Abstract
Our review paper evaluates the impact of plant-based products, primarily derived from plants from Serbia, on P-glycoprotein (P-gp) activity and their potential in modulating drug resistance in cancer therapy. We focus on the role and regulation of P-gp in cellular physiology and its significance in addressing multidrug resistance in cancer therapy. Additionally, we discuss the modulation of P-gp activity by 55 natural product drugs, including derivatives for some of them, based on our team’s research findings since 2011. Specifically, we prospect into sesquiterpenoids from the genera Artemisia, Curcuma, Ferula, Inula, Petasites, and Celastrus; diterpenoids from the genera Salvia and Euphorbia; chalcones from the genera Piper, Glycyrrhiza, Cullen, Artemisia, and Humulus; riccardins from the genera Lunularia, Monoclea, Dumortiera, Plagiochila, and Primula; and diarylheptanoids from the genera Alnus and Curcuma. Through comprehensive analysis, we aim to highlight the potential of natural products mainly identified in plants from Serbia in influencing P-gp activity and overcoming drug resistance in cancer therapy, while also providing insights into future perspectives in this field.
1. Drug Resistance in Cancer Therapy
The primary cause of cancer treatment failure is the development of drug resistance. Intrinsic or innate drug resistance exists before the patient is exposed to anticancer drugs, leading to insufficient effectiveness of the treatment. On the other hand, some tumors initially respond to therapy but eventually become resistant over time [,]. Malignant cells may either be resistant to a single applied drug (individual resistance) or develop cross-resistance to a wide range of structurally and functionally unrelated agents—a phenomenon known as multidrug resistance (MDR) []. This phenomenon is highly significant because most anticancer medications have a narrow therapeutic index, meaning there is a minimal variance between the dosage necessary to generate a therapeutic outcome and the dosage that results in toxicity. Consequently, increasing the dosage after resistance emerges in a clinical setting is useless [].
Numerous mechanisms contribute to the emergence of MDR. These encompass diminished drug accumulation within the cell, alterations to the detoxification system, modifications of target molecules, the rapid repair of DNA damage, and the prevention of apoptosis []. The reduced intracellular accumulation of drugs can be attributed to diminished uptake into the cell and an increased elimination of drugs from the cell. Most chemotherapeutic drugs enter the cell through passive diffusion across the membrane, making changes in the membrane structure capable of affecting drug uptake into the cancer cell. However, the primary reason for reduced accumulation is the effective elimination of chemotherapeutic agents from the cell due to the activity of ATP-binding family (ATP Binding Cassette—ABC) transporters, particularly ABCB1 (P-glycoprotein, MDR1), ABCC1 (MDR protein 1, MRP1), and ABCG2 (breast cancer related protein, BCRP) [,].
1.1. P-gp Role and Regulation in Cellular Physiology
P-glycoprotein (P-gp) is a well-studied member of the ABC transporter family. This transmembrane protein relies on ATP hydrolysis to actively efflux various substrates from cells. P-gp is involved in a range of physiological processes and is distributed in several tissues, including the liver, intestines, kidneys, placenta, endothelial cells of testicular vessels, and the blood–brain barrier (BBB) []. In these tissues, P-gp regulates the cellular uptake rate of xenobiotics, as well as their distribution and excretion, thereby safeguarding vital organs against xenobiotic damage. Moreover, P-gp impacts the effectiveness and bioavailability of medications by modulating their absorption, distribution, metabolism, excretion, and toxicity (ADMET) []. In the epithelial linings of the intestines and liver, P-gp has a protective function by actively removing harmful molecules into the bile duct and intestinal lumen []. Several studies have also shown that P-gp is involved in lipid homeostasis and cholesterol trafficking [,]. In the kidneys, P-gp is specifically localized to the apical surface of the epithelial cells within the proximal tubule. It is postulated that P-gp serves to impede the accumulation of injurious substances in instances of renal injury [,]. As a crucial element of the BBB, P-gp is expressed on the apical surface of capillary endothelial cells, where it prevents the entry of toxic substances into the brain []. In the testis, P-gp interacts with tight-junction proteins and plays an essential role in the restructuring of the blood–testis barrier during spermatogenesis []. P-gp also protects the fetus from maternally transported steroids, toxins and xenobiotics by playing a central role in the placental barrier [].
P-gp consists of two homologous parts, each containing a transmembrane domain (TMD) with six hydrophobic membrane-spanning α-helices and a cytoplasmic nucleotide-binding domain (NBD) []. All 12 α-helices of both TMDs enclose the inner cavity extending from the phospholipid bilayer’s center to the cytoplasm []. The cytoplasmic NBDs bind ATP and have ATPase activity. In the inward-facing conformation with separated NBDs, P-gp has numerous drug-binding sites containing hydrophobic and aromatic residues and polar amino acids. These binding pockets allow for the binding of various compounds with different structures and biological activities, including anticancer drugs, antihistamines, calcium channel blockers, antibiotics and many other drugs []. The binding of ATP molecules to NBDs leads to their dimerization and switch from the inward-facing conformation to the outward-facing conformation. The change in conformation leads to a rotation of the TMDs and a contraction of the inner cavity, allowing the bound substrates to efflux out of the cell []. ATP plays an essential role in stabilizing the outward conformation.
Various transcription factors and signaling pathways regulate the expression level of P-gp. The gene that codes for P-gp, ABCB1, is located on chromosome 7 and has 29 exons. The promoter region of the ABCB1 gene contains an inverse CCAAT element, which interacts with the transcription factors NF-Y and YB-1, as well as a GC element, which interacts with the transcription factors Sp1 and Sp3. These two elements are responsible for the constitutive expression of the ABCB1 gene. However, the activation of the Ras/MEK/ERK signaling pathway leads to the increased binding of the transcription factors Sp1 and Sp3 to the GC element and the increased transcription of the ABCB1 gene []. Similarly, activating the same signaling pathway leads to phosphorylation and the translocation of the transcription factor YB-1 into the nucleus, increasing the expression of P-gp []. Other known inducers of P-gp expression are nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1) []. In response to AKT phosphorylation via the JNK pathway, NF-κB translocates to the nucleus and increases the expression of P-gp []. Conversely, the tumor suppressor p53 binds directly to the promoter of the ABCB1 gene and suppresses its expression. Consequently, mutations in the TP53 gene increase P-gp expression due to the loss of function of the p53 protein []. Numerous studies have shown that microRNAs (miRs) such as miR-27a, miR-145 and miR-331-5p decrease the expression of P-gp by interacting with the 3′ untranslated region of ABCB1 mRNA [,]. miR-137 can also reduce the expression of P-gp by modulating the transcription factor YB-1 [].
1.2. P-gp Role in Cancer Therapy and Efforts to Overcome Multidrug Resistance
In addition to its physiological role, P-gp is also involved in establishing and maintaining a resistant phenotype in cancer cells. The upregulation of P-gp in cancer cells is described as an adaptive response to escape chemotherapy-induced cell death. Due to its broad substrate specificity, many anticancer drugs, including anthracyclines, taxanes, vinca alkaloids, epipodophyllotoxins and various tyrosine kinase inhibitors, are substrates for P-gp [,]. However, given the ability of P-gp to export a variety of substrates, its enhanced expression in cancer stem cells, and increased mitochondrial ATP production, it has been suggested that P-gp not only exports drugs from cells but also transports cell signaling molecules that contribute to tumorigenesis [].
Colorectal, renal and hepatocellular carcinomas originate from tissues that initially have elevated P-gp expression and are often characterized by intrinsic resistance []. In leukemia, lymphoma and multiple myeloma, P-gp is frequently overexpressed after chemotherapy and cancer recurrence []. In acute myeloid leukemia, P-gp is expressed in about 30% of patients at the time of diagnosis and is present in most cases at relapse []. Studies have also shown that intrinsic P-gp expression in non-small cell lung cancer (NSCLC) is similar to normal epithelial tissues []. However, there is a significant upregulation of P-gp expression during chemotherapy, often associated with a lower survival rate [].
In brain tumors, capillary endothelial cells express P-gp in 80% of patients, whereas tumor cells express P-gp in 20%. Remarkably, P-gp is not present in the neovasculature of other primary tumors []. Both low-grade and high-grade gliomas show P-gp vascular staining. Primary neuroblastomas originating from the adrenal glands show a similar vascular staining pattern as high-grade primary brain tumors []. In the study by Demeule et al., P-gp was detected in 60 human brain tumors, including meningiomas, schwannomas and both low- and high-grade gliomas []. P-gp is heterogeneously expressed in the membrane and the cytoplasm in glioma cells. Interestingly, P-gp is rarely found in metastatic brain tumors [,].
P-gp has served as the primary therapeutic target in various strategies to overcome MDR. Over the years, numerous efforts have been made to incorporate P-gp inhibitors into cancer treatment regimens. Regardless of successful efforts to reverse MDR in cell culture, the shortcoming in clinical application is the lack of specificity and high toxicity []. First-generation inhibitors like verapamil, quinidine, and cyclosporine A are compounds already used in the clinic for other indications. These inhibitors were initially identified as P-gp substrates that inhibit P-gp function by competing with other P-gp substrates [,]. However, their clinical application is limited due to their high toxicity and low affinity to P-gp. Second-generation inhibitors, designed as analogues of the first-generation inhibitors, exhibit a significantly higher affinity for P-gp []. These inhibitors also inhibit CYP3A enzymes, which are crucial for the metabolism of many anticancer drugs. This dual action can potentially alter the pharmacokinetics of anticancer drugs, thus increasing chemotherapeutic toxicity []. Subsequently, third-generation inhibitors have been developed with improved specificity for P-gp and a minimal impact on CYP3A enzymes. Important representatives of the third generation of inhibitors are zosuquidar (LY335979), elacridar (GF120918), laniquidar (R101933) and tariquidar (XR9576) []. These newer inhibitors are effective at lower doses and achieve optimal efficacy in the nanomolar range. However, clinical trials with tariquidar have raised significant concerns regarding toxicity, and phase III clinical trials were discontinued []. In addition, a phase III trial of zosuquidar failed to demonstrate improved outcomes in patients with acute myeloid leukemia [].
Despite the initial promise in preclinical and early clinical trials, the setbacks highlight the challenges of developing effective P-gp inhibitors for clinical use. These results underscore the need to research safer and more-efficient strategies to address MDR in cancer treatment. One notable development is the emergence of natural product drugs as fourth-generation P-gp inhibitors. Plants’ vast chemical diversity provides endless opportunities for discovering new P-gp inhibitors, and their derivatization allows for fine-tuning their performance, especially for natural product drugs that can inhibit P-gp while avoiding being transported by it.
2. Modulation of P-gp Activity by Natural Product Drugs
In this review paper, we aim to shed light on several potent classes of natural compounds and their important representatives, which we have been researching for over a decade. These include sesquiterpenoids, especially artemisinin and its derivatives; diterpenoids such as sclareol and its derivatives; and jatrophanes, chalcones, riccardins, and diarylheptanoids (Table 1). Apart from exhibiting strong anticancer activities and selectivity towards cancer cells, these compounds have also demonstrated the ability to affect P-gp and modulate MDR, thus contributing to the ongoing efforts to identify promising candidates for further clinical investigations. Furthermore, we have observed that the chemical derivatization of certain natural compounds discussed herein has led to improved performance against P-gp and enhanced selectivity towards cancer cells.
2.1. Sesquiterpenoids
Sesquiterpenoids have demonstrated strong antitumor properties by inhibiting P-gp, suggesting their potential to overcome MDR in cancer therapy. Among them, artemisinin and its derivatives exhibited significant potential in this direction.
2.1.1. Genus Artemisia
Artemisia is a large, diverse genus of plants belonging to the daisy Asteraceae family, with almost 500 species. Artemisia plants include robust herbaceous varieties and shrubs known for their strong essential oils, which are rich in chemical components. These plants thrive in the temperate climates of the northern and southern hemispheres and generally prefer dry or semi-dry areas. Well-known species are A. vulgaris (common mugwort), A. annua (sagewort), A. tridentata (big sagebrush), A. dracunculus (tarragon), A. absinthium (wormwood), and A. abrotanum (southernwood). Their distinct aroma and bitter taste come from terpenoids and sesquiterpene lactones, which serve as a deterrent against herbivores and may represent an evolutionary advantage. These plants produce small, wind-pollinated flowers [,].
A. annua, or sweet wormwood, is one member of this genus and often grows near roads, gardens, fields, etc. It is found in Asia and southeast Europe and is moderately distributed in Serbia, mainly in the Vojvodina province []. It has been well-known in Chinese traditional medicine for more than 2000 years but became famous in the 1970s, when artemisinin was isolated by Tu Youyou’s group for the first time []. Later, its derivatives artesunate, artemether, and dihydroartemisinin were discovered, in addition to their antimalarial activity together with artemisinin. While the efficacy and low toxicity of artemisinin compounds in the treatment of malaria are well-established, they have also been known to exhibit a range of beneficial effects in other capacities. These include antiviral and fungicidal properties, therapeutic effects in nonmalaria parasitic diseases, anti-inflammatory and antiasthma effects, and potential anticancer activity [].
Artemisinin (Scheme 1), an antimalarial sesquiterpene extracted from A. annua [], and its derivatives have shown significant anticancer activity, including the ability to overcome P-gp-mediated MDR in cancer treatment. While some derivatives are P-gp substrates, many also act as P-gp inhibitors, suggesting that they have the potential to reverse MDR and support the development of new cancer therapies [].
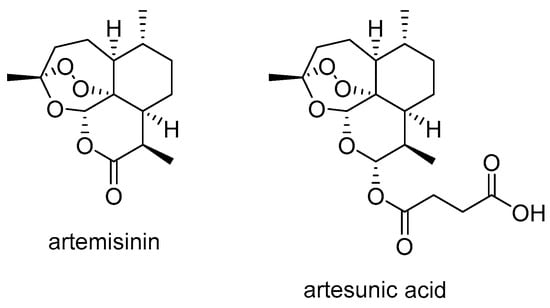
Scheme 1.
Chemical structures of artemisinin and artesunate.
Artesunate (artesunic acid) (Scheme 1) is a semi-synthetic derivative of artemisinin that has attracted considerable attention in cancer research over the last two decades due to its strong antitumor effect. Studies have shown that artesunate and its derivatives not only inhibit tumor growth but also overcome the MDR by inhibiting P-gp activity, thus increasing the efficacy of conventional chemotherapeutic agents.
In a recent study, artesunate was found to increase the cytotoxicity of doxorubicin in doxorubicin-resistant K562 leukemia cells (K562/ADR) []. Artesunate increased the efficacy of doxorubicin by inhibiting glycolysis. In addition, artesunate decreased the expression of the MDR1 and ABCG2 genes and inhibited the activities of P-gp and ABCG2 in K562/ADR cells. Artesunate also promoted apoptosis and decreased cell viability.
The promising activity and facile derivatization of the carboxyl group make artesunate a good candidate for transformation into derivatives (e.g., esters and amides) with better biological activity.
A recent study reported the synthesis of 17 hybrid molecules combining artesunate, a derivative of artemisinin, with synthetic 4-aryl-2-aminopyrimidines (Scheme 2) []. These new compounds were designed to improve the parent molecules’ cytotoxic properties, activity and selectivity. The hybrid molecules, with an ethylenediamine linker, a piperazine linker, and their precursors, including the pyrimidine derivatives artemisinin and artesunate, were tested against susceptible and MDR human non-small cell lung carcinoma cells. The hybrid molecules with the piperazine linker showed high selectivity against cancer cells. These hybrids had a similar anticancer effect to artesunate but exhibited significantly improved selectivity against cancer cells. The hybrid compounds with the piperazine linker bypassed the MDR phenotype, inhibited P-gp activity and increased MDR cancer cells’ sensitivity to doxorubicin. P-gp inhibition by several piperazine-based hybrid compounds was more effective than that by artesunate. Compound 1 (Scheme 2) proved to be the most effective anticancer agent in both sensitive and MDR lung cancer cells.
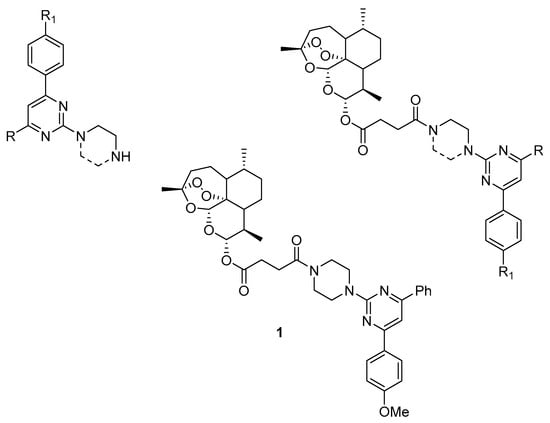
Scheme 2.
Chemical structures of 4-aryl-2-aminopyrimidines and new artesunate-based hybrids.
Artesunate was also found to block rhodamine 123 transport in the Caco-2 cell model and decrease P-gp-mediated digoxin transport []. A new artesunate-podophyllotoxin conjugate 2 (Scheme 3) was found to have strong cytotoxicity against cancer cell lines and reduced drug resistance []. The conjugate decreased P-gp levels in P-gp-overexpressing K562/ADR cells. It also disrupted the microtubule network, caused G2/M cell cycle arrest in K562/ADR cells and induced apoptosis.
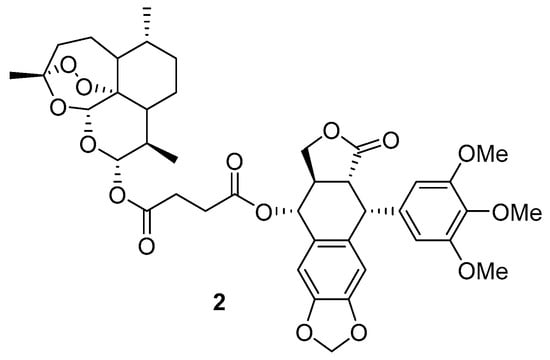
Scheme 3.
Chemical structure of artesunate-podophyllotoxin conjugate.
Natural bicyclic sesquiterpenes, β-caryophyllene (BCP) and β-caryophyllene oxide (BCPO), are present in a large number of plants worldwide, including the genus Artemisia, and are often found in essential oils. BCP is a plant compound, a member of the bicyclic sesquiterpenes. In nature, it mainly occurs as trans-caryophyllene ((E)-BCP) mixed with small amounts of its isomers, (Z)-β-caryophyllene (iso-caryophyllene) and α-humulene (α-caryophyllene), as well as its oxidation derivative BCPO.
BCP and BCPO (Scheme 4) have been shown to enhance the effectiveness of low-dose doxorubicin in treating human hepatoma HepG2 cells []. These sesquiterpenes not only increased the intracellular accumulation of doxorubicin and rhodamine 123 but also interfered with the function of P-gp. Molecular docking studies revealed that BCP has a strong binding affinity to P-gp.
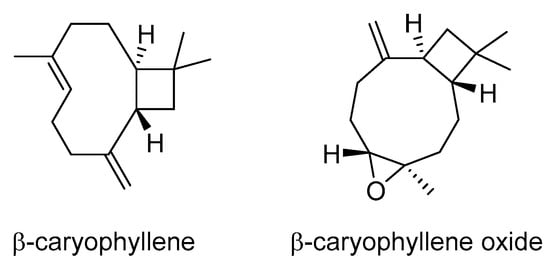
Scheme 4.
Chemical structures of β-caryophyllene and β-caryophyllene oxide.
2.1.2. Genus Curcuma
Curcuma is a genus of plants in the Zingiberaceae family that contains more than 130 species that are native to southeast Asia, southern China, the Indian subcontinent, New Guinea and northern Australia.
Curcuma longa is one of the best-known species within its genus and belongs to the Zingiberaceae family. This perennial herbaceous plant grows from rhizomes and is native to the Indian subcontinent and southeast Asia. It thrives in regions with temperatures between 20 and 30 °C and requires a lot of rainfall each year for optimal growth [].
Turmerones, a group of sesquiterpenoids found in Curcuma longa, were investigated for their impact on the absorption and transport of curcumin in human colorectal adenocarcinoma Caco-2 cells []. The study revealed that the presence of α- and aromatic turmerones significantly enhanced the transport of curcumin into Caco-2 cells. Additionally, α-turmerone (Scheme 5) was found to inhibit the activity of P-gp, potentially explaining the increased curcumin uptake. Conversely, aromatic turmerone increased P-gp mRNA expression.
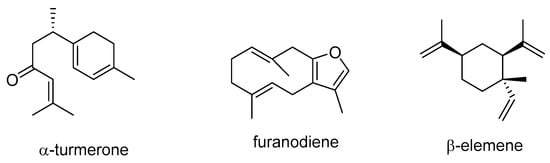
Scheme 5.
Chemical structures of α-turmerone, furanodiene, and β-elemene.
Furanodiene (Scheme 5), a precursor of furan-containing sesquiterpenoids isolated from Rhizoma curcumae, enhanced the anticancer potential of 5-FU, reversing resistance to cisplatin and doxorubicin in xenotransplanted zebrafish cancer models. These effects could be related to its ability to inhibit angiogenesis, induce reactive oxygen species (ROS) production, cause DNA strand breaks, and promote apoptosis by blocking P-gp efflux transport []. β-Elemene (Scheme 5), a sesquiterpenoid extracted from Rhizoma curcumae, affects cellular signaling pathways involved in cell survival and apoptosis. It is a promising candidate for cancer treatment due to its efficacy, safety, and synergistic effects with other substances. A study by Zhang et al. suggested that β-elemene could be used alongside conventional P-gp substrate chemotherapeutics to counteract MDR in leukemia treatment []. Additionally, β-elemene may act as an anti-metastatic agent when combined with doxorubicin in the treatment of MDR gastric cancer. The mechanisms underlying these effects include β-elemene’s ability to enhance the efficacy of doxorubicin by suppressing P-gp expression, increasing intracellular doxorubicin concentrations, and modulating signaling pathways related to Akt phosphorylation, E3 ubiquitin ligases, and miR-1323/Cbl-b/EGFR [,]. Tang et al. found that β-elemene caused the reversal of doxorubicin resistance in MCF-7 cells by decreasing ABC transporter function and ABC gene and protein expression [].
2.1.3. Genus Ferula
Ferula species belong to the Apiaceae family. The genus Ferula, the third-largest genus of the Apiaceae (alt. Umbelliferae) family, is composed of approximately 180 species distributed in central and southwest Asia, the Far East, north India and the Mediterranean. Fifteen of them are endemic to Iran, nine to Turkey, seven to China and one species to Italy, and the rest are indigenous entities of several other countries []. Plants of this genus are rich sources of biologically active natural products such as sesquiterpene coumarins, sulfur-containing compounds and sesquiterpene lactones.
In a study by Kasaian et al., fifteen sesquiterpene coumarins from different Ferula species were examined for their potential to reverse MDR []. When used at very low concentrations, certain sesquiterpene coumarins such as umbelliprenin, farnesiferol B, farnesiferol C, and lehmferin (Scheme 6) significantly enhanced the cytotoxicity of doxorubicin in P-gp-overexpressing MCF-7/ADR cells. These compounds also led to an increased intracellular accumulation of rhodamine 123 in the treated cells. Notably, the ring-opened drimane-type sesquiterpene coumarins, particularly farnesiferol B, farnesiferol C, and lehmferin, demonstrated the strongest inhibitory effect on P-gp efflux, suggesting their potential for further structural modifications to improve their MDR-reversal properties.
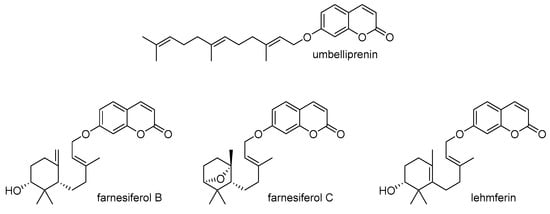
Scheme 6.
Chemical structures of umbelliprenin, farnesiferol B, farnesiferol C, and lehmferin.
2.1.4. Genus Inula
The Inula genus comprises approximately 120 species of flowering plants within the Asteraceae family, originating from Europe, Asia, and Africa. There are 35 species of this genus in Europe and 11 in Serbia. I. helenium can be found in central and southeast Europe and central Asia. It is widely distributed in Serbia. I. oculus-christi L. can be found on the Balkan Peninsula and in surrounding countries, including central and south Russia, the Caucasus Mountains, Armenia and Anatolia. It is also widely distributed in Serbia. I. Britannica L. grows in central and south Europe and Iran. Its habitat is in central Serbia, near roads, forests, and creeks [].
Costunolide (Scheme 7), derived from I. helenium L., demonstrated an antiproliferative effect on various cancer cells and significantly enhanced the antiproliferative efficacy of doxorubicin in drug-resistant cell lines by blocking the PI3K/AKT signaling pathway and reducing P-gp expression [,]. Ergolide (Scheme 7), isolated from I. oculus-christi L., exhibited strong synergistic effects with vincristine against acute lymphoblastic leukemia cells, indicating its potential to overcome P-gp-mediated resistance []. Compound 3 (Scheme 7) from I. japonica was found to inhibit the expression of drug-resistance proteins such as ABCC1, ABCG2, and P-gp. This suggests a potential mechanism for reversing MDR and improving chemotherapy sensitivity in the paclitaxel-resistant human non-small cell lung cancer cell line A549/PTX []. In another study, sesquiterpene lactones isolated from I. britannica from Serbia were screened for cytotoxic activity on four different human cancer cell lines and their multidrug-resistant counterparts, as well as on normal human keratinocytes, and they showed similar cytotoxic activity toward drug-sensitive and drug-resistant cancer cell lines [].
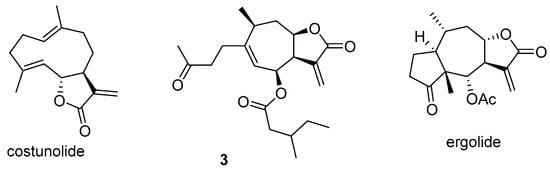
Scheme 7.
Chemical structures of costunolide, sesquiterpenoid 3, and ergolide.
2.1.5. Genus Petasites
Petasites belongs to the Asteraceae family, which is known for its flowering plants. These perennial plants have robust underground rhizomes that spread horizontally, and during their growth phase, they form large, rhubarb-like leaves. Most Petasites species originate from regions in Asia or southern Europe []. Petasites formosanus Kitamura is a perennial herb and the only indigenous Petasites species in Taiwan [].
Two sesquiterpenes, isopetasin and S-isopetasin (Scheme 8), found in Petasites formosanus, have a dual function in cancer therapy. They inhibit P-gp and also have cytotoxic effects []. A study by Abdelfatah et al. demonstrated that these compounds bind with high affinity to key amino acids of the P-gp transporter. This binding leads to increased mitochondrial workload, ATP production, ROS formation, and ultimately cell death in the MDA-MB-231 cell line [].
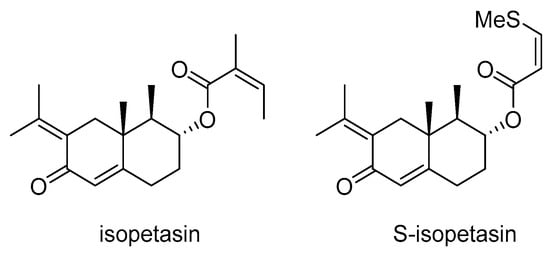
Scheme 8.
Chemical structures of isopetasin and S-isopetasin.
2.1.6. Genus Celastrus
The genus Celastrus (Celastraceae) comprises about 30 species worldwide, consisting of deciduous or evergreen shrubs with cylindrical branches, typically reaching a height of 1–10 m. These plants have a long history in Asia, where they are used in traditional medicine for a variety of ailments, including arthritis, rheumatoid arthritis, cognitive disorders, insomnia, edema, epilepsy, joint and muscle pain [].
Callies et al. created a natural product-based library based on the dihydro-β-agarofurans, previously identified in Celastrus vulcanicola. The library was screened for its ability to inhibit P-gp-mediated daunomycin efflux in MDR cells []. Several analogs showed higher potency than the lead compound and a first-generation P-gp modulator, verapamil, indicating their potential as effective modulators of P-gp-mediated MDR in cancer cells.
2.2. Diterpenoids
Diterpenoids are wide range of plant secondary metabolites formed from four isoprene units found in large number of species. In this section, we will discuss diterpenoids found in some plant species from Serbia and neighboring countries with the potential to inhibit the growth of cancer cells and overcome drug-resistance mechanisms mediated by P-gp.
2.2.1. Genus Salvia
Diterpenoids are wide range of plant secondary metabolites formed from four isoprene units found in a large number of species. Salvia is the largest genus of the Lamiaceae family and includes near 900 species. S. sclarea grows mainly in the Mediterranean region, from Caucasus, Iran and Syria to southern regions of France and northern Africa. In Serbia, it can be found in the eastern and southeastern parts of the country []. S. officinalis habitats are also in the Mediterranean region, from Spain to the northern Balkan regions, eastern Asia, and northern Syria. In Serbia, it is widely distributed, especially in Artemisio-Salvietum officinalis association []. It is cultivated widely across the whole of Europe and North America.
Sclareol (Scheme 9), a natural labdane diterpene, has been extensively studied for its anticancer properties and its ability to reverse resistance to various drugs. Isolated from Salvia sclarea and Salvia officinalis, sclareol has shown promising results in preclinical studies as a cytotoxic agent against cancer cells and, more recently, as a P-gp modulator. These findings suggest its potential as a therapeutic agent in cancer treatment.
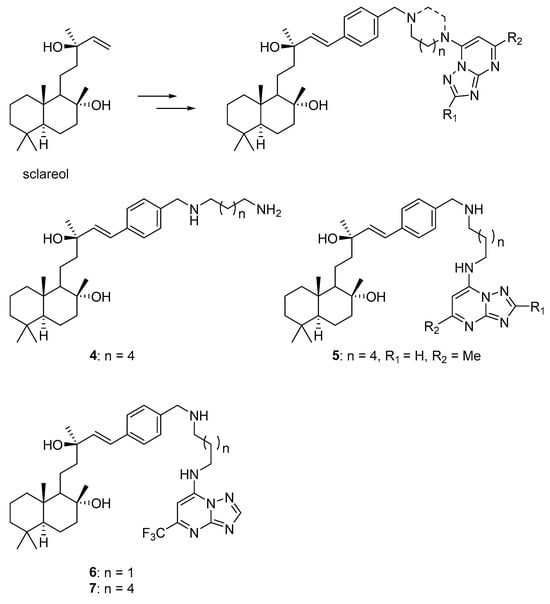
Scheme 9.
Chemical structures of sclareol, its derivatives and hybrids with 1,2,4-triazolo[1,5-a]pyrimidines.
Sclareol has shown potential in reducing cell viability and increasing apoptosis in MCF-7 breast cancer cells []. Combined with cyclophosphamide, sclareol enhanced these anticancer effects. Sclareol increased the expression of p53 and BAX while downregulating Bcl-2. Docking studies revealed an interaction between sclareol and STAT3 and inhibited the role of IL-6 in the modulation of apoptosis-associated genes. Additionally, sclareol treatment in the MKN-45 gastric cancer cell line resulted in a significant reduction in MDR1 gene expression and P-gp levels at lower doses []. Sclareol also reduced the viability of human small-cell lung cancer cells [] and inhibited tumor weight and volume in a xenotransplanted-tumor mouse model.
Sclareol also demonstrated the potential to reverse cisplatin resistance in non-small cell lung cancer cells []. In HeLa cells, sclareol inhibited cell proliferation and induced apoptosis []. By encapsulating doxorubicin and sclareol together, researchers found that a nanostructured lipid carrier (NLC-DOX-SC) is a viable alternative to the DOX:SC combination with fewer side effects [].
Sclareol was found to increase the accumulation of doxorubicin in glioblastoma cells, regardless of the expression and activity of P-gp. This effect was observed in all the glioblastoma cell lines studied, including U87, U87-TxR, and U251 []. The most significant impact was seen in the MDR cell line U87-TxR, which showed an increase in accumulation of over 50%. These findings prompted the development of new hybrid compounds of sclareol and doxorubicin, which exhibited greater selectivity towards cancer cells and displayed reduced resistance compared to doxorubicin in glioblastoma cells [].
Dimas and co-authors published several studies regarding the cytotoxic activity of sclareol. They demonstrated that sclareol induces apoptosis in human leukemic cell lines by downregulating the expression of proto-oncogene c-myc without affecting the expression of the anti-apoptotic protein, Bcl-2 [,], and in human colon tumor cells (HCT116) in vitro by activating both the mitochondrial pathway and the death receptor pathway []. Additionally, the same group reported that sclareol enhances the activity of known anticancer drugs such as doxorubicin, etoposide, and cisplatin against human breast cancer cells [], in addition to inducing apoptosis in human tumor cell lines and suppressing tumor growth in vivo via a p53-independent mechanism of action [].
In a recent study, 24 hybrid molecules were synthesized by combining naturally occurring sclareol and synthetic 1,2,4-triazolo[1,5-a]pyrimidines to improve cytotoxic properties, activity, and selectivity []. Due to the chemoselectivity of the allyl alcohol functional group, the tertiary and tertiary allylic hydroxyl groups of sclareol were preserved during the derivatization, which is essential for the desired biological activity. The concentration-dependent cytotoxicity of these molecules was examined in MDR U87-TxR cells with increased P-gp expression and normal lung fibroblasts (MRC-5). Two hybrid compounds were active in the nanomolar range, and seven compounds were more selective towards glioblastoma cells than sclareol. Additionally, sclareol and several derivatives showed collateral sensitivity. Furthermore, hybrids 5, 6, and 7 (Scheme 9) reduced P-gp activity similarly to tariquidar. Specifically, compound 5 and its precursor 4 (Scheme 9) affected various cellular processes, including cell cycle, cell death, mitochondrial membrane potential, and ROS/RNS levels in glioblastoma cells. The sensitivity of MDR glioblastoma cells was linked to the modulation of oxidative stress and mitochondrial inhibition.
2.2.2. Genus Euphorbia
The genus Euphorbia, also known as spurge, is part of the Euphorbiaceae family and comprises more than 2000 species of annual, biennial, or perennial flowering plants. It is recognized as one of the largest and most diverse genera within the plant kingdom. Studies on the phytochemical composition of these plants have revealed the presence of a wide range of secondary metabolites, including terpenes, glycerols, steroids, cerebrosides, and phenolic compounds. These compounds have been found to exhibit various beneficial activities, such as antiproliferative, cytotoxic, antiviral, antimicrobial, anticancer, and anti-inflammatory properties [,]. Furthermore, the genus Euphorbia is abundant in jatrophanes, which are macrocyclic diterpenes characterized by a unique basic structure and a flexible twelve-membered ring.
Over the past two decades, significant research has focused on the effects of jatrophane diterpenoids on P-gp modulation, resulting in a comprehensive understanding of this area. Numerous jatrophane compounds have emerged as strong P-gp inhibitors. The following section will explore the plant sources of jatrophane diterpenes, extending beyond Serbia and its adjacent regions to include other areas, due to their notable efficacy as P-gp inhibitors.
E. nicaeensis All.
E. nicaeensis is a hardy perennial plant characteristic for the Mediterranean region, also found in Serbia, Romania, and Bulgaria. It grows in dry regions in Serbia such as Deliblato Sand in the Vojvodina province.
Seven newly identified jatrophane diterpenoids named nicaeenin A–G were discovered from the latex of E. nicaeensis in Serbia, in addition to eight known jatrophane diterpenoids—the euphodendrophanes A–C, F, N, O, Q, and S []. Most of the newly identified jatrophanes showed significant potential to inhibit P-gp activity in the two MDR cancer cell lines NCI-H460/R and DLD1-TxR. Among them, nicaeenin F and nicaeenin G (Scheme 10) showed the strongest inhibitory effects. Additionally, nicaeenin G significantly increased the sensitivity of NCI-H460/R cells to doxorubicin compared to Dex-verapamil.
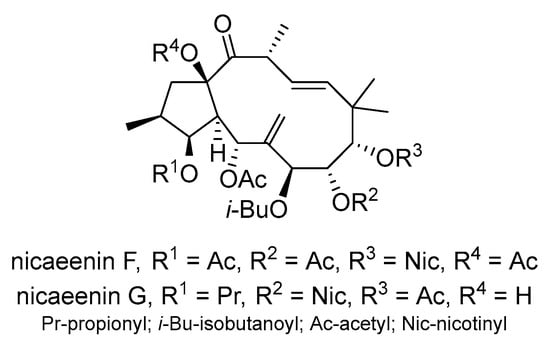
Scheme 10.
Chemical structures of nicaeenin F and G.
Another study by Krstić et al. reported that jatrophane diterpenoids 8 and 9 (Scheme 11), isolated from the roots of E. nicaeensis, effectively inhibited P-gp in MDR cells of non-small cell lung cancer and colorectal carcinoma []. The compounds outperformed the potential of the P-gp inhibitor tariquidar in MDR colorectal carcinoma and glioblastoma cells and served as sensitizing agents that were able to reduce the required drug concentrations for P-gp substrate drugs. Jatrophane 1 also inhibited the cell growth of non-small cell lung cancer and glioblastoma cells, suggesting significant anticancer properties.
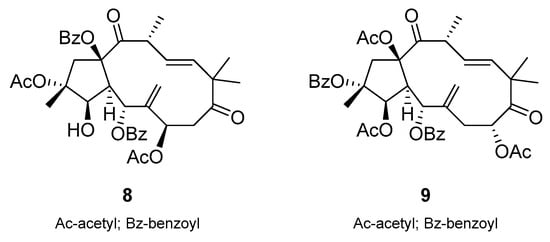
Scheme 11.
Chemical structures of jatrophane diterpenoids 8 and 9.
E. dendroides L.
E. denroides L. is a perennial semisucculent bush with a height up to 3 m. It grows in hilly coastal areas of the Mediterranean, mainly Montenegro, Greece and Albania, on rocky surfaces []. This species is well-known for its use as a biomass for biofuel production [], but it has not been extensively chemically investigated.
Six new jatrophane diterpenoids extracted from the Montenegrin spurge E. dendroides were found to inhibit the growth of four human cancer cell lines: NCI-H460, NCI-H460/R, DLD1, and U87 []. The most potent compounds, euphodendrophane B and the closely related euphodendrophane A (Scheme 12), were tested for their interactions with paclitaxel and doxorubicin, particularly in the MDR non-small lung carcinoma cell line NCI-H460/R. Both compounds demonstrated strong potential to reverse drug resistance by inhibiting P-gp transport, effectively reversing resistance to paclitaxel in NCI-H460/R cells. Additionally, these jatrophanes improved the sensitivity of NCI-H460/R cells to doxorubicin.
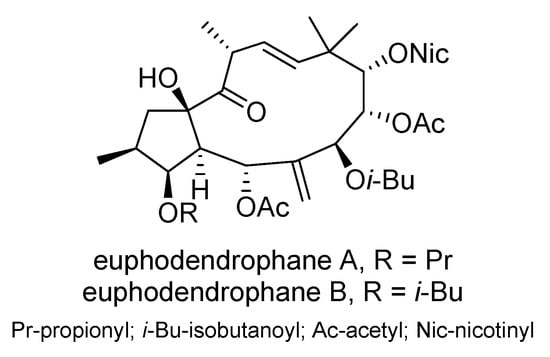
Scheme 12.
Chemical structures of euphodendrophane A and B.
In a study by Jadranin et al., thirteen jatrophane diterpenoids were isolated from the latex of E.dendroides []. The inhibitory effect of these jatrophane diterpenoids on P-gp was investigated. In particular, compounds 10 and 11 (Scheme 13) showed strong P-gp inhibition, outperforming R(+)-verapamil and tariquidar in the colorectal carcinoma MDR DLD1-TxR cells.
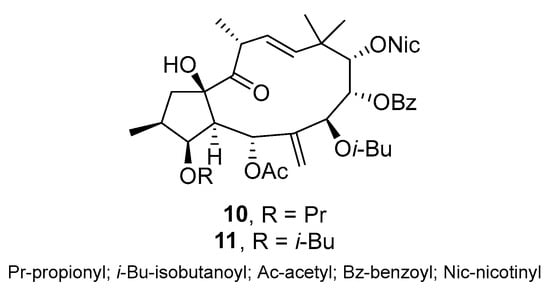
Scheme 13.
Chemical structures of jatrophane diterpenoids 10 and 11.
E. esula L.
E. esula L. (leafy spurge) is a species with a worldwide distribution, from Scandinavia to the Balkan Peninsula (mostly in Serbia), from Spain to mid Russia, and in mid Asia, Iran, Siberia, and east Asia. In Serbia, it can be found in the Fruška Gora Mountains and Gledić Mountains [].
Thirteen new jatrophane diterpenoids, euphoresulanes A-M, and seven known ones were isolated from E. esula []. These compounds were assessed for their ability to reverse MDR in P-gp-overexpressing HepG2/ADR hepatocellular carcinoma cells. Euphoresulan H (Scheme 14) proved to be the most potent MDR modulator, increasing the efficacy of doxorubicin by 33-fold.
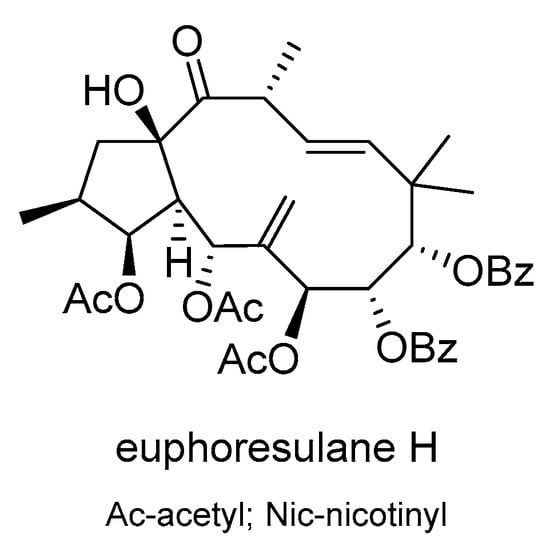
Scheme 14.
Chemical structures of euphoresulan H.
E. helioscopia L.
E. helioscopia L. is a widely distributed Euphorbia species. It can be found across the whole of Europe and in northern Africa and east Asia, but also in other parts of Asia. It is abundant in all regions of Serbia [].
Heliosterpenoids A and B (Scheme 15), two novel jatrophane-derived diterpenoid esters with a unique 5/6/4/6-fused tetracyclic ring structure, were isolated from E. helioscopia []. Both compounds exhibited a strong inhibition of P-gp, with heliosterpenoid A also showing cytotoxicity against the breast cancer cell line MDA-MB-231.
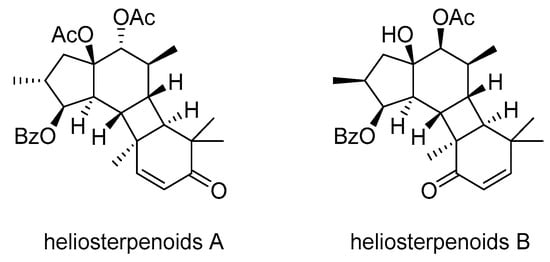
Scheme 15.
Chemical structures of heliosterpenoids A and B.
E. sororia A. Schrenk
ES2 (Scheme 16), a novel jatrophane diterpenoid ester derived from the fruit of E. sororia, a traditional Uighur medicinal plant in China, was evaluated for its potential in reversing P-gp-mediated MDR []. ES2 showed minimal cytotoxicity against both P-gp-overexpressing MDR cells and their non-resistant counterparts but sensitized MDR cells and P-gp-transfected HEK293 cells to chemotherapeutics known to be P-gp substrates. The reversal effect of ES2 was primarily due to the inhibition of the efflux function of P-gp. Additionally, ES2 stimulated the ATPase activity of P-gp in a concentration-dependent manner without altering P-gp expression. Molecular docking analyses revealed the binding affinity of ES2 to the drug-binding site of the P-gp transporter. ES2 significantly enhanced the antitumor efficacy of vinorelbine against KBv200 cell xenografts in nude mice.
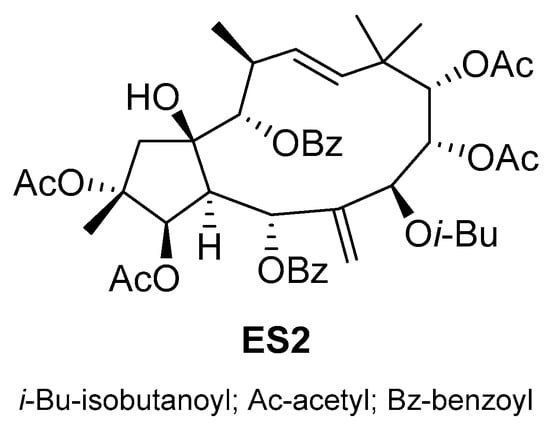
Scheme 16.
Chemical structure of jatrophane diterpenoid ES2.
Eight new jatrophane diterpenoids were found in the fructus of E. sororia, along with fourteen known ones []. These compounds displayed lower cytotoxicity and showed potential in reversing MDR in doxorubicin-resistant human breast cancer MCF-7/ADR cells. Particularly, euphosorophane I (Scheme 17) demonstrated high efficacy in reversing P-gp-mediated resistance to doxorubicin. It increased the intracellular accumulation of rhodamine 123 and doxorubicin in drug-resistant cells, inhibited P-gp-dependent rhodamine 123 efflux, stimulated P-gp ATPase activity, and inhibited doxorubicin transport. Euphosorophane I acted by activating ATPase rather than upregulating P-gp expression, resulting in a better reversal of MDR. Molecular docking analysis confirmed its binding to the drug-binding site of P-gp.
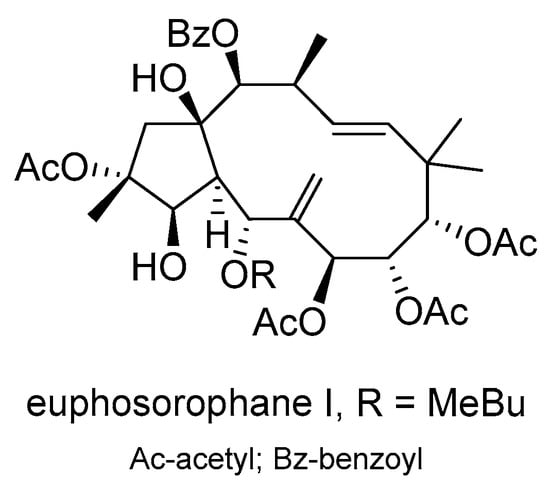
Scheme 17.
Chemical structure of euphosporophane I.
Maimaitijiang et al. conducted a study focusing on the development of novel modulators targeting P-gp to combat MDR in cancer treatment []. They modified compound 12 (Scheme 18), a natural jatrophane from E. sororia, to produce nine derivatives that exhibited enhanced MDR-reversal activity in P-gp-overexpressing MCF-7/ADR cells compared to verapamil. The most effective compound, 13 (Scheme 18), abolished P-gp-mediated resistance to doxorubicin, with low cytotoxicity and a high therapeutic index. Compound 13 not only increased the accumulation of doxorubicin and rhodamine123 in MCF-7/ADR cells but also stimulated P-gp ATPase activity, ultimately increasing doxorubicin sensitivity. The mechanism of action for the reversal activity of compound 13 was through the stimulation of P-gp ATPase activity and not through the direct inhibition of P-gp protein expression. Molecular docking studies confirmed its high binding affinity with the doxorubicin-recognition site on P-gp.
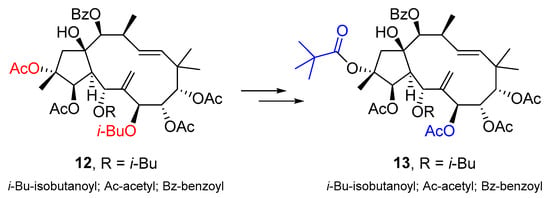
Scheme 18.
Chemical structures of jatrophane diterpenoid 12 and corresponding derivative 13.
E. glomerulans Prokh.
E. glomerulans Prokh. is distributed in northwestern China and the five countries that make up central Asia. A cytotoxic evaluation of jatrophane diterpenoids isolated from the acetone extracts of E. glomerulans on MCF-7/ADR found that several compounds were able to reverse MDR []. Among these, euphoglomeruphane K and L (Scheme 19) showed superior MDR-reversal activity compared to the positive control verapamil.
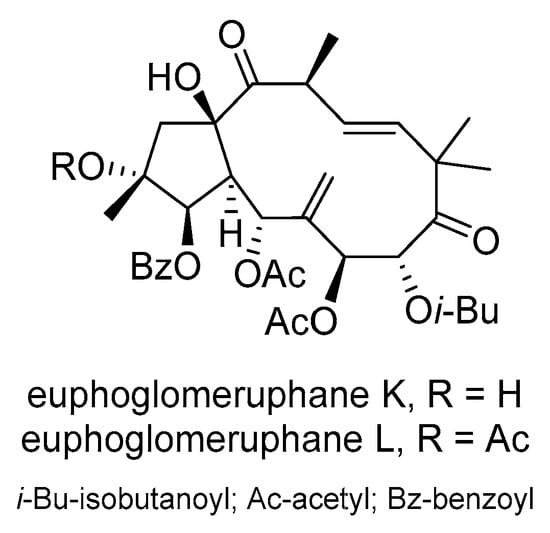
Scheme 19.
Chemical structures of euphoglomueruphane K and L.
Another example of biologically active jatrophane ditrpenoids are those from Pedilanthus tithymaloides (Euphorbia tithymaloides). It is native to tropical and subtropical North America and Central America. Thirteen jatrophane diterpenoids, including eight newly discovered compounds, were isolated from P. tithymaloides []. Modifications of these compounds led to 22 new derivatives, forming a library for screening P-gp-dependent MDR modulators. Compound 14 (Scheme 20) in particular showed exceptional stability and promising antitumor effects, making it a leading candidate for the development of new MDR-reversal agents. The structure–activity relationship (SAR) results for the jatrophane diterpenoids strongly indicate that the position and nature of ester functional group have a key role in the bioactivity of this class of compounds.
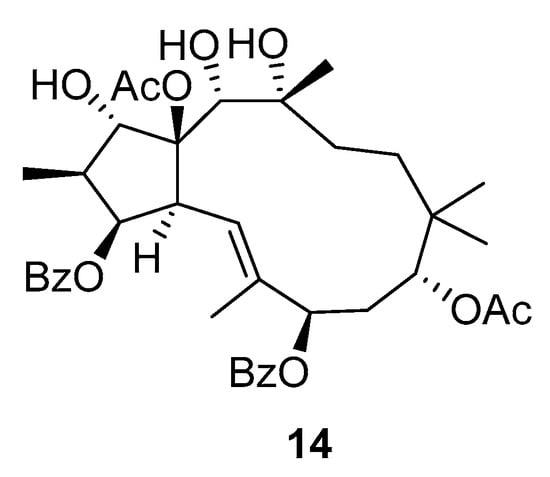
Scheme 20.
Chemical structures of jatrophane diterpenoid 14.
2.3. Chalcones
Chalcones are a group of plant polyphenol compounds that belong to the flavonoid family. Numerous studies have highlighted the potential of natural chalcones to counteract resistance to conventional chemotherapeutics by targeting key multidrug efflux transporters in cancer cells. The ability of naturally occurring chalcones to inhibit transporters such as P-gp has generated great interest in recent years in their use as chemosensitizers to improve drug efficacy and overcome clinical drug resistance in cancer treatment.
2.3.1. Genus Piper
The genus Piper belongs to the Piperaceae family. It contains up to 2000 species of shrubs, herbs, and lianas, many of which are dominant species in their native habitat. The diversification of this taxon is of interest to understanding the evolution of plants. Piper species have a pantropical distribution and are most commonly found in the understory of lowland tropical forests, but they can also occur in clearings and in higher-elevation life zones such as cloud forests [].
Piper methysticum Forster f. (Piperaceae) is a tropical shrub belonging to the Piperaceae family. The common names for P. methysticum extract include ava, ava pepper, awa, intoxicating pepper, kava, kava kava, kava pepper, kawa, kawa kawa, kew, rauschpfeffer, sakau, tonga, wur zelstock, and yangona. The active principles of Kava Kava rootstock are mostly contained in the lipid-soluble resin, which is generally grouped into categories: arylethylene-apyrones, chalcones and other flavanones, and conjugated diene ketones [].
Flavokawain A (Scheme 21), a chalcone compound found in kava, Piper methysticum, has strong anticancer properties. A study by Li et al. investigated the effect of flavokawain A on the viability of paclitaxel-resistant A549/T lung cancer cells and its potential hepatotoxicity in normal liver epithelial cells []. The study found that flavokawain A effectively inhibited the proliferation of A549/T cells and induced apoptosis in a dose-dependent manner. Furthermore, flavokawain A showed no hepatotoxic effects on liver epithelial cells. Treatment with flavokawain A reduced the P-gp expression threefold after 24 h by inhibiting the PI3K/Akt signaling pathway, suggesting that flavokawain A may be a promising treatment option for paclitaxel-resistant lung cancer. Another study showed that flavokawain B (Scheme 21), another chalcone from the kava plant, exhibited cytotoxic and antiproliferative effects on doxorubicin-resistant human colorectal adenocarcinoma cells (LoVo/Dx), although the exact mechanism on the ABCB1 transporter is still unclear [].
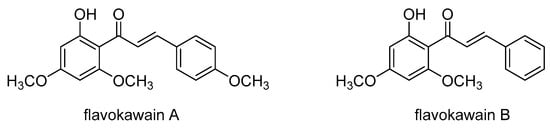
Scheme 21.
Chemical structures of flavokawains A and B.
2.3.2. Genus Helichrysum
The genus Helichrysum comprises about 600 species of flowering plants within the family Asteraceae, commonly known as the sunflower family. These plants are distributed throughout Africa; they are particularly common in South Africa, with 244 species, as well as in Madagascar, Australasia and Eurasia [].
The species Helichrysum zivojinii Černjavski and Soška is an endemic wild-growing plant in the Republic of North Macedonia at Galičica Mountain []. Our recent studies of the extracts obtained from the aerial parts of H. zivojinii revealed their specific cytotoxic action towards malignant cell lines in comparison with healthy immunocompetent peripheral blood mononuclear cells (PBMCs) [] and their potential ability to affect steps in the progression of malignant tumors [].
Chalcone dimers tomoroside A and tomoroside B (Scheme 22) were isolated together from the aerial parts of H. zivojinii []. Tomoroside A inhibited the expression of Topo IIα and HIF-1α and enhanced the anticancer effect of doxorubicin. In contrast, tomoroside B increased HIF-1α expression and likely acts as an antioxidant and redox-status modulator. Tomoroside B also synergized with tipifarnib, suggesting that it may enhance the effect of this chemotherapeutic agent by modulating the MAP kinase pathway. In addition, tomoroside A has the potential to inhibit ABCB1 mRNA expression, which is regulated by HIF-1α and is involved in the development of the MDR phenotype in NCI-H460/R cells. Since P-gp efficiently expels doxorubicin from MDR cancer cells, the enhancement of doxorubicin activity in combination with tomoroside A may be due to the inhibition of ABCB1 mRNA expression induced by tomoroside A.
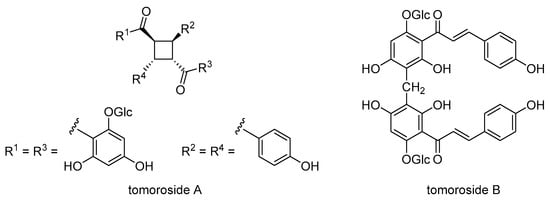
Scheme 22.
Chemical structures of tomoroside A and B.
2.3.3. Genus Glycyrrhiza
Licorice, scientifically known as Glycyrrhiza glabra, belongs to the legume family (Leguminosae or Fabaceae) and is part of a genus comprising over 30 species that are distributed worldwide. It has been historically revered as a highly recommended herb in ancient Egyptian, Roman, Greek, East Chinese, and Western medicinal practices. Licorice root extracts are valued for their many health benefits, e.g., for the treatment of sore throats, tuberculosis, respiratory and liver diseases, as well as for their antibacterial and anti-inflammatory properties. G. glabra, which is widely used in Ayurvedic medicine, grows throughout Asia and some parts of Europe. This medicinal plant is particularly common in regions such as Spain, Italy, Turkey, the Caucasus, central Asia, and western China []. It is found in Serbia in the Vojvodina province and at the Fruška Gora Mountain [].
Licochalcone A (Scheme 23), a chalcone extracted from the roots of the Chinese licorice plant Glycyrrhiza inflata or Glycyrrhiza glabra, is known for its strong anticancer and antioxidant properties. Licochalcone A was found to increase the accumulation of the P-gp substrates daunorubicin and rhodamine 123 in human MDR1 gene-transfected KB/MDR1 cells in a concentration-dependent manner []. Licochalcone A also stimulated the ATPase activity of P-gp and inhibited TNF-α-induced NF-κB activation. Additionally, licochalcone A sensitized KB/MDR1 cells to vinblastine, indicating its potential to reverse MDR. These results suggest that licochalcone A may improve the efficacy of cancer chemotherapy through the dual inhibition of P-gp and NF-κB activation.
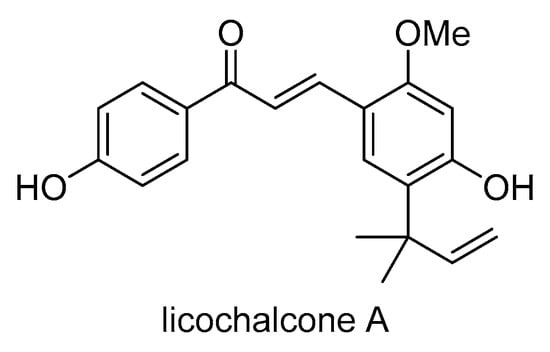
Scheme 23.
Chemical structure of licochalcone A.
2.3.4. Genus Cullen
Cullen, a genus of legumes from the Fabaceae family, is native to tropical, subtropical and arid regions in Africa, Asia and Australia. The genus Psoralea is limited to only 20 species, which are mainly found in the Mediterranean regions and southern Africa, while the remaining species belong to other genera. The genus Cullen has expanded considerably to include the remaining six species of Psoralea found in Africa. A total of 35 Cullen species are now known, whose distribution area extends from India and Sri Lanka to the Philippines and Papua New Guinea [].
Psoralea corylifolia or Cullen corylifolium, is native to northeastern Africa, the southern Arabian Peninsula, and tropical and subtropical Asia. This plant is important in both Indian and Chinese traditional medicine. Its seeds are rich in various coumarins, including psoralen [].
Isobavachalcone (Scheme 24), a natural flavonoid extracted from the medicinal plant Psoralea corylifolia, exhibits significant pharmacological activities on cancer cells via several mechanisms. It induces the production of ROS, inhibits the AKT, ERK and Wnt signaling pathways and promotes apoptosis of cancer cells via mitochondrial or endoplasmic reticulum signaling pathways. Isobavachalcone also has an anti-inflammatory effect by inhibiting the NF-κB signaling pathway and activating the NRF2/HO-1 signaling pathway, thereby reducing the production of various inflammatory mediators []. Isobavachalcone has been shown to interact with cell membranes and inhibit the function of the P-gp transporter []. It increases the intracellular accumulation of doxorubicin in doxorubicin-resistant human adenocarcinoma colon cancer cells (HT29/Dox), although less effectively than verapamil.
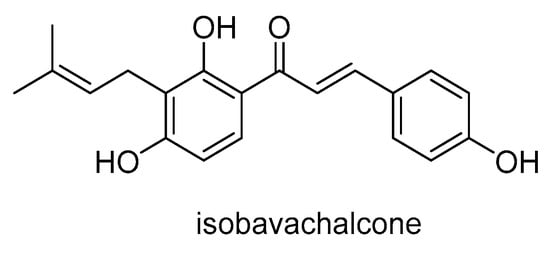
Scheme 24.
Chemical structure of isobavachalcone.
2.3.5. Genus Artemisia
The genus Artemisia is discussed in Section 2.1.1. Artemisia absinthium, also known as common wormwood, is native to North Africa and the temperate regions of Eurasia [] and is widely naturalized in Canada and the northern United States. It is grown as an ornamental plant and used as an ingredient in the spirit absinthe and some other alcoholic beverages. A. absinthum is widespread throughout Serbia and thrives in various habitats such as roadsides, gardens and fields [].
Cardamonin (Scheme 25), a natural chalcone firstly isolated from the flowers of Artemisia absinthium and found in different plants of the Zingiberaceae family, was shown to have an antitumor effect on 5-fluorouracil-resistant gastric cancer cells []. The study found that cardamonin significantly increased the chemosensitivity of 5-fluorouracil in cancer cells by suppressing the Wnt/β-catenin signaling pathway. Cardamonin, alone or in combination with 5-fluorouracil, induced apoptosis and cell cycle arrest in resistant cancer cells and decreased the expression of P-gp, β-catenin and TCF4. It specifically disrupted the β-catenin/TCF4 complex and inhibited TCF4-mediated transcription. In a xenograft mouse model, the co-administration of cardamonin and 5-fluorouracil significantly inhibited tumor growth, suggesting that cardamonin is a potential therapeutic strategy for drug-resistant gastric cancer.
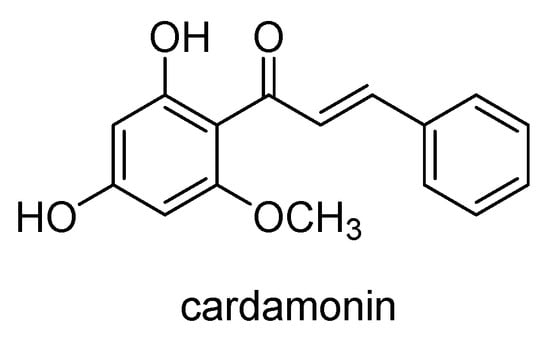
Scheme 25.
Chemical structure of cardamonin.
2.3.6. Genus Humulus
The exact origin of hops is still uncertain, but the presence of three species of the genus Humulus (H. lupulus, H. yunnanensis and H. japonicus) in China suggests that hops likely originated in Asia and later spread eastwards to North America and westwards to Europe [].
Humulus lupulus L., known worldwide as an important ingredient in the brewing industry, uses its female inflorescences, hop cones. These cones are rich in polyphenolic compounds and acylphloroglucides, which are crucial for the preservation of beer and for its distinctive smell and taste. In addition, hop cones have long been used in medicine, particularly as a mild sedative to treat insomnia and to stimulate gastric function [].
Xanthohumol (Scheme 26) is a natural prenylated chalconoid found in the female inflorescences of Humulus lupulus L. Xanthohumol was found to increase the sensitivity of doxorubicin-resistant breast cancer cells (MCF-7/ADR) to doxorubicin by reducing cell viability and stemness []. The study found that xanthohumol inhibits the efflux function of P-gp by acting as its substrate and stimulating its ATPase activity. Xanthohumol binds to the central transmembrane domain of P-gp, which overlaps with the doxorubicin binding site and has a stronger binding affinity than doxorubicin. This binding reduces protein- and ligand-position fluctuation, abolishing drug resistance by inhibiting P-gp-mediated doxorubicin transport. Xanthohumol was also found to have the potential to reverse MDR in human KB carcinoma cells by inhibiting P-gp and NF-κB activation []. In MDR1 gene-transfected KB/MDR1 cells, it increased the accumulation of P-gp substrates, stimulated P-gp ATPase activity, inhibited TNF-α-stimulated NF-κB activation, and sensitized the cells to the cytotoxicity of vinblastine.
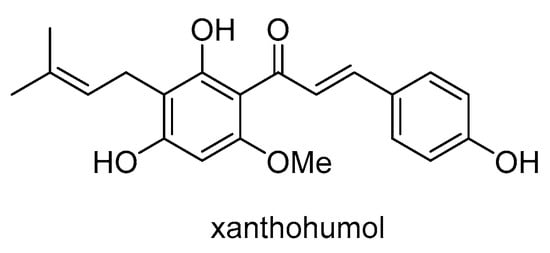
Scheme 26.
Chemical structure of xanthohumol.
2.4. Riccardins
Riccardins are a group of macrocyclic bisbibenzyls extracted from liverworts that have garnered attention for their significant antitumor activity in the literature. They have been found in liverworts such as Monoclea forsteri, Dumortiera hirsuta, Plagiochila cristata, Lunularia cruciata and others []. They were first discovered in higher vascular plants, Primula veris subsp. macrocalyx, in 2007 []. This was the first case of bisbibenzyls being found in higher plants. The same group confirmed these findings [], and later, Novaković et al., also discovered bisbibenzyls including riccardins in Serbian Primula species: P. veris subsp. columnae and P. acaulis [].
Studies have demonstrated that riccardins induce apoptosis and inhibit the proliferation of various cancer cell lines, including MDR variants, by modulating the expression and activity of P-gp. Riccardin D (Scheme 27) is a macrocyclic bisbibenzyl initially isolated from the liverwort Monoclea forsteri and later found in Dumortiera hirsuta and Plagiochila cristata. It has exhibited proliferation inhibitory activity and the ability to induce apoptosis in human leukemia cell lines such as HL-60 and the MDR variant K562/A02 []. Compared to etoposide, it has demonstrated a stronger inhibition of the DNA relaxation activity of topoisomerase II and reduced P-gp expression in resistant cells. Moreover, riccardin D has been shown to inhibit angiogenesis in lung carcinoma cells, induce apoptosis and autophagy in osteosarcoma cells, suppress the NF-kB signaling pathway, cause DNA damage in PC-3 prostate cancer cells, and induce apoptosis in human leukemia cells through the caspase signaling pathway. These findings suggest its potential as a therapeutic agent against various cancers.
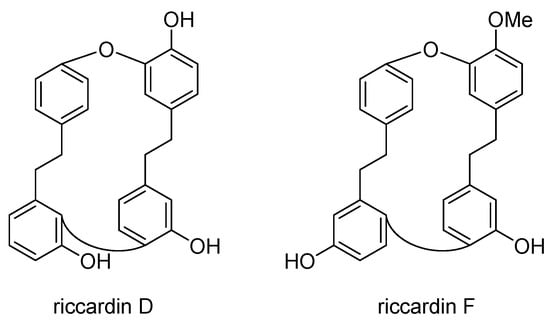
Scheme 27.
Chemical structures of riccardin D and F.
Riccardin F (Scheme 27), a macrocyclic bisbibenzyl isolated from the liverwort Plagiochasma intermedium, showed no direct inhibition of cancer cell growth but significantly reduced the IC50 of doxorubicin in the doxorubicin-induced MDR leukemia cell line K562/A02. At non-cytotoxic concentrations, riccardin F enhanced the accumulation of doxorubicin and increased the retention of rhodamine123 in K562/A02 cells, indicating its potential to modulate P-gp transport activity and reverse P-gp-mediated MDR [].
2.5. Diarylheptanoids
Diarylheptanoids have attracted significant attention for their potential to inhibit the growth of cancer cells and overcome drug-resistance mechanisms mediated by P-gp. There is a growing body of research highlighting the therapeutic potential of diarylheptanoids as valuable agents in cancer treatment. Alnus genus plants are rich in a variety of secondary metabolites such as diarylheptanoids, terpenoids, flavonoids, phenols, steroids, and tannins. Diarylheptanoids are compounds characteristic of the genus Alnus but are also found in the genera Zingiber, Curcuma, Alpinia and Betula. These compounds are natural phenolics characterized by two aromatic rings linked together by a heptane chain. Within this group, both acyclic and cyclic forms exist, with cyclic diarylheptanoids being less common. These compounds are synthesized from two C6-C3 units linked together, with an additional carbon originating from malonyl CoA [,]. The best-known diarylheptanoid is curcumin, which was isolated for the first time in the 19th century from Curcuma longa. Curcumin possesses a wide range of biological activities and is one of the most-studied plant secondary metabolites. Diarylheptanoids exhibit antioxidative [,], anti-inflammatory [], antiviral [], cytotoxic [,,] and anticancer activities [,].
2.5.1. Genus Alnus
The genus Alnus, commonly known as alders, belongs to the family Betulaceae (birch family) and includes over 30 species of trees and shrubs, mainly found in the northern hemisphere and Central America []. The largest species in the genus include the red alder (Alnus rubra) on the west coast of North America and the black alder (Alnus glutinosa), which is native to large parts of Europe and is also cultivated elsewhere. Both species can grow over 30 m high. In contrast, species such as the widespread green alder (Alnus alnobetula) and the endemic Alnus viridis usually grow as compact shrubs that rarely exceed 5 m in height []. Alnus species are well-known for their traditional medicinal use, including their application in treating various diseases such as cancer.
Alnus glutinosa (L.) Gaertn. (black alder, European alder) is a tree found in Europe, the Mediterranean, southeastern Asia, the Caucasus Mountains, and western Siberia. In the Balkan Peninsula, A. glutinosa is found across all Balkan countries—Serbia, Montenegro, Bosnia and Herzegovina, Croatia, and Bulgaria. It typically grows near rivers and creeks and can be found at altitudes up to 1000 m []. It often grows with Fraxinus angustifolia, Ulmus laevis, Quercus rober, Populus alba, Salix alba, Salix cinerea, Rhamus frangula, Viburnum opulus and others. Its Latin name (“glutinosa”) refers to sticky leaves and young branches in spring. A. incana (L.) Moench (gray alder) is a deciduous tree (lower than A. glutinosa) or shrub found across the northern hemisphere and native to large areas of central and northern Europe. In the Balkan region, it is mainly distributed in Serbia and Albania. The bark of old A. incana trees is gray (the reason for the name “gray alder”) and similar to the bark of Fagus sylvatica. A. viridis subsp. viridis is the third Alnus species growing in Serbia. It is a 3–5 m tall shrub that grows from 1300 to 2000 m above sea level at the Stara Planina Mountain in Serbia; in Bulgaria; and in Romania in the Carpathian Mountains []. It can be also found in the Alps [].
The bark of Alnus species is rich source of diarylheptanoids and triterepnes. A. glutinosa and A. incana have very similar diarylheptanoid profile, with oregonin, plathyphyloside, rubranoside A and B, hirsutanonol, hirsutanonol-5-β-D-glucopyranoside, alnuside A and B and hirsutenone as the main constituents []. Oregonin, (5S-1,7-bis(3,4-dihydroxyphenyl)-3-hydroxyheptane-5-O-β-D-xylopyranoside) is an open-chain diarylheptanoid glycoside with 3-carbonyl and 5-xylosyloxy groups and the most-abundant compound in barks of A. glutinosa and A. incana. This diarylheptanoid has antioxidant, antibacterial, anti-inflammatory and anticancer properties; helps combat obesity; and lowers blood cholesterol and triacylglycerol levels [,,,,,,,].
The diarylheptanoid profile of A. viridis bark is different in comparison to A. glutinosa and A. incana. Oregonin, the main diarylheptanoid from black and gray alder, was not found at all in A. viridis subsp. viridis, and the main diarylheptanoids were platyphylloside and p-hydroxydiarylheptanoids. A high number of diarylheptanoid disaccharides were also found. According to the chemical constituents, A. viridis subsp. viridis was more similar to Betula platyphylla var. japonica [].
A study that compared structurally analogous diarylheptanoids from green and black alder barks collected in Serbia revealed that green alder diarylheptanoids induced significant apoptosis in human non-small cell lung carcinoma cells compared to their black alder analogues []. Another study reported the isolation of seven pentacyclic triterpene derivatives (five new) from the bark of green alder from Serbia, and pentacyclic triterpenoids with C-27 hydroxymethyl group were discovered for the first time in genus Alnus. These compounds were cytotoxic toward various cancer cells and were selected for an in silico investigation as potential inhibitors of topoisomerases I and IIα [].
In another study, diarylheptanoids from black alder were found to have chemo-protective effects against doxorubicin in normal keratinocytes []. Additionally, various diarylheptanoids from black alder bark, including oregonin, platyphylloside, alnuside A, and others, showed protective effects against DNA damage in human lymphocytes [].
Furthermore, diarylheptanoids from black alder exhibited protective effects against chemotherapy-induced damage in various cell types, including human non-small cell lung cancer cells and keratinocytes []. Noteworthy diarylheptanoids such as platyphylloside, alnuside A, alnuside B, methylhirsutanonol, and hirsutenone, isolated from Alnus glutinosa, demonstrated significant anticancer potential, especially against MDR non-small cell lung carcinoma NCI-H460/R cells []. While the cytotoxic effects of these diarylheptanoids were not affected by the presence of the MDR phenotype, only alnuside A and (S)-5-O-methylhirsutanonol (Scheme 28) suppressed P-gp activity. These compounds increased the accumulation of the P-gp substrate doxorubicin in a similar manner to curcumin, indicating that they may modulate P-gp function.
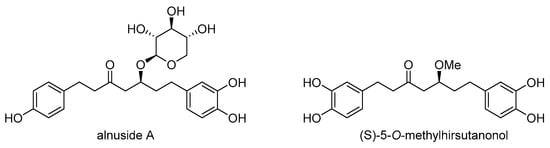
Scheme 28.
Chemical structures of alnuside A and (S)-5-O-methylhirsutanonol.
2.5.2. Genus Curcuma
The genus Curcuma is discussed in Section 2.1.2. Curcumin (Scheme 29) is one of the most extensively investigated plant compounds ever. It originates from the rhizomes of Curcuma longa and has been extensively studied over the past two decades for its anticancer properties. Traditionally used as a spice, pigment, and in cosmetics, curcumin is known for its anti-inflammatory, antiviral, and anticancer effects, as well as its potential benefits in neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune, and neoplastic diseases [,]. It has also been found to prevent liver damage and lower glucose and cholesterol levels. Curcumin exhibits inhibitory effects on P-gp, MRP1, and BCRP activity, overcomes multidrug resistance (MDR) in cancer cells and tumors, and possesses chemosensitizing properties [,,]. Additionally, curcumin reduces the expression of the ABCB1 gene and sensitizes resistant cancer cells to vincristine by promoting apoptosis through the activation of caspase-3 []. Moreover, curcumin is known to modulate the expression of genes involved in DNA repair, inhibit the mTOR signaling pathway, suppress tumor growth, induce apoptosis, and influence the growth and survival of cancer cells [,]. Several delivery methods, such as nanoparticles, liposome encapsulation, and curcumin–phospholipid complexes, have been developed to enhance its bioavailability and efficacy in the treatment of resistant cancers [,,].
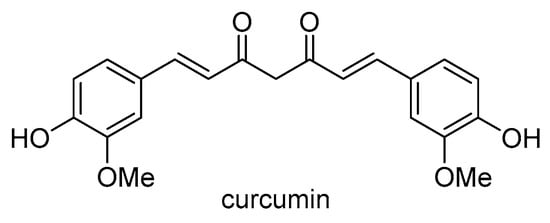
Scheme 29.
Chemical structure of curcumin.
A study found that the compound trans-1,7-diphenyl-5-hydroxy-1-heptene (DHH) (Scheme 30), derived from another Curcuma species, C. comosa, is toxic to drug-resistant human leukemia K562/ADR cells without affecting P-gp expression []. The study revealed that when DHH and doxorubicin were used together, the amount of doxorubicin needed to kill the cells decreased significantly, indicating a change in the MDR characteristics. Furthermore, a low dose of DHH increased cell sensitivity to doxorubicin in resistant cells that express P-gp. DHH’s inhibition of P-gp-mediated drug removal led to increased cell death in K562/ADR cells. These findings suggest that DHH could be a new modifier of P-gp function, enhancing drug accumulation and promoting cell death in doxorubicin-resistant leukemia cells.
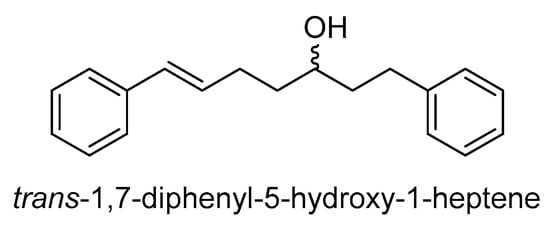
Scheme 30.
Chemical structure of trans-1,7-diphenyl-5-hydroxy-1-heptene.

Table 1.
Natural products and their derivatives with inhibitory activity against P-gp and MDR cancer cells: Classification and genus origin.
Table 1.
Natural products and their derivatives with inhibitory activity against P-gp and MDR cancer cells: Classification and genus origin.
| Classes of Natural Products | P-gp Inhibitors and MDR Modulators | Derivatives | Additional Biological Activities * | Origin |
|---|---|---|---|---|
| sesquiterpenoids |
|
|
| Genus Artemisia |
|
|
| ||
|
| Genus Curcuma | ||
| Genus Ferula | |||
|
| Genus Inula | ||
|
| Genus Petasites | ||
| Genus Celastrus | |||
| diterpenoids |
|
|
| Genus Salvia |
|
| Genus Euphorbia | ||
| chalcones |
|
| Genus Piper | |
|
| Genus Helichrysum | ||
|
| Genus Glycyrrhiza | ||
|
| Genus Cullen | ||
|
| Genus Artemisia | ||
|
| Genus Humulus | ||
| riccardins |
|
| Genera Lunularia, Monoclea, Dumortiera, Plagiochila, and Primula | |
| diarylheptanoids |
|
| Genus Alnus | |
|
| Genus Curcuma |
* Only additional biological activities are reported within this review paper.
3. Novel Perspectives with Naturally Derived P-Glycoprotein Inhibitors
The development of effective chemotherapeutics with strong and selective anticancer activity is an ongoing need to combat drug resistance. P-gp expression in various cancers has led to extensive research into creating efflux inhibitors capable of overcoming MDR in experimental models. Despite significant research efforts, a clinically effective inhibitor has not yet been discovered. This has shifted focus to alternative strategies, such as bypassing the transporters or exploiting the collateral sensitivity of MDR cells.
Recent discoveries have shown that it is possible to reverse resistance and counteract the selective advantage of resistant cells. Compounds selective to MDR have been found to target ABC-transporter-overexpressing MDR cancer cells by exploiting the vulnerabilities caused by transporter overexpression. Certain natural compounds exhibit preferential toxicity against MDR cells [].
The development of hybrid compounds comprising a natural-product scaffold and a drug-like fragment (privileged structural motif) with distinct mechanisms of action holds significant value for enhancing the pharmacokinetic and pharmacodynamic properties of potential drug candidates. Recent studies have shown the promising potential of hybrid compounds in cancer treatment, particularly in addressing MDR and enhancing anticancer effects [,,]. The ability of hybrid compounds to overcome MDR by inhibiting P-gp activity and their potential for targeted drug delivery makes them valuable in developing more-effective cancer therapies. The hybrid compounds also showed promising anticancer activities, demonstrating improved selectivity towards MDR cells, indicating their potential to surpass drug resistance. This is a particularly interesting phenomenon referred to as collateral sensitivity. The dual mechanism of action (collateral sensitivity and P-gp inhibition) makes the hybrid compounds potentially valuable for further development in cancer treatment. Overall, the development of hybrid compounds presents a compelling avenue to advance cancer treatment and improve patient outcomes.
One potential way to successfully introduce P-gp inhibitors into clinical practice is to ensure that these inhibitors affect P-gp function in cancer cells without interfering with the P-gp physiological function in important barriers such as the placenta and the blood–brain barrier. Our recent unpublished data have revealed derivatives of natural products that are capable of acting in this way. Therefore, developing natural-inspired drugs with collateral sensitivity, which can evade the P-gp mechanism of resistance and spare normal P-gp function in the body, holds the potential to create highly efficient anticancer agents. These agents will not only possess anticancer properties but also have the ability to enhance traditional chemotherapy effects.
Author Contributions
Conceptualization, L.L. and M.P.; Investigation, J.D., A.P.-R., M.N., I.O. and M.P.; Writing—original draft preparation J.D., A.P.-R., M.N. and I.O.; Writing—review and editing, J.D. and M.P.; Funding acquisition, L.L. and M.P All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (grant numbers 451-03-66/2024-03/200007, 451-03-47/2024-03/200168, and 451-03-66/2024-03/200026) and the “Belt and Road Initiative” Innovative Talent Exchange Foreign Expert Project (DL2022194002L).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.P. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomes as mediators of drug resistance in cancer. Drug Resist. Updat. 2016, 24, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Ikediobi, O.N. Somatic pharmacogenomics in cancer. Pharmacogenom. J. 2008, 8, 305–314. [Google Scholar] [CrossRef]
- Valente, A.; Podolski-Renic, A.; Poetsch, I.; Filipovic, N.; Lopez, O.; Turel, I.; Heffeter, P. Metal- and metalloid-based compounds to target and reverse cancer multidrug resistance. Drug Resist. Updat. 2021, 58, 100778. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Brozovic, A.; Goncalves, A.C.; Jurkovicova, D.; Line, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updat. 2019, 46, 100645. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updat. 2018, 41, 1–25. [Google Scholar] [CrossRef]
- Juvale, I.I.A.; Abdul Hamid, A.A.; Abd Halim, K.B.; Che Has, A.T. P-glycoprotein: New insights into structure, physiological function, regulation and alterations in disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, V. P-glycoprotein transporter in drug development. EXCLI J. 2016, 15, 113–118. [Google Scholar] [CrossRef]
- Borst, P.; Elferink, R.O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef]
- Bottova, I.; Hehl, A.B.; Stefanic, S.; Fabrias, G.; Casas, J.; Schraner, E.; Pieters, J.; Sonda, S. Host cell P-glycoprotein is essential for cholesterol uptake and replication of Toxoplasma gondii. J. Biol. Chem. 2009, 284, 17438–17448. [Google Scholar] [CrossRef]
- Foucaud-Vignault, M.; Soayfane, Z.; Menez, C.; Bertrand-Michel, J.; Martin, P.G.; Guillou, H.; Collet, X.; Lespine, A. P-glycoprotein dysfunction contributes to hepatic steatosis and obesity in mice. PLoS ONE 2011, 6, e23614. [Google Scholar] [CrossRef]
- Hartmann, G.; Vassileva, V.; Piquette-Miller, M. Impact of endotoxin-induced changes in P-glycoprotein expression on disposition of doxorubicin in mice. Drug Metab. Dispos. 2005, 33, 820–828. [Google Scholar] [CrossRef]
- Heemskerk, S.; Wouterse, A.C.; Russel, F.G.; Masereeuw, R. Nitric oxide down-regulates the expression of organic cation transporters (OCT) 1 and 2 in rat kidney during endotoxemia. Eur. J. Pharmacol. 2008, 584, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Noack, A.; Gericke, B.; von Kockritz-Blickwede, M.; Menze, A.; Noack, S.; Gerhauser, I.; Osten, F.; Naim, H.Y.; Loscher, W. Mechanism of drug extrusion by brain endothelial cells via lysosomal drug trapping and disposal by neutrophils. Proc. Natl. Acad. Sci. USA 2018, 115, E9590–E9599. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Mruk, D.D.; Lui, W.Y.; Lee, W.M.; Cheng, C.Y. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK). Proc. Natl. Acad. Sci. USA 2011, 108, 19623–19628. [Google Scholar] [CrossRef]
- Dunk, C.E.; Pappas, J.J.; Lye, P.; Kibschull, M.; Javam, M.; Bloise, E.; Lye, S.J.; Szyf, M.; Matthews, S.G. P-Glycoprotein (P-gp)/ABCB1 plays a functional role in extravillous trophoblast (EVT) invasion and is decreased in the pre-eclamptic placenta. J. Cell Mol. Med. 2018, 22, 5378–5393. [Google Scholar] [CrossRef]
- Kim, Y.; Chen, J. Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation. Science 2018, 359, 915–919. [Google Scholar] [CrossRef]
- Kodan, A.; Yamaguchi, T.; Nakatsu, T.; Sakiyama, K.; Hipolito, C.J.; Fujioka, A.; Hirokane, R.; Ikeguchi, K.; Watanabe, B.; Hiratake, J.; et al. Structural basis for gating mechanisms of a eukaryotic P-glycoprotein homolog. Proc. Natl. Acad. Sci. USA 2014, 111, 4049–4054. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, K.M.; Hall, M.D.; Moen, J.K.; Chufan, E.E.; Fetsch, P.A.; Shukla, S.; Gill, D.R.; Hyde, S.C.; Xia, D.; Ambudkar, S.V.; et al. Human-Mouse Chimeras with Normal Expression and Function Reveal That Major Domain Swapping Is Tolerated by P-Glycoprotein (ABCB1). Biochemistry 2016, 55, 1010–1023. [Google Scholar] [CrossRef]
- Kodan, A.; Yamaguchi, T.; Nakatsu, T.; Matsuoka, K.; Kimura, Y.; Ueda, K.; Kato, H. Inward- and outward-facing X-ray crystal structures of homodimeric P-glycoprotein CmABCB1. Nat. Commun. 2019, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Scotto, K.W. Transcriptional regulation of ABC drug transporters. Oncogene 2003, 22, 7496–7511. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Xu, W.; Luo, W.; Zhou, L.; Yong, W.; Chen, F.; Wu, C.; Chen, Q.; Han, X. Upregulation of mdr1 gene is related to activation of the MAPK/ERK signal transduction pathway and YB-1 nuclear translocation in B-cell lymphoma. Exp. Hematol. 2011, 39, 558–569. [Google Scholar] [CrossRef]
- Chen, Q.; Bian, Y.; Zeng, S. Involvement of AP-1 and NF-kappaB in the up-regulation of P-gp in vinblastine resistant Caco-2 cells. Drug Metab. Pharmacokinet. 2014, 29, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mu, P.; Qiao, H.; Wen, J.; Deng, Y. JNK-AKT-NF-kappaB controls P-glycoprotein expression to attenuate the cytotoxicity of deoxynivalenol in mammalian cells. Biochem. Pharmacol. 2018, 156, 120–134. [Google Scholar] [CrossRef]
- Feng, D.D.; Zhang, H.; Zhang, P.; Zheng, Y.S.; Zhang, X.J.; Han, B.W.; Luo, X.Q.; Xu, L.; Zhou, H.; Qu, L.H.; et al. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J. Cell Mol. Med. 2011, 15, 2164–2175. [Google Scholar] [CrossRef]
- Ikemura, K.; Yamamoto, M.; Miyazaki, S.; Mizutani, H.; Iwamoto, T.; Okuda, M. MicroRNA-145 post-transcriptionally regulates the expression and function of P-glycoprotein in intestinal epithelial cells. Mol. Pharmacol. 2013, 83, 399–405. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Shen, H.; Li, H.; Long, L.; Hui, L.; Xu, W. miR-137 restoration sensitizes multidrug-resistant MCF-7/ADM cells to anticancer agents by targeting YB-1. Acta Biochim. Biophys. Sin. 2013, 45, 80–86. [Google Scholar] [CrossRef]
- Stankovic, T.; Dinic, J.; Podolski-Renic, A.; Musso, L.; Buric, S.S.; Dallavalle, S.; Pesic, M. Dual Inhibitors as a New Challenge for Cancer Multidrug Resistance Treatment. Curr. Med. Chem. 2019, 26, 6074–6106. [Google Scholar] [CrossRef]
- Dinic, J.; Podolski-Renic, A.; Jeremic, M.; Pesic, M. Potential of Natural-Based Anticancer Compounds for P-Glycoprotein Inhibition. Curr. Pharm. Des. 2018, 24, 4334–4354. [Google Scholar] [CrossRef]
- Begicevic, R.R.; Falasca, M. ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. Int. J. Mol. Sci. 2017, 18, 2362. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Kimchi-Sarfaty, C.; Sauna, Z.E.; Gottesman, M.M. P-glycoprotein: From genomics to mechanism. Oncogene 2003, 22, 7468–7485. [Google Scholar] [CrossRef] [PubMed]
- Leith, C.P.; Kopecky, K.J.; Chen, I.M.; Eijdems, L.; Slovak, M.L.; McConnell, T.S.; Head, D.R.; Weick, J.; Grever, M.R.; Appelbaum, F.R.; et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: A Southwest Oncology Group Study. Blood 1999, 94, 1086–1099. [Google Scholar]
- Berger, W.; Setinek, U.; Hollaus, P.; Zidek, T.; Steiner, E.; Elbling, L.; Cantonati, H.; Attems, J.; Gsur, A.; Micksche, M. Multidrug resistance markers P-glycoprotein, multidrug resistance protein 1, and lung resistance protein in non-small cell lung cancer: Prognostic implications. J. Cancer Res. Clin. Oncol. 2005, 131, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Ishida, T.; Sugio, K.; Inoue, T.; Sugimachi, K. Immunohistochemical evidence that P-glycoprotein in non-small cell lung cancers is associated with shorter survival. Surg. Today 1999, 29, 1141–1147. [Google Scholar] [CrossRef]
- Toth, K.; Vaughan, M.M.; Peress, N.S.; Slocum, H.K.; Rustum, Y.M. MDR1 P-glycoprotein is expressed by endothelial cells of newly formed capillaries in human gliomas but is not expressed in the neovasculature of other primary tumors. Am. J. Pathol. 1996, 149, 853–858. [Google Scholar]
- Henson, J.W.; Cordon-Cardo, C.; Posner, J.B. P-glycoprotein expression in brain tumors. J. Neurooncol. 1992, 14, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Shedid, D.; Beaulieu, E.; Del Maestro, R.F.; Moghrabi, A.; Ghosn, P.B.; Moumdjian, R.; Berthelet, F.; Beliveau, R. Expression of multidrug-resistance P-glycoprotein (MDR1) in human brain tumors. Int. J. Cancer 2001, 93, 62–66. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef]
- Saitoh, H.; Saikachi, Y.; Kobayashi, M.; Yamaguchi, M.; Oda, M.; Yuhki, Y.; Achiwa, K.; Tadano, K.; Takahashi, Y.; Aungst, B.J. Limited interaction between tacrolimus and P-glycoprotein in the rat small intestine. Eur. J. Pharm. Sci. 2006, 28, 34–42. [Google Scholar] [CrossRef]
- Palmeira, A.; Sousa, E.; Vasconcelos, M.H.; Pinto, M.M. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr. Med. Chem. 2012, 19, 1946–2025. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; Ashokraj, Y.; Dey, C.S.; Panchagnula, R. P-glycoprotein inhibitors and their screening: A perspective from bioavailability enhancement. Pharmacol. Res. 2003, 48, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, A.; Ierano, C.; Szakacs, G.; Robey, R.W.; Bates, S.E. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 2011, 50, 209–232. [Google Scholar] [CrossRef]
- Cripe, L.D.; Uno, H.; Paietta, E.M.; Litzow, M.R.; Ketterling, R.P.; Bennett, J.M.; Rowe, J.M.; Lazarus, H.M.; Luger, S.; Tallman, M.S. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: A randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood 2010, 116, 4077–4085. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.; Kelly, J.; Anderson, G. Reproductive Biology of Asteraceae on Oceanic Islands. Bot. Rev. 2023, 90, 67–108. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Wong, Y.K.; Xu, C.; Kalesh, K.A.; He, Y.; Lin, Q.; Wong, W.S.F.; Shen, H.M.; Wang, J. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med. Res. Rev. 2017, 37, 1492–1517. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Mu, L.; Yang, X. Artemisinins as Anticancer Drugs: Novel Therapeutic Approaches, Molecular Mechanisms, and Clinical Trials. Front. Pharmacol. 2020, 11, 529881. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Shang, D.; Efferth, T. Interactions between artemisinin derivatives and P-glycoprotein. Phytomedicine 2019, 60, 152998. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Hu, N.; Zhao, H. Artesunate enhances adriamycin cytotoxicity by inhibiting glycolysis in adriamycin-resistant chronic myeloid leukemia K562/ADR cells. RSC Adv. 2019, 9, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Koračak, L.; Lupšić, E.; Jovanović, N.T.; Jovanović, M.; Novakovic, M.; Nedialkov, P.; Trendafilova, A.; Zlatović, M.; Pešić, M.; Opsenica, I.M. Novel artesunate–pyrimidine-based hybrids with anticancer potential against multidrug-resistant cancer cells. New J. Chem. 2023, 47, 6844–6855. [Google Scholar] [CrossRef]
- Oga, E.F.; Sekine, S.; Shitara, Y.; Horie, T. Potential P-glycoprotein-mediated drug-drug interactions of antimalarial agents in Caco-2 cells. Am. J. Trop. Med. Hyg. 2012, 87, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Zhang, Z.; Chen, Y.; Wang, J. Synthesis and biological evaluation of a novel artesunate-podophyllotoxin conjugate as anticancer agent. Bioorg Med. Chem. Lett. 2016, 26, 38–42. [Google Scholar] [CrossRef]
- Di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of Low-Dose Doxorubicin Cytotoxicity by Affecting P-Glycoprotein through Caryophyllane Sesquiterpenes in HepG2 Cells: An in Vitro and in Silico Study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.; Cheng, S.W.; Yu, H.; Xu, Z.S.; Lee, J.K.; Hon, P.M.; Lee, M.Y.; Kennelly, E.J.; Deng, G.; Yeung, S.K.; et al. The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal Caco-2 cells. J. Med. Food 2012, 15, 242–252. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Guo, D.W.; Lao, Q.C.; Xu, Y.Q.; Meng, Z.K.; Xia, B.; Yang, H.; Li, C.Q.; Li, P. Sensitization and synergistic anti-cancer effects of Furanodiene identified in zebrafish models. Sci. Rep. 2019, 9, 4541. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, X.D.; Li, E.Z.; Luo, Y.; Song, N.; Qu, X.J.; Hu, X.J.; Liu, Y.P. The role of E3 ubiquitin ligase Cbl proteins in beta-elemene reversing multi-drug resistance of human gastric adenocarcinoma cells. Int. J. Mol. Sci. 2013, 14, 10075–10089. [Google Scholar] [CrossRef]
- Deng, M.; Liu, B.; Song, H.; Yu, R.; Zou, D.; Chen, Y.; Ma, Y.; Lv, F.; Xu, L.; Zhang, Z.; et al. beta-Elemene inhibits the metastasis of multidrug-resistant gastric cancer cells through miR-1323/Cbl-b/EGFR pathway. Phytomedicine 2020, 69, 153184. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Zhu, L.X.; Yu, J.D.; Chen, Z.; Gu, M.C.; Mu, C.F.; Liu, Q.; Xiong, Y. Effect of beta-elemene on the kinetics of intracellular transport of d-luciferin potassium salt (ABC substrate) in doxorubicin-resistant breast cancer cells and the associated molecular mechanism. Eur. J. Pharm. Sci. 2018, 120, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Venditti, A.; Sarker, S.D.; Nahar, L.; Akbarzadeh, A. The genus Ferula: Ethnobotany, phytochemistry and bioactivities—A review. Ind. Crops Prod. 2019, 129, 350–394. [Google Scholar] [CrossRef]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Ghandadi, M.; Iranshahi, M. Reversal of P-glycoprotein-mediated multidrug resistance in MCF-7/Adr cancer cells by sesquiterpene coumarins. Fitoterapia 2015, 103, 149–154. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Pesic, M.; Podolski-Renic, A.; Bankovic, J.; de Vos, R.C.H.; Perić, M.; Todorović, S.; Tanic, N. Cytotoxic activity of sesquiterpene lactones from Inula britannica on human cancer cell lines. Phytochem. Lett. 2013, 6, 246–252. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Fan, J.; Lin, X.; Liu, C.; Xu, Y.; Ji, W.; Yan, C.; Su, C. Costunolide and dehydrocostuslactone combination treatment inhibit breast cancer by inducing cell cycle arrest and apoptosis through c-Myc/p53 and AKT/14-3-3 pathway. Sci. Rep. 2017, 7, 41254. [Google Scholar] [CrossRef]
- Cai, H.; Li, L.; Jiang, J.; Zhao, C.; Yang, C. Costunolide enhances sensitivity of K562/ADR chronic myeloid leukemia cells to doxorubicin through PI3K/Akt pathway. Phytother. Res. 2019, 33, 1683–1688. [Google Scholar] [CrossRef]
- Yami, A.; Hamzeloo-Moghadam, M.; Darbandi, A.; Karami, A.; Mashati, P.; Takhviji, V.; Gharehbaghian, A. Ergolide, a potent sesquiterpene lactone induces cell cycle arrest along with ROS-dependent apoptosis and potentiates vincristine cytotoxicity in ALL cell lines. J. Ethnopharmacol. 2020, 253, 112504. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, T.; Chen, T.; Xie, C.; Zhang, Q. Sesquiterpenoids isolated from the flower of Inula japonica as potential antitumor leads for intervention of paclitaxel-resistant non-small-cell lung cancer. Bioorg Chem. 2020, 101, 103973. [Google Scholar] [CrossRef]
- Kulinowski, L.; Luca, S.V.; Minceva, M.; Skalicka-Wozniak, K. A review on the ethnobotany, phytochemistry, pharmacology and toxicology of butterbur species (Petasites L.). J. Ethnopharmacol. 2022, 293, 115263. [Google Scholar] [CrossRef]
- Lin, Y.L.; Mei, C.H.; Huang, S.L.; Kuo, Y.H. Four new sesquiterpenes from Petasites formosanus. J. Nat. Prod. 1998, 61, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, S.; Bockers, M.; Asensio, M.; Kadioglu, O.; Klinger, A.; Fleischer, E.; Efferth, T. Isopetasin and S-isopetasin as novel P-glycoprotein inhibitors against multidrug-resistant cancer cells. Phytomedicine 2021, 86, 153196. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, B.L.; Zhang, Q.X.; Zheng, Y.Z.; Fu, Q. Traditional uses, secondary metabolites, and pharmacology of Celastrus species—A review. J. Ethnopharmacol. 2019, 241, 111934. [Google Scholar] [CrossRef]
- Callies, O.; Sanchez-Canete, M.P.; Gamarro, F.; Jimenez, I.A.; Castanys, S.; Bazzocchi, I.L. Optimization by Molecular Fine Tuning of Dihydro-beta-agarofuran Sesquiterpenoids as Reversers of P-Glycoprotein-Mediated Multidrug Resistance. J. Med. Chem. 2016, 59, 1880–1890. [Google Scholar] [CrossRef]
- Randjelovic, M.; Zlatković, B.; Jovanovic, M.; Miladinovic, B.; Milutinović, M.; Pavlovic, D.; Branković, S.; Kitic, D. Morphological and anatomical analysis of the clary sage herbal drug (Salviae sclareae herba). Lekovite Sirovine 2022, 42, 24–33. [Google Scholar] [CrossRef]
- Jaric, S.; Macukanovic-Jocic, M.; Djurdjevic, L.; Mitrovic, M.; Kostic, O.; Karadzic, B.; Pavlovic, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Afshari, H.; Nourbakhsh, M.; Salehi, N.; Mahboubi-Rabbani, M.; Zarghi, A.; Noori, S. STAT3-mediated Apoptotic-enhancing Function of Sclareol Against Breast Cancer Cells and Cell Sensitization to Cyclophosphamide. Iran. J. Pharm. Res. 2020, 19, 398–412. [Google Scholar] [CrossRef]
- Bordbar-Bonab, S.; Mahbob, S.; Vahed-Jabari, M.; Dolatkhah, H. The effect of Sclareol on the expression of MDR-1 gene and Glycoprotein-P Level in MKN-45 human gastric cancer cells. J. Res. Appl. Basic Med. Sci. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Chen, H.L.; Gong, J.Y.; Lin, S.C.; Li, S.; Chiang, Y.C.; Hung, J.H.; Yen, C.C.; Lin, C.C. Effects of Sclareol Against Small Cell Lung Carcinoma and the Related Mechanism: In Vitro and In Vivo Studies. Anticancer Res. 2020, 40, 4947–4960. [Google Scholar] [CrossRef]
- Pan, C.H.; Chen, S.Y.; Wang, J.Y.; Tsao, S.P.; Huang, H.Y.; Wei-Chen Chiu, P.; Wu, C.H. Sclareol ameliorated ERCC1-mediated cisplatin resistance in A549 human lung adenocarcinoma cells and a murine xenograft tumor model by suppressing AKT-GSK3beta-AP1/Snail and JNK-AP1 pathways. Chem. Biol. Interact. 2020, 332, 109304. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, T.; Cai, P. Sclareol inhibits cell proliferation and sensitizes cells to the antiproliferative effect of bortezomib via upregulating the tumor suppressor caveolin-1 in cervical cancer cells. Mol. Med. Rep. 2017, 15, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.S.M.; Silva, J.O.; Fernandes, R.S.; de Souza, A.M.; Cassali, G.D.; Yoshida, M.I.; Leite, E.A.; de Barros, A.L.B.; Ferreira, L.A.M. Sclareol is a potent enhancer of doxorubicin: Evaluation of the free combination and co-loaded nanostructured lipid carriers against breast cancer. Life Sci. 2019, 232, 116678. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, A.; Terzic Jovanovic, N.; Korac, A.; Zlatovic, M.; Nikolic, I.; Opsenica, I.; Pesic, M. Novel hybrid compounds of sclareol and doxorubicin as potential anticancer nanotherapy for glioblastoma. Biomed. Pharmacother. 2024, 174, 116496. [Google Scholar] [CrossRef]
- Dimas, K.; Kokkinopoulos, D.; Demetzos, C.; Vaos, B.; Marselos, M.; Malamas, M.; Tzavaras, T. The effect of sclareol on growth and cell cycle progression of human leukemic cell lines. Leuk. Res. 1999, 23, 217–234. [Google Scholar] [CrossRef]
- Dimas, K.; Demetzos, C.; Vaos, V.; Ioannidis, P.; Trangas, T. Labdane type diterpenes down-regulate the expression of c-Myc protein, but not of Bcl-2, in human leukemia T-cells undergoing apoptosis. Leuk. Res. 2001, 25, 449–454. [Google Scholar] [CrossRef]
- Dimas, K.; Hatziantoniou, S.; Tseleni, S.; Khan, H.; Georgopoulos, A.; Alevizopoulos, K.; Wyche, J.H.; Pantazis, P.; Demetzos, C. Sclareol induces apoptosis in human HCT116 colon cancer cells in vitro and suppression of HCT116 tumor growth in immunodeficient mice. Apoptosis 2007, 12, 685–694. [Google Scholar] [CrossRef]
- Dimas, K.; Papadaki, M.; Tsimplouli, C.; Hatziantoniou, S.; Alevizopoulos, K.; Pantazis, P.; Demetzos, C. Labd-14-ene-8,13-diol (sclareol) induces cell cycle arrest and apoptosis in human breast cancer cells and enhances the activity of anticancer drugs. Biomed. Pharmacother. 2006, 60, 127–133. [Google Scholar] [CrossRef]
- Mahaira, L.G.; Tsimplouli, C.; Sakellaridis, N.; Alevizopoulos, K.; Demetzos, C.; Han, Z.; Pantazis, P.; Dimas, K. The labdane diterpene sclareol (labd-14-ene-8, 13-diol) induces apoptosis in human tumor cell lines and suppression of tumor growth in vivo via a p53-independent mechanism of action. Eur. J. Pharmacol. 2011, 666, 173–182. [Google Scholar] [CrossRef]
- Stojkovic, P.; Kostic, A.; Lupsic, E.; Jovanovic, N.T.; Novakovic, M.; Nedialkov, P.; Trendafilova, A.; Pesic, M.; Opsenica, I.M. Novel hybrids of sclareol and 1,2,4-triazolo[1,5-a]pyrimidine show collateral sensitivity in multidrug-resistant glioblastoma cells. Bioorg Chem. 2023, 138, 106605. [Google Scholar] [CrossRef]
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed]
- Krstic, G.; Jadranin, M.; Todorovic, N.M.; Pesic, M.; Stankovic, T.; Aljancic, I.S.; Tesevic, V.V. Jatrophane diterpenoids with multidrug-resistance modulating activity from the latex of Euphorbia nicaeensis. Phytochemistry 2018, 148, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Krstić, G.B.; Kostić, A.; Jadranin, M.B.; Pešić, M.; Novaković, M.M.; Aljančić, I.S.; Vajs, V.V. Two new jatrophane diterpenes from the roots of Euphorbia nicaeensis. J. Serbian Chem. Soc. 2021, 86, 1219–1228. [Google Scholar] [CrossRef]
- Dinić, J.; Novaković, M.; Pešić, M. Chapter 9—Potential for cancer treatment: Natural products from the Balkans. In Biodiversity and Biomedicine; Ozturk, M., Egamberdieva, D., Pešić, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 137–159. [Google Scholar]
- Sharma, D.K.; Tiwari, M.; Behera, B.K. A review of integrated processes to get value-added chemicals and fuels from petrocrops. Bioresour. Technol. 1994, 49, 1–6. [Google Scholar] [CrossRef]
- Aljancic, I.S.; Pesic, M.; Milosavljevic, S.M.; Todorovic, N.M.; Jadranin, M.; Milosavljevic, G.; Povrenovic, D.; Bankovic, J.; Tanic, N.; Markovic, I.D.; et al. Isolation and biological evaluation of jatrophane diterpenoids from Euphorbia dendroides. J. Nat. Prod. 2011, 74, 1613–1620. [Google Scholar] [CrossRef]
- Jadranin, M.; Pesic, M.; Aljancic, I.S.; Milosavljevic, S.M.; Todorovic, N.M.; Podolski-Renic, A.; Bankovic, J.; Tanic, N.; Markovic, I.; Vajs, V.E.; et al. Jatrophane diterpenoids from the latex of Euphorbia dendroides and their anti-P-glycoprotein activity in human multi-drug resistant cancer cell lines. Phytochemistry 2013, 86, 208–217. [Google Scholar] [CrossRef]
- Xie, X.L.; Fan, R.Z.; Hu, R.; Luo, S.Y.; Tang, G.H.; Yin, S. Euphoresulanes A-M, structurally diverse jatrophane diterpenoids from Euphorbia esula. Bioorg Chem. 2020, 98, 103763. [Google Scholar] [CrossRef]
- Đurović, S.; Ranimirović, M.; Tomović, G.; Petkovski, G.; Niketic, M. Genus Euphorbia L. (Euphorbiaceae juss.) in Serbia based on herbarium data from the collections BEO and BEOU. Bull. Nat. Hist. Mus. Belgrade 2022, 15, 97–120. [Google Scholar] [CrossRef]
- Mai, Z.P.; Ni, G.; Liu, Y.F.; Li, L.; Shi, G.R.; Wang, X.; Li, J.Y.; Yu, D.Q. Heliosterpenoids A and B, two Novel Jatrophane-Derived Diterpenoids with a 5/6/4/6 Ring System from Euphorbia helioscopia. Sci. Rep. 2017, 7, 4922. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, J.; Zhong, X.; Hu, R.; Gao, J.; Duan, G.; Ji, C.; Chen, L.; Zhang, W.; Miao, C.; et al. ES2 enhances the efficacy of chemotherapeutic agents in ABCB1-overexpressing cancer cells in vitro and in vivo. Pharmacol. Res. 2018, 129, 388–399. [Google Scholar] [CrossRef]
- Yang, H.; Mamatjan, A.; Tang, D.; Aisa, H.A. Jatrophane diterpenoids as multidrug resistance modulators from Euphorbia sororia. Bioorg Chem. 2021, 112, 104989. [Google Scholar] [CrossRef] [PubMed]
- Maimaitijiang, A.; Wang, B.; Yang, H.; Tang, D.; Liu, Y.; Aisa, H.A. Discovery of a novel highly potent and low-toxic jatrophane derivative enhancing the P-glycoprotein-mediated doxorubicin sensitivity of MCF-7/ADR cells. Eur. J. Med. Chem. 2022, 244, 114822. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Liu, G.Y.; Hu, R.; Aisa, H.A. Jatrophane Diterpenoids from Euphorbia glomerulans. J. Nat. Prod. 2019, 82, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, R.; Lou, L.; Li, W.; Tang, G.; Bu, X.; Yin, S. Jatrophane Diterpenoids as Modulators of P-Glycoprotein-Dependent Multidrug Resistance (MDR): Advances of Structure-Activity Relationships and Discovery of Promising MDR Reversal Agents. J. Med. Chem. 2016, 59, 6353–6369. [Google Scholar] [CrossRef]
- Martinez, C.; Carvalho, M.R.; Madrinan, S.; Jaramillo, C.A. A Late Cretaceous Piper (Piperaceae) from Colombia and diversification patterns for the genus. Am. J. Bot. 2015, 102, 273–289. [Google Scholar] [CrossRef]
- Dharmaratne, H.R.; Nanayakkara, N.P.; Khan, I.A. Kavalactones from Piper methysticum, and their 13C NMR spectroscopic analyses. Phytochemistry 2002, 59, 429–433. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Yan, M.; Wu, J.; Liu, Y.; Tian, X.; Jiang, W.; Zhang, L.; Wang, R. Activity and mechanism of flavokawain A in inhibiting P-glycoprotein expression in paclitaxel resistance of lung cancer. Oncol. Lett. 2020, 19, 379–387. [Google Scholar] [CrossRef]
- Palko-Labuz, A.; Kostrzewa-Suslow, E.; Janeczko, T.; Sroda-Pomianek, K.; Pola, A.; Uryga, A.; Michalak, K. Cyclization of flavokawain B reduces its activity against human colon cancer cells. Hum. Exp. Toxicol. 2020, 39, 262–275. [Google Scholar] [CrossRef]
- Akaberi, M.; Sahebkar, A.; Azizi, N.; Emami, S.A. Everlasting flowers: Phytochemistry and pharmacology of the genus Helichrysum. Ind. Crops Prod. 2019, 138, 111471. [Google Scholar] [CrossRef]
- Matić, I.; Aljančić, I.; Vajs, V.; Jadranin, M.; Gligorijevic, N.; Milosavljević, S.; Juranic, Z. Cancer-Suppressive Potential of Extracts of Endemic Plant Helichrysum Zivojinii: Effects on Cell Migration, Invasion and Angiogenesis. Nat. Prod. Commun. 2013, 8, 1291–1296. [Google Scholar] [CrossRef]
- Matic, I.Z.; Aljancic, I.; Zizak, Z.; Vajs, V.; Jadranin, M.; Milosavljevic, S.; Juranic, Z.D. In vitro antitumor actions of extracts from endemic plant Helichrysum zivojinii. BMC Complement. Altern. Med. 2013, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Aljancic, I.S.; Vuckovic, I.; Jadranin, M.; Pesic, M.; Dordevic, I.; Podolski-Renic, A.; Stojkovic, S.; Menkovic, N.; Vajs, V.E.; Milosavljevic, S.M. Two structurally distinct chalcone dimers from Helichrysum zivojinii and their activities in cancer cell lines. Phytochemistry 2014, 98, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, K.; Tomović, G.; Djordjević, V.; Niketić, M.; Stevanović, V. Steppe flora in Serbia—Distribution, ecology, centres of diversity and conservation status. Folia Geobot. 2020, 55, 1–14. [Google Scholar] [CrossRef]
- Nabekura, T.; Hiroi, T.; Kawasaki, T.; Uwai, Y. Effects of natural nuclear factor-kappa B inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed. Pharmacother. 2015, 70, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Toksoy Köseoğlu, S.; Öztürk, M.; Sağıroğlu, M. Phylogenetic and cladistic analyses of the enigmatic genera Bituminaria and Cullen (Fabaceae) in Turkey. Turk. J. Bot. 2015, 39, 60–69. [Google Scholar] [CrossRef]
- Alam, F.; Khan, G.N.; Asad, M. Psoralea corylifolia L: Ethnobotanical, biological, and chemical aspects: A review. Phytother. Res. 2018, 32, 597–615. [Google Scholar] [CrossRef]
- Xing, N.; Meng, X.; Wang, S. Isobavachalcone: A comprehensive review of its plant sources, pharmacokinetics, toxicity, pharmacological activities and related molecular mechanisms. Phytother. Res. 2022, 36, 3120–3142. [Google Scholar] [CrossRef]
- Palko-Labuz, A.; Blaszczyk, M.; Sroda-Pomianek, K.; Wesolowska, O. Isobavachalcone as an Active Membrane Perturbing Agent and Inhibitor of ABCB1 Multidrug Transporter. Molecules 2021, 26, 4637. [Google Scholar] [CrossRef]
- Konowalik, K.; Kreitschitz, A. Morphological and anatomical characteristics of Artemisia absinthium var. absinthium and its Polish endemic variety A. absinthium var. calcigena. Plant Syst. Evol. 2012, 298, 1325–1336. [Google Scholar] [CrossRef][Green Version]
- Ivanov, M.; Gasic, U.; Stojkovic, D.; Kostic, M.; Misic, D.; Sokovic, M. New Evidence for Artemisia absinthium L. Application in Gastrointestinal Ailments: Ethnopharmacology, Antimicrobial Capacity, Cytotoxicity, and Phenolic Profile. Evid. Based Complement. Alternat Med. 2021, 2021, 9961089. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Yuan, X.; Li, Y.; Hou, G.; Liu, X. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/beta-catenin signal pathway. Investig. New Drugs 2020, 38, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hoag, H.; Wu, C.; Liu, H.; Yin, H.; Dong, J.; Qian, Z.; Miao, F.; Liu, M.; Miao, J. Experimental and Simulation Identification of Xanthohumol as an Inhibitor and Substrate of ABCB1. Appl. Sci. 2018, 8, 681. [Google Scholar] [CrossRef]
- Novakovic, M.; Bukvicki, D.; Andjelkovic, B.; Ilic-Tomic, T.; Veljic, M.; Tesevic, V.; Asakawa, Y. Cytotoxic Activity of Riccardin and Perrottetin Derivatives from the Liverwort Lunularia cruciata. J. Nat. Prod. 2019, 82, 694–701. [Google Scholar] [CrossRef]
- Kosenkova, Y.S.; Polovinka, M.P.; Komarova, N.I.; Korchagina, D.V.; Kurochkina, N.Y.; Cheremushkina, V.A.; Salakhutdinov, N.F. Riccardin C, a bisbibenzyl compound from Primula macrocalyx. Chem. Nat. Compd. 2007, 43, 712–713. [Google Scholar] [CrossRef]
- Kosenkova, Y.S.; Polovinka, M.P.; Komarova, N.I.; Korchagina, D.V.; Morozov, S.V.; Vyalkov, A.I.; Kurochkina, N.Y.; Cheremushkina, V.A.; Salakhutdinov, N.F. Fatty-acid composition and secondary metabolites from slightly polar extracts of the aerial part of Primula macrocalyx. Chem. Nat. Compd. 2008, 44, 564–568. [Google Scholar] [CrossRef]
- Novakovic, M.; Ilic-Tomic, T.; Djordjevic, I.; Andjelkovic, B.; Tesevic, V.; Milosavljevic, S.; Asakawa, Y. Bisbibenzyls from Serbian Primula veris subsp. Columnae (Ten.) Ludi and P. acaulis (L.) L. Phytochemistry 2023, 212, 113719. [Google Scholar] [CrossRef]
- Xue, X.; Qu, X.J.; Gao, Z.H.; Sun, C.C.; Liu, H.P.; Zhao, C.R.; Cheng, Y.N.; Lou, H.X. Riccardin D, a novel macrocyclic bisbibenzyl, induces apoptosis of human leukemia cells by targeting DNA topoisomerase II. Investig. New Drugs 2012, 30, 212–222. [Google Scholar] [CrossRef]
- Ji, M.; Shi, Y.; Lou, H. Overcoming of P-glycoprotein-mediated multidrug resistance in K562/A02 cells using riccardin F and pakyonol, bisbibenzyl derivatives from liverworts. Biosci. Trends 2011, 5, 192–197. [Google Scholar] [CrossRef][Green Version]
- Brand, S.; Holscher, D.; Schierhorn, A.; Svatos, A.; Schroder, J.; Schneider, B. A type III polyketide synthase from Wachendorfia thyrsiflora and its role in diarylheptanoid and phenylphenalenone biosynthesis. Planta 2006, 224, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Munde, T.; Brand, S.; Hidalgo, W.; Maddula, R.K.; Svatos, A.; Schneider, B. Biosynthesis of tetraoxygenated phenylphenalenones in Wachendorfia thyrsiflora. Phytochemistry 2013, 91, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ishikado, A.; Nishida, N.; Ninomiya, K.; Fujiwara, H.; Kobayashi, Y.; Yoshikawa, M. Hepatoprotective, superoxide scavenging, and antioxidative activities of aromatic constituents from the bark of Betula platyphylla var. japonica. Bioorg. Med. Chem. Lett. 1998, 8, 2939–2944. [Google Scholar] [CrossRef]
- Tung, N.H.; Kim, S.K.; Ra, J.C.; Zhao, Y.Z.; Sohn, D.H.; Kim, Y.H. Antioxidative and hepatoprotective diarylheptanoids from the bark of Alnus japonica. Planta Med. 2010, 76, 626–629. [Google Scholar] [CrossRef]
- Lai, Y.C.; Chen, C.K.; Lin, W.W.; Lee, S.S. A comprehensive investigation of anti-inflammatory diarylheptanoids from the leaves of Alnus formosana. Phytochemistry 2012, 73, 84–94. [Google Scholar] [CrossRef]
- Tung, N.H.; Kwon, H.J.; Kim, J.H.; Ra, J.C.; Ding, Y.; Kim, J.A.; Kim, Y.H. Anti-influenza diarylheptanoids from the bark of Alnus japonica. Bioorg Med. Chem. Lett. 2010, 20, 1000–1003. [Google Scholar] [CrossRef]
- Choi, S.E.; Kim, K.H.; Kwon, J.H.; Kim, S.B.; Kim, H.W.; Lee, M.W. Cytotoxic activities of diarylheptanoids from Alnus japonica. Arch. Pharm. Res. 2008, 31, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Mshvildadze, V.; Legault, J.; Lavoie, S.; Gauthier, C.; Pichette, A. Anticancer diarylheptanoid glycosides from the inner bark of Betula papyrifera. Phytochemistry 2007, 68, 2531–2536. [Google Scholar] [CrossRef]
- Sun, Y.; Tabata, K.; Matsubara, H.; Kitanaka, S.; Suzuki, T.; Yasukawa, K. New cytotoxic diarylheptanoids from the rhizomes of Alpinia officinarum. Planta Med. 2008, 74, 427–431. [Google Scholar] [CrossRef]
- Ren, B.Q.; Xiang, X.G.; Chen, Z.D. Species identification of Alnus (Betulaceae) using nrDNA and cpDNA genetic markers. Mol. Ecol. Resour. 2010, 10, 594–605. [Google Scholar] [CrossRef]
- Skoczowski, A.; Odrzywolska-Hasiec, M.; Oliwa, J.; Ciereszko, I.; Kornas, A. Ecophysiological Variability of Alnus viridis (Chaix) DC. Green Alder Leaves in the Bieszczady Mountains (Poland). Plants 2021, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Szokala, D. Alpine and subalpine acidophilous vegetation on the eastern side of the Chiprovska Planina Mts. Tuexenia 2023, 43, 109–158. [Google Scholar] [CrossRef]
- Vidaković, V.; Novaković, M.; Popović, Z.; Janković, M.; Matić, R.; Tešević, V.; Bojović, S. Significance of diarylheptanoids for chemotaxonomical distinguishing between Alnus glutinosa and Alnus incana. Holzforschung 2018, 72, 9–16. [Google Scholar] [CrossRef]
- Lee, H.-B.; Lee, H.-K.; Kim, J.-R.; Ahn, Y.-J. Anti-Helicobacter pylori diarylheptanoid identified in the rhizome of Alpinia officinarum. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 367–370. [Google Scholar] [CrossRef]
- Martineau, L.C.; Herve, J.; Muhamad, A.; Saleem, A.; Harris, C.S.; Arnason, J.T.; Haddad, P.S. Anti-adipogenic activities of Alnus incana and Populus balsamifera bark extracts, part I: Sites and mechanisms of action. Planta Med. 2010, 76, 1439–1446. [Google Scholar] [CrossRef]
- Telysheva, G.; Dizhbite, T.; Bikovens, O.; Ponomarenko, J.; Janceva, S.; Krasilnikova, J. Structure and antioxidant activity of diarylheptanoids extracted from bark of grey alder (Alnus incana) and potential of biorefinery-based bark processing of European trees. In Proceedings of the 11th EWLP, Hamburg, Germany, 16–19 August 2010; Volume 65, pp. 623–629. [Google Scholar] [CrossRef]
- Lee, O.; Kim, J.; Choi, Y.W.; Lee, M.; Park, G.; Oh, C. Efficacy of oregonin investigated by non-invasive evaluation in a B16 mouse melanoma model. Exp. Dermatol. 2013, 22, 842–844. [Google Scholar] [CrossRef]
- Novakovic, M.; Stankovic, M.; Vuckovic, I.; Todorovic, N.; Trifunovic, S.; Apostolovic, D.; Mandic, B.; Veljic, M.; Marin, P.; Tesevic, V.; et al. Diarylheptanoids from green alder bark and their potential for DNA protection. Chem. Biodivers. 2014, 11, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Dinic, J.; Randelovic, T.; Stankovic, T.; Dragoj, M.; Isakovic, A.; Novakovic, M.; Pesic, M. Chemo-protective and regenerative effects of diarylheptanoids from the bark of black alder (Alnus glutinosa) in human normal keratinocytes. Fitoterapia 2015, 105, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Stankovic, M.; Vuckovic, I.; Todorovic, N.; Trifunovic, S.; Tesevic, V.; Vajs, V.; Milosavljevic, S. Diarylheptanoids from Alnus glutinosa bark and their chemoprotective effect on human lymphocytes DNA. Planta Med. 2013, 79, 499–505. [Google Scholar] [CrossRef]
- Dinic, J.; Novakovic, M.; Podolski-Renic, A.; Vajs, V.; Tesevic, V.; Isakovic, A.; Pesic, M. Structural differences in diarylheptanoids analogues from Alnus viridis and Alnus glutinosa influence their activity and selectivity towards cancer cells. Chem. Biol. Interact. 2016, 249, 36–45. [Google Scholar] [CrossRef]
- Novakovic, M.; Nikodinovic-Runic, J.; Veselinovic, J.; Ilic-Tomic, T.; Vidakovic, V.; Tesevic, V.; Milosavljevic, S. Bioactive Pentacyclic Triterpene Ester Derivatives from Alnus viridis ssp. viridis Bark. J. Nat. Prod. 2017, 80, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Dinic, J.; Novakovic, M.; Podolski-Renic, A.; Stojkovic, S.; Mandic, B.; Tesevic, V.; Vajs, V.; Isakovic, A.; Pesic, M. Antioxidative activity of diarylheptanoids from the bark of black alder (Alnus glutinosa) and their interaction with anticancer drugs. Planta Med. 2014, 80, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Pesic, M.; Trifunovic, S.; Vuckovic, I.; Todorovic, N.; Podolski-Renic, A.; Dinic, J.; Stojkovic, S.; Tesevic, V.; Vajs, V.; et al. Diarylheptanoids from the bark of black alder inhibit the growth of sensitive and multi-drug resistant non-small cell lung carcinoma cells. Phytochemistry 2014, 97, 46–54. [Google Scholar] [CrossRef]
- Islam, M.R.; Rauf, A.; Akash, S.; Trisha, S.I.; Nasim, A.H.; Akter, M.; Dhar, P.S.; Ogaly, H.A.; Hemeg, H.A.; Wilairatana, P.; et al. Targeted therapies of curcumin focus on its therapeutic benefits in cancers and human health: Molecular signaling pathway-based approaches and future perspectives. Biomed. Pharmacother. 2024, 170, 116034. [Google Scholar] [CrossRef]
- Andjelkovic, T.; Pesic, M.; Bankovic, J.; Tanic, N.; Markovic, I.D.; Ruzdijic, S. Synergistic effects of the purine analog sulfinosine and curcumin on the multidrug resistant human non-small cell lung carcinoma cell line (NCI-H460/R). Cancer Biol. Ther. 2008, 7, 1024–1032. [Google Scholar] [CrossRef][Green Version]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Chearwae, W.; Shukla, S.; Phisalphong, C.; Ambudkar, S.V. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol. Cell Biochem. 2007, 296, 85–95. [Google Scholar] [CrossRef]
- Tang, X.Q.; Bi, H.; Feng, J.Q.; Cao, J.G. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol. Sin. 2005, 26, 1009–1016. [Google Scholar] [CrossRef]
- Yu, S.; Shen, G.; Khor, T.O.; Kim, J.H.; Kong, A.N. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2609–2620. [Google Scholar] [CrossRef]
- Lin, X.; Yang, X.; Yang, Y.; Zhang, H.; Huang, X. Research progress of traditional Chinese medicine as sensitizer in reversing chemoresistance of colorectal cancer. Front. Oncol. 2023, 13, 1132141. [Google Scholar] [CrossRef]
- Guo, S.; Lv, L.; Shen, Y.; Hu, Z.; He, Q.; Chen, X. A nanoparticulate pre-chemosensitizer for efficacious chemotherapy of multidrug resistant breast cancer. Sci. Rep. 2016, 6, 21459. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Lu, P.W.; Lu, E.W.; Lin, C.W.; Yang, S.F. Curcumin and its Analogs and Carriers: Potential Therapeutic Strategies for Human Osteosarcoma. Int. J. Biol. Sci. 2023, 19, 1241–1265. [Google Scholar] [CrossRef] [PubMed]
- Viriyaadhammaa, N.; Duangmano, S.; Saiai, A.; Tungjai, M.; Dejkriengkraikul, P.; Tima, S.; Chiampanichayakul, S.; Krise, J.; Anuchapreeda, S. A Novel Drug Modulator Diarylheptanoid (trans-1,7-Diphenyl-5-hydroxy-1-heptene) from Curcuma comosa Rhizomes for P-glycoprotein Function and Apoptosis Induction in K652/ADR Leukemic Cells. Int. J. Mol. Sci. 2022, 23, 8989. [Google Scholar] [CrossRef]
- Danko, B.; Toth, S.; Martins, A.; Vagvolgyi, M.; Kusz, N.; Molnar, J.; Chang, F.R.; Wu, Y.C.; Szakacs, G.; Hunyadi, A. Synthesis and SAR Study of Anticancer Protoflavone Derivatives: Investigation of Cytotoxicity and Interaction with ABCB1 and ABCG2 Multidrug Efflux Transporters. ChemMedChem 2017, 12, 850–859. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).