Abstract
In order to improve the drug-likeness qualities, the antimalarial endochin-like quinolone (ELQ) scaffold has been modified by replacing the 4-(trifluoromethoxy)phenyl portion with an isoidide unit that is further adjustable by varying the distal O-substituents. As expected, the water solubilities of the new analogs are greatly improved, and the melting points are lower. However, the antimalarial potency of the new analogs is reduced to EC50 > 1 millimolar, a result ascribable to the hydrophilic nature of the new substitution.
1. Introduction
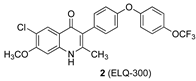
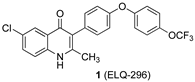
Elaboration of the endochin-like quinolone (ELQ) scaffold has led to the development of several potent and target-selective antimalarials, including the 4-[4-(trifluoromethoxy)phenoxy]phenyl derivatives ELQ-296 and ELQ-300 (1 and 2, respectively, Figure 1) [1]. These compounds target the cytochrome bc1 complex, also known as Complex III, in the Plasmodium mitochondrial electron transport chain [2,3]. However, the drug-likeness qualities of the molecules fall short of what might be required of an effective malaria drug, which includes their poor water solubility, high crystallinity, and limited bioavailability [2,4].
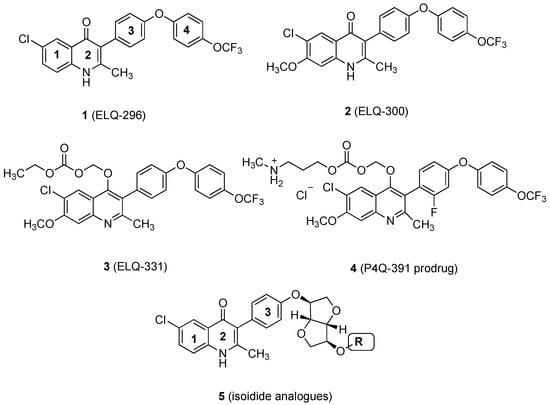
Figure 1.
Structures of bioactive ELQs and a template (5) for isoidide incorporation.
Research efforts to improve the bioavailability of ELQs were largely centered on the preparation of prodrug derivatives of ELQs, such as the O-(ethoxycarboxymethyl derivative, 3 [5], and the methylaminopropoxycarboxymethyl derivative, 4 [6]. Such prodrugs have limitations, however, in that their cleavage characteristics may differ as a function of species, tissue distribution, and local enzymatic or pH environments [7,8,9,10,11]. Adjustment of the drug-likeness properties of the active compound itself offers the potential advantage that its behavior in biological systems is more easily defined and evaluated.
As part of our program to improve drug-likeness properties early in the discovery process for drug candidates, we have found that incorporation or attachment of an isohexide unit to the bioactive scaffold can improve the solubility, permeability, and metabolic stability [12]. In the best examples, this can be accomplished without sacrificing potency or safety from off-target effects [12]. Incorporation of an isohexide unit into the ELQ scaffold might be achieved by attachment at any of several positions, including the quinolone nitrogen (N-1) and the quinolone benzo ring (e.g., C-7), or by replacement of the trifluoromethoxyphenyl substituent (see 5, Figure 1). The quinolone NH is required for efficient target binding [3], so it ought to remain unmodified. Attachment of a (polar) amino-adenine group at C-7 has recently been explored as a means to augment the effectiveness of quinolone antimalarials. Similarly, aza substitution (N for CH) in the central phenyl ring of the 3-aryl substituent (ring 3) has been investigated; the presence of one nitrogen is well tolerated, but double nitrogen substitution is deleterious [1]. There is little published information available on the effect of introducing additional oxygen-containing substituents on rings 3 or 4. However, we chose to incorporate the isoidide (both ring substituents exo) unit as a distal part of the 3-aryl substituent (see Figure 1, structure 5) for three reasons: (1) we could maintain the optimized quinolone portion of the ELQ’s along with the favorable chloro substituent (compare 1) [1,3,13,14], (2) the isoidide substructure could be attached by relatively straightforward and well-precedented Suzuki coupling methods [3,15], and (3) the remaining isoidide hydroxyl could be modified to adjust the size and polarity of the resulting analogs. It remained to be seen whether potency would be sacrificed as a result of these changes.
2. Results and Discussion
2.1. Preparation of the Suzuki Coupling Partners
The elaboration of 4-chloraniline (6, Scheme 1) into 3-iodoquinolone 8 followed the literature procedures [1,2,16] with minor modifications to improve the yields. For the Conrad–Limpach cyclization, nearly quantitative formation of the initially coupled enamine was seen when molecular sieves were used as the dehydrating reagent in toluene solution at room temperature. N-iodosuccinimide (NIS) was found to be a superior reagent for ring iodination [17]. The O-benzyl protecting group was chosen because it can be attached in high yield and offers several possibilities for deprotection under mild conditions. The alkylation product 9 precipitates directly from the reaction mixture. Previous ELQ syntheses used O-methyl or O-ethyl groups [1,2].

Scheme 1.
Synthesis of the Suzuki coupling partners 9 and 12 for isohexide incorporation into ELQs. (a) The product was purified by crystallization or precipitation; (b) the product was purified by chromatography.
Commercial 4-hydroxyphenylboronate pinacol ester (10) was coupled to commercial 4-O-acetylisosorbide by a Mitsunobu reaction in a manner similar to reactions recently described for isosorbide itself [12]. The reaction is slow but efficient; the purification of 12 requires silica chromatography. Cyclopentyl methyl ether (CPME) proved to be superior to THF for the Mitsunobu coupling in that it is higher boiling, easier to keep dry, and peroxide-free [18].
2.2. Suzuki Coupling and Preparation of ELQ Analogues
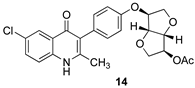
The Suzuki coupling reaction was successful with Pd(dppf)Cl2 as the palladium source [3] (see 13, Scheme 2), requiring only 15 min at 85 °C. Further heating caused partial base-promoted cleavage of the acetyl group. The O-benzyl protecting group was efficiently removed by nucleophilic de-alkylation to give the O-acetyl ELQ analog 14, which precipitated directly from the reaction mixture.

Scheme 2.
Suzuki coupling to form the ELQ framework and further modification of the terminal hydroxy substituent. O-substituents are shown in the red boxes, and the final four ELQ analogs are shown in the green boxes. (a) The product was purified by crystallization or precipitation; (b) the product was purified by chromatography.
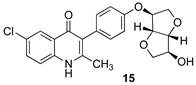
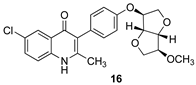
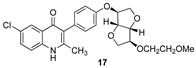
Purposeful deacetylation of the Suzuki product 13 was followed by the removal of the O-benzyl protecting group, this time with dilute HCl, to give the ELQ analog 15. O-methylation of the deacylated compound from 13 followed by debenzylation as before provided the methoxy analog 16, and O-alkylation with bromomethyl ethyl ether followed by debenzylation as before led to the O-methoxyethyl analog 17. In Scheme 2, the distal isoidide substituents (–OAc, –OH, –OMe, and –OCH2CH2OMe, respectively) are indicated by red boxes, and the final four analogs (14–17) are shown in green boxes.
2.3. Evaluation of ELQ Analogues
Data from the physicochemical and in vitro evaluation of ELQs 1 and 2, along with the new ELQ analogs 14–17, are displayed in Table 1. Replacement of the terminal 4-(trifluoromethoxy)phenyl group by the substituted isoidide unit is conducted with very little change in molecular weight (second column). However, the incorporation of the isoidide substructure leads to a dramatic decrease in ClogP, roughly three log units (third column). This is the expected result of the introduction of several new oxygen functionalities and the replacement of the lipophilic aryl ring. There is also a modest but definite reduction in melting point when going from the simple ELQs to the isoidide analogs (fourth column). The non-planar bicyclic ether rings may well reduce pi stacking in the crystal lattice, and a reduced melting point is often predictive of improved solubility [19]. Likewise, the isoidide analogs 15, 16, and 17 show much improved measured kinetic solubility in aqueous buffer over ELQ-300 (sixth column), as expected. None of the new analogs is cytotoxic to human BJ fibroblasts below 100 micromolar (seventh column).

Table 1.
Evaluation of ELQs.
Evaluation of the new analogs 14–17 against the chloroquine-sensitive P. falciparum strain 3D7 (fifth column) indicates that potency below 1 millimolar has been lost as a result of the structural changes to the ELQ scaffold. This disappointing result invalidates our initial assumption that these structural changes far from the quinolone ring could be imposed as a means to improve the drug-like qualities. Evidently, the introduction of polar oxygen functionality in place of the lipophilic 4-(trifluoromethoxy)phenyl subunit is detrimental to binding at the presumed Plasmodium cytochrome bc1 target. Docking studies conducted by Capper et al. [21] on ELQ-structurally-related 4(1H)-pyridones with bovine cyt bc1 suggest that the diphenyl ether substituent extends towards the entrance of the cavity between cytochrome bc1 monomers. While the end of the putative binding channel features several hydrophobic residues, the protein environment next to heme bh is somewhat polar due to the presence of His201, Ser35, and Asp228 residues. Whether new groups introduced into the ELQ scaffold near or beyond the trifluoromethoxy group would be substantially solvent-exposed, a desirable situation for isohexide incorporation to be non-detrimental to target binding, has not been established.
3. Materials and Methods
3.1. General
All commercially available solvents, reagents, and compounds were used as received. Gravity chromatography was performed by using silica gel (Sorbent Technologies 230–400 mesh) as the stationary phase. Silica gel 60 F254 pre-coated plates were used for thin-layer chromatography, and visualization was accomplished with UV light (254 nm) or ninhydrin stain. The LC–MS and high-resolution mass spectrometry (HRMS) analyses were conducted using a Xevo G2-XS QTOF instrument (Waters Corporation, Milford, MA, USA) with electrospray ionization. The LC–MS samples were separated on an Acquity UPLC (Waters Corporation, Milford, MA, USA) C18 1.7 μm column as solutions in water or acetonitrile, prepared at 0.15–0.20 mg/mL concentration. The LC–MS chromatography was carried out with linear gradients of 0.05% formic acid in acetonitrile and 0.05% formic acid in water. 1H, 13C, and HSQC NMR spectra were obtained on a Varian VNMRS 500 or 400 instrument or a Bruker Avance Neo 500 (Bruker USA, San Jose, CA, USA). Chemical shifts (δ) are reported in parts per million (ppm) and are referenced to the residual solvent signal. Coupling constants (J) are reported in hertz (Hz). The usual abbreviations are used to describe multiplicities: s (singlet), d (doublet), t (triplet), q (quartet), br (broad), and app (apparent). Minor impurities such as solvents, water, and grease are not reported in the NMR transcriptions.
3.2. 6-Chloro-2-methylquinolin-4(1H)-one (7)
4-Chloroaniline 6 (3.30 g, 26.3 mmol, 1 equiv) was dissolved in 150 mL of toluene in an oven-dried round bottom flask containing a stir bar and 9 g of activated 4 Å molecular sieves. Ethyl acetoacetate (4.54 mL, 52.6 mmol, 2 equiv) and glacial acetic acid (17.5 mmol, 1.5 equiv) were added to the reaction flask at room temperature, producing a pale-yellow solution. The reaction was stirred for 48 h, at which time NMR analysis indicated the complete disappearance of the starting aniline. The molecular sieves were removed via filtration. The resulting yellow solution was concentrated, and the residue was dissolved in 45 mL of Dowtherm A and transferred with a syringe to a 2-neck oven-dried round bottom flask containing 45 mL of Dowtherm A heated at 250 °C. The reaction mixture was heated at reflux for 25 min. The flask was cooled to room temperature by using a water bath. Upon cooling, a yellow precipitate formed in the dark red solution. The precipitate (6.25 g) was collected via vacuum filtration and washed with cold propionitrile. Crystallization of the resulting yellow solid (contaminated with Dowtherm A) from 1 L of propionitrile afforded a dark yellow solid (3.10 g, 62%): mp 314–317 °C; Lit. [22] 316–318 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.73 (br s, 1H), 7.96 (d, J = 2.5 Hz, 1H), 7.65 (dd, J = 8.5 Hz, 2.5 Hz, 1H), 7.53 (d, J = 9 Hz, 1H), 5.95 (s, 1 H), 2.34 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 175.5, 150.3, 138.7, 131.6, 127.4, 125.5, 123.8, 120.2, 108.6, 19.5; ESI-MS [M + H]+ calc’d for C10H9ClNO, 194.0294; found 194.0352.
3.3. 6-Chloro-3-iodo-2-methylquinolin-4(1H)-one (8)
A suspension of quinolinone 7 (1.261 g, 6.51 mmol, 1 equiv) in acetonitrile (32.6 mL) was stirred at room temperature for 1 h. N-iodosuccinimide (2.197 g, 9.76 mmol, 1.5 equiv) was added to the reaction flask, producing a pale brown suspension within the orange supernatant. The reaction mixture was heated at reflux for 5 h. After cooling, the white precipitate (2.03 g, 98%) was collected by vacuum filtration and washed with cold acetonitrile: mp 236–238 °C; Lit. [22] 237–238 °C; 1H NMR (500 MHz, DMSO-d6) δ 12.31 (br s, 1H), 8.00 (d, J = 2.5 Hz, 1H), 7.71 (dd, J = 8.5 Hz, 2.5 Hz, 1H), 7.59 (d, J = 9 Hz, 1H), 2.63 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 171.9, 151.9, 137.5, 132.1, 128.2, 124.3, 121.4, 120.2, 86.2, 26.2; ESI-MS [M + H]+ calcd for C10H8ClNO, 319.9333; found 319.9346.
3.4. 4-(Benzyloxy)-6-chloro-3-iodo-2-methylquinoline (9)
Iodinated quinolinone 8 (959 mg, 3 mmol, 1 equiv) and cesium carbonate (1.466 g, 4.5 mmol, 1.5 equiv) were combined in a 3-neck oven-dried round bottom flask equipped with a stir bar under a nitrogen atmosphere. Dry acetonitrile (28 mL) was added to the reaction flask, which resulted in a white, cloudy suspension. The reaction mixture was stirred for 5 h. Benzyl bromide (0.428 mL, 3.6 mmol, 1.2 equiv) was added dropwise, and the reaction was stirred for 15 h. The resulting white suspension was collected by vacuum filtration, washed sequentially with cold water and acetonitrile, and then dried under vacuum to afford 9 as an off-white solid (1.077 g, 88%): mp 110–112 °C; 1H NMR (500 MHz, chloroform-d) δ 7.96 (d, J = 9 Hz, 1H), 7.93 (d, J = 2.5 Hz, 1H), 7.64–7.60 (m, 3H), 7.48–7.41 (m, 3H), 5.15 (s, 2H), 2.97 (s, 3H); 13C NMR (125 MHz, chloroform-d) δ 162.3, 162.3, 147.6, 136.0, 132.6, 131.5, 130.7, 129.2, 129.1, 128.7, 123.6, 121.3, 91.6, 76.6, 30.9; ESI-MS [M + H]+ calcd for C17H14ClINO, 409.9803; found 409.9814.
3.5. (3S,3aR,6S,6aR)-6-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy)hexahydrofuro[3,2-b]furan-3-yl Acetate (12)
Diisopropyl azodicarboxylate (1.38 mL, 7.0 mmol, 1.6 equiv) was added to a solution of triphenylphosphine (1.84 g, 7.0 mmol, 1.6 equiv) in cyclopentyl methyl ether (60 mL) in a 100 mL 3-neck round bottom flask at 0–5 °C (ice/water) over a 2 min period under an argon atmosphere. The resulting light yellow suspension was stirred for 15 min at this temperature, and then 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenol 10 (1.0 g, 4.5 mmol, 1 equiv) was added to the reaction mixture. After 2 min, the cooling bath was removed to allow the reaction mixture to warm to room temperature. After 15 min, 2-O-acetyl isosorbide 11 (1.04 g, 5.5 mmol, 1.2 equiv) was added to the reaction mixture with stirring, followed by diisopropylethylamine (1.22 mL, 7.0 mmol, 1.6 equiv). During a 30 min period, all the solids dissolved to form a light brown solution. The reaction mixture was stirred for an additional 30 min, then heated at 90–95 °C for 5 days until TLC analysis indicated the complete disappearance of the starting material. The brown solution was cooled to room temperature and concentrated in vacuo. The residue was chromatographed with 4:1 hexane/ethyl acetate as an eluant to produce 12 (Rf = 0.25) as a viscous yellow oil (1.47 g, 83%). 1H NMR (500 MHz, chloroform-d) δ 7.70 (br d, J = 8.65 Hz, 2H), 6.86 (br d, J = 8.70 Hz, 2 H), 5.16 (d, J = 3.65 Hz, 1H), 4.80 (br t, J = 2.68 Hz, 1 H), 4.68 (d, J = 3.85 Hz, 1H), 4.63 (d, J = 3.85, 1 H), 4.01–4.00 (m, 2H), 3.97 (dd, J = 10.65 Hz, 3.80 Hz, 1H), 3.87 (br d, J = 10.60 Hz, 1H), 2.00 (s, 3H), 1.27 (s, 12H); 13C NMR (125 MHz, chloroform-d) δ 170.3, 159.7, 137.0, 122.0, 114.8, 85.8, 85.8, 84.0, 81.0, 77.9, 72.86, 72.78, 25.2, 21.2; HR-LC–ESI-MS [M + H]+ m/z calcd for C20H27BO7, 391.1922; found, 391.1941.
3.6. (3S,3aR,6S,6aR)-6-(4-(4-(Benzyloxy)-6-chloro-2-methylquinolin-3-yl)phenoxy)hexahydrofuro[3,2-b]furan-3-yl Acetate (13)
Protected iodoquinoline 9 (437.4 mg, 1.1 mmol, 1 equiv) and Pd(dppf)Cl2 (39.1 mg, 0.053 mmol, 0.05 equiv) were combined in a 3-neck round-bottom flask equipped with a stir bar under a nitrogen atmosphere. A solution of phenyl boronic ester isosorbide acetate 12 (500 mg, 1.28 mmol, 1.2 equiv) in 8 mL of DMF under a nitrogen atmosphere was added to the reaction flask, producing an orange–red suspension. Aqueous potassium carbonate (2 N, 2.14 mL, 2.14 mmol, 2 equiv) was added dropwise, leading to the formation of a fluffy white precipitate in the red solution. The reaction was slowly heated to 85 °C and stirred for 15 min until TLC analysis indicated the disappearance of the starting material. The reaction mixture was cooled to room temperature and then decanted into a separatory funnel containing 20 mL of ethyl acetate. The organic layer was washed with saturated aqueous ammonium chloride (3 X 7 mL), dried over anhydrous sodium sulfate, and then concentrated in vacuo. The residue was chromatographed with 5:2 hexane/ethyl acetate as an eluant to produce 13 (Rf = 0.33) as a white sticky solid (402 mg, 69%): mp 51–55 °C; 1H NMR (500 MHz, acetonitrile-d3) δ 8.05 (d, J = 2.4 Hz, 1H), 7.93 (d, J = 8.95 Hz, 1H), 7.65 (dd, J = 8.9 Hz, 2.4 Hz, 1H), 7.37 (d, J = 8.75 Hz, 2H), 7.31–7.28 (m, 3H), 7.12–7.09 (m, 4H), 5.15 (d, J = 3.65 Hz, 1H), 4.94 (d, J = 3.2 Hz, 1H), 4.73 (d, J = 4.05 Hz, 1H), 4.68 (m, 3H), 4.09 (dd, J = 10.45 Hz, 3.9 Hz, 1H), 4.04–3.99 (m, 2H), 3.91 (d, J = 10.7 Hz, 1H), 2.45 (s, 3H), 2.03 (s, 3H); 13C NMR (125 MHz, acetonitrile-d3) δ 170.9, 161.6, 159.4, 157.8, 147.8, 137.5, 132.6, 131.9, 131.5, 131.0, 129.4, 129.38, 129.33, 129.31, 128.1, 124.7, 122.4, 116.6, 86.5, 86.4, 82.3, 78.6, 76.6, 72.9, 25.2, 21.1 (one carbon in the aromatic region is unaccounted for); HR-LC-ESI–MS [M + H]+ m/z calcd for C31H29ClNO6, 546.1677; found, 546.1700.
3.7. (3S,3aR,6S,6aR)-6-(4-(6-Chloro-2-methyl-4-oxo-1,4-dihydroquinolin-3-yl)phenoxy)hexahydrofuro[3,2-b]furan-3-yl Acetate (14)
Acetylated Suzuki coupling product 13 (95 mg, 0.174 mmol, 1 equiv) was dissolved in 3 mL of dry acetonitrile in an oven-dried 3-neck round bottom flask equipped with a stir bar under a nitrogen atmosphere. Sodium iodide (45 mg, 0.300 mmol, 1.7 equiv) and trimethylsilyl chloride (26 μL, 0.211 mmol, 1.2 equiv) were added to the reaction flask, producing a pale-yellow suspension. The reaction was stirred for 8 h at room temperature, whereupon the starting material was consumed according to the TLC analysis. The resulting white suspension was filtered, and the precipitate was washed sequentially with cold water and acetonitrile and then dried under vacuum, affording 14 as an off-white solid (56 mg, 70%): mp 298–299 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.78 (br s, 1H), 7.98 (d, J = 2 Hz, 1H), 7.64 (dd, J = 8.85 Hz, 2.2 Hz, 1H), 7.55 (d, J = 8.85 Hz, 1H), 7.16 (d, J = 8.25 Hz, 2H), 6.97 (d, J = 8.3 Hz, 2H), 5.06 (d, J = 3.25 Hz, 1H), 4.90 (d, J = 2 Hz, 1H), 4.64 (d, J = 4.1 Hz, 1H), 4.61 (d, J = 4.1 Hz, 1H), 4.03 (dd, J = 10.4 Hz, 3.95 Hz, 1H), 3.95 (dd, J = 10.65 Hz, 3.7 Hz, 1H), 3.91 (d, J = 10.25 Hz, 1H), 3.85 (d, J = 10.65 Hz, 1H), 2.21 (s, 3H), 2.01 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 174.0, 169.9, 155.5, 147.4, 138.0, 132.3, 131.7, 128.6, 127.5, 125.4, 124.4, 120.7, 120.2, 114.8, 85.1, 85.1, 80.8, 77.3, 71.8, 71.6, 20.8, 19.1; HR-LC–ESI-MS [M + H]+ m/z calcd for C24H23ClNO6, 456.1208; found, 456.1219.
3.8. 6-Chloro-3-(4-(((3S,3aR,6S,6aR)-6-hydroxyhexahydrofuro[3,2-b]furan-3-yl)oxy)phenyl)-2-methylquinolin-4(1H)-one (15)
Acetylated Suzuki coupling product 13 (100 mg, 0.219 mmol, 1 equiv) was dissolved in 6 mL of dioxane to form a pale-yellow solution. Aqueous potassium hydroxide (1.0 M, 3 mL) was slowly added to the reaction mixture, and the reaction was stirred for 30 min, whereupon TLC analysis indicated the disappearance of the starting material. The solution was concentrated, and the residue was suspended in 12 mL of deionized water and transferred to a separatory funnel. The aqueous layer was extracted with dichloromethane (3 × 5 mL), and the combined extract was dried over anhydrous sodium sulfate and then concentrated to provide the alcohol as a white solid (90.1 mg, 99%): mp 179.5–180 °C; 1H NMR (500 MHz, chloroform-d) δ 8.04 (d, J = 2.3 Hz, 1H), 7.95 (d, J = 8.95 Hz, 1H), 7.6 (dd, J = 8.95 Hz, 2.35 Hz, 1H), 7.33–7.29 (m, 5H), 7.10–7.06 Hz, (m, 4H), 4.87–4.86 (m, 2H), 4.68 (d, J = 3.75 Hz, 1H), 4.62 (s, 2H), 4.44 (br s, 1H), 4.13–4.08 (m, 2H), 3.98 (dd, J = 10.1 Hz, 3.3 Hz, 1 H), 3.93 (d, J = 10 Hz, 1H), 3.02 (s, 3H); 13C NMR (125 MHz, chloroform-d) δ 160.5, 158.9, 156.9, 146.9, 136.3, 131.7, 130.7, 130.2, 128.6, 128.55, 128.51, 128.3, 126.5, 123.8, 121.7, 115.7, 88.2, 85.4, 81.4, 76.1, 75.8, 74.8, 72.5, 25.1 (one isosorbide carbon is unaccounted for); HR-LC–ESI-MS [M + H]+ m/z calcd for C29H27ClNO5, 504.1572; found, 504.1581.
The alcohol from above (20 mg, 0.040 mmol, 1 equiv) was dissolved in 2 mL of glacial acetic acid to form a pale-yellow solution. Aqueous hydrochloric acid (1 M, 2 mL, 2 mmol, 50 equiv) was added slowly. The resulting pale-yellow solution was slowly heated to 55 °C and then stirred for 6 h, whereupon TLC analysis indicated the disappearance of the starting material. The solvent was removed under vacuum, and the yellow residue was suspended in 1 mL of acetonitrile, leading to the separation of the solid product. The solution was decanted, and the remaining precipitate was dried under vacuum to afford 15 as an off-white solid (14 mg, 85%); mp 257 °C (dec); 1H NMR (500 MHz, DMSO-d6) δ 8.0 (br s, 1H), 7.67–7.62 (m, 2H), 7.18 (d, J = 8 Hz, 2H), 6.99 (d, J = 8 Hz, 2H), 5.29 (br s, 1H), 4.86 (d, J = 2 Hz, 1H), 4.60 (d, J = 3.5 Hz, 1H), 4.45 (d, J = 3 Hz, 1H), 4.13 (br s, 1H), 3.98 (dd, J = 10.5 Hz, 2.5 Hz, 1 H), 3.88 (d, J = 10 Hz, 1H), 3.80 (dd, J = 9 Hz, 3 Hz, 1H), 3.70 (d, J = 9.5 Hz, 1H), 2.24 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 174.0, 155.5, 147.6, 138.0, 132.3, 131.5, 128.5, 127.4, 125.3, 124.3, 120.7, 120.3, 114.8, 87.8, 84.8, 80.9, 74.7, 74.3, 71.5, 18.9; HR-LC–ESI-MS [M + H]+ m/z calcd for C29H27ClNO5, 504.1572; found, 504.1581.
3.9. 6-Chloro-3-(4-(((3S,3aR,6S,6aR)-6-methoxyhexahydrofuro[3,2-b]furan-3-yl)oxy)phenyl)-2-methylquinolin-4(1H)-one (16)
The deacetylated Suzuki coupling product, prepared as above (100 mg, 0.200 mmol, 1 equiv), was dissolved in 4 mL of dichloromethane under an argon atmosphere. Tetra-n-butylammonium bromide (19.8 mg, 0.061 mmol, 0.31 equiv) was added to the reaction mixture, followed by 1 mL of an aqueous 50% potassium hydroxide solution. Dimethyl sulfate (25 µL, 0.264 mmol, 1.32 equiv) was added dropwise to the resulting pale-yellow solution. The reaction was stirred for 2.5 h until TLC analysis indicated the complete disappearance of the starting material. The reaction mixture was decanted into the separatory funnel containing 6 mL of dichloromethane and washed with saturated aqueous ammonium chloride (2 × 5 mL). The combined organic layer was dried over anhydrous sodium sulfate and concentrated in vacuo. The residue was chromatographed with 3:2 hexane/ethyl acetate as the eluant to produce the protected methyl ether (Rf = 0.38) as sticky colorless crystals (79.8 mg, 78%): mp 38–41 °C; 1H NMR (500 MHz, acetonitrile-d3) δ 8.05 (d, J = 2.35 Hz, 1H), 7.92 (d, J = 8.95 Hz, 1H), 7.65 (dd, J = 8.95 Hz, 2.4 Hz, 1H), 7.37 (d, J = 8.8 Hz, 2H), 7.30–7.28 (m, 3H), 7.12–7.08 (m, 4H), 4.89 (d, J = 2.25 Hz, 1H), 4.67 (m, 3H), 4.65 (d, J = 4.2 Hz, 1H), 4.05 (dd, J = 10.4 Hz, 3.95 Hz, 1H), 4.00 (dd, J = 10.35 Hz, 1.7 Hz, 1H), 3.90–3.84 (m, 3 H), 3.36 (s, 3H), 2.45 (s, 3H); 13C NMR (125 MHz, acetonitrile-d3) δ 161.6, 159.4, 157.8, 147.7, 137.4, 132.5, 131.9, 131.4, 131.0, 129.3, 129.29, 129.2, 128.0, 124.7, 122.4, 118.3, 116.5, 86.3, 86.2, 85.9, 82.4, 76.6, 72.6, 72.59, 57.4, 25.2; HR-LC–ESI-MS [M + H]+ m/z calcd for C30H29ClNO5, 518.1728; found, 518.1746.
The protected methyl ether from above (19.5 mg, 0.038 mmol, 1 equiv) was dissolved in 2 mL of glacial acetic acid to form a pale-yellow solution. Aqueous hydrochloric acid (1 M, 2 mL, 2 mmol, 52 equiv) was slowly added at room temperature. The resulting solution was slowly heated to 55 °C and stirred for 6 h, whereupon TLC analysis indicated the disappearance of the starting material. The solvent was removed under vacuum, and the yellow residue was suspended in 1 mL of acetonitrile, leading to the separation of the solid product. The solution was decanted, and the precipitate was dried under vacuum to afford 16 as an off-white solid (14.5 mg, 90%): mp 262 °C (dec); 1H NMR (500 MHz, DMSO-d6) δ 8.01 (br s, 1H), 7.67 (app br s, 2H), 7.18 (d, J = 8.6 Hz, 2H), 6.99 (d, J = 8.65 Hz, 2H), 4.87 (br s, 1H), 4.63 (d, J = 4.15 Hz, 1H), 4.56 (d, J = 4.15 Hz, 1H), 4.01 (dd, J = 10.35 Hz, 4.15 Hz, 1H), 3.90 (app br d, J = 12.05 Hz, 2H), 3.85–3.80 (m, 2H), 3.30 (s, 3H), 2.25 (s, 3H); 1C NMR (125 MHz, DMSO-d6) δ 173.7, 155.5, 147.4, 137.9, 132.2, 131.5, 128.5, 127.3, 125.3, 124.2, 120.6, 120.2, 114.7, 85.0, 84.8, 84.3, 80.8, 71.4, 71.2, 56.5, 18.9; HR-LC–ESI-MS [M + H]+ m/z calcd for C23H23ClNO5, 428.1259; found, 428.1291.
3.10. 6-Chloro-3-(4-(((3S,3aR,6S,6aR)-6-(2-methoxyethoxy)hexahydrofuro[3,2-b]furan-3-yl)oxy)phenyl)-2-methylquinolin-4(1H)-one (17)
The deacetylated Suzuki coupling product prepared as above (110 mg, 0.218 mmol, 1 equiv) was dissolved in 5 mL of dry DMF under an argon atmosphere. Sodium hydride (52 mg, 2.17 mmol, 9.9 equiv) was added to the reaction flask to produce a light brown suspension. The reaction was stirred at room temperature for 40 min, and then a solution of bromoethyl methyl ether (0.12 mL, 1.28 mmol, 5.9 equiv) in 0.5 mL of dry DMF was added dropwise. The reaction was stirred for an additional 8 h until TLC analysis indicated the complete disappearance of the starting material. The light brown suspension was cooled to room temperature and concentrated in vacuo. The residue was chromatographed with 2:1 hexane/ethyl acetate as the eluant to produce the protected methoxyethyl ether (Rf = 0.33) as a colorless oil (91.4 mg, 75%). 1H NMR (500 MHz, chloroform-d) δ 8.04 (d, J = 2.3 Hz, 1H), 7.96 (d, J = 8.95, 1H), 7.61 (dd, J = 8.95 Hz, 2.4 Hz, 1H), 7.32–7.28 (m, 5H), 7.11–7.07 (m, 2H), 7.06 (d, J = 8.7Hz, 2H), 4.86 (br d, J = 2.65 Hz, 1H), 4.80 (d, J = 4.05 Hz, 1H), 4.77 (d, J = 4.05 Hz, 1H), 4.62 (s, 2H), 4.14–4.08 (m, 3H), 4.00–3.95 (m, 2H), 3.75–3.66 (m, 2H), 3.55 (t, J = 9.3 Hz, 4.6 Hz, 2H), 3.38 (s, 3H), 2.52 (s, 3H); 13C NMR (125 MHz, chloroform-d) δ160.5, 158.9, 156.9, 146.9, 136.3, 131.7, 130.6, 130.3, 128.6, 128.5, 128.47, 128.4, 128.3, 126.5, 123.7, 121.7, 115.6, 86.0, 85.5, 84.1, 81.4, 75.8, 72.5, 72.3, 72.0, 69.3, 59.3, 25.1; HR-LC–ESI-MS [M + H]+ m/z calcd for C32H33ClNO6, 562.1990; found, 562.2001.
The O-alkylated Suzuki coupling product from above (35.6 mg, 0.063 mmol, 1 equiv) was dissolved in 2 mL of glacial acetic acid. Aqueous hydrochloric acid (1 M, 4 mL, 4 mmol, 63.5 equiv) was slowly added at room temperature. The resulting pale-yellow solution was heated to 55 °C and stirred for 24 h at that temperature, whereupon TLC analysis indicated the disappearance of the starting material. The solvent was removed under vacuum, and the yellow residue was suspended in 1 mL of acetonitrile, leading to the separation of the solid product. The solution was decanted, and the precipitate was dried under vacuum to afford 17 as an off-white solid (26.6 mg, 89%): mp 185 °C (dec); 1H NMR (500 MHz, DMSO-d6, 18 °C) δ 8.13 (d, J = 2.25 Hz, 1H), 7.86 (d, J = 8.9 Hz, 1H), 7.73 (dd, J = 8.85 Hz, 2.3 Hz, 1H), 7.20 (d, J = 8.55 Hz, 2H), 7.00 (d, J = 8.55 Hz, 2H), 4.88 (s, 1H), 4.62 (d, J = 4.1 Hz, 1H), 4.56 (d, J = 4.05 Hz, 1H), 4.01–3.98 (m, 3H), 3.90 (d, J = 10.2 Hz, 1H), 3.84–3.80 (m, 2H), 3.62–3.56 (m, 2H), 3.42 (t, J = 9.35 Hz, 4.65 Hz, 2H), 3.23 (s, 3H), 2.30 (s, 3H); 13C NMR (125 MHz, DMSO-d6) δ 172.3, 155.7, 149.0, 137.9, 132.1, 131.8, 128.0, 127.9, 124.5, 124.1, 120.6, 120.5, 114.9, 85.2, 85.0, 83.1, 80.8, 71.7, 71.4, 71.2, 68.1, 58.1, 19.0; HR-LC–ESI-MS [M + H]+ m/z calcd for C25H27ClNO6, 472.1521; found, 472.1529.
3.11. In Vitro Screening
The ELQs 14–17 were tested against the chloroquine-sensitive Plasmodium falciparum strain 3D7 (MRA-102), as provided by the MR4 Unit of the American Type Culture Collection (ATCC, Manassas, VA, USA). Details of the assay have been previously published [20]. Cytotoxicity IC50 values for 14–17 were obtained on human BJ fibroblasts as previously described [23].
3.12. Kinetic Solubilities
The ELQs 14–17 were evaluated for solubility in pH 7.4 phosphate buffer at 23 °C over a 20 h period according to the method [24] described by AstraZeneca’s Mechanistic Biology and Profiling, Discovery Sciences, R&D group (AstraZeneca, Gothenburg, Sweden).
4. Conclusions
The structural modification of the antimalarial ELQ scaffold, wherein the distal aryl substituent is replaced by an isoidide unit (14–17), has been carried out as a means to improve the drug-likeness qualities of molecules related to 1 and 2. In the course of the syntheses, several modifications to previous methods for assembling ELQs were introduced: a milder method for Conrad–Limpach quinolone synthesis, the use of O-benzyl as a versatile quinolone protecting group, and the use of cyclopentyl methyl ether as a solvent for the slow and water/peroxide-sensitive Mitsunobu reaction. True to expectations, the melting points of the new analogs are reduced relative to 2, and the aqueous solubilities are greatly improved (Table 1). While cytotoxicity levels of 14–17 are acceptable, the antimalarial potency of the new analogs has been lost, undoubtedly due to the hydrophilic nature of the new substituents. Even the option of modifying the terminal hydroxyl to give O-alkyl derivatives (16 and 17) did not lead to active compounds. For modifications of this nature to succeed, it will be necessary to attach the solubilizing group at a different position on the ELQ scaffold, or else more distant from the quinolone ring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29153615/s1. Supporting Information: scanned 1H-NMR and 13C-NMR spectra for 7–9 and 12–17.
Author Contributions
Conceptualization, S.K.; methodology, J.S.; manuscript preparation, J.S. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
We thank Rutgers University for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Acknowledgments
We are pleased to dedicate this paper to David Crich (University of Georgia) on the occasion of his 65th birthday. Crich is an inspiration to lovers and practitioners of carbohydrate chemistry throughout the world. We are grateful to Achyutharao Sidduri (Rutgers University) for the preparation of compound 12, Zoltan Szekely and Jacques Roberge (Rutgers University) for the solubility studies on 14–17, R. Kiplin Guy and Gaurav Shoeran (University of Kentucky College of Pharmacy), and Purnima Bhanot and Kutub Ashraf (Rutgers New Jersey Medical School) for in vitro evaluation of 14–17.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nilsen, A.; Miley, G.P.; Forquer, I.P.; Mather, M.W.; Katneni, K.; Li, Y.; Pou, S.; Pershing, A.M.; Stickles, A.M.; Ryan, E.; et al. Discovery, Synthesis, and Optimization of Antimalarial 4(1H)-Quinolone-3-Diarylethers. J. Med. Chem. 2014, 57, 3818–3834. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, A.; LaCrue, A.N.; White, K.L.; Forquer, I.P.; Cross, R.M.; Marfurt, J.; Mather, M.W.; Delves, M.J.; Shackleford, D.M.; Saenz, F.E.; et al. Quinolone-3-Diarylethers: A New Class of Antimalarial Drug. Sci. Transl. Med. 2013, 5, 177ra37. [Google Scholar] [CrossRef] [PubMed]
- Stickles, A.M. Evaluation of Cytochrome BC1 as a Target for Single-Dose Antimalarial Therapy: A Comparative Assessment of Qi vs. Qo. Ph.D. Thesis, Oregon Health & Science University School of Medicine, Portland, OR, USA, 2014. [Google Scholar]
- Miley, G.P.; Pou, S.; Winter, R.; Nilsen, A.; Li, Y.; Kelly, J.X.; Stickles, A.M.; Mather, M.W.; Forquer, I.P.; Pershing, A.M.; et al. ELQ-300 Prodrugs for Enhanced Delivery and Single-Dose Cure of Malaria. Antimicrob. Agents Chemother. 2015, 59, 5555–5560. [Google Scholar] [CrossRef] [PubMed]
- Frueh, L.; Li, Y.; Mather, M.W.; Li, Q.; Pou, S.; Nilsen, A.; Winter, R.W.; Forquer, I.P.; Pershing, A.M.; Xie, L.H.; et al. Alkoxycarbonate Ester Prodrugs of Preclinical Drug Candidate ELQ-300 for Prophylaxis and Treatment of Malaria. ACS Infect. Dis. 2017, 3, 728–735. [Google Scholar] [CrossRef]
- Monastyrskyi, A.; Brockmeyer, F.; LaCrue, A.N.; Zhao, Y.; Maher, S.P.; Maignan, J.R.; Padin-Irizarry, V.; Sakhno, Y.I.; Parvatkar, P.T.; Asakawa, A.H.; et al. Aminoalkoxycarbonyloxymethyl Ether Prodrugs with a pH-Triggered Release Mechanism: A Case Study Improving the Solubility, Bioavailability, and Efficacy of Antimalarial 4(1H)-Quinolones with Single Dose Cures. J. Med. Chem. 2021, 64, 6581–6595. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Vale, N.; Moreira, R. Cyclization-Activated Prodrugs. Molecules 2007, 12, 2484–2506. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and Clinical Applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap® System. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef]
- Di Consiglio, E.; Darney, K.; Buratti, F.M.; Turco, L.; Vichi, S.; Testai, E.; Lautz, L.S.; Dorne, J.L.C.M. Human Variability in Carboxylesterases and Carboxylesterase-Related Uncertainty Factors for Chemical Risk Assessment. Toxicol. Lett. 2021, 350, 162–170. [Google Scholar] [CrossRef]
- Strickley, R.G.; Oliyai, R. Formulation Challenges of Prodrugs. In Prodrugs; Stella, V.J., Borchardt, R.T., Hageman, M.J., Oliyai, R., Maag, H., Tilley, J.W., Eds.; Springer: New York, NY, USA, 2007; Volume V, pp. 1083–1110. [Google Scholar]
- Sidduri, A.; Dresel, M.J.; Knapp, S. Incorporation of an Isohexide Subunit Improves the Drug-like Properties of Bioactive Compounds. ACS Med. Chem. Lett. 2023, 14, 176–182. [Google Scholar] [CrossRef]
- Cross, R.M.; Monastyrskyi, A.; Mutka, T.S.; Burrows, J.N.; Kyle, D.E.; Manetsch, R. Endochin Optimization: Structure−Activity and Structure−Property Relationship Studies of 3-Substituted 2-Methyl-4(1H)-Quinolones with Antimalarial Activity. J. Med. Chem. 2010, 53, 7076–7094. [Google Scholar] [CrossRef]
- Winter, R.; Kelly, J.X.; Smilkstein, M.J.; Hinrichs, D.; Koop, D.R.; Riscoe, M.K. Optimization of Endochin-like Quinolones for Antimalarial Activity. Exp. Parasitol. 2011, 127, 545–551. [Google Scholar] [CrossRef]
- Pou, S.; Dodean, R.A.; Frueh, L.; Liebman, K.M.; Gallagher, R.T.; Jin, H.; Jacobs, R.T.; Nilsen, A.; Stuart, D.R.; Doggett, J.S.; et al. New Scalable Synthetic Routes to ELQ-300, ELQ-316, and Other Antiparasitic Quinolones. Org. Process Res. Dev. 2021, 25, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.M.; Flanigan, D.L.; Monastyrskyi, A.; LaCrue, A.N.; Sáenz, F.E.; Maignan, J.R.; Mutka, T.S.; White, K.L.; Shackleford, D.M.; Bathurst, I.; et al. Orally Bioavailable 6-Chloro-7-Methoxy-4(1H)-Quinolones Efficacious against Multiple Stages of Plasmodium. J. Med. Chem. 2014, 57, 8860–8879. [Google Scholar] [CrossRef] [PubMed]
- Fandrick, K.R.; Li, W.; Zhang, Y.; Tang, W.; Gao, J.; Rodriguez, S.; Patel, N.D.; Reeves, D.C.; Wu, J.; Sanyal, S.; et al. Concise and Practical Asymmetric Synthesis of a Challenging Atropisomeric HIV Integrase Inhibitor. Angew. Chem. Int. Ed. 2015, 54, 7144–7714. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Cyclopentyl Methyl Ether as a New and Alternative Process Solvent. Org. Process Res. Dev. 2007, 11, 251–258. [Google Scholar] [CrossRef]
- Meanwell, N.A. Improving Drug Candidates by Design: A Focus on Physicochemical Properties as a Means of Improving Compound Disposition and Safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. [Google Scholar] [CrossRef]
- Barrows, R.D.; Hammill, J.T.; Tran, M.C.; Falade, M.O.; Rice, A.L.; Davis, C.W.; Emge, T.J.; Rablen, P.R.; Guy, R.K.; Knapp, S. Evaluation of 1,1-Cyclopropylidene as a Thioether Isostere in the 4-Thiothienopyrimidine (TTP) Series of Antimalarials. Bioorg. Med. Chem. 2020, 28, 115758. [Google Scholar] [CrossRef]
- Capper, M.J.; O’Neill, P.M.; Fisher, N.; Strange, R.W.; Moss, D.; Ward, S.A.; Berry, N.G.; Lawrenson, A.S.; Hasnain, S.S.; Biagini, G.A.; et al. Antimalarial 4(1H)-Pyridones Bind to the Qi Site of Cytochrome Bc1. Proc. Natl. Acad. Sci. USA 2015, 112, 755–760. [Google Scholar] [CrossRef]
- Cross, R.M.; Manetsch, R. Divergent Route to Access Structurally Diverse 4-Quinolones via Mono or Sequential Cross-Couplings. J. Org. Chem. 2010, 75, 8654–8657. [Google Scholar] [CrossRef]
- Carrillo, A.K.; Kadayat, T.M.; Hwang, J.Y.; Chen, Y.; Zhu, F.; Holbrook, G.; Gillingwater, K.; Connelly, M.C.; Yang, L.; Kaiser, M.; et al. Antitrypanosomal Chloronitrobenzamides. J. Med. Chem. 2024, 67, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Wernevik, J.; Bergstrom, F.; Noven, A.; Hulthe, J.; Fredlund, L.; Addison, D.; Holmgren, J.; Stromstedt, P.-E.; Rehnstrom, E.; Lundback, T. A Fully Integrated Assay Panel for Early Drug Metabolism and Pharmacokinetics Profiling. Assay Drug Dev. Technol. 2020, 18, 157–179. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).