An Insight into the Molecular Electronic Structure of Graphene Oxides and Their Interactions with Molecules of Different Polarities Using Quantum Chemical and COSMO-RS Calculations
Abstract
1. Introduction
2. Results and Discussions
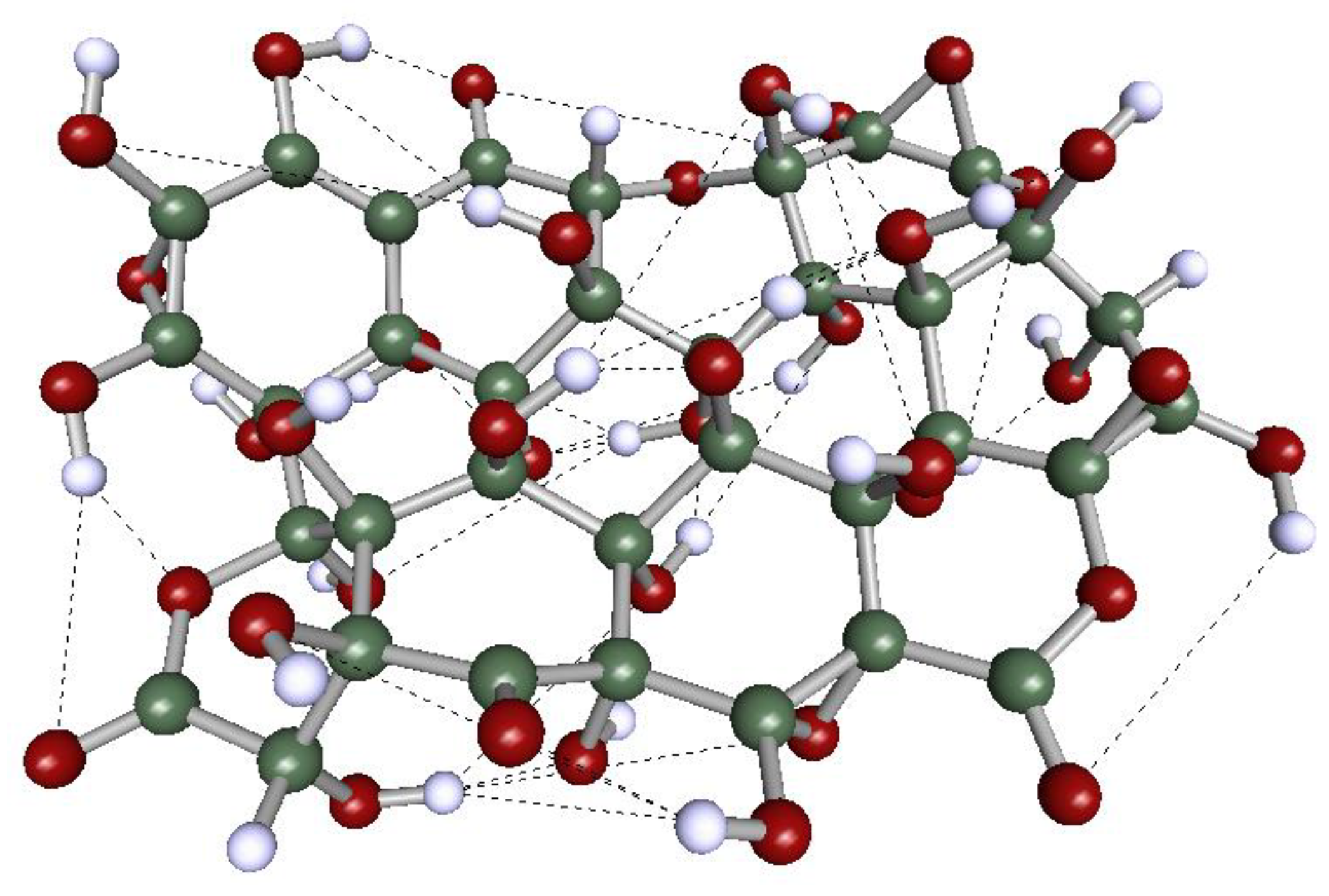
2.1. Molecular and Electronic Structure of the GOs
- The electron density deformations display two distinct shapes. The first shape (delimited by dotted lines in Model 1, Figure 3, for example) resembles the density deformation of graphene (Model 0). It is localized on the planar fragments of the less oxidized GOs (Models 1 and 2) and results from the contribution of the π-electron cloud of the conjugated system. The second one is located on the oxygen-substituted fragments of the molecule, which is a direct consequence of the non-paired, n-electrons of the oxygen atoms. The π-electron cloud remains intact in the non-substituted segments of Models 1 and 2 but practically disappears in the structure of Model 4, where the O-C substitution is the maximum evaluated in this work.
- The electron density deformations show several discontinuities (indicated by arrows in Figure 3) in the GOs studied here. These correspond to regions of the molecular domain where ionic-like bonds (instead of covalent ones) predominate. It arises in the presence of hydrogen atoms strongly polarized positively, involved in H-bond interactions. This phenomenon has been well recognized in DAM studies of H-bond systems. The OH group distribution on the molecular surface of the GOs with high O/C ratios (Model 4, for example) guarantees a quasi-two-dimensional network of H-bonds (Figure 4), which could support strong proton conduction in this kind of material.
2.2. COSMO Analysis of the Charge Distribution on the Molecular Surface of the GOs
2.3. Interaction of GOs with Molecular Species of Different Polarities: Thermodynamic Features
2.4. A Structural Insight to the Interactions of the Polar Solvents with Low-Oxidized GOs
3. Materials and Methods
3.1. Molecular Models
3.2. Quantum Chemical Calculations and Geometry Optimization
3.3. Electronic Structure
3.4. COSMO and COSMO-RS Calculations
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bich Ha, N.; Van Hieu, N. Promising applications of graphene and graphene-based nanostructures. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 023002. [Google Scholar]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Bagri, A.; Grantab, R.; Medhekar, N.V.; Shenoy, V.B. Stability and formation mechanisms of carbonyl- and hydroxyl-decorated holes in graphene oxide. J. Phys. Chem. C 2010, 114, 12053–12061. [Google Scholar] [CrossRef]
- Dideikin, A.T.; Vul, A.Y. Graphene oxide and derivatives: The place in graphene family. Front. Phys. 2019, 6, 149. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Sundqvist, B.; Szabo, T.; Dekany, I.; Dmitriev, V. Pressure-induced insertion of liquid alcohols into graphite oxide structure. J. Am. Chem. Soc. 2009, 131, 18445–18449. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Sun, L. Structure and synthesis of graphene oxide. Chin. J. Chem. Eng. 2019, 27, 2251–2260. [Google Scholar] [CrossRef]
- Sheka, E.F.; Popova, N.A. Molecular theory of graphene oxide. Phys. Chem. Chem. Phys. 2013, 15, 13304–13322. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Romero, A.; Lavin-Lopez, M.P.; Sanchez-Silva, L.; Valverde, J.L.; Paton-Carrero, A. Comparative study of different scalable routes to synthesize graphene oxide and reduced graphene oxide. Mater. Chem. Phys. 2018, 203, 284–292. [Google Scholar] [CrossRef]
- Del Prado Lavin-Lopez, M.; Romero, A.; Garrido, J.; Sanchez-Silva, L.; Luis Valverde, J. Influence of different improved hummers method modifications on the characteristics of graphite oxide in order to make a more easily scalable method. Ind. Eng. Chem. Res. 2016, 55, 12836–12847. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and electronic structure of graphene-oxide. Nano Lett. 2009, 9, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Piner, R.D.; Stadermann, F.J.; Park, S.; Shaibat, M.A.; Ishii, Y.; Yang, D.; Velamakanni, A.; An, S.J.; Stoller, M.; et al. Synthesis and solid-state NMR structural characterization of C-13-labeled graphite oxide. Science 2008, 321, 1815–1817. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N. Measurement of multicomponent solubility parameters for graphene facilitates solvent discovery. Langmuir 2010, 26, 3208–3213. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Elhaes, H.; Abdel-Salam, A.I.; Gomaa, I.; Ibrahim, A.; Yahia, I.S.; Zahran, H.Y.; Ezzat, H.A.; Zahran, M.; Abdel-wahab, M.S.; Refaat, A.; et al. Facile synthesis, structural, morphological and electronic investigation of Mn2O3 nano-rice shape and Mn2O3-rGO hybrid nanocomposite. Opt. Quantum Electron. 2023, 55, 947. [Google Scholar] [CrossRef]
- Jia, R.; Nong, X.-M.; Lu, H.-Q.; Xiong, Y.-S.; Wei, W.; Qin, W.-H.; Li, W. Multidimensional decipherment of interactions in invert sugar-amino acid co-degradation colorants (IACDCs) capture by polyamine co-modified shaddock peel cellulose/graphene oxide aerogel. Sep. Purif. Technol. 2024, 337, 126299. [Google Scholar] [CrossRef]
- Tahir, A.; Liaqat, F.; Saleem, M.; Shaik, M.R.; Adil, S.F.; Siddiqui, M.R.H.; Khan, M. Eco-friendly synthesis of anti-microbial and anti-fungal binary metal oxide decorated reduced graphene oxide nanocomposites with complimenting density functional studies. J. Saudi Chem. Soc. 2023, 27, 101710. [Google Scholar] [CrossRef]
- Lahaye, R.J.W.E.; Jeong, H.K.; Park, C.Y.; Lee, Y.H. Density functional theory study of graphite oxide for different oxidation levels. Phys. Rev. B 2009, 79, 125435. [Google Scholar] [CrossRef]
- Yan, J.A.; Xian, L.D.; Chou, M.Y. Structural and electronic properties of oxidized graphene. Phys. Rev. Lett. 2009, 103, 086802. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.A.; Chou, M.Y. Oxidation functional groups on graphene: Structural and electronic properties. Phys. Rev. B 2010, 82, 125403. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Botello-Mendez, A.R.; Dubois, S.M.M.; Lherbier, A.; Charlier, J.-C. Achievements of DFT for the investigation of graphene-related nanostructures. Acc. Chem. Res. 2014, 47, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Demianenko, E.; Sencha-Hlevatska, K.; Sementsov, Y.; Kartel, M. Quantum-chemical investigation of the superoxide radical scavenging by graphene oxide surface. Low Temp. Phys. 2023, 49, 1088–1092. [Google Scholar] [CrossRef]
- Araujo, W.S.; Rego, C.R.C.; Guedes-Sobrinho, D.; Dias, A.C.; do Couto, I.R.; Bordin, J.R.; de Matos, C.F.; Piotrowski, M. Quantum simulations and experimental insights into glyphosate adsorption using graphene-based nanomaterials. ACS Appl. Mater. Interfaces 2024, 16, 31500–31512. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Liu, C.; Tian, L.; Zhang, Y.; Zhu, M.; Qiao, W.; Wu, J.; Yan, S.; Zhang, H.; et al. Adsorption behaviors and mechanism of phenol and catechol in wastewater by magnetic graphene oxides: A comprehensive study based on adsorption experiments, mathematical models, and molecular simulations. ACS Omega 2024, 9, 15101–15113. [Google Scholar] [CrossRef]
- Tri, N.N.; Ho, D.Q.; Bao, N.T.G.; Trung, N.T. The adsorption of tetracycline, ciprofloxacin on reduced graphene oxide surfaces: Role of intermolecular interaction. Chem. Phys. 2024, 579, 112207. [Google Scholar]
- Murmu, M.; Huda; Mobin, M.; Aslam, R.; Banerjee, P. Adsorption of L-proline nitrate modified graphene oxide on iron surface: Density functional theory and Monte Carlo simulation study. Comput. Mater. Sci. 2024, 242, 113071. [Google Scholar] [CrossRef]
- Berisha, A. Density functional theory and quantum mechanics studies of 2D carbon nanostructures (graphene and graphene oxide) for lenalidomide anticancer drug delivery. Comput. Theor. Chem. 2023, 1230, 114371. [Google Scholar] [CrossRef]
- Adekoya, O.C.; Adekoya, G.J.; Sadiku, R.E.; Hamam, Y.; Ray, S.S. Density Functional Theory interaction study of a polyethylene glycol-based nanocomposite with cephalexin drug for the elimination of wound infection. ACS Omega 2022, 7, 33808. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A. The COSMO and COSMO-RS solvation models. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1338. [Google Scholar] [CrossRef]
- Klamt, A. COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Klamt, A. Conductor-like screening model for real solvents: A new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Palomar, J.; Lemus, J.; Gilarranz, M.A.; Rodriguez, J.J. Adsorption of ionic liquids from aqueous effluents by activated carbon. Carbon 2009, 47, 1846–1856. [Google Scholar] [CrossRef]
- He, H.Y.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Rico, J.F.; Lopez, R.; Ema, I.; Ramifrez, G. Chemical notions from the electron density. J. Chem. Theory Comput. 2005, 1, 1083–1095. [Google Scholar] [CrossRef]
- Rico, J.F.; Lopez, R.; Ema, I.; Ramirez, G. Electrostatic potentials and fields from density expansions of deformed atoms in molecules. J. Comput. Chem. 2004, 25, 1347–1354. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural localized molecular orbitals. J. Chem. Phys. 1985, 83, 1736–1740. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid exfoliation of defect-free graphene. Acc. Chem. Res. 2013, 46, 14–22. [Google Scholar] [CrossRef]
- Liu, W.-W.; Xia, B.-Y.; Wang, X.-X.; Wang, J.-N. Exfoliation and dispersion of graphene in ethanol-water mixtures. Front. Mater. Sci. 2012, 6, 176–182. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Sherwood, J.; De Bruyn, M.; Budarin, V.L.; Ellis, G.J.; Clark, J.H.; Shuttleworth, P.S. Identification of high performance solvents for the sustainable processing of graphene. Green Chem. 2017, 19, 2550–2560. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cui, Y.; Lee, Y.H.; Yang, J.W. Impact of carboxyl groups in graphene oxide on chemoselective alcohol oxidation with ultra-low carbocatalyst loading. Sci. Rep. 2017, 7, 3146. [Google Scholar] [CrossRef]
- Moradi, S.; Taran, M.; Mohajeri, P.; Sadrjavadi, K.; Sarrami, F.; Karton, A.; Shahlaei, M. Study of dual encapsulation possibility of hydrophobic and hydrophilic drugs into a nanocarrier based on bio-polymer coated graphene oxide using density functional theory, molecular dynamics simulation and experimental methods. J. Mol. Liq. 2018, 262, 204–217. [Google Scholar] [CrossRef]
- Silva, A.A.; Stein, R.; Campos, D.; Indrusiak, T.; Soares, B.G.; Barra, G.M.O. Conducting materials based on epoxy/graphene nanoplatelet composites with microwave absorbing properties: Effect of the processing conditions and ionic liquid. Front. Mater. 2019, 6, 156. [Google Scholar] [CrossRef]
- Long, J.; Li, S.; Liang, B.; Wang, Z. Investigation of thermal behaviour and mechanical property of the functionalised graphene oxide/epoxy resin nanocomposites. Plast. Rubber Compos. 2019, 48, 127–136. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, X.; Liu, L.; Weng, L. Improvement of graphene oxide/epoxy resin adhesive properties through interface modification. High Perform. Polym. 2019, 31, 341–349. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hasanzade, Z.; Raissi, H. Density functional theory calculations and molecular dynamics simulations of the adsorption of ellipticine anticancancer drug on graphenc oxide surface in aqueous medium as well as under controlled pH conditions. J. Mol. Liq. 2018, 255, 269–278. [Google Scholar] [CrossRef]
- Hasanzade, Z.; Raissi, H. Solvent/co-solvent effects on the electronic properties and adsorption mechanism of anticancer drug thioguanine on graphene oxide surface as a nanocarrier: Density functional theory investigation and a molecular dynamics. Appl. Surf. Sci. 2017, 422, 1030–1041. [Google Scholar] [CrossRef]
- Lopez, R.; Fernandez Rico, J.; Ramirez, G.; Ema, I.; Zorrilla, D. DAMQT 2.0: A new version of the DAMQT package for the analysis of electron density in molecules. Comput. Phys. Commun. 2015, 192, 289–294. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Carroll, M.T.; Cheeseman, J.R.; Chang, C. Properties of atoms in molecules: Atomic volumes. J. Am. Chem. Soc. 1987, 109, 7968–7979. [Google Scholar] [CrossRef]
- Tomasi, J.; Persico, M. Molecular interactions in solution: An overview of methods based on continuous distribution of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Klamt, A.; Diedenhofen, M. A refined cavity construction algorithm for the conductor-like screening model. J. Comput. Chem. 2018, 39, 1648–1655. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. Software news and updates TmoleX. A graphical user interface for TURBOMOLE. J. Comput. Chem. 2010, 31, 2967–2970. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A.; Koch, L. COSMOThermX, Version 20.0.0. Revision 5273M. Dassault Systems: Vélizy-Villacoublay, France, 2019.












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro, V.R.; Merino, S.; Lopez, R.; Valverde, J.L. An Insight into the Molecular Electronic Structure of Graphene Oxides and Their Interactions with Molecules of Different Polarities Using Quantum Chemical and COSMO-RS Calculations. Molecules 2024, 29, 3839. https://doi.org/10.3390/molecules29163839
Ferro VR, Merino S, Lopez R, Valverde JL. An Insight into the Molecular Electronic Structure of Graphene Oxides and Their Interactions with Molecules of Different Polarities Using Quantum Chemical and COSMO-RS Calculations. Molecules. 2024; 29(16):3839. https://doi.org/10.3390/molecules29163839
Chicago/Turabian StyleFerro, Víctor R., Sonia Merino, Rafael Lopez, and José L. Valverde. 2024. "An Insight into the Molecular Electronic Structure of Graphene Oxides and Their Interactions with Molecules of Different Polarities Using Quantum Chemical and COSMO-RS Calculations" Molecules 29, no. 16: 3839. https://doi.org/10.3390/molecules29163839
APA StyleFerro, V. R., Merino, S., Lopez, R., & Valverde, J. L. (2024). An Insight into the Molecular Electronic Structure of Graphene Oxides and Their Interactions with Molecules of Different Polarities Using Quantum Chemical and COSMO-RS Calculations. Molecules, 29(16), 3839. https://doi.org/10.3390/molecules29163839







