.1 Hz, 1H), 1.48 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.6, 158.5, 148.5, 140.0, 135.1, 132.3, 132.0, 130.2, 129.5, 129.5, 128.3, 128.3, 128.2, 128.1, 127.9, 124.5, 124.2, 121.7, 119.8, 110.6, 72.6, 64.6, 54.0, 32.9, 23.1; HRMS (ESI) m/z: [M + H]+ Calcd for C25H21N2O6S: 477.1115, found 477.1125.
- 10a-Methyl-4b-nitro-10-(thiophen-2-ylsulfonyl)-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3la): white solid; 30.8 mg, 71% yield; m.p. 190.4–191.1 °C; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 7.6 Hz, 1H), 7.76 (d, J = 2.6 Hz, 1H), 7.70 (d, J = 4.9 Hz, 1H), 7.35 (m, 1H), 7.19–7.08 (m, 2H), 6.88 (d, J = 8.2 Hz, 1H), 6.45 (d, J = 10.3 Hz, 1H), 6.16 (s, 1H), 5.58 (d, J = 10.3 Hz, 1H), 3.51 (d, J = 5.7 Hz, 1H), 2.87 (d, J = 18.1 Hz, 1H), 2.63 (dd, J = 18.1, 6.2 Hz, 1H), 1.71 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 192.5, 158.4, 148.3, 143.6, 132.8, 132.7, 132.0, 128.1, 127.8, 127.6, 124.3, 123.9, 119.7, 110.6, 71.6, 65.3, 53.4, 32.9, 22.7; HRMS (ESI) m/z: [M + H]+ Calcd for C19H17N2O6S2: 433.0523, found 433.0524.
- 9-Fluoro-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ab): white solid; 35.0 mg, 76% yield; m.p. 207.8–208.7 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.92 (d, J = 8.2 Hz, 2H), 7.50 (d, J = 8.1 Hz, 2H), 7.43 (m, 1H), 6.96 (m, 1H), 6.82 (s, 1H), 6.76 (d, J = 8.2 Hz, 1H), 6.31 (d, J = 10.4 Hz, 1H), 5.45 (d, J = 10.3 Hz, 1H), 3.46 (d, J = 5.8 Hz, 1H), 2.88 (dd, J = 18.2, 6.1 Hz, 1H), 2.56 (d, J = 18.2 Hz, 1H), 2.44 (s, 3H), 1.52 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.1, 159.8 (d, JCF = 7.5 Hz), 159.1 (d, JCF = 255.2 Hz), 148.7, 144.1, 139.5, 133.6 (d, JCF = 8.9 Hz), 130.1, 127.4, 127.1, 120.5, 112.1 (d, JCF = 20.4 Hz), 111.1 (d, JCF = 20.6 Hz), 106.4 (d, JCF = 3.7 Hz), 69.3, 65.1, 53.1, 32.9, 22.1, 21.1; HRMS (ESI) m/z: [M + H]+ Calcd for C22H20FN2O6S: 459.1021, found 459.1027.
- 8,10a-Dimethyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ac): white solid; 42.2 mg, 93% yield; m.p. 227.7–228.6 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 8.3 Hz, 2H), 7.53 (s, 1H), 7.49 (d, J = 8.2 Hz, 2H), 7.18 (d, J = 8.4 Hz, 1H), 6.80 (d, J = 8.3 Hz, 1H), 6.52 (s, 1H), 6.40 (d, J = 10.3 Hz, 1H), 5.51 (d, J = 10.3 Hz, 1H), 3.57 (d, J = 5.9 Hz, 1H), 2.87 (dd, J = 18.1, 6.1 Hz, 1H), 2.57 (d, J = 18.0 Hz, 1H), 2.44 (s, 3H), 2.30 (s, 3H), 1.45 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.3, 156.2, 148.3, 143.9, 140.2, 132.9, 131.9, 130.1, 127.7, 127.3, 126.7, 125.0, 120.0, 109.7, 71.1, 64.5, 52.6, 32.8, 22.2, 21.0, 20.6; HRMS (ESI) m/z: [M + H]+ Calcd for C23H23N2O6S: 455.1271, found 455.1270.
- 8-(tert-Butyl)-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ad): white solid; 44.8 mg, 90% yield; m.p. 228.9–229.5 °C; 1H NMR (400 MHz, CDCl3) δ 7.93–7.83 (m, 3H), 7.37 (m, 3H), 6.79 (d, J = 8.6 Hz, 1H), 6.48 (d, J = 10.4 Hz, 1H), 6.22 (s, 1H), 5.58 (d, J = 10.4 Hz, 1H), 3.42 (d, J = 5.5 Hz, 1H), 2.84 (d, J = 18.1 Hz, 1H), 2.57 (dd, J = 18.1, 6.1 Hz, 1H), 2.47 (s, 3H), 1.52 (s, 3H), 1.32 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.7, 156.3, 148.5, 147.7, 144.4, 140.4, 130.2, 128.9, 127.8, 126.9, 125.0, 123.9, 120.3, 109.8, 72.4, 64.6, 53.8, 34.8, 32.9, 31.7, 23.2, 21.7; HRMS (ESI) m/z: [M + Na]+ Calcd for C26H28N2O6SNa: 519.1560, found 519.1568.
- 8-Methoxy-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ae): white solid; 43.4 mg, 92% yield; m.p. 223.7–224.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 8.2 Hz, 2H), 7.49 (d, J = 8.1 Hz, 2H), 7.26 (d, J = 2.6 Hz, 1H), 6.95 (m, 1H), 6.85 (d, J = 8.9 Hz, 1H), 6.53 (s, 1H), 6.43 (d, J = 10.4 Hz, 1H), 5.53 (d, J = 10.3 Hz, 1H), 3.73 (s, 3H), 3.56 (d, J = 5.8 Hz, 1H), 2.87 (dd, J = 18.1, 6.1 Hz, 1H), 2.56 (d, J = 18.0 Hz, 1H), 2.44 (s, 3H), 1.44 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.7, 156.1, 152.5, 148.7, 144.4, 140.5, 130.5, 127.8, 127.1, 126.4, 120.7, 117.1, 113.1, 111.0, 71.7, 65.1, 56.1, 53.1, 33.3, 22.7, 21.5; HRMS (ESI) m/z: [M + H]+ Calcd for C23H23N2O7S: 471.1220, found 471.1219.
- 8-Fluoro-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3af): white solid; 39.8 mg, 87% yield; m.p. 209.2–210.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 8.3 Hz, 2H), 7.50 (d, J = 8.2 Hz, 2H), 7.44 (m, 1H), 7.25 (m, 1H), 6.98 (m, 1H), 6.60 (s, 1H), 6.46 (m, 1H), 5.55 (d, J = 10.3 Hz, 1H), 3.59 (d, J = 6.0 Hz, 1H), 2.89 (dd, J = 18.1, 6.2 Hz, 1H), 2.58 (d, J = 18.1 Hz, 1H), 2.44 (s, 3H), 1.45 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 193.2, 158.2 (d, JCF = 238.8 Hz), 154.3 (d, JCF = 1.6 Hz), 148.2, 144.1, 139.9, 130.1, 127.5, 126.8 (d, J = 9.0 Hz), 126.7, 120.4, 118.3 (d, JCF = 24.7 Hz), 114.1 (d, JCF = 26.2 Hz), 111.4 (d, JCF = 8.6 Hz), 70.8 (d, JCF = 2.0 Hz), 64.8, 52.7, 32.8, 22.2, 21.0; HRMS (ESI) m/z: [M + H]+ Calcd for C22H20FN2O6S: 459.1021, found 459.1012.
- 8-Chloro-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ag): white solid; 39.5 mg, 83% yield; m.p. 231.2–231.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 8.3 Hz, 2H), 7.67 (d, J = 2.2 Hz, 1H), 7.50 (d, J = 8.2 Hz, 2H), 7.45 (m, 1H), 7.00 (d, J = 8.7 Hz, 1H), 6.46 (m, 1H), 5.56 (d, J = 10.3 Hz, 1H), 3.59 (d, J = 6.0 Hz, 1H), 2.90 (dd, J = 18.1, 6.2 Hz, 1H), 2.58 (d, J = 18.0 Hz, 1H), 2.44 (s, 3H), 1.46 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.2, 156.9, 148.2, 144.1, 139.8, 131.4, 130.1, 127.5, 127.4, 127.2, 126.7, 120.1, 111.9, 70.6, 64.8, 52.7, 32.7, 22.1, 21.1; HRMS (ESI) m/z: [M + H]+ Calcd for C22H20ClN2O6S: 475.0725, found 475.0733.
- 8-Bromo-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ah): white solid; 43.7 mg, 84% yield; m.p. 214.6–215.1 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.3 Hz, 2H), 7.80 (d, J = 2.0 Hz, 1H), 7.57 (m, 1H), 7.50 (d, J = 8.2 Hz, 2H), 6.95 (d, J = 8.7 Hz, 1H), 6.61 (s, 1H), 6.46 (d, J = 10.3 Hz, 1H), 5.56 (d, J = 10.3 Hz, 1H), 3.59 (d, J = 6.0 Hz, 1H), 2.89 (dd, J = 18.1, 6.2 Hz, 1H), 2.58 (d, J = 18.1 Hz, 1H), 2.44 (s, 3H), 1.46 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 193.2, 157.4, 148.2, 144.1, 139.8, 134.2, 130.1, 130.0, 127.9, 127.5, 126.7, 120.0, 115.0, 112.4, 70.5, 64.8, 52.6, 32.7, 22.1, 21.1; HRMS (ESI) m/z: [M + H]+ Calcd for C22H20BrN2O6S: 519.0220, found 519.0187.
- 10a-Methyl-4b,8-dinitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ai): white solid; 29.7 mg, 61% yield; m.p. 242.3–243.0 °C; 1H NMR (400 MHz, CDCl3) δ 8.78 (s, 1H), 8.31 (d, J = 8.9 Hz, 1H), 7.87 (d, J = 7.8 Hz, 2H), 7.41 (d, J = 7.8 Hz, 2H), 7.01 (d, J = 8.9 Hz, 1H), 6.61 (d, J = 10.3 Hz, 1H), 6.26 (s, 1H), 5.61 (d, J = 10.3 Hz, 1H), 3.47 (d, J = 5.7 Hz, 1H), 2.88 (d, J = 18.1 Hz, 1H), 2.63 (dd, J = 18.1, 6.0 Hz, 1H), 2.49 (s, 3H), 1.54 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.1, 162.5, 148.4, 145.0, 144.9, 139.7, 130.4, 128.6, 128.1, 126.9, 126.1, 124.9, 120.6, 111.1, 71.0, 64.9, 53.6, 32.8, 23.1, 21.7; HRMS (ESI) m/z: [M + Na]+ Calcd for C22H19N3O8SNa: 508.0785, found 508.0792.
- 7,10a-Dimethyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3aj): white solid; 42.4 mg, 93% yield; m.p. 238.0–238.6 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.2 Hz, 2H), 7.60 (d, J = 7.8 Hz, 1H), 7.49 (d, J = 8.2 Hz, 2H), 6.97 (d, J = 7.8 Hz, 1H), 6.75 (s, 1H), 6.50 (s, 1H), 6.40 (d, J = 10.3 Hz, 1H), 5.53 (d, J = 10.3 Hz, 1H), 3.58 (d, J = 5.9 Hz, 1H), 2.88 (dd, J = 18.1, 6.2 Hz, 1H), 2.57 (d, J = 18.0 Hz, 1H), 2.44 (s, 3H), 2.31 (s, 3H), 1.43 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.8, 158.9, 148.7, 144.3, 142.3, 140.6, 130.5, 127.8, 127.7, 127.1, 125.1, 122.6, 120.5, 110.9, 71.3, 64.8, 52.9, 33.2, 22.7, 21.5, 21.5; HRMS (ESI) m/z: [M + H]+ Calcd for C23H23N2O6S: 455.1271, found 455.1270.
- 7-Methoxy-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ak): white solid; 43.1 mg, 92% yield; m.p. 229.6–230.2 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.2 Hz, 2H), 7.60 (d, J = 8.5 Hz, 1H), 7.48 (d, J = 8.2 Hz, 2H), 6.72 (m, 1H), 6.52 (d, J = 2.1 Hz, 1H), 6.46 (s, 1H), 6.43 (d, J = 10.3 Hz, 1H), 5.58 (d, J = 10.3 Hz, 1H), 3.75 (s, 3H), 3.58 (d, J = 6.0 Hz, 1H), 2.88 (dd, J = 18.2, 6.2 Hz, 1H), 2.57 (d, J = 18.1 Hz, 1H), 2.43 (s, 3H), 1.43 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.7, 162.7, 160.0, 148.8, 144.3, 140.6, 130.5, 128.5, 127.7, 127.1, 120.9, 117.3, 110.6, 96.4, 71.1, 64.7, 56.2, 52.9, 33.2, 22.7, 21.5; HRMS (ESI) m/z: [M + H]+ Calcd for C23H23N2O7S: 471.1220, found 471.1222.
- 7-Fluoro-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3al): white solid; 38.1 mg, 83% yield; m.p. 223.0–223.6 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.2 Hz, 2H), 7.74 (m, 1H), 7.49 (d, J = 8.2 Hz, 2H), 7.01 (m, 1H), 6.95 (m, 1H), 6.57 (s, 1H), 6.47–6.38 (m, 1H), 5.58 (d, J = 10.3 Hz, 1H), 3.60 (d, J = 5.9 Hz, 1H), 2.90 (dd, J = 18.2, 6.2 Hz, 1H), 2.58 (d, J = 18.1 Hz, 1H), 2.44 (s, 3H), 1.45 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.17, 163.99 (d, JCF = 246.7 Hz), 159.10 (d, JCF = 13.7 Hz), 148.33, 144.00, 140.01, 130.08, 128.80 (d, JCF = 10.6 Hz), 127.4, 126.7, 121.5 (d, JCF = 2.6 Hz), 120.5, 110.9 (d, JCF = 22.9 Hz), 98.7 (d, JCF = 27.6 Hz), 70.3, 64.5, 52.6, 32.7, 22.2, 21.0; HRMS (ESI) m/z: [M + H]+ Calcd for C22H20FN2O6S: 459.1021, found 459.1013.
- 7-Chloro-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3am): white solid; 39.3 mg, 83% yield; m.p. 253.1–253.5 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.3 Hz, 2H), 7.72 (d, J = 8.2 Hz, 1H), 7.50 (d, J = 8.2 Hz, 2H), 7.25 (m, 1H), 7.15 (d, J = 1.6 Hz, 1H), 6.59 (s, 1H), 6.42 (m, 1H), 5.58 (d, J = 10.3 Hz, 1H), 3.59 (d, J = 6.0 Hz, 1H), 2.90 (dd, J = 18.2, 6.2 Hz, 1H), 2.57 (d, J = 18.1 Hz, 1H), 2.44 (s, 3H), 1.44 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.2, 158.8, 148.3, 144.0, 139.9, 135.6, 130.1, 128.7, 127.5, 126.7, 124.5, 124.2, 120.2, 110.6, 70.3, 64.6, 52.6, 32.7, 22.1, 21.0; HRMS (ESI) m/z: [M + H]+ Calcd for C22H20ClN2O6S: 475.0725, found 475.0731.
- 7-Bromo-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3an): white solid; 43.5 mg, 84% yield; m.p. 253.4–254.1 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.3 Hz, 2H), 7.66 (d, J = 8.2 Hz, 1H), 7.50 (d, J = 8.2 Hz, 2H), 7.39 (m, 1H), 7.26 (d, J = 1.4 Hz, 1H), 6.57 (s, 1H), 6.43 (d, J = 10.3 Hz, 1H), 5.58 (d, J = 10.3 Hz, 1H), 3.59 (d, J = 6.0 Hz, 1H), 2.90 (dd, J = 18.2, 6.2 Hz, 1H), 2.58 (d, J = 18.0 Hz, 1H), 2.44 (s, 3H), 1.44 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.2, 158.8, 148.3, 144.0, 139.9, 130.1, 129.1, 127.5, 127.1, 126.7, 124.9, 123.8, 120.1, 113.4, 70.4, 64.6, 52.6, 32.7, 22.1, 21.1; HRMS (ESI) m/z: [M + H]+ Calcd for C22H20BrN2O6S: 519.0220, found 519.0242.
- 6,10a-Dimethyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ao): white solid; 43.3 mg, 95% yield; m.p. 215.6–216.2 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.2 Hz, 2H), 7.54 (d, J = 7.5 Hz, 1H), 7.48 (s, 2H), 7.21 (d, J = 7.4 Hz, 1H), 7.04 (m, 1H), 6.57 (s, 1H), 6.34 (d, J = 9.0 Hz, 1H), 5.50 (d, J = 10.3 Hz, 1H), 3.57 (d, J = 5.8 Hz, 1H), 2.89 (dd, J = 17.9, 6.0 Hz, 1H), 2.62 (d, J = 17.8 Hz, 1H), 2.44 (s, 3H), 2.06 (s, 3H), 1.43 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.7, 156.6, 148.2, 143.9, 140.2, 132.2, 130.0, 127.3, 126.7, 124.8, 124.5, 123.7, 120.1, 119.4, 71.4, 64.4, 52.8, 33.0, 22.1, 21.0, 14.1; HRMS (ESI) m/z: [M + Na]+ Calcd for C23H22N2O6SNa: 477.1091, found 477.1094.
- 6-(tert-Butyl)-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ap): white solid; 43.8 mg, 88% yield; m.p. 210.6–211.5 °C; 1H NMR (400 MHz, CDCl3) δ 7.81 (d, J = 7.9 Hz, 2H), 7.70 (d, J = 7.5 Hz, 1H), 7.31 (d, J = 7.9 Hz, 2H), 7.23 (d, J = 7.7 Hz, 1H), 7.01 (m, 1H), 6.52 (d, J = 10.3 Hz, 1H), 6.10 (s, 1H), 5.54 (d, J = 10.3 Hz, 1H), 3.39 (d, J = 6.5 Hz, 1H), 2.81 (d, J = 18.8 Hz, 1H), 2.53 (dd, J = 18.8, 6.7 Hz, 1H), 2.40 (s, 3H), 1.44 (s, 3H), 1.24 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.3, 156.2, 149.3, 144.4, 140.5, 134.9, 130.3, 128.8, 128.0, 126.9, 125.9, 125.0, 124.4, 120.0, 71.9, 64.5, 53.3, 34.3, 32.3, 29.9, 23.5, 21.7; HRMS (ESI) m/z: [M + Na]+ Calcd for C26H28N2O6SNa: 519.1560, found 519.1567.
- 6-Methoxy-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3aq): white solid; 44.2 mg, 94% yield; m.p. 232.4–233.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.1 Hz, 2H), 7.49 (d, J = 8.1 Hz, 2H), 7.31 (d, J = 6.8 Hz, 1H), 7.09 (d, J = 6.8 Hz, 2H), 6.57 (s, 1H), 6.36 (d, J = 10.4 Hz, 1H), 5.54 (d, J = 10.3 Hz, 1H), 3.77 (s, 3H), 3.57 (d, J = 5.9 Hz, 1H), 2.86 (dd, J = 18.3, 6.2 Hz, 1H), 2.59 (d, J = 18.2 Hz, 1H), 2.44 (s, 3H), 1.43 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 192.9, 148.1, 146.6, 144.0, 143.9, 140.1, 130.1, 127.3, 126.7, 126.3, 124.6, 120.0, 118.9, 115.1, 71.4, 64.5, 56.4, 52.4, 32.5, 22.3, 21.0; HRMS (ESI) m/z: [M + H]+ Calcd for C23H23N2O7S:471.1220, found 471.1221.
- 6-Ethoxy-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3ar): white solid; 41.5 mg, 86% yield; m.p. 218.9–219.4 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.2 Hz, 2H), 7.49 (d, J = 8.2 Hz, 2H), 7.31 (m, 1H), 7.11–7.00 (m, 2H), 6.57 (s, 1H), 6.36 (d, J = 10.3 Hz, 1H), 5.52 (d, J = 10.3 Hz, 1H), 4.05 (m, 2H), 3.57 (d, J = 5.9 Hz, 1H), 2.85 (dd, J = 18.2, 6.2 Hz, 1H), 2.63 (d, J = 18.2 Hz, 1H), 2.44 (s, 3H), 1.43 (s, 3H), 1.27 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 192.9, 148.0, 147.1, 143.9, 143.0, 140.2, 130.0, 127.3, 126.7, 126.4, 124.6, 119.9, 118.9, 116.5, 71.4, 64.8, 64.4, 52.5, 32.5, 22.3, 21.0, 14.6; HRMS (ESI) m/z: [M + H]+ Calcd for C24H25N2O7S: 485.1377, found 485.1372.
- 6-Fluoro-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3as): white solid; 45.0 mg, 98% yield; m.p. 214.3–214.7 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 8.2 Hz, 2H), 7.56 (d, J = 7.6 Hz, 1H), 7.50 (d, J = 8.2 Hz, 2H), 7.42–7.30 (m, 1H), 7.18 (m, 1H), 6.69 (s, 1H), 6.40 (m, 1H), 5.58 (d, J = 10.3 Hz, 1H), 3.62 (d, J = 5.9 Hz, 1H), 2.90 (dd, J = 18.2, 6.2 Hz, 1H), 2.64 (d, J = 18.1 Hz, 1H), 2.44 (s, 3H), 1.45 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.1, 148.2, 145.8 (d, JCF = 263.4 Hz), 144.6 (d, JCF = 5.1 Hz), 144.1, 139.9, 130.1, 128.8, 127.4, 126.7, 125.1 (d, JCF = 5.4 Hz), 123.2 (d, JCF = 3.6 Hz), 120.4, 118.2 (d, JCF = 15.9 Hz), 71.1 (d, JCF = 2.1 Hz), 64.7, 52.7, 32.7, 22.2, 21.1; HRMS (ESI) m/z: [M + H]+ Calcd for C22H20FN2O6S: 459.1021, found 459.1024.
- 6-Chloro-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3at): white solid; 34.9 mg, 74% yield; m.p. 223.0–223.8 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.2 Hz, 2H), 7.69 (d, J = 7.6 Hz, 1H), 7.57–7.39 (m, 3H), 7.19 (m, 1H), 6.70 (s, 1H), 6.41–6.32 (m, 1H), 5.56 (d, J = 10.3 Hz, 1H), 3.61 (d, J = 5.9 Hz, 1H), 2.89 (dd, J = 18.2, 6.2 Hz, 1H), 2.65 (d, J = 18.2 Hz, 1H), 2.44 (s, 3H), 1.45 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 192.8, 153.8, 148.0, 144.1, 139.9, 131.2, 130.1, 127.5, 127.3, 126.7, 126.2, 125.3, 119.6, 114.4, 71.4, 64.6, 52.5, 32.6, 22.1, 21.0; HRMS (ESI) m/z: [M + H]+ Calcd for C22H20ClN2O6S: 475.0725, found 475.0726.
- 6-Bromo-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3au): white solid; 42.7 mg, 82% yield; m.p. 218.6–219.0 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.95 (d, J = 8.1 Hz, 2H), 7.71 (d, J = 7.6 Hz, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.50 (d, J = 8.1 Hz, 2H), 7.12 (m, 1H), 6.70 (s, 1H), 6.37 (d, J = 10.3 Hz, 1H), 5.55 (d, J = 10.3 Hz, 1H), 3.59 (d, J = 5.8 Hz, 1H), 2.88 (dd, J = 18.3, 6.2 Hz, 1H), 2.63 (d, J = 18.2 Hz, 1H), 2.44 (s, 3H), 1.44 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 192.7, 155.2, 148.0, 144.1, 139.9, 134.1, 130.1, 127.6, 126.9, 126.8, 126.7, 125.6, 119.3, 102.1, 71.6, 64.6, 52.5, 32.5, 22.2, 21.1; HRMS (ESI) m/z: [M + Na]+ Calcd for C22H19BrN2O6SNa: 541.0039, found 541.0041.
- 6,8-Di-tert-butyl-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3av): white solid; 45.4 mg, 82% yield; m.p. 194.4–195.2 °C; 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 7.9 Hz, 2H), 7.77 (s, 1H), 7.38 (d, J = 7.9 Hz, 2H), 7.32 (s, 1H), 6.54 (d, J = 10.3 Hz, 1H), 6.16 (s, 1H), 5.63 (d, J = 10.3 Hz, 1H), 3.46 (d, J = 6.3 Hz, 1H), 2.87 (d, J = 18.7 Hz, 1H), 2.61 (dd, J = 18.8, 6.6 Hz, 1H), 2.47 (s, 3H), 1.55 (s, 3H), 1.32 (s, 9H), 1.31 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.5, 154.0, 149.2, 147.4, 144.3, 140.5, 133.8, 130.2, 128.0, 126.9, 125.9, 124.5, 122.6, 120.3, 72.0, 64.6, 53.2, 35.0, 34.4, 32.3, 31.7, 29.9, 23.7, 21.7; HRMS (ESI) m/z: [M + H]+ Calcd for C30H37N2O6S: 553.2367, found 553.2361.
- 8-Bromo-6-methoxy-10a-methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-one (3aw): white solid; 47.4 mg, 86% yield; m.p. 224.8–225.2 °C; 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 2H), 7.61 (s, 1H), 7.39 (d, J = 7.9 Hz, 2H), 7.03 (s, 1H), 6.57 (d, J = 10.4 Hz, 1H), 6.21 (s, 1H), 5.63 (d, J = 10.4 Hz, 1H), 3.84 (s, 3H), 3.40 (d, J = 5.7 Hz, 1H), 2.90 (d, J = 18.2 Hz, 1H), 2.57 (dd, J = 18.2, 6.2 Hz, 1H), 2.48 (s, 3H), 1.50 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.3, 148.4, 146.2, 145.0, 144.6, 140.1, 130.3, 127.9, 127.1, 126.9, 122.2, 120.1, 118.7, 116.8, 72.2, 64.8, 57.1, 53.7, 32.7, 23.0, 21.7; HRMS (ESI) m/z: [M + Na]+ Calcd for C23H21BrN2O7SNa: 571.0145, found 571.0153.
- 11a-Methyl-7a-nitro-12-tosyl-7a,7b,8,11a,12,12a-hexahydro-9H-naphtho[1′,2′:4,5]furo[3,2-b]indol-9-one (3ax): white solid; 42.3 mg, 86% yield; m.p. 264.7–265.2 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.79 (d, J = 8.5 Hz, 1H), 8.04 (d, J = 9.0 Hz, 1H), 8.00 (d, J = 8.2 Hz, 1H), 7.97 (d, J = 8.3 Hz, 2H), 7.66 (m, 1H), 7.50 (m, 3H), 7.24 (s, 1H), 7.13 (d, J = 8.9 Hz, 1H), 6.02 (d, J = 11.7 Hz, 1H), 5.30 (d, J = 10.3 Hz, 1H), 3.45 (d, J = 5.4 Hz, 1H), 2.91 (dd, J = 18.3, 5.9 Hz, 1H), 2.66 (d, J = 18.2 Hz, 1H), 2.44 (s, 3H), 1.57 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.2, 156.6, 149.1, 144.5, 138.5, 133.7, 130.5, 130.2, 130.0, 129.2, 128.1, 127.6, 127.1, 124.7, 123.2, 121.2, 115.8, 111.2, 71.7, 65.8, 52.8, 32.8, 22.0, 21.1; HRMS (ESI) m/z: [M + H]+ Calcd for C26H23N2O6S: 491.1271, found 491.1262.
- 10a-Methyl-4b-nitro-5,10-ditosyl-4a,4b,5,9b,10,10a-hexahydroindolo[3,2-b]indol-3(4H)-one (3ay): white solid; 35.6 mg, 60% yield; m.p. 206.5–207.4 °C; 1H NMR (400 MHz, CDCl3) δ 7.77 (m, 3H), 7.63 (d, J = 7.8 Hz, 2H), 7.38 (d, J = 8.2 Hz, 1H), 7.35–7.26 (m, 5H), 7.12 (m, 1H), 6.42 (d, J = 10.4 Hz, 1H), 6.21 (s, 1H), 5.45 (d, J = 10.4 Hz, 1H), 4.13 (d, J = 5.1 Hz, 1H), 3.57 (d, J = 18.7 Hz, 1H), 2.70 (dd, J = 18.8, 6.3 Hz, 1H), 2.46 (s, 3H), 2.40 (s, 3H), 1.56 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 193.0, 149.8, 145.7, 144.6, 141.9, 139.5, 134.6, 131.0, 130.3, 129.6, 128.7, 127.8, 127.4, 126.7, 126.6, 125.3, 113.5, 112.1, 75.8, 65.6, 51.7, 34.0, 23.3, 21.8, 21.7; HRMS (ESI) m/z: [M + H]+ Calcd for C29H28N3O7S2: 594.1363, found 594.1366.
3.3. Scale-Up Experiment for Preparation of Compound 3aa
To an ordinary vial charged with a magnetic stirring bar, 1a (2.080 g, 7.5 mmol), 2a (0.816 g, 5.0 mmol), K2CO3 (0.691 g), and CH3CN (100 mL) were added. Then, the mixture was stirred at 65 °C for 72 h. Product 3aa was isolated by flash chromatography on silica gel as a white solid (2.019 g, 92% yield).
3.4. Procedure for Synthesis of Compound 4
In a reactor, MeOH (5.0 mL) was added to substrate 3aa (0.10 mmol) and the mixture was stirred at 0 °C for 10 min. Then, NaBH4 (0.15 mmol) was added in one portion. The mixture was stirred at the same temperature for 15 min, then at 25 °C for 2 h. Upon reaction completion, saturated NH4Cl (5.0 mL) was added and the mixture was extracted with DCM. The organic phase was dried over anhydrous Mg2SO4, filtered, and concentrated to afford the crude product. The crude product was purified by flash chromatography with PE/EA (v/v = 5:1) to provide the desired compound 4 at 95% yield (42.1 mg) as a white solid.
- 10a-Methyl-4b-nitro-10-tosyl-4,4a,4b,9b,10,10a-hexahydro-3H-benzofuro[3,2-b]indol-3-ol (4): white solid; 42.1 mg, 95% yield; m.p. 187.5–188.1 °C; 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 7.6 Hz, 1H), 7.82 (d, J = 7.8 Hz, 2H), 7.35 (m, 3H), 7.14 (m, 1H), 6.95 (d, J = 8.2 Hz, 1H), 6.24 (s, 1H), 5.60 (d, J = 10.4 Hz, 1H), 5.46 (d, J = 10.4 Hz, 1H), 4.01 (s, 1H), 3.07 (d, J = 5.6 Hz, 2H), 2.44 (s, 3H), 2.35 (m, 1H), 2.15 (d, J = 16.6 Hz, 1H), 1.32 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 157.6, 144.0, 140.6, 131.6, 131.4, 130.0, 129.5, 128.6, 126.8, 125.0, 124.4, 120.3, 110.1, 72.0, 65.2, 59.7, 50.1, 26.9, 24.3, 21.6; HRMS (ESI) m/z: [M + Na]+ Calcd for C22H22N2O6SNa: 465.1091, found 465.1100.
3.5. Procedure for Synthesis of Product 5
To a stirred solution of substrate 3aa (0.10 mmol) and NaOH (0.3 mmol) in MeOH (1.0 mL) and THF (1.0 mL), H2O2 solution (30% wt, 5.0 equiv) was added. The mixture was stirred at 30 °C for 24 h. Upon reaction completion, saturated Na2S2O3 (3.0 mL) was added and the mixture was extracted with EA. The organic phase was dried over anhydrous Mg2SO4, filtered, and concentrated to afford the crude product. The crude product was purified by flash chromatography with PE/EA (v/v = 5:1) to provide the desired compound 5 at 98% yield (44.8 mg) as a white solid.
- 9a-Methyl-3b-nitro-9-tosyl-1a,3,3a,3b,8b,9,9a,9b-octahydro-2H-benzofuro[3,2-b]oxireno[2,3-g]indol-2-one (5): white solid; 44.8 mg, 98% yield; m.p. 200.4–200.9 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.98 (d, J = 7.9 Hz, 2H), 7.80 (d, J = 7.5 Hz, 1H), 7.50 (d, J = 7.9 Hz, 2H), 7.46 (m, 1H), 7.23 (m, 1H), 7.03 (d, J = 8.1 Hz, 1H), 6.65 (s, 1H), 3.44 (s, 1H), 3.22 (s, 1H), 2.87 (s, 1H), 2.77 (dd, J = 17.2, 4.5 Hz, 1H), 2.45 (s, 3H), 2.37 (d, J = 17.1 Hz, 1H), 1.47 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 200.3, 156.9, 144.1, 139.9, 131.7, 130.1, 127.8, 126.8, 125.4, 124.3, 119.5, 110.3, 71.7, 62.6, 61.6, 55.6, 53.4, 30.6, 21.1, 19.9; HRMS (ESI) m/z: [M + Na]+ Calcd for C22H20N2O7SNa: 479.0883, found 479.0880.
3.6. Procedure for Synthesis of Product 6
To a stirred solution of substrate 3aa (0.20 mmol) in EtOH (10 mL), Pd/C (10% w.t.) was added. The mixture was stirred at 25 °C for 5 days under hydrogen atmosphere (1 atm). Upon reaction completion, the mixture was filtered then washed with DCM (10 mL). The filtrate was concentrated to afford the crude product, which was purified by flash chromatography with PE/EA (v/v = 5:1) to provide the desired compound 6 at 44% yield (39.1 mg) as a white solid.
- 10a-Methyl-4b-nitro-10-tosyl-1,2,4,4a,4b,9b,10,10a-octahydro-3H-benzofuro[3,2-b]indol-3-one (6): white solid; 39.1 mg, 44% yield; m.p. 184.3–185.1 °C; 1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 7.5 Hz, 1H), 7.79 (d, J = 7.6 Hz, 2H), 7.36 (m, 3H), 7.16 (m, 1H), 7.05 (d, J = 8.1 Hz, 1H), 6.11 (s, 1H), 3.41 (d, J = 7.5 Hz, 1H), 2.67 (d, J = 16.8 Hz, 1H), 2.45 (m, 4H), 2.26–2.10 (m, 2H), 2.00 (d, J = 13.9 Hz, 1H), 1.80 (m, 1H), 1.62 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 204.8, 158.1, 144.1, 140.4, 131.9, 130.1, 128.6, 126.9, 126.6, 124.5, 120.7, 111.3, 71.8, 66.2, 54.5, 36.5, 35.6, 34.4, 23.1, 21.7; HRMS (ESI) m/z: [M + Na]+ Calcd for C22H22N2O6SNa: 465.1091, found 465.1095.
4. Conclusions
In summary, we have developed an efficient dearomative (3 + 2) cycloaddition reaction of para-quinamines and 2-nitrobenzofurans. With the developed protocol, a series of structurally diverse benzofuro[3,2-b]indol-3-one derivatives were smoothly obtained in good to excellent yields (up to 98%) with outstanding diastereoselectivities (all cases > 20:1 dr). In addition, the scale-up synthesis and the versatile transformations of the product also demonstrated the potential synthetic application of this dearomative (3 + 2) cycloaddition reaction. Importantly, this approach represents the first example of the nitrogen nucleophile-triggered (3 + 2) cycloaddition reaction of 2-nitrobenzofurans. Further exploration of the dearomative cycloaddition reaction of electron-deficient heteroarenes for synthesizing polyheterocyclic compounds with structural diversity is ongoing in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29051163/s1, X-ray data for product 3ac; copies of 1H, 13C NMR spectra. References [60,61,62] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, W.-C.Y.; methodology, Z.-H.W. and H.-Y.Z., investigation, H.-Y.Z., Y.-P.Z., Y.Y., J.-Q.Y. and J.-Q.Z.; writing—original draft preparation, Z.-H.W. and W.-C.Y.; writing—review and editing, W.-C.Y.; supervision, Z.-H.W. and M.-Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (Nos. 22171029, 21901024, and 22271027) with financial support from the Sichuan Science and Technology Program (Nos. 2023NSFSC1080).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the required data are reported in the manuscript and Supplementary Materials.
Acknowledgments
This work was performed using the equipment of Chengdu University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Craig, R.A., II; Stoltz, B.M. Polycyclic Furanobutenolide-Derived Cembranoid and Norcembranoid Natural Products: Biosynthetic Connections and Synthetic Efforts. Chem. Rev. 2017, 117, 7878–7909. [Google Scholar] [CrossRef]
- Schinke, C.; Martins, T.; Queiroz, S.C.N.; Melo, I.S.; Reyes, F.G.R. Antibacterial Compounds from Marine Bacteria, 2010–2015. J. Nat. Prod. 2017, 80, 1215–1228. [Google Scholar] [CrossRef]
- Liu, W.; Hong, B.; Wang, J.; Lei, X. New Strategies in the Efficient Total Syntheses of Polycyclic Natural Products. Acc. Chem. Res. 2020, 53, 2569–2586. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.-Q.; Tang, J.-J.; Gao, J.-M. Picrotoxane sesquiterpenoids: Chemistry, chemo- and bio-syntheses and biological activities. Nat. Prod. Rep. 2022, 39, 2096–2131. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-H.; Li, X.; Wang, B.-G. Natural products with 1,2-oxazine scaffold: Occurrence, chemical diversity, bioactivity, synthesis, and biosynthesis. Nat. Prod. Rep. 2023, 40, 1874–1900. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.W.; Rees, D.C. Opportunity Knocks: Organic Chemistry for Fragment-Based Drug Discovery (FBDD). Angew. Chem. Int. Ed. 2016, 55, 488–492. [Google Scholar] [CrossRef]
- Erlanson, D.A.; Fesik, S.W.; Hubbard, R.E.; Jahnke, W.; Jhoti, H. Twenty years on: The impact of fragments on drug discovery. Nat. Rev. Drug Discov. 2016, 15, 605–619. [Google Scholar] [CrossRef]
- Jhoti, H.; Williams, G.; Rees, D.C.; Murray, C.W. The ‘rule of three’ for fragment-based drug discovery: Where are we now? Nat. Rev. Drug Discov. 2013, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, C. Perspectives on Fragment-based Drug Discovery: A Strategy Applicable to Diverse Targets. Curr. Top. Med. Chem. 2021, 21, 1099–1112. [Google Scholar] [CrossRef]
- Troelsen, N.S.; Clausen, M.H. Library Design Strategies to Accelerate Fragment-Based Drug Discover. Chem. Eur. J. 2020, 26, 11391–11403. [Google Scholar] [CrossRef]
- She, Z.; Wang, Y.; Wang, D.; Zhao, Y.; Wang, T.; Zheng, X.; Yu, Z.-X.; Gao, G.; You, J. Two-Fold C–H/C–H Cross-Coupling Using RhCl3·3H2O as the Catalyst: Direct Fusion of N-(Hetero)arylimidazolium Salts and (Hetero)arenes. J. Am. Chem. Soc. 2018, 140, 12566–12573. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Y.; Tang, J.; Zhang, L.; Liu, Q.; Li, Q.; Gao, G.; You, J. Ir-Catalyzed Cascade C–H Fusion of Aldoxime Ethers and Heteroarenes: Scope and Mechanisms. ACS Catal. 2020, 10, 203–209. [Google Scholar] [CrossRef]
- Wilson, A.M.; Waldman, T.E.; Rheingold, A.L.; Ernst, R.D. Ring fusion and polycyclic ring constructions via half-open titanocenes. J. Am. Chem. Soc. 1992, 114, 6252–6254. [Google Scholar] [CrossRef]
- Kiel, G.R.; Bergman, H.M.; Samkian, A.E.; Schuster, N.J.; Handford, R.C.; Rothenberger, A.J.; Gomez-Bombarelli, R.; Nuckolls, C.; Tilley, T.D. Expanded [23]-Helicene with Exceptional Chiroptical Properties via an Iterative Ring-Fusion Strategy. J. Am. Chem. Soc. 2022, 144, 23421–23427. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, V.; Shehadeh, I.; Khanfar, M.A.; Malik, O.G.; Tarazi, H.; Abu-Yousef, I.A.; Sebastian, A.; Baniowda, N.; O’Connor, M.J.; Al-Tel, T.H. One-Pot Synthesis of Diverse Collections of Benzoxazepine and Indolopyrazine Fused to Heterocyclic Systems. J. Org. Chem. 2019, 84, 934–948. [Google Scholar] [CrossRef]
- Manna, S.K.; Das, T.; Samanta, S. Polycyclic Benzimidazole: Synthesis and Photophysical Properties. ChemistrySelect 2019, 4, 8781–8790. [Google Scholar] [CrossRef]
- Chawla, A.S.; Jackson, A.H. Erythrina and related alkaloids. Nat. Prod. Rep. 1989, 6, 55–66. [Google Scholar] [CrossRef]
- Tomita, M.; Okamoto, Y.; Kikuchi, T.; Osaki, K.; Nishikawa, M.; Kamiya, K.; Sasaki, Y.; Matoba, K.; Goto, K. Studies on the Alkaloids of Menispermaceous Plants. CCLIX. Alkaloids of Menispermum dauricum DC. (Suppl. 7). Structures of Acutumine and Acutumidine, Chlorine-Containing Alkaloids with a Novel Skeleton. Chem. Pharm. Bull. 1971, 19, 770–791. [Google Scholar] [CrossRef]
- Min, Z.-D.; Lin, G.; Xu, G.-X.; Munekazu, I.; Toshiyuki, T.; Mizuo, M. Alkaloids of Stephania sinica. Phytochemistry 1985, 24, 3084–3085. [Google Scholar]
- Zhang, H.; Qiu, S.; Tamez, P.; Tan, G.T.; Aydogmus, Z.; Hung, N.V.; Cuong, N.M.; Angerhofer, C.; Soejarto, D.D.; Pezzuto, J.M.; et al. Antimalarial Agents from Plants II. Decursivine, A New Antimalarial Indole Alkaloid from Rhaphidophora decursiva. Pharm. Biol. 2002, 40, 221–224. [Google Scholar] [CrossRef]
- Johnson, G.; Sunderwirth, S.G.; Gibian, H.; Coulter, A.W.; Gassner, F.X. Lithospermum ruderale: Partial characterization of the principal polyphenol isolated from the roots. Phytochemistry 1963, 2, 145–150. [Google Scholar] [CrossRef]
- Celoy, R.M.; VanEtten, H.D. (+)-Pisatin biosynthesis: From (-) enantiomeric intermediates via an achiral 7,2′-dihydroxy-4′,5′-methylenedioxyisoflav-3-ene. Pytochemistry 2014, 98, 120–127. [Google Scholar] [CrossRef]
- Yu, B.-W.; Chen, J.-Y.; Wang, Y.-P.; Cheng, K.-F.; Li, X.-Y.; Qin, G.-W. Alkaloids from Menispermum dauricum. Phytochemistry 2002, 61, 439–442. [Google Scholar] [CrossRef]
- Abd-Elazem, I.S.; Chen, H.S.; Bates, R.B.; Huang, R.C.C. Isolation of two highly potent and non-toxic inhibitors of human immunodeficiency virus type 1 (HIV-1) integrase from Salvia miltiorrhiza. Antiviral Res. 2002, 55, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.; Conan, J.-Y.; Lamar, G. Remarks on the Near W Spectrum of Pterocarpin. Spectrosc. Lett. 1987, 20, 233–247. [Google Scholar] [CrossRef]
- Nair, S.R.; Baire, B. Recent Dearomatization Strategies of Benzofurans and Benzothiophenes. Asian J. Org. Chem. 2021, 10, 932–948. [Google Scholar] [CrossRef]
- Li, Y.-L.; Wang, K.-K.; He, X.-L. Recent Progress of Electron-Withdrawing-Group-Tethered Arenes Involved Asymmetric Nucleophilic Aromatic Functionalizations. Adv. Synth. Catal. 2022, 364, 3630–3650. [Google Scholar] [CrossRef]
- Wang, N.; Ren, J.; Li, K. Dearomatization of Nitro(hetero)arenes through Annulation. Eur. J. Org. Chem. 2022, 2022, e202200039. [Google Scholar] [CrossRef]
- Laviós, A.; Sanz-Marco, A.; Vila, C.; Muñoz, M.C.; Pedro, J.R.; Blay, G. Metal-Free Diastereo- and Enantioselective Dearomative Formal [3 + 2] Cycloaddition of 2-Nitrobenzofurans and Isocyanoacetate Esters. Org. Lett. 2022, 24, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, T.; Li, X.; Wu, J.-D.; Liu, J. Asymmetric decarboxylative [3 + 2] cycloaddition for the diastereo- and enantioselective synthesis of spiro [2.4]heptanes via cyclopropanation. Org. Chem. Front. 2022, 9, 2121–2128. [Google Scholar] [CrossRef]
- Zhou, P.; Yi, Y.; Hua, Y.-Z.; Jia, S.-K.; Wang, M.-C. Dinuclear Zinc Catalyzed Enantioselective Dearomatization [3+2] Annulation of 2-Nitrobenzofurans and 2-Nitrobenzothiophenes. Chem. Eur. J. 2022, 28, e202103688. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Tu, Y.; Zhang, J. Phosphine-Catalyzed Enantioselective Dearomative [3+2]-Cycloaddition of 3-Nitroindoles and 2-Nitrobenzofurans. Angew. Chem. Int. Ed. 2019, 58, 5422–5426. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Yang, L.; Zhou, X.-J.; You, Y.; Wang, Z.-H.; Zhou, M.-Q.; Zhang, X.-M.; Xu, X.-Y.; Yuan, W.-C. Organocatalyzed Dearomative Cycloaddition of 2-Nitrobenzofurans and Isatin-Derived Morita–Baylis–Hillman Carbonates: Highly Stereoselective Construction of Cyclopenta[b]benzofuran Scaffolds. Org. Lett. 2019, 21, 660–664. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.-C.; Xie, M.-S.; Qu, G.-R.; Guo, H.-M. Enantioselective Synthesis of Fused Polycyclic Tropanes via Dearomative [3 + 2] Cycloaddition Reactions of 2-Nitrobenzofurans. Org. Lett. 2020, 22, 164–167. [Google Scholar] [CrossRef]
- Tan, J.-P.; Li, X.; Chen, Y.; Rong, X.; Zhu, L.; Jiang, C.; Xiao, K.; Wang, T. Highly stereoselective construction of polycyclic benzofused tropane scaffolds and their latent bioactivities: Bifunctional phosphonium salt-enabled cyclodearomatization process. Sci. China Chem. 2020, 63, 1091–1099. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Zhou, S.; Wang, Z.-H.; You, Y.; Chen, S.; Liu, X.-L.; Zhou, M.-Q.; Yuan, W.-C. Catalytic asymmetric dearomative [4 + 2] annulation of 2-nitrobenzofurans and 5H-thiazol-4-ones: Stereoselective construction of dihydrobenzofuran-bridged polycyclic skeletons. Org. Chem. Front. 2021, 8, 6330–6336. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, H.-J.; Yue, W.-J.; You, S.-L. Palladium-Catalyzed Highly Stereoselective Dearomative [3 + 2] Cycloaddition of Nitrobenzofurans. Chem 2017, 3, 428–436. [Google Scholar] [CrossRef]
- Zhou, X.-J.; Zhao, J.-Q.; Chen, X.-M.; Zhuo, J.-R.; Zhang, Y.-P.; Chen, Y.-Z.; Zhang, X.-M.; Xu, X.-Y.; Yuan, W.-C. Organocatalyzed Asymmetric Dearomative Aza-Michael/Michael Addition Cascade of 2-Nitrobenzofurans and 2-Nitrobenzothiophenes with 2-Aminochalcones. J. Org. Chem. 2019, 84, 4381–4391. [Google Scholar] [CrossRef]
- Rao, G.A.; Gurubrahamam, R.; Chen, K. Base-Catalysed (4+2)-Annulation Between 2-Nitrobenzofurans and N-Alkoxyacrylamides: Synthesis of [3,2-b]Benzofuropyridinones. Eur. J. Org. Chem. 2022, 2022, e202200657. [Google Scholar] [CrossRef]
- Thopate, S.B.; Phanindrudu, M.; Jadhav, S.B.; Chegondi, R. Site-selective and stereoselective transformations on p-quinols & p-quinamines. Chem. Commun. 2023, 59, 3795–3811. [Google Scholar]
- Al-Tel, T.H.; Srinivasulu, V.; Ramanathan, M.; Soares, N.C.; Sebastian, A.; Bolognesi, M.L.; Abu-Yousef, I.A.; Majdalawieh, A. Stereocontrolled transformations of cyclohexadienone derivatives to access stereochemically rich and natural product-inspired architectures. Org. Biomol. Chem. 2020, 18, 8526–8571. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, B.; Li, H.; He, Y.; Xu, W.; Duan, X.; Sun, H.; Wang, T.; Zhai, H. Synthesis of Hydrobenzoimidazoles from para-Quinamines and 1,3,5-Triazinanes via a Formal [3+2] Annulation Reaction. Adv. Synth. Catal. 2021, 363, 565–569. [Google Scholar] [CrossRef]
- Jin, H.; Lai, J.; Huang, Y. Phosphine-Catalyzed Domino [3 + 3] Cyclization of para-Quinamines with Morita–Baylis–Hillman Carbonates: Access to Hydroquinoline Derivatives. Org. Lett. 2019, 21, 2843–2846. [Google Scholar] [CrossRef]
- Hu, K.-W.; You, X.; Wang, J.-Z.; Wen, X.; Sun, H.; Xu, Q.-L.; Lai, Z. Chiral Phosphoric Acid Catalyzed Asymmetric Desymmetrization of para-Quinamines with Isocyanates: Access to Functionalized Imidazolidin-2-one Derivatives. Org. Lett. 2021, 23, 7873–7877. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Chegondi, R. Diastereoselective Desymmetrization of p-Quinamines through Regioselective Ring Opening of Epoxides and Aziridines. Org. Lett. 2019, 21, 10115–10119. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, H.; Huang, Y. DABCO-mediated [3+3] annulation of para-quinamines: Access to functionalized 1,2,4-triazinone derivatives. Chem. Commun. 2019, 55, 10135–10137. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Dai, C.; Huang, Y. DBU-Catalyzed Desymmetrization of Cyclohexadienones: Access to Vicinal Diamine-Containing Heterocycles. Org. Lett. 2018, 20, 5006–5009. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, Y.; Song, D.; Zhong, Z.; Feng, S.; Xie, X.; Wang, X.; She, X. (3 + 2)-Annulation of p-Quinamine and Aryne: A Strategy To Construct the Multisubstituted Hydrocarbazoles. Org. Lett. 2017, 19, 3600–3603. [Google Scholar] [CrossRef] [PubMed]
- Carreño, M.C.; Ribagorda, M.; Posne, G.H. Titanium-Promoted Stereoselective Synthesis of Hydroindolones from p-Quinamines by Domino Conjugate Additions. Angew. Chem. Int. Ed. 2002, 41, 2753–2755. [Google Scholar] [CrossRef]
- Kishi, K.; Arteaga, F.A.; Takizawa, S.; Sasai, H. Multifunctional catalysis: Stereoselective construction of α-methylidene-γ-lactams via an amidation/Rauhut–Currier sequence. Chem. Commun. 2017, 53, 7724–7727. [Google Scholar] [CrossRef] [PubMed]
- Pantaine, L.; Coeffard, V.; Moreau, X.; Greck, C. Enantioselective Desymmetrization of para-Quinamines through an Aminocatalyzed Aza-Michael/Cyclization Cascade Reaction. Org. Lett. 2015, 17, 3674–3677. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, B.; Jiang, H.; Cheng, Y.; Xiao, W.-J.; Lu, L.-Q. Synthesis of hydroindoles via desymmetric [3+2] cycloadditions of para-quinamines with photogenerated ketenes. Chem. Commun. 2021, 57, 8496–8499. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, R.; Prakash, M.; Samanta, S. Diastereoselective desymmetrization reactions of prochiral para-quinamines with cyclopropenes generated in situ: Access to fused hydroindoline-5-one scaffolds. Org. Biomol. Chem. 2021, 19, 7129–7133. [Google Scholar] [CrossRef]
- Zhou, X.-J.; Zhao, J.-Q.; Lai, Y.-Q.; You, Y.; Wang, Z.-H.; Yuan, W.-C. Organocatalyzed asymmetric dearomative 1,3-dipolar cycloaddition of 2-nitrobenzofurans and N-2,2,2-trifluoroethylisatin ketimines. Chirality 2022, 34, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Dou, P.-H.; Chen, Y.; You, Y.; Wang, Z.-H.; Zhao, J.-Q.; Zhou, M.-Q.; Yuan, W.-C. Organocatalyzed Asymmetric Dearomative [3+2] Annulation of Electron-Deficient 2-Nitrobenzo Heteroarenes with 3-Isothiocyanato Oxindoles. Adv. Synth. Catal. 2021, 363, 4047–4053. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.-Q.; Zhang, Y.-P.; Zhou, M.-Q.; Zhang, X.-M.; Yuan, W.-C. Copper-Catalyzed Asymmetric Dearomative [3+2] Cycloaddition of Nitroheteroarenes with Azomethines. Molecules 2023, 28, 2765. [Google Scholar] [CrossRef]
- Ge, Z.-Z.; Yang, L.; You, Y.; Wang, Z.-H.; Xie, K.-X.; Zhou, M.-Q.; Zhao, J.-Q.; Yuan, W.-C. Asymmetric dearomatization of 2-nitrobenzofurans by organocatalyzed one-step Michael addition to access 3,3′-disubstituted oxindoles. Chem. Commun. 2020, 56, 2586–2589. [Google Scholar] [CrossRef]
- Yang, F.; Rauch, K.; Kettelhoit, K.; Ackermann, L. Aldehyde-Assisted Ruthenium(II)-Catalyzed C-H Oxygenations. Angew. Chem. Int. Ed. 2014, 53, 11285–11288. [Google Scholar] [CrossRef]
- Wales, S.M.; Adcock, H.V.; Lewis, W.; Hamza, D.; Moody, C.J. Nitrogen-Bridged, Natural Product-Like Octahydrobenzofurans and Octahydroindoles: Scope and Mechanism of Bridge-Forming Reductive Amination via Caged Heteroadamantanes. Eur. J. Org. Chem. 2018, 2018, 4696–4704. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).