Abstract
Degenerative conditions, such as neurodegenerative disorders (Alzheimer’s disease (AD), Parkinson’s disease (PD)) and cardiovascular diseases, are complex, multifactorial disorders whose pathophysiology has not been fully elucidated yet. As a result, the available treatment options cannot eliminate these diseases radically, but only alleviate the symptoms. Both inflammatory processes and oxidation are key factors in the development and evolution of neurodegeneration, while acetylcholinesterase inhibitors are the most used therapeutic options against AD. In this work, following the multi-targeting compound approach, we designed and synthesized a series of proline and gamma-aminobutyric acid (GABA) amides with various acidic moieties that possess an antioxidant and/or anti-inflammatory potency. Proline is the pharmacophore of nootropic drugs (e.g., piracetam) used for memory improvement, while GABA is the main inhibitory neurotransmitter in the central nervous system. The designed molecules were subjected to a preliminary screening of their bioactivity in antioxidant and anti-inflammatory assays, as well as against acetylcholinesterase. Most of the synthesized compounds could inhibit lipid peroxidation (IC50 as low as 8 μΜ) and oxidative protein glycation (inhibition of up to 48%) and reduce the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH). In addition, all of the compounds were moderate inhibitors of lipoxygenase (LOX) (up to 46% at 100 μΜ) and could decrease carrageenan-induced paw edema in rats by up to 55%. Finally, some of the compounds were moderate acetylcholinesterase inhibitors (IC50 as low as 219 μΜ). The results confirmed the design rationale, indicating that the compounds could be further optimized as multi-targeting molecules directed against degenerative conditions.
1. Introduction
The term “neurodegenerative disorders” includes a variety of diseases characterized mainly by gradual neuron loss and dysfunction. As the life expectancy increases, so does the possibility of neurodegeneration development, which makes the discovery of new, more effective therapeutic agents necessary [1]. Alzheimer’s disease (AD) is the most common neurodegenerative disorder, accounting for approximately 70% of dementia cases worldwide [2]. Its main symptoms include memory loss, cognitive impairment, and behavioral disorders and its pathophysiology is characterized by tau protein hyperphosphorylation and amyloid precursor protein (APP) decomposition, leading to the formation of neurofibrillary tangles (NFTs) and Aβ amyloid peptide aggregation, respectively [3]. Although significant progress has been made in understanding AD pathophysiology and pathobiochemistry, the mechanisms that contribute to the initiation of neurodegeneration have not been fully elucidated. As a result, there are no available treatment options for eliminating AD radically, but only to alleviate the symptoms.
Oxidative stress has been found to play a key role in the progression of neurodegeneration. Since the brain consumes more than 20% of the respirated oxygen, oxidative stress is highly established in the central nervous system (CNS) [4]. Amyloid peptide aggregation and tau protein hyperphosphorylation are induced by extensive oxidative damage and advanced glycation end-products (AGEs), whereas mitochondrial dysfunction has been found to be of great significance for AD pathogenesis, as it is closely related to cell death and neurodegeneration [5,6,7]. Furthermore, the excitotoxicity produced by increased N-methyl-D-aspartate (NMDA) signaling enhances reactive oxygen species (ROS) formation, resulting in cell malfunction and neurodegeneration [8].
The neuroinflammation induced by microglial cells and astrocyte activation is another key factor in AD pathogenesis, as it is accountable for structural and functional changes in CNS cells, leading to apoptosis and neural loss [9]. Prostaglandins, especially PGE2, inhibit amyloid phagocytosis, thus enhancing the neurotoxicity of microglia, and the lipoxygenase (LOX) activity is closely associated with amyloid plaques and NFT formation [10,11]. Various chronic diseases may have different etiologies; however, prolonged oxidative stress is a common characteristic of these diseases. In aging and age-related conditions, oxidative stress is interrelated with inflammation in a two-way communication. Oxidative stress can induce and aggravate inflammation and vice versa, establishing a vicious cycle [12].
According to the cholinergic hypothesis, a decrease in the cholinergic neurotransmission caused by cholinergic neuron depletion contributes to memory loss and cognitive impairment during AD [13]. Since the acetylcholine (Ach) levels are lowered in the brains of patients with AD, acetylcholinesterase (AchE) inhibitors, which increase the Ach concentration, have been proven to be useful agents for the amelioration of AD symptoms [14]. Nevertheless, AchE inhibitors have failed to treat AD effectively, pointing out that the cholinergic hypothesis needs to be reconsidered and further studies about the relations of AchE with amyloid plaques, NFTs, and other factors taking part in AD pathogenesis have to be carried out [15].
By taking into account all the above-mentioned information and considering the multi-targeting compound approach [16], we designed and synthesized a series of novel compounds by the amidation of proline and GABA with various carboxylic acids. A proline ring is the main pharmacophore of nootropic drugs (e.g., piracetam) used for memory improvement [17], whereas GABA is the main inhibitory neurotransmitter in the CNS [18]. The carboxylic acids used for the synthesis of our derivatives included ((E)-3-methoxy-4-hydroxyphenyl)acrylic acid (ferulic acid) for compound 1c and ((E)-3,5-dimethoxy-4-hydroxyphenyl)acrylic acid (sinapic acid) for compound 2c, which exert antioxidant and neuroprotective activity in cells [19,20]; (E)-3,4-dimethoxy-phenyl)acrylic acid for compound 3c and cinnamic acid for compound 4c, which possess an anti-inflammatory potency due to their cinnamic residue [21]; and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) for compound 5c, which is a well-known water-soluble derivative of tocopherol (vitamin E) with an antioxidant capacity [22]. The bioactivity of these carboxylic acids and some of their derivatives has been extensively reported in the literature. More specifically, ferulic acid and various ferulic derivatives have demonstrated DPPH-reducing activity and a lipid oxidation inhibitory capacity [23]. In addition, Trolox amides have been found to act as radical scavengers (very good DPPH-reducing activity) and as moderate acetylcholinesterase inhibitors [24]. Sinapic ester derivatives have been proven to exert lipid peroxidation inhibitory activity [25], while plant extracts containing phenolic acid derivatives have been proven to inhibit lipid peroxidation and albumin oxidation, as well as reduce DPPH radicals [26,27]. Based on these results, we suggest that compounds with antioxidant and/or anti-inflammatory characteristics could be of great interest for the effective treatment of degenerative conditions, such as neurodegenerative disorders and cardiovascular diseases.
All the compounds (Figure 1) were tested as antioxidants. In particular, their activity against rat hepatic microsomal membrane lipid peroxidation, their interaction ability with the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH), and their potency against fructose-induced protein glycation were evaluated. Moreover, they were examined for their inhibition against AchE, while their anti-inflammatory properties were evaluated by the LOX inhibition capacity and their effect on rat paw edema induced by carrageenan.
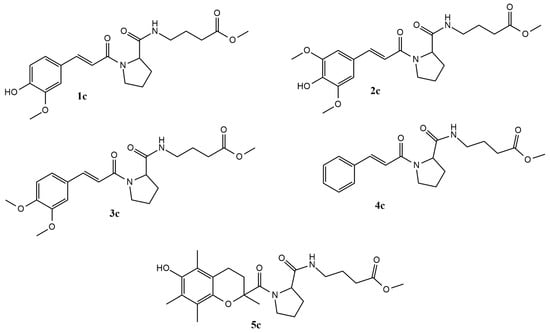
Figure 1.
Depiction of the synthesized molecules.
2. Results
2.1. Synthesis
As shown in Scheme 1, the synthetic process was carried out in three steps. In the first step, the respective carboxylic acids were amidated with proline methyl ester and the produced intermediates (compounds 1a–5a) were hydrolyzed in the second step. Finally, the carboxylic acids obtained from the second step (compounds 1b–5b) were amidated with GABA methyl ester and the final molecules (compounds 1c–5c) were isolated in yields of up to 78%.
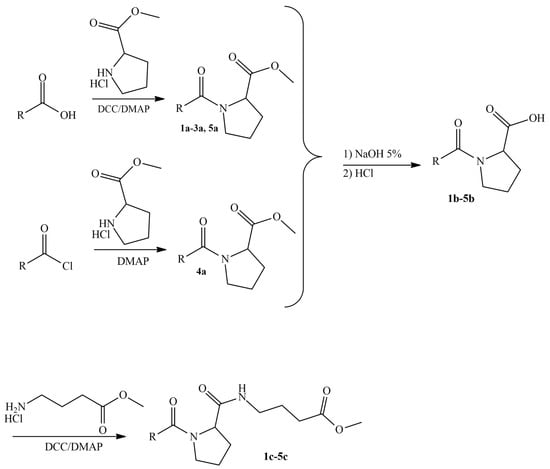
Scheme 1.
Synthetic procedure of compounds 1c–5c.
2.2. Antioxidant Activity
The synthesized compounds were tested for their inhibitory activity against the lipid peroxidation of rat hepatic microsomal membranes. The IC50 values of the compounds after 45 min of incubation are shown in Table 1.

Table 1.
Effect of compounds 1c–5c and Trolox on lipid peroxidation.
The time course of lipid peroxidation, as affected by various concentrations of the most active compound (5c), is shown in Figure 2.
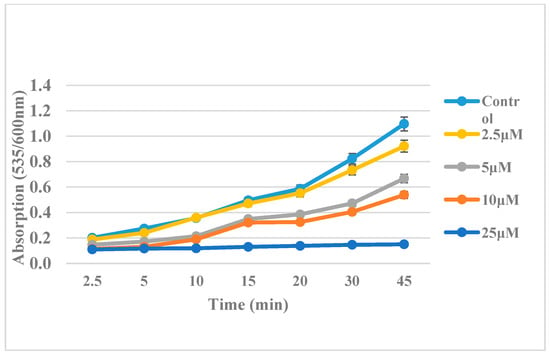
Figure 2.
Effect of various concentrations of compound 2 on time course of lipid peroxidation.
The antioxidant capacity of our compounds was also estimated by their reducing ability for DPPH free radicals (Table 2).

Table 2.
Interaction of compounds 1c–5c and Trolox, at various concentrations, with DPPH (200 μΜ).
The time course of the DPPH reaction with the compounds 1c, 2c, and 5c is shown in Figure 3.
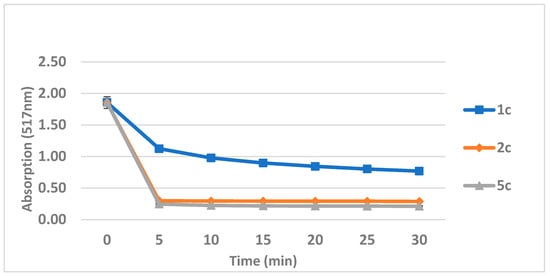
Figure 3.
DPPH interaction with compounds 1c, 2c, and 5c (concentration of 200 μΜ) as a function of time.
Furthermore, the antioxidant potency of the designed compounds was estimated by their inhibitory activity against copper-induced oxidative protein glycation. The compounds were dissolved in water, but a small amount of dimethylsulfoxide (DMSO) was added in some cases due to low water solubility. DMSO was found not to affect the glycation process. The inhibitory activity against the protein glycation of compounds 1–6 is shown in Table 3.

Table 3.
Inhibitory activity of compounds 1c–5c and aminoguanidine (concentration of 1mM) against protein glycation.
2.3. Acetylcholinesterase Inhibition
The IC50 values, as well as the molecular volumes of compounds 1c–5c, are shown in Table 4.

Table 4.
Inhibitory activity of compounds 1c–5c and physostigmine against acetylcholinesterase and their molecular volume, as calculated by Molinspiration.
2.4. Anti-Inflammatory Properties
The inhibitory activity of the designed compounds towards soybean lipoxygenase, as well as their clgoP values, are demonstrated in Table 5.

Table 5.
Inhibitory activity of compounds 1c–5c (concentration of 100 μM) and NDGA against soybean lipoxygenase and their clogP values.
Furthermore, the anti-inflammatory efficacy of the compounds was estimated in vivo by their ability to reduce carrageenan-induced edema in rat paws. The effect of the compounds on paw edema, as well as the respective activity of ibuprofen and naproxen (non-steroidal anti-inflammatory drugs used as references), are shown in Table 6.

Table 6.
Effect of compounds 1c–5c, naproxen, and ibuprofen on carrageenan-induced rat paw edema.
3. Discussion
3.1. Chemistry
The synthesis of our derivatives was carried out in three steps. In the first step, the respective carboxylic acid was amidated with methyl pyrrolidine-2 carboxylate hydrochloride (proline methyl ester hydrochloride) using dicyclohexylcarbodiimide (DCC) as a coupling agent and N,N-dimethyl-aminopyridine (DMAP). For the synthesis of compound 4a, the commercially available cinnamyl chloride was used as the starting material. The reactions were carried out in dichloromethane, using drops of dimethylformamide (DMF) due to the low solubility of the starting carboxylic acid. The expected intermediates were isolated with flash column chromatography in yields of up to 90%. In the second step, ester derivatives 1a–5a were hydrolyzed using a 5% aqueous NaOH solution and 1,4-dioxane as a solvent. The expected carboxylic acids were extracted with ethyl acetate from the aqueous phase after acidification and were obtained in yields of 86–97%. Finally, in the third step, compounds 1b–5b were amidated with methyl-4-aminobutanoate hydrochloride (GABA methyl ester hydrochloride) using DCC as a coupling agent and DMAP. The reactions were performed in dichloromethane and the expected products were purified and received with flash column chromatography in moderate yields, with the maximum reaching up to 78%.
3.2. Antioxidant Activity
As expected, compounds 3c and 4c could not inhibit lipid peroxidation, due to the lack of antioxidant structural characteristics. A group with an easily abstracted hydrogen atom is usually present in antioxidant compounds such as phenols. The phenoxyl radical formed was stabilized by conjugation with the phenyl ring. Compounds 3c and 4c did not have any easily abstracted hydrogen atoms. Compound 5c (Trolox derivative) was the most active, demonstrating an IC50 value of 8 μΜ, about three times more active than Trolox. Trolox is a vitamin E analogue with a strong antioxidant potency; therefore, its derivatives are expected to possess a high level of antioxidant activity. The higher antioxidant activity of compound 5c compared to Trolox can be attributed to its higher lipophilicity, which leads to a more effective approach to the membrane lipids. Compounds 1c and 2c had a lower inhibitory activity against lipid peroxidation, due to both the lower lipophilicity and low electron-donating effect of methoxy substituents, which could not stabilize the phenoxyl radical effectively. Comparing the activity of 1c and 2c, the mesomeric effect of the monomethoxy substituent in compound 1c led to a less stable phenoxyl radical than the dimethoxy substituents, and as a result, the sinapic derivative 2c was a more potent lipid peroxidation inhibitor than the ferulic analogue 1c. The effect of lipophilicity in the inhibitory activity against lipid peroxidation has been confirmed by previous studies in our lab, in which ferulic, sinapic, and Trolox analogues with higher clogP values had a stronger efficacy as lipid peroxidation inhibitors [28].
Moreover, compound 5c was the most active in reducing DPPH. It had a similar activity to that of Trolox at 200 μΜ and 100 μΜ (equal to and half of the DPPH concentration, respectively) and a slightly higher activity at 50 μΜ and 25 μΜ. Compound 2c was highly active at 200 μΜ, but not in lower concentrations, while compound 1c had a lower reducing capacity, even at 200 μΜ. Compounds 2c and 5c could interact rapidly with DPPH since their reaction was completed in the first five minutes, while the interaction between 1c and DPPH was somewhat slower, being completed in about 15 min. The results aligned with the lipid peroxidation inhibition results, pointing out that 5c is a stronger antioxidant agent than compounds 1c and 2c. Since DPPH is a lipophilic free radical, compounds with a high lipophilicity can approach it effectively, thus being able to present a greater DPPH-reducing activity. Indeed, derivatives with a high lipophilicity synthesized previously by us exerted a high level of reducing activity against DPPH [28,29]. Compounds 3c and 4c, with no antioxidant moieties, could not interact with DPPH at all.
Finally, the antioxidant capacity of our molecules was estimated by their inhibitory activity against oxidative protein glycation induced by copper ions. AGEs demonstrate neurotoxic effects, as they increase the APP levels and induce its degradation by beta-secretase. Moreover, they promote apoptosis-related gene expression, leading to neural loss [30]. Despite demonstrating a high antioxidant potency as a lipid peroxidation inhibitor and DPPH reducing agent, compound 5c did not show as high a protein glycation inhibitory activity level as compounds 1c and 2c. Its low water solubility combined with a more rigid structure may not have allowed the molecule to approach bovine albumin effectively, and thus prevented it from inhibiting oxidative albumin glycation. The higher anti-glycation activity of compounds 1c and 2c can be attributed to their acidic properties (phenolic derivatives), since albumin has a great number of basic amino acids, which can bind with acidic molecules [31]. Furthermore, an increased ability to form hydrogen bonds due to their multiple hydrogen acceptor groups, allowing a more effective approach between the molecules and the albumin, resulted in higher inhibitory activity against glycation [32]. Compounds 3c and 4c did not exert any inhibition against protein glycation, due to the absence of acidic substituents and antioxidant structural characteristics.
3.3. Acetylcholinesterase INHIBITION
Acetylcholinesterase (AchE) is the main pharmacological target of most anti-Alzheimer drugs, since cholinergic neuron loss has been closely related to memory loss and cognitive impairment [33]. The anti-AchE activity of the designed compounds was evaluated by their inhibition against acetylthiocholine hydrolysis, induced by AchE and derived from rat brain homogenate (Figure 4).
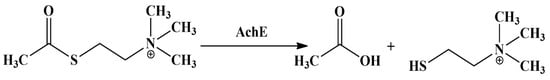
Figure 4.
Acetylthiocholine breakdown.
Compounds 1c–3c exerted moderate inhibitory activity against AchE, which can be attributed to the hydroxy- and methoxy-groups on the benzene ring that may have improved the effective bondage between the compound and the enzyme. No inhibition was observed in the presence of higher concentrations of acetylthiocholine, indicating that the designed compounds could act as competitive AchE inhibitors. The ferulic analogue 1c was slightly more active than 2c and 3c, a result consistent with previous results that demonstrated the anti-AchE activity of various ferulic analogues [34]. The lower efficacy of compounds 2c and 3c may be attributable to their larger molecular volume, which did not allow them to approach the active site of AchE effectively, despite having aromatic substituents contributing to the binding to the enzyme. Compound 5c had an even larger molecular volume, since the chroman ring is a bulky moiety; therefore, it was unable to interact with the active center of AchE and exerted no inhibitory activity. Finally, compound 4c, despite its low molecular volume, did not bind to the enzyme due to the absence of aromatic substituents.
3.4. Anti-Inflammatory Activity
Lipoxygenases catalyze arachidonic acid metabolism using molecular oxygen for the peroxidation of their substrate [35]. Their activity increases with age and is highly associated with AD pathogenesis, promoting amyloid plaque deposition and NFT formation [36,37].
All the compounds demonstrated moderate inhibitory activity against LOX (up to 40% at 100 μΜ), with compounds 2c and 4c being the most potent inhibitors. Despite its strong antioxidant potency, both as a lipid peroxidation inhibitor and as a DPPH radical scavenger, compound 5c was the least active LOX inhibitor. It can be assumed that the inhibitory activity is not attributable to the ability of the compounds to reduce free radicals produced by LOX, but to their ability to bind to the enzyme and prevent substrate binding. No inhibitory activity was observed when higher substrate (linoleic acid) concentrations were added, indicating that the designed compounds acted as weak competitive LOX inhibitors.
Finally, our compounds were tested in vivo for their ability to reduce rat paw edema induced by carrageenan. Carrageenan-induced inflammation causes histamine and serotine release (90 min after the administration) and continues with kinins secretion (2.5 h after the injection). During the last phase (more than 2.5 h after administration), prostaglandins are produced, and pro-inflammatory cytokines are released [38]. In our protocol, the inflammatory process was estimated 3.5 h after the carrageenan administration. Compounds 1c, 2c, and 5c exerted a high activity, which is consistent with their antioxidant potential and may be of great importance for their anti-inflammatory potency, as we have previously reported [39]. The anti-inflammatory properties of the respective carboxylic acids have been previously reported; therefore, these derivatives are also expected to possess anti-inflammatory activity [20,40]. Compound 3c demonstrated the strongest reducing activity against edema (55%), although it had no antioxidant efficacy. This can be explained by the fact that not only oxidative, but also various other biochemical procedures are involved in inflammatory processes. Taking into account the low LOX inhibitory activity of the designed compounds and the marked reduction in edema in vivo, we hypothesize that many other factors may be related to the inflammatory responses induced by carrageenan. All the rats that received the test compounds appeared normal, macroscopically and by autopsy, at the end of the procedure.
4. Materials and Methods
4.1. General
All the commercially available chemicals were purchased from Merck (Kenilworth, NJ, USA) or Sigma (St. Lewis, MO, USA). The NMR spectra (1H-NMR and 13C-NMR) were taken using an AGILENT DD2-500 MHz spectrometer. The IR spectra were recorded on a Perkin Elmer Spectrum BX FT-IR spectrometer (Waltham, MA, USA). The MS method would have been very useful for an additional confirmation of the structure and purity of the synthesized compounds, but unfortunately, we currently do not have access to such an apparatus. An MEL-TEMPII apparatus from Sigma-Aldrich was used for the determination of the melting points. The microanalyses were carried out on a Perkin-Elmer 2400 CHN elemental analyzer (Waltham, MA, USA). The progress of reactions was monitored using thin-layer chromatography (TLC silica gel 60 F24 aluminum sheets, Merck) and the spots were visualized under ultraviolet light.
4.2. Synthesis
4.2.1. Synthesis of Compounds 1a–3a and 5a
The corresponding carboxylic acid (3 mmol) was dissolved in dry dichloromethane using a small amount of DMF (up to 0.5 mL) to increase the solubility. Then, methyl pyrrolidine-2-carboxylate hydrochloride (3.6 mmol) and N,N-dimethylaminopyridine (DMAP, 3.6 mmol), and, subsequently (15 min afterwards), N,N dicyclohexylcarbodiimide (DCC, 3.6 mmol), were added, and the mixture was stirred at ambient temperature overnight. The resulting mixture was purified with filtration and washed with HCl (5%), NaHCO3 (5%), and a saturated NaCl solution, and then dried over Na2SO4. Finally, the solvent was distilled off under a low pressure and the final product was purified via flash column chromatography.
4.2.2. Synthesis of Compound 4a
Cinnamyl chloride (3 mmol) was dissolved in dry dichloromethane and mixed with methyl pyrrolidine-2 carboxylate hydrochloride (3.6 mmol) and DMAP (3.6 mmol). The mixture was stirred at ambient temperature overnight; it was washed successively with HCl (5%), NaHCO3 (5%), and a saturated NaCl solution; and the organic layer was dried over Na2SO4. Finally, the solvent was distilled off under a low pressure, followed by the purification of the final product via flash column chromatography.
4.2.3. Synthesis of Compounds 1b–5b
The corresponding amide of proline methyl ester (1a–5a) synthesized in the previous step was dissolved in 1,4-dioxane (20 mL) and an equal volume of an aqueous NaOH solution (5%) was added. The mixture was stirred for 45 min. Then, dioxane was removed under a reduced pressure, the aqueous phase was acidified using an HCl solution (10%), and it was extracted with ethyl acetate (3 × 20 mL). The combined organic fractions were dried over Na2SO4, and the solvent was evaporated under a reduced pressure.
4.2.4. General Procedure for the Synthesis of Compounds 1c–5c
The corresponding amide of proline (1 mmol) was dissolved in dry dichloromethane, using a small amount of DMF (up to 0.5 mL) to increase the solubility. Then, methyl 4-aminobutanoate hydrochloride (1.2 mmol), N,N-dimethylaminopyridine (DMAP, 1.2 mmol), and N,N dicyclohexylcarbodiimide (DCC, 1.2 mmol, after 15 min) were added, and the mixture was stirred overnight (at room temperature). The resulting mixture was purified with filtration and washed with HCl (5%), NaHCO3 (5%), and a saturated NaCl solution and dried over Na2SO4. Finally, the solvent was distilled off under a low pressure, followed by the purification of the final product via flash column chromatography.
(E)-methyl 1-(3-(4-hydroxy-3-methoxyphenyl)acryloyl)pyrrolidine-2-carboxylate (1a): Flash column chromatography (ethyl acetate/petroleum ether 3/1). Yellow powder, yield of 57%, mp. of 118–121 °C.
IR (nujol): 3405 (O-H), 3217 (N-H), 1708 (C=O ester), 1630 (C=O amide), 1600, 1574 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.64 (d J: 15.4 Hz, 1H, Ph-CH=CH-), 7.10 (d J: 8.2 Hz, 1H, 6-aromatic) 6.98 (s, 1H, 2-aromatic), 6.90 (d J: 8.2 Hz, 1H, 5-aromatic), 6.58 (d J: 15.4 Hz, 1H, Ph-CH=CH-), 4.61 (dd J: 8.0, 3.2 Hz, 1H, 2-pyrrolidine), 3.91 (s, 3H, Ph-OCH3), 3.67–3.73, 3.82–3.89 (m, 2H, 5-pyrrolidine), 3.75 (s, 3H, -OCH3) 2.09–2.26 (m, 2H, 3-pyrrolidine), 1.53–1.79 (m, 2H, 4-pyrrolidine).
13C-NMR (CDCl3): 172.25 (1C, -COOCH3), 165.18 (1C, -CO-N), 147.47 (1C, 4-aromatic), 146.64 (1C, 3-aromatic), 143.09 (1C, Ph-CH=C), 127.99 (1C, 1-aromatic), 122.16 (1C, 6-aromatic), 115.30 (1C, Ph-CH=CH), 114.71 (1C, 5-aromatic), 109.95 (1C, 2 aromatic), 59.02 (1C, 2-pyrrolidine), 55.95 (1C, Ph-OCH3), 52.28 (1C, -OCH3), 46.94 (1C, 5-pyrrolidine), 29.19 (1C, 3-pyrrolidine), 24.86 (1C, 4-pyrrolidine) (See Supplementary File).
(E)-1-(3-(4-hydroxy-3-methoxyphenyl)acryloyl)pyrrolidine-2-carboxylic acid (1b): Yellow powder, yield of 93%, mp. of 169–172 °C).
IR (nujol): 3405 (O-H), 3250 (COO-H), 3206 (N-H), 1732 (C=O carboxylic), 1633 (C=O amide), 1603, 1572 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 10.26 (s, 1H, -COOH), 7.76 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 7.14 (d J: 8.2 Hz, 1H, 6-aromatic), 7.01 (s, 1H, 2-aromatic), 6.94 (d J: 8.2 Hz, 1H, 5-aromatic), 6.54 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 4.74 (dd J: 7.9, 3.1 Hz, 1H, 2-pyrrolidine), 3.94 (s, 3H, Ph-OCH3), 3.69–3.80, (m, 2H, 5-pyrrolidine), 2.05–2.12 (m, 2H, 3-pyrrolidine), 1.83–2.01 (m, 2H, 4-pyrrolidine).
13C-NMR (CDCl3): 171.79 (1C, -COOH), 168.64 (1C, -CO-N), 148.23 (1C, 4-aromatic), 146.75 (1C, 3-aromatic), 145.58 (1C, Ph-CH=C), 126.91 (1C, 1-aromatic), 122.71 (1C, 6-aromatic), 114.90 (1C, Ph-CH=CH), 113.45 (1C, 5-aromatic), 110.31 (1C, 2 aromatic), 60.73 (1C, 2-pyrrolidine), 58.01 (1C, Ph-OCH3), 47.92 (1C, 5-pyrrolidine), 28.85 (1C, 3-pyrrolidine), 24.83 (1C, 4-pyrrolidine).
(E)-methyl 4-(1-(3-(4-hydroxy-3-methoxyphenyl)acryloyl)pyrrolidine-2-carboxamido)butanoate (1c): Flash column chromatography (ethyl acetate/acetone 3/1). Yellow powder, yield of 24%, mp. of 167–170 °C.
IR (nujol): 3405 (O-H), 3215 (N-H), 1712 (C=O ester), 1634, 1627 (C=O amide), 1600, 1572 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.65(d J: 15.3 Hz, 1H, Ph-CH=CH-), 7.44 (s, 1H, -NH) 7.11 (d J: 8.2 Hz, 1H, 6-aromatic), 7.00 (s, 1H, 2-aromatic) 6.92 (d J: 8.2 Hz, 1H, 5-aromatic), 6.58 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 4.72 (dd J: 7.9, 3.1 Hz, 1H, 2-pyrrolidine), 3.93 (s, 3H, Ph-OCH3), 3.60–3.63, 3.73–3.79 (m, 2H, 5-pyrrolidine), 3.64 (s, 3H, -OCH3), 3.21–3.31 (m, 2H, -NH-CH2-CH2-), 2.35 (t J: 7.4 Hz, 2H, -CH2-C=O) 2.09–2.18, 2.23–2.29 (m, 2H, 3-pyrrolidine), 1.77–1.88 (m, 4H, 4-pyrrolidine, -ΝH-CH2-CH2-).
13C-NMR (CDCl3): 173.65 (1C, -COOCH3), 171.29 (1C, -CO-NH), 166.64 (1C, -CO-N), 147.72 (1C, 4-aromatic), 146.27 (1C, 3-aromatic), 143.46 (1C, Ph-CH=C), 127.40 (1C, 1-aromatic), 122.33 (1C, 6-aromatic), 115.20 (1C, Ph-CH=CH), 114.80 (1C, 5-aromatic), 109.99 (1C, 2-aromatic), 60.00 (1C, 2-pyrrolidine), 55.97 (1C, Ph-OCH3), 51.80 (1C, -OCH3), 47.48 (1C, 5-pyrrolidine), 38.77 (1C, -NH-CH2), 31.38 (1C, -CH2-COOCH3), 26.97 (1C, 3-pyrroldine), 25.02 (1C, 4-pyrroldine), 24.72 (1C, -C-CH2-C).
Anal. calculated for C20H26N2O6: C, 61.53; H, 6.71; N, 7.18%. Found: C, 61.93; H, 7.08; N, 6.89%.
(E)-methyl 1-(3-(4-hydroxy-3,5-dimethoxyphenyl)acryloyl)pyrrolidine-2-carboxylate (2a): Flash column chromatography (ethyl acetate). Yellow oil, yield of 68%.
IR (nujol): 3415 (O-H), 3227 (N-H), 1711 (C=O ester), 1638 (C=O amide), 1602, 1575 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.62 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 6.75 (s, 2H, aromatic), 6.57 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 4.62 (dd J: 8.6, 4.1 Hz, 1H, 2-pyrrolidine), 3.91 (s, 6H, Ph-OCH3), 3.69–3.74, 3.85–3.89 (m, 2H, 5-pyrrolidine), 3.75 (s, 3H, -OCH3) 2.01–2.26 (m, 4H, 3,4-pyrrolidine).
13C-NMR (CDCl3): 172.88 (1C, -COOCH3), 165.01 (1C, -CO-N), 147.13 (2C, 3,5-aromatic), 143.27 (1C, Ph-CH=C), 136.70 (1C, 4-aromatic), 126.61 (1C, 1-aromatic), 115.75 (1C, Ph-CH=CH), 104.73 (2C, 2,6-aromatic), 59.01 (1C, 2-pyrrolidine), 56.36 (2C, Ph-OCH3), 52.23 (1C, -OCH3), 46.96 (1C, 5-pyrrolidine), 29.17 (1C, 3-pyrrolidine), 24.86 (1C, 4-pyrrolidine).
(E)-1-(3-(4-hydroxy-3,5-dimethoxyphenyl)acryloyl)pyrrolidine-2-carboxylic acid (2b): Yellow powder, yield of 86%, mp. of 94–98 °C.
IR (nujol): 3405 (O-H), 3275 (COO-H), 3202 (N-H), 1736 (C=O carboxylic), 1632 (C=O amide), 1602, 1570 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.73 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 6.76 (s, 2H, aromatic), 6.52 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 4.72 (dd J: 8.6, 4.1 Hz, 1H, 2-pyrrolidine), 3.91 (s, 6H, Ph-OCH3), 3.76–3.87 (m, 2H, 5-pyrrolidine), 3.70 (s, 3H, -OH) 2.48–2.54 (m, 1H, 3-pyrrolidine), 1.96–2.12 (m, 3H, 3-pyrrolidine, 4-pyrrolidine).
13C-NMR (CDCl3): 172.09 (1C, -COOH), 168.03 (1C, -CO-N), 147.24 (2C, 3,5-aromatic), 145.54 (1C, Ph-CH=C), 137.23 (1C, 4-aromatic), 125.90 (1C, 1-aromatic), 113.95 (1C, Ph-CH=CH), 105.40 (2C, 2,6-aromatic), 60.50 (1C, 2-pyrrolidine), 56.40 (2C, Ph-OCH3), 48.83 (1C, 5-pyrrolidine), 27.20 (1C, 3-pyrrolidine), 24.79 (1C, 4-pyrrolidine).
(E)-methyl 4-(1-(3-(4-hydroxy-3,5-dimethoxyphenyl)-acryloyl)pyrrolidine-2-carboxamido)-butanoate (2c): Flash column chromatography (ethyl acetate/acetone 3/1). Yellow powder, yield of 58%, mp. of 158–159 °C.
IR (nujol): 3408 (O-H), 3223 (N-H), 1704 (C=O ester), 1644, 1631 (C=O amide), 1602, 1578 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.63 (d J:15.3 Hz, 1H, Ph-CH=CH-), 7.42 (s, 1H, -NH) 7.00 (s, 2H, aromatic), 6.57 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 4.70 (dd J: 8.5, 4.1 Hz, 1H, 2-pyrrolidine), 3.92 (s, 6H, Ph-OCH3), 3.73–3.79 (m, 1H, 5-pyrrolidine), 3.64 (s, 3H, -OCH3), 3.19–3.36 (m, 3H, -NH-CH2-CH2-, 5-pyrrolidine), 2.34 (t J: 7.4 Hz, 2H, -CH2-C=O) 1.85–1.89, 2.01, 2.06 (m, 2H, 3-pyrrolidine), 1.77–1.85 (m, 4H, 4-pyrroldine, -ΝH-CH2-CH2-).
13C-NMR (CDCl3): 173.64 (1C, -COOCH3), 171.24 (1C, -CO-NH), 166.50 (1C, -CO-N), 147.20 (2C, 3,5-aromatic), 143.68 (1C, Ph-CH=C), 136.96 (1C, 4-aromatic), 126.35 (1C, 1-aromatic), 115.50 (1C, Ph-CH=CH), 105.09 (2C, 2,6-aromatic), 60.01 (1C, 2-pyrrolidine), 56.41 (2C, Ph-OCH3), 56.35 (1C, -OCH3), 47.55 (1C, 5-pyrrolidine), 38.80 (1C, -NH-CH2), 31.41 (1C, -CH2-COOCH3), 26.94 (1C, 3-pyrrolidine), 25.01 (1C, 4-pyrrolidine), 24.72 (1C, -C-CH2-C).
Anal. calculated for C21H28N2O7: C, 59.99; H, 6.71; N, 6.66%. Found: C, 60.08; H, 6.88; N, 6.54%.
(E)-methyl 1-(3-(3,4-dimethoxyphenyl)acryloyl)pyrrolidine-2-carboxylate (3a): Flash column chromatography (ethyl acetate/petroleum ether 3/1). Colorless oil, yield of 78%.
IR (nujol): 3226 (N-H), 1709 (C=O ester), 1636 (C=O amide), 1601, 1568 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.65 (d J: 15.5 Hz, 1H, Ph-CH=CH-), 7.10 (d J: 8.2 Hz, 1H, 6-aromatic) 7.02 (s, 1H, 2-aromatic), 6.84 (d J: 8.2 Hz, 1H, 5-aromatic), 6.59 (d J: 15.4 Hz, 1H, Ph-CH=CH-), 4.61 (dd J: 8.5, 4.2 Hz, 1H, 2-pyrrolidine), 3.90 (s, 3H, Ph-OCH3), 3.89 (s, 3H, Ph-OCH3) 3.68–3.72, 3.83–3.88 (m, 2H, 5-pyrrolidine), 3.74 (s, 3H, -OCH3) 2.09–2.27 (m, 2H, 3-pyrrolidine), 1.93–2.06 (m, 2H, 4-pyrrolidine).
13C-NMR (CDCl3): 172.88 (1C -COOCH3), 165.11 (1C -CO-N), 150.67 (1C, 4-aromatic), 149.07 (1C, 3-aromatic), 142.91 (1C, Ph-CH=C), 128.07 (1C, 1-aromatic), 122.09 (1C, 6-aromatic), 115.67 (1C, Ph-CH=CH), 111.06 (1C, 2-aromatic), 110.01 (1C, 5-aromatic), 59.02 (1C, 2-pyrrolidine), 55.93 (2C, Ph-OCH3), 52.21 (1C, -OCH3), 46.94 (1C, 5-pyrrolidine), 29.16 (1C, 3-pyrrolidine), 24.84 (1C, 4-pyrrolidine).
(E)-1-(3-(3,4-dimethoxyphenyl)acryloyl)pyrrolidine-2-carboxylic acid (3b): White powder, yield of 95%, mp. of 186–188 °C.
IR (nujol): 3285 (COO-H), 3217 (N-H), 1732 (C=O carboxylic), 1631 (C=O amide), 1599, 1567 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.78 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 7.16 (d J: 8.2 Hz, 1H, 6-aromatic) 7.04 (s, 1H, 2-aromatic), 6.88 (d J: 8.2 Hz, 1H, 5-aromatic), 6.55 (d J: 15.3 Hz, 1H, Ph-CH=CH-), 4.74 (dd J: 8.5, 4.2 Hz, 1H, 2-pyrrolidine), 3.92 (s, 6H, Ph-OCH3), 3.65–3.71, 3.75–3.82 (m, 2H, 5-pyrrolidine), 2.06–2.15 (m, 2H, 3-pyrrolidine), 1.92–2.01 (m, 2H, 4-pyrrolidine).
13C-NMR (CDCl3): 171.49 (1C, -COOH), 168.57 (1C, -CO-N), 148.23 (1C, 4-aromatic), 146.75 (1C, 3-aromatic), 145.58 (1C, Ph-CH=C), 126.91 (1C, 1-aromatic), 122.71 (1C, 6-aromatic), 114.90 (1C, Ph-CH=CH), 113.45 (1C, 2-aromatic), 110.31 (1C, 5-aromatic), 60.73 (1C, 2-pyrrolidine), 58.11 (2C, Ph-OCH3), 47.92 (1C, 5-pyrrolidine), 28.85 (1C, 3-pyrrolidine), 24.83 (1C, 4-pyrrolidine).
(E)-methyl 4-(1-(3-(3,4-dimethoxyphenyl)acryloyl)pyrrolidine-2-carboxamido)butanoate (3c): Flash column chromatography (ethyl acetate/petroleum ether 3/1). Yellow powder, yield of 78% mp. of 158–159 °C.
IR (nujol): 3220 (N-H), 1715 (C=O ester), 1631, 1619 (C=O amide), 1598, 1564 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.68 (d J: 15.4 Hz, 1H, Ph-CH=CH-), 7.45 (s, 1H, -NH) 7.14 (d J: 8.2 Hz, 1H, 6-aromatic), 7.05 (s, 1H, 2-aromatic) 6.88 (d J: 8.2 Hz, 1H, 5-aromatic), 6.61 (d J: 15.4 Hz, 1H, Ph-CH=CH-), 4.72 (d J: 7.9 Hz, 1H, 2-pyrrolidine), 3.93 (s, 6H, Ph-OCH3), 3.92 (s, 6H, Ph-OCH3), 3.73–3.79 (m, 1H, 5-pyrrolidine), 3.65 (s, 3H, -OCH3), 3.20–3.35 (m, 3H, -NH-CH2-CH2-, 5-pyrrolidine), 2.35 (t J: 7.4 Hz, 2H, -CH2-C=O) 2.01, 2.20 (m, 2H, 3-pyrrolidine), 1.75–1.88 (m, 4H, 4-pyrrolidine, -ΝH-CH2-CH2-).
13C-NMR (CDCl3): 173.66 (1C, -COOCH3), 171.27 (1C, -CO-NH), 166.61 (1C, -CO-N), 150.91 (1C, 4-aromatic), 149.16 (1C, 3-aromatic), 143.31 (1C, Ph-CH=C), 127.85 (1C, 1-aromatic), 122.22 (1C, 6-aromatic), 115.52 (1C, Ph-CH=CH), 111.12 (1C, 5-aromatic), 110.06 (1C, 2-aromatic), 60.01 (1C, 2-pyrrolidine, 55.96 (2C, Ph-OCH3), 51.61 (1C, -OCH3), 47.51 (1C, 5-pyrrolidine), 38.77 (1C, -NH-CH2), 31.40 (1C, -CH2-COOCH3), 26.98 (1C, 3-pyrrolidine), 25.03 (1C, 4-pyrrolidine), 24.73 (1C, -C-CH2-C).
Anal. calculated for C21H28N2O6: C, 62.36; H, 6.98; N, 6.93%. Found: C, 62.48; H, 7.36; N, 6.99%.
Methyl 1-cinnamoylpyrrolidine-2-carboxylate (4a): Flash column chromatography (ethyl acetate/petroleum ether 1/1). White powder, yield of 90%, mp. of 88–89 °C.
IR (nujol): 3222 (N-H), 1709 (C=O ester), 1628 (C=O amide), 1601, 1572 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.72 (d J: 15.4 Hz, 1H, Ph-CH=CH-), 7.49–7.53 (m, 2H, aromatic), 7.32–7.43 (m, 3H, aromatic), 6.75 (d J: 15.4 Hz, 1H, Ph-CH=CH-), 4.62 (dd J: 7.7, 3.1 Hz, 1H, 2-pyrrolidine), 3.75 (s, 3H, -OCH3) 3.69–3.73, 3.84–3.88 (m, 2H, 5-pyrrolidine), 2.09–2.17, 2.20–2.27 (m, 2H, 3-pyrrolidine), 1.98–2.08 (m, 2H, 4-pyrrolidine).
13C-NMR (CDCl3): 172.81 (1C, -COOCH3), 164.86 (1C, -CO-N), 142.92 (1C, Ph-CH=C), 135.10 (1C, 1-aromatic), 129.74 (1C, 4-aromatic), 128.77 (2C, 3,5-aromatic), 127.92 (2C, 2,6-aromatic), 117.92 (1C, Ph-CH=CH), 59.04 (1C, 2-pyrrolidine), 52.23 (1C, -OCH3), 46.95 (1C, 5-pyrrolidine), 29.16 (1C, 3-pyrrolidine), 24.95 (1C, 4-pyrrolidine).
1-cinnamoylpyrrolidine-2-carboxylic acid (4b): White powder, yield of 92%, mp. of 180–182 °C.
IR (nujol): 3287 (COO-H), 3214 (N-H), 1732 (C=O carboxylic), 1634 (C=O amide), 1602, 1571 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.62 (d J: 15.5 Hz, 1H, Ph-CH=CH-), 7.44–7.53 (m, 2H, aromatic), 7.29–7.43 (m, 3H, aromatic), 6.75 (d J: 15.5 Hz, 1H, Ph-CH=CH-), 4.54 (dd J: 8.1, 3.3 Hz, 1H, 2-pyrrolidine), 3.57–3.73, 3.76–3.88 (m, 2H, 5-pyrrolidine), 1.99–2.24 (m, 4H, 3,4-pyrrolidine).
13C-NMR (CDCl3): 178.48 (1C, -COOH), 169.69 (1C, -CO-N), 147.21 (1C, Ph-CH=C), 139.72 (1C, 1-aromatic), 134.51 (1C, 4-aromatic), 133.55 (2C, 3,5-aromatic), 132.61 (2C, 2,6-aromatic), 123.02 (1C, Ph-CH=CH), 63.98 (1C, 2-pyrrolidine), 51.84 (1C, 5-pyrrolidine), 33.67 (1C, 3-pyrrolidine), 29.46 (1C, 2-pyrrolidine).
(E)-methyl 4-(1-cinnamoylpyrrolidine-2-carboxamido)butanoate (4c): Flash column chromatography (ethyl acetate). White powder, yield of 75%, mp. of 76–78 °C.
IR (nujol): 3215 (N-H), 1716 (C=O ester), 1634, 1623 (C=O amide), 1605, 1577 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 7.72 (d J: 15.5 Hz, 1H, Ph-CH=CH-), 7.50–7.58 (m, 2H, aromatic), 7.33–7.45 (m, 3H, aromatic), 6.75 (d J: 15.5 Hz, 1H, Ph-CH=CH-), 4.70 (dd J: 8.0, 3.3 Hz, 1H, 2-pyrrolidine), 3.64 (s, 3H, -OCH3), 3.58–3.62, 3.73–3.79 (m, 2H, 5-pyrrolidine), 3.17–3.36 (m, 2H, -NH-CH2-CH2-), 2.34 (t J: 7.4 Hz, 2H, -CH2-C=O), 2.00–2.20 (m, 2H, 3-pyrrolidine), 1.77–1.90 (m, 4H, 4-pyrrolidine, -NH-CH2-CH2-).
13C-NMR (CDCl3): 173.64 (1C, -COOCH3), 171.18 (1C, -CO-NH), 166.31 (1C, -CO-N), 143.28 (1C, Ph-CH=C), 134.86 (1C, 1-aromatic), 129.98 (1C, 4-aromatic), 128.85 (2C, 3,5-aromatic), 127.97 (2C, 2,6-aromatic), 117.83 (1C, Ph-CH=CH), 60.05 (1C, 2-pyrrolidine), 51.59 (1C, -OCH3), 47.51 (1C, 5-pyrrolidine), 38.79 (1C, -NH-CH2), 31.39 (1C, -CH2-COOCH3), 27.05 (1C, 3-pyrrolidine), 25.02 (1C, 4-pyrrolidine), 24.70 (1C, -C-CH2-C).
Anal. calculated for C19H24N2O4: C, 62.53; H, 6.89; N, 4.56%. Found: C, 62.79; H, 7.12; N, 4.66%.
Methyl 1-(6-hydroxy-2,5,7,8-tetramethylchroman-2-carbonyl)pyrrolidine-2-carboxylate (5a): Flash column chromatography (ethyl acetate/petroleum ether 2/3). Colorless oil, yield of 73%.
IR (nujol): 3401 (O-H), 3229 (N-H), 1720 (C=O ester), 1633 (C=O amide), 1608, 1570 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 4.36 (dd J: 11.1, 5.6 Hz, 1H, 2-pyrrolidine), 3.41–3.48, 4.04–4.16 (m, 2H, 5-pyrrolidine), 3.58, 3.71 (s, 3H, -OCH3), 2.49–2.73 (m, 4H, 3,4-chroman), 2.17 (s, 3H, Ph-CH3), 2.16 (s, 3H, Ph-CH3), 2.06, 2.10 (s, 3H, Ph-CH3), 1.68–1.96 (m, 4H, 3,4-pyrrolidine), 1.54, 1.61 (s, 3H, 2-CH3).
13C-NMR (CDCl3): 173.19/172.95 (1C, -COOCH3), 172.79/172.50 (1C, -CO-N), 145.31/145.23 (1C, 9-chroman), 144.68/144.44 (1C, 6-chroman), 121.54/121.50 (1C, 5-chroman), 121.29/121.20 (1C, 8-chroman), 118.94/118.77 (1C, 7-chroman), 118.24/118.20 (1C, 10-chroman), 78.81/78.67 (1C, 2-chroman), 60.80/60.39 (1C, 2-pyrrolidine), 52.01/51.84 (1C, -OCH3), 48.12/47.98 (1C- 5-pyrrolidine), 30.52/30.51 (1C, 3-pyrrolidine), 27.81/27.58 (1C, 3-chroman), 26.06/25.43 (1C, 4-pyrrolidine), 24.96/24.41 (1C, 4-chroman), 21.06/20.67 (1C, 2-CH3), 12.24/12.20 (1C, 5-CH3), 12.16/12.13 (1C, 8-CH3), 11.28/11.25 (1C, 7-CH3).
2-(6-hydroxy-2,5,7,8-tetramethylchroman-2-carbonyl)pyrrolidine-1-carboxylic acid (5b): White powder, yield of 97%, mp. of 135–138 °C.
IR (nujol): 3401 (O-H), 3280 (COO-H), 3220 (N-H), 1741 (C=O carboxylic), 1639 (C=O amide), 1606, 1570 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 4.42 (dd J: 11.1, 5.6 Hz, 1H, 2-pyrrolidine), 3.38–3.50, 4.03–4.09 (m, 2H, 5-pyrrolidine), 2.51–2.73 (m, 4H, 3,4-chroman), 2.16 (s, 3H, Ph-CH3), 2.14 (s, 3H, Ph-CH3), 2.04, 2.08 (s, 3H, Ph-CH3), 1.63–2.01 (m, 4H, 3,4-pyrrolidine), 1.56, 1.61 (s, 3H, 2-CH3).
13C-NMR (CDCl3): 175.58/175.09 (1C, -COOH), 174.80/174.27 (1C, -CO-N), 145.42/145.36 (1C, 9-chroman), 144.51/144.28 (1C, 6-chroman), 121.51 (1C, 5-chroman), 121.40 (1C, 8-chroman), 119.10/119.02 (1C, 7-chroman), 118.08/118.03 (1C, 10-chroman), 78.89/78.78 (1C, 2-chroman), 60.86/60.42 (1C, 2-pyrrolidine), 48.44/48.31 (1C, 5-pyrrolidine), 30.61/30.46 (1C, 3-pyrrolidine), 27.04/26.97 (1C, 3-chroman), 25.94/25.50 (1C, 4-pyrrolidine), 25.01/24.38 (1C, 4-chroman), 21.02/20.67 (1C, 2-CH3), 12.25/12.21 (1C, 5-CH3), 12.15/12.11 (1C, 8-CH3), 11.27/11.18 (1C, 7-CH3).
Methyl 4-(1-(6-hydroxy-2,5,7,8-tetramethylchroman-2-carbonyl)pyrrolidine-2-carboxamido)-butanoate (5c): Flash column chromatography (ethyl acetate/acetone 8/1). Yellow powder, yield of 45%, mp. of 59–61 °C.
IR (nujol): 3401 (O-H), 3227 (N-H), 1712 (C=O ester), 1638, 1628 (C=O amide), 1608, 1570 (C-C aromatic) cm−1.
1H-NMR (CDCl3): 4.42 (dd J: 11.1, 5.6 Hz, 1H, 2-pyrrolidine), 3.66, 3.68 (s, 3H, -OCH3), 3.24–3.29, 3.43–3.52 (m, 2H, 5-pyrrolidine), 2.70–2.79, 3.08–3.16 (m, 2H, -NH-CH2-CH2-) 2.49–2.68 (m, 4H, 3,4-chroman), 2.34 (t J: 7.4 Hz, 2H, -CH2-C=O), 2.18, 2.20 (s, 3H, Ph-CH3), 2.14, 2.15 (s, 3H, Ph-CH3), 2.04, 2.08 (s, 3H, Ph-CH3), 1.89–2.02 (m, 2H, 3-pyrrolidine), 1.66–1.84 (m, 2H, 4-pyrrolidine), 1.58, 1.61 (s, 3H, 2-CH3).
13C-NMR (CDCl3): 174.40 (1C, -COOCH3), 173.64 (1C, -CO-NH), 171.42 (1C, -CO-N), 145.83 (1C, 9-chroman), 144.81 (1C, 6-chroman), 123.45 (1C, 5-chroman), 121.75/121.40 (1C, 8-chroman), 120.68/120.32 (1C, 7-chroman), 118.16 (1C, 10-chroman), 78.75 (1C, 2-chroman), 61.47/60.96 (1C, 2-pyrrolidine), 51.90/51.61 (1C, -OCH3), 48.26/48.17 (1C, 5-pyrrolidine), 38.68/38.50 (1C, -NH-CH2), 31.78/31.30 (1C, -CH2-COOCH3), 31.09/30.85 (3-pyrrolidine), 27.99 (3-pyrrolidine), 25.76/25.52 (4-pyrrolidine), 24.75/24.60 (4-chroman), 24.38/24.29 (1C, -C-CH2-C), 21.48/20.97 (1C, 2-CH3), 12.39 (1C, 5-CH3), 12.25/12.13 (1C, 8-CH3), 11.54/11.28 (1C, 7-CH3).
Anal. calculated for C24H34N2O6: C, 64.55; H, 7.67; N, 6.66%. Found: C, 64.38; H, 7.29; N, 6.13%.
4.3. Biological Evaluation
κ-Carrageenan and soybean lipoxygenase type I-B were purchased from Sigma (St. Louis, MO, USA). For the in vivo experiments, Wistar rats (180–220 g, 3–4 months old) were kept at the Centre of the School of Veterinary Medicine (EL54 BIO42), Aristotle University of Thessaloniki, which is registered by the official state veterinary authorities (presidential degree 56/2013, in harmonization with European Directive 2010/63/EEC). The experimental protocols were approved by the Animal Ethics Committee of the Prefecture of Central Macedonia (No. 270079/2500).
4.3.1. In Vitro Lipid Peroxidation Inhibition
The rat liver microsomal fraction was inactivated with heat at 90 °C for 90 s, and ascorbic acid (0.2 mM) and Fe2SO4 (10 μΜ) were added for the induction of its peroxidation. The compounds dissolved in dimethylsulfoxide (DMSO) were added (concentrations of 1 μΜ–1 mM). Aliquots were removed from the reaction mixture for 45 min (37 °C). The induction of peroxidation was assessed at 535/600 nm as 2-thiobarbituric acid-reactive material. None of the solvents or compounds affected the assay [28].
4.3.2. In Vitro Interaction with the Stable Free Radical 1,1-Diphenyl-2-Picrylhydrazyl (DPPH)
The tested compounds, dissolved in absolute ethanol at final concentrations of 25 up to 200 μΜ, were mixed with DPPH and dissolved in the same solvent at a final concentration of 200 μΜ. The absorbance at 517 nm was recorded every 5 min for 30 min [41].
4.3.3. In Vitro Protein Glycation Inhibition
Bovine serum albumin (BSA, 4 mg/mL) was incubated with fructose (250 mM), Cu2+ (10 μΜ), and NaN3 (0.015%) in a 120 mM phosphate buffer (pH of 7.4) at 37 °C for 72 h. The purification of the glycation product was based on protein precipitation and washing with trichloroacetic acid (TCA). The concentration of the glycation-modified protein was estimated by fluorescence measurements (excitation of 340 nm, emission of 410 nm). Incubations performed under the same conditions, yet in the absence of fructose, were used as a control. All the fluorescence measurements were expressed relatively to the standard quinine sulphate solution (1 μg/mL) [28]. All compounds were stable during the experiment.
4.3.4. In Vitro Evaluation of Acetylcholinesterase Activity
The tested compounds, dissolved in 60% ethanol, were mixed with brain homogenate (20 mg of tissue per mL) from untreated rats (in 0.1 M phosphate buffer with a pH of 8) and acetylthiocholine (0.5 mM). The enzyme activity was assessed by evaluating the reaction product of the liberated thiocholine with 5,5-dithio-bis-(2-nitrobenzoic acid (DTNB) at 412 nm. The solvent system was examined and did not interfere with the assay [42].
4.3.5. In Vitro Evaluation of Lipoxygenase Activity
The reaction mixture (total volume of 3 mL) contained 100 μL of the test compounds (100 μM) or the solvent (absolute ethanol, control), 200 μL of soybean LOX (250 u/mL, in a 0.9% NaCl solution), and 2.6 mL of Tris–HCl buffer with a pH of 9.0. The reaction was initiated by the addition of 100 μL sodium linoleate (100 μM) in the sample mixture (with an equal volume of buffer added to the reference solution). The reaction was followed for 7 min at 28 °C, and the absorbance (234 nm) of a conjugated diene structure was recorded. The performance of the assay was verified using NDGA as a reference [43].
4.3.6. In Vivo Evaluation of Anti-Inflammatory Activity
The examined compounds were suspended in water with a small amount of Tween 80 and administered i.p. (0.15 mmol/kg) to the rats, just after an i.d. injection of 0.1 mL of an aqueous carrageenan solution (1% w/v) in the rear paw of the rats. The produced edema was estimated after 3.5 h as the paw weight increased [44].
4.3.7. Statistical Analysis
All the tests in the in vitro assays were performed in triplicate and the standard deviations were always no more than 10% of the mean values (as calculated using MS Excel 2016). In the in vivo assay, the statistical significance of the results was estimated using p values, which were calculated according to Student’s t-test (https://www.graphpad.com/quickcalcs/ttest1.cfm) (accessed on 21 March 2024).
5. Conclusions
It is evident from the literature that oxidative stress and inflammation play a significant role in the development and progression of neurodegenerative conditions [2,3,4,5,6,7,8,9,10,11,12]. The involvement of various biochemical pathways in the pathogenesis of AD makes its radical treatment difficult, and the current treatment options are useful for alleviating only the symptoms. Multi-acting compounds could be of use in the therapeutic regimens of AD, since they affect many pathways associated with its pathophysiology.
In our work, we suggested the design and synthesis of a series of proline and GABA analogues containing antioxidant and/or anti-inflammatory moieties, such as ferulic acid, cinnamic acid, and Trolox. The Trolox derivative 5c was a very strong lipid peroxidation inhibitor (IC50 of 8 μΜ) and an effective DPPH radical scavenger. Compounds 1c and 2c were weaker antioxidants, as their lipid peroxidation inhibition and DPPH-reducing capacity showed, although they demonstrated a higher inhibitory effect against protein glycation than compound 5c. In addition, compounds 1c–3c were moderate AchE inhibitors, while 4c, with no aromatic substituents, and 5c, with the bulky chroman group, could not inhibit the enzyme. Finally, all the compounds could inhibit LOX up to 40% at 100 μΜ and reduce rat paw edema. Compound 5c, with great antioxidant properties, and 3c, with no antioxidant capacity, were the most active against paw edema, indicating that inflammation is a complex procedure and various signaling pathways are involved in it. In conclusion, our results indicate that compounds with combined molecular characteristics could yield pluripotent derivatives that could act as such or as lead compounds towards the evolution of effective treatments for degenerative conditions such as neurodegenerative disorders.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29163763/s1, NMR spectra.
Author Contributions
Investigation, G.P. and P.T.-N.; supervision, E.A.R.; writing—review and editing, G.P., P.T.-N. and E.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Animal Ethics Committee of the Prefecture of Central Macedonia (No. 270079/2500).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available from the corresponding author upon a relevant request.
Conflicts of Interest
No conflicts of interest are declared by the authors.
References
- Savelieff, M.; Nam, G.; Kang, J.; Jin Lee, H.; Lee, M.; Hee Lim, M. Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev. 2019, 119, 1221–1322. [Google Scholar] [CrossRef] [PubMed]
- Pagali, S.R.; Kumar, R.; LeMahieu, A.M.; Basso, M.R.; Boeve, B.F.; Croarkin, R.E.; Geske, J.R.; Hassett, L.C.; Huston, J.; Kung, S.; et al. Efficacy and safety of transcranial magnetic stimulation on cognition in mild cognitive impairment, Alzheimer’s disease, Alzheimer’s disease-related dementias, and other cognitive disorders: A systematic review and meta-analysis. Int. Psycogeriatrics 2024, 8, 1–49. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef] [PubMed]
- Batkulwar, K.; Godbole, R.; Banarjee, R.; Kassaar, O.; Williams, R.J.; Kulkarni, M.J. Advanced Glycation End Priducts Modulate Amyloidogenic APP Processing and Tau Phosphorylation: A Mechanistic Link between Glycation and the Development of Alzheimer’s Disease. Chem. Neurosci. 2018, 9, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Kamat, K.; Kalani, A.; Rai, S.; Swarknar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2014, 53, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Bai, F. The Association of Tau with Mitochondrial Dysfunction in Alzheimer’s Disease. Front. Neurosci. 2018, 22, 163. [Google Scholar] [CrossRef] [PubMed]
- Binvignat, O.; Olloquequi, J. Excitotoxicity as a Target Against Neurodegenerative Processes. Curr. Phar. Des. 2020, 269120, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Hemonnot, A.-L.; Hua, J.; Ulmann, L.; Hirbec, H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front. Aging Neurosci. 2019, 30, 2033. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Q.; Lv, X.; Gao, F.; Chen, Z.; Shen, Y. Elevated β-secretase 1 expression mediates CD4+ T cell dysfunction via PGE2 signaling in Alzheimer’s disease. Brain Behav. Immun. 2021, 98, 337–348. [Google Scholar] [CrossRef]
- Siddiqui, A.; Akhtar, S.; Skah, Z.; Othman, I.; Kumari, Y. Inflammation Drives Alzheimer’s Disease: Emphasis on 5-lipoxygenase Pathways. Curr. Neuropharmacol. 2021, 19, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Smith, C. Ageing-Associated Oxidative Stress and Inflammation Are Alleviated by Products from Grapes. Oxid. Med. Cell Longev. 2016, 2016, 6236309. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.; Cuello, C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s Disease. Neuropharmacol. 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Majdi, A.; Sadigh-Eteghad, S.; Aghsan, S.R.; Farajdokht, F.; Vatandoust, S.M.; Namvaran, A.; Mahmoudi, J. Amyloid-β, tau, and the cholinergic system in Alzheimer’s disease: Seeking direction in a tangle of clues. Rev. Neurosci. 2020, 31, 391–413. [Google Scholar] [CrossRef] [PubMed]
- Papagiouvannis, G.; Theodosis-Nobelos, P.; Kourounakis, P.N.; Rekka, E.A. Multi-Target Directed Compounds with Antioxidant and/or Anti- Inflammatory Properties as Potent Agents for Alzheimer’s Disease. Med. Chem. 2021, 17, 1086–1103. [Google Scholar] [CrossRef]
- Winblad, B. Piracetam: A Review of Pharmacological Properties and Clinical Uses. CNS Drug Rev. 2005, 11, 169–182. [Google Scholar] [CrossRef]
- Watanabe, M.; Maemura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. GABA and GABA Receptors in the Central Nervous System and Other Organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure–activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Lee, H.E.; Kim, D.H.; Park, S.J.; Kim, J.M.; Lee, Y.W.; Jung, J.M.; Lee, C.H.; Hong, J.G.; Liu, X.; Cai, M.; et al. Neuroprotective effect of sinapic acid in a mouse model of amyloid β1–42 protein-induced Alzheimer’s disease. Pharmacol. Biochem. Behav. 2012, 103, 260–266. [Google Scholar] [CrossRef]
- Godoy, M.E.; Rotelli, A.; Pelzer, L.; Tonn, C.E. Anti-inflammatory Activity of Cinnamic Acid Esters. Molecules 2000, 5, 547–548. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. A review on vitamin e natural analogues and on the design of synthetic vitamin e derivatives as cytoprotective agents. Mini Rev. Med. Chem. 2021, 21, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Zhang, H.Y.; Tsimidou, M.Z. Structure-Antioxidant Relationship of Ferulic Acid Derivatives: Effect of Carbon Side Chain Characteristic Groups. J. Agric. Food Chem. 2003, 51, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, L.; Xia, G.; Zhan, D.; Zhu, J.; Zang, H. Synthesis and Biological Activity of Trolox Amide Derivatives. Braz. J. Pharm. 2020, 58, e18887. [Google Scholar] [CrossRef]
- Ferreira da Silveira, T.F.; Cajaiba, L.M.; Valentin, L.; Barea, B.; Villeneuve, P.; Alves Castro, I. Effect of sinapic acid ester derivatives on the oxidative stability of omega-3 fatty acids rich oil-in-water emulsions. Food Chem. 2020, 309, 125586. [Google Scholar] [CrossRef] [PubMed]
- Chekroun-Bechlaghem, N.; Belyagoubi-Benhamou, N.; Belyagoubi, L.; Gismondi, A.; Nanni, V.; Di Marco, G.; Canuti, L.; Canini, A.; El Haci, I.A.; Atik Bekkara, F. Phytochemical analysis and antioxidant activity of Tamarix africana, Arthrocnemum macrostachyum and Suaeda fruticosa, three halophyte species from Algeria. Plant Biosyst. An. Int. J. Deal. All. Asp. Plant Biol. 2019, 153, 843–852. [Google Scholar]
- Young Kim, H.; Kim, K. Protein Glycation Inhibitory and Antioxidant Activities of Some Plant Extracts in vitro. J. Agric. Food Chem. 2003, 51, 1586–1591. [Google Scholar]
- Papagiouvannis, G.; Theodosis-Nobelos, P.; Tziona, P.; Gavalas, A.; Kourounakis, P.N.; Rekka, E.A. Gabapentin Antioxidant Derivatives with Anti-Inflammatory and Neuro-protective Potency. Let. Drug Des. Dis. 2022, 19, 579–590. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Kourounakis, P.N.; Rekka, E.A. Active anti-inflammatory and hypolipidemic derivatives of lorazepam. Molecules 2019, 24, 3277. [Google Scholar] [CrossRef]
- Ko, S.Y.; Ko, H.A.; Chu, K.H.; Shieh, T.M.; Chi, T.C.; Chen, H.I.; Chang, W.C.; Chang, S.S. The Possible Mechanism of Advanced Glycation End Products (AGEs) for Alzheimer’s Disease. PLoS ONE 2015, 10, e0143345. [Google Scholar] [CrossRef]
- Nishi, K.; Yamasaki, K.; Otagiri, M. Serum Albumin, Lipid and Drug Binding. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and other Body Fluid Proteins; Hoeger, U., Harris, J., Eds.; Springer: Cham, Switzerland, 2020; Volume 94, pp. 383–397. [Google Scholar]
- Intagliata, S.; Spadaro, A.; Lorenti, M.; Panico, A.; Siciliano, E.A.; Barbagallo, S.; Macaluso, B.; Kamble, S.H.; Modica, M.N.; Montenegro, L. In vitro antioxidant and anti-glycation activity of resveratrol and its novel triester with trolox. Antioxidants 2020, 10, 12. [Google Scholar] [CrossRef]
- Patel, A.; Shah, D.; Patel, Y.; Patel, S.; Mehta, M.; Bambharoliya, T. A Review on Recent Development of Novel Heterocycles as Acetylcholinesterase Inhibitor for the Treatment of Alzheimer’s Disease. Curr. Drug Targets 2023, 24, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Wang, K.; Han, X.; Cao, M.; Tan, Z.; Liu, W. Design, synthesis, and evaluation of novel ferulic acid derivatives as multi-target-directed ligands for the treatment of Alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 1008–1024. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Spaziano, G.; Liparulo, A.; Roviezzo, F.; Nabavi, S.M.; Sureda, A.; Filosa, R.; D’ Agostino, B. Recent adnvances in the search for novel 5-lipozygenase inhibitors for the treatment of asthma. Eur. J. Med. Chem. 2018, 153, 65–72. [Google Scholar] [CrossRef]
- Firuzi, O.; Zhuo, J.; Chinnici, C.M.; Wisniewski, T.; Praticò, D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer’s disease. FASEB J. 2008, 22, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Terro, F. Tau protein phosphatases in Alzheimer’s disease: The leading role of PP2A. Aging Res. Rev. 2013, 12, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.J.; Deaver, C.M.; Lewandowski, A.J. Molecular mechanism of action responsible for carrageenan-induced inflammatory response. Mol. Immunol. 2019, 109, 38–42. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Athanasekou, C.; Rekka, E.A. Dual antioxidant structures with potent anti-inflammatory, hypolipidemic and cytoprotective properties. Bioorg. Med. Chem. Lett. 2017, 27, 4800–4804. [Google Scholar] [CrossRef]
- Brenelli de Paiva, L.; Goldbeck, R.; Dantas dos Santos, W.; Squina, F.M. Ferulic acid and derivatives: Molecules with potential application in the pharmaceutical field. Braz. J. Pharm. Sci. 2013, 49, 395–411. [Google Scholar] [CrossRef]
- Di Marco, G.; Gismonti, A.; Panzarella, L.; Canuti, L.; Impei, S.; Leonardi, D.; Canini, A. Botanical Influence on phenolic profile and antioxidant level of Italian honeys. Int. J. Food Chem. 2018, 55, 4042–4050. [Google Scholar] [CrossRef]
- Papagiouvannis, G.; Theodosis-Nobelos, P.; Rekka, E.A. Nipecotic Acid Derivatives as Potent Agents against Neurodegeneration. Molecules 2022, 27, 6984. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Rekka, E.A. Antioxidant serine-(Nsaid) hybrids with anti-inflammatory and hypolipidemic potency. Molecules 2021, 26, 4060. [Google Scholar] [CrossRef] [PubMed]
- Tziona, P.; Theodosis-Nobelos, P.; Papagiouvannis, G.; Petrou, A.; Drouza, C.; Rekka, E.A. Enhancement of the Anti-Inflammatory Activity of NSAIDs by Their Conjugation with 3,4,5-Trimethoxybenzyl Alcohol. Molecules 2022, 27, 2104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).