Stilbenes in Carex acuta and Carex lepidocarpa
Abstract
1. Introduction
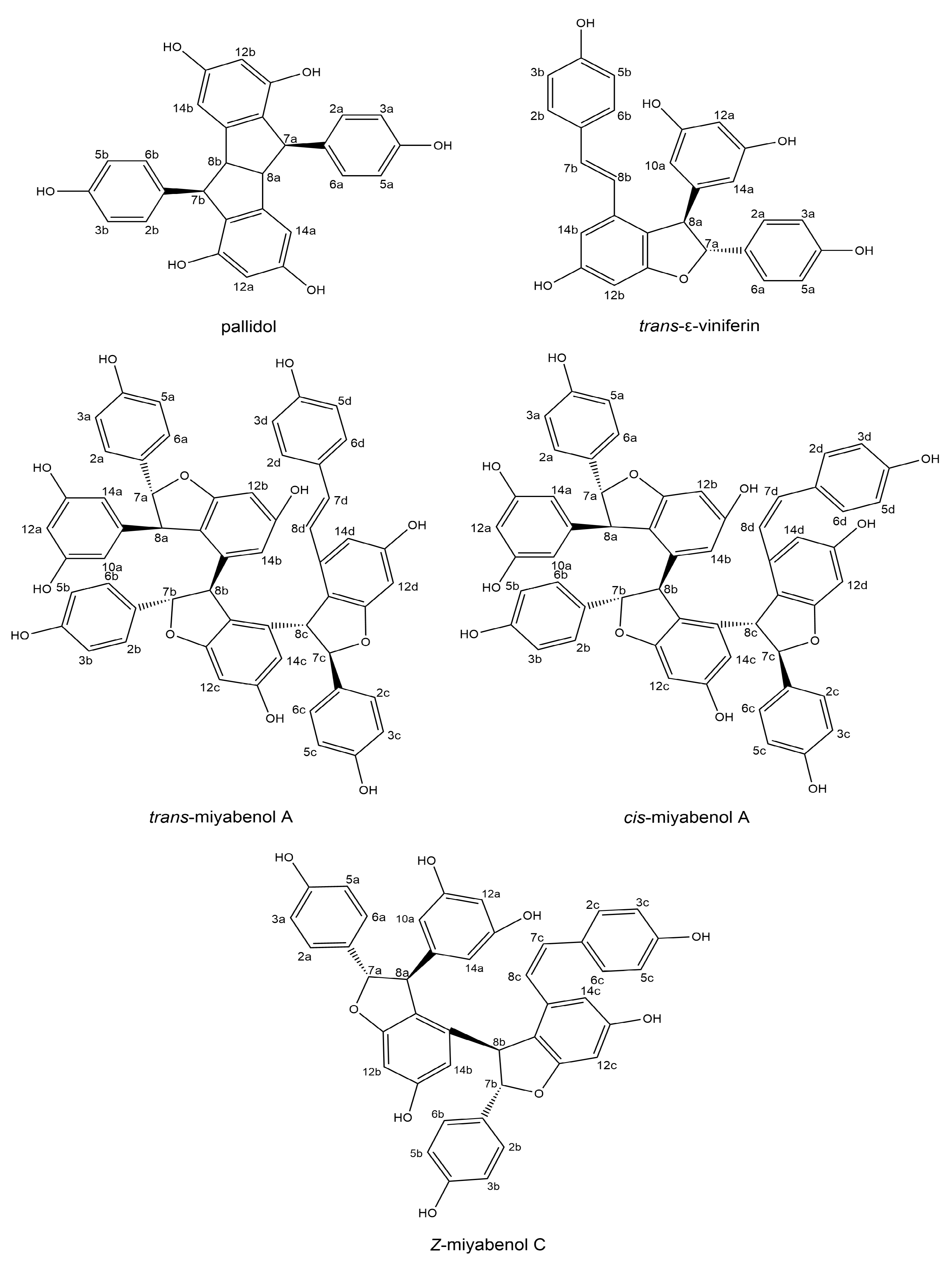
2. Results and Discussion
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals
3.3. HPLC and LC/MS
3.3.1. HPLC
3.3.2. LC/MS
3.3.3. Liquid Chromatography–Nuclear Magnetic Resonance Spectroscopy (LC-NMR)
3.3.4. Determination of Stilbenes by NMR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koopman, J. Carex Europaea. The Genus Carex L. (Cyperaceae) in Europe, 1: Accepted Names, Hybrids, Synonyms, Distribution, Chromosome Numbers; Margraf Publishers: Weikersheim, Germany, 2011. [Google Scholar]
- Schweingruber, F.H.; Kučerová, A.; Adamec, L.; Doležal, J. Anatomic Atlas of Aquatic and Wetland Plant Stems; Springer: Berlin, Germany, 2020. [Google Scholar]
- Hedrén, M. Patterns of allozyme and morphological differentiation in the Carex flava complex (Cyperaceae) in Fennoscandia. Nord. J. Bot. 2002, 22, 257–301. [Google Scholar] [CrossRef]
- Blackstock, N. A reassessment of Yellow Sedges—Carex flava agg. (Cyperaceae) in the British Isles. Ph.D. Thesis, University of Lancaster, Lancaster, UK, 2007. [Google Scholar]
- de Moraes, K.R.; Souza, A.T.; Muška, M.; Hladík, M.; Čtvrtlíková, M.; Draštík, V.; Kolařík, T.; Kučerová, A.; Krolová, M.; Sajdlová, Z.; et al. Artificial floating islands: A promising tool to support juvenile fish in lacustrine systems. Hydrobiologia 2023, 850, 1969–1984. [Google Scholar] [CrossRef]
- Harris, T.; Klimeš, A.; Martínková, J.; Klimešová, J. Herbs are not just small plants: What biomass allocation to rhizomes tells us about differences between trees and herbs. Am. J. Bot. 2023, 110, e16202. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Gromek, S.; Niesen, D.; Seeram, N.P.; Henry, G.E. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food Chem. 2011, 59, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, T.; Gao, Y.; Xu, J.; Jiang, C.; Wang, G.; Bu, G.; Xu, H.; Chen, H.; Zhang, Y. The resveratrol trimer miyabenol C inhibits β-secretase activity and β-amyloid generation. PLoS ONE 2015, 10, e0115973. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, X.; Ding, C.; Zhou, M.; Li, W.; Wang, Y.; You, Q.; Zong, S.; Peng, Q.; Duanmu, D.; et al. Two resveratrol oligomers inhibit cathepsin L activity to suppress SARS-CoV-2 entry. J. Agric. Food Chem. 2023, 71, 5535–5546. [Google Scholar] [CrossRef]
- Arraki, K.; Totoson, P.; Decendit, A.; Badoc, A.; Zedet, A.; Jolibois, J.; Pudlo, M.; Demougeot, C.; Girard-Thernier, C. Cyperaceae species are potential sources of natural mammalian arginase inhibitors with positive effects on vascular function. J. Nat. Prod. 2017, 80, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Arraki, K.; Richard, T.; Badoc, A.; Pédrot, E.; Bisson, J.; Waffo-Téguo, P.; Mahjoub, A.; Mérillon, J.M.; Decendit, A. Isolation, characterization and quantification of stilbenes from some Carex species. Rec. Nat. Prod. 2013, 7, 281–291. [Google Scholar]
- Senda, N.; Kubota, Y.; Hoshino, T.; Nozaki, H.; Hayashi, H.; Nakayama, M. Mass spectra of some stilbene oligomers from Carex species. J. Mass Spectrom. Soc. Jpn. 1995, 43, 45–51. [Google Scholar] [CrossRef]
- Fiorentino, A.; Ricci, A.; D’Abrosca, B.; Pacifico, S.; Golino, A.; Letizia, M.; Picolella, S.; Monaco, P. Potential food additives from Carex distachya roots: Identification and in vitro antioxidant properties. J. Agric. Food Chem. 2008, 56, 8218–8225. [Google Scholar] [CrossRef]
- Suzuki, K.; Shimizu, T.; Kawabata, J.; Mizutani, J. New 3,5,4′-trihydroxystilbene (resveratrol) oligomers from Carex fedia Nees var. miyabei (Franchet) T. Koyama (Cyperaceae). Agric. Biol. Chem. 1987, 51, 1003–1008. [Google Scholar]
- Li, L.; Henry, G.E.; Seerman, N.P. Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J. Agric. Food Chem. 2009, 57, 7282–7287. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Kim, M.; Kim, S.; Mahmud, H.A.; Islam, M.I.; Nam, K.W.; Cho, M.L.; Kwon, H.-S.; Song, H.Y. In vitro activity of alpha-viniferin isolated from the roots of Carex humulis against Mycobacterium tuberculosis. Pulm. Pharmacol. Ther. 2017, 46, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, J.; Ichikawa, S.; Kurihara, H.; Mizunati, J. Kobophenol-A, a unique tetrastilbene from Carex kobomugi Ohwi (Cyperaceae). Tetrahedron Lett. 1989, 30, 3785–3788. [Google Scholar] [CrossRef]
- Meng, Y.; Bourne, P.C.; Whiting, P.; Šik, V.; Dinan, L. Identification and ecdysteroid antagonist activity of three oligostilbenes from the seeds of Carex pendula (Cyperaceae). Phytochemistry 2001, 57, 393–400. [Google Scholar] [CrossRef]
- Kurihara, H.; Kawabata, J.; Ichikawa, S.; Mizutani, J. (-)-ɛ-viniferin and related oligostilbenes from Carex pumita Thunb. (Cyperaceae). Agric. Biol. Chem. 1990, 54, 1097–1099. [Google Scholar]
- Niesen, D.B.; Ma, H.; Yuan, T.; Bach, A.C.; Henry, G.E.; Seeram, N.P. Phenolic constituents of Carex vulpinoidea seeds and their tyrosinase inhibitory activities. Nat. Prod. Commun. 2015, 10, 491–493. [Google Scholar] [CrossRef]
- Dávid, C.Z.; Hohmann, J.; Vasas, A. Chemistry and pharmacology of Cyperaceae stilbenoids: A review. Molecules 2021, 26, 2794. [Google Scholar] [CrossRef]
- Gajbhiye, R.; Sarma, S.S.; Kumar, D.; Singh, S. The treasure trove of the genus Carex: A phytochemical and pharmacological review. Health Sci. Rev. 2024, 10, 100151. [Google Scholar] [CrossRef]
- Biais, B.; Krisa, S.; Cluzet, S.; Costa, G.D.; Waffo-Teguo, P.; Mérillon, J.M.; Richard, T. Antioxidant and cytoprotective activities of grapevine stilbenes. J. Agric. Food Chem. 2017, 65, 4952–4960. [Google Scholar] [CrossRef]
- Soural, I.; Vrchotová, N.; Tříska, J.; Balík, J.; Horník, Š.; Cuřínová, P.; Sýkora, J. Various extraction methods for obtaining stilbenes from grape cane of Vitis vinifera L. Molecules 2015, 20, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Moser, C.; Velasco, R.; Guella, G. Profiling of resveratrol oligomers, important stress metabolites, accumulating in the leaves of hybrid Vitis vinifera (Merzling × Toroldego) genotypes infected with Plasmopara viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef] [PubMed]


| Carex | Part of the Plant | Stilbenes | (m/z) [Da] | Literature |
|---|---|---|---|---|
| C. appessa | seeds | virgatanol | [M + H]+ 471 | [10] |
| resveratrol diglucoside | [M + H]+ 553 | |||
| piceatannol | [M + H]+ 245 | |||
| ε-viniferin | [M + H]+ 455 | |||
| C. buchananii | roots | kobophenol A | [11] | |
| C. capillacea | roots | (E)-miyabenol A | [11] | |
| C. ciliato-marginata | (+)-α-viniferin | [M + H]+ 679 | [12] | |
| pallidol | [M + H]+ 455 | |||
| kobophenol A | [M + H]+ 925 | |||
| C. cuprina | roots | carexinol A | [M + H]+ 941 | [11] |
| kobophenol A | [M + H]+ 925 | |||
| C. distachya | roots | pallidol diglucoside | [13] | |
| C. fedia | roots, rhizomes | ε-viniferin | [14] | |
| miyabenol A | ||||
| miyabenol B | ||||
| miyabenol C | ||||
| C. foliosissima | (+)-α-viniferin | [M + H]+ 679 | [12] | |
| pallidol | [M + H]+ 455 | |||
| kobophenol A | [M + H]+ 925 | |||
| C. folliculata | seeds | pallidol | [15] | |
| kobophenol A | ||||
| C. folliculata | seeds | pallidol | [7] | |
| kobophenol A | ||||
| C. glauca | roots | (E)-miyabenol C | [M + H]+ 681 | [14] |
| (+)-α-viniferin | [M + H]+ 679 | |||
| C. gynandra | aerial part | pallidol | [5] | |
| α-viniferin | ||||
| trans-miyabenol C | ||||
| kobophenol B | ||||
| C. hirta | roots | resveratrol-diglucoside | [M + H]+ 553 | [11] |
| (E)-miyabenol A | [M + H]+ 907 | |||
| C. humilis | roots | α-viniferin | [16] | |
| C. kobomugi | roots | kobophenol A | [17] | |
| C. morrowii | (+)-α-viniferin | [M + H]+ 679 | [12] | |
| pallidol | [M + H]+ 455 | |||
| kobophenol A | [M + H]+ 925 | |||
| C. multufolia | (+)-α-viniferin | [M + H]+ 679 | [12] | |
| pallidol | [M + H]+ 455 | |||
| kobophenol A | [M + H]+ 925 | |||
| C. pendula | seeds | cis-miyabenol A | [M + H]+ 907 | [18] |
| cis-miyabenol C | [M + H]+ 681 | |||
| kobophenol B | [M + H]+ 905 | |||
| C. pumita | roots, rhizomes | (−)-ε-viniferin | [19] | |
| miyabenols A, C | ||||
| C. vulpinoidea | seeds | vulpinoideol A | [M + Na]+ 481 | [20] |
| vulpinoideol B | [M + Na]+ 511 |
| Stilbenes | Retention Time [min] | m/z [M + H]+ [Da] |
|---|---|---|
| Pallidol (peak 1) | 4.02 | 455 |
| trans-resveratrol (peak 2) | 5.51 | 229 |
| Mixture cis+trans miyabenol A (peak 3) | 6.99 | 907 |
| trans-ɛ-viniferin (peak 4) | 7.13 | 455 |
| Z-miyabenol C (peak 5) | 7.45 | 681 |
| Trimer (peak 6) | 7.78 | 681 |
| Stilbenes/Sample Drying | C. acuta 2016 R. t. | C. acuta 2017 Lyof. | C. acuta 2017 R. t. | C. lepidocarpa 2016 R. t. | C. lepidocarpa 2017 Lyof. |
|---|---|---|---|---|---|
| Pallidol (peak 1) | 308 ± 54 | 270 ± 16 | 194 ± 28 | 14,478 ± 976 | 13,087 ± 1755 |
| trans-resveratrol (peak 2) | 88 ± 12 | 78 ± 3 | 84 ± 10 | 70 ± 6 | 66 ± 11 |
| Mixture cis+trans Miyabenol A (peak 3) | 3641 ± 505 | 4572 ± 586 | 2603 ± 227 | 1890 ± 273 | 847 ± 14 |
| trans-ɛ-viniferin (peak 4) | n.d. | n.d. | n.d. | 5035 ± 294 | 3777 ± 332 |
| cis-miyabenol C (peak 5) | 263 ± 5 | 487 ± 55 | 342 ± 32 | 4805 ± 279 | 3209 ± 185 |
| Trimer (peak 6) | 174 ± 18 | 102 ± 7 | 96 ± 9 | 5060 ± 147 | 4065 ± 267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tříska, J.; Vrchotová, N.; Horník, Š.; Sýkora, J.; Kučerová, A. Stilbenes in Carex acuta and Carex lepidocarpa. Molecules 2024, 29, 3840. https://doi.org/10.3390/molecules29163840
Tříska J, Vrchotová N, Horník Š, Sýkora J, Kučerová A. Stilbenes in Carex acuta and Carex lepidocarpa. Molecules. 2024; 29(16):3840. https://doi.org/10.3390/molecules29163840
Chicago/Turabian StyleTříska, Jan, Naděžda Vrchotová, Štěpán Horník, Jan Sýkora, and Andrea Kučerová. 2024. "Stilbenes in Carex acuta and Carex lepidocarpa" Molecules 29, no. 16: 3840. https://doi.org/10.3390/molecules29163840
APA StyleTříska, J., Vrchotová, N., Horník, Š., Sýkora, J., & Kučerová, A. (2024). Stilbenes in Carex acuta and Carex lepidocarpa. Molecules, 29(16), 3840. https://doi.org/10.3390/molecules29163840








