Abstract
The translocator protein (TSPO) is predominately localized on the outer mitochondrial membrane in steroidogenic cells. In the brain, TSPO expression, low under normal conditions, results upregulated in response to glial cell activation, that occurs in neuroinflammation. As a consequence, TSPO has been extensively studied as a biomarker of such conditions by means of TSPO-targeted radiotracers. Although [11C]-PK11195, the prototypical TSPO radioligand, is still widely used for in vivo studies, it is endowed with severe limitations, mainly low sensitivity and poor amenability to quantification. Consequently, several efforts have been focused on the design of new radiotracers for the in vivo imaging of TSPO. The present review will provide an outlook on the latest advances in TSPO radioligands for neuroinflammation imaging. The final goal is to pave the way for (radio)chemists in the future design and development of novel effective and sensitive radiopharmaceuticals targeting TSPO.
1. Introduction
Neuroinflammation is an inflammatory and adaptive response within central nervous system (CNS), which includes the brain and spinal cord. The correct functionality of the brain can be negatively affected by different external agents, including injury, ischemia, infection, toxins, neurotoxic stimuli, autoimmunity and responses to processes that may change neuronal activity. As a consequence, cellular and biochemical responses are generated by the brain cells, predominantly microglia and astrocytes, by activation of what is called “acute neuroinflammation”. This process implies the disruption of toxic agents and irreversible damaged tissue, the protection of surrounding cells and the repairment of tissue, limiting the spread of damage [1]. The neuroinflammatory response aims to mitigate the activation of factors by evoking CNS immunity to defend against potential damage and to maintain and restore homeostasis.
Therefore, neuroinflammation represents a physiological process crucial for the protection of the CNS. However, if inflammation becomes chronic, it can cause damage to healthy brain tissue and contribute to the development of neurological diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS) [2,3].
In this scenario, understanding the mechanisms underlying neuroinflammation is crucial for developing targeted treatments that can regulate inflammation and shield the brain from the adverse effects of excessive or abnormal inflammatory responses.
Interestingly, neuroinflammation seems to be correlated with the overexpression of the translocator protein (TSPO). Therefore, it may be considered a suitable neuroinflammation biomarker as well as a target for positron emission tomography (PET) and single-photon emission computed tomography (SPECT) imaging [4,5,6].
TSPO (18 kDa) is an evolutionarily well-conserved and tryptophan-rich 169-amino-acid protein with 5 trans-membrane domains, each comprised of 21 residues that spread across the whole membrane, with the carboxyl portion outside and the amino one inside the mitochondria. It is predominantly localized at the contact sites between the outer (OMM) and the inner mitochondrial membrane (IMM), where it plays a key role in the translocation of cholesterol, namely the rate-limiting step of steroidogenesis, Figure 1. Furthermore, TSPO actively participates in various physiological processes beyond its primary function, including immunomodulation, mitochondrial metabolism and function. TSPO is also involved in apoptosis, cell respiration, oxidation, cell proliferation and differentiation. Additionally, it contributes to porphyrin transport, heme biosynthesis and protein and ion transport [7,8,9,10].
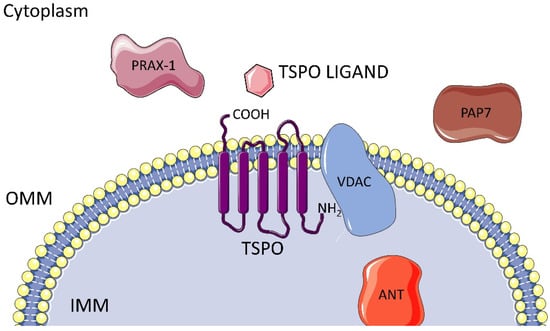
Figure 1.
TSPO localization and associated proteins.
TSPO is associated with the voltage-dependent anion channel (VDAC), the adenine nucleotide translocase (ANT), and other mitochondrial proteins like PRAX-1, PAP7, hence forming the mitochondrial permeability transition pore (MPTP), Figure 1.
Recently, researchers employed different methods to obtain structural insights into TSPO. Jaremko et al. used NMR spectroscopy to determine the high-resolution three-dimensional structure of mouse TSPO (mTSPO) in complex with its high-affinity ligand PK11195 [11,12]. Findings revealed that PK11195 binds to a hydrophobic crevice within the helical bundle of TSPO. Of note, mTSPO structure appeared highly stable when complexed with PK11195. However, subsequent analyses evidenced that the absence of a high-affinity ligand confer a certain conformational flexibility in the mTSPO structure [13].
Shortly after this study, X-ray crystallography was employed to elucidate the crystal structure of TSPO homologues from two bacterial species, namely Rhodobacter sphaeroides (RsTSPO) [14] and Bacillus cereus (BcTSPO) [15]. These investigations proved that monomeric TSPO molecules form dimers within the membrane and that each monomer comprises five transmembrane helices.
Under healthy physiological conditions, TSPO is extensively diffused across various peripheral organs, such as the heart, lungs, kidney, adrenal glands, and nasal epithelium. Steroid-producing tissues exhibit the highest concentration of TSPO, while reduced levels are noted in the liver and in quiescent microglial cells and neurons in the normal brain.
The TSPO expression is altered in a wide range of human diseases [16]. Furthermore, TSPO overexpression has been found in certain cancerous conditions, including breast [17] and colorectal cancer [18], glioma [19] and hepatocellular carcinoma [20]. It has been suggested that TSPO levels may be correlated with the metastatic potential of breast cancer and gliomas. Conversely, reduced levels of TSPO have been identified in patients with generalized anxiety, schizophrenia, panic disorder, post-traumatic stress disorder, and obsessive-compulsive disorder [18]. In the CNS, it is predominantly upregulated in microglia in response to inflammatory stimuli. TSPO overexpression has also been observed in reactive astrocytes. Increased levels of TSPO in glial cells have been associated with several neurodegenerative and neuroinflammatory disorders, including PD, Huntington’s disease, MS, amyotrophic lateral sclerosis, AD, and certain psychiatric conditions [16,21].
Although in vivo structural imaging offers valuable data in preclinical and clinical studies, it is essential to combine morphological information and in vivo molecular imaging to unveil the real-time structures of dynamic physiological processes underlying disease phenomena. Among the tomographic molecular imaging modalities, PET imaging provides more translational possibilities compared to others thanks to its sensitivity combined with its quantitative accuracy. PET is a noninvasive imaging technique offering physiological information by means of radiotracer injection, detection of its radiation, and reconstruction of its distribution. Thus, PET is nowadays a standard diagnosis imaging technique in oncological, neurological, and cardiovascular diseases. This is principally attributed to the clinically useful information it offers about organ and tissue function and status, by using radiolabeled molecular imaging agents. Depending on imaging agent and disease, the type of information obtained may include detection, classification, staging, prognosis, treatment planning, assessing of response to therapy, and surveillance [22].
The radiotracer [11C]PK11195 (1) has been extensively utilized in PET imaging to study neuroinflammation. One of the notable advantages of [11C]PK11195 (1) is its limited susceptibility to a specific TSPO genetic variation known as a single nucleotide polymorphism (SNP) rs6971 in humans. The allelic frequency of the SNP rs6971 is approximately 30% in individuals of European ancestry. This SNP, lacking known clinical significance, exhibits codominant expression, resulting in the formation of high-affinity binders (HABs with two high-affinity alleles), low-affinity binders (LABs with two low-affinity alleles), and mixed-affinity binders (MABs with one low and one high-affinity allele). HABs are homozygous for wild-type TSPO, whereas MABs are heterozygous and LABs are homozygous for the Ala147Thr TSPO [23]. Thus, individuals with the same TSPO density but different genotypes will produce different PET signals. The difference in affinity between the two alleles can be significant for certain radioligands. The relevance of the SNP rs6971 in TSPO to the interpretation of clinical PET data is evident. Although each radioligand has a unique level of sensitivity, studies with TSPO radioligands must take SNP rs6971 into account by doing TSPO genotyping before imaging. This task is an unwelcome extra burden in any PET study. Often, LABs must be excluded because the brain has too little uptake to quantify and studies that include HABs and MABs need a suitable correction for binding levels across genetic groups [24,25,26].
However, [11C]PK11195 (1) presents some drawbacks, such as a high level of nonspecific binding [27,28] and a non-optimal signal-to-noise ratio compared to that in the early years of TSPO PET imaging, complicating its quantification [29]. Although quantification problems have been circumvented [30,31], there is still a need for the development of new TSPO radioligands that can offer improved performance in quantifying TSPO expression while still maintaining low sensitivity to the SNP rs6971 [6].
With respect to our previous overview published in 2022 [32], in this review we make an update on the recent advances in the field of TSPO radioligands for neuroinflammation elucidating concerns with regard to the imaging of TSPO with PET taking into particular account the impact of the SNP rs6971 on second-generation ligand binding, that have permitted to obtain higher TSPO PET brain signal in neuroinflammation diseases compared to control [33], thus suggesting that TSPO is a good target for development of neuroinflammation imaging agents. Furthermore, we give more information about current clinical trials concerning TSPO radioligands for neuroinflammation and we have expanded and updated the section relative to the development of third-generation TSPO radioligands that combine the TSPO-specific signals seen in second-generation ligands with a reduced sensitivity to SNP.
The availability of structural information on mTSPO in complex with its high-affinity ligand (R)-PK11195 by means of NMR spectroscopy [11,12], and the X-ray resolution of the crystal structures of TSPO homologues from two bacteria RsTSPO [14] and BcTSPO [15], together with the most recent advancement in TSPO radioligands for neuroinflammation imaging herein comprehensively reviewed, will help (radio)chemists in the design and development of increasingly specific and effective radiopharmaceuticals targeting TSPO, hopefully leveraging the recent efforts in TSPO radioligand design focusing on the development of ligands with low SNP rs6971 sensitivity and thus definitively cutting out the extra burden in performing TSPO genotyping before any PET study.
2. TSPO Radioligands
The development of radiotracers for PET/SPECT imaging of molecular targets in the CNS is challenging, particularly with regard to quantitation aspects. The first issue to be considered concerns the ability of the radiotracer to cross the blood–brain barrier (BBB). Generally, passive transfer across the BBB is facilitated by low molecular weight (<500 Da), lack of formal charge, low hydrogen-binding capability, and modest lipophilicity (LogD between 2.0 and 3.5) [34]. Once within the CNS, an efficient radioligand has to bind with high affinity and selectivity to its target of interest, which should be of sufficient abundance for detection against a background of nonspecific binding [35].
For reversible radiotracers, specific binding increases over time until reaching an equilibrium defined by Bmax (maximum number of binding sites) and Kd (tracer concentration that binds to half the binding sites at equilibrium) [36]. The ratio Bmax/Kd represents non-displaceable binding potential (BPND), which reflects the specific binding. In particular, BPND less than 1 means low specific signal, while that exceeding 10 means a failure in reaching equilibrium within the time constraints of a dynamic PET scan. In both cases, quantitation becomes challenging.
Two radionuclides are commonly used to label TSPO PET radioligands, that are carbon-11 (11C) and fluorine-18 (18F). The relevant feature of 18F is its longer half-life (t1/2 = 109.8 min) relative to that of 11C (t1/2 = 20.4 min). In addition, PET images with a certain higher spatial resolution can be generated with fluorine-18 than with carbon-11, due to its lower positron energy [37,38]. The widespread occurrence of carbon in natural products and pharmaceuticals makes carbon-11 a valuable and appealing positron-emitting isotope for labeling biologically significant molecules. A major advantage is that 11C-labeled molecules exhibit the same chemical and biological behavior as their unlabeled counterparts. This is crucial as it eliminates concerns about the potential impact of adding an “artificial” PET tag (such as incorporating an 18F atom; vide infra) on the biological properties of the compound of interest. Almost all TSPO radioligands developed so far have a tertiary N-methyl amide position that is usually readily labeled by 11C-methylation. Carbon-11, due to its short half-life, cannot be transported to remote facilities, and the final radiotracer must be produced near the synthesis site. However, an advantage of carbon-11 for local use is that a subject can be injected multiple times on the same day with the same radioligand, and this may be useful for conducting a baseline experiment followed by an experiment in which the same subject undergoes a pharmacological or other type of challenge. In general, the rapid decay of carbon-11 means that the radiation exposure to the subject is well within approved limits. Fluorine-18 is frequently called the “radionuclide of choice” in PET imaging due to its advantageous physical and nuclear properties: a short yet manageable half-life of 110 min, which provides enough time for multistep synthetic labeling reactions, and a short positron linear range in tissue (2.3 mm) that produces the highest resolution PET images among all available positron emitters. The half-life of the 18F isotope is also sufficiently long to enable the transportation of doses to locations several hours away from the site of production. Even though fluorine is typically absent in a TSPO ligand, it can often be inserted at a hydrogen position in many ligands, one of the most common bioisosteric replacements in medicinal chemistry. Replacing hydrogen with fluorine in an organic molecule causes minimal steric disruption due to their similar van der Waals radii (1.35 and 1.20 Å, respectively), but it significantly increases the molecular weight. The strong electron-withdrawing nature of fluorine, as evidenced by the dipole moment of the carbon-fluorine bond, can alter the electronic properties and thus the biological behavior of the molecule. Substituting a hydrogen atom with fluorine is expected to modestly increase the lipophilicity of a molecule, based on the π coefficient of 0.14 for fluorine. However, fluorination does not always lead to an increase in lipophilicity; this bioisosteric substitution can reduce overall lipophilicity in certain structural contexts. Therefore, labeling with fluorine-18 usually results in modifications to the physicochemical properties and biological activity of known ligands. Additionally, replacing hydrogen with fluorine is a well-established approach in medicinal chemistry to modulate metabolism, as the electron-withdrawing properties of fluorine and the high strength of the carbon-fluorine bond make it chemically resistant under most biological conditions. Nonetheless, nearly all PET radioligands undergo significant metabolism during the course of a PET scan, sometimes generating radiometabolites that can cross the BBB and affect brain radioligand quantification. It is important to note that 18F-labeled ligands may be subject to peripheral radiodefluorination, resulting in [18F]fluoride ions in plasma that can be taken up by bone. If the radioactivity in the skull becomes significant, the accurate measurement of radioactivity in brain regions near the skull, such as the neocortex, will be compromised. Although it is difficult to predict the susceptibility of an 18F-labeled compound to radiodefluorination in human subjects, fluorine-18 atoms bound to benzene or pyridine rings are generally resistant to metabolic defluorination. Radioligands labeled with fluorine-18 in an aryl methoxy group are known to be susceptible to radiodefluorination, while fluorine-18 at the terminus of a straight saturated alkyl chain of two or more carbons is usually more resistant to defluorination in human subjects. In the development of TSPO radioligands, the preparation of 18F-labeled ligands is often carried out alongside the synthesis of 11C-labeled analogues. Fluorine-18 is frequently incorporated into a benzene ring, an N-methyl or N-ethyl position, or at the end of aryl fluoroalkoxy groups [37,38].
TSPO radioligands are categorized in three generations, as depicted in Figure 2 and Figure 3 and in Table 1, which lists the most representative compounds implicated in PET neuroinflammation studies.
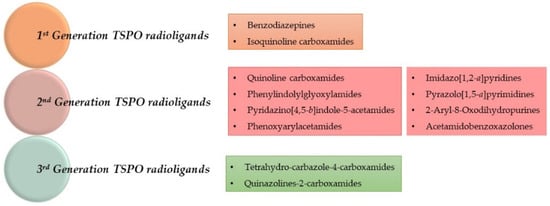
Figure 2.
Classification of TSPO radioligands.
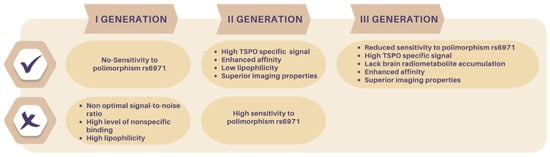
Figure 3.
Advantages and disadvantages of first, second, and third generation of TSPO radioligands.

Table 1.
The most representative compounds in PET imaging of neuroinflammation.
First-generation TSPO radiotracers include isoquinoline carboxamides, like [11C]PK11195 (1), and benzodiazepines, like [11C]Ro5-4864 (2), Figure 4.
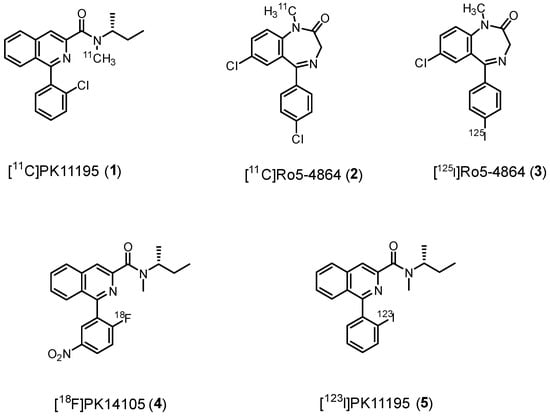
Figure 4.
First-generation TSPO radioligands.
Second-generation TSPO PET radiotracers comprise those compounds developed to overcome the abovementioned [11C]PK11195 (1) limitations. These radioligands have emerged from diverse structural classes and exhibit enhanced affinity and reduced lipophilicity compared to 1, Figure 3. As a result, they demonstrate improved TSPO-specific signals and superior imaging properties. However, at variance with 1, these radioligands suffer from sensitivity to SNP rs6971, Figure 3.
The development of third-generation TSPO radioligands aimed to combine the TSPO-specific signals seen in second-generation ligands with the reduced sensitivity to SNP exhibited by 1 (Figure 3).
2.1. First Generation TSPO Radioligands
2.1.1. Benzodiazepines
Ro5-4864, (7-chloro-5-(4-chlorophenyl)-1-methyl-1,3-dihydro-2H-benzo[e][1,4]diazepin-2-one, Figure 4, Table 1) presents nanomolar affinity for TSPO (Ki = 6.0 nM) and a 1000-fold lower affinity for central benzodiazepine receptor (CBR). For this reason, it was radiolabeled for imaging purposes. However, early PET studies using [11C]Ro5-4864 (2) in humans with gliomas failed to demonstrate elevated radioactivity levels compared to healthy brain regions. Similarly, in vitro studies showed minimal binding of 2 in surgically removed human glioma samples [40]. These disappointing results could be attributed to various factors, such as elevated non-specific binding, high lipophilicity, low brain uptake, as well as species-specific binding [72].
Chlorine was replaced with iodine-125 to give [125I]Ro5-4864 (3), but unfortunately, no successful SPECT imaging was obtained. As a result of these challenges and the discovery of the isoquinolinecarboxamide class of compounds, which proved to be more effective TSPO ligands, benzodiazepines like Ro5-4864 have been deemed unsuitable for further development as tools for TSPO PET imaging.
2.1.2. Isoquinoline Carboxamides
A few years earlier than 2, [11C]PK11195 (1) (1-[2-chlorophenyl]-N-methyl-N-[1-methyl-propyl]-3-isoquinoline carboxamide (Figure 4, Table 1) was synthesized [29] and studied, quickly demonstrating its superiority with respect to 2; 1 exhibited greater overall uptake and higher specific binding in gliomas with respect to 2 [40]. Initially, it was used as a racemate (Ki = 9.3 nM), but subsequent in vivo studies revealed that the (R)-enantiomer showed significantly higher retention in abundant TSPO binding sites. This correlated with in vitro experiments, which showed the (R)-enantiomer to have 2-fold higher affinity for TSPO compared to the (S)-enantiomer (Table 1) [39].
As the prototypical first-generation TSPO radioligand, 1 was widely employed for decades in imaging TSPO in neurological and psychiatric disorders. For instance, it was used in clinical trials to investigate the role of activated microglia in several conditions, including cognitive impairment, psychosis/Schizophrenia, depression, addiction amyotrophic lateral sclerosis, complex regional pain syndrome, Creutzfeldt–Jakob disease, and other neurodegenerative diseases such as synucleinopathies and tauopathies, but also in viral infections, vascular diseases, traumatic brain injury, etc. For a comprehensive review, see Ref. [73]. AD, together with its prodromal condition, mild cognitive impairment (MCI), is the most studied pathology and one of the first pathologies studied apart from glioma, with [11C]PK11195 and (R)-[11C]PK11195 observed in patients since 1995 [74,75,76,77,78].
Despite being extensively used, 1 presents limitations that hinder its suitability as a PET tracer. One major concern is its relatively high lipophilicity (logD = 3.97), not in the ideal range (LogD range = 2.0–3.5) [34,79], leading to significant nonspecific binding, which, together with modest affinity, leads to a poor signal-to-noise ratio. Additionally, high plasma protein binding and low brain permeability further hinder its quantification [80,81]. These factors limit its capability to detect slight changes in TSPO density, making it unsuitable for nuclear imaging in certain diseases [82].
Nevertheless, 1 displays notably low SNP rs6971 sensitivity. It binds with similar affinity in all subjects, which may explain why non-binding has not been reported with this radioligand. Actually, this phenomenon of non-binding may have been overlooked with [11C]-(R)-PK11195 because this radioligand has a low ratio of specific to non-specific binding in the brain. The high non-specific binding of [11C]-(R)-PK11195 may have obscured real differences of specific binding in binders compared to non-binders [27,83].
Later, PK11195-derivatives labeled with fluorine-18, (N-methyl-N-(1-methylpropyl)-1-(2-fluoro-5-nitrophenyl)isoquinoline-3-carboxamide, [18F]PK14105 (4)) [84] or iodine-123, (1-(2-iodophenyl)-N-methyl-N-(1-methylpropyl)isoquinoline-3-carboxamide, [123I]PK11195, (5)) [85] were also synthesized, Figure 4. However, both radioligands displayed a decrease in in vitro affinity compared to 1 and no advantages initially in in vivo studies, rendering such compounds unworthy of further study.
2.2. Second Generation TSPO Radioligands
2.2.1. Quinoline-2-carboxamides
This class of ligands was designed as conformationally restrained analogues of 1 [86]. The most potent representatives as TSPO probes are radiolabeled with carbon-11, (N-(sec-butyl)-N,3-dimethyl-4-phenylquinoline-2-carboxamide, [11C]VC193M (6), IC50 = 2.1 nM), (N-(sec-butyl)-4-(2-fluorophenyl)-N,3-dimethylquinoline-2-carboxamide, [11C]VC198M (7), IC50 = 2.9 nM), and (N-benzyl-N,3-dimethyl-4-phenylquinoline-2-carboxamide, [11C]VC195 (8), IC50 = 2.1 nM), Figure 5, Table 1 [41]. Initially, these derivatives were assessed for their ability to image microglia activation in neurodegenerative conditions [87].
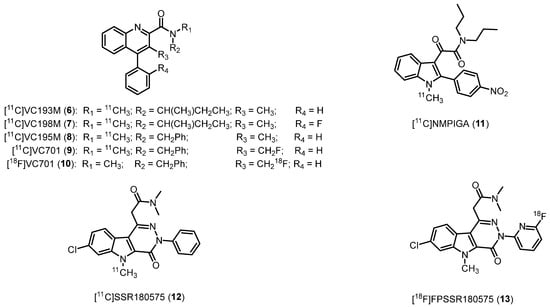
Figure 5.
Second-generation TSPO radioligands.
Among them, the most interesting compound resulted 8, which exhibited results similar to those of 1. Subsequently, the fluoromethyl analogue [11C]VC701 (9) (N-benzyl-3-(fluoromethyl)-N-methyl-4-phenylquinoline-2-carboxamide, Figure 5, Table 1) was synthesized. It displayed an IC50 of 0.11 nM for TSPO, being approximately 20 times more potent with respect to 1. In biodistribution experiments, 9 displayed higher tissue-to-plasma ratios than 8, indicating reduced interaction with plasma proteins [42]. Additionally, VC701 was radiolabeled with fluorine-18 ([18F]VC701, 10, Figure 5) to evaluate its kinetics and pharmacological properties in animals, where it proved to be a TSPO-specific ligand [88]. This fluorinated radioligand 10 was also employed in a study to evaluate neuroinflammation in a mouse MS model (autoimmune encephalomyelitis, EAE): in vivo and ex vivo analyses showed that 18F-VC701 significantly accumulates within the CNS (cortex, striatum, hippocampus, cerebellum, and cervical spinal cord) of EAE compared to control mice, at 2 weeks post-immunization. The increase in 18F-VC701 uptake in EAE mice strongly evidenced the presence of microglia activation in the acute phase of the disease [89].
2.2.2. 2-Phenylindolylglyoxylamides
The rational design of the 2-phenylindolylglyoxylamides (PIGAs) as TSPO ligands consisted in the reduction of the structural flexibility of the indole-3-acetamides, a series of derivatives synthesized by Kozikowski et al. endowed with high TSPO affinity and selectivity [90]. PIGAs exhibited a strong binding affinity for TSPO, ranging from nanomolar to subnanomolar levels [91,92,93]. Among them, the N1-methyl-2-(4′-nitrophenyl)indol-3-yl)glyoxylamide, [11C]NMPIGA (11, Figure 5, Table 1) showed remarkable affinity (Ki = 5.7 nM) and an acceptable lipophilicity (clogP = 3.95). When administered intravenously, 11 readily penetrated the monkey brain, resulting in a significant proportion of reversible specific TSPO binding. However, this novel chemical compound exhibited sensitivity to SNP rs6971. Specifically, 11 demonstrated similarly high affinities for HABs (Ki = 1.57 nM) and MABs (Ki = 1.82 nM) while displaying lower affinity for LABs (Ki = 9.53 nM) [43].
2.2.3. Pyridazino[4,5-b]indole-5-acetamides
Pyridazino[4,5-b]indole-5-acetamides derived by the combination of the indole ring with a pyridazine one [44]. [11C]SSR180575, the 2-(7-chloro-5-methyl-4-oxo-3-phenyl-4,5-dihydro-3H-pyridazino[4,5-b]indol-1-yl)-N,N-dimethylacetamide (12, Figure 5, Table 1) was developed based on high affinity (Ki = 0.83 nM) and selectivity for TSPO of SSR180575, as well as the ability to support neuronal survival and regeneration in animal models of axotomy and neuropathy by facilitating the local synthesis of neurosteroids [44]; 12 exhibited substantial TSPO-specific binding, as demonstrated in a rodent model of acute neuroinflammation. In addition, increased uptake of 12 in the ipsilateral striatum, which exhibited elevated TSPO expression compared to intact tissue in the contralateral striatum, was also evidenced by in vitro autoradiography and in vivo brain PET imaging. Encouraging PET imaging properties of 12 were also observed in non-human primates [94,95].
After that, a series of related compounds was developed, by N3-position functionalization allowing for substitution with fluorine-18. Numerous compounds exhibited potent affinity, with Ki values similar to that of SSR180575. Among them, the derivative featuring a 3-fluoro-2-pyridyl moiety instead of the phenyl ring was selected for labeling, obtaining the 2-(7-chloro-3-(6-fluoropyridin-2-yl)-5-methyl-4-oxo-4,5-dihydro-3H-pyridazino[4,5-b]indol-1-yl)-N,N-dimethylacetamide, [18F]FPSSR180575 (13, Figure 5). In PET imaging evaluation using male Wistar rats bearing gliomas, 13 displayed increased accumulation in the tumor brain tissue relative to the contralateral non-tumor brain, thereby providing outstanding imaging contrast between the tumor and contralateral tissue [96].
2.2.4. Phenoxyarylacetamides
Modification of Ro5-4864, involving the opening of the azepine ring, led to the generation of phenoxyarylacetamides as TSPO ligands. These compounds exhibit strong affinity and selectivity. Among them, the N-(2,5-dimethoxybenzyl)-N-(5-fluoro-2-phenoxyphenyl)acetamide, [11C]DAA1106 (14, Figure 6, Table 1) emerged as the first representative of this class to be radiolabeled [45,46]; 14 demonstrated 5-fold higher affinity and selectivity with respect to 1; in addition, it resulted a species-independent radiotracer, as shown by its subnanomolar affinity in both rat and monkey brains (Ki = 0.043 nM and 0.188 nM, respectively). Furthermore, although it displayed a logD value slightly higher than the ideal range (logD = 3.65), it can cross the BBB [46]. Following its intravenous administration in mice, 14 assembled in regions of the brain with high TSPO density; 14 exhibited greater affinity for microglia compared to 1 in rat models of neuroinflammation [47]. In a study focused on the quantification of TSPO receptors in AD patients, 14 demonstrated heightened uptake in multiple brain regions of patients compared to age-matched healthy individuals [97]. Additionally, 14 was employed in PET studies involving patients affected by schizophrenia to investigate the role of glial cells in the disease’s pathophysiology [98]. It was also used in studies to confirm that smokers exhibit impaired inflammatory function in comparison to non-smokers [99].
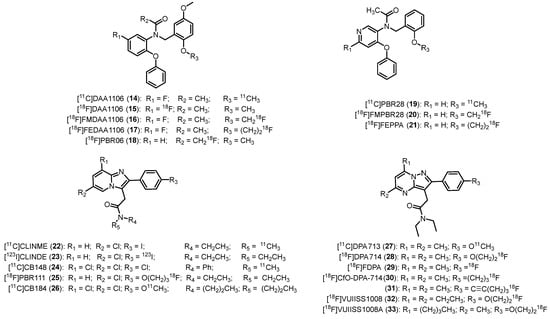
Figure 6.
Second-generation TSPO radioligands.
In parallel with the development of 14, efforts were made to synthesize the [18F]DAA1106 (15) and other 18F-labeled analogues, namely the N-(5-fluoro-2-phenoxyphenyl)-N-(2-(fluoromethoxy)-5-methoxybenzyl)acetamide, [18F]FMDAA1106 (16), and the N-(5-fluoro-2-phenoxyphenyl)-N-(2-(2-fluoroethoxy)-5-methoxybenzyl)acetamide, [18F]FEDAA1106 (17), Figure 6. Next, 16 was obtained by replacing a hydrogen atom in the methoxy group of 15 with fluorine, and 17 was obtained through the elongation of the methoxy chain by CH2 of 16 [100]. Biodistribution analysis of 15 demonstrated minimal radioactivity accumulation in bone, with no significant in vivo defluorination. Analysis of metabolites revealed that over 96% of the total radioactivity in the mouse brain, 60 min after the injection of the radiotracer, was in the form of unmetabolized 15 [101]; 16 showed almost similar affinity for TSPO as 15, but it displayed significant absorption in monkey bones [102].
17 exhibited high TSPO affinity with a Ki value of 0.078 nM, 2-fold higher than 15. Moreover, 17 displayed elevated uptake in the monkey brain, particularly in the occipital cortex, with a radioactivity level 1.5 times higher than 15 [102]. However, a PET study involving control subjects and AD patients with such radioligand indicated that microglial activation does not be involved in this neurodegenerative disease [103].
In an effort to decrease metabolism and defluorination, the labeling position was changed, leading to the development of the [18F]N-fluoroacetyl derivative, namely the N-(2,5-dimethoxybenzyl)-2-fluoro-N-(2-phenoxyphenyl)acetamide, [18F]PBR06 (18, Figure 6) [104]. It demonstrated higher binding affinity than 1, though interspecies variability existed: human tissue exhibited somewhat lower affinity (Ki = 1.0 nM) compared to monkey (Ki = 0.32 nM) and rat (Ki = 0.18 nM). Additionally, 18 displayed high lipophilicity (logD = 4.05) and over 90% of total uptake of specific binding in monkey brains. Human biodistribution analysis showed minimal defluorination with substantial radioactivity observed in metabolism and excretion-related organs, like liver and gallbladder. However, 18 has been extensively studied in various neurological disease models, including Huntington’s disease and stroke-associated neuroinflammation [105,106], showing to be an effective tool for characterizing microglial neuroinflammation over time in a mouse model of stroke [106]. [18F]PBR06 (18) is under study in various clinical trials that are currently recruiting patients; some trials have already been completed, while others are active but have not yet begun recruiting (https://clinicaltrials.gov/). The conditions investigated in these trials mainly include MS, Multiple System Atrophy (MSA), PD, AD, and myocardial infarction.
Phenoxypyridinylacetamides have also been developed as azaisosters of phenoxyphenylacetamides, which should possess a higher hydrophilicity and water solubility than their quite lipophilic counterpart. [11C]PBR28 (19), N-(2-methoxybenzyl)-N-(4-phenoxypyridin-3-yl)acetamide, displayed TSPO binding affinities similar to 18 (Ki = 0.68, 0.94, and 2.5 nM for rat, monkey, and human, respectively) but a more suitable lipophilicity (logD = 3.01), improved signal-to-noise ratio, and high specific binding in the brain (Figure 6, Table 1) [27,48]. This radioligand has been also employed in several studies in human subjects to study the progression of several diseases that involved microglia activation, including AD, MS, Huntington, motor neuron disease (MND), and rheumatoid arthritis diseases [32,73]. Unfortunately, this radioligand showed high sensitivity to the SNP rs6971. It was also recently used to evaluate whether chronic alcohol caused neuroinflammation [107]; 19 binding did not differ between alcohol use disorder (AUD) patients and healthy controls (HC). Nevertheless, when separating by TSPO genotype, MAB AUD participants showed lower 19 binding than HC, while no group differences were observed in HAB [50,108].
[11C]PBR28 (19) is being examined in various clinical trials that are currently recruiting patients; some trials have already been completed, while others are active but have not yet begun recruiting (https://clinicaltrials.gov/). The conditions investigated in these trials primarily include MS, PD, post-traumatic stress disorders (PTSD) and inflammatory arthritis.
Very recently, [11C]PBR28 (19) has been exploited to investigate seasonal changes in TSPO expression [109]. Despite limitations, such as high interindividual variability, test-retest inconsistency, tracer specificity, and a relatively small sample size, results indicate that TSPO levels in the brain are unaffected by light and temperature changes in different seasons [109].
Additionally, for this class of compounds, several efforts were made to synthesize 18F-labeled PBR28 analogues (Figure 6). The fluoromethoxy derivative, named the N-(2-(fluoromethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide, [18F]FMPBR28 (20), showed an affinity measured by competition with [3H]PK11195 in a membrane of human leukocytes and a partition coefficient similar to those of PBR28 (IC50 = 8.28 ± 1.79 nM versus 8.07 ± 1.40 nM; logD = 2.85 vs. 2.82, new value determined by the authors) [110]. When directly compared in the same inflammatory rat model, 20 quickly reached the highest target-to-background ratio at early imaging time (35 min post injection vs. 85 min post injection for 19), suggesting its promising use for imaging acute neuroinflammation. When employed in a rat model of experimental autoimmune myocarditis (EAM), 20 did not show a relevant difference in terms of specific TSPO-uptake between EAM and healthy rats [111]. The fluoroethoxy derivative [18F]FEPPA (21), N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide, displayed higher affinity for TSPO with respect to 19 and 20 and suitable lipophilicity (logP = 2.99) (Figure 6, Table 1). In addition, it showed reduced metabolism and superior brain penetration in rat models [52]. When used for quantifying TSPO binding in the human brain, 21 emerged as a promising PET ligand for assessing neuroinflammation [112,113]. In details, when clinically tested, its binding was significantly higher in AD patients with respect to healthy ones in grey matter and in white matter regions [53]. In the same year, Setiawan et al. [114] evidenced high TSPO distribution volume (VT) during major depressive disorders in the grey matter areas. [18F]FEPPA was employed in several clinical trials to study several pathologies such as synucleopthies, psychosis/schizophrenia, depression and other disorders and to investigate neuroinflammation in participants with depression after the respiratory symptoms of coronavirus disease (COVID-19) have passed (https://clinicaltrials.gov/).
2.2.5. Imidazo[1,2-a]pyridines
This class of compounds was designed as an analogue of alpidem, a compound endowed with nanomolar affinity for both TSPO and CBR [115]. [11C]CLINME (22, Figure 6), the 2-(6-chloro-2-(4-iodophenyl)imidazo[1,2-a]pyridin-3-yl)-N-ethyl-N-methylacetamide was the first compound of this class to be radiolabeled with carbon-11. It was used in acute rodent neuroinflammation, showing higher contrast between the TSPO expression in lesion site and that in the intact one with respect to 1 [116].
Subsequently, [123I]CLINDE (23), N’,N’-diethyl-6-chloro-(4′-[(123)I]iodophenyl)imidazo[1,2-a]pyridine-3-acetamide (Figure 6) has been characterized as SPECT tracer of inflammation, and its permeability across the BBB into the brain and TSPO specificity were showed in different animal models [117]. [123I]23 uptake reflected astrogliosis in brain regions, including caudate putamen, corpus callosum, medium septum and olfactory tubercle [118]. Very recently, Ohshima et al. showed that [123I]CLINDE-SPECT imaging is effective for visualizing neuroinflammation and that the pattern of neuroinflammation differs according to the level of infarction severity [119].
[11C]CB148 (24, Figure 6, Table 1), the 2-(6,8-dichloro-2-(4-chlorophenyl)imidazo[1,2-a]pyridin-3-yl)-N-methyl-N-phenylacetamide represents an interesting alternative to 1, showing subnanomolar TSPO affinity (Ki = 0.20 nM) and high accumulation in TSPO rich regions of the brain, with cerebellum and olfactory bulb (OB) that showed the highest uptake of 24 [55]. Only negligible amounts of metabolites were observed in brain tissue 30 min after intravenous injection of 24.
PBR111, the (2-(6-chloro-2-(4-(3-fluoropropoxy)phenyl)imidazo[1,2-a]pyridin-3-yl)-N,N-diethylacetamide) is a metabolically stable imidazopyridineacetamide with high TSPO affinity (Ki = 3.7 nM) and selectivity and appropriate lipophilicity (LogP = 3.2) [120], rendering it suitable for imaging TSPO expression in neurodegenerative conditions [121]. [18F]PBR111 (25, Figure 6, Table 1) has undergone extensive evaluation in both preclinical experiments [56] and clinical trials to study pathologies such as AD and MS. However, it showed significant sensitivity to SNP rs6971, as confirmed in studies involving healthy individuals, where substantial differences have been observed among HABs, MABs, and LABs [122].
Furthermore, Van Camp and colleagues [123] conducted in vitro autoradiography studies and identified a substantial increase in the binding of 25 within areas of the rat brain subjected to alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) lesions in comparison to the control regions. Additionally, a heightened uptake of 25 in AMPA-lesioned rat brains compared to the uptake of 1 was observed through in vivo PET imaging.
[11C]CB184 (26) (N,N-di-n-propyl-2-[2-(4-methoxyphenyl)-6,8-dichloroimidazol[1,2-a]pyridine-3-yl] acetamide, Figure 6) represents another new candidate TSPO PET tracer endowed with subnanomolar TSPO affinity (Ki = 0.54 nM) and lower lipophilicity (logP = 2.06) with respect to 1 [124]. In mouse brain, 26 exhibited the highest uptake in regions such as the OB, cerebellum, hippocampus, and pons, with stability observed in uptake levels from 30 to 60 min post-injection. Pre-administration of 1 significantly reduced 26 uptake in all brain regions, underscoring its high binding specificity in mouse brain tissue. Metabolic analyses revealed that at 30 min post-injection, 92.7 ± 5.8% of 26 remained unchanged in the brain, and 36.2 ± 15.5% in the plasma [124].
Moreover, 26 PET imaging studies conducted in healthy volunteers revealed rapid brain uptake followed by swift clearance during a 90 min dynamic scan [125]; 26 demonstrated even distribution in gray matter, with the highest accumulation observed in the thalamus, closely followed by the cerebellar cortex and other regions.
2.2.6. Pyrazolo[1,5-a]pyrimidines
This class of compounds was designed as bioisoster of imidazopyridines. The first compounds developed were the carbon-11-labeled [11C]DPA713 (27) (N,N-diethyl-2-(2-(4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide) (Ki = 4.7 nM) [57,58] and the fluorine-18 close analogue [18F]DPA714 (28) (N,N-diethyl-2-(2-(4-(2-fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide) (Ki = 7.0 nM), Figure 6, Table 1 [59].
First, 27 showed a greater difference between healthy and diseased brain in parenchyma in an AMPA-induced rat model of neuroinflammation [58]. It possesses a better signal-to-noise ratio with respect to 1 due to its higher TSPO specific binding. Unfortunately, it suffers from SNP rs6971 sensitivity and radiometabolite accumulation in the human brain.
Next, 28 performed better than 27 in a rat model of acute neuroinflammation with the highest ratio of ipsilateral to contralateral uptake and the highest BPND [126,127]. In 2015, Lavisse and co-workers carried out a study on the pharmacological properties of 28 in the brains of monkeys, showing that [18F]DPA714 is concentrated in the hippocampus, occipital cortex, and the cerebellum [128]. Moreover, the degree of association of 28 with TSPO in the brain was approximately 73%, demonstrating the high specificity of the radioligand to the target in both normal and neurodegeneration-induced models. It has been successfully used for several purposes, such as (i) for image neuroinflammation in human models of AD and MS thanks to the enhanced uptake of [18F]DPA714 co-localized with infarct tissue [60,61]; (ii) to identify specific reactive areas of myeloid cell infiltration in a mouse model of orthotopic glioma in the tumor microenvironment [129]; and (iii) to monitor brown adipose tissue (BAT) activity in tumor-bearing mice in vivo [130]. [18F]DPA714 is under investigation in a number of clinical trials which are recruiting patients; several have been completed and a few are active but recruiting is yet to start (https://clinicaltrials.gov/). Conditions studied in these trials are primarily MS, PD, AD, tumors, myocardial infarction, and autoimmune encephalitis (AIE).
In a very recent study, Zhang et al. evidenced the potential value of 18F-DPA-714 PET tracer in supplementing magnetic resonance imaging (MRI) for AIE detection (clinical trial registration no. NCT05293405) [131].
With defluorination being the primary metabolic pathway in vivo for 28, to overcome these problems, [18F]FDPA (29), the N,N-diethyl-2-(2-(4-fluorophenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide (Figure 6, Table 1)—in which the fluorine atom was directly linked to the aromatic ring, a position that is expected to be resistant to defluorination—was developed [62]. Additionally, 29 exhibited high TSPO affinity (Ki = 1.7 nM) and appropriate lipophilicity (logD = 2.34) as well as the ability to easily cross BBB. In addition, the labeling position on the aromatic moiety imparts a higher stability with regard to in vivo metabolism [63]. In the APP/PS1 mouse model of AD, the radiotracer 29 exhibited an increase to 1.50 ± 0.13 SUV at 3 min post-injection, indicating a 1.6-fold higher uptake and slower washout when compared to age-matched control subjects. Keller et al. [132] demonstrated a substantial age-related elevation in 29 radioactivity in the brains of APP/PS1 mice. Notably, significant variations in binding between wildtype and transgenic animals in vivo at 9 months and ex vivo at 4.5 months were evidenced [132,133]. Moreover, [18F]FDPA can detect changes in TSPO expression after radiotherapy (RT) in head and neck squamous cell carcinoma (HNSCC) in mice. Although the physiological mechanisms behind this RT-induced uptake need to be further evaluated, the pharmacokinetic behavior of [18F]FDPA in HNSCC indicates this tracer to be suitable for TSPO imaging in cancer [134]. Despite these promising results, the transition of PET imaging with 29 to higher species has not been accomplished as of the current status of research.
With the same aim to overcome the metabolism problem associated to 28, a small library of novel pyrazolo[1,5-a]pyrimidines featuring a fluoroalkyl or a fluoroalkynyl moiety at the para-position of the 2-phenyl ring was developed and biologically evaluated as TSPO ligands [135]. All derivatives displayed subnanomolar affinity (Ki = 0.37–0.86 nM) and high selectivity toward TSPO. The most active compounds, which also showed less sensitivity to microsomal metabolism and higher lipophilicity with respect to 28, were radiolabeled with fluorine-18, leading to obtaining compounds 30 (N,N-diethyl-2-(2-(4-(3-fluoropropyl)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide, known as CfO-DPA-714) and 31 (N,N-diethyl-2-(2-(4-(5-fluoropent-1-yn-1-yl)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide), Figure 6. Both compounds were evaluated in vitro and in vivo for their TSPO specific binding and their potential as PET radiotracers in a rodent model of neuroinflammation. For both compounds, brain uptake and accumulation in the region affected by AMPA signaling validated their viability as PET radiotracers for in vivo applications [98].
The pyrazolopyrimidine scaffold was also subjected to optimization studies to explore the effect of the introduction of substituents with various steric bulk at the 5, 6, and 7- positions [136]. SAR analysis highlighted an enhancement in affinity achieved by substituting methyl with ethyl at the 5- and 7- positions of the pyrazolopyrimidine core. A derivative known as VUIIS1008 (2-(5,7-diethyl-2-(4-(2-fluoroethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide displayed subnanomolar affinity (Ki = 0.18 nM), representing a significant 36-fold improvement in binding affinity compared to 28, Figure 6. In addition, this substitution displayed increased lipophilicity (logP7.5 = 2.84 compared to 2.12 of 28), a property deemed suitable for in vivo imaging. For these reasons, it was radiolabeled with fluorine-18 for imaging purposes. [18F]VUIIS1008 (32) showed rapid uptake in TSPO-rich organs. In preclinical PET studies involving both healthy mice and rats with gliomas, 32 demonstrated an enhanced tumor-to-background ratio and a higher BPND in tumors with respect to 28 [137]. Overall, these results highlighted 32 as a promising PET ligand for evaluating TSPO expression in glioma.
Further elongation of the alkyl chain at 7-position by the introduction of a n-butyl group led to derivative 2-(7-butyl-2-(4-(2-fluoroethoxy)phenyl)-5-methylpyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide known as VUIIS1008A, which was radiolabeled with fluorine-18, leading to compound [18F]VUIIS1008A 33, Figure 6 [138]. Such compounds showed picomolar TSPO affinity (IC50 = 16.2 pM), 700-fold higher than 28. Unfortunately, the longer chain was also responsible for an increase in the lipophilicity of the molecule (logD = 3.7). However, compartment modeling of 33 demonstrated a greater tumor-to-background ratio, a higher tumor BP and a lower brain BPND with respect to 28 and 32, thus suggesting 33 as a better candidate for imaging tumors with low TSPO expression [139].
2.2.7. 2-Aryl-8-Oxodihydropurines
In 1999 Murata et al. published a patent reporting a series of high TSPO affinity and selectivity ligands, namely the 2-aryl-8-oxodihydropurines (AOP) [140]. The most active compound, in terms of TSPO affinity (Ki = 0.2 nM) and suitable lipophilicity (logD = 3.3), was radiolabeled with carbon-11, obtaining the [11C]AC5216 (N-benzyl-N-ethyl-2-(7-methyl-8-oxo-2-phenyl-7,8-dihydro-9H-purin-9-yl)acetamide, 34, Figure 7, Table 1) [64]. After injection of 34 into mice, a high radioactivity accumulation was observed in TSPO-rich organs, including lungs, heart, adrenal glands, OB and cerebellum. A PET study on monkey brain evidenced a relatively high uptake in the occipital cortex of 34, an area characterized by high TSPO density in the primate brain. In addition, 34 showed high stability in vivo in the mouse brain [64]. It is of note that in a study performed to visualize microglial activation prompted by amyloid lesions in mouse models of AD, 34 facilitated PET imaging of glial TSPO with notable contrast, outperforming 17 in terms of effectiveness [141]. In 2009, the research group of Yanamoto developed a TSPO PET radioligand characterized by an oxopurine core, namely [11C]DAC (N-benzyl-N-methyl-2-(7-methyl-8-oxo-2-phenyl-7,8-dihydro-9H-purin-9-yl)acetamide) (35, Figure 7) [142]. It showed very similar affinity (Ki = 0.23 nM) for TSPO but lower lipophilicity (logD = 3.0) with respect to 34. In normal mice, 35 displayed the highest radioactivity in the lung and a high level of radioactivity in the brain, evidencing its ability to cross the BBB and enter the brain. In vitro autoradiography and PET in rats subjected to kainic acid (KA)-induced lesions revealed, between 34 and 35, similar levels of brain uptake in the lesioned and nonlesioned striatum. Of note, 35 exhibited a relevant increase of TSPO binding in the lesioned striatum. In addition, displacement experiments indicated high in vivo TSPO specific binding in the injured rat brain. Consequently, 35 proved to be a valuable PET radiotracer for TSPO imaging, and due to its TSPO specific binding, it stood out as a promising new biomarker for brain injury.
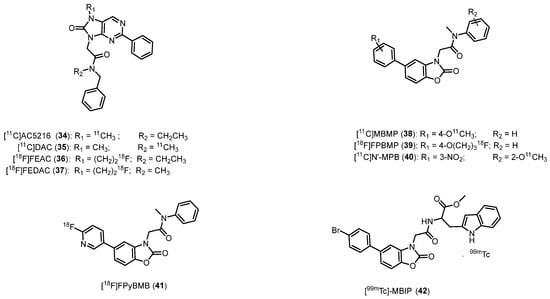
Figure 7.
Second-generation TSPO radioligands.
The same authors, in the following year, showed how high molar activity of 35 (average 4060 GBq/μmol) served as a valuable and sensitive biomarker for visualizing early infarction and characterizing the slightly elevated TSPO expression in the infarcted brain using PET [143,144].
The other two 18F-labeled oxopurines analogues—[18F]FEAC (36) (N-benzyl-N-ethyl-2-(7-(2-fluoroethyl)-8-oxo-2-phenyl-7,8-dihydro-9H-purin-9-yl)acetamide) and [18F]FEDAC (37) (N-benzyl-2-(7-(2-fluoroethyl)-8-oxo-2-phenyl-7,8-dihydro-9H-purin-9-yl)-N-methylacetamide)—were synthesized and evaluated as radioligands (Figure 7) [145,146]. Both compounds showed nanomolar affinity and good selectivity for TSPO (Ki = 0.49 and 1.34 for 36 and 37, respectively). A small-animal PET scan conducted on a rat model of neuroinflammation revealed that 36 and 37 exhibited substantial accumulation of radioactivity in the KA-lesioned striatum. Both radioligands generated signals both in vitro and in vivo, enabling the visualization of the heightened TSPO expression in the rat brain following an infarct. The observed kinetics of both 18F-radiotracers in the monkey brain, along with their tolerance for in vivo metabolism, indicated their potential utility in imaging studies of TSPO in primates [147].
Further in vitro assays and in vivo PET conducted in activated macrophage and inflamed joints of collagen-induced arthritis (CIA) suggested the potential usefulness of 37 imaging in early stages of rheumatoid arthritis (RA) [148].
2.2.8. Acetamidobenzoxazolones
Starting from 2012, a series of acetamidobenzoxazolones (ABOs) was reported as high-affinity TSPO ligands, designed as open analogues of Ro5-4864 [149,150]. The first radioligand of this class developed as a radiotracer for imaging purposes was [11C]MBMP (2-(5-(4-methoxyphenyl)-2-oxobenzo[d]oxazol-3(2H)-yl)-N-methyl-N-phenylacetamide 38, Figure 7, Table 1) [65], selected for radiolabeling because of its nanomolar TSPO affinity (Ki = 0.28 nM) and adequate lipophilicity (LogD = 3.5) [65].
Although 38 demonstrated a higher BPND compared to 1 in the same ischemic model, it may not offer a real advantage over second-generation TSPO radiotracers. Notably, approximately 35% of radiolabeled metabolite from 38 was detected in the mouse brain 60 min post-injection, whereas this proportion was less than 10% for other TSPO radioligands in rodent brains [58,123], and this issue hampered its clinical application.
In the same year, the same research group developed the fluoropropyloxy analogue of 38, the [18F]FPBMP (2-(5-(4-(3-fluoropropoxy)phenyl)-2-oxobenzo[d]oxazol-3(2H)-yl)-N-methyl-N-phenylacetamide, 39, Figure 7) [69], which exhibited high stability, high affinity (Ki = 16.7 nM), equal lipophilicity (logD = 3.5) and TSPO specific binding in an ipsilateral ischemic rat brain in vitro.
Nevertheless, similar to numerous TSPO radiotracers, both 38 and 39 exhibited slow brain kinetics, associated with a lack of substantial reductions in radioactivity throughout the PET scan. This phenomenon was in part ascribed to their lipophilicity. In order to overcome this issue, a new TSPO radiotracer, namely [11C]N′-MPB (N-(2-methoxyphenyl)-N-methyl-2-(5-(3-nitrophenyl)-2-oxobenzo[d]oxazol-3(2H)-yl)acetamide, 40, Figure 7, Table 1), characterized by the presence of a nitro group, was developed [66]; 40 displayed nanomolar TSPO affinity (Ki = 4.9 nM), reduced lipophilicity (logD = 2.08), and improved kinetic and metabolic stability than 38 in visualizing TSPO in a neuroinflammation model.
With the same aim of decreasing lipophilicity and, putatively, of obtaining more rapid brain kinetics, the same research group introduced a pyridine ring in the ABO scaffold, leading to obtaining [18F]FPyBMB (2-(5-(6-fluoropyridin-3-yl)-2-oxobenzo[d]oxazol-3(2H)-yl)-N-methyl-N-phenylacetamide, 41, Figure 7) [151]. Such compound showed nanomolar affinity for TSPO (Ki = 13.4 nM) and modest lipophilicity (logD = 1.92). The higher TSPO binding of 41 on the ipsilateral side with respect to the contralateral one, and improved brain kinetics with respect to the other ABO radiotracers was evidenced by in vitro autoradiography and PET studies of ischemic rat brain. [151].
Finally, a TSPO ligand was developed by combining ABO and indole scaffolds, leading to a compound that was radiolabeled using technetium-99m [99mTc] to be used as SPECT agent, [99mTc]MBIP (methyl (2-(5-(4-bromophenyl)-2-oxobenzo[d]oxazol-3(2H)-yl)acetyl)tryptophanate, 42, Figure 7). It displayed high stability in serum (>91%) and in solution (>94%) after 24 h. In addition, biodistribution analysis assayed on BALB/c mice evidenced significant accumulation of 42 in TSPO-rich organs and appropriate pharmacokinetic properties for a SPECT agent [152].
2.3. Third Generation TSPO Radioligands
2.3.1. 2,3,4,9-Tetrahydrocarbazole-4-carboxamides
Tetracyclic indole has been identified as a promising central core affording TSPO ligands [153]. From this pharmacophore, researchers created the tricyclic derivative N,N-diethyl-9-(2-fluoroethyl)-5-methoxy-2,3,4,9-tetrahydro-1H-carbazole-4-carboxamide [18F]GE180 (43, Figure 8, Table 1) [154]. The (S)-enantiomer exhibited higher affinity (Ki = 0.87 nM, rat; Ki = 9.2 nM, human) and binding specificity for TSPO-rich regions compared to the (R)-enantiomer (Ki = 3.87 nM, rat; Ki = 14.1 nM, human). Moreover, 43 demonstrated enantiomeric stability in vivo, making it a potential candidate for preclinical and clinical studies [67].
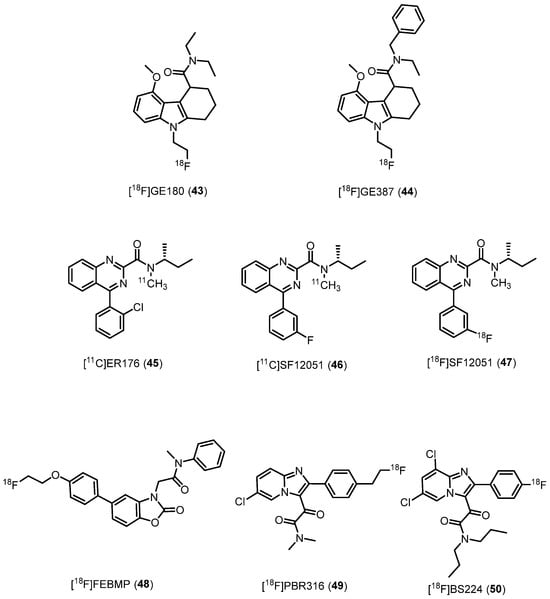
Figure 8.
Third-generation TSPO radioligands.
In preclinical models, 43 proved to be superior to 1 in imaging TSPO-associated neuroinflammation, displaying improved BPND and a longer half-life [155]. Its effectiveness was further demonstrated in studying neuroinflammation during normal aging and AD [156]. Moreover, in human studies, 43 showed low sensitivity to SNP rs6971 [157]; 43 was successfully used in patients with relapsing–remitting MS, detecting microglia/macrophage activation [158]. It also exhibited high tumor-to-background contrast in patients with glioblastoma [159]. Nonetheless, subsequent comparisons with 19 raised concerns about its brain penetration and signal-to-background ratio in diseases without a compromised BBB [160]. As a result, some research groups do not recommend its use [161]. Regardless, a number of clinical trials with 43 are active to study conditions such as AD, ischemic stroke, and glioblastoma (https://clinicaltrials.gov/).
Recently, [18F]GE387 (N-benzyl-N-ethyl-9-(2-fluoroethyl)-5-methoxy-2,3,4,9-tetrahydro-1H-carbazole-4-carboxamide, 44, Figure 8) was developed, featuring a benzyl moiety instead of an ethyl substituent as in 43. The (S)-enantiomer of 44 showed high TSPO affinity and demonstrated low sensitivity to SNP rs6971, similar to 1 [162]. PET scans in rats indicated its ability to enter the brain [163]. However, further biological evaluations of 44 are yet to be reported.
2.3.2. Quinazolines-2-carboxamides
In 2012, the research group of Castellano developed a class of TSPO ligands as azaisoster of 1, namely the quinazolines-2-carboxamides [164,165], that, due to their lower lipophilicity and higher water solubility, should display a better drug-like profile. Some of these quinazolines were chosen for their high affinity and suitable lipophilicity, radiolabeled with carbon-11 and subsequently assessed in monkey as PET radiotracers for visualizing TSPO in brain [25]. Of note, the direct azaisostere of PK11195, now dubbed [11C]ER176 (N-(sec-butyl)-4-(2-chlorophenyl)-N-methylquinazoline-2-carboxamide, 45, Figure 8, Table 1), demonstrated higher affinity (Ki = 3.1 and 9.3 nM for 45 and 1, respectively), lower lipophilicity (logD = 3.55 and 3.97 for 45 and 1, respectively), higher TSPO-specific signal (>80%), higher proportion of TSPO-specific binding in monkey brain, and enhanced suitability for quantification without the presence of challenging radiometabolites with respect to 1. Most importantly, 45 showed very low sensitivity to SNP rs6971 in vitro. In a following study [68], the same group in collaboration with the research group of V. W. Pike from the National Institute of Mental Health of Bethesda (Maryland) carried out whole-body imaging for nine human subjects from HAB, MAB, and LAB. Results evidenced a certain sensitivity of 45 to SNP rs6971 in vivo, in contrast to what was observed in in vitro studies. The reason behind this different sensitivity is unknown but, most probably, may be due to in vivo protein-protein interactions that do not occur in in vitro conditions. Nevertheless, 45 yielded sufficiently high BPND across all rs6971 genotypes, indicating that this radioligand is likely to exhibit greater sensitivity in detecting abnormalities in patients.
A straightforward and reliable HPLC separation technique has been developed for the automated production of [11C]ER176. The entire production process takes approximately 30 min and ensures effective separation from other radiochemical and chemical contaminants, allowing to obtain [11C]ER176 with high radiochemical and chemical purity, as well as excellent molar activity, meeting FDA current good manufacturing practice (CGMP) standards for clinical use [166]. [11C]ER176 is now subjected to clinical trials for neurodegenerative diseases, primarily AD (https://clinicaltrials.gov/).
From a comparison study between [11C]PBR28 19 and 45, the latter performed better showing a higher specific binding and a lower intersubject variability. Together, these results allowed the authors to conclude that such compounds are expected to hold greater statistical significance in clinical studies, necessitating a reduced number of subjects [167].
In a study aimed at quantifying TSPO in healthy humans, 45 demonstrated much greater specific binding than 1. Furthermore, 45 exhibited a distinctive characteristic by not producing radiometabolites capable of entering the brain. For all these reasons, 45 is considered the most promising third generation TSPO radioligand for clinical research developed to date [21,28,73].
Recently [26], 45 analogues were designed by replacing the 2-chloro-phenyl ring with o-, m- and p- fluoro and trifluoromethylphenyl ones. All six compounds exhibited nanomolar affinity for TSPO (Ki between 1.2 and 7.0 nM) and low sensitivity to TSPO genotype in vitro. All six compounds were radiolabeled with carbon-11 and rapidly screened in mice, proving their ability to enter the brain and to provide a sizeable TSPO-specific signal. Among them, the m-fluoro analogue [11C]SF12051 (N-(sec-butyl)-4-(3-fluorophenyl)-N-methylquinazoline-2-carboxamide, 46, Figure 8) displayed the most promising imaging behavior and for this reason was selected to be radiolabeled with fluorine-18, leading to obtaining [18F]SF12051 (47, Figure 8). Preliminary imaging study evidenced that 47 gave comparably promising results in the mouse brain to that of 46.
In vivo studies in the monkey brain of all the six 11C-labeled analogues were also performed. The two in vivo criteria taken into consideration were the BPND and the time stability of total VT, an indirect measure of lack of radiometabolite accumulation in the brain [168]. Results showed that all six 11C-labeled analogues had a good BPND and good time stability of VT. Among them, the o-fluoro, m-trifluoromethyl, and m-fluoro derivatives resulted in the three best candidates being labeled with fluorine-18.
Other third-generation ligands. [18F]FEBMP (2-(5-(4-(2-(fluoro-18F)ethoxy)phenyl)-2-oxobenzo[d]oxazol-3(2H)-yl)-N-methyl-N-phenylacetamide, 48, Figure 8, Table 1), belonging to the ABO family (see Section 2.2.8.) developed by Tiwari et al. [69], displayed high stability, TSPO nanomolar affinity (Ki = 6.6 nM), appropriate lipophilicity (logD = 3.4), and TSPO-specific binding in an ipsilateral ischemic rat brain in vivo. Most importantly, 48 showed low sensitivity to SNP rs6971, as observed in in vitro autoradiography on postmortem human brains [169]. It is still to be understood which is the suitable chemical structure for showing binding to LAB. In fact, even a slight modification in the side chain might greatly affect the binding affinity for LAB. Actually, given a certain core structure such as that of the phenoxyphenylacetamides, the ratio of Ki for LAB to HAB (RKi(L/H)) of [11C]PBR28, [18F]PBR06, and [11C]DAA1106 is greatly different, with values of 55, 17, and 4.7, respectively [19]. Thus, given that the prevalence of Thr147 (low-binding allele) is 30% in Caucasians and 25% in Africans [23], [18F]FEBMP could have a general utility in the sensitive detection of neuroinflammation. Furthermore, the authors conclude, [18F]FEBMP could serve as a core chemical structure for new PET ligands in the entire population, including in individuals with TSPO rs6971 polymorphisms.
[18F]PBR316 (2-(6-chloro-2-(4-(2-(fluoro)ethyl)phenyl)imidazo[1,2-a]pyridin-3-yl)-N,N-dimethyl-2-oxoacetamide, 49, Figure 8, Table 1) belongs to the imidazo[1,2-a]pyridine family. It showed nanomolar affinity (Ki = 6.0 nM) and high selectivity, low sensitivity to SNP rs6971 with a LAB/HAB ratio of 1.5 [70]. Biodistribution in rats of 49 showed a high uptake in organs known to express TSPO such as heart (3.9%) and adrenal glands (7.5% ID per g) at 1 h. [18F]PBR316 entered the brain and accumulated in TSPO-expressing regions and radioactivity was blocked by PK11195 and Ro5-4864 but not flumazenil. Metabolite analysis indicated that radioactivity in adrenal glands and the brain was predominantly due to the intact radiotracer. Due to these characteristics, [18F]PBR316 49 appears suitable for further biological and clinical studies.
[18F]BS224 (50, Figure 8, Table 1), 2-(-2-(4-fuorophenyl)-6,8-dichloroimidazo[1,2-a]pyridin-3-yl)-N,N-dipropylacetamide, is the newest 18F-labeled imidazo[1,2-a]pyridine TSPO radiotracer, endowed with high affinity (Ki = 0.51 nM) and selectivity, suitable lipophilicity (LogD = 2.78), and low sensitivity to SNP rs6971 (RKi(L/H) = 0.76) [71]. Docking studies confirmed that the A147T mutation did not affect the binding mode of 50, supporting the observed in vitro TSPO selectivity of 50 regardless of polymorphisms. PET imaging findings indicated that 50 exhibited a notable target-to-background ratio in the initial imaging stages of brain regions affected by abnormalities in TSPO expression. It enabled a distinct and visible representation of inflammatory lesions in both animal models (LPS-induced inflammatory and ischemic stroke rat models), devoid of interference from skull uptake [71]. Based on these results, authors asserted that [18F]BS224 50 might be a promising next-generation TSPO PET ligand to evaluate neuroinflammatory disease-relevant areas in an ample array of patients irrespective of the common rs6971 polymorphism.
Third-generation TSPO radioligands were developed to have adequately high BPND across all rs6971 genotypes for reliable quantification and to lack the brain radiometabolite accumulation that interferes with the low specific signal in LABs. However, while these novel third-generation ligands may offer improvements over second-generation ones, they still exhibit sensitivity to the A147T variant of TSPO. To make further progress, it is crucial to deepen our understanding of the differing binding requirements between WT and A147T TSPO. As 11C-ER176 is the most promising third-generation TSPO radioligand for clinical research [28,32,168] displaying high specific binding (>80%), an adequately high BPND in LABs, good time stability of total distribution volume (VT) across all genotypes, and no significant amount of radiometabolites accumulating in the brain, future research aimed at resolving the human TSPO crystal structure in complex with this third generation ligand will be crucial in understanding the different binding requirements of WT and A147T TSPO, thus speeding up the development of TSPO PET radioligands that are not affected by the rs6971 genotype [33]. Moreover, it will be essential to investigate the brain permeability and radiometabolite production of identified lead molecules to ensure that radiometabolites do not interfere with the analysis of brain signals. Other challenges to face will be with respect to pharmacokinetics, bioavailability, and safety.
3. Conclusions
TSPO is broadly spread through the whole body, with the highest concentration in tissues that synthesize steroids. A significant increase in TSPO is linked to microglial activation in response to brain injury and neuroinflammation. Consequently, heightened TSPO expression is regarded as a key indicator of neuroinflammation. In this context, TSPO radiotracers have been recognized as potent tools for imaging neuroinflammation.
Despite various challenges related to quantification, allelic binding dependence, microglial phenotype, and incomplete cellular specificity for microglia, TSPO PET imaging of neuroinflammation has remained a focal point in studies of neuroinflammatory diseases. The data collected in this review corroborate the huge efforts in developing more specific tracers during the last forty years.
TSPO PET radiotracers have been classified in three generations. First-generation TSPO radioligands include the isoquinoline carboxamide [11C](R)-PK11195 (1) and the benzodiazepine [11C]Ro5-4864 (2). Even if 1 is still today the most widely used radiotracer for in vivo imaging of TSPO, it suffers from several drawbacks, such as a high level of nonspecific binding and a signal-to-noise ratio that is not optimal, complicating its quantification. Second-generation probes belong to different structural classes endowed with better properties such as enhanced affinity, reduced lipophilicity, improved TSPO-specific signals, as well as superior imaging properties. However, these radioligands suffer from sensitivity to SNP rs6971. Finally, third-generation radiotracers overcame almost all of the limits associated with the second generation and yielded superior outcomes, particularly in preclinical and clinical investigations.
Although these advances in the discovery of less-discriminating TSPO PET ligands, the development of truly non-discriminating ligands will require a better knowledge of the differences characterizing the A147T and WT TSPO structures, and the development of structure–activity relationships through high-throughput screening or artificial intelligence methods such as deep and machine learning that, in the last years, has transformed the approach to drug discovery task by delivering added value in small-molecule drug discovery.
In conclusion, this review aims to offer a perspective on the sequential progress achieved in the development of TSPO PET probes during the past four decades. It also seeks to provide an overview of the currently accessible radioligands for neuroinflammation imaging both in research and in routine clinical settings. This information may assist (radio)chemists in the future design and development of more specific and efficient probes. Indeed, the ongoing advancement of PET radioligands for neuroinflammation imaging presents an appealing and noninvasive method, offering the potential for early diagnosis, disease progression monitoring, and contributing to the rational design and clinical evaluation of patient responses to therapeutic interventions.
Author Contributions
Conceptualization, M.V., S.S., E.B. (Elisabetta Barresi) and F.D.S.; writing—original draft preparation, E.B. (Elisabetta Barresi); writing—review and editing, M.V., S.S., S.T. and F.D.S.; visualization, E.B. (Emma Baglini), S.C., V.P., D.G. and J.C.; supervision, E.B. (Elisabetta Barresi), F.D.S. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Education, Universities and Research (MIUR) by the funding of “projects of national interest“, grant number 2022CF5T454.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Harry, G.J.; Kraft, A.D. Neuroinflammation and Microglia: Considerations and Approaches for Neurotoxicity Assessment. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Guilarte, T.R. TSPO in Diverse CNS Pathologies and Psychiatric Disease: A Critical Review and a Way Forward. Pharmacol. Ther. 2019, 194, 44–58. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator Protein (18 KDa): New Nomenclature for the Peripheral-Type Benzodiazepine Receptor Based on Its Structure and Molecular Function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Guilarte, T.R. Translocator Protein 18 KDa (TSPO): Molecular Sensor of Brain Injury and Repair. Pharmacol. Ther. 2008, 118, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Largeau, B.; Dupont, A.C.; Guilloteau, D.; Santiago-Ribeiro, M.J.; Arlicot, N. TSPO PET Imaging: From Microglial Activation to Peripheral Sterile Inflammatory Diseases? Contrast Media Mol. Imaging 2017, 2017, 6592139. [Google Scholar] [CrossRef]
- Veenman, L.; Gavish, M. The Peripheral-Type Benzodiazepine Receptor and the Cardiovascular System. Implications for Drug Development. Pharmacol. Ther. 2006, 110, 503–524. [Google Scholar] [CrossRef]
- Maaser, K.; Grabowski, P.; Oezdem, Y.; Krahn, A.; Heine, B.; Stein, H.; Buhr, H.; Zeitz, M.; Scherübl, H. Up-Regulation of the Peripheral Benzodiazepine Receptor during Human Colorectal Carcinogenesis and Tumor Spread. Clin. Cancer Res. 2005, 11, 1751–1756. [Google Scholar] [CrossRef][Green Version]
- Hauet, T.; Yao, Z.X.; Bose, H.S.; Wall, C.T.; Han, Z.; Li, W.; Hales, D.B.; Miller, W.L.; Culty, M.; Papadopoulos, V. Peripheral-Type Benzodiazepine Receptor-Mediated Action of Steroidogenic Acute Regulatory Protein on Cholesterol Entry into Leydig Cell Mitochondria. Mol. Endocrinol. 2005, 19, 540–554. [Google Scholar] [CrossRef]
- Ritsner, M.; Modai, I.; Gibel, A.; Leschiner, S.; Silver, H.; Tsinovoy, G.; Weizman, A.; Gavish, M. Decreased Platelet Peripheral-Type Benzodiazepine Receptors in Persistently Violent Schizophrenia Patients. J. Psychiatr. Res. 2003, 37, 549–556. [Google Scholar] [CrossRef]
- Jaremko, Ł.; Jaremko, M.; Giller, K.; Becker, S.; Zweckstetter, M. Structure of the Mitochondrial Translocator Protein in Complex with a Diagnostic Ligand. Science 2014, 343, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, M.; Jaremko, Ł.; Jaipuria, G.; Becker, S.; Zweckstetter, M. Structure of the Mammalian TSPO/PBR Protein. Biochem. Soc. Trans. 2015, 43, 566–571. [Google Scholar] [CrossRef]
- Jaremko, Ł.; Jaremko, M.; Giller, K.; Becker, S.; Zweckstetter, M. Conformational Flexibility in the Transmembrane Protein TSPO. Chem. A Eur. J. 2015, 21, 16555–16563. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, J.; Zheng, Y.; Garavito, R.M.; Ferguson-Miller, S. Crystal Structures of Translocator Protein (TSPO) and Mutant Mimic of a Human Polymorphism. Science 2015, 347, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kalathur, R.C.; Liu, Q.; Kloss, B.; Bruni, R.; Ginter, C.; Kloppmann, E.; Rost, B.; Hendrickson, W.A. Structure and Activity of Tryptophan-Rich TSPO Proteins. Science 2015, 347, 551–555. [Google Scholar] [CrossRef]
- Nutma, E.; Ceyzériat, K.; Amor, S.; Tsartsalis, S.; Millet, P.; Owen, D.R.; Papadopoulos, V.; Tournier, B.B. Cellular Sources of TSPO Expression in Healthy and Diseased Brain. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 146–163. [Google Scholar] [CrossRef]
- Wu, X.; Gallo, K.A. The 18-KDa Translocator Protein (TSPO) Disrupts Mammary Epithelial Morphogenesis and Promotes Breast Cancer Cell Migration. PLoS ONE 2013, 8, e71258. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Yang, W. Inhibiting the Proliferation of Colorectal Cancer Cells by Reducing TSPO/VDAC Expression. Iran. J. Public Health 2023, 52, 1378–1389. [Google Scholar] [CrossRef]
- Ammer, L.M.; Vollmann-Zwerenz, A.; Ruf, V.; Wetzel, C.H.; Riemenschneider, M.J.; Albert, N.L.; Beckhove, P.; Hau, P. The Role of Translocator Protein TSPO in Hallmarks of Glioblastoma. Cancers 2020, 12, 2973. [Google Scholar] [CrossRef]
- Zhang, D.; Man, D.; Lu, J.; Jiang, Y.; Ding, B.; Su, R.; Tong, R.; Chen, J.; Yang, B.; Zheng, S.; et al. Mitochondrial TSPO Promotes Hepatocellular Carcinoma Progression through Ferroptosis Inhibition and Immune Evasion. Adv. Sci. 2023, 10, 2206669. [Google Scholar] [CrossRef]
- Meyer, J.H.; Cervenka, S.; Kim, M.J.; Kreisl, W.C.; Henter, I.D.; Innis, R.B. Neuroinflammation in Psychiatric Disorders: PET Imaging and Promising New Targets. Lancet Psychiatry 2020, 7, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, H.Y. In Vivo Molecular Imaging in Preclinical Research. Lab. Anim. Res. 2022, 38, 31. [Google Scholar] [CrossRef]
- Owen, D.R.; Yeo, A.J.; Gunn, R.N.; Song, K.; Wadsworth, G.; Lewis, A.; Rhodes, C.; Pulford, D.J.; Bennacef, I.; Parker, C.A.; et al. An 18-KDa Translocator Protein (TSPO) Polymorphism Explains Differences in Binding Affinity of the PET Radioligand PBR28. J. Cereb. Blood Flow Metab. 2012, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Guo, Q.; Rabiner, E.A.; Gunn, R.N. The Impact of the Rs6971 Polymorphism in TSPO for Quantification and Study Design. Clin. Transl. Imaging 2015, 3, 417–422. [Google Scholar] [CrossRef]
- Zanotti-Fregonara, P.; Zhang, Y.; Jenko, K.J.; Gladding, R.L.; Zoghbi, S.S.; Fujita, M.; Sbardella, G.; Castellano, S.; Taliani, S.; Martini, C.; et al. Synthesis and Evaluation of Translocator 18 KDa Protein (TSPO) Positron Emission Tomography (PET) Radioligands with Low Binding Sensitivity to Human Single Nucleotide Polymorphism Rs6971. ACS Chem. Neurosci. 2014, 5, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Siméon, F.G.; Lee, J.H.; Morse, C.L.; Stukes, I.; Zoghbi, S.S.; Manly, L.S.; Liow, J.S.; Gladding, R.L.; Dick, R.M.; Yan, X.; et al. Synthesis and Screening in Mice of Fluorine-Containing PET Radioligands for TSPO: Discovery of a Promising 18F-Labeled Ligand. J. Med. Chem. 2021, 64, 16731–16745. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Fujita, M.; Fujimura, Y.; Kimura, N.; Jenko, K.J.; Kannan, P.; Hong, J.; Morse, C.L.; Zoghbi, S.S.; Gladding, R.L.; et al. Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, Two Radioligands for Translocator Protein (18 KDa) in Human and Monkey: Implications for Positron Emission Tomographic Imaging of This Inflammation Biomarker. Neuroimage 2010, 49, 2924–2932. [Google Scholar] [CrossRef]
- Fujita, M.; Kobayashi, M.; Ikawa, M.; Gunn, R.N.; Rabiner, E.A.; Owen, D.R.; Zoghbi, S.S.; Haskali, M.B.; Telu, S.; Pike, V.W.; et al. Comparison of Four 11C-Labeled PET Ligands to Quantify Translocator Protein 18 KDa (TSPO) in Human Brain: (R)-PK11195, PBR28, DPA-713, and ER176—Based on Recent Publications That Measured Specific-to-Non-Displaceable Ratios. EJNMMI Res. 2017, 7, 84. [Google Scholar] [CrossRef]
- Camsonne, R.; Crouzel, C.; Comar, D.; Mazière, M.; Prenant, C.; Sastre, J.; Moulin, M.; Syrota, A. Synthesis of N-(11C) Methyl, N-(Methyl-1 Propyl), (Chloro-2 Phenyl)-1 Isoquinoleine Carboxamide-3 (PK 11195): A New Ligand for Peripheral Benzodiazepine Receptors. J. Label. Compd. Radiopharm. 1984, 21, 985–991. [Google Scholar] [CrossRef]
- Wimberley, C.; Lavisse, S.; Hillmer, A.; Hinz, R.; Turkheimer, F.; Zanotti-Fregonara, P. Kinetic Modeling and Parameter Estimation of TSPO PET Imaging in the Human Brain. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 246–256. [Google Scholar] [CrossRef]
- Yaqub, M.; Van Berckel, B.N.M.; Schuitemaker, A.; Hinz, R.; Turkheimer, F.E.; Tomasi, G.; Lammertsma, A.A.; Boellaard, R. Optimization of Supervised Cluster Analysis for Extracting Reference Tissue Input Curves in (R)-[11C]PK11195 Brain PET Studies. J. Cereb. Blood Flow Metab. 2012, 32, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Viviano, M.; Barresi, E.; Siméon, F.G.; Costa, B.; Taliani, S.; Da Settimo, F.; Pike, V.W.; Castellano, S. Essential Principles and Recent Progress in the Development of TSPO PET Ligands for Neuroinflammation Imaging. Curr. Med. Chem. 2022, 29, 4862–4890. [Google Scholar] [CrossRef]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent Developments in TSPO PET Imaging as a Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 3161. [Google Scholar] [CrossRef] [PubMed]
- Pike, V.W. PET Radiotracers: Crossing the Blood-Brain Barrier and Surviving Metabolism. Trends Pharmacol. Sci. 2009, 30, 431–440. [Google Scholar] [CrossRef]
- Narayanaswami, V.; Dahl, K.; Bernard-Gauthier, V.; Josephson, L.; Cumming, P.; Vasdev, N. Emerging PET Radiotracers and Targets for Imaging of Neuroinflammation in Neurodegenerative Diseases: Outlook Beyond TSPO. Mol. Imaging 2018, 17, 1536012118792317. [Google Scholar] [CrossRef]
- Mintun, M.A.; Raichle, M.E.; Kilbourn, M.R.; Wooten, G.F.; Welch, M.J. A Quantitative Model for the In Vivo Assessment of Drug Binding Sites with Positron Emission Tomography. Ann. Neurol. 1984, 15, 217–227. [Google Scholar] [CrossRef]
- Saxena, P.; Mahmood, T.; Dixit, M.; Gambhir, S.; Ahsan, F. An Exposition of 11C and 18F Radiotracers Synthesis for PET Imaging. Curr. Radiopharm. 2020, 14, 92–100. [Google Scholar] [CrossRef]
- Miller, P.W.; Long, N.J.; Vilar, R.; Gee, A.D. Synthesis Of11C, 18F, 15O, and 13N Radiolabels for Positron Emission Tomography. Angew. Chem. Int. Ed. 2008, 47, 8998–9033. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Hume, S.P.; Pike, V.W.; Ashworth, S.; McDermott, J. Synthesis of the Enantiomers of [N-Methyl-11C]PK 11195 and Comparison of Their Behaviours as Radioligands for PK Binding Sites in Rats. Nucl. Med. Biol. 1994, 21, 573–581. [Google Scholar] [CrossRef]
- Junck, L.; Olson, J.M.M.; Ciliax, B.J.; Koeppe, R.A.; Watkins, G.L.; Jewett, D.M.; McKeever, P.E.; Wieland, D.M.; Kilbourn, M.R.; Starosta-Rubinstein, S.; et al. PET Imaging of Human Gliomas with Ligands for the Peripheral Benzodiazepine Binding Site. Ann. Neurol. 1989, 26, 752–758. [Google Scholar] [CrossRef]
- Matarrese, M.; Moresco, R.M.; Cappelli, A.; Anzini, M.; Vomero, S.; Simonelli, P.; Verza, E.; Magni, F.; Sudati, F.; Soloviev, D.; et al. Labeling and Evaluation of N-[11C]Methylated Quinoline-2-Carboxamides as Potential Radioligands for Visualization of Peripheral Benzodiazepine Receptors. J. Med. Chem. 2001, 44, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Matarrese, M.; Moresco, R.M.; Valenti, S.; Anzini, M.; Vomero, S.; Turolla, E.A.; Belloli, S.; Simonelli, P.; Filannino, M.A.; et al. Synthesis, Labeling, and Biological Evaluation of Halogenated 2-Quinolinecarboxamides as Potential Radioligands for the Visualization of Peripheral Benzodiazepine Receptors. Bioorganic Med. Chem. 2006, 14, 4055–4066. [Google Scholar] [CrossRef] [PubMed]
- Pike, V.W.; Taliani, S.; Lohith, T.G.; Owen, D.R.J.; Pugliesi, I.; Da Pozzo, E.; Hong, J.; Zoghbi, S.S.; Gunn, R.N.; Parker, C.A.; et al. Evaluation of Novel N 1-Methyl-2-Phenylindol-3-Ylglyoxylamides as a New Chemotype of 18 KDa Translocator Protein-Selective Ligand Suitable for the Development of Positron Emission Tomography Radioligands. J. Med. Chem. 2011, 54, 366–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferzaz, B.; Brault, E.; Bourliaud, G.; Robert, J.P.; Poughon, G.; Claustre, Y.; Marguet, F.; Liere, P.; Schumacher, M.; Nowicki, J.P.; et al. SSR180575 (7-Chloro-N,N,5-Trimethyl-4-Oxo-3-Phenyl-3,5-Dihydro-4H-Pyridazino[4,5-b] Indole-1-Acetamide), a Peripheral Benzodiazepine Receptor Ligand, Promotes Neuronal Survival and Repair. J. Pharmacol. Exp. Ther. 2002, 301, 1067–1078. [Google Scholar] [CrossRef]
- Okuyama, S.; Chaki, S.; Yoshikawa, R.; Ogawa, S.I.; Suzuki, Y.; Okubo, T.; Nakazato, A.; Nagamine, M.; Tomisawa, K. Neuropharmacological Profile of Peripheral Benzodiazepine Receptor Agonists, DAA1097 and DAA1106. Life Sci. 1999, 64, 1455–1464. [Google Scholar] [CrossRef]
- Zhang, M.R.; Kida, T.; Noguchi, J.; Furutsuka, K.; Maeda, J.; Suhara, T.; Suzuki, K. [11C]DAA1106: Radiosynthesis and In Vivo Binding to Peripheral Benzodiazepine Receptors in Mouse Brain. Nucl. Med. Biol. 2003, 30, 513–519. [Google Scholar] [CrossRef]
- Venneti, S.; Lopresti, B.J.; Wang, G.; Slagel, S.L.; Mason, N.S.; Mathis, C.A.; Fischer, M.L.; Larsen, N.J.; Mortimer, A.D.; Hastings, T.G.; et al. A Comparison of the High-Affinity Peripheral Benzodiazepine Receptor Ligands DAA1106 and (R)-PK11195 in Rat Models of Neuroinflammation: Implications for PET Imaging of Microglial Activation. J. Neurochem. 2007, 102, 2118–2131. [Google Scholar] [CrossRef]
- Briard, E.; Zoghbi, S.S.; Imaizumi, M.; Gourley, J.P.; Shetty, H.U.; Hong, J.; Cropley, V.; Fujita, M.; Innis, R.B.; Pike, V.W. Synthesis and Evaluation in Monkey of Two Sensitive 11C-Labeled Aryloxyanilide Ligands for Imaging Brain Peripheral Benzodiazepine Receptors In Vivo. J. Med. Chem. 2008, 51, 17–30. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Lyoo, C.H.; McGwier, M.; Snow, J.; Jenko, K.J.; Kimura, N.; Corona, W.; Morse, C.L.; Zoghbi, S.S.; Pike, V.W.; et al. In Vivo Radioligand Binding to Translocator Protein Correlates with Severity of Alzheimer’s Disease. Brain 2013, 136, 2228–2238. [Google Scholar] [CrossRef]
- Imaizumi, M.; Briard, E.; Zoghbi, S.S.; Gourley, J.P.; Hong, J.; Fujimura, Y.; Pike, V.W.; Innis, R.B.; Fujita, M. Brain and Whole-Body Imaging in Nonhuman Primates of [11C]PBR28, a Promising PET Radioligand for Peripheral Benzodiazepine Receptors. Neuroimage 2008, 39, 1289–1298. [Google Scholar] [CrossRef]
- Hirvonen, J.; Kreisl, W.C.; Fujita, M.; Dustin, I.; Khan, O.; Appel, S.; Zhang, Y.; Morse, C.; Pike, V.W.; Innis, R.B.; et al. Increased In Vivo Expression of an Inflammatory Marker in Temporal Lobe Epilepsy. J. Nucl. Med. 2012, 53, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.A.; Garcia, A.; Parkes, J.; McCormick, P.; Stephenson, K.A.; Houle, S.; Vasdev, N. Radiosynthesis and Initial Evaluation of [18F]-FEPPA for PET Imaging of Peripheral Benzodiazepine Receptors. Nucl. Med. Biol. 2008, 35, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Suridjan, I.; Pollock, B.G.; Verhoeff, N.P.L.G.; Voineskos, A.N.; Chow, T.; Rusjan, P.M.; Lobaugh, N.J.; Houle, S.; Mulsant, B.H.; Mizrahi, R. In-Vivo Imaging of Grey and White Matter Neuroinflammation in Alzheimer’s Disease: A Positron Emission Tomography Study with a Novel Radioligand, “18 F”-FEPPA. Mol. Psychiatry 2015, 20, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sagar, A.P.; Kéri, S. Translocator Protein (18 KDa TSPO) Binding, a Marker of Microglia, Is Reduced in Major Depression during Cognitive-Behavioral Therapy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 83, 1–7. [Google Scholar] [CrossRef]
- Sekimata, K.; Hatano, K.; Ogawa, M.; Abe, J.; Magata, Y.; Biggio, G.; Serra, M.; Laquintana, V.; Denora, N.; Latrofa, A.; et al. Radiosynthesis and In Vivo Evaluation of N-[11C]Methylated Imidazopyridineacetamides as PET Tracers for Peripheral Benzodiazepine Receptors. Nucl. Med. Biol. 2008, 35, 327–334. [Google Scholar] [CrossRef]
- Colasanti, A.; Guo, Q.; Muhlert, N.; Giannetti, P.; Onega, M.; Newbould, R.D.; Ciccarelli, O.; Rison, S.; Thomas, C.; Nicholas, R.; et al. In Vivo Assessment of Brain White Matter Inflammation in Multiple Sclerosis with 18F-PBR111 PET. J. Nucl. Med. 2014, 55, 1112–1118. [Google Scholar] [CrossRef]
- James, M.L.; Fulton, R.R.; Henderson, D.J.; Eberl, S.; Meikle, S.R.; Thomson, S.; Allan, R.D.; Dolle, F.; Fulham, M.J.; Kassiou, M. Synthesis and In Vivo Evaluation of a Novel Peripheral Benzodiazepine Receptor PET Radioligand. Bioorganic Med. Chem. 2005, 13, 6188–6194. [Google Scholar] [CrossRef]
- Boutin, H.; Chauveau, F.; Thominiaux, C.; Grégoire, M.C.; James, M.L.; Trebossen, R.; Hantraye, P.; Dollé, F.; Tavitian, B.; Kassiou, M. 11C-DPA-713: A Novel Peripheral Benzodiazepine Receptor PET Ligand for In Vivo Imaging of Neuroinflammation. J. Nucl. Med. 2007, 48, 573–581. [Google Scholar] [CrossRef]
- James, M.L.; Fulton, R.R.; Vercoullie, J.; Henderson, D.J.; Garreau, L.; Chalon, S.; Dolle, F.; Selleri, S.; Guilloteau, D.; Kassiou, M. DPA-714, a New Translocator Protein-Specific Ligand: Synthesis, Radiofluorination, and Pharmacologic Characterization. J. Nucl. Med. 2008, 49, 814–822. [Google Scholar] [CrossRef]
- Golla, S.S.V.; Boellaard, R.; Oikonen, V.; Hoffmann, A.; Van Berckel, B.N.M.; Windhorst, A.D.; Virta, J.; Haaparanta-Solin, M.; Luoto, P.; Savisto, N.; et al. Quantification of [18 F]DPA-714 Binding in the Human Brain: Initial Studies in Healthy Controls and Alzheimer’s Disease Patients. J. Cereb. Blood Flow Metab. 2015, 35, 766–772. [Google Scholar] [CrossRef]
- Hagens, M.H.J.; Golla, S.V.; Wijburg, M.T.; Yaqub, M.; Heijtel, D.; Steenwijk, M.D.; Schober, P.; Brevé, J.J.P.; Schuit, R.C.; Reekie, T.A.; et al. In Vivo Assessment of Neuroinflammation in Progressive Multiple Sclerosis: A Proof of Concept Study with [18F]DPA714 PET. J. Neuroinflamm. 2018, 15, 314. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, R.; Fujinaga, M.; Yang, J.J.; Zhang, Y.; Hatori, A.; Kumata, K.; Yang, J.J.; Vasdev, N.; Du, Y.; et al. A Facile Radiolabeling of [18F]FDPA via Spirocyclic Iodonium Ylides: Preliminary PET Imaging Studies in Preclinical Models of Neuroinflammation. J. Med. Chem. 2017, 60, 5222–5227. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Krzyczmonik, A.; Forsback, S.; Picón, F.R.L.; Kirjavainen, A.K.; Takkinen, J.; Rajander, J.; Cacheux, F.; Damont, A.; Dollé, F.; et al. Radiosynthesis and Preclinical Evaluation of [18F]F-DPA, A Novel Pyrazolo[1,5a]Pyrimidine Acetamide TSPO Radioligand, in Healthy Sprague Dawley Rats. Mol. Imaging Biol. 2017, 19, 736–745. [Google Scholar] [CrossRef]
- Zhang, M.R.; Kumata, K.; Maeda, J.; Yanamoto, K.; Hatori, A.; Okada, M.; Higuchi, M.; Obayashi, S.; Suhara, T.; Suzuki, K. 11C-AC-5216: A Novel PET Ligand for Peripheral Benzodiazepine Receptors in the Primate Brain. J. Nucl. Med. 2007, 48, 1853–1861. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Yui, J.; Fujinaga, M.; Kumata, K.; Shimoda, Y.; Yamasaki, T.; Xie, L.; Hatori, A.; Maeda, J.; Nengaki, N.; et al. Characterization of a Novel Acetamidobenzoxazolone-Based PET Ligand for Translocator Protein (18 KDa) Imaging of Neuroinflammation in the Brain. J. Neurochem. 2014, 129, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Zhang, Y.; Yamasaki, T.; Kumari, N.; Fujinaga, M.; Mori, W.; Hatori, A.; Nengaki, N.; Mishra, A.K.; Zhang, H.; et al. Radiosynthesis and Evaluation of Acetamidobenzoxazolone Based Radioligand [11C]N′-MPB for Visualization of 18 KDa TSPO in Brain. New J. Chem. 2020, 44, 7912–7922. [Google Scholar] [CrossRef]
- Chau, W.F.; Black, A.M.A.; Clarke, A.; Durrant, C.; Gausemel, I.; Khan, I.; Mantzilas, D.; Oulie, I.; Rogstad, A.; Trigg, W.; et al. Exploration of the Impact of Stereochemistry on the Identification of the Novel Translocator Protein PET Imaging Agent [18F]GE-180. Nucl. Med. Biol. 2015, 42, 711–719. [Google Scholar] [CrossRef]
- Ikawa, M.; Lohith, T.G.; Shrestha, S.; Telu, S.; Zoghbi, S.S.; Castellano, S.; Taliani, S.; Da Settimo, F.; Fujita, M.; Pike, V.W.; et al. 11C-ER176, a Radioligand for 18-KDa Translocator Protein, Has Adequate Sensitivity to Robustly Image All Three Affinity Genotypes in Human Brain. J. Nucl. Med. 2017, 58, 320–325. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Fujinaga, M.; Yui, J.; Yamasaki, T.; Xie, L.; Kumata, K.; Mishra, A.K.; Shimoda, Y.; Hatori, A.; Ji, B.; et al. Synthesis and Evaluation of New 18F-Labelled Acetamidobenzoxazolone-Based Radioligands for Imaging of the Translocator Protein (18 KDa, TSPO) in the Brain. Org. Biomol. Chem. 2014, 12, 9621–9630. [Google Scholar] [CrossRef]
- Mattner, F.; Katsifis, A.; Bourdier, T.; Loc’h, C.; Berghofer, P.; Fookes, C.; Hung, T.T.; Jackson, T.; Henderson, D.; Pham, T.; et al. Synthesis and Pharmacological Evaluation of [18F]PBR316: A Novel PET Ligand Targeting the Translocator Protein 18 KDa (TSPO) with Low Binding Sensitivity to Human Single Nucleotide Polymorphism Rs6971. RSC Med. Chem. 2021, 12, 1207–1221. [Google Scholar] [CrossRef]
- Lee, S.H.; Denora, N.; Laquintana, V.; Mangiatordi, G.F.; Lopedota, A.; Lopalco, A.; Cutrignelli, A.; Franco, M.; Delre, P.; Song, I.H.; et al. Radiosynthesis and Characterization of [18F]BS224: A next-Generation TSPO PET Ligand Insensitive to the Rs6971 Polymorphism. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Farges, R.; Joseph-Liauzun, E.; Shire, D.; Caput, D.; Le Fur, G.; Ferrara, P. Site-Directed Mutagenesis of the Peripheral Benzodiazepine Receptor: Identification of Amino Acids Implicated in the Binding Site of Ro5-4864. Mol. Pharmacol. 1994, 46, 1160–1167. [Google Scholar]
- Chauveau, F.; Becker, G.; Boutin, H. Have (R)-[11C]PK11195 Challengers Fulfilled the Promise? A Scoping Review of Clinical TSPO PET Studies. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, A.; Brooks, D.J.; Kennedy, A.M.; Gunn, R.N.; Myers, R.; Turkheimer, F.E.; Jones, T.; Banati, R.B. In-Vivo Measurement of Activated Microglia in Dementia. Lancet 2001, 358, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Parbo, P.; Ismail, R.; Hansen, K.V.; Amidi, A.; Mårup, F.H.; Gottrup, H.; Brændgaard, H.; Eriksson, B.O.; Eskildsen, S.F.; Lund, T.E.; et al. Brain Inflammation Accompanies Amyloid in the Majority of Mild Cognitive Impairment Cases Due to Alzheimer’s Disease. Brain 2017, 140, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Parbo, P.; Ismail, R.; Sommerauer, M.; Stokholm, M.G.; Hansen, A.K.; Hansen, K.V.; Amidi, A.; Schaldemose, J.L.; Gottrup, H.; Brændgaard, H.; et al. Does Inflammation Precede Tau Aggregation in Early Alzheimer’s Disease? A PET Study. Neurobiol. Dis. 2018, 117, 211–216. [Google Scholar] [CrossRef]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The Relationships between Neuroinflammation, Beta-Amyloid and Tau Deposition in Alzheimer’s Disease: A Longitudinal PET Study. J. Neuroinflamm. 2020, 17, 151. [Google Scholar] [CrossRef]
- Parbo, P.; Madsen, L.S.; Ismail, R.; Zetterberg, H.; Blennow, K.; Eskildsen, S.F.; Vorup-Jensen, T.; Brooks, D.J. Low Plasma Neurofilament Light Levels Associated with Raised Cortical Microglial Activation Suggest Inflammation Acts to Protect Prodromal Alzheimer’s Disease. Alzheimer’s Res. Ther. 2020, 12, 3. [Google Scholar] [CrossRef]
- Victor, W.P. Considerations in the Development of Reversibly Binding PET Radioligands for Brain Imaging. Curr. Med. Chem. 2016, 23, 1818–1869. [Google Scholar] [CrossRef]
- Chauveau, F.; Boutin, H.; Van Camp, N.; Dollé, F.; Tavitian, B. Nuclear Imaging of Neuroinflammation: A Comprehensive Review of [11C]PK11195 Challengers. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2304–2319. [Google Scholar] [CrossRef]
- Schuitemaker, A.; van Berckel, B.N.M.; Kropholler, M.A.; Veltman, D.J.; Scheltens, P.; Jonker, C.; Lammertsma, A.A.; Boellaard, R. SPM Analysis of Parametric (R)-[11C]PK11195 Binding Images: Plasma Input versus Reference Tissue Parametric Methods. Neuroimage 2007, 35, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Parente, A.; Feltes, P.K.; Garcia, D.V.; Sijbesma, J.W.A.; Jeckel, C.M.M.; Dierckx, R.A.J.O.; De Vries, E.F.J.; Doorduin, J. Pharmacokinetic Analysis of 11C-PBR28 in the Rat Model of Herpes Encephalitis: Comparison with (R)-11C-PK11195. J. Nucl. Med. 2016, 57, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.J.; Gunn, R.N.; Rabiner, E.A.; Bennacef, I.; Fujita, M.; Kreisl, W.C.; Innis, R.B.; Pike, V.W.; Reynolds, R.; Matthews, P.M.; et al. Mixed-Affinity Binding in Humans with 18-KDa Translocator Protein Ligands. J. Nucl. Med. 2011, 52, 24–32. [Google Scholar] [CrossRef]
- Pascali, C.; Luthra, S.K.; Pike, V.W.; Price, G.W.; Ahier, R.G.; Hume, S.P.; Myers, R.; Manjil, L.; Cremer, J.E. The Radiosynthesis of [18F]PK 14105 as an Alternative Radioligand for Peripheral Type Benzodiazepine Binding Sites. Int. J. Radiat. Appl. Instrum. Part A Appl. Radiat. Isot. 1990, 41, 477–482. [Google Scholar] [CrossRef]
- Gildersleeve, D.L.; Van Dort, M.E.; Johnson, J.W.; Sherman, P.S.; Wieland, D.M. Synthesis and Evaluation of [123I]-Iodo-PK11195 for Mapping Peripheral-Type Benzodiazepine Receptors (Ω3) in Heart. Nucl. Med. Biol. 1996, 23, 23–28. [Google Scholar] [CrossRef]
- Cappelli, A.; Anzini, M.; Vomero, S.; De Benedetti, P.G.; Menziani, M.C.; Giorgi, G.; Manzoni, C. Mapping the Peripheral Benzodiazepine Receptor Binding Site by Conformationally Restrained Derivatives of 1-(2-Chlorophenyl)-N-Methyl-N-(1- Methylpropyl)-3-Isoquinolinecarboxamide (PK11195). J. Med. Chem. 1997, 40, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Belloli, S.; Moresco, R.M.; Matarrese, M.; Biella, G.; Sanvito, F.; Simonelli, P.; Turolla, E.; Olivieri, S.; Cappelli, A.; Vomero, S.; et al. Evaluation of Three Quinoline-Carboxamide Derivatives as Potential Radioligands for the In Vivo Pet Imaging of Neurodegeneration. Neurochem. Int. 2004, 44, 433–440. [Google Scholar] [CrossRef]
- Di Grigoli, G.; Monterisi, C.; Belloli, S.; Masiello, V.; Politi, L.S.; Valenti, S.; Paolino, M.; Anzini, M.; Matarrese, M.; Cappelli, A.; et al. Radiosynthesis and Preliminary Biological Evaluation of [18F]VC701, a Radioligand for Translocator Protein. Mol. Imaging 2015, 14, 7290-2015. [Google Scholar] [CrossRef] [PubMed]
- Belloli, S.; Zanotti, L.; Murtaj, V.; Mazzon, C.; Di Grigoli, G.; Monterisi, C.; Masiello, V.; Iaccarino, L.; Cappelli, A.; Poliani, P.L.; et al. 18 F-VC701-PET and MRI in the In Vivo Neuroinflammation Assessment of a Mouse Model of Multiple Sclerosis. J. Neuroinflamm. 2018, 15, 33. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Ma, D.; Brewer, J.; Sun, S.; Costa, E.; Romeo, E.; Guidotti, A. Chemistry, Binding Affinities, and Behavioral Properties of a New Class of “Antineophobic” Mitochondrial DBI Receptor Complex (MDRC) Ligands. J. Med. Chem. 1993, 36, 2908–2920. [Google Scholar] [CrossRef]
- Primofiore, G.; Da Settimo, F.; Taliani, S.; Simorini, F.; Patrizi, M.P.; Novellino, E.; Greco, G.; Abignente, E.; Costa, B.; Chelli, B.; et al. N,N-Dialkyl-2-Phenylindol-3-Ylglyoxylamides. A New Class of Potent and Selective Ligands at the Peripheral Renzodiazepine Receptor. J. Med. Chem. 2004, 47, 1852–1855. [Google Scholar] [CrossRef] [PubMed]
- Da Settimo, F.; Simorini, F.; Taliani, S.; La Motta, C.; Marini, A.M.; Salerno, S.; Bellandi, M.; Novellino, E.; Greco, G.; Cosimelli, B.; et al. Anxiolytic-like Effects of N,N-Dialkyl-2-Phenylindol-3-Ylglyoxylamides by Modulation of Translocator Protein Promoting Neurosteroid Biosynthesis. J. Med. Chem. 2008, 51, 5798–5806. [Google Scholar] [CrossRef]
- Barresi, E.; Bruno, A.; Taliani, S.; Cosconati, S.; Da Pozzo, E.; Salerno, S.; Simorini, F.; Daniele, S.; Giacomelli, C.; Marini, A.M.; et al. Deepening the Topology of the Translocator Protein Binding Site by Novel N,N-Dialkyl-2-Arylindol-3-Ylglyoxylamides. J. Med. Chem. 2015, 58, 6081–6092. [Google Scholar] [CrossRef] [PubMed]
- Thominiaux, C.; Damont, A.; Kuhnast, B.; Demphel, S.; Le Helleix, S.; Boisnard, S.; Rivron, L.; Chauveau, F.; Boutin, H.; Van Camp, N.; et al. Radiosynthesis of 7-Chloro-N,N-Dimethyl-5-[11C]Methyl-4-Oxo-3- Phenyl-3,5-Dihydro-4H-Pyridazino[4,5-b]Indole-1-Acetamide, [11C] SSR180575, a Novel Radioligand for Imaging the TSPO (Peripheral Benzodiazepine Receptor) with PET. J. Label. Compd. Radiopharm. 2010, 53, 767–773. [Google Scholar] [CrossRef]
- Chauveau, F.; Boutin, H.; Van Camp, N.; Thominiaux, C.; Hantraye, P.; Rivron, L.; Marguet, F.; Castel, M.N.; Rooney, T.; Benavides, J.; et al. In Vivo Imaging of Neuroinflammation in the Rodent Brain with [ 11C]SSR180575, a Novel Indoleacetamide Radioligand of the Translocator Protein (18 KDa). Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 509–514. [Google Scholar] [CrossRef]
- Cheung, Y.Y.; Nickels, M.L.; Tang, D.; Buck, J.R.; Manning, H.C. Facile Synthesis of SSR180575 and Discovery of 7-Chloro-N,N,5-Trimethyl-4-Oxo-3(6-[18F]Fluoropyridin-2-Yl)-3,5-Dihydro-4H-Pyridazino[4,5-b]Indole-1-Acetamide, a Potent Pyridazinoindole Ligand for PET Imaging of TSPO in Cancer. Bioorganic Med. Chem. Lett. 2014, 24, 4466–4471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yasuno, F.; Ota, M.; Kosaka, J.; Ito, H.; Higuchi, M.; Doronbekov, T.K.; Nozaki, S.; Fujimura, Y.; Koeda, M.; Asada, T.; et al. Increased Binding of Peripheral Benzodiazepine Receptor in Alzheimer’s Disease Measured by Positron Emission Tomography with [11C]DAA1106. Biol. Psychiatry 2008, 64, 835–841. [Google Scholar] [CrossRef]
- Takano, A.; Arakawa, R.; Ito, H.; Tateno, A.; Takahashi, H.; Matsumoto, R.; Okubo, Y.; Suhara, T. Peripheral Benzodiazepine Receptors in Patients with Chronic Schizophrenia: A PET Study with [11C]DAA1106. Int. J. Neuropsychopharmacol. 2010, 13, 943–950. [Google Scholar] [CrossRef]
- Brody, A.L.; Gehlbach, D.; Garcia, L.Y.; Enoki, R.; Hoh, C.; Vera, D.; Kotta, K.K.; London, E.D.; Okita, K.; Nurmi, E.L.; et al. Effect of Overnight Smoking Abstinence on a Marker for Microglial Activation: A [11C]DAA1106 Positron Emission Tomography Study. Psychopharmacology 2018, 235, 3525–3534. [Google Scholar] [CrossRef]
- Zhang, M.R.; Maeda, J.; Furutsuka, K.; Yoshida, Y.; Ogawa, M.; Suhara, T.; Suzuki, K. [18F]FMDAA1106 and [18F]FEDAA1106: Two Positron-Emitter Labeled Ligands for Peripheral Benzodiazepine Receptor (PBR). Bioorganic Med. Chem. Lett. 2003, 13, 201–204. [Google Scholar] [CrossRef]
- Kumata, K.; Zhang, Y.; Fujinaga, M.; Ohkubo, T.; Mori, W.; Yamasaki, T.; Hanyu, M.; Xie, L.; Hatori, A.; Zhang, M.R. [18 F]DAA1106: Automated Radiosynthesis Using Spirocyclic Iodonium Ylide and Preclinical Evaluation for Positron Emission Tomography Imaging of Translocator Protein (18 KDa). Bioorganic Med. Chem. 2018, 26, 4817–4822. [Google Scholar] [CrossRef]
- Zhang, M.R.; Maeda, J.; Ogawa, M.; Noguchi, J.; Ito, T.; Yoshida, Y.; Okauchi, T.; Obayashi, S.; Suhara, T.; Suzuki, K. Development of a New Radioligand, N-(5-Fluoro-2-Phenoxyphenyl)-N-(2-[ 18F]Fluoroethyl-5-Methoxybenzyl)Acetamide, for PET Imaging of Peripheral Benzodiazepine Receptor in Primate Brain. J. Med. Chem. 2004, 47, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Varrone, A.; Mattsson, P.; Forsberg, A.; Takano, A.; Nag, S.; Gulyàs, B.; Borg, J.; Boellaard, R.; Al-Tawil, N.; Eriksdotter, M.; et al. In Vivo Imaging of the 18-KDa Translocator Protein (TSPO) with [18F]FEDAA1106 and PET Does Not Show Increased Binding in Alzheimer’s Disease Patients. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Briard, E.; Zoghbi, S.S.; Siméon, F.G.; Imaizumi, M.; Gourley, J.P.; Shetty, H.U.; Lu, S.; Fujita, M.; Innis, R.B.; Pike, V.W. Single-Step High-Yield Radiosynthesis and Evaluation of a Sensitive 18F-Labeled Ligand for Imaging Brain Peripheral Benzodiazepine Receptors with PET. J. Med. Chem. 2009, 52, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.A.; James, M.L.; Belichenko, N.P.; Semaan, S.; Condon, C.; Kuan, J.; Shuhendler, A.J.; Miao, Z.; Chin, F.T.; Longo, F.M. TSPO-PET Imaging Using [18F]PBR06 Is a Potential Translatable Biomarker for Treatment Response in Huntington’s Disease: Preclinical Evidence with the P75NTR Ligand LM11A-31. Hum. Mol. Genet. 2018, 27, 2893–2912. [Google Scholar] [CrossRef] [PubMed]
- Lartey, F.M.; Ahn, G.O.; Ali, R.; Rosenblum, S.; Miao, Z.; Arksey, N.; Shen, B.; Colomer, M.V.; Rafat, M.; Liu, H.; et al. The Relationship Between Serial [18 F]PBR06 PET Imaging of Microglial Activation and Motor Function Following Stroke in Mice. Mol. Imaging Biol. 2014, 16, 821–829. [Google Scholar] [CrossRef]
- Kim, S.W.; Wiers, C.E.; Tyler, R.; Shokri-Kojori, E.; Jang, Y.J.; Zehra, A.; Freeman, C.; Ramirez, V.; Lindgren, E.; Miller, G.; et al. Influence of Alcoholism and Cholesterol on TSPO Binding in Brain: PET [ 11 C]PBR28 Studies in Humans and Rodents. Neuropsychopharmacology 2018, 43, 1832–1839. [Google Scholar] [CrossRef]
- Fujita, M.; Imaizumi, M.; Zoghbi, S.S.; Fujimura, Y.; Farris, A.G.; Suhara, T.; Hong, J.; Pike, V.W.; Innis, R.B. Kinetic Analysis in Healthy Humans of a Novel Positron Emission Tomography Radioligand to Image the Peripheral Benzodiazepine Receptor, a Potential Biomarker for Inflammation. Neuroimage 2008, 40, 43–52. [Google Scholar] [CrossRef]
- Ibrahim, W.; An, J.; Yang, Y.; Cosgrove, K.P.; Matuskey, D. Does Seasonal Variation Affect the Neuroimmune System? A Retrospective [11C]PBR28 PET Study in Healthy Individuals. Neurosci. Lett. 2024, 828, 137766. [Google Scholar] [CrossRef]
- Moon, B.S.; Kim, B.S.; Park, C.; Jung, J.H.; Lee, Y.W.; Lee, H.Y.; Chi, D.Y.; Lee, B.C.; Kim, S.E. Fluoromethyl-PBR28 as a Potential Radiotracer for TSPO: Preclinical Comparison with [11C]PBR28 in a Rat Model of Neuroinflammation. Bioconjug. Chem. 2014, 25, 442–450. [Google Scholar] [CrossRef]
- Kim, G.R.; Paeng, J.C.; Jung, J.H.; Moon, B.S.; Lopalco, A.; Denora, N.; Lee, B.C.; Kim, S.E. Assessment of TSPO in a Rat Experimental Autoimmune Myocarditis Model: A Comparison Study between [18F] Fluoromethyl-PBR28 and [18F]CB251. Int. J. Mol. Sci. 2018, 19, 276. [Google Scholar] [CrossRef] [PubMed]
- Rusjan, P.M.; Wilson, A.A.; Bloomfield, P.M.; Vitcu, I.; Meyer, J.H.; Houle, S.; Mizrahi, R. Quantitation of Translocator Protein Binding in Human Brain with the Novel Radioligand 18 F-FEPPA and Positron Emission Tomography; SAGE Publications: London, UK, 2011; Volume 31, pp. 1807–1816. [Google Scholar]
- Mizrahi, R.; Rusjan, P.M.; Kennedy, J.; Pollock, B.; Mulsant, B.; Suridjan, I.; De Luca, V.; Wilson, A.A.; Houle, S. Translocator Protein (18 KDa) Polymorphism (Rs6971) Explains In-Vivo Brain Binding Affinity of the PET Radioligand [ 18F]-FEPPA. J. Cereb. Blood Flow Metab. 2012, 32, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of Translocator Protein Density, a Marker of Neuroinflammation, in the Brain during Major Depressive Episodes. JAMA Psychiatry 2015, 72, 268–275. [Google Scholar] [CrossRef]
- Langer, S.Z.; Arbilla, S.; Benavides, J.; Scatton, B. Zolpidem and Alpidem: Two Imidazopyridines with Selectivity for Omega 1- and Omega 3-Receptor Subtypes. Adv. Biochem. Psychopharmacol. 1990, 46, 61–72. [Google Scholar]
- Boutin, H.; Chauveau, F.; Thominiaux, C.; Kuhnast, B.; Grégoire, M.C.; Jan, S.; Trebossen, R.; Dollé, F.; Tavitian, B.; Mattner, F.; et al. In Vivo Imaging of Brain Lesions with [11C]CLINME, a New PET Radioligand of Peripheral Benzodiazepine Receptors. Glia 2007, 55, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Mattner, F.; Mardon, K.; Katsifis, A. Pharmacological Evaluation of [123I]-CLINDE: A Radioiodinated Imidazopyridine-3-Acetamide for the Study of Peripheral Benzodiazepine Binding Sites (PBBS). Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 779–789. [Google Scholar] [CrossRef]
- Mattner, F.; Bandin, D.L.; Staykova, M.; Berghofer, P.; Gregoire, M.C.; Ballantyne, P.; Quinlivan, M.; Fordham, S.; Pham, T.; Willenborg, D.O.; et al. Evaluation of [123I]-CLINDE as a Potent SPECT Radiotracer to Assess the Degree of Astroglia Activation in Cuprizone-Induced Neuroinflammation. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1516–1528. [Google Scholar] [CrossRef]
- Ohshima, M.; Moriguchi, T.; Enmi, J.-I.; Kawashima, H.; Koshino, K.; Zeniya, T.; Tsuji, M.; Iida, H. [123I]CLINDE SPECT as a Neuroinflammation Imaging Approach in a Rat Model of Stroke. Exp. Neurol. 2024, 378, 114843. [Google Scholar] [CrossRef]
- Fookes, C.J.R.; Pham, T.Q.; Mattner, F.; Greguric, I.; Loc’h, C.; Liu, X.; Berghofer, P.; Shepherd, R.; Gregoire, M.C.; Katsifis, A. Synthesis and Biological Evaluation of Substituted [18F] Imidazo[1,2-a]Pyridines and [18F]Pyrazolo[1,5-a]Pyrimidines for the Study of the Peripheral Benzodiazepine Receptor Using Positron Emission Tomography. J. Med. Chem. 2008, 51, 3700–3712. [Google Scholar] [CrossRef]
- Callaghan, P.D.; Wimberley, C.A.; Rahardjo, G.L.; Berghofer, P.J.; Pham, T.Q.; Jackson, T.; Zahra, D.; Bourdier, T.; Wyatt, N.; Greguric, I.; et al. Comparison of In Vivo Binding Properties of the 18-KDa Translocator Protein (TSPO) Ligands [18F]PBR102 and [18F]PBR111 in a Model of Excitotoxin-Induced Neuroinflammation. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 138–151. [Google Scholar] [CrossRef]
- Guo, Q.; Colasanti, A.; Owen, D.R.; Onega, M.; Kamalakaran, A.; Bennacef, I.; Matthews, P.M.; Rabiner, E.A.; Turkheimer, F.E.; Gunn, R.N. Quantification of the Specific Translocator Protein Signal of 18F-PBR111 in Healthy Humans: A Genetic Polymorphism Effect on In Vivo Binding. J. Nucl. Med. 2013, 54, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, N.; Boisgard, R.; Kuhnast, B.; Thézé, B.; Viel, T.; Grégoire, M.C.; Chauveau, F.; Boutin, H.; Katsifis, A.; Dollé, F.; et al. In Vivo Imaging of Neuroinflammation: A Comparative Study between [18F]PBR111, [11C]CLINME and [11C]PK11195 in an Acute Rodent Model. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Sekimata, K.; Yamada, T.; Abe, J.; Ito, K.; Ogawa, M.; Magata, Y.; Toyohara, J.; Ishiwata, K.; Biggio, G.; et al. Radiosynthesis and In Vivo Evaluation of Two Imidazopyridineacetamides, [11C]CB184 and [11C]CB190, as a PET Tracer for 18 KDa Translocator Protein: Direct Comparison with [11C](R)-PK11195. Ann. Nucl. Med. 2015, 29, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, J.; Sakata, M.; Hatano, K.; Yanai, S.; Endo, S.; Ishibashi, K.; Wagatsuma, K.; Ishii, K.; Ishiwata, K. Preclinical and First-in-Man Studies of [11C]CB184 for Imaging the 18-KDa Translocator Protein by Positron Emission Tomography. Ann. Nucl. Med. 2016, 30, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, F.; Van Camp, N.; Dollé, F.; Kuhnast, B.; Hinnen, F.; Damont, A.; Boutin, H.; James, M.; Kassiou, M.; Tavitian, B. Comparative Evaluation of the Translocator Protein Radioligands 11C-DPA-713, 18F-DPA-714, and 11C-PK11195 in a Rat Model of Acute Neuroinflammation. J. Nucl. Med. 2009, 50, 468–476. [Google Scholar] [CrossRef]
- Peyronneau, M.A.; Saba, W.; Goutal, S.; Damont, A.; Dollé, F.; Kassiou, M.; Bottlaender, M.; Valette, H. Metabolism and Quantification of [18F]DPA-714, a New TSPO Positron Emission Tomography Radioligand. Drug Metab. Dispos. 2013, 41, 122–131. [Google Scholar] [CrossRef]
- Lavisse, S.; Inoue, K.; Jan, C.; Peyronneau, M.A.; Petit, F.; Goutal, S.; Dauguet, J.; Guillermier, M.; Dollé, F.; Rbah-Vidal, L.; et al. [18F]DPA-714 PET Imaging of Translocator Protein TSPO (18 KDa) in the Normal and Excitotoxically-Lesioned Nonhuman Primate Brain. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 478–494. [Google Scholar] [CrossRef]
- Foray, C.; Valtorta, S.; Barca, C.; Winkeler, A.; Roll, W.; Müther, M.; Wagner, S.; Gardner, M.L.; Hermann, S.; Schäfers, M.; et al. Imaging Temozolomide-Induced Changes in the Myeloid Glioma Microenvironment. Theranostics 2021, 11, 2020–2033. [Google Scholar] [CrossRef]
- Niu, N.; Xing, H.; Wang, X.; Ding, J.; Hao, Z.; Ren, C.; Ba, J.; Zheng, L.; Fu, C.; Zhao, H.; et al. Comparative [18F]FDG and [18F]DPA714 PET Imaging and Time-Dependent Changes of Brown Adipose Tissue in Tumour-Bearing Mice. Adipocyte 2020, 9, 542–549. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, H.; Zhou, Q.; Chunyu, H.; He, L.; Meng, H.; Wang, H.; Wang, Y.; Sun, C.; Xi, Y.; et al. Microglial Activation Imaging Using 18F-DPA-714 PET/MRI for Detecting Autoimmune Encephalitis. Radiology 2024, 310, e230397. [Google Scholar] [CrossRef]
- Keller, T.; López-Picón, F.R.; Krzyczmonik, A.; Forsback, S.; Kirjavainen, A.K.; Takkinen, J.S.; Alzghool, O.; Rajander, J.; Teperi, S.; Cacheux, F.; et al. [18 F]F-DPA for the Detection of Activated Microglia in a Mouse Model of Alzheimer’s Disease. Nucl. Med. Biol. 2018, 67, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; López-Picón, F.R.; Krzyczmonik, A.; Forsback, S.; Takkinen, J.S.; Rajander, J.; Teperi, S.; Dollé, F.; Rinne, J.O.; Haaparanta-Solin, M.; et al. Comparison of High and Low Molar Activity TSPO Tracer [18F]F-DPA in a Mouse Model of Alzheimer’s Disease. J. Cereb. Blood Flow Metab. 2020, 40, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, S.; Keller, T.; Petruk, N.; López-Picón, F.; Eichin, D.; Löyttyniemi, E.; Verhassel, A.; Rajander, J.; Sandholm, J.; Tuomela, J.; et al. Evaluation of [18F]F-DPA as a Target for TSPO in Head and Neck Cancer under Normal Conditions and after Radiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1312–1326. [Google Scholar] [CrossRef]
- Damont, A.; Médran-Navarrete, V.; Cacheux, F.; Kuhnast, B.; Pottier, G.; Bernards, N.; Marguet, F.; Puech, F.; Boisgard, R.; Dollé, F. Novel Pyrazolo[1,5-a]Pyrimidines as Translocator Protein 18 KDa (TSPO) Ligands: Synthesis, in Vitro Biological Evaluation, [18F]-Labeling, and In Vivo Neuroinflammation PET Images. J. Med. Chem. 2015, 58, 7449–7464. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; McKinley, E.T.; Hight, M.R.; Uddin, M.I.; Harp, J.M.; Fu, A.; Nickels, M.L.; Buck, J.R.; Manning, H.C. Synthesis and Structure-Activity Relationships of 5,6,7-Substituted Pyrazolopyrimidines: Discovery of a Novel TSPO PET Ligand for Cancer Imaging. J. Med. Chem. 2013, 56, 3429–3433. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Nickels, M.L.; Tantawy, M.N.; Buck, J.R.; Manning, H.C. Preclinical Imaging Evaluation of Novel TSPO-PET Ligand 2-(5,7-Diethyl-2-(4-(2-[18F]Fluoroethoxy)Phenyl)Pyrazolo[1,5-a]Pyrimidin-3-Yl)-N,N-Diethylacetamide ([18F]VUIIS1008) in Glioma. Mol. Imaging Biol. 2014, 16, 813–820. [Google Scholar] [CrossRef][Green Version]
- Tang, D.; Fujinaga, M.; Hatori, A.; Zhang, Y.; Yamasaki, T.; Xie, L.; Mori, W.; Kumata, K.; Liu, J.; Manning, H.C.; et al. Evaluation of the Novel TSPO Radiotracer 2-(7-Butyl-2-(4-(2-([18F]Fluoroethoxy)Phenyl)-5-Methylpyrazolo[1,5-a]Pyrimidin-3-Yl)-N,N-Diethylacetamide in a Preclinical Model of Neuroinflammation. Eur. J. Med. Chem. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Tang, D.; Li, J.; Nickels, M.L.; Huang, G.; Cohen, A.S.; Manning, H.C. Preclinical Evaluation of a Novel TSPO PET Ligand 2-(7-Butyl-2-(4-(2-[ 18 F]Fluoroethoxy)Phenyl)-5-Methylpyrazolo[1,5-a]Pyrimidin-3-Yl)-N,N-Diethylacetamide (18 F-VUIIS1018A) to Image Glioma. Mol. Imaging Biol. 2019, 21, 113–121. [Google Scholar] [CrossRef]
- Murata, T.; Masumoto, K.; Kondo, K.; Furukawa, K.; Oka, M. 2-Aryl-8-Oxodihydropurinderivater, Process for Preparing Same Medical Compositions Containing the Same and Intermediates Thereof. WO1999028320A1. 1999. Available online: https://patents.google.com/patent/WO1999028320A1/en?oq=+WO+Patent+9928320 (accessed on 2 September 2024).
- Maeda, J.; Zhang, M.R.; Okauchi, T.; Ji, B.; Ono, M.; Hattori, S.; Kumata, K.; Iwata, N.; Saido, T.C.; Trojanowski, J.Q.; et al. In Vivo Positron Emission Tomographic Imaging of Glial Responses to Amyloid-β and Tau Pathologies in Mouse Models of Alzheimer’s Disease and Related Disorders. J. Neurosci. 2011, 31, 4720–4730. [Google Scholar] [CrossRef]
- Yanamoto, K.; Yamasaki, T.; Kumata, K.; Yui, J.; Odawara, C.; Kawamura, K.; Hatori, A.; Inoue, O.; Yamaguchi, M.; Suzuki, K.; et al. Evaluation of N-Benzyl-N-[11C]Methyl-2-(7-Methyl-8-Oxo-2-Phenyl- 7,8-Dihydro-9H-Purin-9-Yl)Acetamide ([11C]DAC) as a Novel Translocator Protein (18 KDa) Radioligand in Kainic Acid-Lesioned Rat. Synapse 2009, 63, 961–971. [Google Scholar] [CrossRef]
- Yui, J.; Hatori, A.; Yanamoto, K.; Takei, M.; Nengaki, N.; Kumata, K.; Kawamura, K.; Yamasaki, T.; Suzuki, K.; Zhang, M.R. Imaging of the Translocator Protein (18 KDa) in Rat Brain after Ischemia Using [11C]DAC with Ultra-High Specific Activity. Synapse 2010, 64, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Yui, J.; Hatori, A.; Kawamura, K.; Yanamoto, K.; Yamasaki, T.; Ogawa, M.; Yoshida, Y.; Kumata, K.; Fujinaga, M.; Nengaki, N.; et al. Visualization of Early Infarction in Rat Brain after Ischemia Using a Translocator Protein (18 kDa) PET Ligand [11C]DAC with Ultra-High Specific Activity. Neuroimage 2011, 54, 123–130. [Google Scholar] [CrossRef]
- Yanamoto, K.; Kumata, K.; Yamasaki, T.; Odawara, C.; Kawamura, K.; Yui, J.; Hatori, A.; Suzuki, K.; Zhang, M.R. [18F]FEAC and [18F]FEDAC: Two Novel Positron Emission Tomography Ligands for Peripheral-Type Benzodiazepine Receptor in the Brain. Bioorganic Med. Chem. Lett. 2009, 19, 1707–1710. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.H.; Lee, B.C.; Lee, S.Y. Design and Synthesis of Phenoxypyridyl Acetamide or Aryl-Oxodihydropurine Derivatives for the Development of Novel Pet Ligands Targeting the Translocator Protein 18 KDa (TSPO). Bull. Korean Chem. Soc. 2016, 37, 1874–1877. [Google Scholar] [CrossRef]
- Yui, J.; Maeda, J.; Kumata, K.; Kawamura, K.; Yanamoto, K.; Hatori, A.; Yamasaki, T.; Nengaki, N.; Higuchi, M.; Zhang, M.R. 18F-FEAC and 18F-FEDAC: PET of the Monkey Brain and Imaging of Translocator Protein (18 KDa) in the Infarcted Rat Brain. J. Nucl. Med. 2010, 51, 1301–1309. [Google Scholar] [CrossRef]
- Chung, S.J.; Yoon, H.J.; Youn, H.; Kim, M.J.; Lee, Y.S.; Jeong, J.M.; Chung, J.K.; Kang, K.W.; Xie, L.; Zhang, M.R.; et al. 18 F-FEDAC as a Targeting Agent for Activated Macrophages in DBA/1 Mice with Collagen-Induced Arthritis: Comparison with 18 F-FDG. J. Nucl. Med. 2018, 59, 839–845. [Google Scholar] [CrossRef]
- Fukaya, T.; Kodo, T.; Ishiyama, T.; Kakuyama, H.; Nishikawa, H.; Baba, S.; Masumoto, S. Design, Synthesis and Structure-Activity Relationships of Novel Benzoxazolone Derivatives as 18 KDa Translocator Protein (TSPO) Ligands. Bioorganic Med. Chem. 2012, 20, 5568–5582. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, T.; Ishiyama, T.; Baba, S.; Masumoto, S. Identification of a Novel Benzoxazolone Derivative as a Selective, Orally Active 18 KDa Translocator Protein (TSPO) Ligand. J. Med. Chem. 2013, 56, 8191–8195. [Google Scholar] [CrossRef]
- Fujinaga, M.; Luo, R.; Kumata, K.; Zhang, Y.; Hatori, A.; Yamasaki, T.; Xie, L.; Mori, W.; Kurihara, Y.; Ogawa, M.; et al. Development of a 18F-Labeled Radiotracer with Improved Brain Kinetics for Positron Emission Tomography Imaging of Translocator Protein (18 KDa) in Ischemic Brain and Glioma. J. Med. Chem. 2017, 60, 4047–4061. [Google Scholar] [CrossRef]
- Srivastava, P.; Kaul, A.; Ojha, H.; Kumar, P.; Tiwari, A.K. Design, Synthesis and Biological Evaluation of Methyl-2-(2-(5-Bromo Benzoxazolone)Acetamido)-3-(1H-Indol-3-Yl)Propanoate: TSPO Ligand for SPECT. RSC Adv. 2016, 6, 114491–114499. [Google Scholar] [CrossRef]
- Okubo, T.; Yoshikawa, R.; Chaki, S.; Okuyama, S.; Nakazato, A. Design, Synthesis, and Structure-Activity Relationships of Novel Tetracyclic Compounds as Peripheral Benzodiazepine Receptor Ligands. Bioorganic Med. Chem. 2004, 12, 3569–3580. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, H.; Jones, P.A.; Chau, W.F.; Durrant, C.; Fouladi, N.; Passmore, J.; O’Shea, D.; Wynn, D.; Morisson-Iveson, V.; Ewan, A.; et al. GE-180: A Novel Fluorine-18 Labelled PET Tracer for Imaging Translocator Protein 18 KDa (TSPO). Bioorganic Med. Chem. Lett. 2012, 22, 1308–1313. [Google Scholar] [CrossRef]
- Dickens, A.M.; Vainio, S.; Marjamäki, P.; Johansson, J.; Lehtiniemi, P.; Rokka, J.; Rinne, J.; Solin, O.; Haaparanta-Solin, M.; Jones, P.A.; et al. Detection of Microglial Activation in an Acute Model of Neuroinflammation Using PET and Radiotracers 11C-(R)-PK11195 and 18F-GE-180. J. Nucl. Med. 2014, 55, 466–472. [Google Scholar] [CrossRef]
- Liu, B.; Le, K.X.; Park, M.A.; Wang, S.; Belanger, A.P.; Dubey, S.; Frost, J.L.; Holton, P.; Reiser, V.; Jones, P.A.; et al. In Vivo Detection of Age-and Disease-Related Increases in Neuroinflammation By18F-GE180 TSPO MicroPET Imaging in Wild-Type and Alzheimer’s Transgenic Mice. J. Neurosci. 2015, 35, 15716–15730. [Google Scholar] [CrossRef] [PubMed]
- Feeney, C.; Scott, G.; Raffel, J.; Roberts, S.; Coello, C.; Jolly, A.; Searle, G.; Goldstone, A.P.; Brooks, D.J.; Nicholas, R.S.; et al. Kinetic Analysis of the Translocator Protein Positron Emission Tomography Ligand [18F]GE-180 in the Human Brain. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Mahler, C.; Vomacka, L.; Lindner, S.; Havla, J.; Brendel, M.; Böning, G.; Ertl-Wagner, B.; Kümpfel, T.; Milenkovic, V.M.; et al. TSPO PET with [18F]GE-180 Sensitively Detects Focal Neuroinflammation in Patients with Relapsing–Remitting Multiple Sclerosis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1423–1431. [Google Scholar] [CrossRef]
- Unterrainer, M.; Fleischmann, D.F.; Diekmann, C.; Vomacka, L.; Lindner, S.; Vettermann, F.; Brendel, M.; Wenter, V.; Ertl-Wagner, B.; Herms, J.; et al. Comparison of 18 F-GE-180 and Dynamic 18 F-FET PET in High Grade Glioma: A Double-Tracer Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 580–590. [Google Scholar] [CrossRef]
- Zanotti-Fregonara, P.; Pascual, B.; Rizzo, G.; Yu, M.; Pal, N.; Beers, D.; Carter, R.; Appel, S.H.; Atassi, N.; Masdeu, J.C. Head-to-Head Comparison of 11C-PBR28 and 18F-GE180 for Quantification of the Translocator Protein in the Human Brain. J. Nucl. Med. 2018, 59, 1260–1266. [Google Scholar] [CrossRef]
- Zanotti-Fregonara, P.; Pascual, B.; Rostomily, R.C.; Rizzo, G.; Veronese, M.; Masdeu, J.C.; Turkheimer, F. Anatomy of 18F-GE180, a Failed Radioligand for the TSPO Protein. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2233–2236. [Google Scholar] [CrossRef]
- Ramakrishnan, N.K.; Hird, M.; Thompson, S.; Williamson, D.J.; Qiao, L.; Owen, D.R.; Brooks, A.F.; Scott, P.J.H.; Bacallado, S.; O’Brien, J.T.; et al. Preclinical Evaluation of (S)-[18F]GE387, a Novel 18-KDa Translocator Protein (TSPO) PET Radioligand with Low Binding Sensitivity to Human Polymorphism Rs6971. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 125–136. [Google Scholar] [CrossRef]
- Qiao, L.; Fisher, E.; McMurray, L.; Milicevic Sephton, S.; Hird, M.; Kuzhuppilly-Ramakrishnan, N.; Williamson, D.J.; Zhou, X.; Werry, E.; Kassiou, M.; et al. Radiosynthesis of (R,S)-[18F]GE387: A Potential PET Radiotracer for Imaging Translocator Protein 18 KDa (TSPO) with Low Binding Sensitivity to the Human Gene Polymorphism Rs6971. ChemMedChem 2019, 14, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Castellano, S.; Taliani, S.; Milite, C.; Pugliesi, I.; Da Pozzo, E.; Rizzetto, E.; Bendinelli, S.; Costa, B.; Cosconati, S.; Greco, G.; et al. Synthesis and Biological Evaluation of 4-Phenylquinazoline-2-Carboxamides Designed as a Novel Class of Potent Ligands of the Translocator Protein. J. Med. Chem. 2012, 55, 4506–4510. [Google Scholar] [CrossRef] [PubMed]
- Castellano, S.; Taliani, S.; Viviano, M.; Milite, C.; Da Pozzo, E.; Costa, B.; Barresi, E.; Bruno, A.; Cosconati, S.; Marinelli, L.; et al. Structure-Activity Relationship Refinement and Further Assessment of 4-Phenylquinazoline-2-Carboxamide Translocator Protein Ligands as Antiproliferative Agents in Human Glioblastoma Tumors. J. Med. Chem. 2014, 57, 2413–2428. [Google Scholar] [CrossRef] [PubMed]
- Li, K.P.; Cai, H. An Improved HPLC Separation Method for TSPO Radioligand [11C]ER176 Clinical Production. J. Label. Compd. Radiopharm. 2024, 67, 273–276. [Google Scholar] [CrossRef]
- Zanotti-Fregonara, P.; Pascual, B.; Veronese, M.; Yu, M.; Beers, D.; Appel, S.H.; Masdeu, J.C. Head-to-Head Comparison of 11C-PBR28 and 11C-ER176 for Quantification of the Translocator Protein in the Human Brain. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1822–1829. [Google Scholar] [CrossRef]
- Lee, J.H.; Siméon, F.G.; Liow, J.S.; Morse, C.L.; Gladding, R.L.; Santamaria, J.A.M.; Henter, I.D.; Zoghbi, S.S.; Pike, V.W.; Innis, R.B. In Vivo Evaluation of 6 Analogs of 11C-ER176 as Candidate 18F-Labeled Radioligands for 18-KDa Translocator Protein. J. Nucl. Med. 2022, 63, 1252–1258. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Ji, B.; Yui, J.; Fujinaga, M.; Yamasaki, T.; Xie, L.; Luo, R.; Shimoda, Y.; Kumata, K.; Zhang, Y.; et al. [18F]FEBMP: Positron Emission Tomography Imaging of TSPO in a Model of Neuroinflammation in Rats, and in Vitro Autoradiograms of the Human Brain. Theranostics 2015, 5, 961–969. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).