Functionalized 2,3′-Bipyrroles and Pyrrolo[1,2-c]imidazoles from Acylethynylpyrroles and Tosylmethylisocyanide
Abstract
1. Introduction
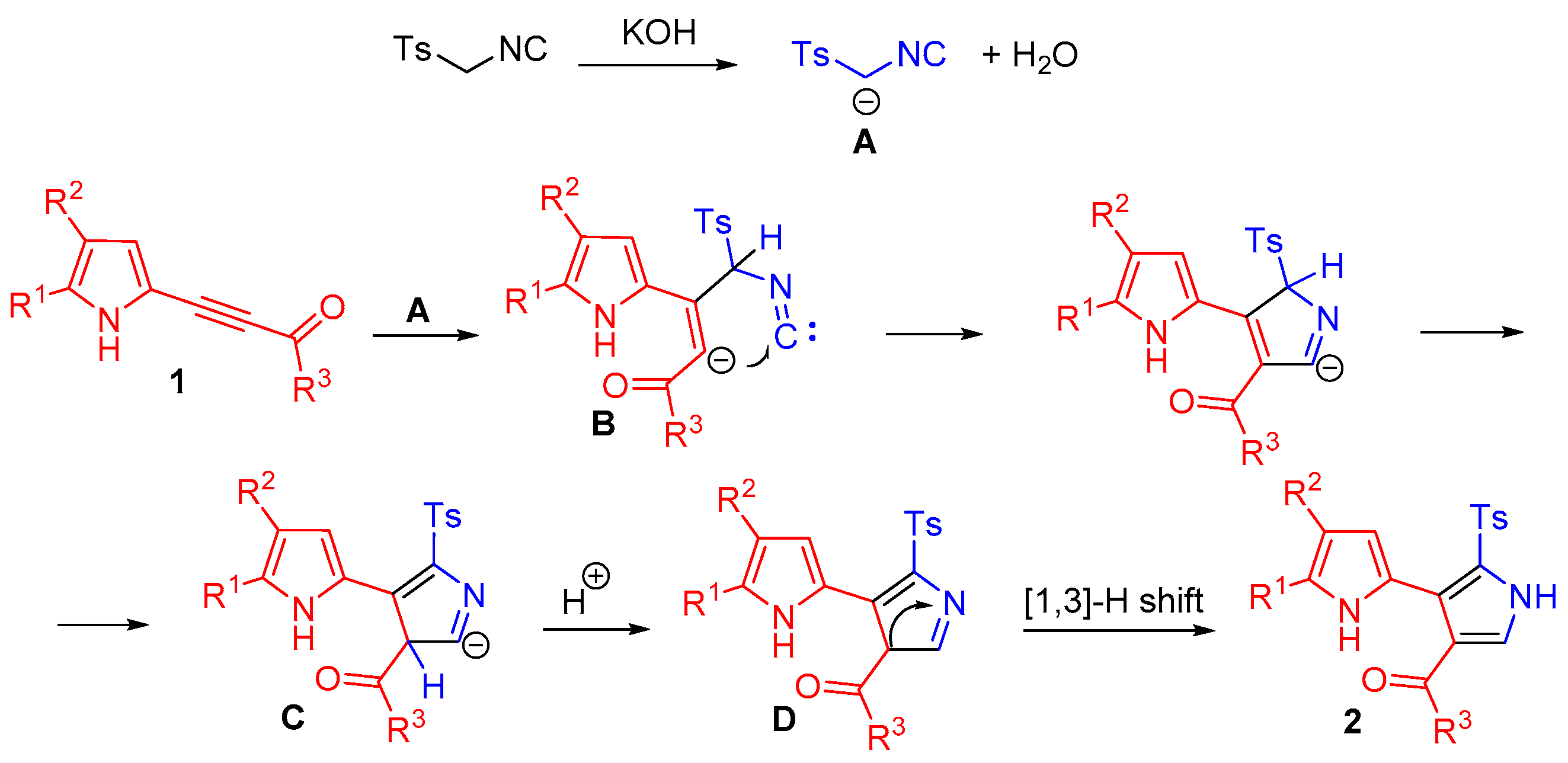
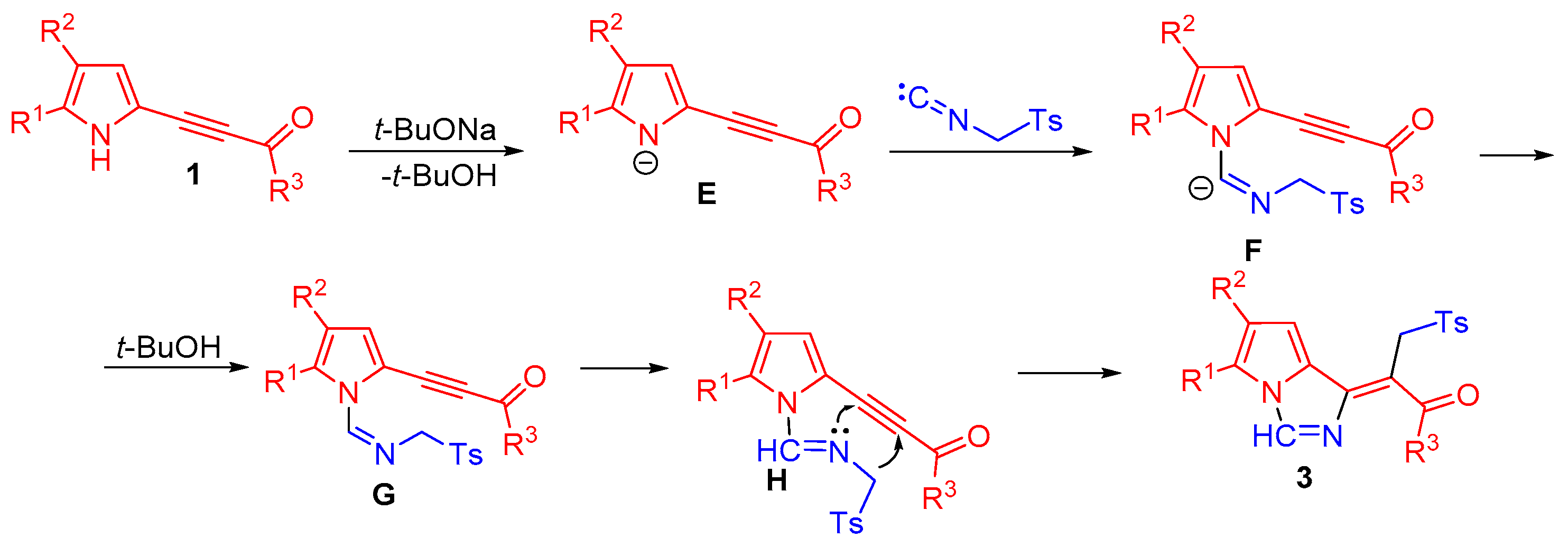
2. Results and Discussion
3. Materials and Methods
3.1. General Information
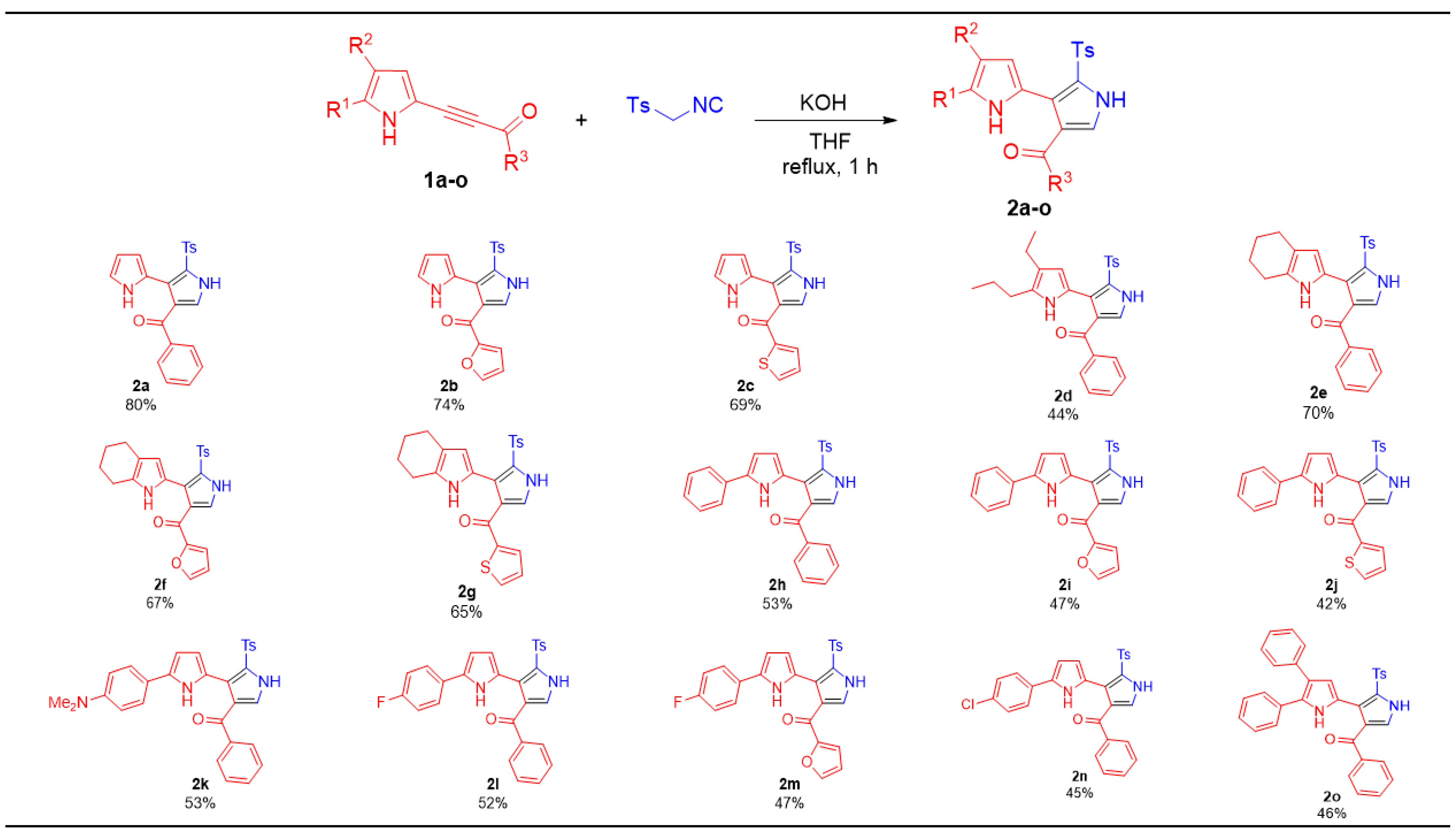
3.2. The Reaction of Acylethynylpyrroles 1a–o with TosMIC in the Presence of KOH: Synthesis of 2,3′-Bipyrroles (2a–o) (General Procedure)
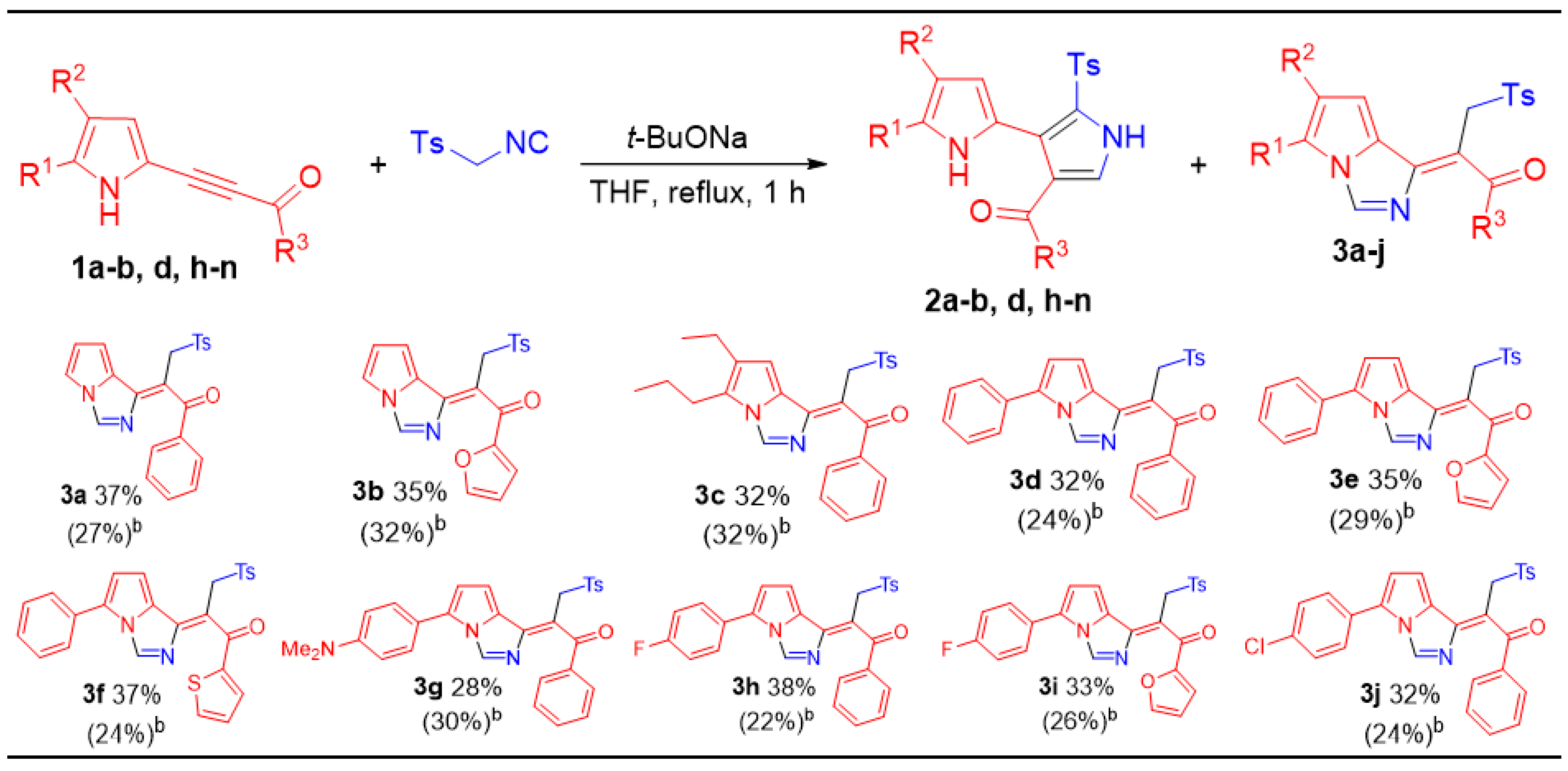
3.3. The Reaction of Acylethynylpyrroles 1a,b,d,h–n with TosMIC in the Presence of t-BuONa: Synthesis of 2,3′-Bipyrroles (2a,b,d,h–n) and Pyrrolo[1,2-c]imidazoles 3a–j (General Procedure)
3.4. Characterization Data of 2,3-Bipyrroles 2a–o and Pyrrolo[1,2-c]imidazoles 3a–j
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, M.; Fathallah, S. Pyrroles and Fused Pyrroles: Synthesis and Therapeutic Activities. Mini-Rev. Org. Chem. 2014, 11, 477–507. [Google Scholar] [CrossRef]
- Kaur, R.; Rani, V.; Abbot, V.; Kapoor, Y.; Konar, D.; Kapil, K. Recent synthetic and medicinal perspectives of pyrroles: An overview. J. Pharm. Chem. Chem. Sci. 2017, 1, 17–32. [Google Scholar]
- Li Petri, G.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M.V.; Barraja, P.; Montalbano, A. Bioactive pyrrole-based compounds with target selectivity. Eur. J. Med. Chem. 2020, 208, 112783. [Google Scholar] [CrossRef]
- Bulumulla, C.; Gunawardhana, R.; Gamage, P.L.; Miller, J.T.; Kularatne, R.N.; Biewer, M.C.; Stefan, M.C. Pyrrole-Containing Semiconducting Materials: Synthesis and Applications in Organic Photovoltaics and Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2020, 12, 32209–32232. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.C.A.D.; Marinho, D.I.L.F.; Hoelz, L.V.B.; Bastos, M.M.; Boechat, N. Pyrroles as Privileged Scaffolds in the Search for New Potential HIV Inhibitors. Pharmaceuticals 2021, 14, 893. [Google Scholar] [CrossRef] [PubMed]
- Rakendu, P.N.; Aneeja, T.; Anilkumar, G. Solvent-Free Synthesis of Pyrroles: An Overview. Asian J. Org. Chem. 2021, 10, 2318–2333. [Google Scholar] [CrossRef]
- Van Vuuren, N.J.; van Rensburg, H.D.J.; Terre’Blanche, G.; Legoabe, L.J. New fused pyrroles with rA1/A2A antagonistic activity as potential therapeutics for neurodegenerative disorders. Mol. Divers. 2022, 26, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.H.; Mir, P.A.; Mohi-ud-din, R.; Sabreen, S.; Maqbool, M.; Shah, A.J.; Shenmar, K.; Raza, S.N.; Pottoo, F.H. A Comprehensive Review on Journey of Pyrrole Scaffold Against Multiple Therapeutic Targets. Anti-Cancer Agents Med. Chem. 2022, 22, 3291–3303. [Google Scholar] [CrossRef]
- Amini, M.; Moghadam, E.S.; Mireskandari, K.; Abdel-Jalil, R. An Approach to Pharmacological Targets of Pyrrole Family from Medicinal Chemistry Viewpoint. Mini-Rev. Med. Chem. 2022, 22, 2486–2561. [Google Scholar] [CrossRef] [PubMed]
- Mateev, E.; Georgieva, M.; Zlatkov, A. Pyrrole as an Important Scaffold of Anticancer Drugs: Recent Advances. J. Pharm. Pharm. Sci. 2022, 25, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.H.; Raj, A.G.; Aruchamy, B.; Nanjan, P.; Drago, C.; Ramani, P. Pyrrole: A Decisive Scaffold for the Development of Therapeutic Agents and Structure-Activity Relationship. ChemMedChem 2024, 19, e202300447. [Google Scholar] [CrossRef]
- Shi, T.; Yin, G.; Wang, X.; Xiong, Y.; Peng, Y.; Li, S.; Zeng, Y.; Wang, Z. Recent advances in the syntheses of pyrroles. Green Synth. Catal. 2023, 4, 20–34. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Stepanova, Z.V.; Sobenina, L.N.; Mikhaleva, A.I.; Ushakov, I.A. Ethynylation of pyrroles with 1-acyl-2-bromoacetylenes on alumina: A formal ‘inverse Sonogashira coupling’. Tetrahedron Lett. 2004, 45, 6513–6516. [Google Scholar] [CrossRef]
- Sobenina, L.N.; Trofimov, B.A. Recent Strides in the Transition Metal-Free Cross-Coupling of Haloacetylenes with Electron-Rich Heterocycles in Solid Media. Molecules 2020, 25, 2490. [Google Scholar] [CrossRef]
- Oparina, L.A.; Belyaeva, K.V.; Kolyvanov, N.A.; Ushakov, I.A.; Gotsko, M.D.; Sobenina, L.N.; Vashchenko, A.V.; Trofimov, B.A. Catalyst-Free Annulation of Acylethynylpyrroles with 1-Pyrrolines: A Straightforward Access to Tetrahydrodipyrrolo[1,2-a:1′,2′-c]imidazoles. J. Org. Chem. 2022, 87, 9518–9531. [Google Scholar] [CrossRef] [PubMed]
- Anguera, G.; Brewster Ii, J.T.; Sánchez-García, D.; Sessler, J.L. Functionalized 2,2’-Bipyrroles: Building Blocks for Pyrrolic Macrocycles. Macroheterocycles 2018, 11, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.; Lopchuk, J.; Song, M.; Butler, B. Synthesis of Heteroaryl-Substituted Pyrroles via the 1,3-Dipolar Cycloaddition of Unsymmetrical Münchnones and Nitrovinylheterocycles. Synthesis 2015, 47, 2776–2780. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Gotsko, M.D.; Saliy, I.V.; Sobenina, L.N.; Ushakov, I.A.; Kireeva, V.V. Functionalized Bipyrroles and Pyrrolyl-Aminopyrones from Acylethynylpyrroles and Diethyl Aminomalonate. Synthesis 2021, 54, 1134–1144. [Google Scholar] [CrossRef]
- Yamawaki, I.; Matsushita, Y.; Asaka, N.; Ohmori, K.; Nomura, N.; Ogawa, K. Synthesis and aldose reductase inhibitory activity of acetic acid derivatives of pyrrolo[1,2-c]imidazole. Eur. J. Med. Chem. 1993, 28, 481–498. [Google Scholar] [CrossRef]
- Van-Gelder, J.M.; Klein, J.Y.; Basel, Y.; Reizelman, A.; Tchilibon, S.; Mouallem, O. Rigidified Compounds for Modulating Heparanase Activity. U.S. Patent 7,795,255, 5 January 2006. [Google Scholar]
- Surivet, J.-P.; Panchaud, P.; Specklin, J.-L.; Diethelm, S.; Blumstein, A.-C.; Gauvin, J.-C.; Jacob, L.; Masse, F.; Mathieu, G.; Mirre, A.; et al. Discovery of Novel Inhibitors of LpxC Displaying Potent in vitro Activity against Gram-Negative Bacteria. J. Med. Chem. 2019, 63, 66–87. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Guodong, C.; Fang, Y.; Feng, H.; Weikang, T. Pyrroloheterocyclic derivative, preparation method therefor, and application thereof in medicine. WO2020200069(A1), 2020. [Google Scholar]
- Zhang, Y.; Zheng, J.; Cui, S. Rh(III)-Catalyzed C–H Activation/Cyclization of Indoles and Pyrroles: Divergent Synthesis of Heterocycles. J. Org. Chem. 2014, 79, 6490–6500. [Google Scholar] [CrossRef] [PubMed]
- Dal Ben, D.; Antonini, I.; Buccioni, M.; Lambertucci, C.; Marucci, G.; Thomas, A.; Volpini, R.; Cristalli, G. Neuropeptide S Receptor: Recent Updates on Nonpeptide Antagonist Discovery. ChemMedChem 2011, 6, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.E.; Carter, P.H.; Tebben, A.J.; Scherle, P.A.; Brown, G.D.; Thompson, L.A.; Xu, M.; Lo, Y.C.; Yang, G.; Liu, R.-Q.; et al. Both 5-arylidene-2-thioxodihydropyrimidine-4,6(1H,5H)-diones and 3-thioxo-2,3-dihydro-1H-imidazo[1,5-a]indol-1-ones are light-dependent tumor necrosis factor-α antagonists. Bioorg. Med. Chem. Lett. 2003, 13, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ding, Y.; Qin, H.-X.; Xu, Z.-G.; Lan, H.-T.; Yang, D.-L.; Yi, C. One-pot synthesis of substituted pyrrole–imidazole derivatives with anticancer activity. Mol. Divers. 2019, 24, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Spyvee, M.; Ladner, R. Hydantoin Containing Deoxyuridine Triphosphatase Inhibitors. U.S. Patent 11,014,924, 21 November 2017. [Google Scholar]
- Mollanejad, K.; Asghari, S.; Jadidi, K. Diastereoselective synthesis of pyrrolo[1,2-c]imidazoles using chiral thiohydantoins, malononitrile, and aldehydes and evaluation of their antioxidant and antibacterial activities. J. Heterocycl. Chem. 2019, 57, 556–564. [Google Scholar] [CrossRef]
- Miguel-Gordo, M.; Gegunde, S.; Jennings, L.K.; Genta-Jouve, G.; Calabro, K.; Alfonso, A.; Botana, L.M.; Thomas, O.P. Futunamine, a Pyrrole–Imidazole Alkaloid from the Sponge Stylissa aff. carteri Collected off the Futuna Islands. J. Nat. Prod. 2020, 83, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Santos, R.M.; Reyes-Gutiérrez, P.E.; Torres-Ochoa, R.O.; Ramírez-Apan, M.T.; Martínez, R. 5,6-Dihydropyrrolo[2,1- a ]isoquinolines as Alternative of New Drugs with Cytotoxic Activity. Chem. Farm. Bull. 2017, 65, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Kalmouch, A.; Radwan, M.A.A.; Omran, M.M.; Sharaky, M.; Moustafa, G.O. Synthesis of novel 2, 3’-bipyrrole derivatives from chalcone and amino acids as antitumor agents. Egypt. J. Chem. 2020, 63, 4409–4421. [Google Scholar]
- Jin, Y.; Bu, P.; He, Q.; Lan, H.-T.; Zhou, F.; Zhang, L.; He, X. Azabicycle Derivatives, Preparation Method and Medical Application. CN105524068, 2014. [Google Scholar]
- Kumar, K. TosMIC: A Powerful Synthon for Cyclization and Sulfonylation. ChemistrySelect 2020, 5, 10298–10328. [Google Scholar] [CrossRef]
- Leusen, D.V.; Leusen, A.M.V. Synthetic Usesof Tosylmethyl Isocyanide (TosMIC). In Organic Reactions; Wiley: Hoboken, NJ, USA, 2001; pp. 417–666. [Google Scholar]
- Ma, Z.; Ma, Z.; Zhang, D. Synthesis of Multi-Substituted Pyrrole Derivatives Through [3+2] Cycloaddition with Tosylmethyl Isocyanides (TosMICs) and Electron-Deficient Compounds. Molecules 2018, 23, 2666. [Google Scholar] [CrossRef] [PubMed]
- Mathiyazhagan, A.D.; Anilkumar, G. Recent advances and applications of p-toluenesulfonylmethyl isocyanide (TosMIC). Org. Biomol. Chem. 2019, 17, 6735–6747. [Google Scholar] [CrossRef] [PubMed]
- Elshina, V.G.; Novokshonov, V.V.; Verochkina, E.A.; Ushakov, I.A.; Rosentsveig, I.B.; Vchislo, N.V. Synthesis of oxazolines and oxazoles by the reaction of propynals with tosylmethyl isocyanide. Mendeleev Commun. 2019, 29, 651–652. [Google Scholar] [CrossRef]
- Pogaku, N.; Krishna, P.R.; Prapurna, Y.L. Iodine-mediated new strategy for the synthesis of 2,5-disubstituted oxazoles from methyl ketones and TosMIC. Synth. Commun. 2018, 48, 1986–1993. [Google Scholar] [CrossRef]
- Van Leusen, A.M.; Oomkes, P.G. One-Step Conversion of Aldehydes to Nitriles. Introduction of a One-Carbon Unit. Synth. Commun. 1980, 10, 399–403. [Google Scholar] [CrossRef]
- Saliy, I.V.; Gotsko, M.D.; Sobenina, L.N.; Ushakov, I.A.; Trofimov, B.A. Chemo- and stereoselective synthesis of E-2-(2-acyl-1-tosylvinyl)pyrroles from tosylmethyl isocyanide (TosMIC) and 2-(acylethynyl)pyrroles. Tetrahedron Lett. 2021, 84, 153432. [Google Scholar] [CrossRef]
- Gotsko, M.D.; Saliy, I.V.; Sobenina, L.N.; Ushakov, I.A.; Trofimov, B.A. Tosyl/pyrrolyl-capped 1,3-enynes via t-BuOK-assisted reaction of TosMIC with acylethynylpyrroles: A new feature of this popular reagent. New J. Chem. 2022, 46, 16646–16650. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Moskva, V.V. The concept of acid and base in organic chemistry. Soros Edu. J. 1996, 12, 33–40. [Google Scholar]


 | |||||
| Entry a | Base | Time, h | Conversion of 1a, % | Yield of 2a, % b | Yield of 3a, % b |
|---|---|---|---|---|---|
| 1 | NEt3 | 24 | 0 | 0 | 0 |
| 2 | DBU | 24 | 0 | 0 | 0 |
| 3 | DABCO | 24 | 0 | 0 | 0 |
| 4 | K2CO3 | 24 | 45 | 15 | 10 |
| 5 | Cs2CO3 | 1 | 100 | 56 | 0 |
| 6 | NaOH | 1 | 100 | 72 | traces |
| 7 | KOH | 1 | 100 | 80 | traces |
| 8 c | t-BuONa | 1 | 100 | 15 | 23 |
| 9 | t-BuONa | 1 | 100 | 27 | 37 |
| 10 c | t-BuOK | 1 | 75 | 43 | traces |
| 11 | t-BuOK | 1 | 100 | 80 | traces |
| 12 d | NaH | 1 | 90 | 17 | 31 |
| 13 c | NaH | 1 | 90 | traces | 39 |
| 14 | NaH | 1 | 100 | traces | 45 |
| 15 e | NaH | 1 | 100 | traces | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotsko, M.D.; Saliy, I.V.; Ushakov, I.A.; Sobenina, L.N.; Trofimov, B.A. Functionalized 2,3′-Bipyrroles and Pyrrolo[1,2-c]imidazoles from Acylethynylpyrroles and Tosylmethylisocyanide. Molecules 2024, 29, 885. https://doi.org/10.3390/molecules29040885
Gotsko MD, Saliy IV, Ushakov IA, Sobenina LN, Trofimov BA. Functionalized 2,3′-Bipyrroles and Pyrrolo[1,2-c]imidazoles from Acylethynylpyrroles and Tosylmethylisocyanide. Molecules. 2024; 29(4):885. https://doi.org/10.3390/molecules29040885
Chicago/Turabian StyleGotsko, Maxim D., Ivan V. Saliy, Igor A. Ushakov, Lyubov N. Sobenina, and Boris A. Trofimov. 2024. "Functionalized 2,3′-Bipyrroles and Pyrrolo[1,2-c]imidazoles from Acylethynylpyrroles and Tosylmethylisocyanide" Molecules 29, no. 4: 885. https://doi.org/10.3390/molecules29040885
APA StyleGotsko, M. D., Saliy, I. V., Ushakov, I. A., Sobenina, L. N., & Trofimov, B. A. (2024). Functionalized 2,3′-Bipyrroles and Pyrrolo[1,2-c]imidazoles from Acylethynylpyrroles and Tosylmethylisocyanide. Molecules, 29(4), 885. https://doi.org/10.3390/molecules29040885







