Abstract
Amidation of lactobionic acid with N,N-dimethylaminopropyltriamine was conducted to obtain N-(3′-dimethylaminopropyl)-lactamido-3-aminopropane (DDLPD), which was quaternized with bromoalkanes of different carbon chain lengths to synthesize double-stranded lactosylamide quaternary ammonium salt N-[N′[3-(lactosylamide)]propyl-N′-alkyl] propyl-N,N-dimethyl-N-alkylammonium bromide (CnDDLPB, n = 8, 10, 12, 14, 16). The surface activity and the adsorption and aggregation behaviors of the surfactants were investigated via equilibrium surface tension, dynamic light scattering, and cryo-electron microscopy measurements in an aqueous solution. The application properties of the products in terms of wettability, emulsification, foam properties, antistatic, salt resistance, and bacteriostatic properties were tested. CnDDLPB exhibited a low equilibrium surface tension of 27.82 mN/m. With an increase in the carbon chain length, the critical micellar concentration of CnDDLPBD decreased. Cryo-electron microscopy revealed that all products except C8DDLPB formed stable monolayer, multi-layer, and multi-compartmental vesicle structures in an aqueous solution. C14DDLPB has the best emulsification performance on soybean oil, with a time of 16.6 min; C14DDLPB has good wetting and spreading properties on polytetrafluoroethylene (PTFE) when the length of carbon chain is from 8 to 14, and the contact angle can be lowered to 33°~40°; CnDDLPB has low foam, which is typical of low-foaming products; C8DDLPB and C10DDLPB both show good antistatic properties. C8DDLPB and C14DDLPB have good salt resistance, and C12DDLPB has the best antimicrobial property, with the inhibition rate of 99.29% and 95.28% for E. coli and Gluconococcus aureus, respectively, at a concentration of 350 ppm.
1. Introduction
Among global environmental issues, carbon emissions have become a key issue that has received a lot of attention [1,2]. Of particular note is China’s high level of carbon emissions between 2009 and 2022 [3]. To address this challenge, China made a solemn commitment at the 75th session of the United Nations General Assembly to adopt dual carbon targets, i.e., carbon peaking and carbon neutrality, as a part of its core carbon reduction strategy. Owing to their green, environmentally friendly, and resource-saving characteristics, biobased chemicals are in alignment with the “dual-carbon” policy development; these chemicals are gradually becoming a new leading industry in the contemporary world of scientific and technological innovation and economic development. China’s “13th Five-Year Plan” and “14th Five-Year Plan” also clearly reveal that focus will be placed on the development of biobased industries [4]. Biobased chemicals, which are derived from renewable resources, are bulk chemicals and fine chemicals prepared from biomass as raw materials, exhibiting several advantages such as carbon reduction and sustainability [5,6,7]. Biobased chemicals produce very little waste during their production process, and most of this waste can be recycled due to their green and healthy characteristics. Their easily degradable nature also provides an effective way to solve the pollution problem of petroleum-based plastics [8,9]. Sugars are typical representatives of biomass raw materials and prepared from natural renewable resources such as starch, exhibiting not only abundant sources but also cost-effectiveness [10]. The use of sugar groups as hydrophilic groups in surfactants endows them with several advantages: (1) Sugar groups endow surfactants with considerably good biodegradation performance and toxicological properties. Moreover, they are mild and non-irritating to the skin and eyes as well as easily biodegradable under anaerobic and aerobic conditions [11,12,13]. (2) Sugar groups contain multiple hydroxyl groups, the oleophobicity and hydrophilicity of which is greater than those of conventional polyoxyethylene ether surfactants; the oleophobicity of a sec-hydroxyl group is 4.5 times greater than that of polyethylene oxide. In oil–water systems, sugar-based surfactants exhibit better interfacial chemistry [14]. (3) Sugar groups exhibit higher resistance to hard water because they can form complexes with metal ions such as Ca2+ to form water-soluble complex ions. (4) Sugar groups also endow surfactants with insecticidal and herbicidal or antimicrobial activity [15,16,17]. Introducing sugar groups into the molecular structure of cationic surfactants may improve their irritation, toxicity, biodegradability, water solubility, compatibility, etc. [18]. Various cationic surfactants derived from sugar groups exhibit advantages, including green characteristics, natural and renewable raw materials, facile biodegradability, and multifunctionality; these surfactants are also safe and mild for human body. Therefore, these surfactants have globally attracted considerable research attention [19,20,21].
Herein, a double-chain lactide amide quaternary surfactant (CnDDLPB), which combines the advantages of a sugar group, an amide bond, and a cationic surfactant, was synthesized via the amine ester reaction of lactobionic acid with an alkyl amine and then quaternized with bromoalkanes (the reaction and synthesis routes are shown in Figure 1), and the structures of the intermediates and products were characterized via Fourier transform infrared (IR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and mass spectrometry(MS). In addition, aggregation behavior and application properties of CnDDLPB were also comprehensively investigated.
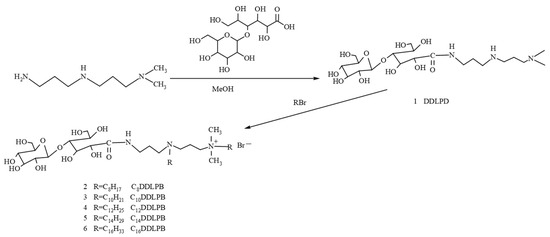
Figure 1.
Synthesis roadmap of double-chain lactosamine quaternary ammonium salts (CnDDLPB).
2. Results and Discussion
2.1. Structure Identification
The chemical structures of the raw materials, synthesized intermediates, and target compounds were characterized using FTIR, 1H NMR, and 13C NMR spectroscopies as well as other characterization methods. The spectra of all products are in the Supporting Information.
Figure S1a shows the FTIR spectrum of N,N-dimethyldipropylenetriamine. The peak observed at 2931 cm−1 corresponds to the symmetrical telescopic vibration of C–H of the methyl group, while the peak observed at 2762 cm−1 corresponds to the symmetrical telescopic vibration of C–H of the methylene group [22]. Figure S1b shows the FTIR spectrum of lactose. A broad and strong absorption peak observed at 3400–3200 cm−1 corresponds to the stretching vibration of the O–H group in lactobionic acid, the absorption peak observed at 1730 cm−1 corresponds to the stretching vibration of the C=O bond in lactobionic acid, and the strong peak observed at 1030 cm−1 corresponds to the C–O bond stretching vibration.
In the spectrum of DDLPD in Figure S1c, absorption peaks corresponding to the O–H group in lactobionic acid and C–H of N, N-dimethyldipropenyltriamine are observed; the two strong characteristic absorption peaks observed at 1650 and 1540 cm−1 correspond to the telescopic vibration of the C=O bond in amide groups and the bending vibration of N–H, respectively, indicating that amide bonds are generated.
2.2. Electrospray Mass Spectrometry (EMS)
The synthesized glycosylamide quaternary ammonium salt was analyzed via ESI-MS, and its composition was analyzed and identified according to its molecular weight: the relative molecular mass of the expected product C12DDLPB is 916. Figure S16 shows the ESI-MS spectrum of the target product C12DDLPB. The quaternary ammonium surfactant C12DDLPB exhibits an (M–H)+ quasimolecular ion peak at 915.35 (m/z). The quasimolecular ion is relatively stable after losing one electron; Br− is bombarded using the positive-ion mode, and a strong [M–Br]+ fragmentation peak is observed at m/z 836.86. The fragmentation peak of [M-Br- OH-OH]+ is observed at m/z 746.88, the fragmentation peak of [M-Br-CH3O-C2H5O2-O-O]+ is observed at m/z 612.55, and the fragmentation peak of [M-Br-CH3O-C2H5O2-OH-OH -H-H]+ is observed at m/z 608.85. The m/z values of the molecular ion peaks of C8DDLPB, C10DDLPB, C14DDLPB, and C16DDLPB are 803.49, 862.51, 974.38, and 1029.45, respectively, which are consistent with the expected molecular masses of the products (Supplementary Material Figures S14–S18) [23].
The combined FTIR spectroscopy, 1H NMR spectroscopy, 13CNMR spectroscopy, and ESI-MS results reveal that the double-stranded lactose amide quaternary surfactants are successfully synthesized.
2.3. Surface Tension
The surface tension of CnDDLPB was determined using the hanging drop method at 25 °C ± 0.1 °C. The surfactant solution was prepared from RO water; the surface tension of RO water was 72 ± 0.3 mN/m. The surface tension versus concentration curve reveals an inflection point in the concentration, corresponding to the critical micellar concentration (CMC). Before the turning point is reached (Figure 2), a linear relationship between the increase in the surfactant concentration and the decrease in surface tension is observed, indicating that the surfactant molecules begin to arrange closely at the interface. After reaching the CMC, the surface tension remains constant.
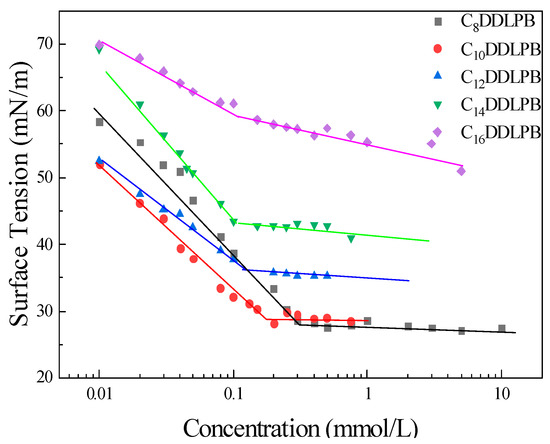
Figure 2.
Surface tension of CnDDLPB in an aqueous solution as a function of concentration.
According to Equations (1)–(3) [24,25], the amount of adsorption at saturation at the gas/liquid interface (Γmax), cross-sectional area per molecule Amin, and surface activity efficiency pC20 can be estimated:
where γ represents the surface tension, mN/m; T represents the absolute temperature, K; and R represents the gas constant, 8.314 J/(mol·K). Moreover, the value of n for an ionic surfactant type 1-1 is considered to be 2, NA represents the Avogadro constant, and C20 represents the concentration of the surfactant required to reduce the surface tension of water by 20 mN/m.
CMC/C20 indicates the difficulty and simplicity of the surfactant adsorption and micellization process. The higher CMC/C20, the easier the tendency for the surfactant to adsorb on the interface than that to form a micelle.
The CMC values of the five double-chain lactose amide quaternary ammonium salts (CnDDLPB) decrease in the order of C8DDLPB > C10DDLPB > C12DDLPB > C14DDLPB > C16DDLPB (Figure 3). Their CMC values decrease linearly with the growth of the hydrophobic carbon chain [26]. This result is attributed to the fact that, when two carbon chains of the surfactant molecule become longer, the space occupied by the molecules arranged at the interface is saturated more rapidly. The larger the space occupied by the two carbon chains of the surfactant molecules, the faster the molecules arranged at the interface reach saturation; at the same time, the longer the carbon chains, the hydrophobicity of the hydrocarbon bonds is gradually enhanced, indicating that the separation formed between the hydrophilic head groups under the action of electrostatic repulsive force is hindered, rendering a significant promotion effect on the development of aggregates in the surfactants. The shorter the carbon chain, the lower the surface tension. The surface tension of the C8DDLPB aqueous solution can be reduced to 27.82 mN/m. The inflection point of CMC on the curve of the change in the surface tension with the C16DDLPB concentration is not extremely clear, which may be attributed to the fact that the two hydrophobic carbon chains are extremely long and lead to poor solubility and a low surface activity.
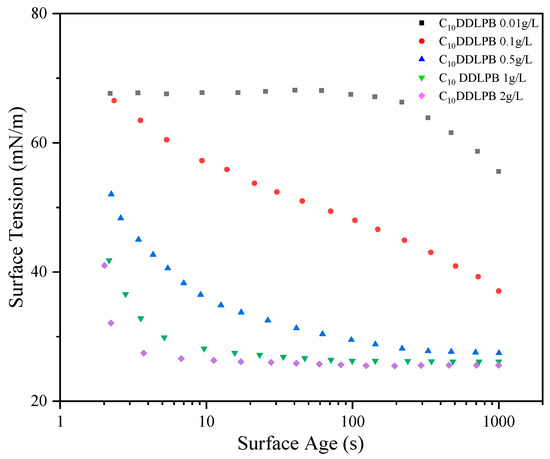
Figure 3.
Dynamic surface tensions vs. surface age for 0.01 g/L–2 g/L CnDDDLPB.
With an increase in the length of the carbon chain, Гmax decreases (Table 1). When the carbon chain becomes longer, the obstruction of the molecules to occupy a position in space increases. The possibility of the dense arrangement of molecules within the unit surface decreases, and the cross-sectional area Amin per molecule increases. The pC20 value of C10DDLPB is the highest, indicative of the higher adsorption efficiency at the interface.

Table 1.
Surface adsorption and aggregation parameters of CnDDLPB in aqueous solution.
2.4. Dynamic Surface Tension
Dynamic surface tension can be used to study the adsorption–diffusion kinetics of surfactants at the air–water interface. In this study, the dynamic surface tension changes in C10DDLPB were measured in the concentration range of 0.01 g/L–2 g/L by the suspended droplet method, and the dynamic surface tension of CnDDLPB with five different carbon chain lengths at a concentration of 1 g/L (25 °C) was measured in detail.
Figure 3 demonstrates the dynamic surface tension curves of C10DDLPB at different concentrations, from which it can be seen that the surface activity increases with increasing concentration, and the higher the concentration, the faster the adsorption rate.
Figure 4 demonstrates the surface tension versus surface age plots of CnDDLPB with different carbon chain lengths at 1 g/L concentration. With the increase in surface age, the surface tension of each surfactant gradually decreased and finally reached a stable value. This indicates that the CnDDLPB molecules undergo the process of diffusion–adsorption, reaching a steady state at the liquid surface. The ability to reduce the surface tension has a tendency to increase with the decrease in carbon chain length, which may be related to the fact that the shorter carbon chain molecules are easier to be arranged and form a compact structure at the interface. However, it is noteworthy that the adsorption rates of the surfactants increased with decreasing carbon chain length, except for C8DDLPB, for which this anomaly may be attributed to the shorter hydrophobic chain.

Figure 4.
Dynamic surface tensions vs. surface age for 1 g/L CnDDDLPB.
2.5. Aggregation Behavior in Aqueous Solutions
The aggregation behavior of CnDDLPB at a concentration of 5 g/L in aqueous solution was observed using cryo-transmission electron microscopy (Cryo-TEM) at a magnification of 92,000 times, as shown in Figure 5. Except for C8DDLPB, which forms ordinary micelles with a diameter of approximately 20 nm in an aqueous solution, the other samples form single-layered, multi-layered, or multi-compartmental vesicle structures, with vesicle diameters ranging from approximately 100 to 400 nm. Among them, C10DDLPB and C12DDLPB formed multi-compartmental vesicles, and C14DDLPB and C16DDLPB formed single-layer vesicle structures. Figure 5b,c and Supporting Information Figure S19b,c show the cryogenic transmission electron microscopy images of C10DDLPB samples kept for 1 month and 24 h, respectively. The magnification of the cryo-electron microscope in the Supporting Information is 45,000×. With an increase in the time of placing the samples, more vesicles are formed, indicating that the vesicle-forming system is more stable with an increase in the sample storage time and that more vesicles are aggregated to form a multi-compartmental vesicle structure. The results reveal that the vesicle-forming system is more stable with an increase in the sample storage time.
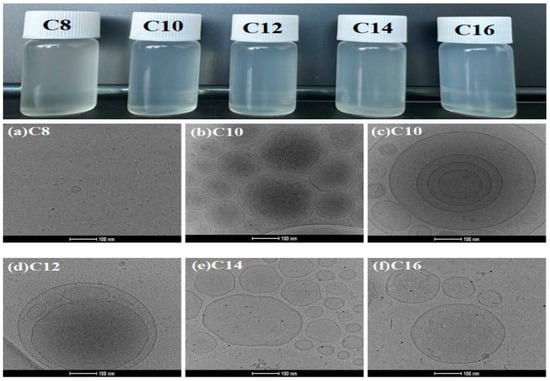
Figure 5.
Aggregation behavior of CnDDLPB under cryo-electron microscopy.
The appearance of the prepared solutions also reveals that C8DDLPB is a translucent light gray solution and that C10DDLPB-C16DDLPB is a light blue solution. During vesicle formation, surfactant molecules aggregate at the water–oil interface and form a closed structure with hydrophobic chains inside and hydrophilic chains outside. The length of the hydrophobic chains is critical to the stability of the vesicles. Surfactant molecular structures with two hydrocarbon chains and large head groups are prone to spontaneous vesicle formation [27,28,29]. The molecular structure of the surfactant synthesized herein (CnDDLPB) is in agreement with such features. However, in case of a short-carbon chain, sufficient hydrophobic forces cannot be provided to maintain the stability of vesicles, resulting in the facile rupture of vesicles. In addition, surfactant molecules with a short-carbon chains exhibit a high degree of expansion in an aqueous solution, indicating that the formation of tight aggregation structures by these short-carbon chains is difficult and that water molecules cannot be wrapped effectively to form complete vesicle structures.
The mechanism of CnDDLPB solution vesicle formation is shown in Figure 6: (1) First, CnDDLPB surfactant molecules are dissolved in water, and the hydrophilic head interacts with water molecules and is surrounded by them, which are dispersed in solution as monomers. (2) Formation of micelles: at the water/oil interface or water/air interface, the hydrophobic tails of the surfactant molecules interact with the oil or air phase, leading to the adsorption of the molecules on the interface. Formation of a stable interface: when the solution concentration reaches the CMC, the active molecules form monolayer micelles [30]. (3) Formation of flexible bilayers: With an increase in the concentration, the micelles begin to disperse and reorganize into flexible bilayer structures. With an increase in the size of the molecular films, they begin to inwardly bend spontaneously to form curved structures to reduce edge energy; this closure can be facilitated by hydrophobic forces between the hydrophobic tails [31,32]. (4) Unstable vesicles: when the bilayer is completely closed, small unstable vesicles are formed [33]. (5) Formation of stable large vesicles: Over time, multiple small vesicles merge with each other to form a stable large vesicle [34,35]. The ultimate stability of a vesicle depends on the balance between hydrophobic effects and surface tension. The stronger the interaction force of the hydrophobic tail, the higher the stability of the vesicle. At the same time, the film formed by surfactant molecules at the interface can also reduce the surface energy of the system and make the vesicle structure more stable. C10DDLPB and C12DDLPB exhibit the best balance of the hydrophobic effect and surface tension, which are more likely to aggregate on the membrane surface and induce the neighboring layers to come closer together, leading to the gradual stacking of the membrane layers to form multi-layer and multi-compartmental structures.
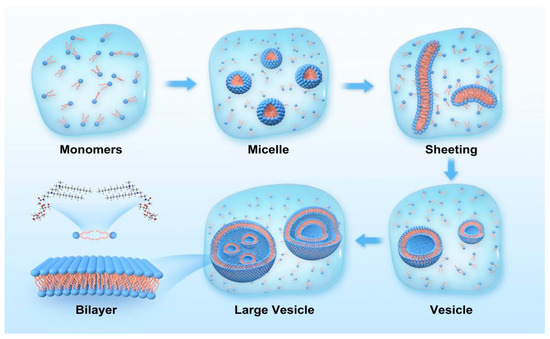
Figure 6.
Schematic diagram of the vesicle formation of CnDDLPB.
Most cationic surfactants cannot spontaneously form vesicles. Therefore, CnDDLPB with a long-carbon chain demonstrates good application prospects in slow-release drug carriers, template agents, biofilm mimicry, microreactors, and the cosmetic and food industries.
Figure 7 shows the particle size distribution of the 5 g/L CnDDLPB sample. CnDDLPB exhibits a single-peak particle distribution, and the overall particle sizes range from 20 nm to 1000 nm. The particle sizes of C16DDLPB and C10DDLPB exhibit a narrower distribution between 50 nm and 400 nm (Figure 7a,d), indicating that the diameters of the formed aggregates are mainly concentrated in this range. The particle size distribution of the C14DDLPB solution becomes narrower between 50 nm and 380 nm (Figure 7b), indicating that the distribution is more concentrated. The particle size distribution shown in Figure 7c is between 50 nm and 700 nm, which is a wider particle size distribution than those of other samples, indicating that the size of the formed aggregates is larger. As shown in Figure 7e, the particle size of this solution is mainly distributed between 20 nm and 80 nm, indicating that larger aggregates are not formed, which is consistent with the TEM results, and the system only forms micelles without vesicle formation.
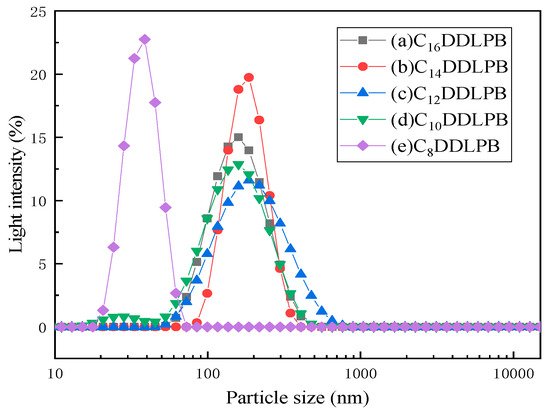
Figure 7.
Particle size distributions of (a) C16DDLPB, (b) C14DDLPB, (c) C12DDLPB, (d) C10DDLPB, and (e) C8DDLPB.
2.6. Wetting Ability
The changes in contact angles between different concentrations of CnDDLPB and PTFE over time are illustrated in Figure 8. It is evident from the graph that the contact angles of CnDDLPB on the surface of PTFE decrease with the increasing concentration.
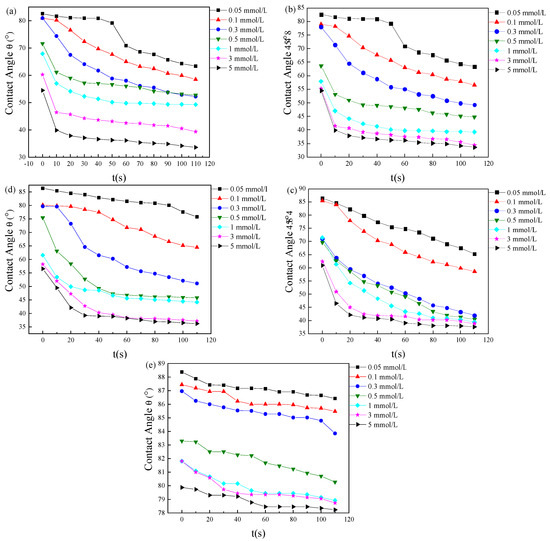
Figure 8.
The effect of surfactant concentration on the contact angle of PTFE surface: (a) C8DDLPB, (b) C10DDLPB, (c) C12DDLPB, (d) C14DDLPB, and (e) C16DDLPB.
In this study, C8DDLPB, C10DDLPB, C12DDLPB, and C14DDLPB have demonstrated favorable wetting properties on the PTFE surface, with contact angles reducing to 33°~38°. Particularly, C8DDLPB and C10DDLPB exhibit the ability to reduce the surface tension of water to 27.82 and 30.27 mN/m, respectively. Conversely, C16DDLPB shows the least wetting ability, with a contact angle only decreasing to 78° within 110 s. Its equilibrium surface tension value is measured at 59 mN/m. These findings suggest that the wetting effect of CnDDLPB on the PTFE surface is closely associated with its capability to lower the surface tension of water. Moreover, the impact of hydrophobic chain length in the hydrophobic group is notable [36,37].
2.7. Emulsifying Ability
The emulsification capacity test results for soybean oil are shown in Figure 9. As the carbon chain length increases, the emulsification capacity initially increases and then decreases. Specifically, C14DDLPB exhibits the best emulsification performance with an emulsification time of 16.6 min, while the emulsification effect is poorest with a carbon chain length of eight. Furthermore, when the hydrophobic chain length becomes too long, as in the case of C16DDLPB, the emulsification ability also weakens. This trend parallels that observed for the single-chain glucose acylamide quaternary ammonium salt CnDGMAPB synthesized by our research group [38]. The hydrophobic chain length of CnDDLPB significantly influences the emulsification capacity of soybean oil. Poor emulsification performance is observed with hydrophobic chain lengths of 8 and 16, whereas a moderate hydrophobic chain length demonstrates good emulsification.
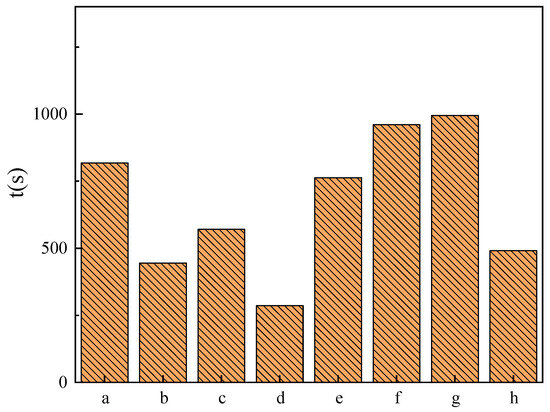
Figure 9.
Emulsification time of soybean oil by surfactants: (a) 1227, (b) 1231, (c) HACC, (d) C8DDLPB, (e) C10DDLPB, (f) C12DDLPB, (g) C14DDLPB, and (h) C16DDLPB.
2.8. Foam Properties
The foam performance of CnDDLPB surfactants in deionized water was evaluated using the Ross–Miles method, and the results are shown in Figure 10. From the graph, it is evident that C12DDLPB surfactant exhibits superior foamability, with a foam height reaching 67 mm. After 30 s, the foam height decreases by only 1 mm, and there is no further change in foam height over the subsequent 5 min, indicating excellent foam stability. However, compared to common anionic surfactants (foam height of >100 mm), it belongs to the category of low-foaming surfactants. C8DDLPB, C10DDLPB, and C14DDLPB initially exhibit similar foam heights of 25 mm, 25 mm, and 22 mm, respectively, indicating relatively poor foamability. On the other hand, the foam generated by C8DDLPB and C16DDLPB dissipates rapidly, classifying them as low-foaming surfactants overall. These surfactants hold promise for applications in low-foam detergents and related products.
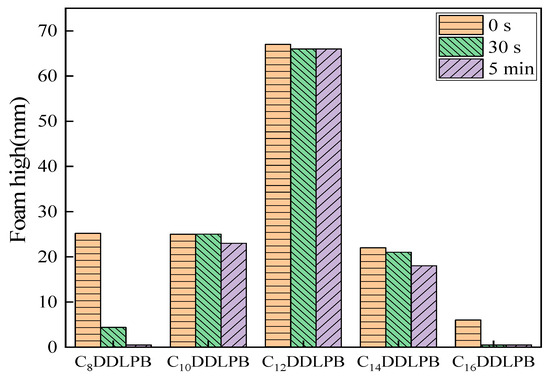
Figure 10.
Graph of the foam properties of CnDDLPB surfactants.
2.9. Antistatic Performance
The surface resistivity of polyester fabric before and after treatment was measured using a high-resistance meter, with the effectiveness of the surfactant’s antistatic properties evaluated based on the magnitude of the decrease in surface resistivity logarithm values (△lgρs). A higher △lgρs value indicates better antistatic performance. A series of synthesized lactose-based quaternary ammonium salt surfactants and commercially available antistatic agent, dihydroxyethyl stearylamine nitrate (SN), were tested, and the experimental results are presented in Table 2: at a concentration of 0.35 g/L, SN > C8DDLPB > C10DDLPB > C12DDLPB > C14DDLPB > C16DDLPB, with C8DDLPB exhibiting the largest △lgρs among the CnDDLPB surfactants, thereby indicating the best antistatic effect. The antistatic effects of C8DDLPB and C10DDLPB are approaching those of SN. Additionally, there is a trend of decreasing antistatic performance with increasing carbon chain length in CnDDLPB.

Table 2.
Antistatic properties of CnDDLPB surfactant.
From Table 2, it can be observed that the maximum adsorption (Гmax) of CnDDLPB decreases with the increase in carbon chain length. Among the synthesized surfactants, C8DDLPB and C10DDLPB exhibit the highest Гmax values, which are 1.890 × 10−10 mol·cm−2 and 1.675 × 10−10 mol·cm−2, respectively. These results suggest that their antistatic effects may be related to their adsorption on the fabric surface.
2.10. Salt-Resistant Performance
This study investigated the salt resistance of DDAC and synthesized CnDDLPB surfactants at different concentrations in various salt environments, as shown in Table 3, Table 4 and Table 5. When the concentration of sodium chloride added was 50 g/L, C8DDLPB and C14DDLPB exhibited transmittance rates of 94.19% and 99.08%, respectively, with excellent transmittance and no precipitation, indicating good salt resistance. In the presence of 35 g/L sodium chloride in the C12DDLPB solution, the transmittance was 93.76%, demonstrating some degree of salt resistance.

Table 3.
Permeability of surfactant solutions with NaCl concentration.

Table 4.
Permeability of surfactant solutions with CaCl2 concentration.

Table 5.
Permeability of surfactant solutions with MgSO4 concentration.
At a concentration of 50 g/L magnesium sulfate, C8DDLPB and C14DDLPB maintained transmittance rates of 99.54% and 86.90%, respectively, indicating strong salt resistance. In the presence of NaCl, CaCl2, and MgSO4, C8DDLPB consistently exhibited excellent salt resistance.
In comparison with DDAC, the series of CnDDLPB synthesized in this study have lactose-based hydrophilic groups containing numerous hydroxyl groups with strong negative polarity. Compared to micelles, these groups can readily attract more metal cations, resulting in a significant reduction in the concentration of unbound counterions in the solution, thereby hindering the formation of precipitates to some extent.
2.11. Antibacterial Performance
In this study, Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli) were used as samples to assess the antibacterial efficacy of C12DDLPB and C14DDLPB. Each group selected an appropriate dilution gradient for antibacterial rate calculation, utilizing the following formula:
where C denotes the average bacterial count of the control sample, and E denotes the average bacterial count of the experimental sample.
Quaternary ammonium salt surfactants carry a positive charge in water, allowing them to interact with negatively charged microbial surfaces, leading to adsorption and the formation of small aggregates that adhere to the cell wall. This process inhibits microbial growth. Additionally, the hydrophobic groups of these surfactants interact with the hydrophilic groups of microbial cells, altering the permeability of the cell membrane. This results in membrane damage and leakage of intracellular substances. Furthermore, the abundance of positive charges can coagulate and denature proteins within microbial cells, affecting cell metabolism and achieving disinfection and antibacterial effects [39].
From Table 6 and Table 7, it can be observed that at a concentration of 350 ppm, C12DDLPB exhibits excellent antibacterial performance against Escherichia coli and Staphylococcus aureus, with antibacterial rates reaching 99.29% and 95.28%, respectively. Conversely, C14DDLPB demonstrates poorer antibacterial efficacy, with antibacterial rates against Escherichia coli and Staphylococcus aureus at 73.20% and 76.91%, respectively. The antibacterial effect is more pronounced when the hydrophobic chain length is 12, which aligns with the previous literature [40]. Therefore, C12DDLPB holds promise as a novel disinfectant product for potential applications.

Table 6.
Inhibition of Escherichia coli by C12DDLPB and C14DDLPB.

Table 7.
Inhibition of Gluconococcus aureus by C12DDLPB and C14DDLPB.
3. Experimental
3.1. Materials and Instruments
Lactobionic acid (99%) was purchased from Aldrich, and chemically pure octyl bromide, decyl bromide, dodecyl bromide, tetradecyl bromide, and hexadecyl bromide were purchased from Shanghai Bohua Biochemical Reagent Co. Ltd., Shanghai, China, and N,N-dimethyl dipropylenetriamine (99.15%) was purchased from Guangdong Swell River Chemical Reagent Co. Ltd., Wengjiang, China. Deuterated dimethyl sulfoxide [DMSO] (99.9%) was purchased from Shanghai McLean Biochemical Technology Co., Shanghai, China. Didecyldimethylammonium chloride [DDAC] (95%) was purchased from Shanghai McLean Biochemical Science and Technology Co., Shanghai, China. Dodecyldimethylbenzylammonium chloride [1227] (99%) and dodecyltrimethylammonium chloride [1231] (99%) were purchased from Shanghai McLean Biochemical Science and Technology Co., Shanghai, China.
FT-IR spectrometer, model VERTEX 70 (Bruker, Saarbrücken, Germany); nuclear magnetic resonance (NMR) instrument, model AVANCE III (Bruker, Germany); electrospray ionization mass spectrometer, model Q Exactive (Thermofisher, Waltham, MA, USA); vacuum drying oven, model DZF-0B (China Yuejin Medical Equipment Co., Ltd., Shanghai, China); automatic surface tension meter, model KRÜSS-Tensíío (KRÜSS, Germany); drop shape analyzer, model DSA25B (KRÜSS, Hamburg, Germany); cryogenic transmission electron microscopy, model Talos F200C (Thermo Fisher, Waltham, MA, USA); and dynamic light scattering instrument, model JEM-1011EX (China Baxter Instruments Co., Ltd., Dandong, China), were utilized.
3.2. Synthesis of the Intermediate N-(3′-dimethylaminopropyl)-lactamido-3-aminop-ropane (DDLPD)
Lactobionic acid (0.1 mol) and N,N-dimethyldipropylenetriamine (0.12 mol) were added to 200 mL of methanol, and the reaction was conducted under reflux conditions for 2 h. After completion of the reaction, the heating was stopped, and the reaction was allowed to cool. The solvent was evaporated using a rotary evaporator, and the resulting product was washed thrice with ether to remove the residual N,N-dimethyldipropylenetriamine, followed by drying under vacuum until a constant weight was obtained, affording the intermediate DDLPD.
3.3. Synthesis of N-[N’[3-(lactosyl amide)]propyl-N’-alkyl]propyl-N,N-dimethyl-N-alkylammonium Bromide (CnDDLPB)
First, DDLPD (0.05 mol), bromoalkane (0.15 mol), and 200 mL of anhydrous methanol were added in a 250 mL three-neck round-bottom flask equipped with a thermometer and a spherical condenser tube. Second, the reaction was performed under reflux conditions for 10 h. After completion of the reaction, the heating was stopped, and the reaction was allowed to cool. The solvent was evaporated using a rotary evaporator, and the product was washed thrice with ether and dried under vacuum until a constant weight was obtained, affording the product CnDDLPB.
3.4. Characterization of CnDDLPB
The samples to be tested were tested using a VERTEX 70 Fourier transform infrared (FTIR) spectral analyzer, and the samples to be tested were pressed with KBr, which was mixed homogeneously with an appropriate amount of the samples to be tested. The raw materials N,N-dimethyldipropylenetriamine, lactobionic acid, DDLPD, and CnDDLPB were scanned in the wavelength range of 500–4000 cm−1, and the structure of the products was characterized by observing the characteristic peaks of the IR spectra generated after scanning.
The synthesized products were subjected to 1H-NMR and 13C-NMR measurements (internal standard was tetramethylsilane (TMS), and solvent was deuterated dimethylsulfoxide (DMSO)) using an AVANCE III NMR spectrometer, and the molecular structure of the synthesized compounds was analyzed by NMR spectroscopy.
The synthesized compounds were detected using Q Exactive electrospray ionization mass spectrometer and identified and analyzed by ESI-MS spectra.
3.5. Surface Tension
The surface tension of CnDDLPB was determined using a completely automated surface tension meter (KRÜSS-Tensíío type) according to the hanging drop method. The prepared solutions to be tested with different concentration gradients were allowed to stand for at least 24 h before the test and then measured after a constant temperature of 20 min was achieved at 25 °C [41].
3.6. Dynamic Surface Tension (DST)
A DSA25B tensiometer (Krüss Company, Hamburg, Germany) was used at 25.0 ± 0.1 °C to record dynamic surface tension (DST) data. The effective surface ages were within the range of 10 ms to 200 s, with an accuracy of ±0.01 mN/m.
3.7. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
First, an aliquot of 3.5 μL solution (3.0 mg/mL or 1.0 mg/mL) was applied to glow-discharged Quantifiol R 2/1 holey carbon grids and blotted for 3 s under a humidity of 100% at 4 °C before being plunged into liquid ethane using a Vitrobot Mark IV (Thermo Fisher Scientific, Waltham, MA, USA). The sample structure was immobilized in ice in a glassy state, and then, the sample attached to the copper mesh was transferred to a cryogenic transfer sample rod, followed by TEM imaging to observe the morphology of the 5 g/L solution of CnDDLPB during a stationary 2-week period [42].
3.8. Dynamic Light Scattering (DLS)
Reverse osmosis (RO) water was used to prepare 5 g/L of CnDDLPB (n = 8, 10, 12, 14, and 16) samples for measurement. The configured solution needed to be stable and homogeneous, and the temperature was set to 25 °C. An appropriate cuvette was selected, the angle of incident light and other parameters of the instrument were set, the solution (height of 1–1.5 cm) to be measured was added into the cuvette, and it was placed into the sample tank to start the measurement [43,44].
3.9. Wettability Study
According to the seated-drop method, the CnDDLPB solutions of various concentrations were aspirated using a micro-syringe at 25 °C and dropped onto a polytetrafluoroethylene (PTFE) film. The contact angle was measured using a contact angle meter, and photographs were taken, and the contact angle was recorded at 10 s intervals [45].
3.10. Emulsifying Performance
Emulsification performance analysis testing is commonly conducted using the cylinder method. In a 500 mL iodine flask, 40 mL of the test solution (1.0 g/L) and an equal volume of edible soybean oil are added. The mixture is vigorously shaken up and down five times, followed by a 1 min static period. This process is repeated five times. After completing the aforementioned steps, the mixture is quickly transferred to a 100 mL graduated cylinder, and timing begins immediately. The timing stops when 10 mL of water appears in the lower layer. The time taken for this process is recorded. This experiment is repeated five times to obtain the average value [46].
3.11. Foam Morphology Characterization
According to the Ross–Miles method, the foam performance of CnDDLPB was evaluated. The prepared solution (1 g/L) was placed in a constant-temperature water bath at (30 ± 0.5) °C for preheating for 30 min. Subsequently, the prepared solution was measured using a foam analyzer. The foam stability was determined based on the initial foam height and the subsequent change in foam height over a specified duration [47].
3.12. Antistatic Performance
The antistatic performance can be tested and analyzed according to GB/T 16801-2013. First, it is necessary to take the fabric finishing agent test solution to pre-treat the polyester fabric samples. Before and after the fabric samples are pre-treated, the surface resistivity is measured using a high resistance meter. The antistatic performance of the fabric finishing agent is evaluated based on the change in surface resistivity or its logarithmic value.
3.13. Salt-Resistant Performance
Prepare a 0.5 g/L solution of the surfactant. Transfer 10 mL of the solution into a test tube, followed by the addition of varying amounts of salt to create solutions of different concentrations. Allow the solutions to stand for 24 h. Subsequently, measure the transmittance of the solutions at 700 nm using a UV-Vis spectrophotometer [48].
3.14. Antibacterial Performance
Prepare a 35% drug stock solution using sterile water and sterilize it by filtration for subsequent use. Streak the tested Staphylococcus aureus and Escherichia coli on TSA plates and incubate them overnight at 37 °C until visible colonies appear. Select an appropriate quantity of colonies and transfer them to 5 mL of TSB liquid medium for incubation at a constant temperature of 37 °C for approximately 7 h, or until reaching an OD600 of approximately 0.6. Once the OD600 reaches 0.6 after adjustment with physiological saline, transfer the culture at a 0.1% ratio to TSB solution and incubate it at 37 °C for 18 h. Following incubation, dilute the treated bacterial suspension with PBS, and apply 100 μL of the diluted suspension onto TSA plates. Incubate the plates at 37 °C for 16 h, then photograph and count the colonies for quantification. Select an appropriate dilution gradient from each group for calculating the antibacterial rate.
4. Conclusions
In this study, double-chain lactobionic amide quaternary ammonium salts were synthesized by the amidation of lactobionic acid with N-N-dimethyldipropyltriamine to obtain glycosylamides, followed by quaternization with bromoalkanes of different chain lengths. The raw materials, intermediates, and target products were analyzed by Fourier transform infrared (FTIR) spectroscopy, proton nuclear magnetic resonance (1H NMR) spectroscopy, (13C NMR) spectroscopy, and electrospray ionization mass spectrometry (ESI-MS). The results indicated the successful synthesis of the target product. Through measurements including equilibrium surface tension, dynamic light scattering, and transmission electron microscopy, the surface activity, adsorption, and aggregation behavior of these compounds in aqueous solutions were investigated. Additionally, their application properties such as wetting ability, emulsification capability, foamability, antistatic performance, salt tolerance, and antibacterial activity were analyzed.
The CMC values of the five double-chain lactose amide quaternary ammonium salts (CnDDLPB) decreased in the order of C8DDLPB > C10DDLPB > C12DDLPB > C14DDLPB > C16DDLPB. With the growth of the carbon chain, the CMC of the lactose amide quaternary ammonium salts (CnDDLPB) decreased, and the products exhibited a good surface activity, which can reduce the surface tension of water to 27.82 mN/m. The solutions of compounds with carbon chain lengths ranging from 8 to 14 demonstrate favorable wetting and spreading properties on PTFE, with contact angles decreasing to 33°~40°.TEM images revealed that, except for C8DDLPB, the other products could form stable vesicle systems in an aqueous solution.
In terms of applications, C14DDLPB exhibits the best emulsification performance on soybean oil, with a time of 16.6 min. The foaming properties of CnDDLPB are generally low, characteristic of typical low-foaming products. Both C8DDLPB and C10DDLPB demonstrate excellent antistatic properties, comparable to the commonly used antistatic agent SN. C8DDLPB and C14DDLPB show good salt tolerance to NaCl, CaCl2, and MgSO4, with light transmittance exceeding 85% at a salt concentration of 50 g/L. Particularly, C12DDLPB displays excellent antibacterial activity against Escherichia coli and Staphylococcus aureus, with inhibition rates reaching 99.29% and 95.28%, respectively, at a concentration of 350 ppm.
Therefore, this product is a novel glucosamine-based cationic surfactant characterized by low foaming, antibacterial properties, antistatic properties, salt resistance, and the ability to form stable vesicular systems. It holds promise pertaining to applications in various fields such as drug delivery carriers, biomimetic membranes, microreactors, daily chemical industry, and food industry in the future.
Supplementary Materials
The following Supporting Information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29122749/s1, Figure S1: FT-IR spectra of (a) N-N-Dimethyldipropyltriamine, (b) Lactobionic Acid, (c) DDLPD; Figure S2: 1H-NMR spectra of DDLPD; Figure S3: 1H-NMR spectra of C8DDLPB ; Figure S4: 1H-NMR spectra of C10DDLPB ; Figure S5: 1H-NMR spectra of C12DDLPB ; Figure S6: 1H-NMR spectra of C14DDLPB ; Figure S7: 1H-NMR spectra of C16DDLPB; Figure S8: 13C-NMR spectra of DDLPD ; Figure S9: 13C-NMR spectra of C8DDLPB ; Figure S10: 13C-NMR spectra of C10DDLPB ; Figure S11: 13C-NMR spectra of C12DDLPB ; Figure S12: 13C-NMR spectra of C14DDLPB ; Figure S13: 13C-NMR spectra of C16DDLPB ; Figure S14: ESI-MS spectra of C8DDLPB ; Figure S15: ESI-MS spectra of C10DDLPB ; Figure S16: ESI-MS spectra of C12DDLPB ; Figure S17: ESI-MS spectra of C14DDLPB; Figure S18: ESI-MS spectra of C16DDLPB; Figure S19: cryo-EM spectra of CnDDLPB.
Author Contributions
Conceptualization, G.W. and Y.W. (Yan Wang); methodology, L.Z.; software, Z.C.; validation, X.L. (Xiaoming Li); formal analysis, X.L. (Xudong Liu); investigation, Y.W. (Yunkai Wang); resources, E.Z.; data curation, Y.H.; writing—original draft preparation, Y.W. (Yunkai Wang); writing—review and editing, Y.W. (Yunkai Wang); visualization, L.Z.; supervision, M.D.S.; project administration, Z.C.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the the Taiyuan University of Science and Technology Scientific Initial Funding (No. 20222010), Taiyuan University of Science and Technology Graduate Education Innovation Programme (No. SY2023023), The Nation Natural Science Foundation of China (No. 22178240) for the financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Author Ying Huang was employed by the company Taiyuan Hengdeyuan Animal Protection Technology Development and Shanxi Livestock and Poultry Breeding. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Du, Q.; Li, Z.; Du, M.; Yang, T. Tianle. Government venture capital and innovation performance in alternative energy production: The moderating role of environmental regulation and capital market activity. Energy Econ. 2024, 129, 107196. [Google Scholar] [CrossRef]
- Yang, T.; Fang, S.; Du, A.; Du, Q. Navigating the nexus: Geopolitical risk, fossil energy prices, and European utility stock returns—Implications for environmental management and energy security in a conflict-ridden global landscape. J. Environ. Manag. 2024, 352, 120086. [Google Scholar]
- Yang, T.; Dong, Q.; Du, Q.; Du, Q.; Du, M. Carbon dioxide emissions and Chinese OFDI: From the perspective of carbon neutrality targets and environmental management of home country. J. Environ. Manag. 2021, 295, 113120. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Hu, Y.; Yu, J. Research Progress on Bio-based Vitrimer Materials. Biomass Chem. Eng. 2023, 57, 37–46. [Google Scholar]
- Xue, X.; Wang, S.; Yang, T.; He, M.; Tang, X. Preparation and photocatalytic performance of Cu/cellulose-based TiO2 composite. New Chem. Mater. 2022, 50, 195–200. [Google Scholar]
- Gericke, M.; Amaral, A.J.R.; Budtova, T.; Tatiana, B.; Pieter, D.; Thomas, G.; Thomas, H.; Herman, H.; Anton, H.; Olli, I.; et al. The European Polysaccharide Network of Excellence (EPNOE) research roadmap 2040: Advanced strategies for exploiting the vast potential of polysaccharides as renewable bioresources. Carbohydr. Polym. 2024, 326, 121633. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Magallanes, L.; Tarditto, L.; María C, P.; María F, G. María. Fatty acids methyl esters from soybean oil for biobased surfactants industry: Obtention C16 C18 concentrate for use as feedstock. Ind. Crops Prod. 2022, 190, 115892. [Google Scholar] [CrossRef]
- Reid, A.G.; Eswara Rao, C.A.; Abhay, A.; Taylor, U.; Ravikumar R, G.; Avantika, S.; Jason S, D.; Gregg T, B.; Eugene, C. Bio-based lactone acrylic plastics with performance and recyclability advantages. Cell Rep. Phys. Sci. 2024, 5, 101938. [Google Scholar]
- Thuy, T.H.N.; Wahyu, S.P.; Jun-Chul, C.; Norihisa, F.; Satoshi, T.; Takehiro, Y.; Nobuo, H.; Sho, K. Design and Evaluation of Bio-Based Industrial Symbiosis System Producing Energy and Chemicals Using Regionally Available Crop Residue. Resour. Conserv. Recycl. 2024, 204, 107509. [Google Scholar]
- Lu, H.; Pezron, I.; Gaudin, T.; Audrey, D. Non-equilibrium micelles formed by sugar-based surfactants under their Krafft temperature. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 167–176. [Google Scholar] [CrossRef]
- Syguda, A.; Ławniczak, Ł.; Wróbel, P.; Filip, W.; Grzegorz, F.; Anna, P.; Marta, W.; Michał, N.; Aleksandra, G.; Łukasz, C. Biodegradable amidequats, derivatives of caprylic and pelargonic acids as cationic surfactants for agricultural applications. J. Mol. Liq. 2023, 391, 123221. [Google Scholar] [CrossRef]
- Siddiqui, U.; Aslam, J.; Ansari, W.; Din, K. Micellization and aggregation behavior of a series of cationic gemini surfactants (m-s-m type) on their interaction with a biodegradable sugar-based surfactant (octyl-β-D-glucopyranoside). Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 164–172. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Yu, H.; JongChoo, L. Synthesis of environment friendly nonionic surfactants from sugar base and characterization of interfacial properties for detergent application. J. Ind. Eng. Chem. 2016, 38, 157–166. [Google Scholar] [CrossRef]
- Guido, V.; Pierluigi, Q.; Claudia, B.; Savarino, P.; Barni, E.; Fisicaro, E. Synthesis and Surface and Antimicrobial Properties of Novel Cationic Surfactants. J. Org. Chem. 2000, 65, 8197–8203. [Google Scholar]
- Wojciech, S.; Natalia, B.; Michal, H. Evaluation of surface active and antimicrobial properties of alkyl D-lyxosides and alkyl L-rhamnosides as green surfactants. Chemosphere 2021, 271, 129818. [Google Scholar]
- Zhi, L.; Zhang, E.; Shi, X. Research progress of glycosyl cationic surfactants. Huaxue Tongbao 2022, 85, 790–801. [Google Scholar]
- Lu, B.; Vayssade, M.; Miao, Y.; Vincen, C.; Eric, G.; Anne, W.; Denis, P.; Audrey, D.; Christophe, E.; Isabelle, P. Physico-chemical properties and cytotoxic effects of sugar-based surfactants: Impact of structural variations. Colloids Surf. B Biointerfaces 2016, 145, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yu, L.; Cheng, G. Synthesis characterization and application of Bola silicone quaternary ammonium salt. CIESC J. 2021, 72, 2837–2848. [Google Scholar]
- Vargas-Ruiz, S.; Soltwedel, O.; Micciulla, S. Sugar Surfactant Based Microemulsions at Solid Surfaces: Influence of the Oil Type and Surface Polarity. Langmuir ACS J. Surf. Colloids 2016, 32, 11928–11938. [Google Scholar] [CrossRef]
- Salomé, V.; Jana, L.; Regine, V.; Thomas, H.; Stefan, W. Wetting of planar solid surfaces by bicontinuous sugar surfactant-based microemulsions. Colloid Polym. Sci. 2017, 295, 2183–2190. [Google Scholar]
- Wang, X.; Hao, J. Ionogels of Sugar Surfactant in Ethylammonium Nitrate: Phase Transition from Closely Packed Bilayers to Right-Handed Twisted Ribbons. J. Phys. Chem. B 2015, 119, 13321–13329. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Sun, Y.; Sun, J. Properties of ethoxylated tris-ester-based quaternary ammonium salts. Deterg. Cosmet. 2014, 37, 69–71. [Google Scholar]
- Dufour, A.; Thiébaut, D.; Ligiero, L.; Matthieu, L.; Vial, J. Chromatographic behavior and characterization of polydisperse surfactants using Ultra-High-Performance Liquid Chromatography hyphenated to High-Resolution Mass Spectrometry. J. Chromatogr. A 2020, 1614, 460731. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 336–367. [Google Scholar]
- Fainerman, V.B.; Miller, R.; Aksenenko, E.V.; Makievski, A.V. 3. Equilibrium adsorption properties of single and mixed surfactant solutions. Stud. Interface Sci. 2001, 13, 189–285. [Google Scholar]
- Wang, T.; Xu, P.; Ma, A. Effect of hydrophobic chain length on surface activity of gemini betaine. Chem. Res. Appl. 2021, 33, 2210–2216. [Google Scholar]
- Aratono, M.; Onimaru, N.; Yoshikai, Y.; Makiko, S.; Ikuyo, K.; Kanda, W.; Akio, O.; Takanori, T.; Belkoura, L.; Reinhard, S.; et al. Spontaneous vesicle formation of single chain and double chain cationic surfactant mixtures. J. Phys. Chem. B 2007, 111, 107–115. [Google Scholar] [CrossRef]
- Johnsson, M.; Wagenaar, A.; Engberts, J.B. Sugar-based gemini surfactant with a vesicle-to-micelle transition at acidic pH and a reversible vesicle flocculation near neutral pH. J. Am. Chem. Soc. 2003, 125, 757–760. [Google Scholar] [CrossRef]
- Yun, Y.; Xiong, W.; Li, X.; Lu, T.; Huang, G.; Li, Z.; Fu, H. Molecular Packing Parameter in Bolaamphiphile Solutions: Adjustment of Aggregate Morphology by Modifying the Solution Conditions. J. Phys. Chem. B 2007, 111, 2225–2230. [Google Scholar] [CrossRef]
- Gong, J.; Song, Y.; Sun, Y.; Sun, Q.; Liu, C.; Tan, J.; Zhao, L.; Xu, B. Vesicle-to-micelle transition in a double chain quaternary ammonium surfactant system: Interfacial behavior and molecular insights. J. Mol. Liq. 2024, 394, 123714. [Google Scholar] [CrossRef]
- Xia, Y.; Goldmints, I.; Johnson, P.; Alan, T.; Arijit, B. Temporal Evolution of Microstructures in Aqueous CTAB/SOS and CTAB/HDBS Solutions. Langmuir ACS J. Surf. Colloids 2002, 18, 3822–3828. [Google Scholar] [CrossRef]
- Hsieh, A.; Franses, E.; Corti, D. Formation of gem-like dispersions of soft crystallites in water by vesicles of a cationic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129822. [Google Scholar] [CrossRef]
- Surajit, G.; Chiranjib, G.; Chiranjib, B.; Sarthak, M.; Jagannath, K.; Nilmoni, S. Spontaneous Transition of Micelle–Vesicle–Micelle in a Mixture of Cationic Surfactant and Anionic Surfactant-like Ionic Liquid: A Pure Nonlipid Small Unilamellar Vesicular Template Used for Solvent and Rotational Relaxation Study. Langmuir ACS J. Surf. Colloids 2013, 29, 10066–10076. [Google Scholar]
- Akihisa, S.; Alan, H. Model for Formation and Growth of Vesicles in Mixed Anionic/Cationic (SOS/CTAB) Surfactant Systems. Langmuir ACS J. Surf. Colloids 2002, 18, 7341–7348. [Google Scholar]
- Zhang, B.; Yao, X.; Li, P.; Guo, C.; Ren, X.; Li, J. Preparation of chitosan sulfate and vesicle formation with a conventional cationic surfactant. Carbohydr. Polym. 2018, 183, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, K.; Wang, Y.; Zheng, Z.; Zhang, C.; Gao, Y.; Du, F. Droplet splash and spread on superhydrophobic lotus leaves: Direct regulation by tuning the chain length of surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129178. [Google Scholar] [CrossRef]
- Katarzyna, M.; Katarzyna, S.; Daniela, G.; Daria, W. Synthesis, Surface and Antimicrobial Activity of New Lactose-Based Surfactants. Molecules 2019, 24, 4010. [Google Scholar]
- Zhi, L. Synthesis and Properties of Novel Gluconamide-Type Surfactants; ShanXi University: Taiyuan, China, 2014. [Google Scholar]
- Jiang, J.; Zou, Y.; Sun, Q. Copolymers functionalized with quaternary ammonium compounds under template chain exhibit simultaneously efficient bactericidal and flocculation properties: Characterization, performance and mechanism. J. Hazard. Mater. 2024, 465, 133476. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X.; Chen, Y.; Zou, W. Bactericidal activity of sugar-based cationic gemini surfactants. China Surfactant Deterg. Cosmet. 2019, 49, 83–86. [Google Scholar]
- Dou, J.; Liu, J.; Wang, G. Surface Activity, Wetting, and Aggregation of a Perfluoropolyether Quaternary Ammonium Salt Surfactant with a Hydroxyethyl Group. Molecules 2023, 28, 7151. [Google Scholar] [CrossRef]
- Ram-On, M.; Cohen, Y.; Talmon, Y. Effect of Polyelectrolyte Stiffness and Solution pH on the Nanostructure of Complexes Formed by Cationic Amphiphiles and Negatively Charged Polyelectrolytes. J. Phys. Chem. B 2016, 120, 5907–5915. [Google Scholar] [CrossRef]
- Matthew, L.L.; Tom, K.; Michael, R.W. Anticipating colloidal instabilities in cationic vesicle dispersions by measuring collective motions with dynamic light scattering. J. Colloid Interface Sci. 2006, 296, 599–607. [Google Scholar]
- Milad, E.; Karin, S.; Stoyan, I.; Nikolay, G.; Kerstin, E. Oppositely charged surfactants and nanoparticles at the air-water interface: Influence of surfactant to nanoparticle ratio. J. Colloid Interface Sci. 2024, 653, 1388–1401. [Google Scholar]
- Shi, X.; Zeng, M.; Xu, X.; Liu, Y.; Kou, J.; Bian, Q.; Song, H.; Zhang, J.; Wang, Q. Promotion of droplet deposition, diffusion-wetting and retention on hydrophobic surfaces by nonionic star-shaped oligomeric surfactants. J. Mol. Liq. 2023, 386, 122521. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, W.; Jiang, Y. Synthesis and properties of a zwitterionic Gemini surfactant. Fine Chem. 2021, 38, 335–340. [Google Scholar]
- Ning, B.; Zhang, M.; Bai, Y.; Wang, W.; Wang, G. Comparison of the properties of perfluoroalkyl polyoxyethylene ether and alkyl polyoxyethylene ether. Colloid Polym. Sci. 2020, 298, 1389–1399. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z.; Li, Y. Synthesis and application performance of branched chain alcohol ether sodium sulfate based on SO3 sulfonation. Text. Aux. 2022, 39, 37–40. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).